Utility of a US Food and Drug Administration (FDA) label indication for condoms for anal sex
Aaron J. Siegler A G , Lauren Ahlschlager A , Elizabeth M. Rosenthal B C , Patrick S. Sullivan B , Colleen F. Kelley D , Eli S. Rosenberg B C , Travis H. Sanchez B , Reneé H. Moore E , C. Christina Mehta E and Michael P. Cecil F
B C , Patrick S. Sullivan B , Colleen F. Kelley D , Eli S. Rosenberg B C , Travis H. Sanchez B , Reneé H. Moore E , C. Christina Mehta E and Michael P. Cecil F
A Department of Behavioral Science and Health Education, Rollins School of Public Health at Emory University, 1518 Clifton Road NE, Atlanta, GA 30322, USA.
B Department of Epidemiology, Rollins School of Public Health at Emory University, 1518 Clifton Road NE, Atlanta, GA 30322, USA.
C Department of Epidemiology and Biostatistics, University at Albany School of Public Health, SUNY One University Place, Rensselaer, NY 12144, USA.
D Emory University School of Medicine, 1648 Pierce Drive NE, Atlanta, GA 30307, USA.
E Department of Biostatistics and Bioinformatics, Rollins School of Public Health at Emory University, 1518 Clifton Road NE, Atlanta, GA 30322, USA.
F TheyFit, LLC, 4140 Tate Street NE, Covington, GA 30014, USA.
G Corresponding author. Email: asiegle@emory.edu
Sexual Health 17(1) 91-95 https://doi.org/10.1071/SH18152
Submitted: 17 August 2018 Accepted: 27 June 2019 Published: 15 October 2019
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Condoms are highly effective for HIV prevention, yet are not currently indicated by the US Food and Drug Administration (FDA) for anal sex. We surveyed a national sample of men who have sex with men to assess whether FDA label indication could affect anticipated condom use, and to determine levels of perceived condom failure for anal sex. We found that 69% of respondents anticipated that a label indication change would increase their likelihood of condom use. Median perceived failure was 15%. We anticipate that these results may aid the FDA in developing standards for a label indication for anal sex.
Additional keywords: HIV, prevention, sexual and gender minorities.
Introduction
Over two of every three new HIV diagnoses in 2015 in the US occurred among men who have sex with men (MSM).1 The US Centers for Disease Control and Prevention and the World Health Organization recommend and promote condom use among MSM,2,3 yet condoms are not currently indicated by the US Food and Drug Administration (FDA) for anal sex. Condoms currently on the market in the US and elsewhere have been evaluated and approved based on clinical data from vaginal sex, not anal sex. The FDA provides guidance for patients in the form of a frequently asked questions section, which includes the hypothetical, ‘Are condoms strong enough for anal intercourse?’ and provides a response of ‘Condoms may be more likely to break during anal intercourse than during other types of sex because of the greater amount of friction and other stresses involved.’4
FDA guidance for condom studies notes a deficiency of data that could be used for an anal sex label indication, and calls for such data to be made available.5 Past FDA condom clearance procedures used total clinical failure (slippage and breakage) performance standards from vaginal sex studies, clearing condoms with <5% total clinical failure. Although smaller datasets can be used to establish FDA 510(k) equivalence for condoms for vaginal sex, a new label indication for anal sex would likely be a larger undertaking and require a large clinical trial. There are several relevant questions regarding a potential FDA label indication of condoms for anal sex, including: (1) is a label indication for anal sex worth pursuing; and (2) what are the levels of patient-reported outcomes (PRO) that can guide label indication. Consumer preferences, such as PRO, are increasingly being used by the FDA to inform regulatory guidelines.6 We sought to answer these questions through a brief survey among a national, online sample of MSM. To assess whether a label indication is worthwhile, the survey explored willingness to use condoms under a hypothetical condition of an FDA condom label indication for anal sex. To provide patient-reported information for condom failure, we documented perceived levels of clinical failure for anal sex.
Methods
Data were collected from September 2015 through April 2016 through the American Men’s Internet Survey (AMIS), an annual Internet survey of MSM in the US. A full overview of the cross-sectional survey methods and study population has been published previously.7 Briefly, participants were recruited primarily through online advertising targeted to MSM. After completing an eligibility screener, participants were consented and asked to complete the survey. To reduce response burden for AMIS participants, supplemental questions such as the ones used in the present study were provided only to a randomly selected subset of the approximately 10 000 annual AMIS participants.
We developed four survey items to assess whether FDA label indications could affect anticipated condom utilisation, as well as perceived and threshold rates of condom failure. Dichotomous items exploring the potential impact of FDA label indications were: (1) ‘Currently there is no condom that is approved by the FDA for use during anal sex. If a condom was FDA-approved for anal sex, would you be more likely to use condoms every time you have anal sex?’; and (2) ‘If a condom was labeled by the FDA as ‘more pleasurable’, would you be more likely to use this condom for anal sex?’ Participants were instructed that condoms ‘are considered to fail when they slip or break’. Participants were then asked to report perceived failure level (‘How often do you think condoms fail [slip or break] when used for anal sex?’) and threshold failure level (‘At what amount of condom failure [slip or break] would you NOT be willing to use condoms for anal sex?’). Perceived and threshold rates of condom failure were assessed using slider bars ranging from 0% to 100%.
Median values and percentiles were used to describe perceived and threshold condom failure levels for anal sex. Bootstrap confidence intervals (CIs) were generated for the overall median values by creating 1000 resampled datasets, obtaining the median value from each of the new datasets and using the 2.5th and 97.5th percentiles of all dataset medians as the upper and lower bounds of the 95% CI. Unadjusted counts and percentages of those responding ‘Yes’ to the items assessing potential behavioural impacts of FDA label indications are reported with bootstrap CIs generated in the manner described above. Associations between descriptive characteristics (demographics, recent HIV prevention behaviours) and study outcomes were assessed with linear and logistic regressions.
Results
Of the 137 608 potential participants who clicked on an advertisement for a men’s health survey, 46 207 (34%) completed the eligibility screen, 25 919 (56%) were eligible to complete the survey and 10 217 (39%) completed the survey. Participants eligible for inclusion in the present analysis were aged ≥15 years, reported having had sex with a man in the past 12 months, resided in the US and provided a valid US zip code. Among the 10 217 participants completing the AMIS survey, 2079 (20%) were randomly selected to be provided with the supplemental questions that comprise the present study.
Of the 2079 participants, 43% (n = 893) were <25 years of age, 28% (n = 587) were aged 25–34 years, 8% (n = 162) were aged 35–44 years and 21% (n = 437) were >44 years of age. Most participants were White (68%; n = 1417); 8% (n = 169) were Black, 15% (n = 307) were Hispanic/Latino and 9% (n = 186) were another race, multiracial or preferred not to answer or had missing values. Most participants self-identified as gay (84%; n = 1685), 15% (n = 306) identified as bisexual and <1% (n = 12) identified as heterosexual or straight. Nearly half the participants had completed college or postgraduate education (n = 1009), one-third had completed some college or an associate or technical degree (n = 675) and 18% (n = 368) had a high school education or less. Most participants reported having anal sex with a man in the past 12 months (86%; n = 1794). Of these, 77% (n = 1377) reported condomless anal sex with a man in the past year and 23% (n = 403) reported always using condoms during anal sex with a man in the past year.
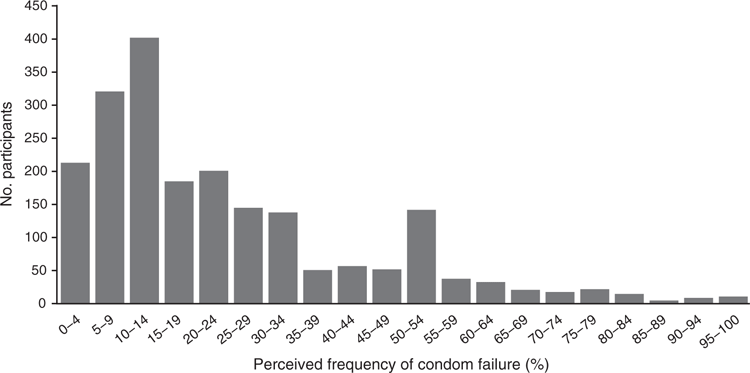
Nearly 69% (95% CI 67–71%, n = 1427) of participants reported being more likely to use condoms each time they had sex if a condom was FDA approved for anal sex. A similar proportion (72%; 95% CI 70–74%, n = 1492) reported being more likely to use a condom for anal sex that was labelled by the FDA as more pleasurable. Fig. 1 describes the distribution of perceptions regarding how often individuals perceive condoms to fail for anal sex. Those at the median perceived that condoms failed at a rate of 15% (95% CI 15–18%). Those at the 5th, 10th, 25th and 75th percentiles perceived the rate of failure to be 2%, 4%, 9% and 32% respectively. For the level of failure at which individuals would not be willing to use condoms (the threshold failure), the median value was 49% (95% CI 43–50%). Those at the 5th, 10th, 25th and 75th percentiles had threshold failure values of 1%, 6%, 20% and 73% respectively.
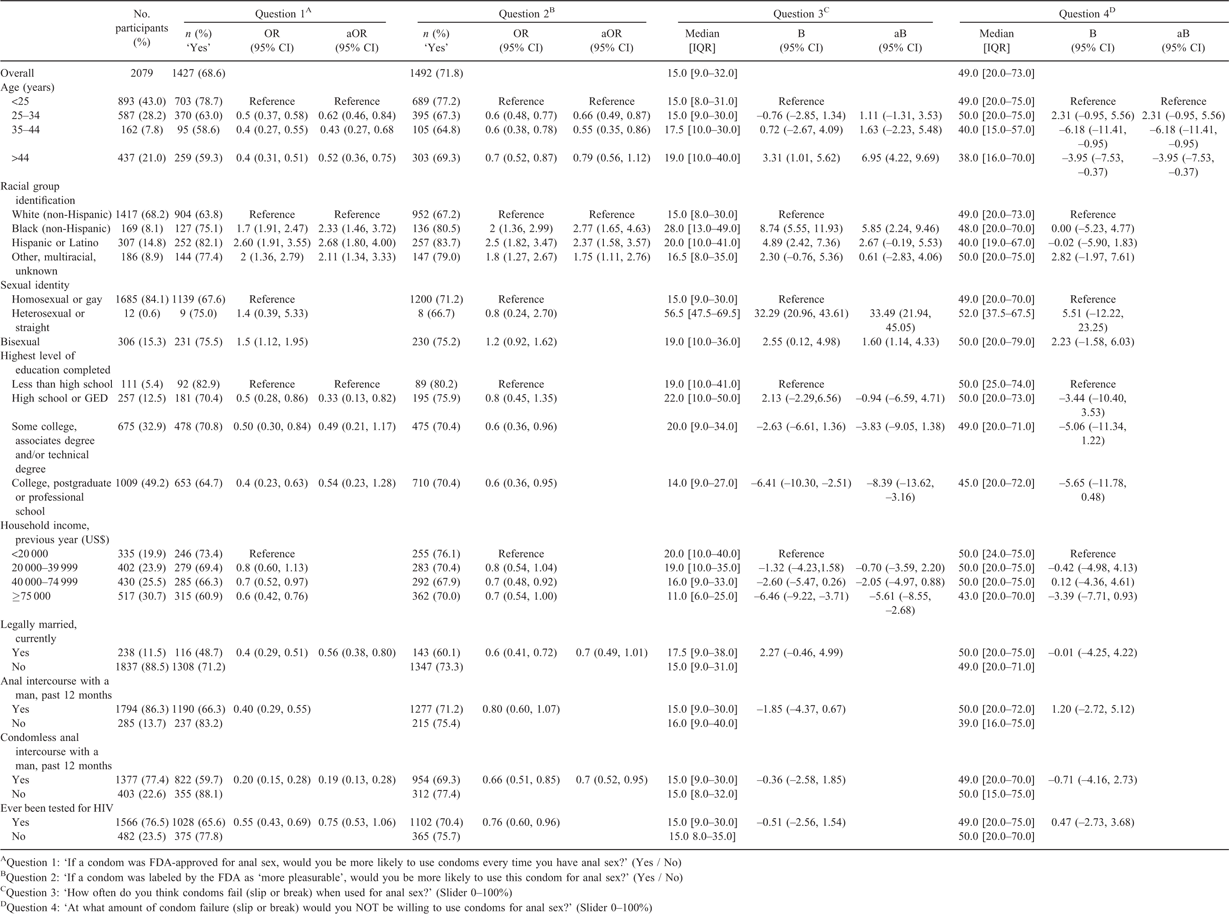
Associations between study population characteristics (demographic and HIV prevention variables) and the four study outcome variables are presented in Appendix 1. Those who were younger, Black, Latino or with lower education levels were more likely to anticipate an increase in their condom use if changes were made to FDA label indications. Those who were older, Black, identified as heterosexual or with an annual income less than US$20 000 were more likely to perceive higher rates of condom failure for anal sex.
Discussion
This study found that FDA label indications for condoms have the potential to affect condom use among MSM. Most MSM (69%) in a national online sample anticipated that FDA label indication of condoms for anal sex would increase their likelihood of using condoms. Demographic groups at higher risk of HIV transmission, such as younger, Black or Latino respondents,1 were more likely to anticipate increases in their condom use. Given that condoms are not explicitly label indicated for anal sex, this study provides evidence that sufficient data should be provided to the FDA to allow for an explicit determination to be made. Condom use among MSM is the product of numerous factors, including personal preference (e.g. fit or feel),8 interpersonal (e.g. family)9 and policy (e.g. low access to appropriate sexual health education among Lesbian, Bisexual, Gay and Transgendered youth).10 Given that structural sexual stigma is associated with decreased use of HIV prevention methods,11 it will be important to make structural changes optimise access to and the use of HIV prevention services.12
Patient-reported outcomes are increasingly being used to inform FDA decision making.6 Most participants (81%) perceived condom failure rates for anal sex as being higher (e.g. >5%) than the current maximum clinical failure level used by the FDA to clear condoms when using data from vaginal sex studies.13 We anticipate these data, along with clinical failure data for anal sex from observational studies and clinical trials, can be used to allow the FDA to determine appropriate levels of condom failure to establish standards for an anal sex indication.
This study is limited in that it used an online sample, the outcomes were based on self-report and some outcomes were based on participant assessments of hypothetical scenarios. Despite these limitations, the magnitude and direction of the findings among a large sample indicate that seeking an FDA label indication for condoms for anal sex is a worthy pursuit.
Conflicts of interest
Michael P. Cecil is the owner of TheyFit LLC. On 26 January 2016, TheyFit LLC sold all assets pertaining to the study aims, including trademarks, intellectual property, inventory, website and regulatory approvals, to Karex Berhad. Michael P. Cecil has no financial interest in Karex Berhad. The remaining authors have no conflicts of interest to declare.
Acknowledgements
The study that provided data for this paper was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (R43HD078154 and R44HD078154), through the Small Business Innovation Research grant mechanism. For the study supported by this grant, the authors, except M. P. Cecil and E. M. Rosenthal, were subcontracted for TheyFit, LLC. The study was facilitated by the Emory Center for AIDS Research (P30AI050409). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
[1] Centers for Disease Control and Prevention. HIV surveillance report. Volume 27. Diagnoses of HIV infection in the United States and dependent areas, 2015. 2016. Available online at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2015-vol-27.pdf [verified 6 September 2019].[2] Centers for Disease Control and Prevention. CDC fact sheet: what gay, bisexual and other men who have sex with men need to know about sexually transmitted diseases. 2017. Available online at: https://www.cdc.gov/std/life-stages-populations/stdfact-msm.htm [verified 24 January 2018].
[3] World Health Organization. Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people: recommendations for a public health approach 2011. 2011. Available online at: http://www.who.int/hiv/pub/guidelines/msm_guidelines2011/en/ [verified 6 September 2019].
[4] U. S. Food and Drug Administration. Condoms and sexually transmitted diseases. 2016. Available online at: https://www.fda.gov/forpatients/illness/hivaids/prevention/ucm126372.htm [verified 22 December 2017].
[5] U.S. Food and Drug Administration (FDA). Testing guidance for male condoms made from new material (non-latex). 1995. Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/testing-guidance-male-condoms-made-new-material-non-latex [verified 6 September 2019].
[6] Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. 2009. Available online at: https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf [verified 6 September 2019].
[7] Zlotorzynska M, Sullivan P, Sanchez T. The annual American Men’s Internet Survey of behaviors of men who have sex with men in the United States: 2015 key indicators report. JMIR Public Health Surveill 2017; 3 e13
| The annual American Men’s Internet Survey of behaviors of men who have sex with men in the United States: 2015 key indicators report.Crossref | GoogleScholarGoogle Scholar | 28356240PubMed |
[8] Hernández-Romieu AC, Siegler AJ, Sullivan PS, Crosby R, Rosenberg ES. How often do condoms fail? A cross-sectional study exploring incomplete use of condoms, condom failures and other condom problems among black and white MSM in southern U.S.A. Sex Transm Infect 2014; 90 602–7.
| How often do condoms fail? A cross-sectional study exploring incomplete use of condoms, condom failures and other condom problems among black and white MSM in southern U.S.A.Crossref | GoogleScholarGoogle Scholar | 25080511PubMed |
[9] Siegler AJ, Voux A, Phaswana-Mafuya N, Bekker LG, Sullivan PS, Baral SD, et al Elements of condom-use decision making among South African men who have sex with men. J Int Assoc Provid AIDS Care 2014; 13 414–23.
| Elements of condom-use decision making among South African men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 24935692PubMed |
[10] Rasberry CN, Condron DS, Lesesne CA, Adkins SH, Sheremenko G, Kroupa E. Associations between sexual risk-related behaviors and school-based education on HIV and condom use for adolescent sexual minority males and their non-sexual-minority peers. LGBT Health 2018; 5 69–77.
| Associations between sexual risk-related behaviors and school-based education on HIV and condom use for adolescent sexual minority males and their non-sexual-minority peers.Crossref | GoogleScholarGoogle Scholar | 29240528PubMed |
[11] Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, Krakower D, Novak DS, Mimiaga MJ, et al State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS 2015; 29 837–45.
| State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States.Crossref | GoogleScholarGoogle Scholar | 25730508PubMed |
[12] Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380 367–77.
| Global epidemiology of HIV infection in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 22819660PubMed |
[13] U.S. Food and Drug Administration Center for Devices and Radiological Health. Letter from the Food and Drug Administration to Penny Northcutt: TheyFit Male Condom 510(k), Approval No. K150072. 2015. Available online at: https://www.accessdata.fda.gov/cdrh_docs/pdf15/K150072.pdf [verified 6 September 2019].
Appendix 1. Characteristics of the study population by key outcomes from a cross-sectional analysis in the US, 2015
OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; pctl, percentile; B, beta; aB, adjusted beta
|