Charophytes of Australia’s Northern Territory – I. Tribe Chareae
Michelle T. Casanova A B C E * and Kenneth G. Karol D
A B C E * and Kenneth G. Karol D
A Royal Botanic Gardens, Melbourne, Birdwood Avenue, South Yarra, Vic. 3141, Australia.
B Federation University, Mount Helen, Vic. 3350, Australia.
C The Natural History Museum, Cromwell Road, London, UK.
D The Lewis B. and Dorothy Cullman Program for Molecular Systematics, New York Botanical Garden, Bronx, NY, USA.
E Present address: 273 Casanova Road, Westmere, Vic. 3351, Australia.
Australian Systematic Botany 36(1) 38-79 https://doi.org/10.1071/SB22023
Submitted: 14 July 2022 Accepted: 2 March 2023 Published: 30 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
This study of Northern Territory charophytes documents 22 species in 3 of the genera in tribe Chareae, family Characeae, including 15 previously described species (Chara benthamii, C. erythrogyna, C. globularis, C. karolii, C. lucida, C. porteri, C. protocharoides, C. setosa, C. submollusca, C. wightii, C. zeylanica, Lamprothamnium capitatum, L. compactum, L. stipitatum, Lychnothamnus barbatus) of which 2 are new for the Australian flora (C. erythrogyna and C. wightii), as well as 5 varieties raised to species rank (C. aridicola, C. arnhemensis, C. bancroftii, C. behriana, C. duriuscula), and 2 newly described species (C. lamprothamniformis, C. schultae). Three previously reported species in the tribe (C. braunii, C. corallina, C. fibrosa) are not recorded from the Northern Territory in this study, as previous records were based on erroneous identifications or localities. Although Northern Territory specimens of Lychnothamnus barbatus have not been seen, it has been included in this treatment, because it occurs in south-eastern Queensland, the Gulf of Carpentaria, Papua New Guinea and Timor-Leste. A key, illustrations and descriptions of all the species are provided.
Keywords: Chara, charophyte, Lamprothamnium, Lychnothamnus, morphology, oospore, taxonomy, Northern Territory.
Introduction
Charophytes are water plants, green macroalgae in family Characeae; they occur in wetlands (lagoons, waterholes, claypans) and riparian areas (rivers, streams, springs) in northern Australia and world-wide, including the Antarctic continent (Schubert et al. 2018). Their growth habit can be diffuse (species of Nitella C.Agardh) or compact (many species of Chara L.), slimy (when there is mucus on the reproductive whorls) or stony (when superficial calcium carbonate is deposited). They grow in deep water, where sufficient light is available (Casanova 2007), and also in shallow, turbid water (Casanova and Porter 2013), and sometimes exist emergent at the edges of rivers, in a thin film of water (Casanova and Nairn 2015). They have been called ‘ecosystem engineers and permanent pioneers’ as a consequence of their multiple and species-specific roles in wetland ecosystems (Schubert et al. 2018). Charophytes often grow in mixed species communities, although single species ‘beds’ or ‘meadows’ can develop under stable, low-nutrient conditions (Casanova 2009). Although charophytes can be diverse and abundant in tropical regions (Zaneveld 1940; Pal et al. 1962), there have been few studies on their diversity and occurrence in northern Australia. The taxonomy of charophytes has been, in the past, complicated by subdivision of species into subspecies, varieties and forms by various authors. In this study, only species names are used, and, in general, taxa recognised in the past at form or variety level are raised to species. This species concept is based on genetic and morphological evidence (Sakayama et al. 2002, 2005, 2006; Karol 2004; Casanova 2005, 2009, 2013a, 2013b; Casanova and Karol 2014). This is one of two related papers, this one concerning the members of tribe Chareae, i.e. members of the genera Chara, Lamprothamnium J.Groves and Lychnothamnus (Rupr.) Leonh. The other genus in tribe Chareae (Nitellopsis Hy) has not been found in Australia. We will deal with tribe Nitelleae (containing Nitella, Sphaerochara Mädler and Tolypella (A.Braun) A.Braun) in a forthcoming paper (M. T. Casanova and K. G. Karol, submitted).
Charophytes consist of stems or axes (singular axis) attached to colourless filamentous structures called rhizoids that anchor them in the soil. Each axis is made up of internodes and nodes, and branchlets arise in whorls at the nodes. Sometimes there are other structures that arise at the nodes: cortex cells (or cortication, visible as linear stripes of tubular cells), stipulodes and reproductive structures (gametangia: female = oosporangium, also referred to as oogonium in many works; male = antheridium). The cortex of charophyte axes is described with reference to the number and relative dominance of the cortical cells, and the placement of spine cells; haplostichous refers to cortical cells the same number as the branchlets in the adjacent whorl. Diptostichous is where the cortical cells are two times the number of branchlets in the adjacent whorl, and triplostichous is where the cortical cells are three times the number of branchlets in the adjacent whorls. Tylacanthous is where the spine cells are on the primary (bigger) cortical cells, and aulacanthous is the condition when the spine cells are on the secondary (smaller) cortical cells, appearing to be in furrows on the cortex. Isostichous is where all cortical cells are approximately the same size, and anisostichous is where the cortical cells differ in size. These characters can most easily be seen in a side view on new growth of the axis, or in a transverse section of the axis viewed under the microscope. Oosporangia have one or more basal cells (1 in Chareae, 1–3 in Nitelleae), five helical cells enveloping a central egg cell, and a crown of smaller cells at the apex (coronula). The coronula is made up of one row of cells in the Chareae (5 cells in total) or two rows of cells in the Nitelleae (10 cells). After fertilisation, the egg cell within the oosporangium develops into an oospore with distinctive helical markings and a darkened multi-layered cell wall, sometimes with a white calcareous covering (gyrogonite).
The first collector of charophytes in the north of Australia was Ferdinand von Mueller (1825–1896) on the North Australian Exploring Expedition of 1855–1856 (Gregory and Gregory 1884). Within tribe Chareae, von Mueller collected specimens of Chara, i.e. C. lucida (A.Braun) Casanova & Karol from Victoria River, Baines Creek and the Gulf of Carpentaria (Casanova and Karol 2014), and C. gymnopitys β duriuscula A.Braun from Victoria River (Nordstedt 1883). He later recorded C. gymnopitys A.Braun and C. gymnopus A.Braun (= C. zeylanica Klein ex Willd.) for Northern Australia (von Mueller 1889). Both Alexander Braun and Otto Nordstedt (Nordstedt 1883) also examined northern Australian material supplied by von Mueller. Zaneveld (1940) examined the material that was used by Braun and Nordstedt, applying the same epithets within the genus Chara (duriuscula, gymnopitys and lucida) at different taxonomic levels (see Casanova and Karol 2014).
Another significant study on northern Australian charophytes was completed by G. O. Allen (Groves and Allen 1935), continuing the pattern of posthumous publication of a mentor’s work by his associate (as was done for Braun by Nordstedt 1883). Although that study was exclusively on Queensland charophytes, there are species in common with the Northern Territory, from the wet tropics, arid, and semi-arid zones. Allen’s taxonomic approach was similar to that of Zaneveld (1940), and 10 species of Chara (including Chara macropogon A.Braun ≡ Lamprothamnium macropogon (A.Braun) Ophel) were recognised.
The next major collector was Ray Specht who visited Arnhem Land and Groote Eylandt during the American–Australian expedition in 1948 (Wood 1958). Specht sent his charophyte material to R. D. Wood in the USA, who identified the tribe Chareae specimens as C. benthamii A.Braun, Chara lucida and ‘C. sp. nov.?’, following the works of Braun (in Nordstedt 1883) and Zaneveld (1940). Wood (1962) and Wood and Imahori (1965) later recorded the same specimens as C. fibrosa var. fibrosa C.Agardh ex Bruzelius, C. corallina var. nobilis f. lucida (A.Braun) R.D.Wood and C. fibrosa var. fibrosa f. arnhemensis R.D.Wood.
Wood attempted a more thorough compilation after collecting in the vicinity of Darwin with Nancy Eddy (Wood 1971). In that treatment, he listed six species in the Chareae for the Northern Territory (C. braunii C.C.Gmel., C. corallina Klein ex Willd., C. fibrosa, C. globularis Thuill., C. setosa Klein ex Willd. and C. zeylanica). He allocated specimens to various subspecies, varieties or forms of those taxa. Northern Territory charophytes were included in works by García and Chivas (2006) and (to genus) in Cowie et al. (2000). Since then, a study on the ecology of the Daly River (Schult et al. 2007) included a report of charophytes collected there, and elsewhere in the Northern Territory, documenting 13 species, some of which were undescribed.
Since the 1970s, more comprehensive collections have been made, particularly by Peter Latz, Peter Dostine, Julia Schult and Simon Townsend, and on Bush Blitz expeditions, greatly increasing the number of specimens and allowing a more comprehensive delineation of known and new species for the Northern Territory.
Materials and methods
Approximately 300 fresh, pressed and spirit-preserved specimens collected in the Northern Territory, deposited in Australian and overseas herbaria and several private collections, were examined for the present study, including type material. In general, each specimen was allocated a letter–number combination (e.g. p###, r###, t###), so that all data obtained from individual specimens (i.e. measurements, photographs, chromosome counts, scanning electron micrographs) could be related back to the specimen. Initial examination involved measurements directly from the specimen (e.g. length of branchlets, number of branchlet segments) and with the use of a microscope (e.g. axis and branchlet diameter, number of cortical cells, number and morphology of stipulodes and bract cells, placement of gametangia). Oospore features were examined and measured with the aid of light microscopy and scanning electron microscopy.
Where possible and appropriate, oospores were removed from the herbarium specimens or obtained from live material. Approximately 50% of the specimens examined had oospores. They were prepared for scanning electron microscope (SEM) examination by cleaning (if required) with a detergent solution, using a modification of the methods of Crawford et al. (2001). Sometimes the enveloping cells were removed by hand, using fine needles. As a general guide, if there were many (>8) oospores on a specimen, approximately three oospores would be used. If there were five to eight oospores present, then no more than two were removed. If there were three to five oospores present, then one was removed. If fewer than three oospores were present, then an oospore was removed only in exceptional circumstances. For type specimens, very old material, or specimens with a single oospore available for examination, the oospores were handled with great care, with minimal manipulation. Oospores were removed from the SEM stubs after microscopy and are stored in alcohol and deposited with the specimen for possible future examination.
Taxonomy
Four genera have been found, or are likely to be found, in the Northern Territory, namely, Chara, Lamprothamnium, Lychnothamnus and Nitella (not dealt with in this paper). Three other charophyte genera exist elsewhere, including Nitellopsis, which occurs in the northern hemisphere and Indonesia, and Sphaerochara and Tolypella, which appear to be restricted to temperate and polar regions. This key applies only to the species in the Northern Territory and will not always be accurate for other regions.
Key to the genera|
1 Coronula consisting of two rows of cells (10 cells), oospores generally a compressed sphere, usually round or oval in side-view, branchlets forked |
|
2 Gametangia arranged side by side at the branchlet nodes, typically one oosporangium with an antheridium on either side |
|
3 Axis and branchlets sometimes corticated, if ecorticate axes at least as wide as the branchlets; stipulodes either in one or two rows, obscure, upright or spreading; where gametangia are conjoined the antheridium is below the oosporangium |
Lychnothamnus (Rupr.) Leonh., Lotos 13: 57, 72 (1863), p.p.
Monoecious. Plant axis corticated or cortex reduced to a few filaments growing down from the axial nodes. Stipulodes in one whorl (haplostephanous), branchlets ecorticate, bract cells elongate (>8 mm) and verticillate. Gametangia arranged on the branchlet nodes side-by-side, usually two antheridia with a central oosporangium. Gametangia originating from different initial cells. Helical cells of the oosporangium terminated by a coronula of five cells. Oospores with a calcified covering (gyrogonite).
Lychnothamnus has one species in Europe, with representatives in China (Han and Li 1994), India (Wood and Imahori 1965), the United States (Karol et al. 2017), and several fossil representatives (Martin-Closas and Ramos 2005).
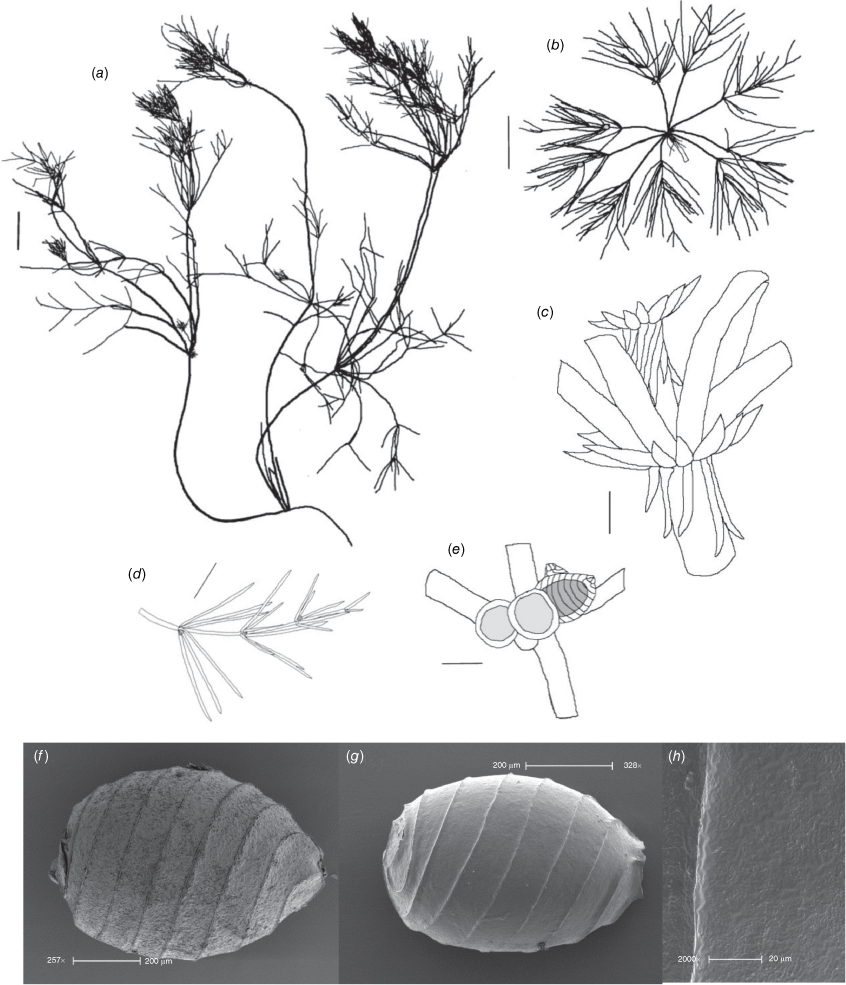
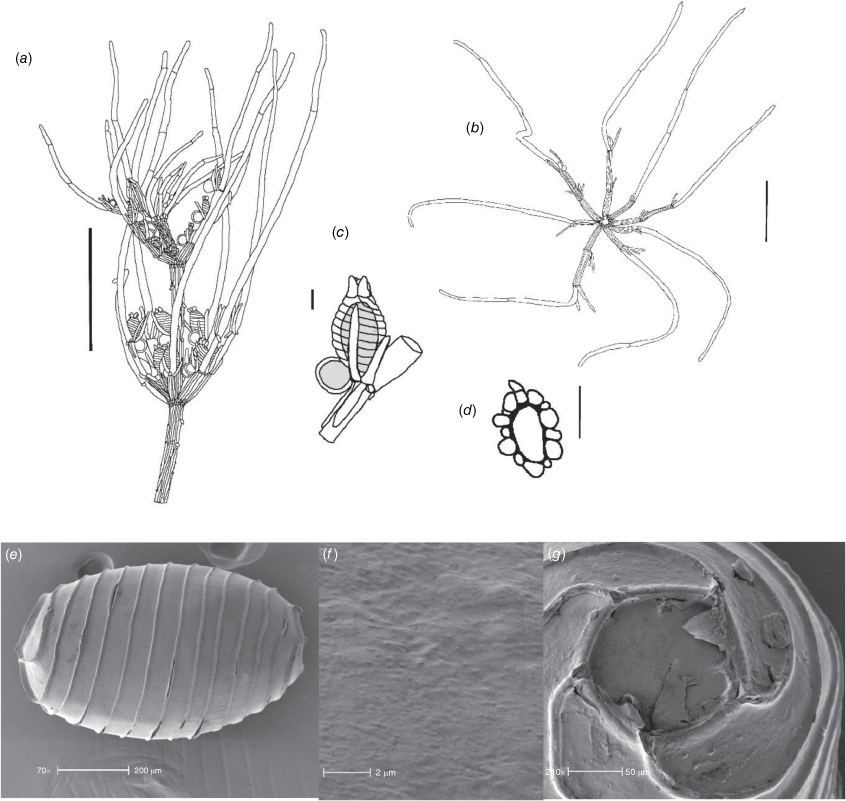
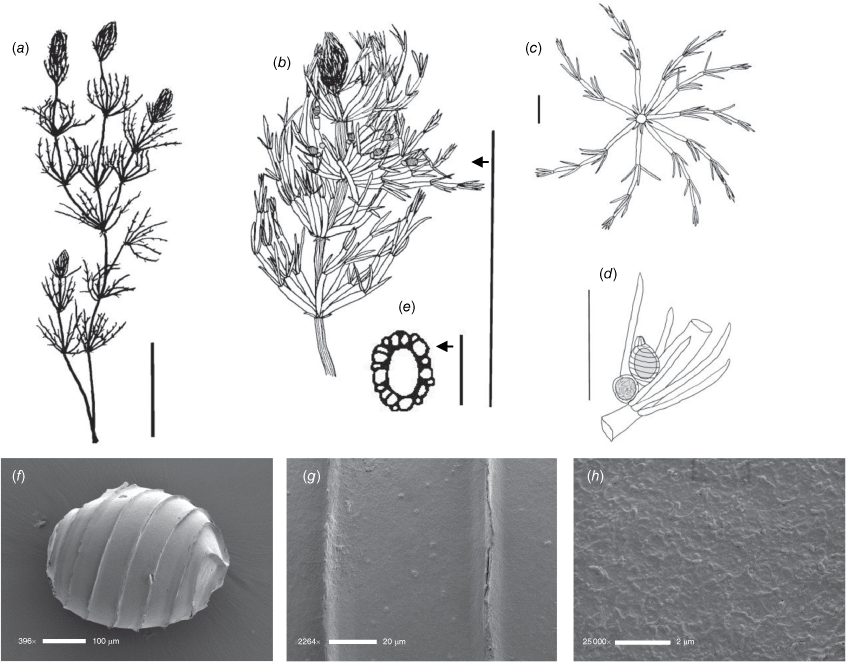
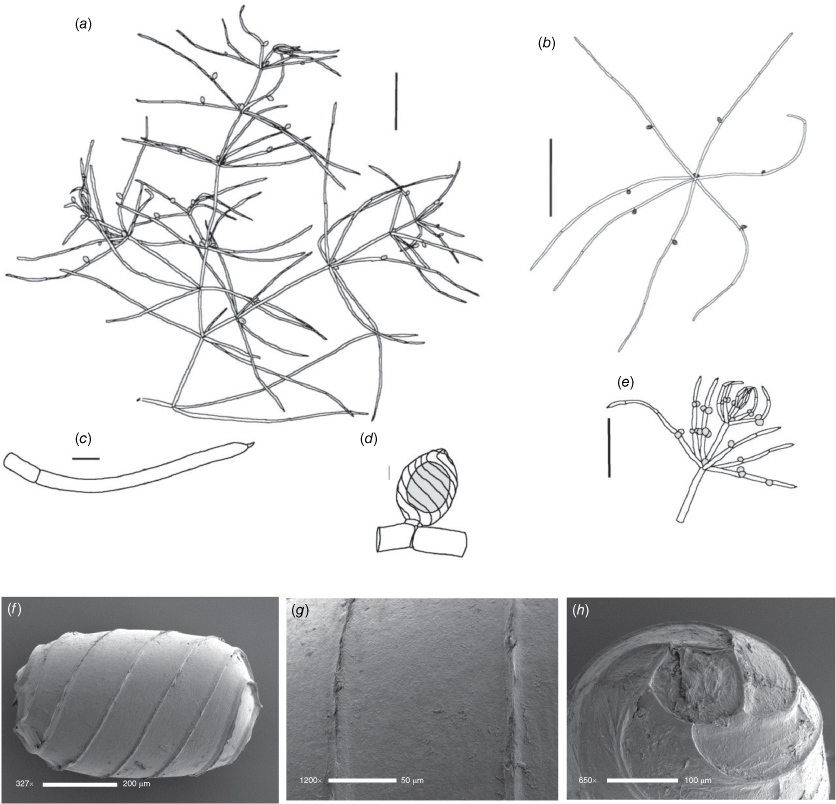
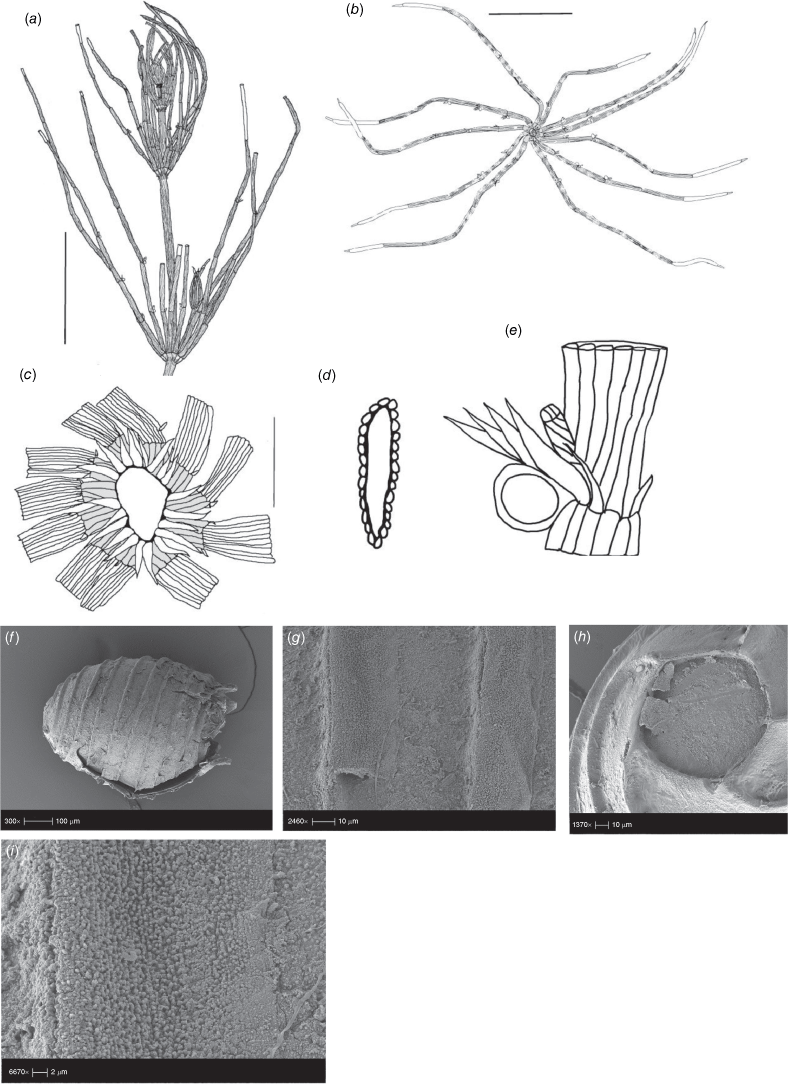
Lychnothamnus barbatus (Meyen) Leonh., Lotos 13: 57, 72 (1863)
Monoecious. Plants from 10 cm up to 1.2 m high, with a shrubby growth habit in shallow water (Fig. 1a), but with elongate stems in deeper, flowing water, occasionally with calcium carbonate deposition on the thallus. Axes up to 600 µm in diameter, the cortex reduced to whiskers of filaments below the stipulodes (Fig. 1c). Spine cells absent or inconspicuous on longer cortical filaments. Stipulodes in one whorl, 2× the number of branchlets, uniform in length in each whorl, but variable on a single plant (Fig. 1c). Branchlets 8–9 in a whorl, up to 30 mm long, 3–5 segments long, uncorticated, terminated by an acuminate end cell (Fig. 1b). Bract cells 4–6, verticillate and elongate (up to 12 mm long) at the branchlet nodes (Fig. 1d), bracteoles shorter than bract cells, occurring below the gametangia. Gametangia arranged side-by-side at the lowest 1–2 branchlet nodes, arising from separate gametangial initials (Fig. 1e). Oosporangia 700–1100 µm long and 500–800 µm wide, with 10–12 stripes of helical cells, coronula up to 100 µm high (Fig. 1e). Oospores brown to black, 700–790 µm long, 450–490 µm wide (Fig. 1g), often with a gyrogonite 800–960 µm long (Fig. 1f) (García 2003). Striae of 9–11 strong ridges, fossa wall minutely verrucate (Fig. 1h). Antheridia up to 350 µm in diameter, 8-scutate (Fig. 1g). Chromosomes n = 28 (Hotchkiss 1963).
Distribution
Occurs in freshwater, permanent lakes and ponds in Europe, North America, Asia, Papua New Guinea, Timor-Leste, and tropical and subtropical Australia. In Australia, specimens have been recorded in freshwater streams and rivers. Fossil records (gyrogonites) from the Holocene occur in Europe, Australia, Asia, North America and Africa (Casanova et al. 2003a; García and Chivas 2006; Sugier et al. 2009; Vicente et al. 2020). The species is included here on the basis of reports from García and Chivas (2006) from the Gulf country in far-northern Queensland (n.v.), its occurrence in PNG (Leach and Osborne 1985; n.v.), and the examination of a specimen from Timor-Leste (D.Cook & T.Lee s.n. (DNA)).
Habitat and ecology
In Europe, Lychnothamnus barbatus occurs usually in temperate lakes, often associated with Nitellopsis obtusa (Desv.) J.Groves (Brzozowski et al. 2021). In Australia, it has been found in deep pools and riffles in freshwater creeks subject to variable flow (0.5–1.5 m deep), and its germination was enhanced in experiments by drying the sediment (Casanova et al. 2003b).
Phenology
Lychnothamnus barbatus is likely to be a perennial species where its habitat is perennial. Australian specimens have been robust and well developed; however, it has been collected only in spring and summer, so its life-history requirements are not known. Reproductive organs and oospores have been found in summer. Oospore germination occurred in soil samples dried over winter and flooded in November (spring) (Casanova et al. 2003b).
Conservation status
This species has been listed as ‘Endangered’ in Australia under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999 and the Queensland Nature Conservation Act 1992 (McCourt et al. 1999; Casanova et al. 2003b). It has also been Red-Listed as endangered or critically endangered in Poland (Siemińska et al. 2006), Lithuania (Balevičius 2000), Germany (Ludwig et al. 1996; Korsch et al. 2013) and the Balkans (Blaženčić et al. 2006).
Etymology
Barbatus meaning ‘bearded’ in Latin. Named for the whiskers of cortical cells that emerge from the axial nodes.
Notes
Lychnothamnus is the only genus (world-wide) with gametangia regularly arranged side-by-side. Sterile specimens of L. barbatus can be distinguished from species of Chara by the incomplete axial cortex and ecorticate branchlets, from Australian species of Lamprothamnium by the incomplete axial cortex and the characteristic bract cells, and from robust species of Nitella by the presence of stipulodes, cortication and monopodial branchlets. Indian material (in BM) is more completely corticated than is Lychnothamnus from other places, with primary cortical cells sometimes fully developed, along with spine cells. Lychnothamnus has not yet been recorded from the Northern Territory, but is likely to occur there in suitable habitat. The Australian material differs genetically from material from Europe (McCourt et al. 1999), but the differences are not sufficient for it to be recognised as a different species.
Specimens examined
QUEENSLAND: Warrill Creek near Aratula, 21 Nov. 1960, R.D.Wood 60-11-21-3 (BM, CANB, L, PC); Wallace Creek at the end of C. Head Road, off the Boonah-Rathdowney Road, in deep pools shaded by Casuarina and Callistemon, 15 Dec. 1996, M.T.Casanova v536 (MEL, NE, NY); Wallaby Creek on the D’Anguilar Highway M.T.Casanova x098 (MEL, BRI, NY). TIMOR-LESTE: Lake Iralalaro–Irasequiro area, 2 Oct. 2009, D.Cook & T.Lee s.n. (DNA). INDIA: Gondah, Oudh, 9 Feb. 1923, G.O.Allen 32 (BM). GERMANY: Obersee bei Lanke, Berlin, 23 June 1872, P.Magnus (BREM); Auf überschwemmerten Moorgrund zwischen Schöneburg u. Willmersdorf bei Berlin, August 1829?, F.Bauer (BREM); Schoneberg, Aug. 1829, F.Bauer (BM).
Lamprothamnium J.Groves, J. Bot. 54: 336 (1916)
Monoecious or dioecious. Plant axis ecorticate, usually narrower than the branchlets. Stipulodes in one whorl (haplostephanous), usually distinctively downward pointing, branchlets ecorticate, bract cells verticillate. Gametangia on the branchlet nodes or arranged internal or external to the axis node at the base of the branchlets. Where conjoined the antheridium usually above the oosporangium. Helical cells of the oosporangium terminated by a coronula of five cells. Oospores with a calcified covering (gyrogonite).
Lamprothamnium has 13 extant species known from Australia (Casanova 2013a) and at least two additional species in Europe, Africa and Asia. All of these species occur in brackish to saline to hypersaline habitats. When compared with ecorticate species of Chara, the species of Lamprothamnium usually have much narrower axes. The current key is largely based on the arrangement of gametangia, so several shoots should be examined and the occurrence and placement of oosporangia and antheridia noted.
Key to species of Lamprothamnium in the Northern Territory|
1 Monoecious (antheridia and oosporangia on the same plant) |
|
2 Oosporangia inside the base of the whorl only, not noticeably stipitate |
Lamprothamnium capitatum Casanova, Austral. Syst. Bot. 26: 274 (2013)
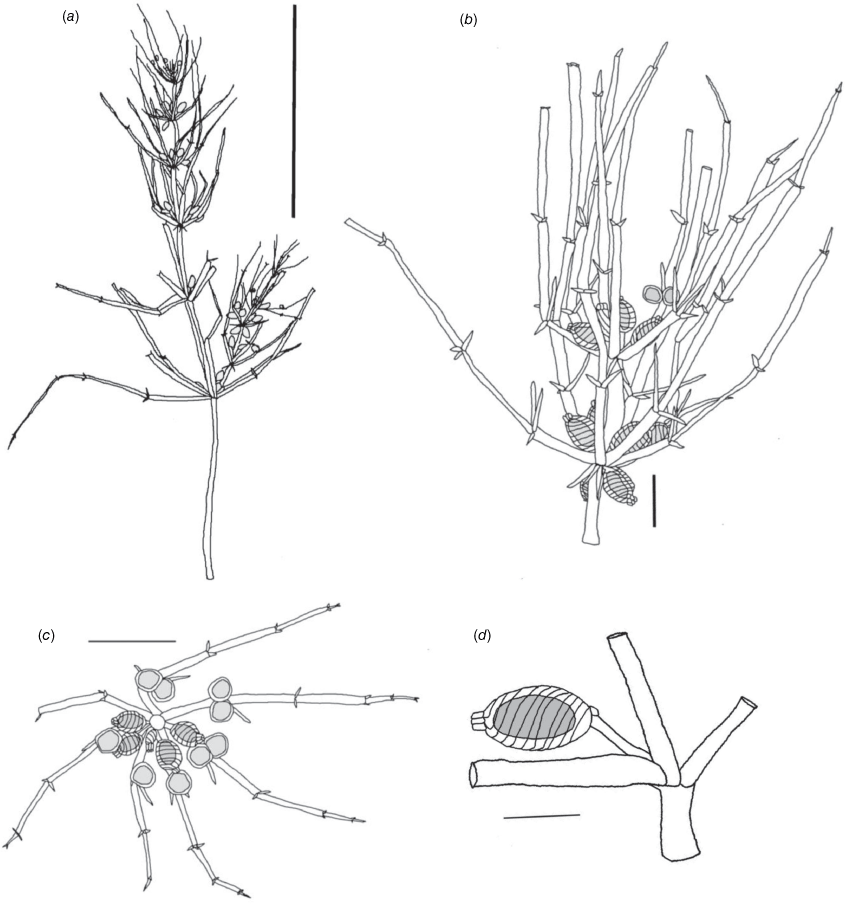
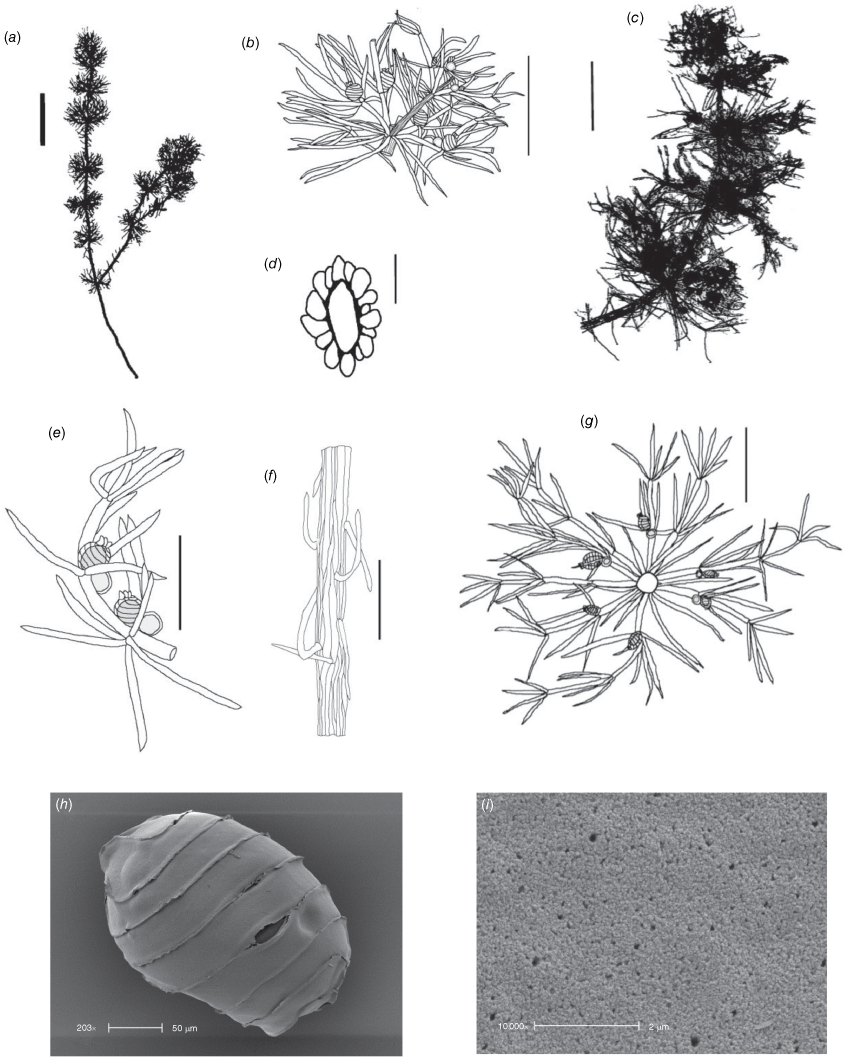
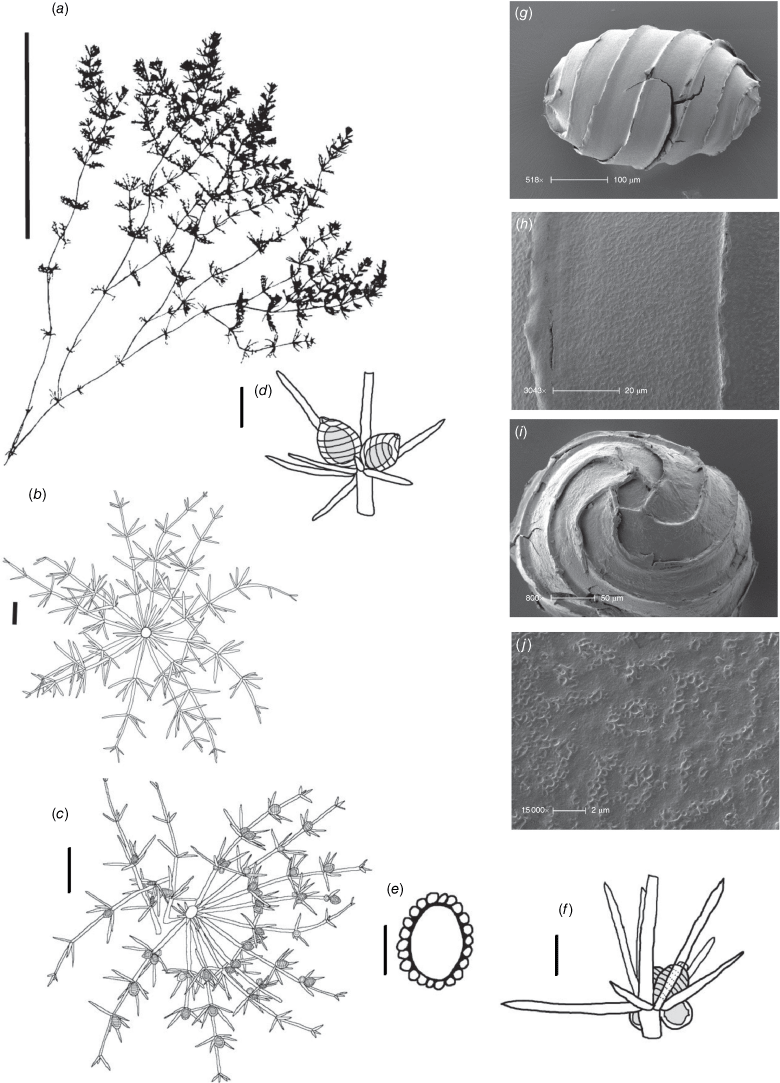
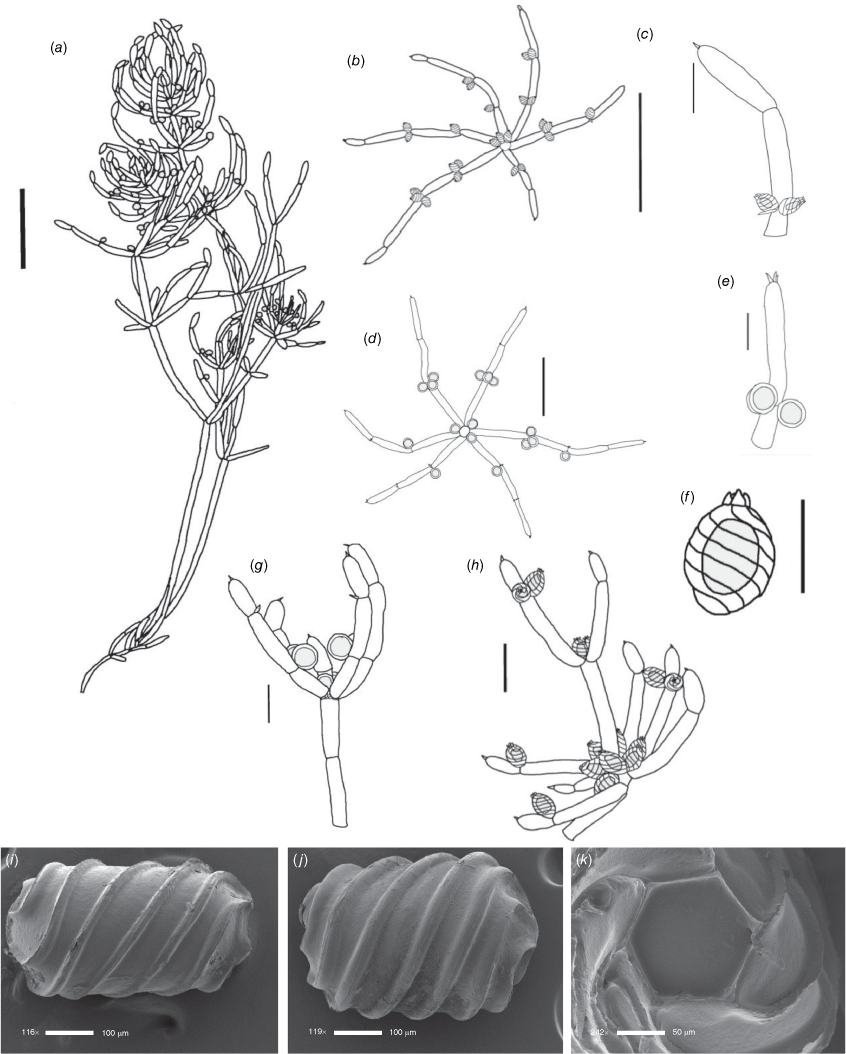
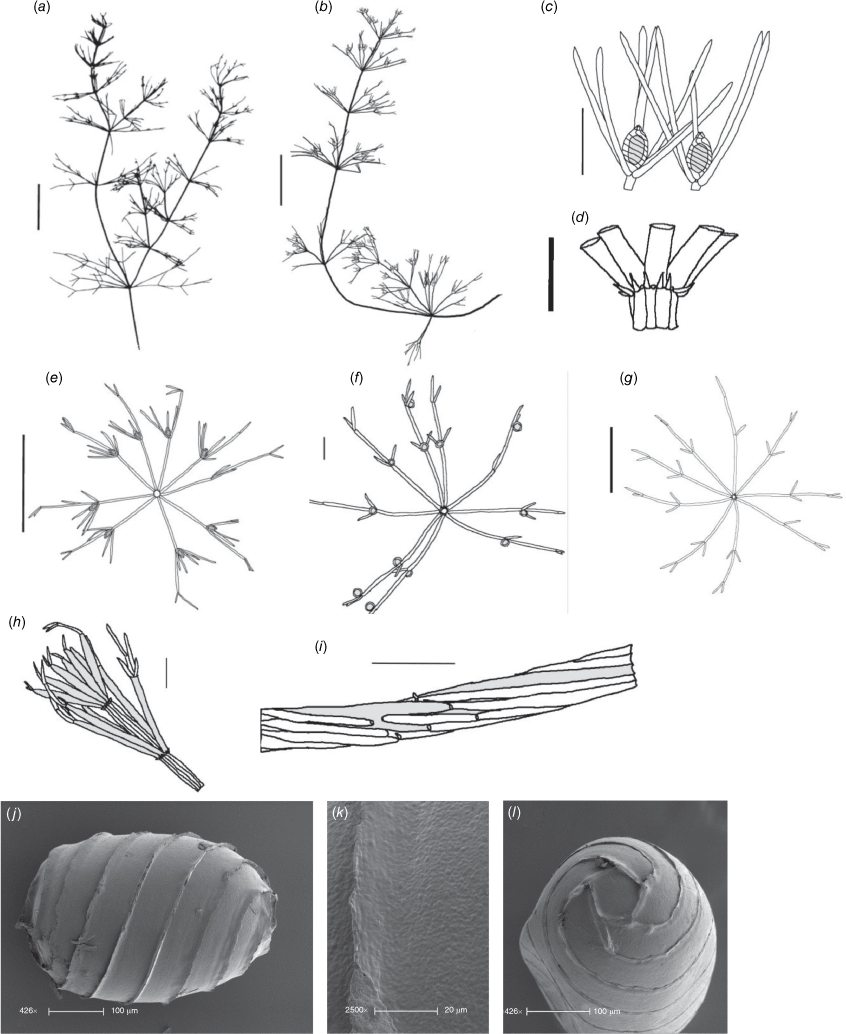
Monoecious. In the Northern Territory, plants occurring in a dense sward. Axes up to 100 μm in diameter, ecorticate, internodes up to 2 cm long (Fig. 2a). Stipulodes in 1 row, opposite the branchlets, 0.2–0.8 mm long, downward pointing. Branchlets 7 or 8 in a whorl, ecorticate, narrow in all parts (Fig. 2c), coalescing into ‘fox-tails’ at the axis apices, basal branchlet cell up to 2 mm long (in southern Australian specimens), branchlet end segments usually 1, bract cells 2–5, verticillate, 0.2–1 mm long, much reduced at upper branchlet nodes (Fig. 2b). Fertile parts contracted into loose foxtails, occurring apically and in the axils of lower branchlets. Gametangia foliar and basal, inside and outside the whorls (Fig. 2c). Oosporangia up to 900 μm long, stipitae inside the base and below the branchlet whorl (Fig. 2d), 10 or 11 stripes of helical cells, coronula up to 85 μm high, of oval cells. Oospores black, 600–740 μm long and 270–285 μm wide with 10 or 11 striae of undulating ridges. Ornamentation smooth to granulate, the grains sometimes arranged in rows. Antheridia up to 300 μm in diameter, solitary or geminate on the first branchlet node (Fig. 2c). Chromosomes not known.
Distribution
Brackish to saline wetlands in coastal and inland Victoria, South Australia and Northern Territory. The specimen from Cotton Island is the first confirmed record of Lamprothamnium in a saline coastal habitat north of the New South Wales–Queensland border in Australia.
Habitat and ecology
Grows in temporary saline waters.
Etymology
From Latin ‘capitatum’, head, referring to the ‘head-like’ appearance of the axial and terminal ‘fox-tails’ in the southern Australian material.
Notes
The specimens listed here most closely align with L. capitatum on the basis of the arrangement of the reproductive structures; however, specimens are less robust, do not appear to have foliar oosporangia, and the oospores are significantly larger. It appears to grow in a low carpet or sward, which differs from the isolated individuals previously examined (Casanova 2013a). Further collections from Northern Territory are required to confirm this determination.
Specimens examined
NORTHERN TERRITORY: Cotton Island, North West Lake, 19 Aug. 1996, C.P.Mangion 287 (DNA); Purnie Bore, Simpson Desert, 17 Sept. 1987, G.Leach 1438 (DNA) [mixed with Chara sp.].
Lamprothamnium compactum Casanova, Austral. Syst. Bot. 26: 276 (2013)
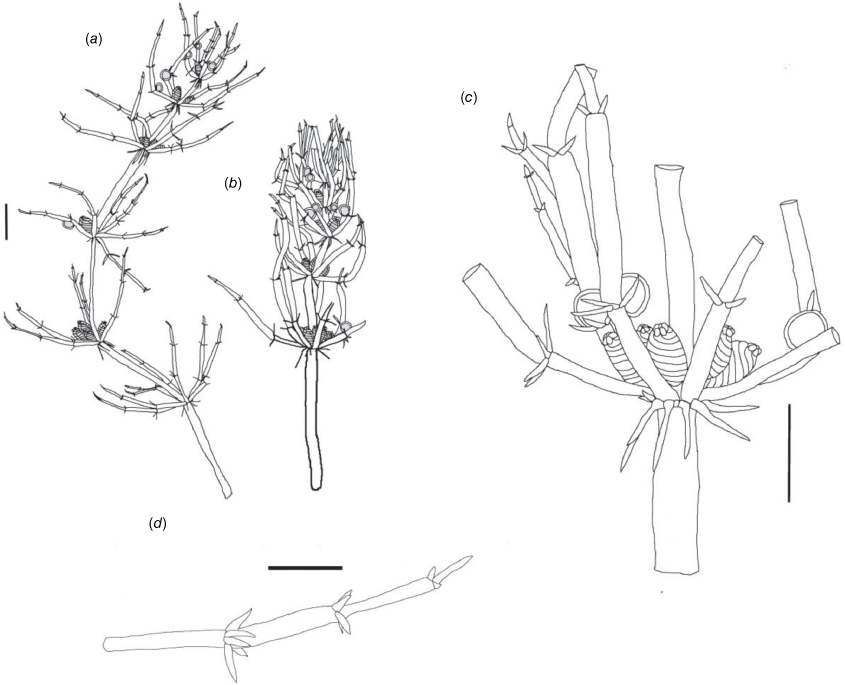
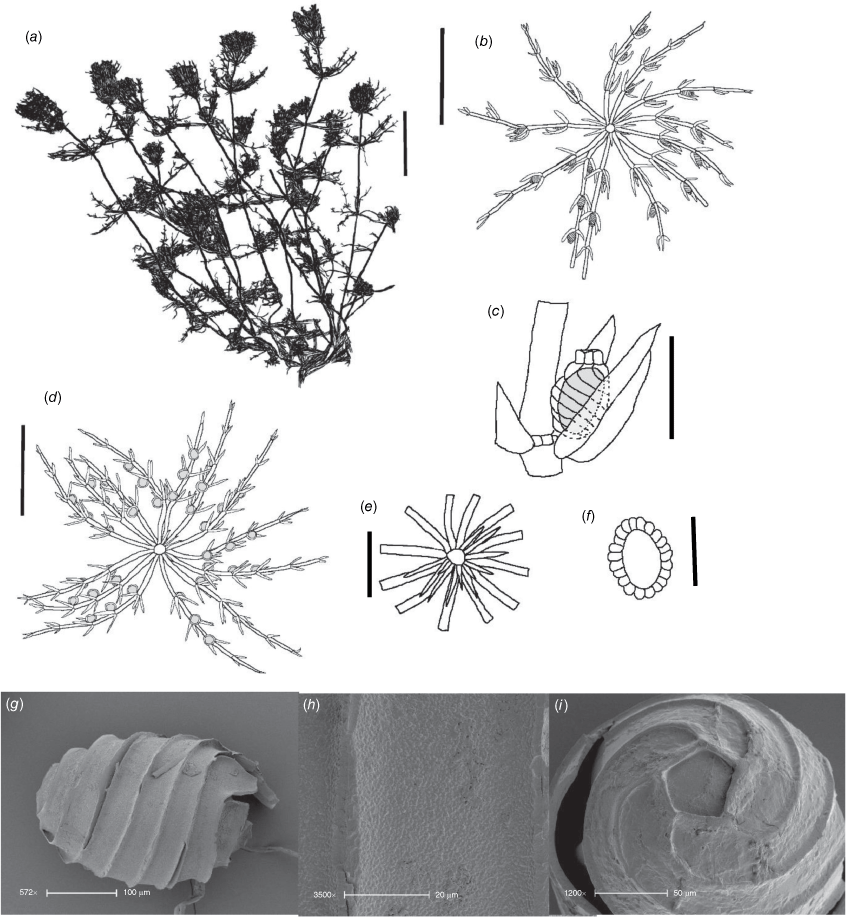
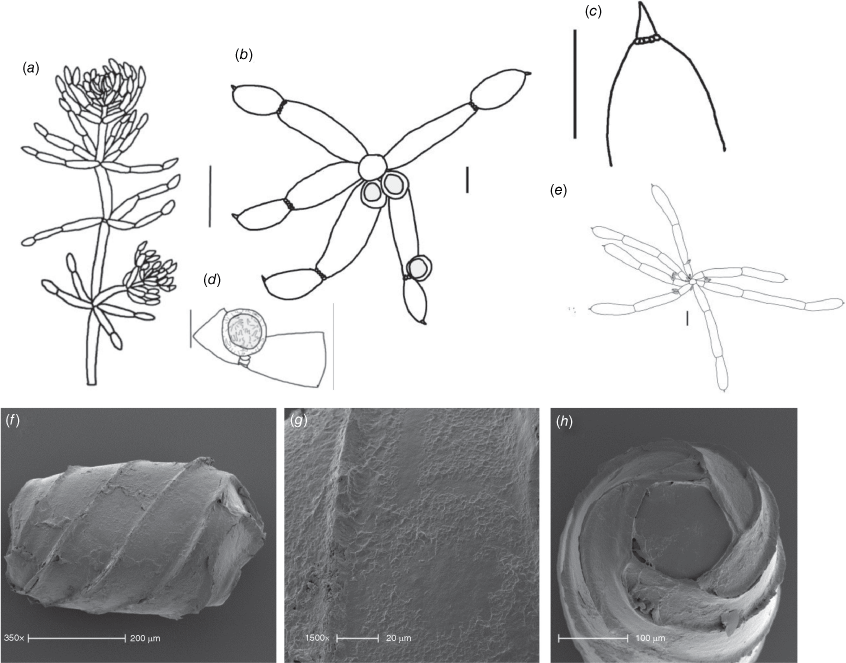
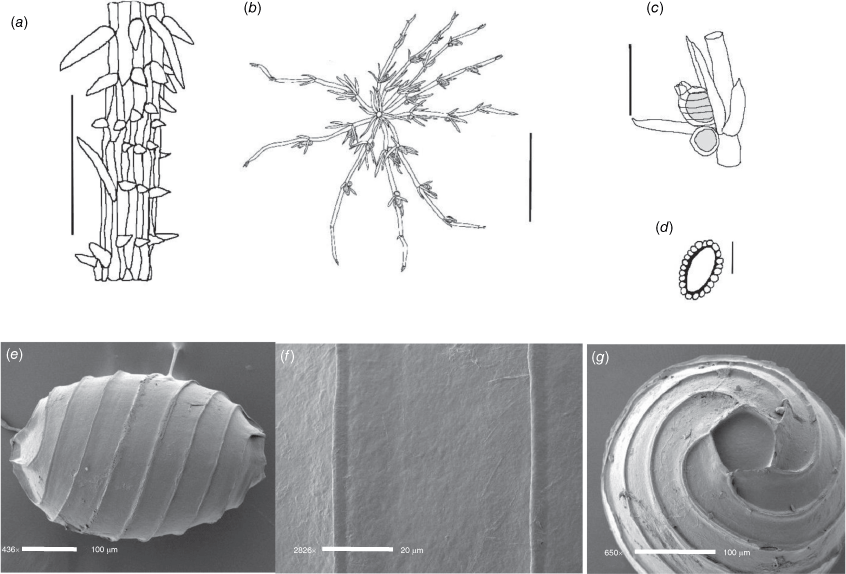
Monoecious. Plants often growing as a ‘lawn’ of closely packed shoots with dense ‘fox-tails’, sometimes with inflated branchlets, or branchlet segments, near the base of the plant, but never inflated in the fertile whorls, smelling somewhat of garlic. Axes up to 400 μm in diameter, ecorticate, internodes up to 4 mm long (Fig. 3a). Stipulodes opposite the branchlets, downward-pointing, long, thin and pointed, up to 3 mm long, and also within the whorls of sterile branchlets (Fig. 3b). Branchlets 6–9 in a whorl, with 4 or 5 cells, short, overlapping and markedly incurved, basal branchlet cell up to 1 mm long, branchlet end segments generally 2 cells long, bract cells long and verticillate, 4 or 5 at each node, up to 2 mm long (shorter in Northern Territory specimens; Fig. 3c). Fertile parts contracted into dense ‘fox-tails’, so that observation of the arrangement of the gametangia requires dissection of the whorls. Gametangia aggregate inside the base of the branchlet whorl and solitary on the branchlets. Oosporangia sessile and aggregate inside the base of the branchlet whorl, rarely 1 on one of the first branchlet nodes, up to 800 μm long with 10 stripes of helical cells, coronula up to 65 μm high. Oospores black, 564–675 μm long, 240–350 μm wide, 11 or 12 striae of low undulating ridges and fossa with granulate ornamentation. Antheridia solitary on the lowest node of the branchlets (Fig. 3b), up to 450 μm in diameter, never at the base of the whorl. Vegetative reproduction by spherical white bulbils attached to the rhizoid nodes. Chromosomes not known.
Distribution
Coastal South Australia including Kangaroo Island, inland salt lakes in southern Northern Territory, coastal and inland salt lakes in western Victoria, northern Tasmania and the south-west of Western Australia.
Habitat
Saline lakes and bores.
Etymology
‘Compactum’ from Latin, compact, here referring to the closely compact whorls of fertile branchlets.
Notes
Lamprothamnium compactum is a low-growing meadow-forming species that forms extensive beds in shallow water down to ~2 m deep. It is distinguished from L. macropogon by a dense fox-tail morphology and the absence of antheridia inside the base of the fertile whorls. Northern Territory material seen so far differs from southern Australian specimens in having smaller and less compact fertile whorls.
Specimens examined
NORTHERN TERRITORY: Fitz Bore, Elkedra Station, 27 June 1984, R.Dance 672 (DNA); loc. Not given, n.d., P.Dostine 12DW23-2 (DNA).
Lamprothamnium stipitatum Casanova, Austral. Syst. Bot. 26: 285 (2013)
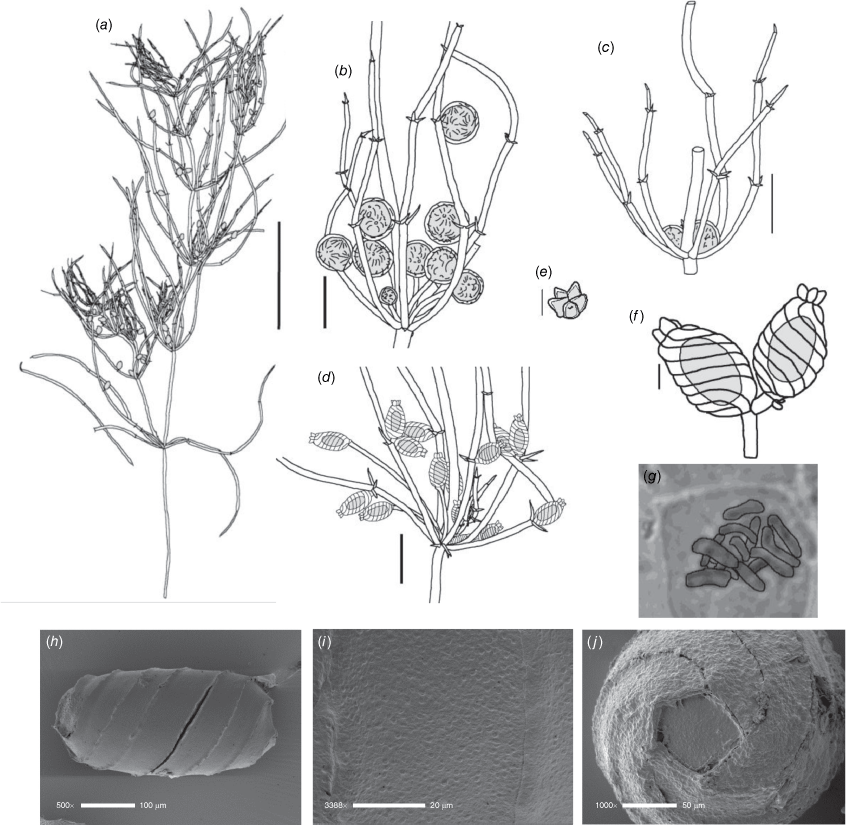
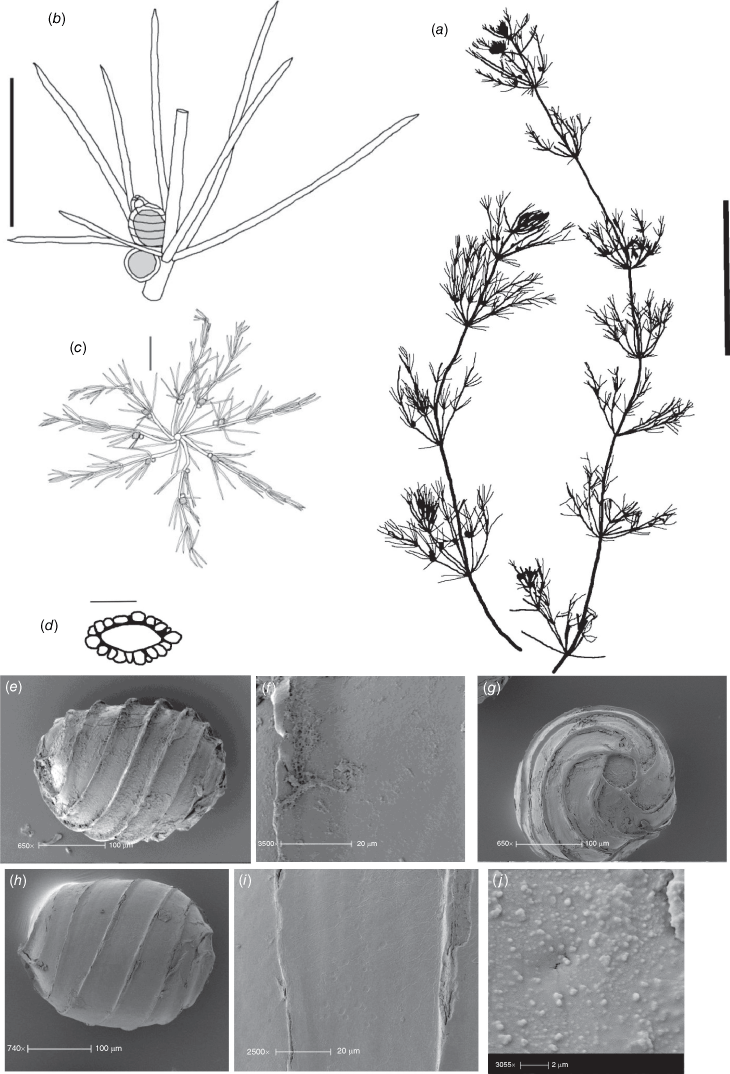
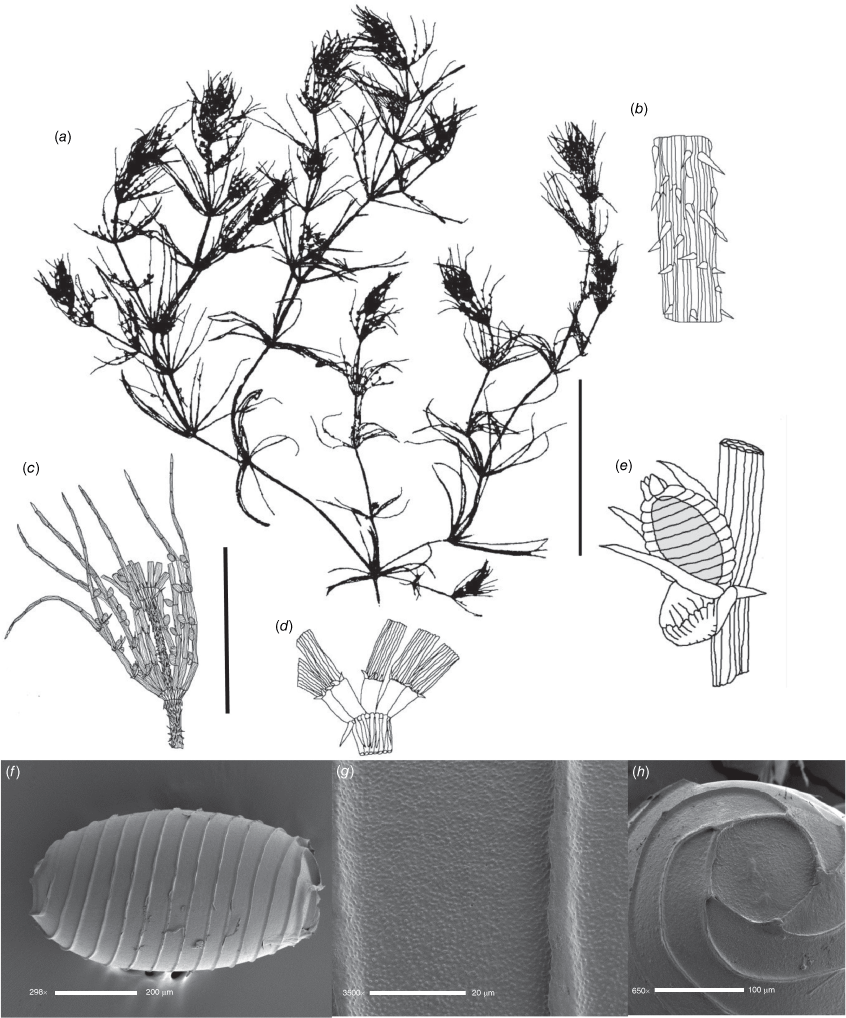
Dioecious. Plants appearing more like a narrow species of Chara than Lamprothamnium (distinguished by the decumbent stipulodes, narrow axes and spreading bract cells, as well as the saline habitat), calcified. Axes 200–300 μm in diameter, ecorticate; internodes up to 5 cm long, a little shorter than the longest branchlets (Fig. 4a). Stipulodes in a single row, opposite the branchlets (Fig. 4d), up to 6 around, but often absent or reduced, downward-pointing, up to 200 μm long. Branchlets 6 in a whorl, up to 45 mm long, usually ~12 mm long, ecorticate, very narrow, up to 100 μm in diameter (Fig. 4b, c), basal branchlet cell up to 2 mm long, branchlet end segments generally 2-celled, the terminal cell much shorter than the others, bract cells verticillate and spreading but usually very short, 100–200 μm long (Fig. 4c). Fertile parts not much contracted, no real distinction between fertile and sterile whorls. Gametangia foliar and internal to the base of the branchlet whorl, sessile and stipitate, solitary and geminate. Oosporangia up to 500 μm long, 300 μm wide, stipitate (stipes up to 2 mm long) and sessile inside the base of the branchlet whorl, sometimes singular, sometimes geminate; on the branchlets, sessile and stipitate (Fig. 4d), sometimes geminate (Fig. 4f). Coronula of triangular cells (star-shaped from above; Fig. 4e). Oospores black, 360–375 mm long, 200–210 μm wide, 8 or 9 striae of low, undulating ridges (Fig. 4h), oospore ornamentation granulate (Fig. 4i), end cell impression up to 65 mm at widest diameter (Fig. 4j). Antheridia large, up to 550 mm in diameter, bright orange, sessile and stipitate inside the base of the branchlet whorl, sessile on the lowest two branchlet nodes (Fig. 4b). Vegetative reproduction not known. Chromosomes n = 14 (Fig. 4g).
Distribution
In brackish temporary wetlands in northern inland Australia: the Pilbara region of Western Australia and the Tanami Desert.
Etymology
‘Stipitatum’ in Latin means provided with a stipe or a stalk, this species being named for the long-stalked gametangia.
Notes
Lamprothamnium stipitatum is the only dioecious Lamprothamnium known from Northern Territory and has very large antheridia. It can be distinguished from dioecious L. heraldii A.García & Casanova on the basis of its stipitate gametangia and paucity of bract cells and stipulodes. This is just the second specimen seen, but it confirms the validity of the species. Lamprothamnium species frequently have gyrogonites, a character that is missing in ecorticate Chara species.
Specimens examined
NORTHERN TERRITORY: Fiddlers Lake, Tanami Desert, 14 Aug. 2001, P.K.Latz 17982 (DNA) [distinctive, but very poor material, intermixed with filamentous algae].
Chara L., Sp. Pl. 2: 115 (1753)
Monoecious or dioecious. Plant axis corticated or ecorticate, stipulodes in one (haplostephanous) or two whorls (diplostephanous), bract cells unilateral or verticillate, branchlets corticated or ecorticate, terminated by a single cell, a cluster of equal-sized cells (corona) or a segment (one or two cells long) surrounded by bract cells. Gametangia sejoined, or conjoined, or on separate plants. When conjoined, the oosporangium is above the antheridium. The helical enveloping cells of the oosporangium are terminated by a coronula of five cells. Oospores with or without a calcified covering (gyrogonite) when mature.
Key to the species of Chara in the Northern Territory|
1 Plants monoecious |
|
2 Plant axis corticated, at least in part |
|
3 Axis diameter greater than 700 µm, plants turgid and upright |
|
4 Plants with no visible bract cells and stipulodes, branchlets straight |
|
5 Axial cortex cells 2× the number of the branchlets in the adjacent whorl |
|
6 Bract cells on the branchlets few, 2 or 3, somewhat unilateral, stipulodes spreading |
|
7 Stipulodes in two rows, gyrogonites present on oospores |
|
8 Stipulodes 1× the number of branchlets, sometimes with more, but never 2× |
|
9 Oogonia >400 µm long, oospores ~300 µm long, spine cells short |
|
10 Gametangia arranged otherwise (sejoined, geminate or clustered) |
|
11 Stipulodes one or two more than the number of branchlets, branchlets short and bract cells abundant, plants without calcification |
|
12 Gametangia conjoined, sometimes geminate and clustered, antheridia 200–500 µm wide |
|
13 Branchlet tips a single cell subtended by bract cells, oospores brown |
|
14 Branchlets fully corticated except the final 1 or 2 segments |
|
15 Branchlet cortex present on a few lower branchlet internodes, gametangia only present where a cortex is present, axis aulacanthous |
|
16 Basal branchlet cell cortex either absent or lacking pigmentation |
|
17 Basal branchlet cell cortex lacking pigment, appearing different from other cortex, plants smooth |
Chara aridicola Casanova & Karol, nom. nov., stat. nov.
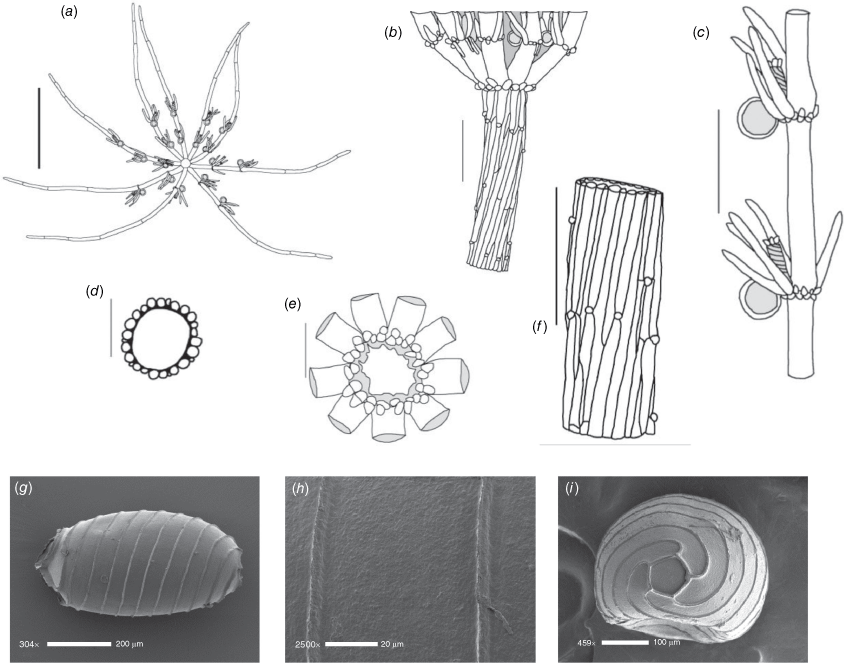
Monoecious. Plants up to ~100 mm high, heavily calcified. Axes 500–1400 µm in diameter (Fig. 5a); 2× corticated, aulacanthous (i.e. spine cells appear to be in furrows because the primary cortical cells are smaller), 18–24 cells around (Fig. 5d). Spine cells globose or conspicuous, 60–200 μm long, 50–70 μm in diameter. Stipulodes inconspicuous, in 2 rows, twice the number in each row as the number of branchlets in the adjacent whorl, uppers 100–300 μm long, lowers 50–150 μm long, downward pointing or globose. Branchlets partly and often incompletely corticated (on the lowest 1–3 branchlet internodes), 8–10 in a whorl, up to 17 mm long, segments 6 or 7, terminated by a single cell (Fig. 5b). Bract cells innermost up to 1.5 mm long, ~2 of them, outer up to 250 μm long, bracteoles 2, shorter than innermost bract cells but longer than the immature oosporangia. Gametangia conjoined singly at the lowest 3 corticated branchlet nodes. Very rarely there are 2 oosporangia above a single antheridium. Oosporangia 1100–1200 μm long, 500–630 μm wide, coronula 150 × 280 μm, somewhat spreading (Fig. 5c). Antheridia up to 500 µm in diameter. Oospores black, 750–820 μm long and 450–500 μm wide with 13–17 ridged striae (Fig. 5e), fossa smooth (Fig. 5f), end-cell impression ~140 μm across (Fig. 5g). Gyrogonites present. Chromosomes n = 28 (specimen Casanova p780).
Distribution
In semi-permanent bores, quarry ponds and springs of the Northern Territory and South Australia; the water chemistry has not been recorded.
Etymology
‘Aridicola’, from Latin aridus, dry, and -cola, dwelling, describing the species’ preference (particularly the type material) for arid zones, referring to its occurrence in arid and semi-arid regions of the Northern Territory, Western Australia and South Australia
Notes
Braun (1853) described this species as a variety in 1853 on the basis of material collected by von Mueller, and speculated that the large oospore size (larger than Chara contraria A.Braun ex Kütz. from Europe) was because of nutrient availability (Nordstedt 1887). Groves in Groves and Allen (1935) recorded one specimen from Queensland and did not refer to material described by Braun (1853). Wood (1962) united all previously recognised varieties and forms of C. contraria with C. vulgaris. This was repeated with reference to Australian material (Wood 1971). The Australian material is different from the type material of C. contraria in Europe, which is tylacanthous (i.e. with spine cells on the larger cortical cells) with conspicuous stipulodes, and C. vulgaris, which usually has brown oospores, conspicuous stipulodes and is more completely corticated on the branchlets. The oospores of C. aridicola also differ in size and ornamentation from those of C. contraria (<750 μm long, somewhat porate) and C. vulgaris (<750 μm long, papillate to verrucate).
The name given by Braun (1853) was C. contraria var. australis. Since the epithet australis was already occupied by a species in subgenus Charopsis, a new epithet has been given, namely, aridicola.
This species has corticated axes and partly corticated branchlets (the lowest 1–3 branchlet internodes) and gametangia only at the branchlet nodes with cortex adjacent to them, large black oospores with gyrogonites. It is similar to C. behriana (A.Braun) Casanova, which has partial or no cortication on the fertile branchlet nodes. The other corticated species of Chara in the Northern Territory have either three times corticated axes (C. setosa Klein ex Willd., C. globularis Thuill., C. zeylanica Klein. ex Willd.) or completely naked branchlets and one row of stipulodes.
Specimens examined
NORTHERN TERRITORY: Barkly Tableland, Brunette Downs, Crows Nest Bore, 11 July 2001, J.A.Risler & C.P.Mangion 835 (DNA, NT). SOUTH AUSTRALIA: Woakwine Quarry, 1 Nov. 2010, M.T.Casanova r846 (BM, MEL, NY).
Chara arnhemensis (R.D.Wood) Casanova & Karol, comb. nov., stat. nov.
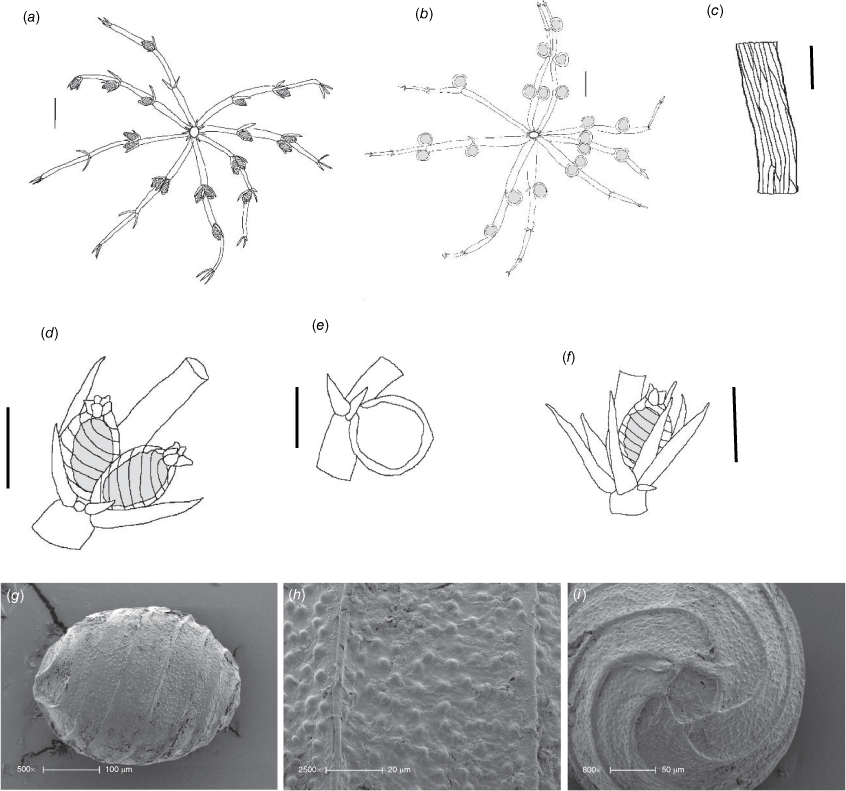
Monoecious. Plants up to 35–150 mm high, with short compact upper internodes in shallow water, and with so many long accessories as to appear prickly, without calcification (Fig. 6a, c). Axes filiform, 100–300 μm wide, internodes 6–12 mm long, essentially 2× corticated, ~16 cells around, anisostichous (i.e. cell sizes variable; Fig. 6d), cortex appearing 1× near the nodes, and rarely appearing 3× in the centre of the internode where upper and lower secondary cells might intermingle. Spine cells abundant, occurring singly, up to 1 mm long and 50–80 μm in diameter, acute, spreading and appressed (Fig. 6f). Stipulodes up to 2.5 mm long and 50–80 µm wide, in a single whorl, 2 per branchlet, acute (Fig. 6b). Branchlets ecorticate, 8–9 in a whorl, 3–5 mm long, 250 μm wide, with 3 or 4 segments, a bunch of bract cells at the apices, basal branchlet cell much shorter than the stipulodes, similar in length to the other branchlet cells (Fig. 6g). Bract cells up to 1.4 mm long, verticillate, 3–6 of them (Fig. 6e). Bracteoles 2, shorter than the bract cells, up to 1.0 mm long. Gametangia conjoined singly at the lowest branchlet node, occasionally at a second node (Fig. 6b, e). Oosporangia 245–300 μm long, 195–250 μm wide, 6 stipes of helical cells, coronula up to 50 µm high, 200 µm wide, cells spreading. Oospores tiny and black, broadly oval, somewhat flanged, 210–250 µm long, 180–210 µm wide with 6–8 striae (Fig. 6h), oospore wall smoothly flocculate, appearing felted and porate at very high magnification (Fig. 6i), fossa up to 60 µm across. Antheridia 150–200 µm in diameter. Chromosomes not known.
Distribution
This species is apparently restricted to freshwaters on Groote Eylandt in the Gulf of Carpentaria.
Etymology
The type material was collected on the 1948 American–Australian expedition to Arnhem Land and the species is named for the area in which the expedition was undertaken.
Notes
This species is distinctive, mostly in relation to the elongate stipulodes, bract cells and long and abundant spine cells. This is the smallest species of Chara in the Northern Territory, with the smallest oospores of any known species of Chara (Wood 1959).
This species was called ʻChara spechtii’ as a manuscript name, although Wood (1962) chose to publish it as Chara fibrosa subsp. fibrosa f. arnhemensis.
Specimens examined
NORTHERN TERRITORY: Little Lagoon, Groote Eylandt, in a freshwater swamp, 18 May 1948, R.L.Specht A29 (AD, NY).
Chara bancroftii (J.Groves) Casanova & Karol, comb. nov., stat. nov.
Dioecious. Plants up to 300 mm high, sometimes lightly calcified (Fig. 7a). Axes 400–500 µm in diameter; 2× corticated, isostichous, 20–28 cells around (Fig. 7f). Spine cells acute and varying in length (up to 500 µm long), or globose and somewhat inconspicuous, ~50 μm in diameter. Stipulodes spreading or conical, in a single whorl, the same number as the number of branchlets in the adjacent whorl (Fig. 7e). Branchlets ecorticate, 10–14 in a whorl, up to 15 mm long, segments 4–6, terminated by a cluster of bract cells (Fig. 7b, d). Bract cells verticillate, up to 1 mm long, 2 or 3 of them, bracteoles 2, similar to bract cells. Bractlet present in place of an antheridium, as long or longer than the oosporangium (Fig. 7c). Gametangia occurring singly or rarely geminate at the lowest 3 branchlet nodes (Fig. 7b, d). Oosporangia up to 500 µm long and 320 µm wide with ~9 stripes of helical cells, coronula up to 50 µm high, cells rod-shaped (Fig. 7c). Oospores very dark brown, 380–410 µm long, 200–250 µm wide. Striae of 8 or 9 flanged ridges (Fig. 7g), fossa wall 40 μm across, smooth to finely granulate (Fig. 7h), end cell impression ~75 μm across (Fig. 7i). Antheridia up to 600 µm in diameter. Chromosomes not known.
Distribution
In freshwater wetlands in tropical and subtropical areas of Queensland and Northern Territory.
Etymology
Named in honour of Thomas Lane Bancroft (1860–1933), a medical naturalist who collected in Queensland.
Notes
Braun amalgamated all dioecious specimens that lack basal gametangia into the taxon Chara dichopitys A.Braun, nom. illeg. (Nordstedt 1883). Groves and Allen (1935) reinstated C. preissii A.Braun and described this taxon with 1× stipulodes (unistipulate) as a variety therein, namely, C. preissii var. bancroftii. Wood and Imahori (1965) amalgamated all the taxa in section Agardhia in Australia into four species (C. fibrosa, C. leptopitys A.Braun, C. submollusca Nordst. and C. ecklonii A.Braun ex Kütz.), synonymising the taxon bancroftii with C. fibrosa f. fibrosa without further recognition. Wood (1971) suggested that f. Bancroftii ‘might be conveniently recognised’ as a form of C. fibrosa (‘like the normal form but with well-developed bract cells’) and provided an illustration of one of the syntypes (Bancroft 47; Wood 1971, p. 31), but no written description.
Chara bancroftii is dioecious, has a corticated axis and naked branchlets. It is distinguished from other dioecious species of Chara in Northern Territory by very large antheridia and abundant bract cells, from C. leptopitys and C. leptopitys subsp. subebracteata Nordst. by the absence of basal gametangia, and from similar dioecious species elsewhere (C. albaniensis (R.D.Wood) Raam, C. hookeri A.Braun, C. mollusca A.Braun, and C. preissii) by the diplostichous cortex and haplostephanous stipulodes.
Specimens examined
NORTHERN TERRITORY: 21 miles [~33.7 km] SE of Elliott, 20 Feb. 1969, P.K.Latz 478 (DNA, NT); 9 km E of Rabbit Flat Road House, 7 July 1980, P.K.Latz 8477 (DNA, NT); Purnie Bore, 11 Sep. 2009, A.Kerezy s.n. (MEL, NY). QUEENSLAND: Pretty Plains, 60 km NW of Valley of Lagoons, 9 Sep. 1976, M.Lazarides 8159 (L, NSW); Pretty Plains Station, near Valley of Lagoons, 9 Sep. 1976, L.G.Adams 8159 (CANB); pond on the road to Carnavon Gorge, 5 July 2010, M.T.Casanova r712 (MEL, NY).
Chara behriana (A.Braun) F.Muell. ex Casanova & Karol, comb. nov., stat. nov.
Monoecious. Plants up to 120 mm high, usually with thick calcium carbonate deposition on the thallus. Axes 600–700 µm in diameter; unevenly 3× corticated (Fig. 8f), secondary and tertiary cells smaller than primary cells, somewhat isostichous to tylacanthous, 26–30 cells around (Fig. 8d). Spine cells globose and somewhat inconspicuous, ~50 μm in diameter (Fig. 8b). Stipulodes somewhat obscure, in 2 whorls, each whorl up to 2× the number of branchlets, upper whorl ~100 μm long, lower whorl ~50–80 μm long (Fig. 8e). Branchlets 9 or 10 in a whorl, up to 12 mm long, segments 5 or 6, terminated by a blunt cell, scarcely corticated, a few cortical filaments on the first branchlet cell, and with cortical initials at the branchlet nodes (Fig. 8a). Bract cells highly reduced abaxially, longer, usually 2 adaxially (to 1000 μm long) at the branchlet nodes, bracteoles 2, ~800 μm long (Fig. 8c). Gametangia conjoined singly at the lowest 2 or 3 branchlet nodes (Fig. 8c). Oosporangia up to 1000 µm long and 400 µm wide with 12 stipes of helical cells, coronula up to 150 µm high, cells peg-shaped, spreading. Oospores brown to black, 530–680 µm long, 350–370 µm wide (Fig. 8g), often with a calcified covering (gyrogonite 700–720 µm long). Striae of 11 or 12 low ridges, fossa wall 60–70 μm across, finely granulate (Fig. 8h), basal cell impression ~80 µm across. Antheridia up to 200 µm in diameter. Chromosomes not known.
Distribution
South Australia, the Victorian Mallee, and into the central part of the Northern Territory.
Etymology
Named in honour of Hans Hermann Behr (1818–1904), German-born botanist who collected in Australia in the 1840s.
Notes
Wood (1962) synonymised C. contraria and all its varieties and forms under C. vulgaris. He followed this treatment for Australian species in this group (Wood 1971) and placed C. contraria var. behriana in synonymy with C. vulgaris var. gymnophylla. Investigation of European material of C. vulgaris indicates that it has well-developed bract cells and brown oospores, which differs from Australian material. European C. contraria also differs significantly (two times corticated axis, evenly corticated branchlets, smaller oospores), so von Mueller’s manuscript name Chara behriana (≡C. contraria var. behriana A.Braun) is here adopted for this species.
Chara behriana has a corticated axis, and largely naked branchlets. It is distinguished from other species with two rows of stipulodes by the scant cortication on the branchlets, black oospores and the occurrence of gametangia on uncorticated nodes, and from species with ecorticate branchlets in subgenus Charopsis by the presence of gyrogonites on the oospores, and by the unilateral development of the bract cells. It differs from C. conimbrigensis A.G.Cunha in having less conspicuous and poorly developed stipulodes and consistently singular conjoined gametangia.
Specimens examined
NORTHERN TERRITORY: Harts Camp Waterhole on the Finke River, Henbury Station. In clear water on sandy bottom, 26 May 2012, R.Breen v053 (MEL); Lake Nash, 23 Oct. 1976, A.S.Mitchell (DNA); Illara Waterhole, 10 km NW of Tempe Downs Station Homestead, 28 Nov. 2001, P.K.Latz 18381 (DNA, NSW, NT); Pioneer Creek, 6 km E of Glen Helen Resort, 17 Oct. 2003, P.K.Latz 19532 (DNA, NT).
Chara benthamii A.Braun in C.F.O.Nordstedt, Abh. Königl. Akad. Wiss. Berlin 1882: 117 (1883), as ʻBenthamiʼ
Monoecious. Plants 120–200 mm high, branching in the lower parts, upper internodes condensed with a ‘fox-tail’ habit, generally without calcification (Fig. 9a, b). Axes 450–550 μm wide, internodes 9–35 mm long, irregularly 2× corticated, primary cortical cells slightly bigger than secondary cortical cells (tylacanthous) or all similar, 18–20 cells around (Fig. 9e). Spine cells sparse, up to 0.8 mm long near the nodes. Stipulodes up to 1 mm long and 200 µm wide, in a single whorl, at least one per branchlet, but fewer than 2 per branchlet (Fig. 9c). Branchlets ecorticate, 8–13 in a whorl, 8–11 mm long, 0.2–0.3 mm wide, with 4–6 segments, the lowest segment the longest, all others decreasing in size, the end segment sometimes 2-celled (Fig. 9c). Bract cells up to 2.6 mm long, somewhat verticillate (internals slightly longer than externals), 2–6 of them, decreasing in size and number at higher branchlet nodes (Fig. 9d). Bracteoles 2, as long as, or shorter than the bract cells, 2 or 3 times as long as the oosporangium. Gametangia conjoined singly at the lowest 2 or 3 branchlet nodes. Oosporangia 500 µm long, 380 µm wide, 8 or 9 stripes of helical cells, coronula 70–80 µm high, 140–170 µm wide, cells somewhat rounded. Oospores black, broadly oval, 330–510 µm long, 253–430 µm wide (dry) with 7 or 8 striae (Fig. 9f), oospore wall smooth to slightly rugose (Fig. 9h), fossa to 54–72 µm in diameter (Fig. 9g), basal cell impression 78 µm wide at the widest point with edges 52 µm long. Antheridia to 360 µm in diameter. Chromosomes not known.
Distribution
South-East Asia and northern Australia, with outliers in southern New South Wales and the south-west of Western Australia, in ditches, drains, rivers and wetlands. The taxon (as C. fibrosa var. benthamii) has also been recorded from Europe (Soulié-Märsche et al. 2013; Mouronval et al. 2015). It appears to be restricted to freshwater.
Etymology
Named for George Bentham (1800–1884), a leading systematic botanist of the 19th century (Jackson 1885).
Notes
Only a few Australian specimens are unequivocally determined as Chara benthamii (on the basis of oospore shape and size and vegetative morphology); many of the specimens allocated to C. benthamii in this study have more bract cells, larger oospores and less tylacanthous cortex. They could possibly be assigned to C. gymnopitys, which is typified on the basis of south-eastern Australia–Tasmanian material. In the absence of a more thorough review of typification of C. gymnopitys and the plethora of form and variety names assigned; Australian monoecious specimens with black oospores, verticillate bract cells and more than one times but fewer than two times stipulodes are here assigned to C. benthamii. In general, Australian specimens have more branchlets (up to 13) than the type specimen.
Chara benthamii was recognised as a species for Queensland (e.g. Bailey 1913) but Groves and Allen (1935) amalgamated it with C. gymnopitys. All the specimens they saw from Queensland conformed to their concept of C. gymnopitys var. benthamii, although the stipulodes (or plants?) were shorter and stouter than for the type of C. benthamii, and plants differed from C. gymnopitys var. gymnopitys by having fewer, shorter and wider ‘bracteal appendages’ (bract cells and bracteoles). Zaneveld (1940) retained C. benthamii as a subspecies of C. fibrosa. Wood and Imahori (1965) and Wood (1971) amalgamated C. benthamii with C. fibrosa and did not recognise it even as a form or ‘microspecies’.
Distinguished on the basis of uneven two times tylacanthous cortex, one times stipulodes, plus several more, but never so many as to be two times, short branchlets, few branchlet cells, the basal cells longest, few, small spine cells, and oval, black oospores. Chara benthamii differs from C. arnhemensis and C. lamprothamniformis Casanova by the overall spiny appearance of those species, from C. duriuscula (A.Braun) Casanova & Karol by having a tylacanthous cortex, verticillate bract cells and consistently more stipulodes. Chara wightii (A.Braun) Casanova is distinguished by oospore colour (brown in C. wightii).
Specimens examined
NORTHERN TERRITORY: Daly River, Camp site 5, 6 Sep. 2010, M.T.Casanova r783 (MEL); Katherine River, low-level site, 7 Nov. 2005, J.Schult A411 (MEL); Yirrkala, in a freshwater stream, 11 Aug. 1948, R.L.Specht A75 (AD-A21397). Carpentaria Highway, Calvert River crossing, 7 July 1998, C.R.Michell & J.Risler 1615 (DNA); 25 km SW of Goyder River Crossing, 16 June 1972, J.Must 1012 (DNA); WESTERN AUSTRALIA: Glen Herring Pool, Pilbara Biological Study site PSW022, 23 May 2004, M.N.Lyons & D.A.Mickle (PERTH); Kalgan Creek pool near Newman, Pilbara Biological Study site PSW066, 24 Sep. 2004, D.A.Mickle & N.Y.Huang 4520 (PERTH); Perry Lake, 13 Oct. 1960, R.D.Wood 60-10-13-4-A (AD-A39012). QUEENSLAND: Moreton Bay, s.dat., C.Stuart 219 (MEL); Broadwater Road, N of Mt Cotton Road, 10 miles [~16 km] S of Brisbane, in a field dam down to 30-cm depth, 24 Nov. 1960, R.D.Wood 60-11-24-1 & A.B.Cribb (AD-A31950). NEW SOUTH WALES: Deua River (Moruya River), Araluen Road, M.T.Casanova r518 (MEL).
Chara duriuscula (A.Braun) Casanova & Karol, comb. nov., stat. nov.
Monoecious. Plants up to 120 mm high, with long branchlets in the basal whorls, upper whorls slightly condensed, lightly to heavily calcified (Fig. 10a). Axes up to 550 µm in diameter, 2–partly 3× corticated, secondary cells (and tertiary cells where present) smaller than the primary (tylacanthous), ~16 cells around (Fig. 10c). Internodes 5–20 mm long. Spine cells solitary, very small but acute, up to 200 µm long, only obvious on the upper, young internodes (Fig. 10g), stipulodes in 1 row, narrow, variable in number and length, mostly 1× the number of branchlets on the adjacent whorl, usually sticking out perpendicular to the axis, 0.4–1.2 mm long (Fig. 10e). Branchlets ~8 in a whorl (Fig. 10f), ecorticate, 4 or 5 cells long, spreading, basal branchlet cell 2.5–3.5 mm long, next two segments equally long (sometimes longer), branchlet end segments very short, exceeded by the subtending bract cells (Fig. 10b), bract cells 1 or 2, internally up to 1 mm long, externally short, up to 0.8 mm long. Bracteoles 2, at least 2× longer than the oosporangium (Fig. 10d). Gametangia conjoined on 2 or 3 branchlet nodes, solitary. Oosporangia to 560–590 µm long, coronula cells appressed. Oospores black, (measured dry) 380–415 µm long, 240–290 µm wide, with 9 or 10 striae of flanged ridges (Fig. 10i), the oospore wall smooth to densely papillate, papillae ~1 μm in diameter, 13–15 across the fossa (Fig. 10j), basal cell impression up to 52 µm in diameter (Fig. 10k). There is evidence of some deposition of calcium carbonate on the surface of oospores (Fig. 10h) and thallus (although no development of a gyrogonite). Antheridia 390–440 µm in diameter. Chromosomes not known.
Distribution
Tropical rivers in the Northern Territory, possibly in Indonesia.
Etymology
From Latin: duriusculus meaning ‘somewhat hard’ perhaps referencing the somewhat calcified nature of the plant.
Notes
When collected, specimens were easily distinguished as a separate taxon (initially tentatively allocated to ʻC. sp. aff. thwaitesiiʼ), and examination of the type material (in LD) allowed allocation of the correct name. This species differs from C. thwaitesii A.Braun by having gametangia conjoined at all branchlet nodes (rather than sejoined and occasionally basal in C. thwaitesii; Wood and Imahori 1965).
The type specimen was designated a variety of C. gymnopitys A.Braun in Nordstedt (1883), distinguished on the basis of oospore size and number of branchlets. Zaneveld (1940) retained it as a variety of C. fibrosa subsp. gymnopitys, occurring in Australia, but not the Malay peninsula. Groves and Allen (1935), Wood (1962), and Wood and Imahori (1965) made no mention of this taxon. Wood (1971) amalgamated it with C. fibrosa f. fibrosa, mentioning it only in synonymy.
Chara duriuscula is a somewhat smooth-looking monoecious charophyte with black oospores, corticated axes and naked branchlets. It has inconspicuous spine cells and few bract cells (mostly internal to the branchlets), and all branchlet segments are long. It differs from C. benthamii (with which it has an overlapping distribution) by having granulate ornamentation on the oospores, more cortical cells, fewer bract cells and gametangia confined to the bottom nodes of the branchlets. It differs from C. drummondii A.Braun by having well-developed stipulodes. It differs from C. gymnopitys in having fewer bract cells and spine cells and smaller oospores. Chara duriuscula is usually well calcified, and sometimes grows semi-emergent along the banks of tropical rivers (Casanova and Nairn 2015).
Specimens examined
Specimens: NORTHERN TERRITORY: Oolloo Crossing, Daly River, 6 Sep. 2010, M.T.Casanova r765 (BM, DNA, MEL, NY), r766 (MEL) r771 (DNA, MEL, NY); Daly River, Camp site 5, 6 Sep. 2010, M.T.Casanova r773 (MEL), r774 (DNA, MEL, NY), r780, r781 (MEL); Barnes Creek, Arnhemland, 1854, F. v. Mueller s.n. (MEL); Arnhemland, Wilton River, 10 Nov. 1987, G.Leach 1662 & C. Dunlop (DNA) [mixed with C. lucida]; Bullo River Station, spring-fed stream, 9 Mar. 2006, D.J.Dixon 1558 (DNA, NSW).
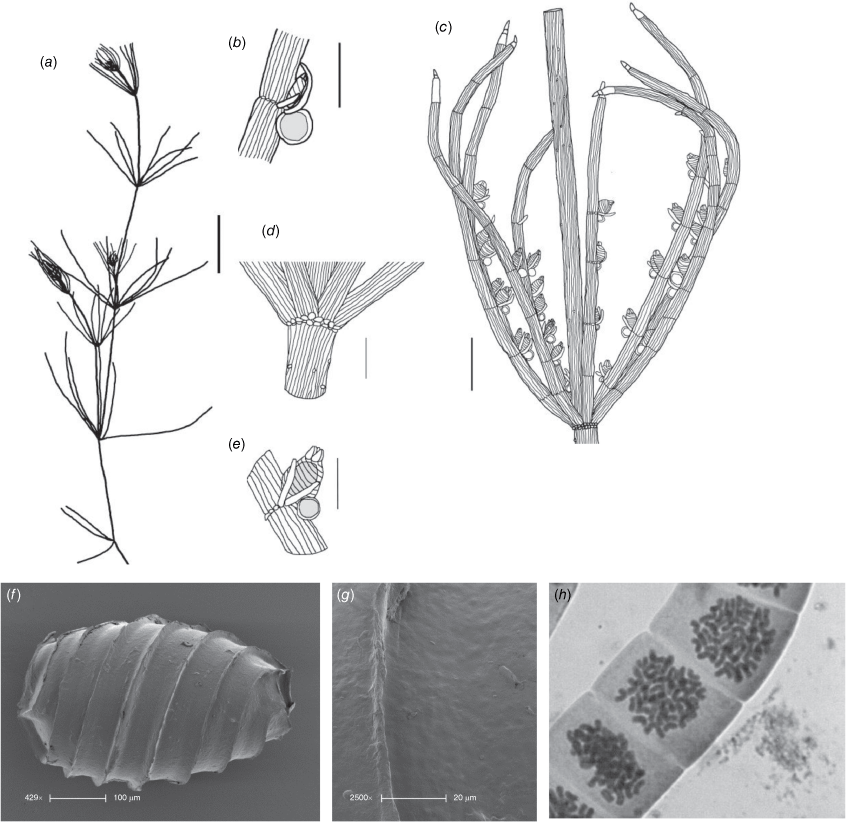
Chara erythrogyna Griff., Not. Pl. Asiat. 2: 278 (1849)
Monoecious. Plants up to 450 mm high, slender, rarely calcified and somewhat spiky in appearance (Fig. 11a). Axes 400–600 µm in diameter, 2× corticated evenly isostichous, 22–24 cells around (Fig. 11e), internodes 15–45 mm long on lower parts, upper parts contracted. Spine cells solitary, only noticable on the younger internodes, rudimentary or absent on older parts, stipulodes in 1 row, up to twice the number of branchlets (18 counted for 12 branchlets), somewhat variable 0.5–1.5 mm long, acute (Fig. 11b). Branchlets 11–14 (or more; Nordstedt 1883) in a whorl, ecorticate, 5 or 6 cells long, up to 20 mm long, tapering, basal branchlet cell the longest, branchlet end segments singular (Fig. 11c), as narrow as the bract cells, bract cells 6–9, verticillate on all branchlet nodes, up to 1.5 mm long, acute (Fig. 11f). Bracteoles 2, shorter than the bract cells. Gametangia conjoined, sejoined, solitary or geminate at the branchlet nodes (Fig. 11d, f). Oosporangia 550–700 µm long, coronula cells very small, apressed, bractlet present when sejoined from antheridia. Oospores black, 375 –420 µm long, 265–290 µm wide, with 8 or 9 striae of prominent ridges (Fig. 11g), the oospore wall smooth or unevenly maculate with rows of small indentations (‘obscurely granulate’ sensu Wood and Imahori 1964) (Fig. 11h, j), basal cell impression 36–76 µm in diameter (Fig. 11i). Antheridia up to 700 µm in diameter, scarce, sejoined or conjoined. Chromosomes not known.
Distribution
In freshwater lakes and wetlands in South-East Asia, Northern Territory around Darwin, Elcho Island.
Etymology
From Greek erythro- ‘red’, and gynos ‘pertaining to female organs’, named for the bright colour of the young oosporangia.
Notes
The type material was collected in South-East Asia, deposited in BM, with fragments in L. The Australian material has similarly geminate, conjoined and sejoined gametangia, similar-sized oosporangia, coronula and oospores and similar vegetative morphology. However, the oospores on Australian material differ from Indian material, which have a minutely figured oospore with a tiny basal cell impression (36 μm). Australian material fits well with Braun’s description of the type material (even to the size of the end segments of branchlets and relative scarcity of antheridia) (Nordstedt 1883).
Braun thought the name Chara erythrogyna was inappropriate (since the gametangia were only red in the juvenile state) and so published the superfluous replacement name Chara griffithii (Braun, unpubl. data, cited in Nordstedt 1883).
Chara erythrogyna is distinguished from all other Australian species with corticated axis and naked branchlets by its gametangial arrangement. This is usually noticeable as frequently geminate oosporangia or oospores, and inconspicuous and rare antheridia, occasionally conjoined with an oosporangium.
Specimens examined
NORTHERN TERRITORY: Yirrkala, 11 Aug. 1948, R.L.Specht A75 (AD); Manton Dam Recreation Area, in water ~50 cm deep, 5 Sep. 2010, M.T.Casanova r752 (MEL, NY); shallow lagoon in Melaleuca–Mimusops thicket, Nightcliff Agricultural Area, 21 Apr. 1961, R.D.Wood & N.Eddy 61-4-21-4A (AD); Elcho Island, 13 July 1975, P.K.Latz 6208 (DNA); Port Darwin, 9 Apr. 1896, T.B.Blow A101 (BM). INDIA: shallow pond, Saharanpur District, United Provinces, 19 Sep. 1926, G.O.Allen 10 (BM); small pond Saharanpur, United Provinces, 31 Oct. 1926, G.O.Allen 158 (BM); Saharanpur United Provinces, G.O.Allen 90 (BM).
Chara globularis Thuill., Fl. Env. Paris 2nd edn, 472 (1799)
Monoecious. Plants up to 200 mm high, with light calcium carbonate deposition on the thallus. Axes narrow, 400–500 µm in diameter (Fig. 12a); 3× corticated, evenly isostichous, ~21 cells around (Fig. 12d). Spine cells absent or inconspicuous. Stipulodes inconspicuous, in two whorls, 2× the number of branchlets in each whorl, globular or rudimentary, clearly present on young nodes, sometimes obscure on old nodes (Fig. 12d). Branchlets 7 or 8 in a whorl, 14–32 mm long, 5–8 segments, corticated with ~12 cortical cells around, terminated by a 2–4-celled uncorticated segment (Fig. 12c). Bract cells obscure and globose, abaxially up to 500 μm long, bracteoles 2, beside gametangia, 100–500 μm long, 50–80 μm wide (Fig. 12b). Gametangia conjoined at the lowest 2–5 branchlet nodes (Fig. 12e). Oosporangia up to 1000 µm long and 400 µm wide, coronula up to 100 µm high, appressed. Oospores black, 600–700 µm long, 300–350 µm wide, usually with ‘basal claws’ and a ‘cage’, often with a thin calcified covering (gyrogonite 800 µm long). Striae of 11 or 12 ridges with a delicate ribbon (flaccid flange) (Fig. 12f), fossa wall 40–50 μm across, minutely papillate (Fig. 12g). Ribbon with nodular structures. Antheridia up to 450 µm in diameter. Chromosomes n = 42 for a Victorian specimen (Fig. 12h) (Casanova 2015).
Distribution
One of the few apparently cosmopolitan species of charophytes, occurring on all continents except Antarctica. Occurs throughout Australia, in shallow, mostly permanent, sometimes turbid, sometimes slightly saline waterbodies. In the Northern Territory, it has been collected only in the arid zone in dams, bores and canyon waterholes.
Habitat and ecology
Chara globularis occurs in a diversity of habitats, from highly turbid to clear running water. It often grows in somewhat eutrophic and modified conditions (e.g. park ponds). It can be a perennial species, but usually reproduces sexually in its first year (Schubert et al., in press). Ripe oospores can be found from October to April in Australian material.
Etymology
Apparently named for the globular stipulodes, bract cells and spine cells.
Notes
Chara globularis has often been referred to as Chara fragilis Desv. [in Loisel., Not. Fl. France: 137. 1810] in the past. This name must be regarded illeg. because the description refers to ‘Chara vulgaris Thuil.’, which was used already for Chara vulgaris L. in Thuillier (1799, p. 471).
Wood (1962) amalgamated a large number of taxa into Chara globularis, sometimes retaining previously published species names at the varietal and form level. Proctor (1971) found that ‘C. globularis’ sensu Wood consisted of many reproductively isolated lineages. Sakayama et al. (2009) investigated the phylogeny of different Japanese specimens that keyed out to Wood’s Chara globularis f. globularis. They found that they could distinguish different genetic entities on the basis of branchlet morphology and oospore characters, describing C. leptospora Sakayama on the basis of the length of the end-segment of the branchlets (1 or 2 cells, up to 3.7 mm long) and elongate oospores with a granulate to papillate oospore wall and a rough nodular pattern on the ribbon structure (flange). The type material of C. globularis has shorter end-segments and broader oospores, with a fine pusticulate ornamentation and fine nodular elements on the ribbon (Sakayama et al. 2009). Northern Territory material falls somewhere in between these, because the oospore ribbon is similar to that of the type material, but oospore shape is similar to that of C. leptospora and the branchlets have an elongate 2- or 3-celled end segment. The name C. globularis is retained for this material pending further investigation.
Chara globularis is smooth-looking and characterised by a distinct foetid or garlic smell when fresh. It is distinguished from C. setosa and C. zeylanica on the basis of its evenly corticated basal branchlet cell, inconspicuous spine cells, and globose stipulodes.
Specimens examined
NORTHERN TERRITORY: Trephina Gorge, East MacDonnell Ranges, 5 Aug. 1957, G.Chippendale s.n. (DNA, MEL); Wigleys waterhole, Alice Springs, Telegraph Station Reserve, 21 Apr. 2012, I.J.Powling v051 (MEL); Alice Springs Primary Industries Research Farm turkey nest tank, 19 Aug. 1968, D.J.Nelson 1735 (DNA); Bottom Bore, Neutral Junction turkey nest, 25 May 1984, R.Dance 735 (DNA); 9 km ESE of Allambi Homestead, 5 Nov. 2001, P.K.Latz 18274 (DNA, NSW, NT); East Mann Range, 90 km W of Amata, 1 Sep. 2009, P.K.Latz 24732 (AD, DNA, NT); No. 4 Bore, 23 km SSE Atula Homestead, 5 Nov. 2008, P.K.Latz 23905 (DNA, NSW, NT). WESTERN AUSTRALIA: Bibra Lake seed bank (cultured), 17 Jan. 2002, M.T.Casanova p473 (MEL); Margaret River seed bank (cultured), 14 Nov. 2009, M.T.Casanova p801 (MEL).
Chara karolii Casanova, Austral. Syst. Bot. 27: 409 (2014)
Monoecious. Plants robust, up to 60 mm high, without calcium carbonate deposition (Fig. 13a). Axes up to 400 µm in diameter, 3-corticate in the upper internodes, isostichous, 24–27 cells around (Fig. 13), ecorticate on the lower internodes; spine cells absent or rudimentary. Stipulodes in 1 whorl, alternate, the same number as the number of branchlets, up to 300 µm long (Fig. 13b). Branchlets 8 or 9 in a whorl, up to 20 mm long, segments 3 or 4, ecorticate (Fig. 13b), terminated by a corona of ~3 cells (Fig. 13e). Bract cells 2 or 3 at branchlet nodes, bracteoles 2, as long as the oosporangia (Fig. 13d). Gametangia conjoined, geminate or clustered at the lowest 2 branchlet nodes (Fig. 13f), as well as basal oosporangia. Oosporangia 500–600 µm long (including coronula), 250–300 µm wide, 8 or 9 stripes of helical cells, coronula up to 150 µm high. Oospores black 480–660 µm long, 280–400 µm wide, 7 or 8 striae of low ridges (Fig. 13g), basal-cell impression 85–103 µm in diameter (Fig. 13j) (25–30% of the diameter of the oospore), oospore wall with a reticulate pattern, when well developed, the reticulum is constructed of small papillae on low ridges, the reticulae ~5 µm in diameter, the papillae ~0.5 µm in diameter on ridges ~0.5 µm high (Fig. 13h, i). Antheridia up to 200 µm in diameter. Chromosomes n = 14 (M.T.Casanova r933) (Fig. 13k).
Distribution
Abundant in temporary wetlands in western Victoria. There is a single specimen collected from the Northern Territory (R.Breen s.n., 25 Aug. 2014 (MEL)), which keys out to Chara karolii (partly corticated axes and geminate gametangia) and is included here pending further study.
Etymology
Named for Kenneth G. Karol from the New York Botanical Garden, who first distinguished this species from Chara muelleri (A.Braun) F.Muell. on the basis of nucleotide analysis.
Notes
Chara karolii has corticated axes and regularly clustered gametangia (i.e. in twos and threes at each node), with the occasional occurrence of basal oosporangia, and granular or reticulately patterned oospores. Cortication is usually absent from the lowest axial internodes and can be restricted to the very youngest internodes. Sometimes cortication is difficult to discern and a thorough check of the upper internodes is necessary. Chara braunii, which might also occur in the Northern Territory, has no cortication whatsoever, and is generally somewhat ‘inflated’ (so that the branchlets appear like a string of sausages, constricted at the nodes). Chara muelleri is distinguished by its singular conjoined gametangia, absence of basal gametangia and more completely corticated axes.
Specimens examined
NORTHERN TERRITORY: creek above John Hayes Rockhole (Trepina), 25 Aug. 2014, R.Breen s.n. (MEL). VICTORIA: Muldoons Sinkhole, Budj Bim Indigenous Protected Area, 28 Mar. 2011, M.T.Casanova r941 (MEL); Streatham–Eurumbeen Road, roadside ditch, 23 Feb. 2011, M.T.Casanova r931 (MEL).
Chara lamprothamniformis Casanova, sp. nov.
Dioecious. Up to 30 cm high, flexible, delicate and narrow, occurring in clumps or swards, not calcified. Axes up to 200 µm in diameter; 2× corticated, all cells similar in size, ~18 cells around (Fig. 14c). Spine cells obscure or small and triangular. Stipulodes in one whorl, regularly 2× the number of branchlets (Fig. 14a, b), short or spreading and downward-pointing. Branchlets 8 or 9 in a whorl, to 8 mm long, 3 or 4 segments, the basal segment the longest (up to half the total branchlet length in female fertile parts), uncorticated, terminated by a cluster of 3–5 bract cells (Fig. 14a, b). Bract cells 6–8, verticillate at the branchlet nodes (Fig. 14f) and elongate on female plants (up to 0.5 mm long), smaller on male plants (Fig. 14e), bracteoles 2, not distinguishable from bract cells. A small bractlet below the oosporangium (Fig. 14d). Gametangia arranged singly or geminate on separate plants (Fig. 14d, f), at the lowest 2 or 3 branchlet nodes. Oosporangia 400 µm long and 350 µm wide, coronula to 40 µm high. Oospores black, 360–410 µm long, 290–305 µm wide (Fig. 14g). Striae of 7 or 8 flanged ridges, fossa wall 40–60 µm across, covered with verrucae 4–5 µm in diameter, 2–10 µm apart, 5–10 of them across the fossa (Fig. 14h), end cell impression very small, up to 50 µm across at the widest part (Fig. 14i). Antheridia up to 450 µm in diameter (Fig. 14e). Chromosomes not known.
Distribution
Occurs in freshwater billabongs associated with the Mainoru River, Arnhem Land, and at Lockhart River, Queensland
Etymology
The epithet lamprothamniformis is derived from the genus name Lamprothamnium and the Latin suffix -formis, resembling, because the distinctively decumbent stipulodes, long bract cells, development of ‘fox-tails’ and verrucate oospore wall in this species resemble those in members of that genus.
Notes
Chara lamprothamniformis can be distinguished from C. schultae Casanova by dioecy, and from other dioecious members of section Agardhia by the distinctive downward pointing stipulodes, absence of spine cells, arrangement of the gametangia and delicate appearance.
Specimens examined
NORTHERN TERRITORY: s. loc, J. Schult k124 (MEL). QUEENSLAND: Lockhart River Community Farm, 4 Oct. 2000, Waterhouse BMW6048 (CANB); Elliott, swamp NE of town, 27 May 1975, T.S.Henshall 979 (DNA).
Chara lucida (A.Braun) Casanova & Karol, Austral. Syst. Bot. 22: 28 (2014)
(Fig. 10.)
Dioecious. Plants flexible, transparent, often in tangled clumps, up to 200 mm tall, uncalcified (Fig. 15a). Axes 0.35–0.7 mm wide (when flat and dried, narrower in fresh material), ecorticate, internodes 8–20 mm long, generally much shorter than the adjacent branchlets (Fig. 15a). Stipulodes in a single row, 6–12 in total, usually fewer apparent, up to 0.5 mm long. Branchlets 6 or 7 in a whorl, ecorticate, 11–50 mm long, 0.3–0.6 mm wide, 4 or 5 cells long, basal branchlet cell up to 10 mm long (Fig. 15b), branchlet end segments small, conical and acute, sometimes subtended by 1 or 2 bract cells, bract cells obscure or short (Fig. 15c). Bracteoles obscure or short, occasionally up to 0.5 mm long. Fertile parts sometimes somewhat contracted with shorter branchlets and internodes. Specimens with only fertile branchlets were distinguished as form tenerior but recent collections (e.g. M.T.Casanova r758) have both long sterile and short fertile branchlets on the same plant. Gametangia sessile inside the base of the whorl and solitary, geminate or clustered at the lowest 1 or 2 branchlet nodes (Fig. 15e). Oosporangia 0.9 mm long, 0.5 mm wide, coronula cells very short, obtuse. Oospores black, cylindrical, 600–670 µm long, 380–400 µm wide, 7 or 8 striae of low ridges (Fig. 15f), ornamentation smooth to minutely granulate (Fig. 15g), basal-cell impression 120–150 µm in diameter at the widest part, edges 95–100 µm long (Fig. 15h). Antheridia from 400 to 850 µm in diameter. Vegetative reproduction not known. Chromosomes not known.
Distribution
Tropical and subtropical Queensland, Western Australia and the Northern Territory in freshwater dams, ponds, still rivers and lagoons. Also occurs in Papua New Guinea.
Etymology
From the Latin ‘lucidus’, meaning shining, clear, transparent. Zaneveld (1940) and Wood (1971) thought this referred to a shiny surface on the pressed specimens, but that feature is neither obvious nor consistent. More likely, it refers to the clear and transparent, almost colourless appearance (Nordstedt 1883) of the pressed type material.
Notes
The species can be distinguished as a totally ecorticate Chara species with narrow axes, distinguished from narrower morphs of C. australis by the short internodes in comparison with the branchlets. Distinguished from C. evanida by dioecy and the single end cells on the branchlet tips. Chara lucida forms tangled masses in warm waters, compared with the upright, turgid stems of C. australis in colder, deeper waters.
Specimens examined
NORTHERN TERRITORY: Mary-Ann Dam, 6 km NNE Tennant Creek, 7 Nov. 1988, P.K.Latz 10993 (DNA, NT); Duckponds Waterhole, 80 km SE of Lajamanu, 12 May 2010, C.Brock 900 (DNA, NT); Swamp SW of Elizabeth Downs Homestead, 23 June 1977, L.A.Craven 4375 (DNA); Arafura Swamp, 20 May 1990, M.J.Clarke 2259 (DNA); Cathedral Billabong, 20 July 1983, P.McBride 8843 (DNA); Connelly’s Camp Waterhole, Benmara Station, 6 May 1984, B.W.Strong 226 (DNA); Lake Ucharonidge, 22 Apr. 1991, D.J.Parsons 136 (DNA); Manton Dam recreation area, 5 Sep. 2010, M.T.Casanova r758 (MEL, NY); roadside borrow-pit on Arnhemland Highway, M.T.Casanova r784 (B, MEL, NY); SW of Elizabeth Downs, Daly River region, 23 June 1977, L.A.Craven 4375 (CANB); Oenpelli, 10 Oct. 1948, R.L.Specht A110 (AD, MEL, NY); Katherine, 14 Feb. 1961, H.S.McKee 8427 (CANB); Arafura Swamp, near Glyde River crossing, 23 Sep. 1998, I.D.Cowie & C.P.Mangion 7985 (DNA); Koolpinyah Station, 8 May 1996, C.R.Michell & J.L.Egan 108 (DNA). QUEENSLAND: in Lagoon, Magoura Station, 18 July 1977, L.A.Craven 4782 (CANB).
Chara porteri Casanova, Austral. Syst. Bot. 22: 30 (2014)
Dioecious. Plants somewhat inflated and turgid, sometimes calcified, up to 150 mm tall, somewhat densely branched, with incurved branchlets in shallow water (Fig. 16a). Axes 0.7 mm wide (up to 1 mm on pressed specimens), ecorticate. Internodes 8–25 mm long, shorter and somewhat contracted at the apices. Rarely internodes appear 2 cells long (Fig. 16g). Stipulodes in a single row when present, frequently obscure or absent, up to 6 in a whorl. Branchlets 6 in a whorl, sometimes made up of a few elongate branchlets and some dwarf branchlets, particularly at the apices, ecorticate, 8–15 mm long, 0.4–0.6 mm wide, 3 or 4 cells long, basal branchlet cell up to 6 mm long, usually the longest cell in the branchlet (Fig. 16b, d), branchlet end segments small, conical and acute, occasionally subtended by bract cells (Fig. 16c, e), bract cells usually obscure, up to 0.2 mm long, or absent. Bracteoles obscure, up to 0.2 mm long, or absent. Gametangia on separate plants, sessile inside the base of the whorl, and solitary, geminate or clustered at the lowest 1 or 2 branchlet nodes (Fig. 16g, h). Oosporangia 0.6–0.75 mm long, 0.5 mm wide, coronula cells short, somewhat spreading, slightly apiculate (Fig. 16f). Oospores black, cylindrical, 470–525 μm long, 250–350 μm wide, 5 or 6 striae of wide ridges (pachygyra) (Fig. 16i), ornamentation smooth to minutely granulate (Fig. 16j), basal-cell impression 100–120 μm in diameter at the widest part, edges 70–76 μm long, rarely 6-sided (Fig. 16k). Antheridia octoscutate, up to 850 μm in diameter (Fig. 16g). Vegetative reproduction not known. Chromosomes n = 14 (M.T.Casanova r416).
Distribution
Occurs in freshwater temporary wetlands (claypans and riparian overflows) in the semi-arid regions in central Australia.
Etymology
Named in honour of John L. Porter, who collected the first herbarium material of this species, from the Paroo region in New South Wales.
Notes
Specimens are distinctly turgid and brightly festooned with gametangia, with incurved branchlets. The oospores have markedly enlarged striae (pachygyra).
Specimens examined
NORTHERN TERRITORY: Joker Gorge, Arltunga, 20 May 1986, G.Leach 736 (DNA); Western Tanami Desert, Wilson Creek floodout, 29 Apr. 2004, I.Cowie & A.Duguid 10241 (DNA, NT); 7 km SSW of Newhaven Homestead in a seasonal freshwater lake, 2 Oct. 2000, P.K.Latz 16815 (DNA NSW, NT); Wonkari Waterhole, S side of Petermann Ranges, 20 Jan. 1968, J.R.Maconochie 685 (DNA, NT); Duck pond outstation at Merrina Waterhole, 5 Sep. 1986, G.Leach 808 (DNA,NT). WESTERN AUSTRALIA: Coolarin Pool, Pilbara Survey PSW033, 18 May 2004, M.N.Lyons & D.A.Mickle 3061 (PERTH).
Chara protocharoides Casanova & Karol, Austral. Syst. Bot. 22: 32 (2014) [non Chara australis R.Br., Prodr. 346 (1810)]
Dioecious. Plants up to 400 mm high, somewhat branched, variably inflated, uncalcified (Fig. 17a). Axes ecorticate, internodes 10–100 mm long, 0.9–1.5 mm in diameter on living plants (1.2–2 mm when flattened in pressing). Stipulodes completely absent. Branchlets 6 or 7 in a whorl, entirely ecorticate, segments swollen or inflated in shallow water (Fig. 17b), elongate and cylindrical in deep-water populations (Fig. 17e), pinched in at the nodes, similar in diameter to the axes, 14–30 mm long, 3 or 4 cells long including end segment, basal branchlet cell variable in size, ~300 µm long in upper, fertile branchlets, similar to second branchlet segments on sterile branchlets, branchlet end segments unicellular, very small and mucronate, up to 200 µm long, with nodal cells at the base (Fig. 17c), bract cells and bracteoles completely absent from all branchlet nodes. Upper axes somewhat contracted. Gametangia on separate plants, singly and geminate on first and second branchlet nodes, occasionally oosporangia inside the base of the branchlet whorl (Fig. 17b, e). Oosporangia 670–900 µm long × 600–780 µm wide with 6 or 7 stripes of helical cells, coronula cells connivent and blunt, 80 µm high. Oospores black, 490–560 µm long and 310–390 µm wide, almost rectangular in side view, with 4 or 5 striae (Fig. 17f), sometimes flanged, fossa smooth to minutely granulate (Fig. 17g), and impression of the basal cell 80 µm in diameter (Fig. 17h). Antheridia 800–1150 µm in diameter, octoscutate (Fig. 17d). Vegetative reproduction not known; appears to be an annual in temporary water bodies. Chromosomes not known.
Distribution
Grows in permanent, seasonal and temporary freshwater water bodies in Western Australia and the Northern Territory.
Etymology
From the genus name Protochara and the Latin suffix -oides, resembling, in reference to the genus Protochara Womersley & Ophel, for which this species remains the type.
Notes
The large axis diameter, and complete absence of stipulodes, bract cells and bracteoles are the main characteristics that allow this species to be distinguished.
Specimens examined
NORTHERN TERRITORY: Kings Canyon, 12 Dec. 1968, P.K.Latz 362 (DNA, NT); Serpentine Gorge, Dec. 1968, M.Fagg s.n. (DNA); 42 km NE of Helen Springs Homestead, 14 June 2002, P.K.Latz & D.E.Albrecht 21953 (DNA, NT); Elcho Island, Melaleuca Swamp, 17 July 1975, C.Dunlop 3936 (DNA); above Reedy Rockhole, 16 July 1981, P.K.Latz 8796 (DNA). WESTERN AUSTRALIA: small lake near inflow of Lake Leschenaltia, 5 Oct. 2010, M.T.Casanova r812 (MEL).
Chara schultae Casanova, sp. nov.
Monoecious. Plants up to 200 mm high, not much branched, elongate and flexible, not calcified (Fig. 18a). Axes 200–400 µm in diameter, 2–3× corticated, somewhat tylacanthous, 21–24 cells around (Fig. 18d). Internodes 5–90 mm long. Spine cells solitary, obscure, acute when visible in the youngest internodes. Stipulodes in 1 row, up to twice the number of branchlets, but sometimes obscure, usually up to 1.5 mm long, spreading and narrow, ~50 µm wide (Fig. 18c). Branchlets 8 or 9 in a whorl, ecorticate, 4 or 5 cells long, basal branchlet cell longest, up to half the branchlet length, branchlet end segment a single cell with long subtending bract cells, bract cells 4–6, verticillate, up to 2 mm long (Fig. 18c). Bracteoles 2, up to 0.5 mm long (Fig. 18b). Gametangia conjoined, solitary at the lowest branchlet nodes, rarely at the second node (Fig. 18b). Oosporangia up to 450 µm long, 300–350 μm wide, coronula appressed. Oospores broadly oval, 300–350 µm long, 210–250 µm wide, with 7 or 8 striae of distinct flanges (up to ~10 µm high) (Fig. 18e), although these might not be present in aged oospores (Fig. 18h), the oospore wall appearing smooth (Fig. 18f, i), but minutely granulate (Fig. 18j), basal cell impression up to 50 µm in diameter (Fig. 18g). Antheridia up to 250 µm in diameter. Chromosomes not known.
Distribution
Tropical northern Australia in shallow freshwater waterbodies, rivers and ponds.
Etymology
Named for Julia Schult, charophyte collector in the Northern Territory.
Notes
Chara schultae is a delicate and flexible monoecious species in section Agardhia. It differs from all others by its very long and narrow bract cells and stipulodes, along with obscure spine cells.
Chara schultae is characterised by monoecy, long primary branchlet segments, and an axial cortex where the number of cortical cells is two to three times the number of branchlets in the adjacent whorl. It differs from the similar monoecious C. arnhemensis on the basis of the relative paucity of spine cells, overall longer and more flexible morphology, and larger oospores.
Specimens examined
NORTHERN TERRITORY: Daly River, 2001, S.Townsend p56[-a] (MEL); Elkedra Station, lagoons on upper Elkedra River, 7 May 1977, T.S.Henshall 1745 (DNA, NT); Hemple Bay, Groote Island, 7 May 1948, R.L.Specht A19 (AD).
Chara setosa Klein ex Willd., Mém. Acad. Roy. Sci. Hist. (Berlin) 1803: 85 (1805)
Monoecious. Plants up to 30 mm high, slightly calcified (Fig. 19a). Axes corticated, cortex cells uniformly 3× the number of branchets in the adjacent nodes, isostichous 26–30 cells around (Fig. 19d), spine cells 75–90 μm long, 60–75 μm wide at the base, internodes 10–80 mm long, up to 0.8 mm in diameter. Stipulodes in 2 tiers, 2 sets per branchlet, uppers longer (to 480 μm long), lower row shorter (250 μm long, 120 μm wide). Branchlets 8–10 in a whorl, corticate, up to 40 mm long, 8–10 cells long including end segment (Fig. 19b), basal branchlet cell shorter than the next cell, corticated, the cortical cells apparently without chlorophyll (‘discoloured’ or ‘diaphanous’), sometimes obscured by the upper row of stipulodes (Fig. 19c), branchlet cells, corticated, sometimes swollen, constricted at the nodes, particularly on young branchlets, branchlet end segments usually 2-celled, ecorticate, bract cells obscure, 6, when present anteriors longer than posteriors (Fig. 19e), bracteoles 2, shorter than the mature oosporangium. Gametangia conjoined singly at second, third and sometimes fourth branchlet node. Oosporangia 670–790 µm long × 550–600 µm wide, with 12 or 13 stripes of helical cells, coronula cells connivent and blunt, ~100 µm high. Oospores not seen on Australian material, Wood and Imahori (1964) reported they are black, 630–675 µm long and 435–495 µm wide, with 9 or 10 rather prominent ridges, fossa 73 µm across; oospore wall dark brown, opaque and smooth. The illustration provided depicts a germinated oospore from the base of a plant collected in Madagascar (Fig. 19f–i). That oospore is ~660 µm long and 520 µm wide with ~10 striae of prominent ridges with a ribbon-structure present on the striae (Fig. 19f). The oospore wall is minutely granulate (Fig. 19g) and the ribbon surface is nodulate (Fig. 19i). Antheridia 200–300 µm in diameter, octoscutate. Vegetative reproduction not known. Chromosomes not known.
Distribution
Chara setosa is a rare species that occurs in lakes and streams in Africa, Madagascar, Asia, Papua New Guinea, and tropical Australia. There is an additonal report of an Australian specimen from the Northern Territory in Wood (1971) lodged in BM (n.v.). Water chemistry has not been recorded.
Etymology
‘Setosa’ in Latin means silky, perhaps referring to the smooth appearance of the species.
Notes
Chara setosa is superficially similar to C. globularis (i.e. with straight and narrow corticated branchlets and axis) but is distinguished by the lack of colouration in the basal branchlet-cell cortex (possibly owing to a lack of chlorophyll). It is similar to C. zeylanica, except that C. zeylanica has no cortex on the basal branchlet cells. The ‘diaphanous’ or ‘discoloured’ basal branchlet cell can be obscured by the upper row of stipulodes, but can be detected by pulling down a branchlet to show the inner surface of the basal cell.
Specimens examined
NORTHERN TERRITORY: Roper River, 16 Jan. 1989, B.G.Thompson 2779 (DNA) [four species were collected from the same locality, under the same collection number, on two sheets; one of these is C. setosa]; WESTERN AUSTRALIA: Harding River Pool (PSW091 T2), 16 May 2006, D.A.Mickle & N.Y.Huang 33-3112 (PERTH). PAPUA NEW GUINEA: Bliri River, Kaiye Village, Aitape, Sepik, 18 July 1961, P.J.Darbyshire 8171 & R.D.Hoogland (B, CANB). MADAGASCAR: Anse NW du lac Itasy (a env. 5 km au S d’Ampefy) Accroche a un radeau flattant (eau profunde de plus de 1 m) [In a bay NW of Lake Itasy (~5 km S of Ampefy), hanging off a floating raft (in water greater than 1 m deep)], 3 Feb. 2000, A.Raynal-Roques, J.Jeremie & M.Grouzis 24910 (BM).
Chara submollusca Nordstedt, Hedwigia 27: 189 (1888)
Dioecious. Plants up to 250 mm high, not much branched, elongate and flexible, not calcified (Fig. 20a, b). Axes 200–555 µm in diameter, irregularly 1× corticated (Fig. 20i). Internodes 9–36 mm long. Spine cells solitary, small, conical with an acute tip, up to 120 µm high, stipulodes in 1 row, 2× the number of branchlets (Fig. 20d), 0.4–1.5 mm long, small and conical. Branchlets 7–10 in a whorl, ecorticate (Fig. 20g), 3 or 4 cells long, basal branchlet cell long, branchlet end segment usually a single cell with some smaller subtending bract cells, sometimes 2 or 3 equally long cells, bract cells 2–6 (Fig. 20e, f), unilateral, internally up to 0.6 mm long, externally small, conical, frequently absent. Bracteoles 2, 1 mm long, bractlet present in place of an antheridium on oosporangial plants. Gametangia on separate plants, solitary at the lowest 2 or 3 branchlet nodes (Fig. 20c). Oosporangia up to 750 µm long, 405–450 μm wide, coronula short. Oospores 360–570 µm long, 290–350 µm wide, with 8 or 9 striae of prominent ridges (Fig. 20j), the oospore wall appearing smooth, somewhat granulate to roughened at high magnification (Fig. 20k), basal-cell impression up to 88 µm in diameter (Fig. 20l). Antheridia up to 600 µm in diameter. Chromosomes not known. Vegetative reproductive organs (bulbils) often present at the apices of the branches (Fig. 20h), consisting of shortened shoots with starch-filled basal branchlet segments.
Distribution
Tropical north and eastern Australia in shallow freshwater waterbodies, rivers and ponds.
Etymology
From Latin ‘sub’ meaning somewhat, and ‘mollis’ meaning soft or flexible.
Notes
Chara submollusca was described as a species by Nordstedt (1888) and included in C. section Agardhia by Wood (1962). However, when Wood examined the Australian charophyte flora he amalgamated C. submollusca with Chara fibrosa var. fibrosa (Wood 1971).
Chara submollusca is characterised by dioecy, haplostichous cortex and very long primary branchlet segments. The presence of specialised opaque branch apices (with starch-filled branchlet segments) is distinctive in this tropical species.
Specimens examined
NORTHERN TERRITORY: Manton Dam Recreation Area, 5 Sep. 2010, M.T.Casanova r756 (MEL, NY); roadside borrow-pit on the Arnhemland Highway, 8 Sep. 2010, M.T.Casanova r786 (MEL, NY); Darwin River Dam, 22 Apr. 1977, J.Must 1467 (DNA); Jabiru Lake, Jabiru, 27 Aug. 1983, I.Cowie s.n. (DNA); Cathedral Billabong, Arnhemland, 29 July 1983, I.D.Cowie s.n. (DNA). QUEENSLAND: Barrow River, 1891, G.Podenzana s.n. (BM); Browns Creek, Pascoe River, 13 July 1948, L.J.Brass 19560 (BM, L).
Chara wightii (A.Braun) Casanova, Austral. Syst. Bot. 26: 293 (2013)
Monoecious. Plants 100–250 mm high, often calcified, somewhat shrubby (Fig. 21a). Axes up to 700 µm in diameter; internodes regularly 2× corticated axis, isostichous or tylacanthous (20–22 cells around) (Fig. 21d). Spine cells small and conical on older axes, longer on young axes, occurring singly, 130–185 μm long and 20–40 μm wide. Stipulodes in a single whorl, the same number as the number as branchlets in each of the whorls (Fig. 21b), 1–2 mm long. Branchlets 9–11 in a whorl, 5–14 mm long, segments 4–6, the basal cell shorter than the second cell in fertile whorls (Fig. 21b). Bract cells 4–6 verticillate at the branchlet nodes, up to 0.4–2.5 mm long, bracteoles 2, similar to bract cells (Fig. 21c). Gametangia conjoined singly or geminate at the lowest 2–4 branchlet nodes. Oosporangia up to 500 µm long and 375 µm wide, 10 or 11 stripes of helical cells, coronula up to 75 µm high, cells apiculate and spreading slightly. Oospores chestnut brown at maturity, 400–470 µm long, 250–310 µm wide (Fig. 21e). Striae of 7–10 low ridges (in Australian material usually 7 or 8), fossa wall 64 μm across (Fig. 21f), basal cell impression ~80 µm wide at the widest point (Fig. 21g). Antheridia up to 500 µm in diameter, octoscutate. Chromosomes not known.
Distribution
Northern Australia, India and South-East Asia (Pal 1932; Pal et al. 1962; Han and Li 1994; Langangen and Leghari 2001). Mostly freshwater.
Etymology
Named for the collector of the type material, Robert Wight (director of the Botanic Garden in Madras [now Chennai] from 1819 to 1853).
Notes
Chara wightii has a corticated axis and naked branchlets and it is often encrusted with calcium carbonate; it is characterised by brown oospores and occasional geminate gametangia, in contrast to C. benthamii and C. duriuscula, which have black oospores and singular gametangia. It can be distinguished from C. erythrogyna (which has sejoined gametangia) by its consistently conjoined gametangia and brown oospores. Australian material tends to be isostichous rather than tylacanthous, but it is included within C. wightii pending further investigation.
Specimens examined
NORTHERN TERRITORY: 20 km SW of Wombungi, Fitzmaurice River area, 15 May 1994, S.McCune 7 & P.Munns (DNA); lagoons on upper Elkedra River, 7 May 1977, T.S.Henshall 1745 (DNA); Port Darwin, 9 Apr. 1896, T.B.Blow A102 (BM). WESTERN AUSTRALIA: Caliwingina Spring, 1 Aug. 2011, M.Curran 1 (PERTH); Arnhem Land, Wilton River, near Bulman, 9 Nov. 1987, G.J.Leach & C.R.Dunlop 1633 (DNA).
Chara zeylanica Klein ex Willd., Mém. Acad. Roy. Sci. Hist. (Berlin) 1803: 86 (1805)
Monoecious. Plants up to 10–50 cm high, compact and often highly calcified in shallow water, but elongate in deeper water (Fig. 22a). Axes up to 400–800 µm in diameter; internodes essentially 3× corticated, (22–30 cells around), 24–65 mm long (Fig. 22b). Spine cells sometimes obscure, where present occurring singly, in pseudo-whorls on the axes, very long and thin up to 800–1000 μm long and 20–40 μm wide (Fig. 22b). Stipulodes in 2 whorls, uppers pointing up (500–600 μm long), lowers pointing down (300–400 μm long), 2× the number as branchlets in each of the whorls (Fig. 22c). Branchlets 9–11 in a whorl, 10–22 mm long, segments 6–8, the basal cell (Fig. 22d) and 1 or2 end cells not corticated (sometimes the basal cell is obscured by the upper row of stipulodes), the remainder corticated with ~14–18 cells around (Fig. 22c). Bract cells 4–6 verticillate at the branchlet nodes, elongate, inner cells up to 0.4–1.2 mm long, outer up to 0.6 mm long, bracteoles 2, up to 0.5–1.3 mm long (Fig. 22e). Gametangia conjoined singly at the lowest 4 or 5 branchlet nodes. Oosporangia 1000–1200 µm long and 600–700 µm wide, 9 stripes of helical cells, coronula up to 100–150 µm high, cells appressed. Oospores black, 850 µm long, 250 µm wide (Fig. 22f), fossa wall with a calcified covering (gyrogonite 1000 µm long). Striae of 10 or 11 flanged ridges, fossa wall 80 μm across, papillate (Fig. 22g), small basal claws present. Basal cell impression rounded, ~100 µm in diameter (Fig. 22h) Antheridia 200–250 µm in diameter, tetrascutate (Fig. 22e). Chromosomes n = 28 (specimen A.T.Hotchkiss, 5 Nov. 1968 (B)).
Distribution
In freshwater streams and rivers in the north of Australia, throughout tropical Asia and most of the tropical regions of the world.
Etymology
Zeylanica is from the Latin Zeylonas, the species being named for its occurrence in Ceylon (Sri Lanka).
Notes
Plants are usually notably coarse and smelly, with abundant reproductive structures visible along the branchlets. Chara zeylanica is one of a few Chara species known with tetrascuate antheridia (with 4 lozenge-shaped shield cells, rather than the 8 triangular shield-cells typical of the genus; Proctor et al. 1971). Sterile plants are distinguished on the basis of the naked (ecorticate) basal branchlet cell, the well-developed stipulodes in two rows, and the axial cortex cells three times the number of branchlets in the adjacent whorl, often with long spine cells. A single specimen from Pullout Bore (P.K.Latz & A.Schubert 28441 (DNA)) has variable development of the axial cortex, similar to Chara brittonii Allen ex C.B.Rob., but is retained in C. zeylanica pending further study.
Specimens examined
NORTHERN TERRITORY: Pullout Bore, 15 km NNW of Amanbidji Settlement, 11 Sep. 2013, P.K.Latz & A.Schubert 28441 (DNA) [specimen without axial cortex in part]; Upper Wickham River, 1 Mar. 1989, Russell-Smith 7734 & Lucas (DNA); Gove Road at Mainoru River Crossing, 21 Oct. 1981, T.S.Henshall 3895 (DNA); Bradshaw Station, 18 Feb. 1999, C.R.Michell 2157 (DNA); Jarong Spring, Wingate Mountains 16 Mar. 1989, Brock 701 & Russell-Smith (DNA); Daly River Mission, 6 Oct. 1992, L.L.V.Williams 296 & G.M.Wightman (DNA); Lake Eames, Vanderlin Island,1 Aug. 1988, P.K.Latz 11084 (BRI, DNA, MEL, NT); Roper River, 2000, S.Townsend R4 (DNA); waterfall pool north of West Baines River Crossing, 5 Nov. 1968, A.T.Hotchkiss x-251 (B); Daly River, 2001, S.Townsend p56 (MEL); Howard Springs, Darwin area, 28 June 1977, L.A.Craven 4472 (CANB); Bulls Run Creek, Tipperary Station, 4 May 1990, G.J.Leach & I.D.Cowie 2852 (DNA, MEL); Mataranka Reserve, 4 May 1977, Must 1487 (CANB, MEL); Daly River crossing,12 Nov. 1982, C.R.Dunlop 6293 (DNA); Amelia Springs, MacArthur River area, 30 Jan. 1976, L.A.Craven 3536 (DNA); Robinson River, approx. 12 km N of Seven Emu, 10 May 1985, G.Leach 631 (DNA, MEL, NT); Kulamindini Swamp, near Elliott, 22 Apr. 1991, D.J.Parsons 137 (DNA); Chambers Bay, Woolner Station, coastal floodplain, 19 June 1995, I.Cowie & R.Griffin 5851 (DNA); Port Darwin, 9 Apr. 1896, T.B.Blow A98 (BM).
Discussion
In the Northern Territory, charophytes occur in all sorts of habitats. In the wet-tropics, they grow in both permanent (lagoon, river) and temporary (wet-season ponds and ephemeral streams) habitats. In the arid zone, they occur in permanent water holes in rivers and canyons, mound-springs and springs, as well as temporary claypans and ponds, in both saline and freshwater. The water is often clear, but can be distinctly turbid (e.g. for Chara porteri).
Of the six Chara species listed by Wood (1971) for the Northern Territory, only three (C. globularis, C. setosa, C. zeylanica) can be confirmed. Wood’s remaining taxa were either misidentifications, or amalgamated taxa based on his monograph (Wood and Imahori 1965). Thus, most of the additional (15) Chara species listed here are either new records (6 spp.), varieties or forms raised to species rank (7 spp.), or species newly described from Northern Territory material (2 spp.). The genus Lamprothamnium has not previously been confirmed for the Northern Territory and the records here extend the distributions of the three species listed. Although no specimens of Lychnothamnus barbatus were seen for this study, it is likely that it will be collected in the Northern Territory because it occurs in adjacent regions.
The charophyte flora of this region can be divided into species of the wet–dry tropics (Chara arnhemensis, C. bancroftii, C. benthamii, C. duriuscula, C. erythrogyna, C. lucida, C. schultae, C. submollusca, C. wightii, C. zeylanica) and species of the semi-arid zones (C. aridicola, C. behriana, C. karolii, C. porteri, C. globularis), with both endemic (i.e. restricted to Australia) and cosmopolitan taxa (C. globularis, C. zeylanica). The species in the wet–dry tropics have affinities with those in South-East Asia (Zaneveld 1940), although there are few studies on the charophytes of our nearest neighbours (East Timor, Papua, Indonesia, Filarszky 1934; New Guinea, Leach and Osborne 1985). Species with a wider distribution are C. lucida (PNG), C. erythrogyna, C. setosa, C. wightii (India), C. benthamii (Malaysia, China and possibly Europe), and C. zeylanica (described as cosmopolitan in the tropics), and all these are monoecious, except C. lucida. Approximately 30% of the Northern Territory species are dioecious, a character that is strongly linked with endemism in the Characeae (Proctor 1980). The occurrence of dioecious Chara lucida in South-East Asia as well as in northern Australia contradicts Proctor’s (1980) contention that dioecious species will be restricted to a single landmass. This could be explained by cryptic species-level diversity between localities, or the close proximity or historical land bridge between continental Australia and Papua New Guinea.
There are relatively few species of charophytes with corticated branchlets compared with those with ecorticate branchlets in Australia, a situation that differs from places where charophytes have been better studied (Schubert et al., in press). It is therefore worth discussing the position of Chara aridicola and C. behriana in comparison to northern hemisphere species with two times cortex and corticated branchlets. The original gatherings of C. aridicola and C. behriana were allocated to varieties of C. contraria on the basis of their black oospores and two times cortex and (partly) corticated branchlets. Wood (1971) amalgamated the Australian entities with C. vulgaris, as he did elsewhere (Wood and Imahori 1965), and placed those with well-corticated branchlets in var. vulgaris and those with few corticated branchlet segments in var. gymnophylla. The modern concept of C. vulgaris is represented by specimens with (mostly) aulacanthous cortex, brown oospores and 2–5 corticated branchlet segments, which rules that taxon out as an identification for these Australian species. Chara contraria in Europe is represented by specimens with (mostly) tylacanthous cortex and black oospores, which usually have 5–7 corticated branchlet segments. The Australian species are distinguished from these by having scarcely corticated branchlets in the usual condition. Additionally, the stipulode rows are usually inconspicuous, and the ecorticate nodes can bear gametangia.
Although this report did not find evidence for Chara braunii in the Northern Territory, the species does occur in the Gawler Ranges in South Australia, and so there is reason to believe that it would be present in similar habitats in Northern Territory.
In general, although charophytes have been collected from a large number of localities in Northern Territory, some of the specimens are virtually unusable for taxonomic work. Good presentation requires a degree of care, decalcification if calcified, floating out onto herbarium sheets in the fresh state, sometimes shaking in water, or submitting to a spray of water to remove debris. ‘Less is more’ when it comes to mounting on sheets. Specimens that are poorly presented are sometimes impossible to determine.
Several species remain problematic. Chara plebeja R.Br. ex A.Braun (as discussed by Casanova and Karol 2014; Casanova 2015) remains an enigma. Only the type material exists, and it is fertile male material (i.e. no oospores). It could be a species of Chara close to C. protocharoides or, given its coastal collection locality, a very ‘stripped down’ species of Lamprothamnium, similar to L. inflatum (Fil. & G.O.Allen) Adr.García & Karol. Further examination of the type material (BM) has not led to new insights; so, more, fertile female material is necessary to resolve that conundrum. A specimen from McDills No. 1 Bore (G.Leach 1510 (DNA)) is apparently dioecious and possibly related to C. muelleri, but is too encumbered with epiphytes to be easily examimed. Other gatherings from bores and springs are similarly calcified or invested with clay; so, some purposeful collecting from these habitats and mound-springs is likely to be rewarding in at least extending the knowledge of distribution of species in the arid zone, and possibly revealing new species.
This study recognises an approximately four-fold increase in the species diversity in tribe Chareae in the Northern Territory. If the rest of Australia has a similar complement of species, there are likely to be more than 150 species on the continent.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Funds for this work were provided by the Australian Biological Resources Study, Bush Blitz grant number TTC216-10 and Charophyte Services.
Acknowledgements
Julia Schult and Simon Townsend are particularly thanked for their assistance with this project. For this study, access to type material in various American and European (B, BM, BP, GFW, GOET, HBG, L, LD, LE, PC, NY and W) and Australian (AD, BRI, CANB, DNA, HO, MEL, NSW, PERTH) herbaria was essential. We thank the directors and staff of these institutions for their kind assistance and permission to remove oospores for examination. Many people have assisted with providing specimens, fresh and preserved, since 2000; some of their names are noted in the specimen lists. This study would have been impossible without their efforts. Beth Williams provided notes, translations and literature in an early part of this study.
References
Bailey FM (1913) ‘Comprehensive Catalogue of Queensland Plants.’ (A. J. Cumming Government Printer: Brisbane, Qld, Australia)Balevičius A (2011) Distribution of Lychnothamnus barbatus community in Lithuania. Biologija 2, 70–73.
Blaženčić J, Stevanović B, Blaženčić Ž, Stevanović V (2006) Red data list of charophytes in the Balkans. Biodiversity and Conservation 15, 3445–3457.
| Red data list of charophytes in the Balkans.Crossref | GoogleScholarGoogle Scholar |
Braun A (1853) Characeae in ‘Plantae Muellerianae: Beitrag zur Flora Südaustraliens, aus den Sammlungen des Dr. Ferd. Müller’. Linnaea 25, 704–709. [In German]
Brzozowski M, Kowalewski G, Szczuciński W, Kaczmarek L, Pełechaty M (2021) Preliminary evidence of an endangered species benefiting from moderate climate warming: a palaeolimnological study of the charophyte Lychnothamnus barbatus. Aquatic Conservation: Marine and Freshwater Ecosystems 31, 2673–2689.
| Preliminary evidence of an endangered species benefiting from moderate climate warming: a palaeolimnological study of the charophyte Lychnothamnus barbatus.Crossref | GoogleScholarGoogle Scholar |
Casanova MT (2005) An overview of Chara L. in Australia (Characeae, Charophyta). Australian Systematic Botany 18, 25–39.
| An overview of Chara L. in Australia (Characeae, Charophyta).Crossref | GoogleScholarGoogle Scholar |
Casanova MT (2007) Charophyceae. In ‘Algae of Australia: Introduction’. (Eds PM McCarthy, AE Orchard) pp. 368–373. (ABRS: Canberra, ACT, Australia; and CSIRO Publishing: Melbourne, Vic., Australia)
Casanova MT (2009) An overview of Nitella (Characeae, Charophyceae) in Australia. Australian Systematic Botany 22, 193–218.
| An overview of Nitella (Characeae, Charophyceae) in Australia.Crossref | GoogleScholarGoogle Scholar |
Casanova MT (2013a) Lamprothamnium in Australia (Characeae, Charophyceae). Australian Systematic Botany 26, 268–290.
| Lamprothamnium in Australia (Characeae, Charophyceae).Crossref | GoogleScholarGoogle Scholar |
Casanova MT (2013b) Review of the species concepts Chara fibrosa and C. flaccida (Characeae, Charophyceae). Australian Systematic Botany 26, 291–297.
| Review of the species concepts Chara fibrosa and C. flaccida (Characeae, Charophyceae).Crossref | GoogleScholarGoogle Scholar |
Casanova MT (2015) Chromosome numbers in Australian charophytes (Characeae, Charophyceae). Phycologia 54, 149–160.
| Chromosome numbers in Australian charophytes (Characeae, Charophyceae).Crossref | GoogleScholarGoogle Scholar |
Casanova MT, Karol KG (2014) A revision of Chara sect. Protochara, comb. et stat. nov. (Characeae: Charophyceae). Australian Systematic Botany 27, 23–27.
| A revision of Chara sect. Protochara, comb. et stat. nov. (Characeae: Charophyceae).Crossref | GoogleScholarGoogle Scholar |
Casanova M, Nairn L (2015) Macroalgae, charophytes and bryophytes. In ‘Vegetation of Australian Riverine Landscapes’. (Eds S Capon, C James, M Reid) pp. 67–86. (CSIRO Publishing: Melbourne, Vic., Australia)
Casanova MT, Porter JL (2013) Two new species of Nitella (Characeae, Charophyceae) from arid-zone claypan wetlands in Australia. Muelleria 31, 53–59.
| Two new species of Nitella (Characeae, Charophyceae) from arid-zone claypan wetlands in Australia.Crossref | GoogleScholarGoogle Scholar |
Casanova MT, García A, Feist M (2003a) The ecology and conservation of Lychnothamus barbatus (Characeae). Acta Micropalaeontologica Sinica 20, 118–128.
Casanova MT, García A, Porter JL (2003b) Charophyte rediscoveries in Australia: what and why? Acta Micropalaeontologica Sinica 20, 129–138.
Cowie ID, Short PS, Osterkamp Madsen M (2000) ‘Floodplain Flora. A Flora of the Coastal Floodplains of the Northern Territory, Australia.’ Flora of Australia Supplementary Series Number 10. (ABRS: Canberra, ACT, Australia)
Crawford SA, Higgins MJ, Mulvaney P, Wetherbee R (2001) Nanostructure of the diatom frustule as revealed by atomic force and scanning electron microscopy. Journal of Phycology 37, 543–554.
| Nanostructure of the diatom frustule as revealed by atomic force and scanning electron microscopy.Crossref | GoogleScholarGoogle Scholar |
Filarszky F (1934) Die Characeen der Deutschen Limnologischen Sunda-Expedition. Archiv für Hydrobiologie 4, 705–726. [In German]
García A (2003) Lychnothamnus barbatus (Meyen) Leonhardi from Australia: statistical analysis of its gyrogonite and comparison with European collections. Acta Micropalaeontologica Sinica 20, 111–117.
García A, Chivas AR (2006) Diversity and ecology of extant and Quaternary Australian charophytes (Charales). Cryptogamie, Algologie 27, 323–340.
Gregory AC, Gregory FT (1884) ‘Journals of Australian Explorations.’ (James Beal, Government Printer: Brisbane, Qld, Australia) Available at https://www.gutenberg.org/files/10461/10461-h/10461-h.htm#seven
Groves J, Allen GO (1935) A review of the Queensland Charophyta. Proceedings of the Royal Society of Queensland 46, 34–59.
| A review of the Queensland Charophyta.Crossref | GoogleScholarGoogle Scholar |
Han FS, Li YY (1994) [‘Flora algarum sinicarum aquae dulcis. Tomus 3. Charophyta.’] (Science Press: Beijing, PR China) [In Chinese with Latin title]
Hotchkiss AT (1963) A first report of chromosome numbers in the genus Lychnothamnus (Rupr.) Leonh. and comparison with other charophyte genera. Proceedings of the Linnean Society of New South Wales 88, 368–372.
Jackson BD (1885) George Bentham. The Botanical Gazette 10, 203–218.
Karol KG (2004) Phylogenetic studies of the Charales: the closest living relatives of land plants. PhD dissertation, University of Maryland, College Park, MD, USA.
Karol KG, Skawinski PM, McCourt RM, Nault ME, Evans R, Barton ME, Berg MS, Perleberg DJ, Hall JD (2017) First discovery of the charophycean green alga Lychnothamnus barbatus (Charophyceae) extant in the New World. American Journal of Botany 104, 1108–1116.
| First discovery of the charophycean green alga Lychnothamnus barbatus (Charophyceae) extant in the New World.Crossref | GoogleScholarGoogle Scholar |
Korsch H, Doege A, Raabe U, van de Weyer K (2013) ‘Rote Liste der Armleuchteralgen (Charophyceae) Deutschlands, 3. Fassung, Stand: Dezember 2012.’ (Thüringische Botanische Gesellschaft e.V.: Jena, Germany) [In German]
Langangen A, Leghari SM (2001) Some charophytes (Charales) from Pakistan. Studia Botanica Hungarica 32, 63–85.
Leach GJ, Osborne PL (1985). ‘Freshwater plants of Papua New Guinea.’ (University of Papua New Guinea Press: Port Moresby, Papua New Guinea)
Ludwig G, Schnittler M, Vollmer I (1996) ‘Rote liste gefährdeter pflanzen Deutschlands.’ (Bundesamt für Naturschutz, Bonn‐Bad Godesberg, Germany) [In German]
McCourt RM, Casanova MT, Karol KG, Feist M (1999) Monophyly of genera and species of Characeae based on rbcL sequences, with special reference to Australian and European Lychnothamnus barbatus (Characeae: Charophyceae). Australian Journal of Botany 47, 361–369.
| Monophyly of genera and species of Characeae based on rbcL sequences, with special reference to Australian and European Lychnothamnus barbatus (Characeae: Charophyceae).Crossref | GoogleScholarGoogle Scholar |
Martin-Closas C, Ramos E (2005) Palaeogene charophytes of the Balearic Islands (Spain). Geologica Acta 3, 39–58.
Mouronval JB, Baudouin S, Borel N, Soulié-Märsche I, Klesczewski M, Grillas P (2015) ‘Guide des Characées de France méditerranéenne.’ (Office National de la Chasse et Faune Sauvage: Paris, France) [In French]
Nordstedt CFO (1883) Fragmente einer Monographie der Characeen. Abhandlungen der Königlichen Akademie der Wissenschaften in Berlin 1882, 1–211. [In German]
Nordstedt CFO (1887) Algologiska småsker. 4. Utdrag ur ett arbete öfver de af Dr. S. Berggren på Nya Sealand och i Australien samlade sötvattensalgerna. Botaniska Notiser 1887, 153–164. [In Swedish]
Pal BP (1932) Burmese Charophyta. Botanical Journal of the Linnean Society. 49, 47–92.
| Burmese Charophyta.Crossref | GoogleScholarGoogle Scholar |
Pal BP, Kundu BC, Sundralingam VS, Venkatraman GS (1962) ‘Charophyta.’ (Indian Council of Agricultural Research: New Delhi, India)
Proctor VW (1971) Chara globularis Thuillier (=C. Vragilis Desvaux): breeding patterns within a cosmopolitan complex. Limnology and Oceanography 16, 422–436.
| Chara globularis Thuillier (=C. Vragilis Desvaux): breeding patterns within a cosmopolitan complex.Crossref | GoogleScholarGoogle Scholar |
Proctor VW (1980) Historical biogeography of Chara (Charophyta): an appraisal of the Braun–Wood classification plus a falsifiable alternative for future consideration. Journal of Phycology 16, 218–233.
| Historical biogeography of Chara (Charophyta): an appraisal of the Braun–Wood classification plus a falsifiable alternative for future consideration.Crossref | GoogleScholarGoogle Scholar |
Proctor VW, Griffin DG, Hotchkiss AT (1971) A synopsis of the genus Chara, series Gymnobasalia (subsection Willdenowia RDW). American Journal of Botany 58, 894–901.
| A synopsis of the genus Chara, series Gymnobasalia (subsection Willdenowia RDW).Crossref | GoogleScholarGoogle Scholar |
Sakayama H, Nozaki H, Kasaki H, Hara Y (2002) Taxonomic re-examination of Nitella (Charales, Charophyceae) from Japan, based on microscopical studies of oospore wall ornamentation and rbcL gene sequences. Phycologia 41, 397–408.
| Taxonomic re-examination of Nitella (Charales, Charophyceae) from Japan, based on microscopical studies of oospore wall ornamentation and rbcL gene sequences.Crossref | GoogleScholarGoogle Scholar |
Sakayama H, Miyaji K, Nagumo T, Kato M, Hara Y, Nozaki H (2005) Taxonomic reexamination of 17 species of Nitella subgenus Tieffallenia (Charales, Charophyceae) based on internal morphology of the oospore wall and multiple DNA marker sequences. Journal of Phycology 41, 195–211.
| Taxonomic reexamination of 17 species of Nitella subgenus Tieffallenia (Charales, Charophyceae) based on internal morphology of the oospore wall and multiple DNA marker sequences.Crossref | GoogleScholarGoogle Scholar |
Sakayama H, Arai S, Nozaki H, Kasai F, Watanabe MM (2006) Morphology, molecular phylogeny and taxonomy of Nitella comptonii (Charales, Characeae). Phycologia 45, 417–421.
| Morphology, molecular phylogeny and taxonomy of Nitella comptonii (Charales, Characeae).Crossref | GoogleScholarGoogle Scholar |
Sakayama H, Kasai F, Nozaki H, Watanabe MM, Kawachi M, Shigyo M, Nishihiro J, Washitani I, Krienitz L, Ito M (2009) Taxonomic reexamination of Chara globularis (Charales, Charophyceae) from Japan based on oospore morphology and rbcL gene sequences, and the description of C. leptospora sp. nov. Journal of Phycology 45, 917–927.
| Taxonomic reexamination of Chara globularis (Charales, Charophyceae) from Japan based on oospore morphology and rbcL gene sequences, and the description of C. leptospora sp. nov.Crossref | GoogleScholarGoogle Scholar |
Schubert H, Blindow I, Bueno NC, Casanova MT, Pełechaty M, Pukacz A (2018) Ecology of charophytes – permanent pioneers and ecosystem engineers. Perspectives in Phycology 5, 61–74.
| Ecology of charophytes – permanent pioneers and ecosystem engineers.Crossref | GoogleScholarGoogle Scholar |
Schubert H, Gregor T, Blindow I, Nat E, Stewart N, Romanowski R, van der Weyer K, Denys L, Korsch, H, Casanova MT (in press) ‘Characeae of Europe.’ (Springer)
Schult J, Townsend SA, Douglas MM, Webster IT, Skinner S, Casanova MT (2007) ‘Recommendations for nutrient resource condition targets for the Daly River.’ (Charles Darwin University: Darwin, NT, Australia)
Siemińska J, Bąk M, Dziedzic J, Gąbka M, Gregorowicz P, Mrozińska T, Pełechaty M, Owsianny PM, Pliński M, Witkowski A (2006) Red list the algae in Poland. In ‘Red List of Plants and Fungi in Poland’. (Eds Z Mirek, K Zarzycki, W Wojewoda, Z Szeląg) pp. 35–52. (W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland)
Soulié-Märsche I, Triboit F, Despréaux M, Rey-Boissezon A, Laffont-Schwob I, Thiéry A (2013) Evidence of Chara fibrosa Agardh ex Bruzelius, an alien species in South France. Acta Botanica Gallica 160, 157–163.
| Evidence of Chara fibrosa Agardh ex Bruzelius, an alien species in South France.Crossref | GoogleScholarGoogle Scholar |
Sugier P, Pełechaty M, Gąbka M, Owsianny PM, Pukacz A, Ciecierska H, Kolada H (2009) Lychnothamnus barbatus: global history and distribution in Poland. Charophytes 2, 19–24.
Thuillier JL (1799) La flore des environs de Paris ou Distribution méthodique des plantes qui y croissent naturellement, faite d’après le systême de Linnée : avec le nom et la description de chacune en latin et en françois [sic]; l’indication de leur lieu natal, de leur durée, du temps de leur floraison, de la couleur de leurs fleurs, et la citation des auteurs qui les ont le mieux décrites ou en ont donné les meilleures figures. Nouvelle édition : revue, corrigée et considérablement augmentee. (Auteur et H.L. Perronneau: Paris, France) [In French]
Vicente A, Sanjuan J, Eaton JG, Villanueva-Amadoz U (2020) The oldest record of North American Lychnothamnus (northeastern Sonora, Mexico): implications for the evolution, ecology, and paleogeographic distribution of the genus. Aquatic Botany 167, 103271
| The oldest record of North American Lychnothamnus (northeastern Sonora, Mexico): implications for the evolution, ecology, and paleogeographic distribution of the genus.Crossref | GoogleScholarGoogle Scholar |
von Mueller F (1889) Notes on the geographical distribution of Australian Characeae. Transactions of the Royal Society of South Australia 12, 149
Wood RD (1958) Some Characeae from Arnhem Land. Records of the American-Australian Scientific Expedition to Arnhem Land 3, 137
Wood RD (1959) Gametangial constants of extant Charophyta for use in micropaleobotany. Journal of Paleontology 33, 186–194.
Wood RD (1962) New combinations and taxa in the revision of Characeae. Taxon 11, 7–25.
| New combinations and taxa in the revision of Characeae.Crossref | GoogleScholarGoogle Scholar |
Wood RD (1971) Characeae of Australia. Nova Hedwigia 22, 1–120.
Wood RD, Imahori KI (1964) Iconograph of the Characeae. In ‘A Revision of the Characeae. Vol. 2’. (Eds RD Wood, KI Imahori) pp. 1–904. (Cramer: Weinheim, Federal Republic of Germany)
Wood RD, Imahori KI (1965) Monograph of the Characeae. In ‘A Revision of the Characeae. Vol. 1’. (Eds RD Wood, KI Imahori) pp. 1–904. (Cramer: Weinheim, Federal Republic of Germany)
Zaneveld JS (1940) The Charophyta of Malaysia and adjacent countries. Blumea 4, 1–223.