A morphological and molecular study supports the recognition of Rhipilia psammophila sp. nov. and Rhipilia baculifera comb. nov. (Halimedaceae, Chlorophyta) from southern Australia
John M. Huisman A * and Heroen Verbruggen
A * and Heroen Verbruggen  B
B
A
B
Australian Systematic Botany 36(6) 427-436 https://doi.org/10.1071/SB23016
Submitted: 31 May 2023 Accepted: 10 October 2023 Published: 31 October 2023
Abstract
Ongoing taxonomic studies of Australian marine algae have led to the recognition of a new species of the green algal genus Rhipilia, here named R. psammophila Huisman & Verbruggen. The new species is unusual for this typically tropical genus in growing in the colder temperate waters of southern Australia and can be distinguished from its congeners by the morphology of its laterally adhering siphons, in addition to unique molecular sequences (rbcL and tufA). In molecular analysis, the new species is sister to the southern Australian Chlorodesmis baculifera, which is here transferred to Rhipilia and represents the third species in the genus with laterally unattached, free siphons, a feature that previously would have excluded it from the genus. Lateral gametangia are described for R. psammophila and represent the first record of reproduction in Rhipilia.
Keywords: algae, Bryopsidales, Chlorodesmis, marine, rbcL, seaweed, taxonomy, tufA.
Introduction
The green algal order Bryopsidales includes a diverse array of morphological forms, united by a siphonous construction in which cellular cross-walls are absent, the entire thallus being essentially a single cell. Thalli can be microscopic or macroscopic, calcified or not, and composed of branched or unbranched siphons that can be free or aggregated into various forms (Huisman 2015). The order includes some of the more conspicuous seaweed genera, such as Caulerpa J.V.Lamour., Codium Kütz. and Halimeda J.V.Lamour.
For the past several years, we have been aware of a flabellate green alga growing in shallow sandy habitats in various locations in southern Western Australia that could not be assigned to any known species. Morphologically, this alga conformed to earlier concepts of the genus Rhipilia Kütz. (Halimedaceae), wherein siphons are generally aggregated to form spongy flabellate or cuneate thalli (Huisman and Verbruggen 2015) and are attached to one another by lateral siphons that terminate in several-pronged tenacula (Millar and Kraft 2001; Verbruggen and Schils 2012). However, recent molecular analyses indicated that this latter character was distributed through various genetic groups within the family Halimedaceae (Cremen et al. 2019; Lagourgue and Payri 2021), and several species of Rhipilia were transferred to a new genus, Kraftalia Lagourgue & Payri, restricting Rhipilia to species with stolons (Lagourgue and Payri 2021). Because this unknown alga lacked a prominent stolon, morphologically it would agree with Lagourgue and Payri’s concept of Kraftalia (Lagourgue and Payri 2021). However, we were reluctant to describe a new species in that genus without molecular support.
Reproductive specimens that were collected in 2008 (PERTH 08188459) represent the first record of fertile material for the Rhipilia–Kraftalia complex. These reproductive structures were highly reminiscent of those found in Chlorodesmis baculifera (J.Agardh) Ducker, an Australian species known only from the colder seas of southern Australia and a geographic outlier in an otherwise warm-water genus. As is typical of Chlorodesmis Harv. & Bailey, the siphons of C. baculifera are not aggregated or laterally attached, and thus it is seemingly generically unrelated to the Rhipilia–Kraftalia complex. It was therefore of interest to undertake a molecular analysis to evaluate whether the newly discovered specimens constitute a previously unrecognised species, and to place these specimens in a molecular phylogenetic context along with other Rhipilia species and Chlorodesmis baculifera.
Materials and methods
Specimens were collected by snorkel and pressed while fresh as herbarium vouchers, preserved in ethanol or dried in silica gel for DNA analysis. Portions for microscopic examination were preserved in 5% formalin/seawater; siphons were teased apart and mounted in seawater for examination and photography, because mounting media caused the siphons to shrink. In situ photographs of the unknown Rhipilia were taken on a Sony RX100iii camera in a Nauticam housing, with twin Inon strobes. Microscope preparations were examined and photographed using either a Nikon SMZ800 stereo microscope or a Nikon Eclipse 80i compound microscope, in both cases with a Nikon DS L4 digital camera. All herbarium sheets and microscopic preparations have been lodged in the Western Australian Herbarium (PERTH). Chlorodesmis baculifera was photographed in the field with an Olympus TG-3 and examined using a Leica DM750 stereo microscope fitted with a Canon EOS 600D camera.
DNA from Bryopsidales samples, including multiple samples of the Western Australian entity as well as Chlorodesmis baculifera and relevant related species, was extracted using a modified CTAB method (Cremen et al. 2016). Molecular data were generated either by PCR amplification and Sanger sequencing of rbcL or tufA genes (cf. Verbruggen et al. 2009a), or by using high-throughput sequencing on the Illumina NovaSeq platform followed by assembly and extraction of the rbcL and tufA genes as previously described (Costa et al. 2016; Marcelino et al. 2016; Cremen et al. 2018). New sequences were deposited in Genbank (OR426990-OR427016).
So as to infer a species tree to evaluate the phylogenetic placement of the unknown Rhipilia and Chlorodesmis baculifera, our strategy consisted of building a phylogeny of the whole Halimedineae suborder of the Bryopsidales. We assembled a dataset including a broad range of species, particularly from the tribes Rhipileae, Rhipiliopsideae and Udoteeae, by using concatenated rbcL and tufA data. Species from suborders Ostreobineae and Bryopsidineae and from order Dasycladales were used as outgroups. Other than the newly generated data, sequences were sourced from previous work (Verbruggen et al. 2009a, 2017; Lagourgue et al. 2018; Cremen et al. 2019; Lagourgue and Payri 2021), and this information is listed in Supplementary Table S1. A maximum-likelihood phylogeny was inferred from the concatenated alignment in IQ-TREE (ver. 2.0.3, see https://github.com/Cibiv/IQ-TREE), by using the model selected as part of the IQ-tree inference procedure (GTR+F+I+G4), and with 100 regular bootstrap replicates to estimate branch support (Minh et al. 2020).
To investigate species boundaries in more detail, we built comprehensive datasets of the Rhipilia genus (sensu Lagourgue and Payri 2021) for the tufA and rbcL marker genes, combining new and previously published data. Sequence alignments were built with MAFFT 7.490 (Katoh and Standley 2013) and trees inferred using BEAST (ver. 1.10.4, see https://github.com/beast-dev/beast-mcmc; Suchard et al. 2018), using a coalescent (constant size) tree prior, a lognormal uncorrelated relaxed clock model and a GTR+G+I model of sequence evolution, with default priors and operators. We ran a chain of 10 million generations, logging every 1000th generation. Following examination of the traces, the first 1 million generations were discarded as burn-in and a maximum clade credibility tree with median node heights was computed from the post-burn-in samples. No outgroups were included in this analysis; instead, the relaxed molecular clock model was allowed to determine the root position, the inferred oldest point of the phylogeny.
Results
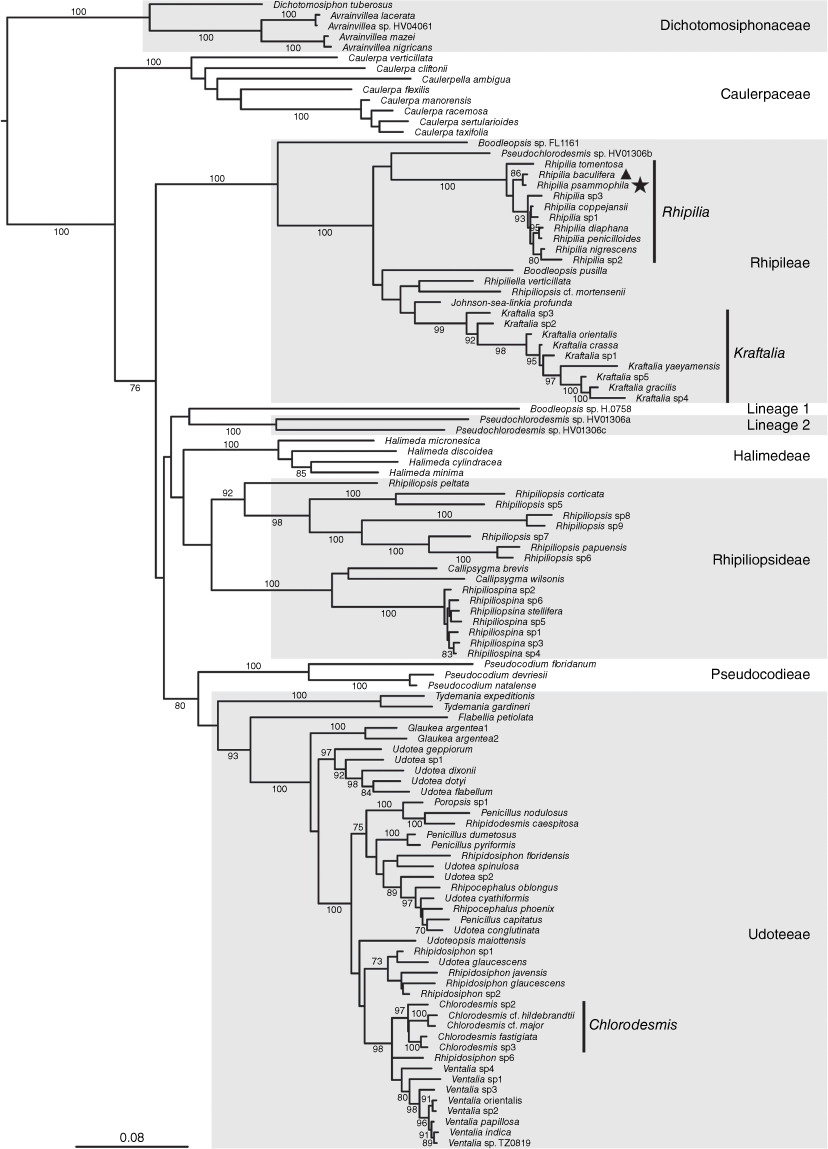
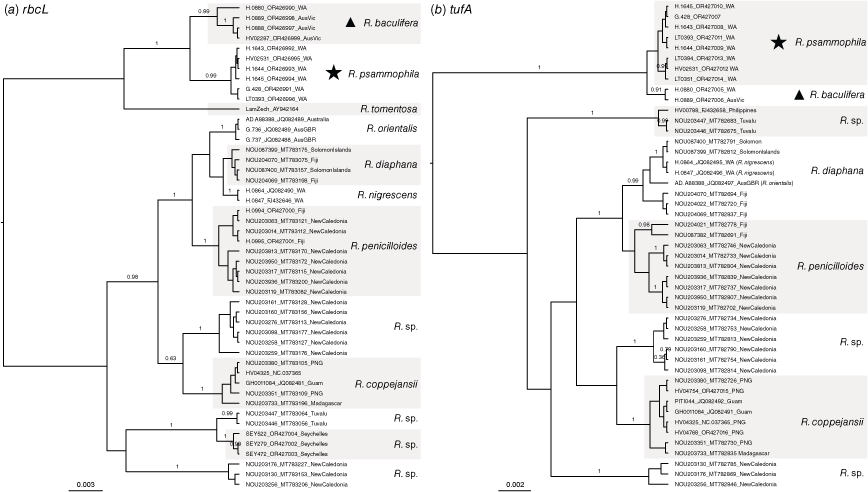
Our broad phylogeny of the suborder Bryopsidineae of the Bryopsidales indicated that the unknown species as well as Chlorodesmis baculifera are found in the tribe Rhipileae, within the genus Rhipilia (Fig. 1, indicated with star and triangle respectively, to indicate the two species). Additional phylogenetic analyses of densely sampled Rhipilia datasets for the rbcL and tufA markers showed that the unknown Rhipilia is a new entity separate from other sequenced Rhipilia species, and is closely related to Chlorodesmis baculifera (Fig. 2). As a consequence of these results and of morphological comparisons with species not represented in molecular libraries, we describe the new species Rhipilia psammophila (Fig. 3) and transfer Chlorodesmis baculifera (Fig. 4) to the genus Rhipilia.
Phylogeny of the Halimedineae suborder of Bryopsidales, showing the placement of Rhipilia baculifera (indicated with a triangle) and Rhipilia psammophila (indicated with a star) in the genus Rhipilia of the tribe Rhipileae. The phylogeny is based on concatenated rbcL and tufA sequences, with bootstrap support indicated at branches (when >70). The scale is in estimated substitutions per site. Outgroups were pruned from the original tree for this visualisation. The concatenated alignment was 94.8% complete, with 7 of 127 sequences missing for rbcL and 6 of 127 sequences missing for tufA. Lineage 1 and 2 annotations refer to the naming of these lineages by Cremen et al. (2019).
Densely sampled gene trees of (a) rbcL and (b) tufA for the genus Rhipilia, including the new species R. psammophila (indicated with a star) and the newly transferred R. baculifera (indicated with a triangle).
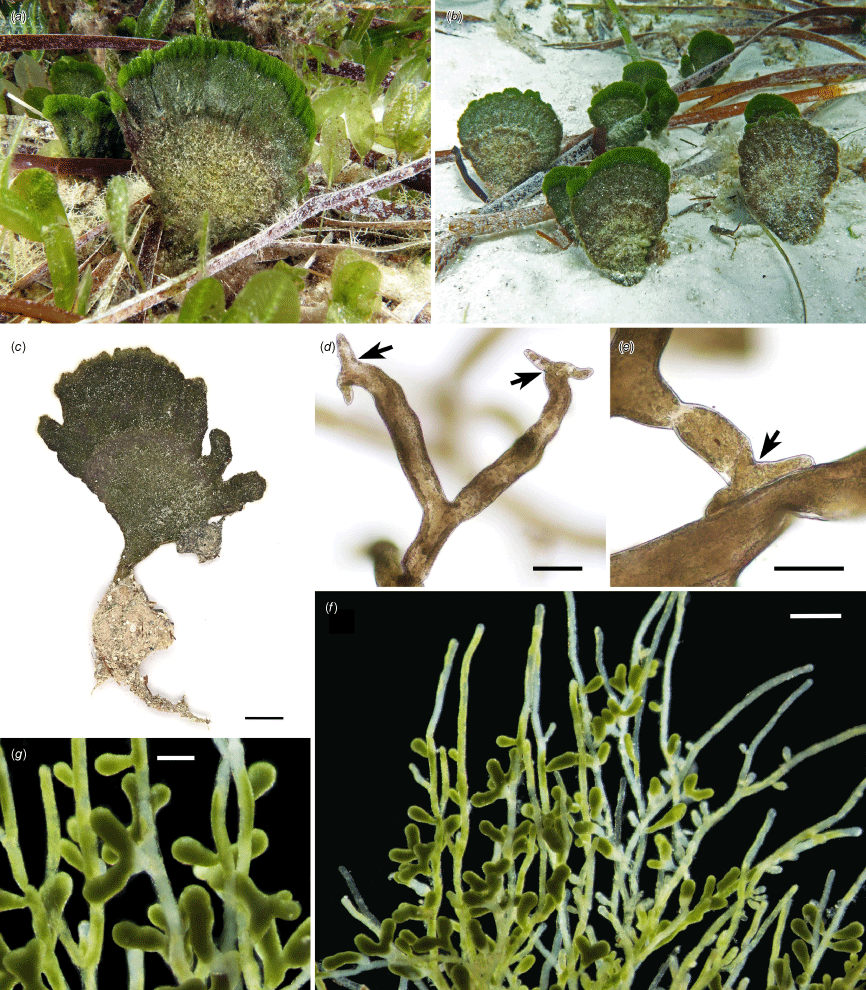
Rhipilia psammophila. (a) Specimen in situ at Two Peoples Bay, Western Australia, growing among seagrasses. (b) Clustered specimens growing in sandy substrata. (c) The pressed holotype specimen (PERTH 09389660). (d) Siphons bearing perpendicular lateral branches with terminal tenacula (indicated by arrows). (e) Tenacula adhering to adjacent filament (indicated by arrow). (f) Fertile filaments with numerous lateral gametangia. (g) Closer view of lateral gametangia. Scale bars: 1 cm (c); 60 µm (d, e); 600 µm (f); 300 µm (g). Vouchers: (a, b, d, e) J.M.Huisman 2.1.22.1 (PERTH [holotype]); (f, g) A.Dekker, C.Nutt, K.Murray & H.Botha s.n. (PERTH 08188459). Photos: J. M. Huisman.
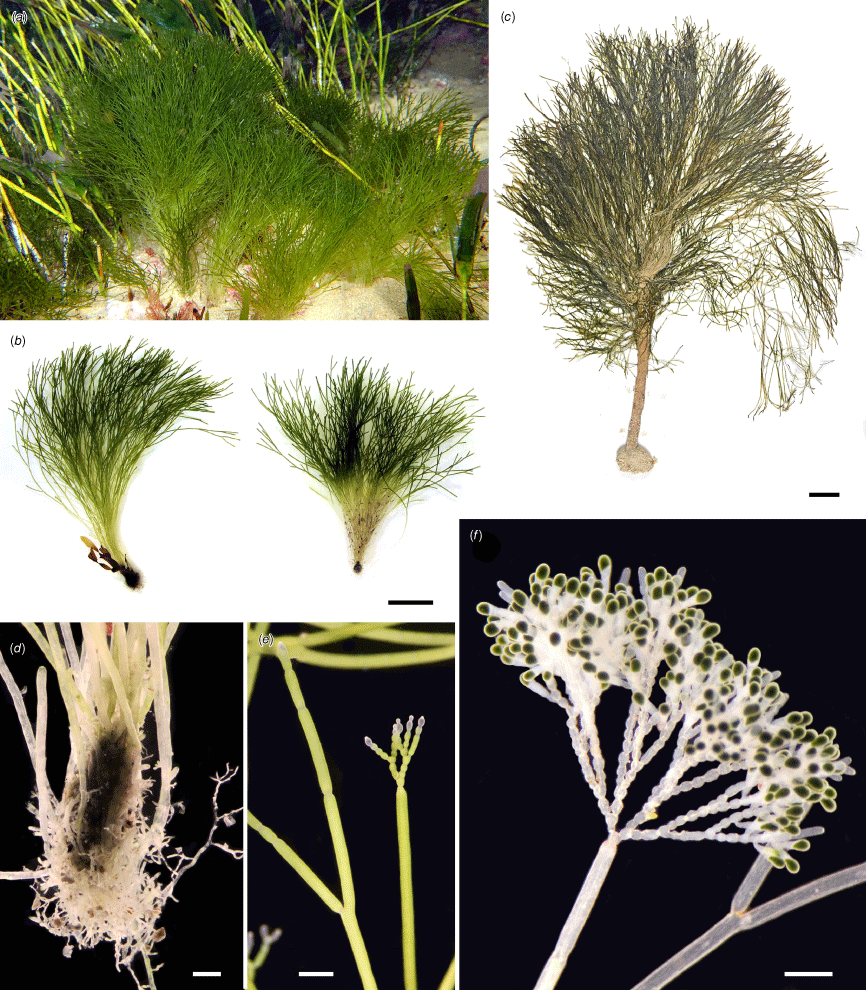
Rhipilia baculifera. (a) Specimen in situ at type locality at Point Lonsdale, Victoria, growing among the seagrass Amphibolis antarctica. (b) Freshly collected specimens, with a limited basal aggregation of siphons. (c) Pressed specimen dredged from deep water, south of Rottnest Island, with a substantial stipe. (d) Closer view of aggregated siphons at base of specimens in Fig. 4b. (e) Free siphons that are dichotomously divided with occasional shallow constrictions. A reproductive tuft is developing at the apex of the right-hand siphon. (f) View of densely branched reproductive tuft, with moniliform lower siphons. Scale bars: 2 cm (b, c); 1 mm (d–f). Voucher: (a, b) H.Verbruggen HV03984 (MELU); (c) C.Sim s.n. (PERTH 08822182); (d–f) living material, vouchers not retained. Photos: H. Verbruggen (a, b, d–f); J. M. Huisman (c).
Taxonomy
Rhipilia psammophila Huisman & Verbruggen, sp. nov.
Type: Two Peoples Bay, Western Australia, in sand at 2 m depth, 2 Jan. 2022, J.M.Huisman 2.1.22.1 (holo: PERTH 09389660 [four specimens are mounted on the sheet; the holotype is the specimen shown in Fig. 3c]; iso: PERTH 09389679 [with four specimens on the sheet]).
Thalli dark green, psammophytic, erect, soft and spongy, up to 10 cm tall (including holdfast) and 7 cm broad, arising from a fibrous matted base, with a subterete short stipe grading into an upper flabellate to cuneate blade. Structure of interwoven terete siphonous filaments, lower filaments 40–70 μm in diameter, upper filaments 60–100 μm in diameter (broadest in fertile filaments), these branched at irregular intervals, with or without shallow constrictions at dichotomies. Lower filaments with short to long lateral branches arising perpendicularly, with constrictions just above the base, forming attachments to adjacent filaments via terminal tenacula, these with 2–4 mostly blunt prongs that occasionally divide once more. Filaments heteroplastic, with numerous round to ellipsoidal chloroplasts 3–5 μm long. Reproduction by profuse basally constricted gametangia arising laterally on upper filaments. Gametangia clavate to obovoid, often once-forked, 145–580 μm long and 45–140 μm broad (Fig. 3).
Growing in sand among seagrasses (Posidonia K.D.Koenig and Amphibolis C.Agardh). At Two Peoples Bay in south-western Western Australia, the species was seen to be locally common, but restricted to sandy habitats between the extensive seagrass beds that dominate the bay (Fig. 1).
Rhipilia psammophila, with its flabellate thallus, structurally with branched siphons attached to one another by tenacula, is typical of what was until recently regarded as Rhipilia (Millar and Kraft 2001; Verbruggen and Schils 2012). However, on the basis of molecular analyses, Lagourgue and Payri (2021) segregated several species of Rhipilia and Rhipiliopsis as their new genus Kraftalia, in doing so accommodating species with and without tenacula in a single genus. However, there was no suggestion that individual species can vary in this respect, so the presence of tenacula remains a useful character for species delimitation.
Our molecular analyses place Rhipilia psammophila in a clade with the type species of Rhipilia (R. tomentosa Kütz.) and other species of the genus as defined by Lagourgue and Payri (2021). Those authors included in Rhipilia species with widely diverging morphologies, but with some consistent features, including the presence of a stolon (although R. tomentosa has been observed without a stolon), siphon dichotomies with a subdichotomous bulge and supra-dichotomous constrictions, and simple tenacula (2 or 3 prongs). Siphon diameters are 20–320 µm. Kraftalia was characterised by Lagourgue and Payri (2021) as having a fan-shaped frond, no stolon, relatively thin siphon diameters (<100 µm in diameter) that are dichotomously divided with or without supra-dichotomous constrictions, and the cohesion of siphons by one or more particular types of structures (direct longitudinal contact, papillae, differentiated siphons, or tenacula). Four previously described Rhipilia and Rhipiliopsis species were transferred to Kraftalia and the authors recognised a further five as-yet undescribed species (none from Australia).
Thus, there is considerable morphological overlap between the two genera, seemingly separable only by the presence of a stolon and potentially larger siphon diameters in Rhipilia. On the basis of morphology, in particular the absence of a stolon and smaller siphon diameters, Rhipilia psammophila appears to align with Kraftalia; however, our molecular analyses clearly align the species with Rhipilia. Although there were no obvious stolons, it is worth noting that the new species often grew in clusters, and there might have been horizontal buried siphons connecting the individual thalli.
Womersley (1984), in his monograph of the green algae of southern Australia, included several species of then Udoteaceae that display some habit similarities with Rhipilia psammophila, including Avrainvillea clavatiramea A.Gepp & E.Gepp, Rhipiliopsis peltata (J.Agardh) A.Gepp & E.Gepp, Rhipiliopsis robusta Womersley, and Rhipilia pusilla (Womersley) Ducker. Of these, only Rhipilia pusilla forms tenacula, but it is a much smaller plant (up to 1.5 cm tall), not compressed, and only the basal siphons are ‘weakly or not attached by lateral tenacula’ (Womersley 1984, p. 247). Lagourgue and Payri (2021) included Rhipilia pusilla in their molecular analyses, but the results were somewhat equivocal, the authors preferring to leave the status of R. pusilla as ‘in question’. Of the known southern Australian taxa, the most similar in appearance are Rhipiliopsis peltata and Rhipiliopsis robusta; however, siphons in these species are attached by circular or papillar fusions (Womersley 1984, pp. 248–251; Kraft 1986, p. 49).
Of the tropical Australian species of Rhipilia documented by Millar and Kraft (2001), Kraft (2007) and Huisman and Verbruggen (2015), only R. tomentosa is described as sand-dwelling. It differs from R. psammophila in the diagnostic regularly curved or bent siphons that attach to adjacent siphons, plus the deep constrictions that are plugged by internal rings of wall thickenings (Millar and Kraft 2001, p. 26). Rhipilia tomentosa has been recorded from eastern Australia (Millar and Kraft 2001), but according to Lagourgue and Payri (2021), the species is restricted to the Caribbean and records from other areas require confirmation with DNA sequences. They noted that Pacific specimens that were morphologically similar to R. tomentosa actually belonged to their new genus Kraftalia on the basis of DNA analyses. However, no species identity was suggested for these Pacific specimens.
Lagourgue and Payri (2021) regarded species in this group to be restricted to specific geographic areas, and on the basis of sequence analyses, morphological differences and the collection locality being far removed geographically and climatically from the distribution of all known species of Rhipilia, we herein recognise the Two Peoples Bay taxon as new.
WESTERN AUSTRALIA. Two Peoples Bay, in sand at 2 m depth, 2 Jan. 2022, J.M.Huisman s.n. (PERTH 09389679); loc. id., southern end of bay, 12 Nov. 2008, A.Dekker, C.Nutt, K.Murray & H.Botha s.n. (PERTH 08188459); Hopetoun, on sand-covered rock, 12 Dec. 2012, H. Verbruggen HV2531 (BR5010111415391V); loc. id., attached in the sand, 12 Dec. 2012, L.Tyberghein LT0351 (BR5010111416428V).
Rhipilia baculifera (J.Agardh) Huisman & Verbruggen, comb. nov.
Bryopsis baculifera J.Agardh, Acta Univ. Lund. 23(2): 21 (1887); Chlorodesmis baculifera (J.Agardh) Ducker, Phycologia 5: 245 (1966). Type citation: ‘ad oras australes Novae Hollandiae; ad Port Phillip legit Br. Wilson!’. Type: Port Phillip Heads, Victoria, John Bracebridge Wilson (holo: Herb Agardh, LD 14898 n.v. (Ducker 1966, p. 245, 1967, p. 156, noted that the type was J.B.Wilson 39, dredged at Port Phillip Heads in 1880)).
Cladophoropsis bulbosa Womersley, Pacific Sci. 9: 391 (1955); Chlorodesmis bulbosa (Womersley) Ducker, Phycologia 4: 149 (1965). Type: Queenscliff, Victoria, s. dat., leg. ign. (holo: MEL 3007).
Thalli medium to dark green, erect, densely tufted, 4–10(–16) cm high, attached by colourless rhizoids and when mature arising from a matted bulbous base up to 2 cm high, formed of entangled rhizoids, in some specimens forming a conspicuous terete stipe up to 6 cm long, 5 mm in diameter. Filaments sparsely branched, of uniform width throughout, (250–)300–500(–600) µm in diameter; wall lamellate, 6–10 µm thick; lateral branches slightly basally constricted; chloroplasts ovoid to lenticular, 2–4 µm long; amyloplasts elongate-ovoid, 6–10 µm long. Reproduction in much branched fertile tufts borne laterally or terminally on the filaments, with each branch of the tuft bearing numerous ovoid laterals that form biflagellate reproductive bodies (probably gametes) that are discharged through the branch apex; fertile in early summer (November–December). (Description modified from Womersley 1955, pp. 391–392 [as Cladophoropsis bulbosa], 1984, p. 244 [as Chlorodesmis baculifera]; Ducker 1965, pp. 150–155 [as Chlorodesmis bulbosa]). (Fig. 4)
Known from Rottnest Island, Western Australia, and from Pearson Island, South Australia, to Waratah Bay, Victoria, and the northern coast of Tasmania.
According to Womersley (1984, p. 244), this species is uncommon and ‘apparently confined to deep water or shaded habitats’. Our recent collections in Victoria have primarily come from shaded areas, but we have also observed it quite commonly in large rock pools among seagrass growing on sand-covered rock. Ducker (1965, pp. 149–150) described similar habitats: ‘littoral rockpools’ and ‘among the dense stand of the marine flowering plant Cymodocea [Amphibolis] antarctica’.
From the Latin baculus ‘rod or staff’ and suffix -fer ‘bearing’, presumably in reference to the thick and firm branches (‘crassitie et firmitate filorum’; Agardh 1887, p. 21).
Our specimens conformed in most respects to descriptions of Chlorodesmis baculifera by Ducker (1965, as C. bulbosa, 1967) and Womersley (1984), differing only in the position of fertile heads, which Ducker (1965) described as ‘modified sidebranches’. In our specimens the fertile heads arose in what appears to be an apical position (Fig. 4e, f), although the bearing filament might be interpreted as an elongate pedicel. Molecular analyses were undertaken with specimens collected from near the Point Lonsdale type locality at Port Phillip Heads, Victoria (e.g. H. Verbruggen, H.0888, MELU), and also with a specimen from Western Australia (J. Huisman, H.0880, MELU) that confirmed the close relationship of plants from this disjunct location. Chlorodesmis baculifera is essentially restricted to southern Australia and is very much a geographical outlier in Chlorodesmis, an otherwise warm-water genus. Our molecular analyses placed this species within the Rhipilia clade and sister to R. psammophila. As such, we here publish the new combination Rhipilia baculifera (J.Agardh) Huisman & Verbruggen.
Discussion
This study provides morphological and molecular support for the recognition of the new species Rhipilia psammophila and the transfer of Chlorodesmis baculifera to Rhipilia, and it also includes the first description of reproduction in the genus. The generic placement of R. psammophila, although clearly supported by molecular analysis, does not conform to the morphological concept of Rhipilia proposed by Lagourgue and Payri (2021) and it appears that no single character, or combination of characters, can distinguish the genus from the closely related Kraftalia. It is evident that further molecular analyses are required.
Although the morphology of R. baculifera might appear to be at odds with the typical flabellate Rhipilia with laterally attached siphons, it is now the third species with free siphons to be attributed to the genus. Both the others, Rhipilia coppejansii Schils & Verbruggen (in Verbruggen and Schils 2012) and R. penicilloides N’Yeurt & Keats (N’Yeurt and Keats 1997), have Chlorodesmis-like morphologies, but molecular analyses place each firmly in Rhipilia. There is the possibility that these species represent a growth form or a life-cycle stage of flabellate taxa that have not yet been sequenced, athough this is unlikely, given that the recent extensive sequencing in this group has not shown any evidence for such an alteration of generations (e.g. Lagourgue and Payri 2022). Interestingly, the three species with free siphons are placed in distinct positions of the phylogeny, suggesting that they have arisen independently from a more structurally complex ancestor. This adds to the growing evidence for reductions in the complexity of bryopsidalean algae, with examples including these three Rhipilia species as well as the genera Chlorodesmis and Poropsis Kütz. in the tribe Udoteeae (Kooistra 2002; Lagourgue and Payri 2022). Although in these lineages the relatively simple morphologies are derived from comparatively more complex organisms, it is worth noting that they represent a small fraction of the simple morphologies seen in the suborder Halimedineae. Many lineages with exceedingly simple body plans including Boodleopsis A.Gepp & E.Gepp and Pseudochlorodesmis Børgesen strains (Verbruggen et al. 2009b; Cremen et al. 2019), and several lineages of endolithic siphons detected through environmental sequencing (Marcelino and Verbruggen 2016), are early-branching in the Halimedineae (see also our Fig. 1), suggesting the ancestor would have been a simple siphon.
Our molecular and morphological analyses, and those of Lagourgue and Payri (2022), have highlighted the complexity of this group, and further study is required to establish morphological features that might distinguish between Rhipilia and Kraftalia, with these presently being separable only by molecular methods. More broadly, clarification of the phylogeny of this group will benefit from a greater representation of taxa with simple morphologies, to further understand the relationships between these and taxa with more complex morphologies.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
John Huisman is an Associate Editor for Australian Systematic Botany but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. Both authors declare that they have no other conflicts of interest.
Acknowledgements
J. M. Huisman thanks Ian McKernan and Alan Kendrick for field support and the staff of the Western Australian Herbarium. H. Verbruggen thanks Riyad Hossen, Tara Jalali and Joanne Birch for assistance.
References
Agardh JG (1887) Till algernes systematik. Nya bidrag. (Femte afdelningen). Acta Universitatis Lundensis 23(2), 1-174 [In Swedish].
| Google Scholar |
Costa JF, Lin SM, Macaya EC, Fernández-García C, Verbruggen H (2016) Chloroplast genomes as a tool to resolve red algal phylogenies: a case study in the Nemaliales. BMC Evolutionary Biology 16, 205.
| Crossref | Google Scholar |
Cremen MCM, Huisman JM, Marcelino VR, Verbruggen H (2016) Taxonomic revision of Halimeda (Bryopsidales, Chlorophyta) in south-western Australia. Australian Systematic Botany 29, 41-54.
| Crossref | Google Scholar |
Cremen MCM, Leliaert F, Marcelino VR, Verbruggen H (2018) Large diversity of nonstandard genes and dynamic evolution of chloroplast genomes in siphonous green algae (Bryopsidales, Chlorophyta). Genome Biology and Evolution 10, 1048-1061.
| Crossref | Google Scholar | PubMed |
Cremen MCM, Leliaert F, West J, Lam DW, Shimada S, Lopez-Bautista JM, Verbruggen H (2019) Reassessment of the classification of Bryopsidales (Chlorophyta) based on chloroplast phylogenomic analyses. Molecular Phylogenetics and Evolution 130, 397-405.
| Crossref | Google Scholar | PubMed |
Ducker SC (1965) The structure and reproduction of the green alga Chlorodesmis bulbosa. Phycologia 4, 149-162.
| Crossref | Google Scholar |
Ducker SC (1966) An earlier name for the green alga Chlorodesmis bulbosa. Phycologia 5, 245-246.
| Crossref | Google Scholar |
Ducker SC (1967) The genus Chlorodesmis (Chlorophyta) in the Indo-Pacific region. Nova Hedwigia 13, 145-182, Plates 25–43.
| Google Scholar |
Katoh K, Standley DM (2013) MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772-780.
| Crossref | Google Scholar | PubMed |
Kooistra WHCF (2002) Molecular phylogenies of Udoteaceae (Bryopsidales, Chlorophyta) reveal nonmonophyly for Udotea, Penicillus and Chlorodesmis. Phycologia 41, 453-462.
| Crossref | Google Scholar |
Kraft GT (1986) The green algal genera Rhipiliopsis A. & E.S. Gepp and Rhipiliella gen. nov. (Udoteaceae, Bryopsidales) in Australia and the Philippines. Phycologia 25, 47-72.
| Google Scholar |
Lagourgue L, Payri CE (2021) Diversity and taxonomic revision of tribes Rhipileae and Rhipiliopsideae (Halimedaceae, Chlorophyta) based on molecular and morphological data. Journal of Phycology 57, 1450-1471.
| Crossref | Google Scholar | PubMed |
Lagourgue L, Payri CE (2022) Large‐scale diversity reassessment, evolutionary history, and taxonomic revision of the green macroalgae family Udoteaceae (Bryopsidales, Chlorophyta). Journal of Systematics and Evolution 60, 101-127.
| Crossref | Google Scholar |
Lagourgue L, Puillandre N, Payri CE (2018) Exploring the Udoteaceae diversity (Bryopsidales, Chlorophyta) in the Caribbean region based on molecular and morphological data. Molecular Phylogenetics and Evolution 127, 758-769.
| Crossref | Google Scholar | PubMed |
Marcelino VR, Verbruggen H (2016) Multi-marker metabarcoding of coral skeletons reveals a rich microbiome and diverse evolutionary origins of endolithic algae. Scientific Reports 6, 31508.
| Crossref | Google Scholar | PubMed |
Marcelino VR, Cremen MCM, Jackson CJ, Larkum AAW, Verbruggen H (2016) Evolutionary dynamics of chloroplast genomes in low light: a case study of the endolithic green alga Ostreobium quekettii. Genome Biology and Evolution 8, 2939-2951.
| Crossref | Google Scholar |
Millar AJK, Kraft GT (2001) Monograph of the green macroalgal genus Rhipilia (Udoteaceae, Halimedales), with a description of R. crassa sp. nov. from Australia and the Philippines. Phycologia 40, 21-34.
| Crossref | Google Scholar |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530-1534.
| Crossref | Google Scholar | PubMed |
N’Yeurt ADR, Keats DW (1997) Rhipilia penicilloides sp. nov. (Udoteaceae, Chlorophyta) from Fiji. Phycologia 36, 172-178.
| Crossref | Google Scholar |
Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A (2018) Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evolution 4, vey016.
| Crossref | Google Scholar | PubMed |
Verbruggen H, Schils T (2012) Rhipilia coppejansii, a new coral reef-associated species from Guam (Bryopsidales, Chlorophyta). Journal of Phycology 48, 1090-1098.
| Crossref | Google Scholar | PubMed |
Verbruggen H, Ashworth M, LoDuca ST, Vlaeminck C, Cocquyt E, Sauvage T, Zechman FW, Littler DS, Littler MM, Leliaert F, De Clerck O (2009a) A multi-locus time-calibrated phylogeny of the siphonous green algae. Molecular Phylogenetics and Evolution 50, 642-653.
| Crossref | Google Scholar | PubMed |
Verbruggen H, Vlaeminck C, Sauvage T, Sherwood AR, Leliaert F, De Clerck O (2009b) Phylogenetic analysis of Pseudochlorodesmis strains reveals cryptic diversity above the family level in the siphonous green algae (Bryopsidales, Chlorophyta). Journal of Phycology 45, 726-731.
| Crossref | Google Scholar | PubMed |
Verbruggen H, Marcelino VR, Guiry MD, Cremen MCM, Jackson CJ (2017) Phylogenetic position of the coral symbiont Ostreobium (Ulvophyceae) inferred from chloroplast genome data. Journal of Phycology 53, 790-803.
| Crossref | Google Scholar | PubMed |
Womersley HBS (1955) New marine Chlorophyta from southern Australia. Pacific Science 9, 387-395.
| Google Scholar |


