Reinstatement of Sabdariffa and new combinations to support a monophyletic concept of Hibiscus (Malvaceae: Hibisceae)
Russell L. Barrett A B * , Vania Nobuko Yoshikawa C , Todd G. B. McLay D E F , Marília Cristina Duarte G , Geoffrey Mwachala H and Margaret M. Hanes I
A B * , Vania Nobuko Yoshikawa C , Todd G. B. McLay D E F , Marília Cristina Duarte G , Geoffrey Mwachala H and Margaret M. Hanes I
A
B
C
D
E
F
G
H
I
Abstract
Recent phylogenetic studies have demonstrated that Hibiscus L. as traditionally defined is grossly polyphyletic. In a major step towards making Hibiscus monophyletic, the genus Sabdariffa (DC.) Kostel. is here reinstated for Hibiscus section Furcaria DC. In total, 123 new combinations are provided (for 117 species and 6 subspecies). Numerous lectotypes are designated. Sabdariffa has a pantropical distribution, with high species diversity in tropical Africa, tropical America and northern Australia. False roselle (Sabdariffa acetosella (Welw. ex Hiern) M.M.Hanes & R.L.Barrett), kenaf (Sabdariffa cannabina (L.) M.M.Hanes & R.L.Barrett) and roselle (Sabdariffa gossypiifolia (Mill.) M.M.Hanes & R.L.Barrett) are all of global economic significance. Ma‘o hau hele (Sabdariffa brackenridgei (A.Gray) M.M.Hanes & R.L.Barrett) is the official state flower of Hawai’i. Full synonymy and type details are included, along with distributions and references to existing descriptions for all species. A series of regional keys to species is presented here with current names in Sabdariffa.
Keywords: classification, false roselle, fibre plants, Hibisceae, Hibiscus section Furcaria, kenaf, Mallows, Malvaceae, roselle, systematics, taxonomy.
We dedicate this paper to the memory of five Malvaceae taxonomists: Antonio Krapovickas (1921–2015), Paul A. Fryxell (1927–2011), F. Douglas Wilson (1928–2022), Lyn A. Craven (1945–214) and Orland ‘Skip’ J. Blanchard Jr (1944–2024). Krapovickas, Fryxell, Wilson and Craven effectively monographed Hibiscus section Furcaria and together named 50 of the currently recognised species. The phylogenetic studies enabling this new classification were championed by Skip Blanchard Jr, who passed away while this paper was in review. Their work was foundational to the treatment we present here.
Introduction
Classifying genera within Malvaceae tribe Hibisceae has provided a daunting challenge since the birth of modern taxonomy (Linnaeus 1753). Over the course of 250 years, at least 80 generic names have been published in the tribe, almost 1 for every 10 species. This great abundance of generic names reflects the vast morphological and species diversity that spans the global tropics. The largest and most variable genus has always been Hibiscus L., typified by H. syriacus L. that therefore became the default dumping ground for Hibisceae species of uncertain affinity. Although distinctive species groups have often been recognised as genera, relationships have remained vague and the utility of segregate genera much debated.
Phylogenetic relationships within the large and morphologically complex tribe Hibisceae, recognised as a natural group by Reichenbach (1832), has recently been clarified with representative molecular data (Hanes et al. 2024). This study articulated the already well-documented gross polyphyly of genus Hibiscus L. (Pfeil et al. 2002, 2004; Pfeil and Crisp 2005; Koopman and Baum 2008) and highlighted the long-standing problem of defining the limits of Hibiscus based on morphological grounds. In parallel, Landrein et al. (2024) have confirmed the inclusion of Thepparatia Phuph. in Hibisceae. Hibiscus as traditionally defined includes at least 20 other genera, among which is the large genus Pavonia Cav. with ~300 species. Therefore Hibiscus cannot be maintained in the current form unless the large genus Pavonia, and several other genera, are similarly included (see supplementary table S1 in Hanes et al. 2024). Expansion of Hibiscus to include all currently recognised species and embedded genera would create a genus of ~850 species and require up to 450 new combinations (Hanes et al. 2024). Transferring species currently included in Hibiscus but not part of the core Hibiscus clade (Hibiscus sens. str.; Hanes et al. 2024) will require ~180 new combinations at species level, with 117 of these combinations made here.
Rather than recognising a gigantic Hibiscus, we believe that smaller units supported as monophyletic in our phylogenetic framework will make groups easier to define morphologically and therefore easier to identify. This approach has been promoted by several authors (e.g. recognition of Talipariti Fryxell 2001d), though a lack of understanding of relationships within the tribe has limited these efforts. Hanes et al. (2024) therefore proposed to move some groups currently placed in Hibiscus to other genera, maintaining Hibiscus for the largest monophyletic group containing the type species, while also retaining most currently recognised genera in the tribe. We consider this the most stable nomenclatural solution that also serves practical identification needs. Proposing a new phylogenetic classification also facilitates and provides structure for a global review of species in tribe Hibisceae. All treatments since Hochreutiner (1900) have only had a regional focus or narrow taxonomic scopes limited to particular sections of Hibiscus.
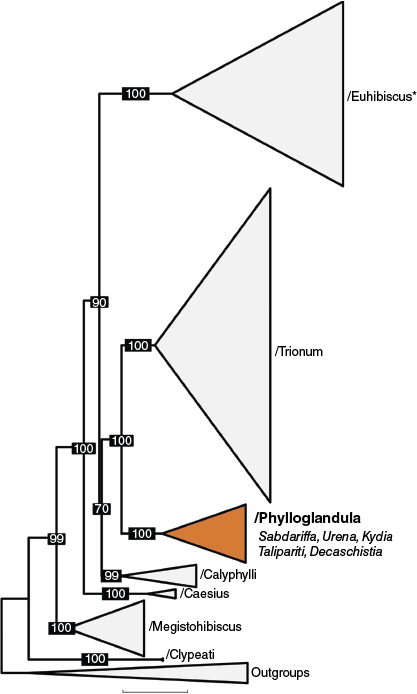
Despite the general pattern of a high ratio of generic names to species names throughout tribe Hibisceae, Hibiscus section Furcaria DC. is the exception. Only three generic names have been validly published for 117 recognised species in Hibiscus section Furcaria and two of these names have only been applied to single species (Canhamo Perini, for Hibiscus radiatus Cav. and Brockmania W.Fitzg. for Hibiscus meraukensis Hochr.). This section is closely affiliated with the genera Decaschistia Wight & Arn., Kydia Roxb., Talipariti and Urena L. in a well-resolved informal clade called /Phylloglandula by Pfeil and Crisp (2005; see Hanes et al. 2024; Fig. 1). A few species from this section were transferred to Abelmoschus Medik. by Walpers (1842) but this proposal was not followed by any other authors. The first validly published name at genus rank for Hibiscus section Furcaria is Sabdariffa (DC.) Kostel. (Kosteletzky 1836), based on Hibiscus section Sabdariffa DC. (de Candolle 1824). Sabdariffa was based on two species, S. digitata (Cav.) Kostel. and S. rubra Kostel., both of which are here recognised as synonyms of Sabdariffa gossypiifolia (Mill.) M.M.Hanes & R.L.Barrett. Hibiscus section Furcaria forms a natural group for recognition at generic rank and contains a significant proportion of the diversity found in tribe Hibisceae (behind Pavonia and Hibiscus, sensu Hanes et al. 2024).
Phylogenetic relationships between major clades within tribe Hibisceae with a focus on the genera Hibiscus (restricted to clade /Euhibiscus* sensu Hanes et al. (2024), including the type of Hibiscus, H. syriacus) and Sabdariffa (within clade /Phylloglandula; coloured orange), modified from Hanes et al. (2024). Numbers represent UFBoot support.
Though a broad global sampling of the section has been undertaken, relatively few species have been included in molecular phylogenies to date. For example, Pfeil et al. (2002) included 17 species and Hanes et al. (2024) included 10 species (five not included in Pfeil et al. 2002). High levels of genome recombination in this clade (octoploids are common) complicate phylogenetic reconstruction using nuclear markers (Hoskins 2016). However, all studies to date have recovered a monophyletic clade for Hibiscus section Furcaria (e.g. Pfeil et al. 2002; Pfeil and Crisp 2005; Hoskins 2016; Champion 2020; Hanes et al. 2024). The consistent monophyly of this group is reflected in the stable sectional affinity with few debates over the circumscription and diagnostic morphology that defines the group (Fryxell 1988; Wilson 2006). Members of this section have distinct calyces with one midrib and two marginal ribs on each lobe that are thickened and rigid (Wilson 1994). The sectional name highlights the bifurcate apex of the epicalyx lobes that are present in some species.
There has not been a global monograph of Hibiscus section Furcaria since the work of Hochreutiner (1900). However, regional treatments and recent species descriptions provide detailed descriptions for almost all species in section Furcaria and the most relevant literature is noted here to facilitate future revisions (Forsskål and Niebuhr 1775; Cavanilles 1787; Walter 1788; Willdenow 1800, 1809; Roxburgh 1814, 1832; de Candolle 1824; Blume 1825; de Saint-Hilaire 1828; Don 1830, 1831; Wight and Arnott 1834; Wight 1839; de Saint-Hilaire and Naudin 1842; Richard 1845, 1847; Garcke 1849a, 1849b, 1849c, 1874, 1880, 1881; Hooker and Bentham 1849, 1872; Hooker 1859; Bentham 1863; Grisebach 1864; Seeman 1865; Kurz 1874; Masters 1874; Dumont 1889; Kuntze 1891a; Kuntze 1891b; Gürke 1892; de Cordemoy 1895; Hochreutiner 1900, 1901, 1902, 1908, 1917a, 1917b, 1924, 1955; de Wildeman and Durand 1901; Smith 1903; Backer 1907; Durand and Durand 1909; Gamble 1915; Fitzgerald 1918; Ulbrich 1919, 1921, 1922, 1924; Britton and Millspaugh 1920; Standley 1923, 1928; Fawcett and Rendle 1926; Domin 1928; Hutchinson and Dalziel 1928, 1931; Uittien 1932; Small 1933; Exell and Mendonça 1937; Yuncker 1943; Guillaumin 1948; von Cufodontis 1948, 1959; Exell 1951, 1961; Parsa 1951; Alain 1953; Berhaut 1954; Hu 1955; Kearney 1955, 1957; Parker 1956; Macbride 1956; León and Alain 1957; Keay 1958; Gonçalves 1961; Roe 1961; Borssum Waalkes 1966; Fosberg and Sachet 1966; Merxmüller 1969; Rakshit and Kundu 1970; Wilson and Byrnes 1970; Bates 1971, 1990, 1999; Ngwe 1971; Stewart 1972; Tackholm 1974; Wilson 1974, 1978, 1983, 1993, 1994, 1995, 1999, 2006; Fryxell 1973, 1988, 1989, 1990, 1992a, 1992b, 1993, 1996, 1997, 2000, 2001a, 2001c, 2007; Riedl 1976; Saldanha and Nicolson 1976; Ali 1977; Abedin 1979; Abedin et al. 1999; Whitmore 1979; Paul and Nayar 1980, 1988; Townsend 1980; Liogier 1981; Smith 1981; Maquet 1983; Matthew 1983; Menzel et al. 1983a; Saldanha 1985; Fryxell and Wilson 1986; Ross 1986; Marais and Friedmann 1987; Naqshi et al. 1988; Webb et al. 1988; Phuphathanaphong et al. 1989; Robertson 1989; Edmonds 1991; Ho 1991; Pradeep and Sivarajan 1991; Cheek 1992; Fuertes 1992; Wheeler 1992; Chang 1993; Paul 1993; Friedman 1994; Green 1994; Edwards et al. 1995; Wilson and Craven 1995; Sivarajan and Pradeep 1996; Herman and Retief 1997; Philcox 1997; Wood 1997; Thulin 1999a, 1999b; Mitchell and Norris 2000; Craven et al. 2003, 2016; Ghazanfar 2003; Florence 2004; Fryxell and Krapovickas 2004; Krapovickas and Fryxell 2004; Pennington et al. 2004; Areces Berazaín 2006; Krapovickas 2006, 2013; Areces Berazaín and Fryxell 2007; Karim and Fawzi 2007; Tang et al. 2007; Lisowski 2009; Lambdon 2012; Cowie et al. 2013; van der Burg 2013; Lopes Esteves et al. 2014; Badry et al. 2015; Blanchard 2015; Badry et al. 2015, 2017; Hanes 2015; Sykes 2016; Fernandes Júnior and Cruz 2018; Wannan 2022, 2024).
Regional checklists often provide summaries of whether species are accepted in particular jurisdictions and often provide useful notes on distribution, misapplications and typification. The following selected references are of particular relevance for Hibiscus section Furcaria (Harvey 1860, 1862; Baillon 1885; Rendle 1899; Prain 1903; de Wildeman 1904; Oliver 1916; Chevalier 1920; Chiovenda 1923; Bremekamp and Obermeyer 1935; Exell 1944; Brenan et al. 1953; Molinari and Fabris 1954; Dwyer 1955; Cavaco 1959; Unzueta et al. 1975; Peyre de Fabregues and Lebrun 1976; Proctor 1982; Brunel et al. 1984; Levin and Moran 1989; Edmonds 1991; Lebrun and Stork 1991; Pool et al. 1993; Jørgensen and Fryxell 1999; Bovini et al. 2001; Germishuizen and Meyer 2003; Acevedo-Rodríguez 2005; Leistner 2005, 2008; Sita and Moutsambote 2005; Akoègninou et al. 2006; Sosef et al. 2006; Krapovickas and Marticorena 2008; Nee 2008; Rondon 2009; Lejoly and Geerinck 2010; Lejoly et al. 2010; Gardner 2011; Acevedo-Rodríguez and Strong 2012; Manning and Goldblatt 2012; Darbyshire et al. 2015; Lima and Conceição 2016; Villaseñor 2016; Mao and Dash 2020; Camargo et al. 2022; Gosline et al. 2023; Rodrigues Costa et al. 2023).
Most currently recognised species are therefore well circumscribed and documented (see Wilson 2006), with modern descriptions available for most species in English, French, Spanish or Portuguese. Many of the species in section Furcaria have been named in the last 20 years and additional species likely await recognition. There are three centres of diversity for the genus, with approximately equal numbers of native species in Africa, tropical Australia and South America.
Rationale for generic concept
Hibiscus section Furcaria has a great deal of recognition as a natural group, with Menzel and Wilson (1969, p. 93) stating that ‘It seems probable that when the large collective genus Hibiscus is better understood, sect. Furcaria will emerge logically as one of several distinct genera now lumped together.’ We agree with the distinctiveness of this section and therefore propose to recognise the group as Sabdariffa at genus level. This represents the largest single-genus change proposed for the reclassification of the Hibisceae (Hanes et al. 2024). Kosteletzky (1836) raised section Furcaria to genus level but unfortunately, the name Furcaria was not actually available (see taxonomy below), therefore we must use the next available name, Sabdariffa, based on the species name for roselle (Hibiscus sabdariffa L.).
A consideration in changing Hibiscus sect. Furcaria to Sabdariffa is that three of the species to be included in this genus have significant economic importance (see further discussion below). Roselle must change both genus and specific epithet as to avoid creating the tautonym ‘Sabdariffa sabdariffa’ (Turland et al. 2018; Art. 23.4). Therefore, the next available species name, published as Hibiscus gossypiifolius Mill. by Miller (1768) in the Gardner’s Dictionary, that we here recombine as Sabdariffa gossypiifolia, must be used. Although this name has very little recognition to users of this plant species, the name ‘sabdariffa’ is still reflected. Kenaf, now Sabdariffa cannabina (L.) M.M.Hanes & R.L.Barrett, has similar issues but the heart of the specific epithet remains, as does false roselle, Sabdariffa acetosella (Welw. ex Hiern) M.M.Hanes & R.L.Barrett. Although it is indisputable that roselle and kenaf are of global significance as cultivated plants (Dalziel 1948; Bates 1965a, 1965b; Wilson 1967; Werber 1993; Goforth and Fuller 1994; Akpan 2007; Magdalita and San Pascual 2022), changing of these important names is considered less disruptive than greatly expanding the concept of Hibiscus. Only a few species are utilised in horticulture (Lawton 2004) and most are poorly known outside natural distributions. Therefore, we do not consider the recombination of 114 other species as being overly disruptive to public knowledge. We consider this solution to be far less disruptive than the inclusion of ~291 Pavonia species (even though none are of economic importance) in an enlarged concept of Hibiscus, one part of an alternate option for creating a monophyletic Hibiscus.
While we seek to provide stable names for all species that we choose to exclude from the newly defined Hibiscus, we have separated this task into several smaller papers due to the nature of the task. Many names are involved and there is still a need to develop more detailed datasets to resolve boundaries in particular groups, and to test the placement of morphologically atypical species. Owing to the long-standing recognition of section Furcaria within Hibiscus and very limited uptake of the available name Sabdariffa, all 117 species we recognise in the genus require new nomenclatural combinations, including 3 economically important species. Provision of names under Sabdariffa provides the highest number of new name combinations for which we are absolutely confident of relationships. This significantly reduces the number of names that remain to be considered to make Hibiscus monophyletic.
Utilisation
At least 16 Sabdariffa species have been documented as important plants for humans with a diversity of uses that include food, medicine, fibre and ornament (Wilson and Menzel 1964; Bates 1965a, 1965b; Dempsey 1975; Duke and duCellier 1993; Perry et al. 1993; Wiersema and León 2013). Utilisation of the three most widely used species, false roselle, kenaf and roselle, has been reviewed in several papers emphasising the economic and cultural importance (Menzel 1986; Edmonds 1991; Akpan 2000, 2007; Magdalita and San Pascual 2022). Roselle is very widely utilised for many purposes around the world including food and beverages, particularly jam and tea (Abdallah et al. 1976; Mitchell 1982; Tsai et al. 2002; Holser and Bost 2004; Qi et al. 2005; Cid-Ortega and Guerrero-Beltrán 2015; Kapepula et al. 2017; Fig. 2). A long history of usage and global dispersal in recent centuries has led to considerable regional variation in the food and chemical properties of a wide range of cultivars of roselle (Juhari et al. 2018). The species is also purported to have numerous medicinal properties and has been utilised widely in traditional medicine (Qi et al. 2005; Wahabi et al. 2010; Al-Snafi 2018; Lautenschläger et al. 2018; Zheoat et al. 2019; Paraiso et al. 2021; Sedillo-Torres et al. 2022; Wang DY et al. 2022). The strong pigmentation found in roselle also has a variety of uses in cosmetic, pharmaceutical and food applications (Jabeur et al. 2017).
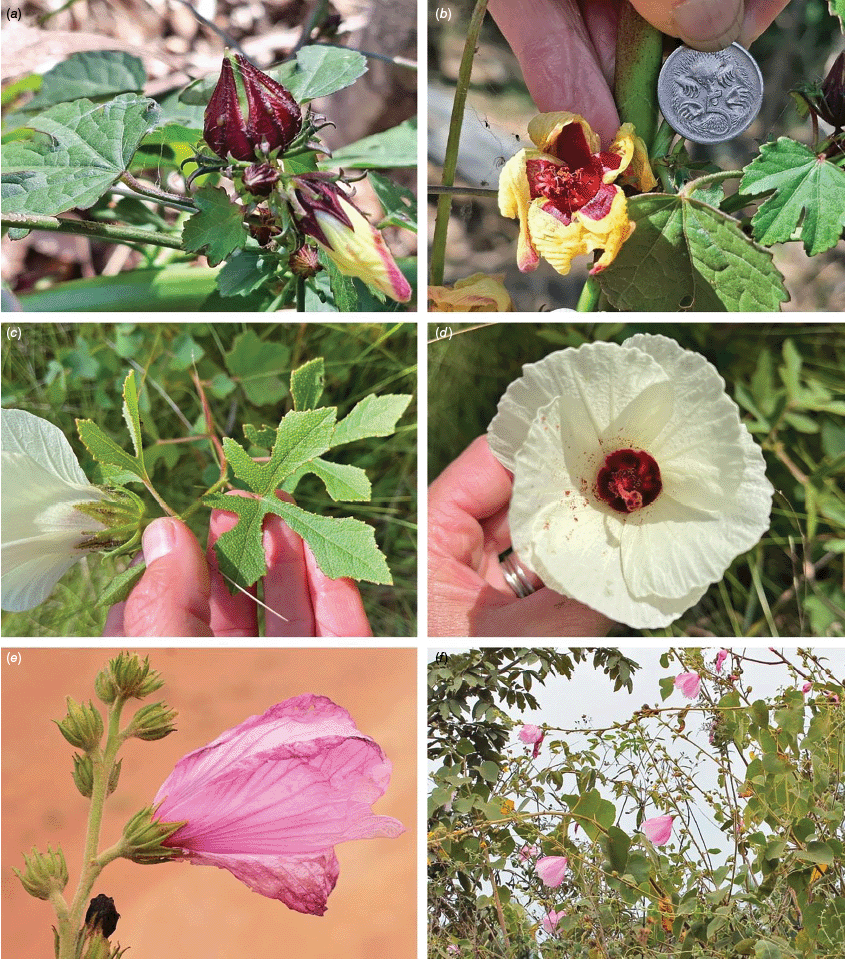
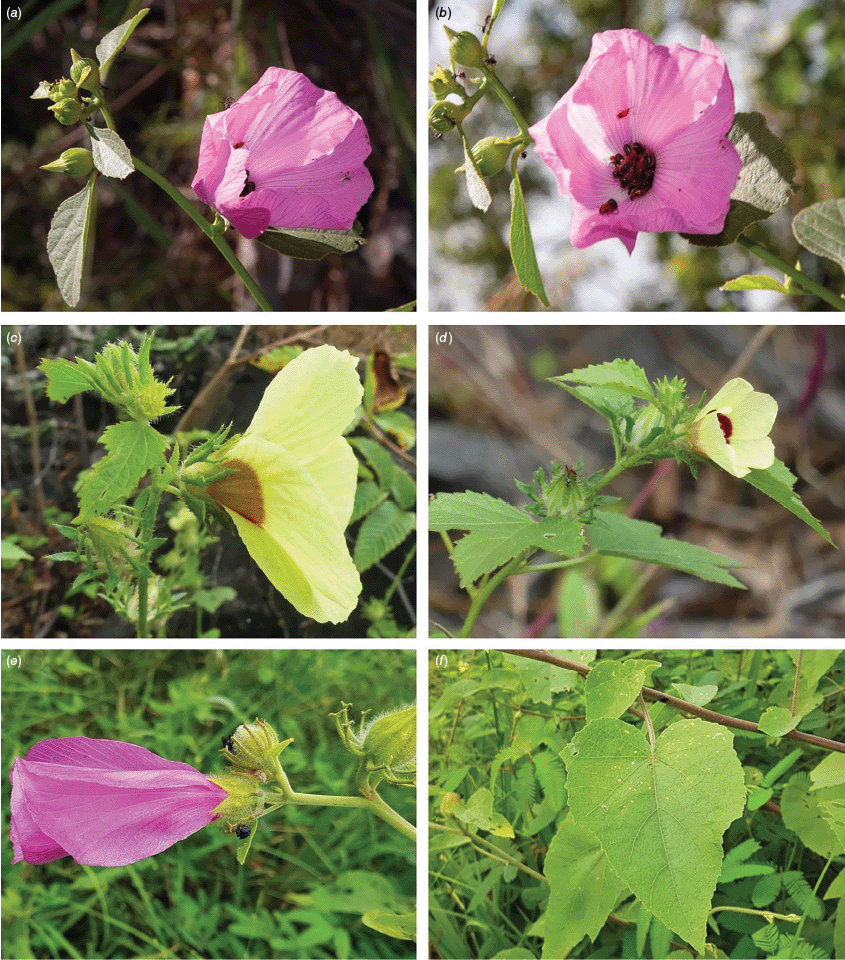
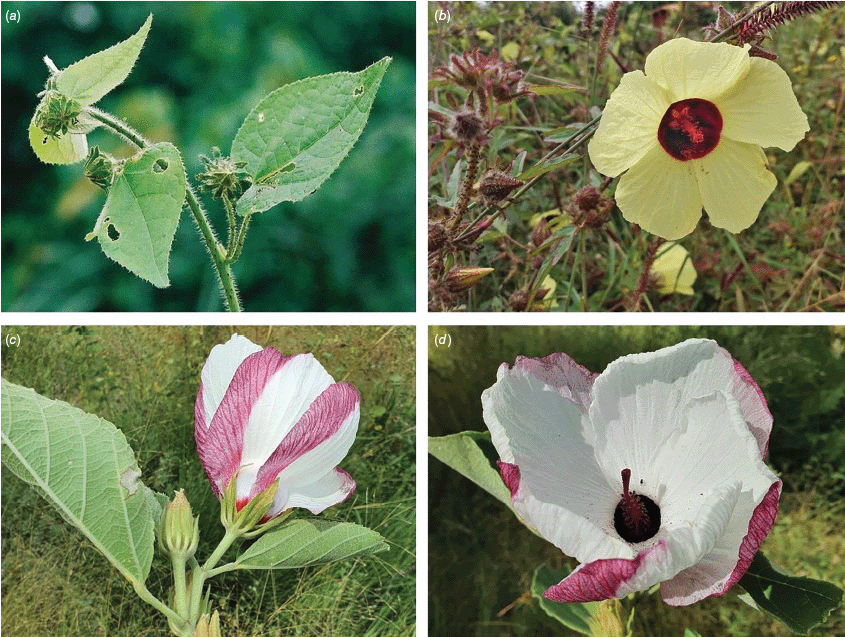
Sabdariffa gossypiifolia (roselle). (a) Traditional collection of fruit in the Northern Province of Sierra Leone. Photograph by Marcia Vermaire, iNaturalist: 199794640, CC BY-NC. (b) Fruit from plant cultivated in Brazil. Photograph by Thales S. Coutinho. (c) Tea made from roselle calyces. (d) Jam made from roselle calyces. Images in c & d licenced from stock.adobe.com.

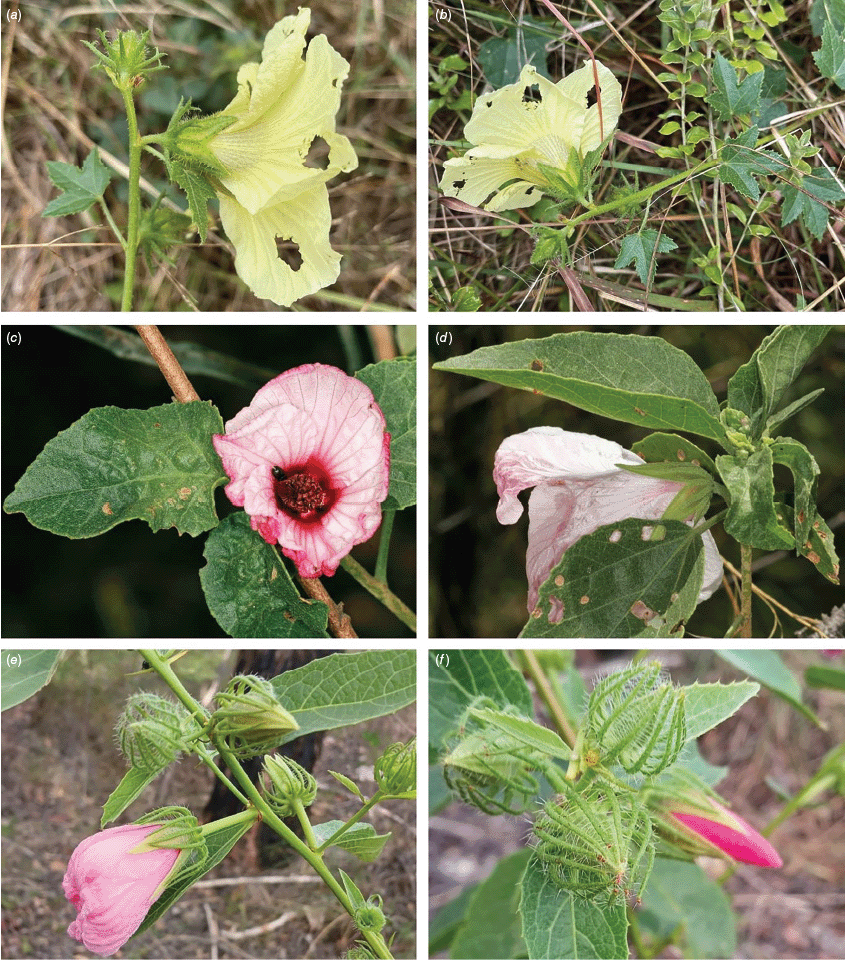
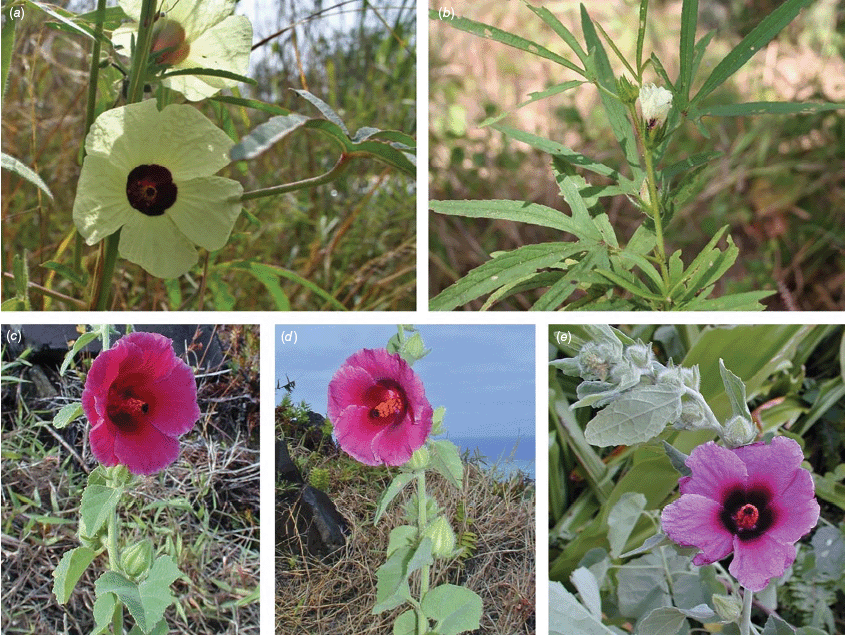
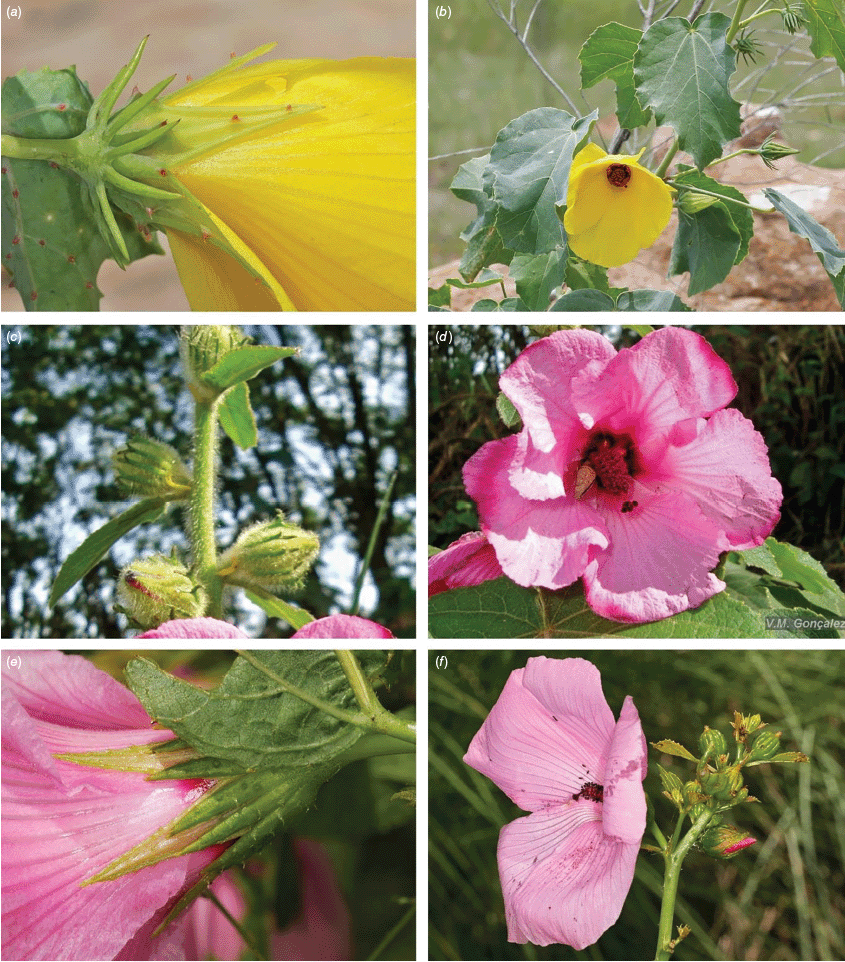
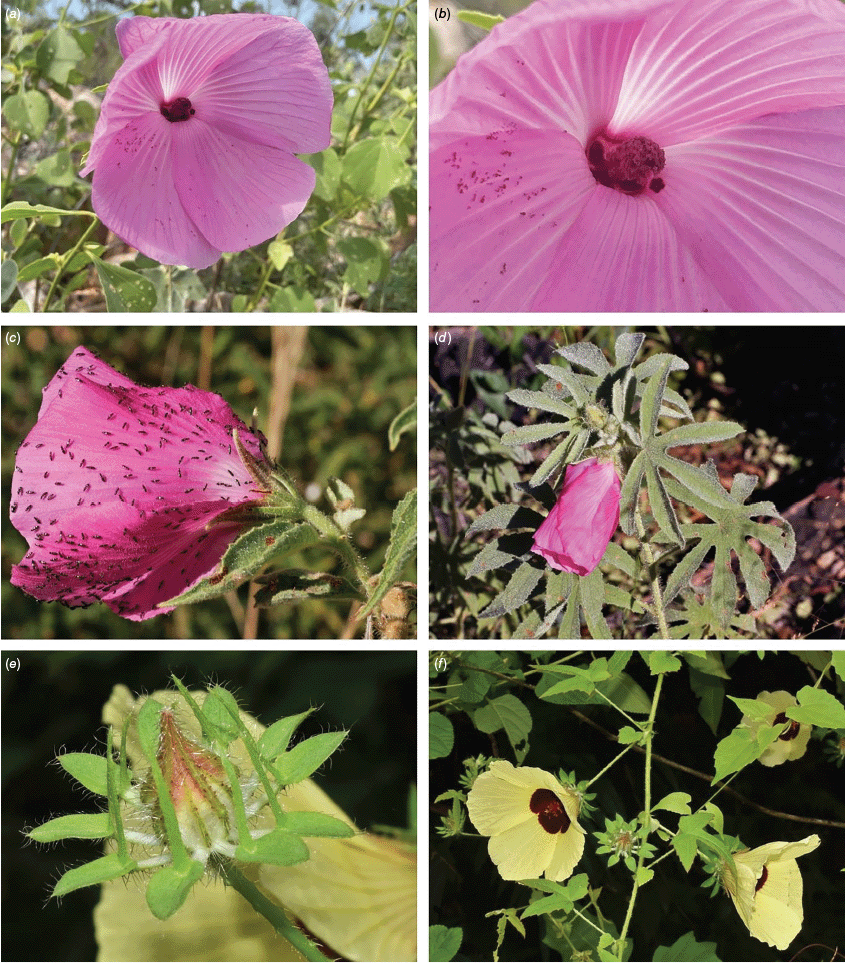
Roselle (Fig. 2), kenaf (Fig. 3), false roselle (Fig. 4) and monarch rose-mallow (Sabdariffa radiata) (Fig. 5) are also very important fibre plants with a very long history of utilisation (Howard and Howard 1911; Wester 1911, 1914; Holland 1918; Crane and Acuna 1945; Crane 1947, 1949; Murdock 1959; Wilson 1967; Taylor 1992; Werber 1993; Goforth. and Fuller 1994; Holser and Willett 2005; Zhang et al. 2020). The long history of cultivation of kenaf in particular makes defining the species challenging, given the range of cultivars and potential wild forms, some of which are illustrated in Fig. 3. There has also been significant historical confusion over the relative definitions of S. cannabina and S. radiata (see discussions under these species below), and a hybrid origin of S. radiata has been proposed by Wilson (1994) that may explain some of the morphological variation present in the species. We note however, that potential wild-type plants have been identified in eastern India, therefore further investigation of their origins is warranted.
Sabdariffa cannabina. (a, b) Commonly cultivated forms. Photographs by (a) Elsa Bussiere, iNaturalist: 201342972, ©, (b) chabiboni1972, iNaturalist: 64100504, CC BY-NC. (c, d) Widespread native form. Photographs by Ian C. Riddell, iNaturalist: 109523824, CC BY. (e, f) Rift Valley native form. Photographs by James Kuria Ndung’u, iNaturalist: 49211951, CC BY-NC.
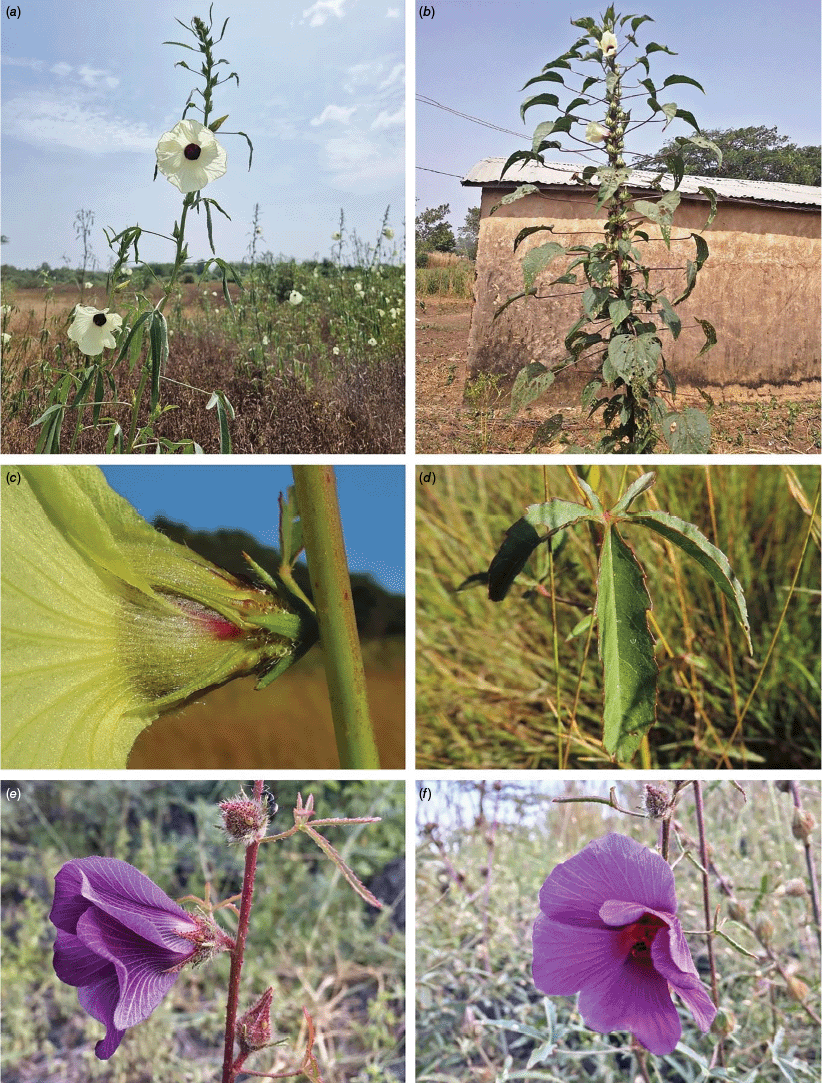
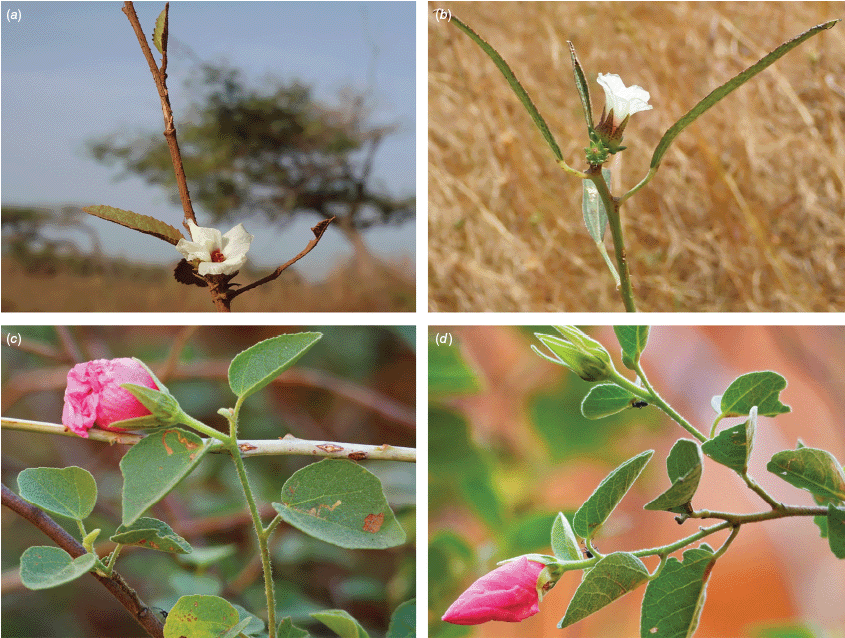
Sabdariffa acetosella. (a–d) Commonly cultivated forms. (a, b) Mindo, Ecuador (naturalised). (c, d) Rajshahi, Bangladesh (naturalised). (e, f) Wild-type (native) form (this plant growing as an introduction in Australia). Photographs by (a, b) Russell Barrett. (c, d) Sabarni Sarker, iNaturalist: 95827800, CC BY-NC-ND. (e, f) Phoebe French.
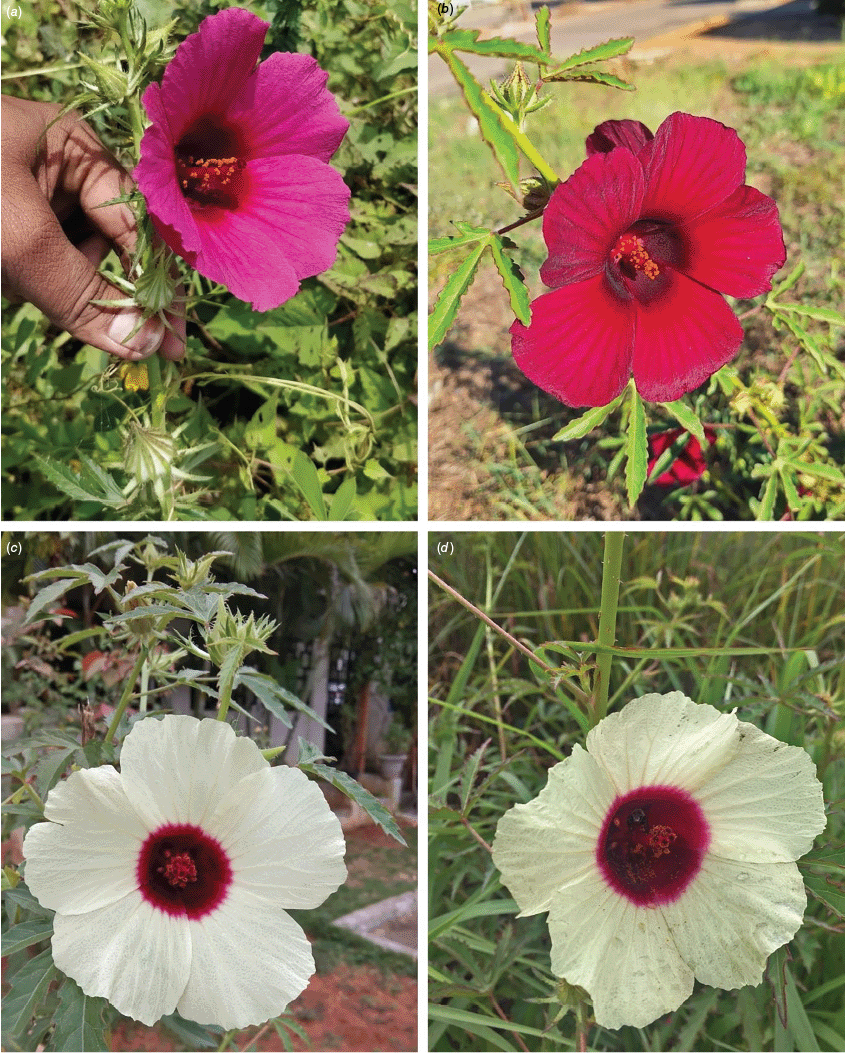
Sabdariffa radiata. (a, b) Commonly cultivated forms. Photographs by (a) Sarvadaman Kulkarni, iNaturalist: 215753567, CC BY-NC. (b) Francisco V. Bezerra Neto, iNaturalist: 211505808, CC BY-NC. (c) Presumed wild-type. Photograph by Swarochi Tathagath, iNaturalist: 62190166, CC BY. (d) Presumed wild-type. Photograph by Kiran Otsarg, iNaturalist: 93619700, CC BY-NC.
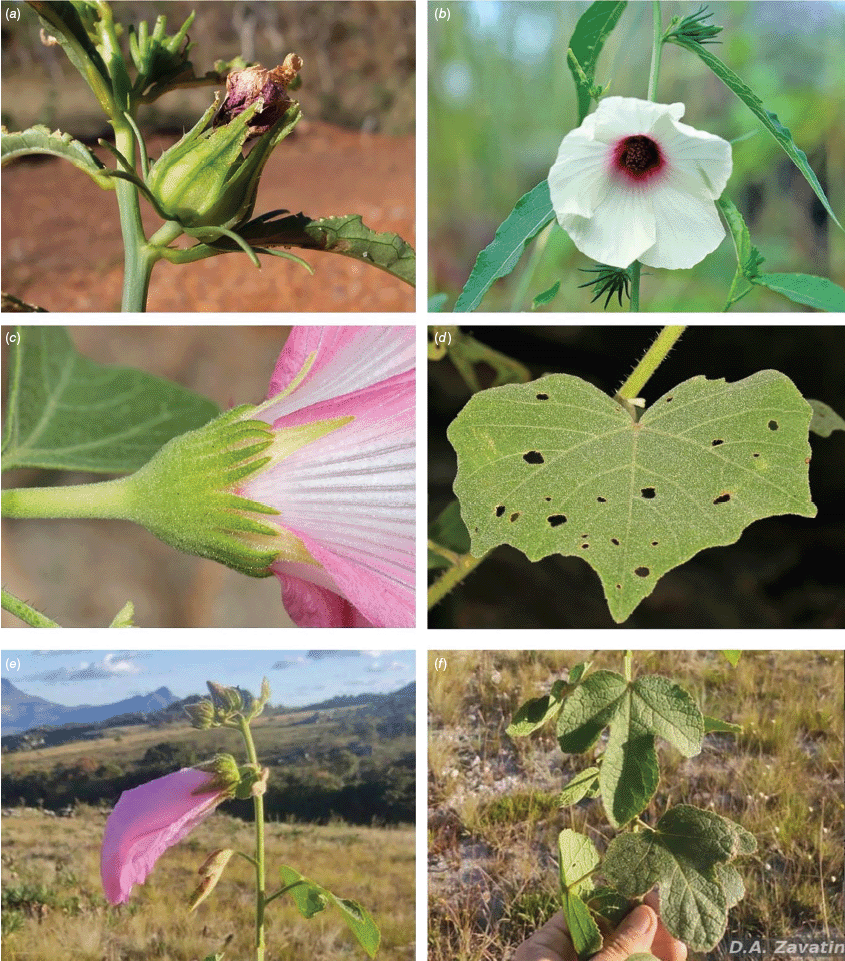
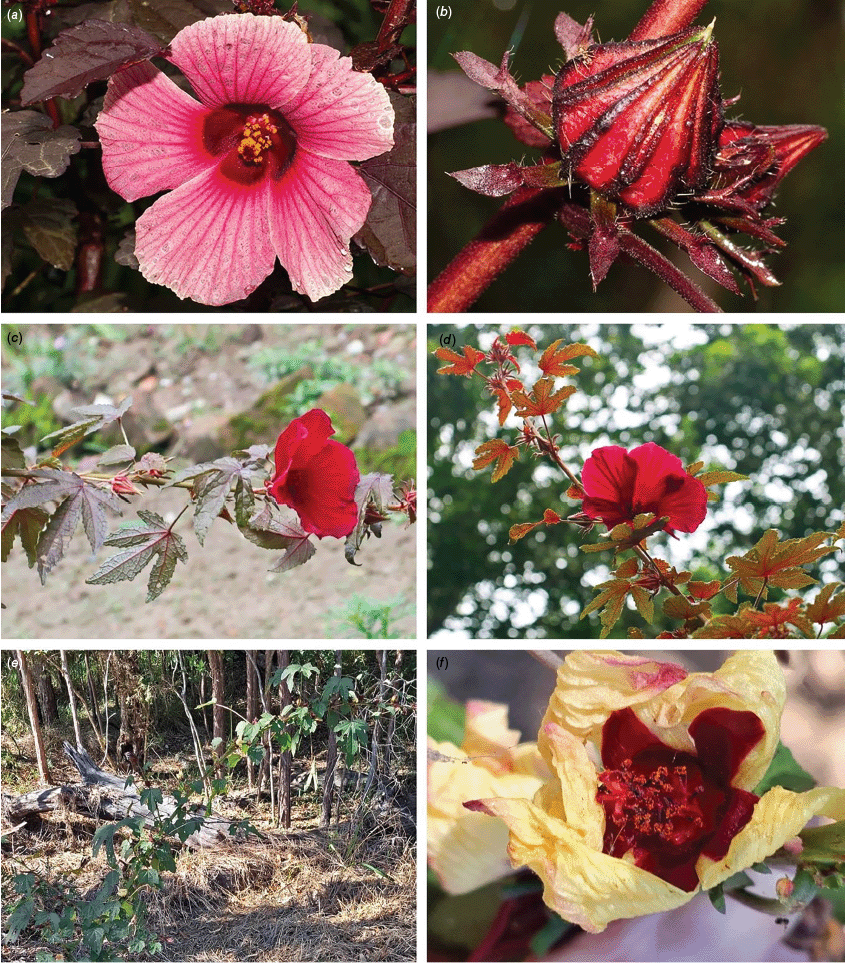
In Australia, the wood of S. heterophylla (Vent.) McLay & R.L.Barrett has been used for making musical instruments (Wilson and Menzel 1964; Mitchell 1982), and the bark was commonly used to make string and fibre products by indigenous Australians (Ahmed and Johnson 2000). Sabdariffa splendens (C.Fraser ex Graham) McLay & R.L.Barrett is recognised to lower male libido and may have potential as a male contraceptive (Dalton 2018). Similar effects have been reported for S. gossypiifolia (Orisakwe et al. 2004). Sabdariffa brackenridgei (A.Gray) M.M.Hanes & R.L.Barrett (Ma‘o hau hele) is a critically endangered (The IUCN Red List of Threatened Species, see https://www.iucnredlist.org/, accessed 21 August 2024) Hawaiian endemic species and the official state flower of Hawai’i (Neal 1965; Kepler 1988; Bates 1990; Whistler 1992). There are three recognised subspecies, one of which (subsp. molokianus (Rock ex Caum) M.M.Hanes & R.L.Barrett) is considered extinct (Wilson 1993).
General biogeography and diversity of Sabdariffa
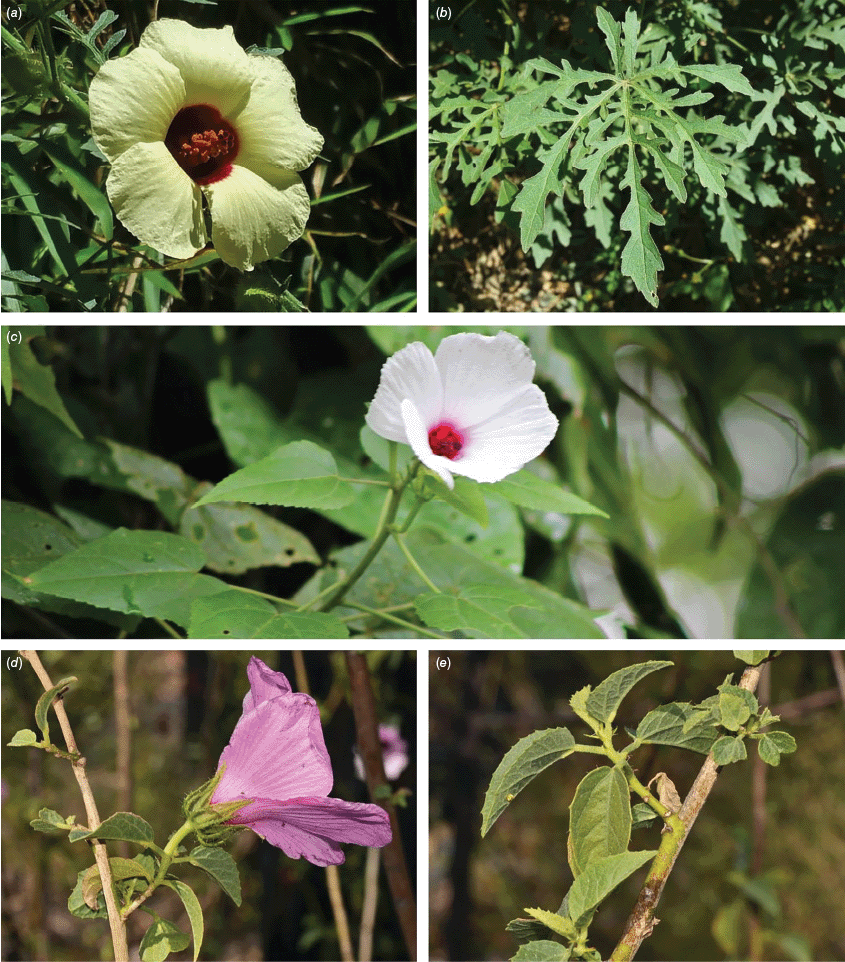
Sabdariffa is pantropical in extent though most species are tropical, occurring in the Americas, Pacific, Australia, Asia and Africa. Hotspots of species diversity in Sabdariffa can be identified in tropical America (especially Brazil), northern Australia and tropical Africa. The greatest proportion of species found in each of these areas constitute localised endemic species and few have naturally broad distributions. For example, most locally endemic species in northern Australia are almost entirely associated with rocky outcrop substrates, especially sandstone (Craven et al. 2003). This suggests that drivers of speciation in northern Australia may be largely edaphic and associated with the natural isolation of the rocky areas in which the species occur. Hybridisation between members of Hibisceae is usually restricted to related species (Janakiram and Patil 2017). However, breeding barriers are clearly present as the co-occurrence of multiple species is not particularly unusual in some regions (Satya 2012; Satya et al. 2012, 2013a, 2013b). Many Australian species of Sabdariffa appear to have allopatric ranges, i.e. for the most part, these are geographically isolated from each other’s presumed closest relatives and co-occurring species are commonly less closely related (Craven et al. 2003; R. L. Barrett and M. D. Barrett, pers. obs.). More closely related species may still possibly hybridise if the distributions happen to overlap (see Wilson 1994) and therefore individual collections with anomalous morphology should be carefully considered to determine if these may be hybrids. Several such anomalous specimens await further assessment.
One notable exception to these patterns of local endemism is currently presumed to be Sabdariffa diversifolia (Jacq.) McLay & R.L.Barrett that has a very broad distribution across Africa (including Madagascar), Asia, to Australia, New Zealand, the Pacific and Central and South America. Although the native range has occasionally been debated (e.g. Gardner 1985; Sykes 1988), fossil pollen records that pre-date human occupation have been found in both New Zealand and the Galápagos, clearly demonstrating the broad natural occurrence in the region (Newnham and Lowe 1991; van Leeuwen et al. 2008). Te Reo Māori names for this species (Aute-Purarangi and Purarangi) are on record from the early 1800s (P. de Lange, pers. comm.). There is also a distinctive morphotype that is restricted to ultramafics at Surville Cliffs in the far north of New Zealand (de Lange et al. 2018). We note that pollen core evidence suggests an introduction to Norfolk Island by Polynesian settlers (Macphail et al. 2001), therefore each case of dispersal should be considered independently. Consideration should perhaps be given to whether this wetland species is able to be ocean-dispersed, as in Talipariti (Yamazaki et al. 2023) or whether the habitat lends itself to associated dispersal by waterbirds. The appearance of a population in a petrel colony on an isolated oceanic rock stack 300 km south of other known occurrences in New Zealand lends weight to some form of facilitated dispersal by these skim-feeding birds (P. de Lange, pers. comm.).
Roselle and kenaf are currently also very widely distributed throughout the global tropics. The wild origins of these species are inferred to be tropical Africa (Wilson 1994; Satya et al. 2013a, 2013b; Hendy 2019) whereas all other occurrences around the world can be inferred by human-mediated dispersal (Alexopoulou et al. 2013; Satya et al. 2013a, 2014). Considerable morphological diversity exists within the native ranges of both roselle and kenaf and numerous morphotypes have been utilised (e.g. Howard and Howard 1911; Fig. 2, 3). Although only a single morphotype is usually found in a given area of introduction, several morphotypes have been identified among introduced populations.
Speciation within section Furcaria has at least in part also been driven by chromosomal rearrangement, in particular polyploidy. Our knowledge in this area is largely due to the work of Margaret Menzel (1924–1987), who undertook investigations spanning a great deal of her lifetime. Extraordinary chromosomal diversity has been documented in the genus (Menzel and Wilson 1961, 1963, 1966, 1969; Menzel 1963, 1986; Wilson and Menzel 1964; Menzel and Martin 1970, 1971, 1974, 1980; Martin and Menzel 1971, 1972; Kachecheba 1972; Fernández 1974; Dasgupta and Bhatt 1981; Menzel and Hancock 1984; Menzel et al. 1983a, 1983b, 1986; Fryxell and Stelly 1993; Fernández et al. 2003) and chromosome numbers are known in a large proportion of species in the genus. Thirteen distinct genome groups have been identified that are largely associated with specific biogeographic regions (e.g. 9 of the 13 genome groups occur in sub-Saharan Africa) but some genome groups are shared across continents in various combinations (Wilson 1994, 2006). The complex genome origins provide significant challenges for reconstructing nuclear genomes and analysis of nuclear data due to paralogy, though there is a growing number of chloroplast genomes that have been published for Sabdariffa and related Hibisceae (Xu et al. 2019; Abdullah et al. 2020; Zhang et al. 2020; Eriksson et al. 2021; Wang ZQ et al. 2022; Kwon et al. 2022, 2023, 2024; Koo et al. 2023; Go et al. 2024). Population-level studies in other Hibisceae genera provide models for future studies on Sabdariffa (Yamazaki et al. 2023; Mashburn 2024).
These data offer some insight into areas of origin for some species, and patterns and processes of speciation within Sabdariffa. However, as noted above, a species-level phylogeny of the genus remains lacking and timing of diversification is unknown. This group will likely prove to be very interesting for exploring the timing and pattern of dispersal around the global tropics, and mechanisms of chromosomal evolution.
Ecology
Although species in Sabdariffa are perennial, most are short-lived (5–10 years), disturbance-following species. A few species can form small trees and the average age expectancy may be more in the order of 20–30 years. These longer-lived species are still considered to be disturbance followers, only with longer lifespans enabled by wetter environments (Craven et al. 2016).
As with many Malvaceae, seeds of Sabdariffa species are commonly long-dormant (likely in the order of 10–50 years) and many species await specific germination cues associated with disturbance before mass germination (Menzel and Wilson 1969; Kak et al. 2015: R. L. Barrett and M. D. Barrett, pers. obs.), whereas some species, including S. gossypiifolia, appear to have no dormancy (Siepe et al. 1997; Anjah et al. 2012). Thus revisiting known populations in which all genetic diversity at the site has been reduced to the soil seedbank is not uncommon. This can make population level studies challenging as specimens are only available under particular conditions, such as specific fire history at a localised scale. Many species therefore remain poorly known in many parts of the world. An advantage however, is that seeds from herbarium specimens can commonly be germinated (Craven et al. 2003, 2016; Krapovickas and Fryxell 2004).
Animal associations with these plants are similarly poorly known. However, at least two hibiscus-flower flies are known to be associated with species of Sabdariffa (Barker 2005) and many more such specific associations are likely to exist. Flower beetles are also commonly observed on species of this genus, the beetles commonly consuming the soft flower parts after these have shrivelled, with beetles often becoming trapped inside (Kirejtshuk and Lawrence 1999; Simon et al. 2021). Whether or not this commonly observed process has an influence on pollination remains largely unknown. However, this would be consistent with other beetle pollination mechanisms in groups including arum lilies, in which beetles may be trapped inside flowers for extended periods and achieve pollination (García-Robledo et al. 2004; Sayers et al. 2021). The closing of the flowers in many African, Asian and Australian Sabdariffa species appears to be primarily associated with self-fertilisation and is described in detail for S. cannabina and S. gossypiifolia by Howard and Howard (1911). Menzel et al. (1983a) concludes that most of the American species are most likely to be bee pollinated, lacking the red basal petal spot found in many other species and lacking the selfing mechanism described above. Menzel et al. (1983a) also notes that S. uncinella (Moç. & Sessé ex DC.) M.M.Hanes & R.L.Barrett appears to be adapted for hummingbird pollination, as the red flowers form a narrow tube and the anthers are clustered towards the style apex. Ruan (2010) and Ruan et al. (2010) provide reviews of the role of style curvature in guiding pollinators to specialised Hibisceae, including S. uncinella.
Species-level taxonomy
Species-level taxonomy of Hibiscus Section Furcaria has been further challenged by difficulties associated with the collection of high quality specimens. Once collected as herbarium specimens, Malvaceae are highly susceptible to mould and insect attacks in tropical environments and therefore the collection of high quality material is often difficult to achieve (Knight et al. 2024). Species in this group are also often very spiny and thus not attractive to collectors. The ephemeral nature of these species in the landscape also means that repeat visits to sites may be required to collect complete specimens and therefore representation in herbaria can remain poor or patchy.
Morphologically there is a great deal of similarity among most of the species in Sabdariffa, with a few morphologically anomalous species that are nevertheless confidently included. These include the enigmatic S. kirstyae (Craven) McLay & R.L.Barrett, known from two very narrow locations in north-western Australia, growing in shallow soils over sandstone ‘pavements’ (Craven et al. 2016). This species differs from all other Australian species in being glaucous and otherwise completely glabrous, and in having yellow flowers, whereas almost all other species in this region have pink flowers. Therefore the superficial differences are very striking. However, the characters of the calyx, aculei, leaves and flowers are all consistent with placement in section Furcaria, where this species was originally named in 2016 (Craven et al. 2016).
This paper mostly accepts the recently published revision papers as authorities for species concepts, with only minimal modifications and addition of recently described species (Wilson 2006; Krapovickas 2006, 2013; Mwachala 2009; van der Burg 2013; Craven et al. 2016; Wannan 2022, 2024). We have attempted to update type citations to link to digitised type specimens where available and add barcodes to specimen citations, to make the location of these materials easier for future workers. Many lectotypes are designated to fix the applications of names. Previously published keys to species in regional areas are updated to include current names in Sabdariffa and additional species not included in the original versions.
Methods
Phylogenetic analyses of clade /Phylloglandula
A summary phylogeny of Hibisceae was made using TreeViewer (ver. 2.2.0, see https://github.com/arklumpus/TreeViewer/releases; Bianchini and Sánchez-Baracaldo 2024) by collapsing nodes in the maximum likelihood phylogeny from Hanes et al. (2024) to represent the major clades of Hibisceae (Fig. 1).
To further investigate phylogenetic relationships within /Phylloglandula, we combined sequences of matK, ndhF, trnQ-rps16 and ycf1 from species available in Hanes et al. (2024) with newly downloaded sequences of these loci from GenBank to incorporate additional species of Sabdariffa. The number of Sabdariffa samples included was maximised by also utilising rbcL and rpl16 sequences (available on GenBank in October 2023), loci that were not utilised by Hanes et al. (2024). The list of taxa included and marker references are presented in Table 1. Alignments of each locus were made separately in Geneious Prime (ver. 2024.0, see https://www.geneious.com) using the MAFFT plugin (ver. 7.450, see https://mafft.cbrc.jp/alignment/software/; Katoh and Standley 2013) and end of alignments were trimmed to reduce raggedness. The combined alignments were checked for duplicate sequences and we maximised sampling for each genus in the clade to estimate relationships between the genera. All alignments were concatenated together and samples represented only by a single locus were removed to reduce the quantity of missing data.
| Species | Source | matK | ndhF | rbcL | rpl16 | trnQ | ycf1 | Original name | |
|---|---|---|---|---|---|---|---|---|---|
| Decaschistia byrnesii | GenBank | AY589066.1 | AY589079.1 | – | – | – | – | ||
| Decaschistia byrnesii | Mitchell2893 | MZ394849 | MZ419903 | – | – | MZ542259 | MZ612135 | ||
| Decaschistia occidentalis | Craven9240 | MZ394850 | MZ419904 | – | – | MZ542273 | MZ612136 | ||
| Hibiscus meeusei A | GenBank | – | AF384627.1 | – | AF384591.1 | – | – | ||
| Hibiscus parvilobus A | Wilson96 | MZ394945 | MZ419990 | – | – | – | MZ612352 | ||
| Hibiscus youngianus A | ND | MZ394986.1 | MZ420030.1 | – | – | MZ542249.1 | MZ612269.1 | ||
| Kydia calycina | R. Neupaney s.n. | EF207261 | EF207293 | – | – | – | – | ||
| Kydia calycina | SuGongWS | MZ395022 | – | – | – | – | MZ612339 | ||
| Papuodendron lepidotum | GenBank | – | AF230247.1 | – | – | – | – | ||
| Sabdariffa aculeata | Abbott 22842 | MZ394860 | – | – | – | – | Hibiscus aculeatus | ||
| Sabdariffa brackenridgei | Blanchard3454 | MZ394877 | MZ419928 | – | – | MZ542079 | MZ612234 | Hibiscus brackenridgei | |
| Sabdariffa cannabina | MK404537 | MK404537 | MK404537 | – | – | MK404537 | MK404537 | Hibiscus cannabinus | |
| Sabdariffa cannabina | SmallSN | EF207259 | EF207290 | – | – | – | – | Hibiscus cannabinus | |
| Sabdariffa cannabina | GenBank | MW446503 | MW446504 | MW446505 | MW446506 | MW446507 | MW446508 | Hibiscus cannabinus | |
| Sabdariffa cannabina | GenBank | MW446504 | MW446505 | MW446506 | MW446507 | MW446508 | MW446509 | Hibiscus cannabinus | |
| Sabdariffa costata | Fryxell 740 | AY589057 | U55323 | – | – | – | – | Hibiscus costatus | |
| Sabdariffa diversifolia | Blanchard3469 | MZ394898 | MZ419948 | – | – | MZ542258 | MZ612295 | Hibiscus diversifolius | |
| Sabdariffa fryxellii | GenBank | – | AF384632.1 | – | AF384584.1 | – | – | Hibiscus fryxellii | |
| Sabdariffa furcellata | Abbott25211 | MZ394909 | – | – | – | MZ542243 | MZ612223 | Hibiscus furcellatus | |
| Sabdariffa furcellata | GenBank | – | AF384629.1 | – | AF384585.1 | – | – | Hibiscus furcellatus | |
| Sabdariffa gossypiifolia | GenBank | JQ693601.1 | – | KU556650.1 | AF384602.1 | – | – | Hibiscus sabdariffa | |
| Sabdariffa gossypiifolia | MZ522720 | MZ522720 | MZ522720 | MZ522720 | MZ522720 | MZ522720 | MZ522720 | Hibiscus sabdariffa | |
| Sabdariffa heterophylla | GenBank | KM894805.1 | AF384631.1 | – | AF384586.1 | – | – | Hibiscus heterophyllus | |
| Sabdariffa mechowii | GenBank | HQ235320.1 | – | KR137549.1 | AY727395.1 | – | – | Hibiscus mechowii | |
| Sabdariffa meraukensis | GenBank | – | AF384633.1 | – | AF384592.1 | – | – | Hibiscus meraukensis | |
| Sabdariffa nigricaulis | GenBank | – | AF384628.1 | – | AF384594.1 | – | – | Hibiscus nigricaulis | |
| Sabdariffa radiata | GenBank | JQ693600 | – | – | AF384600.1 | – | – | Hibiscus radiatus | |
| Sabdariffa splendens | BowlesH95.057 | MZ394973 | MZ420017 | – | – | MZ542169 | MZ612148 | Hibiscus splendens | |
| Sabdariffa splendens | GenBank | KM894510.1 | – | KM895602.1 | AF384606.1 | – | – | Hibiscus splendens | |
| Sabdariffa surattensis | Small s.n | EF207258 | AF384626 | – | – | – | – | Hibiscus surattensis | |
| Sabdariffa surattensis | GenBank | EF207258 | EF207289.1 | – | AF384609.1 | – | – | Hibiscus surattensis | |
| Sabdariffa uncinella | Blanchard3458 | MZ394984 | MZ420028 | – | – | MZ542078 | MZ612268 | Hibiscus uncinellus | |
| Sabdariffa zonata | GenBank | – | AF384630.1 | – | AF384614.1 | – | – | Hibiscus zonatus | |
| Talipariti elatum | GenBank | AB233276.1 | AB233265.1 | – | AB233254.1 | – | – | Hibiscus elatus | |
| Talipariti elatum | Taylor10898 | MZ395106 | MZ420136 | – | – | MZ542076 | MZ612367 | Hibiscus elatus | |
| Talipariti glabrum | GenBank | AB181085.1 | AB181054.1 | – | AB181029.1 | – | – | Hibiscus glaber | |
| Talipariti hamabo | GenBank | AB181099.1 | EF207292.1 | – | AB181051.1 | – | – | Hibiscus hamabo | |
| Talipariti hamabo | GenBank | LC554222 | LC554223 | LC554224 | – | LC554226 | LC554227 | Hibiscus hamabo | |
| Talipariti hamabo | KR259988 | KR259988 | – | – | – | – | Hibiscus hamabo | ||
| Talipariti hastatum | Frank662 | MZ395107 | MZ420137 | – | – | MZ542276 | MZ612404 | Hibiscus hastatus | |
| Talipariti macrophyllum | GenBank | AB181100.1 | AB181076.1 | – | AF384589.1 | – | – | Hibiscus macrophyllus | |
| Talipariti macrophyllum | Craven10202 | MZ395108 | MZ420138 | – | – | MZ542226 | MZ612169 | Hibiscus macrophyllus | |
| Talipariti tiliaceum | GenBank | AB181096.1 | AB181062.1 | – | AF384611.1 | – | – | Hibiscus tiliaceus | |
| Talipariti tiliaceum | GenBank | AB233274.1 | AB233261.1 | – | AB233250.1 | – | – | Hibiscus pernambucensis | |
| Talipariti tiliaceum | GenBank | MN533969 | MN533970 | MN533971 | MN533972 | MN533973 | MN533974 | Hibiscus tiliaceus | |
| Talipariti tiliaceum | BowlesH95.022 | MZ395109 | MZ420139 | – | – | MZ542168 | MZ612170 | Hibiscus tiliaceus | |
| Urena armitiana | Blanchard3455 | MZ395110 | MZ420140 | – | – | MZ542080 | MZ612208 | ||
| Urena armitiana | Clarkson6942 | MZ395111 | MZ420141 | – | – | MZ542264 | MZ612171 | ||
| Urena australiensis | Blanchard3433 | MZ395112 | MZ420142 | – | – | MZ542081 | MZ612347 | ||
| Urena lobata | Beck5143 | MZ395113 | MZ420143 | – | – | – | – | ||
| Urena lobata | Blanchard3449 | MZ395114 | MZ420144 | – | – | MZ542082 | MZ612202 | ||
| Urena lobata | GenBank | EF207260.1 | EF207291.1 | KF724296.1 | KT967071.1 | – | – | ||
| Urena lobata | Hu23840 | MZ395115 | MZ420145 | – | – | MZ542083 | MZ612356 | ||
| Urena procumbens | GenBank | KP094094.1 | – | KP095048.1 | – | – | – |
Missing sequence is represented by an en-dash (–). As all samples are based on previously published sequences (in Hanes et al. 2024 and GenBank) and the original species name is included for posterity in addition to updated names.
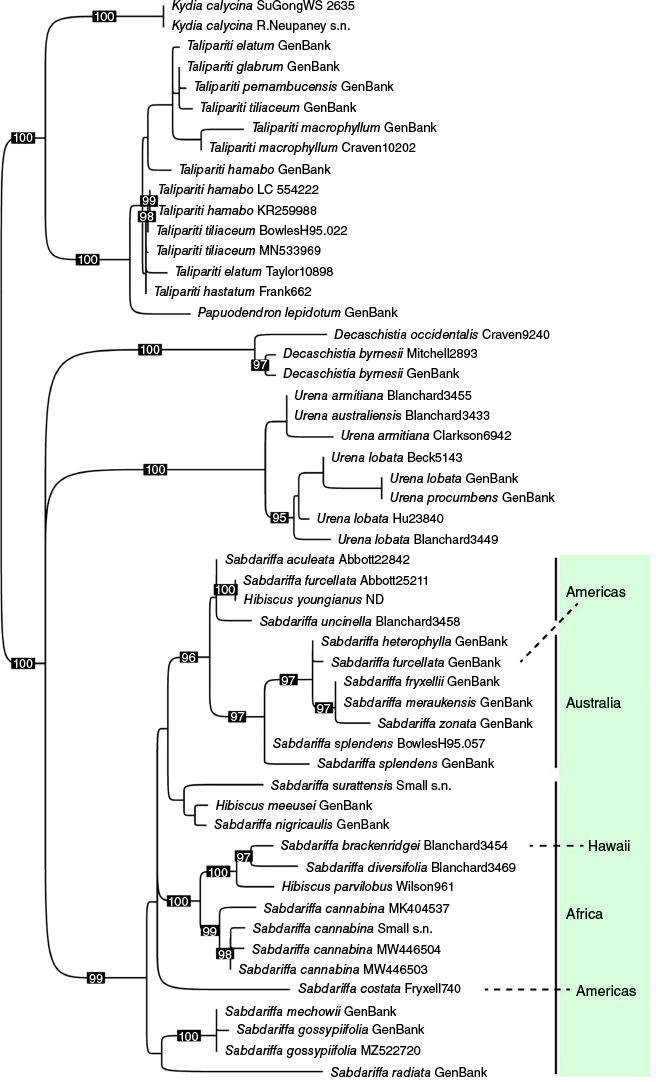
Phylogenetic reconstruction was performed using IQ-TREE2 (ver. 2.23, see https://github.com/iqtree/iqtree2; Minh et al. 2020) to obtain a maximum likelihood phylogeny. The alignment was partitioned by each locus and ModelFinder (see http://www.iqtree.org/ModelFinder/; Kalyaanamoorthy et al. 2017) was used to identify the optimal substitution model for each locus with the Bayesian Information Criterion. Node support was estimated with 1000 ultrafast bootstrap replicates (UFBoot) and the SH-like approximate likelihood ratio test (SH-aLRT; Guindon et al. 2010), with -bnni correction used to reduce the risk of overestimating branch support with UFBoot. The treefile was visualised and edited using TreeViewer (Fig. 6), with the phylogeny rooted following the topology from Hanes et al. (2024). Both the alignment and treefile are available as Supplementary material (SB24013_FileS1.nex; SB24013_FileS2.treefile).
Maximum likelihood phylogeny of /Phylloglandula from IQ-TREE using a partitioned alignment of six plastid loci sourced from Hanes et al. (2024) and GenBank. UFBoot support greater than 95% is shown on the branches. A poorly supported branch was collapsed to a polytomy between Sabdariffa, Urena and Decaschistia. Sabdariffa is highlighted and biogeographical associations are indicated.
Taxonomic review
Although our list of taxa is primarily based on the list provided by Wilson (2006), an extensive range of original literature has been consulted to check usage, application and type specimen information. Access to early literature through online archives has been invaluable for this project and the Biodiversity Heritage Library is particularly acknowledged as a primary source for these documents (see https://www.biodiversitylibrary.org/, accessed 17 April 2024). Distribution statements primarily follow the Plants of the World Online (POWO, Facilitated by the Royal Botanic Gardens, Kew, see https://powo.science.kew.org/, accessed 17 April 2024), supplemented with local knowledge and recent literature.
Although all approaches have potential to cause some confusion, we choose to discuss names under the combinations in Sabdariffa, even when citing earlier authors who naturally discuss these names under the combinations in Hibiscus, for us to clearly assign our concepts to past literature. Similarly, citations of descriptions and illustrations may equally be under Hibiscus combinations or names we consider to be synonyms, or sometimes misapplications, in the original literature we cite.
We have primarily examined digital images only for the purpose of this paper, though during the course of our studies, we have collectively also seen original type specimens for numerous species. We have not specifically indicated where we have seen physical specimens in addition to digital images, except where hyperlinks to these specimens or images are not available and these specimens are subsequently indicated with an exclamation mark (‘!’). Hyperlinks to digitised type specimens are provided where we have been able to locate these, and all have been seen and assessed by the authors. The primary set of links is through the subscription provider JSTOR Plants as a large proportion of readers will have institutional access to this resource but most of these images can notably also be freely accessed through individual institutional websites. In some cases, links have been provided directly from specific open-access websites, including the Australasian Virtual Herbarium (AVH), B, BM, BR, G, K, HBG, Herbario Virtual Austral Americano (HVAA), Indian Virtual Herbarium (IVH), JACQ, MA, Naturalis, NY, P, ReFlora, Sweden’s Virtual Herbarium (SVH), US and Zurich Herbaria (ZH). Numerous typifications have been made where we are confident that we have located all or at least most of the original material. Residual syntypes are listed to fully document the range of original material examined and facilitate access to these specimens.
We have collectively curated the identification of over 7850 records (86%) of Hibiscus section Furcaria on iNaturalist (see https://www.inaturalist.org/, accessed 14 March 2025). Many images presented here are sourced from these verified records, as images posted under creative commons licences and with written permission from the photographers for all rights reserved copyright images. Coding of all taxa in section Furcaria to that section name was undertaken by iNaturalist curator Thomas Mesaglio to facilitate sorting and curation of images (see Mesaglio et al. 2023).
Results
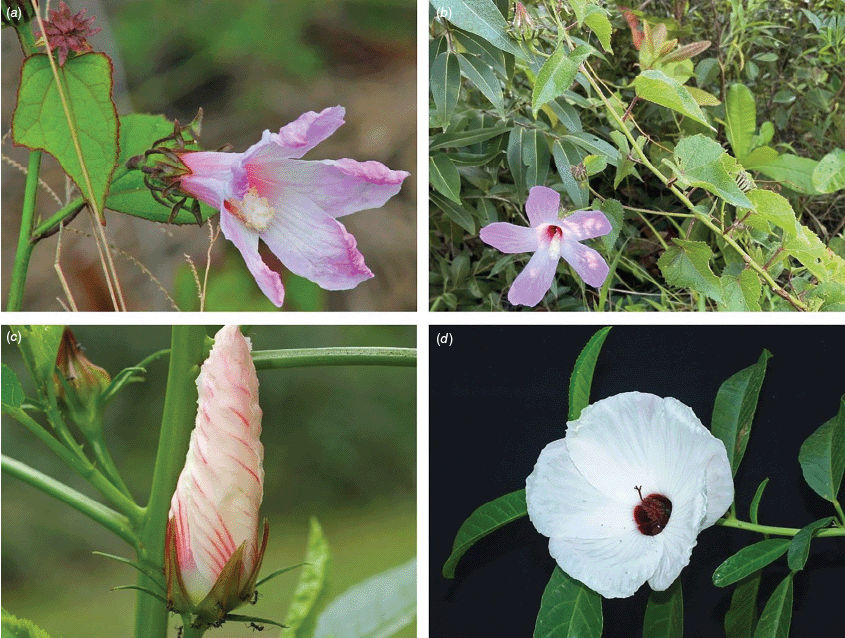
We present the first global revision of Hibiscus sect. Furcaria since Hochreutiner (1900) and make 123 new combinations in the genus Sabdariffa (for 117 species and 6 subspecies). We identified 797 type elements (including illustrations), held in 90 herbaria around the world (A, AD, AJBC, ASU, B, BISH, BJA, BM, BO, BOLO, BR, BRI, BRLU, BSD, C, CAL, CALI, CANB, CAS, CEN, CNS, COI, COL, CTES, DNA, E, EA, ESA, F, FMB, FSU, FT, G, GH, GOET, HBG, HAC, HAL, HNG, JAUM, K, KW, L, LBV, LD, LECB, LIL, LINN, LISC, LMA, LPB, LSU, M, MA, MBM, MEDEL, MEL, MICH, MO, MPU, NBG, NHGC, NSW, NT, NY, P, PAL, PERTH, PH, PRE, R, RB, QRS, S, SI, SJRP, SP, SPF, TEX, TUB, U, UB, UC, UEC, UPS, US, USM, W, WAG, YBI; abbreviations based on Index Herbariorum, New York Botanical Garden’s Virtual Herbarium, see http://sweetgum.nybg.org/ih/). We provide hyperlinks to most available digitised type specimens, lists of references for species descriptions and illustrations, and images for 64 species and 2 additional subspecies.
For the sake of simplifying the task of identifying species, we have divided species diversity in Sabdariffa into six artificial biogeographic regions for which we provide identification keys to taxa. A checklist of taxa in each region is provided in Table 2.
| Africa | Asia | Australia | Pacific | North America & Caribbean | South America | |
|---|---|---|---|---|---|---|
| S. acetosella | *S. acetosella | S. aneuthe | S. australensis | *S. acetosella | *S. acetosella | |
| S. altissima | *S. cannabina | S. aphela | S. brackenridgei subsp. brackenridgei | S. aculeata | S. adscensionis | |
| S. aspera | S. diversifolia subsp. diversifolia | S. arnhemensis | S. brackenridgei subsp. mokuleiana | *S. cannabina | S. amambayensis | |
| S. berberidifolia | S. furcata | S. bacalusia | S. brackenridgei subsp. molokaiana | S. costata | S. andersonii | |
| S. cannabina | S. gossypiifolia | S. byrnesii | *S. cannabina | S. furcellata | S. benensis | |
| S. ceratophora | S. hispidissima | ?*S. cannabina | S. diversifolia subsp. diversifolia | *S. gossypiifolia | S. bifurcata | |
| S. cordofana | S. hoshiarpurensis | S. cummingii | S. fijiensis | S. maculata subsp. maculata | *S. cannabina | |
| S. cuanzensis | S. mastersiana | S. divaricata | S. furcellata | S. maculata subsp. nipensis | S. capitalensis | |
| S. diversifolia subsp. diversifolia | S. meraukensis | S. diversifolia subsp. diversifolia | *S. gossypiifolia | *S. radiata | S. chancoae | |
| S. diversifolia subsp. agioxillos | S. radiata | *S. diversifolia subsp. agioxillos | S. scabricaulis | S. chapadensis | ||
| S. elongatifolia | S. surattensis | S. fallax | S. uncinella | S. commixta | ||
| S. fabiana | S. forsteri | S. conceptionis | ||||
| S. flavorosea | S. fryxellii | S. cucurbitacea | ||||
| S. furcellatoides | *S. gossypiifolia | S. diversifolia subsp. diversifolia | ||||
| S. gilletii subsp. gilletii | S. granitica | *S. diversifolia subsp. agioxillos | ||||
| S. gilletii subsp. hierniana | S. heterophylla | S. ferreirae | ||||
| S. gilletii subsp. lunadaensis | S. inimica | S. flagelliformis | ||||
| S. goossensii | S. kenneallyi | S. furcellata | ||||
| S. gossypiifolia | S. kirstyae | *S. gossypiifolia | ||||
| S. greenwayi | S. marenitensis | S. gregoryi | ||||
| *S. hispidissima | S. menzeliae | S. hassleriana | ||||
| S. holstii | S. meraukensis | S. henningsiana | ||||
| S. mastersiana | S. minutibracteola | S. hilariana | ||||
| S. mechowii | S. mollis | S. hochreutineri | ||||
| S. minkebeensis | S. petherickii | S. itirapinensis | ||||
| S. moxicoensis | S. reflexa | S. kitaibelifolia | ||||
| S. ngokbanakii | S. riceae | S. laxiflora | ||||
| S. nigricaulis | S. sankowskyorum | S. maculata subsp. maculata | ||||
| S. noldeae | S. saponaria | S. manuripiensis | ||||
| S. paolii | S. splendens | S. mariae | ||||
| S. partita | S. squarrulosa | S. matogrossensis | ||||
| *S. radiata | S. stewartii | *S. mechowii | ||||
| S. reekmansii | S. superba | S. multiformis | ||||
| S. rostellata | S. symonii | S. nanuzae | ||||
| S. scotellii | S. thegalea | S. paludicola | ||||
| S. sineaculeata | S. townsvillensis | S. peruviana | ||||
| S. sparsiaculeata | S. zonata | S. pohlii | ||||
| S. subdiversifolia | *S. radiata | |||||
| S. sudanensis | S. saddii | |||||
| S. surattensis | S. sebastianii | |||||
| S. torrei | S. trilineata | |||||
| S. verrucosa | S. wilsonii | |||||
| S. windischii | ||||||
| 42 | 11 | 37 | 9 | 11 | 43 |
Taxa introduced to these regions are indicated by an asterisk (*). Total number of taxa per region (bold) are listed at the bottom of the table.
The final alignment for the /Phylloglandula phylogeny (Fig. 6) included 53 samples (26 Sabdariffa samples and species) and the concatenated length of the six loci was 11,223 bp long (42.8% missing data; see Table 3 for individual locus information, including length of each locus, number of variable sites and the best substitution model used in the phylogenetic analyses). Our expanded sampling further confirms the monophyly of Sabdariffa and sheds light on the strong biogeographic structure within the genus.
| Locus | Number of samples | Aligned length (bp) | Informative/invariable | ModelFinder model for IQ-TREE | |
|---|---|---|---|---|---|
| matK | 46 | 2359 | 76/2233 | HKY + F + G4 | |
| ndhF | 45 | 2101 | 56/2011 | TPM3u + F + I | |
| rbcL | 12 | 1434 | 13/1419 | F81 + F | |
| rpl16 | 22 | 1325 | 33/1252 | TPM3u + F + I | |
| trnQ-rps16 | 13 | 1098 | 35/1052 | F81 + F + R2 | |
| ycf1 | 27 | 2906 | 91/2737 | TPM3u + F + I | |
| Total | 56 | 11,223 | 336/10,698 |
Discussion
Sabdariffa is the third largest genus in tribe Hibisceae after Pavonia and Hibiscus, sensu Hanes et al. (2024). Recent phylogenetic work and phylogenies presented here (Fig. 1, 6) provide evidence for a well-delineated clade for a long-recognised and morphologically distinct group. The /Phylloglandula phylogeny presented here is congruent with Hanes et al. (2024), confirming that each of the five genera in the /Phylloglandula clade are monophyletic with respect to the samples available. Fig. 6 and the phylogenies from Hanes et al. (2024) clearly demonstrate that Talipariti + Kydia form a well-supported clade sister to Sabdariffa + Urena + Decaschistia. The relationships between Sabdariffa, Urena and Decaschistia, however, are not well supported in Fig. 6. Similarly, the ML topology in Hanes et al. (2024) has Sabdariffa + Urena with 83% UFboot support and the BI topology has Urena + Decaschistia with 0.61 posterior probability support.
Although the relationships between Sabdariffa, Urena and Decaschistia remain unclear in our phylogenetic reconstructions, each genus is readily distinguished by morphological characters and appropriately recognised at generic rank. Decaschistia has a depressed, globose capsule with loculicidal dehiscence by (6–)8–10 valves and the style is 8–10 branched. Sabdariffa has an ovoid to ellipsoid capsule with loculicidal dehiscence by 5 valves and the style is 5-branched. Urena has a ±globose schizocarp separating septicidally into 5 dehiscent or indehiscent mericarps and the styles are 10(–15)-branched.
The work presented here moves us substantially closer to a monophyletic Hibiscus. The combinations we make here in Sabdariffa represent over 64% of the species level combinations that we estimate are needed to make Hibiscus monophyletic. Alternate taxonomic solutions are being assessed on a clade-by-clade basis with the Pavonia clade in particular requiring careful consideration and additional sampling. We note that the inclusion of a single sequence of Papuodendron (not included by Hanes et al. 2024) suggests that this genus is more closely related to Talipariti than Hibiscus sens. str. or Sabdariffa. Our work provides clarification across the group and creates a path to further explore species limits in Sabdariffa, especially regarding subspecies status in S. diversifolia and S. gilletii (De Wild.) Mwachala & R.L.Barrett. The strong biogeographic structure we uncover in our phylogenetic work (Fig. 4) will be exciting to compare with our understanding of chromosomal evolution in the group. A species level phylogeny of genus Sabdariffa will be invaluable for understanding dispersal mechanisms, routes, timing of dispersal and to reconstruct the evolutionary history of the economically important species.
Relationships within the genus are often not well resolved due to the conservative nature of the plastid markers. The incongruent position of two samples of S. furcellata is particularly noted (Fig. 6). Unfortunately, there is no overlap in the available sequences between these two samples (Table 2). The ndhF sequence for the sample represented in GenBank is identical to that of S. heterophylla but the rpl16 sequence places this with S. rostellata (Guill. & Perr.) Mwachala & R.L.Barrett, though with only weak support. Similarly, S. rostellata is not represented by ndhF data. Thus, clarifying relationships between these taxa is challenging. Contamination may have also resulted in an incorrect placement for this sample. Currently, resolution within Sabdariffa is too poor to be confident of this and further sampling will be needed.
Taxonomy
Sabdariffa (DC.) Kostel., Allg. Med.-Pharm. Fl. 5: 1857 (1836)
Hibiscus sect. Sabdariffa DC., Prodr. 1: 453 (1824). Type: Hibiscus sabdariffa L. [=Sabdariffa gossypiifolia (Mill.) M.M.Hanes & R.L.Barrett]
Hibiscus sect. Furcaria DC., Prodr. 1: 449 (1824); Furcaria (DC.) Kostel., Allg. Med.-Pharm. Fl. 5: 1856 (1836), nom. illeg., non Desv. (1827), nec Boivin ex Baill. (1858). Type: Hibiscus furcellatus Desr., designated by T.H.Kearney, Amer. Midl. Naturalist 46: 109 (1951) [=Sabdariffa furcellata (Desr.) M.M.Hanes & R.L.Barrett].
Hibiscus [sect. Abelmoschus] subsect. Tuberculatus G.Don, Loudon’s Hort. Brit. 1: 289 (1830), (as ‘Tuberculati’). Type (here designated): Hibiscus diversifolius Jacq. [=Sabdariffa diversifolia (Jacq.) McLay & R.L.Barrett].
Hibiscus sect. Furcaria subsect. Furcaria simplicia Hochr., Ann. Cons. Jard. Bot. Genève 4: 41 (1900), nom. inval.
Hibiscus sect. Furcaria subsect. Furcaria typica Hochr., Ann. Cons. Jard. Bot. Genève 4: 41 (1900), nom. inval.
Canhamo Perini, Prosp. Expl. Notice Cult. Canh. Braz.: 1 (1905). Type: Canhamo braziliensis Perini [=Sabdariffa radiata (Cav.) R.L.Barrett & M.M.Hanes].
Brockmania W.Fitzg., J. & Proc. Roy. Soc. West. Austral. 3: 174 (1918). Type: Brockmania membranacea W.Fitzg. [=Sabdariffa meraukensis (Hochr.) McLay & R.L.Barrett].
Hibiscus sect. Furcaria series Furcaria cannabina Ulbr. in A.Engler (ed.), Die Pflanzenwelt Afrikas 3(2): 400 (1921), nom. inval.
Hibiscus sect. Furcaria series Furcaria furcellata Ulbr. in A.Engler (ed.), Die Pflanzenwelt Afrikas 3(2): 400 (1921), nom. inval.
Hibiscus sect. Furcaria series Furcaria sabdariffa Ulbr. in A.Engler (ed.), Die Pflanzenwelt Afrikas 3(2): 402 (1921), nom. inval.
Hibiscus sect. Cannabini Small, Man. S.E. Fl. 854 (1933). Type: H. cannabinus L. [=Sabdariffa cannabina (L.) M.M.Hanes & R.L.Barrett].
Hibiscus sect. Furcellati Small, Man. S.E. Fl. 854 (1933). Type: H. furcellatus Desr. [=Sabdariffa furcellata (Desr.) M.M.Hanes & R.L.Barrett].
Annual herbs, perennial subshrubs, shrubs, usually erect or spreading, occasionally trailing woody vines (e.g. S. altissima, S. sudanensis, S. surattensis, S. uncinella), rarely prostrate (e.g. S. flagelliformis) or rarely small trees (e.g. S. brackenridgei, S. townsvillensis), 0.3–10 m high. Branchlets commonly with sparse to dense aculei 1.5–6 mm long (sometimes retrorse, sometimes 2- or 3-fid), or stellate hairs, bristles to 3 mm long, hairs whitish, yellowish or brownish, simple or 2-forked hairs to 2 mm long sometimes present, glandular hairs present in some Australian species or branchlets sometimes glabrous. Stipules ±persistent or sometimes caducous, filiform, linear, subulate to ovate (amplexicaul in S. surattensis; sometimes 2-lobed in S. fallax, 3-lobed in S. elongatifolia), 0.75–15(–20) mm long, 0.3–2.0 mm wide, with fine and sometimes coarse bristles or sometimes glabrous. Leaves alternate, lower leaves usually petiolate, apical leaves sometimes sessile and reduced to linear bracts. Mature leaves: petiole 3–180(–220 in S. diversifolia, –240 in S. stewartii) mm long, indumentum similar or dissimilar to that of the branchlet; lamina variable in shape, from simple to 3–7(–9)-lobed even on the same plant (rarely 2- or 4-lobed in S. aspera), (10–)35–260 mm long, (2–)25–260 mm wide, cuneate to obtuse at base, entire to serrate or dentate margin, acute to obtuse apex, concolourous or discolourous, lobes when present elliptic to ovate or narrowly triangular, longer than wide, attenuate to obtuse, adaxial surface indumentum of very sparse to dense simple or 1–4-armed hairs, hairs sessile, sometimes with a few aculei, abaxial surface usually with conspicuous foliar nectaries (0.5–15 mm long) at the base of the midrib and sometimes on lateral ribs (sometimes absent in S. brackenridgei, absent in S. fabiana), indumentum of sparse to dense coarse stellate hairs, 1–4-armed, hairs sessile, the indumentum commonly more dense on the abaxial surface, the abaxial surface with midrib and primary vein indumentum usually dissimilar to the interveinal regions, indumentum whitish to yellowish (rarely lamina glabrous). Flowers usually solitary in leaf axils (sometimes paired), appearing to form racemes when upper leaves reduced (e.g. S. diversifolia) or borne in clusters at the tips of branchlets (e.g. S. mustiae); pedunculate, the peduncle (0.5–)1–100(–130) mm long, often elongating in fruit, sometimes greatly so, commonly with coarse bristles and sparse stellate hairs, sometimes glabrous, articulation usually conspicuous; articulated pedicel (rarely absent), 1–15 mm long, with fine to coarse bristles, pedicels sometimes marginally wider distally, indumentum of peduncle and pedicel commonly distinct and diagnostic; epicalyx commonly with coarse bristles and aculei, 5–22-lobed, (1–2 mm long in S. aphela) (5–)9–37 mm long at anthesis, 0.5–2.5 mm wide, the lobes free or sometimes fused at the base for up to 5 mm, 1- or 3-nerved, linear to subulate (the apex then flattened), entire or distinctly 2-lobed at the apex (the two lobes 0.5–10 mm long), channelled in many species (channelled inside in S. gossypiifolia), commonly appressed to the calyx or spreading to reflexed, with sparse, coarse simple hairs persistent in fruit or glabrous; calyx 5-lobed, 7–35(–55 in S. gossypiifolia) mm long at anthesis (often enlarged in fruit), lobes ±linear, lanceolate, narrowly triangular, triangular or spathulate, free to the base or sometimes fused for 1–7 mm, 10-ribbed at base, with thickened ribs, the midrib of each sepal being raised and from the receptacle to the notch of each sinus, and continuing along each margin of the sepals (ribs not conspicuous in S. goossensii), indumentum of sparse to dense, simple and stellate, whitish, appressed, erect or apically curved hairs or sometimes glabrous, nectary present or absent, calyx persistent and leathery (or fleshy in S. acetosella and S. gossypiifolia) in fruit; corolla usually large, open and showy (salverform in S. uncinella, funnelform in S. greenwayi), sometimes pendulous; petals obovate, (20–)35–95 mm long, 15–65 mm wide, white, creamy yellow, yellow or pale to dark pink (deep magenta in S. australensis to purple-red in S. radiata and red in S. uncinella), prominently veined, with a thin red horizontal stripe at the base or a reddish-brown to deep purple petal spot, glabrous adaxially, glabrous or with sparse stellate hairs abaxially or sometimes with fine simple hairs; staminal column shorter than or equal to the petals, straight, (10–)12–60 mm long, usually the same colour as the petal spot (if present), stamens distributed throughout the column, the filaments 0.5–3.0 mm long, anthers red or yellow; style exserted 1–6 mm beyond apex of staminal column (or rarely included), sparsely hairy (hairs clavate in S. sudanensis), 5-branched, branches 2–12 mm long, stigmas feathery, capitate, wide, the hairs 0.1–0.5 mm long. Capsule 5-celled, loculicidally dehiscent, ovoid to globose, (6–11 in S. scotellii) 12–32 mm long, (6–10 in S. scotellii) 12–23 mm in diameter, shortly beaked, sparse to moderately dense erect or appressed, simple, coarse hairs all over (glandular hairs present in some South American species), glabrescent (or glabrous in S. kirstyae and S. meraukensis). Seeds several per cell, light brown to dark brown, reniform to angular, 2–5 mm long, 2–4 mm wide, sometimes striate, often scaly or variously marked but rarely hairy, with white to cream, light brown or tan funiculus. (n = 18, 36, 54, 72, 90; Skovsted 1944; Menzel and Wilson 1969; Menzel and Hancock 1984; Wilson 1994, 1999; Krapovickas and Fryxell 2004; Lavia and Fernández 2004; Wilson 2006).
Sabdariffa is most readily recognised by the prominence of the calyx nervation, where particularly the midrib and margins of each sepal are raised and rib-like (not raised in most other Hibisceae). Most species bear stout simple hairs on a thickened base on the stems and in some cases (e.g. S. diversifolia) the hairs are thickened into prickles. Nectary glands are present on the abaxial surface of the leaf midrib and on the midrib of the sepals in most species. The fruit valves are usually setose and the fruiting calyx is usually leathery (fleshy in S. acetosella (Welw. ex Hiern) M.M.Hanes & R.L.Barrett and S. gossypiifolia). Forked epicalyx lobe apices are diagnostic when present but this character is only found in selected species.
A genus of 117 species with a primarily tropical distribution, a few species extending to temperate or arid zones. Several species are widely cultivated outside natural ranges for agronomic or horticultural use.
Fryxell (1988) credits Don (1831) in stating that Sabdariffa is a Turkish name for S. gossypiifolia (Hibiscus sabdariffa), though this name does not seem to be in current use in Türkiye (Pezen Özdogan et al. 2011). Drury (1858) also concluded that Sabdariffa was a Turkish name and believed H. sabdariffa to have been an early introduction to India by the Mohammedans. As the name is not classically formed, this is interpreted as feminine in accordance with Kosteletzky (1836) who combined two species names under this genus with feminine terminations. The name ‘Sabdariffa’ appears in classical literature such as de L’Obel (1576, p. 375) but no explanation or origin is provided for the name in that publication.
De Candolle (1824, p. 449) included nine species in Sect. Furcaria. Kearney (1951, p. 109) stated that Sect. Furcaria was ‘typified by H. furcellatus Desr.’ However, Fryxell (1988, p. 195) stated that the concept of the section was first restricted to only three species (Hibiscus surattensis, H. furcatus and H. radiatus) by Kosteletzky (1836, p. 1856) when the section name was raised to generic rank (and thereby taxonomically redefined), excluding H. furcellatus, therefore Fryxell concluded that the first valid selection of a type species was by Borssum Waalkes (1966, p. 57), who designated one of the three species included by Kosteletzky (1836) as the type species. However, the Code does not state that such taxonomic circumscriptions define later choices of type species (Turland et al. 2018), therefore the first known selection, that of Kearney (1951) must stand.
Don’s (1830) subsection Tuberculatus included a broad range of species, many of which are not closely related or even congeneric under our current concepts. Three of the nine original species belong to our concept of Sabdariffa, therefore we consider one of those species to be a logical choice as the type species of subsection Tuberculatus. We here designate H. diversifolius Jacq. as the type species to fix the application of the name.
Infrageneric names published with two words (e.g. ‘subsect. Furcaria simplicia’ and several other infrageneric names published by Ulbrich) are not validly published (see Turland et al. 2018, Art. 21.2, Ex. 2). Hibiscus sect. Furcaria series Friesia Ulbr. (Ulbrich 1921, p. 402) is a synonym of Cravenia McLay & R.L.Barrett (see Hanes et al. 2024).
Keys to Sabdariffa taxa
We present six regional keys providing identification tools for all Sabdariffa taxa recognised here, along with four African taxa of uncertain status that are keyed out under published names in Hibiscus but not recombined in Sabdariffa pending further investigation of the taxonomic distinction. Species designated with an asterisk (*) are introduced in that region.
Africa (42 species and subspecies + 4 uncertain taxa)
Asia (11 species and subspecies)
Australia (37 species and subspecies)
Pacific Islands (9 species and subspecies)
North America and the Caribbean (10 species and subspecies)
South America (43 species and subspecies)
| 1. | Stems aculeate...2 Stems not aculeate...35 |
| 2. | Epicalyx lobes clearly or obscurely bifurcate...3 Epicalyx lobes not bifurcate...18 |
| 3. | Calyx nectary absent...4 Calyx nectary present...16 |
| 4. | Stipules ovate, auriculate, amplexicaul...S. surattensis Stipules linear to subulate, not amplexicaul...5 |
| 5. | Peduncle articulated closer to epicalyx than to base...6 Peduncle articulated near base or apparently unjointed...13 |
| 6. | Calyx lobes with reticulate venation and simple to 4-fid bristles on ribs, otherwise almost glabrous...S. altissima Calyx lobe venation not as above...7 |
| 7. | Lower and mid-plant leaves entire or shallowly lobed...8 Lower and mid-plant leaves deeply palmately lobed...12 |
| 8. | Leaves with patches of lanate pubescence; stems stellate–pubescent...S. furcellatoides Leaves without lanate pubescence; stems hirsute with simple hairs to 3 mm long...9 |
| 9. | Stems and leaves distinctly grey–pubescent; shoots densely aculeate; flowers almost sessile, red...Hibiscus keilii (see Unplaced taxa) Stems and leaves sparsely white or yellowish pubescent; shoots sparsely aculeate; corolla yellow or purple...10 |
| 10. | Plants densely hairy; leaves 3–5-lobed, sinuses to 2 cm deep, thick; leaf lobes at least as broad as long; pedicels 2–13 cm long; calyx ribs weakly thickened and not red fleshy, with swollen-based, bristle-like hairs with 2(–5) arms, intercostal areas densely covered with small, white stellate hairs...S. rostellata Plants sparsely hairy; leaves usually 3-lobed, sinuses 2–4 cm deep; leaf lobes longer than broad; pedicels 1–6 cm long; calyx ribs weakly or strongly thickened, with simple, bristle-like hairs, intercostal areas sometimes with a few simple hairs, otherwise glabrous...11 |
| 11. | Leaves thin, papery; pedicels 1–2.3(–5) cm long; calyx ribs strongly thickened and red fleshy, stems and petiole sparsely hairy with a strong longitudinal band of pubescence...S. fabiana Leaves not papery; pedicels 1–6 cm long; calyx ribs weakly thickened and not red fleshy; stems and petiole glabrous except often with a thin longitudinal line of pubescence...S. holstii |
| 12. | Peduncles to 10(–13) cm long in fruit; stems with stout, retrorse aculei on large bases...*S. hispidissima Peduncles to 5 cm long in fruit; stems more finely aculeate or aculeolate...S. goossensii |
| 13. | Foliar nectary present...14 Foliar nectary absent...15 |
| 14. | Mid-plant leaves not lobed; peduncle articulating near base to 1/3 the distance from base to epicalyx...S. sudanensis Mid-plant leaves deeply 3-lobed, midlobe markedly longer than lateral lobes; peduncle articulating at base or appearing unjointed...S. flavorosea |
| 15. | Stems above glabrous to finely aculeate; leaves glabrous...S. radiata Stems above with stout retrorse aculei and a line of stellate pubescence; leaves scabrous...S. torrei |
| 16. | Epicalyx lobes obscurely bifurcate, outer fork 2 mm long, reflexed, inner fork vestigial or absent; peduncles articulating at base or appearing unjointed, aculeate and stellate–pubescent throughout...S. nigricaulis Epicalyx lobes clearly bifurcate, outer fork 3–9 mm long, not reflexed, inner fork 1–9 mm long, linear to subulate; peduncles articulating above the base...17 |
| 17. | Epicalyx lobes ~10; peduncles rough–aculeate, stellate–pubescent and sometimes simple-pubescent throughout...S. ceratophora Epicalyx lobes 5–9; peduncles fine–aculeate and sparsely puberulent above the joint, densely fine–pubescent below the joint...S. noldeae |
| 18. | Calyx nectary absent...19 Calyx nectary present...21 |
| 19. | Upper 6–11 mm of epicalyx lobes lanceolate; leaves throughout the plant deeply palmately 3–5-lobed...S. mechowii Upper part of epicalyx lobes not lanceolate; leaves entire to shallowly lobed...20 |
| 20. | Peduncle articulating midway between base and epicalyx; leaves densely stellate–pubescent throughout...S. subdiversifolia Peduncle articulating at base or apparently unjointed; leaves with simple and stellate hairs on veins, otherwise almost glabrous...S. berberidifolia |
| 21. | Stems with large stout aculei...22 Stems more finely aculeate or aculeolate...26 |
| 22. | Aculei borne at the base of stem nodes singly, in pairs or in threes; leaves to 3 cm long, to 5 cm wide, shed early...S. sparsiaculeata Aculei borne randomly on the stem; leaves larger, persistent...23 |
| 23. | Calyx densely and finely stellate–pubescent, sparsely aculeate on ribs; midlobes and lateral lobes of lower leaves rotund to spathulate ...S. greenwayi Calyx with rigid bristles; leaf lobes not rotund or spathulate...24 |
| 24. | Calyx aculeate or aculeolate on the ribs; lobes of lower leaves manifestly secondarily lobed...S. partita Calyx not aculeate or aculeolate on the ribs; leaf lobes not manifestly secondarily lobed...25 |
| 25. | Stems usually with one or more longitudinal lines of pubescence; corolla yellow...S. diversifolia subsp. diversifolia Stems more densely and uniformly hairy; corolla reddish to purple...S. diversifolia subsp. ...agioxillos |
| 26. | Some flowers subtended by a linear, flattened, sometimes bifid bract resembling the stipules; leaves deeply 5(–7)-lobed, midlobes and lateral lobes of lower leaves often secondarily lobed...S. reekmansii Flowers without a subtending bract; leaves variously lobed but not secondarily lobed...27 |
| 27. | Epicalyx lobes fleshy, subulate to triangular, length:base width ratio 3:1 or less, upper 3–6 mm channelled on the inner (ventral) surface...S. gossypiifolia Epicalyx lobes not fleshy, generally narrower, length:base width ratio >3:1, inner surface not channelled...28 |
| 28. | Vegetative branches in leaf axils well developed, with up to 7 persistent leaves; newly opened leaves with a gland-like structure at apex of each leaf margin serration; leaves entire to shallowly 3-lobed...S. berberidifolia Vegetative branches not as well developed; gland-like structure not present; leaves variously lobed...29 |
| 29. | Calyx with white, woolly tomentum basally or more uniformly distributed; capsule with long, dense appressed pubescence...S. cannabina Calyx without white woolly tomentum; capsule pubescence various...30 |
| 30. | Epicalyx lobes ±flat outside in dried specimens (may have a central rib inside), marginal ribs absent or indistinct; calyx with aculei or bristles absent or not prominent...31 Epicalyx lobes with distinctly thickened marginal ribs in dried specimens; calyx with very prominent aculei or coarse bristles with large, thickened bases...34 |
| 31. | Leaves 3(–5)-lobed for approximately half the length or 5–7(–9)-lobed almost to the base...32 Leaves entire or very shallowly lobed...33 |
| 32. | Leaves usually 5–7(–9)-lobed almost to the base, the lobes ±lanceolate, often with additional small lateral lobes; aculei on stems very sparse, erect to slightly antrorse, with short, thickened bases; epicalyx and calyx with sparse to moderately dense long, semi-appressed, hispid hairs...S. aspera Leaves usually 3-lobed for approximately half the length, sometimes almost entire, sometimes the central lobe with shallow lateral lobes (therefore blade 5-lobed); aculei on stems erect, moderately dense, with long, thickened bases; epicalyx and calyx with dense, long, semi-appressed, hispid hairs...Hibiscus vanderystii (see S. aspera) |
| 33. | Leaves entire, ±linear with shallow marginal teeth; aculei on stems erect, sparse, with short, thickened bases; epicalyx and calyx with sparse hispid hairs...Hibiscus malangensis (see S. aspera) Leaves usually entire, sometimes very shallowly 3-lobed; aculei on stems antrorse, moderately dense, with short, prominently thickened bases; epicalyx and calyx with dense, long, semi-appressed, hispid hairs...Hibiscus parvilobus (see S. berberidifolia) |
| 34. | Leaves 3–5-lobed for at least half the length; aculei on stems small, scattered; epicalyx and calyx almost glabrous except for large, prominent aculei on margins...S. cordofana Leaves entire, shallowly lobed or deeply lobed ±to the base; aculei on stems large, scattered; epicalyx and calyx mostly glabrous but with long, very prominent aculeli on margins and ribs...S. verrucosa |
| 35. | Epicalyx lobes manifestly to obscurely bifurcate; calyx nectary absent or present...36 Epicalyx lobes not bifurcate; calyx nectary present...46 |
| 36. | Calyx and foliar nectaries present...37 Calyx nectary absent, foliar nectary absent or present...40 |
| 37. | Leaf blade smooth, glabrous to sparsely pubescent, margin irregular, often purple; stipules 12–15 mm long;...S. acetosella Leaf blade densely stellate hairy, margin prominently toothed, green (to brown); stipules 4–5 mm long...38 |
| 38. | Leaves simple, sometimes shallowly irregularly or 3-lobed; blade 15–35 mm long, 10–30 mm wide; epicalyx 7- or 8-lobed; calyx 11–12 mm long; corolla yellow...S. paolii Leaves distinctly 3-lobed or digitately 3–5-lobed almost to the base; blade 50–60 mm long, 50–90 mm wide; epicalyx 9- or 10-lobed; calyx 15–20 mm long; corolla violet or white...39 |
| 39. | Leaves digitately 3–5-lobed almost to the base, even when subtending flowers; corolla violet...S. minkebeensis Leaves palmately lobed or ovate–hastate and 3-lobed or simple when subtending flowers; corolla white...S. ngokbanakii |
| 40. | Foliar nectary absent; leaves glabrous...S. radiata Foliar nectary present; leaves pubescent...41 |
| 41. | Inner fork of epicalyx lobes twice as long as outer fork...42 Inner and outer forks of epicalyx lobes of equal length...44 |
| 42. | Calyx lobes long–acuminate in fruit; length:width ratio of midlobe of mid-plant leaves ~4:1 ...S. gilletii subsp. lundaensis Calyx lobes acute to slightly acuminate in fruit; length:width ratio of midlobe of mid-plant leaves 1:1 to 3:1...43 |
| 43. | Length:width ratio of midlobe of mid-plant leaves ~2:1 to 3:1; outer fork of epicalyx lobes 2–4 mm long, inner fork 6–9 mm long...S. gilletii subsp. gilletii Length:width ratio of midlobe of mid-plant leaves ~1:1; outer fork of epicalyx lobes 1.5–3 mm long, inner fork 4–5 mm long...S. gilletii subsp. hierniana |
| 44. | Stems lanate–pubescent, patchy below, continuous above; lower leaves deeply 3–5-lobed (ratio of base to apex: base to sinus ~5:1)...S. moxicoensis Stems not lanate–pubescent; lower leaves shallowly to moderately 3–5-lobed (ratio of base to apex: base to sinus ~1.3:1 to 2:1)...45 |
| 45. | Stems finely stellate–pubescent and with coarse stellate hairs; lower leaves moderately 5-lobed (ratio of base to apex: base to sinus ~2:1), without a foliar nectary...S. cuanzensis Stems with stiff, simple to 3-fid or stellate pigmented bristles on enlarged bases; lower leaves shallowly 3-lobed (ratio of base to apex: base to sinus ~1.3:1), with a foliar nectary...S. mastersiana |
| 46. | Calyx fleshy in fruit; epicalyx lobes broadly subulate to triangular, upper 3–6 mm channelled on the inner (ventral) surface...S. gossypiifolia Calyx not fleshy in fruit; epicalyx lobes narrower, not channelled...47 |
| 47. | Stipules divided into 2 or 3 filiform or linear segments; epicalyx lobes filiform to narrowly linear; all leaves linear; foliar nectary absent...S. elongatifolia Stipules simple; epicalyx lobes linear to subulate; at least lower leaves broader or palmately lobed; foliar nectary present...48 |
| 48. | Epicalyx lobes subulate, apex apiculate; calyx scattered and finely stellate–pubescent, with simple to stellate stiff bristles on enlarged red bases...S. sineaculeata Epicalyx lobes linear, apex narrowly lanceolate; calyx densely and finely stellate–pubescent, bristles absent...S. scotellii |
| 1. | Stems aculeate...2 Stems not aculeate...9 |
| 2. | Epicalyx lobes clearly or obscurely bifurcate...3 Epicalyx lobes not bifurcate...6 |
| 3. | Stipules foliaceous, ovate, auriculate, amplexicaul...S. surattensis Stipules not foliaceous, linear to subulate, not amplexicaul...4 |
| 4. | Peduncle articulated near base or apparently unjointed...S. radiata Peduncle articulated closer to epicalyx than to base...5 |
| 5. | Peduncles to 10(–13) cm long in fruit; stems with stout, retrorse aculei on large bases...S. hispidissima Peduncles <5 cm long in fruit; stems more finely aculeate or aculeolate...S. furcata |
| 6. | Calyx nectary absent...S. meraukensis Calyx nectary present...7 |
| 7. | Stems with large stout aculei...S. diversifolia subsp. diversifolia Stems more finely aculeate or aculeolate...8 |
| 8. | Epicalyx lobes subulate to triangular, length:base width ratio 3:1 or less, upper 3–6 mm channelled on the inner (ventral) surface...*S. gossypiifolia Epicalyx lobes generally narrower, length:base width ratio >3:1, inner surface not channelled...*S. cannabina |
| 9. | Epicalyx lobes not bifurcate; calyx fleshy in fruit, nectary present...*S. gossypiifolia Epicalyx lobes manifestly to obscurely bifurcate; calyx not fleshy in fruit, nectary absent or present...10 |
| 10. | Calyx and foliar nectaries present; leaves glabrous, sometimes red...*S. acetosella Calyx nectary absent, foliar nectary absent or present; leaves glabrous to pubescent, green...11 |
| 11. | Foliar nectary present; leaves pubescent...S. mastersiana Foliar nectary absent; leaves pubescent or glabrous...12 |
| 12. | Leaves densely stellate hairy; blade simple, margins toothed...S. hoshiarpurensis Leaves glabrous; blade deeply palmately 3–5(–7)-lobed...S. radiata |
| 1. | Epicalyx lobes distinctly bifurcate; calyx red, not fleshy [likely not naturalised]...*S. acetosella Epicalyx lobes not or only obscurely bifurcate; calyx green (or red and fleshy in S. gossypiifolia)...2 |
| 2. | Epicalyx ≤0.6 times as long as the calyx...3 Epicalyx ≥0.7 times as long as the calyx...28 |
| 3. | Calyx lobes between ribs glabrous or nearly glabrous...4 Calyx lobes between ribs moderately to densely stellate–hairy or woolly–hairy...8 |
| 4. | Flowers pedunculate; capsule glabrous or with a few hairs at the apex...5 Flowers not pedunculate; capsule densely hairy...7 |
| 5. | Leaves entire or shallowly lobed; epicalyx 0.3 times as long as the calyx; corolla yellow...S. kirstyae Leaves deeply 2–5-lobed; epicalyx 0.5–1 times as long as the calyx; corolla white...6 |
| 6. | Capsule glabrous or sparsely pubescent at the summit; pedicel glabrous or aculeate...S. meraukensis Capsule sparsely pubescent throughout; pedicel with dense, soft, straight hairs...S. saponaria |
| 7. | Branchlets with stellate hairs...S. marenitensis Branchlets without stellate hairs...S. stewartii |
| 8. | Calyx lobes between ribs woolly–hairy [likely not naturalised]...*S. cannabina Calyx lobes between ribs stellate–hairy...9 |
| 9. | Free portion of epicalyx lobes <6 mm long...10 Free portion of epicalyx lobes >6 mm long...18 |
| 10. | Calyx nectaries usually present; pedicel reflexed in midbud stage...S. reflexa Calyx nectaries always absent; pedicel never reflexed...11 |
| 11. | Branchlets aculeate...12 Branchlets not aculeate...15 |
| 12. | Epicalyx lobes (including fused portion) 2–7 mm long...13 Epicalyx lobes (including fused portion) 9–11 mm long...14 |
| 13. | Epicalyx lobes 2–3 mm long, the segments free at the base...S. squarrulosa Epicalyx lobes 4–7 mm long, the segments connate at the base...S. minutibracteola |
| 14. | Epicalyx and calyx aculeate; branchlets with stellate hairs sparse to scattered...S. fryxellii Epicalyx and calyx usually without aculei (rarely a few present); branchlets with stellate hairs moderate to dense...S. mollis |
| 15. | Climax (apical) leaves deeply 5-lobed...S. superba Climax (apical) leaves usually unlobed (sometimes shallowly 3-lobed)...16 |
| 16. | Epicalyx lobes 1–3 mm long...17 Epicalyx lobes more than 3 mm long...S. zonata |
| 17. | Branchlets with the stellate hairs mostly fine (scattered coarse hairs sometimes present); climax leaves with the petiole indumentum similar to that of the branchlet...S. aphela Branchlets with the stellate hairs coarse; climax leaves with the petiole indumentum dissimilar to that of the branchlet (stellate hairs more dense, an adaxial band of long fine stellate hairs present)...S. squarrulosa |
| 18. | Branchlets aculeate...19 Branchlets not aculeate...24 |
| 19. | Branchlets without stellate hairs...S. stewartii Branchlets with stellate hairs (these sometimes sparse)...20 |
| 20. | Calyx not aculeate (epicalyx rarely with a few aculei)...S. byrnesii Calyx and epicalyx aculeate...21 |
| 21. | Flowers pedunculate...S. kenneallyi Flowers not pedunculate...22 |
| 22. | Epicalyx lobes fused in lower 1/3...S. fryxellii Epicalyx lobes ±free to the base...23 |
| 23. | Aculei tipped with stellate hairs; indumentum very sparse, stellate; deciduous shrub; habitat on sandstone substrates...S. forsteri Aculei tipped with a single hair; indumentum moderately dense, simple and stellate; evergreen tree; habitat on granite substrates...S. townsvillensis |
| 24. | Climax (apical) leaves deeply 5-lobed...S. superba Climax (apical) leaves unlobed to deeply 3-lobed...25 |
| 25. | Epicalyx lobes fused in lower 1/3...S. zonata Epicalyx lobes free...26 |
| 26. | Epicalyx lobes 17–27 mm long...S. sankowskyorum Epicalyx lobes 9–14 mm long...27 |
| 27. | Calyx and epicalyx with both stellate hairs and aculei...S. kenneallyi Calyx with stellate hairs, never aculeate; epicalyx usually not aculeate (rarely a few present)...S. byrnesii |
| 28. | Calyx nectaries present...29 Calyx nectaries absent...32 |
| 29. | Calyx lobes fleshy, bright red...*S. gossypiifolia Calyx lobes neither fleshy nor bright red...30 |
| 30. | Capsule with non-aculeate simple hairs (to 1.2 mm long); stellate hairs absent; pedicels not articulate...S. cummingii Capsule with non-aculeate simple hairs (to 2.5 mm long) and shorter, finer, stellate hairs; pedicels articulate...31 |
| 31. | Stems more densely and uniformly hairy; corolla reddish to purple...*S. diversifolia subsp. agioxillos Stems usually with one or more longitudinal lines of pubescence; corolla yellow...S. diversifolia subsp. diversifolia |
| 32. | Calyx lobes between ribs glabrous or subglabrous...33 Calyx lobes between ribs with moderately dense to very dense stellate or woolly hairs...38 |
| 33. | Flowers pedunculate; capsule glabrous or sparsely pubescent...34 Flowers not pedunculate; capsule moderately or densely pubescent ...36 |
| 34. | Capsule sparsely pubescent throughout; pedicel stellate–pubescent and sometimes with aculei...35 Capsule glabrous or sparsely pubescent at the summit; pedicel glabrous or aculeate...S. meraukensis |
| 35. | Leaf lamina glabrous; peduncles and calyx with bristly hairs on a swollen base...S. divaricata Leaf lamina finely stellate–pubescent; peduncles and calyx with 1- to 4-fid fine hairs...S. saponaria |
| 36. | Leaves mostly glabrous, rarely with a few aculei...S. granitica Leaves stellate–hairy...37 |
| 37. | Epicalyx lobes 9–10 mm long; branchlets with aculei but without stellate hairs...S. stewartii Epicalyx lobes 11–14 mm long; branchlets with aculei and sparse stellate hairs...S. marenitensis |
| 38. | Calyx lobes between ribs woolly–hairy [probably not naturalised]...*S. cannabina Calyx lobes between ribs stellate–hairy...37 |
| 39. | Foliar nectary present on at least some leaves...40 Foliar nectary absent on all leaves...54 |
| 40. | Flowers pedunculate...41 Flowers not pedunculate...45 |
| 41. | Calyx always aculeate...42 Calyx usually not aculeate (sometimes a few aculei present)...43 |
| 42. | Leaf lamina with very dense stellate hairs on the abaxial surface, strongly discolourous (the abaxial surface greyish, often brightly so); 10 epicalyx lobes, 0.8–1 times the length of the calyx...S. inimica Leaf lamina with scattered to moderately dense stellate hairs on the abaxial surface, weakly discolourous (the abaxial surface slightly more brown than the greenish adaxial surface); 14–16 epicalyx lobes, 1.25–1.5 times the length of the calyx...S. aneuthe |
| 43. | Stems densely pubescent; 15–19 epicalyx lobes...S. symonii Stems glabrous, prickly or sparsely pubescent; 8–10 epicalyx lobes...44 |
| 44. | Leaves with nectaries up to 21 mm long; corolla pink; 8 or 9 epicalyx lobes...S. byrnesii Leaves with nectaries absent or up to 10 mm long; 10 epicalyx lobes; corolla white, light pink or yellow...S. heterophylla |
| 45. | Branchlets without stellate hairs...S. stewartii Branchlets with stellate hairs...46 |
| 46. | Branchlets with stellate hairs of two size classes (both fine and coarse hairs present)...47 Branchlets with stellate hairs of one size class only (either fine or coarse hairs present)...53 |
| 47. | Leaf lamina usually strongly discolourous...S. riceae Leaf lamina usually concolourous (if discolourous then weakly so)...48 |
| 48. | Leaf lamina with the indumentum denser on the abaxial surface than on the adaxial surface...49 Leaf lamina with the indumentum generally similar on both surfaces...50 |
| 49. | Stem indumentum of fine stellate hairs and long aculei; calyx lobes 7–13 mm long...S. bacalusia Stem indumentum of fine stellate hairs only; calyx lobes 25–35 mm long...S. sankowskyorum |
| 50. | All leaves simple or occasionally shallowly 3-lobed...51 Most leaves deeply 3–5-lobed (simple below flowers)...52 |
| 51. | Aculei tipped with stellate hairs; indumentum very sparse, stellate; deciduous shrub; habitat on sandstone substrates...S. forsteri Aculei tipped with a single hair; indumentum moderately dense, simple and stellate; evergreen tree; habitat on granite substrates...S. townsvillensis |
| 52. | Branchlets with the coarse stellate hairs usually inserted upon tubercles...S. petherickii Branchlets with the coarse stellate hairs not inserted upon tubercles...S. fallax |
| 53. | Calyx ribs and lobes with moderately dense stellate hairs; branchlets and calyx usually not aculeate...S. arnhemensis Calyx ribs with sparse stellate hairs, calyx lobes with minute glandular hairs, otherwise glabrous; branchlets and calyx aculeate...S. thegalea |
| 54. | Branchlets aculeate...55 Branchlets not aculeate...58 |
| 55. | Flowers pedunculate...56 Flowers not pedunculate...57 |
| 56. | Aculei on stems 1–3.7 mm long; epicalyx lobes 21–31 mm long; petals 70–90 mm long...S. inimica Aculei on stems 0.75–1.5 mm long; epicalyx lobes 16–20(–25) mm long; petals ~60 mm long...S. splendens |
| 57. | Calyx lobes 14–16 mm long at anthesis...S. fallax Calyx lobes 20–21 mm long at anthesis...S. menzeliae |
| 58. | Climax leaves deeply 5-lobed; flowers not pedunculate...S. superba Climax leaves moderately 3-lobed or unlobed; flowers pedunculate...S. symonii |
| 1. | Epicalyx lobes forked at apex...S. furcellata Epicalyx lobes not forked at apex...2 |
| 2. | Stems aculeate...3 Stems not (or sometimes very sparsely and minutely in S. gossypiifolia) aculeate...6 |
| 3. | Aculei fine; leaf nectary absent on climax leaves...S. brackenridgei subsp. mokuleiana Aculei stout, conical; leaf nectary present on upper leaves...4 |
| 4. | Calyx covered with long stiff bristles...5 Calyx with sparse fine stellate and some simple hairs...S. fijiensis |
| 5. | Calyx with a white, woolly tomentum towards the base...*S. cannabina Calyx bristly hairy...S. diversifolia subsp. diversifolia |
| 6. | Stems densely stellate–tomentose...S. australensis Stems almost glabrous to finely stellate–pubescent...7 |
| 7. | Epicalyx and calyx fleshy, red...*S. gossypiifolia Epicalyx and calyx not fleshy, green...8 |
| 8. | Calyx nectary present...S. brackenridgei subsp. brackenridgei Calyx nectary absent...S. brackenridgei subsp. molokaiana |
| 1. | Stems, peduncles and petioles aculeate, sometimes also pubescent...2 Stem, peduncles and petioles without aculei, variously pubescent or glabrous...7 |
| 2. | Corolla twisted, salverform, deep red or scarlet; plants often scandent for 10–20 m...S. uncinella Corolla funnelform, yellow, pale yellow, white or red–purple with a maroon centre; low shrubs...3 |
| 3. | Epicalyx lobes distinctly forked, each ±equal in length...S. aculeata Epicalyx lobes simple or one fork much shorter than the other...4 |
| 4. | Aculei on stems with greatly swollen bases, bases as long as the projecting hairs...S. diversifolia subsp. diversifolia Aculei on stems with slightly swollen bases, bases much shorter than the projecting hairs...5 |
| 5. | Aculei on stems erect, projecting hairs long; leaves simple to shallowly 3(–5)-lobed, margins finely serrate...S. scabricaulis Aculei on stems recurved, projecting hairs short; leaves simple to irregularly 3-lobed, margins coarsely toothed...6 |
| 6. | Leaf blade subglabrous to finely scabrous on both sides with simple and 2–5-radiated trichomes, mainly on the veins and margin; epicalyx lobes undivided...S. maculata subsp. maculata Leaf blade scabrous on both sides with stellate trichomes; epicalyx lobes sometimes ±forked ...S. maculata subsp. nipensis |
| 7. | Epicalyx lobes simple; calyx red, becoming fleshy in fruit...*S. gossypiifolia Epicalyx lobes bifurcate (rarely simple in S. radiata); calyx usually green (reddish in S. acetosella), never fleshy...8 |
| 8. | Calyx nectaries absent...9 Calyx nectaries present...10 |
| 9. | Leaves scabrous, cordate–ovate to 3–5-angulate; foliar nectaries present; corolla lavender...S. costata Leaves glabrous or with a few urticating hairs, deeply palmately 3–5-parted with narrow lobes; foliar nectaries absent; corolla dark red or purplish (sometimes yellow)...*S. radiata |
| 10. | Corolla yellow or yellow suffused with red; leaves usually reddish, glabrous...*S. acetosella Corolla lavender; leaves green, densely pubescent...S. furcellata |
| 1. | Calyx nectary and punctate hairs absent; leaves without nectaries, lamina deeply divided, green...2 Calyx nectary present; leaves with 1–3(–5)-nectaries...3 |
| 2. | Retrorse aculei on stems and petioles; leaves clearly serrated; lobes up to 8 cm long, 1.5 cm wide; epicalyx lobes entire or forked; corolla purple...*S. radiata Antrorse aculei on stems, petioles and main vein on the abaxial surface of the leaf lobes; leaf margin subentire, with tiny teeth; lobes up to 15 cm long, 1 cm wide, epicalyx lobes entire; corolla yellow...*S. mechowii |
| 3. | Calyx with distinctly woolly white tomentum; stems usually aculeate; petiole with antrorse aculei; leaves deeply divided to entire; epicalyx lobes entire; corolla yellow...*S. cannabina Calyx without woolly hairs...4 |
| 4. | Calyx and epicalyx red...5 Calyx and epicalyx green...6 |
| 5. | Foliage reddish or green; epicalyx lobes bifurcated; corolla pink or red (rarely yellow)...*S. acetosella Foliage green; epicalyx lobes entire; corolla yellow or red...*S. gossypiifolia |
| 6. | All leaves with a single nectary on the abaxial surface of the petiole; venation prominent on both sides; fruit glabrous; plants without aculei...7 Nectary at the base of the leaf blade...8 |
| 7. | Calyx powdery, without hispid hairs; epicalyx lobes very small, simple or barely forked, recurved on the fruit...S. wilsonii Calyx hispid; epicalyx lobes somewhat larger, clearly forked, arched...S. windischii |
| 8. | Calyx puberulous or velutinous; petiole distinct...9 Calyx hispid or hirsute...14 |
| 9. | Marginal ribs of calyx lobes united well below the sinus ...10 Marginal ribs of calyx lobes united in the same sinus...12 |
| 10. | Petiole and stem with aculei...S. peruviana Petiole and stem without aculei...11 |
| 11. | Epicalyx lobes longer than the calyx, one apical lobe greatly reduced...S. chancoae Epicalyx lobes shorter than the calyx, apical lobes similar...S. manuripiensis |
| 12. | Calyx lobes with three reddish ribs; epicalyx lobes clearly forked; lower leaves broadly ovate or suborbicular, the upper ones subrhombic; plant rough, with punctate stellate hairs...S. trilineata Calyx lobes without pigmentation on the ribs...13 |
| 13. | Leaves all ovate; stems sparsely stellate–pubescent, with some retrorse aculei; epicalyx lobes forked...S. sebastianii Lower leaves 3–5-angled to 3–5-lobed; the upper leaves ovate; stems densely powdery, without aculei; epicalyx lobes entire...S. henningsiana |
| 14. | Plants aculeate; lower leaves lobed...15 Plants not aculeate...18 |
| 15. | Epicalyx longer than the calyx at anthesis, lobes clearly forked; aculei not distinct...S. bifurcata Epicalyx shorter than the calyx at anthesis, lobes not forked; aculei obvious, with a swollen base...16 |
| 16. | Mature stems only with aculei with a swollen base; corolla dark purple...*S. diversifolia subsp. agioxillos Mature stems with aculei and hairs...17 |
| 17. | Stems with aculei, long simple hairs, and a dense row of small hairs; corolla yellow, petals with a purple basal spot...S. diversifolia subsp. diversifolia Stems densely stellate–pubescent and with aculei; corolla scarlet–red...S. maculata |
| 18. | Basal leaves deeply lobed; epicalyx lobes clearly forked...19 Basal leaves entire or shallowly lobed; epicalyx lobes forked or entire...22 |
| 19. | Leaves with three basal nectaries; stems setose; epicalyx shorter than the calyx; leaves lobed; fruit glabrous...S. kitaibelifolia Leaves with one basal nectary; stems pubescent...20 |
| 20. | Leaves dark green when dry, with irregularly scattered stellate hairs (larger and thicker than those on the stem), fruit glabrous...S. multiformis Leaves light green, with evenly scattered, small stellate hairs (similar to those on the stem); fruit bristly...21 |
| 21. | Stems velutinous; leaves entire or shallowly 3-lobed; calyx without prominent reticulations...S. furcellata Stems hispid; leaves deeply 3-lobed; calyx with prominent reticulations...S. mariae |
| 22. | Inflorescence terminal or flowers axillary; subtending leaves smaller but not changing shape...23 Inflorescence terminal; subtending leaves smaller and distinctly changing shape...31 |
| 23. | Calyx accrescent; fruit oblong, ~2.5 cm long, 2 cm wide, tomentose, covered with small hairs and long appressed hairs; leaves with a cordate base and a well-developed petiole, indumentum similar to that of the stem...24 Calyx not accrescent; fruit ovoid or subglobose...25 |
| 24. | Stem densely pubescent, rarely with a few rigid hairs; leaves angular, sometimes somewhat lobed, somewhat longer than wide; epicalyx lobes forked...S. furcellata Stem with very small, scattered hairs, not concealing the epidermis; leaves wider than long; epicalyx lobes forked...S. commixta |
| 25. | Leaf nectaries 1–5, sometimes varying in number on the same plant, erect or prostrate plants...26 Leaf nectaries always solitary; erect plants...27 |
| 26. | Prostrate plants, with branches several metres long; petioles <1 cm long; leaf nectaries 1–3; pedicels accrescent, surpassing the leaves; epicalyx lobes shortly forked; fruit covered with long, dense hairs...S. flagelliformis Erect plants, with sinuous stems; petioles up to 9 cm long; leaf nectaries 1–5; pedicels only slightly accrescent; epicalyx lobes clearly forked; fruit shortly stellate–pubescent...S. pohlii |
| 27. | Leaves shiny, very reticulated, primary and secondary venation clearly visible on both sides; base slightly cordate; stem with short hairs; epicalyx lobes entire; fruit with short hairs...S. hassleriana Leaves usually dull, densely hirsute, only primary venation clearly visible; other characters not combined as above...28 |
| 28. | Fruit glabrous; stems hispid (hairs 1–2 mm long); leaf base cordate; stipules subulate, <6 mm long; epicalyx lobes entire...S. amambayensis Fruit pilose; other characters not combined as above...29 |
| 29. | Epicalyx lobes clearly forked; fruit with short stellate hairs; stems hirsute (hairs 1 mm or less)...S. chapadensis Epicalyx lobes entire; other characters not combined as above...30 |
| 30. | Stem simple, hirsute and stellate hairs with rays 1–2 mm long; fruit with scattered, simple hairs (<1 mm)...S. cucurbitacea Stems stellate–velutinate, hairs with rays <1 mm long; fruit with a dense layer of small stellate hairs and long, simple, appressed hairs to 1.5 mm long...S. hilariana |
| 31. | Fruits with small, scattered, glandular hairs; basal leaves obtuse, subtrilobed; apical leaves rhombic, subsessile, with the nectary displaced towards the petiole; epicalyx lobes shortly forked...S. laxiflora Fruits without glandular hairs; other characters not combined as above...32 |
| 32. | Fruit glabrous; epicalyx lobes clearly forked...33 Fruit pilose; epicalyx lobes entire or forked...36 |
| 33. | Inflorescence terminal, branched; stems with tiny, scattered stellate hairs; calyx hispid...34 Inflorescence terminal, not branched; other characters not combined as above...35 |
| 34. | Leaves with subentire margin, lower leaves obtuse; petiole to 8 cm long ...S. matogrossensis Leaves with clearly toothed margin, apex acute; petiole 1–3 cm long...S. hochreutineri |
| 35. | Stems hirsute; leaves subsessile, petiole <1 cm long, lamina rough to the touch; calyx with a layer of tiny stellate hairs and larger hairs on the ribs...S. andersonii Stems velutinous; leaves with petioles to 6 cm long, lamina soft to the touch; calyx uniformly hispid...S. nanuzae |
| 36. | Adult leaves with petiole up to 2 cm long...37 Adult leaves with petiole >5 cm long...38 |
| 37. | Stem densely hirsute, stellate hairs with branches 1 mm long; corolla lobes white; leaf base not cordate; epicalyx lobes entire...S. gregoryi Stems pubescent, stellate hairs with branches <0.5 mm long; corolla lobes red; adult leaf base cordate; epicalyx lobes thick, forked ...S. capitalensis |
| 38. | Inflorescence branched...39 Inflorescence simple...40 |
| 39. | Stem densely puberulous; calyx puberulous, ribs hispid, with hairs up to 2 mm long; epicalyx lobes entire or slightly forked...S. paludicola Stem with scattered tiny stellate hairs; calyx puberulous, ribs with scattered, hispid hairs ~0.5 mm long; epicalyx lobes entire, with recurved apex...S. ferreirae |
| 40. | Adult leaves with 5 acuminate lobes; pubescence dense on both sides; petals ~70 mm long; epicalyx lobes clearly forked...S. benensis Adult leaves with 3 obscure, acute lobes; indumentum and petal length not as above; epicalyx lobes entire or forked...41 |
| 41. | Stems with only stellate hairs; epicalyx lobes entire or forked...42 Stems with branched and simple hairs 3–5 mm long; epicalyx lobes clearly forked...43 |
| 42. | Stem densely hirsute, ±smooth, hairs 1–1.5 mm long; leaves with dense, soft pubescence on both sides; epicalyx lobes clearly forked...S. adscensionis Stem with scattered stellate hairs 1.5 mm long; leaves with stellate hairs not obscuring the epidermis; epicalyx lobes entire...S. conceptionis |
| 43. | Leaves with a single nectary, both sides with hairs similar to stems...S. saddii Leaves with 3 nectaries, both sides with very small stellate hairs...S. itirapinensis |
Nomenclature for Sabdariffa species listed in alphabetical order, with recognised species based on Wilson (2006) and subsequent publications
Sabdariffa acetosella (Welw. ex Hiern) M.M.Hanes & R.L.Barrett, comb. nov.
Hibiscus acetosella Welw. ex Hiern, Cat. Afr. Pl. 1: 73–74 (1896). Type citation: ‘Golungo Alto, – In stations clothed with short brushwood, alongside roads, also in neglected fields, and occasionally cultivated by the natives; Varzea do Isidoro, in flower-bud in March 1856, and in May 1855 in fl. near Mussengue. No. 5270. At the moist borders of thickets between Sange and Zanga, fr. Dec. 1854; and in open wooded places on the lower mountains of Queta, Oct. 1855. No. 5271. By fences and thickets near Sange, in seed Dec. 1854. Coll. Carp. 249, 250, 252.’ Type: Angola: Cuanza Norte, Golunga Alta, on the lower mountains of Queta, Oct. 1854, F.M.J.Welwitsch 5271 (lecto: LISU 206160; isolecto: BM 014117363), designated by A.W.Exell & F.A.Mendonça, Consp. Fl. Angol. 1(1): 168 (1937). Residual syn: all Angola: Cuanza Norte, Golunga Alta, near Mussengue, May 1855, F.M.J.Welwitsch 5270 (BM, n.v., LISU, n.v.); Cuanza Norte, near Sange, col. carp. 249 (LISU, n.v.); Cuanza Norte, near Sange, col. carp. 252 (LISU, n.v.).
Hibiscus acetosella Ficalho, Bol. Soc. Geogr. Lisbon, ser. 2: 608 (1881), nom. inval., nom. nud.
Hibiscus eetveldeanus De Wild. & T.Durand in T.Durand & E.A.J.De Wildeman, Bull. Soc. Roy. Bot. Belgique 38(2): 24–25 (1899); Hibiscus surattensis var. eetveldeanus (De Wild. & T.Durand) Hochr., Annuaire Conserv. Jard. Bot. Genève 6: 49–51 (1902). Type citation: ‘Reg. III: env. de Monbanga, 1895 (Alfr. Dewèvre).’ Type: Democratic Republic of The Congo: Monbanga [Mombanga, NW of Monga], 1895, A.Dewèvre 741a (holo: BR, n.v.).
Durand and de Wildeman (1889, pp. 49–51); Ochse and Bakhuizen van den Brink (1960, p. 472, fig. 295); Hauman (1963, pp. 116–117); Bates (1965a, p. 79, fig. 23); Borssum Waalkes (1966, pp. 59–60); Liogier (1981, p. 91); Correll and Correll (1982, p. 931); Marais and Friedmann (1987, p. 36, pl. 11 (7)); Fryxell (1988, p. 200); Edmonds (1991, fig. 1(2), 2(17)); Fryxell (1992b, pp. 100–101); Sivarajan and Pradeep (1996, pp. 101–104, fig. 31, 32); Wilson (1999, pp. 68–69, fig. 2b); Fryxell (2000, p. 14); Krapovickas and Fryxell (2004, pp. 50–51); Leistner (2008, p. 114); Mwachala (2009, p. 32); Lejoly et al. (2010, pp. 170–171); Fayaz (2011, pp. 507–508, fig.); van der Burg (2013, pp. 56–57).
Exell and Mendonça (1937, p. 168) cited ‘Welwitsch 5271 (BM; Lis.U, tipo)’. Examination of the formatting in the full publication indicates that this is a deliberate choice of the sheet at LISU as the type and accepted as inadvertent lectotypification. Wilson (1999, p. 68) suggested that the BM specimen would have been more appropriate, as the best set of Welwitsch’s specimens were retained at BM (Hiern 1896) but the choice of Exell & Mendonça (1937) cannot reasonably be overturned. The earlier publication of Hibiscus acetosella Welw. ex Ficalho (de Ficalho 1881) lacked a description and the name was subsequently validated by Hiern (1896).
The commonly cultivated form has red to purple foliage, calyx and corolla. However, the wild-type has green foliage, a red calyx and yellow corolla with a purple centre. Cultivated forms also have more deeply lobed and more divided leaves, suggesting a possibility that these may be derived through hybridisation with S. radiata but that remains to be critically assessed.
Native to Angola, Burundi, Democratic Republic of The Congo, Zambia and Zimbabwe.
Introduced and commonly naturalised to the Bahamas, Bangladesh, Bénin, Bolivia, Borneo, SE Brazil, Cameroon, Colombia, Costa Rica, Cuba, the Dominican Republic, Ecuador, Florida (USA), Guinea, Gulf of Guinea Islands, Honduras, Ivory Coast, Kenya, Mauritius, Paraguay, Perú, Puerto Rico, Réunion, the Seychelles, Somalia, Sudan, Tanzania, Thailand and Vietnam.
Sabdariffa aculeata (Walter) M.M.Hanes & R.L.Barrett, comb. nov.
(Fig. 7c, d.)
Hibiscus aculeatus Walter, Fl. Carol. [Walter] 177 (1788), non F.Dietr. (1817), nec G.Don (1831), nec Roxb. (1832). Type: United States of America: South Carolina: 3 miles [~4.8 km] S of Lake City, 10 July 1927, K.M.Wiegend & W.E.Manning 1959 (neo, designated by D.B.Ward, J. Bot. Res. Inst. Texas 2: 477 (2008): GH 00247969).
Hibiscus scaber Michx., Fl. Bor. Amer. 2: 45 (1803), nom. illeg., non Lam. (1789). Type: United States of America: Amérique septentrionale, A.Michaux 47-bis (syn: P 02285887, P 02285888).
Walter (1788, p. 177); Michaux (1803, p. 45); Menzel et al. (1983a, pp. 206–207, fig.); Edmonds (1991, p. fig. 2(29)); Blanchard (2015, p. 259); Hanes et al. (2024, fig. 1o, 2e).
Sabdariffa adscensionis (Fryxell & Krapov.) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus adscensionis Fryxell & Krapov., Novon 14: 58, fig. 1 (2004). Type: Bolivia: Santa Cruz: prov. Ñuflo de Chavez, Ascención de Guarayos, 15°40′S, 63°5′W, 26 Apr. 1977, A.Krapovickas A.A.Schinini 31722 (lecto, here designated: CTES 3775B [0001521!]; isolecto: CTES 3775A [0001520!], LPB 0000730!, NY 00021175!, TEX 00208154!).
Fryxell and Krapovickas (2004, pp. 58–59, fig. 1); Krapovickas and Fryxell (2004, pp. 102–103).
Fryxell and Krapovickas cited Krapovickas & Schinini 31722 held at CTES as the holotype of Hibiscus adscensionis. However, this gathering comprises two specimens with separate herbarium accession numbers and barcodes. Therefore, these specimens represent syntypes rather than the holotype and the name is lectotypified here. We selected CTES 3775B (barcode 0001521) over CTES 3775A (barcode 0001520) by the presence of an open flower, showing the disposition of stamens, an important trait to identify some groups within Hibiscus and Sabdariffa.
Sabdariffa altissima (Hornby) Mwachala & R.L.Barrett, comb. nov.
(Fig. 8a, b.)
Hibiscus altissimus Hornby, Proc. & Trans. Rhodesia Sci. Assoc. 41: 55–56 (1946). Type: Mozambique: Zambézia, Vales dos rios Luça e Li-cungo [Lusa River Valley, W of Guruè], ~750 m, 5 July 1942, A.J.W.Hornby 4563 (lecto, here designated): PRE 0282639-0; isolecto: K 000240649, K 000240650, K 000240651, LISC 000600, PRE 0479304-0, WAG 0004652).
Hornby (1946, pp. 55–56); Wilson (1999, p. 53, fig. 1d).
Sabdariffa amambayensis (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus cucurbitaceus var. acuminatus Hassler, Feddes Rep. 7: 378–379 (1909); Hibiscus amambayensis Krapov. & Fryxell, Bonplandia 13(1–4): 79–81, fig. 10 (2004) (non Hibiscus acuminatus Cav. (1787)). Type: Paraguay: Sierra de Amambay, Sep. 1907, E.Hassler 10621, leg. T.Rojas. (lecto, here designated: G 00381134!; isolecto: BM 013839345!, CTES 0001538!, K 000535442!, MPU 016514!, W 1911-0000340!).
Krapovickas and Fryxell (2004, p. 80, fig. 10); Coutinho and Fernandes-Júnior (2024).
Sabdariffa andersonii (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus andersonii Krapov. & Fryxell, Bonplandia 13(1–4): 90–92, fig. 15 (2004). Type: Brazil: Mato Grosso: mun. Barra do Garças, 5 km N de Barra do Garças, 24 Feb. 1982, P.I.Oliveira 444 & W.R.Anderson (holo: MBM 073730!, iso: CTES 0001523!, CTES 0001522!, ESA 2337, NY 00021176!).
Krapovickas and Fryxell (2004, pp. 90–92, fig. 15); Coutinho and Fernandes-Júnior (2024).
The name ‘Hibiscus andersonii’ is sometimes used in popular literature for the cultivar Hibiscus × rosa-sinensis ‘Andersonii’ but we have found no valid publication of the name in that form that might invalidate Hibiscus andersonii Krapov. & Fryxell. The potential for confusion is unfortunate but should be reduced by the transfer of this name to Sabdariffa.
Sabdariffa aneuthe (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
Hibiscus aneuthe Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 188, fig. 1 (2003). Type: Australia: Northern Territory: Kakadu National Park, 0.5 km N of Jim Jim campground, 2 May 1990, A.V.Slee & L.A.Craven 3085 (holo: [mounted on 2 sheets] CANB 407035.1, CANB 407035.2; iso: ASU 0019349, DNA D0180184 (two sheets), L, TEX 00208037).
Craven et al. (2003, pp. 188, fig. 1); Craven (2022).
Sabdariffa aphela (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
(Fig. 8c, d.)
Hibiscus aphelus Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 188, fig. 3 (2003). Type: Western Australia: Middle Spring, 18 km (by air) NW of Kununurra, 8 May 1983, P.A.Fryxell & L.A.Craven 4000 (holo [mounted on 3 sheets]: CANB 642469.1, CANB 642469.2, CANB 642469.3; iso: A 00107053, AD 156512, ASU 0019350, B 10_0199402, BISH, BRI AQ0695705 (2 sheets), CTES, DNA D0171058 (3 sheets), G 00353253, K 000659844, L, MEL 2172255, NSW 623954, P 00640788, PERTH 06486657, TEX 00208002).
Wheeler (1992, pp. 223, fig. 61f, as Hibiscus sp. C); Craven et al. (2003, pp. 188, fig. 3); Craven (2022).
Sabdariffa arnhemensis (F.D.Wilson) McLay & R.L.Barrett, comb. nov.
(Fig. 8e, f.)
Hibiscus arnhemensis F.D.Wilson, Austral. J. Bot. 22: 175, fig. 3, 8 (1974). Type: Australia: Northern Territory: among sandstone boulders along East Alligator River (12°29′S, 132°58′E), 18 May 1968, N.B.Byrnes 812 (holo: DNA A0014527; iso: CANB 246023, PERTH 01599852, US).
Wilson (1974, p. 175, fig. 3, 8); Wilson and McLay (2022).
Sabdariffa aspera (Hook.f.) Mwachala & R.L.Barrett, comb. nov.
(Fig. 9a, b.)
Hibiscus asper Hook.f. in W.J.Hooker, Niger Fl. 228 (1849). Type: Sierra Leone: Miss Turner s.n. (holo: K 000240763).
?Hibiscus vanderystii De Wild., Bull. Jard. Bot. État. Bruxelles 5: 35 (1915). Type citation: ‘Kwango, juillet 1913, H. Vanderyst, n. 1377).’ Type: Angola: Kwango [Cuango River], July 1913, H.J.R.Vanderyst 1377 (holo: BR 0000008952387).
?Hibiscus malangensis Baker f., J. Bot. 77: 22 (1939). Type: Angola: Malange Province, River Cuango, near Xa-Sengue, ~1075 m, 5 Apr. 1937, A.W.Exell & F.A.Mendonça 274 (lecto, here designated: BM 014117362; isolecto: COI 00005060, LISC 000598).
Hooker and Bentham (1849, p. 228); de Wildeman (1915, p. 35); Baker (1939, p. 22); Sprague (1913, pp. 418–419); Wilson (1999, p. 68, in minor part, excl. fig. 2c); ?Leistner (2008, p. 114); van der Burg (2013, pp. 57–59, fig. 16); Lee et al. (2014, pp. 275–276, fig. 1, 3a–f).
Baker (1939) cited material at BM and COI as the type of H. malangensis, therefore a lectotype may be selected. We here designate BM 014117362 as this specimen has a label stating, ‘type’, a copy of the protologue is attached to this sheet and the material is in fertile condition.
The boundaries between Sabdariffa aspera and S. cannabina have been a matter of contention in the literature, especially regarding the placement of synonyms under each name. Fryxell (1990) noted that S. cordofana (Turcz.) Mwachala & R.L.Barrett was likely the same as S. aspera (a view not supported here) and distinct from S. cannabina. Leistner (2008, p. 114) placed H. malangensis Baker f. under S. cannabina but accepted S. aspera. Mwachala (2009) included S. aspera and the associated synonyms under a broad concept of S. cannabina and this has been followed by POWO (see https://powo.science.kew.org/).
Wilson (1999) considered the presence of a white, woolly tomentum at the base of the calyx to be diagnostic for S. cannabina and absent in S. aspera, a conclusion we support. Van der Burg (2013) also considered the two species to be quite distinct, recognising S. aspera as native to Gabon, whereas S. cannabina is only cultivated there. Van der Burg (2013) states that S. aspera can be further distinguished by leaves with 3–5 lobes, the lobed margins being wavy, and the reddish sepals. Assignment of synonyms is made challenging by the considerable morphological variation expressed in the relevant specimens (especially leaf morphology but also calyx and indumentum characters) and further field-based studies are likely to be required to fully resolve these debates. The synonyms listed above do all appear to conform with van der Burg’s concept of S. aspera in lacking the woolly tomentum on the calyx.
Wilson (1999, p. 49) notes that several collections examined appear to be natural hybrids between S. aspera and S. cannabina. These specimens should be further investigated for hybridisation possibly resulting from S. cannabina being introduced into the range of S. aspera or whether these may actually represent a discrete taxon with ‘intermediate’ morphology.
We here consider Wilson’s (1999, p. 68) concept of S. aspera to apply mostly to S. cordofana and S. verrucosa, and only partially to the type concept of S. aspera. Although the delimitation of S. aspera, S. cordofana and S. verrucosa will require further study by local workers, we here distinguish S. aspera from these other two species based on whether the epicalyx lobes are ±flat when dry (S. aspera) or have distinct marginal ribs (S. cordofana and S. verrucosa). The typical form of S. aspera appears to have epicalyx lobes that are almost as long as the calyx lobes at anthesis (v. ~1/2 the length in related taxa). We also tentatively list synonyms under S. aspera based on the same character states, noting that several of these may well warrant recognition as discrete taxa but resolution of these matters is beyond the scope of the current study. We note as an example that specimens at P identified as Hibiscus asper (as at April 2024) are a mix of S. aspera, S. cannabina, S. cordofana and S. verrucosa, with occasional specimens of other species including S. elongatifolia (Hochr.) Mwachala & R.L.Barrett. We have included Hibiscus malangensis and H. vanderystii De Willd. in the key to African species below under those names to encourage further study of these poorly known taxa.
M.Pobegun 1032 from Guinae (P 06721267) notably has deeply lobed lower leaves and simple, almost linear leaves subtending the flowers. A specimen from the Democratic Republic of The Congo (J.Koechlin JK3715; P 06721279) also has simple leaves subtending the flowers.
Specimens from Madagascar previously assigned to S. aspera (e.g. P.B.Phillipson et al. 3890; MO, P 00554877) may represent a distinct new species endemic to Madagascar.
Future studies should investigate consistency of the calyx length relative to the ovary length at anthesis, calyx indumentum (including glands and aculei), corolla colour and relative variation in leaf lobing at different growth stages (e.g. basal v. climax leaves) including lobe margins).
Sabdariffa australensis (Fosberg) R.L.Barrett & M.M.Hanes, comb. nov.
(Fig. 9c, d.)
Hibiscus australensis Fosberg, Micronesica 2: 156 (1966), (as ‘australense’). Type: French Polynesia: Tubuai (Austral) Islands: Rapa Iti: 1/4 mi [~0.4 km] E of Ahurei, top of gravelly sea beach, 1 m, 1 July 1934, H.St.John & F.R.Fosberg 15259 (holo: BISH 1003218).
Fosberg and Sachet (1966, p. 156); Wilson (1993, pp. 280–281); Meyer (2011; 22, pl.).
Sabdariffa bacalusia (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
Hibiscus bacalusius Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 192, fig. 4 (2003). Type: Australia: Northern Territory: Rock Candy Range, ~23 km ESE of Daly River Police Station, 23 June 1985, P.A.Fryxell, L.A.Craven & J.McD.Stewart 4896 (holo [mounted on 2 sheets]: CANB 642040.1, CANB 642040.2; iso: ASU 0019351, DNA D0180026 (2 sheets), K 000381641, L, MEL 2172253, NY 00062129, NY 00689135, TEX 00208003).
Craven et al. (2003, p. 192, fig. 4); Craven (2022).
Sabdariffa benensis (Fryxell & Krapov.) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus benensis Fryxell & Krapov., Novon 14: 59, fig. 2 (2004). Type: Bolivia: Beni: Vaca Diez, 37 km E of Riberalta on road to Guayaramerín, 230 m, 230 m, 21 May 1982, J.C.Solomon 7717 (holo: MO 101459232!; iso: CTES 0001525!, LPB 0000731!, NY 00007086!, NY 00021177!, U 0120139!).
Fryxell and Krapovickas 2004, p. 59, fig. 2); Krapovickas and Fryxell (2004, pp. 101–102).
The protologue cites the holotype of Hibiscus benensis as being held at MO, subsequently (apparently incorrectly) attributed to LPB by Krapovickas & Fryxell (2004, pp. 101–102).
Sabdariffa berberidifolia (A.Rich.) Mwachala & R.L.Barrett, comb. nov.
(Fig. 10a, b.)
Hibiscus berberidifolius A.Rich., Tent. Fl. Abyss. 1: 56 (1847). Type citation: ‘Crescit prope Sankka, in provincia Hedjou (Ant. Petit).’ Type: Abyssinia [Ethiopia]: Sanka Berr, L.R.Quartin Dillon & A.Petit 119 (lecto, designated by F.D.Wilson, Brittonia 35: 175 (1983): P 00151946; isolecto: BR 0000006247713 (fragm.)).
Hibiscus diversifolius var. witteanus Hochr. in W.Robyns, Bull. Jard. Bot. État 18: 276 (1947). Type citation: ‘CONGO BELGE: DISTRICT DES LACS ÉDOUARD ET KIVU: … Tshamugussa, alt. 2500 m., étage des Bambous, fleurs rose violacé, août 1934, G. de Witte 1817* (typus).’ Type: Belgian Congo [Democratic Republic of The Congo]: Tshamugatta, 9 Aug. 1934, G. de Witte 1817 (syn: BR 0000008952356, BR 0000008952769, G 00014202 (3 sheets); G 00014721 (2 sheets)).
Hibiscus parvilobus F.D.Wilson, Bull. Nat. Hist. Mus. London, Bot. 29(1): 67 (1999). Type: Kenya: Rift Valley Prov., Nakuru District, ~9 km NE of Londiani, 2743 m, 8 Feb. 1973, R.W.Spjut & P.D.Ensor 3184 (holo: EA; iso: BR 0000005299447, K 000240691).
Richard (1847, p. 56); Hauman (1963, pp. 112–113); Maquet (1983, p. 383, fig. 121, 2a, b), Wilson (1983, pp. 175–179, fig. 1), Wilson (1999, pp. 61–62, 67, fig. 2a); Mwachala (2009, pp. 46–47).
There are two collections of Hibiscus diversifolius var. witteanus by de Witte at G but Robyns (1947) clarifies that only de Witte 1817 is the type number. de Witte 1320 (BR; G 00014202) is cited by Robyns (1947) but is not a type, in contrast to the citation by Wilson (1983, p. 175) and followed by Mwachala (2009, p. 47). Wilson (1983) may have presumed that the statement ‘typus’ applied to both collections cited but we interpret this as applying only to de Witte 1817.
We follow Mwachala (2009, p. 47) in including Hibiscus parvilobus as a synonym of S. berberidifolia. The type collection appears to differ in the distinctly petiolate leaves that are often somewhat lobed and less prominently toothed at the apex and in a sparser stem indumentum. However, additional collections from near the type locality are currently available at EA and these characteristics are now known to intergrade with those of typical S. berberidifolia.
(a, b) Sabdariffa berberidifolia. Photographs by (a, b) Mike Plagens, iNaturalist: 9563139, CC BY-NC. (c, d) Sabdariffa bifurcata. Photographs by Andre Benedito, iNaturalist: 147298687, ©. (e, f) Sabdariffa brackenridgei subsp. brackenridgei. Photographs by Kathryn Akamine, iNaturalist: 83585479, CC BY-NC.
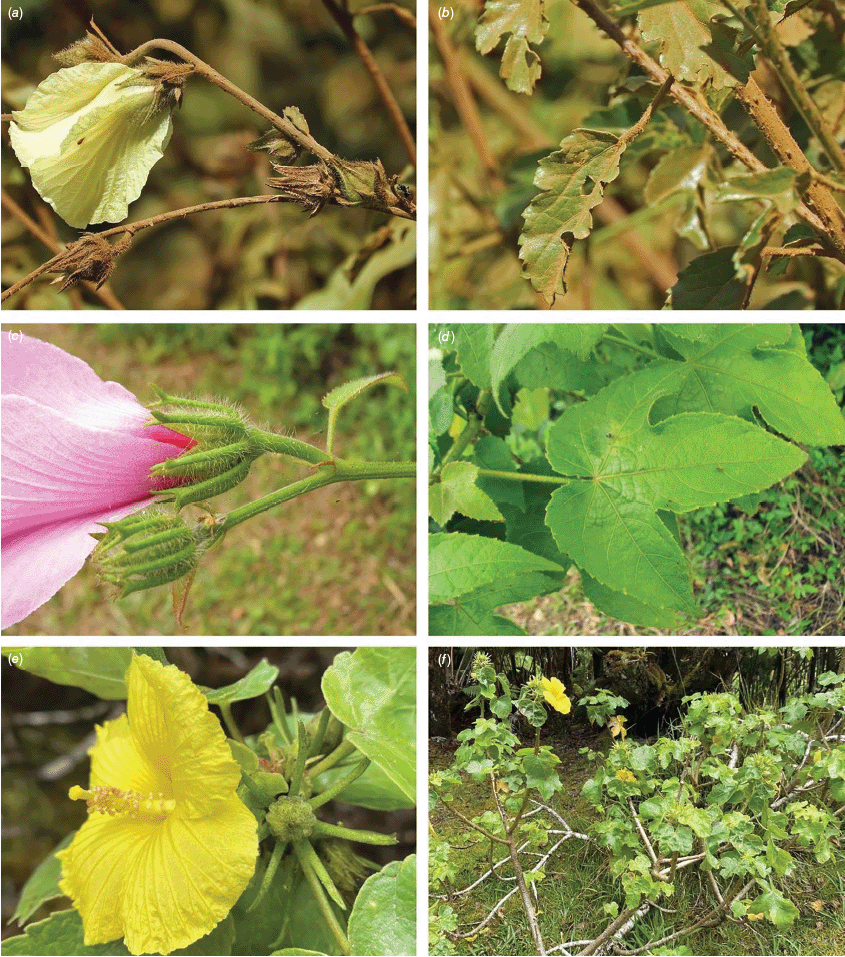
Sabdariffa bifurcata (Cav.) M.C.Duarte & Yoshikawa, comb. nov.
(Fig. 10c, d.)
Hibiscus bifurcatus Cav., Diss. 3: 146–147, t. 51, fig. l (1787). Type citation: ‘Habitat in Brasilia, observatus a Commers. V.S. communicatum a D. Thouin.’ Type: Brazil: circa Rio de Janeiro, July 1767, P.Commerson s.n. (lecto, designated by P.A.Fryxell in R.A.Howard, Fl. Lesser Antilles, Leeward and Windward Isl. 5: 222 (1989), as ‘holo’): P 00673709! [P-JU 12374]; isolecto: C (Herb. Vahl); MA 475799!, MPU 016507!, P 01900220!).
Hibiscus bicornis G.Mey., Prim. Fl. Esseq. 231 (1818); Hibiscus bifurcatus var. bicornis (G.Mey.) Hochr., Ann. Conserv. Jard. Bot. Genève 4: 109 (1900). Type: Guyana: Essequibo River, s. dat., E.K.Rodschied s.n. (holo: GOET 007811).
Hibiscus decipiens A.St.-Hil., Fl. Bras. Merid., 4th edn, 1(7): 247 (1828). Type citation: ‘prope lacum vulgo Sacuarema in provincia Rio de Janeiro.’ Type: Brazil: prov. Rio de Janiero, 1816, A.St.-Hilaire Catal. B2 Sect. 2 n° 475 (lecto, here designated: P 02285901; isolecto: MPU 016510, P 02285902, P 02285903).
Hibiscus fluminensis Vell., Fl. Flumin. 282, n. 7 (1825 [1829]). Type citation: ‘(Tab. 34a T. 7.)’. Type: ‘Hibiscus fluminensis’ in J.V. de M. Vellozo, Fl. Flumin. 7: tab. 34 [BHL] (lecto; original parchment Plate of Florae Fluminensis in the Manuscript Section of the Biblioteca Nacional do Rio de Janeiro [catalogue number mss1198656_040], later published in J.V. de M.Vellozo, Fl. Flumin.: Icon. 7: t. 34 (1831)), designated by Coutinho et al., Brittonia 77(1) (2025).
Hibiscus quinquelobatus Vell., Fl. Flumin. 283, n. 9 (1825 [1829]). Type citation: Habitat locis, et floret mensibus supra citatis. Type: ‘Hibiscus quinquelobatus’ in J.V. de M.Vellozo, Fl. Flumin. 7, tab. 36 [BHL] (lecto: original parchment Plate of Florae Fluminensis in the Manuscript Section of the Biblioteca Nacional do Rio de Janeiro [catalogue number mss1198656_040], later published in J.V. de M.Vellozo, Fl. Flumin. 7: t. 36 (1831)), designated by Coutinho et al., Brittonia 77(1) (2025).
Hibiscus bifurcatus f. glaber Gürke in C.F.P. von Martius et al., Fl. Bras. 12(3): 561 (1892); Hibiscus bifurcatus var. glaber (Gürke) Hochr., Ann. Conserv. Jard. Bot. Genève 4: 109 (1900). Type: Brazil: Rio de Janeiro, Praia Grande, 27 Oct. 1867, A.Glaziou 1310 (lecto, designated by A.Krapovickas & P.A.Fryxell, Bonplandia 13: 61 (2004): R; isolecto: BR 0000013323912!, BR 0000013323929!, C (herb. Warming), P 06642099!). Residual syn: Suriname: Paramaribo, Mar.–Apr. 1844, A.Kappler 1590 (G 00353179!, P 06642110!). Brazil: between S. Ioao & S. Aña, 1868, W.Burchell 9101 (BR 0000013323936!, BR 0000013323943!). Brazil: W.Burchell 9969 (BR 0000013323882!, BR 0000013323899!, BR 0000013323905!). Suriname: 1851, H.R.Wullschlaegel 27 (BR 0000013323868!). Suriname: 1853, H.R.Wullschlaegel 1361 (BR 0000013323875!). Brazil: prov. Minarum: prope villa do Principe etc. C.F.P.Martius (M 0211565!, M 0211566!, M 0211568!).
Hibiscus bifurcatus f. pilosus Gürke in C.F.P. von Martius, Fl. Bras. 12(3): 561 (1892). Type: Brazil: Rio de Janeiro, 1834, C.Gaudichaud 924 (lecto, designated by A.Krapovickas & P.A.Fryxell, Bonplandia 13: 61 (2004): G; isolecto: P 06642098!). Residual syn: Brazil: 16 Aug. 1836, B.Luschnath s.n. [Fl. Bras. no. 1012] (M 0211569!, BR 0000005905065!, BR 0000005905393!, BR 0000005905720!).
Cavanilles (1787, pp. 146–147, t. 51, fig. l); Meyer (1818, p. 231); Vellozo (1827, p. 282, 1831, tab. 34); de Saint-Hilaire (1828, p. 247); Gürke (1892, p. 561); Williams and Cheeseman (1929, p. 88); Uittien (1932, pp. 21–22); Macbride (1956, pp. 469–470); León and Alain (1957, pp. 252–253); Adams (1972, pp. 476–477); Liogier (1981, p. 93); Fryxell (1989, p. 222); Edmonds (1991, fig. 1(14); 2(28)); Fryxell (2000, p. 15); Krapovickas and Fryxell (2004, p. 61); Areces Berazaín (2006, pp. 26–27, fig. 2a, 3a, 4a, f); Rigueiral et al. (2019, p. 4, fig. 1a–c); Coutinho and Fernandes-Júnior (2024).
Cavanilles (1787) worked mostly with material at MA and P. The citation of a specific sheet of Hibiscus bifurcatus at P by Fryxell (1989, p. 232) as ‘holo’ is here accepted as an inadvertent lectotypification.
Although de Saint-Hilaire (1828) did not specify a herbarium holding the type of Hibiscus decipiens, the primary set of specimens associated with this publication is held at P. We here designate the sheet with the most material as lectotype.
No specimens have been traced for Hibiscus fluminensis Vell. or Hibiscus quinquelobatus Vell., therefore the original illustrations (subsequently published 2 years after the protologue but available to Vellozo (1827, 1831) at the time of publication) have been designated as lectotypes by Coutinho et al. (2025). The original artwork for these figures can be viewed through the website of the Biblioteca Nacional (http://bndigital.bn.br/acervodigital; see also Knapp et al. 2015).
Gürke (1892) recognised several formae within S. bifurcata but these are considered to be regional variants of a polymorphic species.
The earlier name Hibiscus strigosus Lindl. has been suggested to be the same as H. bifurcatus but that name clearly applies to an Abelmoschus species. The illustration in the protologue may be an appropriate lectotype for H. strigosus Lindl.
We follow Coutinho et al. (2025) in including Hibiscus quinquelobatus Vell. as a synonym of S. bifurcata.
Widespread in South and Central America, in NE Argentina, Belize, Bolivia, Brazil, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, French Guiana, Guyana, Honduras, Jamaica, Leeward Islands, SE Mexico, Nicaragua, Panamá, Paraguay, Perú, Puerto Rico, Suriname, Trinidad and Tobago, Venezuela and the Windward Islands.
Sabdariffa brackenridgei (A.Gray) M.M.Hanes & R.L.Barrett, comb. nov.
Hibiscus brackenridgei A.Gray, Bot. U.S. Expl. Exped. 15: 175, pl. 12 (1854). Type: United States of America: Hawaii: ‘on a mountain in the west division on Maui’, 1838, Wilkes, U.S. Expl. Exped. s.n. (lecto, designated by M.J.Roe, Pacific Sci. 15: 9 (1961), as ‘holotype’: GH 00052854; isolecto: BISH 1010566, GH, P 00639277, US 00098074).
Roe (1961, p. 9) specifically listed the material at GH as the ‘holotype’ of Hibiscus brackenridgei and this is here accepted as an inadvertent lectotypification, even though species named by Gray (1854) are more commonly lectotypified by material held at US.
This species is currently considered to be endangered (Keir 2018a; The IUCN Red List of Threatened Species, see https://www.iucnredlist.org/).
Sabdariffa brackenridgei subsp. brackenridgei
(Fig. 10e, f.)
Bates (1965a, pp. 79, 87); Roe (1961, pp. 10–11, fig. 4); Bates (1990, pp. 883–884, pl. 123); Wilson (1993, pp. 276–278); Staples and Herbst (2005, p. 388); Fayaz (2011, p. 508, fig.).
Sabdariffa brackenridgei subsp. mokuleiana (M.Roe) M.M.Hanes & R.L.Barrett, comb. nov.
(Fig. 11a, b.)
Hibiscus brackenridgei var. mokuleianus M.Roe, Pacific Sci. 15: 12, fig. 8 (1961), (as ‘mokuleiana’); Hibiscus brackenridgei subsp. mokuleianus (M.Roe) D.Bates, Bishop Mus. Occ. Pap. 29: 105 (1989). Type: United States of America: Hawaii: Oahu, second gulch E of Mt Kaala, 180 m, 16 Feb. 1957, M.J.Roe 210 (holo: BISH 1010569; iso: BISH 1010570).
Roe (1961, pp. 12–13, fig. 8); Menzel and Hancock (1984, fig. 1, 2); Bates (1989, p. 105); Bates (1990, p. 884); Wilson (1993, p. 278); Staples and Herbst (2005, p. 388).
(a, b) Sabdariffa brackenridgei subsp. mokuleianus. Photographs by (a, b) Brandon Najarian, iNaturalist: 120778110, ©. (c, d) Sabdariffa byrnesii. Photographs by (c) Tara L. Crewe, iNaturalist: 40579314, CC BY-NC. (d) Lyn Craven, APII ©. (e, f) Sabdariffa cannabina. Photographs by Jo Floer, iNaturalist: 205616562, CC BY-NC.
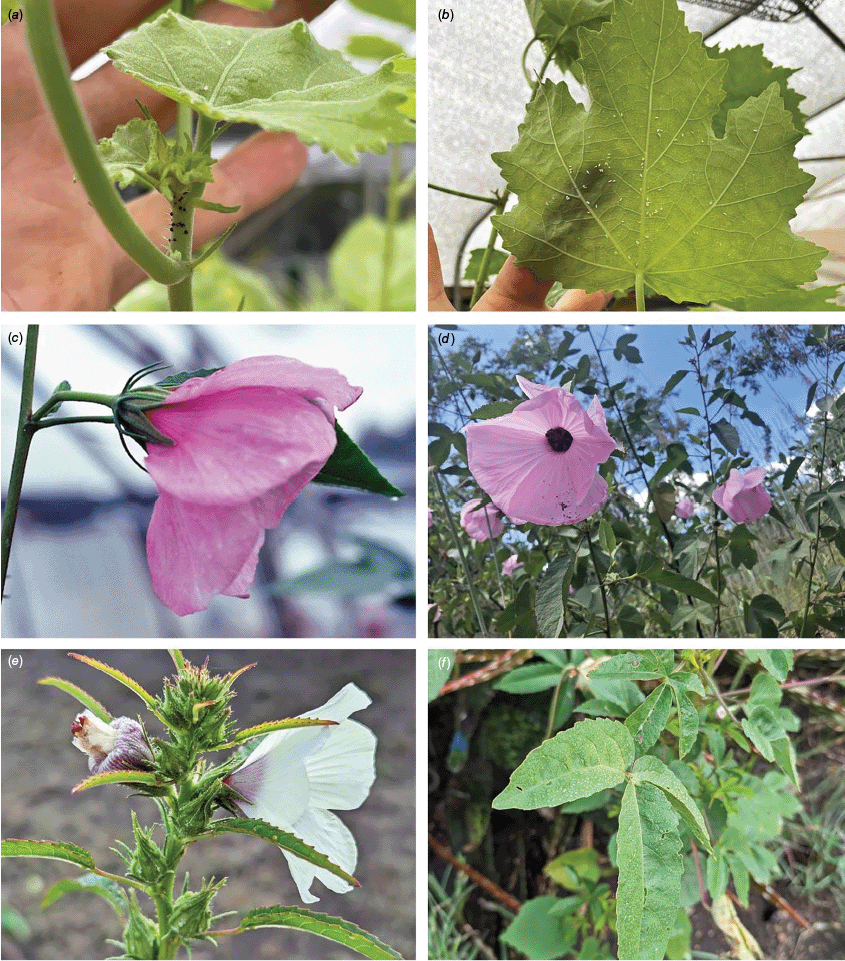
Sabdariffa brackenridgei subsp. molokaiana (Rock ex Caum) M.M.Hanes & R.L.Barrett, comb. nov.
Hibiscus brackenridgei var. molokaianus Rock ex Caum, Bishop Mus. Occ. Pap. 9(5): 4–5, pl. 2, 3 (1930), (as ‘molokaiana’); Hibiscus brackenridgei subsp. molokaianus (Rock ex Caum) F.D.Wilson, Brittonia 45: 278–279 (1993). Type: United States of America: Hawaii: Molokai: western Molokai, Feb. 1920, J.F.C.Rock s.n. (lecto, here designated: BISH 1010572; isolecto: BISH 1010571, BISH 1010573, BISH 1010574, BISH 1010575).
Caum (1930, pp. 17–18, fig.); Roe (1961, pp. 11–12, fig. 6); Wilson (1993, pp. 278–279); Herbst and Wagner (1999, p. 23).
The collection as a whole at BISH was indicated as the type, therefore we here designate BISH 1010572 as lectotype as this sheet has been presumed to be a holotype by previous workers and has the best material and any of the sheets.
This is the only member of Sabdariffa that is currently considered to be extinct (see Wood et al. 2019; Keir 2018b; The IUCN Red List of Threatened Species, see https://www.iucnredlist.org/).
Sabdariffa byrnesii (F.D.Wilson) McLay & R.L.Barrett, comb. nov.
(Fig. 11c, d.)
Hibiscus byrnesii F.D.Wilson, Austral. J. Bot. 22: 177, fig. 4, 9 (1974). Type: Australia: Northern Territory: 57 miles [~91.7 km] E of Pine Creek, 20 Feb. 1969, N.B.Byrnes 1390 (holo: DNA A0014914; iso: ASU 0019352, ASU 0019353, ASU 0019354, NT A0014914, US 00098075).
Wilson (1974, p. 177, fig. 4, 9); Cowie et al. (2013, p. 13, fig. 4); Wilson (2022).
Sabdariffa cannabina (L.) M.M.Hanes & R.L.Barrett, comb. nov.
Hibiscus cannabinus L., Syst. Nat. ed. 10, 2: 1149 (1759). Ketmia glandulosa Moench, Suppl. Meth. 202 (1802), nom. illeg.; Furcaria cannabina (L.) Ulbr. in H.G.A.Engler & O.Drude, Veg. Erde 9 (Pflanzenw. Afrikas); 3(2): 400 (1921); Hibiscus sabdariffa subsp. cannabinus (L.) G.Panigrahi & S.K.Murti, Fl. Bilaspur Dist. 1: 127 (1989). Type: ‘Alcea Bengalensis spinosissima’ in C.Commelijn, Hort. Med. Amstelod. Pl. Rar., 1: 35, t. 18 (1697) (neo: [BHL]), designated by D.O.Wijnands, The Botany of the Commelins 144 (1983) (as ‘lectotype’)).
Hibiscus vitifolius Mill., Gard. Dict., 8th edn, n. 8 (1768), nom. illeg., non L. (1753). Type citation: ‘Ketmia Indica vitis folio, magno flore. Tourn. Inft. R. H. 100.’
?Hibiscus tripartitus Forssk., Fl. Aegypt.-Arab. 126 (1775), non Kuntze (1891); Hibiscus cannabinus var. tripartitus (Forssk.) Chiov., Boll. Soc. Bot. Ital. 1923: 115 (1923). Type: s. loc., s. dat., P.Forsskål s.n. (?holo: LD 1755565).
Hibiscus congener Schumach. & Thonn. in C.F.Schumacher, Beskr. Guin. Pl. 319 (1827). Abelmoschus congener (Schumach. & Thonn.) Walp., Repert. Bot. Syst. 1(2): 308 (1842). Type: Ghana: Volta Region, Keta Lagoon, s. dat., P.Thonning 130 (syn: C 10003981, C 10003982, C 10003983).
Hibiscus obtusatus Schumach. & Thonn. in C.F.Schumacher, Beskr. Guin. Pl. 321 (1827). Type: Ghana: southern part of the country, s. dat., P.Thonning s.n. (syn: C 10003984, C 10003985).
Hibiscus wightianus Wall., Numer. List: n.° 2695A (1831), nom. inval., nom. nud.
Hibiscus cannabinus var. genuinus Hochr., Annuaire Conserv. Jard. Bot. Genève 4: 115 (1900), nom inval.
?Hibiscus cannabinus var. sudanicus Hochr., Annuaire Conserv. Jard. Bot. Genève 10: 20 (1906). Type: Chad: Africa Central, territoire de Chari, Ndelle, s. dat., A.J.B.Chevalier 6914 (syn: G, P, both n.v.).
Hibiscus cannabinus var. simplex A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 16, pl. 2 (left) (1911). Type: ‘Hibiscus cannabinus var. simplex’ in A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 16, pl. 2 (1911) (left-hand illustration).
Hibiscus cannabinus var. purpureus A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 17, pl. 3 (right) (1911). Type: ‘Hibiscus cannabinus var. purpureus’ in A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 16, pl. 3 (1911) (right-hand illustration).
Hibiscus cannabinus var. ruber A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 17, pl. 3 (centre) (1911). Type: ‘Hibiscus cannabinus var. ruber’ in A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 16, pl. 3 (1911) (centre illustration).
Hibiscus cannabinus var. viridis A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 17, pl. 2 (right) (1911). Type: ‘Hibiscus cannabinus var. viridis’ in A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 16, pl. 2 (1911) (right-hand illustration).
Hibiscus cannabinus var. vulgaris A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 17, pl. 3 (left) (1911). Type: ‘Hibiscus cannabinus var. vulgaris’ in A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 16, pl. 3 (1911) (left-hand illustration).
Hibiscus henriquesii Pires de Lima, Brotéria, Sér. Bot. 19: 138 (1921). Type citation: ‘Colhi exemplares floridos e fructificados, nos campos incultos dos arredores de Palma, em 14 de Agosto de 1916. [A. Pires de Lima] No. 24.’ Type: n.v.
Roxburgh (1832, p. 208); Harvey (1860, p. 176); Dalzell and Gibson (1861, p. 20); Masters (1868, p. 204); Trimen (1893, p. 154); Hochreutiner (1900, p. 114); Cooke (1901, p. 109); Prain (1903, p. 267); Baker (1911, p. 27); Ulbrich (1921, p. 400, fig. 188); Popova (1928, p. 493); Andrews (1952, p. 28, fig. 11); Hochreutiner (1955, p. 37, fig. X, 4, 5); Macbride (1956, pp. 470–471); León and Alain (1957, p. 256); von Cufodontis (1959, p. 558); Exell (1961, p. 441); Hauman (1963, pp. 108–110); Bates (1965a; 79, fig. D); Borssum Waalkes (1966, pp. 63–64); Merxmüller (1969, p. 82:14, 17); Adams (1972, p. 476); Gibson (1975, pl. 65, fig. 3); Liogier (1981, pp. 91–93); Blundell (1987, p. 76); Paul and Nayar (1988, pp. 127–128); Fryxell (1989, pp. 222–223); Panigrahi and Murti (1989, p. 127); Edmonds (1991, p. 15, fig. 1(1), 2(15, 16)); Paul (1993, pp. 324–325); Vollesen (1995, p. 198); Sivarajan and Pradeep (1996, pp. 104–106, fig. 33); Philcox (1997, pp. 295–296); Thulin (1999a, p. 44, fig. 25a–f); Wilson (1999, pp. 66–67); Krapovickas and Fryxell (2004, pp. 51–52); Heath and Heath (2009, p. 282, fig.); Mwachala (2009, pp. 41–42); Fayaz (2011, p. 508, fig.); Liu et al. (2013, p. 16, fig. 1–3); Amany et al. (2020, pp. 125–126); Hyde et al. (2024, fig.).
Linnaeus (1759) is not known to have examined a specimen of this widely cultivated species and rather appears to have relied entirely on early literature, hence Wijnands (1983, p. 144) designated an illustration in Commelin (1697) as the ‘lectotype’ of this name (that is to be corrected to neotype as the illustration was not explicitly cited by Linneaus). Whereas the choice is entirely consistent with the protologue and in our opinion entirely appropriate, the neotypification has left lingering doubts about the application of the name, especially as the diagnostic woolly tomentum on the calyx is not evident in this figure. Wijnands (1983, p. 144) suggests that the woolly tomentum is represented by white patches on a coloured original of the figure, however we have now examined images of S. radiata from eastern India with white stripes on the calyx that better match this representation.
We are now convinced that the plant illustrated represents our concept of Sabdariffa radiata, based on the appearance of the stem aculei, the distinct aculei on the leaf petioles, the upper leaves being deeply divided (not simple), the shape of the leaf margins and the white but not woolly calyx. The floral features are ambiguous as presented, especially the representation of the epicalyx, matching neither S radiata nor S. cannabina as we define these, and most likely represent artistic licence (or the forks on the epicalyx may have been entirely overlooked). Although there is extensive historical confusion between the two taxa, we consider the application of this name to the taxon here recognised as S. radiata to be too disruptive, therefore we are preparing a proposal to conserve the name Hibiscus cannabinus with a conserved type to maintain current usage of both names.
There is confusion in the literature regarding the application of the name Hibiscus tripartitus, as different authors either ascribe this to Sabdariffa cannabina (e.g. Wilson 2006; POWO, see https://powo.science.kew.org/) or Fioria vitifolia (L.) Mattei (e.g. El-Hadidi et al. 1999). Forsskål and Niebuhr (1775) cite no specimens and do not refer to any prior literature references, therefore the name is assumed to be based entirely on Forsskål’s own collections (see Hepper and Friis 1994, p. 197). We have only located a single specimen referable to this name, meaning that this is potentially the holotype though further duplicates may be extant. The specimen is in mature fruit, consistent with the protologue and fits the current concept of S. cannabina sens. lat., though this complex requires further review.
Howard and Howard (1911) did not list any specimens for their new varieties in Hibiscus cannabinus and as such the only unambiguous original material we can identify at this stage constitutes the plates published with the protologues. Borssum Waalkes (1966, p. 64) notes that a number of the Howard and Howard (1911) varieties were cultivated at Bogor, with specimens held at BO and L, therefore these may serve as epitypes if the illustrations are insufficient for identification. Wilson (1999, p. 65) considered the varietal names of Howard and Howard (1911) to be cultivar names. However, Howard and Howard (1911, p. 16) state that there are ‘five varieties comprising eight agricultural types’, demonstrating that the varieties were considered to be more than agricultural selections and the names are here considered to fall under the International code of nomenclature for algae, fungi and plants (Turland et al. 2018) rather than the International code of nomenclature for cultivated plants (see https://www.ishs.org/scripta-horticulturae/international-code-nomenclature-cultivated-plants-ninth-edition).
Whether Hibiscus tripartitus, described by Forsskål and Niebuhr (1775), is actually a synonym of S. cannabina is questionalble and the affinity to S. verrucosa should also be considered.
Schumacher and Thonner (in Schumacher 1827) described two additional species from Ghana, H. congener and H. obtusatus but these are considered to be potentially wild forms of this widely cultivated species.
We were unable to locate a type specimen for Hibiscus cannabinus var. sudanicus therefore this name is only tentatively placed here. Wilson (1999, p. 49) examined material at G and P, and considered the type specimens to represent a hybrid between S. cannabina and S. acetosella.
Hibiscus cannabinus var. simplex was recognised by Hauman (1963, pp. 109–110), including var. viridis as a synonym.
We were unable to locate a type specimen for Hibiscus henriquesii (Pires de Lima 1921), therefore application of this name requires confirmation, though the description and type location are consistent with the placement here.
Native to Africa and Arabia, in Angola, Bénin, Botswana, Burkina Faso, Burundi, Cameroon, Central African Republic, Chad, Democratic Republic of The Congo, Eritrea, Ethiopia, The Gambia, Ghana, Guinea, Guinea-Bissau, Ivory Coast, Kenya, Malawi, Mali, Mauritania, Mozambique, Namibia, Niger, Nigeria, Rwanda, Senegal, Sierra Leone, Somalia, South Africa, Sudan, Swaziland, Tanzania, Togo, Uganda, Yemen, Zambia and Zimbabwe.
Introduced and commonly naturalised to Afghanistan, Bangladesh, SE Brazil, Cape Verde, China, Cuba, Dominican Republic, Guatemala, Haiti, Honduras, Hungary, India, Iran, Iraq, Jamaica, Kazakhstan, Kriti, Laos, Leeward Islands, Nepal, Niue, Pakistan, Perú, Puerto Rico, Russia, Sri Lanka, Thailand, Trinidad and Tobago, Turkmenistan, Ukraine, Uzbekistan, Venezuela, Vietnam and the Windward Islands.
Sabdariffa capitalensis (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
(Fig. 12a, b.)
Hibiscus capitalensis Krapov. & Fryxell, Bonplandia 13(1–4): 96–98, fig. 18 (2004). Type: Brazil: Distrito Federal, ~5 km E of Lagoa Paranoa, 25 Nov. 1966, H.S.Irwin, J.W.Grear, R.Souza & R.Reis dos Santos 13146 (holo: UB 199469!; iso: CTES 0001526!, F 0076510F!, NY 00007090!, NY 00021179!, US 00517164!).
Krapovickas and Fryxell (2004, pp. 96–98, fig. 18); Coutinho and Fernandes-Júnior (2024).
Sabdariffa ceratophora (Thulin) Mwachala & R.L.Barrett, comb. nov.
Hibiscus ceratophorus Thulin, Nordic J. Bot. 19: 337, fig. 1 (1999). Type: Somalia: Shabeellaha Dhexe Region, 16 km N of Mogadishu along road to Balcad, 150 m, 15 May 1990, M.Thulin, M.Hedren & A.Dahir 7465 (holo: UPS V-051385; iso: FT, K 000240708).
Hibiscus furcatus var. microcarpus Mattei, Boll. Reale Orto Bot. Palermo 7: 103 (1908). Type: Somalia: Giumbo in reg. Goscia, plant grown from seeds from Macaluso 55, s. dat. (lecto, designated by M.Thulin, Nordic J. Bot. 19: 337 (1999): PAL)
Mattei (1908, p. 103); Thulin (1999a, p. 337, fig. 1); Thulin (1999b, p. 46, fig. 26).
Sabdariffa chancoae (Krapov. & Fryxell) M.C.Duarte & R.L.Barrett, comb. nov.
Hibiscus chancoae Krapov. & Fryxell, Bonplandia 13(1–4): 58, fig. 9 (2004). Type: Perú: San Martín, La Morada, margen del rio Huallaga, 430 m, 23 July 1981, M.Chanco 564 (holo: CTES 0001528; iso: USM).
Krapovickas and Fryxell (2004, p. 58, fig. 6, 9).
Sabdariffa chapadensis (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus chapadensis Krapov. & Fryxell, Bonplandia 13(1–4): 83, fig. 11 (2004). Type: Brazil: Goiás, Chapada dos Veadeiros, ~40 km N of Alto do Paraiso, 1250 m, 24 Mar. 1971, H.S.Irwin, R.M.Harley & G.L.Smith 33113 (holo: UB 00037476!; iso: CAS 0002889!, CTES 0001534!, K 000381197!, MO 1515259!, NY 00689103!, RB 00576929!, SPF 00184546!, TEX 00208120!, US 00955897!).
Krapovickas and Fryxell (2004, p. 83, fig. 11); Coutinho and Fernandes-Júnior (2024).
Sabdariffa commixta (Fryxell & Krapov.) M.C.Duarte & Yoshikawa, comb. nov.
(Fig. 12c, d.)
Hibiscus commixtus Fryxell & Krapov., Novon 14: 61, fig. 3 (2004). Type: Bolivia: La Paz: prov. Iturralde, Luisita, 180 m, Sabana luimeda, W del rio Beni, 29 Feb. 1984, S.G.Beck & R.Haase 10120 (holo: LPB 0000732!; iso: CTES 0001536!, M 0274854!, US 00731457!).
Fryxell and Krapovickas (2004, p. 61); Krapovickas and Fryxell (2004, pp. 76–77).
Sabdariffa conceptionis (Fryxell & Krapov.) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus conceptionis Fryxell & Krapov., Novon 14: 61, fig. 4 (2004). Type: Bolivia: Santa Cruz: prov. Ñuflo de Chavez, Estancia Novicia, ~30 km S de Concepción, 500 m, 1 May 1977, A.Krapovickas & A.Schinini 32114 (holo: CTES 0001537!; iso: LPB 0000733!, NY 00007085!).
Fryxell and Krapovickas (2004, p. 61, fig. 4); Krapovickas and Fryxell (2004, pp. 103–104).
Sabdariffa cordofana (Turcz.) Mwachala & R.L.Barrett, comb. nov.
(Fig. 12e, f.)
Hibiscus cordofanus Turcz., Bull. Soc. Nat. Mosc. 31: 193 (1858). Type: Sudan: Cordofan [Kordofan], 1837–1838, C.G.T.Kotschy 65 (holo: KW 001000983).
Hibiscus verrucosus var. punctatus A.Rich., Tent. Fl. Abyss. 1: 59 (1847); Hibiscus cannabinus var. punctatus (A.Rich.) Hochr., Annuaire Conserv. Jard. Bot. Genève 20: 82 (1917); Hibiscus asper var. punctatus (A.Rich.) Berhaut, Fl. Sénégal 160 (1954). Type citation: ‘Crescit in provincia Chiré (Ant. Petit), et in montibus prope Tchélatchékanné, non procul a convalle fluvii Taccazé, mense Novembre fructus maturos gerens (Schimper).’ Type: Ethiopia: Vallis Taccaze near Djeladjekanne, 29 Sep. 1840, G.W.Schimper 24 (syn: TUB 002650); Ethiopia: in montibus propre Dscheladscheranne, 3 Nov. 1839, G.W.Schimper 717 (syn: BR 0000008403360, TUB 002645, TUB 002646).
Turczaninow (1858, p. 193); Andrews (1952, p. 28); von Cufodontis (1959, p. 557); Wilson (1999, p. 68, p.p., as H. asper).
Hibiscus verrucosus var. punctatus (Richard 1847, p. 59) is here considered to be a synonym of S. cordofana. Application of the name Hibiscus tripartitus Forssk. (currently considered a synonym of S. cannabina) should also be considered in this regard as this might lack a woolly tomentum on the calyx, though the maturity of the type (in fruit) makes this difficult to assess. If H. tripartitus is found to be the same as S. cordofana, H. tripartitus would have priority but on current evidence we retain H. tripartitus under S. cannabina.
Sabdariffa cordofana is allied to S. verrucosa in having epicalyx lobes with distinct marginal ribs when dry but appears to differ consistently from S. verrucosa with climax leaves that are 3–5-lobed for at least half the length (v. variously entire, shallowly lobed or possibly deeply lobed when the plants mature), small (v. large), scattered aculei on the stems, and the epicalyx and calyx glabrous except for large, prominent aculei on the margins (v. epicalyx and calyx with a few stiff hairs and long, very prominent aculeli on margins and ribs).
Some specimens from Sudan (e.g. H.Gillett 77; P 00925200) and Cameroon (e.g. E. de Garine EG925; P 01080937) approach S. aspera in having deeply divided climax leaves but differ markedly in the calyx characters and our present concepts likely still represent an oversimplification of variation in this species complex across tropical Africa
Sabdariffa costata (A.Rich.) M.M.Hanes & R.L.Barrett, comb. nov.
(Fig. 13a, b.)
Hibiscus costatus A.Rich. in R. de la Sagra, Hist. Phys. Cuba, Pl. Vasc. 10(2): 138–139, t. 15 (1845 [1841]). Type: Cuba: ‘Crescit in Vuelta de Abajo’, R.de la Sagra s.n. (lecto, designated by F.Areces Berazaín, Revista Jard. Bot. Nac. Univ. Habana 25/26: 27–28 (2006): P 02285891; isolecto: K 000199693, LIL 000829, P 02285892, P 02285893, US 00098082).
Hibiscus australis J.D.Smith, Enum. Pl. Guatem. 6: 4 (1903), nom. inval., nom. nud.
Hibiscus costatus var. glabellus R.E.Fr., Kongl. Svenska Vetensk. Acad. Handl., ser. 3, 24(2): 30 (1947). Type: Belize: British Honduras, Honey Camp, Coastal Region, 11 Feb. 1929, C.L.Lundell 662 (holo: S 12-17671).
Richard (1845, pp. 138–139); Fries (1947, p. 30); León and Alain (1957, pp. 253–254, fig. 108); Menzel et al. (1983a, p. 207, fig.); Fryxell (1988, p. 205); Edmonds (1991, fig. 1(12); 2(26)); Fryxell (1992b, pp. 103–104, fig. 11 [fig. on p. 85 due to a printing error]); Fryxell (2000, p. 12); Areces Berazaín (2006, pp. 27–28, fig. 1a, 2b, c, 3b, 4b, g).
Sabdariffa cuanzensis (Exell & Mendonça) Mwachala & R.L.Barrett, comb. nov.
Hibiscus cuanzensis Exell & Mendonça in Exell, J. Bot. 74: 136 (1936). Type citation: ‘Hab. Angola: Cuanza Norte, Pungo Adongo, near Cazella, fl. & fr. April, Welwitsch 5241 (Typus in Herb. Mus, Brit.); Calunda Mangue, Welwitsch 5241 (Herb. Mus, Brit.).’ Type: Angola: Cuanza Norte, district Pungo Adongo, near Cazella, Apr. 1857, F.M.J.Welwitsch 5241 (holo: BM 000645511; iso: LISU 206156, MEL 2472896; probable iso: LISU 206155, PRE 0282640-0; possible iso: COI 00005055, G 00014195, K 000240668, P 06655943).
Exell (1936, p. 136); Exell and Mendonça (1937, p. 167); Wilson (1999, p. 71, fig. 3a); Leistner (2008, p. 114).
Collections made by Welwitsch in Angola were grouped under species numbers and variously dispersed (see Albuquerque et al. 2009). In this case, two collections were clearly cited in the protologue of Hibiscus cuanzensis, both assigned the species number ‘Welwitsch 5241’. However, Exell and Mendonça (in Exell 1936, p. 136) were explicit that the collection from ‘near Cazella’ was the ‘Typus in Herb. Mus. Bot.’ and as there is only a single collection at BM matching these details, we accept this as a holotype despite the existence of uncited isotypes. This was also the conclusion of Exell and Mendonça (1937, p. 167) and Wilson (1999, p. 71). Two collections with a derived label ‘Distr. Pungo Andongo’ are likely isotypes but given the aggregation of collections by Welwitsch, we cannot be certain of this. Four specimens simply labelled ‘Iter Angolense’ cannot be reliably assigned to a location, therefore these are listed above as possible isotypes. The specimens from ‘Calunda Mangue’, while constituting original material, are not type specimens.
Sabdariffa cucurbitacea (A.St.-Hil.) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus cucurbitaceus A.St.-Hil., Fl. Bras, Merid. 4th edn, 1(7): 244 (1828); Abelmoschus cucurbitaceus (A.St.-Hil.) Walp., Repert. Bot. Syst. 1: 310 (1842). Type citation: ‘Crescit in parte occidentali desertaque provinciae Minas Geraes, propé Riachara, Corrego do Mathias. Florebat Septembri.’ Type: Brazil: [posiblemente faz. Rancharía, cerca de Coração de Jesus, IX- 1817]. Corgo, do Mathias, p. 612, A.St.-Hilaire Catal. B1 n° 1931 (lecto, here designated: P 02285896!; isolecto: B [photo at F], F 0BN009517!, G, p.p., MPU 016509!, P 02285897!, P 02285898!).
Hibiscus cucurbitaceus var. genuinus Hochr., Ann. Cons. Jard. Bot. Genève 4: 119 (1900), nom. inval.
Hibiscus paulae Krapov., Bonplandia 22(2): 137–139, fig. 1 (2013). Type: Brazil: Minas Gerais, Joaquim Felicio: Serra do Cabral, ~5.9 km da cidade, 13 Nov. 2007, J.Paula-Souza, M.S.Ferrucci & J.G.Rando 9415 (holo: SPF 00195947!; iso: CTES 71537!).
Krapovickas and Fryxell (2004, pp. 81–82); Krapovickas (2013, pp. 137–139, fig. 1); Rigueiral et al. (2019, pp. 6–7, fig. 1d–f); Coutinho and Fernandes-Júnior (2024).
Sabdariffa cummingii (Wannan) R.L.Barrett & McLay, comb. nov.
(Fig. 13c, d.)
Hibiscus cummingii Wannan, Austrobaileya. 14: 28–32, fig. 1–6, 7E, F (2024). Type: Australia: Queensland: North Kennedy District: Hervey Range, near Townsville, 27 Oct. 2010, B.S.Wannan 7198 (holo [mounted on 2 sheets]: BRI AQ1046838).
Wannan (2024, fig. 1–6, 7E, F).
Sabdariffa divaricata (Graham) McLay & R.L.Barrett, comb. nov.
(Fig. 14a, b.)
Hibiscus divaricatus Graham, Edinb. N. Phil. J. 9: 367–8 (1830); Abelmoschus divaricatus (Graham) Walp., Rep. Bot. Syst. 1: 309 (1842). Type citation: ‘…was raised in spring 1829 at the Royal Botanic Garden, from seeds received from New Holland by Mr Goodsir…’. Type: Australia: Queensland: Shoalwater Bay, R.Brown [Iter Austral. No. 5124] (neo, designated by F.D.Wilson, Austral. J. Bot. 22: 170 (1974): BM 013824568).
Hibiscus magnificus F.Muell., Fragm. 2(15): 118–119 (1861). Type citation: ‘In montibus Newcastle Range; ad fluvia Mackenzie et Dawson.’ Type: Australia: Queensland: Dawson River, [1857], F.Mueller s.n. (syn: MEL 18706!); Queensland: Burdekin, 1857, F.Mueller s.n. (K 000659813 (ex MEL)); Newcastle Range, [1857], F.Mueller s.n. (MEL 18707!).
Hibiscus heterophyllus var. flaviflorus F.Muell., Fragm. 6: 170 (1868) (as ‘flaviflorae’). Type citation: ‘haec ad flumen Fitzroy-River nascitur.’ Type: Australia: New South Wales: Marrlaay [Macleay] River, s. dat., H.Beckler s.n. (lecto, here designated: MEL 18721!). Residual syn: Queensland: Herbert River, 16 May 1864, leg. ign. s.n.; (MEL 222502!); Port Denison, s. dat., E.F.A.Fitzlan s.n. (syn: MEL 18725!).
Hibiscus fitzgeraldii F.Muell., Fragm. 8: 242 (1874). Type citation: ‘Ad flumen Bowenii. Species eximie decora, a cl. R. Fitzgerald culta.’ Type: Australia: Queensland: Bowen River, [c. Dec. 1872], R.D.Fitzgerald s.n. (syn: MEL 18670!, MEL 18671!, MEL 18672! (fragm.)).
Hibiscus divaricatus var. luteus Hochr., Annuaire Cons. Jard. Bot. Genève 4: 99 (1900); Hibiscus heterophyllus subsp. luteus (Hochr.) F.D.Wilson, Austral. J. Bot. 22(1): 170, fig. 14 (1974). Type: Australia: Queensland: Rockhampton, 1877, P.A.O’Shanesy s.n. (ex herb. F.Mueller) (syn: G 00353254, G 00353255).
Hibiscus radiatus var. luteus F.Muell. ex Hochr., Annuaire Cons. Jard. Bot. Genève 4: 99 (1900), nom. inval., pro syn.
Hibiscus divaricatus var. genuinus Hochr., Ann. Cons. Jard. Bot. Genève 4: 121 (1900), nom. inval.
Graham (1830a, pp. 367–368); Mueller (1861, pp. 118–119); Bentham (1863, p. 212); Mueller (1868, p. 170, 1874, p. 242); Bailey (1899, p. 126); Wilson (1974, pp. 170–171, fig. 15); Cooper and Cooper (2004, p. 277, fig.); Wilson (2022); Wannan (2024, 7C, D).
The neotype of Hibiscus divaricatus selected by Wilson (1974, p. 170) is in serious conflict with the protologue that clearly refers to a different species, H. diversifolius, therefore we here reject this designation as inappropriate. We refrain from designating a new neotype pending further study and investigation as to whether original material may be extant. This species has also become popular in local horticulture in eastern Australia, therefore proposing a conserved type that maintains the current usage of the name may be advisable, despite the historical nomenclatural confusion dating back at least to Bentham (1863).
For Hibiscus magnificus F.Muell., we refrain from designating a lectotype pending further study. Mueller described flowers but none are present on the two sheets remaining at MEL. A sheet at K from Burdekin is highly likely to be original material and the source of the floral descriptions but that location is not cited in the protologue. Additional syntypes may possibly be present at K that may be more appropriate choices as a lectotype.
There is no material at MEL annotated with the name Hibiscus heterophyllus var. flaviflorus, however there are four sheets annotated by Mueller as varieties, one as ‘var. hypoglauca’ (MEL 2222514) and three as ‘var. lutea’. We conclude that Mueller (1868, p. 170) changed the epithet from ‘lutea’ to ‘flaviforae’, given that the meaning is essentially the same and recognise the three relevant sheets as syntypes of Hibiscus heterophyllus var. flaviflorus. We here choose the only specimen with flowers present as lectotype of this name. We also note that Hochreutiner (1900, p. 99) later validated Mueller’s original variety name Hibiscus divaricatus var. luteus but the two names are treated entirely independently, in part because the identification ‘Hibiscus radiatus var. luteus’ is only annotated on a specimen collected after the publication of Mueller’s epithet ‘flaviflorae’.
There is another collection of Hibiscus divaricatus var. luteus at MEL (Rockhampton, 2 Jan. 1876, P.A.O’Shanesy 1580, MEL 0046761!) that was considered as a possible syntype but the date on the label differs and the collections are not morphologically congruent, therefore we do not consider the MEL sheet to be original material and no duplicate material appears to have been retained at MEL.
A number of synonyms listed above were previously considered to be yellow-flowered forms of S. heterophylla but we prefer to place these under S. divaricata pending further study of this species complex.
(a, b) Sabdariffa divaricata. Photographs by: Russell Barrett, Sydney. (c, d) Sabdariffa diversifolia subsp. diversifolia. Photographs by Thomas Mesaglio, iNaturalist: 176035563, CC BY. (e, f) Sabdariffa diversifolia subsp. agioxillos. Photographs by Fabrício Mil Homens Riella, iNaturalist: 200507731, CC BY.
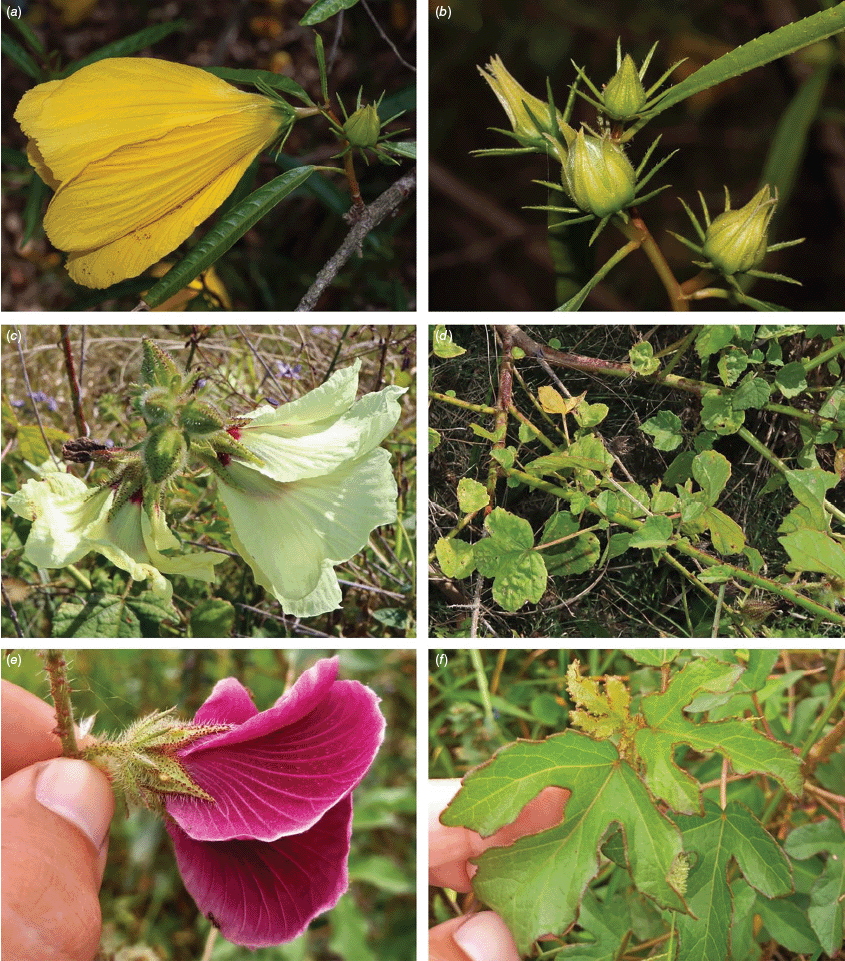
Sabdariffa diversifolia (Jacq.) McLay & R.L.Barrett, comb. nov.
Hibiscus diversifolius Jacq., Obs. Bot. Coll. 2: 307 (1788 [Apr. 1789]); Furcaria diversifolia (Jacq.) Ulbr. in Engler, Pflanzenw. Afrikas 3(2): 402 (1921). Type citation: ‘Speciosa planta communicata mecum suit sub titulo Hibisci ficulnei Commersoni ex India orientali’. Type: ‘Hibiscus diversifolius’ in N.J. von Jacquin, Icon. Pl. Rar. 3: t. 551, 1792, Hb. Jacq. [BHL] (neo designated by P.A.Fryxell, Malvaceae of Mexico 288, 1988).
Hibiscus scaber Lam., Encycl. 3: 350 ([19 Oct.] 1789). Type citation: ‘croit a 1’ Isle de France, & est cultivée depuis long temps au Jardin du Roi’. Type: Îsle de France [Mauritius], s. dat., P.Commerson s.n. (lecto, here designated: P 00287576; isolecto: P 00287575).
Hibiscus biflorus A.Spreng., Tent. Suppl. 19 (1828). Type citation: ‘Uitenhagen C. B. S. Zeyher. (No.241.) V. f.’ Type: South Africa: Uitenhage [Kariega], 1822–1828, C.L.P.Zeyher 241 (lecto, here designated: HAL 0118176. Residual syn: sketch of type at BM (BM 013832976)).
Hibiscus macularis Harv. in W.H.Harvey & O.W.Sonder (eds), Fl. Cap. 1: 171 (1860), nom. inval., nom. nud.
Hibiscus beckleri F.Muell., Fragm. 2: 117–118 (1861). Type citation: ‘In silvis ad fluvium Clarence. Dr. Beckler.’ Type: Australia: New South Wales: Clarence River, H.Beckler s.n. (holo: K 000659866 (ex MEL)).
Hibiscus decaisneanus Hochr., Annuaire Conserv. Jard. Bot. Genève 4: 119 (1900), nom. inval., pro syn.
Hibiscus diversifolius subsp. genuinus Hochr., Annuaire Conserv. Jard. Bot. Genève 4: 119 (1900), nom. inval.
Hibiscus paludosus Merr., Philipp. J. Sci. 3: 151 (1908). Type: Philippines: Mindanao, Lake Lanao, Camp Keithley, July 1907, M.S.Clemens s.n. [874] (syn: BO, G, L 0012950, PH (destroyed), US 00810614).
The sheet selected as lectotype of Hibiscus scaber includes the location details cited in the protologue (de Lamarck and Poiret 1789, p. 307) and the best material. The second sheet is presumed to be a duplicate and here accepted as an isolectotype.
A sketch held at BM has sometimes been referred to as the type of Hibiscus biflorus, presumably because no specimen was located previously, but there is a sheet at HAL and this is here designated as lectotype. No herbarium was cited by Sprengel (1828) but a specimen is stated to have been seen.
POWO (see https://powo.science.kew.org/) lists ‘Hibiscus spicatus Cav., Diss. 3, Tertia Diss. Bot. 163, t. 59, fig. 1 (1787). Type citation: ‘V. S. unicum exemplar apud D. de Jussieu.’ (holo: P-JU; iso: MA 475812)’ as a synonym of H. diversifolius. However, Fuertes and Fryxell (1993, p. 661) determine this specimen as probably being an Alcea species and therefore not a member of the Hibisceae.
Sabdariffa diversifolia (Jacq.) McLay & R.L.Barrett subsp. diversifolia
(Fig. 14c, d.)
Von Jacquin (1789, p. 207, 1795, t. 551); Sprengel (1828, p. 19); Edwards and Ridgeway (1819, t. 381); Ecklon and Zeyher (1835, p. 38); Harvey (1860, p. 171); Mueller (1861, pp. 117–118); Bentham (1863, p. 213); Masters (1868, p. 198); Bailey (1899, p. 127); Hochreutiner (1900, p. 119); Merrill (1908, p. 151); Baker (1911, p. 27); Eyles (1916, p. 415); Ulbrich (1921, p. 402, fig. 187E–G); Exell and Mendonça (1937, p. 173); Garcia (1946, p. 40); Mendonça and Torre (1950, p. 14); Andrews (1952, p. 24, fig. 10); Brenan et al. (1953, p. 225); Hochreutiner (1955, p. 39, fig. 10(6–8)); Exell (1961, p. 443); Hauman (1961, p. 86); Hauman (1963, pp. 110–112); Wilson and Menzel (1964, p. 84, fig. 6, 7, 20, 21); Bates (1965a, p. 79, fig.); Borssum Waalkes (1966, pp. 65–66); Scarth-Johnson (1968, p. 245, fig.); Bates (1971, p. 678); Wilson (1974, p. 164); Maquet (1983, p. 383, fig. 121, 4); Marais and Friedmann (1987, p. 36, fig. 11 (5, 6)); Fryxell (1988, p. 208); Edmonds (1991, p. 17, fig. 1(6, 7); 2(20, 21)); Wilson (1993, pp. 281–282); Vollesen (1995, p. 196); Wilson and Craven (1995, p. 445); Wilson (1999, p. 63); Fryxell (2000, p. 16); Mitchell and Norris (2000, p. 329); Cooper and Cooper (2004, p. 277, fig.); Krapovickas and Fryxell (2004, pp. 65–66); Leistner (2008, p. 114); Mwachala (2009, pp. 45–46); Lejoly et al. (2010, p. 171); Coyne (2011, p. 160, pl.); Badry et al. (2015, pp. 39–45, fig. 3–6); Bredenkamp (2019, p. 1205); Amany et al. (2020, pp. 124–125); McLay (2022); Coutinho and Fernandes-Júnior (2024).
The native range of this species deserves further attention. The origins and native range are commonly accepted as African, occurring in Angola, Botswana, Burkina Faso, Burundi, Cameroon, Chad, Congo, Democratic Republic of The Congo, Egypt, Ethiopia, Gabon, Guinea, Kenya, Liberia, Madagascar, Malawi, Mozambique, Niger, Rwanda, Senegal, South Africa, Sudan, Tanzania, Uganda and Zambia.
POWO (see https://powo.science.kew.org/) considers this subspecies to be introduced in many other regions of the world but we accept this as also native to Asia, Australia, Brazil, the Cook Islands, Costa Rica, Fiji, Galápagos, Mauritius, Mexican Pacific Islands, Mexico, New Caledonia, New Guinea, New Zealand (North Island), Niue, the Philippines, Saint Helena, Tubuai Islands and Vanuatu.
Several lines of evidence support the natural occurrence of S. diversifolia subsp. diversifolia, especially in the Pacific. A primary argument is that the Hawaiian S. brackenridgei is purported to be derived from S. diversifolia (Wilson 1993) and S. brackenridgei is placed as sister to S. diversifolia in our phylogeny (Fig. 6), albeit with very limited sampling. Sabdariffa brackenridgei is clearly in the ‘African’ clade, indicating dispersal at some point. If S. diversifolia is the ancestral origin of S. brackenridgei that has clearly diverged significantly in morphology since arriving in Hawai’i, there was likely a source population of S. diversifolia (or a progenitor) somewhere around the Pacific early enough for dispersal and divergence to occur. The French Polynesian species Sabdariffa australensis is likely to also have been derived from S. diversifolia and the affinity of S. kitaibelifolia should also be investigated.
Specific evidence for the natural occurrence of S. diversifolia in the Pacific comes from fossil pollen discovered in the Galápagos that significantly pre-dates human arrival in these islands (van Leeuwen et al. 2008). Fossil Hibiscus-type pollen is also known from pre-settlement deposits in New Zealand, though this may represent S. diversifolia or Hibiscus trionum (sens. lat.) (Newnham and Lowe 1991; de Lange et al. 2018). We conclude that this is a native species in most countries and islands listed as introduced locations by POWO (see https://powo.science.kew.org/) but some of these, especially Mexico and Brazil, may require more detailed studies to assess the status as a native or adventive species. This species is suggested to be an introduction on Norfolk Island by Polynesian settlers (MacPhail et al. 2001) but even that remains uncertain and a global review is justified. This species is listed for Hawai’i (USA) but is considered to be only in cultivation there.
Sabdariffa diversifolia subsp. agioxillos (Vell.) Mwachala & R.L.Barrett, comb. nov.
(Fig. 14e, f.)
Hibiscus agioxillos Vell., Fl. Flumin. 283 (1825 [1829]). Type citation: ‘(Tab. 35.a T. 7) … Habitat locis supra-citatis.’ Type: ‘Hibiscus agioxillos’ in J.V. de M. Vellozo, Fl. Flumin. 7, tab. 35 [BHL] (lecto: original parchment Plate of Florae Fluminensis in the Manuscript Section of the Biblioteca Nacional do Rio de Janeiro [catalogue number mss1198656_039], later published in J.V. de M.Vellozo, Fl. Flumin. 7: t. 35 (1831)), designated by Coutinho et al., Brittonia 77(1) (2025).
Hibiscus spinulosus Huber, Cat. Gén. 1871 & 1872: 4 (1871), non (W.V.Fitzg.) F.D.Wilson (1974). Type: not designated (see D.J.Mabberley, Taxon 34: 450 (1985).
Hibiscus rivularis Bremek. & Oberm., Ann. Transvaal Mus. 16(3): 424 (1935); Hibiscus diversifolius subsp. rivularis (Bremek. & Oberm.) Exell, Fl. Zambes. 1(2): 444 (1961), nom. superfl. Type: Bechuanaland Prot. [Botswana]: Chobe River, Kabulabula, July 1930, G. van Son s.n., Herb. Transv. Mus. 28936 (lecto, designated by Coutinho et al., Brittonia 77(1) (2025): PRE 0457138-0; iso: PRE 0282642-0BM 000645532, F 0062909F).
Huber (1871, p. 4); Exell (1961, p. 444); Edmonds (1991, p. 17); Wilson (1999, p. 64, fig. 2); Krapovickas and Fryxell (2004, pp. 66–67); Mwachala (2009, p. 46); Badry et al. (2017, pp. 114–118, fig. 1–3); Rigueiral et al. (2019, pp. 8–9, fig. 1g–h); Coutinho and Fernandes-Júnior (2024, fig.); Hyde et al. (2024, fig.); Wannan (2024, 7G, H).
Although the illustration of Hibiscus agioxillos was published 2 years after the description (Vellozo 1831), this was cited in the protologue (Vellozo 1827), therefore Krapovickas and Fryxell (2004, p. 67) considered the illustration to be acceptable as original material and designated this as lectotype of the name. Coutinho et al. (2025) adopted a different view, accepting only the original parchment versions as original material and designated a replacement lectotype. The original artwork for this figure can be viewed through the website of the Biblioteca Nacional do Rio de Janeiro (http://bndigital.bn.br/acervodigital; see also Knapp et al. 2015 and Coutinho et al. 2025).
Although Mwachala (2009) cited the holotype of Hibiscus rivularis as being at BM, Bremekamp and Obermeyer (1935) explicitly stated that the type was held at PRE. As there are two sheets at PRE, Coutinho et al. (2025) designated PRE 0457138-0 as the lectotype based on the quality of the material on the sheet.
Exell (1961, p. 444) reduced Hibiscus rivularis Bremek. & Oberm. to a subspecies of H. diversifolius. However, the name Hibiscus agioxillos Vell. has priority but the application of Vellozo’s name was uncertain when H. rivularis was named by Bremekamp & Obermeyer (1935) and also when recombined by Exell (1961), therefore the priority was not recognised. We here agree with Krapovickas and Fryxell (2004, pp. 66–67) that H. agioxillos can reliably be applied to the taxon most commonly known as Hibiscus diversifolius subsp. rivularis (Bremek. & Oberm.) Exell. As this is the earliest available epithet for this taxon, we adopt this here.
Sabdariffa elongatifolia (Hochr.) Mwachala & R.L.Barrett, comb. nov.
Hibiscus elongatifolius Hochr., Annuaire Conserv. Jard. Bot. Genève 4: 117 (1900). Type: Cameroon: Sanaga, Urwaldgebiet, 1897, G.A.Zenker 1440 (lecto, here designated: P 00151947; isolecto: BM 013723930 (drawing BM 013832980), E 00934006, G 00014188, G 00014189, K 000240745, L 2366875, M 0109182, P 00151948, WAG 0002310).
Hochreutiner (1900, p. 117); Wilson (1999, p. 72, fig. 3g).
We here select the sheet at P with the best material matching the protologue as lectotype of Hibiscus elongatifolius.
Sabdariffa fabiana (Cheek) Mwachala & R.L.Barrett, comb. nov.
(Fig. 15a, b.)
Hibiscus fabiana Cheek, Blumea 65(1): 70 (2020). Type: Guinea: Guinée Forestière, Beyla Prefecture, Mt Kourandou, Sinko, Bronkedou, 1040 m, fl., 28 Oct. 2008, M.Cheek 13904, A.Traore, F.Fofana (holo: HNG; iso: BR 0000019384368 [ID as H. rosa-sinensis], K, MO, ?NHGC, P, WAG).
Cheek et al. (2020, p. 70).
(a, b) Sabdariffa fabiana. Photographs by Erik Simons, iNaturalist: 235727738, CC BY-NC. (c, d) Sabdariffa fallax. Photographs by Sarah Macdonald, iNaturalist: 38168182, CC BY-NC. (e, f) Sabdariffa forsteri. Photographs by (e) Andrew C. Mitchell, iNaturalist: 22404645, CC BY. (f) Jason Searle, iNaturalist: 174644472, CC BY-NC.
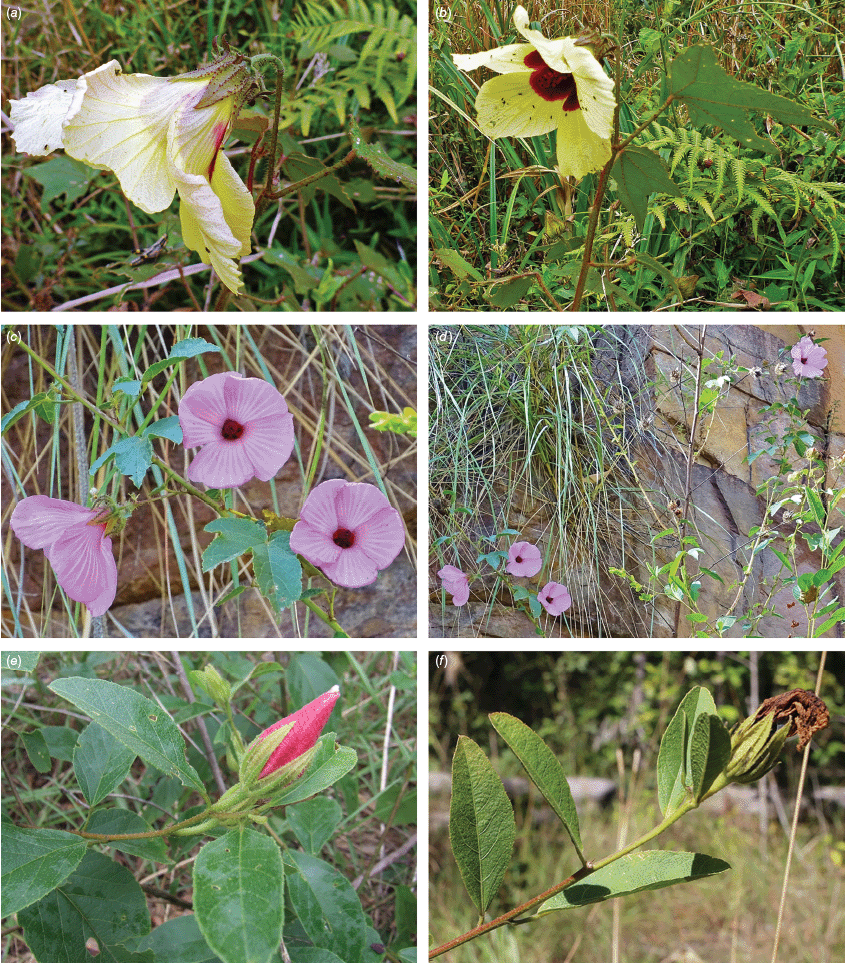
Sabdariffa fallax (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
(Fig. 15c, d.)
Hibiscus fallax Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 192, fig. 5 (2003). Type: Australia: Northern Territory: 6.5 km NE of El Sharana (on tributary of Koolpin Creek), 22 Apr. 1990, A.V.Slee & L.A.Craven 2745 (holo [mounted on 3 sheets]: CANB 397788.1, CANB 397788.2, CANB 397788.3; iso: ASU 0019329, ASU 0019357, ASU 0019358, ASU 0019359, DNA D0180185 (2 sheets), K 000659848, L, MEL 2172252, NY 00743696).
Craven et al. (2003, p. 192, fig. 5); Craven (2022).
Sabdariffa ferreirae (Fryxell & Krapov.) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus ferreirae Fryxell & Krapov., Novon 14: 65–66, fig. 5 (2004). Type: Brazil: Mato Grosso: Vila Bela, Posto Indigena Mamaindé, 25 km W de BR-174, km 449 (~82 km SF de Vilhena), 470 m, 21 May 1985, A.Krapovickas, J.F.Valls, C.Simpson & G.Silva 40148 (holo: CEN 00010487!; iso: CTES 0001540!, CTES 0001541!, K 000815891!, LIL 001383!, MBM 300353!, NY 00021183!, NY 00021187!, NY 00021188!, SI 002644!, SP 378361).
Fryxell and Krapovickas (2004, pp. 65–66, fig. 5); Krapovickas and Fryxell (2004, pp. 99–101, fig. 19); Coutinho and Fernandes-Júnior (2024).
Sabdariffa fijiensis (F.D.Wilson) M.M.Hanes & R.L.Barrett, comb. nov.
Hibiscus fijiensis F.D.Wilson, Brittonia 45: 282–284, fig. 3 (1993). Type: Fiji: Vanua Levu: Mathuata, Seanggangga Plateau, in drainage of Korovuli River, vicinity of Natua, 100–200 m, 25 Nov.–8 Dec. 1947, A.C.Smith 6748 (holo: US 00409515; iso: BISH 1003222, BRI AQ0057169, GH, P 00646013).
Skovsted (1944, p. 12, fig. 4); Wilson (1993, pp. 282–284, fig. 3).
Sabdariffa flagelliformis (A.St.-Hil.) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus flagelliformis A.St.-Hil., Fl. Bras. Merid. 4th edn, 1(7): 243–244 (1828). Type citation: ‘In campis herbidis prope praediolum dictum Paiol chemada, haud longe a monte vulgo Serra da Canastra, in provincia Minas Geraes.’ Type: Brazil: Provence de Minas Gerais, 1816, A.St.-Hilaire Catal. C1 n° 413 (lecto, here designated: P 02285908!; iso: MPU 016511!, P 02285909!, P 02285910!, US 00098086!).
Krapovickas and Fryxell (2004, p. 77); Rigueiral et al. (2019, p. 9, fig. 1i–j); Coutinho and Fernandes-Júnior (2024).
Sabdariffa flavorosea (Baker f.) Mwachala & R.L.Barrett, comb. nov.
Hibiscus flavoroseus Baker f., J. Bot. 77: 20 (1939) (as ‘flavo-roseus’). Type: Angola: Lunda: Saurimo, Vila Henrique de Carvalho, 27 Aug. 1932, R.G.N.Young 646 (holo: BM 000645519).
Baker (1939, p. 20); Wilson (1999, p. 57, fig. 1f); Leistner (2008, p. 114).
Sabdariffa forsteri (F.D.Wilson) McLay & R.L.Barrett, comb. nov.
(Fig. 15e, f.)
Hibiscus forsteri F.D.Wilson in F.D.Wilson & Craven, Austrobaileya 4(3): 439–442 (1995). Type: Queensland: Cook District: 6.8 km from Bromley on the track to Carron Valley, 16 July 1990, J.R.Clarkson 8866 & V.J.Neldner (holo (mounted on 2 sheets): CANB 576920.1, CANB 576920.2; iso: BRI AQ0517379, DNA D0064525, K, L, CNS MBA758, NY, TEX 00208139).
Wilson and Craven (1995, pp. 439–442); Cooper and Cooper (2004, p. 277, fig.); Wilson and Craven (2022).
Sabdariffa fryxellii (Mabb.) McLay & R.L.Barrett, comb. nov.
(Fig. 16a, b.)
Hibiscus zonatus var. spinulosus W.Fitzg., J. & Proc. Roy. Soc. West. Austral. 3: 173 (1918) (as ‘spinulosa’); Hibiscus spinulosus (W.Fitzg.) F.D.Wilson, Austral. J. Bot. 22: 178, fig. 5, 10 (1974), nom. illeg., non Huber (1871); Hibiscus fryxellii Mabb., Taxon 34: 451 (1985), nom. nov. Type: Western Australia: Mount Broome, King Leopold [Wunaamin Miliwundi] Ranges, 20 May 1905, W.V.Fitzgerald 834 (lecto, designated by F.D.Wilson, Austral. J. Bot. 22: 178 (1974): PERTH 06235115; isolecto: NSW 83571).
Fitzgerald (1918, p. 173); Wilson (1974, p. 178, fig. 5, 10); Wheeler (1992, p. 215, fig. 59c); Craven et al. (2003, p. 194); McLay (2022).
Sabdariffa furcata (Willd.) M.M.Hanes & R.L.Barrett, comb. nov.
(Fig. 16c, d.)
Hibiscus furcatus Willd., Enum. Pl. 736 (1809); Hibiscus surattensis var. furcatus (Willd.) Hochr., Annuaire Cons. Jard. Bot. Genève 4: 112 (1900). Type citation: ‘Habitat in India orientali?’ Type: cultivated in Berlin, origin probably India (holo: B -W 12880-01 0).
Wilson (1999, pp. 56–57).
The morphological distinctiveness of the type specimen of H. furcatus Willd. from other Indian Sabdariffa taxa is readily apparent. This species has bifurcate epicalyx lobes, linear to subulate stipules, peduncles <5 cm long in fruit and stems that are relatively finely aculeate or aculeolate.
Pradeep and Sivarajan (1991, p. 635) simply state that ‘Willdenow’s name pertains to a very different species as observed by Paul and Nayar (1980, p. 195, 1988, p. 123).’ Paul and Nayar (1980, p. 195) similarly simply state that H. furcatus and H. hispidissimus are distinct entities, without defining the application of H. furcatus Willd. This discussion is largely repeated by Paul and Nayar (1988, p. 123) under H. aculeatus but again, application of H. furcatus Willd. is not addressed. In the key to species in Paul and Nayar (1988) and Paul (1993), the specimen in question would key to S. hispidissima but is clearly a distinct species.
Willdenow (1809, p. 736) places a question mark after the cited location ‘India orientali?’, indicating doubt over the origin of the material cultivated in Berlin. There is commonly confusion between ‘East India’ and ‘East Indies’ (Indonesia) but the specimen does not readily match any Malesian species either (see Borssum Waalkes 1966).
Wilson (1999, p. 49) states that H. furcatus Willd. ‘occurs in India and Thailand’. Indeed, this name was used in a review of Thai Hibiscus (Phuphathanaphong et al. 1989, pp. 49, 54, fig. 4), however the application was confused there, with H. furcatus Roxb. and H. aculeatus Roxb. included as synonyms and the plant illustrated is S. hispidissima.
Wilson (1999, pp. 56–57) provides the only description that applies to the type concept, considering this a rare species in India and Thailand, citing a small number of collections. While confusion abounds in the literature and in names applied to specimens in herbaria, we here accept that Wilson’s (1999) concept as the type of H. furcatus Willd. does not match any other species.
Photographs that can be matched to the type collection have recently been identified on iNaturalist (observation numbers: 28993434; 64936961; 92749419; 149528955) therefore we can now record the species for Maharashtra, Karnataka and Madhya Pradesh in India. Observations had previously been assigned to S. hispidissima, sometimes with doubt as this has a shrubby habit, quite distinct from the scrambling or climbing habit of S. hispidissima.
Sabdariffa furcellata (Desr.) M.M.Hanes & R.L.Barrett, comb. nov.
(Fig. 16e, f.)
Hibiscus furcellatus Desr. in Lamarck, Encycl. 3: 358 (1789); Furcaria furcellata (Desr.) Ulbr. in Engler, Pflanzenw. Afrikas 3(2): 400 (1921). Type citation: ‘croit dans la Guiane, & nous a été communiquée par M. Stoupy…’. ‘35. H. furcellatus Desrousseaux Lam. diet.’ Type: French Guyana, s. dat., D.Stoupy s.n. (holo: P-LA 00287577).
Hibiscus diodon DC., Prodr. 1: 449 (1824); Hibiscus furcellatus var. diodon (DC.) Uittien in A.Pulle, Fl. Suriname 3: 21 (1932). Type citation: ‘Cayenna’. Type: French Guyana: Cayenne, 1821, G.S.Perrottet 55 (syn: G-DC 00218939, G-DC 00218940).
Hibiscus youngianus Gaud., Voy. Uranie 91 (1827) (as ‘youngiana’), nom. inval., nom. nud.
Hibiscus trilobatus Vell., Fl. Flumin. 281, n° 2 (1825 [1829]). Type citation: ‘silvis maritimis Regii Proedii S.Crucis, ad loca humentia Floret Nov.’. Type: (lecto: original parchment Plate of Florae Fluminensis in the Manuscript Section of the Biblioteca Nacional do Rio de Janeiro [catalogue number mss1198656_033], later published in J.V.de M.Vellozo, Fl. Flumin.: Icon. 7: t. 29 (1831) [BHL], designated by Coutinho et al., Brittonia 77(1) (2025).
Hibiscus youngianus Gaud. ex Hook. & Arn., Bot. Beechey Voy. 79 (1832); Hibiscus furcellatus var. youngianus (Gaud. ex Hook. & Arn.) Hochr., Annuaire Cons. Jard. Bot. Genève 4: 107 (1900). Type: United States of America: Hawaii: Oahu, s. dat., F.W.Beechey s.n. (syn: K 000659885); Hawaii: Oahu, May 1825, J.Macrae s.n. (syn: K 000659884); Hawaii: C.Gaudichaud 279 (syn: P 00646428 (2 sheets); P 00646427).
Hibiscus corylifolius C.Presl, Reliq. Haenk. 2: 133 (1835). Type citation: ‘Habitat in Guayaquil.’ Type: Ecuador: Guayaquil, [locality possibly incorrect], s. dat., T.Haenke s.n. (syn: PR (2 sheets)).
Hibiscus tomentosus A.Stahl, Estud. Fl. Puerto Rico 2: 92 (1884), nom. illeg., non Mill. (1768), nec Kuntze (1891), nom. illeg. Type: Stahl’s watercolour 371, deposited at Sala Manuel María Sama y Auger-Colección Puertorriqueña, General Library, University of Puerto Rico, Mayagüez Campus (lecto, designated by P.Acevedo-Rodríguez, Caribbean J. Sci. 43(2): 194, fig. 3 (2007) [URL]. Residual syn: all Puerto Rico, s. dat., A.Stahl 46 (B, destroyed); ibid., A.Stahl 76 (B, destroyed); ibid., A.Stahl 150 (B, destroyed).
Hibiscus fraternus Sessé & Mociño, Fl. Mex. ed. 2; 159 (1894), non L. (1775). Type: Mexico: In agris de Toa Alta, s. dat., M.Sessé & J.M.Mociño 3539 (?syn: MA 602616); ‘Novae Hispaniiae’, s. dat., M.Sessé & J.M.Mociño 3545bis (?syn: MA 602611 [photo at F]).
Hibiscus furcellatus var. subpalmatus Hochr., Annuaire Cons. Jard. Bot. Genève 4: 107 (1900). Type: Guyane Française [French Guiana]: 1802, A.Gabriel s.n. (lecto, designated by A.Krapovickas & Fryxell, Bonplandia 13: 71 (2004): G 00406219 [photo at F]).
Hibiscus dominicus Hochr. in R.H.Chodat & E.Hassler, Bull. Herb. Boiss. 5: 300 (1905); Hibiscus furcellatus var. dominicus (Hochr.) Hassler, Fedde Rep. 7: 379 (1909). Type citation: ‘Ypacarai, Oct., n. 3364.’ Type: Paraguay: Ad ripam lacus Ypacarai [near Asuncion], Nov. 1898–1899, É.Hassler 3364 (lecto [mounted on 2 sheets], here designated: G 00381130; isolecto: G 00381131, GH 00052823, K 000535441, MPU 016513, P 02285906, P 02285907, UC 941248, W 1902-0002325).
Hibiscus furcellatus var. azuensis Urb. & Helwig, Repert. Spec. Nov. Regni Veg. 24: 236 (1928). Type: Dominican Republic: Hispaniola Island, Azua, Cordillera Central, Loma de Ravine – Pale, on the road from Las Lagunis to Guayajayuco, 18 June 1926, E.L.Ekman H 6412 (syn: S-R-2872, US 00098087).
Hibiscus furcellatus var. afurca Uittien in A.Pulle, Fl. Suriname 3: 21 (1932). Type: Suriname: Maratacca R., savannah of Saparra, [c. 1925], B.W. [Bureau v.h. Boschwezen] 894 (syn: [?B or F] n.v.); Suriname: without locality, [c. 1925], B.W. [Bureau v.h. Boschwezen] 270 (syn: [?B or F] n.v.).
Gaudichaud (1827, p. 91); Presl (1835, p. 133); Stahl (1884, p. 92); Sessé y Lacasta and Mociño (1894, p. 159); Hochreutiner (1900, p. 107); Chodat and Hassler (1905, p. 300); Hassler (1909, p. 379); Williams and Cheeseman (1929, p. 88); Uittien (1932, pp. 20–21); Macbride (1956, pp. 471–472); León and Alain (1957, p. 254); Roe (1961, pp. 6–8, fig. 1, 2); Wilson and Menzel (1964, p. 86, fig. 13, 27); Bates (1965a, p. 79, fig.); Liogier (1981, p. 93); Fryxell (1988, p. 209); Bates (1990, p. 885, pl. 124); Edmonds (1991, fig. 1(13); 2(27)); Fryxell (1992b, pp. 104–106); Wilson (1993, pp. 279–280); Fryxell (2000, p. 15); Krapovickas and Fryxell (2004, pp. 71–76); Areces Berazaín (2006, pp. 29–30, fig. 1b, 2d–f, 3c, 4c, h); Rigueiral et al. (2019, pp. 9–10, fig. 1k–l); Coutinho and Fernandes-Júnior (2024).
No specimens have been traced for Hibiscus trilobatus Vell. but the original illustration (published 2 years after the protologue but available to Vellozo at the time of publication) has been considered original material. The original artwork for this figure has been designated as lectotype of the name by Coutinho et al. (2025) and can be viewed through the website of the Biblioteca Nacional (http://bndigital.bn.br/acervodigital; see also Knapp et al. 2015).
Acevedo-Rodríguez (2005) discusses the work of Augustín Stahl in illustrating the flora of Pueto Rico. Acevedo-Rodríguez (2007) formally designated a lectotype for Hibiscus tomentosus A.Stahl based on an original watercolour by Stahl.
We here designate G 00381130 [mounted on 2 sheets] as the lectotype of H. dominicus as this has an original determination as ‘Hibiscus dominicus sp. nov.’ by Hochreutiner and the material is from Chodat’s herbarium (see Chodat and Hassler 1905).
Hibiscus furcellatus var. azuensis (Urban and Helwig 1928, p. 236) was accepted by Acevedo-Rodríguez and Strong (2012) based on a passing reference in Liogier (1981, p. 93) but Liogier (1981) noted that the variety has only been described, not accepted. We consider the variety to be best included as a synonym of S. furcellata.
Widespread in the Americas south from Florida (USA) and Mexico, in NE Argentina, Belize, Bolivia, Brazil, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, Florida (USA), French Guiana, Guatemala, Guyana, Haiti, Hawai’i (USA), Honduras, Mexico, Nicaragua, Panamá, Paraguay, Perú, Puerto Rico, Suriname, Trinidad and Tobago, Venezuela and the Windward Islands.
Sabdariffa furcellatoides (Hochr.) Mwachala & R.L.Barrett, comb. nov.
Hibiscus furcellatoides Hochr., Annuaire Cons. Jard. Bot. Genève 20: 157–160 (1917). Type: Guinée française [Republic of Guinea]: entre le Konkouré et Timbo, Mar. 1905, A.J.B.Chevalier 12504 (holo: P 00151939).
Hochreutiner (1917b, pp. 157–160); Wilson (1999, p. 53, fig. 1A).
Although POWO (see https://powo.science.kew.org/) includes this species as under S. rostellata, we consider this to be quite distinct based on leaf shape, peduncle length, epicalyx features and calyx indumentum.
Sabdariffa gilletii (De Wild.) Mwachala & R.L.Barrett, comb. nov.
Hibiscus gilletii De Wild., Ann. Mus. Congo Belge, Bot. sér. 5, 1[2]: 166 (1904). Type citation: ‘Kimuenza, mars 1901 (J.Gillet, n. 2057).’ Type: Democratic Republic of The Congo: Bas-Congo, Kimuenza [Kimwenza], Mar. 1901, J.Gillet 2057 (holo: BR 0000008952370).
Hibiscus poggei Gürke ex Engl., Pflanzenw. Ost-Afrikas 3(2): 400 (1921). Type: Angola: Lunda-Kasai Bezirk, [1880s], P.Pogge s.n. (holo: B, n.v., possibly destroyed).
The status of the subspecies deserves further attention as these may warrant recognition at species-level but such an assessment is hampered by the few available collections. We retain these as subspecies for now (Fig. 17a, b).
(a, b) Sabdariffa gilletii. Photographs by Nicholas Wightman, iNaturalist: 261576441, CC BY-NC. (c, d) Sabdariffa gossypiifolia. Photographs by Russell Barrett, cultivated, RBG Sydney. (e, f) Sabdariffa granitica. Photos: Bruce Wannan, ©.

Sabdariffa gilletii subsp. gilletii
De Wildeman (1904, p. 166); Ulbrich (1921, p. 400); Hauman (1963, pp. 120–121); Wilson (1999, p. 69, fig. 3c); Leistner (2008, p. 115).
Sabdariffa gilletii subsp. hierniana (Exell & Mendonça) Mwachala & R.L.Barrett, comb. nov.
Hibiscus hiernianus Exell & Mendonça in Excell, J. Bot. 74: 136–137 (1936). Hibiscus gilletii subsp. hiernianus (Exell & Mendonça) F.D.Wilson, Bull. Nat. Hist. Mus. London, Bot. 29(1): 71 (1999). Type: Angola: Huila, Humpata, Jan. 1860, F.M.J.Welwitsch 4927 (holo: BM 000645505; iso: LSU 206157).
Exell and Mendonça (in Exell 1936, pp. 136–137); Wilson (1999, p. 71); Leistner (2008, p. 115).
Exell and Mendonça (in Exell 1936, p. 137) explicitly cited the material at BM as the type, so this is accepted as a holotype.
Sabdariffa gilletii subsp. lundaensis (Baker f.) Mwachala & R.L.Barrett, comb. nov.
Hibiscus lundaensis Baker f., J. Bot. 77: 21–22 (1939). Hibiscus gilletii subsp. lundaensis (Baker f.) F.D.Wilson, Bull. Nat. Hist. Mus. London, Bot. 29(1): 71 (1999). Type: Angola: Lunda: Cacolo, River Cuilo, 11 Apr. 1937, J.Gossweiler 11821 (holo: BM 000554368).
Baker (1939, pp. 21–22); Wilson (1999, p. 71); Leistner (2008, p. 115).
Sabdariffa goossensii (Hauman) Mwachala & R.L.Barrett, comb. nov.
Hibiscus rostellatus var. goossensii Hauman, Bull. Jard. Bot. État. 31: 88 (1961). Hibiscus goossensii (Hauman) F.D.Wilson, Bull. Nat. Hist. Mus. London, Bot. 29(1): 56, fig. 1c (1999). Type: Democratic Republic of The Congo: District du Bas-Congo, [rec.] 1920, V.G.Goossens 1202 (holo: BR 0000008952707; iso: BR 0000008952349, BR 0000008952011).
Hauman (1961, p. 88); Hauman (1963, p. 118); Wilson (1999, p. 56, fig. 1c).
Sabdariffa gossypiifolia (Mill.) M.M.Hanes & R.L.Barrett, comb. nov.
Hibiscus sabdariffa L., Sp. Pl. 2: 695 (1753), nom. cons; Sabdariffa rubra Kostel., Allg. Med.-Pharm. Fl. 5: 1857 (1836), nom. illeg.; Furcaria sabdariffa (L.) Ulbr. in H.G.A.Engler, Pflanzenw. Afrikas 3(2): 402 (1921). Type citation: ‘Habitat in India.’ Type: Ketmia indica gossypii folio, acetosa, sapore. [apparently cultivated material from the garden of George Clifford III: Hartekamp Garden, Holland], Herb. Clifford (BM-Cliff 000646500), type cons. (see P.A.Fryxell, Taxon 50: 929 (2001)).
Hibiscus gossypiifolius Mill., Gard. Dict. 8th edn, n. 10 (1768). Type: Cultivated. United States of America, Florida, Palm Beach, Everglades Experimental Station, accession A60-234 [grown from seeds received from Santiago de las Vegas, Cuba], 21 Nov. 1962, M.Menzel & F.D.Wilson HV132 (neo, here designated): FSU 000112994).
Hibiscus fraternus L., Pl. Surin. 12 (1775). Type: Suriname: C.G.Dalberg 71 (lecto, designated by P.A.Fryxell, Syst. Bot. Monogr. 25: 225 (1988, as ‘holotype’): LINN-875.36; isolecto: S 09-24429 [? also S 11-9389]).
Hibiscus digitatus Cav., Diss. 3: 151, t. 70, fig. 2 (1787); Sabdariffa digitata (Cav.) Kostel., Allg. Med.-Pharm. Fl. 5: 1857 (1836). Type: Brazil: habitat circa Rio Janeiro, June 1767, P.Commerson s.n. [Wills no. 48] (syn: MA 475802, P-JU 00673711; P 02285905).
Hibiscus acetosus Noronha, Verh. Batav. Genootsch. Kunst. 5(Art. 4): 17 (1790), nom. inval., nom. nud.
Hibiscus digitatus var. kerrianus DC., Prodr. 1: 453 (1824). Type citation: ‘ad Rio-Janerio. H. digitatus Ker. bot. reg. t. 608.’ [Grown at Boyton and provided by Lambert, from seed collected in Brazil near Rio de Janeiro by Bonpland]. Type: ‘Hibiscus digitatus’ in Bot. Reg. 8: t. 608 (1822) [BHL] (lecto, here designated).
Hibiscus cuneatus Bertol. in A.Alessandrini, Nuovi Ann. Sci. Nat. 3: 138 (1840); Hibiscus cruentus Bertol., Nov. Comm. Acad. Sci. Inst. Bonon. 4: 428–429, t. 45 (1840), nom. illeg., nom. superfl., non H. cuneatus Kuntze (1891); Abelmoschus cruentus Walp., Repert. Bot. Syst. 1(2): 310 (1842). Type citation: ‘Esquintla.’ ‘Ex seminibus ab exemplari Guatimalensi eductis oblinui plantas duas, quae tola aestale laete sub dio vixerunt in horto botanico Bononiensi; sed hyeme sequenti perierunt in hipocausto, antequam florerent.’ Type: Guatemala: Escuintla, s.dat. [1836], J.Velásquez s.n. (holo: BOLO 0508032).
Hibiscus sanguineus Griff., Not. Pl. Asiat. 4: 520 (1854), non Franch. (1882). Type citation: ‘Mergue. In aquosis. Ins. Kully Gewen [Myanmar: Tenasserim Province, Mergui Archipelago]: Oct. 1834.’ [W.Griffith] Type: (n.v.).
Hibiscus palmatilobus Baill., Bull. Mens. Soc. Linn. Paris 1(64): 509 (1885). Type citation: ‘Grandidier, no. 49, Mouroundava.’ Type: Madagascar: Mouroundava [Morondava], Apr.–May 1869, A.Grandidier 49 (holo: P 00560072).
Hibiscus masuianus De Wild. & T.Durand in T.A.Durand & E.A.J.De Wildeman, Bull. Soc. Roy. Bot. Belgique 38(2): 20 (1899). Type: Democratic Republic of The Congo: Boma, 1 July 1895, A.Dewèvre 71 (syn: BR 0000008952875; BR 0000008953537).
Hibiscus sabdariffa var. albus A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 32, 36, pl. 6 (left) (1911). Type: ‘Hibiscus sabdariffa var. albus’ in A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: pl. 6 (left -hand illustration).
Hibiscus sabdariffa var. intermedius A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 32, 33, 36, pl. 7 (right) (1911). Type: ‘Hibiscus sabdariffa var. intermedius’ in A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: pl. 7 (right-hand illustration).
Hibiscus sabdariffa var. ruber A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 32, 35, pl. 6 (right) (1911). Type: ‘Hibiscus sabdariffa var. ruber’ in A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: pl. 6 (right-hand illustration).
Hibiscus sabdariffa var. bhaghalpuriensis A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: 33, 36, pl. 7 (left) (1911). Type: ‘Hibiscus sabdariffa var. bhaghalpuriensis’ in A.Howard & G.Howard, Mem. Dept. Agric. India, Bot. Ser. 4: pl. 7 (left-hand illustration).
Hibiscus sabdariffa cultivar ‘Altissima’ Wester, Philipp. Agric. Rev. 7: 268, fig. 4 (1914).
Linnaeus (1737, 1753, p. 695, 1759, p. 1149, in Alm and Linnaeus 1775, p. 12); Edwards (1822, t. 608); Bertoloni (1840, pp. 428–428, fig. 45); Griffith (1854, p. 520); Masters (1874, p. 340); Baillon (1885, p. 509); Durand and de Wildeman (1899, p. 20); Hochreutiner (1900, p. 116); Cooke (1901, p. 110); Williams and Cheeseman (1929, p. 88); Uittien (1932, p. 20); Standley and Steyermark (1949, pp. 351–352); Andrews (1952, p. 28, fig. 12); Hochreutiner (1955, p. 39, fig. 11(3)); Kearney (1955, p. 275); Macbride (1956, pp. 474–475); Ochse and Bakhuizen van den Brink (1977, p. 476, fig. 296); Hauman (1963, pp. 114–115); Bates (1965a, p. 79, fig. 23e); Borssum Waalkes (1966, pp. 64–65); Adams (1972, p. 476); Abedin (1979, p. 10, fig. 2, B, C); Liogier (1981, pp. 94–95); Marais and Friedmann (1987, p. 36, pl. 10 (8, 9)); Fryxell (1988, pp. 225–226); Paul and Nayar (1988, pp. 148–149); Edmonds (1991, p. 21, fig. 1(9, 10); 2(23, 24)); Fryxell (1992b, pp. 117–118); Wheeler (1992, p. 220, fig. 60d); Kenneally et al. (1996, p. 123, pl.); Sivarajan and Pradeep (1996, pp. 114–119, fig. 41); Philcox (1997, pp. 296–297); Wilson (1999, pp. 65–66); Boulos (2000, p. 108); Fryxell (2000, p. 13); Krapovickas and Fryxell (2004, pp. 52–53); van Wyk and Wink (2004, p. 170, fig.); Hussey et al. (2007, p. 184, pl.); Leistner (2008, p. 115); Mwachala (2009, p. 32); Fayaz (2011, p. 510, fig.); van der Burg (2013, pp. 66–67); Cowie et al. (2013, pp. 16–17, fig. 5, pl. 9); Baldini et al. (2019, pp. 212–213, fig. 13); Amany et al. (2020, p. 126); McLay (2022); Coutinho and Fernandes-Júnior (2024, fig.).
No specimens are cited in the protologue for Hibiscus gossypiifolius Mill. Jacek Wajer (BM; pers. comm.) has confirmed that there is no known extant specimen that can be associated with Miller’s description, neither in the main collection of BM nor the Sloane collection, Linnean Society collection or Clifford Herbarium. No illustration can be directly linked to this name and, although Miller cites a polynomial from Tournefort, there is no illustration associated with that polynomial. Although Miller may have observed the species in cultivation in the Apothecaries’ Garden at Chelsea and not preserved a specimen, the source of his description was likely earlier works (e.g. Hughes’s The Natural History of Barbados; Sloane’s Voyage to Jamaica and Natural History of Jamaica and in Browne’s The Civil and Natural History of Jamaica), explaining his presumption that this was a New World species, distinct from ‘true’ roselle from the Old World (Jacek Wajer, pers. comm.). Wester (1911, p. 96) points to Sloane (1707) as the first to record use of the fruit for food in Jamaica and this would seem to be a fairly direct link to Miller’s concept of his species (Miller 1768).
Abedin (1979, p. 10) used the name Hibiscus gossypiifolius for the taxon traditionally called H. sabdariffa (which Abedin applied to H. cannabinus) and cited ‘Holotype: (BM)’ but as this cannot actually be linked to any specimen (as noted above), the statement does not qualify as an inadvertent neotypification.
As no original material can be traced, a neotype must be selected for Miller’s name. We here select a modern collection with a known cytotype to fix the application of Miller’s name to the cultivated form of roselle with large, fleshy, red calyces consistent with the typification of Hibiscus sabdariffa L. by Fryxell (2001b). We choose a cultivated specimen originating from Cuba over any material from Jamaica as the selected neotype has a known chromosome number of 2n = 72. Given the long utilisation of this species and known variation in chromosome number, future workers may wish to formally recognise different cytotypes as distinct taxonomic entities and in such a case, an epitype will subsequently not be required to fix the application of this name.
We here select the cited illustration as lectotype of the name Hibiscus digitatus var. kerrianus DC. as we have not been able to trace any other original material, though a Bonpland collection may possibly be extant somewhere.
Howard and Howard (1911) did not list any specimens for the new varieties under Hibiscus sabdariffa and as such the only unambiguous original material we can identify is here considered to be the plates published with the protologues that are simply recognised as ‘type’ here, in case actual specimens can be located that might more appropriately serve as lectotypes. Wilson (1999, p. 65) considered the varietal names of Howard and Howard (1911) to be cultivar names. Howard and Howard (1911, p. 32) state that ‘Four different forms in all have been isolated by us and these have bred true. As the differences are very distinct and of a morphological nature rather than agricultural, we have formed the following four varieties:’, demonstrating that the varieties were considered to be more than agricultural selections and the names are here considered to fall under the International code of nomenclature for algae, fungi, and plants (Turland et al. 2018), rather than the International code of nomenclature for cultivated plants (see https://www.ishs.org/scripta-horticulturae/international-code-nomenclature-cultivated-plants-ninth-edition).
Bertoloni is commonly considered to have first published 60 new names in his Florula guatimalensis (Bertoloni 1840), a reprint from work published earlier in 1840 in Novi Commentarii Academiae Scientiarum Instituto Bononiensis, including the illegitimate Hibiscus cruentus Bertol. However, Baldini et al. (2019) present a comprehensive review of these names and the associated types, correcting the place of first publication to Bertoloni in Alessandrini (1840) and identifying authentic type material.
Wilson (1999, p. 65) considered the varietal name ‘Altissima’ of Wester (1914) to be a cultivar name and we agree with this conclusion. We list the name here as this is sometimes treated as a variety under the ICN (Turland et al. 2018) in the literature (e.g. Rakshit and Kundu 1970).
As this species has a long history of cultivation, the precise native range is difficult to define. Sabdariffa gossypiifolia is generally considered native to the Central African Republic, Chad, Democratic Republic of The Congo, Gabon, Ghana, Nigeria and Sudan.
Introduced and commonly naturalised in the Andaman Islands, Angola, Australia, Bangladesh, Belize, Bénin, SE Brazil, Burkina Faso, Cambodia, Cameroon, Caroline Islands, Cayman Islands, China, Colombia, Comoros, Cuba, Dominican Republic, Egypt, El Salvador, Fiji, The Gambia, Guatemala, Guinea, Guinea-Bissau, Gulf of Guinea Islands, Haiti, India, Iraq, Jamaica, Laos, Leeward Islands, Madagascar, Malawi, Mali, Marianas, Mauritania, Mauritius, Mexico, Mozambique, Namibia, Nepal, Niger, Pakistan, Perú, Puerto Rico, Réunion, Senegal, Sierra Leone, Solomon Islands, Somalia, South Africa, Sri Lanka, Taiwan, Tanzania, Thailand, Togo, Trinidad and Tobago, Vanuatu, Venezuela, Vietnam, Windward Islands, Zambia and Zimbabwe.
Sabdariffa granitica (Wannan) McLay & R.L.Barrett, comb. nov.
(Fig. 17e, f.)
Hibiscus graniticus Wannan, Austrobaileya 12: 20−21, fig. 1–8 (2022). Type: Australia. Queensland: Cook District: Bonny Glen, Cape York Peninsula, 27 Oct. 2010, B.S.Wannan 5990 & M.Trenerry (holo: BRI AQ880107; iso: CNS, NSW 824525).
Wannan (2022, pp. 20–21, fig. 1–8).
Sabdariffa greenwayi (Baker f.) Mwachala & R.L.Barrett, comb. nov.
(Fig. 18a, b.)
Hibiscus greenwayi Baker f., J. Bot. 75: 99 (1937). Type: Tanzania: NW Usambara, Mnazi, Umba Steppe, 12 Jan. 1930, P.J.Greenway 2034 (holo: EA: iso: BM 013726617 (fragment & drawing), K 000240490; PRE 0501592-0).
Baker (1937, p. 99); Goetz (1980); Edmonds (1991, p. 18); Wilson (1999, pp. 62–63, fig. 2f); Mwachala (2009, pp. 42–44, fig. 6).
Sabdariffa gregoryi (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus gregoryi Krapov. & Fryxell, Bonplandia 13(1–4): 94–96, fig. 17 (2004). Type: Brazil: Goiás, Niquelândia, 10 Apr. 1961, W.C.Gregory, A.Krapovickas & J.Pietrarelli 10190 (holo: CTES 0001542!; iso: LIL).
Krapovickas and Fryxell (2004, pp. 94–96, fig. 17); Coutinho and Fernandes-Júnior (2024).
Sabdariffa hassleriana (Hochr.) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus hasslerianus Hochr., Annuaire Conserv. Jard. Bot. Genève 6: 51–52 (1902). Type citation: ‘Paraguay, in alta planitie ed declivis Sierra de Maracayu, fl. Oct. (Hassler n. 4949).’ Type: Paraguay: Yerbales Mountain, near Ipe Hu, Sierra de Maracaju, Oct. 1898, É.Hassler 4949 (lecto, here designated: G 00381129! [mounted on 2 sheets]; isolecto: B, BM 000545827!, C, F 0BN009518!, G 00381298! [mounted on 2 sheets], G 00381299! [mounted on 2 sheets], GH 00052822!, K 000535438!, LIL 000830!, MICH 1104725!, MO 309103!, MPU 016512!, NY 00221740!, P 02285916!, P 02285917!, S -R-11261!, UC 941190!, W 1902-0002789!).
Hochreutiner (1902, pp. 51–52); Krapovickas and Fryxell (2004, pp. 78–79); Coutinho and Fernandes-Júnior (2024).
Sabdariffa henningsiana (Gürke) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus henningsianus Gürke in C.F.P. von Martius, Fl. Bras. 12(3): 559, tab. 110, fig. l (1892). Type: Brazil: Tocantins: Rio Reason, J.B.E.Pohl 1256 (lecto, first-step designated by A.Krapovickas & P.A.Fryxell, Bonplandia 13: 60 (2004): W; second-step designated here: W 0066239!; isolecto: BR, F 0062908F!, M 0211552!, W 0066240!).
Gürke (1892, p. 559, tab. 110, fig. l); Gottsberger (1972, pp. 468–475, fig. 22–24); Krapovickas and Fryxell (2004, p. 60, fig. 5); Coutinho and Fernandes-Júnior (2024).
When Gürke described H. henningsianus he indicated the type as J.B.E. Pohl 1256 but did not indicate a single herbarium, therefore all original material represents syntypes. Krapovickas and Fryxell (2004) published a first-step lectotypification, selecting the specimen at W. However, the material at W comprises two sheets with distinct barcodes. Therefore, we designate the specimen with W barcode 0066239 as a second-step lectotype (ICN Art. 9, Turland et al. 2018) as this is the best material.
Sabdariffa heterophylla (Vent.) McLay & R.L.Barrett, comb. nov.
(Fig. 18c, d.)
Hibiscus heterophyllus Vent., Jard. Malm. 2: 103, t. 103 (1804), non Griff. (1854); Type: Cultivated. France, Malmaison, from material collected in Australia [most likely originating from New South Wales: Sydney region, 1802–1803, Leschenault or Guichenot s.n.], E.P.Ventinat s.n. (holo [mounted on 3 sheets]: G 00341495).
Hibiscus flabellatus Desf., Tabl. École Bot. 147 (1804). Type citation: ‘…N. Holl.…’. Type: ‘N. Holl.’ [Australia: New South Wales: Sydney region, Baudin Expedition, 1802–1803, J.Leschenault de la Tour or A.Guichenot s.n.] (holo: P 00270074).
Hibiscus procerus Wall., Num. List no. 2692 [p. 91] (1831), nom. inval., nom. nud.
Hibiscus persicifolius Eckl. & Zeyh., Enum. Pl. Afric. Austral. 1: 38 (1835). Type: South Africa: In the Great Karroo; between Graaf Reynet and Uitenhage; near Klipplaatrivier, Dec., C.F.Ecklon & C.L.P.Zeyher 305 (Herb. Sond.) (lecto, designated by F.D.Wilson, Bull. Nat. Hist. Mus. London (Botany) 29(1): 77 (1999): S G-10316; isolecto: MEL, NBG, S 07-10463).
Hibiscus margeriae A.Cunn. ex Benth., Fl. Austral. 1: 213 (1863); Hibiscus heterophyllus var. margeriae (A.Cunn. ex Benth.) Domin, Biblioth. Bot. 22(89): 405 (1928). Type citation: ‘The northern specimens belong mostly to a broader-leaved form, distinguished by A. Cunningham under the name H. Margeriae.’ Type: Australia: Queensland: Brisbane River, Sep. 1829, A.Cunningham 43 (syn: K 000659814); Brisbane River, A.Cunningham 41 (syn: K 000659815).
Hibiscus heterophyllus var. hispidifolius F.Muell., Fragm. 6: 170 (1868) (as ‘hispidifolia’). Type citation: ‘haec ad flumen Fitzroy-River nascitur.’ Type: Australia: Queensland: Fitzroy River, Rockhampton, s. dat., A.Thozet 306 (holo: MEL 18723!).
Hibiscus heterophyllus var. genuinus Hochr., Annuaire Cons. Jard. Bot. Genève 4: 122 (1900), nom. inval.
Hibiscus heterophyllus var. leefei Hochr., Annuaire Cons. Jard. Bot. Genève 4: 122 (1900). Type: Australia: Queensland: scrub near Rockhampton, [c. 1870], Rev. I.E.Leefe 44 (holo: K 000659877).
Hibiscus amaliae Domin, Biblioth. Bot. 22(89): 404 (1928). Type: Australia: Queensland: Rockhampton, A.Deitrich 747 (syn: ?K, MEL 1057692!).
Hibiscus heterophyllus var. discolor Domin, Biblioth. Bot. 22(89): 405 (1928). Type citation: ‘Port Molle, H.M.S. Alert, V. 1881, comm. COPPINGER; Shoalwater Bay, R. Brown Iter Australiense 1802–05 No. 5126.’ Type: Australia: Queensland: Shoalwater Bay, s. dat., R.Brown s.n. [Iter Austral. No. 5126] (lecto, here designated: BM 013824575).
Ventenat et al. (1804, p. 103, t. 103); Desfontaines (1804, p. 147); de Lamarck and Poiret (1804, p. 220); Bailey (1899, p. 127); Hochreutiner (1900, p. 121); Domin (1928, pp. 404, 405); Bates (1965a, pp. 89, 79, fig. 23h); Borssum Waalkes (1966, p. 66); Wilson (1974, p. 168, fig. 13, 170, fig. 14); Wilson and Craven (1995, p. 445); Spencer (1997, p. 379); Cooper and Cooper (2004, pp. 277–278, fig.); McLay (2022).
Hibiscus persicifolius has often been considered a synonym of S. diversifolius. Wilson (1999, p. 77) suggests that the type of Hibiscus persicifolius may have been cultivated or adventive and that the name may actually apply to S. heterophylla. We agree with this conclusion.
The type of Hibiscus heterophyllus var. hispidifolius had not previously been recognised as the protologue information is rather vague but there is only a single collection at MEL annotated with this variety name and we therefore accept this as the holotype.
The Coppinger collection representing Hibiscus heterophyllus var. discolor from Port Molle (Long Island, Whitsundays) has not been traced, therefore a sheet at BM with a favourable amount of material, collected by Robert Brown, is here designated as lectotype of the name Hibiscus heterophyllus var. discolor Domin.
We could not find evidence of a valid description of Hibiscus procerus in Wallich (1831), therefore we consider this to be a nomen nudum. Several specimens appear to be associated with the proposed name but these have no type status: India: 1816, W.Roxburgh s.n. (G 00218926); India: Wallich Cat. 2692 (K 001116821, K 001116822); cultivated from seed from New South Wales [Australia] (BR 0000013466190; BR 0000013466206). Wilson (1999, p. 77) states that Roxburgh’s illustration [Icones Roxburghianae 1506 (K)] is of the white-flowered form of S. heterophylla from Australia. Roxburgh (1832) does not mention the name but does list Hibiscus heterophyllus as a species from New South Wales, cultivated in the botanic garden (represented by BM 013732147), consistent with Wilson’s interpretation.
We here exclude all the yellow-flowered forms from the concept of S. heterophylla, placing these under S. divaricata instead, but further research on this complex is required.
Sabdariffa hilariana (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus hilarianus Krapov. & Fryxell, Bonplandia 13(1–4): 82–83 (2004), non H. cuneifolius Garcke (1849). Hibiscus cucurbitaceus var. cuneifolius A.St.-Hil., Fl. Bras. Merid., 4th edn, 1(7): 244 (1828). Type citation: ‘prope praedium S. Eligii … Minas Geraes. Florebat Septembri.’ Type: Brazil: Minas Gerais: St. Eloi, [SE de Formigas, ahora Montes Claros], 1816, A.St.-Hilaire Catal. C3 n° 694 (lecto, here designated: P 02285900!; isolecto: P 02285899!).
Hibiscus cabralensis Krapov., Bonplandia 15(1–2): 47, fig. 1 (2006). Type: Brazil: Minas Gerais: Mun. Várzea da Palma, Serra do Cabral, estrada Várzea da Palma a Joaquim Felício, 100 m, 5 Dec. 2004, G.Hatschbach & E.Barbosa 78849 (holo: MBM 301210!; iso: CTES 0001532!).
Krapovickas and Fryxell (2004, pp. 82–83); Krapovickas (2006, p. 47); Rigueiral et al. (2019, pp. 10, 12, fig. 4a, b); Coutinho and Fernandes-Júnior (2024).
Sabdariffa hispidissima (Griff.) R.L.Barrett & M.M.Hanes, comb. nov.
(Fig. 19a, b.)
Hibiscus hispidissimus Griff., Not. Pl. Asiat. 4: 521 (1854), non A.Chev. (1940). Type citation: ‘Mergue. [Myanmar: Tenasserim Province, Mergui Archipelago] Ad littoram Ins. Kully Gewen, iner gramina: Oct. 1834. [Griffith].’ Type: India: Kerala, Thanneerpandal, near town of Badagara, A.K.Pradeep 5008 (neo, designated by A.K.Pradeep & V.V.Sivarajan, Taxon 40: 637 (1991): K; isoneo: CALI).
Hibiscus furcatus Roxb. ex DC., Prodr. 1: 449 (1824), nom. illeg., non Willd. (1809), nec Morotzi (1846), nec Mast. (1868), nec Bahadur, Dayal & Raturi (1970); Furcaria roxburghii Kostel., Allg. Med. Pharm. Fl. 5: 1856 (1836). Type citation: in Bengalo. (v.s.). Type: India: Bengal, s. dat., W.Roxburgh s.n. (BM 013730221).
Hibiscus aculeatus Roxb., Fl. Ind. 3rd edn, 206 (1832), nom. illeg., non Walter (1788), nec Bahadur, Dayal & Raturi (1970), nom. illeg. Type citation: ‘I have only observed this in my garden, where it must have been brought from some other place amongst other seeds.’ Type: India: Icones Roxburghianae no. 356 (K).
Hibiscus hamatus Harv. in W.H.Harvey & O.W.Sonder (eds), Fl. Cap. 1: 177 (1860), nom. inval., pro syn.
Roxburgh (1832, p. 204); Wight and Arnott (1834, p. 48); Griffith (1854, p. 521); Dalzell and Gibson (1861, p. 19); Masters (1874, p. 335); Prain (1903, p. 267); Gamble (1915, p. 97); Rakshit and Kundu (1970, p. 161); Saldanha and Nicolson (1976, p. 151); Matthew (1983, p. 113); Paul and Nayar (1980, p. 194); Paul and Nayar (1988, pp. 123–124); Pradeep and Sivarajan (1991, p. 634, fig. 1–6); Paul (1993, pp. 323–324); Sivarajan and Pradeep (1996, pp. 106–110, fig. 34, 35); Philcox (1997, pp. 294–295); Wilson 1999, pp. 55–56).
Historical applications of the names Hibiscus furcatus Roxb. ex DC. and H. aculeatus Roxb. are greatly confused (e.g. by Bahadur et al. 1970) but were clarified by Pradeep and Sivarajan (1991). A problem has been identified here in that Furcaria roxburghii Kostel. is a validly published replacement name for the greatly confused Hibiscus furcatus Roxb. ex DC. This appears to be the earliest name available at species rank for this taxon. The name was presumably overlooked as a valid name as the generic combination Furcaria proposed by Kosteletzky (1836) was an illegitimate homonym, but this does not invalidate the species combination as there was a valid genus Furcaria (Desvaux 1827). However, we refrain from adopting this epithet due to the great confusion surrounding Roxburgh’s concept of ‘Hibiscus furcatus’, and we propose conservation of the name Hibiscus hispidissimus Griff. as a more stable nomenclatural solution.
Sabdariffa hochreutineri (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus hochreutineri Krapov. & Fryxell, Bonplandia 13(1–4): 88–90, fig. 14 (2004). Type: Brazil: Goiás, Rod. Belem-Brasilia (mun. Rianápolis), 23 Mar. 1976, G.Hatschbach & R.Kummrow 38269 (holo: MBM 42520!, iso: C, CTES 0001555!, NY 00007089!).
Krapovickas and Fryxell (2004, pp. 88–90, fig. 14); Coutinho and Fernandes-Júnior (2024).
Sabdariffa holstii (Mwachala) Mwachala & R.L.Barrett, comb. nov.
Hibiscus holstii Mwachala in Verdcourt & Mwachala, Fl. Trop. E. Africa. Malvac. 40 (2009). Type: Tanzania: Lushoto District: Usambaras, [1891–1893], C.H.E.W.Holst 3198 (holo: K).
Mwachala (2009, p. 40).
Sabdariffa hoshiarpurensis (T.K.Paul & M.P.Nayar) R.L.Barrett & M.M.Hanes, comb. nov.
Hibiscus hoshiarpurensis T.K.Paul & M.P.Nayar, Bull. Bot. Surv. India 25(1–4): 188–189, fig. 1–6 (1985). Type: India: Punjab, Dholbah, Hoshiarpur District, 22 Sep. 1970, O.P.Misra 41888[A] (holo: BSD 000000096; iso: CAL 0000006234).
Paul and Nayar (1985, pp. 188–189, fig. 1–6); Paul and Nayar (1988, pp. 131–132); Paul (1993, p. 325); Dhami (2023, p. 132).
Punjab, India, where the species is considered Threatened (Dhami 2023).
Sabdariffa inimica (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
(Fig. 19c, d.)
Hibiscus inimicus Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 197–200, fig. 8 (2003). Type: Australia: Northern Territory: ~37 km SSE of Jabiru, 30 Mar. 1981, L.A.Craven 6639 (holo [mounted on 2 sheets]: CANB 643111.1, CANB 643111.2; iso: DNA D0168580 (2 sheets), K 000380883, L 0599612, TEX 00208005).
Craven et al. (2003, pp. 197–200, fig. 8); Craven (2022).
Sabdariffa itirapinensis (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus itirapinensis Krapov. & Fryxell, Bonplandia 13(1–4): 104–107, fig. 21 (2004). Type: Brazil: Säo Paulo: Itirapina, Fazenda Programa, 12 Nov. 1985, F.M.Martins 16861 (holo: UEC 119160; iso: CTES 0001556, IBGE 21785!).
Krapovickas and Fryxell (2004, pp. 104–107, fig. 21); Rigueiral et al. (2019, pp. 12–13, fig. 4c–e); Coutinho and Fernandes-Júnior (2024).
Sabdariffa kenneallyi (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
(Fig. 19e, f.)
Hibiscus kenneallyi Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 200, fig. 9 (2003). Type: Western Australia: Point of land 3 km E of Middle Osborne Island, 14 June 1985, P.A.Fryxell, L.A.Craven & J.McD.Stewart 4775 (holo [mounted on 3 sheets]: CANB 577133.1, CANB 577133.2, CANB 577133.3; iso: ASU 0019337, DNA D0168603, L 0599611, L 0599610, MEL 2283251, MEL 2283252, NY 00062128, PERTH 06755712).
Wheeler (1992, p. 223, fig. 61e, as Hibiscus sp. B); Craven et al. (2003, p. 200, fig. 9); Craven (2022).
Sabdariffa kirstyae (Craven) McLay & R.L.Barrett, comb. nov.
(Fig. 20a, b.)
Hibiscus kirstyae Craven in L.A.Craven et al., Muelleria 35: 4, fig. 2 (2016). Type: Western Australia: Morgan River, near Theda Station homestead, 17 Feb. 2005, M.D.Barrett 1589 (holo [mounted on 2 sheets]: PERTH 7213425, PERTH 7213433; iso: CANB 527708.1, CANB 527708.2, K).
Craven et al. (2016, p. 4, fig. 2); Craven (2022).
Sabdariffa kitaibelifolia (A.St-Hil.) M.C.Duarte & Yoshikawa, comb. nov.
(Fig. 20c, d.)
Hibiscus kitaibelifolius A.St-Hil., Fl. Bras, Merid. 4th edn, 1(7): 248, pl. 48 (1828). Type citation: ‘Ad ripas rivulorum haud longe ab urbe vulgo S. João del Rey in provincia Minas Geraes. Florebat Martio.’ Type: Brazil: Province de Minas Geraes, Lás João del Rey, 1816–1821, A.St.-Hilaire Catal. D n° 375 (lecto, here designated: P 02285919!; isolecto: P 02285920!, P 02285921!).
Hibiscus furcellatus var. glandulosus Hochr., Annuaire Cons. Jard. Bot. Genève 4: 107–108 (1900). Type citation: ‘Brasil. (Glaziou n. 17466).’ Type: Brazil: près de S. J. d’el Rey, Minas, Env. de Rio de Janeiro, 1889, A.F.M.Glaziou 17466 (lecto, here designated: G 00353182!; isolecto: C, K, P 06612081!, P 06612082!).
De Saint-Hilaire (1828, p. 248, pl. 48); Krapovickas and Fryxell (2004, p. 69); Rigueiral et al. (2019, pp. 13–14, fig. 4f–h); Coutinho and Fernandes-Júnior (2024, fig.).
Most type citations for Hibiscus kitaibelifolius have only listed the type as being at P. Given that there are three sheets, a lectotype is here selected that recognises the sheet P 02285919 as the best material due to the full information in the label.
We here designate G 00353182 as lectotype of Hibiscus furcellatus var. glandulosus as this has an original field label and original annotation by Hochreutiner.
Sabdariffa laxiflora (A.St.-Hil.) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus laxiflorus A.St.-Hil., Fl. Bras. Merid., 4th edn, 1(7): 245 (1828); Abelmoschus laxiflorus Walp., Repert. Bot. Syst. 1: 310 (1842). Type citation: ‘prope pagum Corumbá da parte australi provinciae Goyas. Florebat Junio.’ Type: Brazil: Province de Goyaz [Goiás], [June] 1816, A.St.-Hilaire Catal. C1 n° 679 (lectotype, here designated: P 02285922!; isolectotype: P 02285923!, P 02285924!).
Hibiscus cerradoensis M.Y.Menzel, Fryxell & F.D.Wilson, Brittonia 35: 218–220, fig. 18 (1983). Type: Brazil: Goiás, Mossâmedes, Serra Dourada, ~15 km S of Goiás Velho, 1000 m, 11 May 1973, W.R.Anderson, S.R.Hill, R.Reis dos Santos, & R.Souza 10060 (holo: UB 00037475; iso: NY 00221738, NY 00221739, TEX 00371874, TEX 00371875).
De Saint-Hilaire (1828, p. 245); Menzel et al. (1983a, pp. 218–220, fig. 18); Krapovickas and Fryxell (2004, pp. 83–86, fig. 12); Coutinho and Fernandes-Júnior (2024).
Sabdariffa maculata (Lam.) M.M.Hanes & R.L.Barrett, comb. nov.
Hibiscus maculatus Lam., Encycl. 3: 349–350 (1789), non Bartl. (1837). Type citation: ‘croit dans l’Isle de St. Domingue (V. S. in herb. Juss.).’ Type: Dominican Republic: (lecto, designated by F.Areces Berazaín, Revista Jard. Bot. Nac. Univ. Habana (25/26: 30 (2006): P-JU 12378).
Hibiscus diversifolius var. granatensis Triana & Planch., Fl. Nov. Gran. 1: 165 (1862). Type citation: ‘Province d’Antioquia, ait. 1300 m; dans les endroits où les forêts ont été coupées (Tr.); Cartago, dans les lagunes (Goudol).’ Type: Colombia: prov. Antioquia, alt. 1500, May 1852, J.J.Triana s.n. [herb. Triana No. 5277] (syn: BM 013824619, COL).
Sabdariffa maculata subsp. maculata
De Lamarck and Poiret (1789, pp. 349–350); Triana Silva and Planchon (1862, p. 165); Liogier (1981, p. 94); Krapovickas and Fryxell (2004, pp. 68–69); Areces Berazaín (2006, pp. 30–32, fig. 1c, 2g, 3d, 4d, i).
Sabdariffa maculata subsp. nipensis (Carabia) M.M.Hanes & R.L.Barrett, comb. nov.
Hibiscus nipensis Carabia, Mem. Soc. Cub. Hist. Nat. ‘Felipe Poey’ 17: 15–16 (1943); Hibiscus maculatus subsp. nipensis (Carabia) F.Areces, Revista Jard. Bot. Nac. Univ. Habana 25–26: 32–33 (2006). Type: Cuba: Oriente, Sierra de Ñipe, Arroyo Naranjo, en el nacimiento del arroyo, 18 Apr. 1940, J.P.Carabia 3831 (lecto, designated by F.Areces Berazaín, Revista Jard. Bot. Nac. Univ. Habana (25–26: 32 (2006): NY 000841666; isolecto: AJBC, GH 00052821, HAC, NY 00084165).
Carabia (1943, pp. 15–16); León and Alain (1957, pp. 256–257); Areces Berazaín (2006, pp. 32–33, fig. 1d, 2h, 3e, 4e, j).
The history of specimens from the ‘Atkins’ botanical garden in Cuba, relevant to this species, is discussed by Rodríguez Vázquez and Flores (2020).
Sabdariffa manuripiensis (Krapov.) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus manuripiensis Krapov., Bonplandia 17: 37–40, fig. 3 (2008). Type: Bolivia: Pando: Prov. Manuripi, río Manuripi, subiendo 10–11 km de Pto. Rico, 278 m, 15 Nov. 1996, N.Paniagua & R.Foster 683 (holo: LPB 0000734!; iso: CTES 0004858!).
Krapovickas (2008, pp. 37–40, fig. 3).
Sabdariffa marenitensis (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
(Fig. 20e, f.)
Hibiscus marenitensis Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 203, fig. 11 (2003). Type: Western Australia: Koolan Island, near Acacia Ore Body in central part of island, 2 June 1985, P.A.Fryxell, L.A.Craven & J.McD.Stewart 4606 (holo [mounted on 2 sheets]: CANB 642044.1, CANB 642044.2; iso: ASU 0019338, DNA D0180029, K 000659910, L, NY 00689136, PERTH 06486630, TEX 00208006).
Craven et al. (2003, p. 203, fig. 11); Craven (2022).
Sabdariffa mariae (Krapov.) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus mariae Krapov., Bonplandia 15(1–2): 49, fig. 2 (2006). Type: Brazil: Minas Gerais: Mun. Joaquim Felício, Serra do Cabral, próximo do Rio Preto, 23 Aug. 2002, G.Hatschbach, M.Hatschbach & J.M.Silva 73766 (holo: MBM 272781!; iso: CTES 0001557!).
Krapovickas (2006, p. 49); Rigueiral et al. (2019, p. 14, fig. 4i, j); Coutinho and Fernandes-Júnior (2024).
Sabdariffa mastersiana (Hiern) Mwachala & R.L.Barrett, comb. nov.
(Fig. 21a, b.)
Hibiscus mastersianus Hiern, Cat Afric. Pl. Welw. 1: 71 (1896); Hibiscus surattensis var. mastersianus (Hiern) Hochr., Annuaire Conserv. Jard. Bot. Genève 4: 112 (1900). Type citation: ‘H. furcatus Masters, l.c., p. 201, non Roxb. Pungo Adongo. … in secondary thickets near Caghuy; fl. and fr. May 1857 … No. 5242. Huilla.–In thickets near Ferraõ de Sola in the Lopollo country; … fl. Jan. 1860. No. 4927 … in stoney places at the borders of forests consisting of ‘Panda’ … near Eme; fl. May 1860. No. 4928.’ Type: Mozambique: Lupata, 20 Apr. 1860, J.Kirk s.n. (lecto: K 000240669), designated by E.W.B.H.Milne-Redhead, Bull. Misc. Inform. Kew 1935: 273 (1935). Residual syn: The Gambia, s. dat., Ingram s.n. (n.v.). Angola: Huila Province: near Ferraõ de Sola, Lopolo, Jan. 1860, F.M.J.Welwitsch 4927 (K 000240663); Angola: Huila Province: ‘Eme,’ May 1860, F.M.J.Welwitsch 4928 (K); Angola: Malanje Province: Pungo Adonga, near Gaghuy, May 1857, F.M.J.Welwitsch 5242 (K).
Hibiscus pachmarhicus Haines, Bull. Misc. Inform. 1914: 24–26, fig. 1–7 (1914). Type: India: Central Provinces; Madhya Pradesh, Pachmarhi in the Satpura Range, 900 m, Oct. 1911, H.H.Haines 197P (syn: K 000659776, K 000659777, K 000659778).
Hibiscus beddomei Rakshit & Kundu, Sci. & Cult. 27: 192, fig. 1 (1961). Type: India: South India, without precise locality, Nov. 1856, R.H.Beddome 91 & 92 (holo: CAL 0000006235).
Hiern (1896, p. 71); Haines (1914, pp. 24–26, fig. 1–7); Milne-Redhead (1935, p. 272); Exell (1961, p. 439); Rakshit and Kundu (1961, p. 192, fig. 1); Merxmüller (1969, p. 82:13, 19–20); Paul and Nayar (1988, p. 126); Edmonds (1991, p. 19); Paul (1993, p. 324); Wilson (1999, p. 71–72, fig. 3b); Heath and Heath (2009, p. 284, fig.); Mwachala (2009, pp. 38–39).
Milne-Redhead (1935, p. 273) designated a specimen collected by Kirk in Mozambique as lectotype of Hibiscus mastersianus (K 000240669). This is a valid selection based on the citation of Masters’ (1868) concept of H. furcatus, where this is one of the specimens cited. The Welwitsch specimens directly cited by Hiern (1896) are residual syntypes.
Paul and Nayar (1988, p. 126) and Paul (1993, p. 324) recognised H. beddomei as a distinct species but did not discuss the relationship to S. mastersiana, rather this was distinguished from S. radiata. If Indian populations prove to be distinct from African populations in future, the name Hibiscus pachmarhicus Haines (1914) would have priority for the Indian populations. This species was also possibly an early introduction to India that currently appears native.
Considered to have a naturally disjunct distribution in central and southern Africa and India, occurring in Angola, Botswana, Democratic Republic of The Congo, India, Kenya, Mozambique, Namibia, Rwanda, Tanzania, Zambia and Zimbabwe.
(a, b) Sabdariffa mastersiana. Photographs by (a) Riana Fourie, iNaturalist: 71433047, CC BY-NC. (b) Duncan McKenzie, iNaturalist: 75535507, CC BY-NC. (c) Sabdariffa mechowii. Photograph by bahatiguy, iNaturalist: 156406506, CC BY-NC. (d, e) Sabdariffa menzeliae. Photographs by (d) Geoff Byrne, iNaturalist: 106159528, CC BY-NC. (e) Russell Barrett, Kakadu.
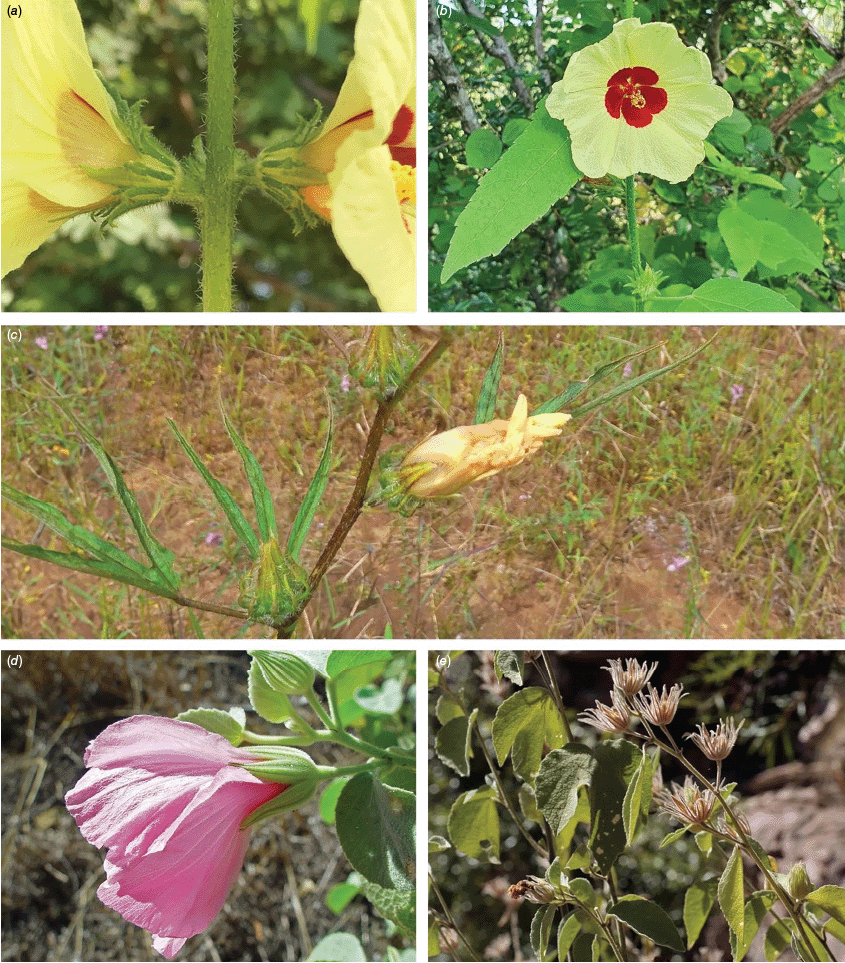
Sabdariffa matogrossensis (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus matogrossensis Krapov. & Fryxell, Bonplandia 13(1–4): 86–88 (2004), non H. scaber Lam. (1789) nec Michx. (1803). Type: Brazil: Mato Grosso: mun. Cáceres, 42 km E de Cáceres (BR-070, km 685), serra das Araras, 30 May 1985, A.Krapovickas, J.F.M.Valls, C.E.Simpson & G.P.Silva 40207 (holo: CEN 00009935!; iso: CTES 0001558!, NY 00007088!, NY 00942240!).
Hibiscus furcellatus var. scaber R.E.Fr., Kongl. Svenska Vetenskapsakad. Handl. 42(1°2): 41 (1908). Type: Brazil: Mato Grosso: Serra do Itapirapuan [Tapirapuan], 20 Apr. 1894, C.A.M.Lindman A. 3303 (lecto, designated by A.Krapovickas & P.A.Fryxell, Bonplandia 13: 86 (2004): S). Residual syn: Brazil: Mato Grosso: inter Coxipó Mirim et Cuyabá [Cuiabá], 17 June 1903, G.O.A.Malme s.n. (UPS V-715111!).
Fries (1908, p. 41); Krapovickas and Fryxell (2004, pp. 86–88, fig. 13); Coutinho and Fernandes-Júnior (2024).
Sabdariffa mechowii (Garcke) Mwachala & R.L.Barrett, comb. nov.
(Fig. 21c, d.)
Hibiscus mechowii Garcke in O.Hoffm., Linnaea 43: 121 (1881). Type citation: ‘Pungo Andongo. Jan.–Apr.’ Type: Angola: Cuanza Norte, s. dat., F.W.A.von Mechow 105 (holo: B, presumed destroyed; drawing at BM 013832979). Neotype (here designated): Angola: Melange Province, Pungo Andongo, Apr. 1857, F.M.J.Welwitsch 5262 (BM 013723928; isoneo: K).
Hibiscus lancibracteatus De Wild. & T.Durand in T.Durand & E.A.J.De Wildeman, Bull. Soc. Roy. Bot. Belgique 38(1): 25 (1899). Type citation: ‘Reg.III: env. de Coquilhatville, 1895 (Alfr. Dewevre).’ Type: Democratic Republic of The Congo: Halte dans une ile située a michamin entre Loukolela et N’Gombi, Mbandaka [Coquilhatville], 17 Nov. 1896, A.Dewèvre 752 (syn: BR 0000008952103; BR 0000008952424, drawing at BM 013832978).
Garcke (1881, p. 121); Durand and de Wildeman (1899, pp. 25–27); de Wildeman and Durand (1901, p. 1, t. 84); Exell (1961, p. 443); Hauman (1963, pp. 113–114); Merxmüller (1969, p. 82:15, 20); Edmonds (1991, p. 19); Wilson (1999, p. 61, fig. 2g); Krapovickas and Fryxell (2004, pp. 48–50, fig. 7); Leistner (2008, p. 115); Mwachala (2009, pp. 44–45).
We here designate a neotype for Hibiscus mechowii as we have been unable to locate any type material and presume this to be destroyed. We have selected a specimen from the same location as the original type to ensure consistency of the species concept. There may be additional duplicates that we have not yet located (see Figueiredo 2008).
Sabdariffa menzeliae (F.D.Wilson & N.Byrnes) McLay & R.L.Barrett, comb. nov.
(Fig. 21e, f.)
Hibiscus menzeliae F.D.Wilson & N.Byrnes, J. Linn. Soc. New South Wales 95: 194–5, t. 11 (1970) (as ‘menzelii’). Type: Australia: Northern Territory: Katherine Gorge, 19 Jan. 1967, N.B.Byrnes 117 (holo: DNA A0014073; iso: NT 0014073, US 00101844).
Wilson and Byrnes (1970, p. 194); Wilson (1974, p. 176); McLay (2022).
Sabdariffa meraukensis (Hochr.) McLay & R.L.Barrett, comb. nov.
(Fig. 22a, b.)
Hibiscus meraukensis Hochr., Annuaire Cons. Jard. Bot. Genève 8: 11–12 (1908). Type: New Guinea: Irian Jaya: Merauke, 30 Aug. 1904, J.W.R.Koch [13] 421 (lecto, designated by J. van Borssum Waalkes, Blumea 14(1): 61 (1966): BO; isolecto: G, L 0012968, L 0012969, L 0012970, L 0012971, L 0012972).
Brockmania membranacea W.Fitzg., Western Mail (Perth) 21(1067); 7, pl. p. 27 (9 June 1906), nom. inval., nom. nud.
Brockmania membranacea W.Fitzg., J. & Proc. Roy. Soc. West. Austral. 3: 174 (1918). Type: Western Australia: Banks of Lennard River, ~10 miles [~16 km] above Wingrah Pass, 8 May 1905, W.V.Fitzgerald 640 (lecto, designated by F.D.Wilson, Austral. J. Bot. 22: 171 (1974): NSW 101036; isolecto: BM 000645648).
Hochreutiner (1908, pp. 11–12); Fitzgerald (1918, p. 174); Hochreutiner (1924, p. 163); Borssum Waalkes (1966, pp. 61–62, fig. 9); Wilson (1974, p. 171); Mitchell (1981, p. 210); Wheeler (1992, p. 217, fig. 59f); Kenneally et al. (1996, p. 122, pl.); Cooper and Cooper (2004, p. 278, fig.); Cowie et al. (2013, p. 14, fig. 4, pl. 8); McLay (2022).
Sabdariffa minkebeensis (Burg) Mwachala & R.L.Barrett, comb. nov.
Hibiscus minkebeensis Burg, Fl. Gabon 45: 59, 62, fig. 17 (2013). Type: Gabon: Woleu-Ntem, Minkébé National Park, southern inselberg area, 686 m, 5 May 2003, L.Ngok Banak 1637, A.Moungazi & P.Mbazza (holo: WAG 0148618; iso: BRLU, HNG, LBV, MO).
Van der Burg (2013, pp. 59, 62, fig. 17).
Sabdariffa minutibracteola (F.D.Wilson) McLay & R.L.Barrett, comb. nov.
Hibiscus minutibracteolus F.D.Wilson, Austral. J. Bot. 22: 178, fig. 6, 11 (1974). Type: Western Australia: Kalumburu, 30 May 1971, N.B.Byrnes 2302 (holo: DNA A0024851; iso: NT A0024851, PERTH 01599879, US 00101845).
Wilson (1974, p. 178, fig. 6, 11); Wheeler (1992, p. 218, fig. 59g); Craven et al. (2003, p. 204); McLay (2022).
Sabdariffa mollis (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, stat. et comb. nov.
(Fig. 22c, d.)
Hibiscus fryxellii var. mollis Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 194, fig. 7 (2003). Type: Western Australia: ~40 km N of Gibb River Homestead on the road to Drysdale Crossing, at crossing of Gibb River, 7 May 1983, P.A.Fryxell & L.A.Craven 3981 (holo [mounted on 3 sheets]: CANB 576816.1, CANB 576816.2, CANB 576816.3; iso: A 00217660, ASU 0019333, BRI AQ0646659 (2 sheets), DNA D0168589 (2 sheets), K 000381794, L 0599609, MEL 2283250, NSW 541865, PERTH 06755704, TEX 00208004).
Craven et al. (2003, p. 194, fig. 7); Craven (2022).
Sabdariffa moxicoensis (Baker f.) Mwachala & R.L.Barrett, comb. nov.
Hibiscus moxicoensis Baker f., J. Bot. 77: 21 (1939). Type: Angola: Moxico: River Luena, near Vila Luzo, ~1240 m, 4 May 1937, A.W.Exell & F.A.Mendonça 1599 (lecto (here designated): BM 000554369; isolecto: BM 000554370, COI 00005093).
Baker (1939, p. 21); Wilson (1999, p. 71, fig. 3e); Leistner (2008, p. 115).
Baker (1939) cited material at BM and COI as the type, therefore a lectotype needs to be selected. We here designate BM 000554369 as the label states that this is the type and a copy of the protologue is attached to this sheet, both of which are absent from the other sheet at BM.
Sabdariffa multiformis (A.St-Hil.) M.C.Duarte & Yoshikawa, comb. nov.
(Fig. 22e, f.)
Hibiscus multiformis A.St-Hil., Fl. Bras. Merid. 4th edn, 1(7): 246 (1828), non Steud. (1840); Hibiscus furcellatus var. multiformis (A.St.-Hil.) Gürke in C.F.P.von Martius et al., Fl. Bras. 12(3): 563 (1892). Type citation: ‘Crescit im paludosis inter vicum vulgo Nossa Sñra da Conçeçao et tabernam vulgo Toporoca. Florebat Martio.’ Type: Brazil: Minas Gerais, Nossa Sñra da Conçeçao, [Mar.] 1816, A.St.-Hilaire Catal. C1 n° 425 (lecto, here designated: P 02285929!; isolecto: MPU 016520!, P 02285927!, P 02285928!). Residual syn: Brazil: Toporoca, 1816, A.St.-Hilaire Catal. C1 n° 890 (syn: MPU 017482!).
De Saint-Hilaire (1828, p. 246); Gürke (1892, p. 563); Krapovickas and Fryxell (2004, p. 70); Rigueiral et al. (2019, pp. 14–15, fig. 4k, l); Camargo et al. (2022, pp. 35–36, fig. 1e); Coutinho and Fernandes-Júnior (2024, fig.).
Krapovickas and Fryxell (2004) designated material at P as the holotype of H. multiformis. However, there are three sheets of the Saint-Hilaire gathering at P (P barcodes 022855927, 022855928 and 022855929). The sheet P 02285929 is here designated as the lectotype as this is the only sheet with a flower at P.
Sabdariffa nanuzae (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus nanuzae Krapov. & Fryxell, Bonplandia 13(1–4): 92–94, fig. 16 (2004). Type: Brazil: Minas Gerais: municipio de Cristália, Morro do Chapéu, ~1200 m, 6 Jan. 1986, J.R.Pirani, C.Kameyama, R.Mello Silva, I.Cordeiro & M.Meguro s.n. [CFCR8923] (holo: SPF 41080!; iso: CTES 0001561!; NY 1043476!).
Krapovickas and Fryxell (2004, pp. 92–94, fig. 16); Rigueiral et al. (2019, p. 15, fig. 6a–c); Coutinho and Fernandes-Júnior (2024).
Sabdariffa ngokbanakii (Burg) Mwachala & R.L.Barrett, comb. nov.
Hibiscus ngokbanakii Burg, Fl. Gabon 45: 62–63, fig. 18 (2013). Type: Gabon: Woleu-Ntem, Oyem, Inselberg Ossapanda, 758 m, 11 Jan. 2003, L.Ngok Banak, J.Lejoly, V.Deman, R.Mboma & S.Lekanga 1357 (holo: LBV; iso: BRLU, WAG 0358239).
Van der Burg (2013, pp. 62–63, fig. 18).
This species is currently considered to be endangered (Ikabanga et al. 2020; The IUCN Red List of Threatened Species, see https://www.iucnredlist.org/).
Sabdariffa nigricaulis (Baker f.) Mwachala & R.L.Barrett, comb. nov.
(Fig. 23a, b.)
Hibiscus nigricaulis Baker f., J. Bot. 77: 19–20 (1939). Type: Angola: Bié: between Coemba and River Cuanza, ~1300 m, 7 May 1937, A.W.Exell & F.A.Mendonça 1759 (lecto, here designated: BM 000554469; isolecto: BM 000554470, COI 00005094).
Hibiscus meeusei Exell, Bol. Soc. Brot., ser. 2, 33: 165 (1959). Type: South Africa: Transvaal, Bronkhorstspruit, 19 Mar. 1959, A.D.J.Meeuse 10646 (holo: BM 000645544; iso: G, NY 221748, P 00389348, PRE 0659511-0, PRE 0285043-0, W 1960-0023394).
Although Baker (1939, p. 20) cited ‘Type in Herb. Mus. Brit.’, there are two sheets of Hibiscus nigricaulis at BM, therefore we here designate BM 000554469 as lectotype as this has the most material on the sheet.
Baker (1939, pp. 19–20); Exell (1959, p. 165); Hauman (1963, p. 110); Merxmüller (1969, p. 82:14, 20); Edmonds (1991, fig. 1(11); 2(31)); Wilson (1999, pp. 60–61, fig. 1e); Leistner (2008, p. 115); Heath and Heath (2009, p. 285, fig.); Bredenkamp (2019, pp. 1205–1206); Hyde et al. (2024, fig.).
Angola, Botswana, Democratic Republic of The Congo, Malawi, Mozambique, Namibia (Caprivi), South Africa, Zambia and Zimbabwe.
Although there is some variation in leaf shape and density of aculei on the calyx across the range of S. nigricaulis, we consider the type of H. meeusei to fall within this variation.
(a, b) Sabdariffa nigricaulis. Photographs by Troos van der Merwe, iNaturalist: 71476923, CC BY-NC. (c, d) Sabdariffa noldeae. Photographs by (c) David J. Harris/RBG Edinburgh, ©. Voucher: Harris 8358 (E). (d) Maël Dewynter, iNaturalist: 222871793, CC BY-NC. (e, f) Sabdariffa paludicola. Photographs by Denise Sasaki/RBG Kew, CC BY. Voucher: D. Sasaki et al. 2286 (K).
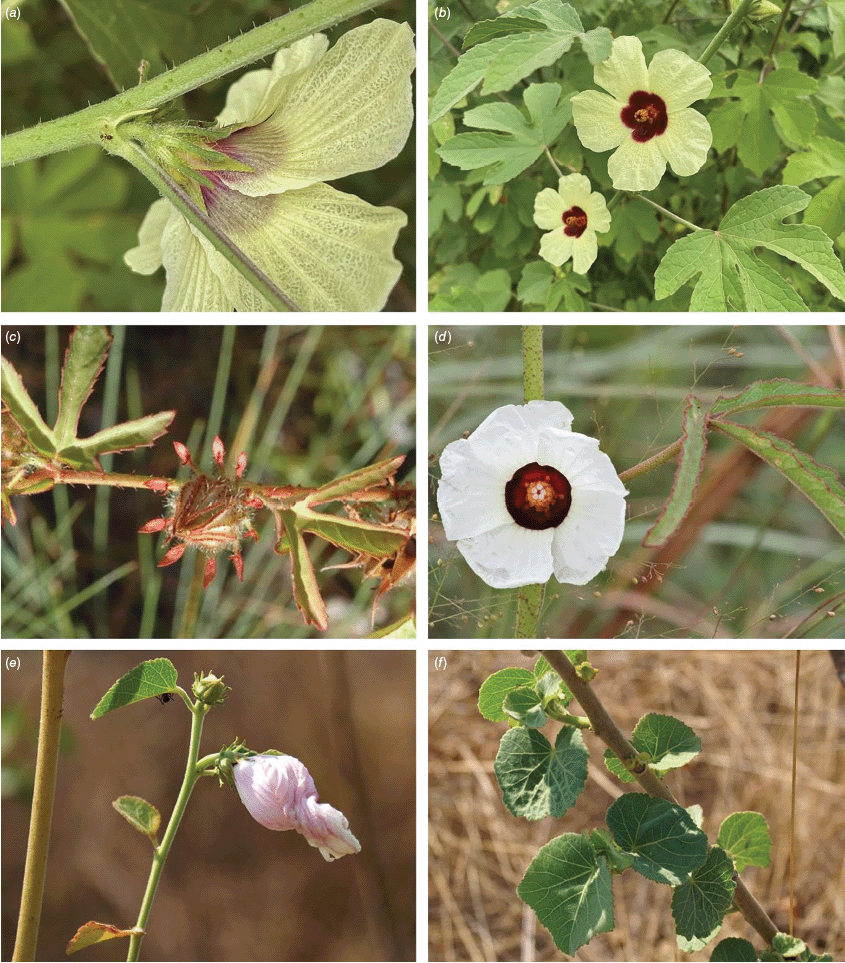
Sabdariffa noldeae (Baker f.) Mwachala & R.L.Barrett, comb. nov.
(Fig. 23c, d.)
Hibiscus noldeae Baker f., J. Bot. 77: 20–21 (1939). Type: Angola: Malange, Quela, Apr. 1938, I. von Nolde 713 (holo: BM 013726657).
Hibiscus eetveldeanus var. asperatus De Wild., Bull. Jard. Bot. État 3: 279 (1911). Type: Democratic Republic of The Congo: Kasai Province, Katola, Apr. 1908, A.Sapin s.n. (lecto, here designated: BR 000000895243; isolecto: BR 000000895214).
De Wildeman (1911, p. 279); Baker (1939, pp. 20–21); Hauman (1963, pp. 119–120); Maquet (1983, p. 383, fig. 121, 6a, b); Wilson (1999, pp. 59–60, fig. 1h); Leistner (2008, p. 115); Mwachala (2009, p. 39); van der Burg (2013, p. 63).
There are two type sheets of Hibiscus eetveldeanus var. asperatus at BR and the sheet with a full flower and original collection slip is here designated as the lectotype.
Mullenders (1954, p. 79) is sometimes attributed as having coined a homonym Hibiscus furcatus Mullend. but this is merely a misapplication of the name, without a description, that actually applies to the concept of S. noldeae.
Sabdariffa paludicola (Fryxell & Krapov.) M.C.Duarte & Yoshikawa, comb. nov.
(Fig. 23e, f.)
Hibiscus paludicola Fryxell & Krapov., Novon 14: 67, fig. 6 (2004). Type: Brazil: Mato Grosso: 3.5 km SE de Barra do Bugres, camino a Cuiabá, 1 June 1985, A.Krapovickas, J.F.Valls, C.Simpson & C.Silva 40211 (holo: CEN 00010491!; iso: CTES 0001562!, CTES 0001563!, K 000581402!. MBM 300350!, NY 00021189!, NY 00888034!, SI 002645!, TEX 00208152!).
Fryxell and Krapovickas (2004, p. 67); Krapovickas and Fryxell (2004, pp. 98–99); Coutinho and Fernandes-Júnior (2024).
Sabdariffa paolii (Mattei) Mwachala & R.L.Barrett, comb. nov.
Hibiscus paolii Mattei in Chiovenda, Result. Sci. Somalia Ital. 1: 32 (1916). Type: Somalia: Bur-Meldàc [=Meel Daaq], 23 July 1913, G.Paoli 699 (holo: FT 002314; iso: PAL [sketch BM 012890]).
Mattei (1916, p. 32); Thulin (1999a, p. 339); Wilson (1999, p. 77).
Wilson (1999, p. 77) only saw a sketch of S. paolii, therefore considered this to be a poorly known taxon related to S. rostellata. Thulin (1999a, p. 339) examined the type at FT (the only known collection) and maintained the species as distinct as this differs significantly in indumentum features.
Sabdariffa partita (Hochr.) M.M.Hanes & R.L.Barrett, comb. nov.
(Fig. 24a, b.)
Hibiscus diversifolius var. partitus Hochr., Annuaire Cons. Jard. Bot. Genève 4: 120 (1900); Hibiscus partitus (Hochr.) F.D.Wilson, Bull. Nat. Hist. Mus. London, Bot. 29(1): 63 (1999). Type: Madagascar: environ de Tananarive, 24 Apr. 1838, J.P.Goudot s.n. (holo: G 00301839).
Hochreutiner (1900, p. 120, 1955, p. 37, fig. X, 9, 10); Wilson (1999, p. 63).
Sabdariffa peruviana (R.E.Fr.) M.C.Duarte & R.L.Barrett, comb. nov.
(Fig. 24c.)
Hibiscus peruvianus R.E.Fr., Kongl. Svenska Vetenskapsakad. Handl. 24(2): 31, Taf. 2, fig. 11–13 (1947). Type: Perú: Stromgebiet des Ucayali von 10°S bis zur Mündung, 1923, G.Tessmann 3072 (syn: B, probably destroyed; S 12-17673).
Fries (1947, p. 31, Taf. 2, fig. 11–13); Macbride (1956, pp. 473–474); Krapovickas and Fryxell (2004, p. 55, fig. 2); Coutinho and Fernandes-Júnior (2024).
Sabdariffa petherickii (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
(Fig. 24d, e.)
Hibiscus petherickii Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 204–206, fig. 13 (2003). Type: Australia: Northern Territory: vicinity of Woolaning Homestead, 4 Apr. 1981, L.A.Craven & C.R.Dunlop 6687 (holo [mounted on 2 sheets]: CANB 642086.1, CANB 642086.2; iso: A 00139250, ASU 0019339, ASU 0019340, DNA D0180179, K 000659893, L, MEL 2172250, TEX 00208007).
Craven et al. (2003, pp. 204–206, fig. 13); Cowie et al. (2013, p. 16, fig. 5); Craven (2022).
Sabdariffa pohlii (Gürke) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus pohlii Gürke in C.F.P.von Martius et al., Fl. Bras. 12(3): 564 (1892). Type citation: ‘Brasilia ad Rio S. Bartholomeo Severino: Pohl n.980, 843…’ Type: Brazil: Mato Grosso: Río S. Bartholomeo Severino, J.B.E.Pohl 980 (lecto: W 0066241!; isolecto: B, destroyed, photo F 0BN009520!), designated by A.Krapovickas & P.A.Fryxell, Bonplandia 13: 77 (2004). Residual syn: Brazil: Río S. Bartholomeo Severino, J.B.E.Pohl 843 (B, probably destroyed, photo: F 9520, n.v.).
Gürke (1892, p. 564); Krapovickas and Fryxell (2004, pp. 77–78); Coutinho and Fernandes-Júnior (2024).
Sabdariffa radiata (Cav.) R.L.Barrett & M.M.Hanes, comb. nov.
Hibiscus radiatus Cav., Diss. 3: 150, t. 54, fig. 2 (1787); Furcaria cavanillesii Kostel., Allg. Med.-Pharm. Flora 5: 1836 (1857), nom. illeg.; Hibiscus cannabinus var. radiatus (Cav.) Chiov., Atti Ist Bot. Univ. Pavia ser. 4, 7: 125–126 (1936). Type: Cultivated, France: Paris, from seeds provided by Banks, 1786, A.J.Cavanilles s.n. (lecto, designated by J. van Borssum Waalkes, Blumea 14(1): 60 (1966, as ‘holotype’): P-JU 12373; isolecto: LECB 0002047, MA 475810).
Hibiscus unidens Lindl., Edward’s Bot. Reg. 11: t. 878 (1825). Hibiscus cannabinus var. unidens (Lindl.) Hochr., Annuaire Conserv. Jard. Bot. Genève 4: 115 (1900). Type citation: ‘Raised at Mr Colvill’s nursery, from Brazilian seed.’ [mid 1824]. Type: ‘Hibiscus unidens’ in Bot. Reg. 11: tab. 878 [BHL].
Hibiscus lindleyi Wall., Pl. Asiat. Rar. 1: 4, t. 4 (1830); Hibiscus radiatus var. lindleyi (Wall.) Kurz, J. Asiat. Soc. Bengal, Pt 2, Nat. Hist. 43: 110 (1874). Type: Burma [Myanmar]: Segaing on Mt Taung Dong, N.Wallich 1895-1 (lecto, designated by J. van Borssum Waalkes, Blumea 14(1): 60 (1966): K-W 001114620; isolecto: G, G, K 000659786, K 001114617; NY 221751).
Hibiscus varians Splitg. ex de Vriese, Nederi. Kruidk. Arch. 1: 338 (1848). Type: Suriname: near Paramaribo, May 1838, F.L.Splitgerber 983 (holo: L 2358281).
Hibiscus heptaphyllus Dalzell & A.Gibson, Bombay Fl. 20–21 (1861). Type citation: ‘Mountain valleys, eastern side of the northern Ghauts.’ (?K, n.v.).
Canhamo braziliensis Perini, Prosp. Expl. Notice Cult. Canh. Braz. 1, plate after title page; fig. 7–12 after page 4; fig. 406 after page 6; fig. after page 8; fig. 4 after page 10; plate after page 12, plate after page 13 (1905); Pavonia perinii Perini, Prosp. Expl. Notice Cult. Canh. Braz. 5 (1905), nom alt. Type: ?K, n.v.
Cavanilles (1787, p. 150, t. 54, fig. 2); Lindley (1825, p. t. 878); Wallich (1830, p. 4, t. 4); Roxburgh (1832, p. 209); de Vriese (1848, p. 338); Hooker (1859, t. 5098); Dalzell and Gibson (1861, pp. 20–21); Kurz (1874, p. 110); Masters (1874, p. 335); Cooke (1901, p. 106); de Perini (1905); Uittien (1932, p. 22); Chiovenda (1936, pp. 125–126); Bates (1965a, p. 79, fig. 19 & 23C); Borssum Waalkes (1966, pp. 60–61); Fryxell (1988, pp. 221–222); Edmonds (1991, fig. 1(4); 2(19); Fryxell (1992b, p. 114); Paul and Nayar (1988, pp. 146–147, fig. 30); Paul (1993, p. 327, fig. 90); Sivarajan and Pradeep (1996, pp. 113–1104, fig. 39); Wilson (1999, p. 59); Fryxell (2000, p. 14); Krapovickas and Fryxell (2004, pp. 44–48); Fayaz (2011, p. 510, fig.).
Canhamo braziliensis Perini has been placed under either Hibiscus cannabinus (e.g. Krapovickas and Fryxell 2004) or H. radiatus (e.g. POWO, see https://powo.science.kew.org/). Hillier (1907, p. 338) assigned the name to ‘Hibiscus radiatus, Sims, not of Benth., doubtfully of Cav.’ based on specimens received at K in April 1907, directly linked to de Perini’s fibre plant. These specimens have not been traced but are likely to be extant at K. Careful examination of the figures included with the protologue suggests that calyx glands are absent, and the leaf lobes are also arranged on one side of the petiole (i.e. spanning ≤180°) and prominently toothed, all characters consistent with S. radiata, therefore we agree with inclusion under this species. A postscript by J. Knight notes that the ‘botanical details’ originally provided in manuscript form were omitted from the final publication (de Perini 1905, [p. 14]) and this has not assisted taxonomic determination of the entity described. De Pereni’s plant is likely identical to the earlier described Hibiscus unidens, also named from material sourced in Brazil.
Considered native to India and Myanmar.
Commonly cultivated and often naturalised in NE Argentina, Bangladesh, SE Brazil, Central African Republic, Colombia, Cuba, Dominican Republic, El Salvador, Galápagos (Ecuador), Honduras, Laos, Leeward Islands, Mexico, Paraguay, Puerto Rico, Thailand, Trinidad and Tobago, southern USA, Venezuela and the Windward Islands.
(a, b) Sabdariffa radiata. Photographs by (a) Frederick Cheong, iNaturalist: 93560526, CC BY-NC. (b) P.S. Sivaprasad, iNaturalist: 198062352, CC BY-NC. (c, d) Sabdariffa reekmansii. Photographs by Bart Wursten, iNaturalist: 109825180. CC BY-NC. (e, f) Sabdariffa riceae. Photographs by Murray Fagg, Northern Territory, APII ©.
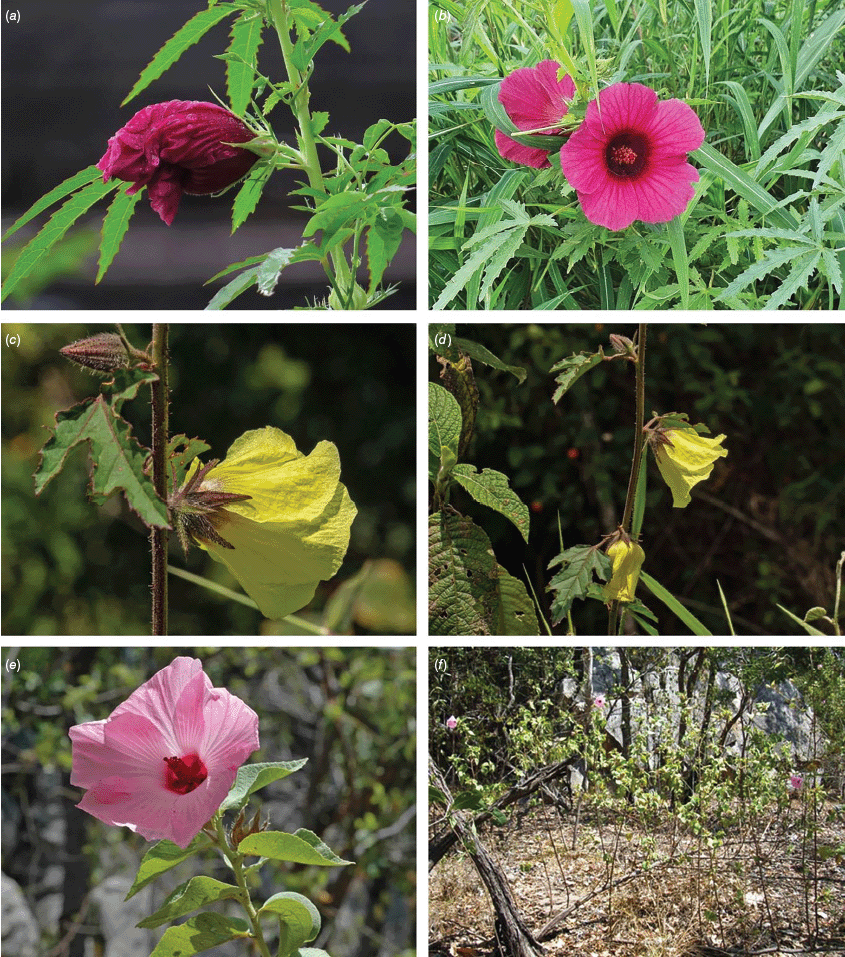
Sabdariffa reekmansii (F.D.Wilson) Mwachala & R.L.Barrett, comb. nov.
(Fig. 25c, d.)
Hibiscus reekmansii F.D.Wilson, Bull. Nat. Hist. Mus. London, Bot. 29(1): 64–65, fig. 2d (1999). Type: Burundi: Muramwya Province, Mptosa road, 17 May 1979, M.Reekmans 7993 (holo: BR 0000005571444; iso: BJA 426026043).
Hibiscus diversifolius var. angustilobus Hauman, Bull. Jard. Bot. Etat. 31: 86 (1961). Type: Democratic Republic of The Congo: Upper Katanga: Sakala Marungu, 2200 m, Apr. 1944, L.Dubois 1157 (holo [mounted on 2 sheets]: BR 0000008952738, BR 0000008952745).
Wilson (1999, pp. 64–65, fig. 2d); Mwachala (2009, p. 42).
Sabdariffa reflexa (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
Hibiscus reflexus Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 206–209, fig. 14 (2003). Type: Western Australia: E shore of Vansittart Bay, 23 May 1993, L.A.Craven, J.McD.Stewart & C.L.Brubaker 9182 (holo [mounted on 2 sheets]: CANB 461648.1, CANB 461648.2; iso: A 00107054, ASU 0019341, BRI AQ0695704, DNA D0180181, G 00353265, K 000659895, L, MEL 2172251, NY, PERTH 06486614).
Wheeler (1992, p. 222, fig. 61d, as Hibiscus sp. A); Craven et al. (2003, pp. 206–209, fig. 14); Craven (2022).
Sabdariffa riceae (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
(Fig. 25e, f.)
Hibiscus riceae Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 209, fig. 15 (2003). Type: Australia: Northern Territory: near Buffalo Spring, Mt Brockman, 4.5 km NNE of Koongarra, 22 May 1980, L.A.Craven 5759 (holo [mounted on 3 sheets]: CANB 309756, CANB 309757, CANB 309758; iso: ASU 0019342, ASU 0019343, DNA D0164059 (2 sheets), K, L 0538642, TEX 00208008).
Craven et al. (2003, p. 209, fig. 15); Craven (2022).
Sabdariffa rostellata (Guill. & Perr.) Mwachala & R.L.Barrett, comb. nov.
(Fig. 26a, b.)
Hibiscus rostellatus Guill. & Perr. in Guill., Perr., & A.Rich., Fl. Seneg. Tent. 1: 55–56 (1831); Hibiscus surattensis var. rostellatus (Guill. & Perr.) Hochr., Annuaire Conserv. Jard. Bot. Genève 4: 113 (1900). Type citation: ‘Crescit in paludosis circà Kounoun in peninsulâ Promontorii-Viridis. Floret à Septembre ad Martium.’ Type: Senegal: G.S.Perrottet (lecto, designated by F.D.Wilson, Bull. Nat. Hist. Mus. London (Botany) 29(1): 53–55 (1999): P 00151940; isolecto: P 00151941). Residual syn: Senegal: Cape Vert, Kounoun, 13 Mar. 1829, G.S.Perrottet 53 & 88 (BM 013726744); Senegal: Cape Vert, Kounoun, 1831, G.S.Perrottet (G); Senegal: 1824, G.S.Perrottet 324 (P 00151942).
Guillemin et al. (1831, pp. 55–56); Andrews (1952, p. 26); Hauman (1963, pp. 117–118); Edmonds (1991, p. 20, fig. 1(8), 2(30)); Wilson (1999, pp. 53–55, fig. 1b); Leistner (2008, p. 115); Mwachala (2009, pp. 39–40); Lejoly et al. (2010, p. 171); van der Burg (2013, pp. 64, 66).
Wilson (1999, p. 53) designated a lectotype for Hibiscus rostellatus at P, stating, “The label on the sheet from P reads as follows: ‘Herbarium Richard [red ink] Hibiscus rostellatus Nob. afigurer [black ink] Senegal, Perottet [red ink]’. Additional sheets at BM and G were considered isolectotypes. Further examination of digital images of these, and additional sheets at the same institutions, shows that more than one collection is involved. The lectotype is accepted here but not the ‘isolectotypes’. An additional sheet at P is accepted as an isolectotype. Leistner (2008, p. 115) lists Welwitsch 5243 (BM, K) as the type but there is no direct evidence that this is original material.
Widespread in tropical Africa, in Angola, Bénin, Burkina Faso, Cameroon, Central African Republic, Chad, Democratic Republic of The Congo, Ethiopia, Gabon, The Gambia, Ghana, Guinea, Guinea-Bissau, Ivory Coast, Kenya, Liberia, Mali, Mozambique, Nigeria, Rwanda, Senegal, Sierra Leone, Sudan, Tanzania, Togo, Uganda and Zambia.
Sabdariffa saddii (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus saddii Krapov. & Fryxell, Bonplandia 13(1–4): 104, fig. 20 (2004). Type: Brazil: Mato Grosso: mun. Chapada dos Guimarães, Casa de Pedra, campo, 30 July 1986, M.Emmerich 6092, E.Santos & E.F.Trinta (holo [mounted on 3 sheets]: R R0001911734!, R0001911734a!, R0001911734b!; iso: CTES 0001565!).
Krapovickas and Fryxell (2004, p. 104, fig. 20); Coutinho and Fernandes-Júnior (2024).
When Krapovickas and Fryxell (2004) described H. saddii, the holotype was simply stated to be preserved at R, however, we located three sheets at R. The three sheets all have the same label with Krapovickas’ handwriting ‘holotype’, the first sheet has an original specimen label and the subsequent two sheets have copies of this label. The barcode numbers associated with these sheets also indicate that these are part of a single ‘specimen’ as the subsequent sheets are designated ‘a’ and ‘b’ respectively. We therefore accept the holotype as being mounted on three sheets, representing three preparations of a single specimen (Art. 8.3; Turland et al. 2018).
Sabdariffa sankowskyorum (Craven) McLay & R.L.Barrett, comb. nov.
(Fig. 26c, d.)
Hibiscus sankowskyorum Craven in Craven et al., Muelleria 35: 6, fig. 1d–f, 3 (2016). Type: Australia: Queensland. Cook District: Brown Creek crossing on the road to Iron Range, on levee of the stream, 9 Aug. l987, J.R.Clarkson 7341 (holo [mounted on 2 sheets]: CANB 572995.1, CANB 572995.2; iso: BRI, L, QRS).
Craven et al. (2016, p. 6, fig. 1d–f, 3); Craven et al. (2022).
Sabdariffa saponaria (Craven) McLay & R.L.Barrett, comb. nov.
(Fig. 27a.)
Hibiscus saponarius Craven in F.D.Wilson & Craven, Austrobaileya 4(3): 442, fig. 2 (1995). Type: Australia: Queensland: Cook District: 4.2 km E of King River on the Edward River to Musgrave road, 3 June. 1989, J.R.Clarkson 8107 & V.J.Neldner (holo [mounted on 2 sheets]: CANB 518986.1, CANB 518986.2; iso: ASU 0019319, BRI AQ0591242, CNS MBA779, K 000659896, K 000659897).
Wilson and Craven (1995, p. 442, fig. 2); Craven (2022); Wannan (2024, 7A, B).
(a) Sabdariffa saponaria. Photograph by Bruce Wannan, ©. Voucher: B.Wannan BW5230 (CNS). (b, c) Sabdariffa sineaculeata. Photographs by Marco Schmidt, iNaturalist: 13515283, CC BY.
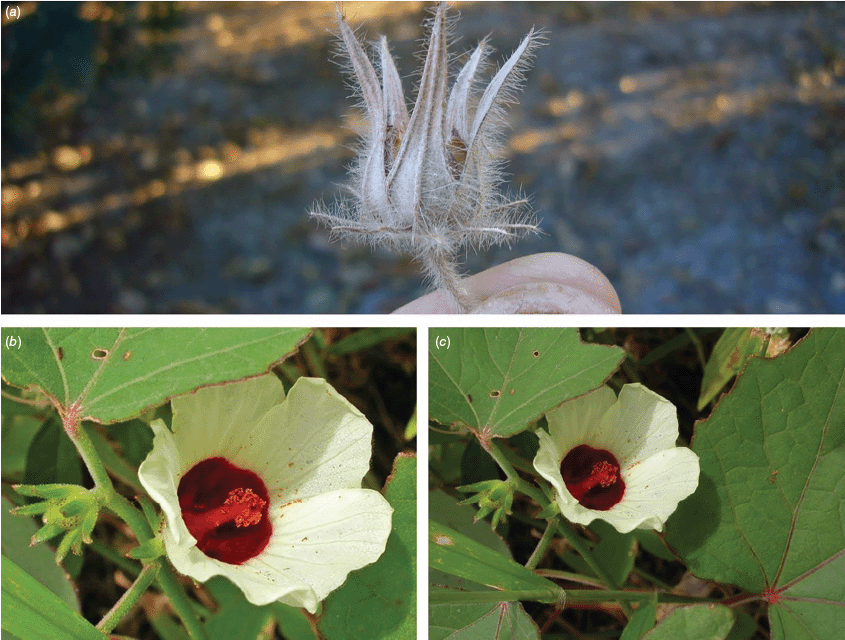
Sabdariffa scabricaulis (Helwig) M.M.Hanes & R.L.Barrett, comb. nov., stat. nov.
Hibiscus furcellatus var. scabricaulis Helwig in Urban, Ark. Bot. 22A(17): 28 (1929). Type: Haiti: Civ. Haiti: Massif de la Hotte, western group, Dame-Marie, Etang-Dérémond, 1 Aug. 1928, E.L.Ekman 10467 (lecto, here designated: S 12-11539; isolecto: K 000535447; S -R-11260).
Helwig (in Urban 1929, p. 28).
We here designate S 12-11539 as the lectotype of Hibiscus furcellatus var. scabricaulis as there is slightly more fertile material on the sheet.
Hibiscus furcellatus var. scabricaulis was accepted by Acevedo-Rodríguez and Strong (2012) based on a passing reference in Liogier (1981, p. 93) but the variety was only noted to have been described, not accepted. This variety is sometimes placed as a synonym of S. maculata Lam. However, the stem bristles in S. maculata have prominently swollen bases and the bristles are usually recurved (bases not or scarcely swollen and bristles straight in H. furcellatus var. scabricualis). Confusion may have arisen as there is a collection of S. maculata by Ekman (P 04698652) from the type location of S. scabricaulis and the assumption may have been made that there would be only one species at this location. The general indumentum and leaf shape in S. scabricaulis are more similar to those in S. costata, though do not appear to directly match that taxon either. Occasionally misspelt in the literature as ‘scabriusculis’.
We have been unable to match the type to any described species and hence choose to recognise this as a distinct taxon at specific rank. This species differs from S. furcellata in stem and calyx indumentum (short stellate hairs with large aculei) and the epicalyx lobes only very slightly bifurcating (commonly appearing simple), similar to S. maculata.
Sabdariffa scotellii (Baker f.) Mwachala & R.L.Barrett, comb. nov.
Hibiscus scotellii Baker f., J. Linn. Soc. Bot. 30: 74–75 (1894). Type citation: ‘on gneissose rocks by Scarcies River, 2 miles [~3.2 km] N of Sasseni, Jan., no. 4535.’ Type: Sierra Leone: Scarcies River, 2 miles [~3.2 km] N of Sasseni, Jan., G.F.Scott-Elliott 4535 (lecto, here designated: K 000240762; isolecto: BM 000645524).
Baker (1894, pp. 74–75); Wilson (1999, p. 73, fig. 3f).
Baker (1894, pp. 65–66) specifies that the first set of specimens collected by Scott-Elliott was retained at K, with duplicates (when available) dispersed to BM, B, HA, CAL and P, in that order of priority. However, this does not constitute designation of holotypes at K, therefore we here select K 000240762 as lectotype as this is an ample sheet with original annotations.
Often confused with S. cannabinna due to a similar habit and highly divided leaves but likely closer to S. aspera and S. verrucosa that share overlapping distributions with S. scotellii. This species differs from all of these taxa in having a short, soft indumentum that suggests a closer relationship with S. sineaculeata.
Sabdariffa sebastianii (Fuertes) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus sebastianii Fuertes, Anales Jard. Bot. Madrid 50(1): 66, fig. 1, 2, 3a–f (1992). Type: Colombia: Caquetá, Sierra de Chiribiquete, Campamento Norte, recorrido a 2 km al SE del campamento, 500–550 m, 12 Dec. 1990, J.Cardiel S.Castroviejo, G.Galeano & F.González 1083 (holo: COL 000002365!; iso: COL 000002362!, COL 000002363!, COL 000002364!, FMB 27723, JAUM 0000213, MA 641943, MEDEL 000195).
Fuertes (1992, p. 65, fig. 1, 2, 3a–f).
Although Fuertes (1992) only cited COL as the location of the holotype, a single sheet at COL is clearly labelled as the holotype by Fuertes and this is considered specific designation, not requiring designation of a lectotype.
Sabdariffa sineaculeata (F.D.Wilson) Mwachala & R.L.Barrett, comb. nov.
(Fig. 27b, c.)
Hibiscus sineaculeatus F.D.Wilson, Bull. Nat. Hist. Mus. London, Bot. 29(1): 72, fig. 3d (1999). Type: Nigeria: Zaria Province, Kan Gimi Veg. mapping area, Dutsen Gwagwa, 20 Oct. 1947, R.W.J.Keay FHI 20117 (holo: K 000580890).
[Hibiscus scotellii Keay, Fl. W. Trop. Afr. (edn 2); 1(2): 347 (1958), non Baker f. (1894), p.p. as to type].
Wilson (1999, p. 72, fig. 3d).
Sabdariffa sparsiaculeata (Baker f.) Mwachala & R.L.Barrett, comb. nov.
(Fig. 28a, b.)
Hibiscus sparsiaculeatus Baker f., J. Bot. 76: 22 (1938). Type: Somalia: Sheik Pass, G.Freemantle s.n. (holo: BM 014117365).
Hibiscus greenwayi var. megensis J.P.Lebrun, Adansonia 15: 379 (1976). Type: Kenya: Nyiro, 4[000]–5000 ft [~1220–1524 m], [1937], A.W.Haylett 12 (holo: K).
Baker (1938, p. 22); Lebrun (1976, p. 379); Edmonds (1991, p. 19); Cheek (1992, fig. 2058); Vollesen (1995, p. 196); Wilson (1999, p. 62, fig. 2h); Thulin (1999b, p. 46, fig. 25g); Mwachala (2009, p. 44).
(a, b) Sabdariffa sparsiaculeata. Photographs by Zarek Cockar, iNaturalist: 206038873, CC BY-NC. (c, d) Sabdariffa splendens. Photographs by (c) Nick Lambert, iNaturalist: 18327064, CC BY-NC-SA. (d) Nathanael Green, iNaturalist: 176241507, CC BY-NC. (e, f) Sabdariffa squarrulosa. Photographs by Russell Barrett, East Kimberley.
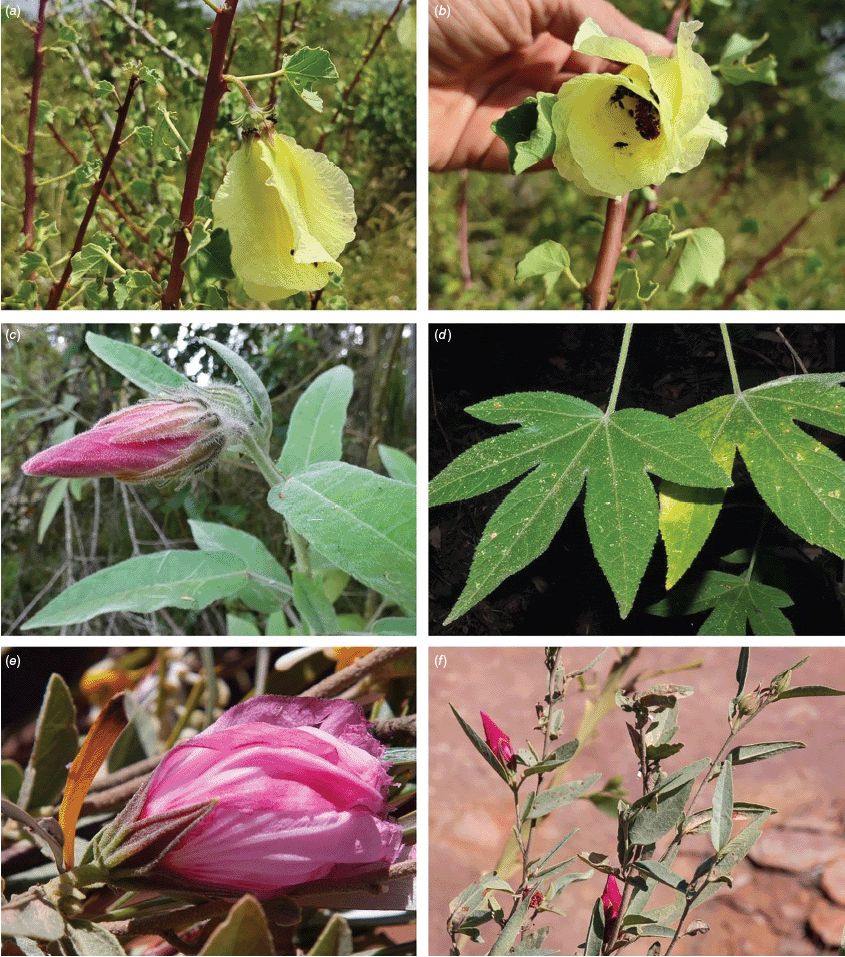
Sabdariffa splendens (C.Fraser ex Graham) McLay & R.L.Barrett, comb. nov.
(Fig. 28c, d.)
Hibiscus splendens C.Fraser ex Graham, Edinburgh N. Phil. J. 175 (1830); Abelmoschus splendens (C.Fraser ex Graham) Walp., Rep. Bot. Syst. 1: 309 (1842); Malvaviscus splendens (C.Fraser ex Graham) Regel, Gartenflora 6: 77 (1857). Type citation: ‘…from New Holland seeds sent by Mr Fraser in November 1828…’ Type: Cultivated, Edinburgh (holo: K 000659876 (photo E 00279411)).
Graham (1830a, p. 175); Graham (1830b, t. 3025); Loddiges (1832, t. 1835); Bentham (1863, p. 213); Bailey (1899, pp. 127–128); Hochreutiner (1900, p. 120); Wilson (1974, p. 165, fig. 1, 16); Wilson and Craven (1995, p. 445); Spencer (1997, p. 383, fig.); Mitchell and Norris (2000, p. 329); Cooper and Cooper (2004, p. 278, fig.); Wilson and Lally (2022).
Although no specific specimen was cited, only a single collection is available matching the protologue and this specimen is clearly the material used for the illustration in Graham, Curtis’s Botanical Magazine 57: t. 3025 (1830) [BHL], noted to be the first recorded flowering in England. As only a single specimen can be identified, this is accepted as a holotype. This matching specimen was overlooked by Wilson (1974) who designated an earlier collection by Fraser as a neotype: [New South Wales:] Hastings River, N. Holl., 1825, C.Fraser s.n. (neotype: E), that was a superfluous designation given that original material is extant, as noted by Lauener and Paul (1985) and Wilson and Craven (1995).
Sabdariffa squarrulosa (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
(Fig. 28e, f.)
Hibiscus squarrulosus Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 212, fig. 17 (2003). Type: Western Australia: Whale Mouth Cave, Osmond Range, 20 July 1991, I.D.Cowie 1919 (holo: CANB 642089; iso: DNA D0059410, PERTH 01823930).
Craven et al. (2003, p. 212, fig. 17); Barrett (2015, fig. 12b); Craven (2022).
Sabdariffa stewartii (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
(Fig. 29a, b.)
Hibiscus stewartii Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 212–213, fig. 18 (2003). Type: Western Australia: S shore of Prince Regent R. (~10 km from mouth), 8 June 1985, P.A.Fryxell, L.A.Craven & J.McD.Stewart 4695 (holo [mounted on 3 sheets]: CANB 642083.1, CANB 642083.2, CANB 642083.3; iso: ASU 0019313, ASU 0019314, DNA D0180028, K 000659898, L, MEL 2172254, NY 00689137, PERTH 06486649, TEX 00208009).
Craven et al. (2003, pp. 212–213, fig. 18); Craven (2022).
Sabdariffa subdiversifolia (Hochr.) M.M.Hanes & R.L.Barrett, comb. nov.
Hibiscus subdiversifolius Hochr., Annuaire Cons. Jard. Bot. Genève 20: 83–85 (1917); Hibiscus diversifolius var. subdiversifolius (Hochr.) Hochr., Fl. Madagascar, Malvac. 40–41, fig. 11, 1–2 (1955). Type citation: ‘Madagascar. loco collector haud indicato (n. 22).’ Type: Madagascar: s. loc. [Près de Marovoay, Boina], s. dat., H.Perrier de la Bâthie 5464 [as 22 at G (see Hanes et al. 2022 for clarification on numbering)] (lecto, here designated: G 00014297; isolecto: P 030950, P 030951).
Hochreutiner (1917a, pp. 83–85); Hochreutiner (1955, pp. 40–41, fig. 11, 1–2); Wilson (1999, p. 61).
Sabdariffa sudanensis (Hochr.) Mwachala & R.L.Barrett, comb. nov.
Hibiscus sudanensis Hochr., Annuaire Cons. Jard. Bot. Genève 10: 18–19 (1906); Hibiscus rostellatus var. sudanensis (Hochr.) Hauman, Fl. Afr. Centr. 10: 119 (1963). Type: Central African Republic: territorie de l’Oubagangi entre le porta de la Noaca et le fort Sibut, 10 Dec. 1903, A.J.B.Chevalier 10757 (lecto, here designated: P 00151944; iso: G 00015130, P 00389358).
Hibiscus sudanensis var. genuinus Hochr., Annuaire Cons. Jard. Bot. Genève 10: 19 (1906), nom. inval.
Hibiscus sudanensis var. glabrescens Hochr., Annuaire Cons. Jard. Bot. Genève 10: 19 (1906). Hibiscus sudanensis f. grandiflorus Hochr., Annuaire Cons. Jard. Bot. Genève 10: 20 (1906), nom. illeg. Type: Central African Republic: Haut Oubangui: rég. de Krébedjé, A.J.B.Chevalier 63xz (lecto, here designated: P 00151945; isolecto: G 00014294, P 00389359, P 00389360).
Hibiscus sudanensis f. minoriflorus Hochr., Annuaire Cons. Jard. Bot. Genève 10: 20 (1906). Type: Central African Republic: territore du Chari, vallèe du Boro, 2 Jan. 1903, A.J.B.Chevalier 7104 (lecto, here designated: P 00151943; isolecto: G 00014295, P 00389361).
Hibiscus rostellatus var. congolanus Hauman, Bull. Jard. Bot. État. 31: 86–87 (1961). Type: Democratic Republic of The Congo: District Forestiere Centrale, Yangambi, vallée marécageuse de la Lilanda, 470 m, 15 Dec. 1937, J.Louis 7032 (holo: BR 0000008953254; iso: BR 0000008953582, K 000240746, WAG 0002316, YBI 177575079).
Hochreutiner (1906, pp. 18–20); Andrews (1952, p. 26); Hauman (1961, pp. 86–87; 1963, p. 119); Wilson (1999, p. 57, fig. 2e); van der Burg (2013, p. 69).
We here designate P 00151944 as lectotype of Hibiscus sudanensis as this is the best material with original labels and annotations by Hochreutiner.
Hochreutiner (1906, p. 19) did not list specimens directly under the variety Hibiscus sudanensis var. glabrescens, rather only under the forms of the variety. However, there is a note specifying that Chevalier 10757 is the type of the species name and var. genuinus, and that Chevalier ‘XZ’ [=63xz] is the type of var. glabrescens (Hochreutiner 1906, p. 20). As Chevalier 63xz is the type of forma grandiflorus, that name is illegitimate.
We here designate P 00151943 as lectotype of Hibiscus sudanensis f. minoriflorus as thisis the best material with original labels and annotations by Hochreutiner.
Hibiscus rostellatus var. congolanus is sometimes included under S. rostellata (e.g. POWO, see https://powo.science.kew.org/) but the leaf shape, indumentum and calyx features are a better match for S. sudanensis. Lejoly et al. (2010, p. 171) accept the variety as named but we do not currently see justification for recognition without a broader review of variation.
Although the species epithet recognises Sudan, this reflects previous political boundaries and the type was collected in what is currently the Central African Republic. The species dubiously occurs in Sudan as currently recognised.
Sabdariffa superba (C.A.Gardner) McLay & R.L.Barrett, comb. nov.
(Fig. 29c, d.)
Hibiscus superbus C.A.Gardner, West. Austral. Forests Dept. Bull. 32: 64, fig. (1923), non Bergmans (1924). Type: Western Australia: West Kimberley, 1901, F.M.House s.n. (lecto, here designated: PERTH 01600311; isolecto: PERTH 01600303).
Gardner (1923, p. 64, fig.); Wheeler (1992, p. 222, fig. 61b); Wilson (1974, p. 179); McLay (2022).
Sabdariffa surattensis (L.) M.M.Hanes & R.L.Barrett, comb. nov.
(Fig. 29e, f.)
Hibiscus surattensis L., Sp. Pl. 2: 696 (1753); Furcaria surattensis (L.) Kostel., Allg. Med. Pharm. Fl. 5: 1856 (1836); Hibiscus involucratus Salisb., Prodr. Stirp. Chap. Allerton 384 (1796), nom. illeg., non (Hochr.) M.M.Hanes, G.E.Schatz & Callm. (2020), nom. illeg.; Hibiscus convolvulaceus Hassk., Abh. Naturf. Ges. Halle 9: 216 (1866), nom. illeg. Type: G.E.Rumphius, Herb. Amboin. 4: 40, t. 16 (1743) [BHL].
Hibiscus trinitarius Noronha, Verh. Batav. Genootsch. Kunst. 5(Art. 4): 17 (1790), nom. inval., nom. nud.
Hibiscus bifurcatus Blanco, Fl. Filip. 545 (1837), nom. illeg., non Cav. (1787). Type: Philippines: Luzon, Bulacan Province, Angat, Dec. 1914, E.Merrill Sp. Blancoana 670 (neo, designated by J. van Borssum Waalkes, Blumea 16(1): 58 (1966): GH; isoneo: BM 013824035, BO, L, P, US).
Hibiscus hypoglossus Harv. & Sond., Fl. Cap. 1: 177 (1860), nom. inval., pro syn.
Hibiscus surattensis var. genuinus Hochr., Annuaire Cons. Jard. Bot. Genève 4: 111 (1900), nom. inval.
Hibiscus surattensis var. villosus Hochr., Annuaire Cons. Jard. Bot. Genève 4: 112 (1900). Type: Nyassaland [Malawi]: ~1200 m, 17 Apr. 1897, H.Johnston per A.Whyte 1896 (lecto, here designated: G 00301841; isolecto: K).
Hibiscus surattensis f. bicolor Backer, Fl. Batavia 1: 129 (1907), nom. inval. [see ICN Art. 26.2 (Turland et al. 2018).
Hibiscus surattensis f. concolor Backer., Fl. Batavia 1: 129 (1907). Type: Indonesia: Java, Tandjungpriok, C.A.Backer 32783 (syn: BO, n.v.).
Hibiscus surattensis f. immaculatus Kurz ex Rakshit & Kundu, Sci. & Cult. 27: 194 (1961) (as ‘immaculata’). Type: Burma: Pegu Yomah, W.S.Kurz 1248 (holo: CAL, n.v.).
Linnaeus (1753, p. 696); Salisbury (1796, p. 384); Blanco (1837, p. 545); Wight (1839, pl. 197); Blanco (1845, p. 380); Hasskarl (1866, p. 74); Blanco (1879, p. 334, t. 347); Trimen (1893, p. 152); Backer (1907, p. 129); Keay (1958, p. 346); Andrews (1952, p. 26); Exell (1961, p. 438, t. 89/4); Rakshit and Kundu (1961, p. 194); Hauman (1963, pp. 121–122); Borssum Waalkes (1966, pp. 58–59); Exell and Gonçalves (1979, p. 25, fig. 8, 4); Gibson (1975, p. 64, fig. 6); Hochreutiner (1955, p. 37, fig. X, 1–3); Marais and Friedmann (1987, p. 37, fig. 11 (2–4)); Paul and Nayar (1988, pp. 153–155, fig. 32); Edmonds (1991, p. 15, fig. 1(3); 2(18)); Pradeep and Sivarajan (1991, p. 636, fig. 7, 8); Chang (1993, p. 742); Paul (1993, pp. 327–329, fig. 91); Vollesen (1995, p. 198); Sivarajan and Pradeep (1996, pp. 119–123, fig. 42); Philcox (1997, pp. 293–294); Wilson (1999, pp. 51–53); Leistner (2008, p. 115); Mwachala (2009, p. 38); Lejoly et al. (2010, p. 171); Fayaz (2011, p. 511, fig.); van der Burg (2013, pp. 69–70, fig. 21); Hyde et al. (2024, fig.).
Jarvis (2007) determined that the sheet commonly cited as the type of Hibiscus surattensis [India: Gujarat, Surat, LINN 875.29] is not original material as ‘the sheet lacks the relevant Species Plantarum number (i.e. ‘12’), and was a post-1753 addition to the collection.’ Jarvis (2007) states that the only original material identified for the name is the plate in Rumphius’ Herb. Amboin. 4: 40, t. 16 (1743).
We designate G 00301841 as lectotype of Hibiscus surattensis var. villosus as this has the most material and original annotations by Hochreutiner.
Widespread in sub-Saharan Africa, also in the Indian subcontinent and south-east Asia, native to Angola, Bangladesh, Bénin, Borneo, Burundi, Cambodia, Cameroon, Central African Republic, southern China, Democratic Republic of The Congo, Ethiopia, Gabon, The Gambia, Ghana, Guinea, Guinea-Bissau, Gulf of Guinea Islands, India, Ivory Coast, Kenya, Laos, Liberia, Malawi, Malaysia, Mozambique, Myanmar, Nigeria, Philippines, Republic of Cabo Verde, Senegal, Sierra Leone, South Africa (Northern Provinces) Sri Lanka, Sudan, Tanzania, Thailand, Togo, Uganda, Vietnam, Zambia and Zimbabwe.
Considered introduced in the Comoros, Madagascar, Mauritius, Réunion and the Seychelles but further study may be warranted.
Sabdariffa symonii (F.D.Wilson & N.Byrnes) McLay & R.L.Barrett, comb. nov.
(Fig. 30a, b.)
Hibiscus symonii F.D.Wilson & N.Byrnes, J. Linn. Soc. New South Wales 95: 195–6, t. 12 (1970). Type. Australia: Northern Territory: adjacent to Alligator River crossing on road to Oenpelli, 11 June 1967, D.E.Symon 5153 (holo: CANB 209244: iso: AD 98674331, US 00101853).
Wilson and Byrnes (1970, p. 195); Wilson (1974, p. 176); Wilson and Lally (2022).
(a, b) Sabdariffa symonii. Photographs by (a) Patsy1955, iNaturalist: 88670951, CC BY-NC. (b) Lyn Craven, APII, ©. (c, d) Sabdariffa townsvillensis. Photographs by Russell Cumming, iNaturalist: 109901123, CC BY-NC. (e, f) Sabdariffa uncinella. Photographs by (e) Juan Carlos López Domínguez, iNaturalist: 103608478, CC BY-NC. (f) Alexis López Hernández, iNaturalist: 155680635, CC BY-NC.
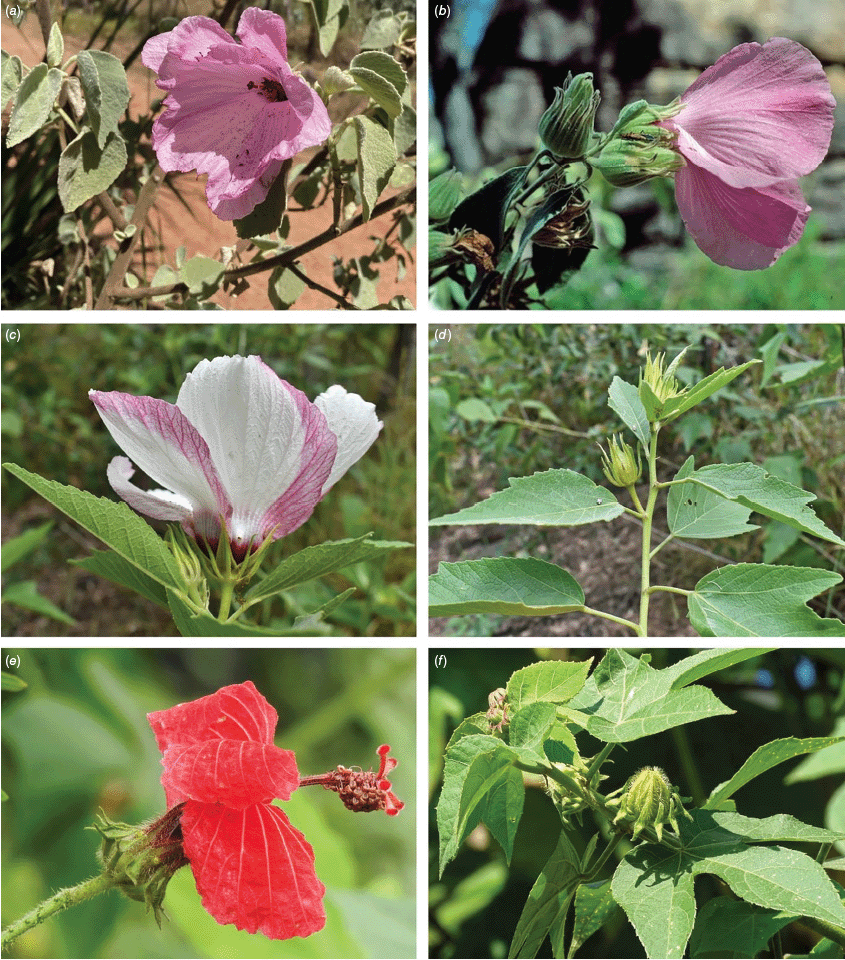
Sabdariffa thegalea (Craven, F.D.Wilson & Fryxell) McLay & R.L.Barrett, comb. nov.
Hibiscus thegaleus Craven, F.D.Wilson & Fryxell, Austral. Syst. Bot. 16: 213–217 (2003). Type: Australia: Northern Territory; 16 km S of Yaimanyi Creek, 24 June 1972, N.B.Byrnes 2687 (holo: CANB 233850; iso: ASU 0019321, DNA A0032757, K, L, NSW, NT A0032757).
Craven et al. (2003, pp. 213–217); Craven (2022).
Sabdariffa torrei (Baker f.) Mwachala & R.L.Barrett, comb. nov.
Hibiscus torrei Baker f., J. Bot. 75: 101 (1937). Type: Mozambique: Niassa, Vila Cabral, July 1934, A.R. da Torre 435 (lecto: COI 00005068; isolecto: BM 000645520, BM 000645521, COI 00005069, G, K 000240670, LMA 0020554-0, LMA 0020554-1).
Baker (1937, p. 101); Wilson (1999, p. 59, fig. 1g).
Baker (1937, p. 101) specifies ‘Type in Herb. Conim., specimen in Herb. Mus. Brit.’ but the location of duplicates precludes the acceptance of a holotype. We here designate COI 00005068 as lectotype as this is an ample specimen with an original label and descriptive working notes attached.
Sabdariffa townsvillensis (Craven) McLay & R.L.Barrett, comb. nov.
(Fig. 30c, d.)
Hibiscus townsvillensis Craven in L.A.Craven et al., Muelleria 35: 9, fig. 1g–i, 4 (2016). Type: Cultivated. Australian Capital Territory: CSIRO glasshouse, Black Mountain, Dec. 2003, L.A.Craven 10469 (holotype (mounted on 6 sheets): CANB 875440.1, CANB 875440.2, CANB 875440.3, CANB 875440.4, CANB 875440.5, CANB 875440.6; isotypes: A, ASU, B, BISH, BRI, CNS, G, K, L, MEL 2417498, NY, P 04023447, US).
Craven et al. (2016, p. 9, fig. 1g–i, 4); Craven et al. (2022).
Sabdariffa trilineata (A.St.-Hil. & Naudin) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus trilineatus A.St.-Hil. & Naudin, Ann. Sci. Nat. Bot. Sér. 2, 18: 39 (1842). Type citation: ‘Prov. Goyaz.- Herb. Deless. (Gardn. 3585).’ Type: Brazil: Province de Goiás: Arrayas [Arraia] et Conceição, Feb. 1840 [rec. at G, K & P in 1841], G.Gardner 3585 (holo: G 00353165! (photo F 23732!); iso: BR 0000013323950!, K 000535440!, K 000535439!, P 02285935!, P 02285936!, W 0066244!, W 1889-0009923!).
De Saint-Hilaire and Naudin (1842, p. 39); Gürke (1892, p. 563); Krapovickas and Fryxell (2004, pp. 58–60, fig. 2); Coutinho and Fernandes-Júnior (2024).
Sabdariffa uncinella (Moç. & Sessé ex DC.) M.M.Hanes & R.L.Barrett, comb. nov.
(Fig. 30e, f.)
Hibiscus uncinellus Moç. & Sessé ex DC., Prodr. 1: 449 (1824). Type: Icones Florae Mexicanae s.n. (lecto, designated by P.A.Fryxell & F.D.Wilson, Brittonia 38: 110 (1986): Torner Collection, no. 6331.1422, Hunt Institute [URL], stem, leaves, and buds only, excluding the corolla).
De Candolle (1824, p. 449); Fryxell and Wilson (1986, p. 110, fig. 1; no full description); Fryxell (1988, pp. 229–232, fig. 55); Fryxell (1992b, pp. 119–122); Fryxell (2000, p. 13).
Sabdariffa verrucosa (Guill. & Perr.) Mwachala & R.L.Barrett, comb. nov.
(Fig. 31a, b.)
Hibiscus verrucosus Guill. & Perr. in D.A.Guillemin et al., Fl. Seneg. Tent. 1: 57 (1831); Abelmoschus verrucosus (Guill. & Perr.) Walp., Repert. Bot. Syst. 1: 308 (1842); Hibiscus cannabinus var. verrucosus (Guill. & Perr.) Garcke, Linnaea 43: 56 (1880). Type citation: ‘Crescit in sabulosis humidis insule fluminis Senegal dictæ Sorr, prope Saint-Louis. Floret mensibus Septembre, Octobre et Novembre.’ Type: Senegambie [Senegal]: Ile de Sorr, Oct. 1825, F.M.R.Leprieur s.n. (lecto, here designated: P 06721189).
Guillemin et al. (1831, p. 57); Wilson (1999, p. 68, fig. 2c, in part, as H. asper).
No authentic material of Hibiscus verrucosus has previously been identified. Mwachala (2009, p. 41) cited a collection by A.Petit at P as a possible holotype but this may not be original material. There may be original material of H. verrucosus in the Gay Herbarium, collected in Senegal prior to the description of H. verrucosus and used in preparation of the Flora Senegambiae Tentamen (see Gillett 1962) but collection details on specimens examined to date in the Gay Herbarium do not match the protologue. A sheet at BM may be original material: Senegal, Feb. 1825, Perrottet 55 (BM 013730377) but this requires confirmation. Searching of digitised collections at P, however, has identified material matching the protologue location and month of collection, and bearing the name Hibiscus verrucosus. Leprieur is also mentioned as a collector in the preface to Guillemin et al. (1831). This specimen (P 06721189) is here designated as lectotype of H. verrucosus as we consider the possibility for any other potential original material to be unequivocally linked to the protologue unlikely, and we consider the fixation of the application of this name with a lectotype to be highly desirable.
Hochreutiner (1900) and many other authors have treated S. verrucosa as a synonym of S. cannabina but usually without knowledge of the type material of S. verrucosa. Wilson (1999) suggested that this may well be an earlier name for S. aspera. Location of original material allows these competing ideas to be critically assessed.
Collections from Richard Toll, Senegal (J.G. Adams 11112; P 02143634) and Auwie, Senegal (J.Trochain 1067; P 06721038) appear to be a good match for the lectotype of S. verrucosa, bearing entire leaves. Another collection from Richard Toll, of significance for understanding variation in this species, has very deeply divided leaves, therefore the entire leaves on the lectotype may reflect young or rapid growth. Specimens from Guinea and Mali with a combination of entire and 3-lobed leaves also fit with our concept of S. verrucosa (e.g. J.G.Adams 11699; P 02143597).
Wilson’s (1999, p. 68) concept of S. aspera includes our concepts of both S. cordofana and S. verrucosa, and our concept of S. aspera. Although the delimitation of taxa in this complex will require further study by local workers, we here distinguish two groups of species based on whether the epicalyx lobes are ±flat (when dried, somewhat fleshy-thickened when fresh) with stiff hispid hairs only (S. aspera) or have distinct marginal ribs and prominent aculei (S. cordofana and S. verrucosa). We recognise S. cordofana from East Africa as a related but disjunct taxon relative to our West African concept of S. verrucosa, both of which have previously been confused with S. aspera.
Sabdariffa verrucosa is closely allied to S. cordofana in having epicalyx lobes with distinct marginal ribs but S. verrucosa has leaves variously entire, shallowly lobed or deeply lobed as the plants mature (v. climax leaves consistently 3–5-lobed for at least half the length), large (v. small) scattered aculei on the stems, and the epicalyx and calyx with a few stiff hairs and long, very prominent aculeli on margins and ribs (v. epicalyx and calyx glabrous except for large, prominent aculei on the margins).
Sabdariffa wilsonii (Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus wilsonii Fryxell, Brittonia 25: 80 (1973). Type: Brazil: Distr. Federal, Córrego Landim, ~25 km N of Brasilia, 850 m, H.S.Irwin, R.Souza & R.Reis dos Santos 12057 (holo: NY 00221746!; iso: CTES 0001568!, CTES 0001569!, F 0076511F!, UB 00037478!, US 00101855!).
Fryxell (1973, p. 80); Krapovickas and Fryxell (2004, pp. 53–54, fig. 1a–c); Rigueiral et al. (2019, p. 18, fig. 6j–l); Coutinho and Fernandes-Júnior (2024).
Sabdariffa windischii (Krapov. & Fryxell) M.C.Duarte & Yoshikawa, comb. nov.
Hibiscus windischii Krapov. & Fryxell, Bonplandia 13(1–4): 54–55, fig. 8 (2004). Type: Brazil: Mato Grosso, mun. Ponte Branca, ~10 km da estrada para Guiratinga, 15 June 1991, P.Windisch & L.Amorim 6157 (holo: SJRP 00006979!; iso: CTES 0001570!).
Krapovickas and Fryxell (2004, pp. 54–55, fig. 8); Coutinho and Fernandes-Júnior (2024).
Sabdariffa zonata (F.Muell.) McLay & R.L.Barrett, comb. nov.
(Fig. 31c, d.)
Hibiscus zonatus F.Muell., Fragm. 1: 221 (1859). Type citation: ‘In clivis rupestribus ad ripas fluviorum Seven Emu et MacArthur River sinus Carpentariae.’ Type: Australia: Northern Territory: Seven Emu River [Creek], 1857, F.Mueller s.n. (lecto, here designated: K 000659823, left-hand piece only). Residual syn: edge of the Landsdown Tableland, Gulf of Carpentaria, 1857, F.Mueller s.n. (MEL 18675); Carpentaria, 1857, F.Mueller s.n. (MEL 18674); Gulf of Carpentaria, 1857, F.Mueller s.n. (K 000659824); Nicholson River, Gulf of Carpentaria, 1857, F.Mueller s.n. (K 000659825, three right-hand pieces on sheet); upper McArthur River, Gulf of Carpentaria, 1857, F.Mueller s.n. (K 000659826).
Hibiscus irritans Hochr., Annuaire Cons. Jard. Bot. Genéve 4: 72 (1900), nom. inval., pro syn.
Hibiscus mustiae F.D.Wilson, Austral. J. Bot. 22: 175, fig. 2, 7 (1974). Type: Australia: Northern Territory: 5 miles [~8 km] N of East Alligator River, 28 June 1972, J.Must 1078 (holo: DNA A0032791; iso: CANB 233870, K, NT A0032791, US 00101846).
Mueller (1859, p. 221); Bentham (1863, p. 215); Bailey (1899, p. 128); Hochreutiner (1900, p. 72); Wilson (1974, p. 174, fig. 17, 18); Cowie et al. (2013, p. 19, fig. 5, pl. 12); McLay (2022).
Mueller’s collection locality details on specimens relative to published protologues are notoriously vague. We here select a single specimen at K (ex MEL) as the lectotype as this can be unambiguously associated with a locality cited in the protologue (Mueller 1859, p. 221). We assign only the left-hand piece to K 000659823, assuming that the remaining three pieces belong to K 000659825.
Unplaced taxa
Hibiscus cannabinus var. chevalieri Hochr., Annuaire Conserv. Jard. Bot. Genève 5: 125 (1901)
Type citation: ‘Koulikoro [?Sarankoro], moyen Niger, terrain rocheux; 6–14 Oct. 1899. Sindou, terres cultivés, parmi les plantations de cotonniers; 10 mai 1899’. Type: n.v.
We have not traced the original material of Hibiscus cannabinus var. chevalieri. Wilson (1999) provided notes on the specimens cited and attributed these to A.J.B.Chevalier but did not see the material either. As we separate H. verrucosus from Wilson’s concept of H. asper, establishment of the taxon of which this variety is most likely to be a synonym is uncertain. The description of the calyx in the protologue suggests that this may be a synonym of H. cannabinus.
Hibiscus elsworthii F.Muell., Fragm. 8(70): 241 (1874)
Type citation: ‘Ad sinum Edgecombe-Bay; Fitzalan’ Type: Queensland: Edgecombe-Bay, E.F.A.Fitzalan (holo: MEL 18677).
Bailey (1913, p. 58).
A name of uncertain application that some workers have suggested may belong in section Furcaria but close examination of the type specimen suggests that this is more likely to be closer to section Lilibiscus Hochr. (B. Wannan, pers. comm.). Wilson (1974, p. 180) also considered this species to fall outside the circumscription of Hibiscus section Furcaria.
Hibiscus keilii Ulbr. in R.E.Fries & T.C.E.Fries, Notizbl. Bot. Gart. Mus. Berlin-Dahlem 8: 681–683 (1924)
Type: Tanzania: (Ostafrika), Usumbura, Luwironsa-Ufer bei Mugera, 4 Aug. 1905, A.Keil 183 (holo: B, probably destroyed; basic illustration of the type at BM 013832977).
Ulbrich (1924, pp. 681–683).
Wilson (1999, p. 75) includes this in an appendix of poorly known species. The distinction from S. diversifolia remains unclear as no authentic material has been located. In addition to the type, Ulbrich (1924, p. 682) also cites a sterile specimen from Kenya (Fries 2049) and POWO (see https://powo.science.kew.org/) records this for the Democratic Republic of The Congo, though the basis for the record is unclear. This is an accepted name in POWO (see https://powo.science.kew.org/) and in the African Plant Database (see https://africanplantdatabase.ch/en/nomen/specie/81556/hibiscus-keilii-ulbr) but we are not confident that H. keilii is distinct from S. diversifolia (and the ‘carmine’ flowers would place this in subsp. agioxillos), therefore we refrain from making a new combination here. We key out H. keilii based on the distinctions noted by Ulbrich (1924, p. 682) but further collections are clearly needed to ascertain the status of this entity. If this taxon does belong under S. diversifolia, then the epicalyx features must have been misinterpreted. A possible relationship with S. rostellata should also be considered.
Hibiscus sect. Furcaria series Saxicolae Ulbr. in A.Engler (ed.) Die Pflanzenwelt Afrikas 3(2): 403 (1921)
Type: Hibiscus saxicola Ulbr.
Hibiscus saxicola Ulbr., Notizbl. Bot. Gart. Mus. Berlin-Dahlem 7: 179–180 (1919)
Type: Cameroon: Bezirk Ebolowa, swischen Posten Sangmelima und Ebolowa, 11 km W of Sangmelima, 700 m, 2 June 1911, J.Mildbraed 5515 (syn: B, possibly destroyed, HBG 509073).
Ulbrich (1919, pp. 179–180).
In the prologue, Ulbrich (1919) placed this species in section Azanza but later (Ulbrich 1921) provided an alternative classification, erecting series Saxicolae under section Furcaria. We are not currently convinced that this species belongs in Sabdariffa and refrain from providing a new combination pending further study.
Data availability
The data that support this study are available in the article or in cited publications.
Conflicts of interest
Dr Russell Barrett is an editor for Australian Systematic Botany but not involved in the peer review or decision-making process for this paper. Australian Systematic Botany encourages in-house editors to publish in the journal but these editors are kept totally separate from the decision-making processes for personal manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
Aspects of this study were funded by a Postdoctoral Fellowship Grant from the Australian Biological Resources Study (ABRS) National Taxonomy Research Grant Program (NTRGP-154) to Sarah Matthews and collaborators at CSIRO, Canberra, that employed T. B. G. McLay.
Acknowledgements
We are grateful to Jacek Wajer for critical insights into the history of specimens associated with Miller’s Gardener’s Dictionary that form the basis of our decision to select a neotype for Hibiscus gossypiifolius Mill. We thank David Mabberley for numerous discussions on Hibiscus nomenclature and offering pragmatic suggestions for resolution of nomenclatural issues. Rebecca Le Get kindly located specimens at MEL that were previously overlooked types. Dr Myriam C. Peichoto (CTES), Dr Marina de Lourdes Fonseca (IBGE), Dr Jordan Teisher (MO), Dr Daniela Sampaio (SJRP) and Dr Stefan Ekman (UPS) sent high quality images of the types. Many additional herbarium staff have assisted us with visits to collections over the last 30 years. Matt Barrett provided considerable field assistance and observations for species from the Kimberley region of Western Australia. Thomas Mesaglio kindly added Section Furcaria to the appropriate taxa in iNaturalist to make sorting and updating images within that web platform simple. We thank Bruce Wannan for discussion of problematic taxon boundaries in eastern Australian species and providing a draft of his publication of Hibiscus cummingii. Miguel Garcia assisted with access to the New South Wales Herbarium library while the building was undergoing renovations. We thank Peter de Lange, Muthama Muasya and an anonymous referee for constructive comments that improved the manuscript. Brendan Lepschi provided a thorough review of the nomenclature presented and provided many comments that improved the manuscript. We thank the following people for permission to include photographs, many of which were posted on iNaturalist under Creative Commons licences as noted in the captions: Kathryn Akamine, Matt Barrett, Tony Bean, Andre Benedito, Alicia Beverage, Francisco V. Bezerra Neto, A. W. Bonné, Elsa Bussiere, Geoff Byrne, Frederick Cheong, Zarek Cockar, Thales Coutinho, Tara L. Crewe, Russell Cumming, Maël Dewynter, Juan Carlos López Domínguez, Murray Fagg, Jo Floer, Riana Fourie, Phoebe French, Rejoice Gassah, Koech Gerald, Victor Gonçalez, Nathanael Green, David J. Harris, Oliver Haumann, Alexis López Hernández, Sarvadaman Kulkarni, Nick Lambert, Paul Latham, Kevin Lester, Jon Luly, Sarah Macdonald, Elizabeth Magne, Mauricio Mercadante, Thomas Mesaglio, Duncan McKenzie, Andrew C. Mitchell, Daniel B. Montesinos-Tubée, Jurga Motiejūnaitė, James Kuria Ndung’u, Ben Newham, Brandon Najarian, Luis Angel Aguilar Orea, Kiran Otsarg, Germaine Alexander Parada, Julien Piolain, Mike Plagens, Franck Rakotonasolo, Juliana Restrepo, William D. Reynolds, Ian C. Riddell, Fabrício Mil Homens Riella, Sabarni Sarker, Denise Sasaki, Marco Schmidt, Jason Searle, Erik Simons, P. S. Sivaprasad, Swarochi Tathagath, Dinesh Valke, Troos van der Merwe, Radha Veach, Marcia Vermaire, Bruce Wannan, Nicholas Wightman, Stuart Worboys, Bart Wursten, Danilo Zavatin; and iNaturalist contributors: chabiboni1972, livexplores and Patsy1955. The late Lyn Craven and John Wrigley provided images to the Australian Plant Image Index and we are grateful to Anna Monro for facilitating permission to use these images.
Author contributions
All authors conceived the study, conducted fieldwork, interpreted results, prepared nomenclature treatments, and wrote and revised the paper.
References
Abdallah MS, Sa’ad F, El-Kholy SA (1976) Taxonomical studies in the flora of Egypt VI. Culinary cultivars of Hibiscus sabdariffa L. in Egypt. Notes Agricultural Research Centre Herbarium Egypt 3, 1-20.
| Google Scholar |
Abdullah MF, Mehmood F, Shahzadi I, Waseem S, Mirza B, Ahmed I, Waheed MT (2020) Chloroplast genome of Hibiscus rosa-sinensis (Malvaceae): comparative analyses and identification of mutational hotspots. Genomics 112(1), 581-591.
| Crossref | Google Scholar | PubMed |
Acevedo-Rodríguez P (2005) Augustín Stahl. In ‘Flora of Puerto Rico and the Virgin Islands’. (National Museum of Natural History: Washington, DC, USA) Available at https://naturalhistory.si.edu/research/botany/research/flora-puerto-rico-and-virgin-islands/agustin-stahl
Acevedo-Rodríguez P (2007) The extant botanical collections and nomenclatural types of Agustín Stahl, Puerto Rican botanist. Caribbean Journal of Science 43, 189-199.
| Crossref | Google Scholar |
Acevedo-Rodríguez P, Strong MT (2012) Catalogue of seed plants of the West Indies. Smithsonian Contributions to Botany 98, 1-1192.
| Crossref | Google Scholar |
Amany M, Eisa SS, Mohamed AA, Al-Sharney I (2020) Taxonomic revision and numerical analysis of Hibiscus L. in Egypt. Arab Universities Journal of Agricultural Sciences 28, 117-130.
| Crossref | Google Scholar |
Ahmed AK, Johnson KA (2000) Horticultural development of Australian native edible plants. Australian Journal of Botany 48, 417-426.
| Crossref | Google Scholar |
Akpan GA (2000) Cytogenetic characteristics and the breeding system in six Hibiscus species. Theoretical and Applied Genetics 100, 315-318.
| Crossref | Google Scholar |
Akpan GA (2007) Hibiscus. In ‘Flower breeding and genetics’. (Ed. NO Anderson) pp. 479–489. (Springer: Dordrecht, Netherlands) 10.1007/978-1-4020-4428-1_17
Alain L, B (1953) Flore de Cuba. III. Contribuciones Ocasionales del Museo de Historia Natural del Colegio “De La Salle” 13, 251-257 [In Spanish].
| Google Scholar |
Albuquerque S, Brummitt RK, Figueiredo E (2009) Typification of names based on the Angolan collections of Friedrich Welwitsch. Taxon 58, 641-646.
| Crossref | Google Scholar |
Alessandrini A (1840) Rendiconto delle sessioni dell’Accademia delle Scienze dell’Istituto di Bologna, Sessione del 21 Febbraio 1839. Nuovi Annali di Scienze Naturali 3, 135-143 [In Italian].
| Google Scholar |
Alexopoulou E, Papatheohari Y, Christou M, Monti A (2013) Origin, description, importance, and cultivation area of kenaf. In ‘Kenaf: a multi-purpose crop for several industrial applications’. (Eds A Monti, E Alexopoulou) Green Energy and Technology Series. pp. 1–15. (Springer: London, UK) 10.1007/978-1-4471-5067-1_1
Alm J, Linnaeus C (1775) D.D. Plantae Surinamenses, quas, venia experient. facult. medicae, in Reg. Acad. Upsaliensi, praeside viro nobiliss. atque generoso D:no Doct. Carolo Linné. Doctoral thesis, Uppsala Universitet. (Typis Edmannianis: [Uppsala, Sweden]) [In Latin] 10.5962/bhl.title.59868
Al-Snafi AE (2018) Pharmacological effects and therapeutic properties of Hibiscus cannabinus – a review. Indo American Journal of Pharmaceutical Sciences 5, 2176-2182.
| Crossref | Google Scholar |
Anjah GM, Ogunsanwo OY, Jimoh SO, Forjoh JN, Tsombou FM (2012) Assessment of regeneration potential of Hibiscus sabdariffa L. under established ecosystems in Cameroon. Journal of Horticulture and Forestry 4(6), 96-102.
| Crossref | Google Scholar |
Areces Berazaín F (2006) Las especies cubanas de Hibiscus sección Furcaria (Malvaceae). Revista del Jardín Botánico Nacional, Universidad de La Habana 25/26, 25-33 [In Spanish].
| Google Scholar |
Badry MO, Tate JA, Sheded MG, El-Naggar SM, Hamed ST (2015) Macro- and micro-morphological characters of Hibiscus diversifolius Jacq. subsp. diversifolius (Malvaceae): a new record for the flora of Egypt. Feddes Repertorium 126, 37-47.
| Crossref | Google Scholar |
Badry MO, Crayn DM, Tate JA (2017) Hibiscus diversifolius subsp. rivularis (Bremek. & Oberm.) Exell (Malvaceae) in Australia. Austrobaileya 10, 113-120.
| Google Scholar |
Bahadur KN, Dayal R, Raturi DP (1970) New plant records for the upper Gangetic Plain. Journal of the Bombay Natural History Society 70, 495-496.
| Google Scholar |
Baillon MH (1885) Liste des plantes de Madagascar. Bulletin mensuel de la Société linnéenne de Paris 1(64), 508-512 [In French].
| Google Scholar |
Baker EG (1894) Hibiscus scotellii. In Elliot GFS, On the botanical results of the Sierra Leone Boundary Commission. Journal of the Linnean Society, Botany 30, 64-100.
| Crossref | Google Scholar |
Baker EG (1911) A contribution to our knowledge of the flora of Gazaland. Dicotyledonae. Polypetalae. Journal of the Linnean Society (Botany) 40, 16-76.
| Crossref | Google Scholar |
Baker EG (1937) Notes on tropical African species of Hibiscus. Journal of Botany 75, 98-102.
| Google Scholar |
Baker EG (1938) Notes from the British Museum herbarium. Journal of Botany 76, 19-22.
| Google Scholar |
Baker EG (1939) New Malvaceae from Angola. Journal of Botany 77, 17-22.
| Google Scholar |
Baldini RM, Macvean AL, Cristofolini G, Daniel TF, Managlia A, Galloni M (2019) Synopsis and typification of the names published by Antonio Bertoloni in Florula Guatimalensis and in preceding publications. Phytotaxa 420, 199-223.
| Crossref | Google Scholar |
Barker JSF (2005) Population structure and host-plant specialization in two Scaptodrosophila flower-breeding species. Heredity 94, 129-138.
| Crossref | Google Scholar | PubMed |
Barrett RL (2015) Fifty new species of vascular plants from Western Australia – celebrating fifty years of the Western Australian Botanic Garden at Kings Park. Nuytsia 26, 3-20.
| Crossref | Google Scholar |
Bates DM (1965a) Notes on the cultivated Malvaceae 1, Hibiscus. Baileya 13(2), 56-96.
| Google Scholar |
Bates DM (1965b) Notes on the cultivated Malvaceae. 1. Hibiscus. Baileya 13(3), 97-130.
| Google Scholar |
Bates DM (1989) Hibiscus brackenridgei. In Wagner WL, Herbst DR, Sohmer SH, Contributions to the flora of Hawai’i. II. Begoniaceae–Violaceae and the monocotyledons. Bishop Museum Occasional Papers 29, 88-130.
| Google Scholar |
Bertoloni A (1840) Florula Guatimalensis. Novi Commentarii Academiae Scientiarum Instituti Bononiensis 4, 403-443 [In Latin].
| Google Scholar |
Bianchini G, Sánchez-Baracaldo P (2024) TreeViewer: flexible, modular software to visualise and manipulate phylogenetic trees. Ecology and Evolution 14, e10873.
| Crossref | Google Scholar | PubMed |
Blanco FM (1845) ‘Flora de Filipinas: segun el sistema sexual de Linneo’, 2nd edn. (M. Sanchez: Manila, Philippines) [In Spanish] 10.5962/bhl.title.48447
Blanco FM (1879) ‘Flora de Filipinas. Vol. 3’, 3rd edn. (Eds A Naves, C Fernández-Villar) (de Plana: Manila, Philippines) [In Spanish] 10.5962/bhl.title.167120
Borssum Waalkes J (1966) Malesian Malvaceae revised. Blumea 14(1), 1-251.
| Google Scholar |
Bovini MG, Carvalho-Okano MR, Vieira MF (2001) Malvaceae A. Juss. no Parque Estadual do Rio Doce, Minas Gerais, Brasil. Rodriguésia 52(81), 17-47 [In Portuguese].
| Crossref | Google Scholar |
Bredenkamp CL (2019) Malvaceae sensu lato. In ‘A flora of the Eastern Cape Province. Volume 2’. (Ed. CL Bredenkamp). Strelitzia 41, 1181-1216 (National Botanical Institute: Pretoria, South Africa).
| Google Scholar |
Bremekamp CE, Obermeyer AA (1935) Sertum Kalahariense. A list of the plants collected. Annals of the Transvaal Museum 16(3), 399-442.
| Google Scholar |
Brenan JPM, Milne-Redhead E, Meikle RD, Ballard F (1953) Plants collected by the Vernay Nyasaland Expedition of 1946. Memoirs of the New York Botanical Garden 8(3), 191-256.
| Google Scholar |
Brunel JF, Hiepo P, Scholz H (Eds) (1984) ‘Flore analytique du Togo phanérogames’. pp. 1–751. (GTZ: Eschborn, Federal Republic of Germany) [In French] 10.2307/3776742
Camargo RP, De Oliveira Bezerra F, Yoshikawa VN, Lopes Esteves G, Duarte MC (2022) Flora da Serra do Cipó, Minas Gerais: Malvoideae (Malvaceae). Boletim de Botânica Universidade São Paulo 39, 31-52 [In Portuguese].
| Crossref | Google Scholar |
Carabia JP (1943) Plantae Cubenses Novae I. Memorias de la Sociedad Cubana de Historia Natural “Felipe Poey” 17, 15-18 [In Spanish].
| Google Scholar |
Caum EL (1930) New Hawaiian Plants. Bishop Museum Occasional Papers 9(5), 1-30.
| Google Scholar |
Cheek M, Haba PK, Cisse S (2020) Hibiscus fabiana sp. nov. (Malvaceae) from the Guinea Highlands (West Africa). Blumea 65, 69-74.
| Crossref | Google Scholar |
Chiovenda E (1923) Note sulla Flora Aegyptiaco-Arabica di Pietro Forskal, pulicata Nel 1775. Bullettino della Società Botanica italiana 1923, 112-117 [In Italian].
| Google Scholar |
Chiovenda E (1936) Flora Somala III. Raccolte somale dei proff. G. Pollacci, L. Maffei, R. Ciferri e N. Puccioni fatte negli anni 1934 e 1935. Atti dell’Istituto Botanico dell’Università di Pavia ser. 4 7, 117-160 [In Italian].
| Google Scholar |
Chodat R, Hassler E (1905) Plantae Hasslerianae soit énumération des plantes récoltées au Paraguay par le Dr Émile Hassler d’Aarau (Suisse) de 1883 à 1902. Bulletin de l’Herbier Boissier, sér. 2 5, 288-305 [In French].
| Google Scholar |
Cid-Ortega S, Guerrero-Beltrán JA (2015) Roselle calyces (Hibiscus sabdariffa), an alternative to the food and beverages industries: a review. Journal of Food Science and Technology 52, 6859-6869.
| Crossref | Google Scholar |
Commelin J (1697) ‘Horti medici Amstelodamensis rariorum. Vol. 1.’ (P. & J. Blaeu: Amstelodami, Dutch Republic) [In Dutch] 10.5962/bhl.title.820
Cooke T (1901) ‘Flora of the Presidency of Bombay. Vol. 1. Part 1. Ranunculaceae to Rubiaceae.’ (Taylor and Francis: London, UK) 10.5962/bhl.title.10884
Coutinho TS, Fernandes-Júnior AJ (2024) Hibiscus. In ‘Flora e Funga do Brasil’. (Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil) Available at https://floradobrasil.jbrj.gov.br/FB9079 [In Portuguese, verified 9 May 2024]
Coutinho TS, Colli-Silva M, Yoshikawa VN, Pastore JFB (2025) Revision of Malvaceae names from Vellozo’s Florae Fluminensis. Brittonia [Published online early 20 February 2025].
| Crossref | Google Scholar |
Cowie ID, Kerrigan RA, Dixon DJ (2013) HIBISCUS L. In ‘Flora of the Darwin Region. Vol. 1’. (Eds PS Short, ID Cowie) pp. 12–19. (Northern Territory Herbarium, Department of Land Resource Management: Darwin, NT, Australia) Available at http://eflora.nt.gov.au/viewfile?file_id=4721 [Verified 16 February 2021]
Crane JC (1947) Kenaf: fiber-plant rival of jute. Economic Botany 1(3), 334-350.
| Crossref | Google Scholar |
Crane JC (1949) Roselle-a potentially important plant fiber. Economic Botany 3(1), 89-103.
| Crossref | Google Scholar |
Crane JC, Acuna JB (1945) Varieties of kenaf (Hibiscus cannabinus), a bast fiber plant, in Cuba. Botanical Gazette 106(3), 349-355.
| Crossref | Google Scholar |
Craven LA (2022) Hibiscus (Section Furcaria). In ‘Flora of Australia’. (Eds TBG McLay, PG Kodela) (Australian Biological Resources Study, Department of Climate Change, the Environment and Water: Canberra, ACT, Australia) Available at https://profiles.ala.org.au/opus/foa/profile/Hibiscus [Verified 19 April 2024]
Craven LA, Wilson FD, Fryxell PA (2003) A taxonomic review of Hibiscus sect. Furcaria (Malvaceae) in Western Australia and the Northern Territory. Australian Systematic Botany 16, 185-218.
| Crossref | Google Scholar |
Craven LA, Barrett RL, Barrett MD (2016) Three new species and one new combination in Hibiscus (Malvaceae). Muelleria 35, 3-14.
| Crossref | Google Scholar |
Craven LA, Barrett RL, Barrett MD (2022) Hibiscus (Section Furcaria). In ‘Flora of Australia’. (Eds TBG McLay, PG Kodela) (Australian Biological Resources Study, Department of Climate Change, the Environment and Water: Canberra, ACT, Australia) Available at https://profiles.ala.org.au/opus/foa/profile/Hibiscus [Verified 19 April 2024]
Dalton A (2018) Native hibiscus dulls male libido, says Indigenous botanist. Australian Geographic, 18 February. Available at https://www.australiangeographic.com.au/topics/science-environment/2018/02/native-hibiscus-dulls-male-libido-says-indigenous-botanist/ [Verified 11 April 2024]
Dalzell NA, Gibson A (1861) ‘The Bombay flora, or, Short descriptions of all the indigenous plants hitherto discovered in or near the Bombay presidency together with a supplement of introduced and naturalised species.’ (Education Society’s Press: Bombay, British India) 10.5962/bhl.title.33061
Dasgupta A, Bhatt RP (1981) Cytotaxonomy of Malvaceae 11. Chromosome numbers and karyotype analysis of Thespesia, Hibiscus, Abelmoschus, Pavonia and Malachra. Cytologia 46, 149-160.
| Crossref | Google Scholar |
de Candolle AP (1824) ‘Prodromus systematis naturalis regni vegetabilis. Vol. 1’. (Treuttel & Wurtz: Paris, France) [In Latin] 10.5962/bhl.title.286
de Ficalho C (1881) Nomes vulgares de algumas plantas Africanus. O Boletim da Sociedade de Geografia de Lisboa, Ser. 2 18, 603-619 [In Portuguese].
| Google Scholar |
de L’Obel M (1576) ‘Plantarum seu stirpium historia.’ (Christophori Plantini: Antverpiae [Antwerp], Belgium) [In Latin] 10.5962/bhl.title.149138
de Lange PJ, Rolfe JR, Barkla JW, Courtney SP, Champion, PD, Perrie LR, Beadel SM, Ford KA, Breitwieser I, Schonberger I, Hindmarsh-Walls R, Heenan PB, Ladley K (2018) ‘Conservation status of New Zealand indigenous vascular plants, 2017’. New Zealand Threat Classification Series 22. (Department of Conservation: Wellington, New Zealand) Available at www.doc.govt.nz/Documents/science-and-technical/nztcs22entire.pdf
de Saint-Hilaire MMA (1828) Tribus III. Malveae. In ‘Flora Brasiliae meridionalis. Vol. 1. Part 2’. (Eds MMA de Saint-Hilaire, A Jussieu, J Cambessedes) pp. 173–278. (A. Belin: Parisiis [Paris, France]) [In Latin] 10.5962/bhl.title.45474
de Saint-Hilaire MMA, Naudin C (1842) Revue de la Flore du Brésil méridional. Deuxième Partie. Annales de Sciences Naturelles. Partie Botanique 18, 24-54 [In Latin].
| Google Scholar |
de Vriese WH (1848) Fred. Lub. Splitgerberi. Religuae botanicae Surinamenses. Nederlandsch Kruidkundig Archief. Verslagen en Mededelingen der Nederlandsche Botanische Vereeniging 1, 314-355 [In Dutch].
| Google Scholar |
de Wildeman É (1911) Decades novarm specierum florae Congolensis. Bulletin du Jardin Botanique de l’État a Bruxelles 3, 253-280 [In Latin].
| Google Scholar |
de Wildeman É (1915) Decades novarm specierum florae Congolensis III. Bulletin du Jardin Botanique de l’État a Bruxelles 5, 1-108 [In Latin].
| Crossref | Google Scholar |
Desfontaines RL (1804) ‘Tableau de l'École de Botanique du Muséum d’Histoire Naturelle.’ (J.A. Brosson: Paris, France) [In French] 10.5962/bhl.title.13828
Desvaux NA (1827) Prodrome de la famille des fougères. Mémoires de la Société Linnéenne de Paris 6, 171-337 [In French].
| Google Scholar |
Dhami KK (2023) Ecological status of three Angiosperms (Alysicarpus bupleurifolius var. hybridus DC, Hibiscus hoshiarpurensis and Ceropegia bulbosa Roxb. var lushii) in Punjab (India). International Journal of Avian and Wildlife Biology 7, 130-133.
| Crossref | Google Scholar |
Domin K (1928) Beiträge zur flora und pflanzengeographie Australiens. I. Teil, 3. Abt. Embryophyta siphonogama, pars II. Dicotyledonae. Bibliotheca Botanica 22(89), 1-763.
| Google Scholar |
Don G (1830) Class XVI. Order 8. Monadelphia. Polyandria. In ‘Loudon’s Hortus Britannicus. A catalogue of all the plants indigenous, cultivated in, or introduced to Britain’, Edn 1. (Ed. JC Loudon) pp. 286–293. (Longman, Rees, Orme, Brown, and Green: London, UK) 10.5962/bhl.title.10320
Don G (1831) ‘A general history of the dichlamydeous plants: comprising complete descriptions of the different orders … the whole arranged according to the natural system. Vol. 1.’ (J.G. & F. Rivington: London, UK) Available at https://www.biodiversitylibrary.org/page/390287#page/510/mode/1up
Dumont A (1889) Recherches sur l’anatomie comparée des Malvacées, Bombacées, Tiliacées, Sterculiacées. Annales des Sciences Naturelles Sér. 7 6, 129-246 [In French].
| Google Scholar |
Durand T, de Wildeman É (1899) Matériaux pour la flore deu Congo. Bulletin de la Société Royale de Botanique de Belgique 38(2), 9-76 [In French].
| Google Scholar |
Dwyer JD (1955) The botanical catalogues of Auguste De St. Hilaire. Annals of the Missouri Botanical Garden 42(2), 153-170.
| Crossref | Google Scholar |
Ecklon CF, Zeyher CLP (1835) ‘Enumeratio plantarum Africae australis extratropicae: quae collectae, determinatae et expositae.’ (Sumtibus auctorum: Hamburgi [Hamburg, German Confederation]) [In Latin] 10.5962/bhl.title.48
Edwards S (1822) Hibiscus digitatus. Cavanilles’s Hibiscus. The Botanical Register 8, t. 608.
| Google Scholar |
Edwards S, Ridgeway J (1819) Hibiscus diversifolius. Various-leaved Hibiscus. The Botanical Register 5, t. 381.
| Google Scholar |
El-Hadidi MN, Hosni HA, El-Hadidy AMH, Araffa S (1999) Malvaceae in the flora of Egypt. 1. Systematic revision of the indigenous taxa. Taeckholmia 19, 127-146.
| Crossref | Google Scholar |
Eriksson JS, Bacon CD, Bennett DJ, Pfeil BE, Oxelman B, Antonelli A (2021) Gene count from target sequence capture places three whole genome duplication events in Hibiscus L. (Malvaceae). BMC Ecology and Evolution 21, 107.
| Crossref | Google Scholar | PubMed |
Exell AW (1936) Notes on the flora of Angola–III. Journal of Botany 74, 132-140.
| Google Scholar |
Exell AW (1959) New and little known species from the Flora Zambesiaca area. IX. Hibisci novi. Boletim da Sociedade Broteriana, Ser. 2 33, 165-181.
| Google Scholar |
Eyles F (1916) A record of plants collected in southern Rhodesia. Transactions of the Royal Society of South Africa 5, 273-523.
| Crossref | Google Scholar |
Fernandes Júnior AJ, Cruz APO (2018) Flora das cangas da Serra dos Carajás, Pará, Brasil: Malvaceae/Flora of the canga of the Serra dos Carajás, Pará, Brazil: Malvaceae. Rodriguésia 69(3), 1237-1254.
| Crossref | Google Scholar |
Fernández A (1974) Recuentos cromosómicos en Malváceas. Boletín de la Sociedad Argentina de Botánica 15, 403-410 [In Spanish].
| Google Scholar |
Fernández A, Krapovickas A, Lavia G, Seijo G (2003) Cromosomas de Malváceas. Bonplandia 12, 141-145 [In Spanish with abstract and keywords in English and Spanish].
| Crossref | Google Scholar |
Fitzgerald WV (1918) The botany of the Kimberleys, north-west Australia. Journal and Proceedings of the Royal Society of Western Australia 3, 102-224.
| Google Scholar |
Fosberg FR, Sachet M-H (1966) Plants of Southeastern Polynesia. 1. Micronesica 2, 153-159.
| Google Scholar |
Fries RE (1908) Studien über die amerikanische Columniferenflora. Kongliga Svenska Vetenskapsakademiens Handlingar, new ser 42(12), 1-67 [In German].
| Google Scholar |
Fries RE (1947) Zur Kenntnis der Süd- und Zentralamerkanischen Malvaceenflora. Kongliga Svenska Vetenskapsakademiens Handlingar 24(2), 1-37 [In German].
| Google Scholar |
Fryxell PA (1973) New species and other notes in the Malvaceae. Brittonia 25, 77-85.
| Crossref | Google Scholar |
Fryxell PA (1988) Malvaceae of Mexico. Systematic Botany Monographs 25, 1-522.
| Crossref | Google Scholar |
Fryxell PA (1990) The Malvaceae published by Turczaninow. Contributions from the University of Michigan Herbarium 17, 173-182.
| Google Scholar |
Fryxell PA (1997) The American genera of Malvaceae – II. Brittonia 49(2), 204-269.
| Crossref | Google Scholar |
Fryxell PA (2000) Malvaceae. In ‘Flora Mesoamericana. Vol. 3(2)’. (Eds G Davidse, MS Sousa, AO Chater, CJ Humphries) pp. 1–162. (Missouri Botanical Garden: Saint Louis, MO, USA; Instituto de Biologia of the National Autonomous University of Mexico: Mexico City, Mexico; and Natural History Museum: London, UK) [Pre-print version]
Fryxell PA (2001b) (1492) Proposal to conserve the name Hibiscus sabdariffa (Malvaceae) with a conserved type. Taxon 50, 929-931.
| Crossref | Google Scholar |
Fryxell PA (2001d) Talipariti (Malvaceae), a segregate from Hibiscus. Contributions from the University of Michigan Herbarium 23, 225-270.
| Google Scholar |
Fryxell PA (2007) Malvaceae. In ‘Manual de plantas de Costa Rica. Volumen IV. Dicotiledoneas (Haloragaceae–Phytolaccaceae). Vol 111’. Monographs in Systematic Botany from The Missouri Botanical Garden. (Eds BE Hammel, MH Grayum, C Herrera, N Zamora) pp. 313–373. (Missouri Botanical Garden Press: Saint Louis, MO, USA)
Fryxell PA, Krapovickas A (2004) Six new species of Bolivian Hibiscus (Malvaceae). Novon 14, 58-69.
| Google Scholar |
Fryxell PA, Stelly DM (1993) Documented chromosome numbers 1993: 2. Additional chromosome counts in the Malvaceae. Sida 15, 639-647.
| Google Scholar |
Fryxell PA, Wilson FD (1986) Clarification of the status of Hibiscus (sect. Furcaria) uncinellus (Malvaceae). Brittonia 38, 107-110.
| Crossref | Google Scholar |
Fuertes J (1992) Estudios botánicos en la Guayana Colombiana. I. Una nueva especie de Hibiscus sección Furcaria (Malvaceae). Anales del Jardin Botanico de Madrid 50, 65-72 [In Spanish].
| Google Scholar |
Fuertes J, Fryxell PA (1993) Nomenclatural notes on some Malvaceae species described by Cavanilles. Taxon 42, 661-664.
| Crossref | Google Scholar |
Garcia JG (1946) Revisão do herbário de Moçambique do Instituto Botânico da Universidade de Coimbra. Boletim da Sociedade Broteriana, Ser. 2 20, 33-42 [In Portuguese].
| Google Scholar |
García-Robledo C, Kattan G, Murcia C, Quintero-Marín P (2004) Beetle pollination and fruit predation of Xanthosoma daguense (Araceae) in an Andean cloud forest in Colombia. Journal of Tropical Ecology 20, 459-469.
| Crossref | Google Scholar |
Garcke A (1849a) Kritische bemerkungen zu der familie der Malvaceen nebst beschreibung neuer arten aus derselben. Botanische Zeitung 7(49), 817-825 [In German].
| Google Scholar |
Garcke A (1849b) Kritische bemerkungen zu der familie der Malvaceen nebst beschreibung neuer arten aus derselben. Botanische Zeitung 7(49), 833-841 [In German].
| Google Scholar |
Garcke A (1849c) Kritische bemerkungen zu der familie der Malvaceen nebst beschreibung neuer arten aus derselben. Botanische Zeitung 7(49), 849-855 Available at https://www.biodiversitylibrary.org/page/33805723#page/449/mode/1up [In German].
| Google Scholar |
Garcke A (1874) Einige worte über die Gattung Hibiscus. Linnaea 38, 673-698 [In German].
| Google Scholar |
Garcke A (1880) Aufzählung der abyssinischen Malvaceen aus der letzten im Jahre 1869 eingesandten Sehimper’schen Sammlung. Linnaea 43, 49-58 [In German].
| Google Scholar |
Garcke A (1881) Malvaceae. In Hoffman O. Plantae Mechowianae. Plantas a cl. de Mechowio adiutore cl. Thenschio in Angola collectas determinat. Linnaea 43, 119-134 [In Latin].
| Google Scholar |
Gardner CA (1923) Botanical notes. Kimberley Division of Western Australia. Forests Department Bulletin 32, 1-106.
| Google Scholar |
Gardner RO (1985) Six plants in New Zealand whose nativity has been doubted. Auckland Botanical Society 40, 41-44.
| Google Scholar |
Gillett JB (1962) Roger dedit’: Plants from Senegal in the Gay Herbarium at Kew. Kew Bulletin 15(3), 431-435.
| Crossref | Google Scholar |
Go S, Koo H, Jung M, Hong S, Yi G, Kim Y-M (2024) Pan-chloroplast genomes for accession-specific marker development in Hibiscus syriacus. Scientific Data 11, 246.
| Crossref | Google Scholar | PubMed |
Gonçalves ML (1961) Revisão das Malvaceae, Bombacaceae e Sterculiaceae de Moçambique, existentes nas herpárias COI, LISC, LM, LMJ e LMM. Estudos de Botânica: Memórias da Junta de Investigações do Ultramar, 2ª série 41, 57-154 [In Portuguese].
| Google Scholar |
Gosline G, Bidault E, van der Burgt X, Cahen D, Challen G, Condé N, Couch C, Couvreur TLP, Dagallier L-PL, Darbyshire I, Dawson S, Doré TS, Goyder D, Grall A, Haba P, Haba P, Harris D, Hind DJN, Jongkind C, Konomou G, Larridon I, Lewis G, Ley A, Lock M, Lucas E, Magassouba S, Mayo S, Molmou D, Monro A, Onana JM, Paiva J, Paton A, Phillips S, Prance G, Quintanar A, Rokni S, Shah T, Schrire B, Schuiteman A, Simões ARG, Sosef M, Stévart T, Stone RD, Utteridge T, Wilkin P, Xanthos M, Nic Lughadha EN, Cheek M (2023) A taxonomically verified and vouchered checklist of the vascular plants of the Republic of Guinea. Scientific Data 10, 327.
| Crossref | Google Scholar | PubMed |
Gottsberger G (1972) Blütenbiologische Beobachtungen an brasilianischen Malvaceen. II. Österreichische Botanische Zeitschrift 120(4/5), 439-509 [In German].
| Crossref | Google Scholar |
Graham R (1830a) Description of several new or rare plants which have lately flowered in the neighbourhood of Edinburgh, and chiefly in the Royal Botanic Garden. The Edinburgh New Philosophical Journal 9, 366-371.
| Google Scholar |
Graham R (1830b) Hibiscus splendens. Splendid Hibiscus. Curtis’s Botanical Magazine 57, t. 3025.
| Google Scholar |
Guillaumin A (1948) Compendium de la flore phanérogamique des Nouvelles Hébrides. Annales de l’Institut Botanico-Geologique de Marseille 6(5–6), 1-56.
| Google Scholar |
Guillemin JBA, Perrottet GS, Richard A (1831) ‘Florae Senegambiae tentamen, seu, Historia plantarum in diversis Senegambiae regionibus a peregrinatoribus Perrottet et Leprieur detectarum.’ (Treuttel et Wurtz: Parisiis [Paris, France]) [In French] 10.5962/bhl.title.595
Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59, 307-321.
| Crossref | Google Scholar | PubMed |
Haines HH (1914) Decades Kewenses. LXXVI. Bulletin of Miscellaneous Information, Royal Gardens, Kew 1914(1), 24-31.
| Crossref | Google Scholar |
Hanes MM, Mashburn B, Callmander MW (2022) Transfer of the endemic Malagasy species of Kosteletzkya to Hibiscus and Perrierophytum (Malvaceae). Candollea 77, 161-171.
| Crossref | Google Scholar |
Hanes MM, Blanchard OJ, Jr, Valencia-D J, McLay TBG, Abbott JR, McDaniel SF, Barrett RL, Mathews S, Neubig KM (2024) Phylogenetic relationships within tribe Hibisceae (Malvaceae) reveal complex patterns of polyphyly in Hibiscus and Pavonia. Systematic Botany 49(1), 77-116.
| Crossref | Google Scholar |
Harvey WH (1862) Addenda et corrigenda to the first volume. In ‘Flora Capensis: being a systematic description of the plants of the Cape colony, Caffraria, & Port Natal (and neighbouring territories). Vol. II. Leguminosae to Loranthaceae’. (Eds WH Harvey, OW Sonder) pp. 583–592. (L. Reeve: Kent, UK)
Hasskarl JC (1866) Neuer schlüssel zu Rumph’s Herbarium Amboinense. Abhandlungen der Naturforschenden Gesellschaft zu Halle 9, 143-366 [In German].
| Google Scholar |
Hassler E (1909) Ex herbario Hassleriano: Novitates paraguarienses. III. Malvaceae. Repertorium Specierum Novarum Regni Vegetabilis 7, 376-383 [In Latin].
| Google Scholar |
Hauman L (1961) Quelques Malvacées nouvelles du Congo et du Ruanda-Urundi. Bulletin du Jardin botanique de l'État a Bruxelles 31(1), 85-90 [In French].
| Crossref | Google Scholar |
Hauman L (1963) Malvaceae. In ‘Flore du Congo et du Ruanda-Urundi: Spermatophytes. Vol. 10’. (Éd. R Boutique) pp. 92–190. (Institut national pour l’étude agronomique du Congo belge: Brussels, Belgium) [In French] 10.5281/zenodo.4415812
Hendy J (2019) Challenging old ideas: how supplemental effects student confidence and learning and adding a key piece to understanding the origin of an important crop, kenaf, Hibiscus cannabinus L. and understanding how supplemental videos effect student confidence and learning. MSc thesis, University of Tennessee, Knoxville, TE, USA. Available at https://trace.tennessee.edu/cgi/viewcontent.cgi?article=6987&context=utk_gradthes
Herbst DR, Wagner WL (1999) Contributions to the flora of Hawai’i: VII. Bishop Museum Occasional Papers 58, 12-36.
| Google Scholar |
Hillier JM (1907) Miscellaneous notes. Bulletin of Miscellaneous Information (Royal Botanic Gardens, Kew) 1907, 325-338.
| Google Scholar |
Hochreutiner BPG (1900) Revision du genre Hibiscus. Annuaire du Conservatoire et du Jardin Botaniques de Genève 4, 23-191 [In Latin].
| Google Scholar |
Hochreutiner BPG (1901) Malvaceae Chevalieranae. Annuaire du Conservatoire et du Jardin Botaniques de Genève 5, 120-126 [In Latin].
| Google Scholar |
Hochreutiner BPG (1902) Malvaceae novae vel minus cognitae. Annuaire du Conservatoire et du Jardin Botaniques de Genève 6, 10-59 [In Latin].
| Google Scholar |
Hochreutiner BPG (1906) Malvaceae et Bombacaceae novae vel minus cognitae. Annuaire du Conservatoire et du Jardin Botaniques de Genève 10, 15-25 [In Latin].
| Google Scholar |
Hochreutiner BPG (1908) Malvaceae et Sterculiaceae novae vel minus cognitae. Annuaire du Conservatoire et du Jardin Botaniques de Genève 11, 1-9 [In Latin].
| Google Scholar |
Hochreutiner BPG (1917a) Malvacées de Madagascar de l’Herbier Perrier de la Bathie. Annuaire du Conservatoire et du Jardin Botaniques de Genève 20, 69-102 [In Latin].
| Google Scholar |
Hochreutiner BPG (1917b) Notulae in Malvaceas. Interjectis descriptionibus specierum et varietatum novarum praesertim ex herbario Delessertiano. Annuaire du Conservatoire et du Jardin Botaniques de Genève 20, 107-172 [In Latin].
| Google Scholar |
Hochreutiner BPG (1924) Genres nouveaux et discutes de la famille des Malvacees. Candollea 2, 79 [In Latin].
| Google Scholar |
Holland JH (1918) Miscellaneous notes: XXIV – Hibiscus cannabinus in Nigeria. Bulletin of Miscellaneous Information (Kew) 1918, 214-215.
| Google Scholar |
Holser RA, Bost G (2004) Hybrid hibiscus seed oil compositions. Journal of the American Oil Chemists Society 81, 795-797.
| Crossref | Google Scholar |
Holser RA, Willett JL (2005) Yield strengths of hybrid Hibiscus canes. Applied Engineering in Agriculture 21, 985-986.
| Crossref | Google Scholar |
Hooker WJ (1859) Hibiscus radiatus. Curtis’s Botanical Magazine, ser. 3 15, t. 5098 Available at https://www.biodiversitylibrary.org/item/14225#page/28/mode/1up.
| Google Scholar |
Hornby AJW (1946) A new species of Hibiscus from Portuguese East Africa. Proceedings and Transactions of the Rhodesia Scientific Association. Bulawayo 41, 55-56.
| Google Scholar |
Hoskins WM (2016) Using phylogenetics to understand the evolutionary relationships of Hibiscus section Furcaria. MSc thesis, University of Tennessee, Knoxville, TE, USA. Available at https://trace.tennessee.edu/cgi/viewcontent.cgi?article=5092&context=utk_gradthes
Howard A, Howard G (1911) Studies in Indian fibre plants 2. On some new varieties of Hibiscus cannabinus L. and Hibiscus sabdariffa L. Memoirs of the Department of Agriculture in India, Botanical Series 4, 1-36.
| Google Scholar |
Hyde MA, Wursten BT, Ballings P, Coates Palgrave M (2024) Flora of Malawi: Genus page: Hibiscus. Available at https://www.malawiflora.com/speciesdata/genus.php?genus_id=942 [Verified 3 April 2024]
Ikabanga DU, Bidault E, Paradis A-H, Texier N, Stévart T (2020) Hibiscus ngokbanakii. In ‘The IUCN Red List of Threatened Species 2020’. e.T172512028A174180595. (International Union for Conservation of Nature and Natural Resources: Gland, Switzerland) Available at https://www.iucnredlist.org/species/172512028/174180595 [Verified 19 August 2024]
Jabeur I, Pereira E, Barros L, Calhelha RC, Soković M, Oliveira M, Ferreira I (2017) Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Research International 100, 717-723.
| Crossref | Google Scholar | PubMed |
Janakiram T, Patil MS (2017) Breeding in Hibiscus: a review. Indian Journal of Agricultural Sciences 87, 159-166.
| Crossref | Google Scholar |
Jørgensen PM, Fryxell PA (1999) Malvaceae. In ‘Catalogue of the vascular plants of Ecuador/Catálogo de las plantas vasculares del Ecuador. Vol. 75’. (Eds PM Jørgensen, S León-Yánez) Monographs in Systematic Botany from the Missouri Botanical Garden, pp. 548–554. (Missouri Botanical Garden Press: Saint Louis, MO, USA)
Juhari NH, Bredie WLP, Toldam-Andersen TB, Petersen MA (2018) Characterization of roselle calyx from different geographical origins. Food Research International 112, 378-389.
| Crossref | Google Scholar | PubMed |
Kachecheba JL (1972) The cytotaxonomy of some species of Hibiscus. Kew Bulletin 27, 425-433.
| Crossref | Google Scholar |
Kak A, Agarwal S, Pandey C, Gupta V (2015) Responses to seed dormancy breaking treatments in wild Hibiscus species. Vegetos – An International Journal of Plant Research 28, 143-147.
| Crossref | Google Scholar |
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587-589.
| Crossref | Google Scholar | PubMed |
Kapepula PM, Kabamba Ngombe N, Tshisekedi Tshibangu P, Tsumbu C, Franck T, Mouithys-Mickalad A, Mumba D, Tshala-Katumbay D, Serteyn D, Tits M, Angenot L, Kalenda PDT, Frédérich M (2017) Comparison of metabolic profiles and bioactivities of the leaves of three edible Congolese Hibiscus species. Natural Product Research 31, 2885-2892.
| Crossref | Google Scholar | PubMed |
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772-780.
| Crossref | Google Scholar | PubMed |
Kearney TH (1951) The American genera of Malvaceae. American Midland Naturalist 46, 93-131.
| Crossref | Google Scholar |
Kearney TH (1955) A tentative key to the North American species of Hibiscus L. Leaflets of Western Botany 7, 274-284.
| Google Scholar |
Kearney TH (1957) A tentative key to the South American species of Hibiscus L. Leaflets of Western Botany 8, 161-168.
| Google Scholar |
Keir M (2018a) Hibiscus brackenridgei. In ‘The IUCN Red List of Threatened Species 2018’. e.T62743A78757481. (International Union for Conservation of Nature and Natural Resources: Gland, Switzerland) Available at https://www.iucnredlist.org/species/62743/78757481
Keir M (2018b) Hibiscus brackenridgei subsp. molokaiana. In ‘The IUCN Red List of Threatened Species 2018’. e.T80091009A8009104. (International Union for Conservation of Nature and Natural Resources: Gland, Switzerland) Available at https://www.iucnredlist.org/species/80091009/80091047
Kirejtshuk AG, Lawrence JF (1999) Notes on the Aethina complex (Coleoptera: Nitidulidae: Nitidulinae), with a review of Aethina (Cleidorura) subgen. nov. and Aethina (Idaethina) Gemminger et Harold. Annales Zoologii 49, 233-254.
| Google Scholar |
Knapp S, Barboza GE, Romero MV, Vignoli-Silva M, Giacomin LL, Stehmann JR (2015) Identification and lectotypification of the Solanaceae from Vellozo’s Flora Fluminensis. Taxon 64(4), 822-836.
| Crossref | Google Scholar |
Koo H, Shin A-Y, Hong S, Kim Y-M (2023) The complete chloroplast genome of Hibiscus syriacus using long-read sequencing: Comparative analysis to examine the evolution of the tribe Hibisceae. Frontiers in Plant Science 14, 1111968.
| Crossref | Google Scholar | PubMed |
Koopman MM, Baum DA (2008) Phylogeny and biogeography of tribe Hibisceae (Malvaceae) on Madagascar. Systematic Botany 33, 364-374.
| Crossref | Google Scholar |
Krapovickas A (2006) Dos especies nuevas de Hibiscus secc. Furcaria (Malvaceae) de Minas Gerais (Brasil). Bonplandia 15(1–2), 47-51 [In Spanish].
| Crossref | Google Scholar |
Krapovickas A (2008) Nuevas especies de Malvaceae. Bonplandia 17(1), 35-45 [In Spanish with summary and keywords in English and Spanish].
| Crossref | Google Scholar |
Krapovickas A (2013) Hibiscus paulae Krapov. (secc. Furcaria), nueva especie de Malvaceae de Minas Gerais (Brasil). Bonplandia 22(2), 137-139 [In Spanish].
| Crossref | Google Scholar |
Krapovickas A, Fryxell PA (2004) Las especies sudamericanas de Hibiscus secc. Furcaria DC. (Malvaceae – Hibisceae). Bonplandia 13(1–4), 35-115 [In Spanish].
| Crossref | Google Scholar |
Krapovickas A, Marticorena A (2008) Malvaceae. In ‘Catálogo de las Plantas Vasculares del Cono Sur (Argentina, Sur de Brasil, Chile, Paraguay y Uruguay). Vol. 3. Dicotyledoneae: Fabaceae (Senna–Zygia)–Zygophyllaceae’. (Eds FO Zuloaga, O Morrone, MJ Belgrano) Monographs in Systematic Botany from the Missouri Botanical Garden Volume 107, 2463–2520. (Missouri Botanical Garden Press: St Louis, MO, USA) [In Spanish]
Kuntze G (1891a) Beiträge zur vergleichenden Anatomie der Malvaceen. Botanisches Centralblatt 45, 229-234 [In German].
| Google Scholar |
Kurz WS (1874) Contributions towards a knowledge of the Burmese flora.—Part I. Journal of the Asiatic Society of Bengal. Part 2. Natural History 43, 39-141.
| Google Scholar |
Kwon SH, Jang YL, Kwon HY (2022) Complete chloroplast genome sequence of Hibiscus trionum Linnaeus 1753 (Malvaceae). Mitochondrial DNA – B. Resources 7, 1902-1903.
| Crossref | Google Scholar | PubMed |
Kwon S-H, Kwon H-Y, Choi Y-I, Shin H (2023) Comprehensive analysis of chloroplast genome of Hibiscus sinosyriacus: evolutionary studies in related species and genera. Forests 14(11), 2221.
| Crossref | Google Scholar |
Kwon S-H, Kwon H-Y, Shin H (2024) Genetic insights into the extremely dwarf Hibiscus syriacus var. micranthus: complete chloroplast genome analysis and development of a novel dCAPS Marker. Current Issues in Molecular Biology 46, 2757-2771.
| Crossref | Google Scholar | PubMed |
Landrein S, Song S-J, Zhang J, Guo Y-J, Shen J-Y, Jiang Q-Y, Low SL (2024) Thepparatia vines (Hibisceae subtribe Trionum) phylogenomics and evolution. Botanical Journal of the Linnean Society 205, 391-402.
| Crossref | Google Scholar |
Lauener LA, Paul H (1985) The type specimens of Robert Graham. Notes from the Royal Botanic Garden, Edinburgh 42, 567-593.
| Google Scholar |
Lautenschläger T, Monizi M, Pedro M, Mandombe JL, Bránquima MF, Heinze C, Neinhuis C (2018) First large-scale ethnobotanical survey in the province of Uíge, northern Angola. Journal of Ethnobiology and Ethnomedicine 14, 51.
| Crossref | Google Scholar | PubMed |
Lavia G, Fernández A (2004) Números cromosómicos en Hibiscus secc. Furcaria DC. (Malvaceae-Hibisceae). Bonplandia 13, 129-130 [In Portuguese].
| Crossref | Google Scholar |
Lebrun J-P (1976) Un curieux Hibiscus d’Éthiopie méridionale. Adansonia 15, 379-380 [In French].
| Crossref | Google Scholar |
Lee C-T, Hsieh S-I, Leou C-S, Lin H-Y, Yeh C-L (2014) A newly naturalized species in Taiwan: Hibiscus asper Hook. f. (Malvaceae). Taiwan Journal of Biodiversity 16, 273-281.
| Google Scholar |
Lejoly J, Geerinck D (2010) Flore de la Tshopo (RD Congo): Familles des Malvaceae, Orchidaceae, Sterculiaceae, Tiliaceae. Taxonomania 29, 1-41 [In French].
| Google Scholar |
Lejoly J, Ndjele M-B, Geerinck D (2010) Catalogue-Flore des plantes vasculaires des districts de Kisangani et de la Tshopo (RD Congo). Taxonomania 30, 1-308 [In French].
| Google Scholar |
Levin GA, Moran R (1989) The vascular flora of Isla Socorro, Mexico. Memoirs of the San Diego Society of Natural History 16, 1-71.
| Google Scholar |
Lima JB, Conceição AS (2016) Malvoideae Burnett (Malvaceae) in the Environmental Protection Area Serra Branca, Raso da Catarina, Jeremoabo, Bahia, Brazil. Biota Neotropica 16(4), e20160187.
| Crossref | Google Scholar |
Lindley J (1825) Hibiscus unidens. One-toothed Hibiscus. The Botanical Register 11, t. 878.
| Google Scholar |
Linnaeus C (1737) ‘Hortus Cliffortianus: plantas exhibens quas in hortis tam vivis quam siccis, Hartecampi in, coluit … Georgius Clifford.’ (Amstelaedami) [In Latin] 10.5962/bhl.title.690
Linnaeus C (1753) ‘Species Plantarum.’ (Impensis Laurentii Salvii: Stockholm) [In Latin] 10.5962/bhl.title.37656
Linnaeus C (1759) ‘Systema Naturae. Vol. 2’, Decima edn. (Impensis Laurentii Salvii: Stockholm) [In Latin] 10.5962/bhl.title.542
Lisowski S (2009) Flore de Guinée. Scripta Botanica Belgica 41, 1-517 [In French].
| Google Scholar |
Liu HY, Wang CM, Tseng YH (2013) Hibiscus cannabinus L. (Malvaceae), a newly naturalized plant in Taiwan. Quarterly Journal of Forest Research 35, 15-22.
| Google Scholar |
Loddiges G (1832) Hibiscus splendens. The Botanical Cabinet 19, t. 1835.
| Google Scholar |
Lopes Esteves G, Duarte MC, Takeuchi C (2014) Sinopse de Hibiscus L. (Malvoideae, Malvaceae) do estado de São Paulo, Brasil: espécies nativas e cultivadas ornamentais. Hoehnea 41, 529-539 [In Portuguese].
| Crossref | Google Scholar |
Macphail MK, Hope GS, Anderson A (2001) Polynesian plant introductions in the Southwest Pacific: initial pollen evidence from Norfolk Island. In ‘The Prehistoric Archaeology of Norfolk Island, Southwest Pacific’. (Eds A Anderson, P White) Records of the Australian Museum, Supplement 27, pp. 123–134. (Australian Museum: Sydney, NSW, Australia)
Magdalita PM, San Pascual AO (2022) Hibiscus (Hibiscus rosa-sinensis): importance and classification. In ‘Floriculture and ornamental plants’. (Eds SK Datta, YC Gupta) Handbooks of crop diversity: Conservation and use of plant genetic resources. pp. 483–522. (Springer Nature: Singapore) 10.1007/978-981-15-3518-5_18
Martin DW, Menzel MY (1971) Identification of the “Gondwanaland” genome found in South American tetraploids of Hibiscus sect. Furcaria. American Journal of Botany 58, 465 [Abstract].
| Google Scholar |
Martin DW, Menzel MY (1972) Identity of the genome shared by African and new species of Hibiscus sect. Furcaria. Journal of Heredity 63, 235-240.
| Crossref | Google Scholar |
Mashburn B (2024) Island hopping in the tropics: conservation genetics, speciation, and biogeography in the iconic flowering plant group Hibiscus sect. Lilibiscus. PhD dissertation, Graduate School of Arts and Sciences, Washington University in Saint Louis, Saint Louis, MO, USA. 10.7936/df5z-rc89
Mattei GE (1908) Contribuzioni alla flora della Somalia italiana. Bollettino del Reale Orto Botanico Giardino Colon. Palermo 7, 85-112 [In Italian].
| Google Scholar |
McLay TBG (2022) Hibiscus (Section Furcaria). In ‘Flora of Australia’. (Eds TBG McLay, PG Kodela) (Australian Biological Resources Study, Department of Climate Change, the Environment and Water: Canberra, ACT, Australia) Available at https://profiles.ala.org.au/opus/foa/profile/Hibiscus [Verified 19 April 2024]
Menzel MY (1963) Cytotaxonomy of twelve species of Hibiscus section Furcaria. American Journal of Botany 50, 262-271.
| Crossref | Google Scholar |
Menzel MY, Hancock JF (1984) Cytotaxonomy of the octoploid and decaploid species of Hibiscus sect. Furcaria (Malvaceae). Nucleus 27, 48-63.
| Google Scholar |
Menzel MY, Martin DW (1970) Genome affinities of four African diploid species of Hibiscus sect. Furcaria. Journal of Heredity 61, 179-184.
| Crossref | Google Scholar |
Menzel MY, Martin DW (1971) Chromosome homology in some intercontinental hybrids in Hibiscus sect. Furcaria. American Journal of Botany 58, 191-202.
| Crossref | Google Scholar |
Menzel MY, Martin DW (1974) Cytotaxonomy of some Australian species of Hibiscus sect. Furcaria. Australian Journal of Botany 22, 141-156.
| Crossref | Google Scholar |
Menzel MY, Martin DW (1980) Evidence for the presence of an intercontinental genome in the Australian hexaploid alliance of Hibiscus sect. Furcaria. Australian Journal of Botany 28, 369-383.
| Crossref | Google Scholar |
Menzel MY, Wilson FD (1961) Chromosomes and crossing behavior of Hibiscus cannabinus, H. acetosella and H. radiatus. American Journal of Botany 48, 651-657.
| Crossref | Google Scholar |
Menzel MY, Wilson FD (1963) Cytotaxonomy of twelve species of Hibiscus sect. Furcaria. American Journal of Botany 50, 262-271.
| Crossref | Google Scholar |
Menzel MY, Wilson FD (1966) Hybrids and genome relations of Hibiscus sabdariffa, H. meeusei, H. radiatus, and H. acetosella. American Journal of Botany 53, 270-275.
| Crossref | Google Scholar |
Menzel MY, Wilson FD (1969) Genetic relationships in Hibiscus sect. Furcaria. Brittonia 21, 91-125.
| Crossref | Google Scholar |
Menzel MY, Fryxell PA, Wilson FD (1983a) Relationships among new world Hibiscus section Furcaria (Malvaceae). Brittonia 35(3), 204-221.
| Crossref | Google Scholar |
Menzel MY, Goetz SG, Adamson WC (1983b) Some pieces of the African genome puzzle in Hibiscus sect. Furcaria. American Journal of Botany 70, 285-297.
| Crossref | Google Scholar |
Menzel MY, Richmond KL, Contilini CS, Huang P (1986) New intergenomic hybrids among some African diploid species of Hibiscus sect. Furcaria. American Journal of Botany 73, 304-309.
| Crossref | Google Scholar |
Merrill ED (1908) New Philippine plants from the collections of Mary Strong Clemens, 1. The Philippine Journal of Science Ser. C, Botany 3, 129-165.
| Google Scholar |
Mesaglio T, Sauquet H, Coleman D, Wenk E, Cornwell WK (2023) Photographs as an essential biodiversity resource: drivers of gaps in the vascular plant photographic record. The New Phytologist 238, 1685-1694.
| Crossref | Google Scholar | PubMed |
Meyer GFW (1818) ‘Primitiae florae essequeboensis adjectis descriptionibus centum circiter stirpium novarum, observationibusque criticis.’ (H. Dieterich: Gottingae [Göttingen, German Confederation]) [In Latin] 10.5962/bhl.title.49397
Meyer J-Y (2011) Rapa, îles Australes. Guide de la flore indigène et endémique. (Direction de l’Environment, Délégation à la Recherche: Papeete, Tahiti) Available at https://www.service-public.pf/diren/wp-content/uploads/sites/17/2019/01/Rapa.pdf [In French]
Michaux A (1803) ‘Flora boreali-americana, sistens caracteres plantarum quas in America septentrionali collegit et detexit Andreas Michaux.’ (Levrault: Parisiis et Argentorati, France) [In Latin] 10.5962/bhl.title.5088
Miller, P (1768) ‘The gardeners dictionary’, 8th edn. (Printed for the author: London, UK) 10.5962/bhl.title.541
Milne-Redhead EWBH (1935) Tropical African plants: XIII. Bulletin of Miscellaneous Information 1935, 271-285.
| Crossref | Google Scholar |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530-1534.
| Crossref | Google Scholar | PubMed |
Mitchell AS (1982) Economic aspects of the Malvaceae in Australia. Economic Botany 36, 313-322.
| Crossref | Google Scholar |
Molinari EP, Fabris HA (1954) Los Hibiscus cultivados en lb República Argentina. Revista de Investigaciones Agrícolas 8, 289-310 [In Spanish].
| Google Scholar |
Mullenders W (1954) La végétation de Kaniama (Entre-Lubishi-Lubilash, Congo belge). Publications de l’Institut National pour l'Étude Agronomique du Congo Belge, Sér. Scientifique 61, 1-499 [In French].
| Google Scholar |
Naqshi AR, Dar GH, Javeid GN, Kachroo P (1988) Malvaceae of Jammu and Kashmir State, India. Annals of the Missouri Botanical Garden 75(4), 1499-1524.
| Crossref | Google Scholar |
Newnham RM, Lowe DJ (1991) Holocene vegetation and volcanic activity, Auckland Isthmus, New Zealand. Journal of Quaternary Science 6, 177-193.
| Crossref | Google Scholar |
Ngwe TM (1971) The Burmese Malvales. Union of Burma Journal of Life Sciences 4, 196-208.
| Google Scholar |
Oliver WRB (1916) The vegetation and flora of Lord Howe Island. Transactions and Proceedings of the New Zealand Institute 49, 94-161.
| Google Scholar |
Orisakwe OE, Husaini DC, Afonne OJ (2004) Testicular effects of sub-chronic administration of Hibiscus sabdariffa calyx aqueous extract in rats. Reproductive Toxicology 18, 295-298.
| Crossref | Google Scholar | PubMed |
Paraiso CM, Januario JGB, Mizuta AG, dos Santos SS, Magon TFD, Ogawa CYL, Silva JVD, Sato F, Visentainer J V, Mikcha JMG, Madrona GS (2021) Comparative studies on chemical stability, antioxidant and antimicrobial activity from hot and cold hibiscus (Hibiscus sabdariffa L.) calyces tea infusions. Journal of Food Measurement and Characterization 15, 3531-3538.
| Crossref | Google Scholar |
Paul TK, Nayar MP (1980) The status of Hibiscus furcatus Roxburgh. Bulletin of the Botanical Survey of India 22, 194-195.
| Google Scholar |
Paul TK, Nayar MP (1985) A new species of Hibiscus Linn. (Malvaceae) from Punjab, India. Bulletin of the Botanical Survey of India 25, 188-189 [Volume year 1983].
| Google Scholar |
Pezen Özdogan F, Nilufer O, Ergun F (2011) Studies on the conformity of Hibiscus sabdariffa L. samples from Turkish market to European Pharmacopeia. FABAD Journal of Pharmaceutical Sciences 36, 25-32.
| Google Scholar |
Pfeil BE, Crisp MD (2005) What to do with Hibiscus? A proposed nomenclatural resolution for a large and well known genus of Malvaceae and comments on paraphyly. Australian Systematic Botany 18, 49-60.
| Crossref | Google Scholar |
Pfeil BE, Brubaker CL, Craven LA, Crisp MD (2002) Phylogeny of Hibiscus and the tribe Hibisceae (Malvaceae) using chloroplast DNA sequences of ndhF and the rpl16 intron. Systematic Botany 27, 333-350.
| Google Scholar |
Pfeil BE, Brubaker CL, Craven LA, Crisp MD (2004) Paralogy and orthology in the Malvaceae rpb2 gene family: investigation of gene duplication in Hibiscus. Molecular Biology and Evolution 21, 1428-1437.
| Crossref | Google Scholar | PubMed |
Phuphathanaphong L, Siriruk P, Nuvongsri G (1989) The genus Hibiscus in Thailand. Thai Forest Bulletin (Botany) 18, 43-79.
| Google Scholar |
Pires de Lima A (1921) Subsídios para o estudo da flora de Moçambique. Espermáfitas do-litoral-norte. Brotéria. Série Botânica;. Revista de Sciencias Naturaes do Collegio de S. Fiel. Lisbon 19, 107-143 [In Portuguese].
| Google Scholar |
Pool A, Brako L, Krapovickas A, Chanco M (1993) Malvaceae. In ‘Catalogue of the flowering plants and gymnosperms of Peru/Catalogo de las angiospermas y gimnospermas del Peru. Vol. 45’. (Eds L Brako, JL Zarucchi) Monograph in Systematic Botany from the Missouri Botanical Garden, pp. 643–664. (Missouri Botanical Garden: Saint Louis, MO, USA)
Popova GM (1928) A contribution to the morphology and biology of Hibiscus cannabinus L. Bulletin of Applied Botany 19, 493-496.
| Google Scholar |
Pradeep AK, Sivarajan VV (1991) Hibiscus hispidissimus, the correct name for H. furcatus DC. non Willd. and H. aculeatus Roxb. non Walter (Malvaceae). Taxon 40, 634-637.
| Crossref | Google Scholar |
Prain D (1903) ‘Bengal plants: a list of the phanerogams, ferns and fern-allies indigenous to, or commonly cultivated in, the Lower provinces and Chittagong, with definitions of the natural orders and genera, and keys to the genera and species. Vol. 1.’ (Botanical Survey of India: Calcutta, British India)
Proctor GR (1982) More additions to the flora of Jamaica. Journal of the Arnold Arboretum 63(3), 199-315.
| Crossref | Google Scholar |
Qi Y, Chin KL, Malekian F, Berhane M, Gager J (2005) Biological characteristics, nutritional and medicinal value of roselle, Hibiscus sabdariffa. Urban Forestry, Natural Resources and Environment Circular 604, 1-2.
| Google Scholar |
Rakshit SC, Kundu BC (1961) New species and varieties of Hibiscus. Science & Culture 27, 192-194.
| Google Scholar |
Rakshit SC, Kundu BC (1970) Revision of the Indian species of Hibiscus. Bulletin of the Botanical Survey of India 12, 151-175.
| Crossref | Google Scholar |
Reichenbach HGL (1832) ‘Flora germanica excursoria ex affinitate regni vegetabilis naturali disposita, sive principia synopseos plantarum in Germania terrisque in Europa media adjacentibus sponte nascentium cultarumque frequentius. Vol. 2. Part 2.’ (Carolum Cnobloch: Lipsiae [Leipzig, German Confederation]) [In Latin]
Richard A (1845) Plantes Vasculaires. In ‘Histoire Physique, Politique et Naturelle de l’Ile de Cuba. Botanique. Vol. 10(2)’. (Ed. R de la Sagra) pp. 115–168. (A. Bertrand: Paris, France) [Publication year on the cover page 1841; in French] 10.5962/bhl.title.51128
Rigueiral LHG, Gonçalez VM, Duarte MC (2019) Espécies nativas de Hibiscus (Malvoideae, Malvaceae) da Região Sudeste do Brasil/Native species of Hibiscus (Malvoideae, Malvaceae) from Southeast Brazil. Rodriguésia 70, e03102017 [In Portuguese].
| Crossref | Google Scholar |
Robyns W (1947) Choripétales nouvelles de la région du Parc National Albert (Congo Belge). Bulletin du Jardin Botanique de l'État a Bruxelles 18(3/4), 261-292 [In French].
| Crossref | Google Scholar |
Rodrigues Costa MT, Bovini MG, Alves LL, de Castro GC, Sobral M (2023) Malvaceae from Serra do Lenheiro, Minas Gerais, Brazil. Rodriguésia 74, e01682021 [In Portuguese].
| Crossref | Google Scholar |
Rodríguez Vázquez PO, Flores FF (2020) Los tipos del herbario “Atkins” del jardín botánico de Cienfuegos, Cuba./Types of the herbarium “Atkins” of the botanical garden of Cienfuegos, Cuba. Centro Agrícola 47(3), 14-22 [In Spanish].
| Google Scholar |
Roe MJ (1961) A taxonomic study of the indigenous Hawaiian species of the genus Hibiscus (Malvaceae). Pacific Science 15, 3-32.
| Google Scholar |
Rondon JB (2009) The subfamily Malvoideae (Malvaceae s.l.) in the western of the Sucre state, Venezuela. UDO Agrícola 9(3), 599-621.
| Google Scholar |
Ruan C-J (2010) Review and advances in style curvature for the Malvaceae. Journal of Plant Developmental Biology 4, 98-111.
| Google Scholar |
Ruan C-J, Teixeira da Silva JA, Qin P (2010) Style curvature and its adaptive significance in the Malvaceae. Plant Systematics and Evolution 288(1/2), 13-23.
| Crossref | Google Scholar |
Salisbury RA (1796) ‘Prodromus stirpium in horto ad Chapel Allerton vigentium.’ (The Author: London, UK) [In Latin] 10.5962/bhl.title.427
Satya P (2012) Prezygotic interspecific hybridization barriers between kenaf (Hibiscus cannabinus L.) and four wild relatives. Plant Breeding 131(5), 648-655.
| Crossref | Google Scholar |
Satya P, Karan M, Sarkar D, Sinha MK, Mahapatra BS (2012) Morphological and molecular characterization of interspecific hybrids of kenaf (Hibiscus cannabinus L.) and false roselle (H. acetosella Welw. ex Hiern). Indian Journal of Genetics 72(2), 234-240.
| Google Scholar |
Satya P, Karan M, Kar CS, Mahapatra AK, Mahapatra BS (2013a) Assessment of molecular diversity and evolutionary relationship of kenaf (Hibiscus cannabinus L.), roselle (H. sabdariffa L.) and their wild relatives. Plant Systematics and Evolution 299(3), 619-629.
| Crossref | Google Scholar |
Satya P, Karan M, Kar CS, Mitra J, Sarkar D, Karmakar PG, Sinha MK, Mahapatra BS (2013b) Development and molecular characterization of interspecific hybrid of Hibiscus cannabinus and H. radiatus. Indian Journal of Biotechnology 12, 343-349.
| Google Scholar |
Satya P, Karan M, Chakraborty K, Biswas C, Karmakar PG (2014) Comparative analysis of diversification and population structure of kenaf (Hibiscus cannabinus L.) and roselle (H. sabdariffa L.) using SSR and RGA (resistance gene analogue) markers. Plant Systematics and Evolution 300, 1209-1218.
| Crossref | Google Scholar |
Sayers TDJ, Johnson KL, Steinbauer MJ, Farnier K, Miller RE (2021) Divergence in floral scent and morphology, but not thermogenic traits, associated with pollinator shift in two brood-site-mimicking Typhonium (Araceae) species. Annals of Botany 128, 261-280.
| Crossref | Google Scholar | PubMed |
Scarth-Johnson V (1968) Some Queensland Hibiscus. Australian Plants 4, 244-245.
| Google Scholar |
Schumacher HCF (1827) ‘Beskrivelse af Guineiske planter: som ere fundne af Danske botanikere, især af etatsraad Thonning.’ (F. Popp: Kjöbenhavn, Denmark) [In Danish] 10.5962/bhl.title.51454
Sedillo-Torres IY, Hernández-Rangel ÁO, Gómez-Y-Gómez Y, Cortés-Avalos D, García-Pérez BE, Villalobos-Rocha JC, Hernández-Rodríguez CH, Zepeda-Vallejo LG, Estrada-de los Santos P, Vargas-Díaz ME, Ibarra JA (2022) Hibiscus acid from Hibiscus sabdariffa L. inhibits flagellar motility and cell invasion in Salmonella enterica. Molecules 27(3), 655.
| Crossref | Google Scholar | PubMed |
Sessé y Lacasta M, Mociño JM (1894) ‘Flora Mexicana, Editio secunda.’ (Oficina Tipográfica de la Secretaría de Fomento: México) [In Spanish] 10.5962/bhl.title.376
Siepe T, Ventrella D, Lapenta E (1997) Evaluation of genetic variability in a collection of Hibiscus cannabinus (L.) and Hibiscus spp (L.). Industrial Crops and Products 6(3–4), 343-352.
| Crossref | Google Scholar |
Simon LD, Ogunwolu EO, Okoroafor E, Ekoja EE (2021) Diversity, relative abundance and temporal spread of insects associated with roselle (Hibiscus sabdariffa L.) at Makurdi, Nigeria. Annals of Tropical Research 43, 1-15.
| Crossref | Google Scholar |
Skovsted A (1944) Some hybridization experiments in the tribe Hibisceae. Comptes-rendus Des Travaux du Carlsberg Laboratoriet; Série Physiologique 24, 1-30.
| Google Scholar |
Small JK (1933) ‘Manual of the southeastern flora: being descriptions of the seed plants growing naturally in Florida, Alabama, Mississippi, eastern Louisiana, Tennessee, North Carolina, South Carolina and Georgia.’ (published by the author: New York, NY, USA) 10.5962/bhl.title.696
Smith JD (1903) ‘Enumeratio Plantarum Guatemalensium: necron Salvadorensium, Hondurensium, Nicaraguensium, Costaricensium. Vol. 6.’ (H.N. Patterson: Oquawkae, IL, USA) [In Latin] 10.5962/bhl.title.827
Sosef MSM, Wieringa JJ, Jongkind CCH, Achoundong G, Azizet Issembk Y, Bedigian D, van den Berg RG, Breteler FJ, Cheek M, Degreef J, Faden RB, Goldblatt P, van der Maesen LJG, Ngok Banak L, Niangadouma R, Nzabi T, Nziengui B, Rogers ZS, Stkvart T, van Valkenburg JLCH, Walters G, de Wilde JJFE (2006) Check-list des plantes vasculaires du Gabon/Checklist of Gabonese vascular plants. Scripta Botanica Belgica 35, 1-438.
| Google Scholar |
Sprague TA (1913) Miscellaneous Notes LXI – Hibiscus asper. Kew Bulletin of Miscellaneous Information 1913, 418-419.
| Google Scholar |
Standley PC (1923) Hibiscus. In ‘Trees and shrubs of Mexico’. Contributions of the United States National Herbarium 23, 777-782.
| Google Scholar |
Standley PC (1928) Flora of the Panama Canal Zone. Contributions of the United States National Herbarium 27, 1-416.
| Google Scholar |
Thulin M (1999a) A new species of Hibiscus (Malvaceae) from Somalia. Nordic Journal of Botany 19, 337-339.
| Crossref | Google Scholar |
Trimen H (1893) ‘A hand-book to the flora of Ceylon: containing descriptions of all the species of flowering plants indigenous to the island, and notes on their history, distribution, and uses: with an atlas of plates illustrating some of the more interesting species. Part 1. Ranunculaceæ–Anacardiaceæ.’ (Dulau & Co.: London, UK) 10.5962/bhl.title.10864
Tsai PJ, McIntosh J, Pearce P, Camden B, Jordan BR (2002) Anthocyanin and antioxidant capacity in roselle (Hibiscus sabdariffa L.) extract. Food Research International 35(4), 351-356.
| Crossref | Google Scholar |
Turczaninow NS (1858) Animadversiones in secundam partem herbarii Turczaninowiani, nunc Universitatis Caesareae Charkowiensis. Bulletin de la Société Impériale Des naturalistes de Moscou 31, 185-250 [In Latin].
| Google Scholar |
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (2018) ‘International code of nomenclature for algae, fungi, and plants (Shenzhen Code)’, adopted by the Nineteenth International Botanical Congress, July 2017, Shenzhen, PR China. Regnum Vegetabile 159. (Koeltz Botanical Books: Glashütten, Germany) 10.12705/Code.2018
Ulbrich E (1919) Einige neue Hibiscus-Arten aus dem tropischen Afrika. Notizblatt des Botanischen Gartens und Museums zu Berlin-Dahlem 7, 179-183 [In Latin with title in German].
| Crossref | Google Scholar |
Ulbrich E (1921) Hibiscus L., Malvaceae. In ‘Die Planzenwelt Afrikas, insbesondere seiner tropischen Gebiete: Grundzge der Pflanzenverbreitung im Afrika und die Charakterpflanzen Afrikas.’ Vegetation der Erde 9. Part 3(2) ’. (Ed. E Engler) pp. 368–408. (W.Engelmann: Leipzig, German Republic) [In German]
Ulbrich E (1922) Species et sectiones africanae novae generis Hibiscus L. Notizblatt des Königl. Botanischen Gartens und Museums zu Berlin 8(72), 157-171 [In Latin].
| Google Scholar |
Ulbrich E (1924) Malvaceae. In Fries RE, Fries TCE. Beiträge zur Kenntnis der Flora des Kenia, Mt Aberdare und Mt Elgon. IV. Notizblatt des Königl. Botanischen Gartens und Museums zu Berlin 8(80), 661-704 Available at https://www.jstor.org/stable/25118178 [In German].
| Google Scholar |
Urban I (1929) Plantae haitienses et domingenses novae vel rariores VII. a cl. E.L. Ekman 1924–1928 lectae. Arkiv för Botanik 22A(17), 1-115 [In Latin].
| Google Scholar |
Urban I, Helwig B (1928) Malvaceae novae et minus cognitae Cubenses atque Domingenses. Repertorium Specierum Novarum Regni Vegetabilis 24, 231-238 [In Latin].
| Crossref | Google Scholar |
van Leeuwen JF, Froyd CA, van der Knaap WO, Coffey EE, Tye A, Willis KJ (2008) Fossil pollen as a guide to conservation in the Galápagos. Science 322(5905), 1206.
| Crossref | Google Scholar | PubMed |
Villaseñor JL (2016) Checklist of the native vascular plants of Mexico. Revista Mexicana de Biodiversidad 87, 559-902.
| Crossref | Google Scholar |
von Cufodontis G (1948) Uber sicht der Africkanischen Hibiscus-Arlen aus der sektion Bomabycella. Annalen des Naturhistorischen Museums in Wien 56, 24-59 [In German].
| Google Scholar |
von Cufodontis G (1959) Supplement: Enumeratio Plantarum Aethiopiae Spermatophyta (Sequentia). Bulletin du Jardin botanique de l’État a Bruxelles 29(1), 533-584 [In Latin].
| Crossref | Google Scholar |
Wahabi HA, Alansary LA, Al-Sabban AH, Glasziuo P (2010) The effectiveness of Hibiscus sabdariffa in the treatment of hypertension: A systematic review. Phytomedicine 17, 83-86.
| Crossref | Google Scholar | PubMed |
Wallich N (1831) ‘Numerical list of dried specimens of plants in the Museum of the Honl. East India Company.’ (Lithographed from the ms.: London, UK) [Publication years on cover page 1828–1849] 10.5962/bhl.title.1917
Wang DY, Nagata M, Matsumoto M, Amen Y, Wang DM, Shimizu K (2022a) Potential of Hibiscus sabdariffa L. and hibiscus acid to reverse skin aging. Molecules 27(18), 6076.
| Crossref | Google Scholar |
Wang ZQ, Yang H, Zhang FJ, Yin YL, Gu CS (2022b) The complete chloroplast genome sequence of Hibiscus coccineus. Mitochondrial DNA – B. Resources 7, 217-218.
| Crossref | Google Scholar |
Wannan BS (2022) Hibiscus graniticus Wannan (Malvaceae), a new species from north-east Queensland. Austrobaileya 12, 19-25.
| Crossref | Google Scholar |
Wannan BS (2024) Hibiscus cummingii Wannan (Malvaceae), a new species from north-east Queensland. Austrobaileya 14, 27-35.
| Crossref | Google Scholar |
Wester PJ (1911) Contributions to the history and bibliography of the roselle. Bulletin of the Torrey Botanical Club 38, 91-98.
| Crossref | Google Scholar |
Wester PJ (1914) New varieties of roselle. Philippine Agricultural Review 7, 266-269.
| Google Scholar |
Wight R (1839) ‘Icones plantarum Indiae Orientalis: or figures of Indian plants. Vol. 1.’ (J.B. Pharoah for the Author: Madras, British India) 10.5962/bhl.title.92
Wilson FD (1967) An evaluation of Kenaf, Roselle and related Hibiscus for fiber production. Economic Botany 21(2), 132-139.
| Crossref | Google Scholar |
Wilson FD (1974) Hibiscus section Furcaria (Malvaceae) in Australia. Australian Journal of Botany 22, 157-182.
| Crossref | Google Scholar |
Wilson FD (1978) Wild kenaf, Hibiscus cannabinus L. (Malvaceae) and related species in Kenya and Tanzania. Economic Botany 32(2), 199-204.
| Crossref | Google Scholar |
Wilson FD (1983) The taxonomic status of Hibiscus (sect. Furcaria) berberidifolius A. Rich. Brittonia 35, 175-179.
| Crossref | Google Scholar |
Wilson FD (1993) Hibiscus section Furcaria (Malvaceae) in islands of the Pacific Basin. Brittonia 45, 275-285.
| Crossref | Google Scholar |
Wilson FD (1994) The genome biogeography of Hibiscus L. section Furcaria DC. Genetic Resources and Crop Evolution 41, 13-25.
| Crossref | Google Scholar |
Wilson FD (1995) Two new species of Hibiscus section Furcaria DC. (Malvaceae) from Queensland. Austrobaileya 4(3), 439-447.
| Crossref | Google Scholar |
Wilson FD (1999) Revision of Hibiscus section Furcaria (Malvaceae) in Africa and Asia. Bulletin of the Natural History Museum London (Botany) 29(1), 47-79.
| Google Scholar |
Wilson FD (2006) A distributional and cytological survey of the presently recognized taxa of Hibiscus section Furcaria (Malvaceae). Bonplandia 15, 53-62.
| Crossref | Google Scholar |
Wilson FD (2022) Hibiscus (Section Furcaria). In ‘Flora of Australia’. (Eds TBG McLay, PG Kodela) (Australian Biological Resources Study, Department of Climate Change, the Environment and Water: Canberra, ACT, Australia) Available at https://profiles.ala.org.au/opus/foa/profile/Hibiscus [Verified 19 April 2024]
Wilson FD, Byrnes N (1970) Two new species of Hibiscus section Furcaria (Malvaceae) from Australia. Proceedings of the Linnean Society of New South Wales 95, 194-197.
| Google Scholar |
Wilson FD, Craven LA (1995) Two new species of Hibiscus section Furcaria DC. (Malvaceae) from northern Queensland. Austrobaileya 4, 439-447.
| Crossref | Google Scholar |
Wilson FD, Lally TL (2022) Hibiscus (Section Furcaria). In ‘Flora of Australia’. (Eds TBG McLay, PG Kodela) (Australian Biological Resources Study, Department of Climate Change, the Environment and Water: Canberra, ACT, Australia) Available at https://profiles.ala.org.au/opus/foa/profile/Hibiscus [Verified 19 April 2024]
Wilson FD, McLay TBG (2022) Hibiscus (Section Furcaria). In ‘Flora of Australia’. (Eds TBG McLay, PG Kodela) (Australian Biological Resources Study, Department of Climate Change, the Environment and Water: Canberra, ACT, Australia) Available at https://profiles.ala.org.au/opus/foa/profile/Hibiscus [Verified 19 April 2024]
Wilson FD, Menzel MY (1964) Kenaf (Hibiscus cannabinus L.) and roselle (H. sabdariffa L.). Economic Botany 18(1), 80-91.
| Crossref | Google Scholar |
Wood KR, Oppenheimer H, Keir M (2019) A checklist of endemic Hawaiian vascular plant taxa from the Hawaiian Islands that are considered possibly extinct in the wild. Technical report. (National Tropical Botanical Garden: Honolulu, HI, USA) Available at https://www.researchgate.net/publication/332933164
Xu XR, Zhou SD, Shi XQ (2019) The complete chloroplast genome of Hibiscus taiwanensis (Malvaceae). Mitochondrial DNA – B. Resources 4, 2533-2534.
| Crossref | Google Scholar | PubMed |
Yamazaki Y, Kajita T, Takayama K (2023) Spatiotemporal process of long-distance seed dispersal in a pantropically distributed sea hibiscus group. Molecular Ecology 32, 1726-1738.
| Crossref | Google Scholar | PubMed |
Yuncker TG (1943) The flora of Niue Island. Bernice P. Bishop Museum Bulletin in Botany 178, 1-126.
| Google Scholar |
Zhang L, Xu Y, Zhang X, Ma X, Zhang L, Liao Z, Zhang Q, Wan X, Cheng Y, Zhang J, Li D, Zhang L, Xu J, Tao A, Lin L, Fang P, Chen S, Qi R, Xu X, Qi J, Ming R (2020) The genome of kenaf (Hibiscus cannabinus L.) provides insights into bast fibre and leaf shape biogenesis. Plant Biotechnology Journal 18(8), 1796-1809.
| Crossref | Google Scholar | PubMed |
Zheoat AM, Gray AI, Igoli JO, Ferro VA, Drummond RM (2019) Hibiscus acid from Hibiscus sabdariffa (Malvaceae) has a vasorelaxant effect on the rat aorta. Fitoterapia 134, 5-13.
| Crossref | Google Scholar | PubMed |