Generic and infrageneric limits of Phebalium and its allies (Rutaceae: Zanthoxyloideae)
Marco F. Duretto A * , Margaret M. Heslewood
A * , Margaret M. Heslewood  A and Michael J. Bayly
A and Michael J. Bayly  B
B
A National Herbarium of New South Wales, Australian Institute of Botanical Science, Royal Botanic Gardens & Domain Trust, Locked Bag 6002, Mount Annan, NSW 2567, Australia.
B School of BioSciences, The University of Melbourne, Parkville, Vic. 3010, Australia.
Australian Systematic Botany 36(2) 107-142 https://doi.org/10.1071/SB22018
Submitted: 1 July 2022 Accepted: 27 March 2023 Published: 21 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
We present a five-locus 129-species phylogeny of Rutaceae from subfamily Zanthoxyloideae, with 193 ingroup samples representing 128 species from all 18 genera in the Eriostemon Group and focus on generic and infrageneric limits in Phebalium and its allies. Maximum parsimony and Bayesian inference analyses were performed using three plastid markers (psbA–trnH, trnL–trnF, rbcL) and two nuclear ribosomal markers (ITS, ETS). Asterolasia, Correa, Diplolaena, Eriostemon, Leionema, Nematolepis and Phebalium are each shown to be monophyletic, reaffirming the results of previous authors. The paraphyly of Rhadinothamnus with respect to Chorilaena is addressed by transferring all taxa of Rhadinothamnus into an expanded Chorilaena and a key to species presented. Microcybe is reduced to synonymy under Phebalium and recognised at a sectional level, with the distinctiveness of M. ambigua recognised by erecting a new monotypic section to accommodate it. The denser sampling of the Eriostemon Group in this study has not improved resolution among genera; there is little support for most relationships among genera, a finding similar to that of previous authors. New sectional classifications with keys are presented for Asterolasia, Leionema, Nematolepis and Phebalium.
Keywords: Asterolasia, Australasia, Chorilaena, Correa, Crowea, Diplolaena, Drummondita, Eriostemon, Geleznowia, Halfordia, Leionema, Microcybe, molecular phylogenetics, Muiriantha, Myrtopsis, Nematolepis, Neoschmidia, Phebalium, Philotheca, plant systematics, Rhadinothamnus, Rutaceae, taxonomy.
Introduction
Rutaceae is a southern hemisphere family (~154 genera, ~2100 spp.) that is well represented in Australia (44 genera, 25 endemic; ~490 spp., ~460 endemic) where eastern and south-western parts of the continent are major centres of diversity (Kubitzki et al. 2011; Wilson 2013, and accounts therein). The subfamilial classification of the family has come under significant scrutiny in the past decade. The first major classification of Rutaceae presented by Engler (1896, 1931) and based largely on fruit characters has been found to be inadequate (see review in Appelhans et al. 2021).
Noting the unsatisfactory nature of the previous classification in Rutaceae, Kubitzki et al. (2011) adopted several informal generic alliances within the largest subfamily, Rutoideae, which were based on morphology but highly correlated to geography and ecology. Most Australasian genera were classified into a number of these alliances. The Boronia Sm. Alliance, which approximately corresponds to tribe Boronieae sensu Engler (1896, 1931), contained most Australasian non-rainforest genera. This alliance was further divided into three generic groups centred on Boronia (3 genera), Correa Andrews (1 genus), and Eriostemon Sm. (14 genera). The Eriostemon Group contained those genera that have flowers with five petals and alternate, simple leaves, as opposed to the genera with four-petalled flowers with opposite and simple or variously compound leaves placed in the Boronia and Correa Groups. Molecular studies have indicated that neither the Boronia Alliance (and so, the Tribe Boronieae), nor any of the three groups can be retained as currently circumscribed (Groppo et al. 2008, 2012; Bayly et al. 2013; Duretto et al. 2020, 2023; Appelhans et al. 2021). The Boronia Alliance, the Boronia Group and Boronia have all proven to be polyphyletic, with Boronia, the recently reinstated genus Cyanothamnus Lindl. (Duretto et al. 2020), Neobyrnesia J.A.Armstr. and Zieria Sm. being more closely related to various Australasian genera placed in the Euodia J.R.Forst. & G.Forst. Alliance than they are to each other (Groppo et al. 2008, 2012; Bayly et al. 2013, 2015; Duretto et al. 2020, 2023; Appelhans et al. 2021). The Eriostemon Group needs to be expanded to include Correa, Halfordia F.Muell., Myrtopsis Engl. and Neoschmidia T.G.Hartley of the Euodia Alliance, which all together form a well-supported clade (Groppo et al. 2008, 2012; Bayly et al. 2013; Duretto et al. 2020; Appelhans et al. 2021). This placement confirms the proposition put forward by Hartley (2003), who, when formally describing Neoschmidia to accommodate the New Caledonian Eriostemon pallidus Schltr., considered his new genus most closely related to Halfordia, although he also considered it to be very similar to Philotheca Rudge.
The apparent non-monophyly of the various formal and informal taxonomic groups discussed above, and the clear need for further research, was why Appelhans et al. (2021), when they proposed a novel formal subfamilial classification for Rutaceae on the basis of molecular and morphological data, did not introduce any formal groups within the largest of the six subfamilies, Zanthoxyloideae. The majority of Australasian genera that were placed in subfamily Rutoideae by Kubitzki et al. (2011) are now placed in subfamily Zanthoxyloideae. Appelhans et al. (2021) reduced subfamily Rutoideae to include only five genera that are confined to the northern hemisphere and southern Africa.
The expanded Eriostemon Group (18 genera, ~210 spp.) represents a significant component of the biodiversity of Rutaceae in Australasia. It is monophyletic and sister to a clade containing Boronia, Euodia and Melicope J.R.Forst. & G.Forst., and their allies (Groppo et al. 2008, 2012; Bayly et al. 2013; Duretto et al. 2020; Appelhans et al. 2021). The Eriostemon Group is largely confined to southern Australia, including Tasmania (Tas.). Chorilaena Endl. (1 sp.), Diplolaena R.Br. (15 spp.), Geleznowia Turcz. (2 spp.), Muiriantha C.A.Gardner (1 sp.), and Rhadinothamnus Paul G.Wilson (3 spp.) are confined to south-western Australia (SW Aust.), Correa (11 spp.) to south-eastern Australia (SE Aust.), including Tasmania, whereas Asterolasia F.Muell. (19 spp.: SE Aust., 14; SW Aust., 5), Crowea Sm. (3 spp.: SE Aust., 2; SW Aust., 1), Microcybe Turcz. (4 spp.: SW Aust., 4; SE Aust., 2) and Nematolepis Turcz. (7 spp.: SE Aust., 6; SW Aust., 1) are found on both sides of the continent. The following four Australian genera have the majority of their species confined to southern parts of the continent, but also have one or a few species in the tropics: Drummondita Harv. (10 spp.: SW Aust., 8; Northern Territory, 1; northern Queensland, N Qld, 1), Eriostemon (2 spp.: New South Wales, NSW, and SE Qld, 1; N Qld, 1), Phebalium Vent. (~35 spp.: southern Aust., ~32; N Qld, 4) and Philotheca (~55 spp.: southern Aust., ~54; N Qld, 1). The following four genera have species outside Australia: Leionema (F.Muell.) Paul G.Wilson (28 spp.) is confined to south-eastern Australia, including Tasmania, except for L. ellipticum Paul G.Wilson (NE Qld) and L. nudum (Hook.) Paul G.Wilson (North Island, New Zealand); Halfordia (1–3 spp.) is widespread from north-eastern New South Wales to northern Queensland and from New Guinea to New Caledonia and Vanuatu (Bayly et al. 2016); and both Myrtopsis (9 spp.) and Neoschmidia (2 spp.) are confined to New Caledonia (Hartley 2003; Kubitzki et al. 2011). The Eriostemon Group can be divided into two subgroups, one centred on Phebalium (see Wilson 1998a; Mole et al. 2004; including Asterolasia, Chorilaena, Diplolaena, Leionema, Microcybe, Nematolepis, Phebalium and Rhadinothamnus) and the other on Eriostemon and Philotheca (including Crowea, Drummondita, Eriostemon, Geleznowia, Muiriantha and Philotheca), plus Correa, Halfordia, Myrtopsis and Neoschmidia.
All Australian genera of the Eriostemon Group have been revised recently (see Flora of Australia accounts in Wilson 2013, and references cited therein), although there are still discrete species complexes to be resolved (e.g. see Bayly et al. 2016; Telford et al. 2019) and new taxonomic concepts, including taxa new to science, have been regularly published (e.g. Telford 2013; Telford and Bruhl 2014, 2020; Bell and Walsh 2015; McDougall et al. 2016; Orme and Duretto 2017; Wege 2017; Copeland and Telford 2018; Duretto 2018; Alvarez and Duretto 2019a, 2019b; Telford et al. 2019; Ford and Duretto 2020; Shepherd and Crawford 2020; Wege and Hislop 2020; Dema et al. 2021).
In some genera of the Eriostemon Group, for example, Leionema and Nematolepis, there are highly disjunct species with distinctive morphological traits that potentially occupy isolated phylogenetic positions, and whose taxonomic placement requires clarification. Leionema ellipticum (NE Qld) differs from the remaining species in the genus by having minutely apiculate anthers (v. retuse) and a deeply grooved gynophore (v. smooth), and Wilson (1998a, 2013a) and Mole et al. (2004) determined that it was sister to the remainder of the genus. Wilson (1998a, 2013a) indicated that it may warrant its own genus or subgenus. Nematolepis phebalioides Turcz. is the only species of that genus found in south-western Australia and the study by Mole et al. (2004) indicated that it is sister to a clade containing the remaining species of the genus that are confined to south-eastern Australia. It differs from all other members of the genus by having pendent flowers with a fused corolla (v. erect flowers and unfused petals) (Wilson 1998a). Pendulous flowers, often associated with bird pollination, are also found in Chorilaena, Diplolaena, Muiriantha and in some species of Philotheca in south-western Australia, and most species of Correa and several species of Leionema in south-eastern Australia. Fused petals are rare in the Eriostemon Group but are usually associated with pendent flowers and are also found in a few species of Philotheca, one species of Leionema, and most species of Correa (see Discussion).
The relationships among the genera of the Eriostemon Group have not previously been discussed in detail. Engler (1896, 1931) placed Halfordia in subfamily Toddalioideae, and the remaining genera in Tribe Boronieae of subfamily Rutoideae. In Engler’s classification, most genera of the Eriostemon Group (viz. Asterolasia, Eriostemon (including the species now placed in Neoschmidia), Crowea, Geleznowia, Microcybe, Phebalium, Philotheca, Pleurandropsis (=Asterolasia)) were placed in subtribe Eriostemoninae, whereas Nematolepis and Chorilaena were the only members of Nematolepidinae, Myrtopsis was placed in Boronieae (comprising six genera, including Boronia and Zieria), and Correa and Diplolaena were the only members of Correinae and Diplolaeninae respectively. Wilson (1970, 1971) discussed possible relationships between the various south-western Australian genera of the Eriostemon Group and the sections of the then broadly circumscribed Eriostemon and Phebalium. The putative relationships were based on seed, anther, and other floral characters. Wilson (1998a) and Bayly (1998) restricted the concept of Eriostemon to the typical section and transferred other species and sections to an expanded Philotheca. Wilson (1998b) also adopted a narrower concept of Phebalium that retained only those species placed in the typical section. He raised Phebalium section Leionema to generic rank and transferred the species of Phebalium section Gonioclados Paul G.Wilson to Rhadinothamnus and species of Phebalium section Eriostemoides Endl. to Nematolepis. Wilson (1970, 1971, 1998b, 2013b, 2013c) considered that Chorilaena and Rhadinothamnus were closely aligned and related to Nematolepis, and that Microcybe was closely related to Phebalium. Correa (Othman et al. 2010) and Philotheca (Wilson 1970, 1998a, 2013d) are the only genera in the Eriostemon Group to have published infrageneric classifications currently in use. The lack of understanding of the relationships between and within these genera is a significant gap in our knowledge of the systematics of Australasian flora and Rutaceae.
Apart from many genera having representatives in broader molecular studies, or studies of single genera or sections (e.g. Correa by Othman et al. 2010 and French et al. 2016; Philotheca section Erionema (F.Muell.) Paul G.Wilson by Batty et al. 2022), only the molecular study of Mole et al. (2004) focussed specifically on the Eriostemon Group, presenting an analysis of the relationships of Phebalium and related genera by using molecular data from the nrDNA ITS region. Mole et al. (2004) showed that Phebalium and Rhadinothamnus were paraphyletic with respect to Microcybe and Chorilaena respectively, and identified a number of species in other genera that were geographically disjunct and taxonomically isolated. Wilson (2013c) reconciled the paraphyly of Phebalium by transferring P. ambiguum C.A.Gardner to Microcybe, noting that it could, alternatively, be placed in a monotypic new genus, or Microcybe could be placed in synonymy under Phebalium. The study by Mole et al. (2004), although insightful, was limited because only a few representatives of most genera were included and the tree was inappropriately rooted using Crowea, as has become apparent from subsequent studies (Bayly et al. 2013; Appelhans et al. 2021). Philotheca is acknowledged to be poly- or paraphyletic (Wilson 1998a, 2013d; Bayly et al. 2013; Duretto et al. 2020; Appelhans et al. 2021) and is the subject of another ongoing study (see, for example, Batty et al. 2022) and so will be represented here by only a few samples for context.
The work we present here is part of a broader project that is investigating the systematics of a large clade of mainly Australasian Rutaceae found in rainforest and sclerophyllous communities corresponding to clade A of Bayly et al. (2013), and Clade C4 of Appelhans et al. (2021). We have constructed a database of ~500 taxa as part of separate investigations into a range of species-rich groups, such as, for example, Boronia, the expanded Eriostemon Group and major clades containing genera found in rainforest. Preliminary analyses of this entire dataset showed the polyphyly of Boronia (see Duretto et al. 2020), and detailed analyses of several clades have already been published in the treatments of Acronychia J.R.Forst. & G.Forst. (Holzmeyer et al. 2015), Boronia and Cyanothamnus (Duretto et al. 2020), and a new subfamily classification of Rutaceae (Appelhans et al. 2021). The data we are presenting here are a subset of the complete dataset, used to address only the issues relevant to the circumscription of the Eriostemon Group (clade C of Bayly et al. 2013; Clade 1 of Duretto et al. 2020; Clade C4e of Appelhans et al. 2021).
The objective of the current study is to test current generic concepts and ascertain relationships mainly within Phebalium and its allies. We aim to do this by producing robust molecular phylogenies based on multiple gene sequences, including all genera and most species in the Eriostemon Group. This work represents a considerable expansion of the Eriostemon Group study published by Mole et al. (2004).
Materials and methods
Taxon sampling
Our dataset comprised 194 accessions of 129 species belonging to 19 genera from subfamily Zanthoxyloideae (sensu Appelhans et al. 2021); most were newly extracted specimens, and these were supplemented with samples from previously published studies. The ingroup corresponds to clade C of Bayly et al. (2013), Clade 1 of Duretto et al. (2020), and Clade C4e of Appelhans et al. (2021) and the outgroup taxon, Boronia pinnata Sm., represents its sister clade (clade D of Bayly et al. 2013; Clade 2 of Duretto et al. 2020; Clade C4f of Appelhans et al. 2021). The taxa sampled are listed in Table 1, along with their voucher details and GenBank accession numbers for all sequences.
|
The ingroup included 193 accessions of 128 species from 18 genera that are a representative sample of the genera found in both rainforest and sclerophyllous communities from clade C (Eriostemon Group), rooted on a representative of clade D (Boronia + Melicope group) of Bayly et al. (2013, fig. 3, =clade B). The numbers in parentheses indicate the number of samples/the number of species sampled/the number of species in that genus. The Eriostemon Group (clade C) is here represented by Asterolasia (30/17/19), Chorilaena (1/1/1), Correa (2/2/11), Crowea (6/3/3), Diplolaena (8/6/15), Drummondita (3/3/10), Eriostemon (3/2/2), Geleznowia (2/1/2), Halfordia (2/1/1–3), Leionema (41/27/28), Myrtopsis (2/2/9), Nematolepis (12/7/7), Neoschmidia (2/1/2), Phebalium (45/30/35) and Philotheca (19/18/55), plus three genera not included in Bayly et al. (2013): Microcybe (8/3/4), Muiriantha (1/1/1) and Rhadinothamnus (6/3/3).
DNA extraction, polymerase chain reaction (PCR), sequencing, alignment
Leaf samples were taken from frozen silica-dried specimens or from herbarium sheets. The plant material was disrupted dry in a TissueLyser II (QIAGEN, Valencia, CA, USA) by using tungsten beads and total genomic DNA was extracted using the Qiagen DNeasy Plant Mini Kit, following the manufacturer’s instructions. The following five DNA regions were sequenced: two nuclear regions, the external (ETS) and internal (ITS) transcribed spacers of the 18S–5.8S–26S ribosomal DNA repeats; and three plastid regions, the psbA–trnH intergenic spacer (psbA–trnH), the trnL–trnF region (including the trnL intron and trnL–trnF intergenic spacer) and, for a subset of 41 ingroup taxa, the rbcL gene. We included the rbcL data, although only a minority of taxa were sampled, in the hope that it would test support for the major clades (see discussion on missing data and tree construction in Johnson et al. 2012). The following primers were used for PCR amplification and sequencing: ETS, myrtF (Lucas et al. 2007) or, in a few cases, Lucas’s modified myrtFb (Bayly et al. 2015) and ETS–18S (Wright et al. 2001); ITS, 18SF and 26SR (Prince 2010) or ITS5 and ITS4 (White et al. 1990), with the former primer pair being proved less likely to co-amplify fungal contaminants in extracts from herbarium material; psbA–trnH, psbAF (Sang et al. 1997) and trnH2 (Tate and Simpson 2003); trnL–trnF region, primers c and f (Taberlet et al. 1991); rbcL, RUTrbcL1F and rbcL1343R (Bayly et al. 2013).
All PCR reactions were performed in 25-μL volumes containing 200 μM of each primer, 200 μM of each dNTP, 0.004% bovine serum albuming (BSA), 2–2.5 mmol MgCl2 and 1 U Taq DNA polymerase. ITS and trnL–trnF amplifications used Promega GoTaq DNA polymerase (Promega Corporation, Madison, WI, USA), whereas amplifications for ETS, rbcL and psbA–trnH utilised Immolase DNA polymerase (Bioline, Luckenwalde, Germany) and a hot start PCR (with an initial cycle of 10 min at 95°C). PCR reactions were subjected to 40 cycles, as follows: denaturation for 30 s at 94°C; annealing for 30 s at 50–58°C; and extension for 1 min at 72°C, with a final extension for 4 min at 72°C. The annealing temperature for ETS, psbA–trnH and trnL–trnF was 53°C, for ITS (Prince) 58°C or (White) 55°C and for rbcL 50°C. Double-stranded PCR templates were purified, and sequencing was performed by Macrogen Inc. (Seoul, South Korea).
Consensus sequences were assembled using ABI software Sequence Navigator (ver. 1.0.1, Applied Biosystems, Inc., Foster City, CA, USA; Parker 1997) and aligned by eye in PAUP* (ver. 4.0a build 169, see http://phylosolutions.com/paup-test/; Swofford 2003). In aligning sequences, gaps were positioned to maximise conformity to known indel types such as simple and inverted duplications of adjacent sequences (Levinson and Gutman 1987; Golenberg et al. 1993). Overlapping indels of different lengths, and insertions of the same length but bearing different relationships to surrounding sequence, were treated as having independent origins, whereas indels of the same length and position and showing minor differences in nucleotide sequence were scored as the same state (Simmons and Ochoterena 2000). Potentially informative indels were scored as additional presence or absence characters and appended to the database. Gaps were treated as missing data in the phylogenetic analyses. Coding sequences of the rbcL gene were translated in MacClade (ver. 4.08a, see http://macclade.org/; Maddison and Maddison 2000) to check for internal stop codons. The full data matrix, including indel characters, is available in File S1 of the Supplementary material. Several small regions that could not be unambiguously aligned and a homoplastic inversion in psbA–trnH (highly incongruent with other characters) were excluded from all analyses.
Phylogenetic analyses
Separate analyses using maximum parsimony or Bayesian inference were run using either individual loci, the concatenated plastid or nuclear loci and the combined plastid and nuclear sequences.
Heuristic searches of the combined or individual datasets were conducted in PAUP* (ver. 4.0a build 166 in the CIPRES Science Gateway, see https://www.phylo.org; Miller et al. 2010) using tree bisection–reconnection branch-swapping to recover all equally most-parsimonious (MP) trees. One thousand replicates of random taxon addition searching were conducted so as to detect multiple islands of trees, with subsequent use of the ‘condense’ option to delete any duplicate trees. Multistate characters were treated as polymorphisms and swapping was performed on best trees. Where searching exhausted computer memory for some datasets, restricted searching was employed saving only 100 trees per replicate for those analyses. Branch supports were calculated using jackknife (JK) rather than bootstrap resampling, following the recommendations of Simmons and Freudenstein (2011). Jackknife analyses utilised faststep searching, in which each replicate was performed using random-sequence addition and no branch swapping, 10 000 replicates and the percentage of characters deleted in each replicate set at one-third. Jackknife values 50–74% were interpreted as weak support for clades, those 75–89% as moderate support, those 90–99% as strong support and those 100% were considered robust.
The MP phylogenies were compared with those obtained using the Markov-chain Monte Carlo (MCMC) method implemented in MrBAYES (ver. 3.2.7a, see https://github.com/NBISweden/MrBayes/; Ronquist et al. 2012) in the CIPRES portal. Most appropriate nucleotide substitution models were determined using the Akaike’s information criterion in MrModeltest (ver. 2.3, J. A. A. Nylander, Uppsala University, Sweden, see https://github.com/nylander/MrModeltest2/releases/tag/v2.3), with data partitioned into the five regions indicated above. ITS fits a symmetrical (SYM) substitution model with roughly equal nucleotide frequencies (nst = 6), a gamma distribution of rate variation among sites with a proportion of invariant sites (SYM + Γ + I model), and State freqpr was set to fixed (equal) for this partition. All other regions fit general time-reversible likelihood (GTR) substitution models (nst = 6) with a gamma distribution of rate variation among sites (GTR + Γ model; trnL–trnF), or also with a proportion of invariant sites (GTR + Γ + I model; ETS, psbA–trnH, rbcL). Scored indels from all regions were combined as an extra partition. Indels were binary encoded and we applied a default two-state Markov model with gamma distribution of rates and coding set to variable (because there were no invariant sites). State freqpr was set to fixed (empirical) to reflect only having two states.
Bayesian posterior probabilities (PP) were estimated using two independent runs of 10 million generations using four chains, with tree sampling every 1000 generations. All parameters were set to be unlinked and with rates being variable between partitions, and all other unstated priors for the analysis were set flat (i.e. as Dirichlet priors). Runs were assessed as sufficient when checked for convergence with Tracer (ver. 1.7.1, see https://github.com/beast-dev/tracer/releases/tag/v1.7.1, accessed 5 March 2020; Rambaut et al. 2018) and when the standard deviation of split frequencies approached 0.001. Trees generated prior to the four Markov chains reaching stationarity (burn-in ~25%) were discarded and the remaining trees were used to construct a 50% majority-rule consensus tree, with nodes assigned posterior probabilities (PP) of 0.95–1.00 considered supported. Clades with 100% JK and PP of 1.00 were considered fully supported.
Results
After exclusion of 44 bp of sequence regions that could not be unambiguously aligned, the analysed dataset of 194 accessions and 129 species comprised 5145 bp, including 1332 parsimony-informative (PI; 182 being scored indels) and 507 variable but parsimony-uninformative characters. The plastid portion comprised 3601 bp, of which 644 were informative, including 98 scored indels, as follows: psbA–trnH, 248 PI (48 indels); rbcL, 86 PI (no indels); trnL–trnF, 310 PI (50 indels). The nuclear portion comprised 1544 bp, of which 688 were informative under parsimony, including 84 scored indels, namely, ETS, 325 PI (39 indels), and ITS, 363 PI (45 indels).
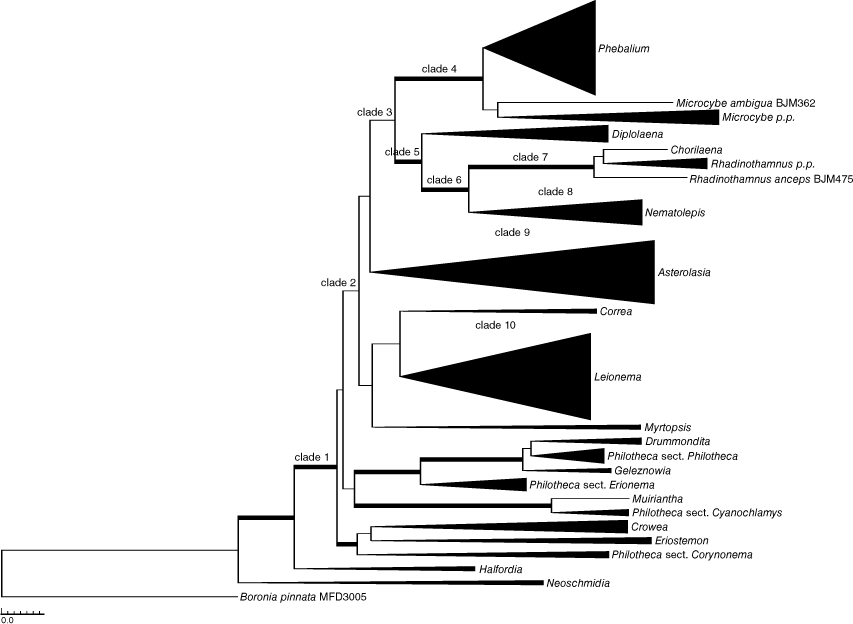
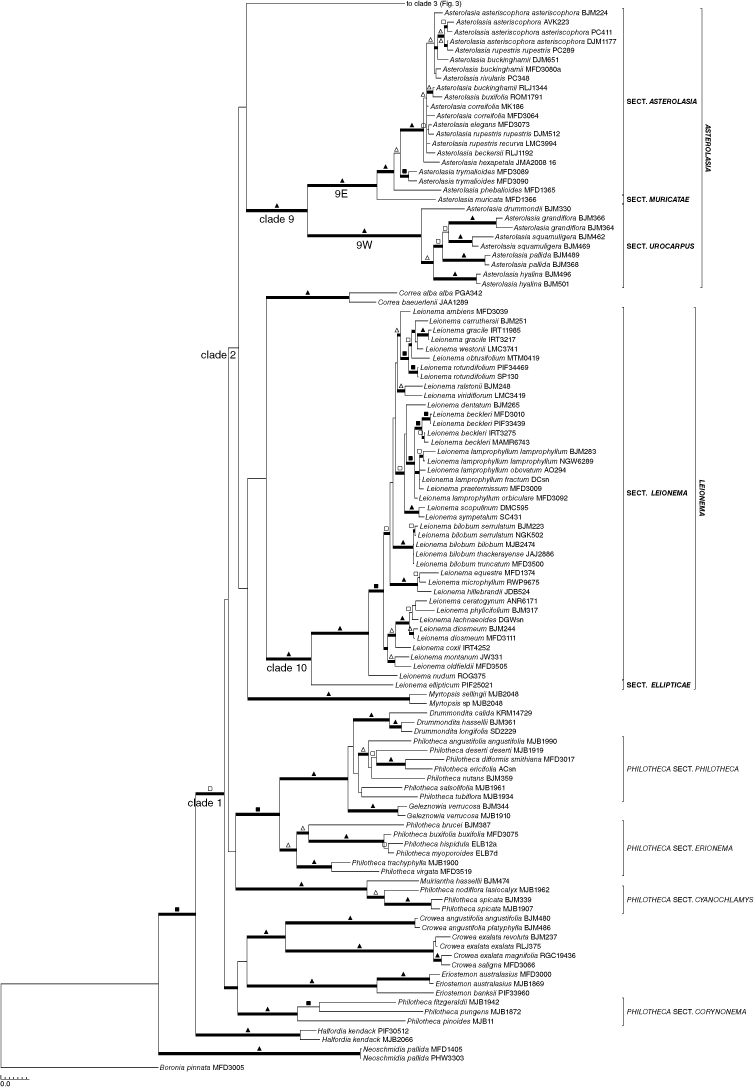
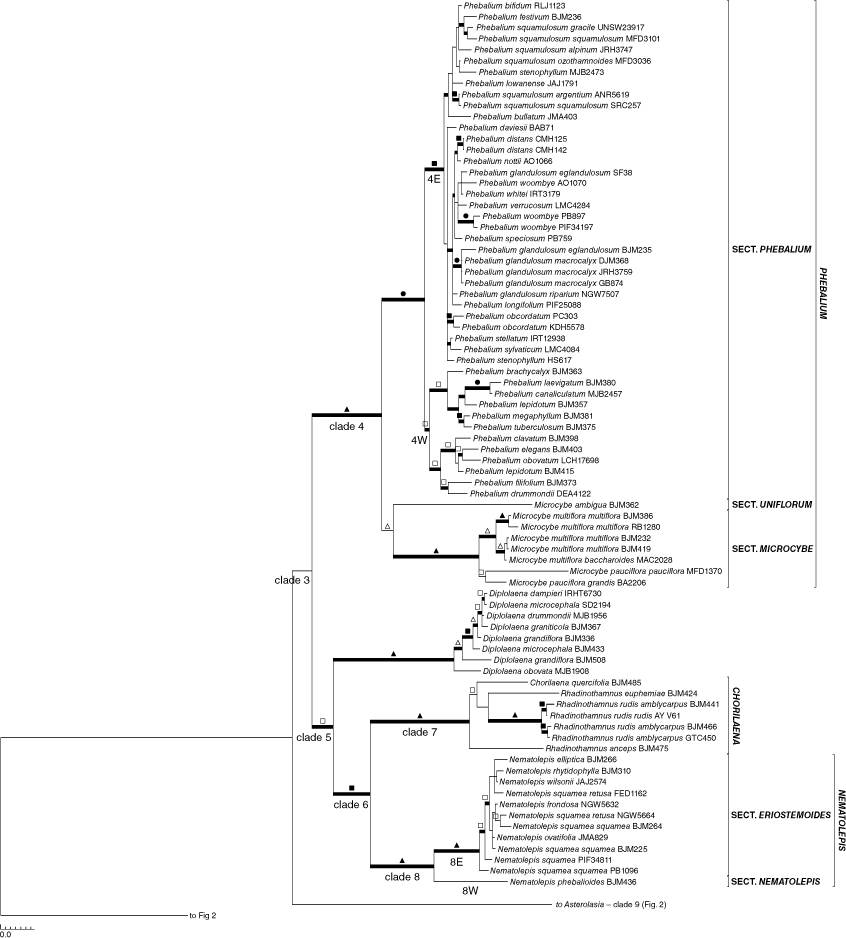
Separate analyses of the nuclear (Supplementary Fig. S1) or plastid (Supplementary Fig. S2) sequences identified minimal phylogenetic conflict among major clades, and those few differences are highlighted in the text below. No substantial changes in structure were seen on the major branches; the only differences were in support values for clades and resolution. On this basis, our final analyses presented here represent the combined molecular data. Fig. 1 is a simplified version of the full phylogenetic tree highlighting relationships among genera. For ease of viewing, we have expanded the complete phylogenetic tree over Fig. 2 and 3 to show details of species relationships. Parsimony and Bayesian analyses showed a high level of congruence, and Fig. 2 and 3 display supports from both jackknife (JK) values >50% and posterior probabilities (PP) on the Bayesian majority-rule consensus tree.
Parsimony analysis of the combined dataset produced 3900 equally most parsimonious trees in a single island of length 5599 steps. Indels in alignments of nuclear loci were mostly of 1 or 2 bp, although longer indels were found, whereas in the plastid loci insertions were predominantly longer repeats of adjacent sequence, with a few instances of very long deletions. A 21-bp inversion in psbA–trnH was highly homoplastic; that region was among those excluded from all analyses and presence or absence of the inversion was scored as an indel.
Neoschmidia and Halfordia occupy early divergent and isolated positions in all analyses, with the remaining genera forming a moderately supported clade (Clade 1: 1.00 PP, 72% JK; only data from combined analyses shown unless otherwise stated) (Fig. 1, 2, S1, S2). In all analyses, the genera Asterolasia, Correa, Diplolaena, Drummondita, Eriostemon, Geleznowia, Halfordia, Leionema, Nematolepis, Neoschmidia, Myrtopsis, and Phebalium were each monophyletic with robust support (all 1.00 PP, 100% JK; Fig. 1–3, S1, S2). The monotypic genera Chorilaena and Muiriantha were each represented by only one sample.
Four genera, namely, Rhadinothamnus, Microcybe, Crowea and Philotheca, were not monophyletic. Rhadinothamnus is paraphyletic with respect to Chorilaena in all analyses and the combined clade has robust support (Clade 7: 1.00 PP, 100% JK; Fig. 1, 3, S1, S2). Rhadinothamnus rudis (Bartl.) Paul G.Wilson constitutes a robust monophyletic lineage (1.00 PP, 100% JK) in combined and separate analyses, but the relationships among R. euphemiae (F.Muell.) Paul G.Wilson, Chorilaena and R. anceps (DC.) Paul G.Wilson are less clear. In the combined analyses, these three latter taxa are successive sister taxa to R. rudis, but without support. In the plastid-only analyses (Fig. S2), R. rudis + R. euphemiae has strong support (1.00 PP, 99% JK), but the sister relationship with Chorilaena is only moderate (80% JK). However, in the nuclear-only analyses (Fig. S1), Chorilaena + R. euphemiae is strongly supported (1.00 PP, 92% JK) as sister to an unsupported clade comprising R. anceps + R. rudis.
Although the core clade of Microcybe (M. multiflora Turcz. and M. pauciflora Turcz.) is robustly supported in all analyses, M. ambigua (C.A.Gardner) Paul G.Wilson occurs as a divergent lineage and is placed as a weakly supported sister to Phebalium (0.93 PP, 86 JK) in the plastid only analyses (Fig. S2), and weakly moderately as sister to the remainder of Microcybe in the combined (0.93 PP, 76% JK; Fig. 3) and nuclear only analyses (1.00 PP, 88% JK) (Fig. 3, S1). The Phebalium + Microcybe clade always has robust support (Clade 4: 1.00 PP, 100% JK; Fig. 1, 3), as does Phebalium.
A similar pattern to Microcybe occurs with Crowea. Although the genus Crowea does have robust support in both combined and nuclear analyses (1.00 PP, 100% JK; Fig. 1, 2, S1), it is not monophyletic in the plastid analyses where C. angustifolia Sm. (SW Aust.) does not group with the clade containing the two south-eastern Australian species; rather, these two lineages are part of a polytomy of three clades that also includes Eriostemon + Philotheca section Corynonema (Paul G.Wilson) Paul G.Wilson with some support (1.00 PP; no JK support; Fig. S2). Philotheca is polyphyletic, with its four sections each resolved as monophyletic in the combined analyses (with weak to strong support), but variously grouping with one or more of the genera Crowea, Drummondita, Eriostemon, Geleznowia and Muiriantha in all analyses. Although support for the polyphyly of Philotheca is strong, some nodes near the base of this group of genera have weak or no support in all analyses (Fig. 1, 2, S1, S2). This group of six genera is the subject of another study and is not dealt with in detail here.
A group of 10 genera, comprising Asterolasia, Chorilaena, Correa, Diplolaena, Leionema, Microcybe, Nematolepis, Myrtopsis, Phebalium and Rhadinothamnus, forms an unsupported clade in the combined analyses (Clade 2: Fig. 1–3) and was the main focus of this study. The lower nodes of this clade also have little or no support. There are some strongly to robustly supported relationships within this large unsupported clade, including Phebalium with Microcybe (Clade 4: 1.00 PP, 100% JK), Chorilaena with Rhadinothamnus (Clade 7: 1.00 PP, 100% JK) and this last clade with Nematolepis (Clade 6: 1.00 PP, 95% JK). In the combined (0.96 PP, 57% JK) and nuclear (0.99 PP, 59% JK) analyses, Diplolaena groups with Clade 6 with some support (Clade 5: Fig. 1, 2, S1) and in the plastid analyses it groups with a poorly supported Clade 6 + Phebalium + Microcybe clade with some support (0.97 PP, no JK support, Fig. S2). This last clade was also retrieved in the combined analyses without support. Asterolasia, Correa, Leionema and Myrtopsis do not form well-supported relationships with any other genera.
Within some genera, there are weakly to robustly supported clades that correlate with geography (Fig. 2, 3). Asterolasia, Crowea and Nematolepis are all divided into robustly supported clades, one confined to south-western Australia (1.00 PP, 100% JK for Asterolasia and Crowea; monotypic for Nematolepis) and the other to south-eastern Australia (including South Australia, SA, and Tas.) (1.00 PP, 100% JK for all) in all analyses. This pattern is repeated in Phebalium, although the south-western clade (Clade 4W, Fig. 3) is only weakly supported in any analysis. Within Leionema, the north Queensland species, L. ellipticum, is sister to a robustly supported clade (1.00 PP, 100% JK) containing the remainder of the species (Fig. 2). Within this latter clade, the New Zealand species, L. nudum, is sister to a strongly supported clade (1.00 PP, 99% JK) containing all remaining species that are from south-eastern Australia including Tasmania.
Discussion
The monophyly of Asterolasia, Correa, Diplolaena, Eriostemon, Leionema, Nematolepis and Phebalium is confirmed, reaffirming the results presented in the more limited ITS analysis of Mole et al. (2004). Rhadinothamnus is paraphyletic with respect to Chorilaena, and Philotheca is polyphyletic with its four sections forming separate clades aligning with the genera Crowea, Drummondita, Eriostemon, Geleznowia and Muiriantha (see further discussion below). The monophyly of both Microcybe, with the inclusion of M. ambigua, and Crowea were supported in only some of the analyses. The lowermost nodes of some genera, as shown in Fig. 2, 3, S1, S2, have a strong geographic correlation as exemplified by the strong east–west divide as seen in Asterolasia, Crowea, Nematolepis and Phebalium, and the more geographically remote species in Leionema, from north-eastern Queensland and New Zealand, being in early divergent positions. This strong east–west pattern, commonly inferred to be a result of vicariant separation, is well documented in speciation patterns in a variety of other plant families and is considered to reflect changes in landform and climate during the increasing aridification of the continent through the Cenozoic (see, e.g., Crisp and Cook 2007; Nge et al. 2020; Binks et al. 2022; Clowes et al. 2022).
There is little robust resolution of relationships among genera apart from Chorilaena with Rhadinothamnus and these with Nematolepis and sometimes also Diplolaena, Phebalium with Microcybe, and Halfordia and Neoschmidia occupying early divergent positions as also presented by Mole et al. (2004), Bayly et al. (2013) and Appelhans et al. (2021). The generic relationships of Asterolasia, Correa, Myrtopsis and Leionema remain unresolved. The relationships within genera and some of the generic groups are discussed below.
Extensive paraphyly of many species on short branches (Fig. 2, 3, S1, S2), particularly in eastern lineages, is perhaps an indicator of recent divergence or ongoing incomplete lineage sorting. Further work with a greater density of sampling within species, and using datasets with more characters, is needed to clarify whether the phylogeny represents distinct species lineages or is confounded by the effects of incomplete lineage sorting or possibly hybridisation. Discussion of interspecific relationships in most genera would be premature on the basis of present sampling. Ground-breaking genome sequencing in progress as part of projects such as the Genomics for Australian Plants (GAP), Australian Angiosperm Tree of Life (AAToL) and Plant and Fungal Tree of Life project (PAFTOL) is examining some lineages of Rutaceae, using more detailed genomic data. This approach, utilising the Angiosperms353 target capture nuclear bait set, including up to 353 genes, provides many more informative characters than does Sanger sequencing of individual loci and might help resolve some of these interspecific relationships. Such data have recently been reported by Reichelt et al. (2021) to greatly improve resolution and support for clades within the speciose and pantropical Zanthoxylum L. (Zanthoxyloideae: Rutaceae).
The remainder of the discussion will focus on specific genera and generic groups followed by a section on pollination.
Asterolasia
Of the 19 species and 4 subspecies recognised in Asterolasia, 17 species and 3 subspecies were included in this study. Asterolasia exasperata P.R.Alvarez & Duretto (NSW) and A. sola Duretto & P.R.Alvarez (Qld), which were recently segregated from A. correifolia (A.Juss.) Benth. (NSW) (Alvarez and Duretto 2019a), were not included in the study, and neither was A. asteriscophora subsp. albiflora B.J.Mole (Victoria, Vic.). Asterolasia is monophyletic (Clade 9: Fig. 1, 2) and its species form two robustly supported subclades, namely, one with all five south-western Australian species (Clade 9W: Fig. 2), and the other with all the south-eastern Australian species (Clade 9E: Fig. 2). In the south-eastern Australian clade, A. muricata J.M.Black (SA) is sister to the remaining species, which form a robustly supported group (1.00 PP, 100% JK), and then in a stepwise fashion A. phebalioides F.Muell. (SA, W Vic.) is sister to an unsupported clade (0.82 PP, 82% JK), and then A. trymalioides F.Muell. (alpine areas, SE Aust.) to a robustly supported clade containing species mainly from non-alpine areas in New South Wales (A. asteriscophora (F.Muell.) Druce is also in eastern Vic.) (1.00 PP, 100% JK), which has little internal support. Morphological evidence indicates that A. exasperata and A. sola are closely related to A. correifolia of this last clade.
Within the south-western Australian clade, there is weak to robust support for the internal structure, with A. drummondii Paul G.Wilson (the most northerly species in this clade) sister to a moderately supported clade (1.00 PP, 82% JK) containing the remainder, and then, going up the tree, A. hyalina (Paul G.Wilson) Wege, A. pallida Benth., and then A. squamuligera (Hook.) Benth. sister to A. grandiflora (Hook.) Benth. Results are consistent with both propositions put forward by Wege (2017), namely that A. nivea (Paul G.Wilson) Paul G.Wilson is a synonym of A. grandiflora, and that the subspecies of A. pallida, as outlined by Wilson (1998c, 2013e), should each be recognised at specific rank. The two samples of A. grandiflora in these analyses represented the forms previously recognised as the two separate species, namely A. grandiflora sens. strict. and A. nivea; the results using molecular data confirmed that at least they are closely related, with the length of the branches like that seen within other species. Asterolasia pallida, if circumscribed in the broad sense with two subspecies, i.e. including samples identified here as A. hyalina, would be paraphyletic.
The two clades of Asterolasia are each robustly supported and clearly distinct on molecular grounds and are here considered worthy of taxonomic recognition. The type species of Asterolasia is A. trymalioides of the south-eastern Australian clade. Generic and subgeneric synonyms of Asterolasia are Actinostigma Turcz. (type: Actinostigma lanceolatum Turcz. = Asterolasia correifolia), Pleurandropsis Baill. (type: P. phebalioides (F.Muell.) Baill. = A. phebalioides), Urocarpus J.Drumm. ex Harv. (type: U. phebalioides J.Drumm. ex Harv. = A. drummondii), Asterolasia section Urocarpus (Drumm. ex Harv.) Benth., Asterolasia sect. Pleurandropsis (Baill.) Kuntze, Phebalium a. Correoides Endl. (containing A. correifolia and A. hexapetala (A.Juss.) Druce), and Phebalium section Correoides (Endl.) Pfeiff. As with Asterolasia, the type species of all these taxa are in the south-eastern Australian clade (see Wilson 1971, 1987, 1998c, 2013e) except that for Urocarpus, which is from south-western Australia. The names Asterolasia and Urocarpus were published at nearly the same time and there has been some conflict over which genus has priority (see Wilson 1971, 1980, 1987). Until 1971, Asterolasia was considered to be the earliest published name. When revising the genus, Wilson (1971) considered Urocarpus to have precedence, believing that it had been published several months earlier in 1855 than was Asterolasia. However, he considered that both genera could be segregated on the basis of carpel number, with Urocarpus sens. strict. having two or three carpels and all south-eastern Australian species, except A. muricata (SA), having five carpels. Wilson (1971) transferred A. muricata and the south-western Australian species that did not already have validly published names in Urocarpus to that genus, leaving the remaining species in Asterolasia and noting that any further nomenclaturial changes must await further taxonomic study. The result was that from 1971, both names were in use. Wilson (1980), when describing U. niveus Paul G.Wilson from south-western Australia, noted that because this species had three or four carpels, there was a gradation of carpel number between Urocarpus and Asterolasia. He concluded the two genera could not be maintained as distinct but did not transfer the south-eastern Australian species of Asterolasia to Urocarpus and so the two generic names remained in use. The issue was later simplified when the publication date for Asterolasia was determined to be in 1854 and not 1855 (Aston 1984; Wilson 1987), thus giving Asterolasia precedence over Urocarpus. Wilson (1987) recognised a broad concept for Asterolasia to cover both south-western and south-eastern Australian species and ensured that all accepted species had validly published names in Asterolasia.
South-western Australian species have one to four carpels, as opposed to species found in south-eastern Australia, which have five carpels, except A. muricata, which has two carpels and is the sister species to the remainder of the south-eastern clade. The name Asterolasia section Urocarpus is available for the south-western clade. There do not seem to be clear morphological apomorphies for either of these geographic clades, even though there is strong molecular support for them. Removing A. muricata from A. section Asterolasia would resolve some issues but would require creation of a third monotypic section without clear apomorphies, or, if placed in A. section Urocarpus, a paraphyletic section. This is a situation similar to that found in Boronia section Boronia where there were clearly demarcated groups on the basis of molecular data, one in south-eastern Australia and another in south-western Australia, but without identifiable morphological apomorphies (Duretto et al. 2023).
The south-eastern Australian species of Asterolasia have yellow or white petals, whereas those in the south-west have flowers with white or pink petals, except for A. squamuligera, which has yellow petals. The character used to distinguish Urocarpus from Asterolasia was having one to four carpels as opposed to five. All south-western species have the reduced number of carpels, as does A. muricata from South Australia, which is the sister species of a robust clade containing all other species from south-eastern Australia. The relationship between A. muricata and the remaining south-eastern species is robust and the branch length between it and the other species is not significantly long. Reduction in carpel number is unusual in Australasian Rutaceae (see also discussion under Phebalium and Microcybe). It could be inferred from the results presented here that a reduction in carpel number is a plesiomorphic state for the genus and having five carpels is a reversion. Alternatively, an equally parsimonious hypothesis is that reduction in carpel number has evolved twice in the genus, i.e. on the branch leading to south-western Australian species and on that leading to A. muricata. Although a reduced carpel number may or may not be an apomorphy for the genus, it is potentially a good diagnostic character to define a section based on Urocarpus, except for a problem regarding the placement of A. muricata. To recognise only two clades (south-eastern and south-western, 9E and 9W in Fig. 2) would mean that neither clade can be defined morphologically. Formally recognising a third monotypic clade comprising A. muricata would enable a formal classification that would be practicable and acknowledge the isolated placement of A. muricata. These taxa being recognised at a sectional level is appropriate, given that the two clades partially defined by a reduced carpel number lack other clear-cut morphological features to separate them. Here we recognise the following three sections for Asterolasia: section Asterolasia (SE Aust., 13 spp.; 5 carpels; yellow or white petals), section Urocarpus (SW Aust., 5 spp.; 1–4 carpels, white, pink or yellow petals, if yellow then stellate hairs with the rays fused so that the hairs look like fimbriate scales and leaves flat), and section Muricatae Duretto & Heslewood (SA, monotypic; 2 carpels, yellow petals with stellate hairs with unfused rays, leaves revolute), which is formally described below.
Chorilaena and Rhadinothamnus
A close relationship between Chorilaena (monotypic, sampled) and Rhadinothamnus (all 3 species and 2 of the 3 subspecies of R. rudis sampled) has been postulated before, because the genera share a unique seed type that has two persistent cartilaginous strands on the adaxial surface between which the aril is attached, as well as the hemispherical calyx, valvate petals, and non-glandular anther apiculum (Wilson 1970, 1971, 1998b, 2013f; Mole et al. 2004; Armstrong 2013). The results presented here (Fig. 1, 3, S1, S2) indicate that retaining the two genera, as currently circumscribed, is not well justified. Mole et al. (2004), referring to this issue, indicated that broadening the concept of Chorilaena to include all species of Rhadinothamnus would create an extremely morphologically diverse genus and their preferred option was to retain Rhadinothamnus and Chorilaena as monotypic and raise the former Phebalium section Gonioclados (containing R. anceps and R. rudis) to generic rank, despite the section not having good support in their analysis. The necessary nomenclatural changes were not made. In the analyses presented here, there is no support for this proposition and no strong support for any species–pair relationships within this clade. The length of the branches in this clade are comparable to that found in other genera. The four species being retained in one genus, an expanded Chorilaena, would create a morphologically diverse genus, in habit, leaf form, and in inflorescence and flower morphology. The inflorescence and flower diversity are, presumably, driven by differences in pollination systems. The morphological diversity seen in the Chorilaena + Rhadinothmanus clade is no more diverse than that seen in other Australasian genera of Rutaceae where various pollination systems appear to have evolved (see discussion below on pollination).
Results presented here and by Mole et al. (2004) indicated that there are the following four taxonomic choices for these four species: (1) acknowledge the close relationship of the four species and expand Chorilaena to include all four; (2) because there are no clear relationships identified among the four species, have four monotypic genera, which would require two novel genera to be formally described; (3) raise Phebalium section Gonioclados to genus level, as proposed by Mole et al. (2004), for the superficially morphologically similar R. anceps and R. rudis, despite this relationship not being supported by molecular data; or 4, recognise that the exact relationships of C. quercifolia Endl. and R. anceps to the other species are not well supported at this stage and maintain the status quo until they are more clearly established. Here, we adopt Option 1 to reflect the data and make the appropriate nomenclatural changes below.
The close relationship of the expanded Chorilaena to Nematolepis is strongly supported (1.00 PP, 95% JK) in our analyses, confirming the results of Mole et al. (2004). Wilson (1970) had also considered Phebalum section Eriostemoides (=Nematolepis) and P. section Gonioclados (=Chorilaena) to be closely related.
Correa
Correa (11 spp.) is represented in the analyses by two accessions that represent each of its two subgenera (see Othman et al. 2010). The monophyly of the genus is confirmed with robust support (Fig. 2, S1, S2). Of note is how the genus resolves with different taxa in each of the different analyses and further work is required to ascertain the relationships of Correa. Correa is also discussed under Pollination below.
Diplolaena
Diplolaena is monophyletic with robust support (1.00 PP, 100% JK; Fig. 1, 3, S1, S2; 6 of 15 species sampled), but its relationships with other genera are inconclusive. Wilson et al. (1998) and Wilson (2013g) indicated that most species grade into each other and offered no discussions of relationships within the genus or between it and other genera. In the combined (0.96 PP, 57% JK) and nuclear (0.99 PP, 58% JK) analyses Diplolaena grouped with Clade 6 (Chorilaena + Rhadinothamnus + Nematolepis) with weak support, although in the plastid analyses (0.97 PP) it was sister to an unsupported clade comprising Clade 6 and the Phebalium + Microcybe clade. No clear groups are identified within Diplolaena and so no formal infrageneric taxonomy is proposed here. Diplolaena is also discussed under Pollination below.
Eriostemon, Philotheca and their relatives (Crowea, Drummondita, Geleznowia, Muiriantha)
The large genus Philotheca is represented here by 18 species (of ~55 total) and all four sections were included in this study. Closely related genera are also well represented in this study, including Eriostemon (both species sampled), Crowea (all three species sampled), Drummondita (3 of 10 species sampled), Geleznowia (1 of 2 species sampled), and Muiriantha (monotypic, sampled). Philotheca is polyphyletic (Fig. 1, 2, S1, S2), confirming results presented by Bayly et al. (2013), Duretto et al. (2020) and Appelhans et al. (2021), although those analyses included fewer species. Each of the four sections was monophyletic and there was strong to robust support for each of the following relationships: species of P. section Philotheca in a clade with Geleznowia and Drummondita (1.00 PP, 100% JK); that clade being sister to P. section Erionema with strong support (1.00 PP, 99% JK); P. section Cyanochlamys (F.Muell.) F.Muell. being sister to the monotypic Muiriantha (1.00 PP, 100% JK); and P. section Corynonema grouping with Eriostemon and Crowea in the combined analyses with more modest support (0.99 PP). Wilson (1998a) discussed the relationships among the sections and noted that P. section Philotheca is similar to Geleznowia in seed morphology. Of note is the clear split between the south-western Australian species of Crowea from the clade containing both south-eastern Australian species. With further study, Crowea may require further division. On the basis of the single exemplars in our molecular analyses, the specific status of C. saligna Andrews is open to question, being embedded within the C. exalata F.Muell. clade with three sampled subspecies. The entire Philotheca assemblage is polyphyletic and the relationships recovered among the larger clades differ between analyses. Philotheca and its relationships are part of another study (see e.g. Batty et al. 2022), with greater taxon sampling and using whole plastid sequences (H. Orel, pers. comm.), and will not be discussed in detail further here. Philotheca section Philotheca is also discussed under Pollination below.
Leionema
Leionema is monophyletic with robust support (Clade 10: 1.00 PP, 100% JK; Fig. 1, 2, S1, S2; 27 of 28 species and all 8 subspecies represented). The only species not represented in this study is L. elatius (F.Muell.) Paul G.Wilson (NE NSW). That species was recently revised by Telford and Bruhl (2020), who determined that the two subspecies previously recognised (see Wilson 1998b, 2013a) each warrant specific rank and that the typical form is a narrow endemic, in contrast to how it had been treated previously. Most material previously assigned to L. elatius subsp. elatius is now included in the more widespread L. beckleri (F.Muell.) I.Telford & J.J.Bruhl. Wilson (1970, 1998b) noted that the relationship of Leionema to other genera in tribe Boronieae is not clear; however he did indicate that it has no close affinity to Phebalium sens. strict., Rhadinothamnus or Nematolepis, a statement supported by this study. Leionema, like Diplolaena, Correa and Myrtopsis, is indisputably part of the Eriostemon Group but has no clearly identifiable close relatives.
Relationships within Leionema have some strong to robust support in all analyses. Leionema ellipticum (NE Qld) is sister to a robust clade (1.00 PP, 100% JK) containing the remaining species, confirming the results of Mole et al. (2004). Within this later clade, L. nudum (New Zealand) is sister to strongly supported clade (1.00 PP, 99% JK) containing all remaining taxa (SE Aust., including Tas.). Within the south-eastern Australian clade, there are a number of species groups. These include the following: (1) a South Australian clade (1.00 PP, 100% JK) containing all three species found in that state, namely, L. equestre (D.A.Cooke) Paul G.Wilson, L. hillebrandii (J.H.Willis) Paul G.Wilson and L. microphyllum (F.Muell.) Paul G.Wilson (also in W Vic.); (2) a Tasmanian clade (1.00 PP, 87% JK) containing both Tasmanian endemic species L. montanum (Hook.) Paul G.Wilson and L. oldfieldii (F.Muell.) Paul G.Wilson, a relationship noted by Wilson (1970), which was not retrieved in the nuclear analyses; (3) a clade containing L. ceratogynum N.G.Walsh, L. diosmeum (A.Juss.) Paul G.Wilson, L. lachnaeoides (A.Cunn.) Paul G.Wilson, L. phylicifolium (F.Muell.) Paul G.Wilson, and L. coxii (F.Muell.) Paul G.Wilson (1.00 PP, 82% JK; L. coxii is not part of this clade in the nuclear analyses); and (4) all four subspecies of L. bilobum (Lindl.) Paul G.Wilson (3 Vic., 1 Tas.) (1.00 PP, 100% JK). The remaining species group with these clades and other species with mixed support. Most species of Leionema were monophyletic where multiply sampled, although there is some paraphyly, which indicates that further investigation is needed of the circumscription of L. lamprophyllum (F.Muell.) Paul G.Wilson and the relationships of its subspecies with both L. beckleri and L. praetermissum P.R.Alvarez & Duretto.
Leionema ellipticum was first collected in 1991 from a mountain top in the Humid Wet Tropics of north-eastern Queensland, an area rich in endemics and taxa with significant phylogenetic isolation and varied geographic connectivity. Wilson (1998b), when describing L. ellipticum, noted that it differed from all other species in the genus by having minutely apiculate anthers that are bluntly mucronulate (v. retuse) and a grooved disc or gynophore divided into 10 parts (v. entire), and suggested that it may not be correctly placed in the genus and may warrant a genus of its own. Mole et al. (2004) considered that L. ellipticum could be placed in either a monotypic genus or a new section of Leionema. Wilson (1998b) and Mole et al. (2004) are correct; the isolated position of L. ellipticum is confirmed and so warrants taxonomic recognition, either as a monotypic genus or a monotypic infrageneric taxon within Leionema. Morphologically the species is similar to the remainder of the genus and this relationship is supported by the predominance of the coumarin osthol in both L. ellipticum and many other species of Leionema (Halstead et al. 2005). To reflect the phylogenetic signal, a new monotypic section is formally described here to accommodate the species (see Taxonomy below).
Wilson (1970) indicated that although Leionema nudum (New Zealand) showed no close affinity to other species in Leionema (E Aust.), it clearly belonged in the genus. No obvious morphological apomorphies can be discerned for the remaining species in the south-eastern Australian clade to distinguish them from L. nudum. Within the south-eastern Australian clade, there are some species groups (see above) but most of the other species do not fall into groups, and the groups themselves are not always easy to define morphologically. In NSW, there are several species that have pendent flowers (L. carruthersii (F.Muell.) Paul G.Wilson, L. ralstonii (F.Muell.) Paul G.Wilson, L. sympetalum (Paul G.Wilson) Paul G.Wilson, L. viridiflorum (Paul G.Wilson) Paul G.Wilson), a character shared with some species in other genera (e.g. Correa and Diplolaena), but only L. ralstonii and L. viridiflorum group together (see also discussion below under Pollination). No formal taxonomic groups will be recognised within the typical section of Leionema.
Myrtopsis
Myrtopsis (9 spp.) is represented in the analyses by two accessions and the genus has robust support (Fig. 1, 2, S1, S2). The relationships of the genus are unresolved and in the different analyses it groups with different taxa. Further work is required to ascertain the relationships of this New Caledonian genus.
Nematolepis
Nematolepis is monophyletic with robust support (Clade 8: 1.00 PP, 100% JK; Fig. 1, 2, S1, S2; see also Mole et al. 2004; all seven species and both subspecies of N. squamea (Labill.) Paul G.Wilson sampled). The genus is most closely related to the Chorilaena + Rhadinothamns clade (= Chorilaena sens. lat.; see above). In all analyses, south-eastern Australian species formed a robust clade (Clade 8E: 1.00 PP, 100% JK) sister to the sole south-western Australian species, N. phebalioides. Nematolepis phebalioides differs from all other species in having pendent flowers with a tubular fused corolla that is red with green or yellow tips (see discussion on Pollination below). The other species have cymose non-pendent inflorescences and flowers with spreading, unfused, white or yellow petals. The south-eastern clade is strongly supported and both clades can be defined on morphological characters, and so they warrant taxonomic recognition.
Wilson (1970) considered that Phebalium (which at the time of his publication included the south-eastern Australian species of Nematolepis) was diverse morphologically and its sections appeared to be more closely related to other genera than they were to each other. He retained Phebalium in the broad sense because he considered that there was gradation between the taxa. Wilson (1970) did note that P. section Eriostemoides, which included all south-eastern Australia species now placed in Nematolepis, shared with N. phebalioides the following characters: two subfloral bracteoles, free and imbricate sepals, glabrous petals, and slightly retuse anthers and seed characteristics. Wilson (1998b) later transferred the species of P. section Eriostemoides to Nematolepis, although he did not make a combination for the section under that genus. The type species of P. section Eriostemoides is N. squamea and, so, a name is available for the south-eastern clade. The rank of section for the two clades is appropriate and a new combination in Nematolepis for P. section Eriostemoides is made below (see Taxonomy). There is weak or no support for relationships within the south-eastern clade. The only species in the genus with multiple accessions, namely N. squamea, was polyphyletic, as were both of its subspecies. Further work is required to determine species limits and relationships of the species in this section.
An apparent absence of speciation in the western lineage, which is monotypic and sister to the eastern clade, is not due to a younger age and there is no evidence that this lineage resulted from a later dispersal. With Rutaceae being poorly represented in the fossil record, we cannot speculate about the possible role that extinction of species might have had on such depauperate lineages. Instead, this pattern could reflect factors such as the greater prevalence of bird pollination (Keighery 1980; Phillips et al. 2010) and a simpler landscape topography maintaining genetic connectivity over broader areas in the west (Phillips et al. 2010).
Phebalium and Microcybe
Phebalium (30 of 35 species, 4 of the 6 subspecies of P. glandulosum Hook., and 5 of the 8 subspecies of P. squamulosum Vent. sampled) and Microcybe (3 of 4 species and all 4 subspecies sampled) form a well-supported clade in all analyses (Clade 4: Fig. 1, 3, S1, S2) supporting the conclusions of Wilson (1970, 1998b, 2013b, 2013c) and Mole et al. (2004). The main contentious issue within the clade is the position of M. ambigua. The species is sister to the remainder of Microcybe in both the combined analyses (0.93 PP, 76% JK) and the analyses based on nuclear data (1.00 PP, 88% JK), although with only moderate support. In the analyses utilising plastid data, M. ambigua is sister to Phebalium with moderate support (0.93 PP, 86% JK). The remainder of Microcybe and all of Phebalium are both robustly supported clades in all analyses, as is the entire Microcybe + Phebalium clade. Missing from this study are M. albiflora Turcz., P. appressum Paul G.Wilson, P. brevifolium Paul G.Wilson, P. calcicola S.Dema & I.Telford, P. cicatricatum A.J.Ford & Duretto, P. graniticola I.Telford & J.J.Bruhl, and P. microphyllum Turcz. Microcybe albiflora is morphologically similar to both M. multiflora and M. pauciflora. The south-western Australian species, i.e. P. appressum, P. brevifolium and P. microphyllum, are morphologically similar to other south-western species such as P. filifolium Turcz. and P. tuberculosum (F.Muell.) Benth. (see also Wilson 1970, 1998b, 2013b), whereas the eastern Australian species, i.e. P. calcicola, P. cicatricatum and P. graniticola, are part of the P. squamulosum group of species (see Telford et al. 2019; Ford and Duretto 2020; Dema et al. 2021).
Microcybe ambigua has had a complicated taxonomic history. It was originally described as M. pauciflora var. uniflora D.A.Herb. (Herbert 1922) and then placed in Phebalium (as P. ambiguum) by Gardner (1943) without much explanation, only to be moved back to Microcybe by Wilson (2013c). Microcybe ambigua shares with the remainder of Microcybe the reduced number or carpels (1–4, v. 5 in Phebalium). It differs from virtually all other species in both genera in having solitary, sessile flowers. All other species of Microcybe have sessile flowers but have inflorescences containing 5–20 flowers. Phebalium clavatum C.A.Gardner is the only species in Phebalium to have solitary and sessile flowers and it also has unusual leaf morphology (Wilson 2013c). That species groups here within a weakly supported clade (1.00 PP, 72% JK), nested well within the south-western Australian clade of Phebalium, that also contains P. drummondii Benth., P. elegans Paul G.Wilson, P. filifolium, P. lepidotum (Turcz.) Paul G.Wilson, and P. obovatum (Paul G.Wilson) Paul G.Wilson (see also Mole et al. 2004, where it forms a clade with P. elegans and P. filifolium).
Wilson (2013c) indicated that Microcybe ambigua was anomalous and discussed two alternative options to his treatment when he transferred it to Microcybe from Phebalium. These were to place it in a monotypic genus or alternatively expand Phebalium to include it and other species of Microcybe. Both these options are consistent with the results presented here. The isolated position of the species in the Microcybe + Phebalium clade certainly requires acknowledgement but the adoption of a monotypic genus that would be defined on few, non-unique, morphological characters seems to be unwarranted. A third option could be to retain M. ambigua in Microcybe but to erect a monotypic section for it, but that is problematic because its close relationship with Microcybe is only moderately supported in some analyses and is not supported by plastid data. We consider that expanding Phebalium to include Microcybe is warranted, as the genera share several apomorphies, including terminal umbels, similar seeds, and having stellate-lepidote trichomes and the relationship is robustly supported here. Reducing Microcybe sens. strict. to the rank of section is appropriate given both clades are robustly supported. The uncertain placement of M. ambigua is recognised by making it the sole member of a newly described and monotypic section (see Taxonomy below). The recognition of sections on the basis of carpel number mirrors our treatment of Asterolasia, although there is not the clear geographical split that is seen in that genus (see above).
The contrasting positions of Microcybe ambigua in this study may suggest a hybrid origin for the species. We saw no signature in the sequences suggestive of this, such as high levels of polymorphism at nuclear loci, which often characterise hybrids. We recorded only two polymorphic sites in ETS and none in ITS. Sequences from M. ambigua show a high number of uninformative substitutions at various loci, indicative of a long period of genetic isolation from both Microcybe sens. strict. and Phebalium. Microcybe ambigua is also lacking most of the distinctive indels of the plastid data that characterised the other two species of Microcybe used in this study.
Phebalium sens. strict. forms two well-supported geographic clades, namely, one from eastern Australia and another one from south-western Australia. A pattern of geographically separated clades is also seen here in Asterolasia, Crowea and Nematolepis and elsewhere in Rutaceae such as in Boronia sections Boronia (Duretto et al. 2023) and Valvatae (Benth.) Engl. (Duretto 1999). In Phebalium, the eastern clade is more strongly supported (Clade 9E: 1.00 PP, 99% JK) than is the south-western clade (Clade 9W: 0.98 PP, 72% JK), which has no support in the analyses utilising plastid data alone. Because morphological characters could not be found to support these clades and because this genus is part of a more comprehensive study by other workers, no formal taxonomic changes within Phebalium sens. strict. are proposed here.
This study has also highlighted the risks associated with sampling only single exemplars or single loci. In Phebalium, there may have been ongoing genetic exchange leading to retention of ancestral polymorphisms in a range of taxa. Almost no taxa represented by multiple accessions were resolved as monophyletic. Further work with a greater density of within-species sampling is needed to determine whether the groupings seen are distinct lineages or whether the phylogenetic signal has been confounded by incomplete lineage sorting. For future studies, it is imperative to include multiple accessions of each taxon to determine whether taxa can be resolved before potentially flawed interpretations are made about relationships.
Pollination
The majority of species in this study have the typical inflorescences and flowers of Australian Rutaceae, i.e. open inflorescences, and erect open flowers with white or yellow petals. These species are entomophilous and pollinated variously by beetles, flies, bees, moths and butterflies (Armstrong 1979). Several species appear to have pollination systems utilising birds where the inflorescence and flowers are pendent, and often with fused petals that are green or red, such as, for example, Chorilaena quercifolia, Correa (all species except C. alba Andrews), Diplolaena (all species), Leionema ralstonii, L. viridiflorum, L. sympetalum, Muiriantha hassellii (F.Muell.) C.A.Gardner, Nematolepis phebalioides, and three species of Philotheca section Philotheca, P. coccinea (C.A.Gardner) Paul G.Wilson, P. nutans (Paul G.Wilson) Paul G.Wilson and P. pachyphylla (Paul G.Wilson) Paul G.Wilson (corolla white). The inflorescence and flowers of Chorilaena quercifolia are, in overall form and colour, remarkably similar to those of L. viridiflorum, a case of convergent evolution. The groups with pendent flowers do not group together and it would appear that pendent flowers and bird pollination have arisen several times in the Eriostemon group (see also Claßen-Bockhoff et al. 1991). Within Leionema itself, pendent flowers appear to have arisen independently several times. The four species of Leionema that have pendent flowers do not group together, although L. ralstonii and L. viridiflorum do so in the combined and plastid analyses and do look similar.
In Diplolaena, the inflorescence is a terminal campanulate pendent head that is surrounded by three or four rows of large and sometimes showy bracts that often exceed the flowers in length. The flowers of Geleznowia are also in terminal clusters surrounded by petaloid bracts but are erect. These two south-western Australian genera are not closely related taxa, and so this is another case of convergent evolution. Other taxa with terminal heads include Phebalium sens. lat. and Philotheca nodiflora (Lindl.) Paul G.Wilson (Philotheca section Cyanochlamys). Another unusual flower type in the Eriostemon Group that differs significantly from the more open flower is that found in Drummondita and Chorilaena euphemiae (F.Muell.) Duretto & Heslewood (previously Rhadinothamnus euphemiae). In these species, the corolla forms an erect tube of unfused petals that barely open. The stamens of Drummondita are also fused. Again, these are not closely related, and this is another case of convergent evolution driven by pollinators. The significant diversity of flower and inflorescence types and assumed pollination syndromes in the Eriostemon group is in sharp contrast to that seen in the very large and diverse Boronia + Melicope clade to which it is sister (see Appelhans et al. 2021, for example) and would be a fertile field for further study.
Taxonomy
With the incorporation of the following taxonomic changes, the Eriostemon group now comprises 16 genera. Nomenclatural novelties and notes are provided below for Asterolasia, Chorilaena (here including Rhadinothamnus), Leionema, Nematolepis, and Phebalium (here including Microcybe). The remaining 11 genera, namely Correa, Crowea, Diplolaena, Drummondita, Eriostemon, Halfordia, Geleznowia, Muiriantha, Myrtopsis, Neoschmidia and Philotheca, are not discussed further here.
Asterolasia F.Muell., Trans. Philos. Soc. Victoria 1: 9 (1854)
A genus of 19 species with five species restricted to south-western Australia and 14 to south-eastern mainland Australia, nine of which are endemic to New South Wales (see Wilson 2013e; Orme and Duretto 2017; Wege 2017; Alvarez and Duretto 2019a).
There are three sections.
Key to sections|
1. Carpels 1–4 (SW Aust., SA) |
|
2. Petals yellow, stellate hairs with unfused rays on abaxial surface; leaves revolute (SA) |
Asterolasia section Asterolasia
Hairs stellate, rays unfused. Leaves flat to revolute. Petals white or yellow. Carpels 5.
A section of 13 species confined to south-eastern Australia, although absent from Tasmania.
Species
Asterolasia asteriscophora (2 subspp.), A. beckersii Orme & Duretto, A. buckinghamii (Blakely) Blakely, A. buxifolia Benth., A. correifolia, A. elegans L.McDougall & Porteners, A. exasperata, A. hexapetala, A. phebalioides, A. rivularis Paul G.Wilson, A. rupestris B.J.Mole (2 subspp.), A. sola, A. trymalioides.
Asterolasia section Urocarpus (J.Drumm. ex Harv.) Benth., Fl. Austral. 1: 350, 352 (1863)
Hairs stellate, rays unfused or fused (on petals) so that the hairs look like scales. Leaves flat to revolute. Petals white, pink, or yellow. Carpels 1–4.
A section of five species confined to south-western Australia.
Species
Asterolasia drummondii, A. grandiflora, A. hyalina, A. pallida, A. squamuligera.
Asterolasia section Muricatae Duretto & Heslewood, sect. nov.
Differs from the typical section by flowers having two carpels (v. 5) and from section Urocarpus by flowers having yellow petals with stellate hairs with unfused rays (v. petals white, pink, or yellow and then hairs on abaxial surface with fused rays so the hairs look like fimbriate scales).
Hairs stellate, rays unfused. Leaves revolute. Petals yellow. Carpels 2.
A monotypic section confined to South Australia.
Etymology
The sectional name is derived from the specific epithet of its type species.
Chorilaena Endl. in S.F.L.Endlicher, E.Fenzl, G.Bentham & H.W.Schott, Enum. Pl. 17 (1837)
Shrubs with a stellate indumentum, often lepidote. Leaves alternate, simple. Inflorescence axillary or rarely terminal, cymose, few-flowered or flowers solitary, or a 6-flowered umbel; pedicles 2- or 4-bracteate. Flowers 5-merous. Sepals deeply lobed or united into a patelliform or hemispherical calyx; margin undulate and lobed. Petals valvate, free, at first coherent in C. euphemiae, lepidote. Stamens 10, free; filaments flat, bearded on adaxial side towards base, otherwise glabrous; anthers obtuse or oblong, with a non-glandular apiculum. Disc small. Carpels free or fused in lower half, glabrous or lepidote, with or without a short sterile apex. Cocci erect, blunt or shortly rostrate. Seeds narrowly reniform or bluntly ellipsoid; aril linear, fleshy, situated between 2 cartilaginous strands, easily detached; outer testa thin, dark brown; sclerotesta smooth; covered by outer testa, only hilum superficial, narrowly elliptic; raphe shrunken, sub-basal or short and shrivelled.
The description and key provided here is largely derived from Wilson (2013f) and Armstrong (2013), and to a lesser extent Kubitzki et al. (2011). For species and subspecies descriptions see Armstrong (2013) and Wilson (2013f).
A genus of four species confined to south-western Australia.
Key to species|
1. Leaves lepidote, margins entire or divergently 2-lobed |
|
2. Petals spreading, free, silvery-lepidote abaxially; leaves elliptic or linear to narrowly or broadly obcordate or suborbicular |
|
3. Leaves 0.7–3.5 cm long, linear to narrowly or broadly obcordate or suborbicular |
Chorilaena anceps (DC.) Duretto & Heslewood, comb. nov.
Chorilaena euphemiae (F.Muell.) Duretto & Heslewood, comb. nov.
Chorilaena quercifolia Endl. in S.F.L.Endlicher, E.Fenzl, G.Bentham & H.W.Schott, Enum. Pl. 17 (1837)
Chorilaena rudis (Bartl.) Duretto & Heslewood, comb. nov.
A species with three subspecies (see Wilson 2013f for a key and descriptions).
Chorilaena rudis subsp. amblycarpus (F.Muell.) Duretto & Heslewood, comb. nov.
Chorilaena rudis subsp. linearis (C.A.Gardner) Duretto & Heslewood, comb. nov.
Leionema (F.Muell.) Paul G.Wilson, Nuytsia 12(2): 270 (1998)
A genus of 28 species; 1 species endemic to north-eastern Queensland, 1 endemic to the North Island of New Zealand, and 26 endemic to south-eastern Australia including Tasmania. See Wilson (2013b), Copeland and Telford (2018), Alvarez and Duretto (2019b) and Telford and Bruhl (2020) for descriptions and keys to species. The genus has two sections.
Key to sections|
1. Anthers minutely apiculate; gynophore with 10 deep grooves (N Qld) |
Leionema (F.Muell.) Paul G.Wilson section Leionema
Anther retuse, without apiculum. Gynophore entire.
A section of 27 species: 26 species endemic to south-eastern Australia, including Tasmania, 1 endemic to the North Island of New Zealand.
Species
Leionema ambiens (F.Muell.) Paul G.Wilson, L. beckleri, L. bilobum (4 subspp.), L. carruthersii, L. ceratogynum, L. coxii, L. dentatum (Sm.) Paul G.Wilson, L. diosmeum, L. elatius, L. equestre, L. gracile (C.T.White) Paul G.Wilson, L. hillebrandii, L. lachnaeoides, L. lamprophyllum (4 subspp.), L. microphyllum (F.Muell.) Paul G.Wilson, L. montanum, L. nudum, L. obtusifolium (Paul G.Wilson) Paul G.Wilson, L. oldfieldii, L. phylicifolium, L. praetermissum, L. ralstonii, L. rotundifolium (A.Cunn. ex Endl.) Paul G.Wilson, L. scopulinum B.M.Horton & Crayn, L. sympetalum, L. viridiflorum (Paul G.Wilson) Paul G.Wilson, L. westonii L.M.Copel. & I.Telford.
Leionema section Ellipticae Duretto & Heslewood, sect. nov.
Differs from the typical section by having minutely apiculate anthers (v. anthers retuse, without apiculum), and the disc or gynophore having 10 deep grooves (v. entire).
A monotypic section confined to one mountain top of the Humid Wet Tropics of north-eastern Queensland.
Etymology
The sectional name is derived from the specific epithet of its type species.
Nematolepis Turcz., Bull. Soc. Imp. Naturalistes Moscou 25(2): 158 (1852)
A genus of seven species confined to southern Australia with two sections. See Wilson (1970, 1998b, 2013h) for descriptions and keys to species and subspecies.
Key to sections|
1. Flowers in cymose inflorescences, not pendent; corolla unfused, spreading, white (SE Aust., Tas.) |
Nematolepis Turcz. section Nematolepis
Flowers solitary, pendent. Corolla fused, red with green or yellow tips.
A monotypic section confined to south-western Western Australia.
Nematolepis section Eriostemoides (Endl.) Duretto & Heslewood, comb. nov.
Flowers in cymose inflorescences, not pendent. Corolla unfused, spreading, white.
A section of six species confined to south-eastern Australia, including Tasmania.
Species
Nematolepis elliptica (Paul G.Wilson) Paul G.Wilson, N. frondosa (N.G.Walsh & Albr.) Paul G.Wilson, N. ovatifolia (F.Muell.) Paul G.Wilson, N. rhytidophylla (Albr. & N.G.Walsh) Paul G.Wilson, N. squamea (with 2 subspp.), N. wilsonii (N.G.Walsh & Albr.) Paul G.Wilson.
Phebalium Vent., Jard. Malmaison 2: t. 102 (1805)
Shrubs or small trees; with stellate-lepidote hairs. Leaves alternate, simple, sessile or shortly petiolate. Inflorescence terminal, sometimes also on short lateral branches, flowers solitary or in umbels or in compact heads, pedicellate or sessile. Bracteoles at base of pedicel or flower when sessile, and usually insignificant. Calyx hemispherical to cup-shaped, 5(–8)-lobed or toothed or entire, or sepals free or united below, lepidote or rarely stellate-pilose abaxially. Petals 5(–10), free, narrowly elliptic, elliptic to obovate or spathulate, white, yellow, or pink to mauve, lepidote abaxially or glabrous. Stamens 10(–16); filaments slender, terete; anthers basifixed, glandular-apiculate. Disc absent. Carpels usually 2–5(–8 in P. nottii (F.Muell.) Maiden & Betche), free or shortly fused at base, lepidote; apically united; style terete at base with branches arising from adaxial medial margin of carpels; stigma small with short spreading lobes; ovules 2 per carpel. Seed oblong-reniform; axial endocarp thin, caducous; aril linear; sclerotesta longitudinally rugulose; hilum linear; raphe small, shrunken.
A genus of 38 species in eastern and southern Australia, classified into 3 sections.
Key to sections|
1. Carpels 2–4; flowers sessile |
|
2. Flowers solitary |
Phebalium Vent. section Phebalium
Species
Phebalium appressum, P. bifidum P.H.Weston & M.J.Turton, P. brachycalyx Paul G.Wilson, P. brevifolium, P. bullatum J.M.Black, P. calcicola, P. canaliculatum (F.Muell. & Tate) J.H.Willis, P. cicatricatum, P. clavatum, P. daviesii Hook.f., P. distans P.I.Forst., P. drummondii, P. elegans, P. festivum Paul G.Wilson, P. filifolium, P. glandulosum (6 subspp.), P. graniticola, P. laevigatum Paul G.Wilson, P. lepidotum, P. longifolium S.T.Blake, P. lowanense J.H.Willis, P. megaphyllum (Ewart) Paul G.Wilson, P. microphyllum, P. nottii, P. obcordatum Benth., P. obovatum, P. speciosum I.Telford, P. squamulosum (8 subspp.), P. stenophyllum (Benth.) Maiden & Betche, P. stellatum I.Telford & J.J.Bruhl, P. sylvaticum I.Telford & J.J.Bruhl, P. tuberculosum, P. verrucosum (Paul G.Wilson) I.Telford & J.J.Bruhl, P. whitei Paul G.Wilson, P. woombye (F.M.Bailey) Domin.
Phebalium section Microcybe (Turcz.) Duretto & Heslewood, comb. nov., stat. nov.
Flowers sessile, clustered in compact terminal heads. Carpels 2–3.
A section of three species, all of which are found in south-western Australia, and two also in south-eastern Australia. For an account of the genus Microcybe and its species see Wilson (2013c).
Species
Phebalium albiflorum (Turcz.) Duretto & Heslewood, comb. nov.
Phebalium multiflorum (Turcz.) Duretto & Heslewood, comb. nov.
Phebalium multiflorum subsp. baccharoides (F.Muell.) Duretto & Heslewood, comb. nov.
Phebalium pauciflorum (Turcz.) Duretto & Heslewood, comb. nov.
Phebalium pauciflorum subsp. grande (Paul G.Wilson) Duretto & Heslewood, comb. nov.
Phebalium section Uniflorum Duretto & Heslewood, sect. nov.
Differs from Phebalium section Phebalium and P. section Microcybe by having the following combination of characters: solitary, sessile flowers with 3 or 4 carpels.
A monotypic section confined to south-western Australia.
Etymology
The sectional name is derived from the Latin, uni- (one) and floris (flower) and refers to the solitary flowers of the sole species.
Supplementary material
Supplementary material is available online.
Data availability
New sequence data for this study are available from GenBank https://www.ncbi.nlm.nih.gov/genbank/: numbers OP481290–OP481809 and OP524196–OP524332. Alignment files are available in Supplementary Data File S1 (SB22018_D1.docx). Other data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
M. Bayly is an editor for Australian Systematic Botany. Despite this relationship, he did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages its editors to publish in the journal and has protocols that keep editors separate from the decision-making processes for their manuscripts. The authors declare that they have no other conflicts of interest.
Declaration of funding
The authors thank the Hermon Slade Foundation for funding this project (Grant HSF13/6).
Acknowledgements
We thank the Directors of AD, BRI, CANB, CNS, DNA, HO, MEL, MELU, NSW and PERTH for access to their herbaria and the loan of material; the Directors of the Royal Botanic Gardens & Domain Trust, Royal Botanic Gardens Victoria, and the Australian National Botanic Gardens for permission to sample their living collections; Erin Batty, Steve Clarke, Paul Forster, Andrew Orme, Matt Renner and Andy Young for collecting material; Brian Mole for a number of the DNA extracts; Erin Batty and Gareth Holmes for assistance with DNA extractions or sequencing. The former Victorian Department of Sustainability and Environment, former Western Australian Department of Conservation and Land Management (later DEC), former New South Wales Department of Planning, Industry and Environment (now DPE), and conservation authorities of the North and South Provinces of New Caledonia (DDEE and DENV), provided permission to collect material in parks and reserves under their control. We thank our reviewers and editor for insightful suggestions that improved this paper.
References
Alvarez PR, Duretto MF (2019a) A reassessment of Asterolasia correifolia (Rutaceae), with descriptions of the newly recognised A. exasperata and A. sola. Telopea 21, 381–389.| A reassessment of Asterolasia correifolia (Rutaceae), with descriptions of the newly recognised A. exasperata and A. sola.Crossref | GoogleScholarGoogle Scholar |
Alvarez PR, Duretto MF (2019b) Leionema praetermissum (Rutaceae), a new restricted endemic for New South Wales. Telopea 22, 67–73.
| Leionema praetermissum (Rutaceae), a new restricted endemic for New South Wales.Crossref | GoogleScholarGoogle Scholar |
Appelhans MS, Bayly MJ, Heslewood MM, Groppo M, Verboom GA, Forster PI, Kallunki JA, Duretto MF (2021) A new subfamily classification of the Citrus family (Rutaceae) based on six nuclear and plastid markers. Taxon 70, 1035–1061.
| A new subfamily classification of the Citrus family (Rutaceae) based on six nuclear and plastid markers.Crossref | GoogleScholarGoogle Scholar |
Armstrong JA (1979) Biotic pollination mechanisms in the Australian flora – a review. New Zealand Journal of Botany 17, 467–508.
| Biotic pollination mechanisms in the Australian flora – a review.Crossref | GoogleScholarGoogle Scholar |
Armstrong JA (2013) Chorilaena (Rutaceae). In ‘Flora of Australia. Vol. 26: Meliaceae, Rutaceae, Zygophyllaceae’. (Ed. A Wilson) pp. 456–458. (CSIRO Publishing: Melbourne, Vic., Australia)
Aston HI (1984) Publication dates of early scientific journals in Victoria. Muelleria 5, 281–288.
| Publication dates of early scientific journals in Victoria.Crossref | GoogleScholarGoogle Scholar |
Batty EL, Holmes GD, Murphy DJ, Forster PI, Neal WC, Bayly MJ (2022) Phylogeny, classification and biogeography of Philotheca sect. Erionema (Rutaceae) based on nrDNA sequences. Australian Systematic Botany 35, 326–338.
| Phylogeny, classification and biogeography of Philotheca sect. Erionema (Rutaceae) based on nrDNA sequences.Crossref | GoogleScholarGoogle Scholar |
Bayly MJ (1998) Notes on the Eriostemon myoporoides (Rutaceae) species complex, including new names and a new generic placement in Philotheca. Muelleria 11, 113–126.
| Notes on the Eriostemon myoporoides (Rutaceae) species complex, including new names and a new generic placement in Philotheca.Crossref | GoogleScholarGoogle Scholar |
Bayly MJ, Holmes GD, Forster PI, Cantrill DJ, Ladiges PY (2013) Major clades of Australasian Rutoideae (Rutaceae) based on rbcL and atpB sequences. PLoS One 8, e72493
| Major clades of Australasian Rutoideae (Rutaceae) based on rbcL and atpB sequences.Crossref | GoogleScholarGoogle Scholar |
Bayly MJ, Duretto MF, Holmes GD, Forster PI, Cantrill DJ, Ladiges PY (2015) Transfer of the New Caledonian genus Boronella to Boronia (Rutaceae) based on analyses of cpDNA and nrDNA. Australian Systematic Botany 28, 111–123.
| Transfer of the New Caledonian genus Boronella to Boronia (Rutaceae) based on analyses of cpDNA and nrDNA.Crossref | GoogleScholarGoogle Scholar |
Bayly MJ, Holmes GD, Forster PI, Munzinger J, Cantrill DJ, Ladiges PY (2016) Phylogeny, classification and biogeography of Halfordia (Rutaceae) in Australia and New Caledonia. Plant Systematics and Evolution 302, 1457–1470.
| Phylogeny, classification and biogeography of Halfordia (Rutaceae) in Australia and New Caledonia.Crossref | GoogleScholarGoogle Scholar |
Bell SAJ, Walsh NG (2015) Leionema lamprophyllum subsp. fractum (Rutaceae); a new and highly restricted taxon from the Hunter Valley of New South Wales. Telopea 18, 505–512.
| Leionema lamprophyllum subsp. fractum (Rutaceae); a new and highly restricted taxon from the Hunter Valley of New South Wales.Crossref | GoogleScholarGoogle Scholar |
Binks RM, Heslewood M, Wilson PG, Byrne M (2022) Phylogenomic analysis confirms polyphyly of Leptospermum and delineates five major clades that warrant generic recognition. Taxon 71, 348–359.
| Phylogenomic analysis confirms polyphyly of Leptospermum and delineates five major clades that warrant generic recognition.Crossref | GoogleScholarGoogle Scholar |
Claßen-Bockhoff R, Armstrong JA, Ohligschlager M (1991) The inflorescences of the Australian genera Diplolaena R.Br and Chorilaena Endl (Rutaceae). Australian Journal of Botany 39, 31–42.
| The inflorescences of the Australian genera Diplolaena R.Br and Chorilaena Endl (Rutaceae).Crossref | GoogleScholarGoogle Scholar |
Clowes C, Fowler RM, Fahey PS, Kellermann J, Brown GK, Bayly MJ (2022) Big trees of small baskets: phylogeny of the Australian genus Spyridium (Rhamnaceae: Pomaderreae), focusing on biogeographic patterns and species circumscriptions. Australian Systematic Botany 35, 95–119.
| Big trees of small baskets: phylogeny of the Australian genus Spyridium (Rhamnaceae: Pomaderreae), focusing on biogeographic patterns and species circumscriptions.Crossref | GoogleScholarGoogle Scholar |
Copeland LM, Telford IRH (2018) Leionema westonii (Rutaceae), a rare new species from north-eastern New South Wales, Australia. Telopea 21, 19–24.
| Leionema westonii (Rutaceae), a rare new species from north-eastern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Crisp MD, Cook LG (2007) A congruent molecular signature of vicariance across multiple plant lineages. Molecular Phylogenetics and Evolution 43, 1106–1117.
| A congruent molecular signature of vicariance across multiple plant lineages.Crossref | GoogleScholarGoogle Scholar |
Dema S, Telford IRH, Andrew RL, Duval DJ, Bruhl JJ (2021) Phebalium calcicola (Rutaceae: Boronieae): a species described as new, restricted to south-eastern South Australia, is proposed as Critically Endangered. Swainsona 35, 43–53.
Duretto MF (1999) Systematics of Boronia section Valvatae sensu lato (Rutaceae). Muelleria 12, 1–131.
| Systematics of Boronia section Valvatae sensu lato (Rutaceae).Crossref | GoogleScholarGoogle Scholar |
Duretto MF (2018) Drummondita borealis Duretto (Rutaceae), a new species from the Northern Territory, and a revised description for D. calida (F.Muell.) Paul G.Wilson from Queensland. Austrobaileya 10, 236–241.
Duretto MF, Heslewood MM, Bayly MJ (2020) Boronia (Rutaceae) is polyphyletic: reinstating Cyanothamnus and the problems associated with inappropriately defined outgroups. Taxon 69, 481–499.
| Boronia (Rutaceae) is polyphyletic: reinstating Cyanothamnus and the problems associated with inappropriately defined outgroups.Crossref | GoogleScholarGoogle Scholar |
Duretto MF, Heslewood MM, Bayly MJ (2023) A molecular phylogeny of Boronia (Rutaceae): placement of enigmatic taxa and a revised infrageneric classification. Australian Systematic Botany 36, 81–106.
| A molecular phylogeny of Boronia (Rutaceae): placement of enigmatic taxa and a revised infrageneric classification.Crossref | GoogleScholarGoogle Scholar |
Engler A (1896) Rutaceae. In ‘Die natürlichen Pflanzenfamilien nebst ihren Gattungen und wichtigeren Arten insbesondere den Nutzpflanzen, III. Teil, Abteilung 4’ [‘The natural plant families together with their genera and more important species, especially the useful plants, III. Part, Section 4’]. (Eds A Engler, K Prantl) pp. 95–201. (Wilhelm Engelmann: Leipzig, German Empire) [In German]
Engler HGA (1931) Rutaceae. In ‘Die natürlichen Pflanzenfamilien Teil 19a’ [‘The natural plant families part 19a’], 2nd edn. (Eds HGA Engler, K Prantl) pp. 187–359. (Wilhelm Engelmann: Leipzig, German Republic) [In German]
Ford AJ, Duretto M (2020) Phebalium cicatricatum (Rutaceae), a newly described and Critically Endangered species from north-eastern Queensland, Australia. Telopea 23, 131–136.
| Phebalium cicatricatum (Rutaceae), a newly described and Critically Endangered species from north-eastern Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
French PA, Brown GK, Bayly MJ (2016) Incongruent patterns of nuclear and chloroplast variation in Correa (Rutaceae): introgression and biogeography in south-eastern Australia. Plant Systematics and Evolution 302, 447–468.
| Incongruent patterns of nuclear and chloroplast variation in Correa (Rutaceae): introgression and biogeography in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Gardner CA (1943) Contributiones Florae Australiae Occidentalis XI. Journal of the Royal Society of Western Australia 27, 165–210.
Golenberg EM, Clegg MT, Durbin ML, Doebley J, Ma DP (1993) Evolution of a noncoding region of the chloroplast genome. Molecular Phylogenetics and Evolution 2, 52–64.
| Evolution of a noncoding region of the chloroplast genome.Crossref | GoogleScholarGoogle Scholar |
Groppo M, Pirani JR, Salatino MLF, Blanco SR, Kallunki JA (2008) Phylogeny of Rutaceae based on two noncoding regions from cpDNA. American Journal of Botany 95, 985–1005.
| Phylogeny of Rutaceae based on two noncoding regions from cpDNA.Crossref | GoogleScholarGoogle Scholar |
Groppo M, Kallunki JA, Pirani JR, Antonelli A (2012) Chilean Pitavia more closely related to Oceania and Old World Rutaceae than to Neotropical groups: evidence from two cpDNA non-coding regions, with a new subfamilial classification of the family. Phytokeys 19, 9–29.
| Chilean Pitavia more closely related to Oceania and Old World Rutaceae than to Neotropical groups: evidence from two cpDNA non-coding regions, with a new subfamilial classification of the family.Crossref | GoogleScholarGoogle Scholar |
Halstead CW, Forster PI, Waterman PG (2005) The occurrence of osthol in Leionema ellipticum supports its assignment to the genus Leionema (Rutaceae). Biochemical Systematics and Ecology 33, 899–903.
| The occurrence of osthol in Leionema ellipticum supports its assignment to the genus Leionema (Rutaceae).Crossref | GoogleScholarGoogle Scholar |
Hartley TG (2003) Neoschmidia, a new genus of Rutaceae from New Caledonia. Adansonia 25, 7–12.
Herbert DA (1922) Contributions to the Flora of Western Australia, no. IV. Journal and Proceedings of the Royal Society of Western Australia 8, 35–40.
Holzmeyer L, Duretto M, Crayn D, Hörandl E, Heslewood M, Jayanthan J, Appelhans MS (2015) Phylogeny of Acronychia (Rutaceae) and first insights into its historical biogeography and the evolution of fruit characters. PLoS One 10, e0136296
| Phylogeny of Acronychia (Rutaceae) and first insights into its historical biogeography and the evolution of fruit characters.Crossref | GoogleScholarGoogle Scholar |
Johnson KA, Holland BR, Heslewood MM, Crayn DM (2012) Supermatrices, supertrees and serendipitous scaffolding: Inferring a well-resolved, genus-level phylogeny of Styphelioideae (Ericaceae) despite missing data. Molecular Phylogenetics and Evolution 62, 146–158.
| Supermatrices, supertrees and serendipitous scaffolding: Inferring a well-resolved, genus-level phylogeny of Styphelioideae (Ericaceae) despite missing data.Crossref | GoogleScholarGoogle Scholar |
Keighery GJ (1980) Bird pollination in south Western Australia: a checklist. Plant Systematics and Evolution 135, 171–176.
| Bird pollination in south Western Australia: a checklist.Crossref | GoogleScholarGoogle Scholar |
Kubitzki K, Kallunki JA, Duretto M, Wilson P (2011) Rutaceae. In ‘The Families and Genera of Vascular Plants. Vol. X. Flowering plants Eudicots: Sapindales, Cucurbitales, Myrtaceae’. (Ed. K Kubitzki) pp. 276–356. (Springer-Verlag: Heidelberg, Germany)
| Crossref |
Levinson G, Gutman GA (1987) Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Molecular Biology and Evolution 4, 203–221.
| Slipped-strand mispairing: a major mechanism for DNA sequence evolution.Crossref | GoogleScholarGoogle Scholar |
Lucas EJ, Harris SA, Mazine FF, Belsham SR, Nic Lughadha EM, Telford A, Gasson PE, Chase MW (2007) Suprageneric phylogenetics of Myrteae, the generically richest tribe in Myrtaceae (Myrtales). Taxon 56, 1105–1128.
| Suprageneric phylogenetics of Myrteae, the generically richest tribe in Myrtaceae (Myrtales).Crossref | GoogleScholarGoogle Scholar |
Maddison WP, Maddison DR (2000) ‘MacClade 4: analysis of phylogeny and character evolution.’ (Sinauer: Sunderland, MA, USA)
McDougall K, Walsh N, Hook C (2016) Recognition of subspecies in Asterolasia trymalioides (Rutaceae: Rutoideae). Muelleria 34, 69–82.
| Recognition of subspecies in Asterolasia trymalioides (Rutaceae: Rutoideae).Crossref | GoogleScholarGoogle Scholar |
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In ‘Proceedings of the Gateway Computing Environments Workshop (GCE)’, 14 November 2010, New Orleans, LA, USA. INSPEC Accession Number 11705685. (IEEE)
| Crossref |
Mole BJ, Udovicic F, Ladiges PY, Duretto MF (2004) Molecular phylogeny of Phebalium (Rutaceae: Boronieae) and related genera based on the nrDNA regions ITS 1+2. Plant Systematics and Evolution 249, 197–212.
| Molecular phylogeny of Phebalium (Rutaceae: Boronieae) and related genera based on the nrDNA regions ITS 1+2.Crossref | GoogleScholarGoogle Scholar |
Nge FJ, Biffin E, Thiele KR, Waycott M (2020) Extinction pulse at Eocene–Oligocene boundary drives diversification dynamics of two Australian temperate floras. Proceedings of the Royal Society of London – B. Biological Sciences 287, 20192546
| Extinction pulse at Eocene–Oligocene boundary drives diversification dynamics of two Australian temperate floras.Crossref | GoogleScholarGoogle Scholar |
Orme AE, Duretto MF (2017) Asterolasia beckersii (Rutaceae), a new species from the Northern Tablelands, New South Wales. Telopea 20, 165–169.
| Asterolasia beckersii (Rutaceae), a new species from the Northern Tablelands, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Othman RNA, Jordan GJ, Worth JRP, Steane DA, Duretto MF (2010) Phylogeny and infrageneric classification of Correa Andrews (Rutaceae) on the basis of nuclear and chloroplast DNA. Plant Systematics and Evolution 288, 127–138.
| Phylogeny and infrageneric classification of Correa Andrews (Rutaceae) on the basis of nuclear and chloroplast DNA.Crossref | GoogleScholarGoogle Scholar |
Parker SR (1997) Sequence Navigator: multiple sequence alignment software. In ‘Sequence Data Analysis Guidebook. Methods In Molecular Medicine, vol. 70’. (Ed. SR Swindell) pp. 145–154. (Springer: Totowa, NJ, USA)
| Crossref |
Phillips RD, Hopper SD, Dixon KW (2010) Pollination ecology and the possible impacts of environmental change in the Southwest Australian Biodiversity Hotspot. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 365, 517–528.
| Pollination ecology and the possible impacts of environmental change in the Southwest Australian Biodiversity Hotspot.Crossref | GoogleScholarGoogle Scholar |
Prince LM (2010) Phylogenetic relationships and species delimitation in Canna (Cannaceae). In ‘Diversity, phylogeny, and evolution in the monocotyledons: Proceedings of the Fourth International Conference on the Comparative Biology of the Monocotyledons and the Fifth International Symposium on Grass Systematics and Evolution’. (Eds O Seberg, G Petersen, AS Barfod, JI Davis) pp. 307–331. (Århus University Press: Århus, Denmark)
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67, 901–904.
| Posterior summarization in Bayesian phylogenetics using Tracer 1.7.Crossref | GoogleScholarGoogle Scholar |
Reichelt N, Wen J, Pätzold C, Appelhans MS (2021) Target enrichment improves phylogenetic resolution in the genus Zanthoxylum (Rutaceae) and indicates both incomplete lineage sorting and hybridization events. Annals of Botany 128, 497–510.
| Target enrichment improves phylogenetic resolution in the genus Zanthoxylum (Rutaceae) and indicates both incomplete lineage sorting and hybridization events.Crossref | GoogleScholarGoogle Scholar |
Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61, 539–542.
| MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space.Crossref | GoogleScholarGoogle Scholar |
Sang T, Crawford DJ, Stuessy TF (1997) Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). American Journal of Botany 84, 1120–1136.
| Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae).Crossref | GoogleScholarGoogle Scholar |
Shepherd KA, Crawford AD (2020) Worthy of love: Geleznowia amabilis (Rutaceae), a stunning new species of ‘Yellow Bells’ from Kalbarri in Western Australia. Nuytsia 31, 89–93.
| Worthy of love: Geleznowia amabilis (Rutaceae), a stunning new species of ‘Yellow Bells’ from Kalbarri in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Simmons MP, Freudenstein JV (2011) Spurious 99% bootstrap and jackknife support for unsupported clades. Molecular Phylogenetics and Evolution 61, 177–191.
| Spurious 99% bootstrap and jackknife support for unsupported clades.Crossref | GoogleScholarGoogle Scholar |
Simmons MP, Ochoterena H (2000) Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology 49, 369–381.
| Gaps as characters in sequence-based phylogenetic analyses.Crossref | GoogleScholarGoogle Scholar |
Swofford DL (2003) ‘PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10.’ (Sinauer: Sunderland, MA, USA)
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17, 1105–1109.
| Universal primers for amplification of three non-coding regions of chloroplast DNA.Crossref | GoogleScholarGoogle Scholar |
Tate JA, Simpson BB (2003) Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Systematic Botany 28, 723–737.
| Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species.Crossref | GoogleScholarGoogle Scholar |
Telford IRH (2013) Phebalium speciosum (Rutaceae: Boronieae), an endangered, narrowly endemic new species of north-eastern New South Wales, Australia. Telopea 15, 51–55.
| Phebalium speciosum (Rutaceae: Boronieae), an endangered, narrowly endemic new species of north-eastern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Telford IRH, Bruhl JJ (2014) Phebalium verrucosum (Rutaceae), new status for a taxon excluded from P. squamulosum on morphological and phytochemical evidence. Telopea 16, 127–132.
| Phebalium verrucosum (Rutaceae), new status for a taxon excluded from P. squamulosum on morphological and phytochemical evidence.Crossref | GoogleScholarGoogle Scholar |
Telford IRH, Bruhl JJ (2020) Morphological data indicate the subspecies of Leionema elatius (Rutaceae) are not conspecific and both should be treated as species. Telopea 23, 197–203.
| Morphological data indicate the subspecies of Leionema elatius (Rutaceae) are not conspecific and both should be treated as species.Crossref | GoogleScholarGoogle Scholar |
Telford IRH, Sadgrove NJ, Bruhl JJ (2019) Three new species segregated from Phebalium squamulosum subsp. squamulosum (Rutaceae) based on morphological and phytochemical data. Muelleria 38, 3–16.
| Three new species segregated from Phebalium squamulosum subsp. squamulosum (Rutaceae) based on morphological and phytochemical data.Crossref | GoogleScholarGoogle Scholar |
Wege JA (2017) Taxonomic notes on Asterolasia (Rutaceae) in Western Australia to inform conservation. Nuytsia 28, 141–146.
| Taxonomic notes on Asterolasia (Rutaceae) in Western Australia to inform conservation.Crossref | GoogleScholarGoogle Scholar |
Wege JA, Hislop M (2020) Few and far between: Philotheca richardsoniana (Rutaceae), a new rarity from Western Australia’s Avon Wheatbelt. Nuytsia 31, 9–13.
| Few and far between: Philotheca richardsoniana (Rutaceae), a new rarity from Western Australia’s Avon Wheatbelt.Crossref | GoogleScholarGoogle Scholar |
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In ‘PCR protocols: a guide to methods and applications’. (Eds M Innis, D Gelfand, J Sninsky, T White) pp. 315–322. (Academic Press: San Diego, CA, USA)
| Crossref |
Wilson A (Ed.) (2013) ‘Flora of Australia. Vol. 26, Meliaceae, Rutaceae, Zygophyllaceae.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Wilson PG (1970) A taxonomic revision of the genera Crowea, Eriostemon and Phebalium (Rutaceae). Nuytsia 1, 3–155.
| A taxonomic revision of the genera Crowea, Eriostemon and Phebalium (Rutaceae).Crossref | GoogleScholarGoogle Scholar |
Wilson PG (1971) Taxonomic notes on the family Rutaceae, principally of Western Australia. Nuytsia 1, 197–207.
| Taxonomic notes on the family Rutaceae, principally of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Wilson PG (1980) A new species of Urocarpus (Rutaceae) from Western Australia. Nuytsia 3, 211–213.
| A new species of Urocarpus (Rutaceae) from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Wilson PG (1987) The names Asterolasia F.Muell. and Urocarpus Harvey (Rutaceae). Nuytsia 6, 7–8.
Wilson PG (1998a) A taxonomic review of the genera Eriostemon and Philotheca (Rutaceae: Boronieae). Nuytsia 12, 239–265.
Wilson PG (1998b) New species and nomenclatural changes in Phebalium and related genera (Rutaceae). Nuytsia 12, 267–288.
Wilson PG (1998c) Nomenclatural notes and new taxa in the genera Asterolasia, Drummondita and Microcybe (Rutaceae: Boronieae). Nuytsia 12, 83–88.
Wilson PG (2013a) Leionema (Rutaceae). In ‘Flora of Australia. Vol. 26: Meliaceae, Rutaceae, Zygophyllaceae’. (Ed. A Wilson) pp. 431–446. (CSIRO Publishing: Melbourne, Vic., Australia)
Wilson PG (2013b) Phebalium (Rutaceae). In ‘Flora of Australia. Vol. 26: Meliaceae, Rutaceae, Zygophyllaceae’. (Ed. A Wilson) pp. 458–480. (CSIRO Publishing: Melbourne, Vic., Australia)
Wilson PG (2013c) Microcybe (Rutaceae). In ‘Flora of Australia. Vol. 26: Meliaceae, Rutaceae, Zygophyllaceae’. (Ed. A Wilson) pp. 480–484, 583. (CSIRO Publishing: Melbourne, Vic., Australia)
Wilson PG (2013d) Philotheca (Rutaceae). In ‘Flora of Australia. Vol. 26: Meliaceae, Rutaceae, Zygophyllaceae’. (Ed. A Wilson) pp. 366–415. (CSIRO Publishing: Melbourne, Vic., Australia)
Wilson PG (2013e) Asterolasia (Rutaceae). In ‘Flora of Australia. Vol. 26: Meliaceae, Rutaceae, Zygophyllaceae’. (Ed. A Wilson) pp. 416–427. (CSIRO Publishing: Melbourne, Vic., Australia)
Wilson PG (2013f) Rhadinothamnus (Rutaceae). In ‘Flora of Australia. Vol. 26: Meliaceae, Rutaceae, Zygophyllaceae’. (Ed. A Wilson) pp. 452–456. (CSIRO Publishing: Melbourne, Vic., Australia)
Wilson PG (2013g) Diplolaena (Rutaceae). In ‘Flora of Australia. Vol. 26: Meliaceae, Rutaceae, Zygophyllaceae’. (Ed. A Wilson) pp. 484–494. (CSIRO Publishing: Melbourne, Vic., Australia)
Wilson PG (2013h) Nematolepis (Rutaceae). In ‘Flora of Australia. Vol. 26: Meliaceae, Rutaceae, Zygophyllaceae’. (Ed. A Wilson) pp. 447–452. (CSIRO Publishing: Melbourne, Vic., Australia)
Wilson PG, Armstrong JA, Griffin EA (1998) Diplolaena (Rutaceae), new taxa and nomenclatural notes. Nuytsia 12, 107–118.
Wright SD, Yong CG, Wichman SR, Dawson JW, Gardner RC (2001) Stepping stones to Hawaii: a trans-equatorial dispersal pathway for Metrosideros (Myrtaceae) inferred from nrDNA (ITS+ETS). Journal of Biogeography 28, 769–774.
| Stepping stones to Hawaii: a trans-equatorial dispersal pathway for Metrosideros (Myrtaceae) inferred from nrDNA (ITS+ETS).Crossref | GoogleScholarGoogle Scholar |