Physical function limitation among gay and bisexual men aged ≥55 years with and without HIV: findings from the Australian Positive and Peers Longevity Evaluation Study (APPLES)
David C. Boettiger A B C * , Md. Hamidul Huque D , Mark Bloch A E , Ian Woolley
A B C * , Md. Hamidul Huque D , Mark Bloch A E , Ian Woolley  F G , David J. Templeton A H I , Matthew G. Law A , Neil Fraser J , Jennifer Hoy F and Kathy Petoumenos A
F G , David J. Templeton A H I , Matthew G. Law A , Neil Fraser J , Jennifer Hoy F and Kathy Petoumenos A
A Kirby Institute, UNSW Sydney, Sydney, NSW, Australia.
B Institute for Health and Aging, University of California, San Francisco, CA, USA.
C Biostatistics Excellence Centre, Faculty of Medicine, Chulalongkorn University, King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
D Neuroscience Research Australia, Sydney, NSW, Australia.
E Holdsworth House Medical Practice, Sydney, NSW, Australia.
F Department of Infectious Diseases, The Alfred Hospital and Monash University, Melbourne, Vic., Australia.
G Monash Infectious Diseases, Monash Health and Centre for Inflammatory Diseases, Monash University, Melbourne, Vic., Australia.
H Department of Sexual Health Medicine, Sydney Local Health District, Sydney, NSW, Australia.
I Discipline of Medicine, Central Clinical School, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia.
J Positive Life NSW, Sydney, NSW, Australia.
Sexual Health 19(6) 533-545 https://doi.org/10.1071/SH22085
Submitted: 20 May 2022 Accepted: 16 August 2022 Published: 12 September 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Background: As people living with HIV now have a life expectancy approaching that of the general population, clinical care focuses increasingly on the management and prevention of comorbidities and conditions associated with aging. We aimed to assess the prevalence of physical function (PF) limitation among gay and bisexual men (GBM) and determine whether HIV is associated with severe PF limitation in this population.
Methods: We analysed cross-sectional data from GBM aged ≥55 years in the Australian Positive and Peers Longevity Evaluation Study who completed a self-administered survey on health and lifestyle factors. PF was measured using the Medical Outcomes Study–Physical Functioning scale. Factors associated with severe PF limitation were assessed using logistic regression.
Results: The survey was completed by 381 men: 186 without HIV and 195 with HIV. Median age was 64.3 years for GBM without HIV and 62.1 years for GBM with HIV. Compared with men without HIV, those with HIV had higher proportions of severe (13.3% vs 8.1%) and moderate-to-severe (26.7% vs 24.2%) PF limitation. Severe PF limitation commonly involved difficulty with vigorous activity (95% with severe PF limitation described being limited a lot), climbing several flights of stairs (68.4% limited a lot), bending, kneeling or stooping (60.5% limited a lot), and walking 1 km (55.0% limited a lot). In a model adjusted for age, body mass index, typical duration of physical activity, psychological distress, and number of comorbidities, we found a significant association between HIV and severe PF limitation (adjusted odds ratio 3.3 vs not having HIV, 95% confidence interval 1.3–8.7).
Conclusions: The biological mechanisms underlying this association require further investigation, particularly given the growing age of the HIV population and inevitable increase in the burden of PF limitation.
Keywords: aging, Australia, gay and bisexual, HIV, men, physical, physical function, physical function limitation.
Introduction
In 2015, the 45 and Up Study reported that 13% of older Australians had severe physical function (PF) limitation.1 PF limitation is a strong predictor of nursing home admission, hospital admission, and death.2 As the Australian population ages,3 measurement and reversal of PF decline have become increasingly important.
PF limitation is similar to, but distinct from, other commonly used terms. The Disablement Model, originally conceptualised by Nagi4 and extended by Verbrugge and Jette,5 describes how pathology may lead to an impairment at the tissue, organ, or body system level, resulting in a PF limitation, and ultimately to disability. Frailty, on the other hand, is a geriatric syndrome caused by dysregulation in multiple body systems.6 Five clinical components are commonly recognised in frailty – slowness, weakness, shrinking, low levels of activity, and exhaustion. PF limitation and frailty are both associated with aging but are not necessarily tightly correlated.6 Management of PF limitation and subsequent disability may include rehabilitation therapy to regain function, and assistive devices to enable completion of activities, thereby preventing further decline.6 However, frailty requires an investigation into the underlying causes and interventions are focused on minimising weight loss and muscle strength, nutritional supplementation, and cardiovascular rehabilitation.6
Although studies have demonstrated an association between HIV and frailty,7–11 the literature on PF limitation among people living with HIV (PLHIV) is less compelling and has used inconsistent and/or poorly validated methodology for assessing PF.12–23 There is no gold standard method for measuring PF; however, the Medical Outcomes Study–Physical Functioning (MOS-PF) scale is well validated in the general population, includes evaluation of both upper and lower body PF, and exhibits sufficient detail to detect variation among those with only minor PF limitation.1,24,25
In the general population, the mechanisms underlying PF limitation are multifactorial and involve the processes of aging and lifestyle factors.26,27 In PLHIV, similar factors are likely to be important, but may be modified or amplified by HIV and HIV-associated comorbidities, coinfections, social behaviours, and medication use. In particular, chronic low-grade inflammation, a well established characteristic of HIV infection,28 contributes to the development of age-associated comorbidities29–31 and may contribute to loss of muscle mass and altered body composition.32–38 HIV and antiretroviral therapy may also impact muscle mass and body composition by causing changes in vitamin D metabolism,39,40 the growth hormone/insulin-like growth factor-1 axis,41 and mitochondrial function,42 along with other as yet undefined pathways.
As PLHIV on antiretroviral therapy now have a life expectancy approaching that of the general population,43 clinical care focuses increasingly on the management and prevention of comorbidities and conditions associated with aging. The purpose of this investigation was to assess the prevalence of PF limitation among Australian gay and bisexual men (GBM) using the MOS-PF scale, and to evaluate whether HIV is associated with severe PF limitation in GBM.
Methods
Participant recruitment
The Australian Positive and Peers Longevity Evaluation Study (APPLES)44,45 recruited GBM with and without HIV aged ≥55 years from a subset of sites involved in the existing Australian HIV Observational Database (AHOD) network.46 Sites included general practice clinics, sexual health clinics, and tertiary referral hospitals from most states and territories in Australia. This analysis includes all participants enrolled in APPLES who completed a self-administered, cross-sectional study survey. The APPLES survey, like the 45 and Up Study questionnaire for men,47 addressed health and lifestyle factors such as personal and family medical history, tobacco, alcohol, and recreational drug use, exercise and diet. Additional HIV-related questions were also included. Comorbidities were based on two specific survey questions: (1) ‘In the last month have you been treated for..’ and included heart disease, thrombosis, any cancer (excluding skin cancer), osteoarthritis, and depression/anxiety; and (2) ‘Has the doctor ever told you that you have..’ and included stroke, diabetes, kidney disease, hepatitis B infection, and hepatitis C infection.
Assessment of physical function limitation
PF limitation was determined using the MOS-PF scale,24,25 consistent with the 45 and Up Study. The following functions are included in the scale: vigorous activity (e.g. running, strenuous sports), moderate activity (e.g. pushing a vacuum cleaner, playing golf), lifting or carrying shopping, climbing flight of stairs, walking, bending, kneeling or stooping, bathing or dressing. Participants are given a choice of three responses for each question with a score allocated for each response: Yes, limited a lot (score = 1); Yes, limited a little (score = 2); No, not limited at all (score = 3). Participants score a minimum of 10 points and a maximum of 30 points, which is then re-scaled to a score between 0 and100, with higher scores indicative of better PF. Scores were categorised as: no limitation (score of 100), minor limitation (score of 90–99), moderate limitation (score of 60–89), and severe limitation (score of 0–59), consistent with earlier work.1
Descriptive analysis
Baseline demographic, clinical and psychosocial characteristics of participants were summarised by HIV status. The proportion of individuals with no, minor, moderate, and severe PF limitation by baseline characteristics were also summarised by HIV status. The extent of limitation for each activity within the MOS-PF scale is described for all individuals with severe PF limitation regardless of HIV status.
Statistical analysis
The association between HIV and severe PF limitation was assessed using logistic regression. Our multivariate model included a priori factors previously described as being associated with PF limitation.14,18,48 These included age (categorised as 55–59 years, 60–69 years, and ≥70 years), body mass index (categorised as underweight if <18.5 kg/m2, healthy if 18.5–24.9 kg/m2, overweight if 25.0–29.9 kg/m2, and obese if ≥30.0 kg/m2), minutes spent active during a typical day defined as moderate or vigorous physical activity and episodes of walking for >10 min (categorised as <150 or ≥150 min), level of psychological distress (categorised as low, moderate, or high based on the Kessler-10 scale49,50), and number of comorbidities (sum of heart disease, thrombosis, cancer [excluding skin cancer], osteoarthritis, depression/anxiety, stroke, diabetes, kidney disease, hepatitis B infection, and hepatitis C infection). Self-reported overall health status and self-reported quality of life were not included in our models due to their clinical similarity with PF. We also conducted a sub-analysis of factors associated with severe PF limitation in GBM with HIV, which included the above-mentioned variables from our main regression analysis, as well as year of HIV diagnosis (categorised as <1996, 1996–2006, or >2006) and latest CD4 cell count (categorised as <500, 500–750, and >750 cells/mm3).
Stata 16.1 (StataCorp, College Station, Texas, USA) was used for all statistical analysis.
Moderate-to-severe physical function limitation
The above descriptive and statistical analyses on severe PF limitation were repeated using moderate-to-severe PF limitation as the outcome.
Ethical approval
This study was approved by St Vincent’s Hospital, Sydney Human Research Ethics Committee, University of New South Wales Human Research Ethics Committee, and all other institutional review boards at participating sites. Written informed consent was obtained from all participants at enrolment.
Results
Baseline
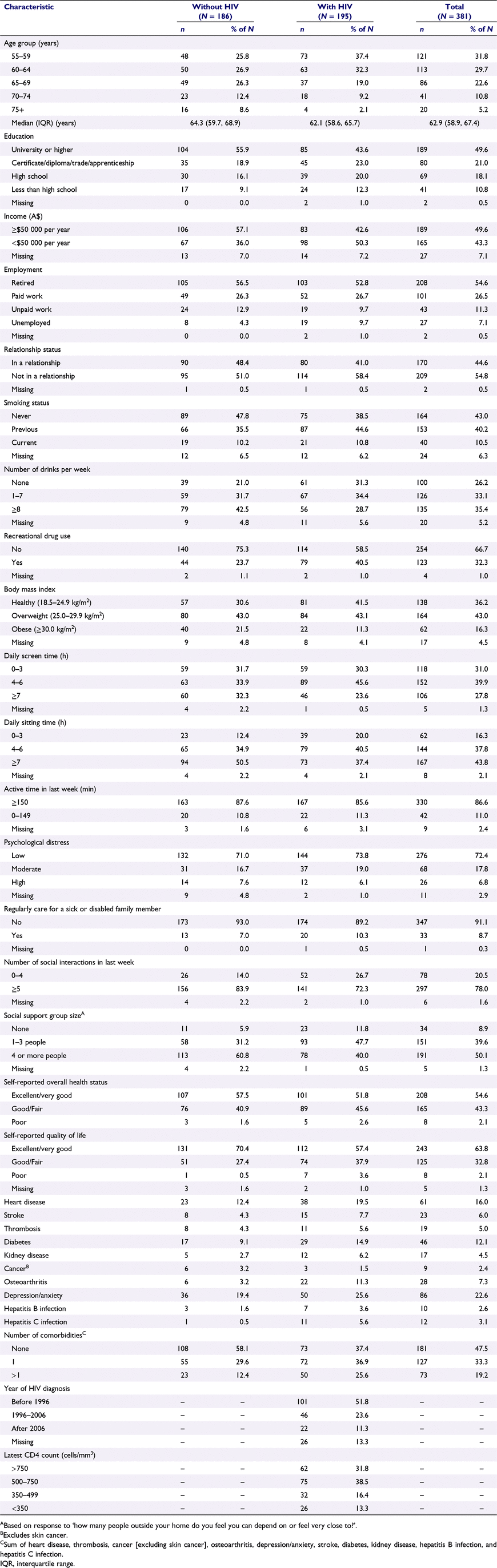
The survey was completed by 389 (87.2%) of the 446 GBM recruited to APPLES. Of these, eight were excluded because they did not answer all questions related to PF. This left a final study population of 381 men: 186 without HIV and 195 with HIV. Median age was 64.3 years for GBM without HIV, 62.1 years for GBM with HIV, and 62.9 years overall. Among GBM with HIV, 99.5% were using antiretroviral therapy and 97.7% were virally suppressed. This is consistent with the prevalence of treatment and virological suppression in the HIV population of Australia.51 Overall health status was reported as excellent or very good by 57.5%, 51.8%, and 54.6% of GBM without HIV, GBM with HIV, and all GBM, respectively. Quality of life was reported as excellent or very good by 70.4%, 57.4%, and 63.8% of GBM without HIV, GBM with HIV, and all GBM, respectively. Other study population characteristics are described in Table 1.
|
Severe physical function limitation
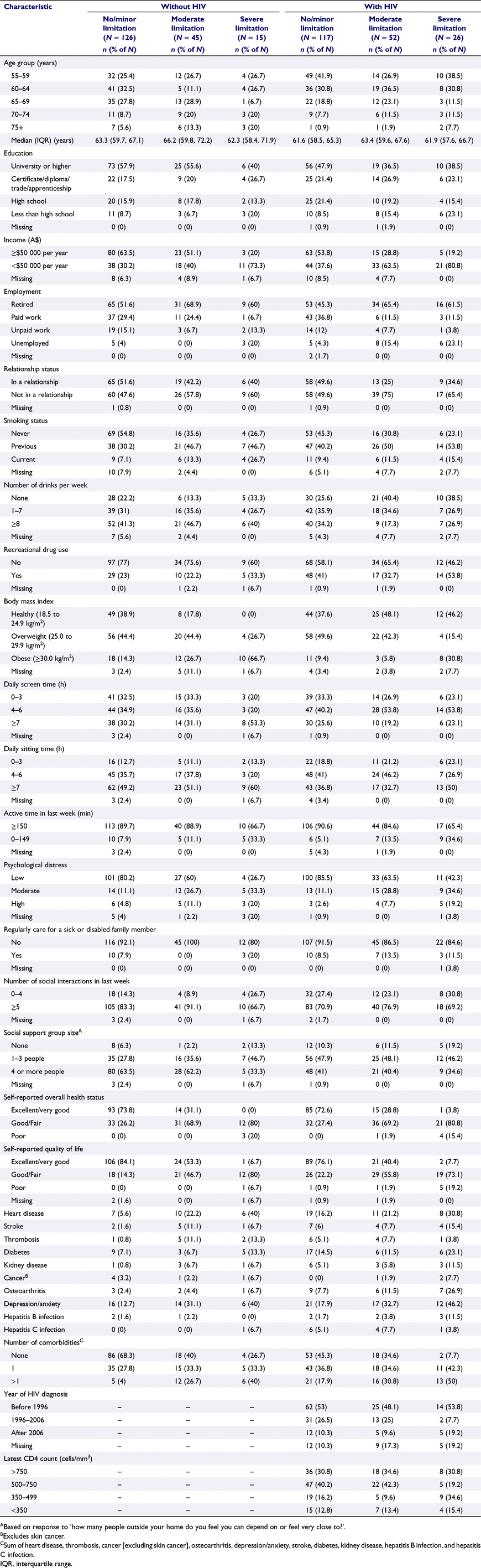
Table 2 shows that, compared with GBM without HIV, those with HIV had higher proportions of severe (13.3% vs 8.1%) and moderate (26.7% vs 24.2%) PF limitation and a lower proportion of no/minor PF limitation (60.0% vs 67.7%). Among GBM with severe PF limitation, those with HIV were more likely than those without HIV to have a healthy body mass index (46.2% vs 0.0%), to have osteoarthritis (26.9% vs 6.7%), and to have multiple comorbidities (50.0% vs 40.0%).
|
Fig. 1 shows severe PF limitation commonly involved difficulty with vigorous activity (95% limited a lot), climbing several flights of stairs (68.4% limited a lot), bending, kneeling or stooping (60.5% limited a lot), and walking 1 km (55.0% limited a lot).
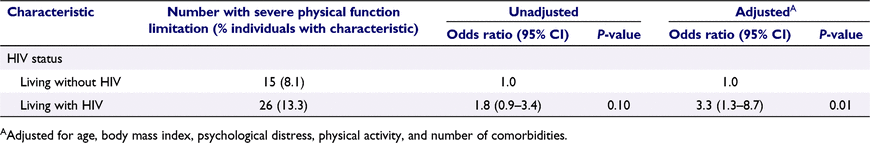
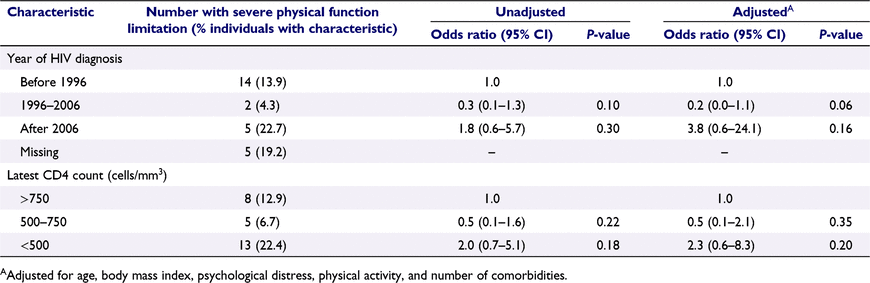
Table 3 shows that having HIV was associated with more than three-fold greater odds of having severe PF limitation (adjusted odds ratio (aOR) 3.3 vs not having HIV, 95% confidence interval (CI) 1.3–8.7, P = 0.01). Among GBM with HIV, year of HIV diagnosis and latest CD4 cell count were not significantly associated with severe PF limitation; however, there were trends towards lower CD4 and earlier HIV diagnosis being associated with severe PF limitation (Table 4).
|
|
Moderate-to-severe physical function limitation
Supplementary Fig. S1 shows that moderate-to-severe PF limitation commonly involved with difficulty with vigorous activity (74.5% limited a lot). For the other activities, <25% of participants with moderate-to-severe PF indicated they were limited a lot.
Supplementary Table S1 shows that having HIV was significantly associated with moderate-to-severe PF limitation (aOR 1.7 vs not having HIV, 95% CI 1.0–3.0, P = 0.05). Among GBM with HIV, year of HIV diagnosis and latest CD4 cell count were not associated with moderate-to-severe PF limitation (Supplementary Table S2).
Discussion
We found that the prevalence of severe PF limitation among GBM with HIV aged ≥55 years was 13.3%, and the prevalence of moderate-to-severe PF limitation was 40.0%. For men with PF limitation, the most frequently affected tasks were vigorous activity and climbing stairs. GBM with HIV were significantly more likely than GBM without HIV to exhibit severe PF alone and moderate-to-severe PF limitation. Among GBM with HIV, the duration of HIV infection and current CD4 cell count were not associated with PF limitation.
We used the MOS-PF scale because it is well validated in the general population, includes evaluation of both upper and lower body PF, and exhibits sufficient detail to detect variation among those with only minor PF limitation.1,24,25 Moreover, the 45 and Up Study, an Australian general population cohort, used this scale, thereby aiding comparison to our findings. In 2015, the 45 and Up Study reported that 13% of older Australians have severe PF limitations.1
Various other PF testing methods have been used in the HIV literature. The Short Physical Performance Battery is an instrument used to objectively measure lower extremity function.52,53 It is designed for a population aged >65 years; however, a modified version has been developed, which allows objective evaluation of PF in a younger population.54 The 6-min walk test, in which the distance walked over 6 min at one’s usual pace is measured,55 is mostly used in studies of cardiovascular, pulmonary, and peripheral vascular disease, but is also used to assess PF. Other less commonly used PF tests include the five-times sit-to-stand test,56,57 hand-grip test,58 physical performance test,59 and upper extremity physical performance battery.60
Despite the range of methods used for measuring PF across studies, earlier findings are broadly similar to our own. Greene et al.14 found that, among 1627 injecting drug users in the United States, HIV infection was associated with a 30% increased risk of reduced physical performance (defined as a Short Physical Performance Battery score of ≤10, a level that prior studies have associated with high risk of immobility and disability52,53,61). The authors also found that reduced physical performance predicted mortality in a dose-response manner among individuals with both well controlled and poorly controlled HIV. An analysis of data from the Multicenter AIDS Cohort Study found HIV-positive men with and without wasting had lower self-reported physical function compared with HIV-negative men, and that HIV-positive men with wasting had poorer grip strength and walk speed than HIV-positive men without wasting and HIV-negative men.20 Richert et al.18 reported that the frequency of poor lower limb performance in the five-times sit-to-stand test in a French HIV cohort was much higher than the expected frequency in the general population (53% vs 2–3%). In a recent systematic review of studies from sub-Saharan Africa by Bernard et al.12, the authors found three studies showing no difference in gait speed between PLHIV and uninfected adults in Uganda, and two studies from Nigeria showing a slower gait speed among PLHIV. They also found three studies reporting information on grip strength: an Ethiopian study showing PLHIV had lower grip strength (−4.2 kg) at antiretroviral therapy initiation than uninfected patients; a South African study showing PLHIV aged ≥50 years had an average grip strength 4.7 kg less than HIV-uninfected individuals; and a study from Senegal reporting no significant difference in grip strength between PLHIV on antiretroviral therapy and age- and sex-matched controls from the general population.
Similar to our findings, prior studies investigating HIV-related factors have not found a strong association between PF limitation and duration of HIV infection or current CD4 cell count.18,21–23,48 However, McInerney et al.48 reported that a longer time on antiretroviral therapy was associated with a higher PF score among PLHIV. This could have been due to the use of older antiretrovirals with adverse effects that impact PF such as muscle wasting, abdominal obesity, lipodystrophy, and peripheral neuropathy. These drugs may have been used by a smaller proportion of our more recent cohort. We explored whether an association between year of HIV diagnosis (as a proxy for antiretroviral therapy duration) and PF limitation was hidden by adjustment for body mass index in our models. However, year of HIV diagnosis remained unassociated with PF limitation, even after removing adjustment for body mass index (results not shown).
There were limitations to this analysis. As a cross-sectional study, it could not evaluate causality of associations or the long-term health outcomes associated with PF limitation. Nevertheless, our findings are aligned with longitudinal studies among PLHIV.14,20,21,23 Although we used a well validated measure of PF that evaluates both upper and lower body activities and can detect minor PF impairment, the inconsistent methodology used in similar studies limits the comparability of our work to that of others. As a self-reported means of measuring PF, the MOS-PF scale may also be more heavily influenced than objective measurements by cultural and environmental factors, as well as the mental health of participants. Despite this, our prevalence estimates for PF limitation are broadly consistent with earlier studies. Comorbidities were self-reported and therefore subject to recall bias, and for some diseases, notably heart disease, there was no strict definition of what form of the disease to include. Further, it was not feasible to cross-check with clinic records as these events may have occurred many years ago and may not have been managed at each patient’s current clinic. However, as these data were reported and collected by similar means for both GBM with and without HIV, any recall bias should not have been differential. Although APPLES is one of the larger studies to evaluate PF limitation in PLHIV, our analysis was not sufficiently powered to compare changes in PF limitation with age among GBM with and without HIV. Future studies should investigate whether HIV is associated with a more rapid decline in PF with age. Finally, GBM without HIV were not population-based controls, but rather recruited via the same treatment clinics from where GBM with HIV were enrolled. As the majority were recruited from general practitioners, it is possible that GBM without HIV in our study were more unwell than the total population of GBM without HIV. However, Australian guidelines recommend that GBM have sexually transmitted infection testing at least annually62 and therefore many of the GBM without HIV recruited to the present study were likely to have been attending the clinic for routine sexually transmitted infection testing rather than for illness.
Despite these limitations, this is one of the most comprehensive studies to-date comparing PF among older GBM with and without HIV. We have used a well validated tool (MOS-PF scale) that assesses both upper and lower body PF and exhibits granularity capable of distinguishing even minor PF limitation. Most importantly, we found GBM with well controlled HIV (i.e. 70.3% with a CD4 count >500 cells/mm3) had a three-fold greater risk of severe PF limitation than GBM without HIV. The biological mechanisms underlying this association require further investigation, particularly given the growing age of the HIV population and inevitable increase in the burden of PF limitation.
Supplementary material
Supplementary material is available online.
Data availability
Data are available upon reasonable request. Please contact Kathy Petoumenos by email: Kpetoumenos@kirby.unsw.edu.au.
Conflicts of interest
DCB is supported by a National Health and Medical Research Council Early Career Fellowship (GNT1140503) and has received grant funding from Gilead Sciences. MGL has received unrestricted grants from Boehringer Ingelhiem, Gilead Sciences, Merck Sharp & Dohme, Bristol-Myers Squibb, Janssen-Cilag, and ViiV HealthCare and consultancy fees from Gilead Sciences and data and safety monitoring board sitting fees from Sirtex Pty Ltd. JH’s institution received reimbursement for her participation in Advisory Boards for Gilead Sciences, MSD Australia and ViiV Healthcare. MB has received reimbursement for participation in Advisory Boards for ViiV Healthcare, Gilead Sciences and Abbvie, consultancy fees from ViiV Healthcare, Gilead Sciences and Janssen, and institutional support for research from ViiV Healthcare, Gilead Sciences, MSD and Abbvie. IW has consulted and participated in clinical trials for Gilead, ViiV and MSD. All other authors report no conflicts of interest.
Declaration of funding
APPLES was funded by an unrestricted research grant from Gilead Sciences Australia. The funder had no role in study design, data collection, analysis, decision to publish or preparation of the manuscript. The Kirby Institute is funded by the Australian Government Department of Health, and is affiliated with the Faculty of Medicine, UNSW Sydney, NSW, Australia. The content is solely the responsibility of the authors, and the views expressed in this publication do not necessarily represent the position of the Australian Government or the official views of any of the governments, institutions or funders mentioned above.
References
[1] Gubhaju L, Banks E, MacNiven R, et al. Physical functional limitations among aboriginal and non-aboriginal older adults: associations with socio-demographic factors and health. PLoS ONE 2015; 10 e0139364| Physical functional limitations among aboriginal and non-aboriginal older adults: associations with socio-demographic factors and health.Crossref | GoogleScholarGoogle Scholar |
[2] Gill TM. Assessment of function and disability in longitudinal studies. J Am Geriatr Soc 2010; 58 S308–S312.
| Assessment of function and disability in longitudinal studies.Crossref | GoogleScholarGoogle Scholar |
[3] Australian Institute for Health and Welfare. Ageing and the health system: challenges, opportunities and adaptations. 2014. Available at https://www.aihw.gov.au/getmedia/19dbc591-b1ef-4485-80ce-029ff66d6930/6_9-health-ageing.pdf.aspx [Accessed 7 May 2021]
[4] Nagi SZ. A study in the evaluation of disability and rehabilitation potential: concepts, methods, and procedures. Am J Public Health Nations Health 1964; 54 1568–1579.
| A study in the evaluation of disability and rehabilitation potential: concepts, methods, and procedures.Crossref | GoogleScholarGoogle Scholar |
[5] Verbrugge LM, Jette AM. The disablement process. Soc Sci Med 1994; 38 1–14.
| The disablement process.Crossref | GoogleScholarGoogle Scholar |
[6] Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB. Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr HIV/AIDS Rep 2014; 11 279–290.
| Functional impairment, disability, and frailty in adults aging with HIV-infection.Crossref | GoogleScholarGoogle Scholar |
[7] Desquilbet L, Jacobson LP, Fried LP, et al. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci 2011; 66A 1030–1038.
| A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men.Crossref | GoogleScholarGoogle Scholar |
[8] Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007; 62 1279–1286.
| HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty.Crossref | GoogleScholarGoogle Scholar |
[9] Desquilbet L, Margolick JB, Fried LP, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. J Acquir Immune Defic Syndr 2009; 50 299–306.
| Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men.Crossref | GoogleScholarGoogle Scholar |
[10] Önen NF, Agbebi A, Shacham E, Stamm KE, Önen AR, Overton ET. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect 2009; 59 346–352.
[11] Terzian AS, Holman S, Nathwani N, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health (Larchmt) 2009; 18 1965–1974.
| Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART.Crossref | GoogleScholarGoogle Scholar |
[12] Bernard C, Dabis F, de Rekeneire N. Physical function, grip strength and frailty in people living with HIV in sub-Saharan Africa: systematic review. Trop Med Int Health 2017; 22 516–525.
| Physical function, grip strength and frailty in people living with HIV in sub-Saharan Africa: systematic review.Crossref | GoogleScholarGoogle Scholar |
[13] Branas F, Jimenez Z, Sanchez-Conde M, et al. Frailty and physical function in older HIV-infected adults. Age Ageing 2017; 46 522–526.
| Frailty and physical function in older HIV-infected adults.Crossref | GoogleScholarGoogle Scholar |
[14] Greene M, Covinsky K, Astemborski J, et al. The relationship of physical performance with HIV disease and mortality. AIDS 2014; 28 2711–2719.
| The relationship of physical performance with HIV disease and mortality.Crossref | GoogleScholarGoogle Scholar |
[15] Mbada CE, Onayemi O, Ogunmoyole Y, Johnson OE, Akosile CO. Health-related quality of life and physical functioning in people living with HIV/AIDS: a case-control design. Health Qual Life Outcomes 2013; 11 106
| Health-related quality of life and physical functioning in people living with HIV/AIDS: a case-control design.Crossref | GoogleScholarGoogle Scholar |
[16] Erlandson KM, Allshouse AA, Jankowski CM, et al. Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials 2012; 13 324–334.
| Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar |
[17] Oursler KK, Sorkin JD, Smith BA, Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses 2006; 22 1113–1121.
| Reduced aerobic capacity and physical functioning in older HIV-infected men.Crossref | GoogleScholarGoogle Scholar |
[18] Richert L, Dehail P, Mercie P, et al. High frequency of poor locomotor performance in HIV-infected patients. AIDS 2011; 25 797–805.
| High frequency of poor locomotor performance in HIV-infected patients.Crossref | GoogleScholarGoogle Scholar |
[19] Negin J, Martiniuk A, Cumming RG, et al. Prevalence of HIV and chronic comorbidities among older adults. AIDS 2012; 26 S55–S63.
| Prevalence of HIV and chronic comorbidities among older adults.Crossref | GoogleScholarGoogle Scholar |
[20] Erlandson KM, Li X, Abraham AG, et al. Long-term impact of HIV wasting on physical function. AIDS 2016; 30 445–454.
| Long-term impact of HIV wasting on physical function.Crossref | GoogleScholarGoogle Scholar |
[21] Brañas F, Sánchez-Conde M, Carli F, et al. Sex differences in people aging with HIV. J Acquir Immune Defic Syndr 2020; 83 284–291.
| Sex differences in people aging with HIV.Crossref | GoogleScholarGoogle Scholar |
[22] Khoury AL, Morey MC, Wong TC, et al. Diminished physical function in older HIV-infected adults in the Southeastern U.S. despite successful antiretroviral therapy. PLoS ONE 2017; 12 e0179874
| Diminished physical function in older HIV-infected adults in the Southeastern U.S. despite successful antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar |
[23] Umbleja T, Brown TT, Overton ET, et al. Physical function impairment and frailty in middle-aged people living with human immunodeficiency virus in the REPRIEVE trial ancillary study PREPARE. J Infect Dis 2020; 222 S52–S62.
| Physical function impairment and frailty in middle-aged people living with human immunodeficiency virus in the REPRIEVE trial ancillary study PREPARE.Crossref | GoogleScholarGoogle Scholar |
[24] Haley SM, McHorney CA, Ware JE. Evaluation of the mos SF-36 physical functioning scale (PF-10): I. Unidimensionality and reproducibility of the Rasch item scale. J Clin Epidemiol 1994; 47 671–684.
| Evaluation of the mos SF-36 physical functioning scale (PF-10): I. Unidimensionality and reproducibility of the Rasch item scale.Crossref | GoogleScholarGoogle Scholar |
[25] Stewart AL, Ware JE. Measuring functioning and well-being: the medical outcomes study approach. Durham and London: Duke University Press; 1992.
[26] Clouston SAP, Brewster P, Kuh D, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev 2013; 35 33–50.
| The dynamic relationship between physical function and cognition in longitudinal aging cohorts.Crossref | GoogleScholarGoogle Scholar |
[27] Colon-Emeric CS, Whitson HE, Pavon J, Hoenig H. Functional decline in older adults. Am Fam Physician 2013; 88 388–394.
[28] Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39 633–645.
| Systemic effects of inflammation on health during chronic HIV infection.Crossref | GoogleScholarGoogle Scholar |
[29] Herrin M, Tate JP, Akgun KM, et al. Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 2016; 73 228–236.
| Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals.Crossref | GoogleScholarGoogle Scholar |
[30] Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation 2018; 138 1100–1112.
| Global burden of atherosclerotic cardiovascular disease in people living with HIV.Crossref | GoogleScholarGoogle Scholar |
[31] Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS 2009; 23 2337–2345.
| HIV infection and the risk of cancers with and without a known infectious cause.Crossref | GoogleScholarGoogle Scholar |
[32] Baranoski AS, Harris A, Michaels D, et al. Relationship between poor physical function, inflammatory markers, and comorbidities in HIV-infected women on antiretroviral therapy. J Womens Health (Larchmt) 2014; 23 69–76.
| Relationship between poor physical function, inflammatory markers, and comorbidities in HIV-infected women on antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar |
[33] Cohen HJ, Pieper CF, Harris T, Rao KMK, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci 1997; 52A M201–M208.
| The association of plasma IL-6 levels with functional disability in community-dwelling elderly.Crossref | GoogleScholarGoogle Scholar |
[34] Erlandson KM, Allshouse AA, Jankowski CM, et al. Association of functional impairment with inflammation and immune activation in HIV type 1–infected adults receiving effective antiretroviral therapy. J Infect Dis 2013; 208 249–259.
| Association of functional impairment with inflammation and immune activation in HIV type 1–infected adults receiving effective antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar |
[35] Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 2000; 51 245–270.
| Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty.Crossref | GoogleScholarGoogle Scholar |
[36] Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 1999; 47 639–646.
| Serum IL-6 level and the development of disability in older persons.Crossref | GoogleScholarGoogle Scholar |
[37] Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res 2004; 16 153–157.
| Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty.Crossref | GoogleScholarGoogle Scholar |
[38] Salter ML, Lau B, Mehta SH, Go VF, Leng S, Kirk GD. Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections. J Acquir Immune Defic Syndr 2013; 64 488–495.
| Correlates of elevated interleukin-6 and C-reactive protein in persons with or at high risk for HCV and HIV infections.Crossref | GoogleScholarGoogle Scholar |
[39] Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged ≥60 y. Am J Clin Nutr 2004; 80 752–758.
| Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged ≥60 y.Crossref | GoogleScholarGoogle Scholar |
[40] Brown TT. Challenges in the management of osteoporosis and vitamin D deficiency in HIV infection. Top Antivir Med 2013; 21 115–118.
[41] Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr 2013; 63 209–215.
| Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection.Crossref | GoogleScholarGoogle Scholar |
[42] Gardner K, Hall PA, Chinnery PF, Payne BAI. HIV treatment and associated mitochondrial pathology: review of 25 years of in vitro, animal, and human studies. Toxicol Pathol 2014; 42 811–822.
| HIV treatment and associated mitochondrial pathology: review of 25 years of in vitro, animal, and human studies.Crossref | GoogleScholarGoogle Scholar |
[43] Edwards JK, Cole SR, Breger TL, et al. Mortality among persons entering HIV care compared with the general U.S. population : an observational study. Ann Intern Med 2021; 174 1197–1206.
| Mortality among persons entering HIV care compared with the general U.S. population : an observational study.Crossref | GoogleScholarGoogle Scholar |
[44] Petoumenos K, Huang R, Hoy J, et al. Prevalence of self-reported comorbidities in HIV positive and HIV negative men who have sex with men over 55 years – The Australian Positive & Peers Longevity Evaluation Study (APPLES). PLoS ONE 2017; 12 e0184583
| Prevalence of self-reported comorbidities in HIV positive and HIV negative men who have sex with men over 55 years – The Australian Positive & Peers Longevity Evaluation Study (APPLES).Crossref | GoogleScholarGoogle Scholar |
[45] Puhr R, Petoumenos K, Huang R, et al. Cardiovascular disease and diabetes in HIV-positive and HIV-negative gay and bisexual men over the age of 55 years in Australia: insights from the Australian Positive & Peers Longevity Evaluation Study. HIV Med 2019; 20 121–130.
| Cardiovascular disease and diabetes in HIV-positive and HIV-negative gay and bisexual men over the age of 55 years in Australia: insights from the Australian Positive & Peers Longevity Evaluation Study.Crossref | GoogleScholarGoogle Scholar |
[46] Australian HIV Observational Database Rates of combination antiretroviral treatment change in Australia, 1997–2000. HIV Med 2002; 3 28–36.
| Rates of combination antiretroviral treatment change in Australia, 1997–2000.Crossref | GoogleScholarGoogle Scholar |
[47] 45 and Up Study Collaborators Cohort profile: the 45 and up study. Int J Epidemiol 2008; 37 941–947.
| Cohort profile: the 45 and up study.Crossref | GoogleScholarGoogle Scholar |
[48] McInerney PA, Ncama BP, Wantland D, et al. Quality of life and physical functioning in HIV-infected individuals receiving antiretroviral therapy in KwaZulu-Natal, South Africa. Nurs Health Sci 2008; 10 266–272.
| Quality of life and physical functioning in HIV-infected individuals receiving antiretroviral therapy in KwaZulu-Natal, South Africa.Crossref | GoogleScholarGoogle Scholar |
[49] Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 2002; 32 959–976.
| Short screening scales to monitor population prevalences and trends in non-specific psychological distress.Crossref | GoogleScholarGoogle Scholar |
[50] Kessler RC, Barker PR, Colpe LJ, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry 2003; 60 184–189.
| Screening for serious mental illness in the general population.Crossref | GoogleScholarGoogle Scholar |
[51] The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia. Annual Surveillance Report 2021. 2021. Available at https://kirby.unsw.edu.au/report/latest-hiv-surveillance-data [Accessed 13 July 2022]
[52] Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995; 332 556–562.
| Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability.Crossref | GoogleScholarGoogle Scholar |
[53] Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49 M85–M94.
| A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission.Crossref | GoogleScholarGoogle Scholar |
[54] Makizako H, Shimada H, Doi T, et al. The modified version of the short physical performance battery for community-dwelling Japanese older adults. Phys Ther Jpn 2017; 44 197–206.
| The modified version of the short physical performance battery for community-dwelling Japanese older adults.Crossref | GoogleScholarGoogle Scholar |
[55] Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985; 132 919–923.
[56] Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci 2002; 57 M539–M543.
| Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people.Crossref | GoogleScholarGoogle Scholar |
[57] Whitney SL, Wrisley DM, Marchetti GF, Gee MA, Redfern MS, Furman JM. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test. Phys Ther 2005; 85 1034–1045.
| Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test.Crossref | GoogleScholarGoogle Scholar |
[58] Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther 2008; 31 3–10.
| Hand-grip dynamometry predicts future outcomes in aging adults.Crossref | GoogleScholarGoogle Scholar |
[59] Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The physical performance test. J Am Geriatr Soc 1990; 38 1105–1112.
| An objective measure of physical function of elderly outpatients. The physical performance test.Crossref | GoogleScholarGoogle Scholar |
[60] Hazuda HP, Dhanda R, Owen SV, Lichtenstein MJ. Development and validation of a performance-based measure of upper extremity functional limitation. Aging Clin Exp Res 2005; 17 394–401.
| Development and validation of a performance-based measure of upper extremity functional limitation.Crossref | GoogleScholarGoogle Scholar |
[61] Vasunilashorn S, Coppin AK, Patel KV, et al. Use of the short physical performance battery score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2009; 64 223–229.
| Use of the short physical performance battery score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study.Crossref | GoogleScholarGoogle Scholar |
[62] Templeton DJ, Read P, Varma R, Bourne C. Australian sexually transmissible infection and HIV testing guidelines for asymptomatic men who have sex with men 2014: a review of the evidence. Sex Health 2014; 11 217–229.
| Australian sexually transmissible infection and HIV testing guidelines for asymptomatic men who have sex with men 2014: a review of the evidence.Crossref | GoogleScholarGoogle Scholar |


