The use of saliva as a lubricant in relation to sexual behavioural patterns in men who have sex with men: an exploratory cross-sectional study
Gaixia Li A , Yi Liu A , Yawu Hu A , Fang Lu A , Bingyang She A , Rui Zhao
A , Fang Lu A , Bingyang She A , Rui Zhao  A , Eric P. F. Chow
A , Eric P. F. Chow  B C D and Lei Zhang
B C D and Lei Zhang  A B C *
A B C *
A
B
C
D
Abstract
We aimed to characterise the sexual practices of MSM who reported using saliva as a lubricant during sexual episodes.
A cross-sectional study on sexual practices of MSM was conducted between February 2022 and September 2022 in Xi’an, China. ‘Saliva use as a lubricant’ was used as a strata in a subgroup analysis.
Among 1142 participants, 225 (19.7%) reported using saliva as a lubricant over the past 3 months. Among them, 114 did so during solo masturbation, whereas 124 did so in 149 sex acts with male partners (45 in providing masturbation, 32 in receiving masturbation, 39 during insertive anal sex and 33 during receptive anal sex). Of the 149 acts, 55.7% (83/149) of participants used their own saliva, whereas 44.3% (66/149) used their partner’s (P = 0.19); 72.4% (108/149) would spit on their hands first before applying to the genitals, whereas only 27.5% (41/149) would spit directly on their own/partners’ genitals (P < 0.001). When comparing sexual practice patterns during the last sexual episode in participants who used saliva as lubricant (213/225) and otherwise (890/917), the former was more likely to solo masturbate (6.0% (13/213) vs 0.8% (7/890), P < 0.001), kiss (9.9% (21/213) vs 27.8% (247/890), P < 0.001) and have more diverse sequential sexual practice (54.9% (117/213) vs 37.4% (333/890), P < 0.001). However, we observed no significant differences in HIV, syphilis, chlamydia and gonorrhoea positivities between the two groups.
MSM who use saliva as a lubricant are more likely to kiss and solo masturbate, with a preference for using their own saliva and spitting it onto the hands first.
Keywords: China, cross-sectional study, gonorrhoea, lubricant, men who have sex with men, risk behaviours, saliva use, sexual practices, STIs.
Introduction
Neisseria gonorrhoeae (NG) infection is a common sexually transmitted infection (STI) that has spread rapidly worldwide since the mid-2000s.1−3 The World Health Organization estimates there are 86.9 million new cases of gonorrhoea worldwide each year.4 Men who have sex with men (MSM) are at a higher risk of STIs than heterosexual males, which is in part related to the high levels of stigma they experience.5 Recent statistics show that the prevalence of gonorrhoea infection among MSM is 1.9% in China, which is almost 10 times higher than the 0.2% in the general population.6
Effective strategies for preventing gonorrhoea in MSM necessitate a comprehensive understanding of the transmission dynamics within this population.7 Although condom use has been regarded as an effective way to prevent transmission through anal intercourse, recent mathematical models have explored the potential role of saliva in transmission, which condoms are highly unlikely to prevent.8 This is particularly relevant in activities such as oral sex, kissing and other acts of saliva exchange, where condoms are not commonly used and are not effective in preventing transmission.9 Further, condom use has been falling among MSM with the rise of more effective HIV-prevention strategies such as pre-exposure prophylaxis (PrEP).10,11 It is therefore important to explore alternative interventions for gonorrhoea prevention.12 Although NG is commonly found in saliva,13 the extent to which saliva contributes to NG transmission remains inconclusive.
One possible way that saliva may facilitate the transmission of NG is through its common use as a lubricant during anal sex or other sexual intercourse.14 Existing evidence has shown that the use of saliva as a lubricant during anal intercourse significantly contributes to the risk of rectal gonorrhoea infection in MSM.15 However, there are other sexual acts, such as rimming followed by anal sex or oral sex followed by anal sex, where residual saliva could be passed into the anus and result in anal gonorrhoea.16 Practices involving saliva – such as its use as a lubricant or its presence during rimming and sequential sexual acts – are common among MSM and may potentially contribute to transmission risk. Given the difficulty of disentangling transmission routes in observational studies, further research into these practices is warranted.17 Our study aimed to characterise the patterns of sexual practices among Chinese MSM who reported using saliva as a lubricant during sexual episodes. With this, we further explore how these sexual patterns involving saliva may contribute to gonorrhoea transmission.
Materials and methods
Study design
We conducted a cross-sectional study among MSM at the Tongjian community centre in Xi’an, Shaanxi Province, China, from February 2022 to September 2022. The Tongjian community centre provides free STI testing and treatment services for MSM. Participants were recruited through snowball sampling. During the study period, MSM visiting the community were invited to complete an online questionnaire to collect information. All men in the study were offered HIV and syphilis serology testing; oropharyngeal swabs, anal swabs and urine samples were collected by community investigators for gonorrhoea and chlamydia testing.
Study population
Participants were eligible for this study if they were aged 18 years or older, were male at birth, had sex with men in the past 3 months, self-reported a HIV-negative status or were unaware of their HIV infection status. Exclusion criteria included anyone unable to give consent or the participants who were unable to complete the questionnaire.
Demographic and behavioural data
We collected demographic and behavioural data via an online questionnaire. We restrict the same IP address during the survey to prevent participants from being surveyed repeatedly. The basic information included sociodemographic information (e.g. age, residence), STI testing history, mouthwash use, alcohol use and the duration of sexual activity over the past 3 months. We also asked participants whether they had used drugs and who their partner was (i.e. the type of sex partner) during the last sexual episode. Participants were allowed to decline to answer any questions and withdraw from the study at any time.
We specifically asked questions related to ‘using saliva as a lubricant’ during sexual episodes over the past 3 months. The specific question about this was: ‘Over the last 3 months, have you used saliva as a lubricant during any of the following sexual acts? (multiple answers possible)’. The sexual acts included solo masturbation (M: solo masturbation), provide masturbation (Mi: you masturbate for the partner), received masturbation (Mr: the partner masturbates for you), insertive anal sex (Ai) and receptive anal sex (Ar). MSM using saliva as a lubricant was defined as having used saliva as a lubricant in these five sexual acts. Other questions were about the source of the saliva (yours or the partner’s) and how saliva was used (your or sexual partner’s penis or anus).
We additionally asked for information related to sequential sexual acts during the participant’s most recent sexual episode with another man. This was intentionally conducted for the most recent sexual episode only as it is impossible for participants to recall sequential sexual acts from all sexual episodes over the past 3 months. The sexual acts recorded from the last sexual episode included kissing (K), solo masturbation (M), provided masturbation (Mi), received masturbation (Mr), insertive oral sex (Oi: you suck other man’s genitals), receptive oral sex (Or: other man sucks your genitals), insertive rimming (Ri: you lick other man’s anus), receptive rimming (Rr: other man licks your anus), insertive anal sex (Ai) and receptive anal sex (Ar). We denoted sequential sexual acts by listing all sexual acts that occurred in the last sexual episode. For example, if a participant answered that kissing was the first step and receptive anal sex was the second step until the end of the sexual episode, his sequential sexual practice would be denoted as ‘K-Ar’.
Biological data
Oropharyngeal swabs, anal swabs and urine samples were collected to test for NG and CT infection using the NG/CT (Neisseria gonorrhoeae/Chlamydia trachomatis) Amplification/Detection Kit (Sansure Biotech, Changsha, China), which comprises a nucleic acid amplification test (NAAT). NG/CT infection was defined as NG/CT positivity at one or more anatomical sites. All tests were performed at the Kingmed Center for Clinical Laboratory. Blood samples were taken for rapid HIV and syphilis testing by colloidal gold methods using the Human Immunodeficiency Virus Antibody (HIV 1/2) Test Kit (Wondfo Biotech, Guangzhou, China) and the Treponema Pallidum (TP) Antibody Test Kit (InTec, Xiamen, China), respectively, with confirmatory testing performed at the Xi’an Lianhu District Centre for Disease Control and Prevention (CDC). Final HIV and syphilis infection status was obtained from the CDC.
Statistical analysis
Sociodemographic characteristics, sexual practices and HIV/STI positivity were reported. The factors associated with the use of saliva as a lubricant were assessed using bivariate tests (Chi-squared tests or Fisher’s Exact Test, see Supplementary Table S1) and univariable logistic regressions. The multivariable logistic regression included significant factors from univariate analysis (P < 0.1). The unadjusted and adjusted odds ratios (ORs) along with 95% CIs were reported. The Chi-squared test was used to compare patterns of sexual practice in the two groups using or not using saliva as a lubricant. The statistical software used for the study analysis was SPSS 26.0 (IBM SPSS, Chicago, IL, USA). P-value < 0.05 was considered statistically significant.
Results
Baseline demographic characteristics of participants
A total of 1332 MSM participated in our study, with 1169 (87.8%) completed questionnaires. Of these, 27 (2.3%) were excluded because of absent NG/CT testing results, leaving 1142 (97.7%) qualified participants in the analysis. The median age of the included participants was 32 years (interquartile range (IQR) 27–39 years), and 55.2% (630/1142) resided in urban areas. A total of 225 (19.7%) MSM reported using saliva as a lubricant for masturbation or anal sex during sexual episodes over the past 3 months, and 917 (80.3%) MSM had not used saliva. Forty (40/1142, 3.5%) MSM tested positive for HIV, 65 (5.7%) for syphilis, 154 (13.4%) for chlamydia and 163 (14.3%) for gonorrhoea. Among the 154 participants with chlamydia infections, 133 (86.4%) had anal infections, 17 (11%) had urethral infections and 12 (7.8%) had oropharyngeal infections. Among the 163 MSM with gonorrhoea infections, 98 (60.1%) had oropharyngeal infections, 63 (38.7%) had anal infections and 18 (11%) had urethral infections. Infections specific to the three anatomical sites of gonorrhoea and chlamydia are shown in Supplementary Figs S1–S2. Notably, there were no statistical differences in HIV and STI positivity between participants who used saliva as a lubricant and those who did not (Tables 1, Tables S2–S4).
| Variables | Number of individuals (n = 1142) | Use of saliva (n = 225) | No saliva use (n = 917) | Unadjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Residence | ||||||||
| Urban | 630 (55.2) | 143 (63.6) | 487 (53.1) | 1 | Ref | |||
| Town | 281 (24.6) | 50 (22.2) | 231 (25.2) | 0.74 (0.52, 1.06) | 0.095 | |||
| Rural | 231 (20.2) | 32 (14.2) | 199 (21.7) | 0.55 (0.36, 0.83) | 0.005 | |||
| Education | ||||||||
| Junior high school or below | 156 (13.7) | 29 (12.9) | 127 (13.9) | 1 | Ref | |||
| Senior high school | 290 (25.4) | 43 (19.1) | 247 (26.9) | 0.76 (0.45, 1.28) | 0.304 | |||
| University | 619 (54.2) | 128 (56.9) | 491 (53.5) | 1.14 (0.73, 1.79) | 0.562 | |||
| Postgraduate or above | 77 (6.7) | 25 (11.1) | 52 (5.7) | 2.11 (1.13, 3.93) | 0.019 | |||
| Sex partner during the most recent sexual episode | ||||||||
| Casual male partner | 507 (44.4) | 79 (35.1) | 428 (46.7) | 1 | Ref | 1 | Ref | |
| Commercial male partner | 22 (1.9) | 8 (3.6) | 14 (1.5) | 2.62 (1.05, 6.58) | 0.040 | 1.19 (0.43, 3.28) | 0.739 | |
| Long-term male partner | 211 (18.5) | 35 (15.6) | 176 (19.2) | 1.11 (0.72, 1.72) | 0.633 | 0.99 (0.62, 1.59) | 0.964 | |
| Regular male partner | 402 (35.2) | 103 (45.8) | 299 (32.6) | 1.98 (1.42, 2.75) | <0.001 | 2.59 (1.79, 3.76) | <0.001 | |
| Duration of sexual activity | ||||||||
| ≤1 h | 1007 (88.2) | 174 (77.3) | 833 (90.8) | 1 | Ref | 1 | Ref | |
| >1 h | 135 (11.8) | 51 (22.7) | 84 (9.2) | 2.91 (1.98, 4.27) | <0.001 | 1.64 (1.06, 2.54) | 0.026 | |
| Having had sex with a partner with HIV over the past 3 months | ||||||||
| No | 911 (79.8) | 132 (58.7) | 779 (85.0) | 1 | Ref | 1 | Ref | |
| Yes | 89 (7.8) | 42 (18.7) | 47 (5.1) | 5.27 (3.35, 8.31) | <0.001 | 3.48 (1.99, 6.11) | <0.001 | |
| Not sure | 142 (12.4) | 51 (22.7) | 91 (9.9) | 3.31 (2.24, 4.88) | <0.001 | 2.15 (1.36, 3.41) | 0.001 | |
| Having had sex with a foreigner over the past 3 months | ||||||||
| No | 1055 (92.4) | 181 (80.4) | 874 (95.3) | 1 | Ref | 1 | Ref | |
| Yes | 46 (4.0) | 27 (12.0) | 19 (2.1) | 6.86 (3.74, 12.61) | <0.001 | 2.87 (1.40, 5.92) | 0.004 | |
| Not sure | 41 (3.6) | 17 (7.6) | 24 (2.6) | 3.42 (11.80, 6.50) | <0.001 | 2.00 (0.94, 4.27) | 0.073 | |
| Antibiotic use over the past 3 months | ||||||||
| No | 1014 (88.8) | 181 (80.4) | 833 (90.8) | 1 | Ref | 1 | Ref | |
| Yes | 128 (11.2) | 44 (19.6) | 84 (9.2) | 2.41 (1.62, 3.59) | <0.001 | 1.61 (1.03, 2.55) | 0.037 | |
| Using drugs A during the most recent sexual episode | ||||||||
| No | 1008 (88.3) | 183 (81.3) | 825 (90.0) | 1 | Ref | 1 | Ref | |
| Yes | 134 (11.7) | 42 (18.7) | 92 (10.0) | 0.46 (0.31, 0.70) | <0.001 | 0.20 (0.94, 4.27) | 0.073 | |
| Mouthwash use over the past 3 months | ||||||||
| No | 828 (72.5) | 119 (52.9) | 709 (77.3) | 1 | Ref | 1 | Ref | |
| Yes | 314 (27.5) | 106 (47.1) | 208 (22.7) | 3.04 (2.24, 4.12) | <0.001 | 1.86 (1.31, 2.63) | 0.001 | |
| Alcohol use over the past 3 months | ||||||||
| No | 564 (49.4) | 64 (28.4) | 500 (54.5) | 1 | Ref | 1 | Ref | |
| Yes | 578 (50.6) | 161 (71.6) | 417 (45.5) | 3.02 (2.20, 4.14) | <0.001 | 2.14 (1.48, 3.10) | <0.001 | |
| Having disclosed their own sexual orientation | ||||||||
| No | 561 (49.1) | 89 (39.6) | 472 (51.5) | 1 | Ref | |||
| Yes | 581 (50.9) | 136 (60.4) | 445 (48.5) | 1.62 (1.20, 2.18) | 0.001 | |||
| Having been diagnosed with STI ever | ||||||||
| No | 812 (71.1) | 133 (59.1) | 679 (74.0) | 1 | Ref | |||
| Yes | 142 (12.4) | 53 (23.6) | 89 (9.7) | 3.04 (2.06, 4.48) | <0.001 | |||
| Not sure | 188 (16.5) | 39 (17.3) | 149 (16.2) | 1.34 (0.90, 1.99) | 0.154 | |||
| Self-reported STI symptoms | ||||||||
| No | 1062 (93.0) | 191 (84.9) | 871 (95.0) | 1 | Ref | |||
| Yes | 80 (7.0) | 34 (15.1) | 46 (5.0) | 3.37 (2.11, 5.39) | <0.001 | |||
| HIV status | ||||||||
| Negative | 1102 (96.5) | 214 (95.1) | 888 (96.8) | 1 | Ref | |||
| Positive | 40 (3.5) | 11 (4.9) | 29 (3.2) | 1.57 (0.77, 3.20) | 0.210 | |||
| Syphilis status | ||||||||
| Negative | 1077 (94.3) | 207 (92.0) | 870 (94.9) | 1 | Ref | |||
| Positive | 65 (5.7) | 18 (8.0) | 47 (5.1) | 1.61 (0.92, 2.83) | 0.10 | |||
| Chlamydia status | ||||||||
| Negative | 988 (86.5) | 200 (88.9) | 788 (85.9) | 1 | Ref | |||
| Positive | 154 (13.5) | 25 (11.1) | 129 (14.1) | 0.76 (0.48, 1.20) | 0.246 | |||
| Gonorrhoea status | ||||||||
| Negative | 979 (85.7) | 198 (88.0) | 781 (85.2) | 1 | Ref | |||
| Positive | 163 (14.3) | 27 (12.0) | 136 (14.8) | 0.78 (0.50, 1.22) | 0.278 | |||
| Oropharyngeal chlamydia | ||||||||
| Negative | 1130 (98.9) | 224 (99.6) | 906 (98.8) | 1 | Ref | |||
| Positive | 12 (1.1) | 1 (0.4) | 11 (1.2) | 0.37 (0.05, 2.86) | 0.368 | |||
| Anal chlamydia | ||||||||
| Negative | 1009 (88.4) | 204 (90.7) | 906 (79.3) | 1 | Ref | |||
| Positive | 133 (11.6) | 21 (9.3) | 112 (12.2) | 0.74 (0.45, 1.21) | 0.229 | |||
| Urethral chlamydia | ||||||||
| Negative | 1125 (98.5) | 221 (98.2) | 904 (98.6) | 1 | Ref | |||
| Positive | 17 (1.5) | 4 (1.8) | 13 (1.4) | 1.26 (0.41, 3.9) | 0.69 | |||
| Oropharyngeal gonorrhoea | ||||||||
| Negative | 1044 (91.4) | 207 (92.0) | 837 (91.3) | 1 | Ref | |||
| Positive | 98 (8.6) | 18 (8.0) | 80 (8.7) | 0.91 (0.54, 1.56) | 0.728 | |||
| Anal gonorrhoea | ||||||||
| Negative | 1079 (94.5) | 216 (96.0) | 863 (94.1) | 1 | Ref | |||
| Positive | 63 (5.5) | 9 (4.0) | 54 (5.9) | 0.67 (0.32, 1.37) | 0.269 | |||
| Urethral gonorrhoea | ||||||||
| Negative | 1124 (98.4) | 224 (99.6) | 900 (98.1) | 1 | Ref | |||
| Positive | 18 (1.6) | 1 (0.4) | 17 (1.9) | 0.24 (0.03, 1.79) | 0.162 | |||
Multivariable analyses demonstrated that MSM whose most recent sex partner was a regular partner were more likely to report ‘using saliva as a lubricant’ over the past 3 months, compared with participants with casual male partners (aOR = 2.59; 95% CI 1.79–3.76). Participants who used mouthwash (aOR = 1.86; 1.31–3.10) and alcohol (aOR = 2.14; 1.48–3.10) were more likely to use saliva as a lubricant. Saliva use was also associated with sexual activity lasting >1 h (aOR = 1.64; 1.06–2.54), having had sex with people living with HIV (aOR = 3.48; 1.99–6.11) or with a foreigner (aOR = 2.87; 1.40–4.27), and using an antibiotic over the past 3 months (aOR = 1.61; 1.03–2.55, Table 1).
The use of saliva as a lubricant over the past 3 months in MSM
Among the 225 MSM who used saliva as a lubricant in the past 3 months, we identified 20 unique patterns of sexual practices where one or more sexual acts that used saliva as a lubricant were reported (Fig. 1).
The UpSet plot shows the sexual acts involved in the use of saliva in 225 MSM who had used saliva as a lubricant over the past 3 months. A total of 263 sexual acts were reported. The most common sexual act when using saliva was solo masturbation (M, 114), followed by masturbating for the partner (Mi, 45), insertive anal sex (Ai, 39), receptive anal sex (Ar, 33) and received masturbation by the partner (Mr, 32).
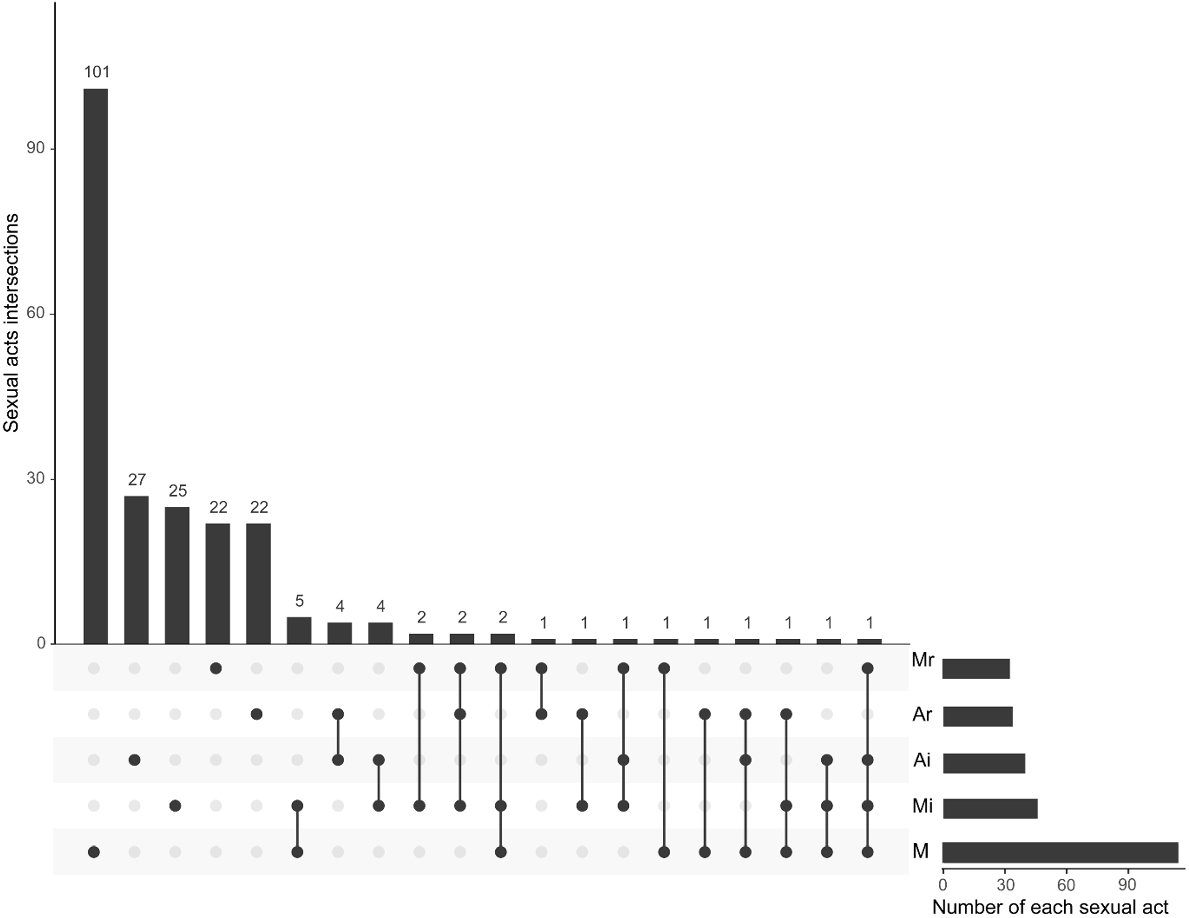
Of the 114 (50.7%, 114/225) MSM who had used saliva as a lubricant during solo masturbation over the past 3 months, 74.6% (85/114) spit saliva on their hands first and then applied it on their penises. In contrast, 124 used saliva as a lubricant in 149 sex acts with male partners. Among these, 45 saliva-use acts occurred when masturbating for the partner (Mi, 20%, 45/225), more than half of which used their own saliva (60%, 27/45), and 70.4% (19/27) of these participants spit saliva on their hands first. Among the 39 saliva-use acts that occurred during insertive anal sex (17.3%, 39/225), 25 used their own saliva (64.1%, 25/39), and 88% (22/25) of them spit saliva on their hands first before applying it to the anus or penis. Among saliva-use acts during receptive anal sex (Ar, 14.7%, 33/225) or received masturbation from the partner (Mr, 14.2%, 32/225), participants were also more likely to spit saliva on the hands first (68.8%, 22/32, Table 2).
| Sex acts (n, %) | Saliva source | Count (%) | Saliva use-pattern | Count (%) | |
|---|---|---|---|---|---|
| M (114, 50.7) | Own saliva | 114 (100.0) | Spit on penis | 29 (25.4) | |
| Spit on hands | 85 (74.6) | ||||
| Mi (45, 20.0) | Partner’s saliva | 18 (40.0) | Spit on partner’s penis | 4 (22.2) | |
| Spit on own hands | 14 (77.8) | ||||
| Own saliva | 27 (60.0) | Spit on partner’s penis | 8 (29.6) | ||
| Spit on own hands | 19 (70.4) | ||||
| Mr (32, 14.2) | Partner’s saliva | 15 (46.9) | Spit on own penis | 5 (33.3) | |
| Spit on partner’s hands | 10 (66.7) | ||||
| Own saliva | 17 (53.1) | Spit on own penis | 5 (29.4) | ||
| Spit on partner’s hands | 12 (70.6) | ||||
| Ai (39, 17.3) | Partner’s saliva | 14 (35.9) | Spit on own penis | 4 (28.6) | |
| Spit on own hands, apply on partner’s anus | 6 (42.9) | ||||
| Spit on own hands, apply on own penis | 4 (28.6) | ||||
| Own saliva | 25 (64.1) | Spit on own penis | 3 (12.0) | ||
| Spit on own hands, apply on partner’s anus | 9 (36.0) | ||||
| Spit on own hands, apply on own penis | 13 (52.0) | ||||
| Ar (33, 14.7) | Partner’s saliva | 19 (57.6) | Spit on partner’s penis | 5 (26.3) | |
| Spit on partner’s hands, apply on own anus | 8 (42.1) | ||||
| Spit on partner’s hands, apply on partner’s penis | 6 (31.6) | ||||
| Own saliva | 14 (42.4) | Spit on partner’s penis | 7 (50.0) | ||
| Spit on partner’s hands, apply on own anus | 4 (28.6) | ||||
| Spit on own hands, apply on partner’s penis | 3 (21.4) |
Note: M, solo masturbation; Mi, masturbating for the partner; Mr, received masturbation by the partner; Ai, insertive anal sex; Ar, receptive anal sex.
Overall, when using saliva as a lubricant during sex with men, MSM tended to use their own saliva (55.7% (83/149) vs 44.3% (66/149) using their partner’s saliva, P = 0.19) and spit on the hands first before applying it (72.4% (108/149) vs 27.5% (41/149) spitting directly on genitals, P < 0.001, Table 2).
Differences in the distribution of individual sexual acts in the last sexual episode
Information on the sequence of sexual acts during the most recent sexual episode was collected from 1103 participants. The participants were divided into saliva-use group and non-saliva group according to the use of saliva as a lubricant over the past 3 months, with 213 MSM who used saliva as a lubricant and 890 MSM who did not.
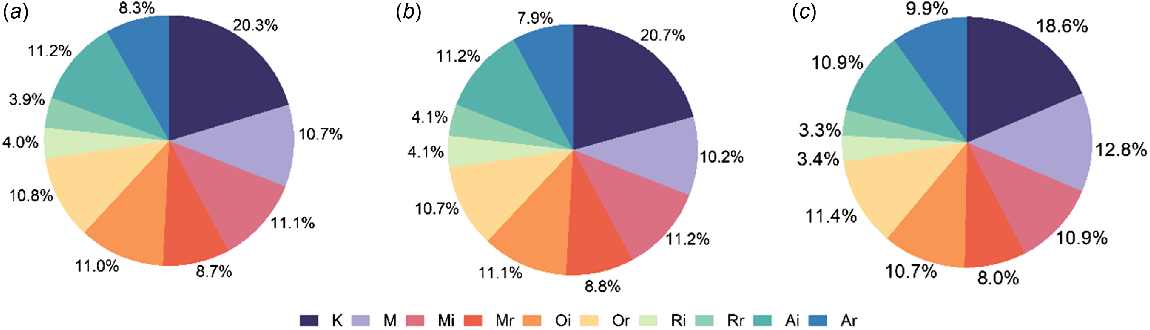
A total of 4694 sexual acts were collected from 1103 sexual episodes. In both groups, kissing was the most frequent sexual act (18.6% vs 20.7%, P = 0.16), followed by the next five common acts, which were solo masturbation (12.8% vs 10.2%, P = 0.023), receptive oral sex (11.4% vs 10.7%, P = 0.541), provided masturbation (10.9% vs 11.2%, P = 0.854), insertive anal sex (10.9% vs 11.2%, P = 0.804) and insertive oral sex (10.7% vs 11.1%, P = 735, Fig. 2). Solo masturbation was the only sexual act with a significant difference between the two groups.
Distribution of sexual acts in MSM during the most recent sexual episode. Information on a total of 850 sexual acts was collected from MSM who used saliva as a lubricant, and a total of 3844 sexual acts were collected from MSM who did not use saliva as a lubricant. In (a), the distribution of sexual acts is described for all participants; (b) shows the distribution for participants who did not use saliva as a lubricant, and (c) shows the distribution for participants who used saliva as a lubricant. (K, kissing; Oi, you suck other man’s genitals; Or, man sucks your genitals; Ri, you lick other man’s anus; Rr, man licks your anus.).
Differences in the stepwise distribution of sexual acts in the last sexual episode
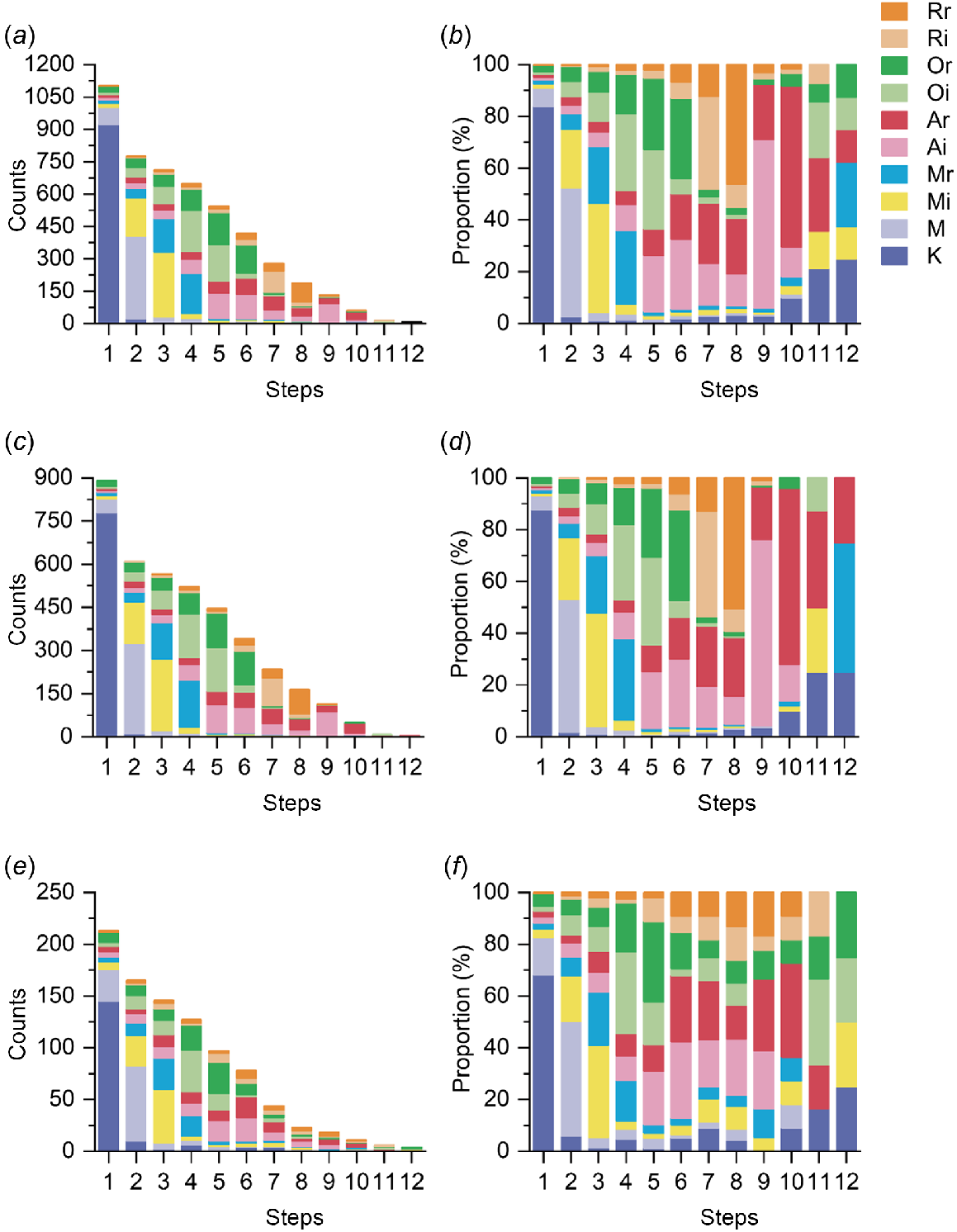
We demonstrated the distribution of sexual acts at each step among participants, with more than half of them having at least four sexual acts during the most recent sexual episode (58.7%, 648/1103). Kissing was the most common first sexual act in a sexual episode (83.9%, 925/1103), and the proportion of kissing increased in the later steps of the sexual episode (Fig. 3b, d, f). Both solo and provided masturbation occurred mainly in the first three steps of a sexual episode. The frequency of insertive rimming and receptive rimming increased and then decreased throughout the steps in a sex episode, peaking at the eighth step. Comparing MSM who had used saliva as a lubricant with those who had not over the past 3 months, the stepwise distribution of sexual acts during the most recent sexual episode showed differences. Received masturbation occurred primarily at the third step among MSM who used saliva, but more frequently at the fourth step among those who did not. The proportion of insertive and receptive oral sex was also higher among participants who used saliva than those who did not after the seventh step (Fig. 3, Table S5).
Sequences of sex acts stacked histogram with percentage stacked histogram for MSM during the most recent sexual episode. (a, b) All participants (n = 1103). (c, d) Participants who did not use saliva as a lubricant (n = 890). (e, f) Participants who used saliva as a lubricant (n = 213). (K, kissing; M, solo masturbation; Mi, masturbating for the partner; Mr, received masturbation by the partner; Ai, insertive anal sex; Ar, receptive anal sex; Oi, you suck other man’s genitals; Or, man sucks your genitals; Ri, you lick other man’s anus; Rr, man licks your anus).
Differences in the sequential sexual practice patterns in the last sexual episode
Considering the sequence of sexual practices, kissing without any further sexual acts was the most frequently occurring sequential sexual practice, accounting for 24.3% (268/1103) of the reported patterns. Participants who did not use saliva demonstrated a higher proportion of ‘kissing only’ (9.9% (21/213) vs 27.8% (247/890), P < 0.001). Notably, participants using saliva as a lubricant showed a higher proportion of ‘solo masturbation (M)’ (6.0% (13/213) vs 0.8% (7/890), P < 0.001). The sequential sexual practice pattern ‘K-M-Mi-Mr-Or-Ai’ was significantly more common among those using saliva as a lubricant than otherwise (1.9% (4/213) vs 0.3% (3/890), P = 0.029). The proportion of ‘other’ sequential sexual practice patterns was significantly higher among MSM who used saliva as a lubricant (54.9% (117/213) vs 37.4% (333/890), P < 0.001, Table S6).
Discussion
Our study identified the differences in sexual practices between MSM who use saliva as a lubricant during sex and otherwise in a Xi’an MSM community in China. Our study demonstrated that 19.7% of participants used saliva as a lubricant during all sexual practices in the past 3 months. In comparison, a study of 1312 MSM in Australia reported that 69% of MSM had used saliva as a lubricant during anal sex over the past 3 months,18 demonstrating a much higher proportion of saliva use than in our study. However, MSM most commonly used saliva as a lubricant during masturbation in our study (50.7%, 114/225), which is consistent with the findings of another Melbourne study of 446 MSM participants.17,19
Our study demonstrated that most MSM who use saliva as a lubricant use their own saliva and spit directly on their hands before applying it to the penis or anus during the provision of masturbation and insertive anal sex. MSM prefer to use their own saliva because of its immediate accessibility and likely limited availability of a gel lubricant, especially in scenarios of quick sex.20 The tendency of participants to spit saliva on their hands first was possibly attributed to its convenience and manoeuvrability when compared with applying saliva directly to the genitals. Importantly, previous studies have shown that the bacterial load of NG is substantially lower in the oropharynx when compared with the anorectum in MSM, and that bacteria cannot survive long ex vivo, suggesting that a substantial amount of saliva is essential for the transmission of gonorrhoea.21,22 In our study, most MSM used saliva as a lubricant during masturbation by spitting it onto the hands first, which may reduce the likelihood of gonorrhoea transmission. This finding was consistent with a previous study by Tran et al.,23 which found no statistically significant association between the use of saliva as a masturbation lubricant and the transmission of gonorrhoea via this route.
Our study showed that MSM who used alcohol were more likely to have used saliva as a lubricant over the past 3 months. Alcohol may affect the ability of MSM to perceive and respond to risks during sexual episodes, potentially leading to practices such as longer duration of sex and having sex with a partner living with HIV or a foreigner.24−26 HIV-infected MSM are less concerned about their partner’s HIV infection status because they are already infected with HIV. As a result, they are freer to use saliva for sexual pleasure and comfort during sexual episodes.27,28 Consequently, the use of saliva as a lubricant may be more common in HIV-infected MSM. We also found that MSM who used saliva as a lubricant had a stronger preference to use mouthwash. Previous studies have revealed that MSM with higher-risk sexual behaviours, such as having more sexual partners, are more likely to use mouthwash frequently,19,29 and a proportion of MSM were willing to adopt the behaviour of using mouthwash to prevent STIs.30 Therefore, MSM may view mouthwash as a self-care measure to mitigate the risks associated with high-risk sexual behaviour and reduce the risk of STIs.
In our study, only 19.7% of MSM had used saliva as a lubricant, and the low proportion may be associated with the discrimination they face.31 Evidence confirms that MSM frequently choose to conceal their saliva use and other sexual risk behaviours because of the experiences of social discrimination and stigma based on their sexual orientation or gender identity.32,33 In the Chinese setting, where hygiene and conservative sexual norms are emphasised,34 saliva use during sexual episodes is often regarded as ‘unhygienic’ and ‘off the boundary’ of regular sexual practices. Consequently, the majority of MSM avoid using saliva as a lubricant to prevent stigma, and the fear of stigmatisation is likely to suppress reporting of saliva use. The small number of individuals who do use saliva may also limit the ability to detect any significant correlation between saliva use and STI transmission. In addition, most of the MSM in our study used saliva during masturbation and spit on the hands first to avoid direct contact between the infected sites and the genitals.28 It is also possible that social factors influence the use of saliva during low-risk activities such as masturbation among MSM. Furthermore, other studies have focused on the use of saliva as a lubricant during anal sex, which makes direct comparison between the studies challenging. There also remains considerable uncertainty regarding the quantity of saliva required for STI transmission, and the flow rate of saliva during sex was not assessed.14,35
Our study suggested that Chinese MSM who use saliva as a lubricant exhibit greater diversity and complexity in sequential practice patterns on the basis of the significantly different sequential sexual practice patterns and different stepwise distribution of sexual acts, as well as an elevated risk of multisite infections. More than half of MSM who used saliva as a lubricant reported patterns of sexual practices that occurred only once, and these sexual practices often included more diverse sexual acts, indicating more complex patterns of sexual practice in these individuals.36 Notably, various combinations and sequential patterns of sexual practices can increase the number of potential routes of transmission between partners across multiple anatomical sites,37,38 amplifying the likelihood of multisite STI transmission.39
Our study had several limitations. First, our study was a single-centre, non-randomised sample with selection bias, which limits the extrapolation of results to some extent. Second, as this was a cross-sectional survey, it was not possible to establish the causality of the association. Third, self-reported data on saliva use and sexual practices induced recall bias. Although we focused on the most recent sexual episode to reduce recall bias, it still did not fully represent the typical sexual practices of an individual. Additionally, although the use of online questionnaires likely reduced social desirability bias, this bias was not completely eliminated and may have affected self-reported data, particularly in relation to sensitive indicators of sexual behaviours. Finally, although our study describes the specific use of saliva as a lubricant, the data were not yet comprehensive. We lack data on the frequency and duration of saliva use, and potential confounding factors such as commercially available lubricants were not taken into account.
In conclusion, we found that ~20% of participants used saliva as a lubricant during any sexual episode, most commonly during masturbation, where they prefer to use their own saliva by spitting on their hands first. MSM who use saliva as a lubricant tend to have more diverse sexual practices. However, we observed no significant differences in HIV, syphilis, chlamydia and gonorrhoea positivities between MSM who use saliva as a lubricant and those who do not. Our study contributes to a growing body of research on saliva use during sexual episodes and its potential role in the transmission of STIs. Further understanding of saliva use during sequential sexual acts can inform the development and promotion of safe sexual practices among MSM.
Data availability
The data supporting this study cannot be publicly shared for ethical or privacy reasons and may be shared upon reasonable request to the corresponding author if appropriate.
Conflicts of interest
Lei Zhang and Eric Chow are Associate Editors for Sexual Health but was not involved in the peer review or decision-making process for this paper.
Declaration of funding
LZ is supported by the National Key R&D Program of China (2022yfc2304900); Outstanding Young Scholars Support Program (3111500001); Xi’an Jiaotong University Basic Research and Profession Grant (xtr022019003, xzy032020032) and Xi’an Jiaotong University Young Scholar Support Grant (YX6J004). EPFC is supported by an Australian National Health and Medical Research Council (NHMRC) Emerging Leadership Investigator Grant (GNT1172873).
Author contributions
Lei Zhang and Gaixia Li conceptualised the study. Rui Zhao performed data collection. Gaixia Li conducted data analysis and prepared the initial draft of the manuscript. All authors reviewed, edited, read and approved the final manuscript.
References
1 Johnson Jones ML, Chapin-Bardales J, Bizune D, et al. Extragenital chlamydia and gonorrhea among community venue–attending men who have sex with men – five cities, United States, 2017. MMWR Morb Mortal Wkly Rep 2019; 68(14): 321-325.
| Crossref | Google Scholar | PubMed |
2 Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE 2015; 10(12): e0143304.
| Crossref | Google Scholar | PubMed |
3 Marley G, Tan RKJ, Wu D, et al. Pay-it-forward gonorrhea and chlamydia testing among men who have sex with men and male STD patients in China: the PIONEER pragmatic, cluster randomized controlled trial protocol. BMC Public Health 2023; 23: 1182.
| Crossref | Google Scholar | PubMed |
4 Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 2019; 97(8): 548-562P.
| Crossref | Google Scholar | PubMed |
5 Dibble KE, Murray SM, Baral SD, et al. Predicting salivary cortisol and sexual behavior stigma among MSM in the American Men’s Internet Survey 2019. Sci Rep 2023; 13(1): 18082.
| Crossref | Google Scholar |
6 Guo Z, Feng A, Zhou Y, et al. Geosocial networking mobile applications use and HIV and other sexually transmitted infections among men who have sex with men in Southern China: a cross-sectional study. Front Public Health 2023; 11: 1063993.
| Crossref | Google Scholar | PubMed |
7 Xu X, Yu Z, Ge Z, et al. Web-based risk prediction tool for an individual’s risk of HIV and sexually transmitted infections using machine learning algorithms: development and external validation study. J Med Internet Res 2022; 24(8): e37850.
| Crossref | Google Scholar | PubMed |
8 Fairley CK, Cornelisse VJ, Hocking JS, Chow EPF. Models of gonorrhoea transmission from the mouth and saliva. Lancet Infect Dis 2019; 19(10): e360-e366.
| Crossref | Google Scholar | PubMed |
9 Tran J, Ong JJ, Bradshaw CS, et al. Kissing, fellatio, and analingus as risk factors for oropharyngeal gonorrhoea in men who have sex with men: a cross-sectional study. eClinicalMedicine 2022; 51: 101557.
| Crossref | Google Scholar | PubMed |
10 Chow EPF, Grulich AE, Fairley CK. Epidemiology and prevention of sexually transmitted infections in men who have sex with men at risk of HIV. Lancet HIV 2019; 6(6): e396-e405.
| Crossref | Google Scholar | PubMed |
11 Kim C-M, Zhao V, Brito De Mello M, et al. Determining the screening frequency for sexually transmitted infections for people who use HIV pre-exposure prophylaxis: a systematic review and meta-analysis. Int J Infect Dis 2023; 129: 181-187.
| Crossref | Google Scholar | PubMed |
12 Luetkemeyer AF, Donnell D, Dombrowski JC, et al. Postexposure doxycycline to prevent bacterial sexually transmitted infections. N Engl J Med 2023; 388(14): 1296-1306.
| Crossref | Google Scholar | PubMed |
13 Chow EPF, Vodstrcil LA, Williamson DA, et al. Incidence and duration of incident oropharyngeal gonorrhoea and chlamydia infections among men who have sex with men: prospective cohort study. Sex Transm Infect 2021; 97(6): 452-458.
| Crossref | Google Scholar | PubMed |
14 Chow EP, Fairley CK. The role of saliva in gonorrhoea and chlamydia transmission to extragenital sites among men who have sex with men: new insights into transmission. J Int AIDS Soc 2019; 22(S6): e25354.
| Crossref | Google Scholar |
15 Cornelisse VJ, Fairley CK, Read TRH, et al. Associations between anorectal chlamydia and oroanal sex or saliva use as a lubricant for anal sex: a cross-sectional survey. Sex Transm Dis 2018; 45(8): 506-510.
| Crossref | Google Scholar | PubMed |
16 Xu X, Chow EPF, Ong JJ, et al. Modelling the potential role of saliva use during masturbation in the transmission of Neisseria gonorrhoeae at multiple anatomical sites. Sex Health 2022; 18(6): 466-474.
| Crossref | Google Scholar | PubMed |
17 Tran J, Fairley CK, Ong JJ, et al. Combinations of sexual activities during a sex episode with recent casual male partner among men who have sex with men: a cross-sectional study. J Sex Res 2024; 61(6): 968-973.
| Crossref | Google Scholar | PubMed |
18 Chow EPF, Cornelisse VJ, Read TRH, et al. Saliva use as a lubricant for anal sex is a risk factor for rectal gonorrhoea among men who have sex with men, a new public health message: a cross-sectional survey. Sex Transm Infect 2016; 92(7): 532-536.
| Crossref | Google Scholar | PubMed |
19 Chow EPF, Williamson DA, Hocking JS, et al. Antiseptic mouthwash for gonorrhoea prevention (OMEGA): a randomised, double-blind, parallel-group, multicentre trial. Lancet Infect Dis 2021; 21(5): 647-656.
| Crossref | Google Scholar | PubMed |
20 Walker S, Bellhouse C, Fairley CK, et al. Pharyngeal gonorrhoea: the willingness of australian men who have sex with men to change current sexual practices to reduce their risk of transmission – a qualitative study. PLoS ONE 2016; 11(12): e0164033.
| Crossref | Google Scholar | PubMed |
21 Quillin SJ, Seifert HS. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol 2018; 16(4): 226-240.
| Crossref | Google Scholar | PubMed |
22 Bissessor M, Tabrizi SN, Fairley CK, et al. Differing Neisseria gonorrhoeae bacterial loads in the pharynx and rectum in men who have sex with men: implications for gonococcal detection, transmission, and control. J Clin Microbiol 2011; 49(12): 4304-4306.
| Crossref | Google Scholar | PubMed |
23 Tran J, Fairley CK, Ong JJ, et al. Association between saliva use for masturbation and urethral gonorrhoea in men who have sex with men: a cross-sectional study. Int J Infect Dis 2024; 148: 107219.
| Crossref | Google Scholar |
24 Vallée A. Sexual behaviors, cannabis, alcohol and monkeypox infection. Front Public Health 2023; 10: 1054488.
| Crossref | Google Scholar | PubMed |
25 Firkey M, Sheinfil A, Ramos J, et al. Cannabis and alcohol co-use and condomless anal sex among men who have sex with men living with HIV: an event-level analysis. AIDS Behav 2021; 25(11): 3770-3781.
| Crossref | Google Scholar | PubMed |
26 Wang Z, Yang X, Mo PKH, et al. Influence of social media on sexualized drug use and chemsex among Chinese men who have sex with men: observational prospective cohort study. J Med Internet Res 2020; 22(7): e17894.
| Crossref | Google Scholar | PubMed |
27 Blondeel K, Dias S, Furegato M, et al. Sexual behaviour patterns and STI risk: results of a cluster analysis among men who have sex with men in Portugal. BMJ Open 2021; 11(1): e033290.
| Crossref | Google Scholar | PubMed |
28 Butler LM, Osmond DH, Graves Jones A, et al. Use of saliva as a lubricant in anal sexual practices among homosexual men. J Acquir Immune Defic Syndr 2009; 50(2): 162-167.
| Crossref | Google Scholar |
29 Hu Y, Zhao R, Li G, et al. Associations between antibacterial mouthwash use with sexual behaviours and sexually transmitted infections among Chinese men who have sex with men: a cross-sectional study. Sex Health 2024; 21(5): SH24083.
| Crossref | Google Scholar |
30 Chow EP, Walker S, Phillips T, et al. Willingness to change behaviours to reduce the risk of pharyngeal gonorrhoea transmission and acquisition in men who have sex with men: a cross-sectional survey. Sex Transm Infect 2017; 93(7): 499-502.
| Crossref | Google Scholar | PubMed |
31 Yu L, Jiang C, Na J, et al. Elevated 12-Month and lifetime prevalence and comorbidity rates of mood, anxiety, and alcohol use disorders in Chinese men who have sex with men. PLoS ONE 2013; 8(4): e50762.
| Crossref | Google Scholar | PubMed |
32 Frye V, Nandi V, Egan J, et al. Sexual orientation- and race-based discrimination and sexual HIV risk behavior among urban MSM. AIDS Behav 2015; 19(2): 257-269.
| Crossref | Google Scholar | PubMed |
33 Fields EL, Bogart LM, Galvan FH, et al. Association of discrimination-related trauma with sexual risk among HIV-positive African American men who have sex with men. Am J Public Health 2013; 103(5): 875-880.
| Crossref | Google Scholar | PubMed |
34 Sun S, Pachankis JE, Li X, et al. Addressing minority stress and mental health among men who have sex with men (MSM) in China. Curr HIV/AIDS Rep 2020; 17(1): 35-62.
| Crossref | Google Scholar | PubMed |
35 Xu X, Chow EPF, Ong JJ, et al. Modelling the contribution that different sexual practices involving the oropharynx and saliva have on Neisseria gonorrhoeae infections at multiple anatomical sites in men who have sex with men. Sex Transm Infect 2021; 97(3): 183-189.
| Crossref | Google Scholar | PubMed |
36 Rosenberger JG, Reece M, Schick V, et al. Sexual behaviors and situational characteristics of most recent male-partnered sexual event among gay and bisexually identified men in the United States. J Sex Med 2011; 8(11): 3040-3050.
| Crossref | Google Scholar | PubMed |
37 Khosropour CM, Coomes DM, Barbee LA. Frequency and combination of sequential sexual acts that may lead to sexually transmitted infections at different anatomic sites within the same person. Arch Sex Behav 2023; 52(2): 823-831.
| Crossref | Google Scholar | PubMed |
38 Janulis P, Goodreau SM, Morris M, et al. Partnership types and coital frequency as predictors of gonorrhea and chlamydia among young MSM and young transgender women. Int J STD AIDS 2023; 34(10): 694-701.
| Crossref | Google Scholar |
39 Fairley CK, Hocking JS, Zhang L, et al. Frequent transmission of gonorrhea in men who have sex with men. Emerg Infect Dis 2017; 23(1): 102-104.
| Crossref | Google Scholar | PubMed |


