Burden of tuberculosis and hepatitis co-infection among people living with HIV in Nepal: a systematic review and meta-analysis
Sulochan GC A B # , Ashok Khanal A C # , Vijay S. GC D * , Suman Bhattarai A C , Suresh Panthee E F , Aashis Khanal C G , Amrit Gaire A , Sagar Poudel A , Rakesh Ghimire A and Sharada P. Wasti H
D * , Suman Bhattarai A C , Suresh Panthee E F , Aashis Khanal C G , Amrit Gaire A , Sagar Poudel A , Rakesh Ghimire A and Sharada P. Wasti H
A Maharajgunj Medical Campus, Institute of Medicine, Tribhuvan University, Kathmandu, Nepal.
B Nepal Pharmacy Students’ Association (NPSA), Kathmandu, Nepal.
C Active Pharmacy Pvt. Ltd., Kathmandu, Nepal.
D Centre for Health Economics, University of York, York, UK.
E Teikyo University Institute of Medical Mycology, Otsuka 359, Hachioji, Tokyo, Japan.
F Sustainable Study and Research Institute, Balaju, Kathmandu-16, Nepal.
G Department of Computer Science, Georgia State University, Atlanta, GA, USA.
H School of Health and Human Sciences, University of Huddersfield, Huddersfield, UK.
Handling Editor: Huachun Zou
Sexual Health 19(5) 406-416 https://doi.org/10.1071/SH21216
Submitted: 28 October 2021 Accepted: 14 May 2022 Published: 23 June 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
People living with HIV (PLHIV) are prone to tuberculosis (TB) and hepatitis co-infections, which cause substantial burden on morbidity and mortality. However, data on the burden of HIV co-infection from a specific low- and middle-income country are limited. To address this gap in evidence, a meta-analysis of published literature and country surveillance report was conducted to estimate the burden of TB, hepatitis B (HBV) and hepatitis C (HCV) co-infection among PLHIV in Nepal. Twenty-three studies, including 5900 PLHIV, were included in the meta-analysis. The pooled prevalence of HIV–TB, HIV–HBV and HIV–HCV co-infection was 19% (95% CI, 10–28%), 3% (2–5%) and 19% (4–33%), respectively. Low CD4 cell count (pooled odds ratio [OR] 4.38, 95% CI 1.11–17.25), smoking (3.07, 1.48–6.37) and alcohol drinking (3.12, 1.52–6.43) were significantly correlated with HIV–TB co-infection. The odds of HCV co-infection was greater in PLHIV, who were male (5.39, 1.54–18.89) and drug users (166.26, 15.94–1734.44). PLHIV who were on antiretroviral therapy had a reduced risk of HCV co-infection (0.49, 0.36–0.66) than the general PLHIV population. The burden of TB and hepatitis co-infection among PLHIV in Nepal was high. Regular screening of PLHIV for co-infections and prompt initiation of treatment are essential to reduce the transmission of infection and improve quality of life.
Keywords: co-infection, hepatitis, HIV, meta-analysis, Nepal, prevalence, systematic review, tuberculosis.
Introduction
Human immunodeficiency virus (HIV) continues to be a significant global public health issue, with an estimated 38 million people living with HIV (PLHIV) in 2019.1 In recent years, due to the improved effectiveness of and increased access to antiretroviral therapy (ART), PLHIV are living longer and healthy lives than ever before.2,3 Despite such progress and global attempts to implement treatment-as-prevention programs every year,4 a significant proportion of PLHIV continue to die from HIV-related co-infections.5 Tuberculosis (TB) remains the most common opportunistic disease and cause of premature death among HIV infected individuals, with an estimated 208 000 deaths globally in 2019.6,7 Since HIV weakens the immune system, PLHIV are at least 20 times more likely to develop TB than people without HIV.8 Among HIV infected individuals, hepatitis B virus (HBV) and hepatitis C virus (HCV) co-infections are not uncommon due to the shared risk of transmission. The global prevalence rates of HCV and HBV co-infections among PLHIV are estimated to be 2.4% and 7.6% respectively,9,10 however this may still be underestimated.11
Although considerable progress in addressing HIV–TB, HIV–HBV and HIV–HCV co-infections have been made by developed nations, the majority of the low- and middle-income countries (LMICs) have not achieved the global targets. LMICs are still facing an overwhelming burden of the HIV epidemic in terms of an increasing number of people living with HIV/AIDS, attributed in part to minimal access to treatment and services availability.12,13
In 2019, the prevalence of HIV was estimated at over 29 000 in Nepal, with a concentrated epidemic in specific sub-populations; people who inject drugs (PWID), men who have sex with men, transgender people, male and female sex workers, and male labour migrants as well as their spouses.14 In 2017, Nepal’s national HIV program implemented the ‘test and treat’ policy which provided ART to all PLHIV regardless of the CD4 cell counts. In line with the World Health Organization’s (WHO) recommendations, all patients with advanced HIV disease in Nepal are offered a package of interventions including screening, treatment and/or prophylaxis for major opportunistic infections, ART and adherence support. Following the national HIV testing and treatment guideline,15 PLHIV with TB are immediately treated for TB, followed by ART as soon as possible. Among PLHIV with HCV, treating both HIV and HCV infections is a priority. However, clinical stabilisation of HIV with ART is advisable before initiating HCV treatment among those with HCV mono-infection. The national treatment protocol recommends treatment of HIV/HBV co-infection with tenofovir disoproxil fumarate with lamivudine or emtricitabine.
The second edition of Nepal’s National HIV Strategic Plan (NHSP) 2016–2021 is entirely aligned with the global commitment of test and treat approach 90–90–90. Subsequently, in line with national commitment and NHSP, Nepal has made substantial progress in reducing HIV, TB and hepatitis infection as part of the Sustainable Development Goals in recent years.16 Despite these significant signs of progress, the global target of 90–90–90 is still far from being achieved since infections with TB, HBV and HCV are now emerging as an increasing cause of morbidity and mortality in HIV infected persons, more specifically in resource-limited settings like Nepal. TB is one of the leading causes of death among PLHIV in Nepal, accounting for 23% of total HIV-related deaths in 2020.17 Likewise, HCV, along with HBV, is considered a growing public health problem in the south-east Asia region,18 and Nepal is not an exception; where in 2016, around 130 000 individuals were infected by HCV.19 The convergence of these infectious diseases poses a significant burden to public health and healthcare systems, particularly in a low-resource nation like Nepal.
Furthermore, there is a need to establish a comprehensive understanding of the national burden of TB and hepatitis co-infection among PLHIV and inform national screening programs and clinical management. Therefore, we undertook this review to provide an overall prevalence of HIV–TB, HIV–HBV and HIV–HCV co-infections and associated risk factors in Nepal.
Materials and methods
This review was conducted and reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure the search process’s quality and adequate reporting.20
Data sources and searches
We searched for articles published from inception to November 2020 using the electronic database PubMed, EMBASE, AMED, MEDLINE (Ovid), Cochrane CENTRAL, PsychINFO, and Nepal Journal Online (NepJOL). After reviewing the titles and abstracts, the reference list of included studies was examined manually to identify further eligible studies. Additionally, free text searching was performed using Google scholar. The search comprised of a combination of keywords HIV (‘human immunodeficiency virus’, or ‘HIV’), co-infection (‘tuberculosis’, ‘TB’, ‘hepatitis B’, ‘HBV’, ‘hepatitis C’, ‘HCV’, ‘co-infection’, or ‘opportunistic infection’) and ‘Nepal’ (see Supplementary material, Appendix A for detailed search strategy).
Study selection
Following the database search and removal of duplicate records, three authors independently screened titles and abstracts for inclusion. We included observational studies that reported estimates of (or sufficient information to derive) the prevalence of TB or hepatitis B or/and hepatitis C among HIV positive individuals. Included studies were limited to primary research reports and those conducted in Nepal. We excluded studies that: (1) purposively selected PLHIV with TB or hepatitis co-infection; (2) did not report TB or hepatitis seroprevalence; (3) did not mention the TB, HBV or HCV diagnostic assays used; or (4) were conferences reports, research letters, editorials, or commentaries.
A positive TB case was defined by a positive result of Acid–Fast Bacillus stained smear or clinical or radiological traits (chest X-ray) suggestive of TB. HBV infection was defined by a positive result of HBV infection markers: hepatitis B surface antigen (HBsAg), hepatitis B e antigen, anti-hepatitis B surface antibody, and anti-hepatitis B core antibody as confirmed by enzyme-linked immunosorbent assay (ELISA) or enzyme immunoassay (EIA). HCV infection was defined by a positive result of the anti-HCV Ab test and confirmed by ELISA or EIA.
Studies identified as potentially eligible or those without an abstract had their full text retrieved, and full texts of the studies were assessed by two reviewers independently. Any discrepancies were resolved through discussion and in consultation with a third reviewer. In some cases, one study resulted in multiple publications. In such a case, we included the most recently published paper with the complete data.
Assessment of methodological quality
The methodological quality of included studies was assessed using an adapted version of the risk of bias tool for prevalence studies, developed by Hoy et al.21 independently by two reviewers. This tool was based on 10 criteria, and each criterion was worth one point; for each item, score 1 indicates low risk, and score 0 shows high risk. Based on the number of Hoy et al. criteria met, studies were categorised into high (0–5), moderate (6–8) or low (9–10) risk of methodological bias (see Appendix B, Supplementary Table S1). A third reviewer compared the assessment and highlighted the disagreements between two reviewers, which were resolved through discussion between the three reviewers. All studies, regardless of their methodological quality, were included. Nineteen studies had a moderate risk of bias (score of 6–8), and four studies had a low risk of bias (score of 9–10).
Data extraction
Using a standardised pro forma, two reviewers extracted data from the included studies. A third reviewer checked the data extraction and highlighted the disagreement between the two reviewers. Any such discrepancies were resolved through discussion between the three reviewers. Data extraction included details of the study such as the first author’s name, the year of publication, information on study type, population sampled, study period, sample size, type of co-infection (TB, HBV or HCV), outcome (prevalence rate), study results for the outcomes of interest (adjusted or unadjusted odds ratios [ORs], raw data) along with associated risk factors of co-infection(s). We chose to use unadjusted ORs preferentially if these data were available.
Data synthesis and statistical analysis
We used a random-effects model to estimate pooled prevalence rate with 95% confidence intervals (CIs).22 The Mantel–Haenszel random-effects model was used to estimate the summary odds ratio and 95% CIs from the included studies. I2 statistics of >50% and Q chi-squared test ≤0.10 were employed to assess the heterogeneity between the studies. The effect sizes of risk factors composed of heterogeneous studies were calculated using the random-effects model. The effect sizes of non-heterogeneous studies were estimated using the fixed-effects model.23 At least two eligible studies per risk factor were needed for the risk factor meta-analysis.
Estimations of publication bias were examined by Egger’s weighted regression method and funnel plot.24 Asymmetry of funnel plot and a P-value < 0.05 was considered indicative of statistically significant publication bias. All analyses were performed with the meta package25 of R statistical software ver. 4.0.2.26 Prevalence rates were reported with the corresponding 95% CI. We performed sensitivity analyses comparing the data from studies with the methodological quality score to assess the robustness of crude findings. Forest plots were used to assess publication bias. Where a significant association was observed, sensitivity analysis was performed to assess the robustness of the result. For this at least two eligible studies were needed.
To estimate the burden, the number of TB, HBV and HCV infections in PLHIV, we used the 2020 data from the UNAIDS27 and the National Centre for AIDS and STD Control (NCASC) Nepal,17 which gives the number of PLHIV.
Results
Search results
The literature search identified 868 potentially relevant records, with an additional 140 records identified through other sources. After removing the duplicates, 356 studies were screened by titles and abstracts and 103 full-text studies were reviewed, with 23 articles included (Fig. 1).
Characteristics of included studies
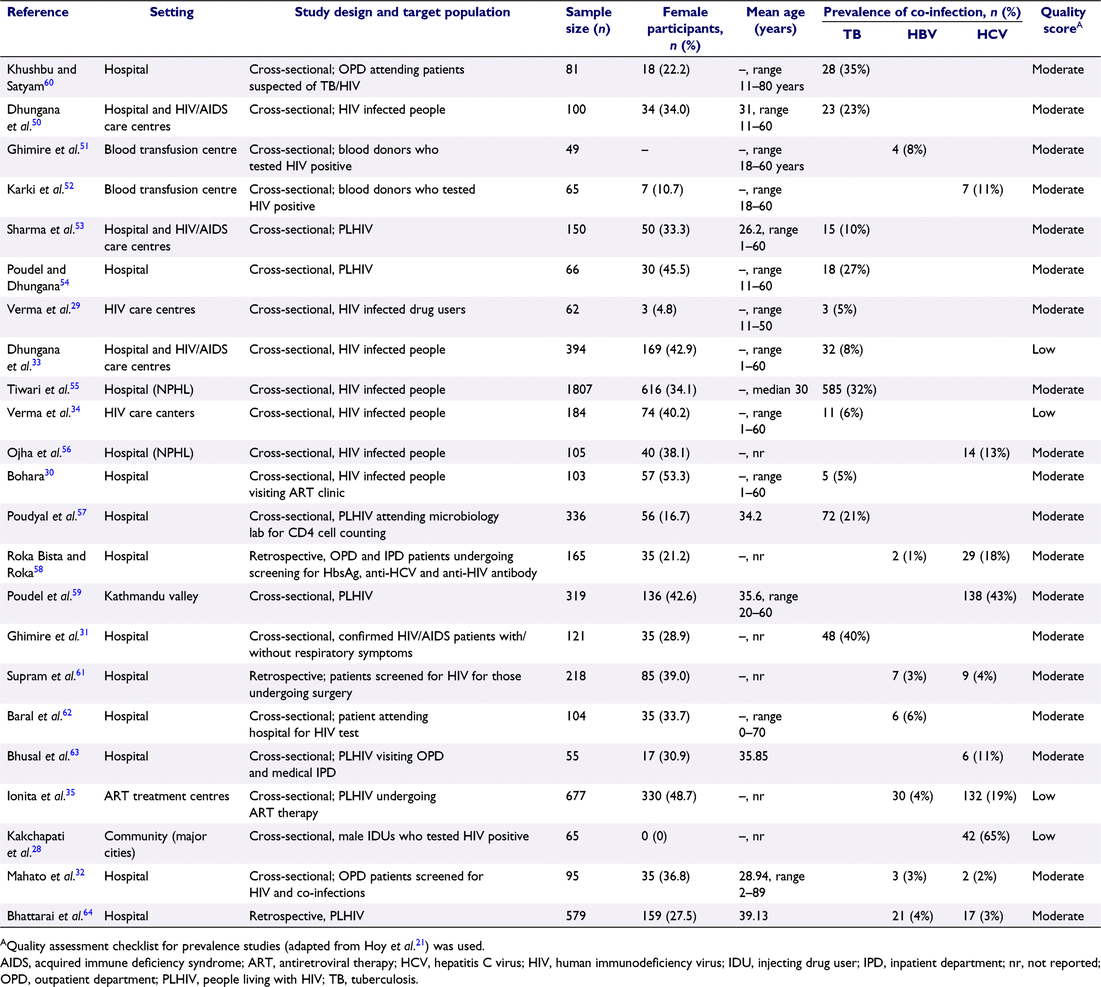
Of the 23 studies, 11 reported TB, 11 were HBV and/or HCV, and one study reported both TB and HCV co-infection in PLHIV. The number of study participants ranged from 49 to 1807 (Table 1). One study included only male participants.28 The proportion of female participants in 22 studies ranged from 4.8% to 53.3%. Of the 23 studies, 20 were cross-sectional studies, and three were retrospective in design. There were 5900 study participants; 3404 with HIV–TB infection, 1887 with HIV–HBV infection and 2343 HIV–HCV infections (Table 1).
|
Prevalence of co-infections in PLHIV
The prevalence of HIV–TB co-infection ranged from 5%29,30 to 40%,31 and pooled prevalence was 19% (95% CI: 10–28%) across 11 included studies. The pooled prevalence of HIV–HBV co-infection in seven studies was 3% (95% CI: 2–5%). The prevalence of HIV–HCV co-infection ranged between 2%32 and 65%,28 and pooled prevalence was 19% (95% CI, 4–33%). Heterogeneity between studies reporting the prevalence of HIV–TB (I2 = 97%, P < 0.01) and HIV–HCV co-infections (I2 = 98%, P < 0.01) was high. While low heterogeneity between studies reporting HIV–HBV co-infection (I2 = 45%, P < 0.1) was observed (Fig. 2).
|
|
Estimates of national cases of TB, HBV and HCV infection in PLHIV
Using our pooled prevalence of TB, HBV and HCV infection in PLHIV and the data on the estimated number of PLHIV in Nepal from the UNAIDS and NCASC reports,17,27 we estimated that there were 5700 (95% CI, 3000–8400) cases of TB, 900 (95% CI, 600–1500) cases of HBV and 5700 (95% CI, 1200–9900) cases of HCV in Nepal.
Co-infection risk factors
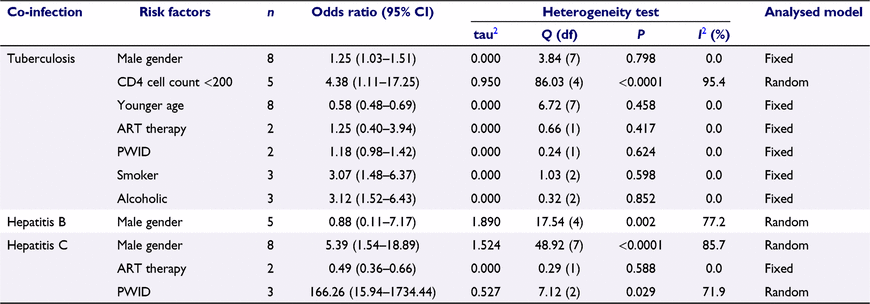
We estimate the pooled OR to examine the association of risk factors with co-infections (Table 2). The risk factors that had a significant association with HIV–TB co-infection were a male gender (pooled OR 1.25, 95% CI: 1.03–1.51), younger age (<30 years old) (OR 0.58, 95% CI: 0.48–0.69), CD4 T-lymphocytes count <200 cells/μ (OR 4.38, 95% CI: 1.11–17.25), smoker (OR 3.07, 95% CI: 1.48–6.37) and alcohol drinker (OR 3.12, 95% CI: 1.52–6.43).
|
However, the male gender was not significantly associated with the HIV–HBV co-infection (OR 0.88, 95% CI: 0.11–7.17). The risk factors that had a significant association with HCV co-infection were male gender (OR 5.39, 95% CI: 1.54–18.89), people who inject drug (OR 166.26, 95% CI: 15.94–1734.44) and taking ART (OR 0.49, 95% CI: 0.36–0.66). We observed greater heterogeneity in some risk factors for HBV and HCV co-infection (Table 2).
Evaluation of publication bias
We generated funnel plots to assess publication bias of the prevalence rate. For the overall prevalence of HIV–TB and HIV–HBV prevalence rates, the asymmetry observed in the funnel plot was minimal (Supplementary material, Appendix C, Fig. S1). We also assessed funnel plot asymmetry using the Egger’s linear regression test. Looking at the funnel plot of HIV–TB prevalence (Supplementary material, Fig. S1), there was a slight evidence of publication bias in terms of smaller studies with minor effect sizes missing at the bottom left corner. Furthermore, Egger’s regression test for publication bias for HIV–TB was non-significant (z = −0.9612, P = 0.827) indicating no evidence of publication bias. No publication bias was obverted in the prevalence estimates for HIV–HBV (z = 1.111, P = 0.402). However, publication bias was observed in the estimates of HIV–HCV prevalence rates (z = 7.572, P = 0.029).
Sensitivity analyses
We performed sensitivity analyses of the co-infection prevalence rates by applying a fixed-effects model, and we found similar prevalence rates between random-effects and fixed-effect models in the overall analysis. We also assessed the prevalence rates by methodological quality. Among the 19 studies with moderate risk of bias (score of 6–8), the pooled prevalence rate of HIV–TB co-infection (22%, 95% CI: 12–32%) was higher, and the prevalence rate of HIV–HCV was low (13%, 95% CI: 2–24%). However, the HIV–HBV prevalence rate was similar (3%, 95% CI: 1–5%). The remaining four studies28,33–35 with a low risk of methodological bias (score >8), had a higher prevalence rate of HIV–HCV (42%, 95% CI: 0–100%), lower rate of HIV–TB (7%, 95% CI: 0–21%), and similar rates for HIV–HBV (4%, 95% CI: 3–6%) co-infection (Supplementary material, Appendix C, Figs S2–S4).
Discussion
Overall, our analysis revealed that the prevalence of HIV–HCV co-infection was more frequent than but not significantly different from HIV–TB co-infection, suggesting that HIV patients appeared to be at greater risk for both HCV and TB infection in Nepal. The prevalence of HIV–TB co-infection (19%) was considerably higher than the 2018 Nepal TB HIV Sentinel Survey finding, i.e. 9.9%.36 Likewise, our estimates of HIV–HCV prevalence (19%) was higher than the UNAIDS estimates for Nepal (7.4%)37 and was about five times higher than the HIV–HCV prevalence reported in other South Asian countries.38 The studies included in this review were primarily conducted in the (tertiary) hospitals, partly explaining the higher prevalence rates. However, the pooled prevalence of HBV infection among PLHIV (3%, 95% CI 2–5%) is significantly lower than the prevalence rate (8.4%) reported by Leumi et al.39 in the WHO south-east Asia region.
Our findings of the significant risk factor of HIV–TB co-infection (being a male, younger adult, CD4 value of <200, tobacco smokers, and alcohol drinkers) and HIV–HCV corroborate previously published evidence that low CD4 cell count and PWID are significantly associated with the development and severity of TB40,41 and HCV10 respectively. The odds of HCV co-infection among PWIDs were higher (175, 50–611) than Platt et al.’s10 study. In their global systematic review, Platt et al. reported lower odds (6.0, 95% CI 4.2–8.7) of HIV–HCV co-infection among PWID. This considerable variation is likely to be due to the small number of studies included in our analysis. The shared transmission routes of both HIV and HCV viruses, unsafe injecting behaviours, larger numbers of injecting partners are believed to be the most common factors that place PWIDs at such an immense risk for HCV transmission.42
We found that the odds of HCV co-infection decreased almost half for those PLHIV on ART, suggesting ART could be beneficial to lower the threat posed by HCV among PLHIV. However, for that to happen, ART has to be started before HCV co-infection since existing co-infection can complicate ART delivery by increasing the risk of drug-induced hepatoxicity and thus influencing the selection of drugs acting dually against HIV and HCV infection.43 Substance use such as drugs, alcohol consumption and cigarette smoking were associated with TB infection among PLHIV, consistent with previous studies conducted in Ethiopia and South India.44,45 In line with previous studies,46–48 in our study, the male gender was a significant determinant of HIV–TB and HIV–Hepatitis C infection relative to females. Surprisingly, we found a higher prevalence of HBV infection in females than in the general PLHIV population, which contradicts the previously reported study.49
To the best of our knowledge, this was the first systematic review and meta-analysis to synthesise the existing evidence on the prevalence and risk factors of TB and Hepatitis (HBV or HCV) co-infection among HIV-infected people in Nepal. Key strengths of our review are the comprehensive search of published literature, including the NepJOL, and the inclusion of common co-infections in PLHIV. Despite this, some limitations do exist in our study. The main limitation of this study was the considerable heterogeneity in the studies in terms of study design, population sampling approach and data collection methods. The quality of studies was also variable, and most studies were of moderate to high risk of bias. Second, due to limited studies, the effect sizes could not be calculated for all risk factors, and the pooled ORs had wide CIs. We only included risk factors that are reported in two or more studies. Further, well-designed population-based studies examining HIV and co-infections would provide better estimates in order to delineate the additive burden, contribution on mortality, early diagnosis and management. Nevertheless, reporting the burden of TB, HBV and HCV co-infection among PLHIV in Nepal is critical in developing strategies to overcome the overall burden posed by HIV.
In this meta-analysis, we found relatively higher TB and HCV infections among PLHIV in Nepal. Preventive interventions such as risk-stratified screening, testing and treating and behavioural interventions are needed for TB and hepatitis control efforts. Besides, strengthening health systems to promote regular ART and integrating TB, hepatitis and HIV prevention, diagnosis and treatment services at a single site would help reduce the burden of TB and hepatitis infection among PLHIV and improve quality of life.
Supplementary material
Supplementary material is available online.
Data availability
All data generated or analysed during this study are included in this article and its supplementary materials.
Conflicts of interest
The authors declare no conflicts of interest.
Declarartion of funding
This study had no specific funding.
References
[1] UNAIDS. Global HIV & AIDS statistics – 2020 fact sheet; 2020. Available at https://www.unaids.org/en/resources/fact-sheet[2] Gulick RM. Antiretroviral treatment 2010: progress and controversies. J Acquir Immune Defic Syndr 2010; 55 S43–8.
| Antiretroviral treatment 2010: progress and controversies.Crossref | GoogleScholarGoogle Scholar | 21045599PubMed |
[3] Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med 2017; 18 256–66.
| Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 27578404PubMed |
[4] Hull M, Lange J, Montaner JSG. Treatment as prevention—where next? Curr HIV/AIDS Rep 2014; 11 496–504.
| Treatment as prevention—where next?Crossref | GoogleScholarGoogle Scholar | 25384357PubMed |
[5] Croxford S, Kitching A, Desai S, Kall M, Edelstein M, Skingsley A, et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health 2017; 2 e35–46.
| Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort.Crossref | GoogleScholarGoogle Scholar | 29249478PubMed |
[6] Swaminathan S, Narendran G. HIV and tuberculosis in India. J Biosci 2008; 33 527–37.
| HIV and tuberculosis in India.Crossref | GoogleScholarGoogle Scholar | 19208978PubMed |
[7] World Health Organization. Global Tuberculosis Report 2020. Geneva; 2020. Available at https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf
[8] UNAIDS. Tuberculosis and HIV; 2020. Available at https://www.unaids.org/en/resources/infographics/tuberculosis-and-hiv
[9] Platt L, French CE, McGowan CR, Sabin K, Gower E, Trickey A, et al. Prevalence and burden of HBV co-infection among people living with HIV: a global systematic review and meta-analysis. J Viral Hepat 2020; 27 294–315.
| Prevalence and burden of HBV co-infection among people living with HIV: a global systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 31603999PubMed |
[10] Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16 797–808.
| Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 26922272PubMed |
[11] Taaffe J, Wilson D. Mobilising a global response to hepatitis: lessons learned from the HIV movement. Glob Public Health 2018; 13 473–88.
| Mobilising a global response to hepatitis: lessons learned from the HIV movement.Crossref | GoogleScholarGoogle Scholar | 27748158PubMed |
[12] The World Bank. World Bank and WHO: half the world lacks access to essential health services, 100 million still pushed into extreme poverty because of health expenses; 2017. Available at https://www.worldbank.org/en/news/press-release/2017/12/13/world-bank-who-half-world-lacks-access-to-essential-health-services-100-million-still-pushed-into-extreme-poverty-because-of-health-expenses.
[13] Rewari BB, Kumar A, Mandal PP, Puri AK. HIV TB coinfection – perspectives from India. Expert Rev Respir Med 2021; 15 911–30.
| HIV TB coinfection – perspectives from India.Crossref | GoogleScholarGoogle Scholar | 33900861PubMed |
[14] UNAIDS. Global AIDS monitoring 2020: country progress report – Nepal; 2020. Available at https://www.unaids.org/sites/default/files/country/documents/NPL_2020_countryreport.pdf
[15] National Centre for AIDS and STD Control. National HIV testing and treatment guidelines. Kathmandu; 2020. Available at http://www.ncasc.gov.np/uploaded/Banner/National-HIV-Testing-Guidelines-May-10-2020-WEB-Version.pdf
[16] UN. The sustainable development goals report 2019; 2019. Available at https://unstats.un.org/sdgs/report/2019/The-Sustainable-Development-Goals-Report-2019.pdf
[17] NCASC. HIV epidemic update of Nepal 2020: National Centre for AIDS and STD Control; 2020. Available at http://ncasc.gov.np/WAD2020/Factsheet-2020-S.pdf
[18] World Health Organization. Viral hepatitis in the WHO South-East Asia region; 2011. Available at https://apps.who.int/iris/bitstream/handle/10665/206521/B4752.pdf
[19] Shrestha A. Viral hepatitis in Nepal: past, present, and future. Euroasian J Hepatogastroenterol 2016; 6 59–61.
| Viral hepatitis in Nepal: past, present, and future.Crossref | GoogleScholarGoogle Scholar | 29201728PubMed |
[20] Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8 336–41.
| Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.Crossref | GoogleScholarGoogle Scholar | 20171303PubMed |
[21] Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012; 65 934–9.
| Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement.Crossref | GoogleScholarGoogle Scholar | 22742910PubMed |
[22] DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7 177–88.
| Meta-analysis in clinical trials.Crossref | GoogleScholarGoogle Scholar | 3802833PubMed |
[23] Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019.
[24] Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315 629–34.
| Bias in meta-analysis detected by a simple, graphical test.Crossref | GoogleScholarGoogle Scholar | 9310563PubMed |
[25] Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22 153–60.
| How to perform a meta-analysis with R: a practical tutorial.Crossref | GoogleScholarGoogle Scholar | 31563865PubMed |
[26] R Core Team. R: a language and environment for statistical computing. 4.0.2 ed. Vienna: R Foundation for Statistical Computing; 2020.
[27] UNAIDS. Country factsheets: Nepal; 2020. Available at https://www.unaids.org/en/regionscountries/countries/nepal
[28] Kakchapati S, Maharjan M, Rawal BB, Dixit SM. Social determinants and risk behaviors associated with prevalent hepatitis C and HIV/HCV co-infection among male injection drug users in Nepal. Arch Public Health 2017; 75 39
| Social determinants and risk behaviors associated with prevalent hepatitis C and HIV/HCV co-infection among male injection drug users in Nepal.Crossref | GoogleScholarGoogle Scholar | 28878895PubMed |
[29] Verma SC, Dhungana GP, Joshi HS, Kunwar HB, Jha RK, Pokhrel AK. Prevalence of pulmonary tuberculosis among HIV infected drug users in Pokhara, Kaski, Nepal. SAARC J Tuber Lung Dis HIV/AIDS 2010; 7 19–25.
| Prevalence of pulmonary tuberculosis among HIV infected drug users in Pokhara, Kaski, Nepal.Crossref | GoogleScholarGoogle Scholar |
[30] Bohara MS. Pulmonary tuberculosis and immunological profile of HIV/AIDS patients in Far West Nepal. J Kathmandu Med College 2014; 3 8–13.
| Pulmonary tuberculosis and immunological profile of HIV/AIDS patients in Far West Nepal.Crossref | GoogleScholarGoogle Scholar |
[31] Ghimire P, Dhungana GR, Bam DS, Rijal BP.. Tuberculosis and HIV co-infection status in United Mission Hospital, Tansen, Western Nepal. SAARC J Tuber Lung Dis HIV/AIDS 2004; 1 32–8.
[32] Mahato S, Mahato A, Yadav J. Prevalence of HIV, HBV and HCV and their co-infection during primary investigation and before ART in Eastern Region of Nepal. IJAMBR 2017; 5 123–31.
| Prevalence of HIV, HBV and HCV and their co-infection during primary investigation and before ART in Eastern Region of Nepal.Crossref | GoogleScholarGoogle Scholar |
[33] Dhungana GP, Sharma S, Khadga P, Verma SC. Surveillance of tuberculosis among HIV infected persons in three different regions of Nepal. Nepal Med Coll J 2013; 15 113–6.
| 24696929PubMed |
[34] Verma SC, Dhungana GP, Joshi HS, Kunwar HB, Pokhrel AK. Prevalence of pulmonary tuberculosis among HIV infected persons in Pokhara, Nepal. J Nepal Health Res Counc 2012; 10 32–6.
| 22929634PubMed |
[35] Ionita G, Malviya A, Rajbhandari R, Schluter WW, Sharma G, Kakchapati S, et al. Seroprevalence of hepatitis B virus and hepatitis C virus co-infection among people living with HIV/AIDS visiting antiretroviral therapy centres in Nepal: a first nationally representative study. Int J Infect Dis 2017; 60 64–9.
| Seroprevalence of hepatitis B virus and hepatitis C virus co-infection among people living with HIV/AIDS visiting antiretroviral therapy centres in Nepal: a first nationally representative study.Crossref | GoogleScholarGoogle Scholar | 28483724PubMed |
[36] National Tuberculosis Center. National Tuberculosis Program Nepal: Annual Report 2074/75 (2018). Kathmandu; 2018. Available at https://bnmtnepal.org.np/wp-content/uploads/2019/11/NTP-Annual-Report-2074-75-Up.pdf
[37] UNAIDS. Country factsheets Nepal. Joint United Nations Programme on HIV/AIDS; 2020. Available at https://www.unaids.org/en/regionscountries/countries/nepal
[38] Martinello M, Amin J, Matthews GV, Dore GJ. Prevalence and disease burden of HCV coinfection in HIV cohorts in the Asia Pacific region: a systematic review and meta-analysis. AIDS Rev 2016; 18 68–80.
| 27196354PubMed |
[39] Leumi S, Bigna JJ, Amougou MA, Ngouo A, Nyaga UF, Noubiap JJ. Global burden of hepatitis B infection in people living with human immunodeficiency virus: a systematic review and meta-analysis. Clin Infect Dis 2020; 71 2799–806.
| Global burden of hepatitis B infection in people living with human immunodeficiency virus: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 31813969PubMed |
[40] Jones BE, Young SMM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis 1993; 148 1292–7.
| Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection.Crossref | GoogleScholarGoogle Scholar | 7902049PubMed |
[41] Jaryal A, Raina R, Sarkar M, Sharma A. Manifestations of tuberculosis in HIV/AIDS patients and its relationship with CD4 count. Lung India 2011; 28 263–6.
| Manifestations of tuberculosis in HIV/AIDS patients and its relationship with CD4 count.Crossref | GoogleScholarGoogle Scholar | 22084539PubMed |
[42] Grassi A, Ballardini G. Hepatitis C in injection drug users: it is time to treat. World J Gastroenterol 2017; 23 3569–71.
| Hepatitis C in injection drug users: it is time to treat.Crossref | GoogleScholarGoogle Scholar | 28611509PubMed |
[43] Kumar R, Singla V, Kacharya Sk. Impact and management of hepatitis B and hepatitis C virus co-infection in HIV patients. Trop Gastroenterol 2008; 29 136–47.
| 19115605PubMed |
[44] Hiregoudar V, Raghavendra B, Karinagannavar A, Khan W, Kamble S, Goud TG. Proportion and determinants of tuberculosis among human immunodeficiency virus-positive patients attending the antiretroviral therapy center attached to a Medical College in South India. J Family Community Med 2016; 23 88–93.
| Proportion and determinants of tuberculosis among human immunodeficiency virus-positive patients attending the antiretroviral therapy center attached to a Medical College in South India.Crossref | GoogleScholarGoogle Scholar | 27186154PubMed |
[45] Dalbo M, Tamiso A. Incidence and predictors of tuberculosis among HIV/AIDS infected patients: a five-year retrospective follow-up study. Adv Infect Dis 2016; 6 70
| Incidence and predictors of tuberculosis among HIV/AIDS infected patients: a five-year retrospective follow-up study.Crossref | GoogleScholarGoogle Scholar |
[46] Alemu YM, Awoke W, Wilder-Smith A. Determinants for tuberculosis in HIV-infected adults in Northwest Ethiopia: a multicentre case-control study. BMJ Open 2016; 6 e009058
| Determinants for tuberculosis in HIV-infected adults in Northwest Ethiopia: a multicentre case-control study.Crossref | GoogleScholarGoogle Scholar | 27084271PubMed |
[47] Choy CY, Ang LW, Ng OT, Leo YS, Wong CS. Factors associated with hepatitis B and c co-infection among HIV-infected patients in Singapore, 2006–2017. Trop Med Infect Dis 2019; 4
| Factors associated with hepatitis B and c co-infection among HIV-infected patients in Singapore, 2006–2017.Crossref | GoogleScholarGoogle Scholar | 31137801PubMed |
[48] Méda ZC, Sombié I, Sanon OWC, Maré D, Morisky DE, Chen Y-MA. Risk factors of tuberculosis infection among HIV/AIDS patients in Burkina Faso. AIDS Res Hum Retroviruses 2013; 29 1045–55.
| Risk factors of tuberculosis infection among HIV/AIDS patients in Burkina Faso.Crossref | GoogleScholarGoogle Scholar | 23517547PubMed |
[49] Chen M, Wong WW, Law MG, Kiertiburanakul S, Yunihastuti E, Merati TP, et al. Hepatitis B and C co-Infection in HIV patients from the TREAT Asia HIV observational database: analysis of risk factors and survival. PLoS One 2016; 11 e0150512
| Hepatitis B and C co-Infection in HIV patients from the TREAT Asia HIV observational database: analysis of risk factors and survival.Crossref | GoogleScholarGoogle Scholar | 26933963PubMed |
[50] Dhungana GP, Ghimire P, Sharma S, Rijal BP. Characterization of mycobacteria in HIV/AIDS patients of Nepal. J Nepal Med Assoc 2008; 47 18–23.
[51] Ghimire P, Thapa D, Rajkarnikar M, Tiwari BR. HIV and Hepatitis B co-infection among volunteer blood donors. J Nepal Health Res Counc 2006; 4 26–8.
[52] Karki S, Ghimire P, Tiwari BR, Shrestha AC, Gautam A, Rajkarnikar M. Seroprevalence of HIV and hepatitis C co-infection among blood donors in Kathmandu Valley, Nepal. Southeast Asian J Trop Med Public Health 2009; 40 66–70.
| 19323036PubMed |
[53] Sharma S, Dhungana GP, Pokhrel BM, Rijal BP. Opportunistic infections in relation to CD4 level among HIV seropositive patients from central Nepal. Nepal Med Coll J 2010; 12 1–4.
| 20677600PubMed |
[54] Poudel BN, Dhungana GP. Scenario of HIV/AIDS patients in a government hospital of Nepal. J Nepal Health Res Counc 2010; 8 103–106.
| 21876573PubMed |
[55] Tiwari BR, Karki S, Ghimire P, Sharma B, Malla S. Factors associated with high prevalence of pulmonary tuberculosis in HIV-infected people visiting for assessment of eligibility for highly active antiretroviral therapy in Kathmandu, Nepal. WHO South East Asia J Public Health 2012; 1 404–11.
| Factors associated with high prevalence of pulmonary tuberculosis in HIV-infected people visiting for assessment of eligibility for highly active antiretroviral therapy in Kathmandu, Nepal.Crossref | GoogleScholarGoogle Scholar | 28615605PubMed |
[56] Ojha CR, KC K, Shakya G. Co-infection of hepatitis C among HIV-infected population with different risk groups in Kathmandu, Nepal. Biomed Res 2013; 24 441–4.
[57] Poudyal N, Gyawali N, Nepal HP, Gurung R, Pandey S, Amatya R, et al. Risk for developing tuberculosis among intravenous drug users with human immunodeficiency virus (HIV) infection. J AIDS HIV Res 2014; 6 104–8.
| Risk for developing tuberculosis among intravenous drug users with human immunodeficiency virus (HIV) infection.Crossref | GoogleScholarGoogle Scholar |
[58] Roka Bista P, Roka K. Seroprevalence of human immunodeficiency virus, hepatitis B surface antigen and hepatitis C antibody in Bir Hospital based population, Kathmandu, Nepal. J NAMS 2014; 14 11–17.
[59] Poudel KC, Palmer PH, Jimba M, Mizoue T, Kobayashi J, Poudel-Tandukar K. Coinfection with hepatitis C virus among HIV-positive people in the Kathmandu Valley, Nepal. J Int Assoc Provid AIDS Care 2014; 13 277–83.
| Coinfection with hepatitis C virus among HIV-positive people in the Kathmandu Valley, Nepal.Crossref | GoogleScholarGoogle Scholar | 24056798PubMed |
[60] Khushbu Y, Satyam P.. Bacteriological profile of lower respiratory tract infection (LRTI) among HIV seropositive cases in central Terai of Nepal. Int J Curr Microbiol App Sci 2015; 4 431–42.
[61] Supram HS, Gokhale S, Sathian B, Bhatta DR. Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) co-infection among HIV infected individuals at tertiary care hospital in western Nepal. Nepal J Epidemiol 2015; 5 488–93.
| Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) co-infection among HIV infected individuals at tertiary care hospital in western Nepal.Crossref | GoogleScholarGoogle Scholar | 26913208PubMed |
[62] Baral SK, Sherchand JB, Parajuli K, Pokhrel BM, Kattel HP, Shrestha D. Human immuno-deficiency virus co-infection with hepatitis B virus and baseline CD4+ T cell count among patients attending a tertiary care hospital, Nepal. J Med Clin Res Rev 2017; 1 1–6.
| Human immuno-deficiency virus co-infection with hepatitis B virus and baseline CD4+ T cell count among patients attending a tertiary care hospital, Nepal.Crossref | GoogleScholarGoogle Scholar |
[63] Bhusal KR, Devkota S, Shrestha M, Khadga P. Profile of Anaemia in HIV positive patients. J Coll Med Sci Nepal 2016; 12 70–3.
| Profile of Anaemia in HIV positive patients.Crossref | GoogleScholarGoogle Scholar |
[64] Bhattarai M, Baniya JB, Aryal N, Shrestha B, Rauniyar R, Adhikari A, et al. Epidemiological profile and risk factors for acquiring HBV and/or HCV in HIV-infected population groups in Nepal. Biomed Res Int 2018; 2018 9241679
| Epidemiological profile and risk factors for acquiring HBV and/or HCV in HIV-infected population groups in Nepal.Crossref | GoogleScholarGoogle Scholar | 29487874PubMed |


