Utilisation of pre-exposure prophylaxis (PrEP) for HIV prevention in the Australian general practice setting: a longitudinal observational study
Kendal Chidwick A * , Allan Pollack A , Doreen Busingye A , Sarah Norman A , Andrew Grulich B , Benjamin Bavinton B , Rebecca Guy B and Nick Medland B
A * , Allan Pollack A , Doreen Busingye A , Sarah Norman A , Andrew Grulich B , Benjamin Bavinton B , Rebecca Guy B and Nick Medland B
A NPS MedicineWise, Level 7/418A Elizabeth Street, Surry Hills, NSW 2010, Australia.
B The Kirby Institute, UNSW Sydney, Level 6, Wallace Wurth Building, High Street, Kensington, NSW 2052, Australia.
Sexual Health 19(2) 101-111 https://doi.org/10.1071/SH21207
Submitted: 21 October 2021 Accepted: 3 March 2022 Published: 26 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Background: Pre-exposure prophylaxis (PrEP) became available through the Australian Pharmaceutical Benefits Scheme (PBS) on 1 April 2018 for HIV infection prevention in patients ≥18 years at medium-to-high HIV risk. The aims were to investigate PrEP utilisation in general practice since PBS listing, and factors associated with discontinuation.
Methods: This longitudinal study included patients aged 18–74 years attending general practices participating in MedicineInsight, a large-scale national primary care database of deidentified electronic health records, between October 2017 and September 2019.
Results: PrEP utilisation increased 10-fold following PBS listing. On average, patients had 9.7 PrEP prescriptions per year; a medication possession ratio of 80.8%. Of 1552 patients prescribed PrEP from April 2018, most were male (98.3%), aged 18–39 years (59.3%), resided in major cities (86.7%) and in the two most socioeconomically advantaged quintiles (70.0%). Almost half (49.1%) of the patients were identified as new to PrEP. At study end, 65.1% were on active PrEP (16.5%, of whom had non-continuous use), 19.2% had discontinued PrEP and 15.7% were lost to follow up. Patients who discontinued were more likely to attend low rather than high PrEP caseload practices (adjusted odds ratio [aOR] 1.7; 95% CI: 1.0–2.8; P = 0.047). The odds of non-continuous therapy was 2.9-fold higher in patients with bipolar disorder (aOR 2.89; 95% CI: 1.10–7.6; P = 0.045).
Conclusions: Following PBS listing, PrEP utilisation increased and stopping therapy was associated with attending low caseload practices. General practice education, particularly among low caseload practices, could help address these disparities.
Keywords: electronic health records, emtricitabine, general practice, MedicineInsight, pharmacoepidemiology, primary health care, prophylaxis, tenofovir disoproxil fumarate drug combination.
Introduction
Pre-exposure prophylaxis (PrEP) is an antiretroviral medicine for the prevention of HIV infection. It is recommended for all people who are at risk, including men who have sex with men (MSM), transgender people, heterosexual men and women at high risk, and people who inject drugs.1 Evidence from clinical trials shows that daily PrEP use, with optimal medication adherence, is a highly effective HIV prevention strategy among people at high risk of HIV.2–6 In addition, on-demand PrEP is highly effective in MSM.7,8 Since 2020, it has been recommended by the World Health Organization9 and the Australasian Society of HIV, Viral Hepatitis and Sexual Health Medicine (ASHM)1 as an HIV prevention option for MSM.
Prior to 2018, about 18 000 Australian adults at risk of HIV received PrEP through state/territory implementation studies.10 Almost 10 000 individuals were enrolled in the Expanded PrEP Implementation in Communities in New South Wales (EPIC-NSW) study between March 2016 and April 2018. The findings from EPIC-NSW demonstrated a 25.1% reduction in HIV diagnoses in NSW in the first 12 months after study enrolment,11 with incidence remaining low long-term, over a 3-year follow-up period.12
In 2018, PrEP was listed on the Australian Pharmaceutical Benefits Scheme (PBS), a government subsidy scheme for prescriptions, for the prevention of HIV in adult patients at medium or high risk of HIV infection. Since 2018, the majority of patients now access PrEP via the PBS;1,13 however, some patients continue to access PrEP via self-importation or as private prescriptions, as cost of self-importation is lower than the PBS-subsidised general patient co-payment (direct cost to the patient, up to A$41 for 1 month’s supply, but lower (A$7) for those eligible on health or financial grounds).1,10 As non-PBS-subsidised PrEP prescriptions are not available in the PBS data, PBS data are likely to underestimate PrEP utilisation.
Questions also remain about how people use PrEP in the real world outside the clinical trial setting and whether the cost of routine care in general practice is a barrier to utilisation. The PrEP in NSW Transition Study, which aimed to determine how people transitioned out of a PrEP implementation trial to receiving PrEP through general practice and standard-of-care prescribing, showed high sustained use and adherence to the PrEP dosing schedule in the 12 months after the end of the EPIC-NSW trial.14 However, there continues to be limited real world data on the utilisation of PrEP in general practice, including among patients new to therapy since the PBS listing and those who access PrEP outside of the PBS.
The MedicineInsight database comprises data for approximately 9% of general practices in Australia and includes both PBS-subsidised and private prescriptions (which may include those obtained through self-importation), along with patient sociodemographic details and conditions, enabling detailed assessment of PrEP utilisation. Using data from MedicineInsight, we describe the: uptake of PrEP in general practice following PBS listing; sociodemographic characteristics of patients prescribed PrEP; patterns of PrEP use; and patient and general practice factors associated with discontinuation and non-continuous use.
Methods
Design and data source
This was a longitudinal observational study, using Australian general practice data from MedicineInsight for the 2 years from 1 October 2017 through to 30 September 2019, and included 6 months of baseline data (1 October 2017–31 March 2018) prior to the 1 April 2018 listing of PrEP on the PBS (Supplementary Fig. S1).
MedicineInsight is a national general practice data program developed and managed by NPS MedicineWise with funding support from the Australian Government Department of Health.15 MedicineInsight extracts and collates longitudinal, de-identified patient health records, including demographics, clinical encounters (excluding progress notes), diagnoses, prescriptions, pathology tests, physical observations, risk factors, adverse reactions, immunisations and billing information from the clinical information systems, MedicalDirector® and Best Practice®. MedicineInsight includes records for over 3.5 million regular patients (approximately 15% of the Australian population) from more than 5000 general practitioners (GPs) in 715 general practices across Australia (as at 1 July 2019). When compared with Medicare Benefits Schedule data, the characteristics of regularly attending MedicineInsight patients are broadly comparable to those of patients who visited a GP in 2017–18 in terms of age, sex and socioeconomic status. However, patients residing in inner regional areas and Tasmania are overrepresented and those in remote areas and South Australia are underrepresented.16 The exclusion of progress notes (for privacy reasons) and the possibility of prescriptions originating outside MedicineInsight practices means that some relevant data may not be available.
Study population
De-identified patient data were obtained from 441 Australian general practice sites that met the standard data quality criteria, described elsewhere.15 The general study population comprised patients who were aged 18–74 years as of 1 July 2017, had valid information for age and sex, had at least two clinical encounters during the study period (1 October 2017–31 September 2019) at an included practice and were not diagnosed with HIV prior to 1 April 2018. Subpopulations included (Fig. 1):
-
The PrEP user population – patients who had at least one prescription for PrEP between 1 April 2018 and 30 September 2019 and were not diagnosed with HIV prior to their first PrEP prescription.
-
The PrEP initiator subpopulations – patients who were identified as being prescribed PrEP for the first time between 1 April 2018 and 30 September 2019 and had a record of attendance at that practice at least 6 months prior to being prescribed PrEP.
The index date was defined for each patient as the date of their first prescription for PrEP during the study period. Patient time (follow up) in the study commenced on the patient’s index date and ended at the earliest of: (i) the end of the study (30 September 2019); (ii) 3 months after the patient’s last visit to the practice (defined as lost to follow up); (iii) date of first HIV diagnosis; or (iv) date of death (defined as the last visit in their year of death).
Definitions
PrEP medicines
PrEP medicines were identified from the ‘Script Item’ table using the ‘medicine active ingredient’ and ‘medicine name’ fields. As these medicines are also used for treating HIV, patients were excluded if they had a diagnosis of HIV recorded before, or 7 days after, the first PrEP prescription or if the term ‘post-exposure prophylaxis (PEP)’ was recorded during therapy with one of the PrEP medicines. Diagnoses were identified from the diagnosis, reason for encounter, reason for prescription and authority indication fields. The count of prescriptions included issued prescriptions plus repeats. For example, an issued prescription with two repeats would be counted as three prescriptions and would be expected to last 3 months, assuming daily dosing. The full search list of PrEP medicines is provided in Supplementary Table S1.
Patterns of PrEP use
Patterns of PrEP use were defined based on dosage instructions recorded by the prescriber (daily or on-demand regimen) and gaps between prescriptions (continuous or non-continuous use).
A patient’s pattern of PrEP use was classified as ‘continuous’ if they never had a >21-day (or >63 days for an issued prescription with two repeats) gap between the expected end of one prescription and the date of the next prescription for PrEP. Patients had a ‘non-continuous’ pattern if they had an on-demand regimen recorded by the prescriber or had one or more gaps of >21 days (or >63 days for an issued prescription with two repeats) between the expected end of one prescription and the date of the next prescription for PrEP. The 21-day gap was chosen as a conservative estimate of the number of days a patient could maintain a protective dose of four pills per week with a 30-day prescription.7,17 Further details of these patterns of PrEP use are provided in Supplementary Table S2.
PrEP status at the end of the study
PrEP status at the end of the study was assessed as active, discontinued, or lost to follow up as defined below:
-
Active: Patient had ‘a current prescription for PrEP’ at 30 September 2019
-
Discontinued: Patient did not have ‘a current prescription for PrEP’ at 30 September 2019 and their last visit at the practice was after their last prescription for PrEP
-
Lost to follow up: Patient did not have a prescription for PrEP at 30 September 2019 and no visit was recorded after the last prescription for PrEP.
A patient was considered to have ‘a current prescription for PrEP’ from the date of their first prescription for a PrEP medicine until the earlier of ‘the date of cessation’ or ‘the end of the study time period’. The date of cessation of PrEP was defined as either the ‘cease date’ if this was recorded by the prescriber, or a derived cease date. A derived cease date was defined as the last prescription date plus the number of days of therapy prescribed plus an additional 90 days to account for missed doses, intermittent use and lag between filling a prescription at the pharmacy. The number of days of therapy prescribed was assumed to be 30 for each issued prescription, multiplied by the number of repeats, where applicable.
Practice caseload
High PrEP caseload practices were defined as those with at least 15 PrEP-user patients and were in the top 5% of all general practices with at least one PrEP user. Low PrEP caseload practices included those with at least one but <15 PrEP-user patients.
Covariates
Sociodemographic characteristics
Sociodemographic characteristics included age (based on year of birth), sex, concession status (healthcare card status, which entitles patients to reduced cost medicines), state/territory, Socio-Economic Indexes for Areas (SEIFA) and remoteness. State/territory, remoteness and SEIFA were based on the patients’ residential postcodes. Remoteness was determined in accordance with the Australian Bureau of Statistics (ABS) geographical framework ‘Remoteness Areas’.18 SEIFA was determined according to the ABS Index of Relative Socio-Economic Advantage and Disadvantage (IRSAD).19 IRSAD is an indicator of relative economic and social advantage/disadvantage position within an area.
Conditions
Mental health conditions20,21 and drug use disorders20,22,23 were assessed as potential factors that might impact adherence or compliance. Conditions assessed included anxiety, depression, bipolar disorder, schizophrenia, opioid use disorder and alcohol use disorder. Patients were defined as having any of these conditions if they had a relevant coded (Docle, Pyefinch) or free-text entry in one of the three diagnosis fields – diagnosis, reason for encounter or reason for prescription – ever recorded at any time from the patient’s earliest record up to the download date. The clinical definitions for the included conditions are shown in Supplementary Table S3.
Statistical analyses
Descriptive statistics were used to describe uptake and patterns of PrEP usage, and the distribution of sociodemographic characteristics including frequencies, percentages and associated 95% confidence intervals (CIs) (adjusted for clustering by practice), means and standard deviations (SDs). Multivariable logistic regression was used to assess association between patterns of use and patient and practice characteristics. The multivariable analyses of discontinued (vs active) and non-continuous use (vs continuous) were adjusted for age, sex and factors found to be significantly associated with discontinuation and non-continuous use, respectively, in univariable analyses. The factors included in both models were age, sex, SEIFA, concession card status, depression, bipolar disorder and practice caseload of PrEP users. To preserve the privacy of individuals, results reported for one to four patients are reported as <5. Data management and analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethics
Approval to conduct this study was granted by the Bellberry Human Research Ethics Committee (application number: 2019-10-849) and the MedicineInsight Independent Data Governance Committee (reference number: 2019-022).
Results
General study population
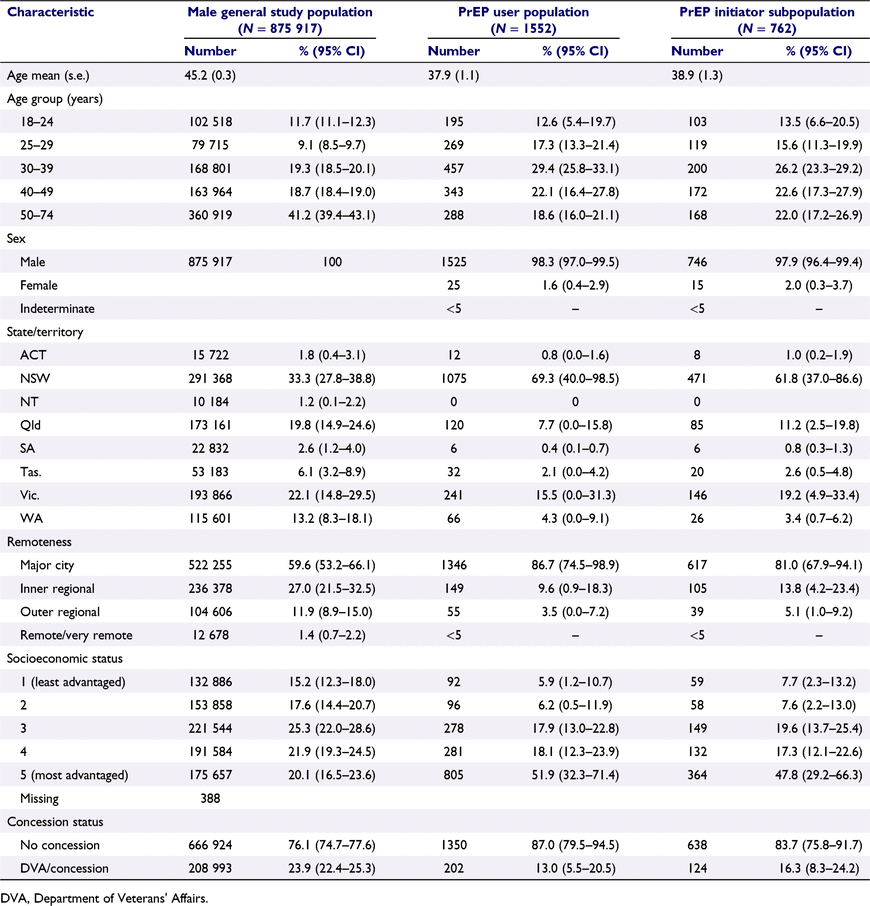
There were 1 999 247 patients eligible for inclusion in the general study population from whom subsequent PrEP user populations were derived (Fig. 1), representing approximately 10.1% of the Australian adult population (≥18 years). The average age for the general study population was 44.3 years, and the majority were female (56.2%) and resided in major cities (61.1%) (Supplementary Table S4). The mean average age for the male general study population was 45.2 years and the majority of males resided in major cities (59.6%) (Table 1).
|
PrEP uptake and patient profiles
Since its listing on the PBS, the uptake of PrEP in general practice increased more than 10-fold from 128 patients prescribed PrEP in April 2018 to 1552 in September 2019 (Fig. 2). The number of patients prescribed PrEP in the first 4 months (n = 504) of the study was twice that of patients prescribed PrEP for the first time in the past 4 months (n = 232) (Fig. 2).
|
|
Of the 1 999 247 general study patients, 1552 (0.1%) patients were prescribed PrEP at least once between 1 April 2018 and 30 September 2019, of whom 762 (49.1%) were prescribed PrEP for the first time (the PrEP initiator subpopulation). The remaining 790 (50.9%) patients were either prescribed PrEP prior to 1 April 2018 or had less than 6 months history at the general practice in order to assess prior use (Fig. 1).
The distribution of sociodemographic characteristics of the PrEP user population and PrEP initiator subpopulation are presented in Table 1. Of the 1552 patients prescribed PrEP, the majority were male (98.3%), aged 18–39 years (59.3%), attended a high caseload practice (63.9%, data not shown) and resided in New South Wales (69.3%), major cities (86.7%) and the two most socioeconomically advantaged area quintiles (70.0%). PrEP users at low PrEP caseload practices were younger and more likely to live in regional and more socioeconomically disadvantaged areas, compared to PrEP users at high caseload practices (Table S5). Among PrEP users at high PrEP caseload practices, only 8.5% had a concession/health care card recorded, compared with 21.1% of PrEP users at low PrEP caseload practices.
Patterns of PrEP utilisation
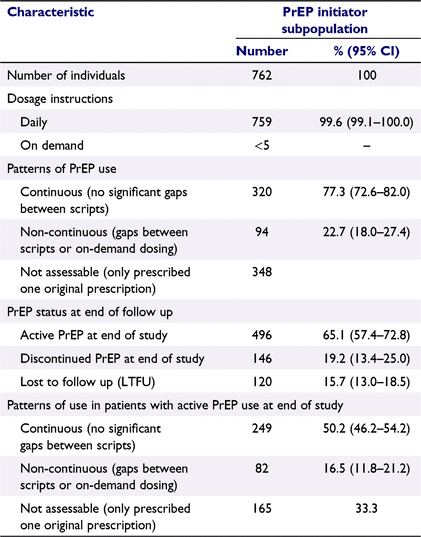
A total of 5025 prescriptions (issued prescription + repeats) for PrEP were recorded for 762 patients in the PrEP initiator subpopulation. The average number of scripts per patient over the 18-month study period was 6.6 (95% CI: 5.9–7.3) or 9.7 scripts (originals and repeats) per person-year, giving a medication possession ratio (MPR) of 80.8% assuming that one prescription equates to 1 month’s supply (Supplementary Table S6). The average duration of PrEP use – from first to last prescription plus 90 days – was 226 days (95% CI: 201–251). Among 94 people identified with a gap of >21 days in PrEP use, the mean average time to first discontinuation of PrEP was 182 days (95% CI: 152–212).
Based on the dosage instructions recorded by GPs on prescriptions, almost all patients (99.6%) in the PrEP initiator subpopulation were prescribed daily PrEP (Table 2); however, analysis of gaps between prescriptions showed that, among the 414 patients with more than one issued prescription for PrEP recorded, 77.3% were on continuous therapy and 22.7% had non-continuous PrEP use (one or more gaps of >21 days between prescriptions).
|
At the end of the analysis period, 65.1% of 762 patients who initiated PrEP were on active therapy, 19.2% had discontinued therapy and 15.7% were lost to follow-up (Table 2). Half of the patients on active therapy at the end of the study had been on PrEP continuously, whereas 16.5% had non-continuous PrEP use and one-third had only one issued prescription for PrEP recorded and, therefore, could not be assessed for gaps in therapy. The sociodemographic characteristics of patients with different patterns of use are presented in Supplementary Table S7.
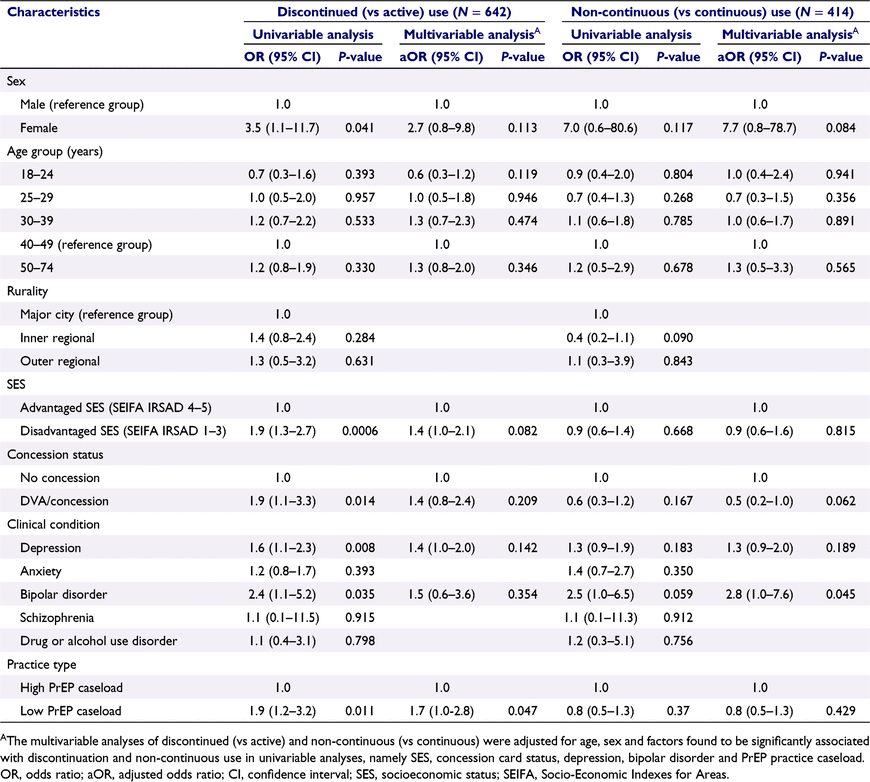
Factors associated with discontinuation and non-continuous use of PrEP
In univariable analyses, patients who discontinued PrEP were more likely to be female, have depression or bipolar disorder, live in a more socioeconomically disadvantaged area, have a concession/healthcare card and attend a low PrEP caseload practice than those with an active prescription at the end of the study (Table 3). After adjusting for age, sex and factors found to be significantly associated with discontinuation in univariable analyses, discontinuing PrEP was associated with attending a low PrEP caseload practice (adjusted odds ratio [aOR] 1.7; 95% CI: 1.0–2.8; P = 0.047) compared to high caseload practices. There was weak evidence that discontinuing PrEP was associated with residing in more disadvantaged socioeconomic status areas (SEIFA 1–3) (aOR 1.4; 95% CI: 1.0–2.1; P = 0.082) compared with the most socioeconomically advantaged areas (SEIFA 4–5) (Table 3).
|
Among patients in the PrEP initiator subpopulation who had more than one issued prescription for PrEP (n = 414), non-continuous therapy was associated with patients with bipolar disorder (aOR 2.8; 95% CI: 1.0–7.6; P = 0.045) (Table 3). There was weak evidence that non-continuous therapy was more likely among females than males. Non-continuous therapy was less likely in patients with a concession/healthcare card (aOR 0.5; 95% CI: 0.2–1.0; P = 0.062) (Table 3).
Discussion
Our findings demonstrate that by September 2019, the uptake of PrEP in the general practice setting had risen 10-fold since its listing on the PBS. Consistent with PBS data where a decline in the number of patients dispensed PrEP for the first time has been reported,10 we observed a 2-fold decrease in the number of patients newly prescribed PrEP in the last 4 months of the study compared to the first 4 months. Patients who discontinued were more likely to attend low rather than high PrEP caseload practices and non-continuous therapy was associated with bipolar disorder and concession card status.
The majority of MedicineInsight patients prescribed PrEP since PBS listing were male (98.3%), aligning with PBS data (98.8% male),10 and resided in major cities (86.7%) and socioeconomically advantaged areas. Our findings also mirror results from the 2344 people in the PrEP in NSW Transition Study of which 98.3% were male, 85.6% resided in major cities and the majority earned a high annual income.14 The main explanation for the low PrEP utilisation among patients residing in socioeconomically disadvantaged and regional areas is the lower prevalence of MSM living in these areas.24 Previous research has shown that 83% of suburbs with a high prevalence of gay residents are in major cities.24,25 These are generally inner city, socioeconomically advantaged suburbs where MSM live because there is less homophobia, where there are general practices familiar with health care for MSM and bisexual men, including PrEP, and where the PrEP health promotion has been targeted. These findings are most likely explained by these more complex sociocultural factors rather than socioeconomic factors.
Just under one-fifth (19.2%) of patients who started PrEP during the study period appear to have discontinued PrEP by the end of the study. A further 15.7% were lost to follow up and were either not currently using PrEP or were prescribed PrEP by a provider outside the MedicineInsight practices. In the EPIC-NSW trial,11 at 12 months after recruitment, 24% of patients did not attend the 12-month scheduled visit for either an HIV test or PrEP prescription, and in the PrEP in NSW Transition Study,14 at around 12 months after leaving the EPIC-NSW trial, 19.7% of those surveyed were not currently using PrEP. If we assume a proportion of patients who were lost to follow up did discontinue PrEP, our findings could indicate a similar or higher discontinuation rate among general practice patients than trial participants.
Our study provides real world insights about patterns of PrEP use, with good adherence to PrEP therapy demonstrated in the general practice setting, with a mean average MPR of 80.8%, similar to the mean MPR (83.1%) reported at 12-months in EPIC-NSW. However, this is lower than self-reported data from the PrEP in NSW Transition Study where, on average, participants reported being about 90% adherent to their dosing schedule,14 possibly because our cohort may have included both patients using daily dosing and on-demand/intermittent PrEP. Consistent with daily PrEP being the most commonly prescribed PrEP regimen in Australia,26 we found that almost all patients who initiated PrEP during the study period were prescribed daily PrEP, according to the recorded ‘directions for use’. The proportion of PrEP initiators (77%) who were on continuous therapy in our study is greater than that reported in a study from Boston, USA, where 60% of patients who initiated PrEP were on continuous therapy,27 but less than that in self-reported data in the PrEP in NSW Transition Study where 85–95% participants intended to use PrEP daily and only 10% reported taking a break from PrEP for at least a week in the past year.14 Potential explanations for the difference in the proportion of people with continuous use of PrEP in this study include different: data sources (electronic health record data in MedicineInsight vs chart review in the Boston study and self-reported survey in the PrEP in NSW Transition Study), patient populations (heterogenous patients attending high and low caseload, and urban and remote, general practices in MedicineInsight vs attendees of one urban specialist community sexual health clinic in the Boston study and EPIC clinical trial participants in the NSW study), and time periods (2017–19 MedicineInsight vs 2011–14 in the Boston study and 2018–20 in the PrEP in NSW Transition study).
Our study showed patients who discontinued PrEP were more likely to attend a low PrEP caseload practice than a high caseload practice. This aligns with a recent PBS data study that found the discontinuation of PrEP was associated with low PrEP caseload of the patients’ prescriber.28 High PrEP caseload practices likely include HIV-specialist GPs who are specially trained in prescribing PrEP. This finding could highlight a need for better education for non-HIV specialist GPs and low caseload practices, to help address this disparity. There was also weak evidence that patients residing in socioeconomically disadvantaged areas were more likely to discontinue PrEP therapy. As socioeconomically disadvantaged areas have a low prevalence of MSM, these are not the localities that HIV prevention campaigns have typically targeted, although a lot of PrEP social marketing now occurs online. This finding may also suggest lower health literacy, poor access to PrEP due to financial constraints and possibly other healthcare access factors in socioeconomically disadvantaged areas. There is evidence from the United States that lack of health insurance and cost of medication are barriers to PrEP initiation and continuation.27,29,30 These findings underscore the need for continued efforts and funding programs to provide widespread, consistent, low-cost PrEP services to underserved communities. In contrast to Australian research based on national PBS data,28 age was not associated with discontinuation in our study.
Non-continuous PrEP use may indicate on-demand dosing/intermittent use or non-adherence to daily dosing, and our data do not enable us to distinguish between these patient groups. Based on the available information on ‘directions for use’, it appears that a small proportion (<0.5%) of the patients who initiated PrEP were prescribed on-demand dosing. Since 2020, the on-demand regimen has been recommended as an alternative option for MSM, particularly those who have sex less than twice a week and can plan ahead for sex at least 2 h in advance,1,9 and has been shown to be highly effective.7,8 The on-demand dosing strategy has the potential to reduce the cost of drugs, pill burden and toxicity, and to improve continuation among those who find daily pill-taking challenging.9
Unlike discontinuation of PrEP, non-continuous PrEP use was not associated with attending a low PrEP caseload practice. Non-continuous use was just as common among patients attending high PrEP caseload practices, where support for using PrEP is arguably the highest. This finding could indicate that the non-continuous users in our study are largely made up of experienced on demand/intermittent users rather than those who are non-adherent. Some individuals may be taking PrEP during periods when they are potentially at risk of HIV, a concept called ‘prevention-effective adherence’ and take fewer pills or cease taking PrEP during periods deemed to be of no or low risk.31,32
We found that patients with a concession card had 50% lower odds of having non-continuous PrEP therapy. As non-continuous PrEP therapy in this study represents a combination of on-demand dosing and non-adherent use, this finding might reflect less knowledge about on-demand dosing among concessional patients and the lower cost of obtaining PrEP for concessional patients (PBS co-payment of A$6.60 for 30 days’ supply, assuming daily dosing) compared with the general patients (A$41.00 for 30 days’ supply).12 The finding that patients with bipolar disorder were 2.9-fold as likely to have non-continuous PrEP use is consistent with prior studies that have found an association between mental health disorders and difficulties with continuing with PrEP.27 This highlights potential subpopulations for whom tailored support for continued PrEP use may be beneficial. We did not detect evidence that any of the other patient characteristics were associated with non-continuous therapy. The ability to detect associations in this analysis may have been limited by the small sample size and the heterogeneity of non-continuous users, which included both patients with on-demand dosing and non-adherent use.
Although the MedicineInsight patient population in this study covers around 10.1% of the Australian adult population, our sample of 1552 patients prescribed PrEP since PBS listing represents approximately 5.9% of all Australian patients ever dispensed PrEP in the first 15 months after PBS listing.10 The exclusion of one high PrEP caseload practice that did not meet the standard data quality criteria, from our study cohort, may be in part responsible for the lower coverage of PrEP users in our study. It also led to lower representation in one of the states compared to PBS data.
The strengths of this study include the substantial sample size and national coverage of the MedicineInsight data. The longitudinal design enabled us to characterise patterns of PrEP use over time and assess factors associated with discontinuation and non-continuous use of PrEP. An important strength of MedicineInsight compared to other datasets is that it comprises data for both PBS-subsidised and private prescriptions. The data have limitations, in addition to those inherent in routinely collected data described elsewhere.15 For privacy reasons, MedicineInsight does not include data from progress notes, which may contain further clinical information. MedicineInsight contains GP prescribing information and it is not known if the medicines are dispensed or used. The data do not incorporate medicines prescribed at non-MedicineInsight practices or by specialists, which may lead to a misclassification of the true patterns of PrEP use. For non-continuous PrEP use, we were not able to distinguish between patients using on-demand dosing and those non-adherent to daily dosing, which might have limited the ability to detect correlates of non-continuous use. Although MedicineInsight is largely representative of Australian general practices, these findings may not be generalisable to all high and low caseload practices across Australia if behaviour varies substantially at non-MedicineInsight practices. Therefore, these findings should be interpreted in consideration of these limitations.
Despite significant steps towards providing access to PrEP for people at substantial risk of HIV through PBS subsidisation, our findings demonstrate differential utilisation of PrEP among patient subgroups and highlight the need for continued efforts to promote widespread availability and uptake of PrEP across all populations in order to significantly reduce HIV transmission in Australia. In particular, this study has identified that people who had stopped taking PrEP were more likely to attend practices with a low number of patients prescribed PrEP and live in socioeconomically disadvantaged areas, both typically outside of inner city high-prevalence gay areas. It is encouraging to see PrEP being prescribed at general practices outside the high prevalence gay areas; however, our findings do highlight some potential quality use of medicines issues that could put patients at ongoing risk of HIV infection. GP education and PrEP health promotion could be increased in these areas to help achieve elimination of HIV transmission.
Supplementary material
Supplementary material is available online.
Data availability
On-provision of the MedicineInsight data (https://www.nps.org.au/medicine-insight) used in this study is not permitted, but organisations may approach NPS MedicineWise to request access to the data, subject to data governance processes and contractual agreements. Data access enquiries can be directed to NPS MedicineWise via DataGovernance@nps.org.au.
Conflicts of interest
KC, AP, DB and SN are all employees of NPS MedicineWise, the custodian of the MedicineInsight data. AG is an Editor of Sexual Health, but was blinded from the peer review process for this paper.
Declaration of funding
The study was funded by the Australian Government Department of Health. The funding body had no role in the design of the study, data collection, analysis or interpretation, nor in writing the manuscript. NM is supported by a NHMRC Fellowship (GNT1158035).
Acknowledgements
We are grateful to the general practices and general practitioners who participate in MedicineInsight and the patients whose de-identified data makes this work possible. We also acknowledge NPS MedicineWise staff, particularly Bronwyn Sim, Lisa Quick and Margaret Wall who contributed to this study.
References
[1] The Australasian Society of HIV, Viral Hepatitis and Sexual Health Medicine. PrEP guidelines update. Prevent HIV by prescribing PrEP. Sydney: ASHM; 2020. Available at https://ashm.org.au/resources/hiv-resources-list/prep-guidelines-2019/ [accessed 7 April 2020][2] Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381 2083–2090.
| Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial.Crossref | GoogleScholarGoogle Scholar | 23769234PubMed |
[3] Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014; 66 340–348.
| HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention.Crossref | GoogleScholarGoogle Scholar | 24784763PubMed |
[4] Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363 2587–2599.
| Preexposure chemoprophylaxis for HIV prevention in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 21091279PubMed |
[5] McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387 53–60.
| Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial.Crossref | GoogleScholarGoogle Scholar | 26364263PubMed |
[6] Grant RM, Mannheimer S, Hughes JP, Hirsch-Moverman Y, Loquere A, Chitwarakorn A, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT Study. Clin Infect Dis 2018; 66 1712–1721.
| Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT Study.Crossref | GoogleScholarGoogle Scholar | 29420695PubMed |
[7] Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373 2237–2246.
| On-demand preexposure prophylaxis in men at high risk for HIV-1 infection.Crossref | GoogleScholarGoogle Scholar | 26624850PubMed |
[8] Molina J-M, Charreau I, Spire B, Cotte L, Chas J, Capitant C, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV 2017; 4 e402–e410.
| Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study.Crossref | GoogleScholarGoogle Scholar | 28747274PubMed |
[9] World Health Organization. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV for men who have sex with men: update to WHO’s recommendation on oral PrEP. Geneva: WHO; 2019. Available at https://apps.who.int/iris/bitstream/handle/10665/325955/WHO-CDS-HIV-19.8-eng.pdf?ua=1 [accessed 7 April 2020]
[10] Kirby Institute. Monitoring HIV pre-exposure prophylaxis in Australia newsletter (Issue 2). Sydney: UNSW Sydney; 2019. Available at https://kirby.unsw.edu.au/sites/default/files/kirby/report/Monitoring-HIV%20pre-exposure-prophylaxis-in-Australia-newsletter_Issue-2.pdf [accessed 7 April 2020]
[11] Grulich AE, Guy R, Amin J, Jin F, Selvey C, Holden J, et al. Population-level effectiveness of rapid, targeted, high-coverage roll-out of HIV pre-exposure prophylaxis in men who have sex with men: the EPIC-NSW prospective cohort study. Lancet HIV 2018; 5 e629–e637.
| Population-level effectiveness of rapid, targeted, high-coverage roll-out of HIV pre-exposure prophylaxis in men who have sex with men: the EPIC-NSW prospective cohort study.Crossref | GoogleScholarGoogle Scholar | 30343026PubMed |
[12] Australian Medicines Handbook. Tenofovir. In ‘Australian Medicines Handbook’. Australian Medicines Handbook: Adelaide, SA, Australia; 2019. Available at https://amhonline.amh.net.au/ [accessed 6 February 2019]
[13] Pharmaceutical Benefits Advisory Committee. Tenofovir with emtricitabine, tablet containing tenofovir disoproxil fumarate 300 mg with emtricitabine 200 mg, TRUVADA. Public Summary Document - July 2017 PBAC Meeting. 2017. Available at http://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2017-07/files/tenofovir-emtricitabine-truvada-psd-july-2017.pdf [accessed 7 April 2020]
[14] Fraser D, Chan C, Vaccher S, Holt M, Prestage G, Zablotska-Manos I, et al. Report on the PrEP in NSW Transition Study, 2018-2020. Sydney: Kirby Institute, UNSW Sydney; 2020. Available at https://kirby.unsw.edu.au/sites/default/files/kirby/report/PrEP-in-NSW-Transition-Study-Report_Kirby-Institute_2020.pdf [accessed 24 February 2021]
[15] Busingye D, Gianacas C, Pollack A, Chidwick K, Merrifield A, Norman S, et al. Data resource profile: MedicineInsight, an Australian national primary health care database. Int J Epidemiol 2019; 48 1741–1741h.
| Data resource profile: MedicineInsight, an Australian national primary health care database.Crossref | GoogleScholarGoogle Scholar | 31292616PubMed |
[16] NPS MedicineWise. General practice insights report July 2017–June 2018. Sydney: NPS MedicineWise; 2019. Available at https://www.nps.org.au/assets/NPS/pdf/General-Practice-Insights-Report_2017-18.pdf [accessed 25 February 2020]
[17] Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4 151ra125
| Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 22972843PubMed |
[18] Australian Bureau of Statistics. The Australian Statistical Geography Standard (ASGS) remoteness structure. 2018. Available at https://www.abs.gov.au/websitedbs/D3310114.nsf/home/remoteness+structure [accessed 23 March 2020]
[19] Australian Bureau of Statistics. Socio-Economic Indexes for Areas. 2018. Available at http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa [accessed 7 November 2019]
[20] Pasipanodya EC, Jain S, Sun X, Blumenthal J, Ellorin E, Corado K, et al. Trajectories and predictors of longitudinal preexposure prophylaxis adherence among men who have sex with men. J Infect Dis 2018; 218 1551–1559.
| Trajectories and predictors of longitudinal preexposure prophylaxis adherence among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 30295803PubMed |
[21] Velloza J, Baeten JM, Haberer J, Ngure K, Irungu E, Mugo NR, et al. Effect of depression on adherence to oral PrEP among men and women in East Africa. J Acquir Immune Defic Syndr 2018; 79 330–338.
| Effect of depression on adherence to oral PrEP among men and women in East Africa.Crossref | GoogleScholarGoogle Scholar | 30063651PubMed |
[22] Monteiro Spindola Marins L, Silva Torres T, Luz PM, Moreira RI, Leite IC, Hoagland B, et al. Factors associated with self-reported adherence to daily oral pre-exposure prophylaxis among men who have sex with man and transgender women: PrEP Brasil study. Int J STD AIDS 2021; 1231–1241.
| Factors associated with self-reported adherence to daily oral pre-exposure prophylaxis among men who have sex with man and transgender women: PrEP Brasil study.Crossref | GoogleScholarGoogle Scholar | 34311605PubMed |
[23] Shuper PA, Joharchi N, Bogoch II, Loutfy M, Crouzat F, El-Helou P, et al. Alcohol consumption, substance use, and depression in relation to HIV pre-exposure prophylaxis (PrEP) nonadherence among gay, bisexual, and other men-who-have-sex-with-men. BMC Public Health 2020; 20 1782
| Alcohol consumption, substance use, and depression in relation to HIV pre-exposure prophylaxis (PrEP) nonadherence among gay, bisexual, and other men-who-have-sex-with-men.Crossref | GoogleScholarGoogle Scholar | 33256651PubMed |
[24] Australian Bureau of Statistics. Same-sex couples in Australia, 2016. 2018. Available at https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/2071.0∼2016∼Main%20Features∼Same-Sex%20Couples∼85 [accessed 24 February 2021]
[25] Callander D, Mooney-Somers J, Keen P, Guy R, Duck T, Bavinton BR, et al. Australian ‘gayborhoods’ and ‘lesborhoods’: a new method for estimating the number and prevalence of adult gay men and lesbian women living in each Australian postcode. Int J Geogr Inf Sci 2020; 34 2160–2176.
| Australian ‘gayborhoods’ and ‘lesborhoods’: a new method for estimating the number and prevalence of adult gay men and lesbian women living in each Australian postcode.Crossref | GoogleScholarGoogle Scholar |
[26] Chan C, Broady T, Bavinton B, Mao L, Bear B, Mackie B, et al. Gay community periodic survey: Sydney 2020. Sydney: UNSW Centre for Social Research in Health, UNSW Sydney; 2020. Available at https://www.arts.unsw.edu.au/csrh/our-projects/gay-community-periodic-surveys [accessed 26 July 2021]
[27] Krakower D, Maloney KM, Powell VE, Levine K, Grasso C, Melbourne K, et al. Patterns and clinical consequences of discontinuing HIV preexposure prophylaxis during primary care. J Int AIDS Soc 2019; 22 e25250
| Patterns and clinical consequences of discontinuing HIV preexposure prophylaxis during primary care.Crossref | GoogleScholarGoogle Scholar | 30768762PubMed |
[28] Medland N, Guy R, Bavinton B, Keen P, Grulich A, Ellard J, et al. Large differences in discontinuation of PBS-subsidised PrEP in Australia: evaluation using national prescription data. Sexual Health 2020; 17 XVII
| Large differences in discontinuation of PBS-subsidised PrEP in Australia: evaluation using national prescription data.Crossref | GoogleScholarGoogle Scholar |
[29] Arnold T, Brinkley-Rubinstein L, Chan PA, Perez-Brumer A, Bologna ES, Beauchamps L, et al. Social, structural, behavioral and clinical factors influencing retention in pre-exposure prophylaxis (PrEP) care in Mississippi. PLoS ONE 2017; 12 e0172354
| Social, structural, behavioral and clinical factors influencing retention in pre-exposure prophylaxis (PrEP) care in Mississippi.Crossref | GoogleScholarGoogle Scholar | 28222118PubMed |
[30] Shover CL, Shoptaw S, Javanbakht M, Lee S-J, Bolan RK, Cunningham NJ, et al. Mind the gaps: prescription coverage and HIV incidence among patients receiving pre-exposure prophylaxis from a large federally qualified health center in Los Angeles, California. AIDS Behav 2019; 23 2730–2740.
| Mind the gaps: prescription coverage and HIV incidence among patients receiving pre-exposure prophylaxis from a large federally qualified health center in Los Angeles, California.Crossref | GoogleScholarGoogle Scholar | 30953305PubMed |
[31] Bavinton BR, Vaccher S, Jin F, Prestage GP, Holt M, Zablotska-Manos IB, et al. High levels of prevention-effective adherence to HIV PrEP: an analysis of substudy data from the EPIC-NSW Trial. J Acquir Immune Defic Syndr 2021; 87 1040–1047.
| High levels of prevention-effective adherence to HIV PrEP: an analysis of substudy data from the EPIC-NSW Trial.Crossref | GoogleScholarGoogle Scholar | 33852503PubMed |
[32] Elsesser SA, Oldenburg CE, Biello KB, Mimiaga MJ, Safren SA, Egan JE, et al. Seasons of risk: anticipated behavior on vacation and interest in episodic antiretroviral pre-exposure prophylaxis (PrEP) among a large national sample of U.S. men who have sex with men (MSM). AIDS Behav 2016; 20 1400–1407.
| Seasons of risk: anticipated behavior on vacation and interest in episodic antiretroviral pre-exposure prophylaxis (PrEP) among a large national sample of U.S. men who have sex with men (MSM).Crossref | GoogleScholarGoogle Scholar | 26538056PubMed |


