Re-evaluation of the genus Englerodendron (Leguminosae–Detarioideae), including Isomacrolobium and Pseudomacrolobium
Manuel de la Estrella A D , Jan J. Wieringa B , Frans J. Breteler B and Dario I. Ojeda
A D , Jan J. Wieringa B , Frans J. Breteler B and Dario I. Ojeda  C
C
A Departamento de Botánica, Ecología y Fisiología Vegetal, Edificio C-4, Celestino Mutis, Campus de Rabanales, Universidad de Córdoba, E-14071 Córdoba, Spain.
B Naturalis Biodiversity Centre, National Herbarium of the Netherlands, Darwinweg 2, NL-2333 CR, Leiden, Netherlands.
C Department of Forest Genetics and Biodiversity, Norwegian Institute of Bioeconomy Research (NIBIO), Høgskoleveien 8, N-1433 Ås, Norway.
D Corresponding author. Email: mdelaestrella@gmail.com
Australian Systematic Botany 32(6) 564-571 https://doi.org/10.1071/SB18075
Submitted: 22 December 2018 Accepted: 8 August 2019 Published: 11 October 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
On the basis of a new phylogeny of the Detarioideae, with a particular focus on Englerodendron Harms, Anthonotha P.Beauv. and related genera, the possible options for delimiting monophyletic genera are discussed. As a result, Isomacrolobium Aubrév. & Pellegr. and Pseudomacrolobium Hauman are synonymised under Englerodendron. The following 12 new combinations are formed within the expanded Englerodendron: E. brachyrhachis (Breteler) Estrella & Ojeda, E. explicans (Baill.) Estrella & Ojeda, E. graciliflorum (Harms) Estrella & Ojeda, E. hallei (Aubrév.) Estrella & Ojeda, E. isopetalum (Harms) Breteler & Wieringa, E. lebrunii (J.Léonard) Estrella & Ojeda, E. leptorrhachis (Harms) Estrella & Ojeda, E. mengei (De Wild.) Estrella & Ojeda, E. nigericum (Baker f.) Estrella & Ojeda, E. obanense (Baker f.) Estrella & Ojeda, E. triplisomere (Pellegr.) Estrella & Ojeda and E. vignei (Hoyle) Estrella & Ojeda. A key to identification of the 17 species now recognised within Englerodendron is presented.
Additional keywords: Africa, Anthonotha, classification, generic delimitation, Macrolobium, monophyly.
Introduction
The tribe Amherstieae Benth. is the most diverse group within subfamily Detarioideae of the Leguminosae, with 50 genera and ~570 species being currently recognised (Legume Phylogeny Working Group 2013, 2017; de la Estrella et al. 2018). Within this tribe, floral morphology is extremely variable and, in many taxa, highly modified, making classification of the group very challenging (Mackinder 2005; de la Estrella et al. 2018). Flowers vary from bilaterally to radially symmetrical, with sepals sometimes entirely lacking but most commonly having 4–5 (rarely up to 10) sepals. Petal number is also extremely variable. Five petals are found in many species and this seems to be the ancestral state (Ojeda et al. 2019); however, several reduction series exist, often resulting in one large adaxial petal with the other four being reduced or absent. In a few genera, all petals have disappeared. In Englerodendron usambarense Harms, an additional one or two petals are present, resulting in six- or seven-merous flowers (Breteler 2008). Stamen numbers in tribe Amherstieae follow trends similar to those seen in the petal number. The ancestral value is likely to be 10, which is reduced in several lineages, often resulting in three fertile stamens and several staminodes, but these staminodes may disappear in a later step (Ojeda et al. 2019). In some cases, the stamen number is increased, being, for example, ~20 in some species of Hymenostegia Harms, and up to 80 in Maniltoa Scheff. Reduction in stamen number, including transformation to staminodes as an intermediate step towards complete loss, appears to be a general trend observed in several groups (Bruneau et al. 2014), potentially being correlated with flower size. Indeed, members of the genus with the largest flowers in the Amherstieae (Berlinia Sol. ex Hook.f.) have 10 stamens and no or partial reduction in the numbers of sepals and petals. However, not all lineages with small flowers have a reduced stamen number. Some genera with small flowers, such as Michelsonia Hauman, Bikinia Wieringa and Zenkerella Taub., have 10 stamens (Cowan and Polhill 1981; Wieringa 1999). One consequence of this variability is that the number of stamens and staminodes appears to be highly homoplasious and does not provide a good basis for classification. Some of the most extreme variation in floral traits within Amherstieae is found within the Berlinia clade (sensu Bruneau et al. 2008), where great plasticity in the number of petals is observed within the same species and among flowers of the same individual (Breteler 2006, 2008, 2010, 2011; Ojeda et al. 2019). For example, Breteler (2008) observed that the corolla in Isomacrolobium Aubrév. & Pellegr. is extremely labile, which hampered any attempt to structure the observed variability into distinct taxonomic entities. The only distinction Breteler (l.c.) could make was between an actinomorphic and a zygomorphic corolla.
The Berlinia clade was first described by Bruneau et al. (2000, 2001) as the ‘Macrolobieae’ clade, and further delimited to include 18 exclusively African genera (Bruneau et al. 2008). The ancestral biome of this clade was reconstructed as primary evergreen rainforest in tropical Africa (de la Estrella et al. 2017), where Detarioideae, and especially members of the Berlinia clade, form the most dominant group of trees in western central Africa (Wieringa 1999; Newbery et al. 2013).
Within the Berlinia clade, several genera have been the focus of recent taxonomic treatments, increasing our knowledge of their diversity and morphological complexity (Wieringa 1999; Mackinder and Pennington 2011; de la Estrella et al. 2012a, 2012b; de la Estrella and Devesa 2014; van der Burgt et al. 2015). Anthonotha P.Beauv., Isomacrolobium and Englerodendron Harms, which show particularly complex floral ontogeny, were recently revised by Breteler (2006, 2008, 2010, 2011).
Anthonotha was first described by Palisot de Beauvois (1806) to accommodate a new species from West Africa, but subsequently described African taxa were initially placed within a pantropical Macrolobium Schreb. (see de la Estrella and Devesa (2014), for a history review of the group). Although it is the most speciose genus within Detarioideae and was previously posited as being non-monophyletic (Mackinder 2005), Macrolobium, as currently circumscribed, is an American genus recently demonstrated to be monophyletic (Murphy et al. 2018). The African taxa previously treated under Macrolobium had already been accommodated by Léonard (1952, 1954, 1955) within a reinstated Anthonotha and the newly published Gilbertiodendron J.Léonard, Paramacrolobium J.Léonard and Pellegriniodendron J.Léonard. Isomacrolobium and Triplisomeris Aubrév. & Pellegr. were subsequently split off from Anthonotha (Aubréville and Pellegrin 1958 and Aubréville 1959 respectively) and Aubréville (1968) added a further segregate genus, namely, Leonardendron Aubrév. Breteler (2006, 2008, 2010, 2011), accepted Anthonotha sensu Aubréville & Pellegrin (Aubréville 1959) but rearranged the other species into Isomacrolobium (zygomorphic flowers) and Englerodendron (non-zygomorphic flowers). Gilbertiodendron and related genera have been the subject of further taxonomic and phylogenetic studies (de la Estrella et al. 2012a, 2014), but relationships among other Berlinia clade genera, especially those of Anthonotha and related genera, have remained obscure (de la Estrella et al. 2017, 2018).
The new phylogenomic framework
With the aim to resolve relationships within Detarioideae, Ojeda et al. (2019) developed a target capture-sequence bait set for the subfamily, by selecting orthologues shared among four representative transcriptomes from the group. Target capture sequence (Hybseq) has proven to be an effective strategy for phylogenetic reconstruction across different taxonomic levels (Vatanparast et al. 2018; Villaverde et al. 2018). With this Detarioideae bait set (ver. 1.0, MYbaits, Arbor Biosciences, Ann Arbor, MI, USA), Ojeda et al. (2019) reconstructed a phylogeny for Anthonotha and closely related genera, which included near-complete species sampling of Anthonotha, Isomacrolobium (except I. sargosii (Pellegr.) Aubrév. & Pellegr. and I. hallei Aubrév.) and Englerodendron, the three genera that had been previously recognised within the group (Bruneau et al. 2000, 2001, 2008; de la Estrella et al. 2018). With near-complete genus-level sampling across the subfamily, the previously unplaced Pseudomacrolobium Hauman, a monospecific African endemic genus from Congo (Kinshasa), was shown to be closely related to Englerodendron and Isomacrolobium (Ojeda et al. 2019). The Ojeda et al. (2019) phylogeny (Fig. 1) shows more robust support across the clades than does any previous study. Two main clades are resolved, one including all Anthonotha species and the other containing the three remaining genera of Englerodendron, Isomacrolobium and Pseudomacrolobium. However, neither Englerodendron nor Isomacrolobium is monophyletic in this phylogeny (Fig. 1), leaving several options for generic re-delimitation.
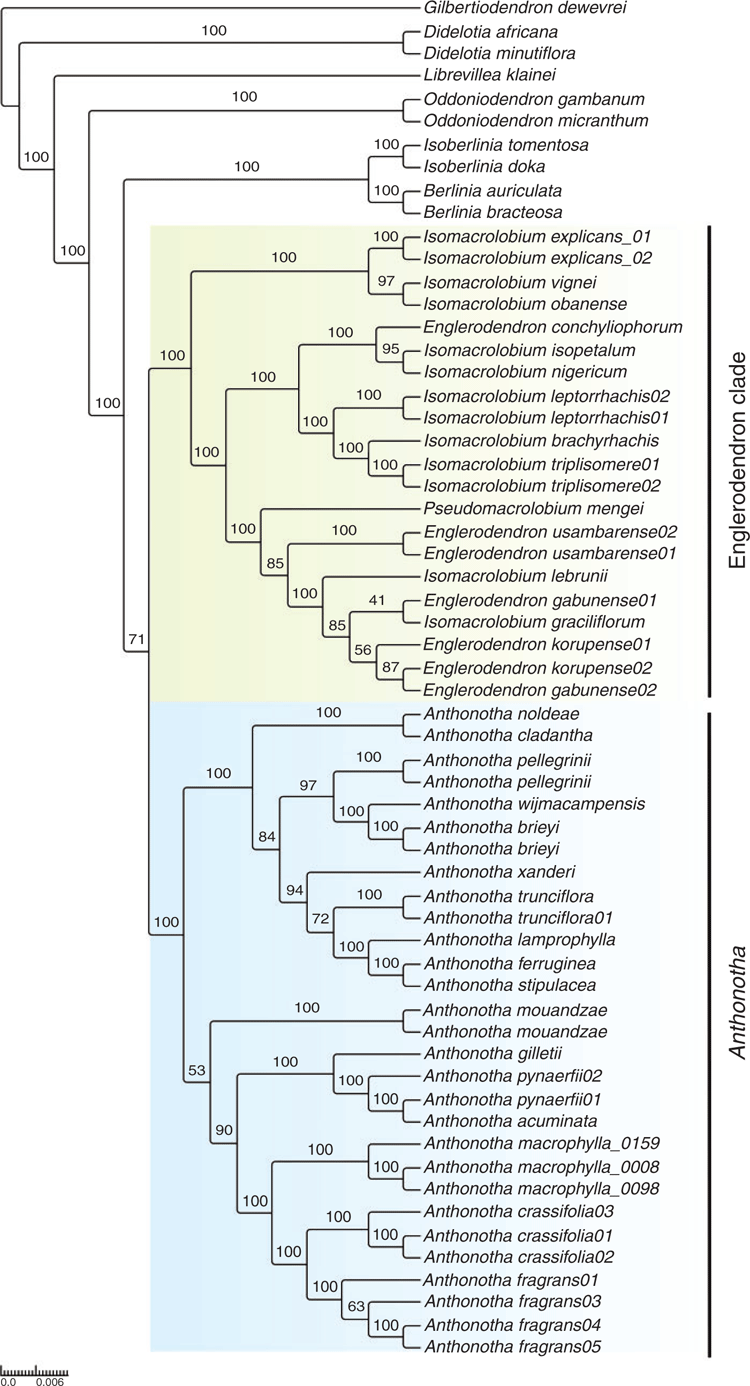
|
Generic circumscription
The Englerodendron clade as shown in Fig. 1 could be split into different recircumscribed genera (Englerodendron, Isomacrolobium, Pseudomacrolobium); however, this would require reinstatement of genera currently placed in synonymy under Isomacrolobium, and the resulting genera would not be diagnosable morphologically. Breteler (2006, 2008, 2011) previously recognised that the members of this group exhibit extensive floral variation, and the phylogenetic evidence (Fig. 1) supports the option, chosen here, of combining these three genera within an enlarged Englerodendron (Ojeda et al. 2019).
Most of the morphological differences given by Breteler (2008) to differentiate Anthonotha from Isomacrolobium apply equally to the newly circumscribed Englerodendron, even though, in that paper, Breteler did not consider Englerodendron because he assumed it to be more closely related to Oddoniodendron De Wild. and Isoberlinia Craib & Stapf ex Holland (Breteler 2006). However, these characters apply to his Englerodendron species as well, just as they do for Pseudomacrolobium, which, because of its many stamens (10–13), has never previously been considered as being related to this clade. Anthonotha is characterised by the presence of a single large petal in the adaxial position, which has a well-developed, inrolled, gutter-shaped claw that is at least half as long as the lamina and the lamina is always bilobed or divided into two. The other four petals may be reduced in various stages, or absent. Englerodendron in this new circumscription has (1–)2–5 large petals, whereas the other petals may be reduced in various stages or be absent. Where only a single petal remains (possible in E. vignei (Hoyle) Estrella & Ojeda), it is not located in the adaxial position. Petals may be clawed, but the claw is often short and not inrolled into a solid support for the laminae, and the lamina of the adaxial petal may be lobed or split, although not as strongly as it is in Anthonotha. In addition to these floral differences, Anthonotha can be distinguished by its dense, usually persistent (except in A. trunciflora (Harms) J.Léonard), appressed indumentum on the lower leaflet surfaces, whereas the leaflets of Englerodendron are sparsely hairy to glabrous, the indumentum not obscuring the lower leaflet surfaces.
Taxonomic treatment
Englerodendron Harms, Bot. Jahrb. Syst. 40(1): 27–30, fig. 2 (1907)
Type species: Englerodendron usambarense Harms.
Type species: Pseudomacrolobium mengei (De Wild.) Hauman.
Type species: Isomacrolobium leptorrhachis (Harms) Aubrév. & Pellegr.
Type species: Triplisomeris explicans (Baill.) Aubrév. & Pellegr.
Type species: Leonardendron gabunense (J.Léonard) Aubrév.
Trees or shrubs, up to 36 m tall and 85-cm diameter at breast height. Stipules free or united at base. Leaves paripinnate, 1–7-jugate, leaflets opposite to subopposite, glabrous to sparsely hairy below. Inflorescence of most species a compound raceme up to 1.25 m long, but, in some species, shorter and not pendulous. Bracteoles covering the flower in bud. Flowers 5-merous (6- or 7-merous in E. usambarense). Sepals 5(–7), or 4 when the two adaxial ones are united. Petals (1–)2–5(–7), base often narrowed into a claw, but claw not stiff or inrolled. Fertile stamens 3–7 or 10(–13), supplemented by up to 7 staminodes. Ovary 2–8 ovulate. Pods leathery to woody, (explosively) dehiscent or only dehiscent once on the forest floor, laterally flattened, in general oblong, depauperate pods also (ob)ovate.
Seventeen species, distributed in rain forest of tropical Africa from Guinea to Congo (Kinshasa) and one disjunct species in the Usambara Mountains of Tanzania (Englerodendron usambarense) (Robyns 1952; Breteler 2006, 2008, 2011; van der Burgt et al. 2007).
1. Englerodendron brachyrhachis (Breteler) Estrella & Ojeda, comb. nov.
Type: Gabon, 50 km south-east of Achouka, A.M.Louis, Breteler & de Bruijn 729 (holo: WAG [3 sheets]!; iso: BR!, K!, LBV!, MA!, MO!, P!, PRE!).
2. Englerodendron conchyliophorum (Pellegr.) Breteler, Adansonia 28(1): 109 (2006)
Type: Gabon, near Lastoursville, Micouma, 23 Nov. 1929, M.G. Le Testu 7680 (lecto: P[2 sheets]!; isolecto: BM, BR [2 sheets]!, WAG [3 sheets]!), fide F.Pellegrin, Mém. Inst. Étud. Centrafr. 1: 45 (1949) and, subsequently, J.Léonard, Mém. Cl. Sci. Acad. Roy. Sci. Belgique (8vo) sér. 2, 30(2): 224 (1957)).
3. Englerodendron explicans (Baill.) Estrella & Ojeda, comb. nov.
Type: Guinea, Fouta Djallon, 1837, Heudelot 738 (holo: P [3 sheets]!; iso: BM!, BR!, HBG, K [2 sheets]!, OXF!, WAG [2 sheets]!).
Note
In the first part of his paper (Adansonia 5: 361), Baillon stated that he was studying the herbarium of the ‘Musée des Colonies Françaises’; this herbarium is now part of P.
Type: Guinea, Fouta Djallon, 1837, Heudelot 738 (syn-: BM!, BR!, K [2 sheets]!, P [3 sheets]!, OXF!, WAG [2 sheets]!).
Note
Bentham referred to the sheet in the Hooker herbarium (then still private, now part of K), but another sheet was part of his own herbarium that was incorporated in K before publication. Both got annotated with observations and were clearly used for the description; hence, all Heudelot 738 sheets should be regarded as syntypes.
4. Englerodendron gabunense (J.Léonard) Breteler, Adansonia 28(1): 109 (2006)
Type: Gabon, Roungassa, 21 Dec.1929, G.M.P.C. Le Testu 7808 (holo: BR!; iso: BM, BR!, K, LISC, P!, WAG!). The isotype at BR arrived in BR only after publication of the basionym; hence, the other BR sheet is the holotype as indicated in the protologue.
5. Englerodendron graciliflorum (Harms) Estrella & Ojeda, comb. nov.
Type: Equatorial Guinea, Nkolentangan, Dec. 1907, Tessmann B.57 (lecto: K, isolecto: BR (fragment from K), B (destroyed) fide F.J.Breteler, Pl. Ecol. Evol. 144 (1): 71 (2011)).
6. Englerodendron hallei (Aubrév.) Estrella & Ojeda, comb. nov.
Type: Gabon, Abanga, chantier C.E.F.A., bord riv. Lano, Jun. 1963, N.Hallé 2195 (holo: P!).
7. Englerodendron isopetalum (Harms) Breteler & Wieringa, comb. nov.
Type: Cameroon, Bipindi, 1905, G.Zenker 3384 (lecto: BR!; isolecto: BM!, E, G!, HBG, GOET, K, L!, M, MO!, P!, S, US, fide J.Léonard, Mém. Cl. Sci. Acad. Roy. Sci. Belgique (8vo) sér. 2, 30(2): 224 (1957)).
8. Englerodendron korupense Burgt, Adansonia 29 (1): 60 (2007)
Type: Cameroon, South-west Province, Korup National Park, north-western plot near P transect, Subplot 44LN, 6 Apr. 2005, X.M. van der Burgt 741 (holo: WAG!; iso: BR, G, K!, MO, P, SCA, WAG!, YA).
9. Englerodendron lebrunii (J.Léonard) Estrella & Ojeda, comb. nov.
Type: D.R.Congo, between Dekese and Bumbuli, Oct. 1932, J-P.A.Lebrun 6497 (holo: BR[3 sheets]!; iso: K!, WAG!, YBI).
10. Englerodendron leptorrhachis (Harms) Estrella & Ojeda, comb. nov.
Type: Cameroon, Bipindi, Sep. 1901, G.A,Zenker 2445 (lecto: P!; isolecto: B (destroyed), BR!, G!, GOET, K!, L!, MO!, WAG!, fide F.J.Breteler, Pl. Ecol. Evol. 144 (1): 73 (2011)).
11. Englerodendron mengei (De Wild.) Estrella & Ojeda, comb. nov.
Type: Congo (Kinshasa), Djombo, 5 Mar. 1913, A.Mengé 88 (holo: BR).
12. Englerodendron nigericum (Baker f.) Estrella & Ojeda, comb. nov.
Type: Nigeria, Oban, 1911, Talbot 582 (lecto: BM[2 sheets]!; isolecto: K!), fide F.Pellegrin, Bull. Soc. Bot. France 104: 498 (1958))
13. Englerodendron obanense (Baker f.) Estrella & Ojeda, comb. nov.
Type: Nigeria, Oban, 1911–1912, Mr. & Mrs. Talbot 1428 (lecto-: BM!,; isolecto- BR! (fragment), K!) fide F.Pellegrin, Bull. Soc. Bot. France 104: 498 (1958)).
Type: Sierra Leone, Pujehun, 16 Feb. 1914, C.E.Lane-Poole 161 (holo: K[2 sheets]!; iso: BR).
Type: Liberia, Monrovia, 5 Feb. 1922, Dinklage 2805 (syn-: B (destroyed), HBG, P! [two sheets]). Breteler (2011) selected a neotype (Jongkind 5706, WAG), only to discover later that some isotypes were extant.
14. Englerodendron sargosii Pellegr., Bull. Soc. Bot. France 68: 11 (1921)
Type: D.R. Congo, Kouilou River, R.Sargos 101 (holo: P!; iso: BR!, WAG!). Both BR and WAG are fragments from the holotype obtained after the publication of the protologue.
15. Englerodendron triplisomere (Pellegr.) Estrella & Ojeda, comb. nov.
Type: Gabon, Nyanga Mount, between l’Onoij and Mouila, 26 Nov. 1925, G.M.P.C. Le Testu 5792 (lecto-: P!; isolecto: BM!, WAG!), fide J.Léonard, Mém. Cl. Sci. Acad. Roy. Sci. Belgique (8vo) sér. 2, 30(2): 226 (1957)).
16. Englerodendron usambarense Harms, Bot. Jahrb. Syst. 40(1): 28 (1907)
Type: Tanzania, Lushoto District, between Amani and Bomole, Oct. 1905, H.G.A.Engler 3436 (syn- B(destroyed), B! [in Herb. A.Peter 48011, photo: K!]). Harms also mentioned a second gathering (W. Busse 2210) that is assigned to the species with doubt (‘Offenbar’), rendering it unclear whether this should be considered syntype material as well.
17. Englerodendron vignei (Hoyle) Estrella & Ojeda, comb. nov.
Type: Ghana, Dompim, May 1930, C.Vigne 1968 (lecto-: K!; isolecto: BR, FHO!, WIS). fide A.Aubréville & F.Pellegrin, Bull. Soc. Bot. France 104: 498 (1958)). Although Hoyle marked the K sheet as holotype, the protologue cites both Kew and Oxford, rendering them syntypes.
Key to the species of EnglerodendronAdapted from previous treatments for the included genera (Cowan and Polhill 1981; Breteler 2006, 2008, 2010, 2011; van der Burgt et al. 2007).
|
1. |
|
1: |
|
2. |
|
3. |
|
3: |
|
4. |
|
4: |
|
5. |
|
5: |
|
2: |
|
6. |
|
6: |
|
7. |
|
7: |
|
8. |
|
8: |
|
9. |
|
9: |
|
10. |
|
10: |
|
11. |
|
11: |
|
12. |
|
13. |
|
13: |
|
12: |
|
14. |
|
14: |
|
15. |
|
15: |
|
16. |
|
16: |
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Manuel de la Estrella was funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement number 659152 (GLDAFRICA).
Acknowledgements
The authors thank staff of the following herbaria for their support on our visit and loan of material: BM, BR, BRLU, K, LBV, MA, MT, P and WAG, Félix Forest and Olivier Hardy for their support, and Prof. John McNeill for nomenclatural comments. We are indebted to the editors, Colin Hughes and Anna Monro, and two anonymous reviewers for their comments on an earlier version of the manuscript.
References
Aubréville A (1959) ‘La flore forestière de la Côte d’Ivoire.’ (Centre Technique Forestier Tropical: Paris, France)Aubréville A (1968) Les Césalpinioidées de la flore Camerouno-Congolaise, considérations taxonomiques, chorologiques, écologiques, historiques et évolutives. Adansonia 8, 147–175.
Aubréville A, Pellegrin F (1958) De quelques Césalpiniées africaines. Bulletin de la Société Botanique de France 104, 495–498.
| De quelques Césalpiniées africaines.Crossref | GoogleScholarGoogle Scholar |
Breteler FJ (2006) Novitates Gabonenses 56. Two Anthonotha species from Gabon transferred to Englerodendron (Fabaceae, Caesalpinioideae). Adansonia 28, 105–111.
Breteler FJ (2008) Anthonotha and Isomacrolobium (Leguminosae, Caesalpinioideae): two distinct genera. Systematics and Geography of Plants 78, 137–144.
Breteler FJ (2010) Revision of the African genus Anthonotha (Leguminosae, Caesalpinioideae). Plant Ecology and Evolution 143, 70–99.
| Revision of the African genus Anthonotha (Leguminosae, Caesalpinioideae).Crossref | GoogleScholarGoogle Scholar |
Breteler FJ (2011) Revision of the African genus Isomacrolobium (Leguminosae, Caesalpinioideae). Plant Ecology and Evolution 144, 64–81.
| Revision of the African genus Isomacrolobium (Leguminosae, Caesalpinioideae).Crossref | GoogleScholarGoogle Scholar |
Bruneau A, Breteler FJ, Wieringa JJ, Gervais GYF, Forest F (2000) Phylogenetic relationships in tribes Macrolobieae and Detarieae as inferred from chloroplast trnL intron sequences. In ‘Advances in Legume Systematics 9.’ (Eds PS Herendeen, A Bruneau) pp. 121–149. (Royal Botanic Gardens, Kew: London, UK)
Bruneau A, Forest F, Herendeen PS, Klitgaard BB, Lewis GP (2001) Phylogenetic relationships in the Caesalpinioideae (Leguminosae) as inferred from chloroplast trnL intron sequences. Systematic Botany 26, 487–514.
Bruneau A, Mercure M, Lewis GP, Herendeen PS (2008) Phylogenetic patterns and diversification in the caesalpinioid legumes. Botany 86, 697–718.
| Phylogenetic patterns and diversification in the caesalpinioid legumes.Crossref | GoogleScholarGoogle Scholar |
Bruneau A, Klitgaard BB, Prenner G, Fougere-Danezan M, Tucker SC (2014) Floral evolution in the Detarieae (Leguminosae): phylogenetic evidence for labile floral development in an early-diverging legume lineage. International Journal of Plant Sciences 175, 392–417.
| Floral evolution in the Detarieae (Leguminosae): phylogenetic evidence for labile floral development in an early-diverging legume lineage.Crossref | GoogleScholarGoogle Scholar |
Cowan RS, Polhill R (1981) Tribe 5. Amherstieae Benth emend. J. Léonard (1957). In ‘Advances in Legume Systematics.’ (Eds R Polhill, PH Raven) Vol. 1, pp. 135–142. (Royal Botanic Gardens, Kew: London, UK)
de la Estrella M, Devesa JA (2014) The genus Gilbertiodendron (Leguminosae–Caesalpinioideae) in western Africa. Systematic Botany 39, 160–192.
| The genus Gilbertiodendron (Leguminosae–Caesalpinioideae) in western Africa.Crossref | GoogleScholarGoogle Scholar |
de la Estrella M, Devesa JA, Wieringa JJ (2012a) A morphological re-evaluation of the taxonomic status of the genus Pellegriniodendron (Harms) J.Léonard (Leguminosae–Caesalpinioideae–Detarieae) and its inclusion in Gilbertiodendron J.Léonard. South African Journal of Botany 78, 257–265.
| A morphological re-evaluation of the taxonomic status of the genus Pellegriniodendron (Harms) J.Léonard (Leguminosae–Caesalpinioideae–Detarieae) and its inclusion in Gilbertiodendron J.Léonard.Crossref | GoogleScholarGoogle Scholar |
de la Estrella M, van der Burgt XM, Mackinder BA, Devesa JA, James MS, Hawthorne WD (2012b) Gilbertiodendron tonkolili sp. nov. (Leguminosae–Caesalpinioideae) from Sierra Leone. Nordic Journal of Botany 30, 136–143.
| Gilbertiodendron tonkolili sp. nov. (Leguminosae–Caesalpinioideae) from Sierra Leone.Crossref | GoogleScholarGoogle Scholar |
de la Estrella M, Wieringa JJ, Mackinder BA, van der Burgt XM, Devesa JA, Bruneau A (2014) Phylogenetic analysis of the African genus Gilbertiodendron J.Léonard and related genera (Leguminosae–Caesalpinioideae–Detarieae). International Journal of Plant Sciences 175, 975–985.
| Phylogenetic analysis of the African genus Gilbertiodendron J.Léonard and related genera (Leguminosae–Caesalpinioideae–Detarieae).Crossref | GoogleScholarGoogle Scholar |
de la Estrella M, Forest F, Wieringa JJ, Fougère-Danezan M, Bruneau A (2017) Insights on the evolutionary origin of Detarioideae, a clade of ecologically dominant tropical African trees. New Phytologist 214, 1722–1735.
| Insights on the evolutionary origin of Detarioideae, a clade of ecologically dominant tropical African trees.Crossref | GoogleScholarGoogle Scholar | 28323330PubMed |
de la Estrella M, Forest F, Klitgård B, Lewis GP, Mackinder BA, de Queiroz LP, Wieringa JJ, Bruneau A (2018) A new phylogeny-based tribal classification of subfamily Detarioideae, an early branching clade of florally diverse tropical arborescent legumes. Scientific Reports 8, 6884
| A new phylogeny-based tribal classification of subfamily Detarioideae, an early branching clade of florally diverse tropical arborescent legumes.Crossref | GoogleScholarGoogle Scholar | 29720687PubMed |
Legume Phylogeny Working Group (2013) Towards a new classification system for legumes: progress report from the 6th International Legume conference. South African Journal of Botany 89, 3–9.
| Towards a new classification system for legumes: progress report from the 6th International Legume conference.Crossref | GoogleScholarGoogle Scholar |
Legume Phylogeny Working Group (2017) A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66, 44–77.
| A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny.Crossref | GoogleScholarGoogle Scholar |
Léonard J (1952) Notulae systematicae XII. Les genres Macrolobium Schreb. et Gilbertiodendron J. Léonard au Congo Belge. Bulletin du Jardin Botanique de l’État 22, 185–191.
| Notulae systematicae XII. Les genres Macrolobium Schreb. et Gilbertiodendron J. Léonard au Congo Belge.Crossref | GoogleScholarGoogle Scholar |
Léonard J (1954) Notulae systematicae XIV. Les genres Macrolobium Schreb. et Gilbertiodendron J.Léonard en Afrique Tropicale. Bulletin du Jardin Botanique de l’État 24, 57–61.
| Notulae systematicae XIV. Les genres Macrolobium Schreb. et Gilbertiodendron J.Léonard en Afrique Tropicale.Crossref | GoogleScholarGoogle Scholar |
Léonard J (1955) Notulae systematicae XVII. Les genres Anthonotha P.Beauv. et Pellegriniodendron J.Léonard en Afrique tropicale (Caesalpinaceae). Bulletin du Jardin Botanique de l’État 25, 201–203.
| Notulae systematicae XVII. Les genres Anthonotha P.Beauv. et Pellegriniodendron J.Léonard en Afrique tropicale (Caesalpinaceae).Crossref | GoogleScholarGoogle Scholar |
Mackinder B (2005) Detarieae sensu lato. In ‘Legumes of the World’. (Eds GP Lewis, B Schrire, B Mackinder, M Lock) pp. 69–109. (Royal Botanic Gardens, Kew: London, UK)
Mackinder B, Pennington RT (2011) Monograph of Berlinia (Leguminosae). Systematic Botany Monographs 91, 1–117.
Murphy B, de la Estrella M, Schley R, Forest F, Klitgaard B (2018) On the monophyly of Macrolobium Schreb., an ecologically diverse neotropical tree genus (Fabaceae–Detarioideae). International Journal of Plant Sciences 179, 75–86.
| On the monophyly of Macrolobium Schreb., an ecologically diverse neotropical tree genus (Fabaceae–Detarioideae).Crossref | GoogleScholarGoogle Scholar |
Newbery DM, Van Der Burgt XM, Worbes M, Chuyong GB (2013) Transient dominance in a central African rain forest. Ecological Monographs 83, 339–382.
| Transient dominance in a central African rain forest.Crossref | GoogleScholarGoogle Scholar |
Ojeda DI, Koenen E, Cervantes S, de la Estrella M, Banguera-Hinestroza E, Janssens SB, Migliore J, Demenou B, Bruneau A, Forest F, Hardy OJ (2019) Phylogenomic analyses reveal an exceptionally high number of evolutionary shifts in a florally diverse clade of African legumes. Molecular Phylogenetics and Evolution 137, 156–167.
| Phylogenomic analyses reveal an exceptionally high number of evolutionary shifts in a florally diverse clade of African legumes.Crossref | GoogleScholarGoogle Scholar | 31075505PubMed |
Palisot de Beauvois AMFJ (1806) ‘Flore d’Oware et de Benin en Afrique, I.’ (Fain et Compagnie: Paris, France)
Robyns W (1952) ‘Flore du Congo Belge. Vol. 3. Caesalpioideae.’ (Publications de l’Institute National pour l’Étude Agronomique du Congo Belge: Brussels, Belgium)
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313.
| RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies.Crossref | GoogleScholarGoogle Scholar | 24451623PubMed |
van der Burgt XM, Eyakwe MB, Newbery DM (2007) Englerodendron korupense (Fabaceae, Caesalpinioideae), a new tree species from Korup National Park, Cameroon. Adansonia 29, 59–65.
van der Burgt XM, Mackinder BA, Wieringa JJ, de la Estrella M (2015) The Gilbertiodendron ogoouense species complex (Leguminosae: Caesalpinioideae), central Africa. Kew Bulletin 70, 29
| The Gilbertiodendron ogoouense species complex (Leguminosae: Caesalpinioideae), central Africa.Crossref | GoogleScholarGoogle Scholar |
Vatanparast M, Powell A, Doyle JJ, Egan AN (2018) Targeting legume loci: a comparison of three methods for target enrichment bait design in Leguminosae phylogenomics. Applications in Plant Sciences 6, e1036
| Targeting legume loci: a comparison of three methods for target enrichment bait design in Leguminosae phylogenomics.Crossref | GoogleScholarGoogle Scholar | 29732266PubMed |
Villaverde T, Pokorny L, Olsson S, Rincón-Barrado M, Johnson MG, Gardner EM, Wickett NJ, Molero J, Riina R, Sanmartín I (2018) Bridging the micro- and macroevolutionary levels in phylogenomics: Hyb–Seq solves relationships from populations to species and above. New Phytologist 220, 636–650.
| Bridging the micro- and macroevolutionary levels in phylogenomics: Hyb–Seq solves relationships from populations to species and above.Crossref | GoogleScholarGoogle Scholar | 30016546PubMed |
Wieringa JJ (1999) Monopetalanthus exit: a systematic study of Aphanocalyx, Bikinia, Icuria, Michelsonia and Tetraberlinia (Leguminosae, Caesalpinioideae). Wageningen Agricultural University Papers 99, 1–320.


