Lifetime cost of HIV management in Australia: an economic model†
Megumi Lim A , Angela Devine
A , Angela Devine  A B , Richard T. Gray C , Jisoo A. Kwon C , Jolie L. Hutchinson C and Jason J. Ong
A B , Richard T. Gray C , Jisoo A. Kwon C , Jolie L. Hutchinson C and Jason J. Ong  A D E F *
A D E F *
A Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Vic., Australia.
B Global and Tropical Health Division, Menzies School of Health Research, Charles Darwin University, Darwin, NT, Australia.
C The Kirby Institute, UNSW Sydney, Sydney, NSW, Australia.
D Melbourne Sexual Health Centre, The Alfred Hospital, Melbourne, Vic., Australia.
E Central Clinical School, Monash University, Melbourne, Vic., Australia.
F Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, UK.
Sexual Health 19(6) 517-524 https://doi.org/10.1071/SH21250
Submitted: 25 December 2021 Accepted: 27 July 2022 Published: 30 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Background: Antiretroviral therapy (ART) for HIV has significantly reduced morbidity and mortality, but the drugs can be expensive. This study aimed to estimate the lifetime cost of HIV management from the Australian healthcare perspective.
Methods: A Markov cohort model, consisting of 21 health states based on CD4 count and line of ART, simulated disease progression over the lifetime of persons living with HIV. We reported costs using 2019 Australian dollars (A$) at a discount rate of 3.5% per annum. One-way sensitivity analysis was used to assess the impact of model inputs, and probabilistic sensitivity analyses were conducted to calculate the 95% confidence intervals for the lifetime cost estimate.
Results: The average discounted lifetime cost of HIV management was A$282 093 (95% CI: $194 198–421 615). The largest proportion of lifetime cost was due to ART (92%). The lifetime cost was most sensitive to third- and second-line ART costs, followed by the probability of failing third-line therapy for those with a CD4 count of <200 cells/μL. A 20% or 50% reduction in patented ART costs would reduce the lifetime cost to A$243 638 and A$161 400, respectively. Replacing patented ART drugs with currently available generic equivalents reduced the lifetime cost to A$141 345.
Conclusion: The relatively high lifetime costs for managing HIV mean that ongoing investment will be required to provide care and treatment to people living with HIV, and supports the urgent need to avert new infections. Reducing the price of ARTs (including consideration of generic drugs) would have the most significant impact on lifetime costs.
Keywords: antiretroviral therapy, Australia, health economics, HIV/AIDS, lifetime cost, living with HIV, management cost, mathematical models.
Introduction
The Joint United Nations Programme on Human Immunodeficiency Virus and Acquired Immune Deficiency Syndrome (UNAIDS) has set an ambitious goal of 95:95:95 by 2030 to end Acquired Immune Deficiency Syndrome (AIDS) as a public health threat.1 This means 95% of people living with human immunodeficiency virus (PLHIV) will know their status, 95% of people who know their status will be on antiretroviral therapy (ART), and 95% of people on treatment will have a suppressed viral load.1 This strategy aims to control the HIV pandemic to reach a HIV-free world.2 Australia aims to achieve the 95:95:95 targets by 2022.3 As of 2020, 90% of PLHIV in Australia were aware of their status, 89% of those diagnosed received treatment, and 95% of those on treatment have suppressed viral loads.4 Collectively, 73% of PLHIV in Australia have suppressed viral loads,4 which is contributing to ongoing reductions of new HIV diagnoses in recent years. However, there remain challenges to improve the diagnosis of those unaware of their HIV infection and their access to ART, particularly among overseas-born individuals.5
Accessibility to testing services and treatment needs to be improved to meet the 95:95:95 targets by 2022. However, due to the extensive resources required to realise these ambitions (which includes access to HIV testing, procurement of drugs, and sufficient resources to keep people on treatment for life), an accurate estimation of the lifetime cost of managing HIV is essential for policymakers who are involved in healthcare budget planning and decision-making.2 Many aspects of HIV management have substantially changed in the last decade, affecting underlying cost inputs. With the emergence of newer and highly effective ART drugs with less toxicity, less HIV-related complications, and less complicated drug dosing schedules, PLHIV are now surviving longer and with less morbidity.6,7 Further improvements have been achieved with earlier commencement of therapy (i.e. starting ART for newly diagnosed PLHIV regardless of CD4 count) and improvements in monitoring viral load and ART resistance.8
Similar to many high-income countries, Australia’s management for PLHIV has improved with less morbidity and mortality.9 Thus, regularly updated cost estimates are essential to ensure accurate health budgeting. In the past 20 years, only two estimates of the lifetime cost of HIV management in Australian adults have been published.10 Gray et al. estimated a lifetime cost of A$424 844 (discounted at 3.5%, 2015 A$),11 and the study by Tilden et al. reported a lifetime cost of A$146 300 (discounted at 5%, 2010 A$).12 However, since the publication of these two estimates, Australia has made changes related to commonly used antiretroviral regimens (e.g. access to cheaper single-tablet regimens) and patient management (e.g. 6-monthly review for most PLHIV rather than 3-monthly). Hence, this study aimed to provide an updated estimate of the lifetime cost of HIV care from the Australian healthcare provider perspective.
Methods
Analytic overview and model structure
Using TreeAge Pro 2020, R2. (TreeAge Software, Williamstown, MA, USA), a Markov cohort model was built to simulate disease progression for PLHIV based on their CD4 T-lymphocyte counts (categorised as >500 cells/μL; 351–500 cells/μL; 201–350 cells/μL; 50–200 cells/μL; <50 cells/μL) and line of treatment (no ART, first, second, and third line of ART; Fig. 1). Disease progression was modelled as 6-monthly transitions between these health states. A cycle length of 6 months was chosen to reflect the frequency of clinical review for most PLHIV in Australian clinical practice.13 In each cycle, simulated individuals probabilistically experience infection control (i.e. HIV is virologically suppressed, which is defined as <200 copies/mL), treatment failure, CD4+ decline, and death according to their CD4+ health state and age. In the higher CD4 count categories (>500 cells/μL and 351–500 cells/μL) of the Markov model, once a person is on treatment, we assumed they remained in the same CD4 count category unless they had a virologic failure or were not on ART (when they would have a probability of going to a lower CD4 category).
|
|
Treatment efficacy, disease progression and probability of death
The model parameters for the proportion of the population in each health state at the start of the model (Supplementary material Table S1), relative mortality rate, and disease progression based on line of ART (Table S2) were taken primarily from the Australian HIV Observational Database (AHOD), which has the most comprehensive surveillance data of PLHIV in Australia.14 The AHOD is a prospective cohort study established in 1999 to monitor treatment uptake and outcomes of PLHIV under routine clinical care around Australia.14 To ensure a more contemporary cohort, data from the AHOD was restricted to those participants whose first 3-drug regimen was on-going as of 31 March 2018, or ended at some point after 1 January 2010 prior to changing to another 2+-drug regimen (N = 1753) (J. Hutchinson, pers. comm., 31 July 2020). The age-related baseline probability of death was taken from the 2016–18 Australian Bureau of Statistics life tables and weighted according to the proportion of males and females among PLHIV (Table S3).15 Other literature sources on HIV progression were used for the relative probability of death (for CD4 count <50 cells/μL), probability of starting ART and probability of hospitalisation.16–19
As we aimed to model the cross-section of existing PLHIV in Australia, those not receiving ART or are yet to be diagnosed were also accounted for in the model in the starting population.
Cost estimation
The lifetime HIV-related patient care and treatment costs were calculated from the Australian healthcare provider’s perspective and discounted at 3.5%, as required by the Pharmaceutical Benefits Advisory Committee Guidelines.20 We included direct HIV-related clinical costs (e.g. consultations and laboratory testing), ART drugs, prophylaxis for opportunistic infections, and hospitalisations for those with CD4 counts <200 cells/μL (Table S4). A half-cycle correction was applied to all costs. To determine the proportion of the lifetime cost estimate attributable to each cost item, seven cost categories were created: first-line ART, second-line ART, third-line ART, prophylaxis drugs, consultations, laboratory tests, and hospitalisation.
The choice of first-, second- and third-line ART included in the cost calculations were decided based on commonly prescribed medications following the Australian HIV guidelines.13 In the base case analysis, the costs of ART drugs and prophylaxis drugs for opportunistic infections (antibiotics) were taken from published dispensing prices on the Pharmaceutical Benefits Scheme (Table S4).21 Details of the breakdown of the cost items can be found in Table S5.
HIV-related hospitalisation costs were taken from the Australian Refined Diagnosis Related Groups (AR-DRG) V8.0, the last published data from the Independent Hospital Pricing Authority that included HIV-related codes.22 HIV-related codes have since been removed from the AR-DRG. Because of the limited data on how the AR-DRG complexity categories were defined, the three AR-DRG codes’ average cost was used as the total cost of hospitalisation (Table S6). The costs (minor, intermediate and major complexity) were inflated from 2017 to 2019 using the Australian Consumer Price Index.23 These hospitalisation costs were multiplied by corresponding probabilities of hospitalisation in each CD4 health state in the model (Table 1). The costs of routine medical consultation and laboratory tests were taken from the Medicare Benefits Schedule (Tables S7–S9).24
|
Sensitivity and scenario analyses
We conducted one-way sensitivity analyses to explore which variables were the main drivers of lifetime cost. We evaluated all input parameters, such as costs (routine medical consultations, laboratory testing and hospitalisation and ART prophylaxis drugs) and transition probabilities. Where available, 95% confidence intervals were used as input range values, and where not available (i.e. cost inputs and life tables), the parameter ranges were assumed to vary by ±30% (Table S10). We also evaluated the impact of discounting at 0 and 5%.
Probabilistic sensitivity analysis (PSA) accounted for joint uncertainty of the most influential input parameters. We included the top 10 cost parameters and top 10 probability parameters from the univariate sensitivity analysis in the PSA (Table S11). We used gamma distributions to describe cost parameters and beta distributions for transition probabilities, according to best practice. The PSA was run with 10 000 iterations using Monte Carlo simulation.
Scenario analyses were conducted to explore the following:
-
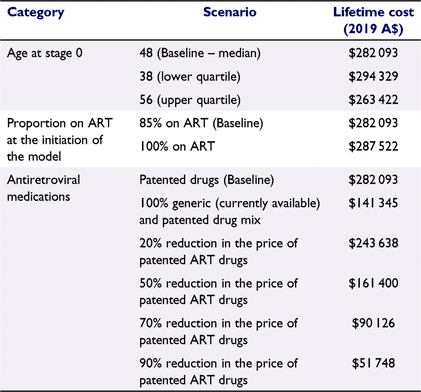
The impact of changing the starting age of the cohort from 48 years to 38 and 56 years (IQR for patient age from the AHOD study).
-
Increasing the proportion of the cohort on ART at the initiation of the model from 85% to 100%.
-
Switching all ART to generics where available.25
-
Reducing the price of patented ART by 20–90%. Patented drug prices from the PBS were used where generic equivalents were not available (Table S5). The cost of prophylaxis for opportunistic infections was taken from the PBS as generic equivalents’ online prices were more expensive than PBS prices.
Results
Base case
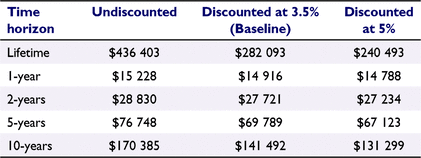
The mean lifetime cost was A$282 093 (95% CI: $194 198–421 615; Table 1) at the baseline discount rate of 3.5%. The undiscounted lifetime cost was A$436 403 (95% CI: 293 466–699 883). When increasing the discount rate to 5%, the lifetime cost decreased to A$240 493 (95% CI: 166 285–351 252). Table 2 shows the lifetime cost according to a range of time horizons and discounting rates.
|
In the one-way sensitivity analysis, we found that the cost of third-line ART, at A$160 405, was the primary driver of the lifetime cost estimate, contributing 56.9% of the costs (Fig. 2). This was followed by the second- and first-line ART at A$57 053 (20.2%) and A$41 591 (14.7%), respectively. Lifetime costs from laboratory tests, prophylaxis medications, and consultation costs were A$15 161 (5.4%), A$5773 (2.0%) and A$1625 (0.6%), respectively. The mean direct HIV-related lifetime hospitalisation cost of A$395 only constituted 0.1% of lifetime treatment costs.
|
|
Sensitivity and scenario analyses
The parameters that had the largest impact on lifetime cost were third- and second-line ART drugs, followed by the probability of failure of third-line ART for those whose CD4 counts were <200 cells/μL (Fig. 3).
|
|
From our scenario analysis, if the cohort of PLHIV in Australia were at the lower quartile age of the AHOD cohort (38 instead of 48 years), the average lifetime cost would increase by A$12 236 (J. Hutchinson, pers. comm., 31 July 2020; Table 2). Conversely, if the cohort of PLHIV in Australia were at the upper quartile age of the AHOD cohort (56 years), the average lifetime cost would reduce by A$18 671. The lifetime cost associated with replacing patented drugs with generic drugs (where available) was A$141 345, which is nearly half of the lifetime cost estimate using patented medications from the PBS.
Discussion
This study estimated the lifetime cost of HIV management to be A$282 093 from the Australian healthcare provider perspective. This study adds to the global body of literature on estimating the lifetime cost of HIV management, providing a comparison to lifetime cost estimates in other countries and a reference for future Australian lifetime cost models for healthcare budgeting.10 We also add to the international literature by underscoring the relatively high contribution of the cost of ART and thus the potential cost-savings from using generic drugs. When multiplying the lifetime cost estimate by the estimated number of PLHIV in Australia (for the Australian population of PLHIV at the start of 2019), it would cost the government an estimated A$7.9 billion over the next 61 years.4 This projected financial impact does not account for the hundreds of new HIV infections in Australia each year. Additionally, because our objective was to estimate the cost from the Australian healthcare provider perspective, our lifetime cost estimate does not include productivity losses and out-of-pocket costs incurred by patients.
There have only been two studies that attempted to estimate the lifetime cost of HIV management in adults living with HIV in Australia.11,12 Our estimate of A$282 093 lies between the estimates of the other two studies from Australia (A$175 263–A$454 215) when inflated to 2019 A$.11,12 The differences in this study’s cost estimates are likely due to the differences in methodology and cost of the previous studies’ ART regimes. For example, the estimate (A$424 822) proposed by Gray et al. was calculated based on the average cost per year from the start of treatment, and included more expensive drug regimens (it was assumed that the average cost of first-line ART in the first 9 years to be A$10 685 per annum, followed by 14 years on the second-line at A$19 364 per annum, 3 years on third-line ART at A$31 411 per annum, and 14 years on the fourth-line at A$28 162 per annum) and monitoring regimes (annual undiscounted monitoring costs were A$4000 regardless of viral load).11 In contrast, our study’s annual undiscounted monitoring costs were lower at A$954 and A$3388 for those with controlled viral loads and uncontrolled viral loads, respectively. The study by Gray et al. did not use a decision model, but used cross-sectional averages to produce an annual cost estimate.11 The second study by Tilden et al. reported discounted (5%) lifetime costs to be A$146 300 (using Raltegravir as first-line ART).12 Although generated with a Markov model, the findings by Tilden et al. were summarised in a conference abstract, and the complete methodology was not published, so it was not possible to understand the assumptions in their model.12
In our study, all three lines of ART combined took up the largest proportion of lifetime treatment costs (92%) (Fig. 2). This is consistent with other studies in the systematic review, which found that the majority of projected lifetime cost (54–88%) was attributable to ART costs.10,26,27 Combined with the difference in lifetime cost estimates from replacing patented ART drugs with generic drugs (Table 2), this supports the findings from the univariate sensitivity analysis (Fig. 3) that costs of ARTs are the most important factor in the accrued lifetime cost of HIV management. The contribution of ART drugs to lifetime cost is higher in this study than other studies in the systematic review.10 Although this may be attributable to differences between healthcare systems and cost items across papers, the relatively high proportion of lifetime cost contributed by ART drugs in our study is more likely to be a reflection of reduced complications (and need for hospitalisation) as a consequence of drug developments. This increase in contribution of ART drugs to lifetime costs was also observed over the years of publication in the systematic review.10
The lifetime estimate from completely replacing patented ART drugs with currently available generic ART drugs resulted in a 50% reduction in lifetime cost. Further, in our hypothetical scenario analyses, a 50% reduction in patented ART drugs prices resulted in a 43% reduction in lifetime cost, whereas a 90% reduction in patented ART drugs prices resulted in an 82% reduction in lifetime cost. In replacing the cost of patented ART drugs with the cost of generic drugs in the model, there was an inherent assumption that generic drugs result in the same adherence level and hence efficacy as patented drugs. We did not account for potential differences in the effectiveness of generic ART from patented ART, such as increased pill burden with generic ARTs resulting in poorer adherence, lowered viral suppression, and increased frequency of HIV mutations leading to ART resistance. These factors could be modelled if future empirical data demonstrate significant differences in effectiveness with the use of generic ART.
Our study has several limitations. First, we used data from the 2019 New South Wales HIV strategy report (where 94% of people were diagnosed with a CD4 count of <500 cells/μL, and 82% were diagnosed with a CD4 count of >500 cells/μL were reported to be on ART, respectively) to identify the proportion of people in each CD4 category not on ART.28 Applying the on-treatment proportions using the AHOD cohort (J. Hutchinson, pers. comm., 31 July 2020) by CD4 count, starting proportion on ART in our model was only 85%, which is less than the 89% estimated in the annual surveillance report.4 However, our univariate sensitivity analysis found that the proportion of PLHIV on ART at the start of the model had minimal impact on the lifetime cost estimate. Second, our model’s rate of hospitalisation was not age-specific due to a lack of available data; however, this is unlikely to impact our findings significantly as hospitalisation only accounted for 0.1% of the lifetime cost estimate. Third, ART switches in the AHOD data were only for people experiencing virological failure. In the clinical setting, switches can also occur due to the development of side-effects, pill burden, development of comorbidities, lack of compliance to ART and loss to follow up, so these may be underestimated in our model. Loss to follow up may also be more likely among younger patients, people who inject drugs, those with a higher baseline viral load, or with prior episodes of loss to follow up.29 A future microsimulation model (using individual data) could account for these factors and confirm the impact of including these model parameters on the estimated lifetime cost of HIV management. Finally, collecting costs from a societal perspective was beyond the scope of our study. However, future studies may consider using a societal perspective to better capture the broad range of costs related to people living with HIV.
Despite these limitations, to our knowledge, this study provides the most robust estimate of lifetime cost of HIV management in Australia, modelled using the latest available data of PLHIV in Australia and the latest recommended ART regimes. Future models could improve the existing Markov model by exploring the impact of comorbidities and other co-infections associated with HIV (such as Hepatitis C) and ART switches for other reasons (apart from virological failure). Additionally, estimating lifetime costs for different sub-populations (such as men who have sex with men, intravenous drug users, the non-Medicare eligible, and members of the culturally and linguistically diverse community) is also important for health policy decision-makers due to variation in health service utility between these communities.30 For example, there are important differences of equitable access to HIV diagnosis and treatment among migrant populations, compared to their native-born counterparts.5 Regular updating of lifetime costs should be undertaken with any future changes in HIV management, particularly when related to ART costs.
Conclusion
Adequate financing is essential to reach the UNAIDS 95:95:95 targets, for ongoing management of people living with HIV and investments in programs to avert new infections. From the Australian healthcare providers’ perspective, the estimated lifetime cost of HIV care was A$282 093. This high lifetime cost estimate provides evidence for the cost-effectiveness of interventions seeking to prevent HIV acquisition, highlighting the need to strengthen investments into these programs. Our study also demonstrates the impact on lifetime costs by replacing patented ART drugs with generic equivalents. Although we acknowledge it would not be realistic to use generic ART for all patients, increasing the proportion of PLHIV using generic ART or reducing the price of patented ART drugs could result in substantial cost savings.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
Jason J. Ong is the Special Issues Editor for Sexual Health and was blinded from the peer-review process for this paper.
Declaration of funding
Jason J. Ong is supported by an Australian National Health and Medical Research Council (NHMRC) Emerging Leader Investigator Grant (GNT1193955). The funding body did not influence the research or decision to publish. The Kirby Institute is funded by the Australian Government Department of Health, and is affiliated with the Faculty of Medicine, UNSW Sydney, Australia.
References
[1] UNAIDS. Fast-Track: ending the AIDS epidemic by 2030. Geneva, Switzerland: UNAIDS; 2014.[2] Gray G. HIV, AIDS and 90-90-90: what is it and why does it matter? 2016. Available at https://theconversation.com/hiv-aids-and-90-90-90-what-is-it-and-why-does-it-matter-62136 [Accessed 22 November 2020]
[3] Australian Government, Department of Health. Eighth national HIV strategy 2018–2022. 2018. Available at https://www.health.gov.au/resources/publications/eighth-national-hiv-strategy-2018-2022 [Accessed 24 August 2022]
[4] Australian Federation of AIDS Organisations. HIV in Australia 2020. 2020. Available at https://www.afao.org.au/wp-content/uploads/2019/11/2725_afao_infographic_9.pdf [Accessed 24 August 2022]
[5] Marukutira T, Gunaratnam P, Douglass C, et al. Trends in late and advanced HIV diagnoses among migrants in Australia; implications for progress on Fast-Track targets: a retrospective observational study. Medicine (Baltimore) 2020; 99 e19289
| Trends in late and advanced HIV diagnoses among migrants in Australia; implications for progress on Fast-Track targets: a retrospective observational study.Crossref | GoogleScholarGoogle Scholar |
[6] Shoko C, Chikobvu D, Bessong PO. A Markov model to estimate mortality due to HIV/AIDS using viral load levels-based states and CD4 cell counts: a principal component analysis approach. Infect Dis Ther 2018; 7 457–71.
| A Markov model to estimate mortality due to HIV/AIDS using viral load levels-based states and CD4 cell counts: a principal component analysis approach.Crossref | GoogleScholarGoogle Scholar |
[7] University of New South Wales. Mapping HIV outcomes: geographical and clinical forecasts of numbers of people living with HIV in Australia. Sydney: University of New South Wales; 2015.
[8] Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17 e235–e79.
| Sexually transmitted infections: challenges ahead.Crossref | GoogleScholarGoogle Scholar |
[9] Kirby Institute. National update of HIV, viral hepatitis and sexually transmissible infections in Australia 2009–2018. 2020. Available at https://data.kirby.unsw.edu.au/hiv [Accessed 24 August 2022]
[10] Tran H, Saleem K, Lim M, et al. Global estimates for the lifetime cost of managing HIV: a systematic review. AIDS 2021; 35 1273–81.
| Global estimates for the lifetime cost of managing HIV: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[11] Gray RT, Watson J, Cogle AJ, et al. Funding antiretroviral treatment for HIV-positive temporary residents in Australia prevents transmission and is inexpensive. Sex Health 2018; 15 13–9.
| Funding antiretroviral treatment for HIV-positive temporary residents in Australia prevents transmission and is inexpensive.Crossref | GoogleScholarGoogle Scholar |
[12] Tilden D, Jackson D, LeReun C, et al. A modeled economic evaluation of raltegravir compared to standard practice in Australia for treatment naïve patients with HIV. Value Health 2010; 13 A550
| A modeled economic evaluation of raltegravir compared to standard practice in Australia for treatment naïve patients with HIV.Crossref | GoogleScholarGoogle Scholar |
[13] Medland N, Cornelisse V, Smith D. Antiretroviral drugs and other therapies in HIV patients. 2019. Available at https://hivmanagement.ashm.org.au/antiretroviral-drugs/ [Accessed 12 December 2020]
[14] Kirby Institute. The Australian HIV observational database (AHOD). 2020. Available at https://kirby.unsw.edu.au/project/ahod [Accessed 24 August 2022]
[15] Australian Bureau of Statistics. Life tables, states, territories and Australia: 2016–2018. 2019. Available at https://www.abs.gov.au/statistics/people/population/life-tables/2016-2018 [Accessed 24 August 2022]
[16] Moore CL, Grulich AE, Prestage G, et al. Hospitalisation rates and associated factors in community-based cohorts of HIV-infected and -uninfected gay and bisexual men. HIV Med 2016; 17 327–39.
| Hospitalisation rates and associated factors in community-based cohorts of HIV-infected and -uninfected gay and bisexual men.Crossref | GoogleScholarGoogle Scholar |
[17] Brogan AJ, Talbird SE, Davis AE, Wild L, Flanagan D. Is increased screening and early antiretroviral treatment for HIV-1 worth the investment? An analysis of the public health and economic impact of improvement in the UK. HIV Med 2019; 20 668–80.
| Is increased screening and early antiretroviral treatment for HIV-1 worth the investment? An analysis of the public health and economic impact of improvement in the UK.Crossref | GoogleScholarGoogle Scholar |
[18] Kwon JA, Anderson J, Kerr CC, et al. Estimating the cost-effectiveness of needle-syringe programs in Australia. AIDS 2012; 26 2201–10.
| Estimating the cost-effectiveness of needle-syringe programs in Australia.Crossref | GoogleScholarGoogle Scholar |
[19] Kerr CC, Stuart RM, Gray RT, et al. Optima: a model for HIV epidemic analysis, program prioritization, and resource optimization. JAIDS J Acquir Immune Defic Syndr 2015; 69 365–76.
| Optima: a model for HIV epidemic analysis, program prioritization, and resource optimization.Crossref | GoogleScholarGoogle Scholar |
[20] Australian Government, Department of Health and Aged Care. 3A.1 Overview and rationale of the economic evaluation. 2016. Available at https://pbac.pbs.gov.au/section-3a/3a-1-overview-and-rationale-of-economic-evaluation.html [Accessed 24 August 2022]
[21] Australian Government, Department of Health and Aged Care. Pharmaceutical Benefits Scheme (PBS). 2020. Available at https://www.pbs.gov.au/pbs/home [Accessed 24 August 2022]
[22] Independent Hospital Pricing Authority. National hospital cost data collection, AR-DRG cost weight tables V8.0x, Round 21 (Financial year 2016–17). 2019. Available at https://www.ihpa.gov.au/publications/national-hospital-cost-data-collection-ar-drg-cost-weight-tables-v80x-round-21 [Accessed 12 December 2020]
[23] Galarraga O, Kuo C, Mtukushe B, Maughan-Brown B, Harrison A, Hoare J. iSAY (incentives for South African youth): stated preferences of young people living with HIV. Soc Sci Med 2020; 265 113333
| iSAY (incentives for South African youth): stated preferences of young people living with HIV.Crossref | GoogleScholarGoogle Scholar |
[24] Australian Government, Department of Health and Aged Care. MBS online: Medicare Benefits Schedule. 2020. Available at http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home [Accessed 24 August 2022]
[25] Gambone GF, Feldman MB, Thomas-Ferraioli AY, Shubert V, Ghose T. Integrating financial incentives for viral load suppression into HIV care coordination programs: considerations for development and implementation. J Public Health Manag Pract 2020; 26 471–80.
| Integrating financial incentives for viral load suppression into HIV care coordination programs: considerations for development and implementation.Crossref | GoogleScholarGoogle Scholar |
[26] Hornberger J, Green J, Wintfeld N, et al. Cost-effectiveness of enfuvirtide for treatment-experienced patients with HIV in Italy. HIV Clin Trials 2005; 6 92–102.
| Cost-effectiveness of enfuvirtide for treatment-experienced patients with HIV in Italy.Crossref | GoogleScholarGoogle Scholar |
[27] Peng S, Tafazzoli A, Dorman E, Rosenblatt L, Villasis-Keever A, Sorensen S. Cost-effectiveness of DTG + ABC/3TC versus EFV/TDF/FTC for first-line treatment of HIV-1 in the United States. J Med Econ 2015; 18 763–76.
| Cost-effectiveness of DTG + ABC/3TC versus EFV/TDF/FTC for first-line treatment of HIV-1 in the United States.Crossref | GoogleScholarGoogle Scholar |
[28] New South Wales Government. NSW HIV Surveillance Data Reports: Fourth quarter and annual data report 2019. 2019. Available at https://www.health.nsw.gov.au/endinghiv/Publications/q4-2019-and-annual-hiv-data-report.pdf [Accessed 24 August 2022].
[29] McManus H, Petoumenos K, Brown K, et al. Loss to follow-up in the Australian HIV Observational Database. Antivir Ther 2015; 20 731–41.
| Loss to follow-up in the Australian HIV Observational Database.Crossref | GoogleScholarGoogle Scholar |
[30] Hutchinson AB, Farnham PG, Dean HD, et al. The economic burden of HIV in the United States in the era of highly active antiretroviral therapy: evidence of continuing racial and ethnic differences. JAIDS J Acquir Immune Defic Syndr 2006; 43 451–7.
| The economic burden of HIV in the United States in the era of highly active antiretroviral therapy: evidence of continuing racial and ethnic differences.Crossref | GoogleScholarGoogle Scholar |
† A preprint version of this article is available at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3868096. Data presented at the following meetings: International Health Economics Association (iHEA) 2021 Congress, virtual, 13 July 2021; and Joint Australasian Sexual Health+ HIV&AIDS (ASHM) Conference 2021, virtual, 9 September 2021.


