Comparing the effectiveness of secondary distribution of HIV/syphilis dual self-testing to testing card referral in promoting HIV testing among gay, bisexual, and other men who have sex with men in Guangzhou, China: a quasi-experimental study
Yongjie Sha A , Yuan Xiong A , Jason J. Ong
A , Yuan Xiong A , Jason J. Ong  B C , Yehua Wang A , Mengyuan Cheng A , Yuxin Ni A , Ying Lu A , Joseph D. Tucker A B D and Weiming Tang A D *
B C , Yehua Wang A , Mengyuan Cheng A , Yuxin Ni A , Ying Lu A , Joseph D. Tucker A B D and Weiming Tang A D *
A University of North Carolina Project – China, 7 Lujing Road, Guangzhou 510095, China.
B Faculty of Infectious and Tropical Diseases, London School of Hygiene & Tropical Medicine, London, UK.
C Central Clinical School, Monash University, Melbourne, Vic., Australia.
D Institute for Global Health and Infectious Diseases, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Sexual Health - https://doi.org/10.1071/SH21176
Submitted: 13 September 2021 Accepted: 3 November 2021 Published online: 26 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing
Abstract
Background: Social network approaches to testing allow individuals (indexes) to distribute tests to social networks (alters). This quasi-experimental study compared two social network-based testing strategies in promoting human immunodeficiency virus (HIV) testing among Chinese gay, bisexual, and other men who have sex with men (GBMSM).
Methods: GBMSM aged ≥18 years were recruited from Guangzhou, China. From May to September 2019, indexes could distribute blood-based HIV/syphilis dual self-testing kits to people within their social network. Indexes recruited from October 2019 to January 2020 could send HIV testing cards to their social networks for free facility-based tests. Alters were encouraged to upload a photo verification of test results. Indexes and alters received incentives during both periods.
Results: There were 245 participants who were assessed for eligibility and 208/245 (84.9%) were eligible. 106 and 102 indexes were recruited in the secondary distribution and testing card arms respectively. 154/208 (74.0%) completed follow up at 1 month. 92 indexes in the secondary distribution arm self-reported distributions to 179 unique alters, and 62 in the testing card arm to 26 unique alters. An average of 1.95 (standard deviation [s.d.] = 1.90) HIV/syphilis dual self-tests and 0.42 (s.d. = 0.78) HIV testing cards were distributed, generating a risk difference of 1.53 (95% confidence interval [CI] 1.09, 1.96). Indexes self-identifying as gay (P = 0.007) or having previously tested (P = 0.02) distributed more tests. Secondary distribution cost less per alter tested (USD120 vs USD9408).
Conclusions: Secondary distribution engaged more GBMSM to distribute tests and reached more GBMSM to test compared to referral cards, suggesting advantage in facilitating testing uptake among Chinese GBMSM.
Keywords: China, HIV, men who have sex with men, secondary distribution, self-testing, social network, syphilis, testing uptake.
Introduction
Gay, bisexual and other men who have sex with men (GBMSM) account for approximately one-quarter of new HIV infections in China (as of 2018).1 The national prevalence of HIV from 2001 to 2018 among Chinese MSM was estimated to be 5.7% (95% CI: 5.4–6.1%).2 HIV testing presents a crucial entry point into the HIV care continuum. However, approximately 30% of people living with HIV in China are unaware of their serostatus and 33.2% of MSM reported no HIV test in their lifetime.3,4 Using data from the national HIV surveillance system, a study suggested that HIV testing rate (tested in the last year) among Chinese MSM ranged between 43.2 and 49.0%.5 Common barriers to HIV testing among MSM in China include stigma concerning homosexuality and HIV, uncertainty of testing locations, and fear of privacy violation during testing.6
HIV self-testing (HIVST), recommended by the World Health Organization, may complement facility-based HIV testing with potentials to increase HIV testing coverage.7 Self-testing involves individuals collecting their own specimen (oral fluid or blood), performing a test and interpreting the results themselves, permitting privacy, convenience, and confidentiality.7 HIVST is acceptable among many key populations.8 Global studies conducted among MSM and ciswomen found that oral-fluid-based HIVST increased testing coverage among these populations and their sexual partners.9–11 However, most experiments used oral-fluid-based self-tests. More evidence is needed on the feasibility of the blood-based test in order to offer choice in type of test kits to reach more people.
Blood-based HIVST creates opportunities to combine STI testing in HIV self-testing. Compared to oral-fluid-based HIVST, blood-based HIVST has higher accuracy but is less easy to use.12 Although previous studies indicated preference for an oral-fluid-based test among HIVST users, preference for a blood-based test increased if the test also offered STI testing.13 Studies in China found that blood-based HIV/syphilis dual self-testing expanded HIV/syphilis testing among MSM and their sexual partners;14 however, although both oral-fluid-based and blood-based self-tests have shown acceptability among key populations, HIVST uptake remains low in many countries.15,16 Optimising the delivery of a HIVST service would be crucial to increase testing uptake.
Social network-based strategies leverage large social networks to promote HIV care uptake.17 Social network-based strategies can improve HIV testing, referral, adherence, and retention among MSM.18,19 Secondary distribution is a social network-based strategy where multiple HIV testing cards or HIVST kits are provided to individuals (referred to as ‘indexes’) for distributions to their sexual and social contacts (referred to as ‘alters’).20 Previous studies found that peer referral of HIV testing increased test uptake among high-risk individuals and prevents HIV transmission.21–24 Although many studies have focused on a social network-based method, limited data exist to evaluate the effectiveness of different strategies among key populations, which could be helpful to inform the scale-up of HIVST. This quasi-experimental study evaluated two social network-based approaches (secondary distribution of HV/syphilis dual self-testing kits vs HIV testing card referral) in promoting HIV testing among Chinese GBMSM.
Methods
Study design and setting
This quasi-experimental study was conducted in Guangzhou, China, from May 2019 to January 2020 to compare the effectiveness of secondary distribution (intervention) with testing card referral (control) in promoting HIV testing. The secondary distribution program was implemented between 1 May and 7 October 2019, at the Dermatology Hospital of Southern Medical University (DH-SMU). Study staff at the site handled the study procedures, including testing, introducing the program, and offering HIV/syphilis dual self-testing kits to participants. The self-test used in this study is the SD Bioline HIV/syphilis Duo rapid test kit (Standard Diagnostics, Inc., Gyeonggi-do, South Korea), which is a blood-based finger-prick-based HIV self-test that allows concurrent testing for syphilis using a single sample. The sensitivity and specificity are 91.7% and 99.5%, respectively, when compared to health clinic results.25 The testing card referral was implemented between 14 October 2019 and 17 January 2020 at three sites – a GBMSM community-based HIV/STI testing clinic, a GBMSM community-based HIV/STI testing weekend clinic at DH-SMU, and a municipal-level Centers for Disease Control and Prevention (CDC) HIV testing clinic. Testing card referral was embedded in their routine testing services. GBMSM volunteers or public health staff handled the same study procedures as that the intervention arm. Both self-testing and the key population-led HIV testing delivery model are major strategies in China to promote HIV testing in addition to traditional medical facility-based testing.26 These facilities in our study were selected to reflect the preferences of the GBMSM community for HIV testing. The two arms were implemented one at a time to compare the intended outcomes in similar catchment areas without having people choose between the two. No significant temporal fluctuations were identified in HIV testing uptake by month. Eligibility was defined as age ≥18 years, male sex assigned at birth, ever had sex with men, were willing to participate in a follow-up interview at 1 month, and willing to provide a phone number.
HIVST secondary distribution program
We posted banner ads on WeChat and Blued, the most used social media applications among the Chinese and Chinese GBMSM, respectively. Interested men scheduled an appointment with study staff located at the DH-SMU for free HIV/syphilis testing and an opportunity to apply for free HIV/syphilis dual self-testing kits for distribution to people in their social network. At the site, indexes were given a dual HIV/syphilis test by the study staff using the same test in the self-testing kit. After indexes were introduced to the study and how to use the self-test in person, those who agreed to distribute could sign up for up to five kits. Each kit additionally included instructions for use, a QR code for test result upload, and a unique ID number to link indexes with their alters. Indexes were requested to distribute the kits in a month and were allowed to access kits multiple times (Fig. 1).
|
|
After using the self-testing kit, alters were encouraged to upload their test result with the kit’s numeric ID to a database located at Sojump by scanning the QR code. Alters who uploaded a reactive result were contacted to recommend confirmatory testing at a district-level CDC or local hospital. All uploaded results were verified by study staff. Indexes received an incentive of USD3 after completing the baseline survey and USD5 for a follow-up survey. Alters and corresponding indexes both received an additional USD3 when alters uploaded their test results.
HIV testing card
Men visiting the three clinics for HIV testing were invited to participate after community-based volunteers or public health staff introduced the project in person at the site. Eligible men could sign up for up to five testing referral cards. Each testing card contained a QR code to upload test results when the alter completed an HIV test either in a testing facility or using a self-purchased self-test, and a unique numeric ID linking alters with indexes. Indexes were encouraged to distribute the testing cards in a month.
Alters who received testing cards were encouraged to take an HIV test however they preferred. If they took a facility-based test, they were encouraged to upload a picture of their test result report to the study database. If they self-tested, they were encouraged to upload a picture of their self-test result. All uploads were verified. The incentive structure was the same as in the secondary distribution arm.
Data collection
We collected data from all indexes who agreed to distribute and all alters who uploaded their test results. Indexes were requested to complete an online baseline survey at the site and an online follow-up survey at 1 month. Baseline survey items included sociodemographic information, sexual behaviors in the past month, testing experience, social network data and respondents’ phone number to identify duplicates. Sociodemographic information included age, education, marital status, income, sexual orientation and gender identity, and disclosure (defined as disclosing gay/bisexual identity or having sexual activities with another male to/with anyone). Data on sexual behaviors included previous sex with men, the number of sex partners and condomless anal sex in the past month. Data on HIV testing behaviors included previous HIV tests, previous self-tests, testing locations, and comparison between self-testing and clinic-based testing. Social network data were characterised by requesting indexes to list their social contacts, describe their relationships and answer whether they would distribute to each specific contact. Responders may not skip or refuse to answer survey questions.
A follow-up survey asked about indexes’ relationships to the recipients, the number of self-testing kits/testing cards distributed, sexual experiences in the past month, and distributing experiences including point of sex.
Alters were also requested to complete an online survey when uploading test results. The survey instrument collected the same data as that for the indexes. Additional data on alters’ experience receiving and using self-tests were gathered.
Economic cost data were collected for all expenditures from organisations in the two arms using a health provider perspective. Fixed costs consist of building rent, office equipment, and personnel. Variable costs include consumables, telephone bills, and transport (Supplementary File S1). The cost for the HIVST arm was collected from 1 May to 30 September 2019, over which this arm was implemented. The cost for the referral card arm was collected from 14 October 2019 to 14 January 2020. The costs are reported in US dollars (2020). We report the total economic cost of the two arms, the cost per alter tested, the cost per alter diagnosed with HIV, and their corresponding incremental cost-effectiveness ratios.
Outcomes
The primary outcomes of this study were test uptake measured by: (1) the proportion of indexes self-reporting distributions to social networks; and (2) the mean number of distributions self-reported. Secondary outcomes were subgroup analyses based on age, sexual orientation, disclosure of sexual orientation or same-sex sexual behavior, sex in the past month, and prior HIV testing. We further compared the cost-effectiveness of secondary distribution to the testing card referral reporting incremental cost-effectiveness ratio (ICER).
Data analysis
We examined the hypothesis that the secondary distribution would increase HIV test uptake compared to the distribution of the testing card. We reported risk ratios of participants in each group who reported distributions, risk differences of the numbers of distributions participants reported, with estimated 95% confidence interval (CI). Risk ratios were calculated by unconditional maximum likelihood estimation (Wald) and were adjusted for a small sample. CIs were calculated using normal approximation (Wald). We assessed effect modification using a linear probability model based on five subgroups: age (no older than age 30 years and >30 years), sexual orientation (self-identified as gay and self-identified as other than gay), disclosure of sexual orientation or same-sex sexual behavior to anyone (yes or no), recent sex in the past month (yes or no), and prior experience of HIV testing (ever tested for HIV or never tested for HIV). We used descriptive statistics to examine sociodemographic and behavioral characteristics of indexes. We used self-reported distributions at follow up for analysis. Missing data of distributions for not distributing to any type of recipients were treated as the event not detected and counted as 0. All analyses were performed in R 3.6.3 (R Core Team, USA).
Ethical approval and informed consent
The study protocol was approved by the ethics review committees at the University of North Carolina at Chapel Hill. Verbal informed consent was obtained from each index participant. Online informed consent was accessed from all participants by them checking a box on a self-administered online consent form indicating their agreement to participate in the study.
Results
From May 2019 to January 2020, 106 index participants in the secondary distribution group and 102 in the testing card referral group were recruited (Fig. 2). The intervention group’s participation rate/response rate was 91.4% (106/116) and 79.1% (102/129) for the control group. No duplicated participant was identified. At 1 month, 92 (86.8%) participants who accessed HIV/syphilis self-testing kits and 62 (60.8%) who accessed testing cards completed a follow-up survey. In the referral testing card arm, participants who received or opted out of follow-up were similar in sociodemographic characteristics and risk except for sexual orientation (see Supplement 2).
At follow up, 92 indexes in the HIV/syphilis self-testing arm self-reported having distributed 179 kits to alters, whereas 62 indexes in the testing card arm self-reported 26 distributions. We received 142 test result uploads from the HIV/syphilis self-testing group, among which 139 were from unique alters, one was from an index participant, and two were submitted after the program completion. Three test results were uploaded from the testing card referral group; one was from a unique alter, and two were from index participants. In the HIV/syphilis self-testing arm, 14 (six indexes, eight alters) received a reactive HIV result and five (three indexes, two alters) received a reactive syphilis result. Among the eight alters who uploaded their test results, except that one participant who was confirmed to be false-positive, two lost contact, and seven already been living with HIV, the remaining four participants received positive confirmatory test results for HIV. Participants who received a reactive syphilis test were recommended to take a confirmation test at their local hospital. Two index participants in the testing card arm received a reactive HIV result and were already aware of their infection status at the time of enrollment.
Index participants' characteristics
Demographic characteristics were mostly similar between the secondary distribution and the testing card referral groups (Table 1). The mean age of the participants was 27.0 years (s.d. = 6.74) for the HIVST group and 28.7 years (s.d. = 7.33) for the testing card group. The majority of participants were self-identified men (98%), self-identified gay (74% [self-testing] vs 77% [testing card]), unmarried (88% vs 92%), had a monthly income of >USD458 (76% vs 82%), had a higher education degree (75% vs 71%), and had anal sex in the past month (60% vs 57%). A higher proportion of participants in the testing card arm have ever tested for HIV than participants in the HIVST arm (78% vs 89%, P = 0.039).

|
Distributions from index participants to alters
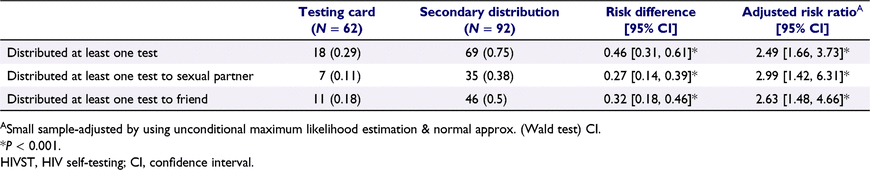
More participants in the secondary distribution group reported distributing at least one test (75% vs 29%, P < 0.001), had given at least one test to their sexual partner (38% vs 11%, P < 0.001), and had given at least one test to their friend (50% vs 18%, P < 0.001) than those in the testing card group (Table 2).
|
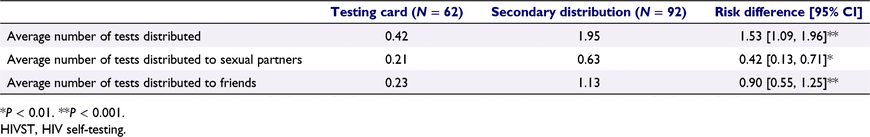
Participants in the secondary distribution group reported having distributed more self-tests to social contacts (1.95 vs 0.42, P < 0.001), more self-tests to sexual partners (0.63 vs 0.21, P < 0.01), and more self-tests to friends (1.13 vs 0.23, P < 0.001), compared to participants distributing testing cards (Table 3).
|
Distributions by subgroups
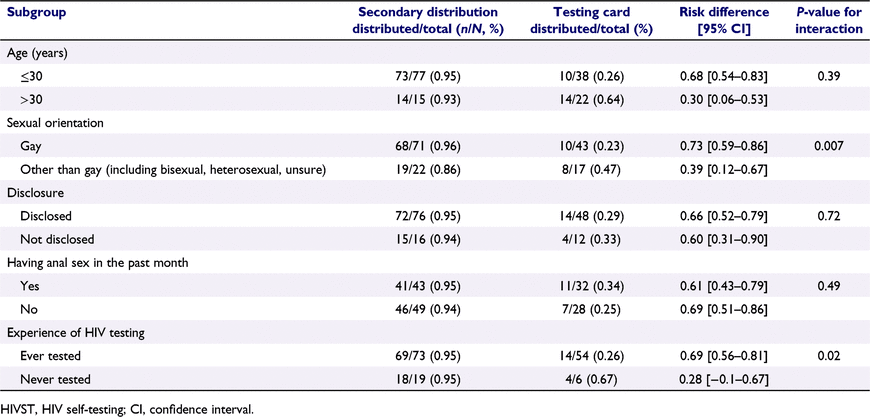
The effect of secondary distribution is modified by self-identified sexual orientation and prior experience of testing. Participants in the secondary distribution group who are self-identified as gay (P = 0.007) and who ever tested for HIV were significantly more likely to have distributing behaviors compared to other participants across the two groups. There was no significant effect modification between groups of different age, different disclosure status, or different sexual behaviors in the past month (Table 4).
|
Costing analysis
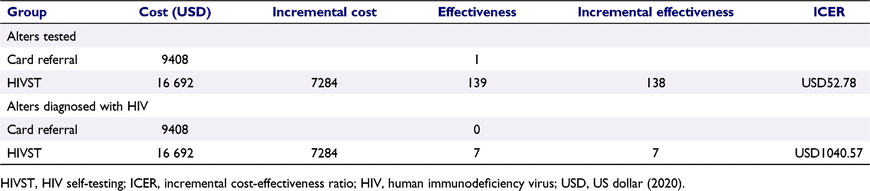
The total economic cost was USD16 692 for secondary distribution and USD9408 for testing card referral. The cost per alter tested for testing card referral was USD9408, and for secondary distribution, it was USD120. The ICER per alter tested was USD52.78. Additionally, the cost per alter who tested positive in the HIVST secondary distribution group was USD2384. There was no newly diagnosed person in the card referral group. The ICER per alter who tested positive was USD1041 (Table 5).
|
Discussion
Promoting HIV testing among key populations is essential to reach the UNAIDS ‘95–95–95’ targets. We assessed the effectiveness of GBMSM distributing HIVST compared to referral testing cards. Our data suggest the feasibility of using blood-based HIV/syphilis self-tests to support the HIV self-testing strategy among GBMSM in China. We also found that secondary distribution of HIVST engaged more social networks to encourage testing behaviors compared to testing card referral. Our findings extend the existing literature by focusing on blood-based self-testing and social network-based testing approaches in China and including an economic evaluation.
Although both approaches leveraged social networks to expand testing coverage, indexes who distributed blood-based HIV/syphilis dual self-testing were more likely to have peers receive the test than indexes referring testing cards. Our data are consistent with prior findings suggesting self-testing could circumvent barriers to facility-based testing and increase testing coverage among key populations.8,27 Compared to peer referral, secondary distribution could not only utilise peer influence to encourage testing in a similar way as peer referral of testing23, but also enable recipients to test in a setting of their choice with potentially more confidentiality and less stigma.28 In contrast testing card referral still requires the participants to actively seek testing themselves. Our findings also indicated that blood-based self-testing can be integrated into social network-based testing strategies to promote testing among Chinese GBMSM, given that blood-based self-testing is harder to perform compared to oral fluid-based self-testing, but is regarded to have higher accuracy, and participants in previous studies showed varying preference for blood-based and oral fluid-based testing kits.11,29,30 Participants in our study have cited the high accuracy of blood-based self-testing results as one major factor that facilitated their distribution (unpublished), reflecting the importance of credibility in social network-based approaches. Including syphilis self-testing into HIV self-testing may have also contributed to participants’ willingness to access and distribute the self-tests, as suggested by prior studies that testers preferred to have options for other STI testing.11,30 In China, only 56.4% of Chinese MSM living with HIV were aware of their status as of 2020.31 Blood-based HIV self-testing that incorporates STI testing and secondary distribution of self-testing could be helpful to increase testing uptake among key populations in China.
We also found that participants who previously tested for HIV in the HIVST group were more likely to distribute tests to their social contacts. Retesting is an important strategy to identify new cases.32 Previous evidence has shown that direct provision of self-testing could facilitate retesting for HIV among key populations.33 Our data suggest that GBMSM who retest for HIV were also willing to distribute HIV/syphilis dual tests to their social networks. This finding suggests that secondary distribution could be combined with HIV retesting strategies and sexual health care to increase HIV and syphilis testing uptake among GBMSM in China.
Secondary distribution was cheaper at increasing test uptake among GBMSM. The cost per alter tested in the HIVST arm was USD120 compared with USD9408 in testing card referral arm. We also found that although a new HIV diagnosis costs USD2384, this may still be worthwhile, as earlier identification can avert new HIV cases from the ongoing transmission of an undiagnosed and untreated person. This would be cost-saving, as it averts the much higher lifetime costs for managing a person living with HIV.34 Consistent with other economic evaluations from low- and middle-income countries, implementing HIVST programs had higher economic costs than facility-based programs.35–37 In our study, personnel cost comprised the majority (59%) of the total cost as our secondary distribution program was implemented by the research team in a facility-based setting where participants came to the site for HIV testing and signed up for free HIVST kits. In addition to personnel, categories with the biggest discrepancies between secondary distribution and testing card referral are promotion and consumables related to the self-tests (Supplementary File S1). Future implementation of secondary distribution could reduce the need for participants to attend facilities or automate the process whereby participants could access HIVST kits without extra personnel. Policy may also consider funding community based organisations to implement a HIVST strategy in China to reduce costs related to promotion and purchasing self-tests.
Our study has research and policy implications. First, our study outcomes suggested that secondary distribution of blood-based HIV/syphilis dual self-tests can reach more GBMSM for testing than testing card referral. Future research is needed to identify barriers and facilitators of distribution to optimise this approach and to engage different stakeholders (e.g. government organisations, community based organisations) for scale-up. In particular, as the barriers and facilitators of distribution may vary depending on the order of preferences of local participants, it is important to understand how the effectiveness of secondary distribution changes in different regions in order to optimise this strategy globally.32 Second, we found that self-identifying as gay and having prior HIV testing experience facilitated secondary distribution. Future implementation could take advantage of those identifications; for example, research could identify, include, and train those people for secondary distribution and study their social network and distributing behaviors to maximise their effectiveness to distribute tests. Third, secondary distribution can have higher economic costs due to personnel. Future implementation may explore ways to reduce the involvement of personnel to decrease overall costs related to secondary distribution, such as automating the process whereby the index participants obtain the self-tests.
Our study has several limitations. First, analyses of distributions were based on self-reports. This could introduce bias; however, our computerised self-administered survey allowed a high degree of anonymity. Moreover, self-reports also reflected the real-world setting because of its efficiency to obtain responses at a low cost. Many HIV self-testing programs rely on self-report to confirm use of self-tests.32,38 Using self-report in our study would help understand, assess, and inform the real-world implementation of secondary distribution. Second, photo verification of self-reported outcomes was only feasible during the HIV/syphilis self-testing period. Only 1/62 of alters verified their facility-based outcomes. Index participants may have overreported distributions; however, because the cognitive and situational factors influencing the validity of self-reports remained similar between the two arms, reporting between groups would not be different.39 The much lower verification rate in the test card referral arm could suggest that alters who received testing card referrals may be less willing to take facility-based tests or share information about their facility-based testing, as testing card referral maintains barriers typically associated with facility-based testing. Third, 13% (14/106) of indexes in the intervention group and 38% (39/102) in the control group were lost to follow up, which could introduce bias. However, those who received or opted out of follow up were similar in terms of sociodemographic characteristics and risk behaviors, except for sexual orientation (Supplementary File S2). Finally, the sample size reported in our study was relatively small. This could potentially impact the costing analysis as only one outcome was reported in the control condition.
Conclusion
Secondary distribution engaged more GBMSM to distribute tests to their social network and reached more GBMSM to test, suggesting the feasibility and advantage of secondary distribution of blood-based HIV/syphilis self-testing in promoting testing behaviors among Chinese GBMSM. GBMSM who self-identify as gay or who have previously tested for HIV were more effective at distributing tests. More implementation research is needed to expand network approaches and integrate them within existing health services.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
Dr. Jason J. Ong and Dr. Joseph D. Tucker are Editors of this Sexual Health Special Issue, but were blinded from the peer review process for this paper.
Declaration of funding
This work was supported by the National Key Research and Development Program of China [grant number 2017YFE0103800]; Academy of Medical Sciences and the Newton Fund [grant number NIF\R1\181020]; the National Institutes of Health [grant numbers NIAID 1R01AI114310-01, NIAID K24AI143471, R25 AI140495]; UNC Center for AIDS Research [grant number NIAID 5P30AI050410]; National Science and Technology Major Project of China [grant number 2018ZX10101-001-001-003]; the National Nature Science Foundation of China [grant numbers 81903371, 81772240]; and the Zhuhai Medical and Health Science and Technology Plan Project [grant number 20181117A010064].
Acknowledgements
The authors thank all GBMSM who participated in this study and the staff at the other three sites who contributed to the study.
References
[1] Wu Z, Chen J, Scott SR, McGoogan JM. History of the HIV Epidemic in China. Curr HIV/AIDS Rep 2019; 16 458–66.| History of the HIV Epidemic in China.Crossref | GoogleScholarGoogle Scholar | 31773405PubMed |
[2] Dong MJ, Peng B, Liu ZF, Ye QN, Liu H, Lu XL, et al. The prevalence of HIV among MSM in China: a large-scale systematic analysis. BMC Infect Dis 2019; 19 1000
| The prevalence of HIV among MSM in China: a large-scale systematic analysis.Crossref | GoogleScholarGoogle Scholar | 31775654PubMed |
[3] National Health Commission of the People’s Republic of China. The State Council Information office of the People’s Republic of China. 2021. Available at http://www.scio.gov.cn/m/xwfbh/gbwxwfbh/xwfbh/wsb/Document/1642083/1642083.htm
[4] Li X, Lu H, Raymond HF, Sun Y, Jia Y, He X, et al. Untested and undiagnosed: barriers to HIV testing among men who have sex with men, Beijing, China. Sex Transm Infect 2012; 88 187–93.
| Untested and undiagnosed: barriers to HIV testing among men who have sex with men, Beijing, China.Crossref | GoogleScholarGoogle Scholar | 22158932PubMed |
[5] Tang S, Tang W, Meyers K, Chan P, Chen Z, Tucker JD. HIV epidemiology and responses among men who have sex with men and transgender individuals in China: a scoping review. BMC Infect Dis 2016; 16 588
| HIV epidemiology and responses among men who have sex with men and transgender individuals in China: a scoping review.Crossref | GoogleScholarGoogle Scholar | 27765021PubMed |
[6] Xu W, Zheng Y, Kaufman MR. Predictors of recent HIV testing among Chinese men who have sex with men: a barrier perspective. AIDS Patient Care STDS 2018; 32 408–17.
| Predictors of recent HIV testing among Chinese men who have sex with men: a barrier perspective.Crossref | GoogleScholarGoogle Scholar | 30234365PubMed |
[7] World Health Organization. WHO recommends HIV self-testing: evidence update and considerations for success: policy brief. 2021. Available at https://www.who.int/publications/i/item/WHO-CDS-HIV-19.36
[8] Figueroa C, Johnson C, Verster A, Baggaley R. Attitudes and acceptability on HIV self-testing among key populations: a literature review. AIDS Behav 2015; 19 1949–65.
| Attitudes and acceptability on HIV self-testing among key populations: a literature review.Crossref | GoogleScholarGoogle Scholar | 26054390PubMed |
[9] De Boni RB, Veloso VG, Fernandes NM, Lessa F, Corrêa RG, Lima RS, et al. An internet-based HIV self-testing program to increase HIV testing uptake among men who have sex with men in Brazil: descriptive cross-sectional analysis. J Med Internet Res 2019; 21 e14145
| An internet-based HIV self-testing program to increase HIV testing uptake among men who have sex with men in Brazil: descriptive cross-sectional analysis.Crossref | GoogleScholarGoogle Scholar | 31373276PubMed |
[10] Amstutz A, Lejone TI, Khesa L, Muhairwe J, Bresser M, Vanobberghen F, et al. Home-based oral self-testing for absent and declining individuals during a door-to-door HIV testing campaign in rural Lesotho (HOSENG): a cluster-randomised trial. Lancet HIV 2020; 7 e752–61.
| Home-based oral self-testing for absent and declining individuals during a door-to-door HIV testing campaign in rural Lesotho (HOSENG): a cluster-randomised trial.Crossref | GoogleScholarGoogle Scholar | 33045193PubMed |
[11] Choko AT, Fielding K, Johnson CC, Kumwenda MK, Chilongosi R, Baggaley RC, et al. Partner-delivered HIV self-test kits with and without financial incentives in antenatal care and index patients with HIV in Malawi: a three-arm, cluster-randomised controlled trial. Lancet Glob Health 2021; 9 e977–88.
| Partner-delivered HIV self-test kits with and without financial incentives in antenatal care and index patients with HIV in Malawi: a three-arm, cluster-randomised controlled trial.Crossref | GoogleScholarGoogle Scholar | 34143996PubMed |
[12] Tan YR, Kaur N, Ye AJ, Zhang Y, Lim JXZ, Tan RKJ, et al. Perceptions of an HIV self-testing intervention and its potential role in addressing the barriers to HIV testing among at-risk heterosexual men: a qualitative analysis. Sex Transm Infect 2021; 97 514–20.
| Perceptions of an HIV self-testing intervention and its potential role in addressing the barriers to HIV testing among at-risk heterosexual men: a qualitative analysis.Crossref | GoogleScholarGoogle Scholar | 33452131PubMed |
[13] Balán I, Frasca T, Ibitoye M, Dolezal C, Carballo-Diéguez A. Fingerprick versus oral swab: acceptability of blood-based testing increases if other STIs can be detected. AIDS Behav 2017; 21 501–4.
| Fingerprick versus oral swab: acceptability of blood-based testing increases if other STIs can be detected.Crossref | GoogleScholarGoogle Scholar | 27439457PubMed |
[14] Zhang C, Koniak-Griffin D, Qian HZ, Goldsamt LA, Wang H, Brecht ML, et al. Impact of providing free HIV self-testing kits on frequency of testing among men who have sex with men and their sexual partners in China: a randomized controlled trial. PLoS Med 2020; 17 e1003365
| Impact of providing free HIV self-testing kits on frequency of testing among men who have sex with men and their sexual partners in China: a randomized controlled trial.Crossref | GoogleScholarGoogle Scholar | 33035206PubMed |
[15] Indravudh PP, Hensen B, Nzawa R, Chilongosi R, Nyirenda R, Johnson CC, et al. Who is reached by HIV self-testing? individual factors associated with self-testing within a community-based program in rural Malawi. J Acquir Immune Defic Syndr 2020; 85 165–73.
| Who is reached by HIV self-testing? individual factors associated with self-testing within a community-based program in rural Malawi.Crossref | GoogleScholarGoogle Scholar | 32501815PubMed |
[16] Shrestha R, Alias H, Wong LP, Altice FL, Lim SH. Using individual stated-preferences to optimize HIV self-testing service delivery among men who have sex with men (MSM) in Malaysia: results from a conjoint-based analysis. BMC Public Health 2020; 20 1777
| Using individual stated-preferences to optimize HIV self-testing service delivery among men who have sex with men (MSM) in Malaysia: results from a conjoint-based analysis.Crossref | GoogleScholarGoogle Scholar | 33238941PubMed |
[17] McCree DH, Millett G, Baytop C, Royal S, Ellen J, Halkitis PN, et al. Lessons learned from use of social network strategy in HIV testing programs targeting African American men who have sex with men. Am J Public Health 2013; 103 1851–56.
| Lessons learned from use of social network strategy in HIV testing programs targeting African American men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 23948017PubMed |
[18] Ghosh D, Krishnan A, Gibson B, Brown S-E, Latkin CA, Altice FL. Social network strategies to address HIV prevention and treatment continuum of care among at-risk and HIV-infected substance users: a systematic scoping review. AIDS Behav 2017; 21 1183–207.
| Social network strategies to address HIV prevention and treatment continuum of care among at-risk and HIV-infected substance users: a systematic scoping review.Crossref | GoogleScholarGoogle Scholar | 27125244PubMed |
[19] Wang K, Brown K, Shen SY, Tucker J. Social network-based interventions to promote condom use: a systematic review. AIDS Behav 2011; 15 1298–308.
| Social network-based interventions to promote condom use: a systematic review.Crossref | GoogleScholarGoogle Scholar | 21811843PubMed |
[20] Thirumurthy H, Masters SH, Mavedzenge SN, Maman S, Omanga E, Agot K. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study. Lancet HIV 2016; 3 e266–74.
| Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study.Crossref | GoogleScholarGoogle Scholar | 27240789PubMed |
[21] Nyondo AL, Choko AT, Chimwaza AF, Muula AS. Invitation cards during pregnancy enhance male partner involvement in prevention of mother to child transmission (PMTCT) of human immunodeficiency virus (HIV) in Blantyre, Malawi: a randomized controlled open label trial. PLoS ONE 2015; 10 e0119273
| Invitation cards during pregnancy enhance male partner involvement in prevention of mother to child transmission (PMTCT) of human immunodeficiency virus (HIV) in Blantyre, Malawi: a randomized controlled open label trial.Crossref | GoogleScholarGoogle Scholar | 25734485PubMed |
[22] Koo K, Makin JD, Forsyth BWC. Where are the men? Targeting male partners in preventing mother-to-child HIV transmission. AIDS Care 2013; 25 43–8.
| Where are the men? Targeting male partners in preventing mother-to-child HIV transmission.Crossref | GoogleScholarGoogle Scholar | 22670795PubMed |
[23] Ong’wen P, Samba BO, Moghadassi M, Okoko N, Bukusi EA, Cohen CR, et al. Chain peer referral approach for HIV testing among adolescents in Kisumu County, Kenya. AIDS Behav 2020; 24 484–90.
| Chain peer referral approach for HIV testing among adolescents in Kisumu County, Kenya.Crossref | GoogleScholarGoogle Scholar | 31267295PubMed |
[24] Glasman LR, Dickson-Gomez J, Lechuga J, Tarima S, Bodnar G, de Mendoza LR. Using peer-referral chains with incentives to promote HIV testing and identify undiagnosed HIV infections among crack users in San Salvador. AIDS Behav 2016; 20 1236–43.
| Using peer-referral chains with incentives to promote HIV testing and identify undiagnosed HIV infections among crack users in San Salvador.Crossref | GoogleScholarGoogle Scholar | 26687093PubMed |
[25] Holden J, Goheen J, Jett-Goheen M, Barnes M, Hsieh YH, Gaydos CA. An evaluation of the SD bioline HIV/syphilis duo test. Int J STD AIDS 2018; 29 57–62.
| An evaluation of the SD bioline HIV/syphilis duo test.Crossref | GoogleScholarGoogle Scholar | 28661234PubMed |
[26] Yang F, Janamnuaysook R, Boyd MA, Phanuphak N, Tucker JD. Key populations and power: people-centred social innovation in Asian HIV services. Lancet HIV 2020; 7 e69–74.
| Key populations and power: people-centred social innovation in Asian HIV services.Crossref | GoogleScholarGoogle Scholar | 31818717PubMed |
[27] Johnson CC, Kennedy C, Fonner V, Siegfried N, Figueroa C, Dalal S, et al. Examining the effects of HIV self-testing compared to standard HIV testing services: a systematic review and meta-analysis. J Int AIDS Soc 2017; 20 21594
| Examining the effects of HIV self-testing compared to standard HIV testing services: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 28530049PubMed |
[28] den Daas C, Geerken MBR, Bal M, de Wit J, Spijker R, Op de Coul ELM, PREVENT study group Reducing health disparities: key factors for successful implementation of social network testing with HIV self-tests among men who have sex with men with a non-western migration background in the Netherlands. AIDS Care 2020; 32 50–6.
| Reducing health disparities: key factors for successful implementation of social network testing with HIV self-tests among men who have sex with men with a non-western migration background in the Netherlands.Crossref | GoogleScholarGoogle Scholar | 31416354PubMed |
[29] Obiezu-Umeh C, Gbajabiamila T, Ezechi O, Nwaozuru U, Ong JJ, Idigbe I, et al. Young people’s preferences for HIV self-testing services in Nigeria: a qualitative analysis. BMC Public Health 2021; 21 67
| Young people’s preferences for HIV self-testing services in Nigeria: a qualitative analysis.Crossref | GoogleScholarGoogle Scholar | 33413246PubMed |
[30] Ong JJ, Nwaozuru U, Obiezu-Umeh C, Airhihenbuwa C, Xian H, Terris-Prestholt F, et al. Designing HIV testing and self-testing services for young people in Nigeria: a discrete choice experiment. Patient 2021; 14 815–26.
| Designing HIV testing and self-testing services for young people in Nigeria: a discrete choice experiment.Crossref | GoogleScholarGoogle Scholar | 33942248PubMed |
[31] UNAIDS. Country Factsheets | China | 2020. 18 October 2021. Available at https://www.unaids.org/en/regionscountries/countries/china
[32] Chamie G, Napierala S, Agot K, Thirumurthy H. HIV testing approaches to reach the first UNAIDS 95% target in sub-Saharan Africa. Lancet HIV 2021; 8 e225–36.
| HIV testing approaches to reach the first UNAIDS 95% target in sub-Saharan Africa.Crossref | GoogleScholarGoogle Scholar | 33794183PubMed |
[33] Ortblad K, Kibuuka Musoke D, Ngabirano T, Nakitende A, Magoola J, Kayiira P, et al. Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: a cluster-randomized controlled health systems trial. PLoS Med 2017; 14 e1002458
| Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: a cluster-randomized controlled health systems trial.Crossref | GoogleScholarGoogle Scholar | 29182634PubMed |
[34] Tran H, Saleem K, Lim M, Chow EPF, Fairley CK, Terris-Prestholt F, et al. Global estimates for the lifetime cost of managing HIV: a systematic review. AIDS 2021; 35 1273–81.
| Global estimates for the lifetime cost of managing HIV: a systematic review.Crossref | GoogleScholarGoogle Scholar | 33756510PubMed |
[35] Cambiano V, Ford D, Mabugu T, Napierala Mavedzenge S, Miners A, Mugurungi O, et al. Assessment of the potential impact and cost-effectiveness of self-testing for HIV in low-income countries. J Infect Dis 2015; 212 570–77.
| Assessment of the potential impact and cost-effectiveness of self-testing for HIV in low-income countries.Crossref | GoogleScholarGoogle Scholar | 25767214PubMed |
[36] George G, Chetty T, Strauss M, Inoti S, Kinyanjui S, Mwai E, et al. Costing analysis of an SMS-based intervention to promote HIV self-testing amongst truckers and sex workers in Kenya. PLoS ONE 2018; 13 e0197305
| Costing analysis of an SMS-based intervention to promote HIV self-testing amongst truckers and sex workers in Kenya.Crossref | GoogleScholarGoogle Scholar | 29979704PubMed |
[37] Mangenah C, Mwenge L, Sande L, Ahmed N, d’Elbée M, Chiwawa P, et al. Economic cost analysis of door-to-door community-based distribution of HIV self-test kits in Malawi, Zambia and Zimbabwe. J Int AIDS Soc 2019; 22 e25255
| Economic cost analysis of door-to-door community-based distribution of HIV self-test kits in Malawi, Zambia and Zimbabwe.Crossref | GoogleScholarGoogle Scholar | 30907499PubMed |
[38] Tahlil KM, Ong JJ, Rosenberg NE, Tang W, Conserve DF, Nkengasong S, et al. Verification of HIV self-testing use and results: a global systematic review. AIDS Patient Care STDS 2020; 34 147–56.
| Verification of HIV self-testing use and results: a global systematic review.Crossref | GoogleScholarGoogle Scholar | 32324482PubMed |
[39] Brener ND, Billy JOG, Grady WR. Assessment of factors affecting the validity of self-reported health-risk behavior among adolescents: evidence from the scientific literature. J Adolesc Health 2003; 33 436–57.
| Assessment of factors affecting the validity of self-reported health-risk behavior among adolescents: evidence from the scientific literature.Crossref | GoogleScholarGoogle Scholar | 14642706PubMed |


