Towards resolution of the Prostanthera ovalifolia R.Br. assemblage (Lamiaceae: Prostantheroideae): clarification of P. cineolifera R.T.Baker & H.G.Sm. and description of three new species
Ruth L. Palsson A , Rose L. Andrew
A , Rose L. Andrew  A , Ian R. H. Telford
A , Ian R. H. Telford  A , Robert T. Miller B and Jeremy J. Bruhl
A , Robert T. Miller B and Jeremy J. Bruhl  A *
A *
A
B
Abstract
The circumscription and application of Prostanthera ovalifolia R.Br. appears to have been based largely on leaf shape, leading to a geographically and morphologically diverse concept of the species. This approach has completely or partially subsumed other species (namely P. atriplicifolia A.Cunn. ex Benth., P. latifolia (Benth.) Domin, P. lanceolata Domin and P. cineolifera R.T.Baker & H.G.Sm.), and included considerable variation hypothesised by some authors as distinct species (viz. P. sp. Hawkesbury (B.J. Conn 2591), P. sp. Olney State Forest (R.L. Palsson 166) and P. sp. Oxley Wild Rivers National Park (J.B. Williams s.n. NE 91044)). Morphological and molecular data, supported by phytochemical data, are used to partially resolve the taxonomy of this group. The circumscription of Prostanthera cineolifera is clarified and the name lectotypified. Three new species from New South Wales are recognised: Prostanthera dyarubbin Palsson & R.T.Mill., P. faucicola Palsson & I.Telford and P. milleri Palsson & J.J.Bruhl. Prostanthera milleri fits the criteria of Critically Endangered. We also lectotypify Prostanthera ovalifolia.
Keywords: DNA, morphology, new species, Prostanthera dyarubbin, Prostanthera milleri, Prostanthera faucicola, single-nucleotide polymorphism, SNP, species delimitation, taxonomy, typification.
Introduction
Robust and reliable definition of species is an ongoing, urgent and important task for systematists and imperative for conservation. Knowing that a population of species ‘x’ constitutes small numbers of individuals of restricted geographic distribution affects the conservation status of a species, relative to an abundant, widely distributed species ‘y’. However, what if species ‘y’ is better classified as multiple species including rare, restricted species ‘a’, ‘b’, ‘c’, etc.? We consider that the last case is a common condition in the systematics of vascular plants. Examples in Australia of such ‘hidden’ plant biodiversity have been revealed by recent taxonomic revisions including those of Xerochrysum bracteatum (Vent.) Tzvelev (Collins et al. 2022), Phebalium Vent. (Telford et al. 2019; Dema et al. 2021), Netrostylis R.L.Barrett, J.J.Bruhl & K.L.Wilson (Barrett et al. 2021) and Prostanthera lasianthos Labill. (Conn et al. 2021).
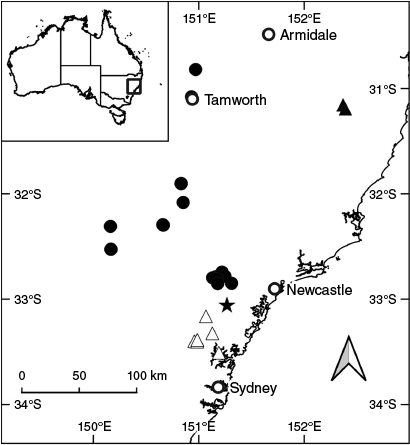
Here we focus on the genus Prostanthera Labill. (Lamiaceae: Prostantheroideae), an Australian endemic genus of mostly shrubs that includes 114 described species and 14 accepted tag-named entities (Wilson et al. 2019; Conn et al. 2021; O’Donnell et al. 2021, 2023; Australian Plant Name Index, see https://biodiversity.org.au/nsl/services/search/names), some of which are treated as species complexes with morphological variability and wide distributions. One such case is Prostanthera ovalifolia R.Br. sens. lat. (Brown 1810; Althofer 1978), supposedly characterised by leaves ‘narrow-ovate to ovate’ (Conn 1992 in PlantNET) but treated as including plants with a wide range of leaf shapes; e.g. see images currently determined as P. ovalifolia at Herbarium NSW (see https://herbariumcollection.botanicgardens.org.au/#/result/genus:Prostanthera%20AND%20specificEpithet:Ovalifolia, accessed 27 May 2025). Prostanthera ovalifolia is currently treated as one species ranging from the tropics south to cool temperate Australia (Fig. 1); subsuming P. atriplicifolia A.Cunn. ex Benth. and usually including Prostanthera sp. Hawkesbury (B.J.Conn 2591) NSW Herbarium (see https://biodiversity.org.au/nsl/services/apc-format/display/233589). Specimens of the Prostanthera ovalifolia complex have been misidentified as various other species, as shown by a study focused on P. cineolifera (Palsson et al. 2024).
Distribution of Prostanthera ovalifolia according to Australasian Virtual Herbarium (AVH) data (https://avh.ala.org.au/, accessed 18 March 2025). Records labelled or unambiguously inferred as cultivated or naturalised and those with spurious geocodes have been removed. All records in South Australia are either cultivated or naturalised and are therefore not included here. Likewise, VicFlora (see https://vicflora.rbg.vic.gov.au/flora/taxon/f55795c1-9f8e-4161-9f3c-8b896224f224, accessed 19 March 2025) considers all specimens west of East Gippsland to be naturalised and these are also not shown. The arrow indicates the type location of P. ovalifolia.
Prostanthera ovalifolia was collected by Robert Brown in 1803 from Mount Westall in coastal central Queensland ~112 km north of Rockhampton, in the tropics. That gathering is still the only collection of this species from the type location. There are several instances, particularly in South Australia and Victoria, where P. ovalifolia has become naturalised as a ‘garden escape’ (see notes associated with; e.g. MEL 2428494A, in South Australia and NSW457559 in Victoria; https://avh.ala.org.au/, accessed 18 March 2025). VicFlora (see https://vicflora.rbg.vic.gov.au/flora/taxon/f55795c1-9f8e-4161-9f3c-8b896224f224, accessed 19 February 2025) considers P. ovalifolia to be introduced in Victoria, except for plants east of Mallacoota that are likely to be an undescribed species native to far eastern Victoria and south-eastern New South Wales. These plants are still determined as P. ovalifolia sens. lat. The distribution of indigenous (i.e. native at location; excluding cultivated and clearly naturalised occurrences) P. ovalifolia sens. lat. is currently considered to extend from the type location, Mount Westall near Rockhampton in Queensland, south to East Gippsland in Victoria (Fig. 1).
Since the description of P. ovalifolia and P. atriplicifolia, P. latifolia (Benth.) Domin, based on P. ovalifolia var. latifolia Benth. and P. lanceolata Domin (Domin 1928) have been described. Although Australian Plant Census (APC) accepts recognition of P. lanceolata, PlantNET (see https://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=sp&name=Prostanthera~lanceolata, accessed 4 June 2025) notes that ‘The taxonomic status of this taxon is uncertain’. The Queensland Herbarium does not accept P. lanceolata as distinct from P. ovalifolia and P. latifolia is treated as a name of uncertain application by APC (Council of Heads of Australasian Herbaria 2010). Miller (1988) informally described the ‘Hawkesbury River’ and ‘Wattagan State Forest’ variants of P. ovalifolia in the ‘Prostanthera and Westringia Study Group Newsletter 14’ that we refer to as P. sp. Hawkesbury (B.J.Conn 2591) NSW Herbarium and P. sp. Olney State Forest (R.L. Palsson 166) R.L. Palsson respectively.
The three samples treated as P. ovalifolia in the phylogenetic study of Wilson et al. (2012) were all members of a ‘Clade J’ (Wilson et al. 2012, fig. 3) but were not recovered as a monophyletic group, highlighting to us the ongoing issue with their broadconcept of P. ovalifolia. Based on comparison of morphology and morphometric clustering, herbarium specimens referred to by Wilson et al. (2012) as ‘P. ovalifolia 1 and 2’ were reidentified by us as P. lanceolata Domin, and ‘P. ovalifolia 3’ as an undescribed new species with the APC-accepted tag name P. sp. Hawkesbury (B.J.Conn 2591) NSW Herbarium (Palsson et al. 2024; R. L. Palsson, R. L. Andrew, I. R. H. Telford and J. J. Bruhl, unpubl. data).
In the context of the P. ovalifolia assemblage, a further tag name recognised at NE required testing: Prostanthera sp. Oxley Wild Rivers National Park (J.B. Williams s.n. NE 91044) I.Telford. This putative new taxon is known from several sites on the Northern Tablelands of New South Wales, geographically isolated from the morphologically similar P. sp. Hawkesbury and P. sp. Olney State Forest.
Prostanthera cineolifera was also a member of ‘Clade J’ (Wilson et al. 2012, fig. 3). There appears to have been considerable uncertainty as to the identity of specimens from the Hunter Valley and Lower Hawkesbury of New South Wales, variously identified as P. cineolifera (e.g. NSW 848980), P. lanceolata (e.g. NSW 406732) and P. ovalifolia (e.g. NSW 206177). Uncertainty about specimens of P. cineolifera from the Hunter Valley identified as P. ovalifolia was also apparent (e.g. NSW 243352, NSW 454917, NSW 891464 and NSW 228346).
Materials and methods
Study group and materials
Field work involved collection of herbarium vouchers and metadata including soil descriptions, digital images, leaf material for phytochemical (placed in envelopes and dried at 16°C and 16% relative humidity prior to storage at −25°C) and morphological analyses, material stored in silica gel (Si gel), and material for cultivation.
At each site in the field, one plant was sampled for herbarium vouchers, while leaves were sampled for DNA analysis from each plant and dried on silica gel. Multiple individuals, separated by at least 5 m, were sampled. Flowers were collected from several plants and preserved in 70% ethanol for morphological study. Groups of plants separated by more than ~100 m were usually treated as separate collections. Material from each site was allocated a single collection number and single herbarium accession number, with an alphabetical suffix identifying material from separate individuals. Voucher specimens were lodged at NE, with duplicates allocated to BRI, NSW and CANB as appropriate. Herbarium codes follow Index Herbariorum (see http://sweetgum.nybg.org/ih/, accessed 17 July 2022). Relevant images were examined from Global Plants on JSTOR (see https://plants.jstor.org/) and individual herbarium databases (e.g. https://data.kew.org/).
Phylogenetic analysis
Molecular samples and DArTseq data were prepared as described in Palsson et al. (2024). The filtered data consisted of 3915 single-nucleotide polymorphisms (SNPs) scored for 110 individuals, with an average of 0.92% missing data and a mean read depth of 13.93. Basic population-level statistics were calculated. Phylogenetic analysis of 130 samples of Prostanthera was performed on SNP data to further examine species limits of the study group; particularly of the P. sp. Hawkesbury group (P. sp. Hawkesbury, P. sp. Olney State Forest and P. sp. Oxley Wild Rivers National Park). Samples of P. rotundifolia sens. str. were used as the outgroup, and samples of other Prostanthera species and putative species were included for context (Supplementary Table S1).
Using the dartR package (ver. 2.9.7, see https://cran.r-project.org/package=dartR; Gruber et al. 2018; Mijangos et al. 2022) in R (ver. 4.0.2, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/), DArTseq SNPs were filtered using minimum call rates of 95% for loci and 90% for individuals, and minimum reproducibility of 99%. Sites at which the minor allele was only detected in one sample were removed, as singletons may result from sequence error (Johnson and Slatkin 2008; O’Leary et al. 2018). SNP data were exported in FASTA format with heterozygous positions replaced by standard ambiguity codes for maximum likelihood analysis with IQ-TREE (ver. 2.4.0, see http://www.iqtree.org/; Nguyen et al. 2015). ModelFinder (see http://www.iqtree.org/ModelFinder/; Kalyaanamoorthy et al. 2017) was used to determine the best substitution model with a correction for ascertainment bias, as the data consisted of a concatenated SNP matrix and IQ-TREE first removed sites evaluated as invariant, leaving 4865 parsimony-informative sites.
The best-fit model according to the Bayesian Information Criterion was SYM + ASC + R4, indicating a symmetric model with four categories of rates and equal base frequencies (Zharkikh 1994; Yang 1995; Soubrier et al. 2012), and an ascertainment bias correction. Branch support was evaluated using 1000 ‘ultrafast bootstraps’ (Hoang et al. 2017) that have a higher threshold for confidence (95%) than conventional bootstraps.
Morphology and morphological analyses
Morphological analyses were carried out as described in Palsson et al. (2024). Some populations of all species considered were sampled, either from field collections or herbarium vouchers, in both drought and wetter-than-average years. This allowed informal comparison of morphological characteristics across dry and wet seasons. Calyx and corolla sizes, the length of the internodes in an inflorescence and the number of flowers were all reduced in drought affected plants (R. L. Palsson, pers. obs.). Such reductions in size and number with water stress are well documented (e.g. Burkle and Runyon 2016; Gallagher and Campbell 2017). Specimens of most of the study group used to construct comparative descriptions included those collected during both drought and non-drought years.
Adult and juvenile leaves were treated separately, to avoid conflating different homologies. We found that the distinction between adult and juvenile leaves needed to be based on morphological criteria (cf. Conn 1992 in PlantNET, where juvenile leaves are described as different from adult leaves) and not fertility of branches, as we found some shoots to be neotenous; i.e. juvenile-like toothed leaves present on flowering branchlets. Growth after heavy browsing was frequently of juvenile form (R. L. Palsson, J. J. Bruhl and I. R. H. Telford, pers. obs.).
Many sources seem to confound or not distinguish ‘bract’, ‘bracteole’ and ‘prophyll’ (see Bruhl 1995). Here we avoid the term ‘bracteole’, as used in by Conn (1992) in PlantNET (e.g. ‘bracteoles not persistent’ for P. cineolifera). Rather, we use ‘prophyll’ that is strictly defined as the first two lateral (foliar) organs on a vegetative or reproductive branch (Briggs and Johnson 1979). In Prostanthera, the floral prophylls, paired bract-like organs below each flower, vary in shape, size and persistence; in some cases these are shed extremely early, i.e. caducous. We use ‘floral bract’ (=pherophylls of Briggs and Johnson 1979; see also Conn 1984, 1988) for the single bract below the floral prophylls. Both vary in shape, size and persistence.
In Prostanthera, both glandular and non-glandular trichomes (=hairs) are present on most vegetative and floral parts (including branchlets, leaves, petioles, calyces and corollas) (e.g. Conn 1984; Harley et al. 2004). Non-glandular trichomes are hereinafter referred to as non-glandular hairs, except for the seemingly non-glandular hairs of the anther appendages that are simply referred to as hairs. The multicellular peltate subsessile glandular trichomes, with a diameter of ~0.1 mm, are referred to as glandular trichomes. In many papers on Prostanthera (e.g. Conn 1984; Conn et al. 2021), density of non-glandular hairs was used to measure ‘hairiness’ (B. J. Conn, pers. comm., 2024).
Anthers possess an appendage (apiculum) that usually bears non-glandular trichomes or hairs. There are differences between the anther appendage and associated hairs in the study group. Measurement of the full length of the appendage can be problematic, therefore a pragmatic approach was taken. The length of the appendage, including the length of any hairs, was measured from the apex of the anther, i.e. only that portion of the appendage that was able to be measured clearly and reliably.
Morphological characters were scored from representative specimens from our field collections and from material held in BRI, CANB, NE and NSW. One element of the type gathering of P. ovalifolia, CANB 278989.1 was directly available and scored but as there was a great deal of missing data for this sample, the data were not included in the final analysis. Material from Mount Stanley, ~200 km SSE of Mount Westall, has been used as a proxy for typical P. ovalifolia (NE 106213, NE 106220, NE 106222, NE 106354). The Mount Stanley material was the best proxy available, geographically closest to the type location and seemingly most similar morphologically. See Supplementary Tables S2 and S3 for the character list and data scored for the morphological analysis respectively.
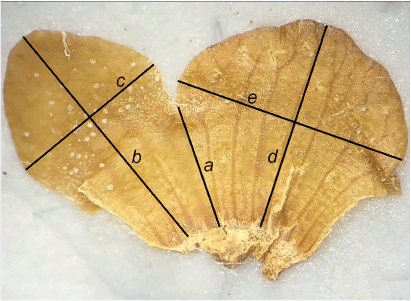
Measurements were taken from herbarium specimens, from flowers stored in 70% ethanol or flowers from herbarium specimens following rehydration with water with a drop of detergent. The possible small size difference between material stored in 70% ethanol and rehydrated material is considered insignificant given the variation in size of floral parts within one entity. Herbarium specimens were used for the leaf characters and calyx length. Calyces were dissected out and flattened from flowers stored in 70% ethanol (Fig. 2). Corollas stored in 70% ethanol were used to measure corolla adaxial tube length and corolla lobe dimensions. Corolla adaxial tube length was measured as in Wilson et al. (2017, fig. 1). Corolla lobes were measured in the same manner as the calyx lobes; after dissecting and flattening out the corolla.
Flattened calyx with abaxial calyx lobe on left, showing measurement protocol. Abaxial calyx lobe length = b − a, abaxial calyx lobe width = c, adaxial calyx lobe length = d − a, adaxial calyx lobe width = e.
Measurements were taken using either a calibrated graticule on the microscope or by photographing structures together with a steel rule and calculating the length using ImageJ (ver. 1.54, W. S. Rasband, US National Institutes of Health, Bethesda, MD, USA, see https://imagej.nih.gov/ij/; Schneider et al. 2012). Styles and stylar lobes were measured in the same manner as the calyx lobes after dissecting and straightening out the pistil. Microscopes used were Leica Wild MZ8 and a Leica MZ8 at NE, and a Leica EZ4. Multiple measurements (three to six) were taken per specimen and per population, sometimes constrained by the number of suitable flowers available. To measure the density of glandular trichomes on the abaxial and adaxial surfaces of leaves, fully developed leaves from herbarium specimens were imaged with the Nikon SMZ25 stereomicroscope and Nikon DS-Ri2 camera at University of New England (UNE), and the density calculated using NIS-Elements imaging (ver. 4.50, Nikon Instruments Inc., Tokyo, Japan, see https://www.microscope.healthcare.nikon.com/products/software/nis-elements). For each species, 10–15 leaves were imaged from a range of specimens and populations. Diaspores, i.e. one-seeded partial fruits or mericarps, were similarly imaged using the Nikon SMZ25.
Phytochemical analysis
Sadgrove et al. (2020) reported results of gas chromatographic analysis of essential oils for many samples of Prostanthera. We reinterpret these data in the context of the taxonomic hypotheses of our study. Phytochemical data for samples of the study group (CIA-1, CIA-2, CIA-3, OFS-1, OFS-2, OFS-3 and OVA-1) (CIA is P. cineolifera, OFS-1 and OFS-2 are P. sp. Olney State Forest, OSF-3 is P. sp. Hawkesbury and OVA-1 is P. ovalifoliai were selected from tables 2 and 4 of Sadgrove et al. (2020) and combined. Compounds that were not detected in the samples of interest were removed as were compounds with trace quantities (<0.6% g g–1 of fresh leaves). In the final table (Supplementary Table S4), compounds were grouped according to presence in the species of interest. Note: a labelling error in Sadgrove et al. (2020) meant that the sample, R.L.Palsson 172, was mislabelled as OSF-3 and P. sp. Olney State Forest instead of P. sp. Hawkesbury from Bicentenary Road, Webbs Creek.
Taxonomy
In the species descriptions, the order of the characters follows the ‘Flora of Australia’ (Australian Biological Resources Study 2019). Description of geographic distribution uses Interim Biogeographic Regionalisation for Australia (IBRA) regions (see https://data.gov.au/data/dataset/interim-biogeographic-regionalisation-for-australia-ibra-version-7-regions), whereas in the citation of specimens, botanical or pastoral districts in use by the relevant herbaria are used to facilitate curation. In the citation of specimens, those examined physically are indicated !; those seen as images in herbarium databases are indicated an asterisk (*) following the specific herbarium code; and images viewed on JSTOR Global Plants (see https://plants.jstor.org/) are indicated by *JSTOR. Suggested conservation status is based on International Union for Conservation of Nature Red List Categories and Criteria (version 3.1; International Union for Conservation of Nature 2019).
Results
Phylogenetic analysis
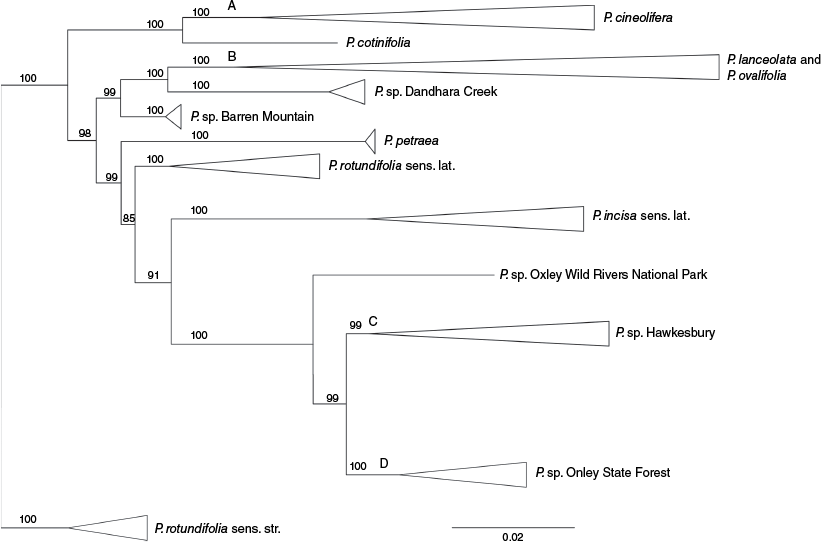
The phylogenetic analysis of SNP data recovered a tree with very strong bootstrap support across all major branches (Fig. 3, Supplementary Fig. S2). All samples of P. cineolifera formed a clade (Fig. 3, Clade A). Samples of P. lanceolata and P. ovalifolia constituted clade B, but neither was monophyletic (Fig. 3, Clade B). After filtering of the SNP data, the single sample remaining of P. sp. Oxley Wild Rivers National Park (Fig. 3) was strongly supported as sister to the clade composed of samples of P. sp. Hawkesbury (Fig. 3, Clade C) and samples of P. sp. Olney State Forest (Fig. 3, Clade D).
Phylogenetic tree from IQ-TREE of 130 samples of Prostanthera. Clade A shows all samples of P. cineolifera, Clade B shows all samples of P. ovalifolia and P. lanceolata, Clade C includes all samples of P. dyarubbin (P. sp. Hawkesbury) and Clade D includes all samples of P. milleri (P. sp. Olney State Forest). The taxon labelled here as P. sp. Oxley Wild Rivers National Park is described herein as P. faucicola Palsson & I.Telford. Numbers on branches are ‘ultrafast bootstrap support’. See Supplementary Fig. S2 for the phylogenetic tree showing all branches.
Morphological analysis
Morphological characters that displayed discontinuities across species and entities being tested are presented in Table 1.
| Characters | P. ovalifolia | P. cineolifera | P. dyarubbin | P. milleri | P. faucicola | |
|---|---|---|---|---|---|---|
| Abaxial leaf gland density (mm−2) | 30–60 | 25–70 | 15–30 | 25–45 | 60–100 | |
| Adaxial leaf gland density (mm−2) | 30–45 | 15–60 | 1–10 | 10–25 | 25–70 | |
| Floral prophyll persistence | Caducous at late bud development | Caducous at late bud development | Persistent | Persistent | Persistent | |
| Floral prophyll scar | Conspicuous | Conspicuous | – | – | – | |
| Floral prophyll length (mm) | 0.8–1.3 | 2.2–3.0 | 1.6–3.6 | 1.3–2.3 | 2.0–3.5 | |
| Floral prophyll width (mm) | ~0.1 | ~0.1 | 0.2–0.6 | 0.2–0.4 | 0.5–1.0 | |
| Calyx lobe displacement relative to corolla at anthesis | Reflexed, ~90° | Adpressed | Recurved | Recurved | Recurved | |
| Outer corolla glands | Moderately dense | Very sparse | Sparse | Sparse | Dense | |
| Anther colour | Cream | Cream to pink | Purple to deep purple | Mauve | Unknown | |
| Anther appendage (number of trichomes) | 8–12 | 12–50 | 15–35 | 6–12 | 3–6 | |
| Mericarp length (mm) | Not available | 1.4–1.8 | ~1.5 | ~1.8 | Not available |
P. ovalifolia data are based on specimens from Mount Stanley.
Phytochemical analysis
Prostanthera ovalifolia sampled from Mount Stanley contained no essential oils unique to the sample. The essential oils found in P. cineolifera but not in P. ovalifolia (OVA-1), P. sp. Hawkesbury (OSF-3) or P. sp. Olney State Forest (OSF-1 and OSF-2) were bicyclogermacrene, caryophyllene oxide and α-pinene. Prostanthera sp. Olney State Forest (OSF-1 and OSF-2) contained a large quantity of the essential oil 1,8-cineole (30.6–51.8% g g–1 of fresh leaf weight) and also E-verbenol and menth-3-en-8-ol, which are essential oils not found in P. ovalifolia sampled from Mount Stanley, P. cineolifera or P. sp. Hawkesbury. Prostanthera sp. Hawkesbury (OSF-3) contained small quantities of 1,8-cineole (5.5% g g–1 of fresh leaf weight) but also four oils not found in samples of P. ovalifolia, P. cineolifera or in P. sp. Olney State Forest, namely, γ-cadinene, 1-hexanol, 2-hexanol and (E)-2-hexenal (Supplementary Table S4).
Discussion
This study is the first to combine morphological, molecular and phytochemical data to resolve some of the relationships and taxonomy of the P. ovalifolia assemblage within Clade J in the phylogenetic study of Wilson et al. (2012), leading to the recognition of three new species previously hypothesised on the basis of morphological characteristics.
Prostanthera ovalifolia and P. lanceolata
Specimens determined by us a priori in the field or in the herbarium to be P. lanceolata or P. ovalifolia clustered in one group in analyses of morphological and molecular data (Palsson et al. 2024, fig. 3, 4, Group C), and formed a single clade in our phylogenetic analysis (Fig. 3, Clade B). Given that neither species was recovered as monophyletic (Supplementary Fig. S2), further study of these is warranted. To allow thorough testing of the limits of the species, collection of material from the type location of P. ovalifolia should be a priority. Only the original gathering by Robert Brown exists from this area. The type location is within Shoalwater Military Training Area that has presented obstacles to collection to date. We are again in the process of seeking permission to collect from the site.
Status of Prostanthera cineolifera
Previously, morphometric (Palsson et al. 2024, fig. 2a), and molecular and phytochemical analyses (Sadgrove et al. 2020, fig. 3; Palsson et al. 2024, fig. 2b, 3a, 4) supported the recognition of P. cineolifera. The monophyly, distinctive morphological and phytochemical characteristics reported here (Table 1, Fig. 3–5) corroborate the status as a distinct species.
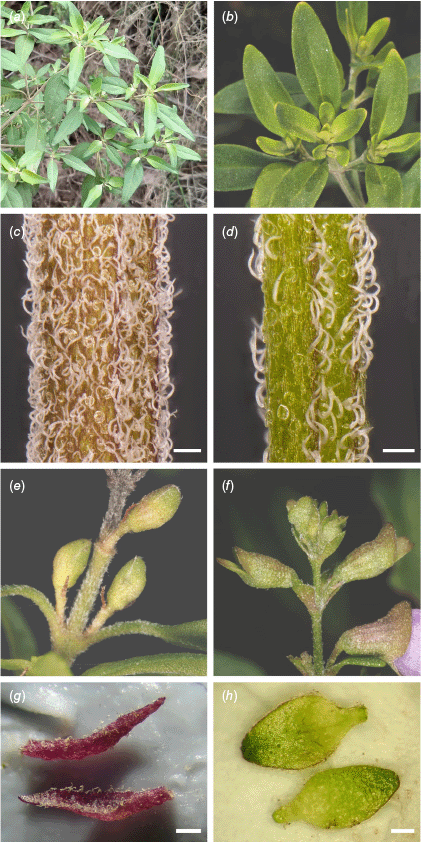
Comparisons of selected morphological characteristics in Prostanthera cineolifera (Column 1) and P. ovalifolia (Column 2). Leaves (a, b): (a) Sawpit Road, Cedar Creek NSW, unvouchered, (b) R.L. Palsson 195. Stem indumentum (c,d), scale bars: 0.25 mm: (c) R.L. Palsson 153, (d) R.L. Palsson 200. Flower buds (e, f): (e) R.L. Palsson 157, (f) R.L. Palsson 200. Floral bracts (g, h), scale bars: 0.5 mm: (g) R.L. Palsson 212, (h) R.L. Palsson 201. Photographs by R. L. Palsson (a, c, d, g, h) and J. J. Bruhl (b, e, f).
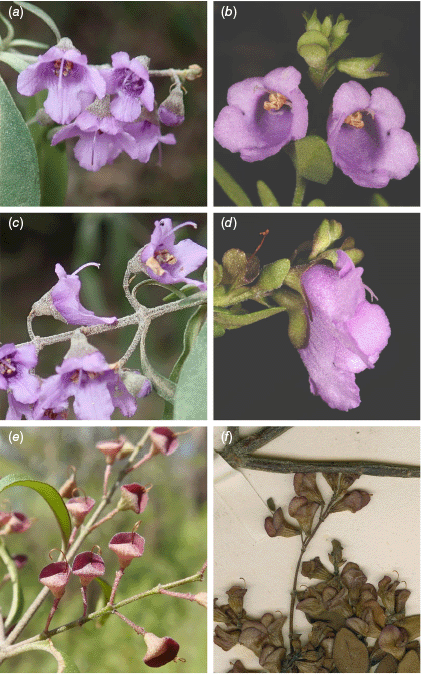
Comparison of selected morphological characteristics in Prostanthera cineolifera (Column 1) and P. ovalifolia (Column 2). Front view of inflorescence (a, b): (a) R.L. Palsson 138, (b) R.L. Palsson 200. Lateral view of a flower showing disposition of calyx lobes (c, d): (c) R.L. Palsson 138, (d) R.L. Palsson 201. Fruiting calyces (e, f): (e) R.L. Palsson 485 A, (f) R. Brown s.n. (BM001041052, lectotype). Image reproduced with kind permission from the Trustees, Natural History Museum. Image cropped from https://data.nhm.ac.uk/object/d1b31585-4779-46c1-9029-fd487ac5b63b/1748251284062, accessed 10 June 2025. Other photographs by R. L. Palsson (a, c, e) and J. J. Bruhl (b, d).
Putative new species
The three putative new species, P. sp. Hawkesbury, P. sp. Olney State Forest and P. sp. Oxley Wild Rivers National Park, that share relatively long, persistent hairy floral prophylls and recurved calyx lobes, clustered as three separate subgroups using morphometric data (Palsson et al. 2024, fig. 2a). These three entities have sufficient morphological differences (Table 1) to warrant recognition at the rank of species.
Recognition of the three entities is corroborated here by phylogenetic analysis (Fig. 3), with the reciprocally monophyletic P. sp. Hawkesbury and P. sp. Olney State Forest form the strongly supported sister clade to the single sample of P. sp. Oxley Wild Rivers National Park. The last is represented in the phylogeny by one of two samples submitted for analysis because only one survived filtering of the DArT seq data. Clearly, further searching in the vicinity of the remote location for additional plants and populations is desirable. Although P. sp. Olney State Forest was represented by multiple samples, these were from the only known location for the species. Other populations in the vicinity and further afield in similar habitats were searched for without success. Narrowly endemic species known from only one population are known in Prostanthera, e.g. P. conniana T.C.Wilson (Wilson et al. 2015), P. gilesii Althofer ex B.J.Conn & T.C.Wilson (Conn and Wilson 2015), P. makinsonii B.J.Conn & T.C.Wilson (Conn and Wilson 2015) and P. volucris R.P.O’Donnell (O’Donnell et al. 2023). We consider that further such species await circumscription, e.g. P. sp. Barren Mountain (L.M. Copeland 2256) NE Herbarium.
In Sadgrove et al. (2020, tables 2, 4) the specimen labelled OSF-3 was a mislabelling for a specimen here determined as P. sp. Hawkesbury. Given this amendment, the essential oil profiles of P. sp. Hawkesbury and P. sp. Olney State Forest differ and corroborate morphological (Table 1) and molecular evidence (Fig. 3) of two new species. In the field, P. sp. Olney State Forest can readily be distinguished from P. sp. Hawkesbury and other members of the study group by a strongly resinous smell on crushing the leaves and young stems. This resinous odour relates to the high levels of 1,8-cineole together with E-verbenol and menth-3-en-8-ol (Supplementary Table S4). There are examples of phytochemistry as a taxonomically informative source of data. In the Lamiaceae, Moshari-Nasirkandi et al. (2024) found that 20 Iranian Salvia L. species each contained a distinct phytochemical profile. Bruňáková et al. (2021) suggested that phytochemical profiling should be included in the integrated plant authentication system for Hypericum L. (Hypericaceae). Both Salvia and Hypericum contain pharmacologically important compounds. Phytochemical analysis is not always useful taxonomically (e.g. Yang et al. 2014; Jiawei et al. 2020) but in our study, despite the small sample sizes, phytochemistry appears to be taxonomically informative given that the results are corroborated by both morphological and DNA data.
Despite superficial similarity, P. sp. Oxley Wild Rivers National Park is morphologically and genetically distinct from P. sp. Hawkesbury and P. sp. Olney State Forest. The two known populations of P. sp. Oxley Wild Rivers in the Macleay Gorges subregion of the IBRA NSW North Coast bioregion (Department of Climate Change Energy the Environment and Water 2020) are ~12 km apart and separated geographically from both P. sp. Hawkesbury and P. sp. Olney State Forest by over 200 km. The flowering of P. sp. Oxley Wild Rivers National Park occurs later in the season (November) than that of P. sp. Hawkesbury and P. sp. Olney State Forest (September and October respectively).
Conclusions
Multiple lines of evidence including morphological, molecular, phylogenetic and phytochemical evidence support the recognition of two of the three putative new species, namely P. sp. Hawkesbury and P. sp. Olney State Forest. Morphological and phylogenetic evidence support the recognition of the third, P. sp. Oxley Wild Rivers National Park. For these three species, we propose taxonomic recognition at rank of species, namely, P. sp. Hawkesbury (B.J.Conn 2591) as P. dyarubbin Palsson & R.T.Mill., P. sp. Olney State Forest as P. milleri Palsson & J.J.Bruhl. and P. sp. Oxley Wild Rivers National Park (J.B. Williams s.n. NE 91044) as P. faucicola Palsson & I.Telford, described below.
The circumscription and distribution of P. cineolifera have been clarified and the name is here lectotypified. The species is currently known from the Hunter Valley and two historical gatherings in the Peel Valley. Vegetative and floral features allow practical recognition and identification of P. cineolifera, P. lanceolata and three new species mentioned below.
This contribution to the taxonomy of Prostanthera continues the recent integrated analytical approach to the delimitation of species in the genus (O’Donnell et al. 2021, 2023; Conn et al. 2021). This sound, evidence-based approach using multiple lines of corroborating data has replaced the intuitive approach that was based largely on morphological characteristics and very limited samples dating from Bentham’s (1870) broad concept of species.
Future work should include full testing of the taxonomic and geographical limits of P. ovalifolia but awaits securing new collections from and near the type location. Variation in morphological characteristics that appears to covary with geographic distribution suggests to us that some of the mapped records of P. ovalifolia are likely undescribed species and therefore merit testing (e.g. those from the Wollongong–Bodalla and from Nadgee–Mallacoota areas).
Taxonomy
Modification to the key to Prostanthera in ‘Flora of New South Wales’ (PlantNET, Royal Botanic Gardens and Domain Trust, Sydney, NSW, Australia, see https://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=gn&name=Prostanthera):
| 15 | Calyx lobes adpressed to the corolla in developing bud and fully opened flower; branchlet indumentum of mostly sinuate hairs...Prostanthera cineolifera |
| 15* | Calyx lobes reflexed away from the corolla in developing bud and fully opened flower; branchlet indumentum of curved hairs...Prostanthera lanceolata |
Further modification beyond the amendment presented here is beyond the scope of our study.
Prostanthera cineolifera R.T.Baker & H.G.Sm., J. Proc. Roy. Soc. New South Wales 46(1): 105, t. 1 (1912)
Type citation: ‘Singleton (Howitz). Siberia, Broke, (C. H. Cheesebrough [sic]). Cedar Creek, Millfield (C. F. Laseron)’. Type: New South Wales. Siberia, Broke, Mar. 1912, C.H. Cheeseborough [sic] s.n. (lecto, here designated: NSW 134514! Residual syn: New South Wales. Singleton, 27 Oct. 1908, Mr Howitz s.n. (BRI AQ0522596*); New South Wales. Cedar Creek, Millfield, s. dat., C.F. Laseron s.n. (no specimen located, n.v.)).
Shrub, single or multi-stemmed to 1–4(–6) m tall, 1–3 m wide. Branchlets quadrangular, grooved below leaf insertions, covered with scattered glandular trichomes and densely covered with simple non-glandular hairs, each hair usually sinuate (sometimes with a simple curl), mostly ±antrorse, 0.2–0.4 mm long, white. Juvenile leaves (including leaves on water shoots) larger than adult leaves, margins often dentate with up to six small teeth per leaf, one known population retains a few dentate teeth on some mature plants. Leaves mid-green above, paler below; petiole 3–8 mm long; lamina ovate to lanceolate, 14–30 mm long (to 48 mm on juvenile leaves), 5–14 mm wide (to 16 mm on juvenile leaves), length to width ratio 2.5–4.0, length of maximum width from base to total lamina length ratio 0.3–0.6, base attenuate to cuneate, margin entire, apex obtuse, non-glandular hairs absent except for prominent midrib on abaxial surface (there densely distributed, ±antrorse, sinuate, 0.1–0.2 mm long), in depressed midrib on adaxial surface (depressed for ~2/3 length, dense to sparse antrorse non-glandular hairs to 0.25 mm long) and usually proximal third of margin (antrorse simple non-glandular hairs up to 0.25 mm long); abaxial surface moderately to densely glandular (15–60 mm−2), secondary veins often visible; adaxial surface densely glandular (25–70 mm−2), secondary veins indistinct. Inflorescences: thryses to ~13 cm long with dichasia usually reduced to single flowers. Floral bracts early caducous, narrowly naviculate, ~2.5 mm long, ~0.75 mm wide; apex obtuse; abaxially sparsely to densely glandular; adaxially non-glandular hairs mostly absent but margin ciliate with non-glandular hairs to 0.15 mm long. Pedicel 1.25–4.75 mm long, moderately covered with non-glandular hairs, antrorse, ~0.1 mm long, glandular trichomes moderately common. Floral prophylls caducous, a1 axis to anthopodium ratio 1.0–3.0, opposite or subopposite, narrowly naviculate, 2.2–3.0 mm long, ~0.1 mm wide, margin entire with curled non-glandular hairs to 0.25 mm long; abaxial surface with scattered to dense glands; glandular trichomes rare on adaxial surface; venation not visible in fresh specimens. Calyx green to maroon, bilobed, both lobes adpressed to the corolla; outer surface with a moderate to dense cover of glandular trichomes, with scattered non-glandular hairs becoming ±glabrescent with age, non-glandular hairs sometimes remaining on abaxial half of calyx tube only; inner surface without non-glandular hairs but non-glandular hairs densely distributed towards margin of lobes, up to 0.2 mm long; margin densely ciliate, with many white non-glandular hairs up to 0.25 mm long; tube 2.0–2.5 mm long, sometimes ridged, particularly in dried specimens; abaxial lobe elliptic, 1.0–2.0 mm long, 1.5–3.0(–4.0) mm wide at base; apex usually rounded; adaxial lobe transversely elliptical, 0.5–1.0(–1.7) mm long, 2.5–4.0(–6.0) mm wide at base; apex usually rounded, adaxial lobe length to abaxial lobe length ratio 0.3–0.8. Corolla pink–mauve; adaxial corolla tube length 3.5–6 mm long; outer surface moderately hairy distally (antrorse non-glandular hairs up to 0.2 mm long); margin usually without non-glandular hairs, sometimes scattered minute non-glandular hairs, glandular trichomes occasional; inner surface of tube and lobes with non-glandular hairs or with scattered very short antrorse non-glandular hairs to 0.1 mm, glandular trichomes occasional; abaxial median lobe broadly elliptic to circular, 1.5–4 mm long, 1.3–3.5 mm wide, 0.8–3.25 mm wide below distal lobing, length to width ratio 0.9–1.3; apex rounded; margin slightly irregular and slightly undulate, occasionally a shallow sinus up to 0.25 mm deep; lateral lobes very broadly ovate to ovate, 1.5–2.75 mm long, 1.0–3.25 mm wide, length to width ratio 0.9–1.5; apex rounded; margin regular; adaxial median lobe-pair broadly depressed ovate, 0.9–2.5 mm long, 1–2.5 mm wide, length to width ratio 0.8–1.1; margin regular. Stamens inserted 0.7–1.6 mm above corolla base; abaxial stamens with filament 1.2–2.4 mm long; adaxial stamens with filament 1.2–2.0 mm long; anthers cream to pink, locules 0.6–0.9 mm long; connective extended to form 1 or 2 basal appendages (one appendage usually shorter than the other), extending beyond base of anther and terminating with 12–~50 translucent conical hairs; length beyond the anther of appendage including hairs up to ~0.25 mm; hairs to 0.1 mm. Pistil 4–7 mm long; ovary cylindrical obovoid, 1.1–1.3 mm long, diameter at base 0.7–0.9 mm, lobes 0.5–0.6 mm long; style 3.25–5.75 mm long; stigmatic lobes 0.4–0.5 mm long. Fruiting calyx only slightly enlarged, adaxial lobe surrounds the folded-over abaxial lobe that conceals developing mericarps. Mericarps maturing to off-white or cream to charcoal (often mottled), 1.4–1.8 mm long, wrinkled and minutely papillose, distally 0.6–0.9 mm extended beyond base of style.
The main essential oils in P. cineolifera are 1,8-cineole, prostantherol and p-cymene. Bicyclogermacrene, caryophyllene oxide and α-pinene are essential oils found in P. cineolifera but not in P. ovalifolia sens. lat. (from Mount Stanley), P. dyarubbin sp. nov. or P. milleri sp. nov. (tables 2, 4 in Sadgrove et al. 2020; Supplementary Table S4). Aromatic with a scent of 1,8-cineole when crushed.
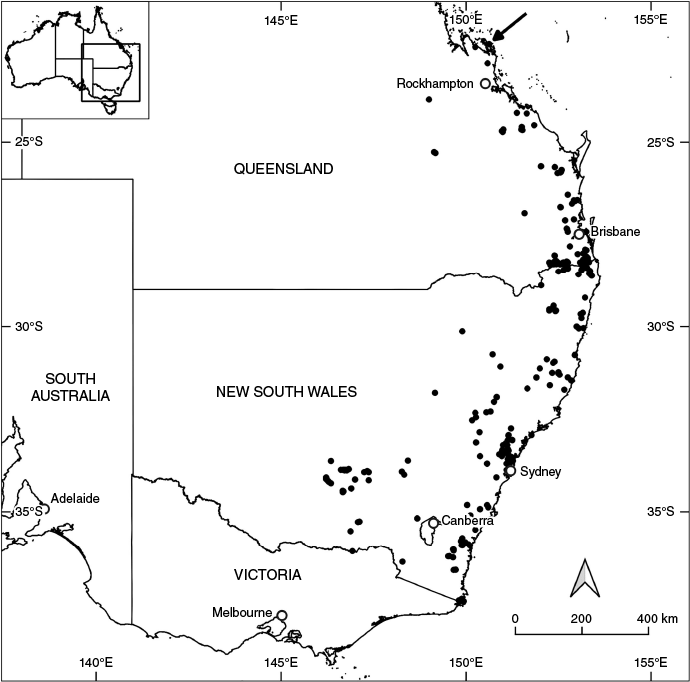
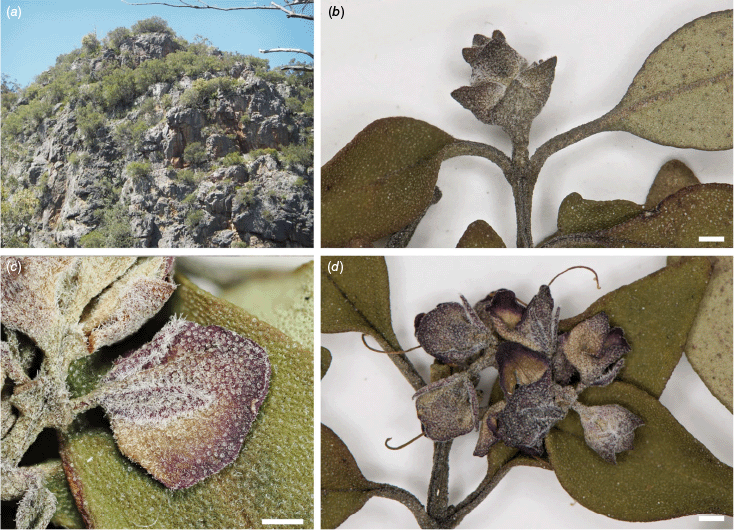
Extant populations of P. cineolifera occur in the Hunter, Kerrabee and Yengo subregions of the Sydney Basin bioregion on the western and southern sides of the Hunter Valley from Wingen Maid north-west of Scone south-east to Bellbird and as far west as Goulburn River National Park. There are historical specimens in New South Wales from Manilla and Upper Moore Creek in the Nandewar bioregion (Fig. 6).
The species grows in skeletal sandy soil at altitudes of up to 630 m on sandstones and conglomerates of the Narrabeen Group (Colquhoun et al. 2022), often associated with ridges, cliff lines and extending down gullies. At the western limit of the range, P. cineolifera is confined to gully bottoms. The vegetation consists of eucalypt open forest or woodland with dominant species Angophora costata, Eucalyptus deanei, E. fibrosa, E. punctata or E. sparsifolia.
Our extensive fieldwork (J. J. Bruhl, I. R. H. Telford, R. L. Andrew and especially R. L. Palsson) has primarily indicated that of the populations known to us, P. cineolifera is currently protected in Pokolbin State Forest (Bees Nest Ridge and Yellow Rock Road, and parts of Sawpit Road and Broken Back Trail), Singleton Military Area, Wingen Maid Nature Reserve, Wollemi National Park (Cousins Creek), Goulburn River National Park and in the 635-ha Cedar Creek biodiversity offset, now Cedar Creek Nature Reserve (S. A. J. Bell, unpubl. data, 2017).
Only the small population at Wallaby Rocks appears to be threatened. In 2011, Driscoll collected P. cineolifera (NSW 891464) from ‘top of Wallaby Rocks’. Our two searches have not yielded any plants on top of Wallaby Rocks. Currently, the population is confined to south-western cliff-lines; ‘pushed’ to cliff lines possibly by fire or grazing pressure. Grazing pressure is likely an important factor, as several heavily browsed plants were found on lower parts of cliff-lines. This possibly threatened population is genetically similar to the larger protected Wingen Maid population (Palsson et al. 2024, fig. 3b) that unfortunately does have the lowest genetic diversity of the P. cineolifera populations (Palsson et al. 2024, table 4). We recommend that effort be applied to feral goat control at Wallaby Rocks to protect this small, threatened population.
Frequent fire is a potential threat to P. cineolifera, as this species is considered fire sensitive (Department of the Environment 2008). The largest known populations are in the Pokolbin area on Bees Nest Ridge and Yellow Rock Road, an area that was severely affected by the 2019–20 fires. Fortunately, most populations occurred in areas in which the canopy was unburnt. Six months post fire, there was evidence of large burnt stands of P. cineolifera, some resprouting from trunks and seedling recruitment (Luke Foster, Department of Planning, Industry and Environment, pers. comm., 2021).
Prostanthera cineolifera is currently listed as Vulnerable under both Commonwealth and NSW legislation (Office of Environment and Heritage 2019). As of 2018, the number of individuals was <10,000 with Extent of Occurrence (EOO) of 14,000 km2 and Area of Occupancy (AOO) of 44 km2 (R. L. Palsson, R. L. Andrew, I. R. H. Telford and J. J. Bruhl, unpubl. data). Since 2018, several more populations of >1000 individuals and two more locations have been discovered along with recruitment observed by Foster. Also, the Broken Back Range populations were burnt in the summer of 2019–20 and 18 months later mass recruitment was observed by R. L. Palsson et al. The status of Vulnerable may still be met for P. cineolifera but discovery of yet more populations in little surveyed areas such as Wollemi National Park would likely enable the status of ‘Least Concern’ (International Union for Conservation of Nature 2019) to be met for this species.
Three geographically separate gatherings are cited in the protologue (Baker and Smith 1912). Only two specimens of these three syntypes could be located; i.e. C.H. Cheeseborough [sic] s.n. (NSW 134514) and Mr Howitz s.n. (BRI AQ0522596). The NSW specimen was selected as the lectotype as this is a fruiting specimen that was seen by R. T. Baker. The sheet bears the original ‘Technological Museum, Sydney’ label with R. T. Baker’s name (Fig. 7). By comparison, the syntype of P. cineolifera collected by Howitz is a sterile specimen that does have a ‘Technological Museum, Sydney’ label but does not bear Baker’s name, [handwriting] and therefore we treat the Howitz sheet as a residual syntype. There is a specimen of P. cineolifera at the Queensland Herbarium (BRI AQ0336458) that was collected by C.H. Cheesebrough [sic] from the type location that bears two separate annotations in addition to the original label from the Technological Museum, Sydney. One, in red printing has ‘PART OF TYPE COLLECTION’ and the other a Queensland Herbarium specimen note ‘syntype Prostanthera cineolifera R.T.Baker & H.B.Sm. J. Proc. Roy. Soc. NSW 46(1): 105 (1912).’ However, the date of collection on the original label is ‘June 1915’, 3 years after the publication of the species. Therefore, we consider this material a topotype of no nomenclatural status and not part of the original material for this name.
The spelling of the collector of the specimens from Siberia, Broke, is problematic. Both ‘Cheesebrough’ and ‘Cheeseborough’ are given on specimen labels and the specimen citation in Baker and Smith (1912). Neither of these spellings appears to have been recorded as a family name in Australia. By contrast, the first mention of the collector’s name in Baker and Smith (1912, p. 104) was ‘In March of this year a quantity of this plant was forwarded to the Museum, by Mr C. H. Cheesbrough of Siberia, Broke, near Singleton…’. So, ‘Cheesbrough’ appears to be the correct spelling for the collector.
Prostanthera cineolifera is distinguished from other species of Prostanthera in the Hunter and Hawkesbury River catchments by a loose open inflorescence, a stem indumentum of sinuate ± antrorse non-glandular hairs and both calyx lobes are firmly adpressed to the corolla in both the developing bud and fully expanded flower.
Prostanthera cineolifera has been confused with P. dyarubbin, P. milleri, P. lanceolata and P. ovalifolia. Prostanthera cineolifera can be distinguished from P. lanceolata and P. ovalifolia by the calyx lobes of the latter two species being ±perpendicular to the corolla in both the developing bud and fully expanded corolla. Prostanthera cineolifera can be distinguished from P. dyarubbin and P. milleri that both have more compact inflorescences, persistent hairy floral prophylls and reflexed calyx lobes. In both P. dyarubbin and P. milleri, the ±shield-shaped floral bracts are more often observed, whereas in P. cineolifera, the lanceolate and naviculate floral bracts are only observed on early buds. Vegetative material of P. cineolifera has been confused with P. discolor; both species have similar stem indumenta but the entire leaf margins of P. discolor are ornamented with minute teeth (visible using a hand lens), whereas the proximal third of the leaf margins of P. cineolifera usually have antrorse non-glandular hairs.
NEW SOUTH WALES: North Western Slopes: Manilla, Oct. 1912, B.J. Moss s.n., (NSW 232116!); Upper Moore Creek, Tamworth, 1933, E. Wyndham s.n. (NSW 134411!); Central Western Slopes: Scone, 31 Aug. 1907, R.H. Cambage s.n. (NSW 227969!); Wingen Maid Nature Reserve, north of Salisbury Trig, 09 Oct. 1997, J.R. Hosking 1498 (CANB, MEL, NE!, NSW); Goulburn River National Park, Morrisons Flat Trail, 11 Oct. 2022, R.L. Palsson 539, J.L. Griffiths, P. Bussell & E. Gill (NE!, NSW!); Wallaby Rocks, Wybong, 8 Sep. 2012, N.J. Sadgrove 291 (CANB, MEL, NE!, NSW); Above Cousins Creek, NW Wollemi National Park, 22 Aug. 1988, S.A.J. Bell s.n. (NSW 424206!). North Coast: Bellbird, Limestone Creek, 7 Oct. 2016, L.M.C. Foster s.n. (NE 106633!); Pokolbin State Forest, Bees Nest Ridge Rd, 10 Oct. 2017, R.L. Palsson 155, R.L. Andrew, J.J. Bruhl & I.R. Telford (NE!); Pokolbin State Forest, Broken Back Trail, 5 Oct. 2017, R.L. Palsson 134 & M.R. Donald, (NE!); Pokolbin State Forest, Yellow Rock Road, 26 Oct. 2021, R.L. Palsson 483, L.M.C. Foster, E. Gill, (NE!, NSW!); Pokolbin State Forest, Sawpit Rd, 1.8 km from junction with Cedar Creek Rd, 6 Oct. 2017, R.L. Palsson 139 & M.R. Donald (NE!, NSW!).
Prostanthera dyarubbin Palsson & R.T.Mill., sp. nov.
Type: New South Wales. Central Coast: Bicentenary Rd, 3.2 km SW of Wisemans Ferry, 1 Sep. 2017, R.L. Palsson 100 & M.R. Donald (holo: NSW!; iso: AD!, BRI!, CANB!, K!, MEL!, MO!, NE 106387!).
Prostanthera sp. 10 (Hawkesbury, B.J.Conn 2591), J.D.Briggs & J.H.Leigh, Rare or Threatened Austral. Pl. 81 (1996).
Prostanthera sp. Hawkesbury (B.J.Conn 2591) NSW Herbarium, CHAH, Australian Plant Census, https://id.biodiversity.org.au/tree/51689388/51241805 [accessed 10 Oct 2024].
Prostanthera dyarubbin is characterised by persistent floral prophylls 1.6–3.6 mm long, with non-glandular hairs, recurved calyx lobes, a compact inflorescence and no scent of 1,8-cineole when crushed, and differs from P. cineolifera and P. ovalifolia in the loose open inflorescence, adpressed calyx lobes, caducous floral prophylls and strong 1,8-cineole scent of these species.
Shrub single- to multi-stemmed to (1–)4–6 m tall, up to 2 m wide. Branchlets quadrangular, distinctly 4-ridged, covered with scattered glandular trichomes ~0.1 mm in diameter and densely covered with antrorse non-glandular hairs in rows on the leaf decurrencies, non-glandular hairs 0.15–0.3 mm long, white. Juvenile leaves larger than adult leaves, margins often dentate with up to six small teeth per leaf, some populations retain few to many dentate leaves on mature plants. Leaves dark green above, sometimes tinged with purple, paler below; petiole 2–10 mm long; lamina lanceolate, 8–30 mm long, 4–12 mm wide, length to width ratio 2.0–5.0, length of maximum width from base to total lamina length ratio 0.3–0.4, base attenuate to cuneate, margin entire, apex obtuse, non-glandular hairs absent except for prominent midrib on abaxial surface (non-glandular hairs distributed densely, ±antrorse, 0.05–0.2 mm long) and usually proximal third of margin (antrorse simple non-glandular hairs up to 0.2 mm long); abaxial surface moderately to densely glandular (15–30 mm−2); adaxial surface sparsely glandular (1–10 mm−2), secondary veins indistinct. Inflorescences ±narrow, compact thryses to ~6 cm long with dichasia usually reduced to single flowers. Floral bracts caducous, ±obtrullate and concave, vary in shape along axis of inflorescence, central floral bracts 2.5–5.0 mm long, 2.0–3.5 mm wide; abaxially scattered ±antrorse non-glandular hairs 0.05–0.2 mm long, sparsely to densely glandular; adaxially non-glandular hairs absent, occasional glands; margins ciliate with non-glandular hairs to 0.2 mm long. Pedicel 0.5–2.7 mm long, scattered non-glandular hairs ~0.1 mm long, moderately glandular. Floral prophylls persistent pink to maroon, a1 axis to anthopodium ratio 3–4 (in some populations floral prophylls inserted immediately below calyx), opposite, narrowly elliptic, 1.6–3.6 mm long, 0.2–0.6 mm wide; margin and abaxial surface moderately to densely covered by non-glandular hairs to 0.3 mm long; adaxial surface with non-glandular trichomes absent, with scattered glands. Calyx usually maroon, bilobed, both lobes recurved; outer surface hairy mainly on the tube (non-glandular hairs to 0.1 mm), densely glandular; inner surface proximally without non-glandular hairs, lobes variable from ±without non-glandular hairs to densely hairy towards margin of lobes; margin sparsely to densely ciliate, with white non-glandular hairs up to 0.1 mm long; tube 1.5–2.5 mm long; abaxial lobe broadly ovate, 1.0–4.0 mm long, 1.5–4.0 mm wide; adaxial lobe broadly depressed ovate, 0.7–2.3 mm long, 2.0–4.5 mm wide; apex usually rounded; fruiting calyx somewhat enlarged in fruit. Corolla mauve–purple; adaxial corolla tube length 3.5–5 mm long; outer surface sparsely to moderately hairy, antrorse non-glandular hairs up to 0.2 mm long, scattered glands; margin usually densely ciliate, non-glandular hairs up to 0.1 mm long; inner surface usually with scattered antrorse non-glandular hairs up to 0.15 mm, particularly on the lobes, scattered glands; abaxial median lobe broadly oblong to ovate, 1.4–3.3 mm long, 1.4–3.5 mm wide at widest; lateral lobes ovate, 1.7–3.3 mm long, 1.2–3.5 mm wide, apex rounded to ±acute; adaxial median lobe-pair broadly ovate, each lobe 1.2–2.75 mm long, 0.75–1.5 mm wide. Stamens with filaments 0.8–1.3 mm long; anthers purple to deep purple; locules 0.75–1.0 mm long; connective extended to form 1 or 2 basal appendages (one appendage usually shorter than the other), extending beyond base of anther and terminating with 15–35 translucent purple conical hairs; length beyond the anther of appendage including hairs 0.1–0.3 mm; hairs to 0.1 mm. Pistil 4.5–6 mm long; ovary cylindrical obovoid, 0.8–1.0 mm long, diameter at base 0.6–0.8 mm, lobes 0.4–0.5 mm long; style 3.5–4.5 mm long; stigmatic lobes to 0.5 mm long. Mericarps maturing to dark brown, ~1.5 mm long, wrinkled and minutely papillose, distally ~0.8 mm extended beyond base of style.
Although P. dyarubbin contains small quantities of 1,8-cineole and prostantherol, this species also contains four oils not found in P. ovalifolia sens. lat., P. cineolifera or P. milleri, namely, cadilene, 1-hexanol, 2-hexanol and (E)-2-hexenal (tables 2, 4 in Sadgrove et al. 2020; Supplementary Table S4).
Known populations of P. dyarubbin are in the Pittwater and Yengo subregions of the Sydney Basin bioregion, in the environs of the lower Hawkesbury River from Mogo Creek southwards to near Hornsby and from near Webbs Creek eastwards to Ku-ring-gai Chase National Park (Fig. 6).
The species grows on dry ridges, rocky slopes, along cliff lines and in gullies at altitudes up to 150 m on sandstones of the Narrabeen Group and Hawkesbury Sandstone (Colquhoun et al. 2022). The vegetation is eucalypt shrubby open forest with Eucalyptus punctata, Angophora bakeri, A. costata and Corymbia eximia recorded as dominants. Associated species include Allocasuarina littoralis, Astrotricha longifolia, Grevillea buxifolia, G. sericea and Actinotus helianthi.
The species is conserved in Dharug National Park, Ku-ring-gai Chase National Park, Parr State Conservation Area, Popran National Park, Wisemans Ferry Historic Site and Yengo National Park. There are undoubtedly many more populations of P. dyarubbin that remain undiscovered within the relatively unexplored areas of the distribution. We recommend a status of ‘Least Concern’ (International Union for Conservation of Nature 2019).
The species epithet is the traditional Dharug (Darug/Daruk) name for the Lower Hawkesbury area. There are several Indigenous spellings for the name of the river (Dyarubbin, Dyarrubin, Deerubbin, Deerubbun). We have selected Dyarubbin, the most commonly used spelling that is also used by the Darug Custodian Aboriginal Corporation (see https://www.dyarubbin.com/).
Prostanthera dyarubbin is morphologically similar to P. milleri Palsson & J.J.Bruhl and P. faucicola Palsson & I.Telford, sharing relatively large persistent hairy floral prophylls, recurved calyx lobes and a compact inflorescence. The known distribution of P. dyarubbin is remote from the known distribution of P. faucicola in the Macleay Gorges (Fig. 6). Prostanthera dyarubbin differs from P. milleri in the lack of the scent of 1,8-cineole in freshly crushed leaves of the former. The short anther appendages of P. dyarubbin have 15–35 conical hairs v. 6–12 in P. milleri. The leaves of P. dyarubbin tend to be tougher and narrower that the leaves of P. milleri (length to width ratio 2.0–5.0 for P. dyarubbin v. 2.0–3.4 in P. milleri). The anthers of P. dyarubbin are purple to deep purple v. mauve for P. milleri.
A horticultural selection of the species was brought into cultivation by Henry Parry, Floralands Nursery, Maghoney Creek, New South Wales, under the name Prostanthera ‘Violet Beauty’. The selection ‘differs from the typical form in the intensity of the leaf and calyx colouration that is deeper than the normal colour range during flowering’ (Miller 1988).
NEW SOUTH WALES: Central Coast: Eastern Dyarrubin, 12 June 1996, A. Bofeldt s.n. (NSW 888576!); Wisemans Ferry, ~15 km N towards St. Albans, 4 Oct. 1984, T.A. James 568 (NSW!); Wisemans Ferry, 0.1 km S on Old Northern Road, 22 Sep. 1987, B.J. Conn 2591 (NSW!); Kuring-gai Chase, Coal and Candle Creek Road, 12 Oct 1964, O.D. Evans s.n. (NSW 71554!); Bar Point, ~3 km along Pacific Motorway from N end of Hawkesbury River bridge, 5 Oct. 2017, R.L. Palsson 133 & M.R. Donald (NE!, NSW!); Hawkesbury River, Oct. 1916, H.M.R. Rupp s.n. (NSW 228302!); Brisbane Water National Park, Mullet Creek, Nov. 1890 L. Stephenson s.n. (NSW 134072!).
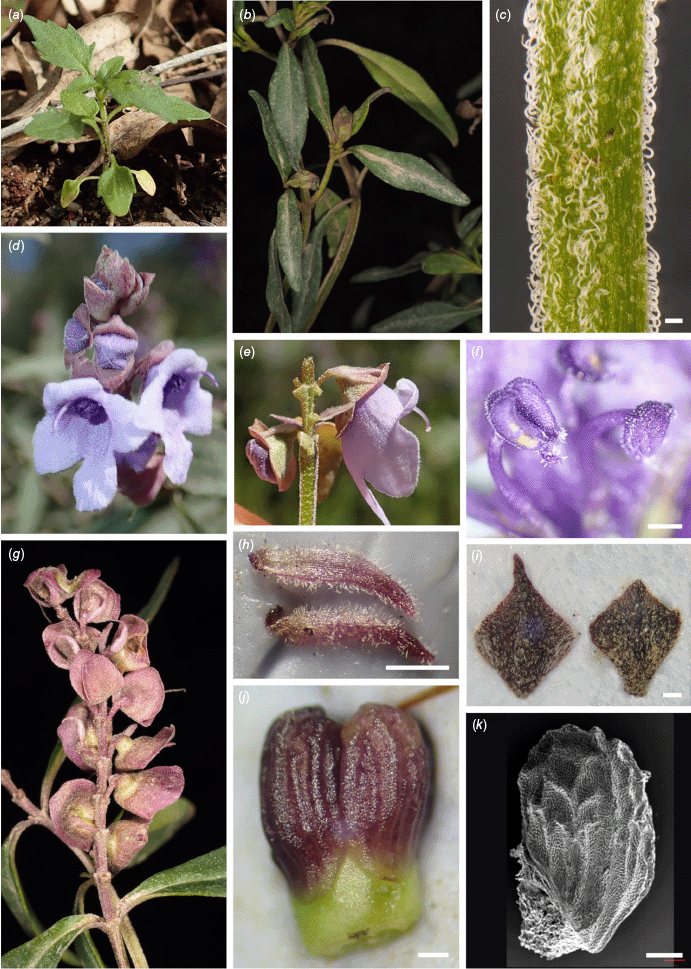
Prostanthera dyarubbin (P. sp. Hawkesbury). (a) Seedling, Upper Mangrove, not vouchered. (b) Leaves R.L. Palsson 168. (c) Stem indumentum, scale bar: 0.25 mm, R.L. Palsson 206 a. (d) Inflorescence, including buds with floral bracts, R.L. Palsson 100. (e) Lateral view of a flower showing disposition of calyx lobes and persistent hairy floral prophylls, R.L. Palsson 536 a. (f) Anther with short anther appendage, scale bar: 0.5 mm, R.L. Palsson 100. (g) Fruiting calyces, R.L. Palsson 169. (h) Floral prophylls, scale bar: 0.5 mm, R.L. Palsson 132. (i) Floral bracts, a representative sample, scale bar: 0.5 mm, R.L. Palsson 132. (j) Developing mericarps, scale bar: 0.25 mm, R.L. Palsson 173. (k) Scanning electron micrograph of mericarp, scale bar: 0.25 mm, R.L. Palsson 175. Photographs by R. L. Palsson (a, c, d–f, h–k) and J. J. Bruhl (b, g).
Prostanthera faucicola Palsson & I.Telford, sp. nov.
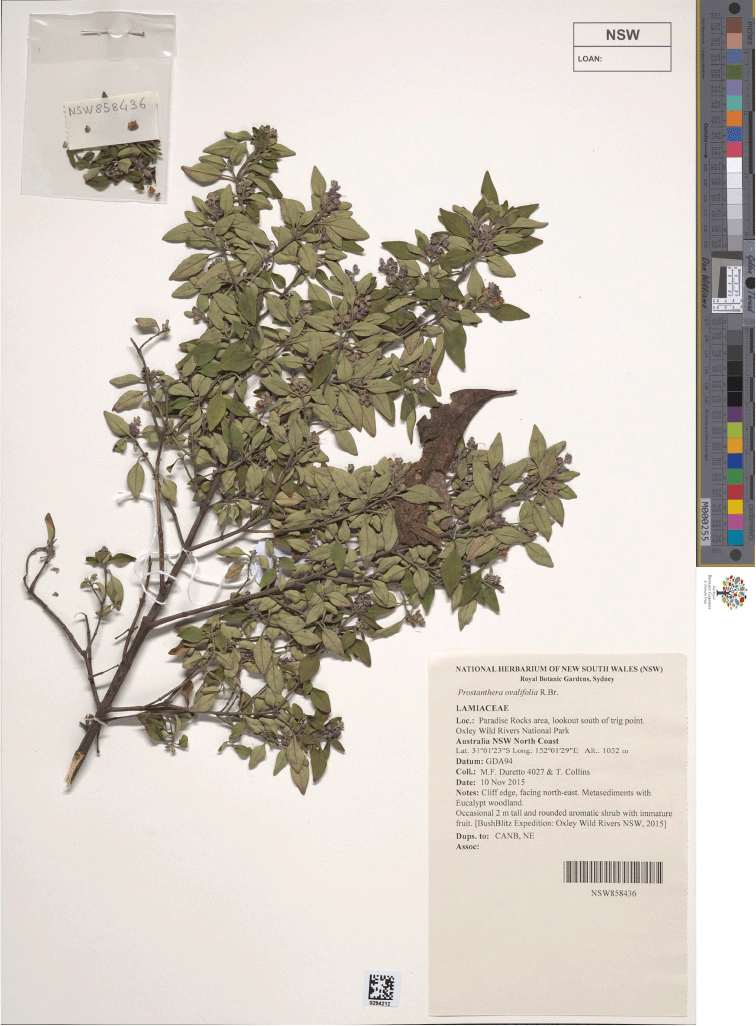
Type: New South Wales. Northern Tablelands: Paradise Rocks area, lookout S of trig point, Oxley Wild River National Park, 10 Nov. 2015, M.F. Duretto 4027 & T.L. Collins (holo: NSW 858436*; iso: CANB!, NE 107073!).
Prostranthera faucicola is morphologically similar to P. dyarubbin and P. milleri but differs in the floral prophylls usually being asymmetrical and wider; short anther appendages in P. faucicola with 3–6 conical hairs v. 15–35 in P. dyarubbin and 6–12 in P. milleri (Table 1).
Shrub to 2 m tall. Branchlets quadrangular, with scattered glandular trichomes and densely distributed antrorse non-glandular hairs in rows on the leaf decurrencies, non-glandular hairs ~0.15 mm long, white. Juvenile leaves not seen, toothed leaves not noted on mature plants. Leaves discolourous, darker above; petiole 2.5–8.0 mm long; lamina ovate to lanceolate, 12–27 mm long (longer leaves appear to be on a water shoot), 5–10 mm wide (wider leaves appear to be on a water shoot), length to width ratio 2.1–3.1, length of maximum width from base to total lamina length ratio 0.3–0.5, base cuneate, sometimes attenuate, margin entire, apex usually obtuse, mostly without non-glandular hairs except for occasional non-glandular hairs on prominent midrib on abaxial surface, in depressed midrib on adaxial surface(depressed for ~2/3 length with sparse antrorse non-glandular hairs to 0.15 mm long) and for up to the proximal 2/3 of margin (antrorse simple non-glandular hairs up to 0.15 mm long); abaxial surface densely glandular (60–100 mm−2), secondary veins sometimes visible; adaxial surface moderately to densely glandular (25–70 mm−2). Inflorescences compact thryses to ~1.5 cm long with dichasia usually reduced to single flowers. Floral bracts caducous, broadly scutiform and concave, apex rounded, vary in shape along axis of inflorescence, central floral bracts 2.75–4.0 mm long, 2.75–3.25 mm wide, size depends on position in inflorescence and maturity; abaxially scattered non-glandular hairs and scattered to densely glandular, glandular trichomes and non-glandular hairs becoming sparse towards distal margin, non-glandular hairs to 0.15 mm long; adaxially without non-glandular hairs; margin ciliate, non-glandular hairs to 0.05 mm long on distal margin, up to 0.2 mm elsewhere on margin. Pedicel 1.5–1.75 mm long. Floral prophylls persistent, opposite, usually inserted at base of calyx, narrowly elliptic, 2.0–3.5 mm long, 0.5–1.0 mm wide, length to width ratio 3–5, not always symmetrical; abaxial surface non-glandular hairy with non-glandular hairs to 0.2 mm long, moderately densely glandular; adaxial surface without non-glandular hairs; margin ciliate to densely ciliate (non-glandular hairs up to 0.25 mm), apex ±without non-glandular hairs. Calyx colour not recorded, bilobed, both lobes recurved, outer surface moderately to densely glandular, scattered non-glandular hairs particularly on the tube, non-glandular hairs up to 0.1 mm long; inner calyx tube and inner abaxial calyx lobe without non-glandular hairs; inner adaxial calyx lobe moderately to densely hairy, non-glandular hairs up to 0.05 mm long; margin ciliate, white non-glandular hairs, 0.02–0.05 mm long; tube ~1.5 mm long, sometimes ridged in dried specimens; abaxial lobe elliptic, ~2.4 mm long, ~2.2–2.7 mm wide at base, apex rounded; adaxial lobe oblate, ~1.2 mm long, 1.9–2.5 mm wide at base, apex rounded; adaxial lobe length to abaxial lobe length ratio ~0.5. Corolla colour not recorded; adaxial corolla tube length ~2.5 mm long; outer surface moderately hairy beyond calyx, ±antrorse non-glandular hairs up to 0.1 mm long, moderately densely glandular on lobes; corolla margin, particularly on adaxial lobes, with white non-glandular hairs 0.02–0.05 mm long; inner surface tube and abaxial lobe without non-glandular hairs, adaxial lobe hairy to densely hairy, non-glandular hairs to 0.05 mm long; abaxial median lobe broadly elliptic, 1.6–1.9 mm long, 1.2–1.8 mm wide, 1.2–1.5 mm wide below distal lobing when present, length to width ratio 1.0–1.4; apex rounded; lateral lobes usually shallowly triangular to deltate, 1.5–2.0 mm long, 1.5–2.2 mm wide, length to width ratio 0.8–1.3; apex rounded; adaxial median lobe-pair depressed ovate, sometimes distal lobing, ~1.0 mm long, 1.2–1.8 mm wide, length to width ratio 0.5–0.8. Stamens inserted 0.5–0.8 mm above corolla base; filaments 0.6–1.0 mm long; anther locules ~0.75 mm long; anthers, colour not seen; connective extended to form 1 or 2 basal appendages (one appendage usually shorter than the other), extending beyond base of anther and terminating in 3–6 translucent conical hairs; length beyond the anther of appendage including hairs ~0.4 mm, hairs to 0.05 mm. Pistil ~6.0 mm long; ovary cylindrical obovoid, 0.6–0.8 mm long, diameter at base 0.5–0.6 mm, lobes 0.3–0.5 mm long; style ~5.0 mm long; stigmatic lobes ~0.6 mm long. Fruiting calyx slightly accrescent. Mericarps, mature mericarps not seen.
Prostanthera faucicola occurs in the Macleay Gorges subregion of the New South Wales North Coast bioregion where the species is known from two populations in Oxley Wild Rivers National Park, east of Walcha, New South Wales (Fig. 6).
The species grows in shallow skeletal soil on rock outcrops and cliff tops of gorge rims at altitudes of 980–1055 m. Vegetation in which the species occurs is recorded as Eucalyptus woodland and heath with Leptospermum variable and Leionema beckleri.
On the basis of three specimens known from two collection sites of unknown population size, we suggest that a ‘Data Deficient’ status is appropriate under the International Union for Conservation of Nature (2019).
The specific epithet is from the Latin fauces (throat or gorge) and -cola (dwelling) referring to the habitat of the species.
The informal tag name Prostanthera sp. Oxley Wild Rivers National Park (J.B. Williams s.n. NE 91044) has been used at NE for curatorial purposes and this study.
NEW SOUTH WALES: Northern Tablelands: Paradise Rocks, ~50 km E of Walcha, 3 Dec. 1978, J.B. Williams s.n. (NE 91044!); Oxley Wild Rivers National Park (2009 addition), on southern side of large rocky outcrop 1.6 km NNW of ‘Garibaldi’ homestead, 21 Dec. 2008, L.M. Copeland 4355 (NE!).
Prostanthera faucicola (P. sp. Oxley Wild Rivers National Park). (a) Habitat. (b) Buds with floral bracts, scale: 1 mm, M.F. Duretto 4027. (c) One of the often asymmetrical floral prophylls, scale: 1 mm, M.F. Duretto 4027. (d) Inflorescence, note paired persistent hairy floral prophylls, scale: 1.0 mm, M.F. Duretto 4027. Photographs by Lachlan Copeland (a), Tareg Shaldoom (b, d) and J. J. Bruhl (c).
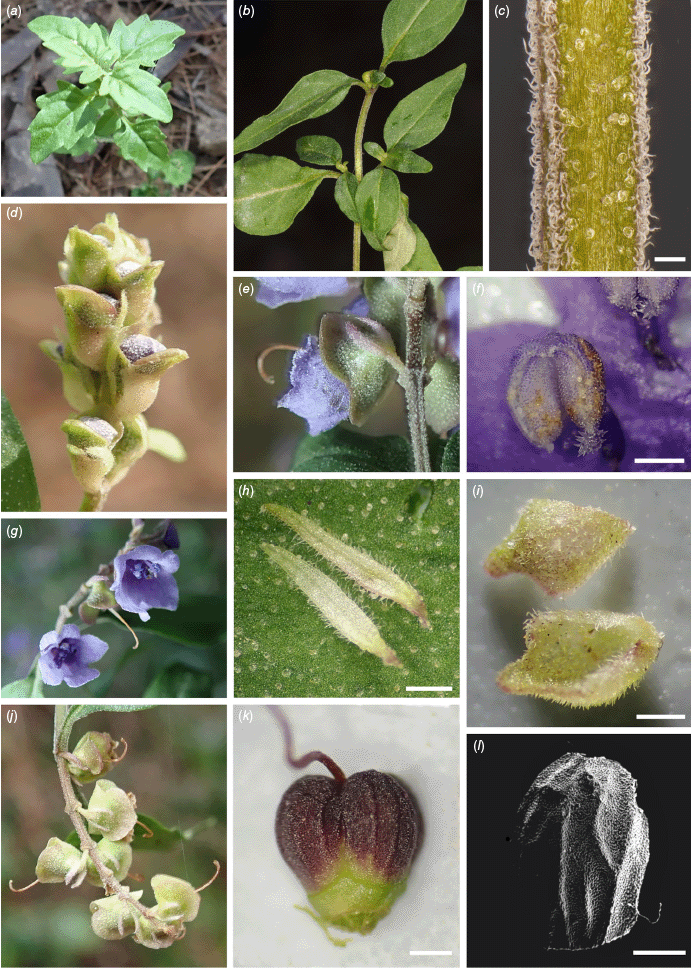
Prostanthera milleri Palsson & J.J.Bruhl, sp. nov.
Type: New South Wales. Central Coast: Olney State Forest, intersection of Walkers Ridge Rd, ~5.1 km from junction with Wollombi Forest Rd, Watagan State Forest, 29 Sep. 1987, B.J. Conn 2613 & B. Timmis (holo: NSW 228852!; iso: CANB, MEL 2523242*, NE 110202!, ?RSA).
Prostanthera milleri is morphologically similar to P. dyarubbin and P. faucicola but differs in the strong scent of 1,8-cineole in freshly crushed leaves in P. milleri, absent in P. dyarubbin and P. faucicola; leaves of P. milleri softer and wider than the leaves of P. dyarubbin (length to width ratio 2.0–3.4 in P. milleri v. 2.0–5.0 for P. dyarubbin); anthers of P. milleri cream to mauve v. purple to deep purple in P. dyarubbin.
Shrub, single to multi-stemmed, 1–4 m tall, 1–2 m wide, covered with glandular trichomes (~0.1 mm in diameter). Branchlets quadrangular, distinctly 4-ridged, with scattered glandular trichomes and densely distributed antrorse non-glandular hairs in rows on the leaf decurrencies, non-glandular hairs ~0.15 mm long, white. Juvenile leaves dentate with up to six small teeth per leaf. Leaves strongly discolourous, mid-green above, paler below; petiole 3–15 mm long; lamina ovate to lanceolate, 16–47 mm long, 6–16 mm wide, length to width ratio 2.0–3.4, length of maximum width from base to total lamina length ratio 0.2–0.4, base cuneate, sometimes attenuate; margin entire, apex usually obtuse, without non-glandular hairs except for prominent midrib on abaxial surface (sparsely to moderately densely hairy, non-glandular hairs ±antrorse, to 0.15 mm long), in depressed midrib on adaxial surface (depressed for ~2/3 length and with scattered ±antrorse non-glandular hairs to 0.15 mm long) and usually proximal fifth of margin (antrorse simple non-glandular hairs up to 0.15 mm long); abaxial surface moderately to densely glandular (25–45 mm−2); adaxial surface moderately glandular (10–25 mm−2), secondary veins often visible. Inflorescences ±narrow, ±compact thryses to ~1.5 cm long with dichasia usually reduced to single flowers. Floral bracts caducous, scutiform and concave, can vary in shape along axis of inflorescence, central floral bracts 1.7–2.6 mm long, 1.7–2.3 mm wide; abaxial surface with scattered non-glandular hairs to 0.15 mm long, scattered to densely distributed glands; adaxial surface without non-glandular hairs, occasional glands; margin ciliate, non-glandular hairs to 0.2 mm long. Pedicel 1.5–2.25 mm long, scattered non-glandular hairs ~0.15 mm long, moderately to densely glandular. Floral prophylls persistent, pale green to pale pink, a1 axis to anthopodium ratio 2–5, opposite, narrowly elliptic, 1.3–2.3 mm long, 0.2–0.4 mm wide, length to width ratio 4–7; abaxially hairy, scattered glands; margin ciliate to densely ciliate (non-glandular hairs up to 0.2 mm long); adaxially without non-glandular hairs, occasional glands; midrib visible in specimens stored in spirits. Calyx green, green with maroon lobes or all maroon, bilobed, both lobes recurved from corolla; outer surface moderately to densely glandular, scattered non-glandular hairs; margin sparsely to densely ciliate with slightly crisped or adpressed white non-glandular hairs up to 0.1 mm long; inner surface sparsely to densely hairy towards margin of lobes with scattered glandular trichomes, non-glandular hairs up to 0.15 mm long; tube 1.5–2.5 mm long, sometimes ridged in dried specimens; abaxial lobe transversely elliptic to oblate, (1.0–)1.4–2.3 mm long, 1.6–3.0(–3.8) mm wide at base, apex rounded or occasionally retuse; adaxial lobe transversely elliptic, 0.7–1.7 mm long, 2.0–3.4(–4.1) mm wide at base; apex rounded and margin often shallowly sinuate; adaxial lobe length to abaxial lobe length ratio 0.3–1.2. Corolla blue-purple; adaxial corolla tube length 3.0–4.5 mm long; outer surface moderately hairy beyond calyx, ±antrorse non-glandular hairs up to 0.1 mm long, sparsely glandular; inner surface tube without non-glandular hairs, sparsely glandular, abaxial lobe usually without non-glandular hairs, adaxial lobe hairy to densely hairy, non-glandular hairs to 0.1 mm long, lateral lobes ±without non-glandular hairs to hairy, non-glandular hairs to 0.1 mm long; corolla margin densely to very densely ciliate with crinkled white non-glandular hairs, 0.04–0.15 mm long; abaxial median lobe oblate to elliptic, 1.5–3.0 mm long, 1.6–3.0 mm wide, 1.0–2.2 mm wide below distal lobing, length to width ratio 0.7–1.5, apex rounded and margin regular in fresh specimens; lateral lobes usually shallowly triangular to deltate, sometimes oblate, 1.4–3.0 mm long, 1.5–3.4 mm wide, length to width ratio 0.7–1.1, apex rounded, margin regular in fresh specimens; adaxial median lobe-pair depressed ovate, 0.6–1.3 mm long, 1.4–3.0 mm wide, length to width ratio 0.3–0.7, apex rounded, margin regular in fresh specimens. Stamens abaxial stamens inserted 0.6–1.5 mm above corolla base, filaments 1.0–2.5(–4.2) mm long; adaxial stamens inserted 0.5–1.1 mm above corolla base; filaments 0.9–2.3 mm long; anther locules 0.7–1.3 mm long; anthers cream to mauve, connective extended to form 1 or 2 basal appendages (one appendage usually shorter than the other), extending beyond base of anther and terminating with 6–12 translucent mauve conical hairs, length beyond the anther of appendage including hairs to ~0.2 mm, hairs to 0.1 mm. Pistil 4–7.5 mm long; ovary cylindrical obovoid, 0.6–0.7 mm long, diameter at base ~0.5 mm, lobes ~0.3 mm long; style 3.5–5(–6.8) mm long; stigmatic lobes 0.2–0.4 mm long. Fruiting calyx accrescent. Mericarps, mature colour unknown, to ~1.8 mm long, wrinkled and minutely papillose.
Although P. milleri contains large quantities of the essential oil 1,8-cineole and some p-cymene, this species also contains E-verbenol and menth-3-en-8-ol that are essential oils not found in P. ovalifolia sens. lat., P. cineolifera or P. dyarubbin (tables 2, 4 in Sadgrove et al. 2020; Supplementary Table S4) and the latter chemical gives the crushed leaves a distinctive sent.
Prostanthera milleri occurs in Olney State Forest, Watagan Mountains in the Wyong subregion of the Sydney Basin bioregion (Fig. 6).
This species grows on ridges and slopes at altitudes of ~450 m. This site is on Hawkesbury Sandstone but downslope of a small basalt remnant (Colquhoun et al. 2022). The vegetation is recorded as eucalypt shrubby open forest–shrubby tall open forest with dominant species Eucalyptus piperita, Corymbia eximia and Syncarpia glomulifera, and an E. agglomerata plantation. Associated species include Allocasuarina torulosa, Acacia elata, Astrotricha latifolia, Zieria smithii, Smilax australis and Calochlaena dubia.
There is only one known population of P. milleri, with >1000 plants recorded in 2017, in an AOO <10 km2. In 2022, this population had decreased by at least 70%, likely as a result of the severe drought that ended in early 2020. The population is ±a single age cohort with some recruitment close to the road where the leaf litter is relatively shallow. Downslope where there is leaf litter up to 20 cm deep, no recruitment was noted in 2022. Based on the AOO and severe decline in the number of mature individuals, we suggest a ‘Critically Endangered’ status is appropriate under the International Union for Conservation of Nature (2019), fulfilling Criteria B2 (a) and (b)(iv). The known population occurs within a section of state forest marked as ‘forest management zone 2- a permanent exclusion to harvesting’ (Mark Drury, Forestry Corporation of NSW, pers. comm., 2022). Nearby ridges in Olney State Forest have been searched unsuccessfully for further populations of P. milleri.
Honouring Robert T. Miller, who informally documented and provided extensive descriptions for the ‘Hawkesbury River’ and ‘Wattagan State Forest’ variants of P. ovalifolia (Miller 1988). Miller was a very active member of the ‘Prostanthera and Westringia Study Group’ of the Society for Growing Native Plants from the early 1980s, becoming leader of the study group from 1992 until closure in 2006.
The closest known population of P. dyarubbin to the known population of P. milleri is the Mogo Creek population, 25 km to the south-west. We have propagated cuttings of P. milleri and distributed plants to the Australian National Botanic Gardens.
NEW SOUTH WALES: Central Coast. Olney State Forest, intersection of Walkers Ridge Rd and Murrays Forest Rd, 11 Oct. 2017, R.L. Palsson 166, R.L. Andrew, J.J. Bruhl & I.R. Telford (AD!, BRI!, CANB!, K!, M!, MEL!, MO!, NE!, NSW!, US!); Olney State Forest, intersection of Walkers Ridge Rd and Murrays Forest Rd, 11 Oct. 2017, R.L. Palsson 161, R.L. Andrew, J.J. Bruhl & I.R. Telford (NE!); Olney State Forest, intersection of Walkers Ridge Rd and Murrays Forest Rd, 18 Nov. 2017, R.L. Palsson 215 & M.R. Donald (CANB!, MEL!, NE!, NSW!).
Prostanthera milleri (P. sp. Olney State Forest). (a) Seedling, R.L. Palsson 533. (b) Leaves, R.L. Palsson 166. (c) Stem indumentum, scale bar: 0.25 mm, R.L. Palsson 162. (d) Buds with floral bracts, R.L. Palsson 534a. (e) Lateral view of a flower showing disposition of calyx lobes and persistent hairy floral prophylls, R.L. Palsson 125. (f) Anther with short anther appendage, scale bar: 0.5 mm, R.L. Palsson 125. (g) Front view of inflorescence, R.L. Palsson 125. (h) Floral prophylls, scale bar: 0.5 mm, R.L. Palsson 124. (i) Floral bracts, scale bar: 0.5 mm, R.L. Palsson 161. (j) Fruiting calyces, R.L. Palsson 125. (k) Developing mericarps, scale bar: 0.25 mm, R.L. Palsson 214. (l) Scanning electron micrograph of mericarp, scale bar: 0.25 mm, R.L. Palsson 213. Photographs by R. L. Palsson (a, c–l) and J. J. Bruhl (b).
Prostanthera ovalifolia R.Br., Prodr.: 509. 1810
Type: [‘(T.) v.v.’]: Australia, Queensland. Port Curtis: ‘[pencil] Genus Labiatorum [sic] Scutellariae proximum [ink] Shoal Bay Passage III, 26 Aug. 1802, R.Brown s.n. (lecto, here designated: BM001041052*JSTOR. Residual syntypes: BM001041053*JSTOR, CANB278989.1!, DAO 411303*JSTOR, E00066062*JSTOR, FI011221*JSTOR. MEL 43469*JSTOR).
Mabberley and Moore (2022, p. 555) considered BM001041052 as a ‘GCFL’ (a good candidate for lectotypification). We agree. This sheet has Brown’s original field tag as cited above (Fig. 14). The location ‘Shoal Bay’ on ‘Aug 26 – 1802’ fits Mount Westall and the date given in Brown’s diary (Vallance et al. 2001, p. 254). We treat all other syntypes cited above as residual syntypes.
Lectotype of Prostanthera ovalifolia R.Br. (R.Brown s.n.; BM001041052). Image reproduced with kind permission from the Trustees, Natural History Museum. Image from https://data.nhm.ac.uk/object/d1b31585-4779-46c1-9029-fd487ac5b63b/1748251284062, accessed 10 June 2025.

Putative new taxon: Prostanthera sp. Mangrove Creek (D.S. Gibbons NE 49860) R.L.Palsson
The single known gathering of this putative new taxon was collected from the currently inundated Mangrove Creek Dam site, 19 km SW of the Olney State Forest site. This specimen shares leaf size, leaf shape and floral prophyll characters with P. milleri. In the material from the Mangrove Creek Dam site, the floral bracts are wider with a blunt apex unlike the attenuate apex on scutiform floral bracts in Olney State Forest material. We consider this plant to represent a distinct species and as such, has been excluded from the description and range of P. milleri.
Data availability
Morphological data are available in the article and in the accompanying Supplementary materials. The SNP datasets generated during or analysed during the current study are available in the RUNE repository at https://hdl.handle.net/1959.11/58744 (doi:10.25952/rgw2-sc64).
Conflicts of interest
Jeremy Bruhl is an associate editor for Australian Systematic Botany but was not involved in the peer review or decision-making process for this paper. Australian Systematic Botany encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
This research was funded by the Office of Environment and Heritage (now Department of Planning and Environment) acting for and on behalf of the State of New South Wales, Australia. R. L. Palsson received student research funds from UNE.
Acknowledgements
We thank Saving Our Species (New South Wales Department of Planning and Environment) and UNE for funding; National Parks and Wildlife Service (New South Wales), Forestry Corporation New South Wales and various Rangers of National Parks for access to reserved lands (permit: SL100305) and State Forests (permit: RES100107); Luke Foster (New South Wales Department of Planning and Environment) as coordinator of the Saving Our Species project, for comments on related reports and assistance in the field; Officer in Charge Singleton Military Area (SMA), Australian Army, for facilitating access; Peter Bussell, Kaye Durham, Elizabeth Gill, Robert Gibson (New South Wales Department of Planning and Environment), Joan Griffiths, Aaron Hedger, David Martin, Hilary Pearl, Judith Roland, Brigitte Stievermann, Bob Ross and Grant Woolcock (Central Coast Council) for assistance with fieldwork; John Nevin and Phil Rose for assistance with propagation of cuttings and repotting of specimens; Penelope Sinclair for assistance with mounting specimens; Margaret Donald for assistance with field work, giving R. L. Palsson a good grounding in R and for the use of her Leica M60 microscope; Jody McNally of the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Chiswick for assistance with freeze drying material for DNA extraction; Tracey Howie of the GuriNgai community for advice on the preferred spelling of the aboriginal name for the Hawkesbury River; Grant Webster and Jake Cassar for connecting us with Tracey Howie; Andrew Thompson and Alex Karsten for allowing access to their properties; directors and staff of AD, BRI, CANB and NSW, for help during visits; staff, including Monique Grol, Melinda Laidlaw and Michael Mathieson (BRI), Ranee Prakash (BM), Brendan Lepschi (CANB), Nimal Karunajeewa (MEL) and Lachlan Copeland for images; Karen Wilson (NSW) and Peter Stevens (MO) for discussion on nomenclatural and taxonomic terms; David Mabberley for help with and comments on the lectotypification of P. ovalifolia; UNE for access to facilities, in particular, the N.C.W. Beadle Herbarium (NE) where this study was based; Ian Simpson, Lindsey Frost and Rhyan Gorman for assisting with watering in the glasshouse; Tareg Shaldoom for technical help with images and Endnote; Margaret Williams for proofreading, Trish Waters, Margaret and Chris Cooper, Jeremy Bruhl and family, Laurel Arthur and family, and Shelley and Jamie Rowntree for providing accommodation for R. L. Palsson on visits to Armidale and Canberra; and Trevor Wilson for assistance at NSW and valuable discussions with R. L. Palsson. We thank W.R. (Bill) Barker for assistance at AD. We thank Trevor Wilson, an anonymous referee, Michael (Mike) Bayly and Brendan Lepschi for detailed comments on the manuscript.
References
Australian Biological Resources Study (2019) Flora of Australia. Guide to contributors. (Commonwealth of Australia: Canberra, ACT, Australia) Available at https://ausfloradotnet.files.wordpress.com/2019/11/contributor-guidelines_flora-of-australia_2019.pdf
Baker RT, Smith HG (1912) On a new species of Prostanthera and its essential oils. Journal and Proceedings of the Royal Society of New South Wales 46, 103-110 Available at https://www.biodiversitylibrary.org/page/41676470#page/133/mode/1up.
| Google Scholar |
Barrett RL, Bruhl JJ, Wilson KL (2021) Netrostylis, a new genus of Australasian Cyperaceae removed from Tetraria. Telopea 24, 53-60.
| Google Scholar |
Briggs BG, Johnson LAS (1979) Evolution in the Myrtaceae - Evidence from inflorescence structure. Proceedings of the Linnean Society of New South Wales 102, 157-256 Available at https://biostor.org/reference/67846.
| Google Scholar |
Bruhl JJ (1995) Sedge genera of the world: relationships and a new classification of the Cyperaceae. Australian Systematic Botany 8, 125-305.
| Crossref | Google Scholar |
Bruňáková K, Bálintová M, Henzelyová J, Kolarčik V, Kimáková A, Petijová L, Čellárová E (2021) Phytochemical profiling of several Hypericum species identified using genetic markers. Phytochemistry 187, 112742.
| Crossref | Google Scholar | PubMed |
Burkle LA, Runyon JB (2016) Drought and leaf herbivory influence floral volatiles and pollinator attraction. Global Change Biology 22, 1644-1654.
| Crossref | Google Scholar | PubMed |
Council of Heads of Australasian Herbaria (2010) Prostanthera sp. Hawkesbury (B.J.Conn 2591). In ‘Vasular Plants’. (Australian Plant Census) Available at https://id.biodiversity.org.au/tree/51689388/51241805 [Verified 10 October 2024]
Collins TL, Schmidt-Lebuhn AN, Andrew RL, Telford IRH, Bruhl JJ (2022) There’s gold in them thar hills! Morphology and molecules delimit species in Xerochrysum (Asteraceae; Gnaphalieae) and reveal many new taxa. Australian Systematic Botany 35, 120-185.
| Crossref | Google Scholar |
Colquhoun GP, Hughes KS, Deyssing L, Ballard JC, Folkes CB, Phillips G, Troedson AL, Fitzherbert JA (2022) NSW Geology Data Package: Catalogue Number: 9309; Map Sheet Code: N/A; Scale: Statewide; 2025 - Version 2.5 (Current). New South Wales Seamless Geology dataset, version 2.2. (Geological Survey of New South Wales, Department of Primary Industries and Regional Development: Maitland, NSW, Australia) Available at https://search.geoscience.nsw.gov.au/product/9232 [Digital dataset]
Conn BJ (1984) A taxonomic revision of Prostanthera Labill. section Klanderia (F.v. Muell.) Benth. (Labiatae). Journal of the Adelaide Botanic Gardens 6, 207-348.
| Google Scholar |
Conn BJ (1988) A taxonomic revision of Prostanthera Labill. section Prostanthera (Labiatae). 1. The species of the Northern Territory, South Australia and Western Australia. Nuytsia – the Journal of the Western Australian Herbarium 6, 351-411.
| Crossref | Google Scholar |
Conn BJ, Wilson TC (2015) Two new species of Prostanthera (Lamiaceae) in New South Wales. Telopea 18, 464-466.
| Crossref | Google Scholar |
Conn BJ, Henwood MJ, Proft KM, Scott JA, Wilson TC, Howes RS (2021) An integrative taxonomic approach resolves the Prostanthera lasianthos (Lamiaceae) species complex. Australian Systematic Botany 34, 438-476.
| Crossref | Google Scholar |
Dema S, Telford IRH, Andrew RL, Duval DJ, Bruhl JJ (2021) Phebalium calcicola (Rutaceae: Boronieae): a species described as new, restricted to south-eastern South Australia, is proposed as Critically Endangered. Swainsona 35, 43-53.
| Google Scholar |
Department of Climate Change Energy, the Environment and Water (2020) Interim Biogeographic Regionalisation for Australia (Subregions - States and Territories) v. 7 (IBRA) Available at https://datasets.seed.nsw.gov.au/dataset/interim-biogeographic-regionalisation-for-australia-ibra-version-7-regions [ESRI shapefile]
Department of the Environment (2008) Approved Conservation Advice for Prostanthera cineolifera. Available at http://www.environment.gov.au/biodiversity/threatened/species/pubs/11233-conservation-advice.pdf
Domin K (1928) Beitrage zur Flora und Pflanzengeographie Australiens. Bibliotheca Botanica 22, 937–1317. Available at https://www.biodiversitylibrary.org/item/315467#page/450/mode/1up [In German and Latin]
Gallagher MK, Campbell DR (2017) Shifts in water availability mediate plant–pollinator interactions. New Phytologist 215, 792-802.
| Crossref | Google Scholar | PubMed |
Gruber B, Unmack PJ, Berry OF, Georges A (2018) dartR: an R package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Molecular Ecology Resources 18(3), 691-699.
| Crossref | Google Scholar |
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2017) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35, 518-522.
| Crossref | Google Scholar |
Jiawei W, Jie W, Haiqing Y, Gang F, Yang H (2020) Correlation analysis between genetic and chemical differences of Nardostachys jatamansi from different habitats in Ganzi Tibetan Autonomous Prefecture, Sichuan Province, China. Biochemical Systematics and Ecology 92, 104133.
| Crossref | Google Scholar |
Johnson PLF, Slatkin M (2008) Accounting for bias from sequencing error in population genetic estimates. Molecular Biology and Evolution 25, 199-206.
| Crossref | Google Scholar | PubMed |
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587-589.
| Crossref | Google Scholar | PubMed |
Mabberley DJ, Moore DT (2022) The Robert Brown Handbook. A guide to the life and work of Robert Brown (1773–1858) Scottish botanist. Regnum Vegetabile 160, 1-624.
| Google Scholar |
Mijangos JL, Gruber B, Berry O, Pacioni C, Georges A (2022) dartR v2: an accessible genetic analysis platform for conservation, ecology and agriculture. Methods in Ecology and Evolution 13, 2150-2158.
| Crossref | Google Scholar |
Miller R (1988) The genus Prostanthera in the Sydney Region. Prostanthera & Westringia Study Group Newsletter 14, 25-32 Available at https://anpsa.org.au/wp-content/uploads/prostanthera14.pdf.
| Google Scholar |
Moshari-Nasirkandi A, Iaccarino N, Romano F, Graziani G, Alirezalu A, Alipour H, Amato J (2024) Chemometrics-based analysis of the phytochemical profile and antioxidant activity of Salvia species from Iran. Scientific Reports 14, 17317.
| Crossref | Google Scholar | PubMed |
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32, 268-274.
| Crossref | Google Scholar | PubMed |
O’Donnell RP, Wilson TC, Andrew RL, Telford IRH, Taaseki G, Zimmer HC, Bruhl JJ (2021) Molecular phylogenetic analysis of the Prostanthera phylicifolia (Lamiaceae) assemblage resolves relationships of the ‘Critically Endangered’ P. gilesii and other putative new species. Telopea 24, 359-376.
| Crossref | Google Scholar |
O’Donnell RP, Bruhl JJ, Telford IRH, Wilson TC, Zimmer HC, Taseski GM, Andrew RL (2023) Molecular and morphological analyses support recognition of Prostanthera volucris (Lamiaceae), a new species from the Central Tablelands of New South Wales. Australian Systematic Botany 36, 1-20.
| Crossref | Google Scholar |
Office of Environment and Heritage (2019) Singleton Mint Bush – profile. (NSW OEH) Available at https://threatenedspecies.bionet.nsw.gov.au/profile?id=10672
O’Leary SJ, Puritz JB, Willis SC, Hollenbeck CM, Portnoy DS (2018) These aren’t the loci you’re looking for: principles of effective SNP filtering for molecular ecologists. Molecular Ecology 27, 1-14.
| Crossref | Google Scholar |
Palsson RL, Telford IRH, Bruhl JJ, Andrew RL (2024) Population genetic structure and range limits of Prostanthera cineolifera (Lamiaceae), a vulnerable shrub with a patchy distribution. Population Genetics 25, 1231-1251.
| Crossref | Google Scholar |
Sadgrove NJ, Padilla-González GF, Telford IRH, Greatrex BW, Jones GL, Andrew R, Bruhl JJ, Langat MK, Melnikovova I, Fernandez-Cusimamani E (2020) Prostanthera (Lamiaceae) as a ‘cradle of incense’: chemophenetics of rare essential oils from both new and forgotten Australian ‘mint bush’ species. Plants 9, 1570.
| Crossref | Google Scholar | PubMed |
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671-675.
| Crossref | Google Scholar | PubMed |
Soubrier J, Steel M, Lee MSY, Der Sarkissian C, Guindon S, Ho SYW, Cooper A (2012) The influence of rate heterogeneity among sites on the time dependence of molecular rates. Molecular Biology and Evolution 29, 3345-3358.
| Crossref | Google Scholar | PubMed |
Telford IRH, Sadgrove NJ, Bruhl JJ (2019) Three new species segregated from Phebalium squamulosum subsp. squamulosum (Rutaceae) based on morphological and phytochemical data. Muelleria 38, 3-16.
| Google Scholar |
Wilson TC, Conn BJ, Henwood MJ (2012) Molecular phylogeny and systematics of Prostanthera (Lamiaceae). Australian Systematic Botany 25, 341-352 https://doi.org/10.1071/SB12006.
| Google Scholar |
Wilson TC, Elkan L, Henwood MJ, Murray L, Renner MAM, Wardrop C (2015) Prostanthera conniana (Lamiaceae, Westringieae), a new species from the Southern Tablelands, New South Wales, Australia. Telopea 18, 520-526.
| Crossref | Google Scholar |
Wilson TC, Conn BJ, Henwood MJ (2017) Great expectations: Correlations between pollinator assemblages and floral characters in Lamiaceae. International Journal of Plant Sciences 178, 170-187.
| Crossref | Google Scholar |
Wilson TC, Carmen P, Hook C (2019) Recircumscription of Prostanthera denticulata R.Br. (Lamiaceae, Westringieae) and the new species P. crocodyloides T.C.Wilson. Telopea 22, 75-87.
| Crossref | Google Scholar |
Yang Z (1995) A space-time process model for the evolution of DNA sequences. Genetics 139, 993-1005.
| Crossref | Google Scholar | PubMed |
Yang H, Pei H, Gang F, Saima A, Cheng P (2014) Comprehensive analyses of molecular phylogeny and main alkaloids for Coptis (Ranunculaceae) species identification. Biochemical Systematics and Ecology 56, 88-94.
| Crossref | Google Scholar |
Zharkikh A (1994) Estimation of evolutionary distances between nucleotide sequences. Journal of Molecular Evolution 39, 315-329.
| Crossref | Google Scholar | PubMed |