Liming effect on soil chemical and biological properties, pests and diseases, and crop yields in robusta coffee and black pepper in Vietnam
Long Nguyen Van A B C , Laetitia Herrmann A C , Thao Le Dinh D , Chung Nguyen Van D , Liem Nguyen Van D , Aydin Enez A E , Lambert Brau A E and Didier Lesueur
D , Liem Nguyen Van D , Aydin Enez A E , Lambert Brau A E and Didier Lesueur  A C F G H *
A C F G H *
A
B
C
D
E
F
G
H
Abstract
Vietnam is the global leading producer of robusta coffee and black pepper. However, expanding coffee and pepper cultivation and intensive farming practices have led to soil acidification and increased pest and pathogen pressures. Agricultural liming applications could sustainably alleviate acidification, modify soil physicochemical parameters, restore microbial ecosystems, and suppress soil pathogens.
To address this issue, field trials were conducted in Gia Lai province in acidic soil within coffee and pepper plantations.
Two treatments were applied: 2.5 t ha−1 of dolomite lime and a no-lime control. The trials assessed soil chemical and biological properties, soilborne pests and diseases, and crop yield.
The results indicated no significant yield differences between the lime-treated and control crops. However, application of lime effectively raised soil pH by around 0.5 units for coffee and 0.4 units for pepper, compared to the free-lime treatment. In contrast, soil pH in the control plots decreased by 0.3 units (6.8%) for coffee and 0.2 units (3.8%) for pepper plantations compared to the pre-application values. In coffee plantations, lime application led to significant enhancements in organic matter and exchangeable K+, Ca2+, and Mg2+ by 17.4%, 26.1%, 103.6%, and 243.7%, respectively. It also decreased exchangeable Fe3+ and Al3+ by 9.7% and 30.3%, respectively, compared to the control. Additionally, lime application significantly improved root mycorrhization by arbuscular mycorrhizal fungi. In pepper farms, liming considerably improved available NH4+ and NO3− and exchangeable Ca2+ and Mg2+ by 7.5%, 9.8%, 35.1%, and 132.8%, respectively. Exchangeable Fe3+ and Al3+ decreased by 29.8% and 29.0%, respectively. However, for both commodities, no positive effects of liming were observed for populations of pathogenic fungi, oomycetes, and nematodes.
Lime had positive effects on soil chemical properties and colonisation by arbuscular mycorrhizal fungi but did not significantly affect soilborne pathogens and crop yield.
Sustainable soil acidity mitigation and improvement of soil fertility could be undertaken by annual lime application. Alternative practices, including biological and ecological approaches, should be explored in conjunction with the use of lime.
Keywords: acidification, black pepper, crop yields, lime application, robusta coffee, soilborne pathogens, soil properties, sustainable practices.
Introduction
Robusta coffee (Coffea canephora var. robusta) and black pepper (Piper nigrum L.) play a vital role in enhancing the socio-economic development in Vietnam, particularly in the Central Highlands (CH). Vietnam is the world’s largest exporter of robusta coffee beans and peppercorns, attributable to extensive cultivation and improved farming practices including irrigation, fertilisation, weed and disease management over the past few decades (Hong 2017). Vietnam cultivates 650,000 ha of robusta coffee and 120,000 ha of black pepper, accounting for around 53% and 58% of the global supply, respectively (ICO 2022; IPC 2022). Approximately 91% of the coffee and 61% of the pepper are grown in slightly acidic soils (Arcisols and Rhodic Ferralsols) in the CH region and are mainly cultivated by smallholder farmers (Dung et al. 2019; DCP 2023; Rigal et al. 2023).
Excessive use of nitrogen (N) fertilisers is common to maintain high yields but has been identified as the primary cause of soil acidification (Zhou et al. 2014; Hao et al. 2020; Zhang et al. 2022). Soil acidification is caused mainly by accumulation of H+ in soil (Barak et al. 1997). Excessive N application promotes soil nitrification and hydrolysis, which releases H+ into the soil (Cai et al. 2015; Zhao et al. 2020). Reduction in soil pH is a major concern in agricultural production (Zamanian et al. 2018; Hao et al. 2020), and the consequences of soil acidification include an increase in toxic heavy metals (Al and Mn) and a decrease in the availability of soil nutrients. This leads to adverse changes in soil physiochemical and biological properties and disrupts the balance of soil microorganisms. Consequently, these cumulative negative effects lead to the gradual accumulation of soilborne pests and diseases (SBPDs), causing damage to plant and soil productivity (Hao et al. 2020; Zhang et al. 2023; Deng et al. 2024). Several authors have described a relationship of nematodes, fungi, and bacterial abundance with soil acidification. Furthermore, acidification reduces beneficial microbiomes and weakens plant resistance to disease (Mulder et al. 2005; Fan et al. 2018; Zhang et al. 2022). In contrast, acidic soils stimulate the growth and reproduction of harmful nematodes (Wang et al. 2009; Rahayu and Sari 2017; Nisa et al. 2021).
Acidic soils with low nutrient availability harm bean yield in coffee-producing countries such as Ethiopia, Colombia, and Brazil (Cyamweshi et al. 2014; Dibaba et al. 2020; Parecido et al. 2021). Profitable yields depend on soil pH, supply of Ca and Mg, and neutralisation of Al3+ by lime addition (Chaves et al. 1984; Parecido et al. 2021). In Vietnam, the average optimal soil pH value for coffee and pepper growth in the Acrisol and Rodic Ferralsol soils was around 5.0 (4.5–5.5) and 5.5 (5.0–6.0), respectively (MARD 2015; Bo et al. 2017; Dung et al. 2019). However, the soils of most coffee and pepper farms are strongly acidic, with averages of approximately pH 4.4 and 4.8, respectively (Dung et al. 2019) and acidification significantly affects soil health properties in these farms, with increased Al and Fe bioavailability by 41% and 27% (Ha 2016), and decreased Ca and Mg by 78–93% and 83–95%, respectively (Dung et al. 2019). Acidic soils also harbour harmful pathogens such as Meloidogyne root-knot and Pratylenchus root-rot nematodes, Fusarium, and oomycetes, posing a serious threat to plant growth and productivity (Khoa et al. 2014; Huyen et al. 2018; Dung et al. 2019). These pathogens are responsible for significant losses in coffee and pepper cultivation (Khoa et al. 2014; Dung et al. 2019; PPD 2019). To keep yields as high as possible, smallholders tend to use excessive chemical inputs to manage these diseases despite the significant environmental impacts (Nysanth et al. 2022). Some agronomists suggested that increasing soil pH could help reduce the populations of SBPDs that affect both coffee and pepper plantations and make production more sustainable in Vietnam.
Traditionally, agricultural liming materials are universally used in agricultural production to alleviate acidification, modify soil physicochemical parameters, and restore microbial ecosystems, subsequently improving soil and plant health as well as suppressing SBPDs (Shen et al. 2018; Bolan et al. 2023; Deng et al. 2024). Lime neutralises and displaces H+ and adds base cations such as Ca2+ and Mg2+ to the soil solution, subsequently improving soil pH and fertility (Mahmud and Chong 2022). The addition of lime via abiotic and biotic processes also enhances the mobility and availability of plant nutrients and the immobilisation of toxic heavy metals in soil (Li et al. 2019a; Silva et al. 2019). Furthermore, liming regulates soil chemical parameters, which directly decreases growth of SBPDs by modifying the soil microbiome, enriching the population of beneficial microorganisms, and inhibiting pathogen growth (Takamoto et al. 2023; Deng et al. 2024). However, some previous studies reported that increased soil pH by lime application did not significantly influence the population of pathogens (Pennanen et al. 1998; Pawlett et al. 2009; Yin et al. 2021).
The impact of lime treatment can vary widely according to factors such as lime doses, soil properties, agricultural practices, and environmental conditions (Bolan et al. 2003; Pagani and Mallarino 2015; Li et al. 2019a). Recommendations for suitable lime application rates in field conditions for growing coffee and pepper range within 0.5–4.0 t ha−1 year−1 (Corrêa et al. 2008; MARD 2015; Parecido et al. 2021). For instance, due to the same range of soil pH, soil types, and climate conditions in Vietnam, the Ministry of Agriculture and Rural Development of Vietnam recommends the annual application of 0.5–1.0 t lime ha−1 year−1 for intensive coffee and pepper cultivation (MARD 2015). However, these rates could be insufficient because soil pH continuously decreases over time (Dung et al. 2019). Another study revealed that the optimal lime application rate for the highest coffee yield is 2.5 t ha−1 (Chaves et al. 1984). Additionally, based on investigation on pepper plantation soils in CH, 2.5–5.0 t lime applied annually was advised to sustainably improve soil pH and soil properties, and it requires application of 25 t lime ha−1 to raise one unit of soil pH (unpublished work). Excessive lime application can negatively affect soil pH, nutrient balance, and cost-effectiveness and result in Ca toxicity (Wang et al. 2010; Hamilton et al. 2012; Rhodes et al. 2018; Yan et al. 2021; Norberg and Aronsson 2022). Therefore, it is crucial to carefully define the appropriate lime addition rate to ensure sufficient nutrient and income balances for crops.
Similarly, the diverse initial soil characteristics and levels of soil acidification in coffee and pepper cultivation can lead to varying effects of lime application. Additionally, there is limited knowledge about the ability of lime to mitigate soil acidification and suppress SBPDs in acidic and intensive coffee and pepper farms in Vietnam. This is an important consideration for sustainable coffee and pepper production. Thus, our objective is to assess the effects of lime (2.5 t ha−1) on soil chemical and biological properties, SBPDs, and crop yields in different acidic robusta coffee and black pepper farming systems to determine the best strategy to produce both crops sustainably.
Materials and methods
Study site description
The study involved experiments on 10 productive robusta coffee (TRS1 variety) and 10 productive black pepper plantations (Vinh Linh variety) in the Dak Doa district, Gia Lai province, Vietnam (Fig. 1). The target district is the capital of cultivated coffee and pepper areas in Gia Lai, with a total production area of 28,742 and 2000 ha, respectively (GSO 2022). Monoculture and conventional practices were used. The average mineral fertiliser application was 343 kg N, 184 kg P, and 213 kg K ha−1 year−1 for robusta coffee; and 193 kg N, 95 kg P, and 441 kg K ha−1 year−1 for black pepper plantations. The density of coffee and pepper was 1100 and 1600 trees ha−1, respectively. The farms are flat to slightly sloped (3–5°), with an elevation of 600–700 m above sea level.
Location of Gia Lai province in Vietnam, and the farm sites in Dak Doa district, Gia Lai. BP.01, BP.02 … BP.10 – black pepper farms; CF.01, CF.02 … CF.10 – robusta coffee farms.

The coffee and pepper crops were around 8–10 years old and were grown in Rodic Ferralsol soil. The initial soil pH, organic matter (OM) content, total N (%N), and phosphorus (P) availability of the experimental farms varied. The average soil pH was 4.7 (range 4.4–4.9) in coffee farms and 5.2 (range 4.7–5.7) in pepper farms. Total N content was 0.19% (range 0.16–0.24%) and 0.22% (range 0.21–0.25%) in coffee and pepper plantations, respectively. Additionally, the OM and P were 4.5% (range 2.24–6.5%) and 224 mg kg−1 (range 57–436 mg kg−1) in coffee plantations and 5.5% (range 4.24–6.5%) and 144 mg kg−1 (range 77–290 mg kg−1) in pepper plantations, respectively.
The research area experiences two seasons: rainy season (May–November) and dry season (December–April). The annual average rainfall is 1900–2700 mm, and temperature averages about 23°C (GSO 2021, 2022).
Experimental design
Ten productive robusta coffee and 10 black pepper plantations were used for experiments separately over 15 and 18 months, respectively. Each farm was considered as a replicate and consisted of two treatments (plots) representing limed treatment (2.5 t lime ha−1) and control (no lime).
Each plot had an area of 500 m2 comprising 60 coffee plants (10 trees in each row × 6 rows) or 80 pepper trees (10 trees in each row × 8 rows). Plots were separated from each other by three untreated plant rows.
Commercial dolomitic lime [CaMg(CO3)2] with CaO content exceeding 90% was uniformly broadcast over the soil surface during the rainy season, without incorporation into the topsoil.
Soil and root sampling and analyses
For each farm (replicate), five plants (samples) per treatment were randomly selected and sampled, with chosen trees being at the centre of the treatment and equidistant from each other, avoiding the borders. For the target tree, the soil was collected in four positions, corresponding to four directions at the top layer (0–30 cm depth) where most of the roots of coffee and pepper are, and 0.8–1 m away from the tree foot (MOST 2007), using a trowel to dig a hole with standard dimensions (20 cm × 20 cm × 30 cm). The soil was homogenised and split into two subsamples (0.5 kg sample−1) for analysis of soil properties and soil pathogens. At the same time, roots of coffee and pepper plants were also collected and used for nematode extraction and mycorrhiza colonisation detection.
Soil property assessment
Soil properties analysed included soil pH (H2O) (1:2.5 soil:water suspension), OM content using the Walkley and Black (1934) method, bio-available N (NH4+ and NO3−) using the Kjeldahl (1883) method, P (Olsen and Sommers 1982), and potassium (K) using the Chapman (1965) method. Exchangeable cations including Ca2+, Mg2+, Al3+, and Fe3+ were measured using various methods (Sokolov 1939; Chapman 1965; Hartwig and Loeppert 1993).
The densities of Fusarium spp. and Rhizoctonia spp. in the soil were measured using the soil dilution plate technique described by Burgess et al. (2008). Assessment of zoospore density of Phytophthora in soil samples was modified from the method of Masago et al. (1976). The population of plant parasitic nematodes (Meloidogyne and Pratylenchus spp.) in the soil was sampled using a modified tray method from the Baermann funnel method (Hooper 1986, 1990). To extract nematodes from roots, a 5 g aliquot was selected and processed for analysis as described in Hooper (1986, 1990).
The roots of coffee and pepper plants were dried at room temperature to check for the presence and intensity of AMF colonisation. The roots were stained using the ink and vinegar technique (Vierheilig et al. 1998) and bleached following the method of Koske and Gemma (1989). Fifteen random root fragments (1 cm each) per sample were observed under a microscope (×40 magnification) and scored on a 0–5 scale to assess the presence and intensity of AMF colonisation in each fragment. This allowed for determining the frequency (F%) and intensity (M%) of AMF colonisation (Trouvelot 1986).
Coffee and pepper yield measurement
The harvest season concluded at 15 months for coffee and 18 months for pepper after applying lime. The cherry yield of coffee and pepper (t ha−1) was determined by weighing all freshly harvested cherries from five selected trees, which were previously sampled for soil characteristics and SBPDs. Cherries from five coffee or pepper trees of treatments were pooled into a final sample (20 kg) and were then sent to the Pepper Research and Development Center (Pleiku, Vietnam) for processing to calculate the ratio of fresh cherry to green coffee beans and peppercorns. The ratio (fresh cherry/bean or peppercorns) = fresh cherry coffee or pepper/green coffee bean or peppercorn. The yield of coffee bean or peppercorns (t ha−1) = total cherry yield of coffee or pepper (t ha−1)/the ratio. The yield (t ha−1) was estimated for 1100 robusta trees and 1600 black pepper trees per hectare.
Statistical analysis
All data were analysed using Microsoft® Excel and XLSTAT software ver. 2023.1.5 (Lumivero 2023). The Mann–Whitney U test (non-parametric statistic) was used to estimate the distribution of parameters and statistically test the two independent treatments. Correlation coefficients of the independent variables were determined by Pearson test at P < 0.05.
Results
Soil physicochemical parameters
After 12 months of applying lime to the soil, positive effects on soil parameters were measured. The highest pH values in the lime-treated farms were 5.2 for coffee and 5.8 for pepper, while the control farms had corresponding pH values of 4.7 and 5.5 (Supplementary Tables S1 and S2). Overall, applying lime increased the average pH by 4.3% for coffee (from 4.7 to 4.9) and by 3.8% for pepper (from 5.2 to 5.4) compared to the pre-application values. Additionally, compared with the control, soil pH in the lime plots was significantly higher, approximately 0.5 units (11.4%) for coffee and 0.4 units (8%) for pepper (Table 1). On the other hand, the average pH value in the control farms (without lime addition) decreased by 0.3 units (6.8%) from 4.7 to 4.4 for coffee and by 0.2 units (3.8%) from 5.2 to 5.0 for pepper farms.
| Crops | Treatment | Statistics | pHH2O | OM (%) | NH4+ | NO3− | P | K | Ca2+ | Mg2+ | Fe3+ | Al3+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/kg) | |||||||||||||
| Robusta coffee | Lime application | Mean | 4.9a | 5.4a | 34.3a | 16.7a | 251a | 140a | 501a | 57.4a | 380b | 85b | |
| Standard error of mean | 0.03 | 0.22 | 0.62 | 0.42 | 25.68 | 10.12 | 41.18 | 9.25 | 20.02 | 6.10 | |||
| Minimum | 4.6 | 2.7 | 30.7 | 13.3 | 42.6 | 54.0 | 212.6 | 4.4 | 88.9 | 24.6 | |||
| Maximum | 5.2 | 7.2 | 40.3 | 21.9 | 608.5 | 246.7 | 661.4 | 118.1 | 500.2 | 135.9 | |||
| Control (no-lime) | Mean | 4.4b | 4.6b | 34.0a | 16.2a | 215a | 111b | 246b | 16.7b | 421a | 122a | ||
| Standard error of mean | 0.05 | 0.17 | 0.70 | 0.41 | 19.97 | 7.89 | 16.60 | 2.25 | 20.19 | 6.78 | |||
| Minimum | 4.2 | 2.4 | 28.3 | 13.3 | 40.6 | 34.4 | 112.0 | 2.2 | 98.4 | 62.8 | |||
| Maximum | 4.7 | 6.5 | 39.6 | 20.3 | 455.8 | 178.7 | 426.7 | 35.3 | 526.4 | 187.8 | |||
| P-value | <0.001 | 0.006 | 0.678 | 0.329 | 0.345 | 0.050 | <0.001 | <0.001 | 0.023 | <0.001 | |||
| Black pepper | Lime application | Mean | 5.4a | 6.0a | 34.4a | 22.5a | 189a | 230a | 866a | 149a | 391b | 37.4b | |
| Standard error of mean | 0.04 | 0.12 | 0.77 | 0.76 | 15.42 | 15.86 | 75.85 | 22.53 | 24.96 | 3.56 | |||
| Minimum | 5.1 | 5.1 | 27.6 | 17.2 | 94.0 | 108.7 | 345.5 | 47.7 | 255.7 | 14.8 | |||
| Maximum | 5.8 | 7.0 | 39.9 | 29.6 | 418.3 | 446.0 | 1952.6 | 403.8 | 709.3 | 73.4 | |||
| Control (no-lime) | Mean | 5.0b | 5.7a | 32.0b | 20.5b | 151a | 195a | 641b | 64b | 557a | 52.7a | ||
| Standard error of mean | 0.06 | 0.09 | 0.72 | 0.81 | 9.01 | 12.76 | 65.60 | 10.50 | 25.14 | 3.62 | |||
| Minimum | 4.8 | 5.1 | 26.4 | 15.2 | 88.9 | 100.9 | 220.9 | 18.7 | 359.5 | 27.2 | |||
| Maximum | 5.5 | 6.4 | 40.1 | 29.0 | 224.0 | 383.1 | 1737.4 | 243.5 | 765.3 | 86.9 | |||
| P-value | <0.001 | 0.069 | 0.024 | 0.035 | 0.092 | 0.163 | 0.014 | <0.001 | <0.001 | 0.001 | |||
For each variable, mean values followed by different letters in the column within the crop and treatments are significantly different at α = 0.05 following non-parametric statistical test of the two independent treatments (Mann–Whitney test). The average data were determined from 10 farms (replicates) with a total of 50 samples per treatment for each crop. Commercial dolomitic lime was applied as broadcast uniformly over the soil surface with a dose of 2.5 t ha−1.
In coffee plantations, the OM range was 2.7–7.2% in treated and 2.4–6.5% in control areas (Table S1). The average OM content significantly increased by 17.4% and 20.0% compared to the control (Table 1) and pre-application values, respectively. For pepper farms, the OM content range was 5.1–7.0% in treated and 5.1–6.4% in control areas (Table S2). The average OM content increased by 5.3% and 9.1% compared to the control and pre-application values, respectively. The lime application positively affected bio-available N, P, and exchangeable K in coffee and pepper farms. Notably, significant improvements were observed in availability of NH4+ (7.5%) and NO3− (9.8%) in pepper farms following lime application. The addition of lime also considerably enhanced K in coffee (26.1%) compared to the control (P < 0.05). The highest P availability due to lime treatment was 609 mg kg−1 in coffee (farm 04) and 418 mg kg−1 in pepper (farm 09) (Tables 1, S1, and S2). Furthermore, lime caused a 12.1% increase in P availability for coffee and a 31.3% increase for pepper compared to their initial values.
Soil Ca2+ and Mg2+ values significantly increased with lime application compared to the free lime treatment (Table 1). The average soil Ca2+ content in coffee and pepper treatments increased by 103.6% and 35.1% compared to the control, respectively. Moreover, soil Mg2+ content increased by 243.7% in coffee and 132.8% in pepper. On the other hand, soil Fe3+ and Al3+ respectively decreased by 9.7% and 30.3% in coffee, and by 29.8% and 29.0% in pepper plantations compared to the control.
AMF root colonisation
In the case of AMF colonisation in coffee roots, both F% and M% were significantly enhanced by lime application. The average F% and M% were 92.0% and 23.9%, respectively, in the limed plots, and 79.0% and 18.4% in control plots (Fig. 2 and Table S3). Due to unsuitable root size, we were unable to examine AMF colonisation on pepper roots.
Effect of AMF root colonisation on robusta coffee tree after 12-month lime application. The significant difference between the control and lime treatment was tested by non-parametric Mann–Whitney test of the two independent treatments at α = 0.05. The average data were derived from 10 farms (replicates) with 50 samples per treatment. F%, frequency of arbuscular mycorrhizal fungal colonised on the root; M%, intensity of arbuscular mycorrhizal fungal colonised on the root. Commercial dolomitic lime was broadcast uniformly over the soil surface with a dose of 2.5 t ha−1. *Significant at level alpha = 0.05.

Soilborne pests and diseases
Fusarium spp. were detected in all farms, and Rhizoctonia spp. in over 90% of plantations. The average density of Fusarium spp. in the limed treatment was 9.2 × 102 and 1.1 × 103 colony forming units (cfu) g−1 of soil in coffee and pepper plantations, respectively, compared to 1.1 × 103 and 9.9 × 102 cfu g−1 of soil in the control (Table 2). Of pepper farms, 80% showed the presence of Phytophthora spp., and the number of zoospores was lower in the limed treatment compared to the control. However, the population of pathogenic fungi remained almost the same across treatments and crops, suggesting that lime application might not lead to significant changes in pathogenic soil fungi.
| Crops | Treatment | Fusarium spp. | Rhizoctonia spp. | Phytophthora spp. | Plant parasitic nematodes (Meloidogyne and Pratylenchus spp.) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil | Root | |||||||||||
| F (%) | Mean (cfu g−1 soil) | F (%) | Mean (cfu g−1 soil) | F (%) | Mean (zoospores g−1 soil) | F (%) | Mean (individuals 100 g−1) | F (%) | Mean (individuals 5 g−1) | |||
| Robusa coffee | Lime | 100 | 9.2 × 102a (2.0 × 102−1.7 × 103) | 90 | 2.0 × 102a (0–6.5 × 102) | Not assessed | 70 | 132a (0–1248) | 70 | 143a (0–760) | ||
| Control | 100 | 1.1 × 103a (4.0 × 102−2.3 × 103) | 90 | 2.1 × 102a (0–7.0 × 102) | 72 | 174a (0–2748) | 76 | 161a (0–754) | ||||
| Black pepper | Lime | 100 | 1.1 × 103a (3.0 × 102−2.4 × 103) | 94 | 1.9 × 102a (0–9.0 × 102) | 80 | 2835a (0–134,400) | 86 | 225a (0–1633) | 88 | 205a (0–1314) | |
| Control | 100 | 9.9 × 102a (1.5 × 102−2.0 × 103) | 90 | 2.0 × 102a (0–5.5 × 102) | 80 | 3544a (0–148,300) | 82 | 207a (0–1983) | 88 | 180a (0–2155) | ||
For each variable, mean values followed by the same letters in the column within the crop and treatments are not significantly different at α = 0.05 following non-parametric test of the two independent treatments (Mann–Whitney test). The average data were derived from 10 farms (replicates) with a total of 50 samples per treatment for each crop. Data in brackets show the lowest to highest values. F, frequency of occurrence; cfu, colony forming units. Commercial dolomitic lime was applied as broadcast uniformly over the soil surface with a dose of 2.5 t ha−1.
The percentage of plant parasitic nematode occurrence in the soils and roots of coffee and pepper was 70–76% and 82–88%, respectively (Table 2). The average number of nematodes in the soil and roots of coffee plantations was 132 individuals 100 g−1 of soil and 143 individuals 5 g−1 of root in the lime treatment, slightly lower than the control (174 and 161 individuals, respectively). In contrast, the population of nematodes in the limed treatment of the pepper plantations was higher than in the control, with 225 and 205 individuals in soil and roots, compared to 207 and 180 individuals. Our results did not show any significant difference in nematode densities under coffee and pepper plantations, regardless of crops or treatments. Specific data for treatments in individual farms of each crop are provided in Tables S4 and S5.
Coffee and pepper yield
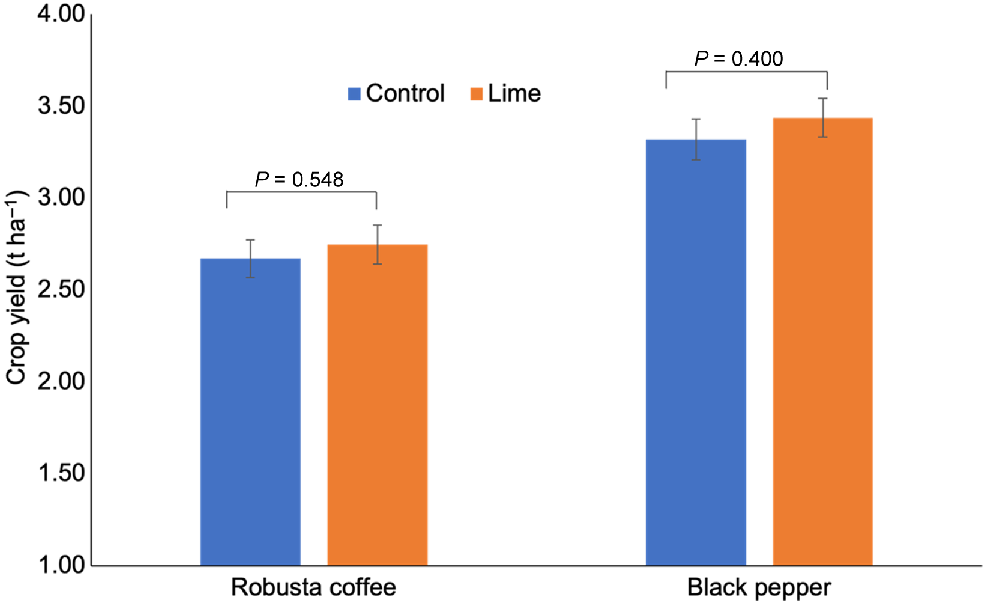
The impact of lime on crop yields is shown in Fig. 3. The average yield of green coffee beans in the treatment with lime was similar to the control without lime, at around 2.7 t ha−1. Similarly, the average yield of dry peppercorn in the control group was 3.3 t ha−1, which was 3.03% lower than for the lime-treated group (3.4 t ha−1). This suggests no significant differences (P > 0.05) in yield between treatments for both crops. The specific yield data for individual farms for both crops are provided in Table S6.
Effect of lime application on robusta coffee bean and peppercorn after 15- and 18-month experiments, respectively. The average yield for each crop was derived from 10 farms (replicates) with 50 samples per treatment. Coffee and pepper were harvested 15 and 18 months after lime application, respectively. The computed P-value is greater than the significant level α = 0.05, suggesting no significant difference (P > 0.05) between treatments according to the non-parametric Mann–Whitney test. Error bars indicate standard error.
Correlation of soil pH with soil parameters and SBPDs
Pearson’s correlations between soil properties of robusta coffee plantations are shown in Table 3. Soil pH was significantly and positively correlated with OM (0.293), NO3 (0.238), P (0.207), K (0.450), Ca (0.419), and Mg (0.278), but negatively correlated with Fe (−0.147), Al (−0.166), Fusarium (−0.239), and nematode density on coffee roots (−0.025). The AMF root colonisation and soil pH were positively but not strongly correlated. However, AMF symbiosis was strongly correlated with K and Ca concentration, with correlation coefficients of 0.205 and 0.406, respectively, and negatively correlated with Al concentration (−0.214). Rhizoctonia fungi were significantly correlated with OM, NO3, P, K, Al, and the nematode population in roots. In addition, nematodes in roots were strongly correlated with P (0.240), Al (0.264), Rhizoctonia (0.225), and nematodes in soil (0.422), but negatively and non-significantly correlated with soil pH, Ca, and AMF colonisation.
| r-values | P-values | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | OM | NH4 | NO3 | P | K | Ca | Mg | Fe | Al | Fusarium | Rhizoctonia | Nematodes (soil) | Nematodes (roots) | AMF (F%) | AMF (M%) | ||
| pH | 0.003 | 0.179 | 0.017 | 0.039 | <0.001 | <0.001 | 0.005 | 0.146 | 0.099 | 0.017 | 0.168 | 0.455 | 0.807 | 0.077 | 0.140 | ||
| OM | 0.293 ** | 0.477 | <0.001 | <0.001 | <0.001 | <0.001 | 0.327 | 0.024 | <0.001 | <0.001 | 0.001 | 0.520 | 0.212 | 0.277 | 0.893 | ||
| NH4 | 0.135 | 0.072 | 0.504 | 0.612 | 0.001 | 0.047 | 0.105 | 0.002 | 0.215 | 0.062 | 0.462 | 0.591 | 0.656 | 0.139 | 0.375 | ||
| NO3 | 0.238 * | 0.348 ** | 0.068 | 0.005 | <0.001 | 0.210 | 0.468 | 0.049 | 0.051 | 0.107 | 0.031 | 0.478 | 0.929 | 0.860 | 0.612 | ||
| P | 0.207 * | 0.738 ** | −0.051 | 0.281 ** | 0.010 | 0.313 | 0.173 | 0.001 | <0.001 | <0.001 | 0.001 | 0.572 | 0.016 | 0.190 | 0.086 | ||
| K | 0.450 ** | 0.409 ** | 0.321 ** | 0.454 ** | 0.256 ** | <0.001 | 0.455 | 0.451 | 0.058 | 0.003 | 0.001 | 0.749 | 0.883 | 0.041 | 0.128 | ||
| Ca | 0.419 ** | 0.419 ** | 0.199 * | 0.127 | 0.102 | 0.404 ** | <0.001 | 0.365 | 0.132 | 0.013 | 0.396 | 0.070 | 0.180 | <0.001 | 0.001 | ||
| Mg | 0.278 ** | 0.099 | −0.163 | −0.073 | −0.137 | 0.076 | 0.403 ** | 0.004 | <0.001 | 0.051 | 0.475 | 0.983 | 0.780 | 0.096 | 0.413 | ||
| Fe | −0.147 | −0.225 * | 0.309 ** | 0.197 * | −0.342 ** | 0.076 | −0.092 | −0.282 ** | 0.091 | 0.597 | 0.053 | 0.172 | 0.028 | 0.187 | 0.344 | ||
| Al | −0.166 | 0.384 ** | 0.125 | 0.196 | 0.494 ** | 0.190 | −0.152 | −0.378 ** | −0.170 | 0.067 | 0.034 | 0.815 | 0.008 | 0.032 | 0.133 | ||
| Fusarium | −0.239 * | −0.425 ** | −0.187 | −0.162 | −0.358 ** | −0.298 ** | −0.249 * | 0.196 | −0.053 | −0.184 | 0.171 | 0.068 | 0.794 | 0.151 | 0.178 | ||
| Rhizoctonia | 0.139 | 0.315 ** | −0.074 | 0.215 * | 0.323 ** | 0.327 ** | 0.086 | 0.072 | −0.194 | 0.212 * | −0.138 | 0.309 | 0.024 | 0.609 | 0.753 | ||
| Nematodes (soil) | 0.076 | −0.065 | −0.054 | −0.072 | −0.057 | −0.032 | −0.182 | 0.002 | −0.138 | −0.024 | 0.184 | 0.103 | 0.000 | 0.910 | 0.331 | ||
| Nematodes (roots) | −0.025 | 0.126 | 0.045 | −0.009 | 0.240 * | 0.015 | −0.135 | −0.028 | −0.219 * | 0.264 ** | 0.026 | 0.225 * | 0.422 ** | 0.157 | 0.103 | ||
| AMF (F%) | 0.178 | 0.110 | 0.149 | 0.018 | −0.132 | 0.205 * | 0.406 ** | 0.167 | 0.133 | −0.214 * | −0.145 | −0.052 | 0.011 | −0.143 | <0.001 | ||
| AMF (M%) | 0.149 | 0.014 | 0.090 | 0.051 | −0.172 | 0.153 | 0.320 ** | 0.083 | 0.096 | −0.151 | −0.136 | −0.032 | −0.098 | −0.164 | 0.681 ** | ||
Significant correlations are shown in italics. P- and r-values are indicated in the upper and lower halves of table, respectively.
*Significantly different from 0 at α = 0.05.
**Significantly different from 0 at α = 0.01.
In pepper plantations, similarly, soil pH was closely correlated with P, K, and Ca, with correlation coefficients of 0.253, 0.249, and 0.340, respectively, and negatively with Fe (−0.249) and Al (−0.226) at α = 0.05. Nutrient elements (NO3, P, K, Ca, and Mg) were significantly negatively correlated with Fe in the soil solution (Table 4). Nematode densities in soil and root were strongly correlated with Fe (0.237 and 0.244, respectively) and Al (0.203 and 0.321) but negatively with OM (−0.268 and −0.320), K (−0.335 and −0.261), and Ca (−0.276 and −0.232). In addition, Fusarium, Rhizoctonia, and Phytophthora in soil were negatively but non-significantly correlated with soil pH (α = 0.05).
| r-values | P-values | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | OM | NH4 | NO3 | P | K | Ca | Mg | Fe | Al | Fusarium | Rhizoctonia | Phythopthora | Nematodes (soil) | Nematodes (roots) | ||
| pH | 0.662 | 0.065 | 0.558 | 0.011 | 0.012 | 0.001 | 0.060 | 0.013 | 0.023 | 0.469 | 0.682 | 0.429 | 0.401 | 0.544 | ||
| OM | 0.044 | 0.470 | 0.440 | 0.859 | 0.017 | 0.106 | <0.001 | 0.684 | 0.698 | 0.307 | 0.068 | 0.176 | 0.007 | 0.001 | ||
| NH4 | 0.186 | 0.073 | 0.129 | 0.010 | 0.001 | 0.076 | 0.070 | 0.802 | 0.695 | 0.605 | 0.774 | 0.203 | 0.462 | 0.134 | ||
| NO3 | 0.059 | −0.078 | 0.153 | 0.001 | 0.013 | 0.002 | 0.138 | <0.001 | 0.681 | 0.014 | 0.364 | 0.933 | 0.050 | 0.171 | ||
| P | 0.253 * | −0.018 | 0.257 ** | 0.323 ** | 0.001 | 0.383 | 0.395 | <0.001 | 0.073 | 0.584 | 0.875 | 0.568 | 0.182 | 0.492 | ||
| K | 0.249 * | 0.238 * | 0.342 ** | 0.247 * | 0.317 ** | <0.001 | <0.001 | <0.001 | 0.704 | 0.321 | 0.231 | 0.001 | 0.001 | 0.009 | ||
| Ca | 0.340 ** | 0.163 | 0.178 | 0.310 ** | 0.088 | 0.662 ** | <0.001 | 0.003 | 0.025 | 0.193 | 0.306 | 0.378 | 0.005 | 0.020 | ||
| Mg | 0.189 | 0.382 ** | 0.182 | 0.149 | 0.086 | 0.614 ** | 0.513 ** | 0.019 | 0.633 | 0.586 | 0.637 | 0.068 | 0.149 | 0.265 | ||
| Fe | −0.249 * | −0.041 | −0.025 | −0.395 ** | −0.353 ** | −0.403 ** | −0.292 ** | −0.235 * | 0.003 | 0.999 | 0.024 | 0.227 | 0.018 | 0.014 | ||
| Al | −0.226 * | −0.039 | −0.040 | −0.042 | 0.180 | 0.038 | −0.224 * | 0.048 | 0.293 ** | 0.194 | 0.371 | 0.964 | 0.043 | 0.001 | ||
| Fusarium | −0.073 | 0.103 | 0.052 | 0.246 * | 0.055 | 0.100 | 0.131 | 0.055 | 0.000 | 0.131 | 0.038 | 0.122 | 0.593 | 0.352 | ||
| Rhizoctonia | −0.041 | 0.183 | −0.029 | −0.092 | −0.016 | −0.121 | −0.103 | 0.048 | 0.226 * | 0.090 | 0.208 * | 0.891 | 0.431 | 0.307 | ||
| Phytophthora | −0.080 | 0.136 | 0.128 | −0.009 | 0.058 | 0.325 ** | 0.089 | 0.183 | −0.122 | 0.005 | −0.156 | 0.014 | 0.446 | 0.660 | ||
| Nematodes (soil) | 0.085 | −0.268 ** | −0.074 | −0.197 * | −0.135 | −0.335 ** | −0.276 ** | −0.145 | 0.237 * | 0.203 * | 0.054 | −0.080 | −0.077 | <0.001 | ||
| Nematodes (root) | 0.061 | −0.320 ** | −0.151 | −0.138 | −0.069 | −0.261 ** | −0.232 * | −0.113 | 0.244 * | 0.321 ** | 0.094 | −0.103 | −0.044 | 0.879 ** | ||
Significant correlations are shown in italics. P- and r-values are indicated in the upper and lower halves of table respectively.
*Significantly different from 0 at α = 0.05.
**Significantly different from 0 at α = 0.01.
Discussion
Coffee and pepper yields
Our findings showed that the treatments had no significant effect on yield of crops, confirming the results of Parecido et al. (2021). These authors discovered no difference in green coffee bean yield between control and treatments with broadcast applied lime at 2.1 and 4.2 t ha−1 over 5-year harvests due to no significant increases in soil pH or lowered micronutrient deficiency in coffee trees. However, another previous study showed a positive effect of liming on yields. Chaves et al. (1984) found that application of 2.5 t ha−1 dolomitic lime in a field experiment showed the best coffee yield, whereas adding 5 and 10 t ha−1 decreased yield. The improved yield from liming resulted from decreasing exchangeable Al and adjusting levels of exchangeable Ca and Mg in the soil (Chaves et al. 1984). Conversely, due to intensive practices, plants remained free of nutrient stress throughout their cycle, and soils were still acidic, which could explain why the yields were similar for treatment and control in the present study, aligning with the statement by Byrareddy et al. (2019). The current lime rate did not significantly raise soil pH, impacting available nutrients for plant uptake to increase productivity. Moreover, the present study assessed crop yields in a short period after lime application (15–18 months), which may be insufficient time for significant changes in soil nutrients, and consequently fewer positive effects on yield.
Importantly, application of 2.5 t lime ha−1 helped maintain pH values at threshold levels, which are preferable for coffee and pepper trees, whereas soil pH decreased without lime addition. The reduction of soil pH in control farms could be caused by the accumulation of H+, which is produced by nitrification and hydrolysis resulting from intensive mineral fertilisers, especially N fertilisers. Low soil pH, in conjunction with limited nutrient availability, can cause a serious decrease in crop growth and productivity (Hamza et al. 2007; Cyamweshi et al. 2014; Zu et al. 2014; Dibaba et al. 2020; Parecido et al. 2021). Therefore, the improvement of soil properties by continuous liming promises to greatly improve plant growth and yield in the following years, contributing to the reduction of fertilisation and sustainable coffee and pepper cultivation.
Soil physicochemical properties
Multiple studies have shown that adding lime to soil can increase its pH, particularly in acidic soils (Chairiyah et al. 2021; Parecido et al. 2021; Teshale et al. 2021; Fitria and Soemarno 2022). Under greenhouse conditions, application of 1.6–4.8 t lime ha−1 increased soil pH by 0.6–1.0 units (Dibaba 2021). Similarly, in field conditions, pH was enhanced by 0.4 and 1.3 units with the application of 2.1 and 4.2 t lime ha−1, respectively (Parecido et al. 2021). Mahmud and Chong (2022) found that application of dolomitic lime neutralises and displaces H+ from the soil solution through the involvement of Ca and Mg with H+ on the exchange complex, resulting in pH increases.
The average optimal soil pH value for coffee and pepper growth in Acrisols and Rodic Ferralsol soils in Vietnam was found to be around 5.0 (range 4.5–5.5) and 5.5 (range 5.0–6.5), respectively (MARD 2015; Bo et al. 2017; Dung et al. 2019). Similarly, in some coffee-producing countries such as Colombia, the soil pH threshold was identified as a range of 4.9–5.7 (Sadeghian and Diaz 2020) and 5.0–5.9 in Brazil (Parecido et al. 2021). The optimal soil pH for pepper growth and development was found within the range of 4.8–6.25, with maximum growth at pH 5.5 (Hamza et al. 2007; Zu et al. 2014). The present study found that liming efficiently raised soil pH, with ranges of 4.6–5.2 for coffee and 5.1–5.8 for pepper, which are suitable for crop growth and development. Furthermore, 50% of coffee and 40% of pepper farms reached the optimum pH values (Tables S1 and S2). Conversely, the pH values of 50% of coffee and 40% of pepper farms in the control were lower than the requirements, suggesting the urgent need for higher liming to raise soil pH significantly.
The study also indicated that increasing pH through lime application enhanced the availability of essential nutrients and reduced metal toxicity, consistent with previous studies such as Dibaba (2021), Parecido et al. (2021), Teshale et al. (2021) and Fitria and Soemarno (2022). These improvements could be explained by the direct provision of Ca and Mg into the soil by lime (Silva et al. 2019), which promotes abiotic and biotic processes (Li et al. 2019a; Silva et al. 2019). These processes include the control of basic cation leaching (Neina 2019), increased P availability and mobility (Bailey and Laidlaw 1999; Hamilton et al. 2012), decreased exchangeable cations (Al3+ and Fe3+) (Gessa et al. 2005; Neina 2019), as well as the restoration of the soil microbial ecosystem, particularly improved abundance and activity of beneficial microorganisms (Li et al. 2019a; Silva et al. 2019).
Changes in soil OM in our study could be directly and indirectly linked to shifts in microbial communities and stimulated fungal activities. Soil OM dynamics were influenced by increased soil pH, which in turn affected microbial biomass and enzyme activities (Maestrini et al. 2014; Brassard et al. 2016; Herrmann et al. 2019). In fact, there was a significant correlation between soil pH and OM in coffee plantations (Table 3). Furthermore, the enhancement of OM could be related to increases in plant carbon inputs, which were caused by soil pH increases (Pietri and Brookes 2008). Regardless of treatments, the current levels of OM, as well as macro and secondary nutrients (including N, P, K, Ca, and Mg), were identified as optimal for coffee and pepper growth and productivity (Hamza et al. 2004; Bo et al. 2017; Dung et al. 2019). This suggests that the main issue with intensive coffee and pepper cultivation in Vietnam is acidic soil rather than nutrient deficiency and imbalance, indicating a need for further studies on the reduced inputs of mineral fertilisers due to liming effects.
AMF root colonisation
The AMF colonisation was significantly affected by lime application, consistent with previous studies (Andrade et al. 2009; Hazard et al. 2013; Jansa et al. 2014; Beenhouwer et al. 2015). These authors emphasised that soil pH is the most important driver of AMF community composition in coffee roots, positively impacting spore germination due to the preference of the fungi for near-neutral or alkaline soils. Growth of AMF is hindered by low pH and high P concentrations (Toljander et al. 2008; Helgason and Fitter 2009; Qin et al. 2015). As suggested by Hammer et al. (2011) and Chen et al. (2014), there was no significant difference in available P between the treatments and controls in our study, suggesting that P may not be the main factor influencing AMF colonisation in coffee roots. Along with pH, the significant enhancement of AMF colonisation in our study could be attributed to increased OM, available K, exchangeable Ca and Mg, and low concentrations of heavy metals (Sessitsch et al. 2001; Qin et al. 2015).
The application of lime and agronomic practices can lead to significant variability in soil properties. The effectiveness of lime in increasing soil pH depends on factors such as soil type (Li et al. 2019a), rainfall (Li et al. 2019b), types of lime, quality and dosage of lime (Li et al. 2019a; Nunes et al. 2019; Mahmud and Chong 2022), irrigation (Azam and Gazey 2021), agrochemical inputs, and experimental conditions (Li et al. 2019a; Nunes et al. 2019). For example, under coffee plantations, there was no significant improvement in available NH4+ and NO3− but there was a notable increase in K; in contrast, in pepper plantations, the trends were the opposite. This could be due to the high levels of N provided for coffee, while more K was supplied to pepper. As a result, soils in both coffee and pepper farms remained acidic, indicating that higher lime rates should be considered. However, it is important to note that applying higher lime rates can lead to a non-significant increase in soil pH (Norberg and Aronsson 2022), increased production costs, soil nutrient imbalance, and Ca toxicity, all of which can restrict crop growth and yield (Wang et al. 2010; Hamilton et al. 2012; Rhodes et al. 2018; Yan et al. 2021). Pilot laboratory studies showed that to increase soil pH by 1 unit (from 5.2 to 6.2) requires 25 t lime ha−1 (unpublished work). Therefore, the higher lime rate recommendation could be impractical and inconvenient for coffee and pepper production in Vietnam.
Soilborne pests and diseases
Lime application has shown inhibitory effects on growth of microbial pathogens and disease incidence (Gatch and Toit 2017). For instance, Takamoto et al. (2023) discovered that the addition of dolomite led to a lower severity of soilborne diseases compared to the control. Increasing soil pH through lime amendment can suppress soilborne pathogens through various mechanisms. It can directly inhibit the growth and reproduction of plant parasitic nematodes, particularly root-knot nematodes (Wang et al. 2009; Nisa et al. 2021). Additionally, improving soil pH and nutrients through lime application can restore the diversity of soil microorganisms, enrich the population of beneficial bacteria, and reduce abundance of pathogenic fungi (Deng et al. 2024). However, our results showed no effects of lime application on population of SBPDs. This finding was consistent with the studies of Pennanen et al. (1998), Pawlett et al. (2009), and Yin et al. (2021). This could be attributed to the soil remaining acidic, which may not effectively suppress soilborne pathogens, improve the soil microbial ecosystem, and promote the growth of soil antagonists. Furthermore, the study was conducted over a 1-year period, which might have been insufficient time to build up soil biodiversity, beneficial organisms, and suppression of SBPDs (Gatch and Toit 2017; Wan et al. 2019). For example, significant suppression of Fusarium wilt after annual limestone application required three continuous years of lime application (Gatch and Toit 2017).
Nevertheless, the edaphic conditions required by soilborne pathogens and coffee and pepper trees might be similar. Optimal soil pH values for coffee and pepper plants also represent suitable conditions for the growth and survival of pathogenic fungi (Bhai et al. 2010; Watanabe et al. 2011; Cruz et al. 2019) and nematodes (Souza 2008). Moreover, intensive N application has been associated with increased plant disease severity and promotion of pathogen establishment (Fagard et al. 2014; Martinez et al. 2021). Parasitic and biotrophic plant pathogenic microbial species may thrive in high residual soil N (Lekberg et al. 2021). Available N in the soil was significantly higher in unhealthy compared to healthy pepper plants, resulting in microbial community differentiation (Obieze et al. 2023).
The threshold background of pathogenic fungi and nematodes affecting coffee and pepper in Vietnam has not yet been officially published. However, crops could remain safe if the nematode density is below 100 individuals 100 g−1 soil (Hoa et al. 2016; Dung et al. 2019). Our study found that over 70% of coffee and pepper plantations exceeded this threshold value, highlighting the potential damage caused by nematodes on these farms. Based on the discussion above, managing soilborne pathogens by raising soil pH in traditional coffee and pepper farming systems in Vietnam may not be effective, and suggests that other sustainable practices, in addition to lime, that can alleviate soil acidification and control soilborne diseases should be considered.
Conclusion
We investigated the short-term effects of using lime to improve soil quality and crop yield in areas of intensive coffee and pepper cultivation in CH, Vietnam. Applying lime had positive effects on soil pH, fertility, colonisation by AMF, and levels of exchangeable Al and Fe. The specific changes in soil pH and other indicators varied depending on the particular farming practices at each farm. Interestingly, the presence of soilborne pathogens and crop yield were not significantly affected by the use of lime. Our findings suggest that regular lime application can help sustainably reduce soil acidity and enhance soil nutrients in acidic coffee and pepper farming soils to maintain high yields. Additionally, to effectively control soilborne pathogens in intensive farming systems, it is advisable to explore other alternative practices such as biological control and ecological approaches (e.g. use of cover crops, intercropping, and OM application) in conjunction with the use of lime.
Data availability
Data that support this study are available in the article and accompanying supplementary materials.
Declaration of funding
This work was funded by the ACIAR project: ‘Enhancing smallholder livelihoods in the Central Highlands of Viet Nam through improving the sustainability of coffee and black pepper farming systems and value chains,’ and Deakin University.
Author contributions
All authors participated in the conceptualization, original draft preparation, review, and editing. The authors have read and approved the final manuscript.
Acknowledgements
We gratefully acknowledge the valuable support of the Pepper Research and Development Center’s team in conducting sampling as well as the robusta coffee and black pepper farmers who allowed field experiments to be run on their farms.
References
Andrade SAL, Mazzafera P, Schiavinato MA, Silveira APD (2009) Arbuscular mycorrhizal association in coffee. The Journal of Agricultural Science 147(2), 105-115.
| Crossref | Google Scholar |
Azam G, Gazey C (2021) Slow movement of alkali from surface-applied lime warrants the introduction of strategic tillage for rapid amelioration of subsurface acidity in south-western Australia. Soil Research 59(1), 97-106.
| Crossref | Google Scholar |
Bailey J, Laidlaw A (1999) The interactive effects of phosphorus, potassium, lime and molybdenum on the growth and morphology of white clover (Trifolium repens L.) at establishment. Grass and Forage Science 54(1), 69-76.
| Crossref | Google Scholar |
Barak P, Jobe BO, Krueger AR, Peterson LA, Laird DA (1997) Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant and Soil 197, 61-69.
| Crossref | Google Scholar |
Beenhouwer MD, Van Geel M, Ceulemans T, Muleta D, Lievens B, Honnay O (2015) Changing soil characteristics alter the arbuscular mycorrhizal fungi communities of Arabica coffee (Coffea arabica) in Ethiopia across a management intensity gradient. Soil Biology and Biochemistry 91, 133-139.
| Crossref | Google Scholar |
Bhai RS, Raj S, Kumar A (2010) Influence of soil pH and moisture on the biocontrol potential of Trichoderma harzianum on Phytophthora capsici-black pepper system. Journal of Biological Control 24(2), 153 157.
| Crossref | Google Scholar |
Bolan N, Adriano DC, Curtin D (2003) Soil acidification and liming interactions with nutrient and heavy metal transformation and bioavailability. Advances in Agronomy 78(21), 215-272.
| Crossref | Google Scholar |
Bolan N, Sarmah AK, Bordoloi S, Bolan S, Padhye LP, Van Zwieten L, Sooriyakumar P, Khan BA, Ahmad M, Solaiman ZM, Rinklebe J, Wang H, Singh BP, Siddique KHM (2023) Soil acidification and the liming potential of biochar. Environmental Pollution 317, 120632.
| Crossref | Google Scholar | PubMed |
Brassard P, Godbout S, Raghavan V (2016) Soil biochar amendment as a climate change mitigation tool: key parameters and mechanisms involved. Journal of Environmental Management 181, 484-497.
| Crossref | Google Scholar | PubMed |
Burgess LW, Phan HT, Knight TE, Tesoriero L (2008) ‘Diagnostic manual for plant diseases in Vietnam. ACIAR Monograph 129.’ (Australian Centre for International Agricultural Research: Canberra). Available at https://repositorio.fedepalma.org/handle/123456789/80808
Byrareddy V, Kouadio L, Mushtaq S, Stone R (2019) Sustainable production of robusta coffee under a changing climate: a 10-year monitoring of fertilizer management in coffee farms in Vietnam and Indonesia. Agronomy 9(9), 499.
| Crossref | Google Scholar |
Cai Z, Wang B, Xu M, Zhang H, He X, Zhang L, Gao S (2015) Intensified soil acidification from chemical N fertilization and prevention by manure in an 18-year field experiment in the red soil of southern China. Journal of Soils and Sediments 15(2), 260-270.
| Crossref | Google Scholar |
Chairiyah RR, Ramija KE, Batubara SF (2021) Liming of acid soil and the interaction with soil pH and corn productivity. IOP Conference Series: Earth and Environmental Science 807, 042071.
| Crossref | Google Scholar |
Chapman HD (1965) Cation-exchange capacity. In ‘Methods of soil analysis: part 2 chemical and microbiological properties, Vol. 9’. (Ed. AG Norman) pp. 891–901. (American Society of Agronomy). 10.2134/agronmonogr9.2.c6
Chaves J, Pavan M, Igue K (1984) Response of coffee to lime. Pesquisa Agropecuária Brasileira 19(5), 573-582.
| Google Scholar |
Chen Y-L, Zhang X, Ye J-S, Han H-Y, Wan S-Q, Chen B-D (2014) Six-year fertilization modifies the biodiversity of arbuscular mycorrhizal fungi in a temperate steppe in Inner Mongolia. Soil Biology and Biochemistry 69, 371-381.
| Crossref | Google Scholar |
Corrêa JB, Reis THP, Pozza AAA, Guimarães PTG, de Carvalho JG (2008) Basis saturation index effects on nutrition and beans yield of coffee trees. Coffee Science 2(2), 159-167 Available at https://coffeescience.ufla.br/index.php/Coffeescience/article/view/62.
| Google Scholar |
Cruz DR, Leandro LFS, Munkvold GP (2019) Effects of temperature and pH on Fusarium oxysporum and soybean seedling disease. Plant Disease 103(12), 3234-3243.
| Crossref | Google Scholar | PubMed |
Cyamweshi RA, Nabahungu NL, Mukashema A, Ruganzu V, Gatarayiha MC, Nduwumuremyi A, Mbonigaba JJ (2014) Enhancing nutrient availability and coffee yield on acid soils of the central plateau of southern Rwanda. Global Journal of Agricultural Research 2(2), 44-55.
| Google Scholar |
Deng W, Gong J, Peng W, Luan W, Liu Y, Huang H, Mei X, Yang M, Zhu S (2024) Alleviating soil acidification to suppress Panax notoginseng soil-borne disease by modifying soil properties and the microbiome. Plant and Soil 502, 653-669.
| Crossref | Google Scholar |
Dibaba BT (2021) Dry matter yield and nutrient uptakes of arabica coffee seedlings as influenced by lime and coffee husk compost amendments at Western Ethiopia. Pelita Perkebunan (a Coffee and Cocoa Research Journal) 37(2), 97-106.
| Crossref | Google Scholar |
Dibaba TB, Kufa T, Regassa A (2020) Effects of lime and coffee husk compost on growth of coffee seedlings on acidic soil of Haru in Western Ethiopia. Journal of Degraded and Mining Lands Management 8(1), 2391-2400.
| Crossref | Google Scholar |
Dung ND, Lai NX, Truc HC, Tien TM, Ha NT, Hiep NV, Hoai NTT, Quyen NT (2019) Characteristic and changing of soil fertility under Coffee and Black pepper cultivation in Central Highlands. Science and Technology of Agriculture and Rural Development 10, 3-9.
| Google Scholar |
Fagard M, Launay A, Clement G, Courtial J, Dellagi A, Farjad M, Krapp A, Soulie M-C, Masclaux-Daubresse C (2014) Nitrogen metabolism meets phytopathology. Journal of Experimental Botany 65(19), 5643-5656.
| Crossref | Google Scholar | PubMed |
Fan K, Weisenhorn P, Gilbert JA, Shi Y, Bai Y, Chu H (2018) Soil pH correlates with the co-occurrence and assemblage process of diazotrophic communities in rhizosphere and bulk soils of wheat fields. Soil Biology and Biochemistry 121, 185-192.
| Crossref | Google Scholar |
Fitria L, Soemarno S (2022) Effects of lime and compost on chemical characteristics and soil hydraulic conductivity of alfisols at ATP jatikerto coffee plantation. Caraka Tani: Journal of Sustainable Agriculture 37(1), 48-61.
| Crossref | Google Scholar |
Gatch EW, Toit LJD (2017) Limestone-mediated suppression of fusarium wilt in spinach seed crops. Plant Disease 101(1), 81-94.
| Crossref | Google Scholar | PubMed |
Gessa CE, Mimmo T, Deiana S, Marzadori C (2005) Effect of aluminium and pH on the mobility of phosphate through a soil-root interface model. Plant and Soil 272, 301-311.
| Crossref | Google Scholar |
GSO (2021) ‘Statistical yearbook of Vietnam 2021.’ (General Statistics Office (GSO), Statistical Publishing House) Available at https://www.gso.gov.vn/wp-content/uploads/2022/08/Sach-Nien-giam-TK-2021.pdf
GSO (2022) ‘Statistical yearbook of Vietnam 2022.’ (General Statistics Office (GSO), Statistical Publishing House) Available at https://www.gso.gov.vn/wp-content/uploads/2023/06/Sach-Nien-giam-TK-2022-update-21.7_file-nen-Water.pdf
Ha LV (2016) Study on chemical properties of basaltic soil for coffee growing at highland Di Linh, Lam Dong. Journal of Vietnam Agricultural Science and Technology 01(62), 30-35 Available at https://tapchi.vaas.vn/vi/tap-chi/7159/so-01-62-nam-2016.
| Google Scholar |
Hamilton EJ, Miles RJ, Lukaszewska K, Remley M, Massie M, Blevins DG (2012) Liming of two acidic soils improved grass tetany ratio of stockpiled tall fescue without increasing plant available phosphorus. Journal of Plant Nutrition 35(4), 497-510.
| Crossref | Google Scholar |
Hammer EC, Nasr H, Wallander H (2011) Effects of different organic materials and mineral nutrients on arbuscular mycorrhizal fungal growth in a Mediterranean saline dryland. Soil Biology and Biochemistry 43(11), 2332-2337.
| Crossref | Google Scholar |
Hamza S, Sadanandan A, Srinivasan V (2004) Influence of soil physico-chemical properties on productivity of black pepper (Piper nigrum L.). Journal of Spices and Aromatic Crops 13(1), 6-9 Available at https://core.ac.uk/download/pdf/236023195.pdf.
| Google Scholar |
Hamza S, Srinivasan V, Dinesh R (2007) Nutrient diagnosis of black pepper (Piper nigrum L.) gardens in Kerala and Karnataka. Journal of Spices and Aromatic Crops 16(2), 77-81 https://core.ac.uk/download/pdf/236023608.pdf.
| Google Scholar |
Hao T, Zhu Q, Zeng M, Shen J, Shi X, Liu X, Zhang F, De Vries W (2020) Impacts of nitrogen fertilizer type and application rate on soil acidification rate under a wheat-maize double cropping system. Journal of Environmental Management 270, 110888.
| Crossref | Google Scholar | PubMed |
Hazard C, Gosling P, van der Gast CJ, Mitchell DT, Doohan FM, Bending GD (2013) The role of local environment and geographical distance in determining community composition of arbuscular mycorrhizal fungi at the landscape scale. The ISME Journal 7(3), 498-508.
| Crossref | Google Scholar | PubMed |
Helgason T, Fitter AH (2009) Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). Journal of Experimental Botany 60(9), 2465-2480.
| Crossref | Google Scholar | PubMed |
Herrmann L, Lesueur D, Robin A, Robain H, Wiriyakitnateekul W, Bräu L (2019) Impact of biochar application dose on soil microbial communities associated with rubber trees in North East Thailand. Science of the Total Environment 689, 970-979.
| Crossref | Google Scholar | PubMed |
Hoa NX, Phong NH, Dan CT, Van TNT, Trang NTT, Phi LV (2016) Effect of nematode on yellow leaf and root rot diseases of replanting coffee. Journal of Vietnam Agricultural Science and Technology 02(63), 78-83.
| Google Scholar |
Hong T (2017) The variation of basaltic soil fertility growing coffee in Western Highlands. Journal of Vietnam Agricultural Science and Technology 09(82), 104-110.
| Google Scholar |
Hooper D (1986) Laboratory methods for work with plant and soil nematodes. Reference book 402, Vol. 2. Ministry of Agriculture, Fisheries and Food Technical Bulletin, pp. 133–154. Available at https://cir.nii.ac.jp/crid/1570572699492725120
Huyen PTT, Giang PQ, Van Toan N, Trinh PT (2018) Correlation between the distribution of nematodes and soil physicochemical characteristics in coffee rejuvenation areas. EnvironmentAsia 11(1), 141-156.
| Crossref | Google Scholar |
ICO (2022) Exports of all forms of coffee by exporting countries to all destinations from Feb 2020 to Jan. 2021. International Coffee Organization. Available at https://ico.org/prices/m1-exports.pdf [retrieved 26 July]
IPC (2022) Pepper statistical yearbook 2021. International Pepper Community. Available at https://www.ipcnet.org/statistics/
Jansa J, Erb A, Oberholzer H-R, Šmilauer P, Egli S (2014) Soil and geography are more important determinants of indigenous arbuscular mycorrhizal communities than management practices in Swiss agricultural soils. Molecular Ecology 23(8), 2118-2135.
| Crossref | Google Scholar | PubMed |
Khoa LĐ, Ha PV, Dan CT, Long HH, Nuong NK (2014) Studying on evaluation of the recent status of replanted coffee plantations in Central Highland. Journal of Vietnam Agricultural Science and Technology 03(45), 3-11.
| Google Scholar |
Kjeldahl J (1883) A new method for the determination of nitrogen in organic matter. Zeitschrift Fur Analytische Chemie 22, 366-382.
| Google Scholar |
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycological Research 92(4), 486-488.
| Crossref | Google Scholar |
Lekberg Y, Arnillas CA, Borer ET, Bullington LS, Fierer N, Kennedy PG, Leff JW, Luis AD, Seabloom EW, Henning JA (2021) Nitrogen and phosphorus fertilization consistently favor pathogenic over mutualistic fungi in grassland soils. Nature Communications 12(1), 3484.
| Crossref | Google Scholar | PubMed |
Li Y, Cui S, Chang SX, Zhang Q (2019a) Liming effects on soil pH and crop yield depend on lime material type, application method and rate, and crop species: a global meta-analysis. Journal of Soils and Sediments 19(3), 1393-1406.
| Crossref | Google Scholar |
Li GD, Conyers MK, Helyar KR, Lisle CJ, Poile GJ, Cullis BR (2019b) Long-term surface application of lime ameliorates subsurface soil acidity in the mixed farming zone of south-eastern Australia. Geoderma 338, 236-246.
| Crossref | Google Scholar |
Lumivero (2023) XLSTAT statistical and data analysis solution. Available at https://www.xlstat.com/en/papers
Maestrini B, Herrmann AM, Nannipieri P, Schmidt MWI, Abiven S (2014) Ryegrass-derived pyrogenic organic matter changes organic carbon and nitrogen mineralization in a temperate forest soil. Soil Biology and Biochemistry 69, 291-301.
| Crossref | Google Scholar |
Mahmud MS, Chong KP (2022) Effects of liming on soil properties and its roles in increasing the productivity and profitability of the oil palm industry in Malaysia. Agriculture 12(3), 322.
| Crossref | Google Scholar |
MARD (2015) Decision no. 730/QĐ-BNN-TT: technical process of planting, cultivating and harvesting black pepper. The Ministry of Agriculture and Rural Development-MARD. Available at https://thuvienphapluat.vn/van-ban/Linh-vuc-khac/Quyet-dinh-730-QD-BNN-TT-2015-quy-trinh-trong-cham-soc-va-thu-hoach-ho-tieu-373656.aspx
Martinez DA, Loening UE, Graham MC, Gathorne-Hardy A (2021) When the medicine feeds the problem; do nitrogen fertilisers and pesticides enhance the nutritional quality of crops for their pests and pathogens? Frontiers in Sustainable Food Systems 5, 701310.
| Crossref | Google Scholar |
Masago H, Yoshikawa M, Fukada M, Nakanishi N (1976) Selective inhibition of Pythium spp. on a medium for direct isolation of Phytophthora spp. from soils and plants. Phytopathology 67, 425-428.
| Crossref | Google Scholar |
MOST (2007) Soil quality – sampling – part 4: guidance on the procedure for the investigation of natural, near-natural, and cultivated sites. Ministry of Science and Technology (MOST). Available at https://caselaw.vn/van-ban-phap-luat/254768-tieu-chuan-quoc-gia-tcvn-7538-4-2007-iso-10381-4-2003-ve-chat-luong-dat-lay-mau-phan-4-huong-dan-qui-trinh-dieu-tra-cac-vung-tu-nhien-ban-tu-nhien-va-vung-canh-tac-nam-2007
Mulder C, Van Wijnen HJ, Van Wezel AP (2005) Numerical abundance and biodiversity of below-ground taxocenes along a pH gradient across the Netherlands. Journal of Biogeography 32(10), 1775-1790.
| Crossref | Google Scholar |
Neina D (2019) The role of soil pH in plant nutrition and soil remediation. Applied and Environmental Soil Science 2019, 5794869.
| Crossref | Google Scholar |
Nisa RU, Tantray AY, Kouser N, Allie KA, Wani SM, Alamri SA, Alyemeni MN, Wijaya L, Shah AA (2021) Influence of ecological and edaphic factors on biodiversity of soil nematodes. Saudi Journal of Biological Sciences 28(5), 3049-3059.
| Crossref | Google Scholar | PubMed |
Norberg L, Aronsson H (2022) Mitigating phosphorus leaching from a clay loam through structure liming. Acta Agriculturae Scandinavica, Section B – Soil & Plant Science 72(1), 987-996.
| Crossref | Google Scholar |
Nunes MR, Denardin JE, Vaz CMP, Karlen DL, Cambardella CA (2019) Lime movement through highly weathered soil profiles. Environmental Research Communications 1, 115002.
| Crossref | Google Scholar |
Nysanth NS, Divya S, Nair CB, Anju AB, Praveena R, Anith KN (2022) Biological control of foot rot (Phytophthora capsici Leonian) disease in black pepper (Piper nigrum L.) with rhizospheric microorganisms. Rhizosphere 23, 100578.
| Crossref | Google Scholar |
Obieze CC, George PBL, Boyle B, Khasa DP (2023) Black pepper rhizomicrobiome: spectrum of plant health indicators, critical environmental factors and community compartmentation in Vietnam. Applied Soil Ecology 187, 104857.
| Crossref | Google Scholar |
Pagani A, Mallarino AP (2015) On-farm evaluation of corn and soybean grain yield and soil pH responses to liming. Agronomy Journal 107(1), 71-82.
| Crossref | Google Scholar |
Parecido RJ, Soratto RP, Perdoná MJ, Gitari HI, Dognani V, Santos AR, Silveira L (2021) Liming method and rate effects on soil acidity and arabica coffee nutrition, growth, and yield. Journal of Soil Science and Plant Nutrition 21(4), 2613-2625.
| Crossref | Google Scholar |
Pawlett M, Hopkins DW, Moffett BF, Harris JA (2009) The effect of earthworms and liming on soil microbial communities. Biology and Fertility of Soils 45, 361-369.
| Crossref | Google Scholar |
Pennanen T, Fritze H, Vanhala P, Kiikkilä O, Neuvonen S, Bååth E (1998) Structure of a microbial community in soil after prolonged addition of low levels of simulated acid rain. Applied and Environmental Microbiology 64(6), 2173-2180.
| Crossref | Google Scholar | PubMed |
Pietri JCA, Brookes PC (2008) Relationships between soil pH and microbial properties in a UK arable soil. Soil Biology and Biochemistry 40(7), 1856-1861.
| Crossref | Google Scholar |
Qin H, Lu K, Strong PJ, Xu Q, Wu Q, Xu Z, Xu J, Wang H (2015) Long-term fertilizer application effects on the soil, root arbuscular mycorrhizal fungi and community composition in rotation agriculture. Applied Soil Ecology 89, 35-43.
| Crossref | Google Scholar |
Rahayu DS, Sari NP (2017) Development of pratylenchus coffeae in biochar applied soil, coffee roots and its effect on plant growth. Pelita Perkebunan 33(1), 24-32.
| Crossref | Google Scholar |
Rhodes R, Miles N, Hughes JC (2018) Interactions between potassium, calcium and magnesium in sugarcane grown on two contrasting soils in South Africa. Field Crops Research 223, 1-11.
| Crossref | Google Scholar |
Rigal C, Tuan D, Cuong V, Le Van B, Trung HQ, Long CTM (2023) Transitioning from monoculture to mixed cropping systems: the case of coffee, pepper, and fruit trees in Vietnam. Ecological Economics 214, 107980.
| Crossref | Google Scholar |
Sadeghian KS, Diaz MC (2020) Soil acidity correction: effects on the initial coffee growth. Cenicafé 71, 21-31.
| Google Scholar |
Sessitsch A, Weilharter A, Gerzabek MH, Kirchmann H, Kandeler E (2001) Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Applied and Environmental Microbiology 67(9), 4215-4224.
| Crossref | Google Scholar | PubMed |
Shen G, Zhang S, Liu X, Jiang Q, Ding W (2018) Soil acidification amendments change the rhizosphere bacterial community of tobacco in a bacterial wilt affected field. Applied Microbiology and Biotechnology 102(22), 9781-9791.
| Crossref | Google Scholar | PubMed |
Silva EMB, Costa AS, José JV, Ferraz APF, Damasceno APAB, da Silva TJA (2019) Correction of acidity of a brazilian cerrado oxisol with limestone and wood ash on the initial growth of cowpea. Agricultural Sciences 10(7), 841-851.
| Crossref | Google Scholar |
Sokolov A (1939) Determination of active aluminum in soils. Chemical Society of Agriculure 7,.
| Google Scholar |
Takamoto A, Takahashi T, Togami K, Hishinuma A (2023) Responses of soybean, Glycine max (L.) Merr. to dolomite and calcite fertilization in an upland field converted from a paddy field. Plant Production Science 26(3), 259-272.
| Crossref | Google Scholar |
Teshale E, Kufa T, Regassa A (2021) Effects of lime on phosphorus availability and nutrient uptake of hybrid coffee (Coffea arabica L.) seedlings under acidic nursery soil. Agriculture, Forestry and Fisheries 10(1), 21-27.
| Crossref | Google Scholar |
Toljander JF, Santos-Gonzalez JC, Tehler A, Finlay RD (2008) Community analysis of arbuscular mycorrhizal fungi and bacteria in the maize mycorrhizosphere in a long-term fertilization trial. FEMS Microbiology Ecology 65(2), 323-338.
| Crossref | Google Scholar | PubMed |
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Microbiology 64(12), 5004-5007.
| Crossref | Google Scholar | PubMed |
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science 37(1), 29-38.
| Google Scholar |
Wan S, Liu Z, Chen Y, Zhao J, Ying Q, Liu J (2019) Effects of lime application and understory removal on soil microbial communities in subtropical Eucalyptus L’Hér. Plantations. Forests 10(4), 338.
| Crossref | Google Scholar |
Wang C, Bruening G, Williamson VM (2009) Determination of preferred pH for root-knot nematode aggregation using pluronic F-127 gel. Journal of Chemical Ecology 35(10), 1242-1251.
| Crossref | Google Scholar | PubMed |
Wang Y-H, Zhang L-X, Sun Q-Y (2010) Effects of excessive calcium fertilization on photosynthetic characteristics and chloroplast ultra-structure of tea tree. Plant Nutrition and Fertilizer Science 16(2), 432-438.
| Google Scholar |
Watanabe K, Matsui M, Honjo H, Becker JO, Fukui R (2011) Effects of soil pH on rhizoctonia damping-off of sugar beet and disease suppression induced by soil amendment with crop residues. Plant and Soil 347(1–2), 255-268.
| Crossref | Google Scholar |
Yan P, Zou Z, Zhang J, Yuan L, Shen C, Ni K, Sun Y, Li X, Zhang L, Zhang L, Fu J, Han W (2021) Crop growth inhibited by over-liming in tea plantations. Beverage Plant Research 1(1), 9.
| Crossref | Google Scholar |
Yin C, Schlatter DC, Kroese DR, Paulitz TC, Hagerty CH (2021) Responses of soil fungal communities to lime application in wheat fields in the Pacific Northwest. Frontiers in Microbiology 12, 576763.
| Crossref | Google Scholar | PubMed |
Zamanian K, Zarebanadkouki M, Kuzyakov Y (2018) Nitrogen fertilization raises CO2 efflux from inorganic carbon: a global assessment. Global Change Biology 24(7), 2810-2817.
| Crossref | Google Scholar | PubMed |
Zhang Y, Ye C, Su Y, Peng W, Lu R, Liu Y, Huang H, He X, Yang M, Zhu S (2022) Soil Acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: Evidence from literature review and field trials. Agriculture, Ecosystems & Environment 340, 108176.
| Crossref | Google Scholar |
Zhang Z, He P, Hao X, Li L-J (2023) Long-term mineral combined with organic fertilizer supports crop production by increasing microbial community complexity. Applied Soil Ecology 188, 104930.
| Crossref | Google Scholar |
Zhao H, Yu L, Yu M, Afzal M, Dai Z, Brookes P, Xu J (2020) Nitrogen combined with biochar changed the feedback mechanism between soil nitrification and Cd availability in an acidic soil. Journal of Hazardous Materials 390, 121631.
| Crossref | Google Scholar | PubMed |
Zhou J, Xia F, Liu X, He Y, Xu J, Brookes PC (2014) Effects of nitrogen fertilizer on the acidification of two typical acid soils in South China. Journal of Soils and Sediments 14(2), 415-422.
| Crossref | Google Scholar |
Zu C, Li Z, Yang J, Yu H, Sun Y, Tang H, Yost R, Wu H (2014) Acid soil is associated with reduced yield, root growth and nutrient uptake in black pepper (Piper nigrum L.). Agricultural Sciences 5(5), 466-473.
| Crossref | Google Scholar |


