A new species of Phryssonotus (Diplopoda: Synxenidae) from north-eastern New South Wales, Australia
Cuong Huynh A * and Anneke A. Veenstra
A * and Anneke A. Veenstra  A
A
A
Abstract
Phryssonotus borealis is a new synxenid species found in north-eastern New South Wales, Australia. This species differs from other Phryssonotus species by its white tergal scale trichomes and its genetic make-up.
ZooBank: urn:lsid:zoobank.org:pub:9BA52C7B-C1E3-421C-A37D-4A3D6FD39EEB
Keywords: 18S, COI, northern, parthenogenesis, phylogenetic analysis, scale trichome patterns.
Introduction
The family Synxenidae (Polyxenida) comprises two genera: Phryssonotus Scudder, established in 1885 (Scudder 1885), and Condexenus, described by Nguyen Duy-Jacquemin (2006). One species, Phryssonotus novaehollandiae, endemic to Australia, was first identified by Silvestri (1923) from a single juvenile specimen in the stadium V, featuring eight pairs of legs and 10 ommatidia, collected at Mount Lofty, South Australia. Diagnostic features for juvenile Phryssonotus, based on the number of ommatidia, were subsequently applied to adult specimens, leading to confusion in distinguishing between P. novaehollandiae and P. capensis, both of which possess 10 ommatidia. In 1984, this resemblance led Condé and Nguyen Duy-Jacquemin (1984) to mistakenly identify specimens from Australia and Papua New Guinea as P. capensis. Short and Huynh (2009) later confirmed that P. novaehollandiae was the only species present in Australia; this species possesses 11 ommatidia in the adult stage, which is clearly different from P. capensis. Despite this, Phryssonotus populations collected from different locations in Australia are biologically and morphologically distinct, displaying variations in sexual and asexual populations and in the patterns of tergal scale trichomes. This diversity led to the description of two distinct species, Phryssonotus australis and P. occidentalis, by Huynh and Veenstra (2017). Recently a new Phryssonotus species collected from north-eastern New South Wales exhibited distinct white colour patterns on its tergites, contrasting with the black colouration observed in its predecessors (Fig. 1). The combination of colour pattern characteristics and genetic evidence supports the description of Phryssonotus borealis, sp. nov. as a novel species within the family Synxenidae.
Materials and methods
Collection
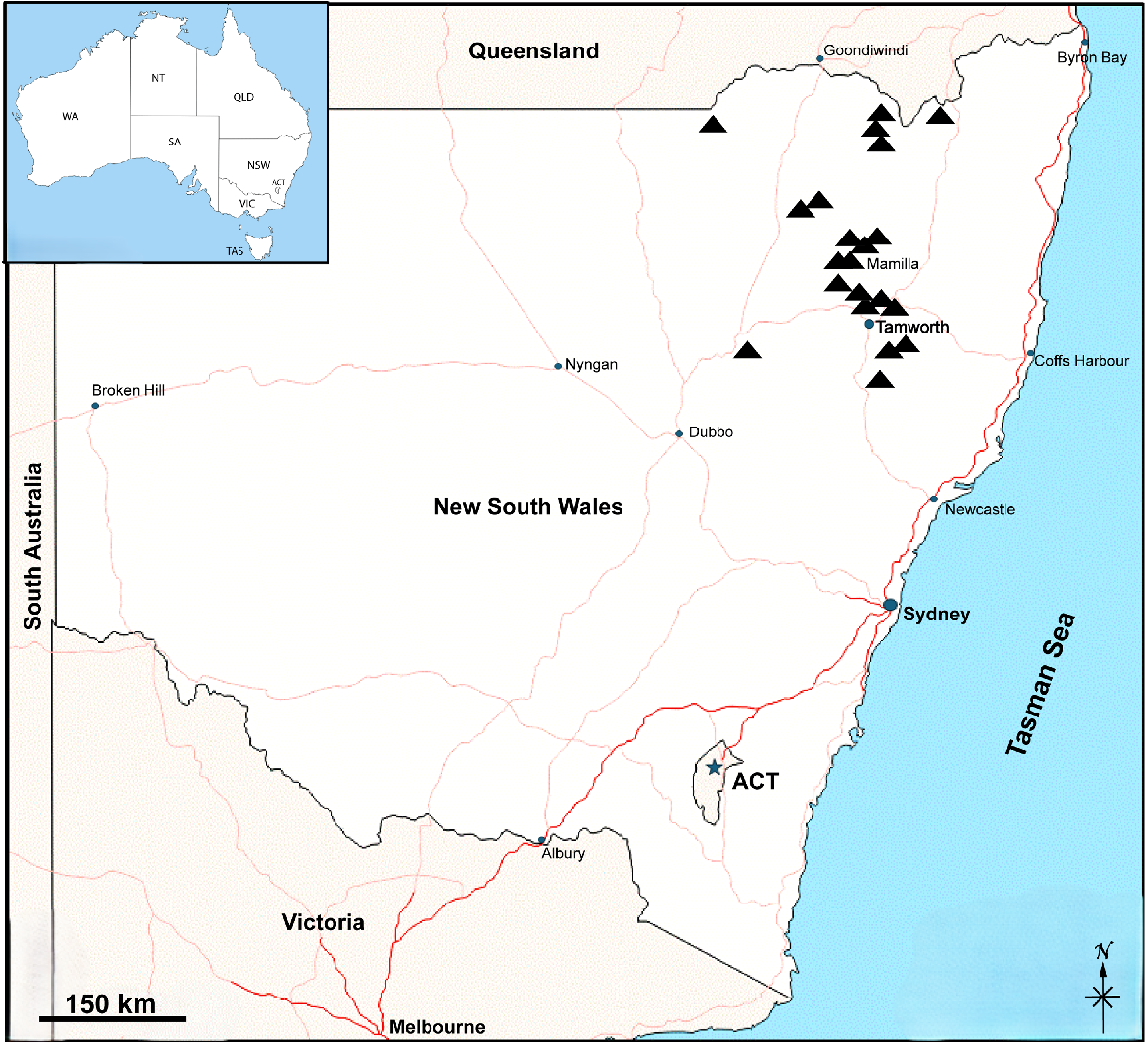
Fifteen live Phryssonotus specimens, subadult stage with 14 pairs of legs, were collected from leaf litter at the base of a river red gum tree (Eucalyptus camaldulensis), approximately 100 m from the entrance of the Manilla Rivergum Caravan Park (30.743830S, 150.729166E; elevation 361 m) in Manilla, New South Wales, Australia, on 20 October 2022, by author Cuong Huynh (Fig. 2). The live specimens were subsequently reared to adult – 17 pairs of legs stage – to observe the dorsal arrangement patterns and colour variations of their tergal scale trichomes. Additionally, moulted exoskeletons were collected and used for detailed scale trichome measurements.
Phryssonotus specimen collection sites (▲) in the State of New South Wales, the location within Australia indicated on the inserted map (map source: d-maps.com).
Additional specimens
Preserved Phryssonotus specimens in collections from the north-eastern regions of New South Wales, Australia, at the Australian Museum were examined. In total, 31 vials were examined, including from Tamworth (accession numbers KS.87381–3, KS.87385, KS.87388, KS.87392–4, KS.87396–7, KS.87409, KS.87411, KS.87413, KS.87415, KS.87416); Linton National Park (KS.87386–7, KS.87399); Warrabah (KS.87414); Altholwood (KS.87389); Woodsreff (KS.87390, KS.87395); Manilla (KS.87398, KS.87405, KS.87412); Tenterfield (KS.87401); Inverell (KS.87402); Asford (KS.87404); Narrabri (KS.87403, KS.87407); Rowena (KS.87707) (Table 1).
| Museum accession numbers | Town | Latitude | Longitude | Specimens | Location | Collector | Date | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | KS.87381 | Tamworth | 31 04 30S | 151 01 40E | 1af | Crownland road side reserve, corner New England Highway & old Tamworth Rd | G. Carter | 6-Dec-2001 | |
| 2 | KS.87382 | Tamworth | 31 40 48S | 150 49 00E | 2af | Chikotts Creek Road, North of Murnrurdi, NSW | Wilky & Smith | 6-Dec-2001 | |
| 3 | KS.87383 | Tamworth | 30 14 19S | 150 06 17E | 1af | Mount Kaputar National Park, 600 m below end of Bullawa Creek Road, NE Narrabri | Wilky & Smith | 11-Dec-2001 | |
| 4 | KS.87365 | Tamworth | 31 04 30S | 151 01 40E | 2af | Crownland road side reserve, corner New England Highway & old Tamworth Rd | G. Carter | 6-Dec-2001 | |
| 5 | KS.87388 | Tamworth | 29 02 26S | 151 42 15E | 10f 10L | Crown Reserve, 2.5 km along Gibraltar Road, off Bruxner Highway | G. Carter | 6-Dec-2001 | |
| 6 | KS.87392 | Tamworth | 31 04 05S | 151 02 04E | 8af | Crown Reserve, 200 m past tip between Kootingal and Tamworth | G. Carter | 6-Dec-2001 | |
| 7 | KS.87393 | Tamworth | 31 04 05S | 151 02 04E | 12af | Crown Reserve, 200 m past tip between Kootingal and Tamworth | G. Carter | 6-Dec-2001 | |
| 8 | KS.87394 | Tamworth | 31 04 05S | 151 02 04E | 6af | Crown Reserve, 200 m past tip between Kootingal and Tamworth | G. Carter | 6-Dec-2001 | |
| 9 | KS.87396 | Tamworth | 31 21 29S | 151 08 40E | 1af + 1/2 body | Crown Reserve, 8 km south of Woolomin, SE Tamworth | Wilky & Smith | 6-Dec-2001 | |
| 10 | KS.87397 | Tamworth | 31 04 30S | 151 01 40E | 3af | Crownland road side reserve, corner New England Highway & old Tamworth Rd | G. Carter | 6-Dec-2001 | |
| 11 | KS.87409 | Tamworth | 31 18 51S | 151 09 20E | 1af | Crown Reserve, SE Tamworth | Wilky & Smith | 15-Dec-2001 | |
| 12 | KS.87411 | Tamworth | 30 58 33S | 150 55 48E | 1af | Attunga State Forest, NW Tamworth | G. Carter | 7-Dec-2001 | |
| 13 | KS.87413 | Tamworth | 31 03 34S | 150 55 49E | 4af | Crown Reserve, 1.7 km Forrest Rd, 40 m right along track slope on hill, Tamworth | Doherty & Eliot | 6-Dec-2001 | |
| 14 | KS.87415 | Tamworth | 31 03 34S | 150 55 49E | 1af | Crown Reserve, 1.7 km Forrest Rd, 400 m right along track, mid-slope on hill, Tamworth | Doherty & Eliot | 6-Dec-2001 | |
| 15 | KS.87416 | Tamworth | 30 58 33S | 150 55 48E | 1af damaged | Attunga State Forest, NW Tamworth | G. Carter | 7-Dec-2001 | |
| 16 | KS.87386 | Linton NP | 30 27 51S | 150 53 31E | 2af | Linton Nature Reserve, 300 m from reserve entrance from Warrabeh | Doherty & Eliot | 9-Dec-2001 | |
| 17 | KS.87387 | Linton NP | 30 26 56S | 150 51 SSE | 1af 16L | Linton Nature Reserve, 500 m past fork in road, NW side of reserve. 100 m East from road | Doherty & Eliot | 9-Dec-2001 | |
| 18 | KS.87399 | Linton NP | 30 26 56S | 150 51 58E | 1af | Linton Nature Reserve, 700 m West of entrance | Doherty & Eliot | 9-Dec-2001 | |
| 19 | KS.87414 | Warrabah | 30 27 51S | 150 53 1E | 1af | Linton Nature Reserve, 300 m from reserve entrance from Warrabeh. | Doherty & Eliot | 9-Dec-2001 | |
| 20 | KS873S9 | Atholwood | 29 07 04S | 151 08 02E | 1af+ 4L | Seven State Forest, Atholwood loop Rd | Wilky & Smith | 13-Dec-2001 | |
| 21 | KS.87390 | Woodsreef | 30 23 39S | 150 44 08E | 8L | Crown Reserve Woods reef, between road and Nangharah Creek | Doherty & Eliot | 9-Dec-2001 | |
| 22 | KS.87395 | Woodsreef | 30 23 39S | 150 44 08E | 1af | Crown Reserve Woods reef, between road and Nangharah Creek | Doherty & Eliot | 9-Dec-2001 | |
| 23 | KS.87398 | Manilla | 30 4731S | 150 29 40E | 5af + 123L | Dowe State Forest, West Manilla | Wilky & Smith | 14-Dec-2001 | |
| 24 | KS.87412 | Manilla | 30 4731E | 150 29 40E | 1af + 23L | Dowe State Forest, West Manilla | Wilky & Smith | 14-Dec-2001 | |
| 25 | KS.87405 | Manilla | 30 4731S | 150 29 40E | 2af + 10L | Dowe State Forest, South of Central trail, West of Manila | Wilky & Smith | 14-Dec-2001 | |
| 26 | KS.87401 | Tenterfield | 29 02 26S | 151 42 15E | 1af | Crown Reserve, 2.5 km along Gibraltar Rd, off Bruxner Hwy. West Tenterfield | Wilky & Smith | 13-Dec-2001 | |
| 27 | KS.87402 | Inverell | 29 25 59S | 151 04 18E | 6L | Crown Reserve, 8.9 km along Bukkula-Ashford Rd. North lnverell | Wilky & Smith | 13-Dec-2001 | |
| 28 | KS.87404 | Asford | 29 10 46S | 151 00 18E | 4af | Kwiambal National Park, East side of Park,150 m South of road. North West Asford | Doherty & Eliot | 13-Dec-2001 | |
| 29 | KS.87407 | Narrabri | 30 14 21S | 150 05 31E | 1af | Mt Kaputar National Park, end Bullawa Creek track, 50 m NE picnic area, NE Narrabri | Wilky & Smith | 11-Dec-2001 | |
| 30 | KS.87403 | Narrabri | 30 14 15S | 150 05 23E | 2af | Mt Kaputar National Park, end Bullawa Creek track, 1.1 km past Foggy Dell turnoff. NE Narrabri | Wilky & Smith | 11-Dec-2001 | |
| 31 | KS.87707 | Rowena | 29 48 57S | 148 56 50E | 1af | Cameron Lane, 4.6 km West of Burren-Pokataroo Rd junction. East Rowena | Wilky & Smith | 20-Dec-1999 |
a, adult; f, female; L, leg.
Morphological study
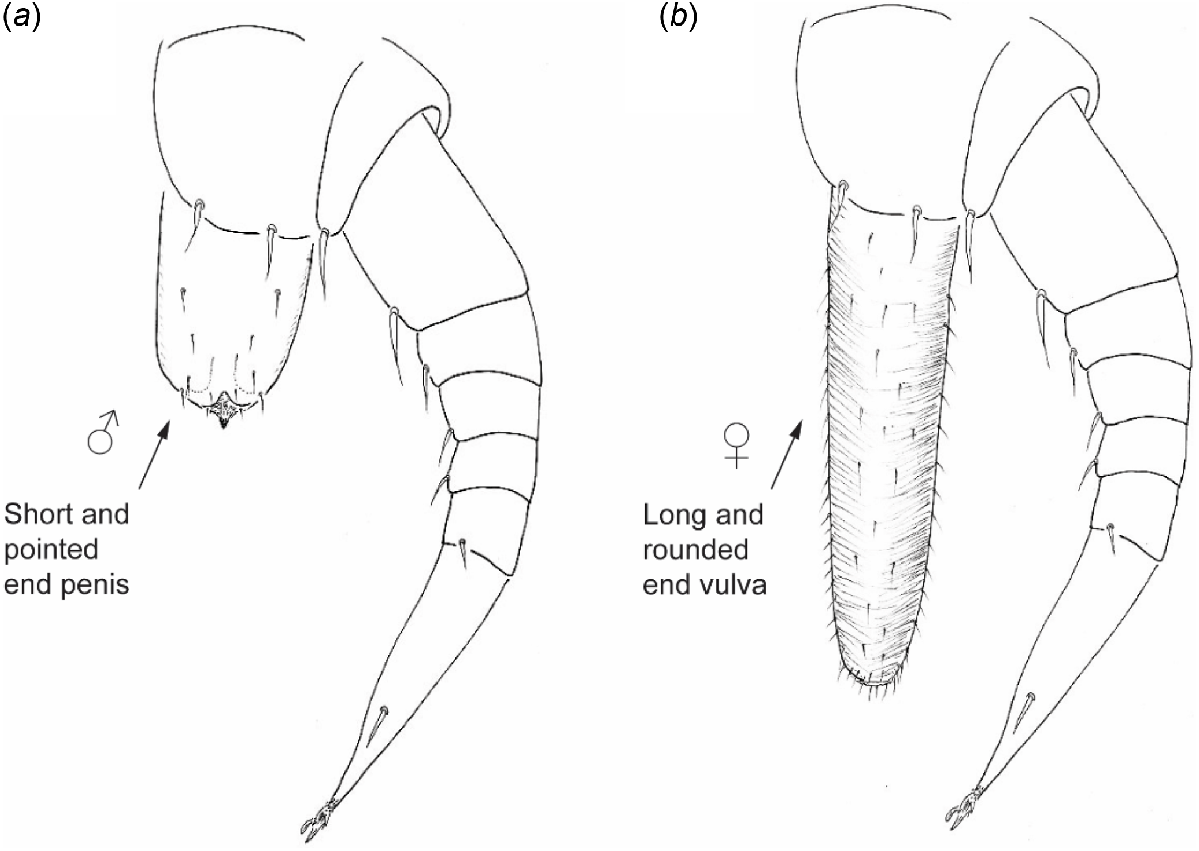
Morphological characteristics of Phryssonotus specimens were examined following the methods of Huynh and Veenstra (2017). The sex of adult specimens (17 pairs of legs) was determined by the presence of reproductive organs on the second coxal plates: vulva in females and penes in males (Fig. 3a, b). Body length (mm) was measured from the vertex of the head to the tip of the telson. The tergal scale trichomes were measured for length-to-width ratios (μm), and their patterns and colour were recorded photographically (Figs 1 and 5).
The second legs of Phryssonotus species showing the sex organs. (a) Male sex organ – a short and pointed end penis; (b) female sex organ – a long and rounded end vulva.
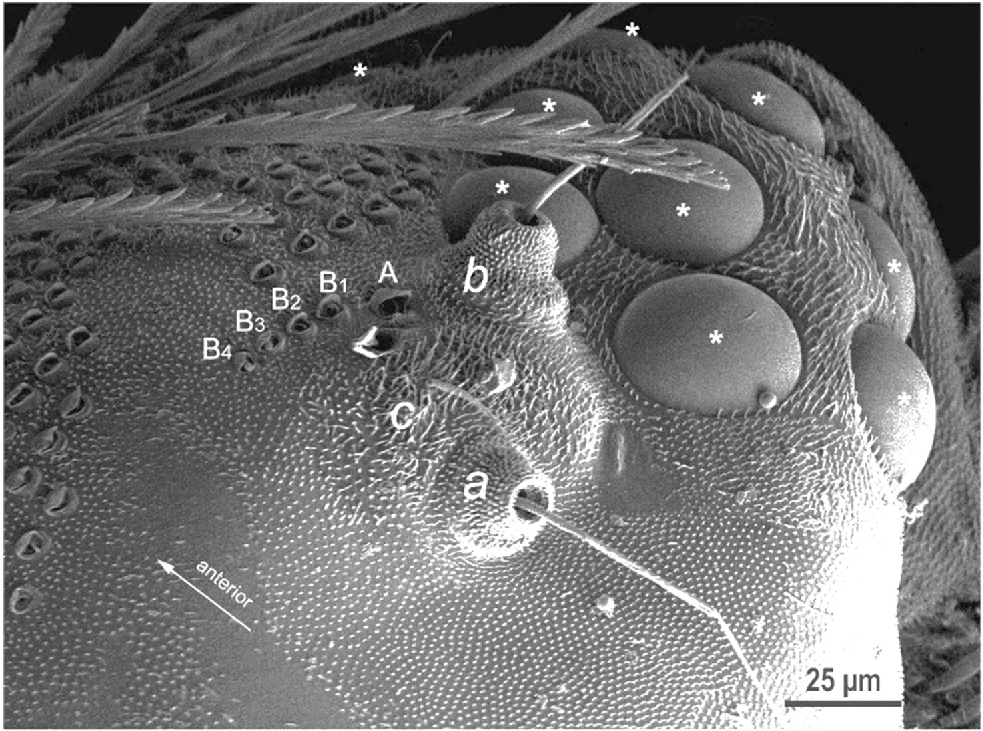
The taxonomic classification of the species was based on the presence of a long frontal trichome A and the short frontal trichome B sockets next to the sensory organs – trichobothria located near the eye region of the head (Fig. 4), following the classification criteria of Short and Huynh (2009).
Genetic study
The specimen preparation technique of Short and Huynh (2010) was modified to facilitate DNA extraction. Preserved specimens were transferred from 100% ethanol onto a microscope slide, where the scale trichomes were carefully stripped. A drop of DPX mounting medium was applied, and a coverslip was placed over the scale trichomes for microscopic analysis of characteristics. The stripped specimens were then used for DNA extraction using the DNeasy Blood and Tissue Kit (Qiagen, https://www.qiagen.com.au). Genomic DNA was preserved for further study. Two gene markers were targeted for analysis: The 18S small subunit ribosomal RNA gene (using primers 1F and 5R: White et al. 1990) and the mitochondrial cytochrome c oxidase subunit I gene (CO1) (using primers dgLCO1490 and dgHCO2198: Meyer 2003). These primers are conserved and widely used for molecular identification at the genus and species levels (Huynh and Veenstra 2018; Huynh et al. 2023). The CO1 region was included due to its standard use in the Barcode of Life project (2010–2025; https://boldsystems.org) for species identification. The polymerase chain reaction (PCR) protocol followed that of Huynh and Veenstra (2017). DNA sequences of Phryssonotus borealis, sp. nov. were deposited in GenBank under the accession numbers PQ346279 (18S) and PQ344739 (CO1). BLAST searches (http://www.ncbi.nlm.nih.gov) were conducted to identify sequences of closely related species. Sequence alignments were performed using BioEdit (Hall 1999), and MEGA 11 (Kumar et al. 2016) was used to construct a bootstrap maximum-likelihood tree for phylogenetic analysis. Bootstrap values were indicated above the branches. GenBank accession numbers for sequences from other penicillate millipede species and outgroups are provided in Table 2.
| Family | Species | Collection location | GenBank accession number | ||
|---|---|---|---|---|---|
| 18S rRNA | CO1 | ||||
| Polyxenidae | Monographis sp. | China | AY596371 | - | |
| Monographis queenslandicus | Boodall Park, Qld, Australia | KF147166 | - | ||
| Monographis dongnaiensis | Trang Bom, Dong Nai, Vietnam | KP255446 | - | ||
| Unixenus mjobergi | Masthead Island, Qld, Australia | MF592755 | - | ||
| Unixenus karajinensis | Pilbara, WA, Australia | MF592754 | - | ||
| Unixenus corticolus | Deep Lead, Vic, Australia | - | MF592722 | ||
| Polyxenus lagurus | Europe | AY859596 | - | ||
| Polyxenus lagurus | Europe | - | HQ966144 | ||
| Polyxenus fasciculatatus | Europe | AF173235 | - | ||
| Eudigraphis Takakuwai nigricans | Japan | - | LC010907 | ||
| Synxenidae | Phryssonotus australis (Parthenogenetic, coastal population) | Point Addis, Vic, Australia | KY820871 | KY820869 | |
| Phryssonotus borealis (Parthenogenetic, north-east NSW population) | Manilla, NSW, Australia | PQ346279 | PQ344739 | ||
| Phryssonotus novaehollandiae (Sexual, inland population) | Stawell, Vic, Australia | KY820870 | KY820867 | ||
| Phryssonotus occidentalis (Sexual population) | Fitzgerald River National Park, WA, Australia | KY820872 | KY820868 | ||
| Outgroups | Sphaeromimus musicus | Europe | FJ409961 | - | |
| Procyliosoma leae | Australia | FJ409955 | - | ||
| Glomeridella minima | Europe | - | JN271878 | ||
| Pogonostermum sp. | Australia | - | KU745274 | ||
NSW, New South Wales; Qld, Queensland; Vic, Victoria; WA, Western Australia.
Results
Both the live Phryssonotus specimens and those from the museum consisted exclusively of females, suggesting that this population is parthenogenetic in this geographic region. Adult Phryssonotus specimens showed diagnostic morphological features, including 11 ommatidia, a sensory organ – a long frontal trichome A, and short frontal trichomes B (B1–B5) are adjacent to trichobothrium c(Fig. 4). The characteristics of these structures align with taxonomically important morphological features previously identified in Australian Phryssonotus species, consistent with findings of Short and Huynh (2009). The body length of live specimens ranged from 3.2 to 3.4 mm (n = 14), closely matching the size of Phryssonotus occidentalis (3.4 mm) from western Australia but slightly shorter than P. australis (range: 3.6–3.8 mm), parthenogenetic populations, from Victoria and Tasmania, and P. novaehollandiae (3.8 mm) from Victoria and South Australia (Huynh and Veenstra 2017). Additionally, adult Phryssonotus specimens showed scale trichome patterns differing from those of P. australis, P. novaehollandiae and P. occidentalis, with distinctive tergal scale trichomes forming a white medial trapezoid band, with small white stripes laterally on the body (Fig. 5).
Variation in scale trichome patterns on live specimens and illustrations of tergal scale trichome patterns seen in Phryssonotus species in Australia. (a) P. borealis, sp. nov., (b) P. australis, (c) P. novaehollandiae, (d) P. occidentalis.

A comparison of scale trichome length-to-width ratios in this new Phryssonotus species gave a ratio of 1:60 (n = 100 scale trichomes), which resembles that of P. occidentalis (1:60) but is smaller than those of P. australis (1:64) and P. novaehollandiae (1:75) (Huynh and Veenstra 2017).
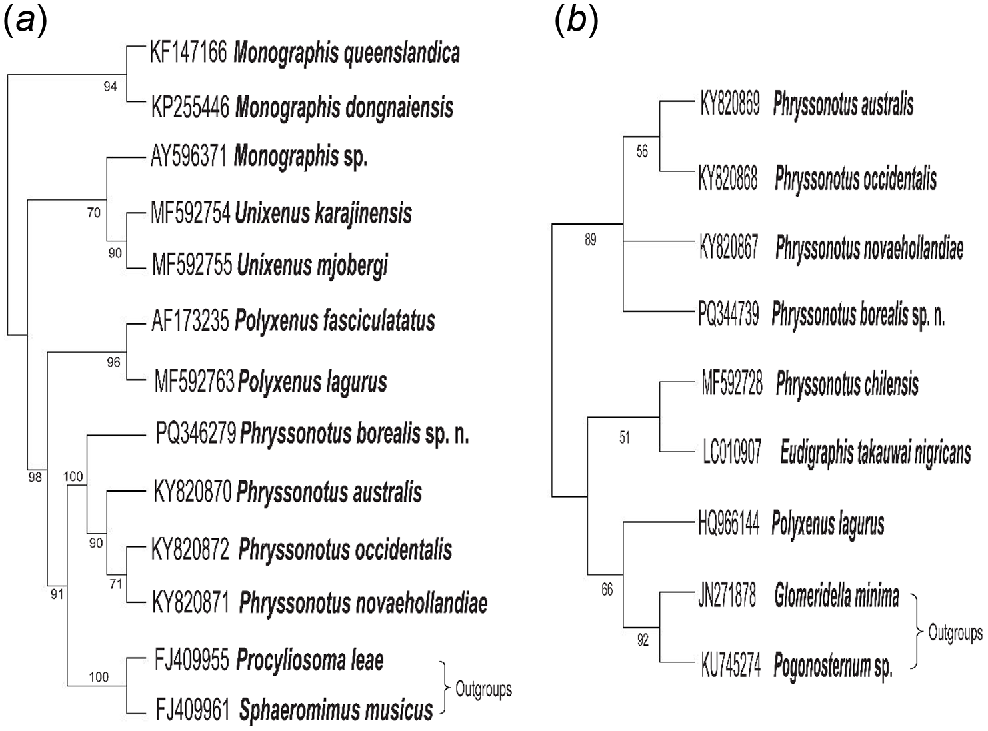
The 18S maximum-likelihood phylogenetic tree, constructed using 1000 bootstrap replications, yielded a strongly supported clade comprising four Phryssonotus species, confirming their classification within the same genus (Fig. 6a). A similar analysis of the CO1 region also supported this grouping with high bootstrap values, linking Phryssonotus borealis, sp. nov. to sequences from three Phryssonotus species in the same clade (Fig. 6b). Pairwise genetic distances based on the 18S region showed minimal divergence for P. borealis, sp. nov. relative to P. novaehollandiae (0.19%), P. australis (0.23%), and P. occidentalis (0.23%). In contrast, CO1 sequences revealed significantly greater divergence: P. borealis, sp. nov. differed from P. novaehollandiae (11%), P. australis (11%), and P. occidentalis (10%) (Table 3).
The 18S (a) and CO1 (b) bootstrapping maximum-likelihood trees of Phryssonotus borealis, sp. nov. were generated with 1000 bootstrap replications. Support values >50% are shown on the nodes.
| Species | Phryssonotus borealis, sp.nov. | ||
|---|---|---|---|
| Pairwise distance | |||
| 18S | CO1 | ||
| Phryssonotus australis | 0.0023 | 0.11 | |
| Phryssonotus novaehollandiae | 0.0019 | 0.11 | |
| Phryssonotus occidentalis | 0.0023 | 0.1 | |
The values indicate the percentage of genetic similarity between Phryssonotus borealis, sp. nov. with the other three Phryssonotus species.
Taxonomy
Genus Phryssonotus Scudder, 1885
Synxenus Silvestri, 1990.
Kubanus Attems, 1926.
Koubanus Attems, 1928.
Schindalmonotus Attems, 1926.
Lophonotus Menge, 1854, preoccupied, nonStephens (1829).
Kaubanus (sic) Attems, 1929, misprint byJones (1937).
Schindelmonatus (sic) Attems, 1929, misprint byJones (1937).
Genus Phryssonotus is characterised by 17 pairs of legs, 15 normal pairs of walking legs and 2 pairs of pushing legs caudally; 11 tergal plates and a telson; 10 pleural projections; body covered with scale trichomes and long barbate trichomes laterally; 8–11 ommatidia; a long frontal trichome A and short frontal trichomes B (B1–B5) are adjacent to trichobothrium c.
Type species
Phryssonotus novaehollandiae Silvestri, 1923.
Synxenus novaehollandiaeSilvestri, 1923: 14–15.
Phryssonotus borealis, sp. nov.
ZooBank: urn:lsid:zoobank.org:pub:9BA52C7B-C1E3-421C-A37D-4A3D6FD39EEB
Material examined
HOLOTYPE. A female adult, 17 pairs of legs, was collected from Manilla (30.743830S, 150.729166E, elevation 361 m), New South Wales, Australia. Australian Museum accession number: KS 131816. PARATYPES. 10 adults, 17 pairs of legs, were collected in the same location and date as holotype. KS 131817–28. The types were deposited in the Australian Museum, Sydney, Australia. GenBank accession numbers: PQ346279 for 18S and PQ344739 for CO1.
Diagnosis
Phryssonotus borealis, sp. nov. has the general characteristics of Australian Phryssonotus species, with 11 ommatidia, a typical long frontal trichome A and five short frontal trichomes B (B1–B5) (Fig. 4), body length ~3.4 mm. Parthenogenetic population. Body with distinctive tergal scale trichomes forming a white medial trapezoid band and small lateral white stripes (Figs 1, 5a).
Discussion
The Australian Phryssonotus species show high speciation and diversity. Despite their similarities in taxonomic characteristics, these species differ significantly in both morphology and genetic makeup. Notably, the variation in tergal scale trichome colouration serves as a key distinguishing feature. Most previously described Phryssonotus species are found in South Australia, Victoria and Western Australia, these regions collectively being considered part of southern Australia. The species have black tergal scale trichomes that stand out against the white background of the sides of the body (Fig. 5). In contrast, Phryssonotus borealis has scale trichomes forming a white medial trapezoid band and small lateral strips, forming the white pattern against the black background of its body. This species is found in north-eastern regions of New South Wales, extending to the border of Queensland. Because of its tergal trapezoid-shaped patterns it has a similar appearance to P. australis, a southern species primarily found in coastal habitats in Victoria and northern Tasmania. However, they differ in colouration and in the length–width ratios of the scale trichomes. Phryssonotus borealis is slightly shorter in body length and has smaller-scale trichome ratios than P. australis, and its measurements are similar to those of P. occidentalis from Western Australia. Notably, the length–width ratios of the scale trichomes among these Phryssonotus species are not a reliable characteristic to differentiate among these species, as body length of the species significantly influences the outcome of the scale trichome ratio. Instead, the colouration of the tergal scale trichomes serves as a distinct morphological character that differentiates among these Phryssonotus species.
Most Phryssonotus populations consist of both males and females. However, there is an exception in reproduction, as seen in the case of Phryssonotus australis, which reproduces through parthenogenesis. P. borealis is another example of an asexual population found in Australia. Both these species are parthenogenetic, consisting of a female-only population. Parthenogenesis is an effective survival strategy, allowing these species to adapt and maintain population stability in geographic isolation where habitats are favourable despite the absence of gene flow. This reproductive mode enables them to occupy diverse habitats across vast areas of Australia.
In this study, Phryssonotus species showed genetic differences. Phylogenetic trees indicated that P. borealis belongs to the genus Phryssonotus based on 18S analysis (Fig. 6a), and CO1 gene analysis (Fig. 6b) indicated significant genetic divergence from other Phryssonotus species. The genetic distance of P. borealis compared to its closest relatives (Table 3) exceeds the accepted species level in millipede taxonomy and validates its distinctiveness.
In conclusion, a combination of taxonomic and genetic studies confirmed that Phryssonotus borealis is a newly identified species. Its morphological and genetic differences from related species demonstrated ongoing speciation and adaptation within the genus Phryssonotus in Australia.
Acknowledgement
A special thank you to Dr Matt Shaw, Arachnology Collection Manager of the Australian Museum, who provided a loan of the collection of penicillate millipedes from New South Wales; and Mr Thomas Wong for helping and supporting in the field, Tamworth, NSW, Australia, during October 2022.
References
Condé B, Nguyen Duy-Jacquemin M (1984) Diplopodes pénicillates de Papouasie et de Bornéo. Revue Suisse de Zoologie 91, 47-55.
| Crossref | Google Scholar |
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Window 95/98/NT. Nucleic Acid Symposium Series 41, 95-98.
| Google Scholar |
Huynh C, Veenstra AA (2017) Two new species of Phryssonotus (Diplopoda: Synxenidae) from southern and western Australia. Australian Journal of Zoology 65(4), 248-256.
| Crossref | Google Scholar |
Huynh C, Veenstra A (2018) Two new Lophoturus species (Diplopoda, Polyxenida, Lophoproctidae) from Queensland, Australia. ZooKeys 741, 133-154.
| Crossref | Google Scholar |
Huynh C, Veenstra AA, Likhitrakarn N (2023) First records of penicillate millipedes (Diplopoda, Polyxenidae) from Thailand, with descriptions of two new species. Zootaxa 5383(4), 514-536.
| Crossref | Google Scholar | PubMed |
Jones S (1937) On two new South Indian pselaphognathous Diplopods. Zoologischer Anzeiger 119(5–6), 138-146.
| Google Scholar |
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870-1874.
| Crossref | Google Scholar | PubMed |
Meyer CP (2003) Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biological Journal of the Linnean Society 79, 401-459.
| Crossref | Google Scholar |
Nguyen Duy-Jacquemin M (2006) Condexenus, a new genus of the millipede family Synxenidae (Diplopoda, Polyxenida) from Namibia. Norwegian Journal of Entomology 53, 237-248.
| Google Scholar |
Short M, Huynh C (2009) Phryssonotus novaehollandiae Silvestri, 1923: the sole Australian representative of the millipede Family Synxenidae. Soil Organisms 81, 695-700.
| Google Scholar |
Short M, Huynh C (2010) A technique for examination of diagnostic characters of penicillate millipedes. Memoirs of Queensland Museum: Nature 55, 231-234.
| Google Scholar |
Silvestri F (1923) Notizia della presenza del genere Synxenus (Myriapoda, Diplopoda) in Catalogna e descrizione di quattro species. Treballs del Museu de Ciències Naturals de Barcelona 4, 5-15.
| Google Scholar |