Genetic structure and new occurrence records of the iconic Tasmanian mountain shrimp Anaspides tasmaniae (Thomson, 1893) (Anaspidesidae : Anaspidacea) reveal relictual distribution in southern Tasmania
Christoph G. Höpel A C , Shane T. Ahyong B and Stefan Richter A
A C , Shane T. Ahyong B and Stefan Richter A
A Allgemeine and Spezielle Zoologie, Institut für Biowissenschaften, Universität Rostock, Universitätsplatz 2, 18055 Rostock, Germany.
B Australian Museum Research Institute, Australian Museum, 1 William Street, Sydney, NSW 2010, Australia, and School of Biological, Earth and Environmental Sciences, University of New South Wales, Kensington, NSW 2052, Australia.
C Corresponding author. Email: christoph.hoepel@uni-rostock.de
Australian Journal of Zoology 68(1) 45-53 https://doi.org/10.1071/ZO20100
Submitted: 23 December 2020 Accepted: 11 March 2021 Published: 9 April 2021
Abstract
The iconic ‘mountain shrimps’ of the genus Anaspides Thomson, 1894, are endemic to Tasmania, inhabiting various freshwater habitats such as mountain tarns and creeks, as well as streams inside caves. They are often labelled as ‘living fossils’ because of their close resemblance to their Triassic relatives. Prior to 2015, only two species were recognised but recent studies have uncovered a total of at least seven species. The type species of Anaspides, A. tasmaniae (Thomson, 1893), was previously believed to occur throughout Tasmania, but following a review in 2016, this species was confirmed only from a small range on the east and south-east side of Mt Wellington, with Anaspides from other parts of Tasmania referable to other species. We herein provide a detailed assessment of the distribution and genetic structure of A. tasmaniae based on extensive field surveys throughout the ranges of all species of Anaspides. The distribution of A. tasmaniae is extended to include four separate localities in and around the Mt Field National Park, 50 km north-west of Mt Wellington. The recovered genetic structure of A. tasmaniae based on 48 specimens indicates that the disjunct distribution is unlikely to be the result of artificial translocation but, instead, probably reflects postglacial relictualisation of a formerly continuous range present during Pleistocene glacial maxima. Of particular interest is the record of syntopy in Anaspides, observed at the entrance of Khazad Dum cave, where both A. tasmaniae and A. swaini inhabit the inflow stream.
Keywords: Anaspidacea, Anaspidesidae, Anaspides tasmaniae, conservation, endemic, genetic structure, glaciation, mountain shrimp, relictual distribution, Tasmania.
Introduction
The mountain shrimps of the genus Anaspides Thomson, 1894 (Anaspidesidae: Ahyong and Alonso-Zarazaga 2017) are endemic to Tasmania and are probably the most iconic freshwater invertebrates of the island (Ahyong 2016; Ahyong and Alonso-Zarazaga 2017). Anaspides occurs in a variety of freshwater surface habitats such as mountain tarns, creeks, streams, and pools, as well as subterranean streams in caves all over Tasmania. Although Anaspides spp. are found in surface waters between 150 m and approximately 1500 m above sea level (asl), they mainly occur in high-altitude areas above 700–800 m asl. Anaspides is often referred to as a ‘living fossil’ because of its close resemblance to Triassic relatives. The first species of Anaspides was discovered in 1892 by G. M. Thomson, who collected specimens on Mt Wellington, which he initially named Anaspis tasmaniae Thomson, 1893 (but later corrected to Anaspides tasmaniae) (Thomson 1894). In 1908, Smith enlarged the known distribution of Anaspides, finding populations on the Hartz Mts, Mt Field and Mt Read. A second species of Anaspides, A. spinulae Williams, 1965, was described from Lake St Clair (Williams 1965a). In the same year, Williams reported the first records of Anaspides from caves (Williams 1965b). Further investigations in caves and surface waters revealed further occurrences and higher morphological variation in Anaspides, as well as molecular evidence for undiscovered diversity (Goede 1967; Lake and Coleman 1977; O’Brien 1990; Jarman and Elliott 2000).
Our current taxonomic knowledge is based significantly on the investigations of Ahyong (2015, 2016), which led to recognition of five new species: A. clarkei Ahyong, 2015, A. jarmani Ahyong, 2015, A. swaini Ahyong, 2015, A. eberhardi Ahyong, 2016 and A. richardsoni Ahyong, 2016. Anaspides richardsoni occurs in the northern half of Tasmania from the West Coast Range, east to Cradle Mountain, Gunns Plains and Mole Creek, across the Central Plateau, and south to Mt Field, whereas A. swaini has a more central-south-western distribution, being reported from the Wellington Range over the Snowy Range and Arthur Range to the flanks of lakes Pedder and Gordon, and north to the vicinity of Lake St Clair. Moreover, Ahyong (2016) reported morphological differences within these widespread species, especially within A. swaini, recognising three subtly different, geographically discrete forms, which might represent separate species (currently under study). The remaining species have narrow ranges and are morphologically rather consistent. Anaspides spinulae occurs only within Lake St Clair, A. eberhardi is present only in caves of the Junee–Florentine karst and A. jarmani and A. clarkei are found in just a few localities in south-east Tasmania. Finally, A. tasmaniae, formerly believed to be widespread over the whole island in surface waters and caves, now appeared to be limited to a small range on the east and south-east side of Mt Wellington above 450 m asl with no known cave occurrences. Richter et al. (2018), using molecular data, largely corroborated the major species clades of Ahyong (2016) and recovered a monophyletic A. tasmaniae, with different sister group relationships depending on the gene fragment used.
Anaspides tasmaniae is readily distinguished from other species of the genus by the combination of well developed eyes, the presence of two antennular clasping spines in adult males, the elongated telson with an evenly rounded posterior margin lined with closely set spinules and a male pleopod I, in which the retinacular lobe is visible in lateral view (Ahyong 2016). In particular, the elongated telson with an evenly rounded posterior margin and the form of the antennular inner flagellum makes it easy to distinguish from other Anaspides species.
We herein provide a detailed assessment of distribution and genetic structure of A. tasmaniae based on extensive recent field surveys throughout the ranges of all species of Anaspides, extend the known distribution of A. tasmaniae to an area in the Junee–Florentine Karst, 50 km north-west of Mt Wellington and provide evidence of sympatry of two species of Anaspides at the entrance of the Khazad Dum cave.
Material and methods
Specimens were collected during two extensive field surveys in February 2017 and March 2019, sampling widely throughout the range of all species of Anaspides. In total, 37 specimens of A. tasmaniae were collected and newly sequenced for this study (see Table S1, Supplementary Material). In addition, a total of 9 COI, 11 16S rRNA and one 28S rRNA sequence from the study by Richter et al. (2018) were included. The newly sequenced specimens were collected using hand nets and preserved directly in 80–95% ethanol. Specimens were identified based on morphology following Ahyong (2015, 2016).
Tissue was removed from the 8th thoracopod or from the pleopods of the specimens. The DNA extraction was performed with the innuPrep Forensic Kit (Analytik Jena) and the DNeasy Blood and Tissue Kit (Qiagen) following the manufacturer’s instruction. Three markers were partially sequenced: mitochondrial cytochrome c oxidase subunit I (COI) and 16S rRNA as well as the nuclear 28S rDNA. The polymerase chain reaction (PCR) had a total volume of 30 μL containing 3 μL of each primer (each 10 μm), 3 μL 10 × buffer (Sigma Aldrich), 3 μL dNTP mix (2 mm, Biozym), 2.58 μL MgCl2 (25 mm, Sigma Aldrich), 0.12 μL Taq-polymerase (Sigma Aldrich), 10.71 μL ultrapure water and 4.5 μL of the DNA extract. Primers were LCO2 (5′-TCN ACH AAY CAT AAA GAY ATT GGA AC-3′) (Richter et al. 2018), HCO2198 (5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′) (Folmer et al. 1994) for COI, 16Sa (5′- CGC CTG TTT ATC AAA AAC AT-3′), 16Sb (5′-CTC CGG TTT GAA CTC AGA TCA-3′) (Xiong and Kocher 1991) for 16S and rd4.8a (5′-ACC TAT TCT CAA ACT TTA AAT GG-3′) and rd7b1 (5′-GAC TTC CCT TAC CTA CAT-3′) (Richter et al. 2007) for 28S rDNA. PCR amplification programs comprised an initial denaturation step at 94°C for 1 min, followed by 40 amplification cycles (94°C for 30 s, 50°C for 30 s, 72°C for 60 s) for COI, 38 amplification cycles (94°C for 30 s, 48°C for 30 s, 72°C for 60 s) for 16S, 35 amplification cycles (94°C for 30 s, 55°C for 30 s, 72°C for 60 s) for 28S, and a final elongation step at 72°C for 5 min. All PCR reactions were performed with a Mastercycler gradient (Eppendorf). PCR products were visualised by gel electrophoresis, using 5 μL of the PCR product with 1.5 μL loading buffer DNA b II (AppliChem) on a 1.2% agarose/TAE gel stained with 5 μL Rotisafe (Carl Roth). PCR products were purified using paramagnetic beads (High Prep PCR, Magbio) following the manufacturer’s instruction with a finale volume of 25 μL. Sequencing of PCR products was performed in Lightrun96 well plates by the company Eurofins Genomics as well as using the dideoxy chain termination method and cycle sequencing using an ABI Prism® Big Dye® Terminator V.1.1 Cycle Sequencing Kit. Cycle sequencing products were analysed by using capillary separation on an ABI Genetic Analyzer 3130 xl (Applied Biosystems/Hitachi). The resulting chromatograms were manually checked and adjusted with Geneious 2019.1.3 (Biomatters Limited). All sequences were deposited in GenBank (Benson et al. 2003) (accession numbers: MW584899–MW584904, MW581074–MW581077, MW581544–MW581580).
The sequences of all three gene fragments were separately aligned using ClustalW (Thompson et al. 1994) implemented in Geneious Prime 2019.1.3 (Biomatters Limited). Pairwise distances were calculated using MEGA X (Kumar et al. 2018). For pairwise distance analyses the uncorrected p-distances were calculated. Phylogenetic networks were calculated with Network 5.0.1.1 (Fluxus Technologies) separately for COI, 16S, and 28S alignments by using the median-joining method with the parameter epsilon set to 10.
Results
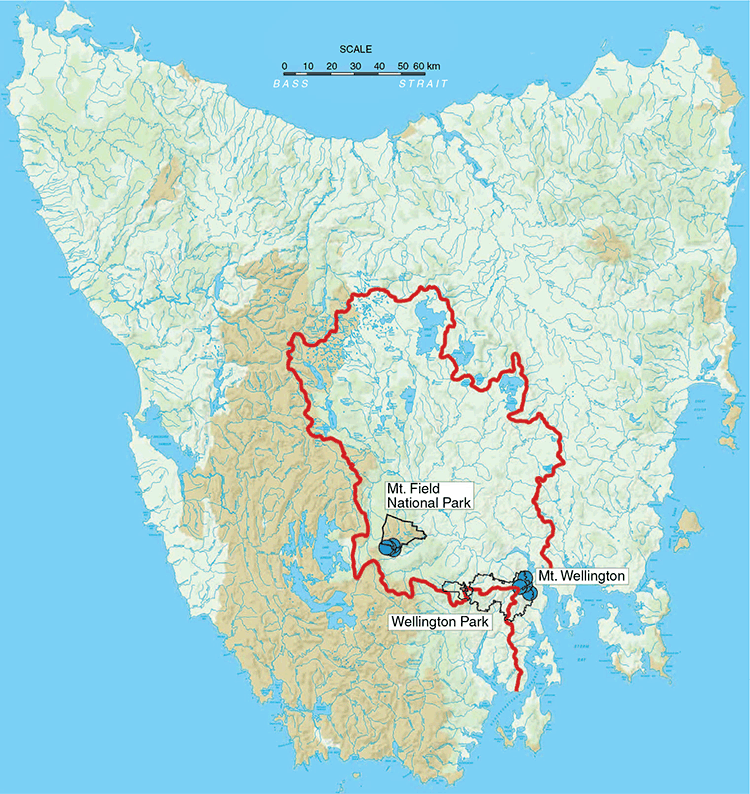
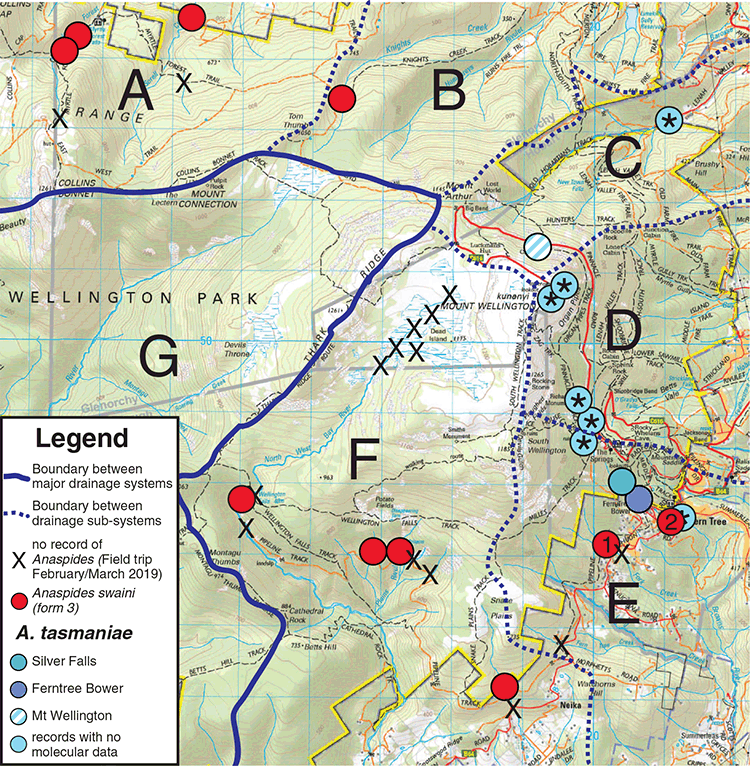
We found Anaspides tasmaniae at three different localities east and south-east of Mt Wellington, within the known range of the species: at a small runnel near The Chalet, Mt Wellington (970 m asl); the Browns River at Silver Falls (520 m asl); and Fern Tree Bower (470 m asl) (Figs 1 and 2). Extensive searches of the Mt Wellington plateau in the head waters of North West Bay River, where Anaspides has been historically reported to occur (Ahyong 2016), recovered no evidence of Anaspides. Also considering the records in Ahyong (2016), true A. tasmaniae has been confirmed only from three different drainages in the east and south-east of Mt Wellington: the catchments of the New Town Rivulet, the Guy Fawkes Rivulet and the Browns River (Fig. 2). The neighbouring drainages, all in Wellington Park, i.e. North West Bay River catchment, Knights Creek catchment and Myrtle Forest Creek/Sorell Creek catchment, are inhabited by Anaspides swaini (form 3 of Ahyong 2016) (Figs 1 and 2).
|
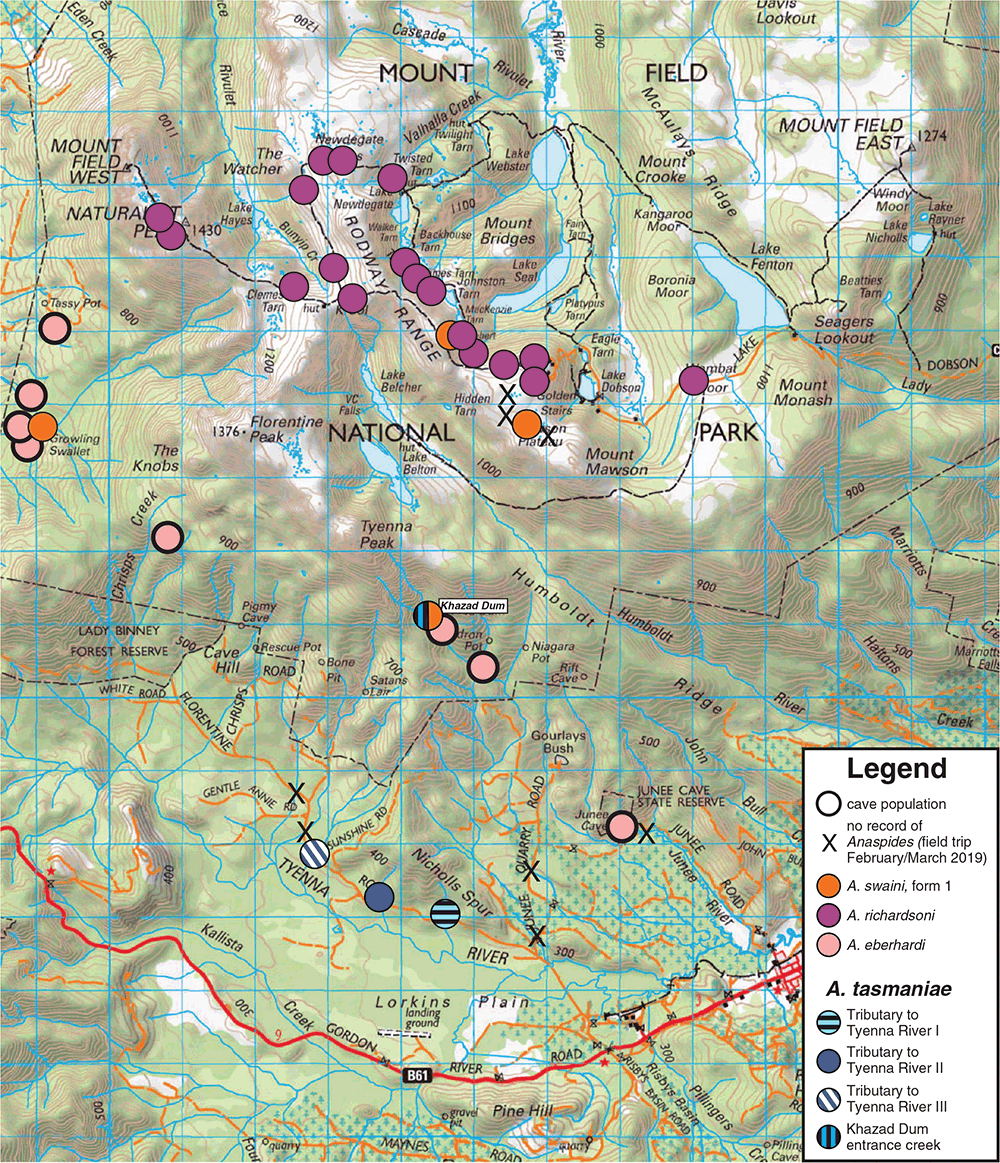
Anaspides tasmaniae, however, was discovered within and near the Mt Field National Park. In Mt Field National Park, we recorded four species of Anaspides: A. richardsoni, A. eberhardi, A. swaini (supporting the general distribution shown in Ahyong 2016) as well as A. tasmaniae. Anaspides richardsoni inhabits the tarns and runnels on the plateau from Wombat Moor and the north side of the Mawson Plateau over the tarn shelf, Newdegate Pass and K Col to the waters of the Mt Field West plateau (Figs 1 and 3). The troglobitic species, A. eberhardi, occurs only in caves of the Junee–Florentine Karst at the edges of the Mt Field National Park and Junee Cave State Reserve. We observed A. eberhardi in Niggly Cave, Khazad Dum, Porcupine Pot and Junee Cave. Further records of A. eberhardi are from cave JF-104, Welcome Stranger Cave, Gormenghast Cave, Pendant Pot, Growling Swallet and Settlement Cave (Ahyong 2016). Anaspides swaini occurs in the inflow creeks of Growling Swallet (Garths Creek) and Khazad Dum. Although, A. swaini has been recorded from Robert Tarn and the Mawson Plateau in the past (1970, 1975; see Ahyong 2016), Anaspides swaini is apparently now absent on the plateaus of the Mt Field National Park. Although Robert Tarn is currently inhabited by A. richardsoni only, our searches on the Mawson Plateau in March 2019 revealed no signs of any Anaspides, with the tarns being completely dried out.
|
Anaspides tasmaniae was found at four separate localities. Three of these localities lie outside the Mt Field National Park, alongside the Florentine Road, with two creeks draining from Nicholls Spur and one creek draining from Tyenna Peak. All of these three creeks are direct tributaries of the Tyenna River (Fig. 3). These creeks were situated in a tree fern rainforest, with low to no water flow at the time of sampling; Anaspides were mainly found in small seemingly isolated pools. All of these sites are in low-altitude areas at 320–340 m asl. The creek drainages (Tributary to Tyenna River I and II) originate at Nichols Spur with a maximum altitude of 515 m asl, but how far upstream Anaspides occurs is unknown. The neighbouring two tributaries to the Tyenna River crossing the Florentine Road as well as two tributaries to the Tyenna River crossing the Junee Quarry Road had no A. tasmaniae (see Fig. 3). The fourth locality is within the Mt Field National Park, where A. tasmaniae occurs in the inflow creek to the Khazad Dum cave (760 m asl) (Fig. 3). Here, A. tasmaniae was found sympatrically with A. swaini (form 1) and although A. tasmaniae and A. swaini were found outside the cave entrance, as well as in the twilight zone (up to 30 m inside the cave), both species showed no signs of cave adaptation.
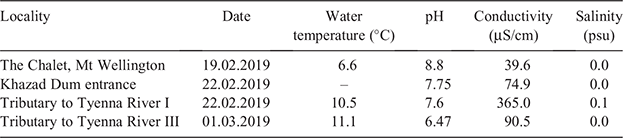
Water quality parameters were taken from four different collection sites of A. tasmaniae: The Chalet, Mt Wellington; Khazad Dum cave; Tributary to the Tyenna River I and III (Table 1). The water temperatures ranged from 6.6°C at Mt Wellington to 10.5–11.1°C at the other collection sides. The pH ranged from slightly acidic (6.47 at Tributary to the Tyenna River III) to alkaline (8.8 at Mt Wellington, 7.75 at Khazad Dum cave and 7.6 at Tributary to the Tyenna River I). The conductivity within the localities was always low, ranging from 39.6 µS/cm at Mt Wellington over 74.9 µS/cm at Khazad Dum and 90.5 µS/cm at Tributary to the Tyenna River I to 365 µS/cm at Tributary to the Tyenna River III. The salinity ranged in all localities between 0.0 and 0.1 psu.
|
Analyses of COI and 16S sequences of Anaspides tasmaniae from each of the studied localities recovered three haplotype lineages with six different haplotypes in COI and three haplotypes in 16S (Fig. 4). The first lineage comprises specimens found on the east and south-east side of Mt Wellington, whereas the specimens of the remaining two lineages are from within and adjacent to the Mt Field National Park. Specimens of the second lineage are from the entrance creek of Khazad Dum and specimens of the third lineage are from tributaries of the Tyenna River I, II and III draining from Nicholls Spur and Tyenna Peak (Fig. 3). Between the lineages we found only slight genetic differentiation (uncorrected p-distances: COI: 1.1–1.6%, 16S: 0.3–0.6%). In 28S, individuals of all three lineages share the same haplotype (Fig. 4).
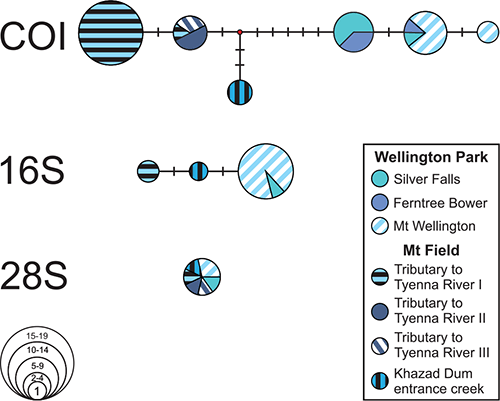
|
Discussion
After the revision of Anaspides by Ahyong (2016), the type species of the genus, A. tasmaniae, was shown not to be widespread throughout Tasmania, as had long been believed. It has a much more restricted range, confirmed by Ahyong (2016) only from the east and south-east side of Mt Wellington. We now provide the first records of true A. tasmaniae from outside of Mt Wellington. Anaspides tasmaniae is present in three tributaries of the Tyenna River near Mt Field National Park as well as in the inflow waters of Khazad Dum (Fig. 5b, g). These localities are more than 50 km from the localities at which A. tasmaniae occurs at Mt Wellington and the water bodies are connected only via the Tyenna and Derwent rivers, in which Anaspides, however, does not occur (Fig. 1). Notably, A. tasmaniae occurs together with A. swaini (form 1) in the inflow creek of Khazad Dum, which is one of the few documented instances of sympatry in Anaspides and the only known locality where two Anaspides species actually occur in syntopy. There is no evidence for gene flow between these two species (unpubl. data).
Ahyong (2016) reported specimens from Khazad Dum cave (Ahyong 2016, fig. 34E–H) with a more rounded posterior telson margin as in A. tasmaniae, armed pleonite 5–6 terga and usually armed pleura as well, but tentatively considered these specimens to be aberrant A. swaini. We now reidentify these specimens as A. tasmaniae. Our specimens showed no apparent cave adaptations, which was also noted by Ahyong (2016). Interestingly, Ahyong (2016) mentioned also anomalous specimens similar to those from Khazad Dum from Risby Basin cave (located at the foot of the Maydena Range south of Gordon River Road, 8 km from Khazad Dum and draining as well into the Tyenna River), which potentially could also represent A. tasmaniae.
Furthermore, our searches ‘confirmed’ the absence of Anaspides on the Wellington Plateau. Anaspides was historically recorded from tarns on the plateau but has not been recorded there since major bushfires swept the area in the 1930s, permanently altering the habitat, resulting in the loss of tarns and swamp flora (O’Brien 1990). Ahyong (2016) also mentioned that Manton (1930) observed two colour forms in Anaspides on Mt Wellington: a dark brown to olive-green form found on the Mt Wellington plateau, and a light brown form occurring on the slopes. Manton’s dark form probably refers to Anaspides swaini (form 3), and the light brown form, to A. tasmaniae. Our observations support these hypotheses with A. tasmaniae ranging from light to medium brown (Fig. 5d–f, h) and Anaspides swaini (form 3) being much darker, ranging from dark brown to dark olive green. In the Browns River catchment, we found only A. tasmaniae, although specimens of A. swaini (form 3) were collected there in the early 20th century, having presumably been translocated from the North-west Bay catchment via a now defunct aqueduct (Ahyong 2016). Evidently, the translocated A. swaini population has not survived.
The discovery of A. tasmaniae at independent localities around Mt Field, all distant from Mt Wellington, begs the question of whether the observed distribution is artificial or natural. Although A. swaini appears to have been translocated as a result of water-supply engineering in the early 20th century, no similar activities have occurred between Mt Wellington and Mt Field, nor are the localities in question subject to fishery stocking or other plausible interventions. Either way, if the Mt Field populations of A. tasmaniae were the result of artificial translocation from Mt Wellington, shared haplotypes would be expected. However, the genetic divergence between Mt Wellington and Mt Field populations indicates that the disjunct distribution is more likely to be natural, and we hypothesise that it relates to glacial events. The main limiting factor for the distribution of Anaspides appears to be water temperature (Williams 1965a; Swain and Reid 1983; O’Brien 1990). The species prefer cold water, although they are able to temporarily tolerate elevated water temperatures (Williams 1965a; Swain and Reid 1983). Also, the concentration of dissolved solids and turbidity could play a role in their distribution (Williams 1965a; Swain and Reid 1983; O’Brien 1990). Smith (1908) described the waters in which Anaspides occurs as ‘ice-cold waters of absolute clarity’, which is consistent with our field observations. Considering this, and that temperatures during glacial periods in the Pleistocene were 6–10°C lower than today, in combination with a lowered snowline (~1000–1500 m lower than current) and a lowered sea level (~120 m, 25 000 years ago) (Colhoun 1985; Lambeck and Chappell 2001; Thrush 2008; Colhoun et al. 2010), the Tyenna and Derwent rivers were potentially favourable habitats at that time. Thus, during glacial periods, A. tasmaniae may have occupied a wider range including lower altitudes that encompassed the Tyenna and Derwent River catchments during the Pleistocene glaciations. The top of the mountains might not have been inhabited during glaciations although it is not clear whether the plateau of Mt Wellington had an ice cap (Davies 1958) whereas the plateaus of the Mt Field National Park were glaciated several times (Kiernan 1990; Mackintosh et al. 2006; Colhoun et al. 2010). The increasing interglacial temperatures would have led to contractions of suitable Anaspides habitat throughout Tasmania. In the case of A. tasmaniae, we suggest it occupied a wider continuous range during the Pleistocene glacials, and with postglacial warming became restricted to the higher and colder areas at Mt Wellington and in the Mt Field area, where they are relictual today.
Conservation
Although our research extends the known distribution (four new collection sites) of A. tasmaniae as well as its known genetic diversity through discovery of three distinct haplotype clusters in COI and 16S, the overall geographic range is still limited and the populations are vulnerable. This is especially the case for the three tributaries of the Tyenna River, which are outside the protected area of the Mt Field National Park in low-altitude areas. Additionally, the presence of Anaspides swaini (form 3) in adjacent drainages at Mt Wellington suggests that A. tasmaniae is indeed limited to the three water catchments at the east and south-east side of Mt Wellington. As Ahyong (2016) stated, its conservation status requires reassessment to precisely determine its current distribution and population size, especially within the Mt Field National Park.
The Mt Field National Park and the Junee–Florentine Karst area can be considered as a hotspot of Anaspides diversity, with four species and several different genetic lineages occurring in that area. Furthermore, Mt Field National Park and the adjacent landscapes have great importance for the populations of A. tasmaniae, which is found only in a small range at the east and south-east of Mt Wellington and in four different localities in and alongside the Mt Field National Park. Current climatic trends will probably see further contraction of suitable habitats of Anaspides to higher altitudes in the future, making conservation planning even more poignant. It is uncertain if the Mawson Plateau or Mount Wellington Plateau will again become suitable habitats for Anaspides in the future. For the protection of this iconic species we suggest formal protection of the habitat of A. tasmaniae in the Mt Field area, perhaps by extension of the Mt Field National Park south over the Nicholls Spur to the Florentine Road between Junee Quarry Road and Drewhurst Road.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Collection of specimens in 2017 was funded by the German Science Foundation (DFG RI 837/22-1) and in 2019 was partly funded by the DAAD (Nr. 57438025, Kurzstipendien für Doktoranden, 2019/2020) and the University of Rostock (Promotionsstipendienprogramm, ‘Unsere Besten promovieren in Rostock’). The present project is funded by the German Science Foundation (DFG RI 837/22-2).
Acknowledgements
We are grateful to Alastair Richardson, Michael Driessen, Arthur Clarke, Cathy Plowman, David Butler, and Stefan Eberhard for enthusiastic support of our Anaspides research. We further thank the University of Tasmania, Institute for Zoology, especially David Green, for providing us with scientific equipment for our field trips. We are thankful to Markus Grams and Marian Reinhardt for assistance in the field and to Andrew Palfreyman for providing a new record on Mt Wellington. Specimens were collected under Tasmanian Department of Primary Industries, Parks, Water and Environment permit nos TFA16347 and FA18250. Ralf Bastrop (University of Rostock) provided the infrastructure for sequencing some of the data. We are grateful to the reviewers for their comments and suggestions.
References
Ahyong, S. T. (2015). Preliminary diagnoses of three new species of Tasmanian mountain shrimps, Anaspides Thomson, 1894 (Syncarida, Anaspidacea, Anaspididae). Zootaxa 3957, 596–599.| Preliminary diagnoses of three new species of Tasmanian mountain shrimps, Anaspides Thomson, 1894 (Syncarida, Anaspidacea, Anaspididae).Crossref | GoogleScholarGoogle Scholar | 26249099PubMed |
Ahyong, S. T. (2016). The Tasmanian mountain shrimps, Anaspides Thomson, 1894 (Crustacea, Syncarida, Anaspididae). Records of the Australian Museum 68, 313–364.
| The Tasmanian mountain shrimps, Anaspides Thomson, 1894 (Crustacea, Syncarida, Anaspididae).Crossref | GoogleScholarGoogle Scholar |
Ahyong, S. T., and Alonso-Zarazaga, M. A. (2017). Anaspidesidae, a new family for syncarid crustaceans formerly placed in Anaspididae Thomson, 1893. Records of the Australian Museum 69, 257–258.
| Anaspidesidae, a new family for syncarid crustaceans formerly placed in Anaspididae Thomson, 1893.Crossref | GoogleScholarGoogle Scholar |
Benson, D. A., Karsch-Mizrachi, I., Lipman, D. J., Ostell, J., and Wheeler, D. L. (2003). GenBank. Nucleic Acids Research 31, 23–27.
| GenBank.Crossref | GoogleScholarGoogle Scholar | 12519940PubMed |
Colhoun, E. A. (1985). Glaciations of the west coast range, Tasmania. Quaternary Research 24, 39–59.
| Glaciations of the west coast range, Tasmania.Crossref | GoogleScholarGoogle Scholar |
Colhoun, E. A., Kiernan, K., Barrows, T. T., and Goede, A. (2010). Advances in Quaternary studies in Tasmania. In ‘Australian Landscapes’. (Eds P. Bishop, and B. Pillans.) pp. 165–183. (Geological Society: London.)
Davies, J. L. (1958). The cryoplanation of Mount Wellington. Papers and Proceedings of the Royal Society of Tasmania 92, 151–154.
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294–299.
| 7881515PubMed |
Goede, A (1967). Tasmanian cave fauna: character and distribution. Helictite 5, 71–86.
Jarman, S. N., and Elliott, N. G. (2000). DNA evidence for morphological and cryptic Cenozoic speciations in the Anaspididae, ‘living fossils’ from the Triassic. Journal of Evolutionary Biology 13, 624–633.
| DNA evidence for morphological and cryptic Cenozoic speciations in the Anaspididae, ‘living fossils’ from the Triassic.Crossref | GoogleScholarGoogle Scholar |
Kiernan, K. (1990). The extent of late Cenozoic glaciation in the Central Highlands of Tasmania, Australia. Arctic and Alpine Research 22, 341–354.
| The extent of late Cenozoic glaciation in the Central Highlands of Tasmania, Australia.Crossref | GoogleScholarGoogle Scholar |
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35, 1547–1549.
| MEGA X: molecular evolutionary genetics analysis across computing platforms.Crossref | GoogleScholarGoogle Scholar | 29722887PubMed |
Lake, P. S., and Coleman, D. J. (1977). On the subterranean syncarids of Tasmania. Helictite 15, 12–17.
Lambeck, K., and Chappell, J. (2001). Sea level change through the last glacial cycle. Science 292, 679–686.
| Sea level change through the last glacial cycle.Crossref | GoogleScholarGoogle Scholar | 11326090PubMed |
Mackintosh, A. N., Barrows, T. T., Colhoun, E. A., and Fifield, L. K. (2006). Exposure dating and glacial reconstruction at Mt. Field, Tasmania, Australia, identifies MIS 3 and MIS 2 glacial advances and climatic variability. Journal of Quaternary Science 21, 363–376.
| Exposure dating and glacial reconstruction at Mt. Field, Tasmania, Australia, identifies MIS 3 and MIS 2 glacial advances and climatic variability.Crossref | GoogleScholarGoogle Scholar |
Manton, S. M. (1930). 36. Notes on the habits and feeding mechanisms of Anaspides and Paranaspides (Crustacea, Syncarida). Proceedings of the Zoological Society of London 100, 791–800.
| 36. Notes on the habits and feeding mechanisms of Anaspides and Paranaspides (Crustacea, Syncarida).Crossref | GoogleScholarGoogle Scholar |
O’Brien, D. P. (1990). The conservation status of the mountain shrimp (Anaspides tasmaniae and Anaspides spinulae): a report on its distribution, ecology and taxonomy, including recommendations for management. Department of Parks, Wildlife and Heritage, Hobart.
Richter, S., Olesen, J., and Wheeler, W. C. (2007). Phylogeny of Branchiopoda (Crustacea) based on a combined analysis of morphological data and six molecular loci. Cladistics 23, 301–336.
| Phylogeny of Branchiopoda (Crustacea) based on a combined analysis of morphological data and six molecular loci.Crossref | GoogleScholarGoogle Scholar |
Richter, S., Schwentner, M., Wirkner, C. S., and Ahyong, S. T. (2018). Phylogeny and species diversity of Tasmanian mountain shrimps and their relatives (Crustacea, Anaspidesidae). Zoologica Scripta 47, 84–105.
| Phylogeny and species diversity of Tasmanian mountain shrimps and their relatives (Crustacea, Anaspidesidae).Crossref | GoogleScholarGoogle Scholar |
Smith, G. W. (1908). Preliminary account of the habits and structure of the Anaspididae, with remarks on some other fresh-water Crustacea from Tasmania. Proceedings of the Royal Society of London 80, 465–473.
Swain, R., and Reid, C. I. (1983). Observations on the life history and ecology of Anaspides tasmaniae (Thomson) (Syncarida: Anaspididae). Journal of Crustacean Biology 3, 163–172.
| Observations on the life history and ecology of Anaspides tasmaniae (Thomson) (Syncarida: Anaspididae).Crossref | GoogleScholarGoogle Scholar |
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22, 4673–4680.
| CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice.Crossref | GoogleScholarGoogle Scholar | 7984417PubMed |
Thomson, G. M. (1893). Notes on Tasmanian Crustacea, with descriptions of new species. Papers & Proceedings of the Royal Society of Tasmania , 45–76.
Thomson, G. M. (1894). III. On a freshwater schizopod from Tasmania. Transactions of the Linnean Society of London, 2nd Series. Zoology 6, 285–303.
Thrush, M. N. (2008). The Pleistocene glaciations of the Cradle Mountain region, Tasmania. Ph.D. Thesis, University of Newcastle, Newcastle.
Williams, W. D. (1965a). Ecological notes on Tasmanian Syncarida (Crustacea: Malacostraca), with a description of a new species of Anaspides. Internationale Revue der Gesamten Hydrobiologie und Hydrographie 50, 95–126.
| Ecological notes on Tasmanian Syncarida (Crustacea: Malacostraca), with a description of a new species of Anaspides.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1965b). Subterranean occurrence of Anaspides tasmaniae (Thomson) (Crustacea, Syncarida). International Journal of Speleology 1, 333–337.
| Subterranean occurrence of Anaspides tasmaniae (Thomson) (Crustacea, Syncarida).Crossref | GoogleScholarGoogle Scholar |
Xiong, B., and Kocher, T. D. (1991). Comparison of mitochondrial DNA sequences of seven morphospecies of black flies (Diptera: Simuliidae). Genome 34, 306–311.
| Comparison of mitochondrial DNA sequences of seven morphospecies of black flies (Diptera: Simuliidae).Crossref | GoogleScholarGoogle Scholar | 2055453PubMed |