A review on the bioweathering and bioremediation of asbestos containing waste materials in soils
Santanu Mukherjee A * , Shailja Sharma B , Shiv Bolan C D E , Liuwei Wang F , Terri-Ann Berry G , Shannon L. Wallis H , Dan Blanchon I J , Deyi Hou F , Valerie A. Geoffroy K , Kadambot H. M. Siddique D and Nanthi Bolan C D E *
A * , Shailja Sharma B , Shiv Bolan C D E , Liuwei Wang F , Terri-Ann Berry G , Shannon L. Wallis H , Dan Blanchon I J , Deyi Hou F , Valerie A. Geoffroy K , Kadambot H. M. Siddique D and Nanthi Bolan C D E *
A
B
C
D
E
F
G
H
I
J
K
Handling Editor: Claudio Bini
Abstract
Asbestos is a silicate mineral that occurs naturally and is made up of flexible fibres that are resistant to heat, fire, and chemicals and do not conduct electricity. Both anthropogenic disturbance and natural weathering of asbestos-containing waste materials (ACWMs) can result in the emission of asbestos fibre dust, which when breathed, can cause asbestosis, a chronic lung illness that happens due to prolonged exposure of such fibre dust, and can cause ‘mesothelioma’ cancer. Although asbestos mining and its utilisation had been banned in many countries, there is still a significant issue of ACWMs disposal in the built environment and abandoned sites. It is neither practical nor economical to safely eliminate ACWMs from the built environment, and it is estimated that globally, 4 billion metric tonnes of ACWMs require safe management strategies. The toxicity of inhaled asbestos fibre relies on its surface properties, and in particular the distribution of iron, which serves a critical role in pathogenicity by forming reactive free radicals that damage DNA, thereby trigging cancer. Examining the usefulness of higher plants and microbes in the bioremediation of soil contaminated with ACWMs is the prime aim of the review. Higher plants and microorganisms such as lichens, fungi, and bacteria often play a major role in the remediation of soil contaminated with ACWMs by facilitating the bioweathering of asbestos and the removal of iron to mitigate the toxicity of asbestos.
Keywords: asbestos, bioremediation, bioweathering, humans, lung disease, mesothelioma cancer, pathogenicity, toxicity.
Introduction
The serpentine and amphibole families of minerals include the six fibrous forms of main silicate minerals found in asbestos, such as tremolite, actinolite, crocidolite, anthophyllite, chrysotile, and amosite. Among these six minerals, five minerals belong to the inosilicate (calcium, magnesium, and iron silicates) group (amphibole sub-group) and one to the phyllosilicate (magnesium silicates) group (serpentine sub-group). Table 1 provides detailed information about various types of asbestos, their origins, and mineralogical classifications. ‘Asbestiform’ crystal formation usually can be found in this category of silicate minerals (Obmiński 2021). In the past, asbestos was mostly used in the building industry as a powerful insulator. Virta (2002) have reported that asbestos materials, when combined with textiles, paper, cement, and plastic materials, then helped to improve the structural stability. Although mining of asbestos and its commercial use had been prohibited in most countries, significant volumes of fibres remain in historical sites occupied by defunctioning asbestos mining and processing units. Remediation of asbestos-containing waste materials (ACWMs) in defunct asbestos mines and processing industries is more challenging than safe removal and containment asbestos coverings from buildings and the freely-dispersed asbestos fibres can readily become airborne when they are disturbed by anthropogenic influences. It is estimated that worldwide, there is about 4 billion tonnes of ACWMs that require safe disposal and management though most governmental agencies in developed nations have specific regulations on how asbestos can be managed and ultimately disposed.
| Mineral groups | Asbestos types | Colour | Origin | Chemical formula | Characteristics/Properties | References | |
|---|---|---|---|---|---|---|---|
| Phyllosilicate (Serpentine) | Chrysotile | White, green, grey, and yellowish | Formed in serpentinised ultramafic rocks (e.g. peridotite) via hydrothermal alteration. | Mg₃Si₂O₅(OH)₄ | IARC (2012) and Jöns and Bach (2014) | ||
| Inosilicate (Amphibole) | Actinolite | Green | Occurs in low-grade metamorphic settings (e.g. greenschist facies) or altered mafic igneous formations | [Ca2(Mg,Fe2+)5Si8O22(OH)2]n | Bevins (1994) and Deer et al. (2013) | ||
| Tremolite | White to pale green | Metamorphic origin in calcium-rich rocks (e.g. metamorphosed dolomite or limestone). | [Ca2Mg5Si8O22(OH)2]n | Bevins (1994) and IARC (2012) | |||
| Anthophyllite | Grey, white, green, and brown-grey | Forms in metamorphosed ultramafic rocks (e.g. talc schists) or magnesium-rich environments. | [(Mg, Fe2+)7Si8O22(OH)2]n | IARC (2012) and Deer et al. (2013) | |||
| Amosite (Grunerite) | Brown, greenish, and grey | Originates in iron-rich metamorphic rocks (e.g. banded iron formations). | [(Mg,Fe2+)7Si8O22(OH)2]n | IARC (2012) and Deer et al. (2013) | |||
| Crocidolite (Riebeckite) | Lavender and blue green | Found in sodium-rich metamorphic environments (e.g. altered ironstone or volcanic rocks). | [NaFe2+3Fe3+2Si8O22(OH)2]n |
| IARC (2012) and Deer et al. (2013) |
Asbestos dust fibres can be released when human disturbance or weathering of ACWMs occurs. If breathed or consumed, these fibres can remain lodged in the body indefinitely (Nayak 2016). Heart failure, lung cancer, and asbestosis can result from exposure to asbestos dust fibres in the workplace or the environment. Numerous countries including Australia, Brazil, and Canada have outlawed the mining and use of asbestos due to the grave health risks posed by asbestos fibres. Constant asbestos fibre (>8 μm length and <0.25 μm diameter) exposure can cause lung damage and inflammation, which can result into the interstitial fibrosis/chronic lung illness known as ‘asbestosis’, aggressive lung cancer and pleural ‘mesothelioma’ (Pott et al. 1972; Slavin 2006; Gray et al. 2016).
Globally, a significant legacy of in situ distribution of ACWMs has been observed in the built environment, and also in defunct asbestos mines and processing industries, and disposal sites (LaDou 2004; Militello et al. 2021). It has been demonstrated that it is safer to allow ACWMs to remain undisturbed or stabilised in situ by encapsulating with a sealant that was also found to offer environmental advantages though potential strategies for integrating bioremediation with established encapsulation methods needs to be examined more thoroughly (Bose et al. 2022; Wallis 2023). However, under some circumstances, it is necessary to remove ACWMs in the built environment and develop strategies for their effective management. The elimination of ACWMs is one of the most pressing environmental and health concerns in asbestos risk management. Researchers have found that bioremediation methods can also serve as an effective strategy for managing ACWMs in soils though this method requires significant time (Schapira et al. 2023; Durczak et al. 2024).
For the safe elimination of hazards stemming from ACWMs in soils, different strategies, like stabilisation, destruction, inertisation, or containment can be used (Ervik et al. 2021). The majority of ACWMs that remain in the built environment can be securely enclosed in a physically secured form, which reduces the chances of health hazards from airborne asbestos fibres that are harmful to human health. Nevertheless, hazardous asbestos fibres may also be released due to the weathering of exposed ACWMs (Kempton and McCarthy 2017; Bolan et al. 2023). Although safe removal and eradication of ACWMs from these areas is highly preferred, stabilisation and encapsulation may also provide a workable and safe alternative in cases where safe removal of ACWMs is not possible, i.e. places where ACWMs are subjected to disturbance or any other weathering events. Łuniewski et al. (2024) have discussed the possibilities of the integration of encapsulation with bioremediation methods which highlight the practical implementation strategies for applying the bioweathering and bioremediation methods as effective long-term solutions.
Currently, the only legal permissible fate for ACWMs is the constructed landfills in the majority of nations (Promentilla and Peralta 2003; Favero-Longo et al. 2009a; Bhattacharya et al. 2015). Thermal, thermo-chemical, and bioremediation methods are proven to be efficient for the inactivation of ACWMs in landfills (Pickin et al. 2015; Necasova and Buchta 2019; Wallis et al. 2020). However, bioremediation, including bioweathering and phytoremediation, has mostly been demonstrated at laboratory experiments. In contrast, thermal and thermochemical treatments for the inertisation of ACWMs have been applied at large-scale industrial operations (Kusiorowski et al. 2012; Pira et al. 2018; Paolini et al. 2019). Bioremediation of asbestos that is occurring under natural conditions encompasses a number of processes that include (David et al. 2020a; Babu et al. 2023): (1) photo stabilisation, which provides vegetation cover over exposed/buried asbestos that prevents the dispersal of asbestos dust; (2) bioweathering, which breaks down asbestos minerals via biological processes involving plants, lichens, bacteria, and fungi; and (3) phytoextraction, which is the uptake of iron by higher plants and soil microbial community, thereby reducing the toxicity of asbestos fibre. Therefore, plants can degrade asbestos per type in a certain period of time, and often the degraded entity has been found as no longer a fibre, as reported by Gopishankar et al. (2022) and Wallis et al. (2020).
The toxicity of asbestos fibres inhaled in the lung depends on both physical and chemical characteristics of fibres. The pathogenicity of asbestos fibres is largely determined by their surface features, particularly by the distribution of iron (David et al. 2020a). For instance, crocidolite, which is one of the asbestos varieties thought to be most carcinogenic, has significant amounts of redox-active iron at the surface in a weak coordination state. This type of iron eventually causes cancer by generating extremely reactive free radicals that harm DNA. By absorbing iron from asbestos, soil-borne microorganisms such as lichens, bacteria, and fungus have been shown to be beneficial in reducing the toxicity of asbestos and promoting the bioremediation of soil containing asbestos. Similar to this, higher plants may take up iron from asbestos, which makes it easier to phytoremediate soil that contains ACWMs. Researchers have found that Aspergillus niger, Candelariella vitellina, Acidithiobacillus thiooxidans, Lecanora rupicola, Saccharomyces cerevisiae, and Xanthoparmelia tinctina are some of the specific microbial species that were found to be effective for application in soils (Bose et al. 2022; Choi et al. 2023; Schapira et al. 2023). Some of the plant species like Cynodondactylon, Cymbopogon citratus, Silene nutans, Cajanus cajan, Chrysopogon zizanioides, and Acacia concinnia have been reported as beneficial plants for bioremediation of ACWMs in soils (Wallis 2023). Furthermore, it has been found that chelating substances such as siderophores and polycarboxylic acids, which solubilise and render the iron in asbestos accessible, are released by higher plants as well as soil microbes including bacteria, fungus, and lichen.
There is little published information regarding the significance of studying bio-weathering and bioremediation of asbestos waste, despite the rising frequency of ACWM dispersal at legacy sites and the uncontrolled release of hazardous asbestos fibres into the environment though all the asbestos types in those sites do not make up of the same percentage of asbestiform structures. The emission of asbestos fibres and the hazardous effects on human health have been the primary topics of a small number of studies and technical papers on ACWMs (Valenzuela et al. 2016; Wallis et al. 2020; Thives et al. 2022).The primary aim of this review is to critically scrutinise and synthesise literature data on the bioweatheirng, bioremediation, and phytoremediation of ACWMs to provide introduction of the review’s methods.
In addition to promoting the development of bioremediation and phytoremediation strategies for the sustainable management of ACWM contamination in the environment, the review will attempt to bridge the knowledge gaps regarding the emission of asbestos fibres from ACWMs into soil and atmospheric environments, thereby mitigating the toxicity of ACWMs to humans and ecosystems. The paper also attempts to suggest future research directions for managing the pollution of ACWMs through phytoremediation and bioremediation.
Methodology
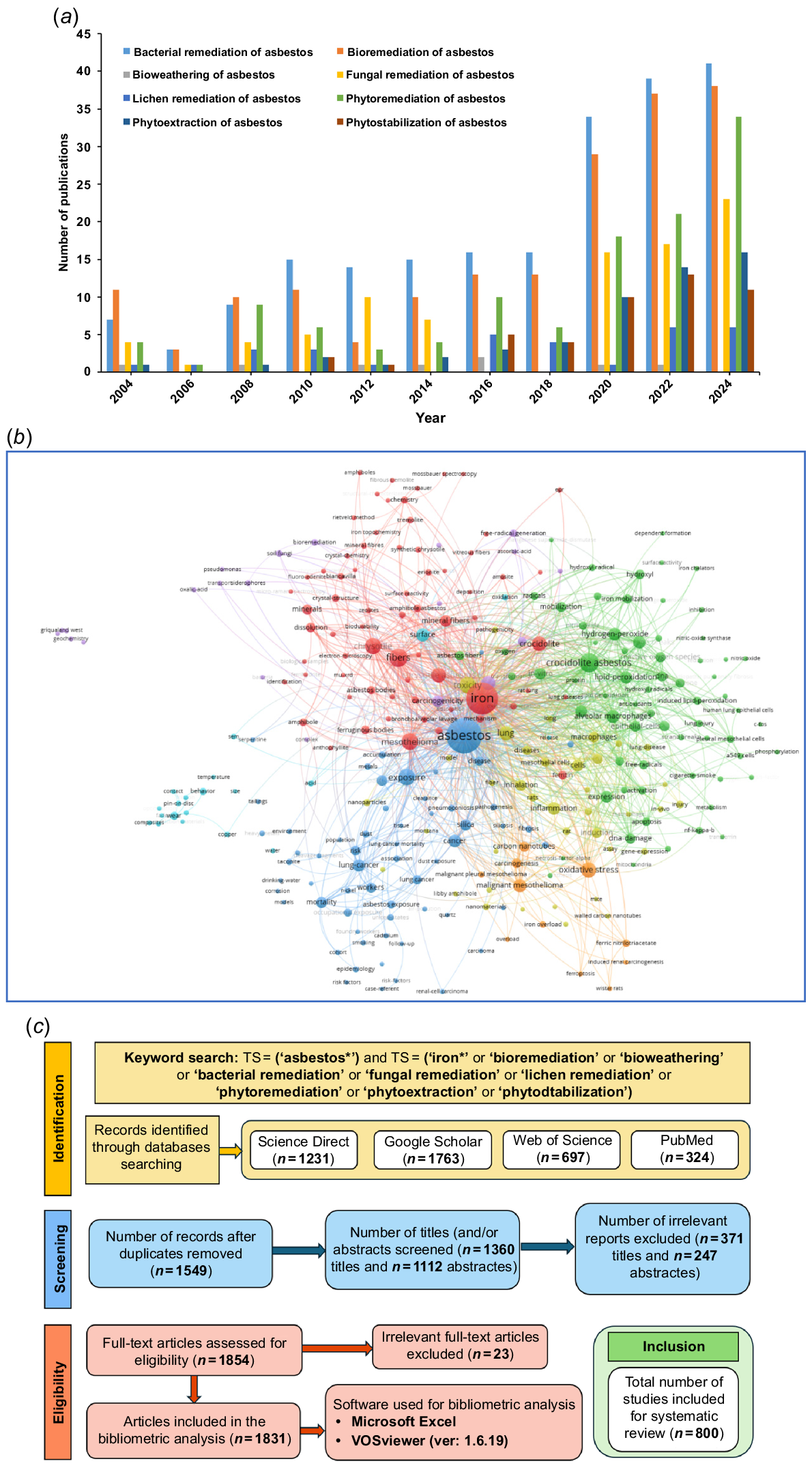
A thorough literature search was conducted in Web of Science Core Collections with the following searching terms: TS = (‘asbestos*’) AND TS = (‘iron*’ OR ‘bioremediation’ OR ‘bioweathering’ OR ‘bacterial remediation’ OR ‘fungal remediation’ OR ‘lichen remediation’ OR ‘phytoremediation’ OR ‘phytoextraction’ OR ‘phytostabilisation’). The explanations of key words such as ‘Bioweathering’, ‘Bioremediation’, ‘Phytostabilisation’, ‘Phytoextraction’, and ‘Siderophore’ have already been provided by the authors elsewhere (David et al. 2020a, 2020b, 2021; Berry et al. 2022, 2024; Bolan et al. 2023). A total of 800 results were obtained. The software VOSviewer (ver. 1.6.19) was used to visualise the results and used for in-depth study of selecting the papers that were subsequently reviewed (Fig. 1). PRISMA flow chart is used to highlight the review’s content and improve its quality by establishing a rigorous methodological framework and providing a more thorough synthesis of relevant studies (Fig. 1). PECO (Population, Exposure, Comparator, and Outcome) framework can also be used to clarify the present manuscript’s scope and inclusion criteria. The study design for a review along with inclusion/exclusion of the literature can be obtained from such methodological framework.
Sources of asbestos
Asbestos mining
The use of asbestos can be traced back as far as the Stone Age, some 750,000 years ago. However, it was not until the late 1800s and the start of the industrial revolution that asbestos manufacturing became a thriving industry (King 2024). Following the establishment of the first commercial asbestos mines in Canada and Russia, asbestos industries developed in Scotland, Germany, England, Italy, and Australia. From the early 19th Century, South Africa discovered amosite asbestos, Finland mined anthophyllite asbestos, and Switzerland and Zimbabwe extensively mined chrysotile asbestos for the global market (King 2024). Other notable locations for asbestos extraction include Brazil and China (Haley and Pizzolitto 2016). Asbestos production reached a peak in the 1970s (Radetzki 2010), driven by its valuable physical and chemical attributes, such as heat and fire resistance, insulating capabilities, chemical inertness, and strength (Godish 1989). The peak global production of asbestos occurred around 1977 and was estimated to be close to 4.8 million tons per year, which was contributed by 25 countries (King 2024).
However, global awareness of the health hazards associated with asbestos exposure led to a reduction in use of asbestos and the closure of many asbestos mines. Strict regulations and bans on asbestos use have been implemented across nations to mitigate health risks. The decline in asbestos mining activities reflects a collective recognition of the importance of safeguarding public health. Many industrialised nations have stopped producing asbestos by the 21st century; the top producers were Kazakhstan, Brazil, China, and Russia, with annual production estimated to be nearly 2 million tonnes (USGS 2013). It is anticipated that almost 1 million tonnes of asbestos were mined worldwide in the last decade (Flanagan 2022) (see Supplementary Fig. S1a). While 69 countries have banned the mining, import, and use of asbestos (Kazan-Allen 2022), low- to middle income nations (like India, Brazil, Russia) that need rapid industrial expansion and may have poor environmental regulations still import and use asbestos (Leong et al. 2015). At current rates, Russia could continue to produce raw asbestos fibres for more than 100 years (Ramazzini 2010) and many countries that have banned the use of all other forms still allow the controlled use of chrysotile asbestos. Although safer (synthetic) alternatives may be available, the imposition of tariffs by certain countries serves as a hindrance to the cessation of asbestos use.
Asbestos in the built environment
In particular, the malleable properties of asbestos-containing materials (ACMs) have been utilised as an important building, binding, and strengthening material (King 2024). The Health and Safety Executive (HSE) of the United Kingdom has published a survey guide for the determination of asbestos containing building materials, which contains information on the likely percentage of asbestos present per material (Table 2; Young 2016). Although, extremely useful, this does not cover all of the materials produced for this industry, but include information about specific asbestos type or allow for regional variations. In addition to the long-term public health impact of exposure to these diverse and widespread materials (discussed in the following section), their use continues to contribute substantially to the quantities of hazardous waste disposed of to landfills. As a result of the incorporation of asbestos into a wide variety of products; i.e. because asbestos was once widely used in the construction of both residential and commercial structures, significant amounts of it are still present today (Ramazzini 2010).Waste products containing asbestos from removal or demolition are frequently dumped in controlled landfills. Although this method might work in the short run, it does not encourage recycling or sustainable land usages, nor does it totally prevent future fibre release (Godish 1989; Spasiano et al. 2017).
| Material types | Asbestos content (%) | ||
|---|---|---|---|
| Loose insulation | Bulk loose fill (e.g. ‘jiffy bag’, mattresses) | 100 | |
| Sprayed coatings | 55–85 | ||
| Thermal insulation | 6–85 | ||
| Asbestos boards | Millboard | 37–97 | |
| Insulating board | 15–40 | ||
| Paper, felt, and cardboard | 100 | ||
| Textiles | Ropes and yarns | ≈100 | |
| Cloth | ≈100 | ||
| Gaskets and washers | 90 | ||
| Strings | ≈100 | ||
| Friction products | Resin-based materials | 30–70 | |
| Cement products | Profiled sheets | 10–15 | |
| Other encapsulated materials | Textured coatings | 3–5 | |
| PVC flooring | 7 | ||
| Thermoplastic floor tiles | ≤25 | ||
| Reinforced PVC | 1–10 | ||
| Reinforced plastic | 1–10 | ||
| Reinforced resin composites (reinforced with woven chrysotile cloth) | 20–50 | ||
Instead of being disposed in its pure fibrous state, asbestos is frequently mixed with a wide range of building materials. Compared to raw fibres, asbestos has a substantially larger volume in landfills because it is incorporated in a matrix rather than existing as loose fibres. Wallis et al. (2020) have estimated that around 4 billion tonnes of contaminated material needs to be disposed of based on current usage patterns and an average asbestos concentration of 5%. This problem is aggravated by the degradation of construction materials containing asbestos and the continued usage of asbestos in some nations, leading to significantly higher than permissible levels of asbestos in soil and in products containing asbestos. There isn’t yet a clear long-term plan to deal with the legacy of multiple polluted sites (both identified and unmarked) or to manage the growing volumes of trash. When disturbed, even materials like contaminated soils that have very little asbestos (<1% by weight) can cause serious health concerns (Carlin et al. 2015).
Asbestos exposure pathways
Since the inception of the asbestos mining industry, a diverse range of human exposure pathways have developed. These have been discussed in terms of waves of exposure (Fig. S1b), which was first proposed by Landrigan and Kazemi (1991). An additional fourth wave of exposure was added more recently; however, this remains a simplified picture of exposure pathways that are highly complicated and often interconnected. For example, although many countries have ceased asbestos mining operations, the legacy of past mining activities poses ongoing environmental concerns. Abandoned mines may continue to pose risks to nearby communities and ecosystems. Therefore, the remediation of these sites is essential for preventing potential asbestos exposure hazards (Berman and Crump 2008). In regions where asbestos mining persists, stringent safety measures and regulatory frameworks are imperative to ensure the well-being of workers and prevent adverse environmental impacts. In South Africa, very little mineralogical or geochemical data are available about the existing hazardous geological materials from derelict asbestos mines. There are around 6000 derelict mines in South Africa, Of these, 249 are abandoned asbestos mines and of which 16% have been rehabilitated (Schapira et al. 2023). It appears that first wave of exposure due to raw asbestos handling continues to provide potential human health hazards.
Although the second wave of exposure has been limited geographically by the increase in the number of countries banning its use and import, there are still many countries using this low cost and robust mineral in products. In 2022, the top five users were India (424,000 tonnes), China (261,000 tonnes), Russia (230,000 tonnes), Uzbekistan (108,000 tonnes), and Indonesia (104,000 tonnes) (Kazan-Allen 2022). The third wave of exposure, characterised by repairs, renovations, and the removal of asbestos, is closely linked with the built environment. Exposure pathways can be diverse, including occupational exposure from the non-asbestos industry such as trade workers (carpentry, plumbing) and handling asbestos-containing waste building materials, and domestic (home-based) and/or para-occupational exposure, which can affect homeowners and relatives of those exposed in the workplace (Berry et al. 2022). A large number of communities worldwide have been affected by asbestos exposure in the environment; the reports of which have been found in South Africa, the United Kingdom, Australia, Japan, Italy, the Netherlands, Denmark, Canada, and the United States. Additionally, asbestos exposure can arise from abandoned mines, as previously mentioned, or from localised industrial activity (Magnani et al. 2023). This might also involve building deterioration, demolition, exposure to asbestos-containing rocks or substrates in the environment (Fig. 2), and emergency situations like fires and earthquakes as described by the fourth wave (Fig. S1b).
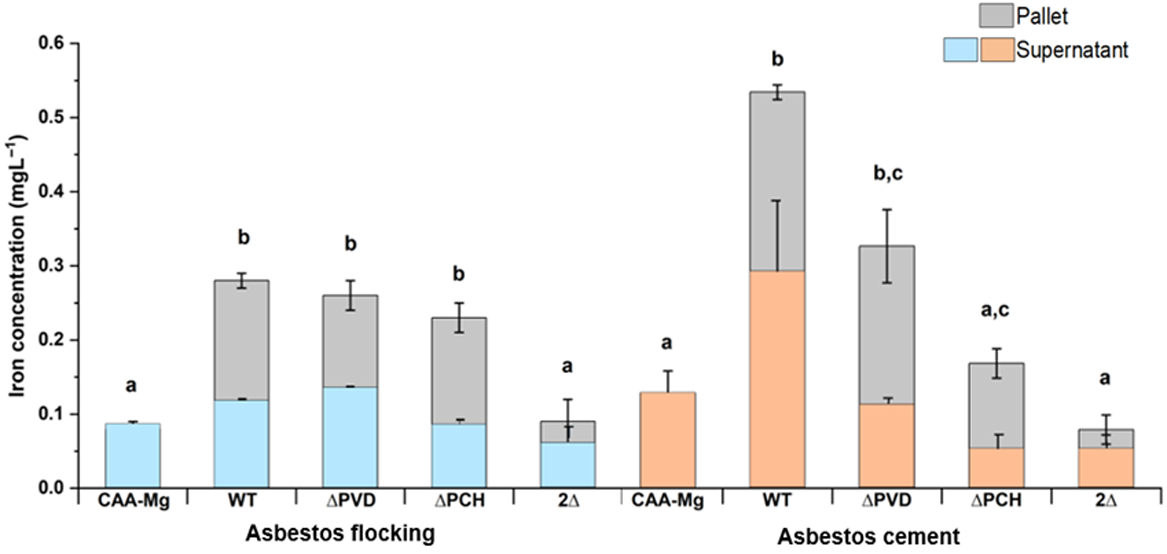
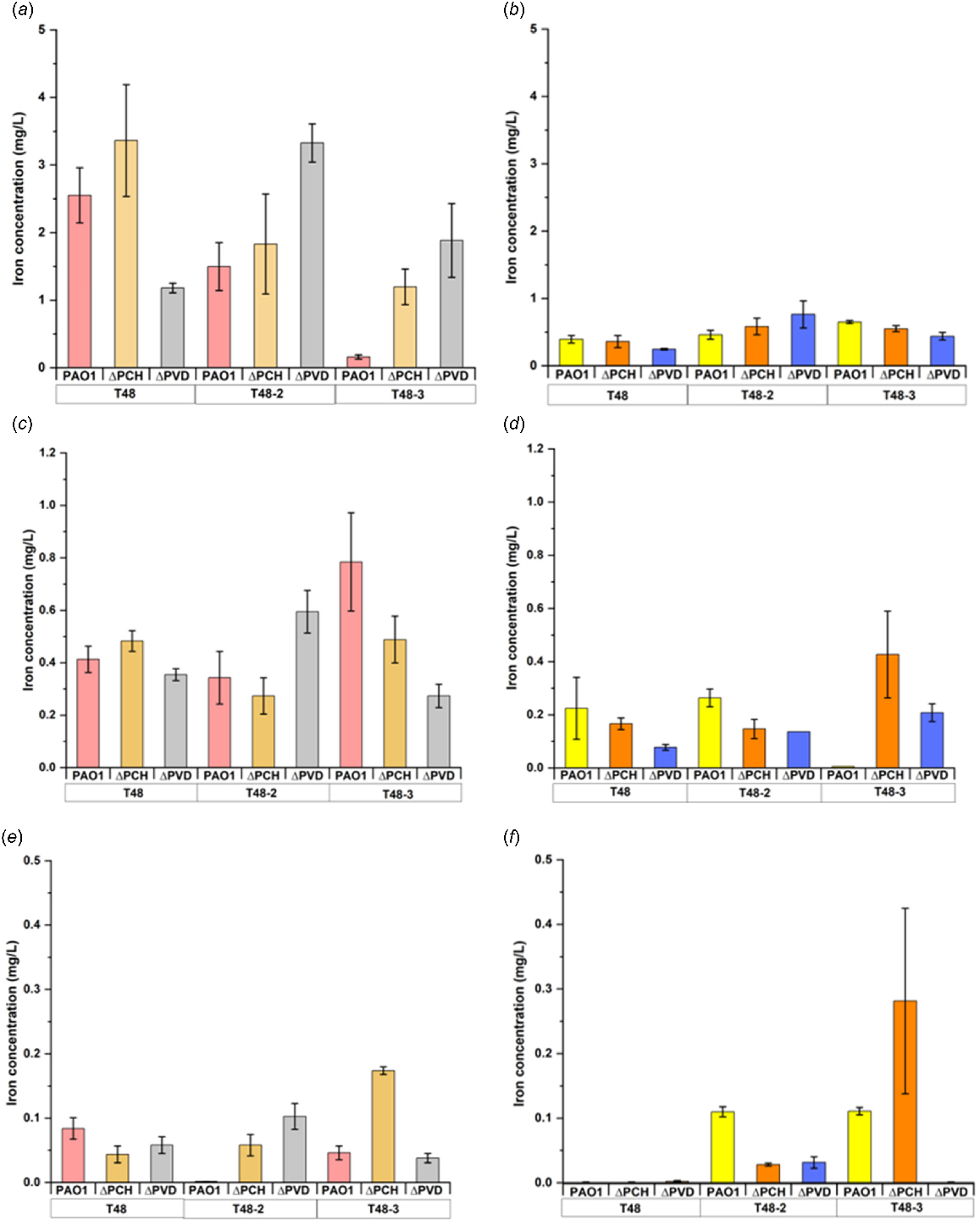
Graphical presentation showing the dissolved iron concentration from either asbestos cement or flocking waste after 18 or 40 h, respectively, undercontrolled condition (casamino acids without magnesium (CAA-Mg)) or inoculated with the following strains of Pseudomonas aeruginosa. WT, wild-type; ΔPVD, pyoverdine-deficient; ΔPCH, pyochelin-deficient; 2Δ pyoverdine- and pyochelin-deficient (modified after David et al. 2020a, 2021).
Malignant mesothelioma (MM), which affects the pleural, peritoneal, pericardial, and testicular membranes, is considered as a severe form of cancer with a very low survival rate, despite the fact that asbestos can cause a wide range of deadly and non-fatal diseases (Amin et al. 2018). The global incidence of MM is rising despite the restricted use of asbestos and it was reported that over the past 19 years (2000–2019) it had been increased from 372,112 disability-adjusted life years (DALY) to 586,970 DALY (Kwak et al. 2021). In Italy, where asbestos has been prohibited for several decades, the lengthy incubation period (between exposure and illness onset) has led to the registration of excess mortality in municipalities and areas affected by asbestos (Magnani et al. 2023).
Since the 1990s, MM incidence and mortality have been estimated with an aim to determine peak mortality in various countries. Peak mortalities predicted in the following countries have varied considerably, for example; during 2000–2004, 2007–2016, 2015, 2017, 2019, and 2020 in USA, Spain, Denmark, Netherlands, Italy, and UK, respectively (Magnani et al. 2023). With the exception of USA, all of these countries are listed on the International Ban Asbestos Secretariat (IBAS) website (Kazan-Allen 2022). However, some data sources claims that the USA was one of the first nations to regulate some products containing asbestos; for instance, in 1973, the use of spray-applied surface asbestos-containing material for fireproofing and insulating reasons was banned (Kazan-Allen 2022). Mortality peaks have not yet occurred in several of the nations that implemented bans in the 21st century. For instance, the Republic of Korea (South Korea) outlawed the use of asbestos in all of the forms in 2009, but a new study forecast that asbestos use will increase steadily over the following 20 years without reaching a peak. This implies that the number of MM cases may rise beyond 2038 (Kwak et al. 2021).
Bioweathering and bioremediation of asbestos
Bacterial and fungal weathering and remediation
This section aims to provide an overview of some of the most recent research on the impact of bacteria, fungi, or lichen on the characteristics of asbestos (Table 3; Fig. 3). Detailed explanations of their specific roles and effects in the bioweathering and bioremediation processes have also been discussed in this section. The core mechanisms of complex processes such as bioweathering, phytoremediation, and bioremediation are discussed in this section. Microorganisms have been the subject of greater investigation in recent years due to their propensity to change asbestos fibres. Besides magnesium, asbestos fibres contain iron, an essential micronutrient for most organisms that scavenge this element by various mechanisms such as: (1) the production of siderophores defined by organic ligands with high affinity and specificity for iron; (2) the reduction; and (3) the production of organic and inorganic acids or chelating molecules. Siderophore-mediated asbestos bioweathering in soils depends on siderophore concentrations, which vary in bulk soil depending on soil pH and will accelerate with increasing cell density in the rhizosphere (Völker and Wolf-Gladrow 1999), being even higher in biofilms (Liermann et al. 2000). Asbestos fibres can be transported through soil by groundwater flow but mechanisms governing this mobility including particle size, shape, and soil pore size are complex event (Wallis et al. 2020). Soil microorganisms have developed, thanks to their adaptation in contact with these raw silicate minerals, different weathering mechanisms and would therefore be potential candidates for bioremediation process (Fig. 4).
| Location | Organism(s) | Methodology | Key findings | References | |
|---|---|---|---|---|---|
| Asbestos mines (Italy) | Fungi (Verticillium leptobactrum, Fusarium oxysporum) | Dilutions were prepared in sterile water at ratios of 1:500 or 1:1000 (weight/volume). Rock fragments underwent vortexing in sterile water for 15 min. The resulting suspensions were then cultured on a medium of 2% malt extract agar supplemented with gentamycin and streptomycin at concentrations of 4 × 105 g mL−1 and 3 × 105 g mL−1, respectively. | Solubilisation of magnesium and silicon, along with the evaluation of the Mg/Si ratio in asbestos fibres post-exposure to fungal mycelia, suggested significant bio-weathering activity of Verticillium leptobactrum on chrysotile. | Daghino et al. (2009) | |
| Balangeromine (Italy) | Fungi (V. leptobactrum,Fusarium oxysporum, Paecilomyces lilacinus, and Aspergillus fumigatus) | Soil samples were diluted in sterile water at ratios of 1:500 or 1:1000 (w/v). Rock fragments were submerged in sterile water, and all samples were then subjected to vigorous mixing for 15 min using a vortex. The resulting suspensions were subsequently plated onto 2% malt agar media supplemented with gentamycin and streptomycin at concentrations of 4 × 105 g mL−1 and 3 × 105 g mL−1, respectively. | In the culture media with chrysotile fibres, F. oxysporum, V. leptobactrum, and P. lilacinus exhibited a notable rise in solubilised iron, contrasting with the controls. Conversely, A. fumigatus showed no significant variations in solubilised iron levels. | Daghino et al. (2008) | |
| Asbestos mines (Rajasthan, India) | Fungi (Aspergillus tubingenesis and Coemansia reversa) | Soil samples were dispersed in sterile water at different concentrations, ranging from 1:50 to 1:500 (w/v). Following that, all suspensions were agitated using a vortex for 10–15 min and applied onto potato dextrose agar plates supplementedwith 4 × 105 g mL−1 gentamicin and 3 × 105 g mL−1 streptomycin. | A. tubingenesis resulted in a moderate yet significant extraction of iron, resulting in an Fe/Si ratio of 0.21. Meanwhile, Coemansiareversa further reduced this ratio to an impressive value of 0.16. | Bhattacharya et al. (2016) | |
| Asbestos mines (Rajasthan, India) | Not mentioned | Soil samples were prepared by suspending them in sterile water at varying concentrations ranging from 1:50 to 1:500 (w/v). Each suspension underwent vigorous mixing (vortexing) for 10–15 min before being plated onto nutrient agar plates. These plates were then incubated for 24 h at a constant temperature of 37°C. | Scanning Electron Microscopy-Energy Dispersive X-ray (SEM-EDX) was employed to assess the reduction in iron content within asbestos fibres. The results indicated a significant decrease in asbestos iron content with the bacterial isolates. | Bhattacharya et al. (2015) | |
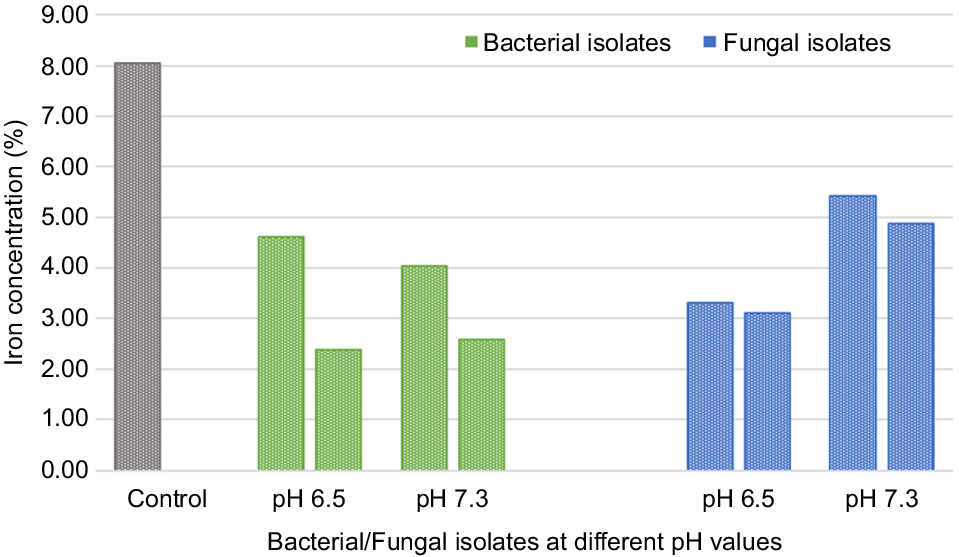
| Asbestos mines (Rajasthan, India) | Fungi (A. tubingensis and Coemansia reversa), and bacteria (Bacillus atrophaeus and Bacillus subtilis) | Soil samples were prepared as suspensions in sterile water at various concentrationsranging from 1:50 to 1:500 (w/v). After vortexing for 10–15 min, the suspensions were plated onto nutrient agar plates, and the temperature range for experimentation was set between 35°C and 45°C. | At pH of 6.5, the fungal isolates exhibited a 58.9% reduction in iron content and showed a 61.2% reduction. At pH 7.3, compared to the control, the fungal isolates and demonstrated reductions of 32.6% and 39.4% in asbestos iron content, respectively. | Bhattacharya et al. (2021) | |
| Asbestos mines (Western Alps) | Fungi (V. leptobactrum) | Verticillium leptobactrum was cultured in a liquid medium containing chrysotile fibres | The absence of lichen symbiosis had caused a notable decline in the magnesium oxide (MgO) content and a rise in the silica to magnesium oxide (SiO2: MgO) ratio of chrysotile fibres. | Daghino et al. (2010) | |
| Asbestos-cement roofs (Torino, Italy) | Lichen (Acarospora cervina), Candelariella aurella and Candelariella vitellina) | Adhesive tape was applied to sampled areas, and WinRHIZO was utilised for the analysis to quantify lichen colonisation. Specifically, V. leptobactrum was cultivated in a liquid medium in the presence of chrysotile fibres. | A lichen cover of 25% altered the characteristics of asbestos-cement sheets with chrysotile and crocidolite fibres. Combining pull-up tests and image analysis of linear structures, researchers observed that fibre loss was notably reduced (approximately 30%) in areas where lichens grew, acting as a physical barrier to fibre detachment. | Favero-Longo et al. (2009a) | |
| Asbestos mine (Kahurangi National Park, New Zealand) | Fungi (Cladosporium cladosporioides, Leucosporidium scottii, Pseudopithomyces chartarum) and bacteria (Actinomycetospora and Sphingomonas) | A small 0.25 g portion was taken, processed in a Nucleopore filter unit (NPU), and frozen within 24 h for subsequent DNA extraction. | Samples of asbestos fibres collected from soil, biofilm-covered asbestos-containing material (ACM), and lichens growing on ACM exhibited positive signs of degradation. However, raw fibres obtained directly from asbestos mine did not show any evidence of degradation. | Berry et al. (2024) | |
| Asbestos mine (Arizona) | Fungal siderophore (Ferrichrome) and bacterial siderophore (Desferrioxamine) | The chrysotile samples, fibrous bundles were manually separated from rock impurities using a hammering technique. The selected fibers were then dry ground in a high-energy vibratory ball mill for a duration of 15 min. | Fungal and bacterial siderophores demonstrated similar effectiveness in iron chelation. However, the fungal siderophore displayed a greater ability to reduce reactive oxygen species, potentially resulting in a stronger mitigation of chrysotile toxicity. | Mohanty et al. (2018) | |
| Asbestos removal site of (Jussieu, University of Paris) | Bacteria (Pseudomonas aeruginosa) | Asbestos samples (0.2 g each) were autoclaved at 121°C for 20 min. Following this, the samples were incubated at 70°C for 14 days to guarantee complete sterilisation and remove any residual water for accurate weighing. | The study observed a substantial rise in iron extraction when siderophores were present. Additionally, the absence of one siderophore could be compensated for by the other. P. aeruginosa utilised asbestos waste as a source of iron and magnesium. | David et al. (2020a) | |
| Asbestos-contaminated soil and pieces of asbestos roof cement in soil (Netherlands) | Fungi (Penicillium grancanariae) | In the dilution method, 1 g of the sample was mixed with 9 mL of 0.9% NaCl, shaken for 30 s, and 100 μL of each dilution (101, 103, and 105) was spread onto agar plates in triplicates. The direct method involved placing around 10 mg of various samples on agar plates in duplicates. Incubation occurred at 25°C. | Among the tested strains, P. grancanariae demonstrated the greatest ability to extract iron from crocidolite fibres. Additionally, all bacterial strains successfully removed magnesium from the same fibres. The combination of bacteria and fungi in a synergistic approach significantly enhanced cation removal, potentially providing a method for decontaminating asbestos-contaminated soils. | Wong et al. (2023) |
Comparative chart indicating iron content of asbestos after fungal and bacterial treatments at two different pH levels (Source: Bhattacharya et al. 2021).
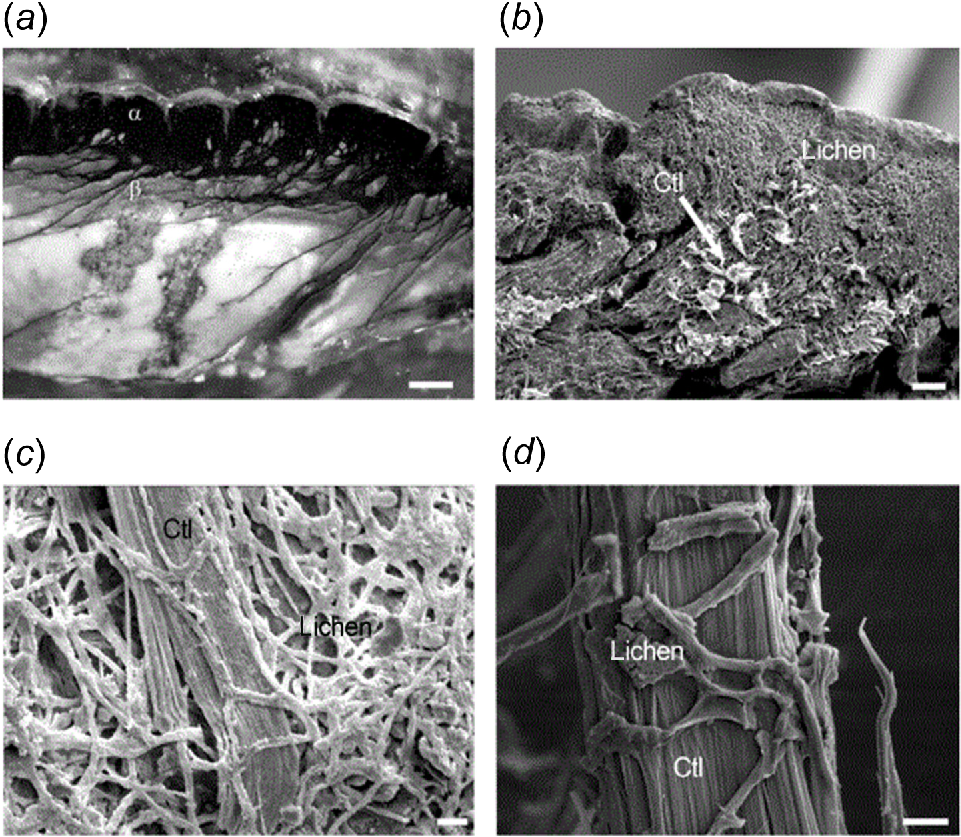
Lichens growing on asbestos-rich serpentinites are depicted in (a) the microphotograph and (b–d) the SEM pictures. Scale bars are: (a) 1 mm; (b) 100 mm; (c) 10 mm; and (d) 2 mm (Source: Favero-Longo et al. 2005a).
Among 98 fungi species isolated from two dismissed asbestos mines in Western Alps area, Aspergillus fumigates, Verticillium leptobactrum, and Paecilomyces lilacinus were the three dominated while Penicillium, Mortierella, Myrothecium, and Cladosporium were abundant in one of the two mines (Daghino et al. 2008). V. leptobactrum and P. lilacinus were able to extract iron from crocidolite while V. leptobactrum removed magnesium from chrysotile fibres (Daghino et al. 2009). Wasserbauer et al. (1988) found nitrifying bacterial population on roofing materials; however, Bhattacharya et al. (2015) isolated bacterial species from asbestos-contaminated soils and asbestos rocks from closed mines in India that might lower the iron content of asbestos. Microbial activities from these natural environments may hold the key to identify potential bioremediators of asbestos.
Although the number of asbestos involving bioremediation studies has increased significantly in recent years, the efficacy of siderophores, whether bacterial or fungal, in dissolving iron from asbestos fibres has been tested on a limited number of siderophores compared to over 500 known structures (Hider and Kong 2010). Moreover, although chrysotile is the asbestos species most frequently found in waste, few studies have focused on the alteration of amphibolic forms of asbestos such as amosite or crocidolite, which can also be encountered in waste. Lund and Aust (1990) suggested that low-molecular-weight intracellular iron chelator such as ascorbate, citrate, ADP, and ferrozine can mobilised iron from asbestos (crocidolite, amosite, chrysotile, and tremolite). First evidences of the removal of iron by the commercial siderophore desferrioxamine B were shown on crocidolite and amosite (Chao and Aust 1994; Werner et al. 1995). Some fungi are well adapted to these mineral substrates, such as the species Fusarium oxysporum and V. leptobactrum, isolated from naturally occurring serpentinic rocks (Martino et al. 2004). In soil, some are mycorrhizal fungi providing inorganic nutrients to their plant hosts while the others saprophytic fungi could therefore be considered as biofertilisers due to their potential of mineral leaching, providing essential nutrients for plants (Hoffland et al. 2004). The role of siderophores produced by various fungi such as Geomyces pannorum, Mortiella hyaline, Oidio dendronmaius, Oidiodendron griseum, and F. oxysporum in iron extraction was studied on crocidolite fibres, with F. oxysporum, M. hyalina and Oidiodendron maius being the most effective in iron solubilisation from fibres (Martino et al. 2003). The most interesting F. oxysporum was able to dissolve iron from the three kind of asbestos fibres (chrysotile, amosite, and crocidolite) due to chelators release with the ranking of the total iron release was chrysotile > crocidolite > amosite, considering the accessibility of the chelators in the crystal structure and differences in the iron percentage/composition among them (Daghino et al. 2005).
Recent research comparing the release of siderophores has highlighted that both fungal and bacterial species, including Aspergillus tubingenesis and Coemansia reversa, as well as Bacillus subtilis and Bacillus atrophaeus, are efficient at producing siderophores, which may lessen the toxicity of asbestos due to their capacity for iron chelation (Bhattacharya et al. 2021). Likewise, Pseudomonas species that produce siderophores were investigated by David et al. (2020a, 2021) in relation to the biodeterioration of asbestos cement, asbestos waste from flocking, and raw asbestos (crocidolite, amosite, chrysotile) (Fig. 5). The release of siderophores such as pyoverdine and pyochelin by Pseudomonas or their purified forms were found to be effective in enhancing the solubilisation and bioavailability of iron from asbestos fibres. Moreover, raw asbestos or asbestos-containing waste can serve as nutrient source for the soil bacteria Pseudomonas since a growth stimulation was observed due to iron and magnesium dissolution processes (David et al. 2020a). They have showed for the first time the repression of the pyoverdine and pyochelin pathway by the addition of asbestos fibres due to iron release. The efficiency of the siderophore-driven bioweathering varies depending on the type of pyoverdine, concentration and time (David et al. 2020b, 2020c). Besides the siderophore-driven mechanism, fungi can dissolve asbestos fibres by the production of organic acids, which helped the mechanical mechanisms by introducing their hyphae in existing cavities, cracks, fissures and by active penetration (Daghino et al. 2008; Mohanty et al. 2018). The most efficient acid for reducing the positive charge in chrysotile asbestos was oxalic acid, according to Holmes and Lavkulich (2014), who had also described how this affected the material’s surface characteristics gradually.
Impact of biological degradation at varying concentrations of asbestos cement tiles (ACT) utilising (a) Aspergillus niger and (b) Acidithiobacillus thiooxidans in solid-state culture (SSC) (modified after Borges et al. 2022).
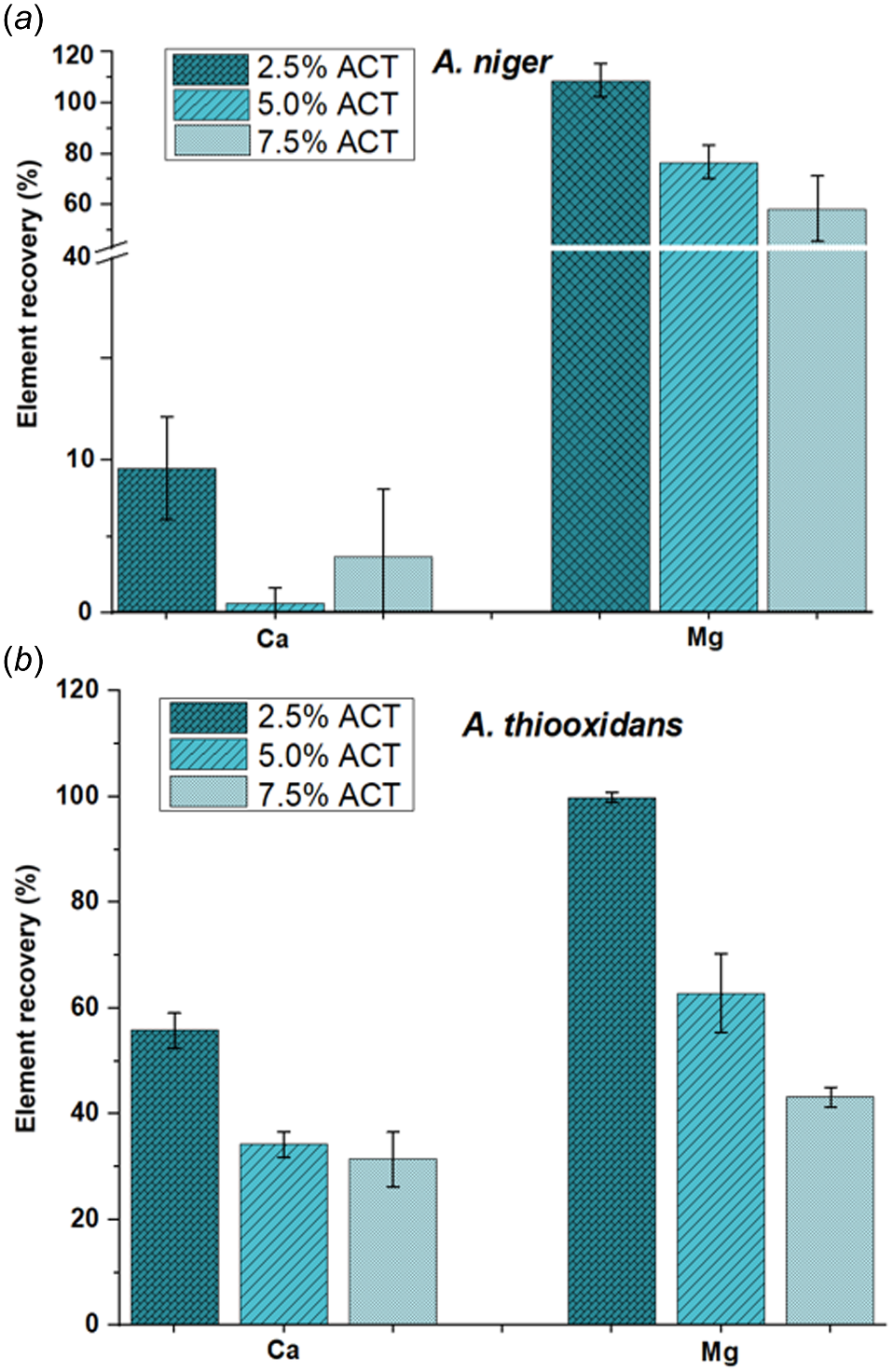
However, the actual challenge is to degrade ACW where fibres are embedded in matrices such as cement, plastic or tar phase depending on waste type, which represent a societal problem to face. Balducci et al. (2012) revealed that the lactic acid produced by Lactobacilli grown in milk whey induced a strong dissolution of the cement phase of asbestos-cement wastes. Recently, David and Geoffroy (2022) have improved this process by inoculating milk whey with lactic acid bacteria, thereby compensating for the rise in pH associated with the dissolution of mineral matrices such as cement, and maintaining an acid pH that favours the dissolution of chrysotile, the species most commonly found in waste (David and Geoffroy 2022). Another innovative anaerobic biological process, the dark fermentation of biodegradable waste biomasses, based on acidic dissolution of the cement matrix of a glass-fibre conglomerate, simulating a cement asbestos composite, was evidenced by Spasiano et al. (2017). The acidic effluent was due to the presence of acetic, propionic and butyric acid, volatile fatty acids. Recently, Borges et al. (2022) demonstrated for the first time the high potential of the sulphuric acid producer A. thiooxidans in raw chrysotile and asbestos cement tile dissolution by microbial filtrate, submerged and solid-state cultivation. Corrosion of asbestos cement pipes by acid producing bacteria, slime forming and heterotrophic aerobic bacteria have already been reported earlier (Wang and Cullimore 2010). Finally, the last mechanism studied the Fe (III) reduction where Fe (III) is used as a terminal electron acceptor during respiration of anaerobic thermophilic chemolitho autotrophic microorganisms. Microbial respiration of iron dissolved from crocidolite by Deferrisoma palaeochroriense allowed microbial growth at 60°C and iron release from asbestos fibres with a higher rate compared to the one obtained by soil fungi (Choi et al. 2023). Thermovibrio ammonificans showed evidence of biosilicification when grown with chrysotile or tremolite-actinolite whereas the silicium and magnesium release was also higher compared to the one with fungi (Choi et al. 2023).
Lichen weathering and remediation
Lichens are communities, where a fungus (usually an Ascomycete, less commonly a Basidiomycete) forms a symbiosis with one or more photosynthetic organisms (green algae and/or cyanobacteria). The fungus creates a thallus structure which is usually made up of layers of fungal hyphae, the uppermost of which may be fused together with polysaccharides to form a resistant cortex. The algae or cyanobacteria are generally found below the cortex in the medulla layer, an area of loosely-woven fungal hyphae (Jahns 1988). Some lichens are epilithic, broadly adhering to a rock surface with hyphae or rhizines, others are endolithic, growing partly or completely within a mineral substrate (Chen et al. 2000). Penetration of rock structures by hyphae is usually 1–2 mm, but penetration to 10 mm is known to occur (de la Rosa et al. 2013). The lichen symbiosis can be complex. Some lichens have both cyanobacteria and green algae as photosynthetic partners, others have a second fungus present (e.g. basidiomycete yeasts (Spribille et al. 2016). A complex microbiome of endophytic or parasitic fungi and bacteria may also be present within the thallus or between the thallus and substrate (Adamo and Violante 2000; Spribille et al. 2022).
Biogeophysical weathering of rocks occurs when rhizines or individual fungal hyphae penetrate into crevices and mineral cleavage planes, followed by expansion and contraction of the fungal tissue when wetting and drying and/or freezing and thawing occur, or when the deposition of organic and inorganic compounds causes swelling (Adamo and Violante 2000; Chen et al. 2000). In addition, detached particles of the rock substrate are often incorporated into the lichen thallus (Chen et al. 2000). Biogeochemical weathering occurs via the production of a range of primary and secondary metabolites, which dissolve minerals and chelate metal cations (Favero-Longo et al. 2005a, 2005b), resulting in mineral surface corrosion and precipitation of mineral salts at the lichen-rock interface, or within the lichen thallus (Gadd et al. 2014).
Despite being a relatively inhospitable substrate for plants, natural asbestos deposits and asbestos cement roofs can support a diverse range of lichen species. For example, Gilbert (1970) recorded 20 lichen species on asbestos cement roofs in north-eastern England, Christensen (2004) reported 22 epilithic lichen species on asbestos cement roofs in Denmark, Favero-Longo et al. (2006) noted 24 foliose and crustose lichens covering up to 70% of serpentinite rock walls in a disused asbestos mine in Italy, and Doyle et al. (2023) reported species of Protoblastenia, Caloplaca, Physcia, Xanthoparmelia, and Verrucaria as being present on asbestos cement roofs in New Zealand.
Investigations into the weathering effects of lichens on asbestos have largely focused on chrysotile and asbestos cement. Evidence of weathering of chrysotile in naturally-occurring serpentinite has been reported in association with Lecanora atra (Wilson et al. 1981), Caloplaca spp. and Ochrolechia patella (Adamo et al. 1993), C. vitellina (Favero-Longo et al. 2005a; Turci et al. 2007), L. rupicola, Xanthoparmelia pulla and X. tinctina (Favero-Longo et al. 2005a), Lecidea atrobrunnea, Rhizocarpon geographicum, and Sporastria testudinea (Favero-Longo et al. 2005b). Focusing on bioweathering of asbestos cement roofs, Brown (1998) noted that over time asbestos cement roofing material degraded to leave a loose, asbestos-rich layer associated with lichens and algae. Favero-Longo et al. (2009b) found that fibres of chrysotile and crocidolite were modified in roofing material under Acarospora cervina and Candelariella spp. Berry et al. (2024) found that samples of the lichen Xanthoparmelia scabrosa on asbestos cement roofs had incorporated and partially modified bundles of chrysotile fibrils.
Evidence of weathering by lichens includes the appearance of etching and corrosion of the rock surface (Adamo et al. 1993), penetration of hyphae into the serpentinite, disaggregation of the mineral, and surrounding of individual chrysotile fibres (Wilson et al. 1981; Favero-Longo et al. 2005a, 2013), incorporation of chrysotile fibres and/or other lithic materials into the lichen thallus (Favero-Longo et al. 2005b; Berry et al. 2024), depletion in the relative percentage of magnesium and/or iron in the chrysotile fibres (Wilson et al. 1981; Favero-Longo et al. 2005a, 2009b), deposition of metallic oxalates in the lichen-rock interface, or within the lichen itself (Ervik et al. 2021), and surface modification or partial dissolution of the chrysotile fibres themselves (Berry et al. 2024).
Oxalic acid is one of the main metabolites produced by the fungal partner of most lichens (Gadd et al. 2014), and the presence of metallic oxalates between lichens and their substrate supports the idea that oxalic acid is largely responsible for biogeochemical weathering. Experiments where chrysotile fibres are incubated in oxalic acid solutions have resulted in the destruction of fibre structure and reduction in Fenton activity (Favero-Longo et al. 2005b). Follow up studies using the isolated and cultured mycobiont fungus from X. tinctina, found that the isolated fungus produced oxalic acid, its hyphae surrounded the chrysotile fibres and a depletion in magnesium occurred (Favero-Longo et al. 2007). This finding is important because it confirms that the main fungal partner in the lichen is able to produce oxalic acid and degrade chrysotile fibres. Not all lichens produce oxalic acid, instead producing other organic acids or secondary metabolites such as depsides or depsidones, some of which have the ability to form complexes with metal cations (Chen et al. 2000). There is some evidence that some of these may be involved in bioweathering, for example Turci et al. (2007) found that pulvinic acid derived from Candelariella species were capable of weakly modifying the surface of chrysotile fibres in vitro over a 5-week period. In addition, as mentioned earlier, a lichen is a community of different species, including photobionts (green algae and/or cyanobacteria), basidiomycete yeasts in the cortex (Spribille et al. 2016), and filamentous non-lichenised fungi (Bjelland and Ekman 2005), and bacteria (Uroz et al. 2009; Bjelland et al. 2011) inside or under the thallus, all of which may have a role in bioweathering of asbestos.
Phytoremediation of asbestos
Another effective bioremediation approach is phytoremediation, which uses plants and/or associated rhizobacteria to bioaccumulate, degrade, or stabilise contaminants (Hou et al. 2023; Wang and Hou 2023). There are generally two primary methods for phytoremediating asbestos-contaminated soils: phytostabilisation and phytoextraction (Table 4). Phytostabilisation relies on plant roots to stabilise heavy metals, ultimately reducing their mobility and bioavailability (Bolan et al. 2011; Wang et al. 2020, 2021). Additionally, the established vegetation cover on degraded soil surfaces effectively mitigates soil erosion (Bolan et al. 2011; Kumari and Maiti 2022; Zhao et al. 2022), which in turn can limit asbestos exposure through airborne pathways. Successes have been achieved in ecologically restoring asbestos-contaminated mining sites, where plant cover has been established to impede the migration of asbestos fibres and associated heavy metals. For example, 15~40% vegetation cover cultivated on asbestos-rich serpentine soils reduced asbestos fibre dispersion up to 50% (Favero-Longo et al. 2009b). The grass-legume layer of Cynodon dactylon, Sorghastrum nutans, Acacia concinna, and C. cajan successfully thrived on asbestos-contaminated soils and effectively hindered the migration of heavy metals, including Cr and Ni (Kumar et al. 2017). Beyond higher plants, lichens have also been utilised to achieve phytostabilisation (Favero-Longo et al. 2005a, 2005b).
| Plant species | Amendment assisting phytoremediation | Major processes and mechanisms for asbestos remediation | Asbestos remediation performance | Reference | |
|---|---|---|---|---|---|
| Mixture of bluegrass (Poa pratensis), perennial ryegrass (Lolium perenne), tall fescue (Schedonorus arundinaceus), and alsike clover (Trifolium pratense) | 180 tonnes per ha compost (Interval/Foster Farm), 56 tonnes per ha gypsum, 0.224 tonnes per ha NPK fertiliser, 22 tonnes per ha limestone | Soil amendment boosts to obstruct the dispersion of asbestos fibres, thus promoting phytostabilisation. | The yield of grass and clover showed a significant increase, with the total yield in the two remediation groups rising from 0.11 tonnes per ha to 2.39 tonnes per ha and 2.91 t/ha, respectively. | Chaney et al. (2011) | |
| Leptoplax emarginata | 1.8 mL per column Ni-resistant bacterial strains | Leptoplax emarginata increased dissolution of Ni-bearing mineral phase in the rhizosphere, thus aiding in hyperaccumulation. | L. emarginata enhanced the dissolution of chrysotile by over double and resulted in the bioaccumulation of 88% of the solubilised nickel. | Chardot-Jacques et al. (2013) | |
| Minuartia and Thymus species | Not mentioned | Utilising the method of establishing vegetation cover helps impede the migration of asbestos fibres into the air. | A vegetation canopy ranging from 15% to 40% on serpentine soils rich in asbestos led to a decline of asbestos fibre dispersion by as much as 50%. | Favero-Longo et al. (2009b) | |
| Lichens (Candelariella vitellina, Leacanora rupicola, and Xanthoparmelia tinctina). | Not mentioned | Lichens can leach cations (positively charged ions) from chrysotile fibres, which subsequently aids in promoting hyperaccumulation post-dissolution. | Significant MgO content reduction in lichen-colonised chrysotile; transformation to non-toxic amorphous silica. | Favero-Longo et al. (2005a) | |
| Oats (Avena sativa) and mix of Timothy grass (Phleum pratense), red clover, alsike clover | 300 tonnes per ha municipal biosolids and 900 tonnes per ha de-inking sludges | Soil amendment promotes plant survival, strengthening the plant’s barrier mechanism to prevent the dispersion of asbestos fibres. | CO2 emissions showed a notable reduction after the first year, indicating rapid carbon stabilisation in the soil of this rehabilitated asbestos mine due to the biosolids. | Khlifa et al. (2023) | |
| Grass–legume cover (Cynodondactylon, Sorghastrum nutans, Acacia concinna, and Cajanus cajan) | Not mentioned | Utilising plants with metal-scavenging properties can accumulate metals and, more importantly, serve as barriers to prevent their spread. | The grass-legume association was found to be effective in accumulating metals, acting as a potential barrier to further contamination and facilitating the phytoremediation process. | Kumar et al. (2017) | |
| Lolium perenne, Poa pratensis, Elymus junceus, Bromus inermis, Medicago sativa, Trifolium hybridum, Melilotus alba | 10 tonnes per ha NPK fertiliser, 40 tonnes per ha farmyard manure or sawdust | Utilising plants such as Lolium perenne and Poa pratensis, which flourish in metal-rich soils, to create a sustained plant cover that prevents the spread of asbestos fibres | Vegetation cover was sustained for 3 years with the mentioned amendments and selected plant species. | Moore and Zimmermann (1977) | |
| Melinis repens and other pioneer grasses | 100 tonnes per ha CaNO₃, 50 tonnes per ha KNO₃, 175 tonnes per ha 2:3:2 NPK fertiliser, 4000 tonnes per ha well matured kraal manure, 300 tonnes per ha flower of sulfur | Adjusting the soil conditions and nutrient status can enhance the sustainability of a locally adapted grass cover, thereby preventing the spread of asbestos fibres. | Significant 8-year vegetation composition change; improved nutrient status in rehabilitated areas. | Morgenthal et al. (2004) |
It’s worth noting that asbestos mine wastes are typically rich in magnesium silicates but severely lacking in calcium, nitrogen, phosphorus, and organic matter (Meyer 1980; Chaney and Mahoney 2014). Another study also found that asbestos itself did not have a toxic effect on plant growth (Gonneau et al. 2017). Instead, the hindrance to plant growth in asbestos-enriched soils was attributed to heavy metals and a deficiency of nutrients (Gonneau et al. 2017). Therefore, to enhance the survival rate of plant cover, soil amendment with limestone, fertilisers, biochar, or manure is crucial for the phytostabilisation process (Kumar and Maiti 2015; Dvořáčková and Dvořáček 2023; Wang et al. 2023; Ramteke et al. 2024).
Moore and Zimmermann (1977) had used NPK fertilisers and farmyard manure to promote plant growth on asbestos mine wastes. This study demonstrated that plants survived over a 3-year period but showed slight deficiencies in calcium and nitrogen at the end of the second growing season. Another study utilised a combination of amendments, including gypsum, manure compost, limestone, and fertilisers to improve an asbestos-contaminated mine site soil in Vermont, USA, encouraging plant growth and phytostabilisation. Dense vegetation successfully thrived even on steep slopes, with plant roots growing over 25 cm within just 1 year, while no plants survived in the unamended control soils (Chaney et al. 2011). In a long-term ecological restoration project, biosolids were used as a soil amendment at an application rate of 1200 million g per ha to rehabilitate asbestos-contaminated soils in Quebec, Canada (Fig. S2) (Khlifa et al. 2023). Such soil amendments were used to address the nutrient deficiency problem in asbestos- contaminated soils. The vegetation cover on this site not only thrived but also provided additional benefits by absorbing atmospheric carbon dioxide and storing carbon in the ground (Khlifa et al. 2023). Another effective approach to ensure the survival of vegetation cover is to use local species adapted to asbestos (Morgenthal et al. 2004). An 8-year field trial found that local perennial grasses, including Hyperthelia dissoluta, Cymbopogon excavatus, Themeda triandra, and Heteropogon contortus survived despite the abnormally high magnesium and low calcium concentration in chrysotile asbestos mine tailings (Morgenthal et al. 2004).
However, it is essential to consider that asbestos remains in the soil. Phytostabilised soils should be continuously monitored to control asbestos exposure to organisms. For example, even a well-rehabilitated asbestos-contaminated mine site in South Africa showed signs of exposed asbestos where livestock grazed on vegetated waste dump soils (Freemantle et al. 2022). Another viable approach is to utilise plants for iron removal from asbestos-contaminated soil, thereby reducing the toxicity of asbestos. Higher plants and many soil microorganisms rely on iron for their metabolic processes, and as a result, some have developed highly efficient mechanisms for scavenging it from their environment (Morrissey and Guerinot 2009). Non-graminaceous plants employ phenolics to chelate ferrous ions, leading to the reduction of ferric chelates at the root surface, ultimately enabling the extraction of the ferrous ions (Kobayashi and Nishizawa 2012). Graminaceous plants release potent chelators like the mugineic acid family phytosiderophores, which effectively solubilise ferrous iron and render it bioavailable without the need for reduction (Kobayashi and Nishizawa 2012).
A promising asbestos solubilisation effect was observed when using the nickel hyper accumulator Leptoplax emarginata for phytoextraction. This approach promoted the dissolution of chrysotile asbestos by a two-fold increase and resulted in the bioaccumulation of 88% of the solubilised nickel (Chardot-Jacques et al. 2013). Notably, the chrysotile asbestos fibres were discovered to be attached to the roots of the hyperaccumulator, providing direct evidence of the interaction between plants and minerals (Fig. S3). Furthermore, no mineralogical changes were observed, suggesting that dissolution played a dominant role in the process (Chardot-Jacques et al. 2013).
Summary and conclusions
ACWMs can weather and release asbestos fibre dust after getting disturbed by anthropogenic activities. Breathing of asbestos fibres can cause asbestos-related lung diseases such as asbestosis and mesothelioma cancer. The surface distribution of free iron, which forms highly reactive free radicals that damage DNA and eventually cause cancer, is connected to the toxic consequences of inhaled asbestos fibres. By enabling the bioweathering of asbestos and the extraction of iron to lessen asbestos toxicity, microorganisms such as bacteria, fungi, lichens, and higher plants aid in the remediation of soil contaminated with ACWMs. Rock-dwelling lichens are able to weather mineral substrates through physical and chemical means (Fig. S4). Microorganisms and higher plants produce siderophores and low molecular organic acids, thereby promoting the solubilisation of iron from asbestos fibres and its subsequent absorption and assimilation (Fig. 6). The extraction of iron from asbestos fibres alleviates asbestos toxicity.
Graphs showing that the iron concentration was determined in the supernatant following bacterial growth (b, d, f) or in the presence of each bacterial strain’s supernatant (a, c, e) following three 48-h renewal cycles at 30°C. (a, b) Raw chrysotile, (c, d) raw crocidolite, and (e, f) raw amosite (modified after David et al. 2020c).
The following study directions are advised in light of the crucial role that higher plants and microbes play in the bioweathering and bioremediation of ACWMs, as well as the information gaps that currently exist regarding the mechanisms underlying the bioweathering of asbestos and the biosequestration of iron:
Examination of the microorganism-induced and higher plants-induced bioweathering of ACWM under different environmental conditions.
Understanding the mechanisms involved in bioweathering using advanced spectroscopic techniques.
Examination of the biochemical processes involved in the biosequestration of iron using molecular techniques.
Monitoring the extent of bioweathering and biostabilisation of ACWMs under the impact of environmental factors and conditions (e.g. temperature, moisture, microbial species, population density and oxygen levels).
Author contributions
Terri-Ann Berry, Shannon L. Wallis, Dan Blanchon, Deyi Hou, Valerie A. Geoffroy, Kadambot H.M. Siddique and Nanthi Bolan: conceptualization and contributed to the interpretation of the discussion of various sections and provided critical revision and editing of the article. Shiv Bolan, Santanu Mukherjee, Shailja Sharma, Liuwei Wang: writing – original skeleton of the entire draft, contributed different sections and data extraction for Tables and prepared Figures. All authors read and approved the final manuscript.
Acknowledgements
We would like to acknowledge the Healthy Environments and Lives (HEAL) National Research Network, National Health and Medical Research Council (NHMRC) (Grant No. 2008937).
References
Adamo P, Violante P (2000) Weathering of rocks and neogenesis of minerals associated with lichen activity. Applied Clay Science 16, 229-256.
| Crossref | Google Scholar |
Adamo P, Marchetiello A, Violante P (1993) The weathering of mafic rocks by lichens. The Lichenologist 25, 285-297.
| Crossref | Google Scholar |
Amin W, Linkov F, Landsittel DP, Silverstein JC, Bashara W, Gaudioso C, Feldman MD, Pass HI, Melamed J, Friedberg JS, Becich MJ (2018) Factors influencing malignant mesothelioma survival: a retrospective review of the National Mesothelioma Virtual Bank cohort. F1000 Research 7, 1184.
| Crossref | Google Scholar |
Babu B, Thirumoorthy A, Gopinath P, Ramalingam R, Yasminebegum A, Anusha M (2023) Tribological and mechanical properties investigations on polymer based composites reinforced with bio fillers. Materials Today: Proceedings
| Crossref | Google Scholar |
Balducci G, Foresti E, Lelli M, Lesci IG, Marchetti M, Pierini F, Roveri N (2012) Process for treating an asbestos containing material. EU patent: EP2428254 B1. Available at https://hdl.handle.net/11585/145828
Berman DW, Crump KS (2008) Update of Potency factors for asbestos-related lung cancer and mesothelioma. Critical Reviews in Toxicology 38, 1-47.
| Crossref | Google Scholar | PubMed |
Berry T-A, Belluso E, Vigliaturo R, Gieré R, Emmett EA, Testa JR, Steinhorn G, Wallis SL (2022) Asbestos and other hazardous fibrous minerals: potential exposure pathways and associated health risks. International Journal of Environmental Research and Public Health 19, 4031.
| Crossref | Google Scholar |
Berry T-A, Wallis S, Doyle E, de Lange P, Steinhorn G, Vigliaturo R, Belluso E, Blanchon D (2024) A preliminary investigation into the degradation of asbestos fibres in soils, rocks and building materials associated with naturally occurring biofilms. Minerals 14, 106.
| Crossref | Google Scholar |
Bhattacharya S, John PJ, Ledwani L (2015) Bacterial weathering of asbestos. Silicon 7, 419-431.
| Crossref | Google Scholar |
Bhattacharya S, John PJ, Ledwani L (2016) Fungal weathering of asbestos in semi arid regions of India. Ecotoxicology and Environmental Safety 124, 186-192.
| Crossref | Google Scholar | PubMed |
Bhattacharya S, John PJ, Ledwani L (2021) Microbial siderophores an envisaged tool for asbestos bioremediation – a microcosm approach. Materials Today: Proceedings 43, 3110-3116.
| Crossref | Google Scholar |
Bjelland T, Ekman S (2005) Fungal diversity in rock beneath a crustose lichen as revealed by molecular markers. Microbial Ecology 49, 598-603.
| Crossref | Google Scholar | PubMed |
Bjelland T, Grube M, Hoem S, Jorgensen SL, Daae FL, Thorseth IH, Øvreås L (2011) Microbial metacommunities in the lichen–rock habitat. Environmental Microbiology Reports 3, 434-442.
| Crossref | Google Scholar | PubMed |
Bolan NS, Park JH, Robinson B, Naidu R, Huh KY (2011) Phytostabilization. In ‘Advances in agronomy’. (Ed. DL Sparks) pp. 145–204. (Academic Press) 10.1016/b978-0-12-385538-1.00004-4
Bolan S, Kempton L, McCarthy T, Wijesekara H, Piyathilake U, Jasemizad T, Padhye LP, Zhang T, Rinklebe J, Wang H, Kirkham MB, Siddique KHM, Bolan N (2023) Sustainable management of hazardous asbestos-containing materials: containment, stabilization and inertization. Science of the Total Environment 881, 163456.
| Crossref | Google Scholar |
Borges R, Klaic R, Farinas CS, Ribeiro C (2022) Biological treatment of asbestos cement wastes by Aspergillus niger and Acidithiobacillus thiooxidans. Applied Clay Science 216, 106375.
| Crossref | Google Scholar |
Bose VG, Shreenidhi KS, Malik JA (2022) Phytoremediation of PAH-contaminated areas. In ‘Advances in bioremediation and phytoremediation for sustainable soil management’. (Ed. JA Malik) pp. 141–156. (Springer: Cham) 10.1007/978-3-030-89984-4_9
Brown S (1998) Physical properties of asbestos-cement roof sheeting after long-term exposure. Journal of Occupational Health and Safety Australia and New Zealand 14, 129-134.
| Google Scholar |
Carlin DJ, Larson TC, Pfau JC, Gavett SH, Shukla A, Miller A, Hines R (2015) Current research and opportunities to address environmental asbestos exposures. Environmental Health Perspectives 123, A194-A197.
| Crossref | Google Scholar | PubMed |
Chao CC, Aust AE (1994) Effect of long-term removal of iron from asbestos by desferrioxamine B on subsequent mobilization by other chelators and induction of DNA single-strand breaks. Archives of Biochemistry and Biophysics 308, 64-69.
| Crossref | Google Scholar | PubMed |
Chardot-Jacques V, Calvaruso C, Simon B, Turpault M-P, Echevarria G, Morel J-L (2013) Chrysotile Dissolution in the Rhizosphere of the Nickel Hyperaccumulator Leptoplax emarginata. Environmental Science & Technology 47, 2612-2620.
| Crossref | Google Scholar | PubMed |
Chen J, Blume H-P, Beyer L (2000) Weathering of rocks induced by lichen colonization – a review. Catena 39, 121-146.
| Crossref | Google Scholar |
Choi JK, Vigliaturo R, Gieré R, Pérez-Rodríguez I (2023) Microbe-mineral interactions between asbestos and thermophilic chemolithoautotrophic anaerobes. Applied and Environmental Microbiology 89, e0204822.
| Crossref | Google Scholar |
Christensen S (2004) Epilithic lichen vegetation of corrugated asbestos-cement roof tiles in the Copenhagen area. Graphis Scripta 15, 7-13.
| Google Scholar |
Daghino S, Martino E, Fenoglio I, Tomatis M, Perotto S, Fubini B (2005) Inorganic materials and living organisms: surface modifications and fungal responses to various asbestos forms. Chemistry – A European Journal 11, 5611-5618.
| Crossref | Google Scholar | PubMed |
Daghino S, Martino E, Vurro E, Tomatis M, Girlanda M, Fubini B, Perotto S (2008) Bioweathering of chrysotile by fungi isolated in ophiolitic sites. FEMS Microbiology Letters 285, 242-249.
| Crossref | Google Scholar | PubMed |
Daghino S, Turci F, Tomatis M, Girlanda M, Fubini B, Perotto S (2009) Weathering of chrysotile asbestos by the serpentine rock-inhabiting fungus Verticillium leptobactrum. FEMS Microbiology Ecology 69, 132-141.
| Crossref | Google Scholar | PubMed |
Daghino S, Martino E, Perotto S (2010) Fungal weathering and implications in the solubilization of metals from soil and from asbestos fibres. In ‘Current research, technology and education topics in applied microbiology and microbial biotechnology. Vol. 1’. (Ed. A Mendez-Vilas) pp. 329–338. (Formatex Research Center)
David SR, Fritsch S, Forster A, Ihiawakrim D, Geoffroy VA (2020a) Flocking asbestos waste, an iron and magnesium source for Pseudomonas. Science of The Total Environment 709, 135936.
| Crossref | Google Scholar |
David SR, Ihiawakrim D, Regis R, Geoffroy VA (2020b) Efficiency of pyoverdines in iron removal from flocking asbestos waste: an innovative bacterial bioremediation strategy. Journal of Hazardous Materials 394, 122532.
| Crossref | Google Scholar |
David SR, Ihiawakrim D, Regis R, Geoffroy VA (2020c) Iron removal from raw asbestos by siderophores-producing Pseudomonas. Journal of Hazardous Materials 385, 121563.
| Crossref | Google Scholar |
David SR, Jaouen A, Ihiawakrim D, Geoffroy VA (2021) Biodeterioration of asbestos cement by siderophore-producing Pseudomonas. Journal of Hazardous Materials 403, 123699.
| Crossref | Google Scholar |
de la Rosa JPM, Warke PA, Smith BJ (2013) Lichen-induced biomodification of calcareous surfaces: bioprotection versus biodeterioration. Progress in Physical Geography: Earth and Environment 37, 325-351.
| Crossref | Google Scholar |
Doyle E, Blanchon D, Wells S, de Lange P, Lockhart P, Waipara N, Manefield M, Wallis S, Berry T-A (2023) Internal transcribed spacer and 16S Amplicon sequencing identifies microbial species associated with asbestos in New Zealand. Genes 14, 729.
| Crossref | Google Scholar |
Durczak K, Pyzalski M, Brylewski T, Juszczyk M, Leśniak A, Libura M, Ustinovičius L, Vaišnoras M (2024) Modern methods of asbestos waste management as innovative solutions for recycling and sustainable cement production. Sustainability 16, 8798.
| Crossref | Google Scholar |
Dvořáčková H, Dvořáček J (2023) Manure application followed by biochar application increases plant production regardless of soil dehydrogenase activity. Soil Use and Management 39, 1557-1569.
| Crossref | Google Scholar |
Ervik T, Eriksen Hammer S, Graff P (2021) Mobilization of asbestos fibers by weathering of a corrugated asbestos cement roof. Journal of Occupational and Environmental Hygiene 18, 110-117.
| Crossref | Google Scholar | PubMed |
Favero-Longo SE, Turci F, Tomatis M, Castelli D, Bonfante P, Hochella MF, Piervittori R, Fubini B (2005a) Chrysotile asbestos is progressively converted into a non-fibrous amorphous material by the chelating action of lichen metabolites. Journal of Environmental Monitoring 7, 764.
| Crossref | Google Scholar |
Favero-Longo SE, Castelli D, Salvadori O, Belluso E, Piervittori R (2005b) Pedogenetic action of the lichens Lecidea atrobrunnea, Rhizocarpon geographicum gr. and Sporastatia testudinea on serpentinized ultramafic rocks in an alpine environment. International Biodeterioration & Biodegradation 56, 17-27.
| Crossref | Google Scholar |
Favero-Longo SE, Siniscalco C, Piervittori R (2006) Plant and lichen colonization in an asbestos mine: spontaneous bioattenuation limits air dispersion of fibres. Plant Biosystems - An International Journal Dealing with All Aspects of Plant Biology 140, 190-205.
| Crossref | Google Scholar |
Favero-Longo SE, Girlanda M, Honegger R, Fubini B, Piervittori R (2007) Interactions of sterile-cultured lichen-forming ascomycetes with asbestos fibres. Mycological Research 111, 473-481.
| Crossref | Google Scholar | PubMed |
Favero-Longo SE, Castelli D, Fubini B, Piervittori R (2009a) Lichens on asbestos–cement roofs: bioweathering and biocovering effects. Journal of Hazardous Materials 162, 1300-1308.
| Crossref | Google Scholar | PubMed |
Favero-Longo SE, Matteucci E, Siniscalco C (2009b) Plant colonization limits dispersion in the air of asbestos fibers in an abandoned asbestos mine. Northeastern Naturalist 16, 163-177.
| Crossref | Google Scholar |
Favero-Longo SE, Turci F, Fubini B, Castelli D, Piervittori R (2013) Lichen deterioration of asbestos and asbestiform minerals of serpentinite rocks in Western Alps. International Biodeterioration & Biodegradation 84, 342-350.
| Crossref | Google Scholar |
Freemantle GG, Chetty D, Olifant M, Masikhwa S (2022) Assessment of asbestos contamination in soils at rehabilitated and abandoned mine sites, Limpopo Province, South Africa. Journal of Hazardous Materials 429, 127588.
| Crossref | Google Scholar |
Gadd GM, Bahri-Esfahani J, Li Q, Rhee YJ, Wei Z, Fomina M, Liang X (2014) Oxalate production by fungi: significance in geomycology, biodeterioration and bioremediation. Fungal Biology Reviews 28, 36-55.
| Crossref | Google Scholar |
Gilbert OL (1970) Further studies on the effect of sulphur dioxide on lichens and bryophytes. New Phytologist 69, 605-627.
| Crossref | Google Scholar |
Godish D (1989) Asbestos exposure in schools. Journal of School Health 59, 362-363.
| Crossref | Google Scholar | PubMed |
Gonneau C, Miller K, Mohanty SK, Xu R, Hwang W-T, Willenbring JK, Casper BB (2017) Framework for assessment and phytoremediation of asbestos-contaminated sites. Environmental Science and Pollution Research International 24, 25912-25922.
| Crossref | Google Scholar | PubMed |
Gopishankar T, Baraiya Divyeksha H, Vasantha VL, Praveen N (2022) Bioremediation and detoxification of asbestos from soil. In ‘Advances in bioremediation and phytoremediation for sustainable soil management’. (Ed. JA Malik) pp. 211–228. (Springer) 10.1007/978-3-030-89984-4_14
Gray C, Carey RN, Reid A (2016) Current and future risks of asbestos exposure in the Australian community. International Journal of Occupational and Environmental Health 22, 292-299.
| Crossref | Google Scholar | PubMed |
Haley P, Pizzolitto AC (2016) Asbestos in Eastern Townships, Quebec, Canada: a 30-year mining legacy. Environmental Geochemistry and Health 38, 791-808.
| Google Scholar |
Hider RC, Kong X (2010) Chemistry and biology of siderophores. Natural Product Reports 27, 637-657.
| Crossref | Google Scholar | PubMed |
Hoffland E, Kuyper TW, Wallander H, Plassard C, Gorbushina AA, Haselwandter K, Holmstrom S, Landeweert R, Lundstrom US, Rosling A, Sen R, Smits MM, van Hees PAW, van Breemen N (2004) The role of fungi in weathering. Frontiers in Ecology and the Environment 2, 258-264.
| Crossref | Google Scholar |
Holmes EP, Lavkulich LM (2014) The effects of naturally occurring acids on the surface properties of chrysotile asbestos. Journal of Environmental Science and Health, Part A 49, 1445-1452.
| Crossref | Google Scholar |
Hou D, Al-Tabbaa A, O’Connor D, Hu Q, Zhu Y-G, Wang L, Kirkwood N, Ok YS, Tsang DCW, Bolan NS, Rinklebe J (2023) Sustainable remediation and redevelopment of brownfield sites. Nature Reviews Earth & Environment 4, 271-286.
| Crossref | Google Scholar |
Jöns N, Bach W (2014) Serpentinization. In ‘Encyclopedia of marine geosciences’. (Eds J Harff, M Meschede, S Petersen, J Thiede) pp. 1–12. (Springer: Dordrecht) 10.1007/978-94-007-6644-0_119-1
Kazan-Allen L (2022) Chronology of national asbestos bans. International Ban Asbestos Secretariat. Available at http://www.ibasecretariat.org/chron_ban_list [accessed 23 August 2024]
Kempton L, McCarthy T (2017) A review of asbestos stabilisation and containment practices final report 18th May 2017. Sustainable Buildings Research Centre, Commonwealth of Australia and University of Wollongong. Available at https://tinyurl.com/y3jzr57w [accessed 23 August 2024]
Khlifa R, Rivest D, Grimond L, Bélanger N (2023) Stability of carbon pools and fluxes of a Technosol along a 7-year reclamation chronosequence at an asbestos mine in Canada. Ecological Engineering 186, 106839.
| Crossref | Google Scholar |
King D (2024) History of asbestos around the globe. Available at https://www.asbestos.com/asbestos/history/ [accessed 23 August 2024]
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annual Review of Plant Biology 63, 131-152.
| Crossref | Google Scholar | PubMed |
Kumar A, Maiti SK (2015) Effect of organic manures on the growth of Cymbopogon citrates and Chrysopogonzizanioides for the phytoremediation of chromite-asbestos mine waste: a pot scale experiment. International Journal of Phytoremediation 17, 437-447.
| Crossref | Google Scholar | PubMed |
Kumar A, Maiti SK, Tripti , Prasad MNV, Singh RS (2017) Grasses and legumes facilitate phytoremediation of metalliferous soils in the vicinity of an abandoned chromite–asbestos mine. Journal of Soils and Sediments 17, 1358-1368.
| Crossref | Google Scholar |
Kumari S, Maiti SK (2022) Nitrogen recovery in reclaimed mine soil under different amendment practices in tandem with legume and non-legume revegetation: a review. Soil Use and Management 38, 1113-1145.
| Crossref | Google Scholar |
Kusiorowski R, Zaremba T, Piotrowski J, Adamek J (2012) Thermal decomposition of different types of asbestos. Journal of Thermal Analysis and Calorimetry 109, 693-704.
| Crossref | Google Scholar |
Kwak K, Cho S-I, Paek D (2021) Future incidence of malignant mesothelioma in South Korea: updated projection to 2038. International Journal of Environmental Research and Public Health 18, 6614.
| Crossref | Google Scholar |
LaDou J (2004) The asbestos cancer epidemic. Environmental Health Perspectives 112, 285-290.
| Crossref | Google Scholar | PubMed |
Landrigan PJ, Kazemi H (1991) The third wave of asbestos disease: exposure to asbestos in place: public health control. New York Academy of Science. Available at https://nyaspubs.onlinelibrary.wiley.com/toc/17496632/1991/643/1
Leong SL, Zainudin R, Kazan-Allen L, Robinson BW (2015) Asbestos in Asia. Respirology 20, 548-555.
| Crossref | Google Scholar | PubMed |
Liermann LJ, Barnes AS, Kalinowski BE, Zhou X, Brantley SL (2000) Microenvironments of pH in biofilms grown on dissolving silicate surfaces. Chemical Geology 171, 1-16.
| Crossref | Google Scholar |
Lund LG, Aust AE (1990) Iron mobilization from asbestos by chelators and ascorbic acid. Archives of Biochemistry and Biophysics 278, 60-64.
| Crossref | Google Scholar |
Łuniewski S, Rogowska W, Łozowicka B, Iwaniuk P (2024) Plants, microorganisms and their metabolites in supporting asbestos detoxification – a biological perspective in asbestos treatment. Materials 17, 1644.
| Crossref | Google Scholar |
Magnani C, Mensi C, Binazzi A, Marsili D, Grosso F, Ramos-Bonilla JP, Ferrante D, Migliore E, Mirabelli D, Terracini B, Consonni D, Degiovanni D, Lia M, Cely-García MF, Giraldo M, Lysaniuk B, Comba P, Marinaccio A (2023) The Italian experience in the development of mesothelioma registries: a pathway for other countries to address the negative legacy of asbestos. International Journal of Environmental Research and Public Health 20, 936.
| Crossref | Google Scholar |
Martino E, Prandi L, Fenoglio I, Bonfante P, Perotto S, Fubini B (2003) Soil fungal hyphae bind and attack asbestos fibers. Angewandte Chemie International Edition 42, 219-222.
| Crossref | Google Scholar | PubMed |
Martino E, Cerminara ST, Prandi L, Fubini B, Perotto S (2004) Physical and biochemical interactions of soil fungi with asbestos fibers. Environmental Toxicology and Chemistry 23, 938-944.
| Crossref | Google Scholar | PubMed |
Meyer DR (1980) Nutritional problems associated with the establishment of vegetation on tailings from an asbestos mine. Environmental Pollution Series A, Ecological and Biological 23, 287-298.
| Crossref | Google Scholar |
Militello GM, Gaggero L, La Maestra S (2021) Asbestiform amphiboles and cleavage fragments analogues: overview of critical dimensions, aspect ratios, exposure and health effects. Minerals 11, 525.
| Crossref | Google Scholar |
Mohanty SK, Gonneau C, Salamatipour A, Pietrofesa RA, Casper B, Christofidou-Solomidou M, Willenbring JK (2018) Siderophore-mediated iron removal from chrysotile: implications for asbestos toxicity reduction and bioremediation. Journal of Hazardous Materials 341, 290-296.
| Crossref | Google Scholar | PubMed |
Moore TR, Zimmermann RC (1977) Establishment of vegetation on serpentine asbestos mine wastes, Southeastern Quebec, Canada. The Journal of Applied Ecology 14, 589-599.
| Crossref | Google Scholar |
Morgenthal T, Maboeta M, van Rensburg L, Bredenkamp GJ (2004) Revegetation of heavy metal contaminated mine dumps using locally serpentine-adapted grassland species. South African Journal of Botany 70, 784-789.
| Crossref | Google Scholar |
Morrissey J, Guerinot ML (2009) Iron uptake and transport in plants: the good, the bad, and the ionome. Chemical Reviews 109, 4553-4567.
| Crossref | Google Scholar | PubMed |
Nayak L (2016) The mineral fibre: asbestos-its manufacture, properties, toxic effects and substitutes. Nature Environment and Pollution Technology 15, 477-482.
| Google Scholar |
Obmiński A (2021) Asbestos waste recycling using the microwave technique – benefits and risks. Environmental Nanotechnology, Monitoring & Management 16, 100577.
| Crossref | Google Scholar |
Paolini V, Tomassetti L, Segreto M, Borin D, Liotta F, Torre M, Petracchini F (2019) Asbestos treatment technologies. Journal of Material Cycles and Waste Management 21, 205-226.
| Crossref | Google Scholar |
Pira E, Donato F, Maida L, Discalzi G (2018) Exposure to asbestos: past, present and future. Journal of Thoracic Disease 10, S237-S245.
| Crossref | Google Scholar | PubMed |
Pott F, Huth F, Friedrichs KH (1972) Tumors of rats after i.p. injection of powdered chrysotile and benzo(a)pyrene. Zentralbl Bakteriol Orig B 155, 463-469.
| Google Scholar | PubMed |
Promentilla MAB, Peralta GL (2003) An evaluation of landfill disposal of asbestos-containing waste and geothermal residues within a risk-assessment framework. Journal of Material Cycles and Waste Management 5, 13-21.
| Crossref | Google Scholar |
Radetzki M (2010) Peak oil and other threatening peaks – chimeras without substance. Energy Policy 38, 6566-6569.
| Crossref | Google Scholar |
Ramazzini C (2010) Asbestos is still with us: repeat call for a universal ban. Odontology 98, 97-101.
| Crossref | Google Scholar | PubMed |
Ramteke P, Gabhane V, Kadu P, Kharche V, Jadhao S, Turkhede A, Gajjala RC (2024) Long-term nutrient management effects on organic carbon fractions and carbon sequestration in Typic Haplusterts soils of Central India. Soil Use and Management 40, e12950.
| Crossref | Google Scholar |
Schapira JS, Bolhar R, Master S, Wilson AH (2023) Mineralogical, petrological and geochemical characterisation of chrysotile, amosite and crocidolite asbestos mine waste from Southern Africa in context of risk assessment and rehabilitation. Minerals 13, 1352.
| Crossref | Google Scholar |
Slavin G (2006) Mineral dusts are dangerous: asbestos and disease. Nercian Geologist 16, 173-184.
| Google Scholar |
Spasiano D, Luongo V, Petrella A, Alfè M, Pirozzi F, Fratino U, Piccinni AF (2017) Preliminary study on the adoption of dark fermentation as pretreatment for a sustainable hydrothermal denaturation of cement-asbestos composites. Journal of Cleaner Production 166, 172-180.
| Crossref | Google Scholar |
Spribille T, Tuovinen V, Resl P, Vanderpool D, Wolinski H, Aime MC, Schneider K, Stabentheiner E, Toome-Heller M, Thor G, Mayrhofer H, Johannesson H, McCutcheon JP (2016) Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 353, 488-492.
| Crossref | Google Scholar | PubMed |
Spribille T, Resl P, Stanton DE, Tagirdzhanova G (2022) Evolutionary biology of lichen symbioses. New Phytologist 234, 1566-1582.
| Crossref | Google Scholar | PubMed |
Thives LP, Ghisi E, Thives Júnior JJ, Vieira AS (2022) Is asbestos still a problem in the world? A current review. Journal of Environmental Management 319, 115716.
| Crossref | Google Scholar |
Turci F, Favero-Longo SE, Tomatis M, Martra G, Castelli D, Piervittori R, Fubini B (2007) A biomimetic approach to the chemical inactivation of chrysotile fibres by lichen metabolites. Chemistry – A European Journal 13, 4081-4093.
| Crossref | Google Scholar | PubMed |
Uroz S, Calvaruso C, Turpault M-P, Frey-Klett P (2009) Mineral weathering by bacteria: ecology, actors and mechanisms. Trends in Microbiology 17, 378-387.
| Crossref | Google Scholar | PubMed |
USGS (2013) Mineral resource program – asbestos production by country. Available at http://www.indexmundi.com/minerals/?product=asbestos [accessed 27 April 2023]
Valenzuela M, Giraldo M, Gallo-Murcia S, Pineda J, Santos L, Ramos-Bonilla JP (2016) Recent scientific evidence regarding asbestos use and health consequences of asbestos exposure. Current Environmental Health Reports 3, 335-347.
| Crossref | Google Scholar | PubMed |
Völker C, Wolf-Gladrow DA (1999) Physical limits on iron uptake mediated by siderophores or surface reductases. Marine Chemistry 65, 227-244.
| Crossref | Google Scholar |
Wallis SL, Emmett EA, Hardy R, Casper BB, Blanchon DJ, Testa JR, Menges CW, Gonneau C, Jerolmack DJ, Seiphoori A, Steinhorn G, Berry T-A (2020) Challenging global waste management - bioremediation to detoxify asbestos. Frontiers in Environmental Science 8, 20.
| Crossref | Google Scholar |
Wang D, Cullimore DR (2010) Bacteriological challenges to asbestos cement water distribution pipelines. Journal of Environmental Sciences 22, 1203-1208.
| Crossref | Google Scholar |
Wang L, Hou D (2023) Plant-based strategies for the sustainable management of soil contamination. In ‘Soil constraints product’. (Eds N Bolan, MB Kirkham) pp. 25–44. (CRC Press) 10.1201/9781003093565-2
Wang L, Hou D, Shen Z, Zhu J, Jia X, Ok YS, Tack FMG, Rinklebe J (2020) Field trials of phytomining and phytoremediation: a critical review of influencing factors and effects of additives. Critical Reviews in Environmental Science and Technology 50, 2724-2774.
| Crossref | Google Scholar |
Wang L, Rinklebe J, Tack FMG, Hou D (2021) A review of green remediation strategies for heavy metal contaminated soil. Soil Use and Management 37, 936-963.
| Crossref | Google Scholar |
Wang L, Deng J, Yang X, Hou R, Hou D (2023) Role of biochar toward carbon neutrality. Carbon Research 2, 2.
| Crossref | Google Scholar |
Wasserbauer R, Zadák Z, Novotný J (1988) Nitrifying bacteria on the asbestos-cement roofs of stable buildings. International Biodeterioration 24, 153-165.
| Crossref | Google Scholar |
Werner AJ, Hochella MF, Guthrie GD, Hardy JA, Aust AE, Rimstidt JD (1995) Asbestiform riebeckite (crocidolite) dissolution in the presence of Fe chelators; implications for mineral-induced disease. American Mineralogist 80, 1093-1103.
| Crossref | Google Scholar |
Wilson MJ, Jones D, McHardy WJ (1981) The weathering of serpentinite by Lecanora Atra. The Lichenologist 13, 167-176.
| Crossref | Google Scholar |
Wong L, Patyshakuliyeva A, Rodenburg U, Korthals GW, Pover J, Koerten H, Kuramae E, Bodelier PLE (2023) Significant expansion of bacterial and fungal strains to remove iron and magnesium from asbestos. Available at https://dx.doi.org/10.2139/ssrn.4333171
Young W (2016) Imports and exports (Asbestos-containing products) prohibition order 2016. New Zealand Government, Wellington 26. Available at https://www.legislation.govt.nz/regulation/public/2016/0218/10.0/whole.html
Zhao S, Qin L, Wang L, Sun X, Yu L, Wang M, Chen S (2022) Soil bacterial community responses to cadmium and lead stabilization during ecological restoration of an abandoned mine. Soil Use and Management 38, 1459-1469.
| Crossref | Google Scholar |