Hepatitis C virus infection in HIV-infected men in Singapore, 2006–2018: incidence and associated factors
Li Wei Ang A G , Chiaw Yee Choy B , Oon Tek Ng B C D , Yee Sin Leo B C D E F and Chen Seong Wong B C D
A G , Chiaw Yee Choy B , Oon Tek Ng B C D , Yee Sin Leo B C D E F and Chen Seong Wong B C D
A National Public Health and Epidemiology Unit, National Centre for Infectious Diseases, 16 Jalan Tan Tock Seng, 308442, Singapore.
B Department of Infectious Diseases, National Centre for Infectious Diseases, 16 Jalan Tan Tock Seng, 308442, Singapore.
C Department of Infectious Diseases, Tan Tock Seng Hospital, 11 Jalan Tan Tock Seng, 308433, Singapore.
D Lee Kong Chian School of Medicine, Nanyang Technological University, 11 Mandalay Road, 308232, Singapore.
E Saw Swee Hock School of Public Health, National University of Singapore, 16 Medical Drive, 117597, Singapore.
F Yong Loo Lin School of Medicine, National University of Singapore, 10 Medical Drive, 117597, Singapore.
G Corresponding author. Email: Li_Wei_Ang@ncid.sg
Sexual Health 18(3) 221-231 https://doi.org/10.1071/SH20197
Submitted: 31 October 2020 Accepted: 23 February 2021 Published: 21 June 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Abstract
Background: The epidemiology of hepatitis C virus (HCV) infection in people living with HIV has been evolving, with increasing evidence of permucosal (sexual) transmission identified predominantly in HIV-positive men who have sex with men (MSM). The aim of this study was to estimate the incidence rate and elucidate epidemiological factors associated with HCV infection among HIV-infected men in Singapore from 2006 to 2018. Methods: A retrospective cohort study was conducted using a clinical database maintained by the Clinical HIV Program at the National Centre for Infectious Diseases, Singapore. Factors associated with incident HCV infections were identified using Cox proportional hazards regression analyses. Results: Among 1348 HIV-infected male patients who were HCV seronegative at baseline, 64 (4.7%) subsequently tested positive for HCV, giving an incidence of 0.88 per 100 person-years of follow-up (PYFU) (95% confidence interval (CI) 0.69–1.13). The incidence rate of HCV seroconversion increased from 0.33 (95% CI 0.12–0.71) per 100 PYFU in 2010–2012 to 1.93 (95% CI 1.36–2.67) in 2016–2018. Independent factors associated with incident HCV infection were younger age groups at HIV diagnosis versus ≥45 years, HIV acquisition via MSM or via both sexual contact and intravenous drug use versus heterosexual transmission, HIV diagnosis in later periods versus 2006–2009, and recent syphilis acquisition. Conclusions: An increasing trend of incident HCV infection was seen in HIV-infected men, particularly for MSM. Preventive and behavioural interventions should be targeted at HIV-infected individuals engaged in high-risk sexual behaviour.
Keywords: co-infections, HIV/AIDS, men who have sex with men, syphilis, hepatitis C virus infection, epidemiology, risk factors, preventive intervention.
Background
HIV and hepatitis C virus (HCV) share similar routes of transmission. HIV-infected persons may be at higher exposure risk to HCV.1 Globally, about 2.3 million people living with HIV (PLHIV) are coinfected with HCV, and the highest prevalence of HCV co-infection in PLHIV is in people who inject drugs (PWID) (82.4%), followed by men who have sex with men (MSM) (6.4%).2 Compared with HCV mono-infected patients, the risk of hepatocellular carcinoma and hepatic decompensation may no longer be higher in patients co-infected with HIV and HCV as previously thought, due to the availability and efficacy of combination antiretroviral therapy (ART) and direct-acting antivirals (DAAs).3 However, the overall mortality risk remains higher in HIV-infected patients coinfected with HCV, compared with HCV mono‐infected patients3 and HIV mono-infected patients.4 Internationally, HCV infection remains a pressing public health issue, and the World Health Organization has set an ambitious target to eliminate hepatitis B and C by 2030.5
Since the early 2000s, there have been reports implicating sexual contact as an important route for HCV transmission among HIV-infected MSM.6 Rising numbers of HCV infections and evidence of sexual transmission of HCV have been reported among HIV-infected gay men and other MSM in large cities in Europe,7–16 Australia,17,18 the United States19,20 and Asia.21–24 Changes in sexual behaviour and the concomitant surge of other sexually transmitted infections (STIs) such as syphilis appear to have fuelled the recent outbreaks of sexually-acquired acute HCV infections.25
In Singapore, an estimated 6.5% of HIV-infected persons are co-infected with HCV.26 Sexual transmission (97%) has been the predominant route of HIV infection among Singapore residents, and the majority of the newly-diagnosed cases (>90%) are men. As of the end of 2018, there were 8295 Singapore residents diagnosed with HIV infection, of whom 2034 had died.27
Reports of recent increases in the number of HCV infections among PLHIV have highlighted the need for a multipronged strategy that involves screening, prompt diagnosis and linkage to care. Integrated testing of hepatitis B virus, HCV and STIs among HIV-infected persons plays an important role in preventing onward transmission via appropriate treatment following diagnosis. Although the introduction of DAAs has revolutionised the HCV treatment landscape, their high cost remains a barrier to treatment access in Singapore.28 Since 2016, there has been a reduction in the price of DAAs.29
To date, no studies have investigated the incidence rate of HCV infection among HIV-infected persons in Singapore, and whether there are differences among HIV transmission risk groups. In this study, we examined the temporal trends and identified epidemiological factors associated with seroconversion of HCV among HIV-infected men seen at the largest referral centre for HIV care in Singapore.
Methods
Study design and population
We conducted a retrospective cohort study of HIV-infected patients who had ever received care under the Clinical HIV Program at the National Centre for Infectious Diseases (NCID), the largest centre for HIV care in Singapore. Of 5592 newly diagnosed cases of HIV infections notified to the Singapore Ministry of Health between 2006 and 2018, 3472 (62.1%) had ever sought HIV care at this centre during the 13-year period. The majority of HIV-infected patients on follow-up at NCID are screened for co-infections upon HIV diagnosis.26
We analysed the clinical database maintained under the Program, which included demographic characteristics, virologic and immunologic parameters, co-infections and opportunistic infections, antiretroviral therapy and monitoring, and laboratory records of HIV-infected patients seen at NCID. Analyses were restricted to HIV-infected men as they comprise the majority of reported HIV cases in Singapore.
We selected Singapore male residents diagnosed with HIV who had at least one visit for HIV care between 2006 and 2018 and at least two HCV-related tests during the 13-year period with a negative result at baseline. The baseline for our analyses on incidence of HCV infection referred to the date of first antibody HCV (anti-HCV) test using electrochemiluminescence immunoassay (ECLIA). For patients with subsequent positive HCV antibody test result, their HCV diagnoses were confirmed by real-time polymerase chain reaction (PCR) test and/or HCV viral load detection. The first positive anti-HCV test was taken to be the estimated date of HCV seroconversion due to the wide variation in the interval between tests. A sensitivity analysis was performed by estimating the date of HCV seroconversion as the midpoint between the last negative and first positive HCV test dates (midpoint approach). Incidence of HCV infection was defined as the first positive HCV confirmatory test following baseline negative anti-HCV test. HCV diagnoses that could not be verified were excluded from our analyses. Chronic HCV infection was characterised by persistent RNA (viral load) for at least 6 months.
Ethics approval for use of the clinical data was obtained from the Singapore National Healthcare Group Domain Specific Review Board (NHG DSRB reference number 2012/00438). Informed consent was not obtained as the clinical data collected was used as part of the care management of HIV patients. All data analysed for the study were anonymised.
Statistical methods
Follow-up time was calculated from the baseline negative anti-HCV test date to the date of first positive anti-HCV test for those with HCV diagnoses verified by confirmatory HCV test, or last negative anti-HCV test.15,30,31 For time trend analyses, we defined four distinct calendar periods of follow-up: 2006–2009, 2010–2012, 2013–2015, and 2016–2018. The person-time incidence rate of HCV infection for each time interval and the entire study period from 2006 to 2018 was calculated as the number of incident HCV infections divided by the number of person-years of follow-up (PYFU). Confidence intervals (CIs) of the estimated incidence rates were calculated based on assumption of Poisson distribution.
Recent syphilis acquisition was defined as having a positive result based on rapid plasma reagin (RPR) or venereal disease research laboratory (VDRL) test within ±6 months of HCV seroconversion or within ±6 months of the last anti-HCV test.24
Baseline demographic and clinical characteristics were compared between HIV-infected patients with and without incident HCV infection. Chi-square or Fisher’s exact test was used for categorical variables where appropriate, and Mann–Whitney U test for continuous variables between these two groups. Kaplan-Meier curves were used to compare the cumulative incidence of HCV infection by time from HIV diagnosis to HCV seroconversion across four calendar year periods of HIV diagnosis.
Crude and adjusted hazard ratios (HR) together with their 95% CIs were calculated based on Cox proportional hazards regression models. Multivariable analysis was used to determine independent factors for seroconversion of HCV. We included age at HIV diagnosis and HIV transmission risk group as variables selected a priori based on scientific literature of similar epidemiological studies. Other variables with P < 0.10 in the univariable regression analyses were entered in the multivariable model. We checked the proportional hazards assumption using statistical tests and graphical diagnostics, and no violation was observed for the covariates in the multivariable Cox model.
All P values reported were two-sided and statistical significance was taken as P < 0.05. Statistical analyses were performed using Stata version 16 (StataCorp, College Station, TX, USA) and R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population and incidence of HCV infection
Between 2006 and 2018, 3472 HIV-infected patients were seen under the Clinical HIV Program. We first excluded 2119 from the analysis on incidence of HCV infection for the following reasons: 233 were women, 119 men did not have any HCV test, 122 men were tested positive on their initial HCV test, and 1645 men did not have any subsequent HCV test after their initial negative result (Fig. 1). A total of 1353 HIV-infected male patients had at least a second anti-HCV test following their negative baseline test during the 13-year period. As confirmatory HCV tests were not done for five HIV-infected men, we excluded them from the study. We included 1348 HIV-infected male patients in the study, and they contributed 7239 person-years during the follow-up period. The median follow-up time per patient was 5.17 years (interquartile range (IQR) 2.71–7.72).
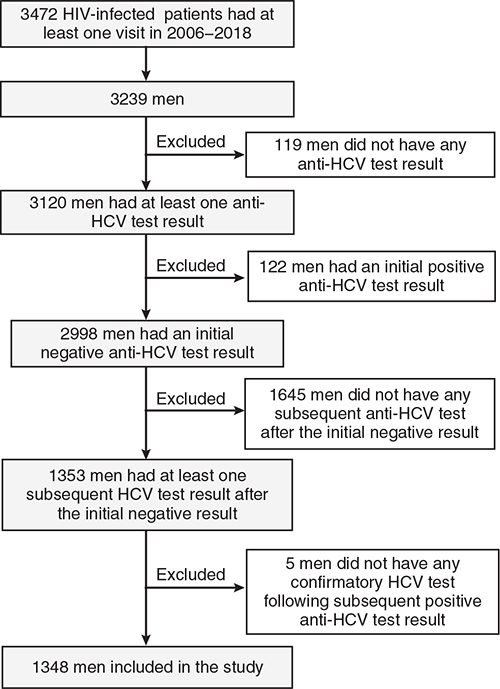
|
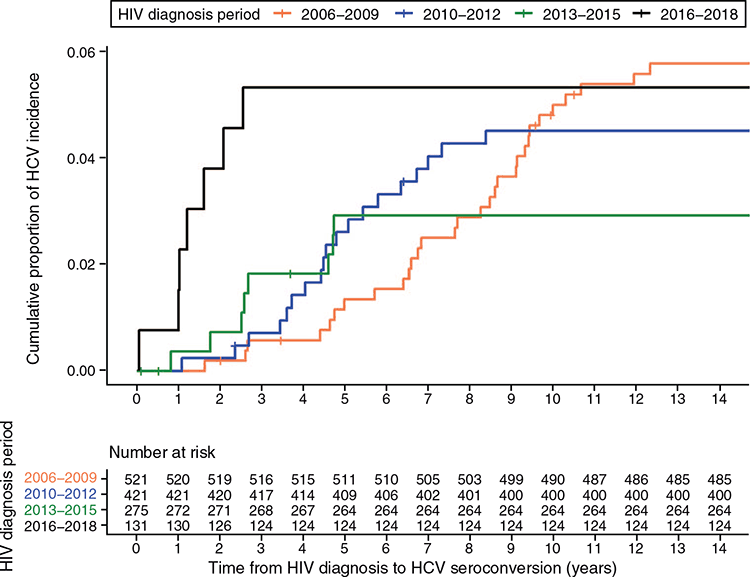
Between 2006 and 2018, 75 (5.6%) HIV-infected men were found to have a positive anti-HCV test after one or more negative anti-HCV tests, and among them, 11 (14.7%) had undetectable HCV RNA and were considered as false-reactive HCV antibody response. Overall, 64 incident HCV infections occurred over 7239 PYFU, giving an overall incidence of 0.88 (95% CI 0.69–1.13) per 100 PYFU. The median time from last negative to first positive anti-HCV test for those with incident HCV infections was 4.03 years (IQR 1.56–6.69). The median time from HIV diagnosis to HCV seroconversion was 4.89 years (IQR 2.66–8.11). At 2 years after HIV diagnosis, the cumulative proportion of HCV incidence was 3.8% in those diagnosed with HIV in 2016–2018, compared with 0.2% in 2006–2009 (Fig. 2).
Using the midpoint approach to estimate the date of HCV seroconversion, the overall incidence was 0.90 (95% CI 0.70–1.16) per 100 PYFU in the 13-year period. Supplementary Fig. S1 depicted the trends of annual HCV incidence rate based on the two approaches for estimation of date of HCV seroconversion. The increase in HCV incidence rate in 2017 and 2018 based on the midpoint approach was less steep compared with the method of using first positive anti-HCV test as the estimated date of HCV seroconversion.
Among the 64 patients with incident HCV infection, 59 (92.2%) had persistent viral load for at least 6 months. Of these 59 patients with chronic HCV infection, 46 (78.0%) were treated and all except one who defaulted subsequent follow-up had been cured (sustained virologic response defined as undetectable RNA level 12 weeks after completion of treatment) while 13 (22.0%) did not have any record of treatment in NCID. Spontaneous HCV clearance was observed in two patients (3.1%), and they were not treated during the acute phase of the infection. Three patients with incident HCV infection (4.7%) did not have subsequent viral load results. The majority of those who were treated for HCV infection received sofosbuvir/velpatasvir, sofosbuvir/daclatasvir, or ledipasvir/sofosbuvir, while pegIntron/ribavirin or ombitasvir/paritaprevir/ritonavir were administered to two patients each. The median interval from positive HCV confirmatory test to administration of anti-HCV therapies was 16 months (IQR 10–29).
Characteristics of HIV-infected men with and without incident HCV infection
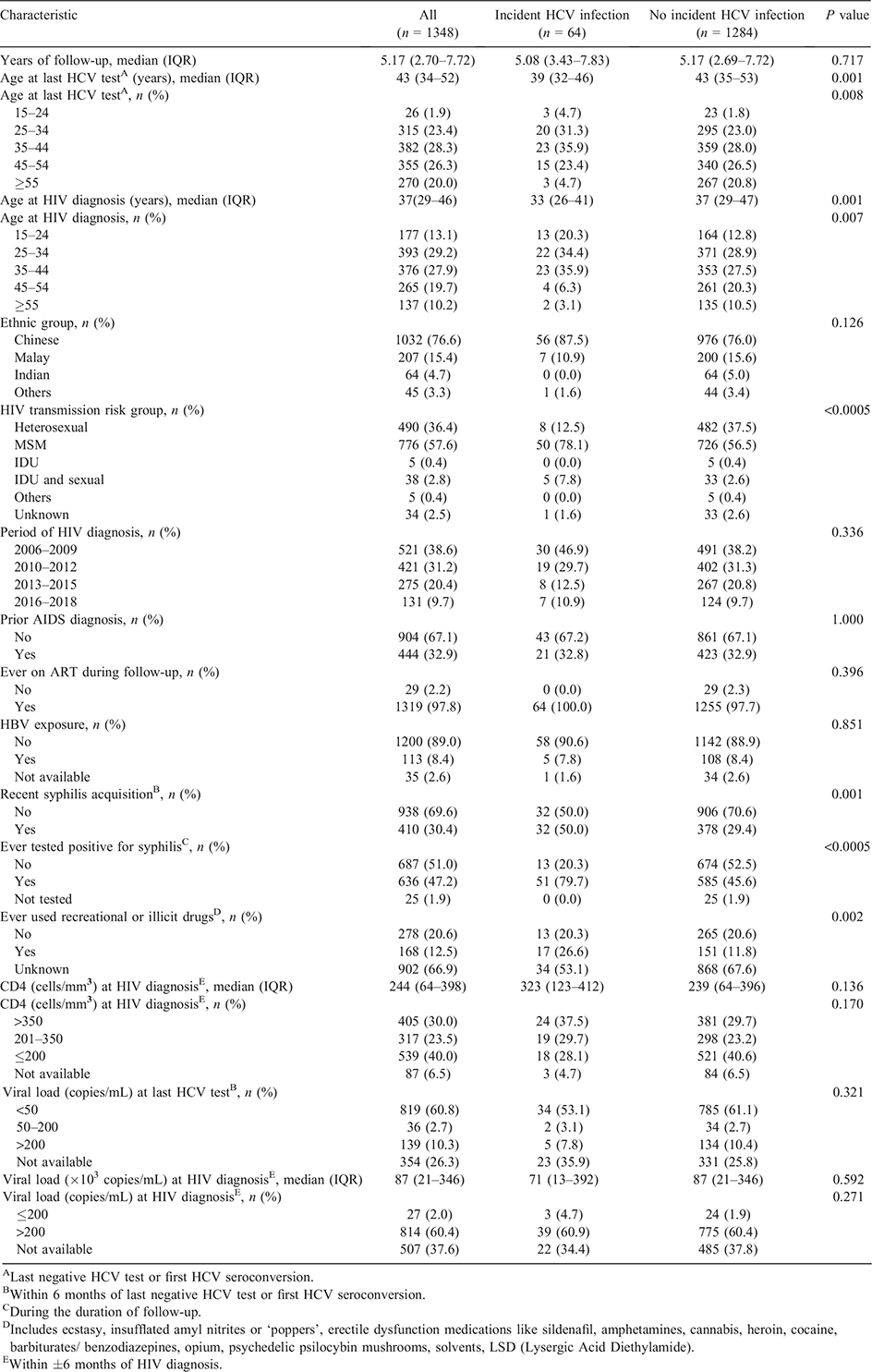
The mean age at the last HCV test during the follow-up period was 44 years (standard deviation 12, range 19–78). HIV-infected men aged 25–44 years at HIV diagnosis comprised 51.7% of the study sample (Table 1). The mean age at HIV diagnosis was 38 years (standard deviation 12, range 17–77). About three-quarters (76.6%) were Chinese. The majority (96.7%) were infected with HIV via sexual transmission. About 69.9% were diagnosed with HIV in the first two calendar periods of 2006–2009 and 2010–2012. Over two-thirds (67.1%) of the HIV-infected men did not have prior AIDS diagnosis. Almost all HIV-infected male patients (97.9%) had ever received ART. Three in 10 men (30.4%) had recent syphilis acquisition. Information on whether the patients had ever used recreational or illicit drugs was not available for about two-thirds (66.9%) of the HIV-infected men. More than half (53.6%) had CD4 >200 cells/mm3. About 26.3% of the HIV-infected male patients did not have HIV viral load measurements at the time of their last HCV test during the follow-up period, while among 994 with known HIV viral load within 6 months of last negative HCV test or first HCV seroconversion, 819 (82.4%) had undetectable HIV viral load (<50 copies/mL).
Compared with HIV-infected men who did not have HCV seroconversion, a significantly higher proportion of those with incident HCV infection were aged 15–44 years at HIV diagnosis (90.6% vs 69.2%), acquired HIV via MSM exposure (78.1% vs 56.5%), had recent syphilis acquisition (50.0% vs 29.4%) and had ever tested positive for syphilis during their follow-up (79.7% vs 45.6%) (all P < 0.001) (Table 1). Among 994 HIV-infected men who had HIV viral load measurements within 6 months of their last negative HCV test or first HCV seroconversion, there were no significant differences in the proportions with undetectable viral load between the two groups with and without incident HCV infection (82.9% vs 82.4%) (P = 0.927).
Comparison of HIV-infected male patients included and excluded from the study
Compared with 1891 male patients that we had excluded from the analyses, a significantly higher proportion of those included in this study were aged between 15–34 years at HIV diagnosis (42.3% vs 31.0%), infected with HIV via MSM exposure (57.6% vs 50.1%), diagnosed with HIV in 2010–2012 (31.2% vs 21.4%), did not have prior AIDS diagnosis (67.1% vs 61.4%), had ever received ART (97.9% vs 93.9%), had ever been tested for syphilis (98.2% vs 92.0%), and had CD4 >200 cells/mm3 at the time of HIV diagnosis (53.6% vs 47.4%) (all P < 0.001) (Supplementary Table S1). A significantly lower proportion of the patients included in our study were infected with HIV via heterosexual transmission (36.4% vs 41.7%), and via intravenous drug use (IDU) and sexual transmission (2.8% vs 4.2%), and were diagnosed in 2016–2018 (9.7% vs 20.1%) (all P < 0.05) (Supplementary Table S1).
Temporal trends of incident HCV infection rate
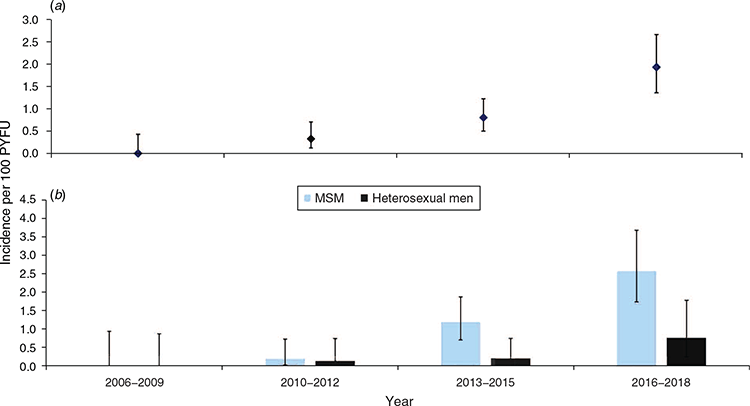
The incidence of HCV infection increased significantly from 0.33 (95% CI 0.12–0.71) per 100 PYFU in 2010–2012 to 1.93 (95% CI 1.36–2.67) in 2016–2018 (Fig. 3a). The overall HCV seroconversion was 1.23 (95% CI 0.94–1.63) per 100 PYFU in MSM, significantly higher than that of heterosexual men, which was 0.29 (95% CI 0.14–0.58). The incidence among MSM in the most recent period of 2016–2018 was 2.59 (95% CI 2.74–3.69) per 100 PYFU, 10 times that of 0.20 (95% CI 0.02–0.73) in 2010–2012 (Fig. 3b). Among men infected with HIV via heterosexual transmission, the increase in incidence from 0.13 per 100 PYFU in 2010–2012 to 0.77 in 2016–2018 was not statistically significant (incidence rate ratio 5.73, 95% CI 0.67–48.84).
Factors associated with incident HCV infection
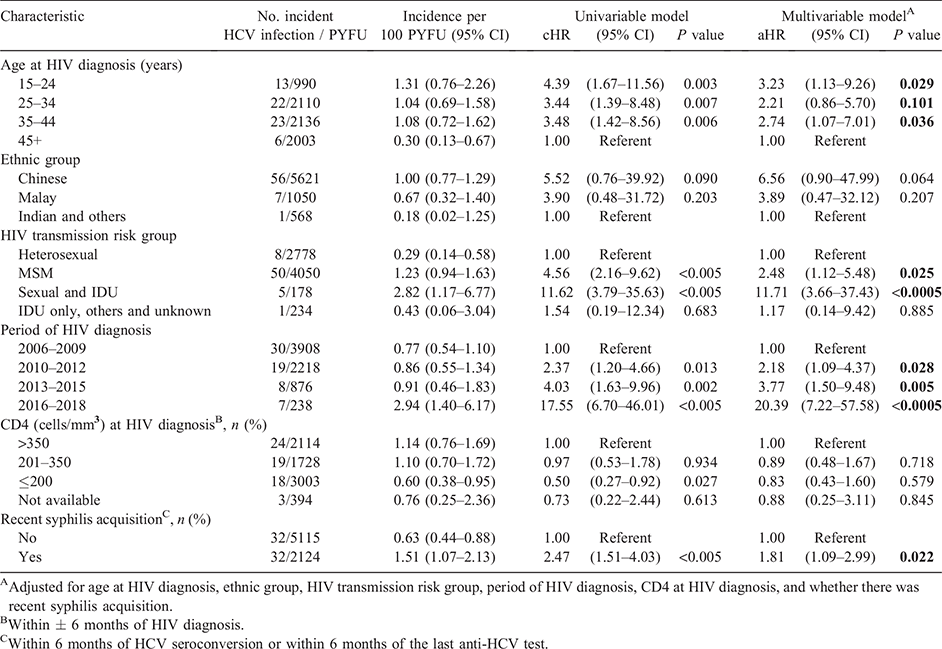
Univariate Cox regression analyses indicated that men in younger age groups below 45 years at HIV diagnosis, those with HIV exposure via MSM or via both sexual contact and IDU, diagnosed with HIV in the periods after 2006–2009 and recent syphilis acquisition were at higher risk of incident HCV infection (Table 2).
In the multivariable Cox model, factors associated with incident HCV infection were younger age at HIV diagnosis (15–24 years: adjusted HR (aHR) 3.23, 95% CI 1.13–9.26; 35–44 years: aHR 2.74, 95% CI 1.07–7.01) versus ≥45 years, HIV acquisition via MSM exposure (aHR 2.48, 95% CI 1.12–5.48) or via both sexual contact and IDU (aHR 11.71, 95% CI 3.66–37.43) versus heterosexual transmission, HIV diagnosis in later calendar periods (2010–2012: aHR 2.18, 95% CI 1.09–4.37; 2013–2015: aHR 3.77, 95% CI 1.50–9.48; 2016–2018: aHR 20.39, 95% CI 7.22–57.58) versus 2006–2009, and recent syphilis acquisition (aHR 1.81, 95% CI 1.09–2.99) (Table 2).
Discussion
The incidence rate of HCV infection in HIV-infected men was on an increasing trend, and this was primarily attributed to MSM transmission. Marked increases in HCV infection among MSM have been observed in HIV cohort studies conducted in Japan,21 Taiwan,24 France,32 Switzerland,15 and the Netherlands33 (Supplementary Table S2). In Singapore, the incidence of HCV infection among HIV-infected men in the most recent period of 2016–2018 was 1.93 (95% CI 1.36–2.67) per 100 PYFU (Fig. 3a). Of the 37 new HCV infections in the most recent 3-year period, 30 (81.1%) occurred among HIV-infected MSM, giving an incidence of 2.59 (95% CI 1.74–3.69) per 100 PYFU, which was higher than that reported in Taiwan (1.92 in 2011–2018),24 South Korea (0.66 in 2010–2014),23 Australia (1.0 in 2015),34 France (0.92 in 2016),32 and the United States (1.26 in 2011–2013),20 and it was comparable with that of Tokyo, Japan (2.49 in 2011–2012)21 and Switzerland (4.09 in 2011)15 (Supplementary Table S2).
Our study revealed recent syphilis acquisition as an independent factor associated with HCV seroconversion. Concomitant or past syphilis infection15,24,34 and history of inconsistent condom use15 had been identified as independent risk factors in a number of studies. The increase in HCV incidence coincided with the rising trend in the incidence of syphilis infection among HIV-infected men who had ever sought HIV care at the largest referral centre in Singapore.35 In the Swiss HIV cohort study, the significant increase in syphilis acquisition among MSM mirrored that of HCV infection incidence.15,36 In the United States, Western Europe, Australia and China, recent upsurges of syphilis infections have also been reported, particularly among high-risk groups such as gay, bisexual and other MSM.37–39 Our results corroborated these findings that suggest the rising HCV incidence may be driven by transmission via the sexual route in HIV-infected MSM in conjunction with higher testing rates in recent years.
In Singapore, promoting consistent condom use in casual and steady sexual relationships among heterosexual men who buy sex and MSM remains a priority, though results have been mixed.40–42 Changes in risk perception and high-risk sexual behaviour, possibly due to risk compensation in response to the availability of highly effective HCV DAA therapies and/or HIV pre-exposure prophylaxis (PrEP),43 has been postulated as the main cause for the surge in HCV incidence among HIV-infected MSM, although these findings are not universal. A Swiss study found that MSM with suppressed HIV viral load were more likely to engage in unprotected sex,44 which elevated the risk of acquiring HCV infection.13,15 In a review and meta-analysis of studies on HIV-positive MSM who were not PWID, a large proportion of infections in the HCV seroconverters were attributable to mucosally traumatic sex and sex while under the influence of methamphetamine.45 Phylogenetic analyses have identified HCV transmission networks among HIV-infected MSM engaging in high-risk sexual behaviour in the United States,46 United Kingdom,10,47 the Netherlands,33 France,48 Germany and Australia,49 and Taiwan.24 In addition, more frequent testing in 2015 due to DAA availability was deemed to be a potential contributing factor to the steep increase in HCV incidence among HIV-infected MSM in San Diego, CA, USA.50
Among HIV-infected men seen at NCID in 2006–2018, the overall testing rate based on the presence of any anti-HCV or HCV PCR tests for each individual per calendar year was 29.6 per 100 PYFU (95% CI 28.8–30.4) (data not shown). There was a significant increase in the HCV testing rate from 18.8 per 100 PYFU (95% CI 16.9–21.0) in 2013 to 41.7 per 100 PYFU (95% CI 38.8–44.8) in 2018 (Supplementary Fig. S2). Among those with at least two HCV tests over the study period, the median interval between tests was 21 months (IQR 10–53). The time from HIV diagnosis to HCV infection appeared to have shortened in recent years (Fig. 2). This was also reflected in the higher risk of HCV incidence in more recent period of HIV diagnosis (Table 2), which could be partially attributed to corresponding increase in testing rate of newly diagnosed HIV patients for HCV (Supplementary Fig. S2). A significant decrease in time from HIV seroconversion to HCV infection over calendar period of HIV seroconversion was observed in a study involving cohorts of HIV-positive MSM from the CASCADE Collaboration across Europe, Australia and Canada, which underscored the need for routine and continued surveillance following HIV diagnosis.16 To maximise the benefits of HIV treatment, testing services and linkage to care are crucial for appropriate management of HIV/HBV and HIV/HCV coinfections.5 According to the guidelines from the National HIV Program, Singapore,51 and the Infectious Disease Society of America and the US Centers for Disease Control and Prevention (CDC),52 all HIV-infected persons should be screened for HCV infection upon entry into HIV care, and for at-risk HCV-seronegative persons, HCV antibody testing is recommended annually or as indicated by risk exposure. In the STI guidelines published by the US CDC in 2015, HIV-infected MSM are recommended to be screened at least yearly and more frequently depending on specific circumstances such as local HCV prevalence and incidence, high-risk sexual behaviour, and concomitant ulcerative STIs or STI-related proctitis.53 In 2010, the European AIDS Treatment Network recommended routine screening for incident HCV infection every 6 months using serum alanine aminotransferase (ALT) and every 12 months with HCV antibody test among HIV-infected MSM engaging in unprotected anal sex, and screening within 3 months of diagnosis of a new STI or IDU exposure.54 Based on the findings of a prospective observational cohort study to assess recent HCV seroconversion in Taiwan, Sun et al. suggested that in addition to routine testing, patients with unexplained abnormal liver function or those recently diagnosed with STIs should also be tested for HCV infection.55 However, as acute HCV reinfections can occur in persons with normal levels of liver enzymes, anti-HCV testing should not only be triggered by clinical suspicion or the presence of significantly elevated levels of ALT.56,57 A study in London, UK revealed that as many as 25% of HIV-positive MSM who had been treated for HCV infection would become re-infected within 2 years, which underscores the need for increased sexual education, surveillance and preventive intervention.58 A modelling study found that routine periodic screening for newly acquired HCV infection in HIV-infected MSM would prolong life expectancy and is cost effective in well-resourced settings.59 The optimal screening strategy is highly dependent on the incidence rate of HCV infection.
We acknowledged several limitations when interpreting our study findings. About half of the HIV-infected male patients seen at NCID did not have another HCV test following their initial negative test (Fig. 1); hence, they had to be excluded from the analysis. Patients who are suspected of HCV infection are more likely to have follow-up HCV tests, resulting in overestimation of HCV incidence in our cohort. We assumed that HCV infection occurred close to the date of the positive test. However, the majority of HIV-infected patients are not periodically screened for HCV during the course of HIV care at NCID; hence, some infections may have occurred earlier. The estimation of HCV seroconversion is affected by frequency of testing and the proportions of patients who are asymptomatic and those who have been lost to follow-up. New HCV infections are usually asymptomatic. A proportion of infected persons (15%–45%) spontaneously clear the virus within 6 months of infection without any treatment.60 Hence, the rate of HCV seroconversion might have been under- or overestimated. In addition, patients might have reinfections that are not detected by serological testing or have late or absent seroconversion.61 In this study, we did not take into consideration HCV reinfection after HCV clearance with anti-HCV therapy or spontaneous clearance. Patients with acute spontaneous resolving HCV infection could experience a rapid short-term decline of antibody values.62 A study investigating anti-HCV dynamics among HIV-infected MSM revealed that about one-quarter of those with sustained virologic response had seroreversion.63 For these patients, the incidence might have been underestimated. Although recent syphilis infection was associated with HCV seroconversion, patients included in the study were more likely to have been tested for syphilis than those who were excluded (Supplementary Table S1). Another caveat was the different frequency and time interval of tests for HCV and syphilis. The overall testing rate per 100 PYFU among HIV-infected men seen at NCID in 2006–2017 was higher at 35.4 (95% CI 34.5–36.4) for syphilis35 compared with 28.3 (95% CI 27.5–29.1) for HCV (data not shown). As our study was based on a retrospective observational design, the presence of unmeasured confounding could not be ruled out. Sexual behaviour such as the type and number of sexual partners and history of other types of STIs may be potential risk factors for incidence of HCV seroconversion but this information was not collected. Illicit drug use was likely to be under-reported (Table 1). In view of the potential selection bias and targeting of HIV-infected patients for HCV and syphilis testing based on clinicians’ assessment, further epidemiological investigations are needed to shed light on the specific drivers of HCV infections in HIV-infected men.
Despite the limitations of our study, the incidence of HCV infection among HIV-infected MSM in Singapore appears to be on the rise, which is consistent with numerous reports on the increasing trend of HCV infection in MSM in the last decade. Our study findings underscore the need for more frequent HCV testing in high-risk HIV-infected patients, particularly those who engage in risky sexual behaviour and/or have concomitant STIs such as syphilis in addition to the traditional risk group of IDU. A targeted approach comprising preventive interventions such as risk-stratified screening, regular testing for early diagnosis and prompt treatment, and counselling on safe sexual practices and risk reduction, should be incorporated in the provision of comprehensive HIV care.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Dr Ng Oon Tek was supported by NMRC Clinician Scientist Award (MOH-000276). Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not reflect the views of MOH/NMRC. This research did not receive any specific funding.
Acknowledgements
The authors thank the patients and staff in the NCID Clinical HIV Program who made this study possible.
References
[1] National Institutes of Health. HIV and opportunistic infections, coinfections, and conditions. 2020. Available at https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-and-hepatitis-c [accessed 24 October 2020].[2] Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16 797–808.
| Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 26922272PubMed |
[3] Salmon-Ceron D, Nahon P, Layese R, Bourcier V, Sogni P, Bani-Sadr F, et al Human immunodeficiency virus/hepatitis C virus (HCV) co-infected patients with cirrhosis are no longer at higher risk for hepatocellular carcinoma or end-stage liver disease as compared to HCV mono-infected patients. Hepatology 2019; 70 939–54.
| Human immunodeficiency virus/hepatitis C virus (HCV) co-infected patients with cirrhosis are no longer at higher risk for hepatocellular carcinoma or end-stage liver disease as compared to HCV mono-infected patients.Crossref | GoogleScholarGoogle Scholar | 30569448PubMed |
[4] Chen TY, Ding EL, Seage Iii GR, Kim AY. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis 2009; 49 1605–15.
| Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression.Crossref | GoogleScholarGoogle Scholar | 19842982PubMed |
[5] World Health Organization. Combating hepatitis B and C to reach elimination by 2030. 2016. Available at https://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/ [accessed 10 December 2019].
[6] Jin F, Matthews GV, Grulich AE. Sexual transmission of hepatitis C virus among gay and bisexual men: a systematic review. Sex Health 2017; 14 28–41.
| Sexual transmission of hepatitis C virus among gay and bisexual men: a systematic review.Crossref | GoogleScholarGoogle Scholar | 27712618PubMed |
[7] Browne R, Asboe D, Gilleece Y, Atkins M, Mandalia S, Gazzard B, et al Increased numbers of acute hepatitis C infections in HIV positive homosexual men: is sexual transmission feeding the increase? Sex Transm Infect 2004; 80 326–7.
| Increased numbers of acute hepatitis C infections in HIV positive homosexual men: is sexual transmission feeding the increase?Crossref | GoogleScholarGoogle Scholar | 15295139PubMed |
[8] Gambotti L, Batisse D, Colin-de-Verdiere N, Delaroque-Astagneau E, Desenclos JC, Dominguez S, et al Acute hepatitis C infection in HIV positive men who have sex with men in Paris, France, 2001–2004. Euro Surveill 2005; 10 535
| Acute hepatitis C infection in HIV positive men who have sex with men in Paris, France, 2001–2004.Crossref | GoogleScholarGoogle Scholar |
[9] Götz HM, van Doornum G, Niesters HG, den Hollander JG, Thio HB, de Zwart O. A cluster of acute hepatitis C virus infection among men who have sex with men: results from contact tracing and public health implications. AIDS 2005; 19 969–74.
| A cluster of acute hepatitis C virus infection among men who have sex with men: results from contact tracing and public health implications.Crossref | GoogleScholarGoogle Scholar | 15905679PubMed |
[10] Danta M, Brown D, Bhagani S, Pybus OG, Sabin CA, Nelson M, et al Recent epidemic of acute hepatitis C virus in HIV positive men who have sex with men linked to high-risk sexual behaviours. AIDS 2007; 21 983–91.
| Recent epidemic of acute hepatitis C virus in HIV positive men who have sex with men linked to high-risk sexual behaviours.Crossref | GoogleScholarGoogle Scholar | 17457092PubMed |
[11] Giraudon I, Ruf M, Maguire H, Charlett A, Ncube F, Turner J, et al Increase in diagnosed newly acquired hepatitis C in HIV-positive men who have sex with men across London and Brighton, 2002–2006: is this an outbreak? Sex Transm Infect 2008; 84 111–5.
| Increase in diagnosed newly acquired hepatitis C in HIV-positive men who have sex with men across London and Brighton, 2002–2006: is this an outbreak?Crossref | GoogleScholarGoogle Scholar | 17932125PubMed |
[12] Urbanus AT, van de Laar TJ, Stolte IG, Schinkel J, Heijman T, Coutinho RA, et al Hepatitis C virus infections among HIV infected men who have sex with men: an expanding epidemic. AIDS 2009; 23 F1–7.
| Hepatitis C virus infections among HIV infected men who have sex with men: an expanding epidemic.Crossref | GoogleScholarGoogle Scholar | 19542864PubMed |
[13] Rauch A, Rickenbach M, Weber R, Hirschel B, Tarr PE, Bucher HC, et al Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study. Clin Infect Dis 2005; 41 395–402.
| Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study.Crossref | GoogleScholarGoogle Scholar | 16007539PubMed |
[14] Bottieau E, Apers L, Van Esbroeck M, Vandenbruaene M, Florence E. Hepatitis C virus infection in HIV-infected men who have sex with men: sustained rising incidence in Antwerp, Belgium, 2001-2009. Euro Surveill 2010; 15 19673
| Hepatitis C virus infection in HIV-infected men who have sex with men: sustained rising incidence in Antwerp, Belgium, 2001-2009.Crossref | GoogleScholarGoogle Scholar | 20929655PubMed |
[15] Wandeler G, Gsponer T, Bregenzer A, Günthard HF, Clerc O, Calmy A, et al Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic. Clin Infect Dis 2012; 55 1408–16.
| Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic.Crossref | GoogleScholarGoogle Scholar | 22893583PubMed |
[16] van Santen DK, van der Helm JJ, Del Amo J, Meyer L, D’Arminio Monforte A, Price M, et al Lack of decline in hepatitis C virus incidence among HIV-positive men who have sex with men during 1990–2014. J Hepatol 2017; 67 255–62.
| Lack of decline in hepatitis C virus incidence among HIV-positive men who have sex with men during 1990–2014.Crossref | GoogleScholarGoogle Scholar | 28412290PubMed |
[17] Matthews GV, Hellard M, Kaldor J, Lloyd A, Dore GJ, et al Further evidence of HCV sexual transmission among HIV-positive men who have sex with men: response to Danta. AIDS 2007; 21 2112–3.
| Further evidence of HCV sexual transmission among HIV-positive men who have sex with men: response to Danta.Crossref | GoogleScholarGoogle Scholar | 17885306PubMed |
[18] Matthews GV, Hellard M, Haber P, Yeung B, Marks P, Baker D, et al Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C. Clin Infect Dis 2009; 48 650–8.
| Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C.Crossref | GoogleScholarGoogle Scholar | 19191653PubMed |
[19] Luetkemeyer A, Hare CB, Stansell J, Tien PC, Charlesbois E, Lum P, et al Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J Acquir Immune Defic Syndr 2006; 41 31–6.
| Clinical presentation and course of acute hepatitis C infection in HIV-infected patients.Crossref | GoogleScholarGoogle Scholar | 16340470PubMed |
[20] Samandari T, Tedaldi E, Armon C, Hart R, Chmiel JS, Brooks JT, et al Incidence of hepatitis C virus infection in the Human Immunodeficiency Virus Outpatient Study Cohort, 2000–2013. Open Forum Infect Dis 2017; 4 ofx076
| Incidence of hepatitis C virus infection in the Human Immunodeficiency Virus Outpatient Study Cohort, 2000–2013.Crossref | GoogleScholarGoogle Scholar | 28616444PubMed |
[21] Nishijima T, Shimbo T, Komatsu H, Hamada Y, Gatanaga H, Oka S. Incidence and risk factors for incident hepatitis C infection among men who have sex with men with HIV-1 infection in a large urban HIV clinic in Tokyo. J Acquir Immune Defic Syndr 2014; 65 213–7.
| Incidence and risk factors for incident hepatitis C infection among men who have sex with men with HIV-1 infection in a large urban HIV clinic in Tokyo.Crossref | GoogleScholarGoogle Scholar | 24185533PubMed |
[22] Lin AWC, Wong KH, Chan K. More safer sex intervention needed for HIV-positive MSM with higher education level for prevention of sexually transmitted hepatitis C. J Int AIDS Soc 2014; 17 19663
| More safer sex intervention needed for HIV-positive MSM with higher education level for prevention of sexually transmitted hepatitis C.Crossref | GoogleScholarGoogle Scholar |
[23] Lee S, Lee SH, Lee SJ, Kim KH, Lee JE, Cho H, et al Incidence and risk factors of hepatitis C virus infection among human immunodeficiency virus (HIV) patients in a large HIV clinic in South Korea. Korean J Intern Med 2016; 31 772–8.
| Incidence and risk factors of hepatitis C virus infection among human immunodeficiency virus (HIV) patients in a large HIV clinic in South Korea.Crossref | GoogleScholarGoogle Scholar | 27117318PubMed |
[24] Ho SY, Su LH, Sun HY, Huang YS, Chuang YC, Huang MH, et al Trends of recent hepatitis C virus infection among HIV-positive men who have sex with men in Taiwan, 2011–2018. EClinicalMedicine 2020; 24 100441
| Trends of recent hepatitis C virus infection among HIV-positive men who have sex with men in Taiwan, 2011–2018.Crossref | GoogleScholarGoogle Scholar | 32637905PubMed |
[25] Nijmeijer BM, Koopsen J, Schinkel J, Prins M, Geijtenbeek TB. Sexually transmitted hepatitis C virus infections: current trends, and recent advances in understanding the spread in men who have sex with men. J Int AIDS Soc 2019; 22 e25348
| Sexually transmitted hepatitis C virus infections: current trends, and recent advances in understanding the spread in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 31468692PubMed |
[26] Choy CY, Ang LW, Ng OT, Leo YS, Wong CS. Factors associated with hepatitis B and C co-infection among HIV-infected patients in Singapore, 2006–2017. Trop Med Infect Dis 2019; 4 87
| Factors associated with hepatitis B and C co-infection among HIV-infected patients in Singapore, 2006–2017.Crossref | GoogleScholarGoogle Scholar |
[27] Ministry of Health Singapore. Update on the HIV/AIDS situation in Singapore 2018 (June 2019). 2019. Available at https://www.moh.gov.sg/resources-statistics/infectious-disease-statistics/hiv-stats/update-on-the-hiv-aids-situation-in-singapore-2018-(june-2019) [accessed 30 September 2019].
[28] Wong YJ, Cheen MH, Hsiang JC, Kumar R, Tan J, Teo EK, et al Economic evaluation of direct-acting antivirals for the treatment of genotype 3 hepatitis C infection in Singapore. JGH Open 2019; 3 210–6.
| Economic evaluation of direct-acting antivirals for the treatment of genotype 3 hepatitis C infection in Singapore.Crossref | GoogleScholarGoogle Scholar | 31276038PubMed |
[29] Soh BYM, Kumar R, Ekstrom VSM, Lin CYH, Thangaraju S, Tan HH, et al Prevalence of hepatitis C virus infection and the IL28B genotype polymorphism among blood donors and high-risk populations. Singapore Med J 2019; 60 34–9.
| Prevalence of hepatitis C virus infection and the IL28B genotype polymorphism among blood donors and high-risk populations.Crossref | GoogleScholarGoogle Scholar | 29926111PubMed |
[30] Chaillon A, Sun X, Cachay ER, Looney D, Wyles D, Garfein RS, et al Primary incidence of hepatitis C virus infection among HIV-infected men who have sex with men in San Diego, 2000–2015. Open Forum Infect Dis 2019; 6 ofz160
| Primary incidence of hepatitis C virus infection among HIV-infected men who have sex with men in San Diego, 2000–2015.Crossref | GoogleScholarGoogle Scholar | 31041355PubMed |
[31] Gamage DG, Read TR, Bradshaw CS, Hocking JS, Howley K, Chen MY, et al Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis 2011; 11 39
| Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study.Crossref | GoogleScholarGoogle Scholar | 21291565PubMed |
[32] Pradat P, Huleux T, Raffi F, Delobel P, Valantin M, Poizot-Martin I, et al Incidence of new hepatitis C virus infection is still increasing in French MSM living with HIV. AIDS 2018; 32 1077–82.
| Incidence of new hepatitis C virus infection is still increasing in French MSM living with HIV.Crossref | GoogleScholarGoogle Scholar | 29438195PubMed |
[33] van de Laar TJ, Van der Bij AK, Prins M, Bruisten SM, Brinkman K, Ruys TA, et al Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis 2007; 196 230–8.
| Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission.Crossref | GoogleScholarGoogle Scholar | 17570110PubMed |
[34] Medland NA, Chow EP, Bradshaw CS, Read TH, Sasadeusz JJ, Fairley CK. Predictors and incidence of sexually transmitted Hepatitis C virus infection in HIV positive men who have sex with men. BMC Infect Dis 2017; 17 185
| Predictors and incidence of sexually transmitted Hepatitis C virus infection in HIV positive men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 28253838PubMed |
[35] Ang LW, Wong CS, Ng OT, Leo YS. Incidence of syphilis among HIV-infected men in Singapore, 2006–2017: temporal trends and associated risk factors. Sex Transm Infect 2020; 96 293–9.
| Incidence of syphilis among HIV-infected men in Singapore, 2006–2017: temporal trends and associated risk factors.Crossref | GoogleScholarGoogle Scholar | 31371448PubMed |
[36] Thurnheer MC, Weber R, Toutous-Trellu L, Cavassini M, Elzi L, Schmid P, et al Occurrence, risk factors, diagnosis and treatment of syphilis in the prospective observational Swiss HIV Cohort Study. AIDS 2010; 24 1907–16.
| Occurrence, risk factors, diagnosis and treatment of syphilis in the prospective observational Swiss HIV Cohort Study.Crossref | GoogleScholarGoogle Scholar | 20616699PubMed |
[37] Abara WE, Hess KL, Neblett Fanfair R, Bernstein KT, Paz-Bailey G. Syphilis trends among men who have sex with men in the United States and Western Europe: a systematic review of trend studies published between 2004 and 2015. PLoS One 2016; 11 e0159309
| Syphilis trends among men who have sex with men in the United States and Western Europe: a systematic review of trend studies published between 2004 and 2015.Crossref | GoogleScholarGoogle Scholar | 27556925PubMed |
[38] Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2017. Sydney: Kirby Institute, UNSW Sydney; 2017. Available at https://kirby.unsw.edu.au/sites/default/files/kirby/report/SERP_Annual-Surveillance-Report-2017_compressed.pdf [accessed 16 January 2020].
[39] Tucker JD, Yin Y-P, Wang B, Chen X-S, Cohen MS. An expanding syphilis epidemic in China: epidemiology, behavioural risk and control strategies with a focus on low-tier female sex workers and men who have sex with men. Sex Transm Infect 2011; 87 ii16–8.
| An expanding syphilis epidemic in China: epidemiology, behavioural risk and control strategies with a focus on low-tier female sex workers and men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 22110145PubMed |
[40] Action for AIDS Singapore. Press Room: MSM outreach HIV testing project round 6 (2013). 2013. Available at https://afa.org.sg/portfolio-item/msm-outreach-hiv-testing-project-round-6/ [accessed 15 February 2019].
[41] Wee S, Barrett ME, Lian WM, Jayabaskar T, Chan KW. Determinants of inconsistent condom use with female sex workers among men attending the STD clinic in Singapore. Sex Transm Infect 2004; 80 310–4.
| Determinants of inconsistent condom use with female sex workers among men attending the STD clinic in Singapore.Crossref | GoogleScholarGoogle Scholar | 15295132PubMed |
[42] Wong ML, Sen P, Wong CM, Tjahjadi S, Govender M, Koh TT, et al Human immunodeficiency virus (HIV) prevention education in Singapore: challenges for the future. Ann Acad Med Singap 2012; 41 602–9.
| 23303119PubMed |
[43] Hoornenborg E, Achterbergh RCA, Schim van der Loeff MF, Davidovich U, Hogewoning A, de Vries HJC, et al MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection. AIDS 2017; 31 1603–10.
| MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection.Crossref | GoogleScholarGoogle Scholar | 28657964PubMed |
[44] Hasse B, Ledergerber B, Hirschel B, Vernazza P, Glass TR, Jeannin A, et al Frequency and determinants of unprotected sex among HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2010; 51 1314–22.
| Frequency and determinants of unprotected sex among HIV-infected persons: the Swiss HIV cohort study.Crossref | GoogleScholarGoogle Scholar | 21034200PubMed |
[45] Hagan H, Jordan AE, Neurer J, Cleland CM. Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men. AIDS 2015; 29 2335–45.
| Incidence of sexually transmitted hepatitis C virus infection in HIV-positive men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 26258525PubMed |
[46] Centers for Disease Control and Prevention (CDC) Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men – New York City, 2005–2010. MMWR Morb Mortal Wkly Rep 2011; 60 945–50.
| 21775948PubMed |
[47] van de Laar T, Pybus O, Bruisten S, Brown D, Nelson M, Bhagani S, et al Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 2009; 136 1609–17.
| Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 19422083PubMed |
[48] Serpaggi J, Chaix ML, Batisse D, Dupont C, Vallet-Pichard A, Fontaine H, et al Sexually transmitted acute infection with a clustered genotype 4 hepatitis C virus in HIV-1-infected men and inefficacy of early antiviral therapy. AIDS 2006; 20 233–40.
| Sexually transmitted acute infection with a clustered genotype 4 hepatitis C virus in HIV-1-infected men and inefficacy of early antiviral therapy.Crossref | GoogleScholarGoogle Scholar | 16511416PubMed |
[49] van de Laar T, Pybus O, Bruisten S, Brown D, Nelson M, Bhagani S, et al Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 2009; 136 1609–17.
| Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 19422083PubMed |
[50] Chaillon A, Sun X, Cachay ER, Looney D, Wyles D, Garfein RS, et al Primary incidence of hepatitis C virus infection among HIV-infected men who have sex with men in San Diego, 2000–2015. Open Forum Infect Dis 2019; 6 ofz160
| Primary incidence of hepatitis C virus infection among HIV-infected men who have sex with men in San Diego, 2000–2015.Crossref | GoogleScholarGoogle Scholar | 31041355PubMed |
[51] National Centre for Infectious Diseases (NCID). Recommendations for the use of antiretroviral therapy in adults living with HIV in Singapore. Singapore HIV Congress, 30 November 2019. Available at https://www.ncid.sg/About-NCID/OurDepartments/Documents/ART%20Recommendations.pdf [accessed 23 January 2020].
[52] Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. 2020. Available at https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Adult_OI.pdf [accessed 7 December 2020].
[53] Workowski KA, Bolan GA, Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64 1–137. [Erratum in MMWR Recomm Rep 2015; 64(33): 924]
| 26042815PubMed |
[54] European AIDS Treatment Network (NEAT) Acute Hepatitis C Infection Consensus Panel Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. AIDS 2011; 25 399–409.
| Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference.Crossref | GoogleScholarGoogle Scholar | 21139491PubMed |
[55] Sun HY, Chang SY, Yang ZY, Lu CL, Wu H, Yeh CC, et al Recent hepatitis C virus infections in HIV-infected patients in Taiwan: incidence and risk factors. J Clin Microbiol 2012; 50 781–7.
| Recent hepatitis C virus infections in HIV-infected patients in Taiwan: incidence and risk factors.Crossref | GoogleScholarGoogle Scholar | 22189113PubMed |
[56] Bottieau E, Apers L, Van Esbroeck M, Vandenbruaene M, Florence E. Hepatitis C virus infection in HIV-infected men who have sex with men: sustained rising incidence in Antwerp, Belgium, 2001–2009. Euro Surveill 2010; 15 19673
| Hepatitis C virus infection in HIV-infected men who have sex with men: sustained rising incidence in Antwerp, Belgium, 2001–2009.Crossref | GoogleScholarGoogle Scholar | 20929655PubMed |
[57] Reiberger T. Acute hepatitis C virus infection in HIV-infected men who have sex with men: should we change our screening practice? Clin Infect Dis 2014; 59 1694–5.
| Acute hepatitis C virus infection in HIV-infected men who have sex with men: should we change our screening practice?Crossref | GoogleScholarGoogle Scholar | 25186589PubMed |
[58] Martin TC, Martin NK, Hickman M, Vickerman P, Page EE, Everett R, et al Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM. AIDS 2013; 27 2551–7.
| Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM.Crossref | GoogleScholarGoogle Scholar | 23736152PubMed |
[59] Linas BP, Wong AY, Schackman BR, Kim AY, Freedberg KA. Cost-effective screening for acute hepatitis C virus infection in HIV-infected men who have sex with men. Clin Infect Dis 2012; 55 279–90.
| Cost-effective screening for acute hepatitis C virus infection in HIV-infected men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 22491339PubMed |
[60] World Health Organization. Hepatitis C. 2019. Available at www.who.int/news-room/fact-sheets/detail/hepatitis-c [accessed 13 January 2020].
[61] Thomson EC, Nastouli E, Main J, Karayiannis P, Eliahoo J, Muir D, et al Delayed anti-HCV antibody response in HIV-positive men acutely infected with HCV. AIDS 2009; 23 89–93.
| Delayed anti-HCV antibody response in HIV-positive men acutely infected with HCV.Crossref | GoogleScholarGoogle Scholar | 19050390PubMed |
[62] Strasak AM, Kim AY, Lauer GM, de Sousa PS, Ginuino CF, Fernandes CA, et al Antibody dynamics and spontaneous viral clearance in patients with acute hepatitis C infection in Rio de Janeiro, Brazil. BMC Infect Dis 2011; 11 15
| Antibody dynamics and spontaneous viral clearance in patients with acute hepatitis C infection in Rio de Janeiro, Brazil.Crossref | GoogleScholarGoogle Scholar | 21226945PubMed |
[63] Vanhommerig JW, Thomas XV, van der Meer JT, Geskus RB, Bruisten SM, Molenkamp R, et al Hepatitis C virus (HCV) antibody dynamics following acute HCV infection and reinfection among HIV-infected men who have sex with men. Clin Infect Dis 2014; 59 1678–85.
| Hepatitis C virus (HCV) antibody dynamics following acute HCV infection and reinfection among HIV-infected men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 25186590PubMed |