Response of wheat to phosphorus-enriched ironstone gravel
David Weaver A , David Rogers A , Ronald Master A , Peta Richards B , Robert Summers
A , David Rogers A , Ronald Master A , Peta Richards B , Robert Summers  C and Simon Clarendon
C and Simon Clarendon  A *
A *
A
B
C
Abstract
Gravel fractions (>2 mm) in soil are almost always excluded from laboratory analysis and glasshouse experiments as they are considered to be inert; however, the >2 mm fraction is always present in field experiments.
To determine whether the >2 mm fraction of ironstone gravel (IG) soil enriched with phosphorus (P) can supply P to wheat (Triticum aestivum L.).
An IG soil was separated into different size fractions (<2, 2–4, 4–6, 6–8 and 8–10 mm), and adsorption and desorption experiments, volumetric moisture measurements and glasshouse experiments were conducted. Each of the >2 mm fractions were enriched with P to different levels and added to a sand culture, or to the enriched <2 mm fraction in different amounts (25%, 50% and 75% IG). Wheat was grown in pots and growth correlated to P added from enriched soil fractions, weighted Colwell P, soil solution P concentrations and volumetric water content.
The <2 mm fraction of the IG soil adsorbed more P than the >2 mm fraction of the IG soil likely due to its greater specific surface area. Volumetric water content decreased as gravel amount increased. Wheat was more responsive to P for larger compared to smaller gravel sizes. The P-enriched IG was able to support the growth of wheat in the absence of any other P source. For the same level of P enrichment, dry matter decreased as gravel amount increased.
The IG influences wheat growth through P retention and release and soil moisture. Volumetric water content can be reduced significantly by high gravel contents, leading to reduced wheat growth despite sufficient P fertility.
Depending on the nature of the soil matrix, soils with high amounts (~50%) of larger IG are likely to require lower P applications to optimise crop yield. Soil sampling strategies and laboratory testing need to consider how to practically include the >2 mm fraction during sample collection and analysis.
Keywords: adsorption, desorption, ironstone gravel, phosphorus, responsiveness, volumetric water content, wheat, yield.
Introduction
Ironstone gravel (IG) soils are a dominant feature across the south-west agricultural region of Western Australia (SWWA), spanning more than 2 million hectares and representing approximately 15% of the 14 million hectares used for grain production in the state (Holmes et al. 2021). These soils, commonly referred to as ‘forest gravels’ or ‘gravelly duplex soils,’ are typically acidic, highly weathered and contain significant amounts of iron (Fe) and aluminium (Al) oxides, which occur in both the fine (<2 mm) and coarse (>2 mm) soil fractions (Weaver et al. 2022). The chemical and physical nature of these IG soils presents several agronomic challenges, including variable nutrient retention and limited water-holding capacity.
One of the critical constraints in IG soils is their phosphorus (P) deficiency, particularly in newly cleared soils (Wild 1958). Due to intense weathering and leaching, these soils are typically low in essential nutrients including nitrogen (N), potassium (K), sulfur (S) and a range of micronutrients (Robson and Gilkes 1980). Although P deficiency has been overcome to some degree by fertilisation (Weaver and Wong 2011), P dynamics and supply from different size fractions to crops, particularly from the >2 mm fraction that is excluded from laboratory analysis is poorly understood. Phosphorus is of particular interest, given its high fixation and poor mobility when Fe and Al oxides are present. Phosphorus has a central role in crop establishment, root development and tillering in cereals such as wheat (Triticum aestivum). Consequently, P fertilisation can be necessary for achieving profitable crop yields on these soils (Bowden and Bennett 1974).
Soil testing and fertiliser advice for P is based on the <2 mm fraction, and on the assumption that this fraction is the chemically ‘reactive’ portion of the soil. The >2 mm fraction is routinely discarded from soil chemical analysis and in controlled experiments such as pot trials, under the long-standing assumption that it is chemically inert and merely dilutes the nutrient-rich fine fraction (Miller and Guthrie 1984). However, emerging evidence suggests that this assumption may overlook important contributions of the >2 mm fraction to soil nutrient dynamics. Several studies have reported that the >2 mm fraction, particularly when composed of IG, can be more chemically reactive than the <2 mm fraction (Tiessen et al. 1993) and may even possess a higher total nutrient content (Abekoe and Tiessen 1998). Furthermore, the >2 mm fraction plays a role in altering soil physical properties including bulk density, porosity and water movement, all of which can influence nutrient transport and availability (Anamosa et al. 1990; Brouwer and Anderson 2000; Ritz and Young 2004). Tokunaga et al. (2003) and Weaver et al. (2022) have also shown that gravels may provide more surface area for chemical interactions than previously assumed.
These findings challenge the traditional approach of disregarding the >2 mm fraction in both laboratory analyses and glasshouse experiments and highlight a disconnect between experimental conditions and field reality. Although the >2 mm fraction is typically excluded in controlled studies, it is ubiquitous in the field where it can exert both direct and indirect effects on plant growth and nutrient availability. This disconnect complicates the interpretation of P fertilisation strategies developed from the <2 mm fraction under laboratory or glasshouse conditions and their applicability to field scenarios where the soil also includes the >2 mm fraction in different amounts, size distribution and mineralogy (Holmes et al. 2021).
Several studies have attempted to examine the relationship between gravel content and crop growth, with mixed results. Sanidanya (2015) conducted a pot trial in which IG (8 mm in size) was mixed with red sand in varying proportions, with the sand pre-treated with P to establish differing P concentrations in the <2 mm fraction. They found that wheat biomass was positively correlated with P concentration in the fine fraction, and that gravel content per se did not influence yield. These findings are consistent with the diffusion-limited concept of P availability (Barber 1962), suggesting that when only the <2 mm fraction is fertilised, gravels may act primarily as inert diluents.
However, the interpretation of these results is limited by the fact that P was added solely to the fine fraction prior to mixing. This approach precludes any potential interaction between added P and the coarse fraction, including adsorption–desorption dynamics or potential P contributions from the gravels themselves. The relatively large gravel size (8 mm) may also limit chemical reactivity due to reduced surface area (Weaver et al. 2022). Such pot experiments may underestimate the potential of the >2 mm fraction to retain and release P, particularly under conditions where P is added to the whole soil matrix, as occurs during fertilisation in the field.
Field trials, in contrast, suggest that gravel content may influence crop response to P in more complex ways. Moody and Bolland (1999) reported that critical Colwell P values – based on analysis of the <2 mm fraction – increased with higher gravel content in trials from Badgingarra, WA. For example, one site showed an increase in critical Colwell P from 10 mg kg−1 (0% gravel) to 22 mg kg−1 (30–50% gravel), indicating that higher P levels were needed in the fine fraction to achieve equivalent yield in gravel-rich soils. These results suggest a potential ‘dilution effect’ of gravels on P supply but may also reflect reduced P accessibility or altered soil–plant interactions in the presence of high gravel content.
Other studies provide further evidence that gravel content can influence nutrient dynamics and plant performance in non-trivial ways. Ercoli et al. (2006) and Masoni et al. (2008) found that increasing gravel content (up to 30%) reduced total N and P uptake and decreased wheat biomass in Italian soils, although nutrient concentrations in plant tissue remained unaffected. The reduction in biomass was attributed to reduced soil volume available for root growth and impaired soil–root–water interactions, rather than nutrient deficiency per se. Similarly, Tiessen et al. (1993) demonstrated that ferruginous nodules (0.5–2 mm) from northern Ghana exhibited hysteresis in P adsorption–desorption and required higher initial P applications to achieve similar P saturation as finer soil fractions. These studies collectively highlight the complexity of P dynamics in gravelly soils and underscore the need to consider the chemical reactivity and physical implications of coarse fractions in nutrient management strategies.
Despite these findings, key knowledge gaps remain. Most studies have either focused on the <2 mm fraction or have applied P solely to this fraction, limiting our understanding of how gravel properties – such as size, proportion and P enrichment – affect P availability and plant response. In particular, there is a need to determine whether P-enriched gravels can act as a functional P source for crop growth, and how the physical and chemical properties of the gravel influence this capacity. Recent work by Weaver et al. (2022) has shown that IG can retain plant-available P that can be measured by Colwell bicarbonate extraction, challenging the assumption that P adsorbed to coarse particles is biologically inaccessible. In addition to challenges associated with how crops respond to nutrients in soils containing high gravel content, it has long been recognised that soil moisture (French and Schultz 1984) amongst a range of factors can limit crop production (Hochman and Horan 2018). Ironstone gravel soils introduce additional challenges since soil water storage capacity is usually found to decrease when the content of rock fragments increases (Poesen and Lavee 1994; Cousin et al. 2003; Baetens et al. 2009; Chen et al. 2011; Tetegan et al. 2011; Carrick et al. 2013; Sanidanya 2015).
As global demand for P increases and concerns over the sustainability of P fertiliser use intensify (Dhillon et al. 2017), improving P use efficiency becomes a pressing goal. A more nuanced understanding of P dynamics in gravelly soils is essential to developing fertiliser strategies that are both agronomically effective and environmentally sustainable. Addressing the potential contribution of the >2 mm fraction to P supply is a critical step towards this objective.
This study aims to evaluate the P adsorption and desorption characteristics of different size fractions of an IG soil and to explore the extent to which the >2 mm fraction – when enriched with P – can contribute to wheat growth. Specifically, the study seeks to do the following:
Compare the P adsorption and desorption characteristics of the <2 and >2 mm fractions of an IG soil.
Determine whether wheat growth can be sustained when P-enriched IG is the sole source of P.
Quantify the effect of varying levels of P enrichment, gravel size and gravel proportion on wheat biomass.
Examine the relationship between IG particle size and wheat responsiveness to P-enriched gravel.
These objectives are designed to test the following hypotheses:
The >2 mm fraction of IG will adsorb less P than the <2 mm fraction, but both will exhibit similar desorption rates with differences in magnitude.
Wheat biomass will increase with the level of P enrichment and the proportion of P-enriched IG, with growth supported even when P-enriched IG is the sole P source.
Wheat responsiveness to P-enriched IG will vary with gravel size, though no difference is expected between P accessed from the <2 and >2 mm fractions.
Wheat was selected as the test crop in these studies due to its agronomic relevance in the region. In SWWA, wheat is the dominant winter cereal, with over 4 million hectares sown annually, contributing more than AUD3 billion to the state’s economy. The overlap between wheat production areas and the distribution of IG soils makes it an ideal species for this study. Furthermore, wheat is highly responsive to P supply during early development, with root growth and tillering strongly affected by P availability. Its relatively short lifecycle, uniform growth habit and well-characterised response to P fertilisation also make it well-suited to controlled pot trials. The availability of P-efficient genotypes and standardised experimental protocols enhances the reproducibility and interpretability of results, facilitating comparison across studies.
In summary, this study addresses a key gap in our understanding of P dynamics in gravelly soils by investigating the role of the >2 mm fraction in P retention and availability. By evaluating P adsorption–desorption characteristics and crop response to P-enriched gravels, the research aims to challenge conventional assumptions about the inertness of the coarse fraction and contribute to the development of more accurate soil testing, interpretation and fertiliser recommendations for IG soils.
Materials and methods
Soils and properties
An IG soil (0–30 cm) was collected from West Dale (−32.327233 S 116.509133 E), around 90 km south east of Perth, WA (Weaver et al. 2022). Under the Western Australian soil groups classification (Schoknecht and Pathan 2013) it is described as a very gravelly deep sandy soil, and is colloquially known as Yalanbee soil. It is typical of 45% of the 2.7 million ha of the gravelly soils used for crop and pasture production in SWWA. The soil is described as a sesqui-nodular tenosol (Isbell and National Committee on Soil and Terrain 2021) using the Australian soil classification and is classified as a Endopetric Pisoplinthic Plinthosol (Arenic) (IUSS Working Group WRB 2015) under the World Reference Base. The soil had not been previously fertilised nor used for agricultural production and was collected from a cleared area amongst remnant vegetation [Eucalyptus accedens (powderbark wandoo) with an understory of Banksia sessilis (parrotbush)].
Soil preparation
The IG soil (soil A; Table 1) was sieved using <2, 2–4, 4–6, 6–8 and 8–10 mm fractions in preparation for use in experiments. The >2 mm fractions of the IG soil were gently washed with water and air dried prior to use. In addition, a white, low P retention, nutrient deficient washed sand (soil B; Table 1) (https://www.richgro.com.au/product/soft-washed-play-sand/) was used as the <2 mm fraction or supporting soil matrix (sand culture) for combination with >2 mm ironstone gravels in pot experiments. The <2 mm fractions were analysed for Colwell P (Colwell 1963), Colwell K (Rayment and Lyons 2011), P buffering index (PBI; Burkitt et al. 2002), pH (CaCl2) (Rayment and Lyons 2011), KCl-40S (Blair et al. 1991), particle size (Indorante et al. 1990) and specific surface area (SSA) using N adsorption (Brunauer et al. 1938) and are shown in Table 1. Ammonium oxalate extractable Fe and Al (Tamm 1922) of the <2 mm fraction were estimated using an established pedotransfer function for Fe (Weaver and Summers 2021) and the following pedotransfer function for Al (log ammonium oxalate Al = 0.799 × logPBI + 148; n = 3484; R2 = 0.54). Other properties of the <2 and >2 mm fractions of the IG soil (soil A) are described in Weaver et al. (2022).
| Property | Soil A | Soil B | |
|---|---|---|---|
| Munsell colour | 10YR 7/3 | N9 | |
| %>2 mm | 81 | 0 | |
| Coarse sand (200–2000 μm) | 61.3 | 93.8 | |
| Fine sand (20–200 μm) | 27.6 | 4.3 | |
| Silt (2–20 μm) | 4.0 | – | |
| Clay (<2 μm) | 7.0 | 1.9 | |
| pH (CaCl2) | 5.4 | 6.1 | |
| PBI | 45.4 | 2.5 | |
| Colwell P (mg kg−1) | <2 | <2 | |
| Colwell K (mg kg−1) | 29 | <15 | |
| KCl40S (mg kg−1) | 0.8 | 2.3 | |
| Surface area (m2 g−1) | 31.2 | 0.2 | |
| Ammonium oxalate extractable Fe (mg kg−1) | 550 | 71 | |
| Ammonium oxalate extractable Al (mg kg−1) | 639 | 63 |
Phosphorus adsorption and desorption
Adsorption and desorption of P was examined for each size fraction of the IG soil A (<2, 2–4, 4–6, 6–8 and 8–10 mm). The P adsorption isotherms were developed at a 10:1 solution:soil ratio by incubating each size fraction in triplicate, and quiescently, with occasional gentle mixing to avoid abrasion of gravel surfaces (Weaver et al. 2022). Initial P concentrations of 0, 5, 10, 20, 40, 100 and 400 mg P L−1 were used for the <2 mm fraction, and 0, 5, 10, 20, 40 and 100 mg P L−1 for the >2 mm fractions. At a 10:1 solution:soil ratio this resulted in P application of 50, 100, 300, 400, 1000 and 4000 mg P kg−1 for initial P concentrations of 5, 10, 20, 40, 100 and 400 mg P L−1, respectively. The P solutions were prepared using potassium dihydrogen orthophosphate (KH2PO4), and final concentrations were determined after 1, 2, 5, 8 and 16 days of adsorption. Following the adsorption phase, each sample was air dried at ambient temperature and subjected to five sequential extractions of 1 day duration with de-ionised (DI) water at a 10:1 ratio. All solutions were filtered at <0.45 μm prior to analysis for filterable reactive P (Murphy and Riley 1962). Adsorption isotherms were constructed by plotting P adsorbed vs equilibrium P concentration and fitting the Freundlich equation. Desorption isotherms were constructed by plotting P that remained adsorbed (by difference) vs equilibrium P concentration and fitting the Freundlich equation.
Volumetric water content
To assist with interpretation of the results from pot experiments, volumetric water content (VWC) measurements were determined on oven dried (105°C) mixes of soil B (Table 1) and IG 2–4, 4–6, 6–8 and 8–10 mm in size. These VWC measurements were determined at drained upper limit (DUL) or field capacity (Cassel and Nielsen 1986). The VWC was determined for gravel contents of 0, 25, 50, 75 and 100% by volume following cessation of drainage under gravity (Govindasamy et al. 2023), an approach shown to be comparable to the pressure plate method (Cresswell et al. 2008). Estimated gravimetric water contents were then adjusted to VWC using bulk density of each of the mixes (Moorberg and Crouse 2017).
Pot experiments
A series of five pot experiments were undertaken to test the stated hypotheses. This involved the preparation of P-enriched IG (IG+P) of different size ranges and mixing IG+P or IG without P enrichment (IG−P) in different proportions with a <2 mm fraction (Table 1). Following enrichment with P, the IG was air dried prior to mixing with the <2 mm fraction. Dependent on the experiment, the <2 mm fraction may also have been enriched with P, but separately from the IG. All incubations of <2 and >2 mm fractions with P were undertaken using KH2PO4, and pre- and post-incubation P concentrations were measured (Weaver et al. 2022). The test crop used in all pot experiments was wheat (var. Cobalt). This variety was selected for its consistent growth and high yield. More contemporary varieties are favoured for their relevance to current agricultural conditions and yield potential (Anderson et al. 2015).
All pot experiments used the same basal nutrients to supply nutrients other than P. These were prepared as separate mixtures of salts to avoid precipitation of undesirable compounds. Mixture 1 contained copper sulfate (CuSO4·5H2O), zinc sulfate (ZnSO4·7H2O), magnesium sulfate (MgSO4·7H2O), cobalt sulfate (CoSO4·7H2O) and manganese sulfate (MnSO4·H2O) so that a 5-mL aliquot applied at commencement delivered 4.4, 3.4, 90.8, 0.2 and 13.9 mg of Cu, Zn, Mg, Co and Mn per pot, respectively. Mixture 2 contained potassium sulfate (K2SO4) so that a 5-mL aliquot provided 145 mg K per pot at commencement and at 7–10 day intervals. Mixture 3 contained sodium molydbate (NaMoO4·2H2O) and boric acid (H3BO4) so that a 5-mL aliquot applied at commencement delivered 3.2 and 0.07 mg of Mo and B per pot, respectively. Mixture 4 contained calcium chloride (CaCl2·2H2O) so that a 5-mL aliquot provided 68 mg Ca per pot at commencement. Mixture 5 contained ammonium nitrate (NH4NO3) so that a 5-mL aliquot provided 114 mg N per pot at commencement and at 7–10 day intervals. Mixture 7 contained EDTA ferric sodium salt (C10H12N2NaFeO8) so that a 5-mL aliquot provided 3.8 mg Fe per pot at commencement. Gypsum (CaSO4·2H2O) was also applied at 0.35 g to each pot at commencement.
In all five pot experiments, the pot base was lined with geotextile fabric to eliminate washout of the <2 mm fraction through pot drainage holes, but to allow the pots to be free draining. When IG was used, the <2 and >2 mm treatments (IG+P or IG−P) were mixed to achieve a specified gravel percentage and then assembled in layers. One-third of the mixture was placed in the pot base, followed by a thin layer of sand (1 cm deep) to house and protect a rhizon (www.rhisosphere.com) inserted horizontally into the thin layer of sand 62 mm above the base. The inserted rhizon was used to extract soil solution. The remaining two-thirds of the soil mixture were placed in the pot above the rhizon layer and basal solutions were applied. Pots were watered to 75% of DUL and 10 wheat seeds were sown in each pot. Pots were misted until germination and thinned to five even plants. Automated overhead irrigation delivered equal volumes to all pots at each watering event, resulting in VWC slightly above field capacity immediately after watering. Some additional manual watering of pots was required at unspecified intervals dependent on observations of plant performance and extractions of soil moisture using rhizons. Soil solution was extracted on a weekly basis and analysed for filterable reactive P (Murphy and Riley 1962).
The response of wheat to P supplied from IG incubated with the same concentration of P for three gravel size fractions was explored in a sand culture medium. The experimental design comprised three replicates, two levels of P enrichment, three gravel sizes and three gravel quantities, and an additional control treatment with 100% soil B, resulting in 57 pots.
The IG+P was prepared in a fluvarium (Clarendon et al. 2019) where 39.6 kg of 2–4, 4–6 and 6–8 mm washed IG was placed, and 396 L of 100 mg P L−1 was cycled continuously over the gravel for 3.3 days. Following analysis of P in solution (Murphy and Riley 1962) at commencement and after 3.3 days, the 2–4, 4–6 and 6–8 mm IG+P retained 76.6, 56.3 and 59.3 mg P kg−1, respectively. In triplicate 25%, 50% and 75% by weight of IG+P and IG−P for each of the 2–4, 4–6 and 6–8 mm fractions was mixed with soil B (Table 1), respectively. Following pot assembly and management using the described design and treatments, plant tops were harvested once at the end of 45 days growth [mid to late tillering Z27–Z29; Zadoks et al. (1974)], oven dried at 60°C for 48 h and weighed.
The response of wheat to P supplied from IG (>2 mm) that was enriched with P to different levels was explored in a sand culture medium. The experimental design comprised three replicates, five levels of P enrichment and four gravel sizes for each of the IG+P treatments for 25% IG+P mixed with soil B (60 pots), and five levels of P enrichment and four gravel sizes for each of the IG+P treatments for 50% IG+P mixed with soil B (20 pots) (Table 1). Triplicate control treatments consisting of 100% soil B with and without P applied to achieve maximum growth were included (six pots), resulting in a total of 86 pots. To achieve each level of P enrichment, 12 kg of each of 2–4, 4–6, 6–8 and 8–10 mm washed IG was incubated with 120 L of 5, 10, 20, 40 and 100 mg P L−1 for 10 days in 190-L plastic drums.
Following pot assembly and management using the described design and treatments, plant tops were harvested after 42 days of growth, oven dried at 60°C for 48 h, weighed and the pots left to dry. After the pots were left to dry, any plant regrowth was cut and returned to the pots. Pots were subsequently reseeded for a second harvest after 54 days when the plant tops were harvested, oven dried at 60°C for 48 h, weighed, and the pots left to dry. Pots were again reseeded for a third harvest after an additional 42 days when the plant tops were harvested, oven dried at 60°C for 48 h and weighed. All three harvests were made at mid to late tillering Z27–Z29 (Zadoks et al. 1974). Basal nutrients were applied during each growth cycle, but the only P applied was via the IG+P at the start of the experiment. Control treatments consisting of 100% soil B received P applications prior to the first and third growth cycles. In addition to the collection of dry matter (DM) weights and rhizon soil solution P concentrations, harvested plant material was also analysed for P content (McQuaker et al. 1979).
The response of wheat to P application for the <2 mm fraction of the IG soil (soil A) and the white sand (soil B) (Table 1) in the absence of >2 mm fractions was explored. The experimental design comprised two soils, 10 P application rates and two replicates, resulting in 40 pots. The P was applied to duplicates of 3 kg of the <2 mm fraction of the IG soil (soil A) to achieve 0, 39.4, 78.8, 118.1, 157.5, 196.9, 236.3, 275.6, 315.0 and 354.4 mg P pot−1. The P was applied to duplicates of 6 kg of the <2 mm fraction of the white sand soil (soil B) to achieve 0, 21.1, 42.2, 63.3, 84.4, 105.5, 126.6, 147.7, 168.8 and 189.8 mg P pot−1. Following pot assembly and management using the described design and treatments, plant tops were harvested after 6–7 weeks growth [late tillering Z27–Z29; Zadoks et al. (1974)], oven dried at 60°C for 48 h and weighed.
The response of wheat to P supplied from IG (>2 mm) that was enriched with P to achieve similar levels of surface P concentration was explored in a sand culture medium. The experimental design comprised three replicates, four gravel sizes and three P concentrations to achieve the IG+P treatments (36 pots), and triplicate control treatments consisting of 100% soil B with and without P applied to achieve maximum growth (six pots), resulting in a total of 42 pots.
To prepare the IG+P treatments, washed gravel in the 2–4, 4–6, 6–8 and 8–10 mm ranges was incubated with 150 L of 2, 20 and 100 mg P L−1 for 10 days in 190-L plastic drums at solution:gravel ratios of 43:1, 24:1, 20:1 and 13:1, respectively. These ratios were selected to achieve the same estimated surface P concentration on each IG at each of the incubating P concentrations for the incubating concentrations of 2, 20 and 100 mg P L−1, on assumptions of median size and sphericity using mass–volume relationships (Pennell 2016). Subsequent direct measurements of SSA of each gravel size indicated that ratios of 21.5:1, 18.7:1, 15.8:1 and 13:1 for the 2–4, 4–6, 6–8 and 8–10 mm fractions, respectively, should have been used (Weaver et al. 2022).
Based on SSA measurements from Weaver et al. (2022), solution:gravel ratios and concentrations of P before and after incubation, actual surface P concentrations of IG were on average 0.0006, 0.0015 and 0.0049 mg P m−2 for the incubating concentrations of 2, 20 and 100 mg P L−1, respectively, with a tendency for mg P m−2 to decline as gravel size increased. Control pots comprising 100% soil B with no P applied and 190 mg P pot−1 were also included.
Given estimates of SSA from physical measurement for each IG size, each of the IG+P treatments were mixed with sand (soil B) in different proportions in an attempt to achieve similar quantities of P for each pot. From total P analysis of IG+P the amount of P added per pot was 51, 87, 128 and 125 mg for the 2–4, 4–6, 6–8 and 8–10 mm fractions incubated with 2 mg P L−1, respectively; 74, 108, 128 and 142 mg for the same fractions incubated with 20 mg P L−1; and 133, 174, 156 and 192 mg for the same fractions incubated with 100 mg P L−1. The different proportions of IG+P added were 15%, 27%, 32% and 49% by weight for the 2–4, 4–6, 6–8 and 8–10 mm fractions, respectively. Following pot assembly and management using the described design and treatments, plant tops were harvested at 44 days at early stem elongation [Z31–Z33; Zadoks et al. (1974)], oven dried at 60°C for 48 h and weighed. In addition to the collection of DM weights, rhizon soil solution P concentrations were also determined.
This experiment explored the response of wheat to P from mixes of separately P-enriched <2 and >2 mm fractions of soil A. The experimental design comprised three replicates, four gravel sizes, two gravel amounts, three levels of >2 mm fraction fertility and three levels of <2 mm fraction fertility (216 pots). In addition, there were three replicates of control pots comprising the <2 mm fraction at three levels of fertility (nine pots) to bring the total number of pots to 225.
The <2 and >2 mm fractions of the IG soil (soil A) were separately incubated with P to achieve 0, 50% or 95% of relative yield (RY) when different proportions of different size fractions, <2 or >2 mm, were present. Incubating P concentrations were based on results from experiments 1, 2 and 3 and previous P sorption experiments (Weaver et al. 2022). For IG, 19 kg of 2–4, 4–6, 6–8 and 8–10 mm washed gravel was incubated with 170 L of P solutions of varying concentration for 9 days to achieve IG+P with different levels of P enrichment. Selected P concentrations for 2–4 mm gravel were 11, 22, 67 and 179 mg P L−1, for 4–6 mm gravel were 11, 34, 112 and 358 mg P L−1, for 6–8 mm gravel were 11, 22, 89 and 324 mg P L−1, and for 8–10 mm gravel were 11, 34, 112 and 402 mg P L−1. These levels of enrichment allowed the addition of 25% or 50% IG+P so that 50% or 95% RY could be achieved. Resulting Colwell P of IG+P for the 2–4 mm gravel was 10 and 5 mg kg−1 to achieve 50% RY at 25% and 50% gravel content, respectively, and correspondingly 57 and 27 mg kg−1 to achieve 95% RY. Resulting Colwell P of IG+P for the 4–6 mm gravel was 11 and 6 mg kg−1 to achieve 50% RY at 25% and 50% gravel content, respectively, and correspondingly 48 and 21 mg kg−1 to achieve 95% RY. Resulting Colwell P of IG+P for the 6–8 mm gravel was 6 and 4 mg kg−1 to achieve 50% RY at 25% and 50% gravel content, respectively, and correspondingly 34 and 15 mg kg−1 to achieve 95% RY. Resulting Colwell P of IG+P for the 8–10 mm gravel was 6 and 3 mg kg−1 to achieve 50% RY at 25% and 50% gravel content, respectively, and correspondingly 22 and 13 mg kg−1 to achieve 95% RY.
For the <2 mm fraction (soil A), P was applied with moisture to achieve 50% of DUL on the day prior to pot construction. The amount of P applied was 23.7 or 93.2 mg P kg−1 of <2 mm fraction, resulting in an estimated Colwell P of 8 and 22 mg kg−1 which would achieve 50% and 95% RY, respectively, from the <2 mm fraction if this was the only fraction present. Triplicates of 25% or 50% of each gravel size enriched to achieve 0%, 50% or 95% of RY were mixed with the <2 mm fraction of IG soil (soil A) also enriched to achieve 0%, 50% or 95% of RY. Additional control treatments consisting of 100% of the <2 mm fraction of IG soil (soil A) to achieve 0%, 50% or 95% RY were included for a total of 225 pots. Following pot assembly and management using the described design and treatments, plant tops were harvested after 46 days of growth [late tillering Z29; Zadoks et al. (1974)], oven dried at 60°C for 48 h and weighed. The plants were allowed to regrow for a further 90 days until maturity [Z92; Zadoks et al. (1974)] when the plant tops were harvested, and DM and grain weights were recorded following oven drying at 60°C for 48 h. In addition to the collection of DM weights, harvested plant material for harvest 1 was also analysed for P content (McQuaker et al. 1979), and rhizon soil solution P concentrations were determined.
During the first 46 days of growth, soil moisture was logged in 15-min intervals in specific pots using an EC-5 soil moisture sensor (https://edaphic.com.au/products/soils/ec-5-soil-water-content-sensor/). Logged treatments included pots with 0% gravel with sufficient P to achieve 0%, 50% and 95% RY; pots with 25% gravel with sufficient P to achieve 50% and 95% RY; and pots with 50% gravel with sufficient P to achieve 50% and 95% RY. Raw mV readings from EC-5 sensors were correlated with manually collected calibrated soil moisture measurements from a handheld ProCheck (www.decagon.com) soil moisture meter to estimate timeseries of VWC.
Data analysis and curation
The available data were presented as box and violin plots (McGill et al. 1978; Hintze and Nelson 1998) and scatterplots, stratified and symbolised by IG amount, size, weighted Colwell P and total P added to experimental pots. Weighted Colwell P was calculated from separate Colwell P of gravel and matrix fractions and the mass of these fractions within a pot. In addition, linear, non-linear regression, analysis of variance (ANOVA), analysis of covariance (ANCOVA) and Spearman rank correlation were undertaken using the exploratory data analysis and presentation tools in RStudio (rstudio.com), DataDesk 8.3 (datadesk.com) and Igor Pro v9 (wavemetrics.com). The threshold level of significance was set at P < 0.05.
The responsiveness of wheat to applied P, either added as IG+P for different gravel size fractions or directly to the <2 mm fraction, was examined using the Mitscherlich equation (Eqn 1):
where y is the yield of DM (g pot−1), X is the amount of P applied (mg P pot−1), a is maximum DM (g pot−1), b quantifies the yield response (, where Y0 is DM when X = 0; b ranges within 0–1) and c describes the curvature of the response. As c increases, the response curve moves to the left, becomes steeper and less P is required to produce the same relative yield. Hence smaller values of c are regarded as less responsive than larger values of c. The DM responses were re-expressed as RY by dividing y by a, refitting Eqn 1 and setting a = 100 and b = 1 which resolves to Eqn 2.
Parallel curves analysis using accumulated ANOVA in Genstat V22.1 from VSN International Ltd was used to determine whether equation parameters for different treatments were justified (Powers 2021). Treatment differences explored included whether more than or less than 50% of the weighted Colwell P or total P added to a pot was derived from gravel or matrix, or whether treatments included 25% or 50% gravel.
Results
Phosphorus adsorption and desorption
The amount of P adsorbed increased in the size order 8–10, 6–8, 4–6, 2–4 and <2 mm. The amount of P adsorbed increased with increasing contact time for all size fractions at all incubating P concentrations (Fig. 1a–e). The amount of P adsorbed increased at a faster rate over time as the incubating P concentration increased.
Net P adsorption and desorption dynamics for the (a) <2, (b) 2–4, (c) 4–6, (d) 6–8 and (e) 8–10 mm fractions of soil A. Closed symbols show adsorption phase for 1, 2, 5, 8 and 16 days at 5, 10, 20, 40, 100 and 400 mg P L−1 initial P concentrations. Open symbols show desorption of five sequential 24 h extractions at the end of the adsorption phase. Thick dashed grey line and grey shading show fitted adsorption curve (Freundlich) after 16 days with 95% confidence band. Thin solid black lines connect treatments with the same initial P concentration through increasing time for the adsorption phase, followed by sequential extracts through the desorption phase (thin dashed black line).
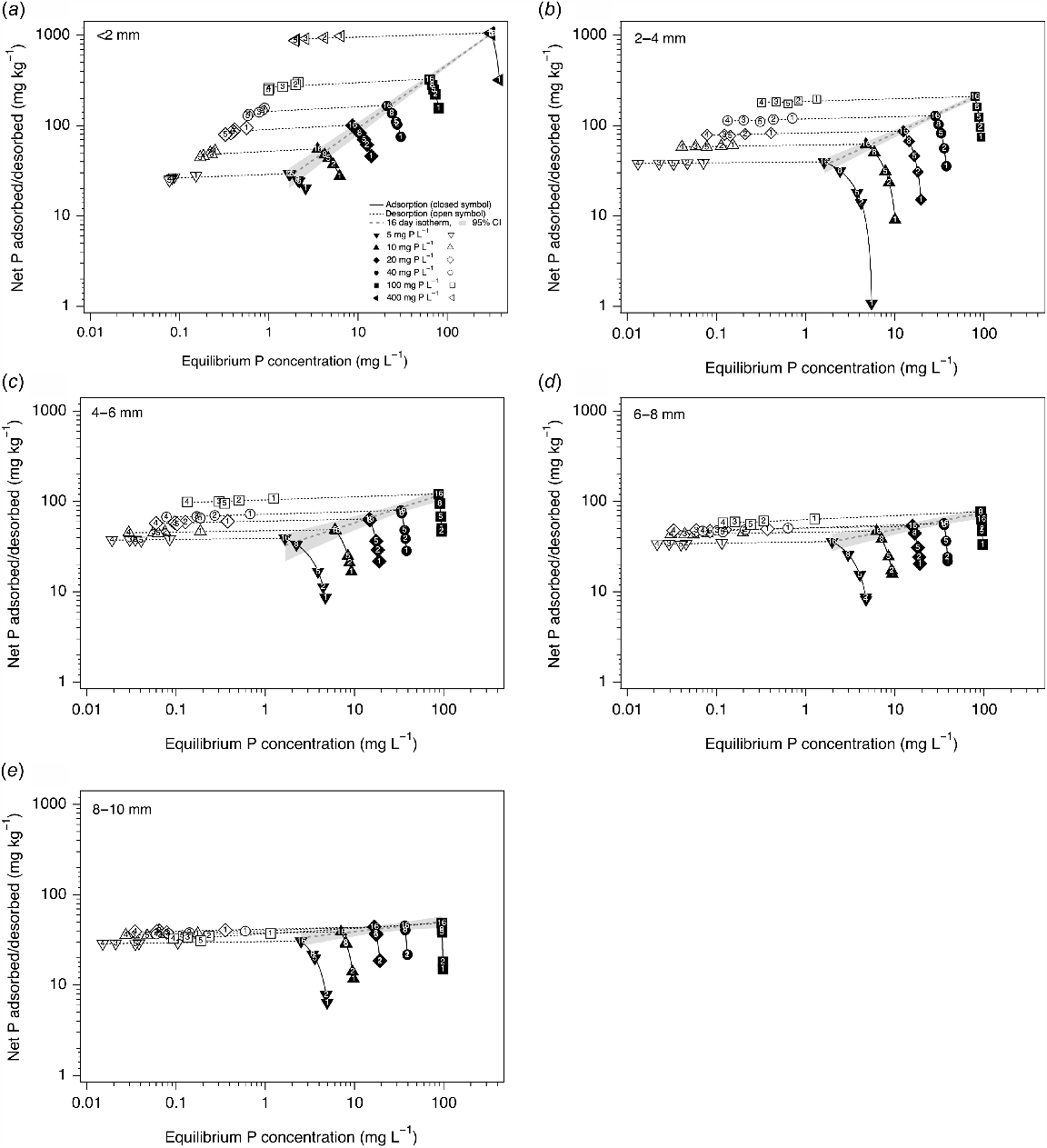
More P was adsorbed than desorbed. The <2 mm fraction adsorbed 29–1050 mg P kg−1 after 16 days and desorbed 5–172 mg P kg−1 after five sequential extractions depending on the amount of P applied. Correspondingly, the 2–4 mm fraction adsorbed 40–210 mg P kg−1 and desorbed 2–36 mg P kg−1, the 4–6 mm fraction adsorbed 40–120 mg P kg−1 and desorbed 2–25 mg P kg−1, the 6–8 mm fraction adsorbed 36–77 mg P kg−1 and desorbed 2.5–21 mg P kg−1, and the 8–10 mm fraction adsorbed 31–49 mg P kg−1 and desorbed 2–18 mg P kg−1.
There was a significant interaction between size, incubate P concentration and extraction on the amount of P desorbed. Most P was desorbed in the first extraction for all size fractions, and the amount of P desorbed decreased with increasing size. The amount of P desorbed increased with increasing incubate P concentration in the following size order: 8–10, 6–8, 4–6, 2–4 and <2 mm.
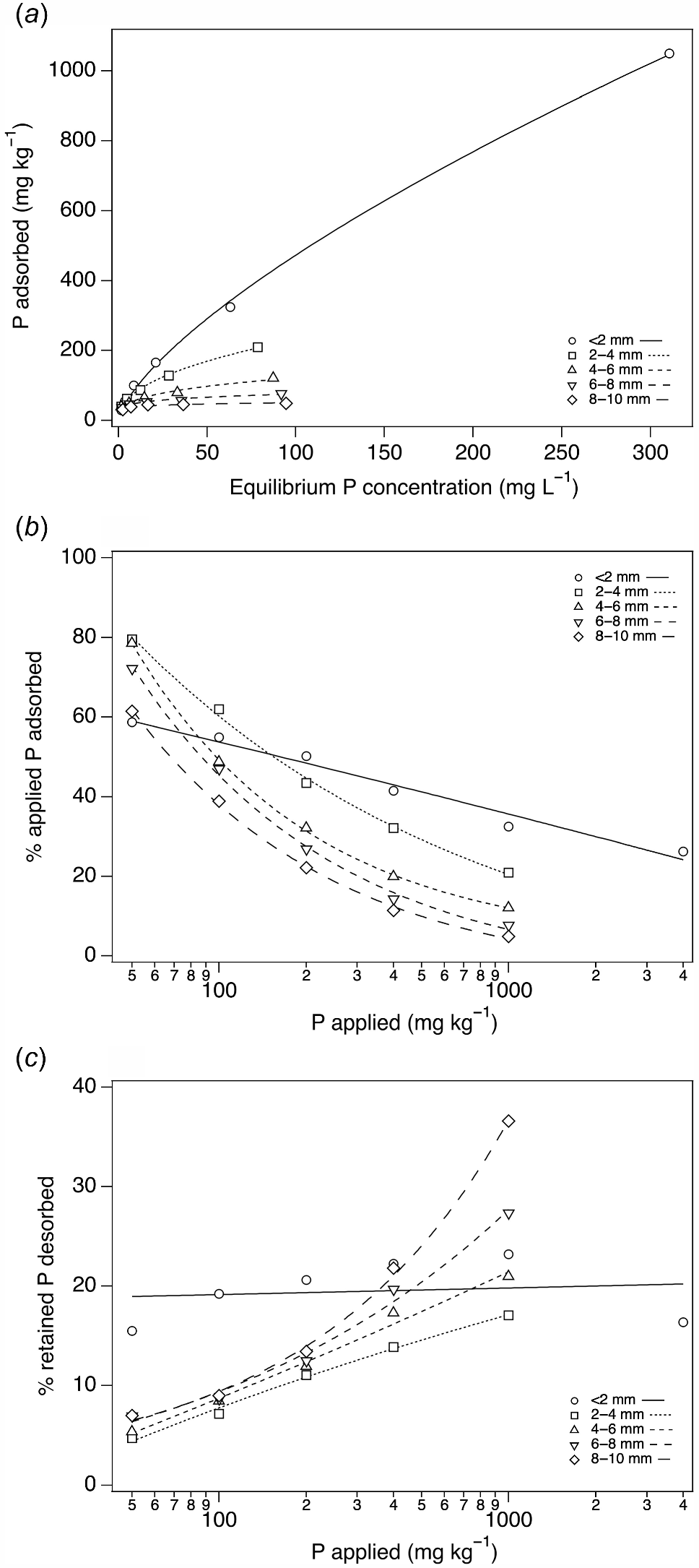
The P adsorption decreased with increasing particle size (Fig. 2a), with most P adsorbed by the <2 mm fraction and the least by the 8–10 mm fraction of soil A. The percentage of applied P retained after 16 days decreased with increasing P applied and ranged within 5–80% (Fig. 2b). The 2–4, 4–6, 6–8 and 8–10 mm fractions retained more P (60–80%) than the <2 mm fraction (60%) at the lowest rate (50 mg P kg−1) of P application. The rate of decline in the percentage of P adsorbed with P applied was greater for the >2 mm fractions than the <2 mm fraction. At 1000 mg P kg−1 applied, the >2 mm fractions retained 5–20% of the applied P, whilst the <2 mm fraction retained about 35%. The percentage of adsorbed P that was subsequently desorbed remained constant (~20%) with P applied for the <2 mm fraction (Fig. 2c). In contrast, the percentage of adsorbed P that was subsequently desorbed increased with increasing P applied and with increasing size for the >2 mm fractions, and ranged within 5–35%. At 1000 mg P kg−1 applied, the 4–6, 6–8 and 8–10 mm fractions desorbed a greater percentage of the retained P than the <2 mm fraction, whilst for below 400 mg P kg−1 applied the >2 mm fractions desorbed a smaller percentage (5–20%) of the retained P than the <2 mm fraction.
(a) Freundlich isotherms fitted to 16-day P adsorption data for the <2, 2–4, 4–6, 6–8 and 8–10 mm fractions. Fitted Freundlich curves (Padsorbed = A × Equilibrium P concentrationB) had the following A and B coefficients and R2 values: A = 18.06, B = 0.72, R2 = 0.96; A = 29.08, B = 0.45, R2 = 0.99; A = 25.13, B = 0.35, R2 = 0.99; A = 19.85, B = 0.29, R2 = 0.98; and A = 19.37, B = 0.20, R2 = 0.93 for the <2, 2–4, 4–6, 6–8 and 8–10 mm fractions, respectively. (b) Percentage of applied P adsorbed after 16 days for the <2, 2–4, 4–6, 6–8 and 8–10 mm fractions for different amounts of P applied. (c) Percentage of the P retained after 16 days that was desorbed after five extractions with DI water for the <2, 2–4, 4–6, 6–8 and 8–10 mm fractions for different amounts of P applied.
Volumetric water content
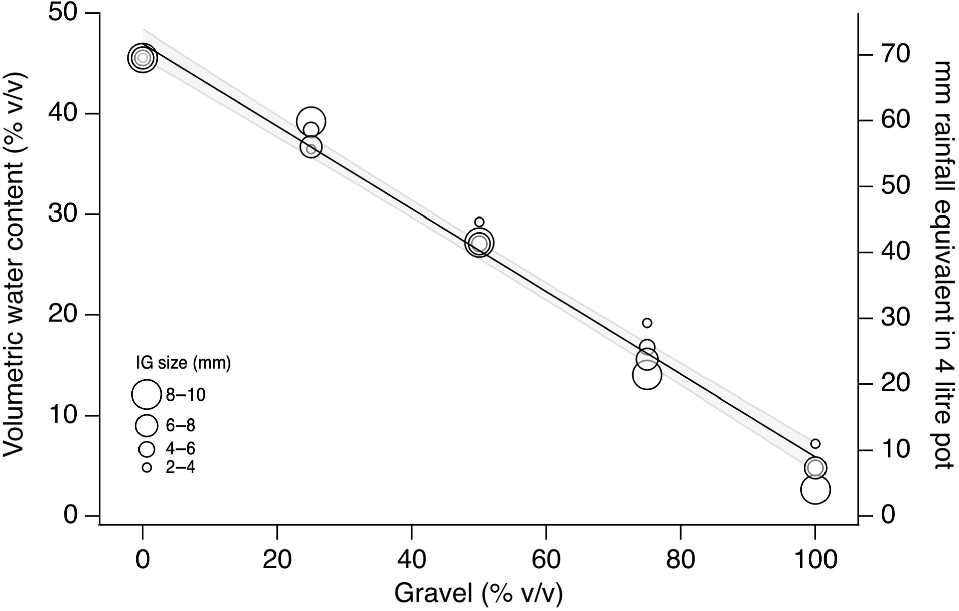
When soil B was used as the fine fraction, VWC decreased linearly with increasing gravel percentage. The ANCOVA showed that the effect of gravel size was not significant (P > 0.05), and there was no significant difference in intercept among the groups. Therefore, a single linear equation described VWC as a function of gravel percentage (Fig. 3). The VWC decreased from around 40% (equivalent to 65 mm rainfall in a 4-L pot) when no gravel was present, to <5% (equivalent to <10 mm rainfall) when the sample was 100% gravel.
Pot experiments
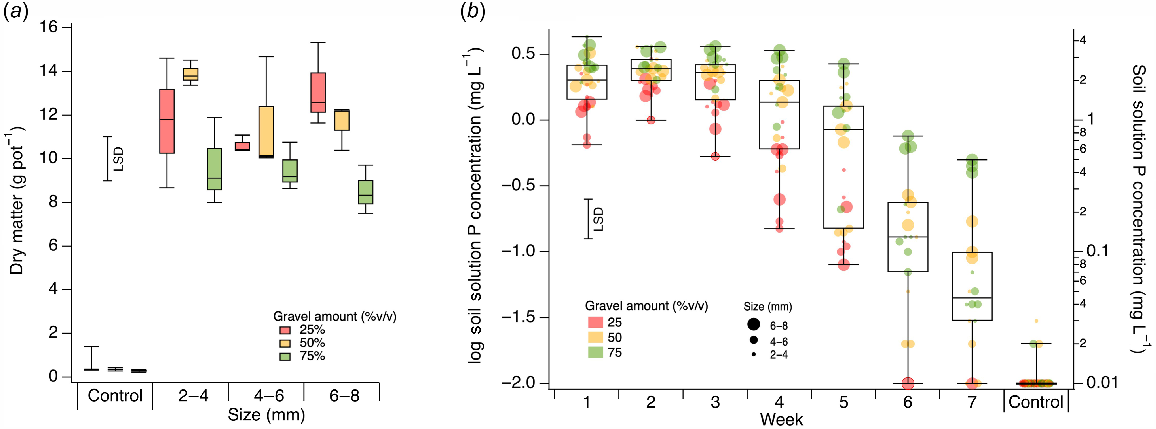
The treatment effect of IG+P had a 30-fold increase in g DM pot−1 (11.1) over IG−P (0.37). There was no significant treatment effect of gravel size on DM (P = 0.47). However, there was a significant effect (P < 0.001) of gravel amount (Fig. 4a), with 75% IG+P producing lower amounts of DM than both 25% and 50% IG+P. For DM, there was significant interaction (P < 0.001) between gravel amount and gravel treatment (IG+P, IG−P), but not between gravel size and gravel treatment (P = 0.31). Soil solution P concentrations of the IG+P treatments were much greater (mean of 0.65 mg P L−1) than the IG−P treatments (<0.01 mg P L−1) (Fig. 4b). Soil solution P concentrations decreased significantly over time (P < 0.001) and with IG+P amount (P < 0.001), but no significant effect of gravel size was identified (P = 0.85). Measured P concentrations were roughly in proportion to the mass of IG+P present.
(a) Boxplots of dry matter (g pot−1) for 2–4, 4–6 and 6–8 mm IG+P and IG−P (control) treatments at 25%, 50% and 75% (v/v). (b) Boxplots of the weekly change in soil solution P concentration (mg L−1) for for 2–4, 4–6 and 6–8 mm IG+P and IG−P (control) treatments at 25%, 50% and 75% (v/v). The LSD is based on a four way interaction between treatment, size, amount and sampling time.
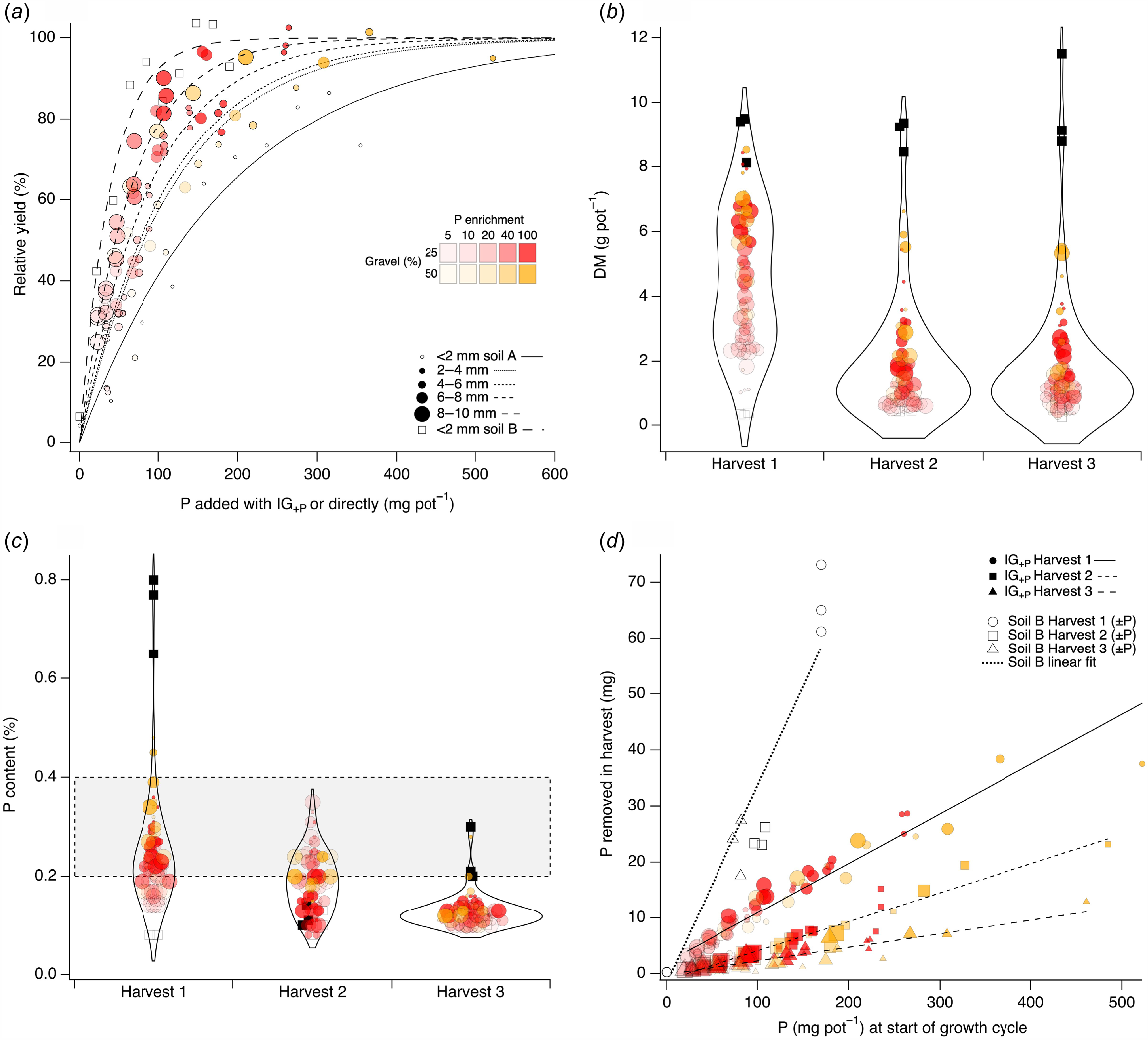
The combined RY from the first harvest of Experiments 2 and 3 is shown in Fig. 5a. Wheat grown on 100% sand (soil B; Table 1) was the most responsive to P application, whilst wheat grown on 100% <2 mm fraction (soil A; Table 1) was the least responsive to P application. Between soils B and A, wheat response to P application systematically decreased with decreasing gravel size. The c coefficients of the fitted curves were 0.0257, 0.0153, 0.0113, 0.0091, 0.0085 and 0.0053 for the sand and 8–10, 6–8, 4–6, 2–4 and <2 mm fractions, respectively. For IG of the same size, greater levels of RY and DM were achieved when either IG+P was present in greater amounts relative to the <2 mm fraction, or the IG+P was enriched with a higher P concentration (Fig. 5a, b). Wheat DM was greater in the first harvest than in subsequent harvests. Some treatments from the first harvest, particularly IG+P either with smaller size (2–4 and 4–6 mm) and a higher level of P enrichment (100 mg P L−1), or higher gravel amount (50%) of any size with higher levels of P enrichment (40 and 100 mg P L−1) could support as much DM as soil B with P applied to maximise wheat growth (Fig. 5b). The mean DM pot−1 of harvests 2 and 3 fell to 37% and 33%, respectively, of harvest 1. The P content of harvested plant material fell with successive harvests (Fig. 5c). For harvest 1 of experiment 2, plant tissue P content increased significantly with increasing P enrichment (P < 0.0001), gravel amount (P < 0.0001) and decreasing gravel size (P < 0.0001). From harvest 1, the highest tissue P contents were found in sand treatments with P applied to maximise growth, followed by treatments with 50% IG+P enriched to the highest level and with smaller sizes of IG (2–4 and 4–6 mm), and then treatments with 25% IG+P, with the lowest P content for wheat grown in soil B with no P added. By harvest 3, the P contents of wheat grown on all IG+P treatments were similarly low and below the whole-shoots critical range estimated from Reuter and Robinson (1997) for all treatments except where soil B had P applied to achieve maximum growth. For IG treatments, the amount of P removed in harvested biomass decreased with consecutive harvests for similar levels of P in the pot at the start of a growth cycle (Fig. 5d), as indicated by the decreasing slope of the relationship for consecutive harvests. This reduction occurred despite the relatively small removal of P in harvested biomass (tens of milligrams) compared to the quantity of P available at the start of each growth cycle (hundreds of milligrams). The amount of P removed in harvest for IG treatments was also smaller than for wheat grown in soil B. The slope of the relationship between P removed in harvested biomass and P in the pot at the start of the growth cycle (Fig. 5d) was highest when P was added in a soluble form to soil B, than when P was added in the form of IG+P.
(a) Relationship between the RY (%) of wheat to the added mg P pot−1 either as IG+P or directly for the <2 mm fraction of the IG soil ( ; c = 0.0053), and for 2–4 mm (●; c = 0.0085), 4–6 mm (●; c = 0.0091), 6–8 mm (
; c = 0.0053), and for 2–4 mm (●; c = 0.0085), 4–6 mm (●; c = 0.0091), 6–8 mm ( ; c = 0.0113), 8–10 mm (
; c = 0.0113), 8–10 mm ( ; c = 0.0153) gravels, and for sand (□; c = 0.0257). The c coefficient from curvefit (Eqn 2) in parenthesis following symbol. Data compiled from the first harvest of Experiments 1, 2 and 3. (b) Violin plots of DM from three sequential harvests (Experiment 2) compared to DM of wheat grown in sand without (□) and with P (■) to maximise growth. (c) Violin plots of DM P content from three sequential harvests (Experiment 2) compared to DM of wheat grown in soil B without (□) and with P (
; c = 0.0153) gravels, and for sand (□; c = 0.0257). The c coefficient from curvefit (Eqn 2) in parenthesis following symbol. Data compiled from the first harvest of Experiments 1, 2 and 3. (b) Violin plots of DM from three sequential harvests (Experiment 2) compared to DM of wheat grown in sand without (□) and with P (■) to maximise growth. (c) Violin plots of DM P content from three sequential harvests (Experiment 2) compared to DM of wheat grown in soil B without (□) and with P ( ) to maximise growth. Grey shaded area shows typical whole shoot critical tissue concentrations for wheat from Reuter and Robinson (1997). (d) P removed in harvested material as a function of the P available at the start of each growth cycles (closed coloured symbols) for three sequential harvests (harvest 1:
) to maximise growth. Grey shaded area shows typical whole shoot critical tissue concentrations for wheat from Reuter and Robinson (1997). (d) P removed in harvested material as a function of the P available at the start of each growth cycles (closed coloured symbols) for three sequential harvests (harvest 1:  , harvest 2: ■, harvest 3: ▲) compared to soil B (open symbols) without (origin) and with P to maximise growth. Linear fit through combined soil B data for three harvests also shown. Symbol size shows gravel size, and colour and colour intensity represent gravel % (v/v) and concentration of P enriching solution (mg P L−1), respectively.
, harvest 2: ■, harvest 3: ▲) compared to soil B (open symbols) without (origin) and with P to maximise growth. Linear fit through combined soil B data for three harvests also shown. Symbol size shows gravel size, and colour and colour intensity represent gravel % (v/v) and concentration of P enriching solution (mg P L−1), respectively.
The DM and soil solution P concentrations from Experiment 4 are shown in Fig. 6. Most DM was produced in soil B when P was applied, and the least was produced in soil B when P was not applied. The IG+P treatments with the largest quantity of P per pot and greatest surface concentration of P also showed high DM production (Fig. 6a). However, within each treatment and size fraction, the range of DM values remained relatively narrow. The ANOVA for the IG+P treatments separated into different IG sizes showed significant increases in DM as the amount of P per pot increased and concentration of P on the surface of IG increased (Fig. 6a). Further, for a fixed level of P per pot and IG+P surface P concentration, DM amount varied little and showed small but significant differences.
(a) Boxplots of dry matter (g pot−1) for soil B with and without P, IG+P of various sizes incubated with 2 mg P L−1 (green), 20 mg P L−1 (orange) and 100 mg P L−1 (red) at different solution:soil ratios to achieve similar surface P concentrations. Quantity of P added with differing amounts of IG+P or as KH2PO4 for each treatment following incubation shown in mg P pot−1. (b) Boxplots of log soil solution P for soil B with and without P, IG+P of various sizes incubated with 2 mg P L−1 (green), 20 mg P L−1 (orange) and 100 mg P L−1 (red) at different solution:soil ratios to achieve similar surface P concentrations. Week of sampling shown by symbol size. Treatments with the same letter are not significantly different and increase alphabetically.
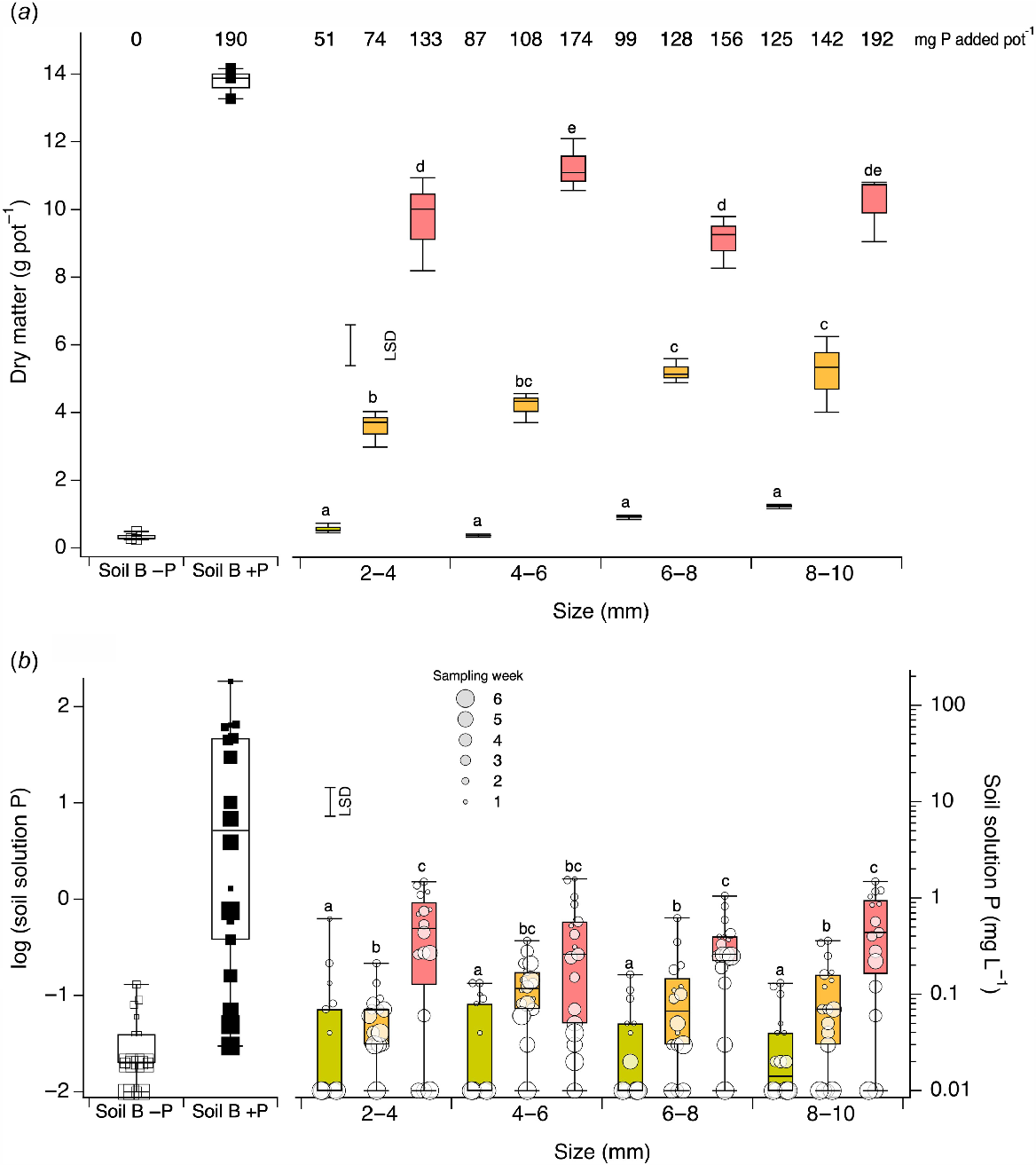
Like DM, soil solution P concentrations were highest for soil B with P applied, and lowest for soil B with no P applied (Fig. 6b). The highest soil solution P concentrations for IG+P treatments occurred with the largest quantity of P per pot and highest surface concentration of P, and lowest soil solution P concentrations occurred for the lowest quantity of P per pot and surface concentration of P. For a fixed level of P per pot and IG+P surface P concentration, soil solution P concentrations varied little and showed some small but significant differences. The ANOVA showed no significant effect of IG size (P = 0.97), but there was a significant effect due to the quantity of P per pot and surface P concentration (P < 0.0001). Soil solution P concentrations decreased with increasing time (P < 0.0001) (Fig. 6b).
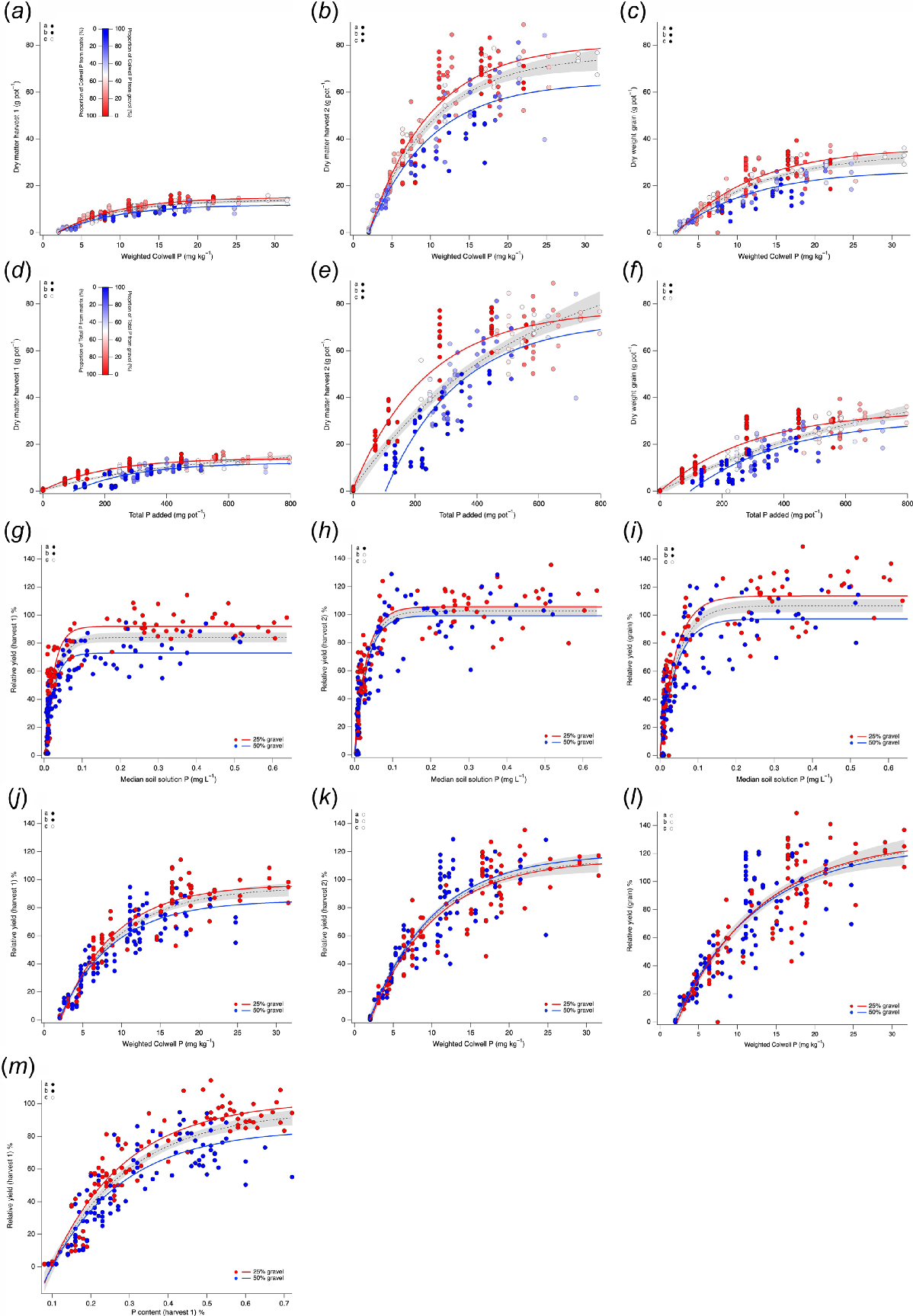
The DM for harvest 1, harvest 2 and grain followed Mitscherlich responses when expressed as a function of weighted Colwell P (Fig. 7a–c) and total P added per pot (Fig. 7d–f). Much more DM was observed at harvest 2 and grain yield compared to harvest 1. While typical Mitscherlich responses were followed, differentiation by the proportion of weighted Colwell P or total P added per pot showed systematic increases in the amounts of DM when a greater proportion of the P was supplied by the soil matrix (<2 mm) than by the gravel (>2 mm). Separation of the data into groups where less than or more than 50% of the P derived from the gravel showed significant increases in DM when <50% of the P was derived from gravel (>50% of the P was derived from the soil matrix). Relationships of RY as a function of median soil solution P for harvest 1 (Fig. 7g), harvest 2 (Fig. 7h) and grain yield (Fig. 7i) followed a Mitscherlich response; however, greater RY was achieved for 25% than for 50% gravel treatments. Similarly, relationships of RY as a function of weighted Colwell P for harvest 1 (Fig. 7j), harvest 2 (Fig. 7k) and grain yield (Fig. 7l) followed a Mitscherlich response. However, greater RY was achieved at 25% than at 50% gravel only for harvest 1, whilst the fitted curves could not be separated for harvest 2 or grain and were not significantly different (P > 0.05). Relationships of RY as a function of P content measured in harvested biomass for harvest 1 followed a Mitscherlich response (Fig. 7m). For the same P content, greater RY was achieved for 25% than 50% gravel.
Dry matter (g pot−1) as a function of weighted Colwell P (mg kg−1) for (a) harvest 1, (b) harvest 2 and (c) grain; dry matter (g pot−1) as a function of total P added (mg pot−1) for (d) harvest 1, (e) harvest 2 and (f) grain. Relative yield as a function of median soil solution P (mg L−1) for (g) harvest 1, (h) harvest 2 and (i) grain. Relative yield as a function of weighted Colwell P (mg kg−1) for (j) harvest 1, (k) harvest 2 and (l) grain. Relative yield as a function of P content (%) for (m) harvest 1. For a–f point colour represents the proportion of weighted Colwell P (a–c) or total P added (d–f) derived from gravel or soil matrix. Dashed black line and grey shading show the fitted curve and 95% confidence band for the full dataset. For a–f red solid line shows the fitted curve for points where the proportion of weighted Colwell P (a–c) or total P (d–f) added from gravel is <50% (matrix > 50%), and the blue solid line shows the fitted curve for points where the proportion of weighted Colwell P (a–c) or total P (d–f) added from gravel is >50% (matrix < 50%). For g–i (median soil solution P) and j–l (weighted Colwell P) and m (P content) red points and line from pots with 25% gravel and blue points and line for pots with 50% gravel. Top left corner of each panel indicates whether curve parameters (a, b or c from Eqn 1) are significantly different (●) or not significantly different (○) between groups (red and blue lines).
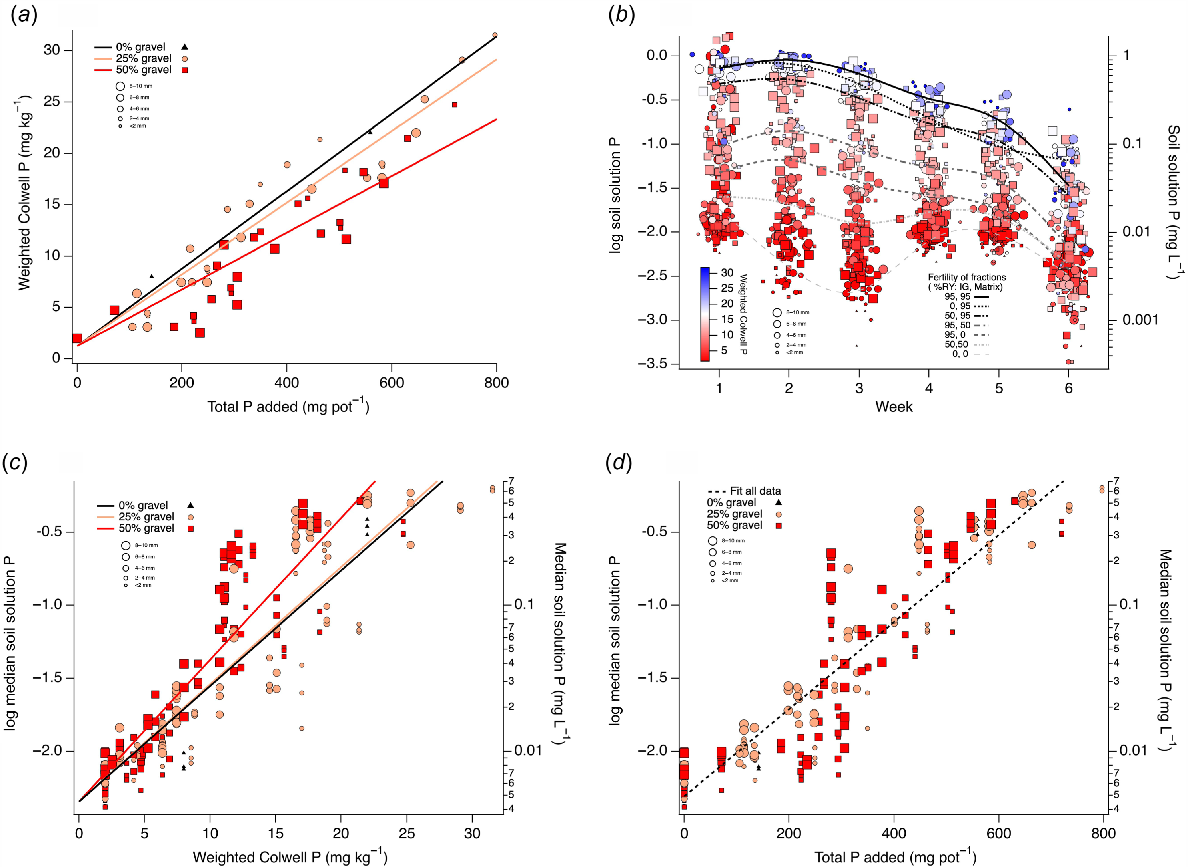
Weighted Colwell P was linearly related with total P added (Fig. 8a), and the slope of these relationships decreased as the percentage of gravel increased. Soil solution P collected in rhizons during the first 46 days decreased weekly, and much higher concentrations were found in treatments with higher weighted Colwell P (Fig. 8b). The highest mean concentrations were in treatments where both IG and the soil matrix were enriched to achieve 95% RY, and the lowest observed mean concentrations where neither IG nor the soil matrix had any P enrichment. Mean soil solution P concentrations were higher when the soil matrix was enriched to achieve 95% RY and IG 0% RY than other RY combinations of IG and soil matrix, such as 50% IG 95% soil matrix, 95% IG 50% soil matrix, 95% IG 0% soil matrix and 50% IG 50% soil matrix (Fig. 8b). Median soil solution P from rhizons was linearly related with weighted Colwell P, and the slope of this relationship was greater for treatments with 50% gravel than for 0% or 25% gravel (Fig. 8c). Median soil solution P from rhizons was linearly related with total P added; however, this relationship was not differentiated by gravel percentage (Fig. 8d).
(a) Linear relationships between weighted Colwell P (mg kg−1) and total P added (mg pot−1) for treatments with 0% (black triangles), 25% (orange circles) or 50% (red squares) gravel by weight per pot. Size fraction increases with increasing symbol size. Fitted lines based on significant terms from ANCOVA. (b) Changes in soil solution P (mg L−1) over sampling week. Gaussian noise added in x direction to reveal overlapping data points. Colour scale shows weighted Colwell P (mg kg−1) of treatment at commencement. Triangles show treatments with 0% gravel, circles with 25% gravel and squares with 50% gravel. Size fraction increases with increasing symbol size. Overlays of cubic splines fitted to mean values for treatments where IG and soil matrix had been incubated with P prior to being combined in a pot to achieve 95% RY from IG and 95% RY from matrix (solid black line), 0% RY from IG and 95% RY from matrix (dotted black line), 50% RY from IG and 95% RY from matrix (black dash dot dot line), 95% RY from IG and 50% RY from matrix (dark grey dash dot line), 95% RY from IG and 0% RY from matrix (dark grey dashed line), 50% RY from IG and 50% RY from matrix (light grey short dash dotted line), and 0% RY from IG and 0% RY from matrix (light grey long dashed line). (c) Log median soil solution P as a function of weighted Colwell P (mg kg−1) of treatment at commencement. Black triangles show treatments with 0% gravel, orange circles with 25% gravel and red squares with 50% gravel. Size fraction increases with increasing symbol size. Fitted lines based on significant terms from ANCOVA. (d) Log median soil solution P as a function of total P added (mg pot−1) of treatment at commencement. Black triangles show treatments with 0% gravel, orange circles with 25% gravel and red squares with 50% gravel. Size fraction increases with increasing symbol size. Fitted lines based on significant terms from ANCOVA.
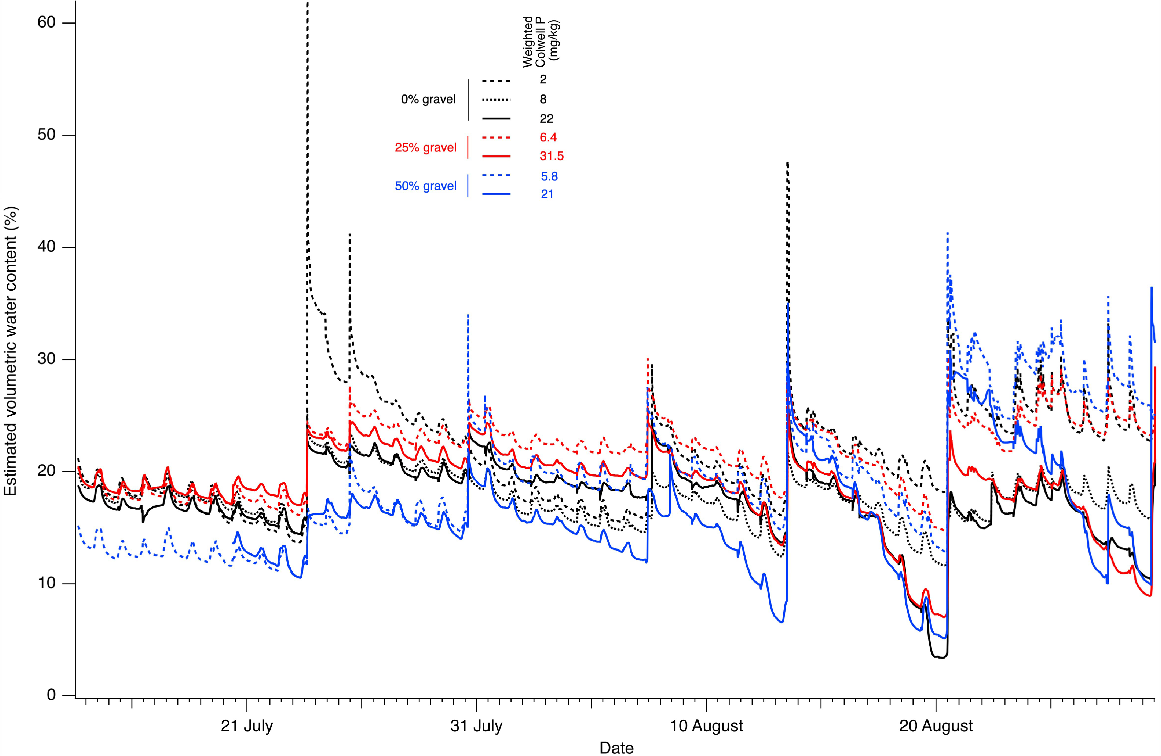
Estimated VWC varied over time during the first 46 days of plant growth according to plant growth stage, watering regime, percentage gravel and weighted Colwell P of each treatment (Fig. 9). Small-scale diurnal changes in VWC were observed in all treatments due to daily automated applications of water and evapotranspiration. Large-scale changes in VWC were observed due to larger manual applications of water when plant observations and extractions of soil moisture from rhizons indicated that additional moisture was required. For a given gravel percentage, VWC decreased as weighted Colwell P increased. Pots containing both high gravel content and high weighted Colwell P levels exhibited the lowest VWC and the most rapid declines in VWC between manual watering events.
Discussion
The experiments conducted in this study aimed to increase understanding of the role of IG in P retention and release, and its subsequent availability for wheat growth. The results indicate that IG in soils can retain and release P, and supply P to wheat. The dynamics of this supply are impacted by IG size, amount and P enrichment. In addition, IG amount influences available soil moisture (Figs 3, 9), which exerts an overriding control on wheat productivity compared to P fertility, with various implications for agricultural practices, especially in regions with IG rich soils.
Phosphorus retention and release
Our findings demonstrate that the trends in P adsorption and desorption of the IG fractions (>2 mm) and fine earth fraction (<2 mm) are similar but vary in degree (Figs 1, 2). The adsorption capacity of IG was lower due to their small surface area (Weaver et al. 2022), while the desorption behaviour exhibited similar trends to the fine earth fraction but tended to be lower for IG than fine earth fraction. In addition, the percentage of added P that was retained (Fig. 2b), and the percentage of retained P that was desorbed (Fig. 2c) were similar to values reported by Clarendon et al. (2019). The results from this work support hypothesis 1, confirming that the larger size and lower surface area of IG reduces their P adsorption capacity and potential for P desorption.
This behaviour aligns with previous research indicating that, dependent on mineralogy, larger soil particles (such as gravel) have a reduced capacity for P adsorption due to their lower surface area-to-volume ratio (Clarendon et al. 2019; Weaver et al. 2022). This characteristic makes IG less efficient in retaining applied P (Fig. 2b), leading to lower overall P retention in soils where IG is the dominant soil fraction. In addition, IG enriched with high P concentrations showed a greater percentage of retained P desorbed. This indicated that the IG surface of larger fractions with lower surface area was likely saturated with P that could be more rapidly desorbed. Despite lower overall P retention, IG can be sufficiently enriched to provide a significant proportion of the Colwell P and DM from a soil (Figs 7, 8a). In support of hypothesis 3, wheat can take up P from the <2 or >2 mm fractions. However, reduced RY when IG contents are 50% compared to 25% (Fig. 7) suggests some negative impacts due to the presence of more IG. While sufficient P may be supplied, other factors such as reduced VWC may override the growth potential that P fertility from IG may provide.
Soil solution concentrations
The presence of IG+P influenced soil solution P concentrations in the following ways. As the amount of similarly enriched IG+P increased, the P concentration in the soil solution increased and was maintained at higher levels for longer (Fig. 4b), likely due to the greater surface area enriched with P exposed to soil water. These increases in soil solution P led to commensurate increases in DM, supporting hypothesis 2 that wheat growth can be supported when P-enriched IG is the only source of P, and that wheat biomass will increase with increases in P enrichment of IG or the amount of P-enriched gravel present. While soil solution P concentrations are supported to some degree by IG+P, the soil matrix seems to support higher soil solution P concentrations than IG. This is evident in Fig. 8b where much higher soil solution P concentrations were maintained when the whole soil comprised soil matrix (<2 mm fraction of soil A) enriched to 95% RY and IG not enriched compared to soil matrix (<2 mm fraction of soil A) not enriched and IG enriched to 95% RY. This is consistent with the P adsorption and desorption behaviour of the different size fractions shown in Fig. 1, and similar to those reported by Clarendon et al. (2019). The soil matrix was able to retain and release more P than IG, and hence whole soils containing P-enriched fine fractions are more likely to support higher soil solution P concentrations than P-enriched IG. However, even when higher soil solution P concentrations can be achieved, DM can decrease with high gravel amounts (Fig. 7g–i). Presumably this occurs because of lower soil moisture, and because higher soil solution P concentrations will encourage more biomass and evapotranspiration, depleting soil moisture even more rapidly. The IG+P enriched with higher initial P concentrations led to higher soil solution P concentrations (Fig. 6b), but these concentrations tended to be lower than when a soluble P source was applied directly to soil B. This points to differences in short- and long-term P retention and release characteristics of IG compared to soil B and the form in which P was added to pots. In the case of IG+P added to a pot, P was pre-adsorbed onto the IG and must then be released into soil solution via desorption processes (Fig. 1) whereas soluble P was added directly to soil B as a control. Soil B had a lower PBI than IG, and was less influenced by adsorption–desorption processes, hence P was more likely to move to plant roots via mass flow. In contrast, when IG+P is the P source, P is likely to move to plant roots via diffusion (Bhat and Nye 1973) as its release is controlled by desorption processes.
In addition, the reduction in the slope of the positive relationship between the log of median soil solution P and weighted Colwell P as gravel content decreased (Fig. 8c) suggests that whole-soil PBI is influenced by gravel content. Weaver et al. (2023) demonstrated that the slope of linear relationships between calcium chloride extractable P (Rayment and Lyons 2011), the benchmark measure of dissolved reactive P, and Colwell P decreased as soil PBI increased. The PBI of the >2 mm fractions examined ranged within 5–10 (Weaver et al. 2022) compared to the <2 mm fraction of soil A which had a PBI of 45.4 (Table 1). Increasing soil gravel content would reduce whole-soil PBI, leading to higher soil solution concentrations for the same weighted Colwell P in comparison to a soil with a low gravel content and consistent with the findings of Weaver et al. (2023).
Impact on wheat growth
Wheat growth experiments revealed that higher gravel content generally resulted in lower absolute plant biomass, despite providing sufficient P fertility. This reduction in biomass is primarily attributed to low plant available water (Figs 3, 6, 7 and 9). In support of hypothesis 3, gravel size influences P responsiveness of wheat (Fig. 5a) in a similar way that soil PBI (Burkitt et al. 2002) influences responsiveness of pastures to P (Gourley et al. 2019; Weaver et al. 2024). Soils with low PBI have response curves with large curvature coefficients (c value), and soils with high PBI have small c values. This effect is evident in Fig. 5a where soil B has a low PBI, a low surface area and is coarse textured (Table 1). In comparison, soil A has a much higher PBI and comprises finer textured materials. Ranging between these extremes are the mixtures of soil B and IG fractions in which the c value systematically increases as gravel size increases. Both surface area and mineralogy are key factors influencing soil PBI, and PBI of IG increases as gravel size decreases (Weaver et al. 2022). Hence the amount of gravel, and the size distributions of gravel in a soil, along with PBI of each size fraction will influence the overall P retention of the whole soil and its responsiveness to P applications.
The decline in DM and P content of plant tissue in sequential harvests for Experiment 2 (Fig. 5b, c) is indicative of removal of P in plant tissue along with P that may have been leached from the pot. In addition, the decline in slope of the relationship between P removed in harvest and the amount of P at the start of the growth cycle for IG treatments compared to the much greater slope for the same relationship for soil B where P was supplied in a soluble form, points to both differences in short- and longer-term P retention and release characteristics of soil materials in different treatments, and the form in which P was added. For example, soil B has a PBI of 2.5 (Table 1) compared to PBI values of 4.7–10.6 for intact IG dependent on size (Weaver et al. 2022). These slightly higher PBI values will lead to ongoing adsorption and penetration of P into the minerals on the gravel surface (Barrow 2021). The incubation process to prepare IG+P materials and time for plant growth for each harvest would contribute to ongoing adsorption and penetration of P. This is evident in Fig. 5d through the reduction in slope in subsequent harvests. In comparison, when soluble P is added to soil B with a lower PBI, adsorption and penetration of P is unlikely because surface area is lower, and soil B does not contain minerals that contribute to these processes. Hence soil B does not provide competition for P resources for plant growth, whilst the addition of IG+P to soil B in sufficient quantity provides P in an adsorbed form subject to ongoing slow reactions (Barrow 1974; Barrow and Shaw 1975) that competes with plant growth for P resources.
The lower DM and RY in the presence of 50% compared to 25% gravel (Experiment 5) (Fig. 7) is indicative of reduced soil moisture, leading to lower biomass for the same level of weighted Colwell P or total P added. This is reinforced by the relationship between RY and wheat P content for harvest 1 of Experiment 5 (Fig. 7m). Reduced RY when 50% gravel is present compared to 25% gravel for the same P content in the plant biomass is suggestive of concentration effects due to differences in biomass when 25% or 50% gravel is present. Previous research indicates that drought-induced biomass reduction can lead to elevated concentrations of P in plant tissues, even when the total amount of P absorbed by the plant decreases. Reduced biomass can lead to a higher concentration of P within the remaining tissue (Liu et al. 2021; Jiang et al. 2023). These drought-induced effects may help to explain some of the variability in relationships in Fig. 7, and why the relationship between RY and P content (Fig. 7m) could be differentiated by gravel content using parallel curves analysis.
Reduced volumetric water content
High gravel content significantly affects the soil’s physical properties, particularly its ability to retain water (Figs 3, 9). Gravelly soils typically have larger pore spaces, which leads to increased drainage and reduced water-holding capacity. This reduced VWC can limit the availability of water to plant roots, especially during critical growth stages. It is well established that plant available water capacity (PAWC) reflects soil texture variations and subsequent constraints on water and nutrient uptake (He et al. 2021). Even though sufficient P may be present, the lack of adequate soil moisture can impede the plant’s ability to absorb and utilise nutrients effectively, leading to reduced biomass. The pot experiments show that despite sufficient P supply from IG, plant biomass and RY were diminished when higher gravel contents were present. For example, in Experiment 1 the least biomass resulted from 75% IG+P which generated the highest soil solution concentrations compared to 25% and 50% IG+P (Fig. 4). In Experiment 5 (Fig. 7), RY was lower in the presence of 50% compared to 25% gravel, and when more than 50% of the Colwell P or total P added was derived from the gravel fraction for the same weighted Colwell P, or total P added (Fig. 8). Hence, despite sufficient P available in a pot measured either as weighted Colwell P, total P added or median soil solution P, RY was lower for higher gravel amounts or when IG was supplying most P. To supply most P, gravel must either have higher P contents than the matrix or contribute most of the mass. Adsorption experiments (Fig. 1) showed that IG fractions retain less P than the matrix, hence it is more likely for gravel to supply most P when gravel amounts are high, which will lead to lower PAWC (Figs 3, 9) and reduced plant biomass.
The amount of IG seems to act as a control on VWC (Figs 3, 9) and subsequent plant growth even when P supply from IG and/or the fine earth fraction maintains high soil solution P concentrations (Fig. 4) or soil Colwell P (Fig. 7).
Reduced soil moisture retention in gravelly soils poses a challenge for crop growth. Water is a crucial medium for desorption, diffusion and hence nutrient transport to plant roots, and its scarcity can limit the plant’s capacity to take up essential nutrients, including P. The decreased water availability in gravel-rich soils may lead to water stress, further inhibiting plant growth and productivity (He and Dijkstra 2014; Suriyagoda et al. 2014). In addition, the fluctuating water availability can create an environment where nutrients are either leached away during heavy rainfall or become unavailable during dry periods, complicating nutrient management strategies.
Practical implication on agriculture
The study underscores the importance of considering soil IG content and its influence on P fertility and transient VWC over and above water limited yield potential (French and Schultz 1984). Depending on the nature of the soil matrix, soils with higher gravel content may have lower soil P retention than is estimated from soil tests on the soil matrix alone. Since soils with lower P retention are more P responsive due to lower c values, and lower P application rates are likely required to optimise crop yield.
The lack of influence of gravel content on Mitscherlich responses of RY as a function of weighted Colwell P (Fig. 7k and l) suggests that whole-soil Colwell P is a more suitable measure than soil solution P to estimate RY of wheat across a range of soil moisture conditions, whether that be influenced by climatic conditions and/or gravel content.
The impact of reduced VWC on wheat growth highlights the need for strategies that account for the increased drainage in gravel-rich soils. Techniques such as mulching or incorporating organic matter could be explored to improve soil moisture retention in these conditions. Mechanical separation of the gravel into the upper soil profile may act as a mulch and protect evaporation of the separated underlying matrix. Organic matter can enhance soil structure, and may lead to an increase in water-holding capacity, although this is uncertain (Minasny and McBratney 2018). Mulching can help reduce water evaporation from the soil surface, maintaining higher soil moisture levels and mitigating the adverse effects of high gravel content.
Future research should explore the potential benefits of mulching gravelly soils to improve soil moisture retention so that nutrient supply from IG can be optimised. Long-term field trials could provide valuable insights into the practical applications of these strategies and their impact on crop yield and sustainability. Additionally, investigating the interactions between gravel content, soil moisture and nutrient dynamics can help develop more effective soil management practices tailored to specific soil compositions and crop requirements.
The experiments conducted here included a <2 mm fraction with PBI values classified as extremely low to medium P sorption capacity (Gourley et al. 2019). Choosing a soil with a <2 mm fraction with a higher PBI than was used here may exaggerate some findings and moderate others. For example, a <2 mm fraction with higher PBI would be less P responsive and have lower c values, requiring greater additions of P to achieve RY targets. Given the stronger P retention of the <2 mm fraction of a higher PBI soil, whole-soil PBI would likely be dominated by the P sorption capacity of the <2 mm fraction despite dilution of whole-soil PBI by addition of gravel with low PBI characteristics, reducing soil solution concentrations. Despite this, increased P fertilisation should allow critical Colwell P levels to be achieved without a deficit in RY targets. Ultimately, the PBI of the soil’s constituent parts, the amounts of each and the P fertility of each fraction will determine the potential response of wheat to the characteristics of the whole soil. But these factors may be inconsequential if the whole-soil characteristics result in yield limitation by a soil moisture deficit. Furthermore, these experiments used soil fractions with very low P fertility that were artificially enriched with P, achieving Colwell P levels up to 30 ppm for the <2 mm fraction, probably slightly higher than critical level for a soil with PBI of 45 (Bell et al. 2013). Choosing soil fractions that have been enriched with P under standard agricultural practices, separating these into different size fractions and recombining in different ways may provide additional understanding of the effects of different soil P fertility levels, PBI, soil organic carbon and influences of non-wetting. This may lead to improved understanding of P dynamics in relation to gravel content and assist to refine fertilization practices for sustained P availability and optimal plant growth.
Further effort is required to find practical solutions to apply these findings to soil sampling strategies and commercial laboratory settings where the >2 mm fraction is routinely discarded, yet the wider industry is reliant on soil test results from laboratories to make evidence-based fertiliser decisions. Foremost, however, simpler and routine methodologies are required to assess and understand how plant available water capacity may limit growth even when soil fertility is adequate (Foley 2017).
It may be necessary to reconsider soil sampling approaches that consider the collection of samples that are representative of whole soils, notwithstanding the spatial variability of soil gravel content at a range of scales (Weaver et al. 1992; Holmes et al. 2021).
After the collection of representative samples, laboratory processes need to be considered. Previous research has shown that for some measures such as PBI, grinding of soil greatly increases P retention (Clarendon et al. 2019; Weaver et al. 2022) as does end-over-end tumbling of gravels, but may be a suitable approach for measures like Colwell P. Introduction of additional preparation (splitting, grinding of some size fractions and not others) and analytical steps is unlikely to be favoured by commercial laboratories, hence other approaches, including adaptation of current, or new testing procedures need consideration. This may include upscaling (greater sample mass and extractant volumes) of diffuse gradient thin film technology (Mason et al. 2013, 2010) to cater for increased variability and heterogeneity introduced by whole-soil samples containing gravel (Buchter et al. 1994).
This study highlights the agronomic and analytical importance of accounting for gravel content in soil assessments. For farmers, recognising the influence of gravel amount, size and P enrichment on P availability and soil moisture retention can support more targeted fertilisation and water management strategies, ultimately improving wheat yield outcomes. For policymakers, including those with oversight of guiding laboratory analysis, soil test interpretation and decision support systems, the findings underscore the need to revisit soil sampling and testing protocols to ensure they reflect whole-soil properties. This includes gravel fractions typically excluded from routine analysis. Consideration also needs to be given to temporally variable characteristics such as rainfall, which along with soil PBI and pH have been shown to be of critical importance influencing soil moisture, P availability and RY (Scanlan et al. 2024). Incorporating these insights can enhance the reliability of soil test interpretations and inform evidence-based nutrient management, ensuring that both agronomic advice and policy guidelines are better tailored to the realities of gravelly agricultural soils.
Conclusion
This study provides valuable insights into the complex interactions between soil composition, particularly IG content, IG properties such as surface area and mineralogy, and P dynamics in agricultural soils. Our experiments highlight the dual role of IG in both P retention and release, and VWC. The presence of IG can influence soil solution P concentrations and soil moisture, leading to changes in wheat growth. These findings suggest that tailored fertilisation strategies considering gravel content could enhance agricultural productivity and sustainability.
Future research should focus on long-term field trials to validate these findings and explore potential for mechanical separation of the gravel as a surface mulch to reduce evaporation of the separated underlying matrix. Additionally, investigating the interactions of gravel content with other soil properties and nutrients could provide a more comprehensive understanding of its influence on cereal production. The development of practices that mitigate the effects of reduced VWC in gravel-rich soils could further support optimal crop growth and yield.
Furthermore, soil sampling strategies and laboratory testing need to consider how to practically include the >2 mm fraction during sample collection, analysis and interpretation of the results.
Declaration of funding
This research was supported by the Royalties for Regions project ‘Boosting Grains R and D’ (Project code FFPjP12) and the Western Australian Department of Primary Industries and Regional Development.
Acknowledgements
Numerous current and past staff of the WA Department of Primary Industries and Regional Development are acknowledged for their contribution to collecting and collating the dataset reported here. They include, but are not limited to Craig Scanlan, Eric Dobbe, John Grant, Paul Matson and Ian Rose. Royalties for Regions (Project FFFPjP12) and the WA Department of Primary Industries and Regional Development are acknowledged for their funding support.
References
Abekoe M, Tiessen H (1998) Fertilizer P transformations and P availability in hillslope soils of northern Ghana. Nutrient Cycling in Agroecosystems 52(1), 45-54.
| Crossref | Google Scholar |
Anamosa PR, Nkedi-Kizza P, Blue WG, Sartain JB (1990) Water movement through an aggregated, gravelly oxisol from cameroon. Geoderma 46(1–3), 263-281.
| Crossref | Google Scholar |
Anderson G, Chen W, Bell RW, Brennan R (2015) Making better fertiliser decisions for cropping systems in Western Australia. Soil test – crop response relationships and critical soil test values and ranges. WA Department of Primary Industries and Regional Development, Perth. Available at https://library.dpird.wa.gov.au/bulletins/42
Baetens JM, Verbist K, Cornelis WM, Gabriels D, Soto G (2009) On the influence of coarse fragments on soil water retention. Water Resources Research 45(7), W07408.
| Crossref | Google Scholar |
Barber SA (1962) A diffusion and mass-flow concept of soil nutrient availability. Soil Science 93(1), 39-49.
| Crossref | Google Scholar |
Barrow NJ (1974) The slow reactions between soil and anions. 1. Effects of time, temperature, and water content of a soil on the decrease in effectiveness of phosphate for plant growth. Soil Science 118, 380-386.
| Crossref | Google Scholar |
Barrow NJ (2021) Comparing two theories about the nature of soil phosphate. European Journal of Soil Science 72(2), 679-685.
| Crossref | Google Scholar |
Barrow NJ, Shaw TC (1975) The slow reactions between soil and anions. 2. Effect of time and temperature on the decrease in phosphate concentration in the soil solution. Soil Science 119(2), 167-177.
| Crossref | Google Scholar |
Bell R, Reuter D, Scott B, Sparrow L, Strong W, Chen W (2013) Soil phosphorus–crop response calibration relationships and criteria for winter cereal crops grown in Australia. Crop & Pasture Science 64(5), 480-498.
| Crossref | Google Scholar |
Bhat KKS, Nye PH (1973) Diffusion of phosphate to plant roots in soil. Plant and Soil 38(1), 161-175.
| Crossref | Google Scholar |
Blair GJ, Chinoim N, Lefroy RDB, Anderson GC, Crocker GJ (1991) A soil sulfur test for pastures and crops. Soil Research 29(5), 619-626.
| Crossref | Google Scholar |
Brouwer J, Anderson H (2000) Water holding capacity of ironstone gravel in a typic plinthoxeralf in Southeast Australia. Soil Science Society of America Journal 64, 1603-1608.
| Crossref | Google Scholar |
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. Journal of the American Chemical Society 60(2), 309-319.
| Crossref | Google Scholar |
Buchter B, Hinz C, Flühler H (1994) Sample size for determination of coarse fragment content in a stony soil. Geoderma 63(3–4), 265-275.
| Crossref | Google Scholar |
Burkitt LL, Moody PW, Gourley CJP, Hannah MC (2002) A simple phosphorus buffering index for Australian soils. Australian Journal of Soil Research 40(3), 497-513.
| Crossref | Google Scholar |
Carrick S, Palmer D, Webb T, Scott J, Lilburne L (2013) Stony soils are a major challenge for nutrient management under irrigation development. In ‘Accurate and efficient use of nutrients on farms’. (Eds LD Currie, CL Christensen). Occasional Report No. 26. (Fertilizer and Lime Research Centre, Massey University: Palmerston North, New Zealand). Available at https://flrc.massey.ac.nz/workshops/13/Manuscripts/Paper_Carrick_1_2013.pdf
Chen H, Liu J, Wang K, Zhang W (2011) Spatial distribution of rock fragments on steep hillslopes in karst region of northwest Guangxi, China. CATENA 84(1–2), 21-28.
| Crossref | Google Scholar |
Clarendon SDV, Weaver DM, Davies PM, Coles NA (2019) The influence of particle size and mineralogy on both phosphorus retention and release by streambed sediments. Journal of Soils and Sediments 19(5), 2624-2633.
| Crossref | Google Scholar |
Colwell JD (1963) The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Australian Journal of Experimental Agriculture and Animal Husbandry 3(10), 190-197.
| Crossref | Google Scholar |
Cousin I, Nicoullaud B, Coutadeur C (2003) Influence of rock fragments on the water retention and water percolation in a calcareous soil. CATENA 53(2), 97-114.
| Crossref | Google Scholar |
Cresswell HP, Green TW, McKenzie NJ (2008) The adequacy of pressure plate apparatus for determining soil water retention. Soil Science Society of America Journal 72(1), 41-49.
| Crossref | Google Scholar |
Dhillon J, Torres G, Driver E, Figueiredo B, Raun WR (2017) World phosphorus use efficiency in cereal crops. Agronomy Journal 109(4), 1670-1677.
| Crossref | Google Scholar |
Ercoli L, Masoni A, Mariotti M, Arduini I (2006) Dry matter accumulation and remobilization of durum wheat as affected by soil gravel content. Cereal Research Communications 34(4), 1299-1306.
| Crossref | Google Scholar |
Foley JA (2017) Rapid field and laboratory methods for measuring plant available water capacity and water retention curves. DNRME Technical Note 2017. Department of Natural Resources Mines and Energy, Toowoomba, Qld, Australia. Available at https://www.apsim.info/wp-content/uploads/2021/01/Methods-for-PAWWRC-estimation_DNRME.pdf
French RJ, Schultz JE (1984) Water use efficiency of wheat in a Mediterranean-type environment. I. The relation between yield, water use and climate. Australian Journal of Agricultural Research 35(6), 743-764.
| Crossref | Google Scholar |
Gourley CJP, Weaver D, Simpson RJ, Aarons SR, Hannah MM, Peverill KI (2019) The development and application of functions describing pasture yield responses to phosphorus, potassium and sulfur in Australia using meta-data analysis and derived soil-test calibration relationships. Crop & Pasture Science 70, 1065-1079.
| Crossref | Google Scholar |
Govindasamy P, Mahawer SK, Mowrer J, Bagavathiannan M, Prasad M, Ramakrishnan S, Halli HM, Kumar S, Chandra A (2023) Comparison of low-cost methods for soil water holding capacity. Communications in Soil Science and Plant Analysis 54(2), 287-296.
| Crossref | Google Scholar |
He M, Dijkstra FA (2014) Drought effect on plant nitrogen and phosphorus: a meta-analysis. New Phytologist 204(4), 924-931.
| Crossref | Google Scholar | PubMed |
He D, Oliver Y, Wang E (2021) Predicting plant available water holding capacity of soils from crop yield. Plant and Soil 459(1), 315-328.
| Crossref | Google Scholar |
Hintze JL, Nelson RD (1998) Violin plots: a box plot-density trace synergism. The American Statistician 52(2), 181-184.
| Crossref | Google Scholar |
Hochman Z, Horan H (2018) Causes of wheat yield gaps and opportunities to advance the water-limited yield frontier in Australia. Field Crops Research 228, 20-30.
| Crossref | Google Scholar |
Holmes KW, Griffin EA, van Gool D (2021) Digital soil mapping of coarse fragments in southwest Australia: targeting simple features yields detailed maps. Geoderma 404, 115282.
| Crossref | Google Scholar |
Indorante SJ, Hammer RD, Koenig PG, Follmer LR (1990) Particle-size analysis by a modified pipette procedure. Soil Science Society of America Journal 54(2), 560-563.
| Crossref | Google Scholar |
Isbell RF, National Committee on Soil and Terrain (2021) ‘The Australian soil classification.’ (CSIRO Publishing) Available at https://www.publish.csiro.au/book/8016;SITEKEY=main
Jiang S, Tang Y, Fan R, Bai S, Wang X, Huang Y, Li W, Ji W (2023) Response of Carex breviculmis to phosphorus deficiency and drought stress. Frontiers in Plant Science 14, 1203924.
| Crossref | Google Scholar |
Liu AN, Zhang Y, Hou ZF, Hui Lü G (2021) Allometric scaling of biomass with nitrogen and phosphorus above- and below-ground in herbaceous plants varies along water-salinity gradients. AoB PLANTS 13(4), plab030.
| Crossref | Google Scholar |
Mason S, McNeill A, McLaughlin M, Zhang H (2010) Prediction of wheat response to an application of phosphorus under field conditions using diffusive gradients in thin-films (DGT) and extraction methods. Plant and Soil 337(1–2), 243-258.
| Crossref | Google Scholar |
Mason SD, Mclaughlin MJ, Johnston C, Mcneill A (2013) Soil test measures of available P (Colwell, resin and DGT) compared with plant P uptake using isotope dilution. Plant and Soil 373(1), 711-722.
| Crossref | Google Scholar |
Masoni A, Ercoli L, Mariotti M, Pampana S (2008) Nitrogen and phosphorus accumulation and remobilization of durum wheat as affected by soil gravel content. Cereal Research Communications 36(1), 157-166.
| Crossref | Google Scholar |
McGill R, Tukey JW, Larsen WA (1978) Variations of box plots. The American Statistician 32, 12-16.
| Crossref | Google Scholar |
McQuaker NR, Brown DF, Kluckner PD (1979) Digestion of environmental materials for analysis by inductively coupled plasma-atomic emission spectrometry. Analytical Chemistry 51(7), 1082-1084.
| Crossref | Google Scholar |
Minasny B, McBratney AB (2018) Limited effect of organic matter on soil available water capacity. European Journal of Soil Science 69(1), 39-47.
| Crossref | Google Scholar |
Moorberg CJ, Crouse DA (2017) ‘Soils laboratory manual, K-State Edition.’ (NPP eBooks 15. New Prairie Press) Available at https://newprairiepress.org/ebooks/15
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27, 31-36.
| Crossref | Google Scholar |
Poesen J, Lavee H (1994) Rock fragments in top soils: significance and processes. CATENA 23(1), 1-28.
| Crossref | Google Scholar |
Powers SJ (2021) Regression analysis in the context of designed experiments: Neglect not thy opportunity to test for position and parallelism. Annals of Applied Biology 179(1), 4-11.
| Crossref | Google Scholar |
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods – Australasia.’ (CSIRO Publishing: Collingwood, Vic, Australia). Available at https://www.publish.csiro.au/book/6418/
Ritz K, Young IM (2004) Interactions between soil structure and fungi. Mycologist 18(2), 52-59.
| Crossref | Google Scholar |
Scanlan CA, Malik R, Boitt G, Gherardi M, Easton J, Rengel Z (2024) Phosphorus buffering determines how soil properties and rainfall influence wheat (Triticum aestivum) yield response to phosphorus fertiliser. Crop & Pasture Science 75(12), CP24295.
| Crossref | Google Scholar |
Schoknecht NR, Pathan S (2013) Soil groups of Western Australia : a simple guide to the main soils of Western Australia (4th edn). Department of Agriculture and Food, Western Australia, Perth. Available at http://researchlibrary.agric.wa.gov.au/rmtr/348/
Tamm O (1922) Eine Methode zur Bestimmung de der anorganischen Komponente des Bodens. Meddelanden fran Statens skogsforsoksanstalt Stockholm 19, 387-404 Available at https://pub.epsilon.slu.se/10138/1/medd_statens_skogsforskningsanst_027_01.pdf.
| Google Scholar |
Tetegan M, Nicoullaud B, Baize D, Bouthier A, Cousin I (2011) The contribution of rock fragments to the available water content of stony soils: proposition of new pedotransfer functions. Geoderma 165(1), 40-49.
| Crossref | Google Scholar |
Tiessen H, Abekoe MK, Salcedo IH, Owusu-Bennoah E (1993) Reversibility of phosphorus sorption by ferruginous nodules. Plant and Soil 153(1), 113-124.
| Crossref | Google Scholar |
Tokunaga TK, Olson KR, Wan J (2003) Moisture Characteristics of Hanford Gravels. Vadose Zone Journal 2, 322-329.
| Crossref | Google Scholar |
Weaver D, Summers R (2021) Phosphorus status and saturation in soils that drain into the Peel Inlet and Harvey Estuary of Western Australia. Soil Research 59(7), 699-714.
| Crossref | Google Scholar |
Weaver DM, Wong MTF (2011) Scope to improve phosphorus (P) management and balance efficiency of crop and pasture soils with contrasting P status and buffering indices. Plant and Soil 349(1), 37-54.
| Crossref | Google Scholar |
Weaver DM, Ritchie GSP, Gilkes RJ (1992) Phosphorus sorption by gravels in lateritic soils. Australian Journal of Soil Research 30(3), 319-330.
| Crossref | Google Scholar |
Weaver D, Summers R, Schweizer S, Leopold M, Scanlan C (2022) Valuable phosphorus retained by ironstone gravels can be measured as bicarbonate extractable P. Geoderma 418, 115862.
| Crossref | Google Scholar |
Weaver D, Summers R, Neuhaus A (2023) Agronomic soil tests can be used to estimate dissolved reactive phosphorus loss. Soil Research 61(7), 627-646.
| Crossref | Google Scholar |
Weaver D, Rogers D, Dobbe E, et al. (2024) Validation of critical soil-test phosphorus values from the better fertiliser decisions for pastures meta-analysis. Crop & Pasture Science 75(1), CP23194.
| Crossref | Google Scholar |
Wild A (1958) The phosphate content of Australian soils. Australian Journal of Agricultural Research 9(2), 193-204.
| Crossref | Google Scholar |
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Research 14(6), 415-421.
| Crossref | Google Scholar |