Translating mouthwash use for gonorrhoea prevention into a public health campaign: identifying current knowledge and research gaps
Eric P. F. Chow A B C , Kate Maddaford A , Sabrina Trumpour A B and Christopher K. Fairley A B
A B C , Kate Maddaford A , Sabrina Trumpour A B and Christopher K. Fairley A B
A Melbourne Sexual Health Centre, Alfred Health, 580 Swanston Street, Carlton, Vic. 3053, Australia.
B Central Clinical School, Monash University, 99 Commercial Road, Melbourne, Vic. 3004, Australia.
C Corresponding author. Email: eric.chow@monash.edu
Sexual Health 16(5) 433-441 https://doi.org/10.1071/SH18237
Submitted: 20 December 2018 Accepted: 13 February 2019 Published: 17 May 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
The gonorrhoea rate among gay and bisexual men who have sex with men (MSM) has been increasing rapidly in many Western countries. Furthermore, gonorrhoea is becoming increasingly resistant to antibiotics and only limited options remain for treatment. Recent evidence suggests that the oropharynx may play an important role in gonorrhoea transmission. It is hypothesised that reducing the prevalence of oropharyngeal gonorrhoea will also reduce the population incidence of gonorrhoea. Mouthwash has been proposed as a novel non-antibiotic intervention to prevent oropharyngeal gonorrhoea; hence, reducing the probability of antibiotic resistance developing. However, its efficacy is yet to be confirmed by a randomised controlled trial – the findings of which will be available in 2019. If the trial shows mouthwash is effective in preventing gonorrhoea, this finding could potentially be translated into a public health campaign to increase the mouthwash use in the MSM population. This article summarises the current evidence of the effectiveness of mouthwash against gonorrhoea and discusses the potential literature gaps before implementing the mouthwash intervention at a population level.
Additional keywords: gay men, intervention, men who have sex with men, Neisseria gonorrhoeae, prevention, sexually transmitted diseases, sexually transmissible infections.
Introduction
Gonorrhoea is increasing at an alarming rate among gay and bisexual men who have sex with men (MSM) not only in Australia but also in many western countries.1–4 The rise of antimicrobial resistance in Neisseria gonorrhoeae in recent years is of particular concern.5 Recently, the first cases of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin were found in Australia6 and the UK, and herald the prospect of gonorrhoea becoming substantially more difficult to treat.7 Clinical trials on three new antibiotics to treat gonorrhoea (solithromycin, zoliflodacin and gepotidacin) are currently underway.8 A phase II clinical trial has demonstrated that the microbiologic cure rate of a single dose of zoliflodacin for anorectal gonorrhoea was 100% and for urogenital/cervical gonorrhoea, it was 96%; however, it was less effective for oropharyngeal gonorrhoea (50–82%).9
The control of oropharyngeal gonorrhoea is complicated because it has a relatively short duration of infection, up to 3–4 months.10,11 Individuals with oropharyngeal gonorrhoea are almost always asymptomatic;12 therefore, the current recommended annual screening would have missed most oropharyngeal cases. The importance of oropharyngeal gonorrhoea has been highlighted in a recent hypothesis and mathematical models that suggest it may play an important role in transmission and control of gonorrhoea in MSM.13–15 Oral sex is common among MSM. MSM can acquire oropharyngeal gonorrhoea from condomless oral sex (fellatio),16 but condom use for oral sex among MSM is relatively low.17 A US study surveyed 871 sexually active MSM and found that only 9% used condoms for oral sex consistently in the last 12 months.18 Recent qualitative research has indicated that men do not like the loss of sensation or the taste of condoms during oral sex and thus they are not willing to use condoms for fellatio.19,20 Nevertheless, condomless anal sex has also been increasing among MSM over the last few years in the era of pre-exposure prophylaxis (PrEP) for HIV.3,21 In addition, a mathematical model has predicted that gonorrhoea cannot be eliminated, even in a scenario with 100% condom use for anal sex.22 A recent hypothesis and other data suggest that oropharyngeal gonorrhoea may be transmitted via kissing in addition to condomless fellatio and possibly rimming (i.e. oro-anal contact).15,23–29 In the context of this new data and the falling rate of condom use, novel interventions for gonorrhoea prevention that targets the oropharynx are required if successful control is to be realised. It has been suggested that an antibacterial mouthwash could be used to prevent oropharyngeal gonorrhoea.30,31 If mouthwash is proven to be effective against Neisseria gonorrhoeae, this can be translated into an important public health campaign to increase mouthwash use at a population level. However, there are several unknown factors that need to be solved if mouthwash is to be implemented for gonorrhoea prevention. The aim of this paper is to: (1) discuss the current knowledge and understanding of mouthwash use for gonorrhoea prevention; and (2) identify what future studies are required before translating mouthwash use as a public health campaign to prevent gonorrhoea at a population level.
Current evidence on mouthwash to prevent oral diseases
Clinical studies have demonstrated that the use of antibacterial mouthwashes can effectively prevent plaque and gingivitis.32–34 Addy et al. (1991) conducted an observational study on 10 healthy young adults and showed that there was a large reduction in bacterial counts in saliva for up to 7 h after rinsing with chlorhexidine-containing mouthwashes.35 Other studies have confirmed these findings and shown a significant reduction in levels of aerobic and anaerobic bacteria in both saliva and mucosal samples after using antimicrobial mouthwash.36,37
The antibacterial properties of mouthwash prompted investigators to propose more than 30 years ago that mouthwash be used for post-exposure prophylaxis for STIs, particularly among sex workers.38–40 Recent evidence is consistent with this proposition because high bacterial loads of Neisseria gonorrhoeae bacterial are present in the saliva among men with oropharyngeal gonorrhoea.27 Therefore, it was hypothesised that the use of mouthwash can effectively reduce the bacterial load of Neisseria gonorrhoeae in saliva and thus prevent ongoing transmission. However, until recently, there had been very limited scientific evidence to support the proposition that mouthwash may prevent an STI. Recently, an in vitro study has demonstrated that two alcohol-containing Listerine® products can inhibit the growth of Neisseria gonorrhoeae to less than 102 colony forming units (CFU) per mL.30 Another in vitro study has shown that Chlorhexidine 0.2% mouthwash can also inhibit the growth of Neisseria gonorrhoeae to less than 102 CFU per mL, and authors revealed the inhibitory effect is stronger with a longer exposure time.41 Both in vitro studies used phosphate buffered saline as a control and displayed no inhibitory effect in the control group.
To date, there has been only one published randomised controlled trial (RCT) examining the effect of mouthwash against Neisseria gonorrhoeae.30 This RCT randomised 58 MSM with untreated oropharyngeal gonorrhoea into either the mouthwash or saline group, and participants were instructed to rinse and gargle the allocated solution for 60 s. Results showed almost half (48%) of MSM cleared the infection in the mouthwash group, compared with only 16% in the saline group (P = 0.013). The clearance effect is more profound at the tonsillar fossae compared with the posterior oropharynx, suggesting it is important for mouthwash to reach the posterior oropharynx.
A novel intervention on mouthwash use to prevent oropharyngeal gonorrhoea
Based on the findings from previous in vitro studies and the single RCT, it has been suggested that mouthwash may lead to a novel intervention preventing gonorrhoea in the oropharynx,30,41 and findings from the OMEGA (Oral Mouthwash use to Eradicate GonorrhoeA) study will answer the question about whether this novel approach may work.31 In brief, the OMEGA trial is a large multicentre double-blind RCT conducted in Australia, which has recently completed. The OMEGA trial recruited more than 500 MSM in Melbourne and Sydney and examined whether daily use of mouthwash over a 3-month period can effectively prevent gonorrhoea in the oropharynx. The trial used two different types of alcohol-free mouthwash – one active mouthwash, which has an inhibitory effect against Neisseria gonorrhoeae in vitro, and one control mouthwash, which has no inhibitory effect against Neisseria gonorrhoeae, which aimed to examine whether daily use of active mouthwash for 3 months could prevent oropharyngeal gonorrhoea. It is anticipated that the results of the OMEGA trial will be available in 2019.
A potential public health campaign on mouthwash use to prevent and control gonorrhoea
If the results from the OMEGA trial show that daily use of the ‘active’ mouthwash can prevent oropharyngeal gonorrhoea in MSM, it would be reasonable to translate this finding into a public health campaign that recommends daily mouthwash use as a preventive strategy for oropharyngeal gonorrhoea. Increasing mouthwash use in the community could potentially reduce the overall incidence of gonorrhoea at a population level and thus reduce the potential for resistant strains to develop. Daily mouthwash use has also been reported to be an acceptable and easy intervention for gonorrhoea among the MSM population.19,20,42 A mathematical model has predicted that a 50% coverage of daily mouthwash use that had the effect of increasing the daily untreated clearance of gonorrhoea in the oropharynx would result in a seven-fold reduction in the prevalence of gonorrhoea at all sites in MSM.15 However, there are several research questions that need to be answered before recommending or implementing mouthwash for gonorrhoea prevention if it were shown to work in the OMEGA trial.13,3143
To date, the recommendations about mouthwash use have been for the prevention of oral diseases such as gingivitis and not gonorrhoea prevention. Based on the clinical trial conducted by Ross et al. (1993), the recommendation of mouthwash for preventing gingivitis is to use it twice daily as a mouthwash rinse for 30 s.44 There are also no recommendations about how to use a mouthwash to prevent gonorrhoea except from the OMEGA trial (i.e. rinse and gargle 20 mL of mouthwash for 60 s every day).31
It is likely that the use of mouthwash to prevent gonorrhoea will be different to gingivitis; for example, the site of the required action is likely to be different. Oropharyngeal Neisseria gonorrhoeae is primarily detected in both the tonsils and posterior oropharynx by nucleic acid amplification test (NAAT); however, a small proportion (6–10%) of infection is only detected at either the tonsils or the posterior oropharynx.45 If mouthwash is effective in preventing gonorrhoea, it is important to have specified guidelines for use that enable the mouthwash to reach both the tonsils and posterior oropharynx. How this is best achieved is not known.
Which mouthwash works?
Another important question is to identify the types of mouthwash that have inhibitory effects against Neisseria gonorrhoeae. To date, only one small clinical trial has demonstrated Listerine® Cool Mint (with 21.6% alcohol) could inhibit the growth of Neisseria gonorrhoeae,30 and the OMEGA trial used alcohol-free mouthwashes (product names withheld until publication).31 The alcohol-free mouthwash in the intervention arm of the OMEGA trial has been tested in vitro and has been found that it could inhibit the growth of Neisseria gonorrhoeae (D. A. Williamson, unpubl. data). This suggests that alcohol is not the key protective factor against Neisseria gonorrhoeae. Nevertheless, there are more than 100 different commercial mouthwash products in the market. Further laboratory testings will be required to identify which mouthwash has an inhibitory effect against Neisseria gonorrhoeae.
Mouthwash dosing
The OMEGA trial recommends using mouthwash at least once a day31 and it is currently the only clinical trial examining the effectiveness of mouthwash against oropharyngeal gonorrhoea. If mouthwashes can be used as prophylaxis for gonorrhoea, it is hypothesised mouthwash can also be taken in several other ways rather than daily and this concept is similar to PrEP.46,47 Further research and trials are required to explore the role and effectiveness if mouthwash is taken periodically or during episodic risk-taking (e.g. after sexual exposure, overseas travel).
How to use a mouthwash
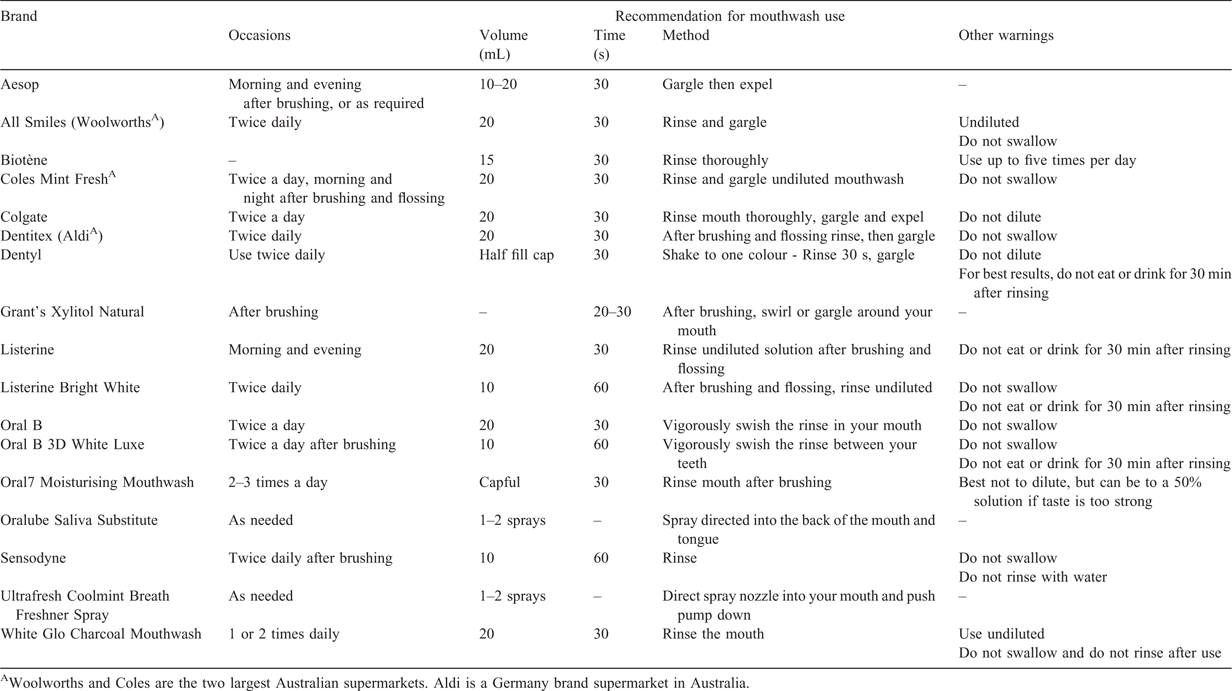
The way mouthwash is used may also influence its effect. If it is diluted, this may also reduce the antibacterial effect against Neisseria gonorrhoeae. Some commercial mouthwashes, particularly those alcohol-containing mouthwashes, cause a burning sensation, which creates discomfort. Although none of the manufacturers recommend their consumers dilute their mouthwash for best use (Table 1), it is estimated that a small proportion of individuals (7%) in the general population in the UK dilute their mouthwash for use.48 A small pilot study on 10 MSM showed no individual (0%) diluted their mouthwash.42
|
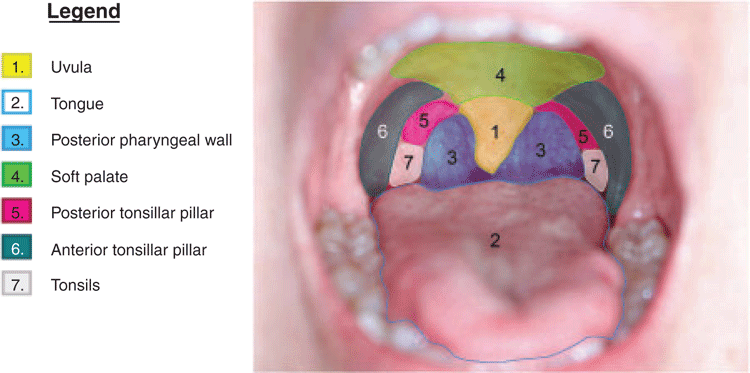
The way mouthwash is used will also influence what areas it reaches. Most of the manufacturers recommend the users rinse the mouthwash solution thoroughly so that the solution can cover the entire oral cavity and only a few manufacturers recommend the users both rinse and gargle the solution (Table 1). The site of oropharyngeal gonorrhoea infection is often at the posterior oropharynx;45 and therefore, it is reasonably hypothesised that the act of gargling would be important and required for the mouthwash to reach the back of the oropharynx to clear gonorrhoea infection. Lin and Raman (2012) conducted a pilot study on 10 individuals assessing the efficacy of three different methods (oral rinse 15 mL for 30 s, oral gargle 15 mL for 30 s and oral spray four squirts) to treat a sore throat.49 The authors examined the visualisation of food dye in seven areas of the oral cavity, this includes the uvula, tongue, posterior pharyngeal wall, soft palate, posterior tonsillar pillar, anterior tonsillar pillar and tonsils (Fig. 1). Results showed that both oral gargle and oral spray have similar efficacy in reaching the oropharynx and both were better than oral rinse. This highlights the importance of oral gargle or oral spray if mouthwash is used for preventing gonorrhoea. This is also consistent with a recent study on 20 individuals in Melbourne.50 Across all three different methods, most people prefer oral spray followed by oral rinse then oral gargle.50 Furthermore, other formulations of mouthwash such as tablets and strips should also be explored because some people may have difficulty in gargling a mouthwash, especially individuals with very sensitive gag reflexes. Gargling was reported as the least preferred method, described as ‘more difficult’ and ‘uncomfortable’ compared with rinse and spray that are ‘quick and easy’.50
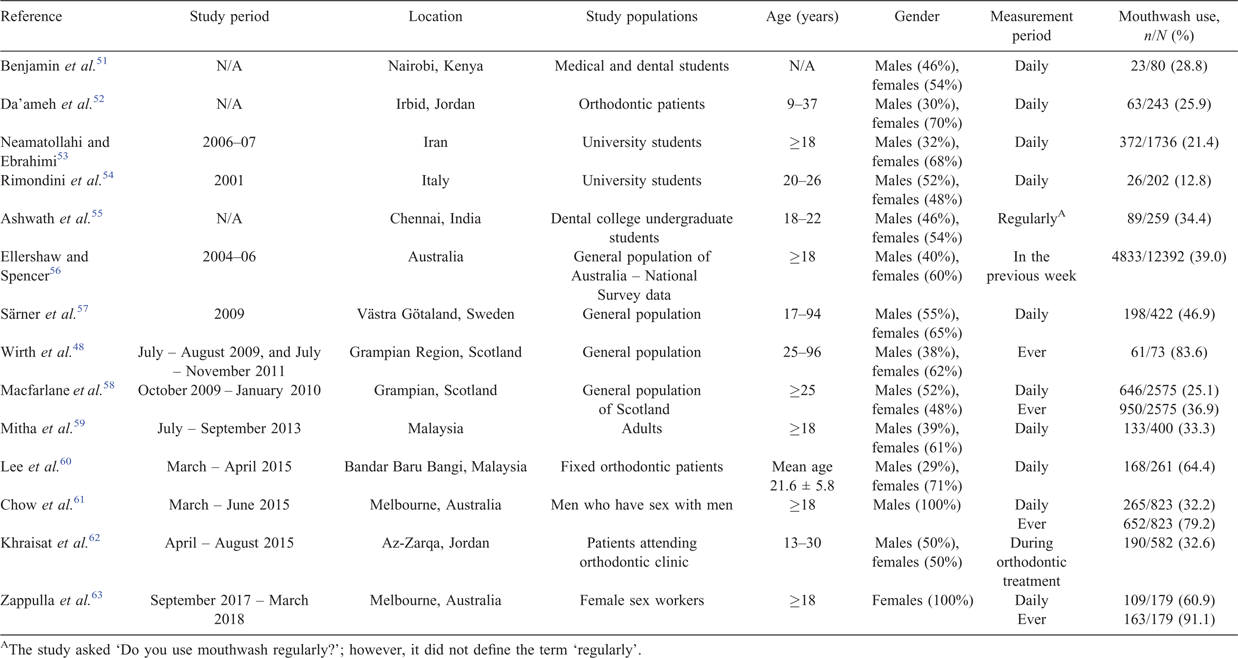
The optimal frequency, time and volume of mouthwash use are also not known. Most mouthwashes are recommended two times a day and do not specify the maximum number of times per day, except Biotène®, which recommends not using their product more than five times a day. Table 2 shows the proportion of daily mouthwash use varies in different settings and populations, ranging from 13% among university students in Italy54 to 64% among orthodontic patients in Malaysia.60 Most mouthwash users use mouthwash to prevent bad breath or avoid tooth decay.48,57 There are very few studies examining mouthwash use in specific populations that are stratified by sexual risk. One study in Melbourne reported 32% of MSM used mouthwash daily.61 Another study reported 61% of female sex workers (FSWs) in Melbourne used mouthwash daily.63
|
Mouthwash use also varies by different sociodemographic characteristics. Several studies have found that more females use mouthwash than males.55,57,58 Mouthwash use also varies by age, but findings from published studies are not consistent and the relationship is not fully understood; for example, Macfarlane et al. found that the proportion of mouthwash use decreases with increasing age in the general population in Scotland.58 Phillips et al., however, found that the proportion of mouthwash use increases with increasing age among MSM in Melbourne.64 Furthermore, Särner et al. did not find any significant association between age and mouthwash use in the Swedish population.57 Phillips et al. have further demonstrated that mouthwash use is not associated with an increasing number of kissing or oral sex partners among MSM.64 Further research is required to understand why mouthwash use is not common among young adults, especially young MSM, because they are at a higher risk of gonorrhoea.65
Optimal time of mouthwash use
The small RCT and the OMEGA trial recommended participants to use mouthwash for 60 s, although most commercial mouthwashes only recommend use for 30 s (Table 1). One study found that rinsing for 30 s is sufficient for the mouthwash to prevent plaque compared with 60 and 90 s rinsing periods.66 However, the optimal time of mouthwash use for gonorrhoea prevention is still unknown.
The optimal volume of mouthwash use is also important to determine whether it provides sufficient coverage to inhibit the growth of Neisseria gonorrhoeae in the oropharyngeal area. A small volume of mouthwash may be difficult to gargle,67 but a large volume of mouthwash may also be difficult to gargle due to the creation of bubbling from the mouthwash. The manufacturer’s recommendation of the volume of mouthwash for each use varies across different brands, ranging from 10 mL to 20 mL (Table 1). One survey conducted in Scotland estimated that about 89% of adults rinsed with one mouthful of mouthwash (i.e. a mouthwash was defined as 20 mL in the survey).48 Keukenmeester et al. compared the different volumes of mouthwash for rinsing and they concluded that 15 mL of mouthwash appears to be the most acceptable and comfortable volume for each use.68
Mouthwash use after sexual exposure
Harm-reduction programs recommend FSWs to use mouthwash after performing fellatio with their clients but not to brush their teeth.40 Currently, it is still unclear whether mouthwash should be used straight after sexual exposure to maximise its effectiveness. A small pilot study has found that most MSM use mouthwash as part of their daily routine dental care, but rarely before or after oral sex.42 This contrasts with FSWs where mouthwash is mainly used before and after each client.63 Further studies examining the changes in oral microbiome before and after mouthwash use will be required to inform the safety of mouthwash use in relation to sexual exposure.
Modes of delivery of public health campaign
The correct modes of delivery of the public health campaign need to be identified and explored to maximise the effectiveness of the campaign and also increase the coverage of the targeted population. Evidence has shown that sexual health messages are well delivered to the MSM population through social marketing approaches such as advertising material on the websites, in the media, at public events and via dating websites.69 Digital media interventions using social media platforms and smartphone dating applications have also been found to be effective in promoting sexual health messages, particularly to young adults.70,71 As with other public health interventions such as the human papillomavirus vaccine or HIV PrEP, the monitoring and roll out of mouthwash use intervention will require similar long-term surveillance and measurement of the intervention in the targeted population at a population level.
Other concerns
There have been concerns about whether alcohol-containing mouthwash would increase the risk of oropharyngeal cancer.72,73 To date, the concentration of alcohol in commercial mouthwashes varies, ranging from 5% to 26%.73 Gandini et al. (2012) conducted a meta-analysis and concluded that there was no association between oral cancer and frequent mouthwash users, including those who use mouthwash up to three times per day.74 Oral cancer is more likely to be associated with poor oral hygiene.75,76 Moreover, there has been no effect on the breath alcohol levels after using the alcohol-containing mouthwash.77
Conclusion
Gonorrhoea is on the rise in many Western countries and several health organisations have indicated that antimicrobial resistance to Neisseria gonorrhoeae is a major public health threat globally. Oropharyngeal gonorrhoea is common and may play an important role in gonorrhoea transmission in MSM. Mouthwash has been proposed as a novel preventive strategy for gonorrhoea in the OMEGA trial, but the results of the OMEGA trial will not be available until 2019. If these and other studies show it is effective, mouthwash use can be translated into a public health campaign for gonorrhoea prevention and control. However, several questions remain and need to be solved before rolling it out as a community intervention to maximise the effectiveness of the campaign.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
E. P. F. Chow was supported by the Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (number 1091226) at the time when this manuscript was written.
References
[1] Callander D, Guy R, Fairley CK, McManus H, Prestage G, Chow EPF, Chen M, O’Connor CC, Grulich AE, Bourne C, Hellard M, Stoove M, Donovan B. Gonorrhoea gone wild: rising incidence of gonorrhoea and associated risk factors among gay and bisexual men attending Australian sexual health clinics. Sex Health 2018;| Gonorrhoea gone wild: rising incidence of gonorrhoea and associated risk factors among gay and bisexual men attending Australian sexual health clinics.Crossref | GoogleScholarGoogle Scholar | 30409244PubMed |
[2] Mohammed H, Blomquist P, Ogaz D, Duffell S, Furegato M, Checchi M, Irvine N, Wallace LA, Thomas DR, Nardone A, Dunbar JK, Hughes G. 100 years of STIs in the UK: a review of national surveillance data. Sex Transm Infect 2018; 94 553–8.
| 100 years of STIs in the UK: a review of national surveillance data.Crossref | GoogleScholarGoogle Scholar | 29654061PubMed |
[3] Chow EPF, Grulich AE, Fairley CK. Epidemiology and prevention of sexually transmitted infections in men who have sex with men at risk of HIV. Lancet HIV 2019;
| Epidemiology and prevention of sexually transmitted infections in men who have sex with men at risk of HIV.Crossref | GoogleScholarGoogle Scholar | 31006612PubMed |
[4] Blank S, Daskalakis DC. Neisseria gonorrhoeae – rising infection rates, dwindling treatment options. N Engl J Med 2018; 379 1795–7.
| Neisseria gonorrhoeae – rising infection rates, dwindling treatment options.Crossref | GoogleScholarGoogle Scholar | 30403946PubMed |
[5] Day MJ, Spiteri G, Jacobsson S, Woodford N, Amato-Gauci AJ, Cole MJ, Unemo M, Balla E. Stably high azithromycin resistance and decreasing ceftriaxone susceptibility in Neisseria gonorrhoeae in 25 European countries, 2016. BMC Infect Dis 2018; 18 609
| Stably high azithromycin resistance and decreasing ceftriaxone susceptibility in Neisseria gonorrhoeae in 25 European countries, 2016.Crossref | GoogleScholarGoogle Scholar | 30509194PubMed |
[6] Whiley DM, Jennison A, Pearson J, Lahra MM. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect Dis 2018; 18 717–8.
| Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin.Crossref | GoogleScholarGoogle Scholar | 29976521PubMed |
[7] Public Health England. UK case of Neisseria gonorrhoeae with high-level resistance to azithromycin and resistance to ceftriaxone acquired abroad. Health Protection Report Advanced Access Report. 2018. Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/694655/hpr1118_MDRGC.pdf [verified 6 March 2019].
[8] Alirol E, Wi TE, Bala M, Bazzo ML, Chen XS, Deal C, Dillon JAR, Kularatne R, Heim J, Hooft van Huijsduijnen R, Hook EW, Lahra MM, Lewis DA, Ndowa F, Shafer WM, Tayler L, Workowski K, Unemo M, Balasegaram M. Multidrug-resistant gonorrhea: a research and development roadmap to discover new medicines. PLoS Med 2017; 14 e1002366
| Multidrug-resistant gonorrhea: a research and development roadmap to discover new medicines.Crossref | GoogleScholarGoogle Scholar | 28746372PubMed |
[9] Taylor SN, Marrazzo J, Batteiger BE, Hook EW, Sena AC, Long J, Wierzbicki MR, Kwak H, Johnson SM, Lawrence K, Mueller J. Single-dose Zoliflodacin (ETX0914) for treatment of urogenital gonorrhea. N Engl J Med 2018; 379 1835–45.
| Single-dose Zoliflodacin (ETX0914) for treatment of urogenital gonorrhea.Crossref | GoogleScholarGoogle Scholar | 30403954PubMed |
[10] Chow EP, Camilleri S, Ward C, Huffam S, Chen MY, Bradshaw CS, Fairley CK. Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: a systematic review. Sex Health 2016; 13 199–204.
| Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: a systematic review.Crossref | GoogleScholarGoogle Scholar | 26886136PubMed |
[11] Wallin J, Siegel MS. Pharyngeal Neisseria gonorrhoeae: coloniser or pathogen? BMJ 1979; 1 1462–3.
| Pharyngeal Neisseria gonorrhoeae: coloniser or pathogen?Crossref | GoogleScholarGoogle Scholar | 111754PubMed |
[12] Kinghorn G. Pharyngeal gonorrhoea: a silent cause for concern. Sex Transm Infect 2010; 86 413–4.
| Pharyngeal gonorrhoea: a silent cause for concern.Crossref | GoogleScholarGoogle Scholar | 20798397PubMed |
[13] Fairley CK, Zhang L, Chow EPF. New thinking on gonorrhoea control in MSM: are antiseptic mouthwashes the answer? Curr Opin Infect Dis 2018; 31 45–9.
| New thinking on gonorrhoea control in MSM: are antiseptic mouthwashes the answer?Crossref | GoogleScholarGoogle Scholar | 29176445PubMed |
[14] Fairley CK, Hocking JS, Zhang L, Chow EP. Frequent transmission of gonorrhea in men who have sex with men. Emerg Infect Dis 2017; 23 102–4.
| Frequent transmission of gonorrhea in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 27983487PubMed |
[15] Zhang L, Regan DG, Chow EPF, Gambhir M, Cornelisse V, Grulich A, Ong J, Lewis DA, Hocking J, Fairley CK. Neisseria gonorrhoeae transmission among men who have sex with men: an anatomical site-specific mathematical model evaluating the potential preventive impact of mouthwash. Sex Transm Dis 2017; 44 586–92.
| Neisseria gonorrhoeae transmission among men who have sex with men: an anatomical site-specific mathematical model evaluating the potential preventive impact of mouthwash.Crossref | GoogleScholarGoogle Scholar | 28876289PubMed |
[16] Morris SR, Klausner JD, Buchbinder SP, Wheeler SL, Koblin B, Coates T, Chesney M, Colfax GN. Prevalence and incidence of pharyngeal gonorrhea in a longitudinal sample of men who have sex with men: the EXPLORE study. Clin Infect Dis 2006; 43 1284–9.
| 17051493PubMed |
[17] Rosenberger JG, Reece M, Schick V, Herbenick D, Novak DS, Van Der Pol B, Fortenberry JD. Sexual behaviors and situational characteristics of most recent male-partnered sexual event among gay and bisexually identified men in the United States. J Sex Med 2011; 8 3040–50.
| Sexual behaviors and situational characteristics of most recent male-partnered sexual event among gay and bisexually identified men in the United States.Crossref | GoogleScholarGoogle Scholar | 21883941PubMed |
[18] Glynn TR, Operario D, Montgomery M, Almonte A, Chan PA. The duality of oral sex for men who have sex with men: an examination into the increase of sexually transmitted infections amid the age of HIV prevention. AIDS Patient Care STDS 2017; 31 261–7.
| The duality of oral sex for men who have sex with men: an examination into the increase of sexually transmitted infections amid the age of HIV prevention.Crossref | GoogleScholarGoogle Scholar | 28530499PubMed |
[19] Walker S, Bellhouse C, Fairley CK, Bilardi JE, Chow EP. Pharyngeal gonorrhoea: the willingness of Australian men who have sex with men to change current sexual practices to reduce their risk of transmission – a qualitative study. PLoS One 2016; 11 e0164033
| Pharyngeal gonorrhoea: the willingness of Australian men who have sex with men to change current sexual practices to reduce their risk of transmission – a qualitative study.Crossref | GoogleScholarGoogle Scholar | 27992427PubMed |
[20] Chow EP, Walker S, Phillips T, Fairley CK. Willingness to change behaviours to reduce the risk of pharyngeal gonorrhoea transmission and acquisition in men who have sex with men: a cross-sectional survey. Sex Transm Infect 2017; 93 499–502.
| Willingness to change behaviours to reduce the risk of pharyngeal gonorrhoea transmission and acquisition in men who have sex with men: a cross-sectional survey.Crossref | GoogleScholarGoogle Scholar | 28676558PubMed |
[21] Holt M, Lea T, Mao L, Kolstee J, Zablotska I, Duck T, Allan B, West M, Lee E, Hull P, Grulich A, De Wit J, Prestage G. Community-level changes in condom use and uptake of HIV pre-exposure prophylaxis by gay and bisexual men in Melbourne and Sydney, Australia: results of repeated behavioural surveillance in 2013–17. Lancet HIV 2018; 5 e448–56.
| Community-level changes in condom use and uptake of HIV pre-exposure prophylaxis by gay and bisexual men in Melbourne and Sydney, Australia: results of repeated behavioural surveillance in 2013–17.Crossref | GoogleScholarGoogle Scholar | 29885813PubMed |
[22] Hui B, Fairley CK, Chen M, Grulich A, Hocking J, Prestage G, Walker S, Law M, Regan D. Oral and anal sex are key to sustaining gonorrhoea at endemic levels in MSM populations: a mathematical model. Sex Transm Infect 2015; 91 365–9.
| Oral and anal sex are key to sustaining gonorrhoea at endemic levels in MSM populations: a mathematical model.Crossref | GoogleScholarGoogle Scholar | 25596192PubMed |
[23] Barbee LA, Khosropour CM, Dombrowski JC, Manhart LE, Golden MR. An estimate of the proportion of symptomatic gonococcal, chlamydial and non-gonococcal non-chlamydial urethritis attributable to oral sex among men who have sex with men: a case-control study. Sex Transm Infect 2016; 92 155–60.
| An estimate of the proportion of symptomatic gonococcal, chlamydial and non-gonococcal non-chlamydial urethritis attributable to oral sex among men who have sex with men: a case-control study.Crossref | GoogleScholarGoogle Scholar | 26297719PubMed |
[24] Cornelisse VJ, Walker S, Phillips T, Hocking JS, Bradshaw CS, Lewis DA, Prestage GP, Grulich AE, Fairley CK, Chow EPF. Risk factors for oropharyngeal gonorrhoea in men who have sex with men: an age-matched case-control study. Sex Transm Infect 2018; 94 359–64.
| Risk factors for oropharyngeal gonorrhoea in men who have sex with men: an age-matched case-control study.Crossref | GoogleScholarGoogle Scholar | 29358525PubMed |
[25] Templeton DJ, Jin F, McNally LP, Imrie JC, Prestage GP, Donovan B, Cunningham PH, Kaldor JM, Kippax S, Grulich AE. Prevalence, incidence and risk factors for pharyngeal gonorrhoea in a community-based HIV-negative cohort of homosexual men in Sydney, Australia. Sex Transm Infect 2010; 86 90–6.
| Prevalence, incidence and risk factors for pharyngeal gonorrhoea in a community-based HIV-negative cohort of homosexual men in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 19841003PubMed |
[26] Cornelisse VJ, Zhang L, Law M, Chen MY, Bradshaw CS, Bellhouse C, Fairley CK, Chow EPF. Concordance of gonorrhoea of the rectum, pharynx and urethra in same-sex male partnerships attending a sexual health service in Melbourne, Australia. BMC Infect Dis 2018; 18 95
| Concordance of gonorrhoea of the rectum, pharynx and urethra in same-sex male partnerships attending a sexual health service in Melbourne, Australia.Crossref | GoogleScholarGoogle Scholar | 29486706PubMed |
[27] Chow EP, Tabrizi SN, Phillips S, Lee D, Bradshaw CS, Chen MY, Fairley CK. Neisseria gonorrhoeae bacterial DNA load in the pharynges and saliva of men who have sex with men. J Clin Microbiol 2016; 54 2485–90.
| Neisseria gonorrhoeae bacterial DNA load in the pharynges and saliva of men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 27413195PubMed |
[28] Chow EP, Lee D, Tabrizi SN, Phillips S, Snow A, Cook S, Howden BP, Petalotis I, Bradshaw CS, Chen MY, Fairley CK. Detection of Neisseria gonorrhoeae in the pharynx and saliva: implications for gonorrhoea transmission. Sex Transm Infect 2016; 92 347–9.
| Detection of Neisseria gonorrhoeae in the pharynx and saliva: implications for gonorrhoea transmission.Crossref | GoogleScholarGoogle Scholar | 26622046PubMed |
[29] Hutt DM, Judson FN. Epidemiology and treatment of oropharyngeal gonorrhea. Ann Intern Med 1986; 104 655–8.
| Epidemiology and treatment of oropharyngeal gonorrhea.Crossref | GoogleScholarGoogle Scholar | 2938529PubMed |
[30] Chow EP, Howden BP, Walker S, Lee D, Bradshaw CS, Chen MY, Snow A, Cook S, Fehler G, Fairley CK. Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study. Sex Transm Infect 2017; 93 88–93.
| Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study.Crossref | GoogleScholarGoogle Scholar | 27998950PubMed |
[31] Chow EPF, Walker S, Hocking JS, Bradshaw CS, Chen MY, Tabrizi SN, Howden BP, Law MG, Maddaford K, Read TRH, Lewis DA, Whiley DM, Zhang L, Grulich AE, Kaldor JM, Cornelisse VJ, Phillips S, Donovan B, McNulty AM, Templeton DJ, et al A multicentre double-blind randomised controlled trial evaluating the efficacy of daily use of antibacterial mouthwash against oropharyngeal gonorrhoea among men who have sex with men: the OMEGA (Oral Mouthwash use to Eradicate GonorrhoeA) study protocol. BMC Infect Dis 2017; 17 456
| A multicentre double-blind randomised controlled trial evaluating the efficacy of daily use of antibacterial mouthwash against oropharyngeal gonorrhoea among men who have sex with men: the OMEGA (Oral Mouthwash use to Eradicate GonorrhoeA) study protocol.Crossref | GoogleScholarGoogle Scholar | 28659133PubMed |
[32] Van der Weijden FA, Van der Sluijs E, Ciancio SG, Slot DE. Can chemical mouthwash agents achieve plaque/gingivitis control? Dent Clin North Am 2015; 59 799–829.
| Can chemical mouthwash agents achieve plaque/gingivitis control?Crossref | GoogleScholarGoogle Scholar | 26427569PubMed |
[33] Supranoto SC, Slot DE, Addy M, Van der Weijden GA. The effect of chlorhexidine dentifrice or gel versus chlorhexidine mouthwash on plaque, gingivitis, bleeding and tooth discoloration: a systematic review. Int J Dent Hyg 2015; 13 83–92.
| The effect of chlorhexidine dentifrice or gel versus chlorhexidine mouthwash on plaque, gingivitis, bleeding and tooth discoloration: a systematic review.Crossref | GoogleScholarGoogle Scholar | 25059640PubMed |
[34] Alshehri FA. The use of mouthwash containing essential oils (LISTERINE®) to improve oral health: a systematic review. Saudi Dent J 2018; 30 2–6.
| The use of mouthwash containing essential oils (LISTERINE®) to improve oral health: a systematic review.Crossref | GoogleScholarGoogle Scholar | 30166864PubMed |
[35] Addy M, Jenkins S, Newcombe R. The effect of some chlorhexidine-containing mouthrinses on salivary bacterial counts. J Clin Periodontol 1991; 18 90–3.
| The effect of some chlorhexidine-containing mouthrinses on salivary bacterial counts.Crossref | GoogleScholarGoogle Scholar | 2005231PubMed |
[36] DePaola LG, Minah GE, Overholser CD, Meiller TF, Charles CH, Harper DS, McAlary M. Effect of an antiseptic mouthrinse on salivary microbiota. Am J Dent 1996; 9 93–5.
| 9002796PubMed |
[37] Fine DH, Furgang D, Sinatra K, Charles C, McGuire A, Kumar LD. In vivo antimicrobial effectiveness of an essential oil-containing mouth rinse 12 h after a single use and 14 days’ use. J Clin Periodontol 2005; 32 335–40.
| In vivo antimicrobial effectiveness of an essential oil-containing mouth rinse 12 h after a single use and 14 days’ use.Crossref | GoogleScholarGoogle Scholar | 15811048PubMed |
[38] Donovan B. Medico-social aspects of a house of prostitution. Med J Aust 1984; 140 272–5.
| 6546599PubMed |
[39] Donovan B. The repertoire of human efforts to avoid sexually transmissible diseases: past and present. Part 2: strategies used during or after sex. Sex Transm Infect 2000; 76 88–93.
| The repertoire of human efforts to avoid sexually transmissible diseases: past and present. Part 2: strategies used during or after sex.Crossref | GoogleScholarGoogle Scholar | 10858708PubMed |
[40] Rekart ML. Sex-work harm reduction. Lancet 2005; 366 2123–34.
| Sex-work harm reduction.Crossref | GoogleScholarGoogle Scholar | 16360791PubMed |
[41] Kenyon C, Cuylaerts V, Crucitti T. P5.10 Inhibitory effect of chlorhexidine antiseptic mouthwash against Neisseria gonorrhoeae? An in-vitro study. Sex Transm Infect 2017; 93 (Suppl 2) A239
[42] Cornelisse VJ, Fairley CK, Walker S, Young T, Lee D, Chen MY, Bradshaw CS, Chow EPF. Adherence to, and acceptability of, Listerine® mouthwash as a potential preventive intervention for pharyngeal gonorrhoea among men who have sex with men in Australia: a longitudinal study. Sex Health 2016; 13 494–6.
| Adherence to, and acceptability of, Listerine® mouthwash as a potential preventive intervention for pharyngeal gonorrhoea among men who have sex with men in Australia: a longitudinal study.Crossref | GoogleScholarGoogle Scholar | 28636870PubMed |
[43] Chow EPF, Fairley CK. Could antiseptic mouthwash inhibit pharyngeal Neisseria gonorrhoeae? Further research is required. Sex Transm Infect 2017; 93 403
| Could antiseptic mouthwash inhibit pharyngeal Neisseria gonorrhoeae? Further research is required.Crossref | GoogleScholarGoogle Scholar | 28827305PubMed |
[44] Ross NM, Mankodi SM, Mostler KL, Charles CH, Bartels LL. Effect of rinsing time on antiplaque-antigingivitis efficacy of listerine. J Clin Periodontol 1993; 20 279–81.
| Effect of rinsing time on antiplaque-antigingivitis efficacy of listerine.Crossref | GoogleScholarGoogle Scholar | 8473539PubMed |
[45] Bissessor M, Whiley DM, Lee DM, Snow AF, Fairley CK, Peel J, Bradshaw CS, Hocking JS, Lahra MM, Chen MY. Detection of Neisseria gonorrhoeae isolates from tonsils and posterior oropharynx. J Clin Microbiol 2015; 53 3624–6.
| Detection of Neisseria gonorrhoeae isolates from tonsils and posterior oropharynx.Crossref | GoogleScholarGoogle Scholar | 26292303PubMed |
[46] Hampel B, Reinacher M, Fehr JS. HIV pre-exposure prophylaxis (PrEP): is it time to rethink HIV prevention in travelers? Travel Med Infect Dis 2018; 25 6–7.
| HIV pre-exposure prophylaxis (PrEP): is it time to rethink HIV prevention in travelers?Crossref | GoogleScholarGoogle Scholar | 29929002PubMed |
[47] Namey E, Agot K, Ahmed K, Odhiambo J, Skhosana J, Guest G, Corneli A. When and why women might suspend PrEP use according to perceived seasons of risk: implications for PrEP-specific risk-reduction counselling. Cult Health Sex 2016; 18 1081–91.
| When and why women might suspend PrEP use according to perceived seasons of risk: implications for PrEP-specific risk-reduction counselling.Crossref | GoogleScholarGoogle Scholar | 27093238PubMed |
[48] Wirth T, Kawecki MM, Reeve J, Cunningham C, Bovaird I, Macfarlane TV. Can alcohol intake from mouthwash be measured in epidemiological studies? Development and validation of mouthwash use questionnaire with particular attention to measuring alcohol intake from mouthwash. J Oral Maxillofac Res 2012; 3 e1
| Can alcohol intake from mouthwash be measured in epidemiological studies? Development and validation of mouthwash use questionnaire with particular attention to measuring alcohol intake from mouthwash.Crossref | GoogleScholarGoogle Scholar | 24422013PubMed |
[49] Lin CT, Raman R. Comparison of the efficacy between oral rinse, oral gargle, and oral spray. J Prim Care Community Health 2012; 3 80–2.
| Comparison of the efficacy between oral rinse, oral gargle, and oral spray.Crossref | GoogleScholarGoogle Scholar | 23803449PubMed |
[50] Maddaford K, Fairley CK, Trumpour S, Chung M, Chow EPF. The sites in the oropharynx reached by mouthwash using oral rinse, oral gargle and oral spray: clinical implication for oropharyngeal gonorrhoea prevention. Presented at the IUSTI Asia Pacific Sexual Health Congress; 1–3 November 2018; Auckland, New Zealand.
[51] Benjamin SN, Gathece LW, Wagaiyu EG. Knowledge, attitude and use of mouthwash among dental and medical students of the University of Nairobi. Int J Dent Oral Health 2016; 2 1–6.
| Knowledge, attitude and use of mouthwash among dental and medical students of the University of Nairobi.Crossref | GoogleScholarGoogle Scholar |
[52] Da’ameh MD, Al-Shorman I, Al-Shdeifat N, Fnaish NM. Oral hygiene measures in orthodontic treatment in Northern Jordan. Pak Oral Dent J 2011; 31 336–9.
[53] Neamatollahi H, Ebrahimi M. Oral health behavior and its determinants in a group of Iranian students. Indian J Dent Res 2010; 21 84–8.
| Oral health behavior and its determinants in a group of Iranian students.Crossref | GoogleScholarGoogle Scholar | 20427913PubMed |
[54] Rimondini L, Zolfanelli B, Bernardi F, Bez C. Self-preventive oral behavior in an Italian university student population. J Clin Periodontol 2001; 28 207–11.
| Self-preventive oral behavior in an Italian university student population.Crossref | GoogleScholarGoogle Scholar | 11284532PubMed |
[55] Ashwath B, Vijayalakshmi R, Malini S. Self-perceived halitosis and oral hygiene habits among undergraduate dental students. J Indian Soc Periodontol 2014; 18 357–60.
| Self-perceived halitosis and oral hygiene habits among undergraduate dental students.Crossref | GoogleScholarGoogle Scholar | 25024551PubMed |
[56] Ellershaw AC, Spencer AJ. Dental attendance patterns and oral health status. Dental statistics and research series no. 57. Cat. No. DEN 208. Canberra: Australian Institute of Health and Welfare; 2011.
[57] Särner B, Sundin E, Abdulrahman S, Birkhed D, Lingstrom P. Use of different mouthrinses in an adult Swedish population. Swed Dent J 2012; 36 53–60.
| 22611905PubMed |
[58] Macfarlane TV, Kawecki MM, Cunningham C, Bovaird I, Morgan R, Rhodes K, Watkins R. Mouthwash use in general population: results from adult dental health survey in Grampian, Scotland. J Oral Maxillofac Res 2011; 1 e2
| 24421979PubMed |
[59] Mitha S, Elnaem MH, Koh M, En C, Babar MG, Siddiqui J, Jamshed S. Use and perceived benefits of mouthwash among Malaysian adults: an exploratory insight. J Adv Oral Res 2016; 7 7–14.
| Use and perceived benefits of mouthwash among Malaysian adults: an exploratory insight.Crossref | GoogleScholarGoogle Scholar |
[60] Lee JH, Abdullah AAA, Yahya NA. Oral hygiene practices among fixed orthodontic patients in a university dental setting. Int J Oral Dent Health 2016; 2 027
| Oral hygiene practices among fixed orthodontic patients in a university dental setting.Crossref | GoogleScholarGoogle Scholar |
[61] Chow EPF, Walker S, Read TRH, Chen MY, Bradshaw CS, Fairley CK. Self-reported use of mouthwash and pharyngeal gonorrhoea detection by nucleic acid amplification test. Sex Transm Dis 2017; 44 593–5.
| Self-reported use of mouthwash and pharyngeal gonorrhoea detection by nucleic acid amplification test.Crossref | GoogleScholarGoogle Scholar | 28876323PubMed |
[62] Khraisat HM, Al-Shdeifat NA, Al-Alawneh AM, Al-Zyood AI, Al-Maani MO. Oral hygiene practices among fixed orthodontic patients in Az-Zarqa Pak Oral Dental J 2016; 36 404–7.
[63] Zappulla A, Fairley CK, Donovan B, Guy R, Bradshaw CS, Chen MY, Phillips T, Maddaford K, Chow EPF. Frequency of tongue kissing, oral, vaginal and anal sex among female sex workers with male clients in Melbourne Australia. Presented at the IUSTI Asia Pacific Sexual Health Congress; 1–3 November 2018; Auckland, New Zealand.
[64] Phillips T, Fairley CK, Walker S, Chow EPF. Associations between oral sex practices and frequent mouthwash use in men who have sex with men: implications for gonorrhoea prevention. Sex Health 2018;
| Associations between oral sex practices and frequent mouthwash use in men who have sex with men: implications for gonorrhoea prevention.Crossref | GoogleScholarGoogle Scholar | 30558710PubMed |
[65] Chow EP, Tomnay J, Fehler G, Whiley D, Read TR, Denham I, Bradshaw CS, Chen MY, Fairley CK. Substantial increases in chlamydia and gonorrhea positivity unexplained by changes in individual-level sexual behaviors among men who have sex with men in an Australian sexual health service from 2007 to 2013. Sex Transm Dis 2015; 42 81–7.
| Substantial increases in chlamydia and gonorrhea positivity unexplained by changes in individual-level sexual behaviors among men who have sex with men in an Australian sexual health service from 2007 to 2013.Crossref | GoogleScholarGoogle Scholar | 25585066PubMed |
[66] Paraskevas S, Danser MM, Timmerman MF, Van der Velden U, van der Weijden GA. Optimal rinsing time for intra-oral distribution (spread) of mouthwashes. J Clin Periodontol 2005; 32 665–9.
| Optimal rinsing time for intra-oral distribution (spread) of mouthwashes.Crossref | GoogleScholarGoogle Scholar | 15882228PubMed |
[67] Amornyotin S, Srikureja W, Chalayonnavin W, Kongphlay S, Chatchawankitkul S. Topical viscous lidocaine solution versus lidocaine spray for pharyngeal anesthesia in unsedated esophagogastroduodenoscopy. Endoscopy 2009; 41 581–6.
| Topical viscous lidocaine solution versus lidocaine spray for pharyngeal anesthesia in unsedated esophagogastroduodenoscopy.Crossref | GoogleScholarGoogle Scholar | 19588284PubMed |
[68] Keukenmeester RS, Slot DE, Rosema NA, Van der Weijden GA. Determination of a comfortable volume of mouthwash for rinsing. Int J Dent Hyg 2012; 10 169–74.
| Determination of a comfortable volume of mouthwash for rinsing.Crossref | GoogleScholarGoogle Scholar | 23046005PubMed |
[69] Pedrana A, Hellard M, Guy R, El-Hayek C, Gouillou M, Asselin J, Batrouney C, Nguyen P, Stoovè M. Stop the drama Downunder: a social marketing campaign increases HIV/sexually transmitted infection knowledge and testing in Australian gay men. Sex Transm Dis 2012; 39 651–8.
| Stop the drama Downunder: a social marketing campaign increases HIV/sexually transmitted infection knowledge and testing in Australian gay men.Crossref | GoogleScholarGoogle Scholar | 22801349PubMed |
[70] Wadham E, Green C, Debattista J, Somerset S, Sav A. New digital media interventions for sexual health promotion among young people: a systematic review. Sex Health 2019; 16 101–123.
| New digital media interventions for sexual health promotion among young people: a systematic review.Crossref | GoogleScholarGoogle Scholar | 30819326PubMed |
[71] Gabarron E, Luque LF, Schopf TR, Lau AYS, Armayones M, Wynn R, Serrano JA. Impact of Facebook ads for sexual health promotion via an educational web app: a case study. Int J E-Health Med Commun 2017; 8 18–32.
| Impact of Facebook ads for sexual health promotion via an educational web app: a case study.Crossref | GoogleScholarGoogle Scholar |
[72] Winn DM, Blot WJ, McLaughlin JK, Austin DF, Greenberg RS, Preston-Martin S, Schoenberg JB, Fraumeni JF. Mouthwash use and oral conditions in the risk of oral and pharyngeal cancer. Cancer Res 1991; 51 3044–7.
| 2032242PubMed |
[73] McCullough MJ, Farah CS. The role of alcohol in oral carcinogenesis with particular reference to alcohol-containing mouthwashes. Aust Dent J 2008; 53 302–5.
| The role of alcohol in oral carcinogenesis with particular reference to alcohol-containing mouthwashes.Crossref | GoogleScholarGoogle Scholar | 19133944PubMed |
[74] Gandini S, Negri E, Boffetta P, La Vecchia C, Boyle P. Mouthwash and oral cancer risk quantitative meta-analysis of epidemiologic studies. Ann Agric Environ Med 2012; 19 173–80.
| 22742785PubMed |
[75] Orbak R, Bayraktar C, Kavrut F, Gündogdu C. Poor oral hygiene and dental trauma as the precipitating factors of squamous cell carcinoma. Oral Oncology Extra 2005; 41 109–13.
| Poor oral hygiene and dental trauma as the precipitating factors of squamous cell carcinoma.Crossref | GoogleScholarGoogle Scholar |
[76] Oji C, Chukwuneke F. Poor oral hygiene may be the sole cause of oral cancer. J Maxillofac Oral Surg 2012; 11 379–83.
| Poor oral hygiene may be the sole cause of oral cancer.Crossref | GoogleScholarGoogle Scholar | 24293926PubMed |
[77] Modell JG, Taylor JP, Lee JY. Breath alcohol values following mouthwash use. JAMA 1993; 270 2955–6.
| Breath alcohol values following mouthwash use.Crossref | GoogleScholarGoogle Scholar | 8254857PubMed |