Sand characteristics do not influence hatching success of nests at the world’s largest green turtle rookery
David T. Booth A * , Melissa N. Staines
A * , Melissa N. Staines  A and Richard D. Reina B
A and Richard D. Reina B
A School of Biological Sciences, The University of Queensland, Brisbane, Qld 4072, Australia.
B School of Biological Sciences, Monash University, Melbourne, Vic. 3800, Australia.
Australian Journal of Zoology 69(4) 113-124 https://doi.org/10.1071/ZO21050
Submitted: 1 December 2021 Accepted: 21 March 2022 Published: 5 May 2022
© 2021 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Raine Island, located in the northern Great Barrier Reef, hosts the largest green turtle nesting aggregation in the world, but typically experiences low hatching success (20–60%, depending on the number of females visiting the island to nest). To determine whether the low hatching success of green turtle eggs at Raine Island might be explained by local sand characteristics, we investigated the physical properties of Raine Island sand and compared it to sand from other eastern coast Australian sea turtle nesting beaches that have high hatching success (>80%). We also measured the water, salt and organic material content of sand within nests at Raine Island to see whether any of these variables were correlated with the proportion of early embryo death or hatching success. The physical characteristics of Raine Island sand were similar to those of other eastern coast Australian nesting beaches, so it seems unlikely that inherent physical sand properties, water content, salt or organic matter explain the relatively low hatching success observed on Raine Island compared to other Australian green turtle nesting beaches. However, we found that nests that were inundated twice with seawater during spring high tides at the end of their first week of incubation experienced greater early development mortality and lower hatching success than did non-inundated nests, suggesting that embryos drowned during the inundation. Last, we found that hatching success declined towards the end of the nesting season, suggesting that the beach sand in the nesting areas of Raine Island changes in some way, and/or that egg quality decreases as the nesting season progresses.
Keywords: biogenic sand, Chelonia mydas, embryonic mortality, green turtle, incubation success, nest inundation, Raine Island, sand composition.
Introduction
On Raine Island and other high-density sea turtle-nesting beaches such as ‘arribada’ beaches in central America, high early embryo death rates are common compared with lower nesting-density beaches (Valverde et al. 1998; Booth et al. 2020a). Eggs are highly viable when laid, so nest conditions are most likely to be responsible for embryo death. Nest conditions such as temperature, substrate moisture, substrate salt content, and oxygen partial pressure influence embryonic development and hatchling outcomes of sea turtle eggs (Ackerman 1997). Of these variables, temperature has been extensively studied, and is known to influence hatching success, hatchling morphology including sex, and hatchling locomotion performance (Yntema and Mrosovsky 1980; Howard et al. 2014; Booth 2017; Hays et al. 2017). Sea turtle embryos develop successfully only at incubation temperatures between approximately 25°C and 33°C (Ackerman 1997; Howard et al. 2014), although embryos can survive temperatures up to 36°C during the latter stages of their development (Booth 2017). The influence of substrate moisture and oxygen partial pressure on embryonic development are less well studied, but under extreme conditions are associated with decreased hatching success (McGehee 1990; Ackerman 1997; Bézy et al. 2014, 2015; Gatto and Reina 2022), and in the case of moisture content, can also influence hatchling size (McGehee 1990; Erb et al. 2018), and sex, although the moisture effect on sex may be a consequence of the moisture content on incubation temperature (Lolavar and Wyneken 2017). In theory and in practise, both water and oxygen availability to the developing embryos are dependent on the physical properties of the nest substrate, particularly substrate particle size (Mortimer 1990).
Water exchange between the nest substrate and the developing embryo depends on differences in water potential between the embryo’s body fluid and the substrate, water moving from regions of higher (less negative) water potential to regions of lower (more negative) water potential (Ackerman 1991). Sea turtle embryo fluids are expected to be similar to those of hatchlings, which have an osmolarity of ∼370 mmol (Reina and Cooper 2000), which corresponds to a water potential of ∼−950 kPa (Ackerman 1997). Nest substrate has a highly variable water potential, because it depends on both substrate water content (typically reported as a fraction or percentage on a mass/mass or volume/volume basis; e.g. 100 × (gram of water per gram of substrate), where substrate mass is measured on a dry mass basis), and the substrate particle-size distribution (Mortimer 1990; Ackerman 1991, 1997). Typically, sea turtle nest sands have water contents between 2% and 10% (McGehee 1990; Wood and Bjorndal 2000; Suss et al. 2012; Hill et al. 2015; Erb et al. 2018), so that substrate water potential is >−300 kPa, conditions that place sea turtle embryos in positive water balance. Only in very dry sands (<1% water) does the water potential become more negative than −950 kPa, which would place embryos in negative water balance, and, in such dry sands, hatching success is reduced (Mortimer 1990). The other important factor that affects water potential in sea turtle nest is the salt content of the sand (Mortimer 1990). Because sea turtle nests are constructed close to the sea, the salt content of sand can vary considerably (Mortimer 1990) as salt can be deposited by sea spray, or over-wash of seawater at high tides or during storm surges. Seawater has a water potential ∼−2000 kPa, so sea turtle eggs in complete contact with sand wetted with seawater rapidly lose water to the substrate causing embryo death due to dehydration (Bustard and Greenham 1968).
Oxygen is exchanged between developing embryos that consume oxygen and the air above the sand via diffusion through the sand substrate (Ackerman 1977; O’Connor et al. 2009; Booth and Dunstan 2018). The rate of this exchange depends on the difference in partial pressure of oxygen between the embryos in the nest and the air above the sand and the gas conductance of the sand (Ackerman 1977; O’Connor et al. 2009; Suss et al. 2012). The partial pressure of oxygen in air (∼20 kPa) remains more or less constant throughout incubation, but in sea turtle nests, oxygen decreases to 10–15 kPa by the end of incubation (Ackerman 1977; Maloney et al. 1990; Ralph et al. 2005; Honarvar et al. 2008; O’Connor et al. 2009; Chen et al. 2010; Bézy et al. 2014, 2015; Stewart et al. 2019). These studies have found that the magnitude of the decrease in oxygen partial pressure experienced by embryos in the nest depends on the number and size of embryos in the nest, the amount of microbial respiration in the surrounding sand, the density of nests, and the sand gas conductance. Sand gas conductance is determined by the volume of the interstitial gas spaces between the sand particles (O’Connor et al. 2009), which in turn is dependent to some extent on the sand particle size, with coarser sands having greater interstitial volumes and, therefore, greater gas conductance (Mortimer 1990; Ackerman 1977; Suss et al. 2012; Stewart et al. 2019).
As well as consuming oxygen and lowering the oxygen availability in the sand (Valverde et al. 1998; Honarvar et al. 2008; Chen et al. 2010; Bézy et al. 2014, 2015), some beach sand microorganisms may be pathogenic to developing sea turtle embryos and, thus, cause an increase in embryonic mortality if the microorganisms are in high abundance (Valverde et al. 1998; Honarvar et al. 2011; Bézy et al. 2014, 2015). Higher abundance of sand microorganisms such as bacteria and fungi has been associated with greater organic material content within the sand on sea turtle nesting beaches (Valverde et al. 1998; Honarvar et al. 2011; Bézy et al. 2014, 2015) and has been associated with greater sea turtle embryonic mortality (Bézy et al. 2014, 2015). Thus, high sand organic material content is thought to be a possible cause of elevated sea turtle embryo death at high density sea turtle beaches (Bézy et al. 2014, 2015). Periodic storm activity which removes and replaces highly contaminated beach sand is probably an important moderator of long-term low hatching success at such beaches (Bézy et al. 2014, 2015).
Raine Island hosts the largest nesting aggregation of green turtles (Chelonia mydas) in the world (Limpus et al. 2003), but has a relatively low hatching success even when clutches are protected against destruction by subsequent nesting females, with the majority of embryo mortality occurring very early in incubation (Booth and Dunstan 2018; Booth et al. 2020a). Hatching success varies inversely with the number of nesting turtles from a high of ∼60% in low female-visitation years (<5000) to a low of ∼20% in high female-visitation years (>100 000; Coffee and Robertson 2021). In high-visitation years, each night female density on the beach is so high, it is possible to step from one turtle to another without touching the beach sand (Limpus et al. 2003). High mortality early in development also occurs at high density olive ridley (Lepidochelyes olivacea) ‘arribada’ nesting beaches (Valverde et al. 1998). Recent investigations have concluded that neither low egg viability (Booth et al. 2021), high nest temperatures (Booth et al. 2020a, 2020b) or low nest oxygen partial pressure (Booth et al. 2020b) can explain high early stage embryo mortality typical of nests laid at Raine Island. Here, so as to determine whether sand composition plays a role in embryonic mortality, we examine the physical properties of sand from Raine Island, which is principally composed of foraminifera tests (Dawson et al. 2014), and compare it to sand from other eastern coast Australian sea turtle nesting beaches that have a high hatching success rate. Additionally, we investigate the initial water, salt and organic matter content of sand in the nest chamber to determine whether any of these variables is correlated with the relatively high early embryo mortality and low hatching success of green turtle clutches laid on Raine Island.
Materials and methods
Collecting and processing sand samples
Sand samples were collected from six sea turtle nesting beaches along the eastern coast of Queensland, Australia (Fig. 1). Samples were collected from the following three mineral-sand beaches: Wreck Rock beach (24.3157°S, 151.9654°E), an important loggerhead turtle (Caretta caretta) and minor green turtle and flatback turtle (Natator depressus) nesting beach; Mon Repos beach (24.7961°S, 152.4416°E), a major loggerhead turtle and minor green and flatback turtle nesting beach; Peregian beach (26.4774°S, 153.0990°E), an occasional green and loggerhead turtle nesting beach, and three biogenic carboniferous island beaches: Raine Island (11.5904°S, 144.0352°E), a major green turtle nesting beach, Sand bank No. 8 (13.3690°S, 143.9659°E), a minor green turtle nesting beach and Heron Island (23.4422°S, 151.9146°E), a minor green turtle, and occasional loggerhead turtle nesting beach. Three sand samples from each beach of approximately 1 kg were collected from 70 cm below the sand surface (approximate nest depth of green turtles) by digging a hole by hand and then taking samples in 1 L plastic jars that were immediately sealed with a screw-top. Samples were taken from the berm region (Raine Island and Sand bank No. 8) or the first dune from the hightide mark (other beaches), which are the areas that support most frequent sea turtle nesting activity. The sample from Sand bank No. 8 had only its organic matter content determined. These sand samples were collected so that the physical properties of the sand from different nesting beaches could be compared, and to discover whether Raine Island sand had properties different from the sand collected from beaches with high hatching success. In addition, sand samples were collected from Raine Island nests during egg laying in October 2018, December 2018, and February 2019. Nest sand was collected in 60 mL plastic specimen vials from immediately adjacent to eggs as they were being added to nests. These samples were used to test for possible relationships between water content, salt content and organic matter content and the frequency of early embryo death and hatching success. At this time, temperature loggers (iButtonTM Maxim, Model DS1922L, resolution of 0.06°C, accuracy ± 0.2°C) were placed among the eggs for a different study (Booth et al. 2020a). However, we used the temperature traces from these loggers to infer whether nests were inundated with water (indicated by a sudden decrease in temperature) during the spring high-tides that occurred in mid-February 2019. Sand samples were transported to a laboratory at the University of Queensland, St Lucia Campus, and stored at room temperature until processed.
Sand grain-size distribution
Grain-size distribution was determined by air drying sand on a laboratory bench top at ∼25°C for 2 weeks, before weighing out triplicate 200 g samples and passing them through a series of standard sorting sieves (53, 125, 250, 500, 1000, 2000 μm) and weighing the proportion in each sieve to calculate percentage contribution of each grain size.
Sand water characteristic curves
Triplicate subsamples of each sand sample were used to determine the water content–water potential characteristic curve by using the pressure-plate method (Klute 1986). Samples were placed inside sample rings, saturated with water and placed on a pressure plate before being exposed to the measurement pressure for 7 days in a room maintained at 20°C. After this period, the water content of samples was measured by mass-loss before and after drying at 105°C for 24 h. Water potential was then calculated in kPa.
Sand oxygen permeability
Sand gas conductance can be quantified by measuring its Krogh’s diffusion constant (Schmidt-Nielsen 1997). Krogh’s diffusion constant was measured in a vertically mounted 15 cm long, 3 cm diameter PVC tube, plugged at one end with a rubber stopper that had a fibre-optic oxygen probe (Pyroscience robust probe OXROB3, Germany) inserted through it, along with an 18 G needle fitted with a three-way stopcock. The probe was connected to a fibre-optic oxygen metre (FirestingO2, Pyroscience, Germany) and calibrated with nitrogen gas (0% O2) and room air (20.95% O2) at each measurement. The oxygen diffusion rate was measured in air, first by flushing the tube via the 18 G needle with nitrogen gas until the probe read zero, and then shutting the nitrogen off and allowing oxygen to diffuse down the tube. The oxygen concentration at the probe was logged once every 30 s until the oxygen concentration reached approximately 20%, then the tube was flushed with room air to check the span calibration (20.95%) of the probe. The tube was then filled with lightly compacted sand, and the same procedure was repeated. Barometric pressure was recorded at the time of each measurement by accessing The University of Queensland weather station (https://www.wunderground.com/dashboard/pws/IBRISBAN110). This procedure was repeated three times on the same sand sample. The sand was then emptied from the tube, and the procedure was repeated using a different sand sample from the same location.
To calculate Krogh’s oxygen diffusion constant from these trials, oxygen concentration recorded at the probe was converted to oxygen partial pressures (PO2) by multiplying the oxygen concentration by barometric pressure after subtracting the saturated water vapour pressure at the measurement temperature of 25°C. A plot of ln(room air PO2 − probe PO2) versus time was created, and the slope of a regression line fitted to this plot was used to calculate a constant that was directly proportional to Krogh’s oxygen diffusion constant. This procedure was repeated six times for room air and each of the three sand samples, with a mean calculated from all six trials. To convert the value calculated from the regression slope to units of Krogh’s diffusion constant (m2 Pa−1 s−1 × 10−12), the constant was multiplied by a conversion factor (16.45/constant for room air), where 16.45 m2 Pa−1 s−1 × 10−12 is Krogh’s oxygen diffusion constant for air (Buggren and Roberts 1991).
Determining water and organic material content of sand samples
All samples were subsampled and analysed in triplicate. A quantity of 2–5 g of sand was added to pre-weighed porcelain crucibles, and dried in an oven set at 105°C for 24 h and when re-weighed to ±0.001 g on a digital balance (A&D balances, Australia). Sand samples were then combusted in a muffle furnace set at 430°C for 24 h and then re-weighed. Sand samples used for inter-beach comparisons were then re-combusted in the muffle furnace set at 550°C for a further 1 h and then re-weighed. Water content was calculated on a dry-mass basis as
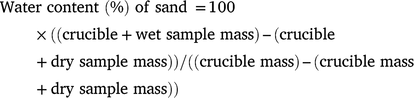
A standard method for determining soil organic matter content is to combust a sample of soil previously dried at 105°C for 24 h at 500–550°C for a further 24 h (Klute 1986). This method has been used previous for determining sand organic matter content at sea turtle nesting beaches (Bézy et al. 2014, 2015). However, this method is not recommended for biogenic sand (i.e. those composed of skeletal material of organisms such as forams, corals and molluscs) because such a high ignition temperature also combusts some or all of the carbonate in these biogenic sands and results in an over-estimation of the sand organic matter content (Salehi et al. 2011). The beaches of coral cays and some islands in the Great Barrier Reef system are composed principally of biogenic carboniferous sands (Dawson et al. 2014), for which a more appropriate method to estimate organic matter content is to combust at a lower temperature such as 430°C (Salehi et al. 2011).


Determining salt content of sand
Salt content of nest sand was measured in triplicate by placing a known mass of dry sand into a 50 mL volumetric flask and adding pure reverse osmosis (RO) water to the 50 mL mark. The resultant mixture was then vigorously shaken to dissolve salts in the sand, and allowed to stand for at least 30 min. Then, the solution was poured into a 50 mL specimen jar and its salinity was measured with a salinity meter (TPS, Brisbane, Australia) calibrated with pure RO water and a precision 200 mg L−1 sodium chloride solution. The mass of salt in the sample was calculated by first determining the volume of water added to the dry sand sample, using a density of 1.55 g mL−1 (as measured by determining the mass of 500 mL of sand in a measuring cylinder), as Volume = 50 − (sand dry mass)/1.55, and then using the formula
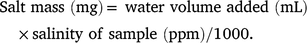
The salt content of the dry sand was then calculated:
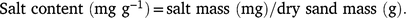
Assessing hatching success
During the 2018–2019 nesting season on Raine Island, we determined hatching success of nests laid between 21 October 2018 and 29 October 2018 (October 2018), 2 December 2018 and 9 December 2018 (December 2018), and 12 February 2019 and 14 February 2019 (February 2019). We set up both natural and relocated nests on the south-facing beach for monitoring for experiments described in a previous study (Booth et al. 2020a), but used the sand samples taken then in the current study. All nests of both types were located in the berm and back berm area of the beach where the greatest concentration of nest occurs. For natural nests, when a female finished egg chamber construction and began laying eggs, a sand sample was removed from the bottom of the egg chamber with care not to touch the tail or disturb the egg-laying process. Once the female had finished egg-laying and back-filling of the nest, three 50 × 50 × 1800 mm wooden stakes were hammered in around the nest to a depth of 1 m, to protect the nest from disturbance by subsequent nesting females. For the relocated nests, we dug a nest hole 70 cm deep by hand, then collected all of the eggs from a nearby laying turtle as they were being laid and relocated them into the hand-dug nests. A sand sample was taken from the bottom of the hand-dug nest before eggs were added to it. Sand was then used to backfill the nest and three wooden stakes inserted around the nest as described for natural nests. We relocated clutches and completed artificial nest construction within 30 min of the end of egg-laying.
On a subsequent field trip, typically 1 or 2 weeks after the average 55-day incubation period, the nests were excavated, the temperature-data loggers recovered, and the number of empty shells (=hatched eggs) and unhatched eggs were counted. We calculated hatchling success using the following equation (Miller 1999):
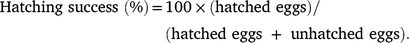
Because the greatest proportion of embryo death on Raine Island occurs very early in embryonic development, before any obvious sign of an embryo is visible to the naked eye (i.e. within the first 7 days of incubation (Booth et al. 2020a), which corresponds to Phase 1 of development according to the 6-phase field development guide described in Booth et al. (2020a)), we opened any unhatched eggs to determine the proportion of Phase 1 deaths, which was quantified as (Miller 1999):
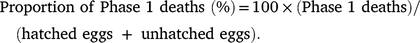
It is likely that embryos within these eggs died after laying as opposed to the eggs being inviable at laying because 97% of Raine Island green turtle eggs are viable at laying (Booth et al. 2021).
Statistical analysis
Sand water content, organic material content, and salt content are presented in boxplots as medians and 5th, 10th, 25th, 75th, 90th and 95th percentiles. Medians rather than means are reported and non-parametric statistics used because data are reported as proportions (percentages) rather than numbers and therefore violate the assumptions of the normal distribution. Spearman rank correlation was used to test for relationships among these variables. Mann–Whitney U-tests were used to compare organic matter determined by combustion at 550°C and at 430°C, and organic matter content between mineral sands and biogenic carboniferous sands. Mann–Whitney U-tests were used to compare both proportion of Phase 1 deaths and hatching success of nests set up in February that were inundated during the spring tide events with those what were set up at the same time but were not inundated (‘dry’ nests).
Mann–Whitney U-tests were used to compare the proportion of Phase 1 death and hatching success of natural and artificial nests. Kruskal–Wallis ANOVA by ranks and multiple-comparison tests was used to test for differences in organic content from sand samples collected from different beaches. Kruskal–Wallis ANOVA by ranks and multiple-comparison tests was used to test for differences in water content, organic matter content, and sand salt content of nest sand samples collected from Raine Island during October 2018, December 2018 and February 2019. Multiple regression analysis was used to investigate whether an interaction among sand water content, organic matter content, and salt content was associated with Phase 1 death or clutch hatching success. All statistical tests were performed using Statistica© ver. 13.2 Software (TIBCO, Palo Alto, CA, USA).
Results
Sand particle-size distribution
Sand particle-size analysis was performed on two mineral and two biogenic carboniferous sand beaches. All samples were classified as sands because no particles under 250 μm were detected. The sand particle sizes of the two mineral sands (Mon Repos beach and Peregian beach) were generally smaller and well sorted, with all particles within the 225–500 μm size range (Fig. 2). In contrast, the two biogenic carboniferous sands (Raine Island and Heron Island) had a larger proportion of particle size in the 250 to >2000 μm size range (Fig. 2) and were less well sorted.
|
|
Sand water content–water potential characteristic curves
The two mineral sands (Mon Repos beach and Peregian beach) bound water more strongly than the two biogenic carboniferous sands (Raine Island and Heron Island), as indicated by the mineral sand curves being located to the left of biogenic carboniferous sand curves in the water content–water potential plot (Fig. 3). Water potential was greater at a lower water content for the two mineral sands than for the two biogenic carboniferous sands, but the decrease in water potential as the sand continued to dry was much greater once the lower threshold of the plateau region of the curve was reached (Fig. 3).
|
|
Krogh’s oxygen diffusion constant for the different sand samples
Krogh’s oxygen diffusion constant was not significantly different (median value 6.5 × 10−12 m2 Pa−1 s−1) among all sands (H(4, N = 30) = 0.000, P = 1.000) and was approximately one-third of that of air (Fig. 4).
Sand organic material content
Sand organic content determined by combustion at 550°C was always greater than that determined at 430°C (Mann–Whitney U = 0.00, Z = 8.153, P < 0.001; Fig. 5), but the difference was smaller for samples from mineral sand beaches (0.26%) than for samples from biogenic carboniferous sand beaches (1.34%; Fig. 5). Sand organic matter content determined at 550°C was highly correlated with that determined at 430°C (rs = 0.89, t(N – 2) = 16.94, P < 0.001, N = 79); so, only organic matter content determined at 430°C is reported. Sand organic matter content varied across beaches (Kruskal–Wallis H(5, N = 79) = 66.58, P < 0.001), with the biogenic carboniferous sands from Raine Island, Sand bank No. 8 and Heron Island having similar medians (∼2.3%), which were greater (P < 0.01) than those of the mineral sands from Wreck Rock beach, Mon Repos Beach, and Peregian Beach, which had similar medians (∼1.0%; Fig. 5).
Raine Island nests
Of the 25 nests set up on 12 and 13 February, 10 were inundated by seawater rising from beneath the nest during the peak tides on 18 and 19 February, as indicated by a distinct rapid decrease in nest temperature during these events (Fig. 6). None of the nests set up in October 2018 and December 2018 experienced seawater inundation. The inundated nests experienced greater Phase 1 mortality (medians: inundated = 78%, dry = 36%; Z = 2.2025, P = 0.043), and lower hatching success (medians: inundated = 10%, dry = 51%; Z = −2.524, P = 0.012) than did ‘dry’ nests, and, for this reason, were excluded from all subsequent analyses. Phase 1 mortality (medians: natural = 18.1%, relocated = 18.3%; z = −0.408, P = 0.683) and hatching success (medians: natural = 70.2%, relocated = 76.8%; z = 0.694, P = 0.488) of artificial and natural nests did not differ; so, the data from both types of nests were pooled for all subsequent analysis. Hatching success was not correlated with sand water content (Fig. 7), sand organic matter content (Fig. 8), or sand salt content (Fig. 9). The proportion of Phase 1 embryo death was not correlated with sand water content (Fig. 7), or sand organic matter content (Fig. 8), but was negatively correlated with sand salt content (Fig. 9). Multiple regression analysis indicated that sand water content, organic matter content, and salt content did not interact to affect either the proportion of Phase 1 deaths (r2 = 0.06, F(3,96) = 1.1352, P = 0.339) or clutch hatching success (r2 = 0.03, F(3,96) = 0.8476, P = 0.471). The median sand organic matter content (2.1%) was similar across the nesting season (H(2, N = 100) = 2.951, P = 0.123; Fig. 10). Sand water content increased as the nesting season progressed from a median of 0.4% in October to a median of 4.2% in February (H(2, N = 100) = 59.51, P < 0.001; Fig. 10). Sand salt content was highly variable, but with a similar median (0.1 mg/g) in October and December, but was much less variable with a smaller median (0.05 mg/g) in February (H(2, N = 100) = 28.96, P < 0.001; Fig. 10). The proportion of Phase 1 embryo death was similar in October and December, with a median of 12.5%, but increased to a median of 36.1% by February (H(2, N = 100) = 14.99, P = 0.001; Fig. 10). Hatching success was similar in October and December, with a median of 72.0%, but decreased to a median of 45.7% by February (H(2, N = 100) = 12.24, P = 0.002; Fig. 10).
|
|
Discussion
Our study failed to detect any major differences between the physical properties of Raine Island sand and sand from high hatching-success eastern coast Australian nesting beaches that might have been implicated in the lower hatching success of Raine Island nests. The sand of Raine Island and Heron Island sampled from the green turtle nesting areas is composed primarily of biogenically derived carbonaceous particles and had a greater proportion of larger-sized particles than did the mineral sand beaches. The difference in particle size between Raine and Heron Islands and the mainland mineral sand beaches is probably due to the source of particles that make up the sand. The mainland beach mineral sands are derived from decaying inland rock formations that are transported by rivers to the sea (Geoscience Australia 2021). These particles must be of a relatively small size to be transported from their inland creation site to the beach. In contrast, the biogenic island sand particles are sourced from the breakdown of corals, mollusc shells and foraminifera tests from the reefs surrounding the islands. Because of their larger particle size, the island sands were expected to have a lower water-binding affinity because of their lower relative surface area of all particles combined than for mainland beach sands. This prediction was borne out, and the island sands have lower water-binding properties that are more similar to sandy loam soils than the fine-grained mineral sands (Ackerman 1991). Despite these differences, given that the water content of sand on sea turtle nesting beaches is usually greater than 2% (McGehee 1990; Wood and Bjorndal 2000; Suss et al. 2012, Hill et al. 2015; Erb et al. 2018), and that the sand water potential at 2% is greater than −950 kPa (Fig. 2), embryos will be experiencing a positive water-balance environment, conditions that should lead to high hatching success (Ackerman 1991, 1997). Even if sand water content was to fall to very low levels that would place embryos into a negative water budget situation, the large volume of water contained within a clutch of sea turtle eggs should buffer such water loss and still result in high clutch hatching success (Ackerman 1997; Smith et al. 2021). The waterbinding characteristics of Raine Island sand are very similar to those of Heron Island sand, which hosts a high hatching success of green turtle clutches (∼80%, Limpus 2008); so, we conclude that the low hatching success of green turtle clutches on Raine Island cannot be attributed to the sand having unusual water-binding properties.
The larger particle sizes of Raine Island and Heron Island sands were expected to have slightly higher Krogh’s diffusion constants because of their greater interstitial gas space than that for the mineral sand beaches. However, no difference was detected in Krogh’s oxygen diffusion constant, indicating that oxygen permeability was similar in all the sands examined. This is most likely because the difference in interstitial gas space among sands is too small to cause a detectable difference by our measurement method. Using the ratio of oxygen diffusivity in air to that in the sand, we estimated that the proportion of interstitial gas volume of the sands we tested was ∼0.4 of the entire volume of sand, being similar to that previously reported for sea turtle nesting sand (Ackerman 1977; Mortimer 1990). Such porosity means that nesting beach sands are usually porous enough that diffusion rate of gases is unlikely to affect hatching success (Mortimer 1990). The sand particle-size range of all beaches we sampled was well within the range found globally across green turtle nesting beaches (Mortimer 1990). Although data from biogenic sand from Ascension Island suggested that hatching success is negatively correlated with the mean particle size (Mortimer 1990), we found that the mean particle size of nesting sand from Heron Island is larger than that from Raine Island, yet Heron Island has a generally higher hatching success. To conclude, the oxygen diffusion characteristic of Raine Island sand is very similar to all of the sea turtle nesting sands sampled; so, is unlikely to be responsible for the low hatching success of Raine Island green turtle nests.
The loss of mass was greater in all sands when combusted at 550°C than at 430°C, indicating that there was some carbonaceous material in all sand samples (Salehi et al. 2011; Sato et al. 2014). However, the difference in mass loss between 430°C and 550°C was greater for the island sands than for the mainland mineral sands, indicating a higher carbonaceous content. Our analysis suggested that the absolute organic carbon content of beach sand cannot be determined by combustion at 430°C, but previous work has indicated that mass loss after combustion at 300–430°C is tightly correlated with absolute organic carbon content (Salehi et al. 2011; Sato et al. 2014) and, therefore, mass loss after combustion at 430°C can be used as a proxy for absolute organic carbon content for correlation analysis. As we found no relationship between the proportion of Phase 1 death or hatching success with sand mass loss after combustion at 430°C, there does not appear to be a direct relationship between nest sand organic matter content and embryo mortality. This finding is in contrast to the high-density olive ridely turtle Ostional beach in Costa Rico where nests with higher organic matter content tended to have a lower hatching success (Bézy et al. 2014, 2015).
All of our organic matter estimates are well above those reported for green turtle nesting beaches around the world (0.05–2.4%; Mortimer 1990). Although the characteristics of sand (i.e. predominantly mineral or biogenic) of the high-density olive ridley turtle nesting at Ostional beach in Costa Rica was not reported, combustion at 550°C indicated an organic matter content of 3–4% (Bézy et al. 2014, 2015), being similar to the values we found at our biogenic sand beaches at this combustion temperature. Ostional beach sand is predominately mineral in origin, but its organic matter content is two to three times greater than that of the mineral sands from eastern coast Australian beaches, being consistent with the conclusion that high-density olive ridley beaches have a high organic matter content as a result of the accumulation of sea turtle egg-related by-products and associated microorganisms (Bézy et al. 2014, 2015). Clearly, the method for estimating the organic content of sea turtle nesting sand needs to be standarised to a system that is inexpensive and does not produce toxic waste material. Salehi et al. (2011) suggested that combustion of samples at 360°C is a good compromise because it burns most organic carbon, destroys less inorganic carbon, and causes less structural water loss from clay particles.
We anticipated an increase in sand organic matter towards the end of the nesting season, owing to the accumulation of hatched eggshells and meconium, as well as dead egg contents. We also expected greater embryo mortality as a result of this accumulation of organic matter in sand as the nesting season progressed. Although we did observe greater embryo mortality at the end of the season, we did not find a greater sand organic matter content or a correlation between sand organic matter content and early embryo death or clutch hatching success. This might be because the 2018–2019 nesting season supported a very low number of nesting females (∼2700, compared with an average 30 000 annually; Dunstan et al. 2020); so, the amount of organic material added to the sand was only a small fraction compared with that already present in the sand at the beginning of the nesting season. The sand organic matter content at Raine Island was similar to that at Heron Island, which has a high clutch hatching success; so, there is no evidence to suggest that a high organic sand content per se is the cause of high early embryo death or low hatching success of green turtle clutches on Raine Island. Besides a possible increase in pathogenic microbes in the sand towards the end of the nesting season causing a decrease in hatching success, other factors could cause an increase in early embryo death. Such causes might be a decrease in female heath, resulting in production of poor-quality eggs, or a decrease in sperm potency owing to a long storage time in the female’s reproductive tract, resulting in a decrease in egg fertility.
Seawater over-wash and sea-spray can increase the salt content of sand, and high sand salt content can cause a rapid loss of water by osmosis and result in embryo death in sea turtle eggs that are surrounded by sand wetted with seawater (Bustard and Greenham 1968). However, there is spatial variation in the microenvironment experienced by eggs within a nest (Wallace et al. 2004), which can result in spatially variable hatching success within a nest (Ralph et al. 2005). The arrangement of sea turtle eggs within the nest means that the eggs are not individually surrounded by sand, but rather laid in large clutches that have a large combined water reserve that can buffer the osmotic forces experienced by the peripheral eggs that are in contact with sand (Smith et al. 2021). Hence, it is not surprising that we found that sand salt content did not influence clutch hatching success. However, there was a weak correlation between sand salt content and the proportion of Phase 1 embryo deaths, and, surprisingly, nests with a greater sand salt content experienced lower Phase 1 embryo death. It has been suggested that sand with a higher salt content might supress to some degree the abundance of beach sand microorganisms (Cornelius et al. 1991, Honarvar et al. 2011, Bézy et al. 2014); so, if early embryo death is associated with the abundance of soil microorganisms, the lower death rate in nests with high sand salt content might be explained by this mechanism.
The nests that experienced inundation by seawater during spring tides in February were underwater for several hours on two consecutive days. The resulting increase in embryo death was most probably caused by embryos drowning because early stage embryos are suspectable to asphyxiation when immersed in water (Limpus et al. 2020).
The rainfall pattern on Raine Island during our study can explain why water content increased, and salt content decreased in nests sampled in February compared with nests sampled in October and December. Early in the egg-laying season, little rainfall occurs and the surface sand is relatively dry. The higher water content of nest sand at this time is caused by wave over-wash of seawater, resulting in both an increase in sand water and salt content. Seasonal rains typically start in mid- to late December and continue through until March (Laloë et al. 2021). Hence, by February, the sand has been thoroughly rinsed by rain water, which not only increases the sand water content, but washes always the salt deposited by the sea, resulting in a lower overall sand salt content.
Conclusions
We found that the physical characteristics of Raine Island nesting beach sand with respect to grain size, water-binding characteristic, water, oxygen permeability and organic matter were similar to that of Heron Island and, therefore, cannot explain the high embryo mortality found at Raine Island. We did find that tidal inundation of water at the end of the first week of incubation increased early development mortality and, thereby, resulted in decreased hatching success, and that the proportion of early development mortality increased and hatching success decreased in nests that were laid towards the end of the nesting season. This suggests that the beach sand in the nesting area changes in some way during the nesting season, possibly by an increase in the abundance of beach sand microorganisms, so that it is less suitable for successful incubation of sea turtle eggs. Alternatively, egg quality might decrease towards the end of the nesting season because of a decrease in female health or a decrease in the quantity/quality of sperm stored in the female’s reproductive tract.
Data availability
The original data are available from the corresponding author upon request.
Conflicts of interest
All authors declare no conflicts of interest.
Declaration of funding
This study was funded by the Raine Island Recovery Project, which is a 5-year, AU$7.95 million collaboration between BHP, the Queensland Government, the Great Barrier Reef Marine Park Authority, Wuthathi and Meriam Nation (Ugar, Mer, Erub) Traditional Owners and the Great Barrier Reef Foundation to protect and restore the island’s critical habitat to ensure the future of key marine species.
Acknowledgements
All Raine Island field work was approved by the Raine Island Scientific Advisory Group. We thank Jamie Tedeschi and the many people from the Raine Island recovery program that assisted us in the Raine Island field work. We thank the traditional owner of Raine Island, Wuthathi and Meriam Nation (Ugar, Mer, Erub), for allowing access to Raine Island. Animal ethics approval was granted for this study by the University of Queensland NEWMA animal ethics committee, approval number SBS/267/17.
References
Ackerman, RA (1977). The respiratory gas exchange of sea turtle nests (Chelonia, Caretta). Respiration Physiology 31, 19–38.| The respiratory gas exchange of sea turtle nests (Chelonia, Caretta).Crossref | GoogleScholarGoogle Scholar | 918411PubMed |
Ackerman RA (1991) Physical factors affecting the water exchange of buried reptile eggs. In ‘Egg Incubation: its Effects on Embryonic Development in Birds and Reptiles’. (Eds DC Deeming, MWJ Ferguson) pp. 193–211. (Cambridge University Press: Cambridge, UK)
Ackerman RA (1997) The nest environment and the embryonic development of sea turtles. In ‘The Biology of Sea Turtles’. (Eds PL Lutz, JA Musick, J Wyneken) pp. 83–106. (CRC Press: Boca Raton, FL, USA)
Bézy, VS, Valverde, RA, and Plante, CJ (2014). Olive ridley sea turtle hatching success as a function of the microbial abundance and the microenvironment of in situ nest sand at Ostional, Costa Rica. Journal of Marine Biology 2014, 351921.
| Olive ridley sea turtle hatching success as a function of the microbial abundance and the microenvironment of in situ nest sand at Ostional, Costa Rica.Crossref | GoogleScholarGoogle Scholar |
Bézy, VS, Valverde, RA, and Plante, CJ (2015). Olive ridley sea turtle hatching success as a function of the microbial abundance in nest sand at Ostional, Costa Rica. PLoS ONE 10, e0118579.
| Olive ridley sea turtle hatching success as a function of the microbial abundance in nest sand at Ostional, Costa Rica.Crossref | GoogleScholarGoogle Scholar | 25714355PubMed |
Booth, DT (2017). Influence of incubation temperature on sea turtle hatchling quality. Integrative Zoology 12, 352–360.
| Influence of incubation temperature on sea turtle hatchling quality.Crossref | GoogleScholarGoogle Scholar | 28054446PubMed |
Booth, DT, and Dunstan, A (2018). A preliminary investigation into the early embryo death syndrome (EEDS) at the world’s largest green turtle rookery. PLoS ONE 13, e0195462.
| A preliminary investigation into the early embryo death syndrome (EEDS) at the world’s largest green turtle rookery.Crossref | GoogleScholarGoogle Scholar | 29694365PubMed |
Booth, DT, Dunstan, A, Bell, I, Reina, R, and Tedeschi, J (2020a). Low male production at the world’s largest green turtle rookery. Marine Ecology Progress Series 653, 181–190.
| Low male production at the world’s largest green turtle rookery.Crossref | GoogleScholarGoogle Scholar |
Booth, DT, Archibald-Binge, A, and Limpus, CJ (2020b). The effect of respiratory gases and incubation temperature on early stage embryonic development in sea turtles. PLoS ONE 15, e0233580.
| The effect of respiratory gases and incubation temperature on early stage embryonic development in sea turtles.Crossref | GoogleScholarGoogle Scholar | 33264278PubMed |
Booth, DT, Dunstan, A, Robertson, K, and Tedeschi, J (2021). Egg viability of green turtles nesting on Raine Island, the world’s largest nesting aggregation of green turtles. Australian Journal of Zoology 69, 12–17.
| Egg viability of green turtles nesting on Raine Island, the world’s largest nesting aggregation of green turtles.Crossref | GoogleScholarGoogle Scholar |
Buggren W, Roberts JL (1991) Respiration and metabolism. In ‘Environmental and Metabolic Animal Physiology’. (Ed. C Ladd-Prosser) pp. 353–436. (John Wiley & Sons Inc: New York, NY, USA)
Bustard, HR, and Greenham, P (1968). Physical and chemical factors affecting hatching in the green sea turtle, Chelonia mydas (L.). Ecology 49, 269–276.
| Physical and chemical factors affecting hatching in the green sea turtle, Chelonia mydas (L.).Crossref | GoogleScholarGoogle Scholar |
Chen, C-L, Wang, C-C, and Cheng, I-J (2010). Effects of biotic and abiotic factors on the oxygen content of green sea turtle nests during embryogenesis. Journal of Comparative Physiology B 180, 1045–1055.
| Effects of biotic and abiotic factors on the oxygen content of green sea turtle nests during embryogenesis.Crossref | GoogleScholarGoogle Scholar |
Coffee OI, Robertson K (2021) Raine Island Recovery Project: 2020–2021 Season technical report to the Raine Island Scientific Advisory Committee and Raine Island Reference Group. Department of Environment and Science, Queensland Government, Brisbane, Qld, Australia.
Cornelius SE, Ulloau MA, Castro JC, Mata del Valle M, Robertson DC (1991) Management of olive ridely sea turtles (Lepidochelys olivvacea) nesting at Playas Nancite and Ostional, Costa Rica. In ‘Neotropical Wildlife Use and Conservation. Vol. 1’. (Eds J Robinson, K Redford) pp. 111–135. (University of Chicago Press: Chicago, IL, USA)
Dawson, JL, Smithers, SG, and Hua, Q (2014). The importance of large benthic foraminifera to reef island sediment budget and dynamics at Raine Island, northern Great Barrier Reef. Geomorphology 222, 68–81.
| The importance of large benthic foraminifera to reef island sediment budget and dynamics at Raine Island, northern Great Barrier Reef.Crossref | GoogleScholarGoogle Scholar |
Dunstan, AJ, Robertson, K, Fitzpatrick, R, Pickford, J, and Meager, J (2020). Use of unmanned aerial vehicles (UAVs) for mark-resight nesting population estimation of adult female green sea turtles at Raine Island. PLoS ONE 15, e0228524.
| Use of unmanned aerial vehicles (UAVs) for mark-resight nesting population estimation of adult female green sea turtles at Raine Island.Crossref | GoogleScholarGoogle Scholar |
Erb, V, Lolavar, A, and Wyneken, J (2018). The role of sand moisture in shaping loggerhead sea turtle (Caretta caretta) neonate growth in Southeast Florida. Chelonian Conservation and Biology 17, 245–251.
| The role of sand moisture in shaping loggerhead sea turtle (Caretta caretta) neonate growth in Southeast Florida.Crossref | GoogleScholarGoogle Scholar |
Gatto, CR, and Reina, RD (2022). A review of the effects of incubation conditions on hatchling phenotypes in non-squamate reptiles. Journal of Comparative Physiology B 192, 207–233.
| A review of the effects of incubation conditions on hatchling phenotypes in non-squamate reptiles.Crossref | GoogleScholarGoogle Scholar |
Geosience Australia (2021) Mineral sands. Available at https://www.ga.gov.au/education/classroom-resources/minerals-energy/australian-mineral-facts/mineral-sands. [Accessed 14 November 2021]
Hays, GC, Mazaris, AD, Schofield, G, and Laloë, J-O (2017). Population viability at extreme sex-ratio skews produced by temperature-dependent sex determination. Proceedings of the Royal Society B: Biological Sciences 284, 20162576.
| Population viability at extreme sex-ratio skews produced by temperature-dependent sex determination.Crossref | GoogleScholarGoogle Scholar | 28179520PubMed |
Hill, JE, Paladino, FV, Spotila, JR, and Tomillo, PS (2015). Shading and watering as a tool to mitigate the impacts of climate change in sea turtle nests. PLoS ONE 10, e0129528.
| Shading and watering as a tool to mitigate the impacts of climate change in sea turtle nests.Crossref | GoogleScholarGoogle Scholar | 26030883PubMed |
Honarvar, S, O’Connor, MP, and Spotila, JR (2008). Density-dependent effects on hatching success of the olive ridley turtle, Lepidochelys olivacea. Oecologia 157, 221–230.
| Density-dependent effects on hatching success of the olive ridley turtle, Lepidochelys olivacea.Crossref | GoogleScholarGoogle Scholar | 18481091PubMed |
Honarvar, S, Spotila, JR, and O’Connor, MP (2011). Microbial community structure in sand on two olive ridley arribada nesting beaches, Playa La Flor, Nicaragua and Playa Nancite, Costa Rica. Journal of Experimental Marine Biology and Ecology 409, 339–344.
| Microbial community structure in sand on two olive ridley arribada nesting beaches, Playa La Flor, Nicaragua and Playa Nancite, Costa Rica.Crossref | GoogleScholarGoogle Scholar |
Howard, R, Bell, I, and Pike, DA (2014). Thermal tolerances of sea turtle embryos: current understanding and future directions. Endangered Species Research 26, 75–86.
| Thermal tolerances of sea turtle embryos: current understanding and future directions.Crossref | GoogleScholarGoogle Scholar |
Klute A (1986) Water retention: laboratory methods. In ‘Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods’. (Ed. A Klute) pp. 687–734. (Soil Society of America: Madison, WI, USA)
Laloë, J-O, Tedeschi, JN, Booth, DT, Bell, I, Dunstan, A, Reina, RD, and Hays, GC (2021). Extreme rainfall events and cooling of sea turtle clutches: implications in the face of climate warming. Ecology and Evolution 11, 560–565.
| Extreme rainfall events and cooling of sea turtle clutches: implications in the face of climate warming.Crossref | GoogleScholarGoogle Scholar | 33437451PubMed |
Limpus CJ (2008) A biological review of Australian marine turtle species. 2. Green turtle, Chelonia mydas (Linnaeus). Queensland Environmental Protection Agency, Brisbane, Qld, Australia
Limpus, CJ, Miller, JD, Parmenter, CJ, and Limpusr, DJ (2003). The green turtle, Chelonia mydas, population of Raine Island and the northern Great Barrier Reef: 1843–2001. Memoirs of the Queensland Museum 49, 349–440.
Limpus, CJ, Miller, JD, and Pfaller, JB (2020). Flooding-induced mortality of loggerhead sea turtle eggs. Wildlife Research 48, 142–151.
| Flooding-induced mortality of loggerhead sea turtle eggs.Crossref | GoogleScholarGoogle Scholar |
Lolavar, A, and Wyneken, J (2017). Experimental assessment of the effects of moisture on loggerhead sea turtle hatchling sex ratios. Zoology 123, 64–70.
| Experimental assessment of the effects of moisture on loggerhead sea turtle hatchling sex ratios.Crossref | GoogleScholarGoogle Scholar | 28764866PubMed |
Maloney, JE, Darian-Smith, C, Takahashi, Y, and Limpus, CJ (1990). The environment for development of the embryonic loggerhead turtle (Caretta caretta) in Queensland. Copeia 1990, 378–387.
| The environment for development of the embryonic loggerhead turtle (Caretta caretta) in Queensland.Crossref | GoogleScholarGoogle Scholar |
McGehee, MA (1990). Effects of moisture on eggs and hatchlings of loggerhead sea turtles (Caretta caretta). Herpetologica 46, 251–258.
Miller JD (1999) Determining clutch size and hatching success. In ‘Research and Management Techniques for the Conservation of Sea Turtles’. (Eds KL Eckert, KA Bjorndal, FA Abreu-Grobois, M Donnelly) pp. 124–129. IUCN/SSC Marine Turtle Specialist Group Publication No. 4
Mortimer, JA (1990). The influence of beach sand characteristics on the nesting behavior and clutch survival of green turtles (Chelonia mydas). Copeia 1990, 802–817.
| The influence of beach sand characteristics on the nesting behavior and clutch survival of green turtles (Chelonia mydas).Crossref | GoogleScholarGoogle Scholar |
O’Connor, MP, Honarvar, S, Sotherland, PR, and Spotila, JR (2009). Biophysical factors affecting gas exchange in sea turtle nests. Integrative and Comparative Biology 49, E124.
Ralph, CR, Reina, RD, Wallace, BP, Sotherland, PR, Spotila, JR, and Paladino, FV (2005). Effect of egg location and respiratory gas concentrations on development success in nests of leatherback turtles, Dermochelys coriacea. Australian Journal of Zoology 53, 289–294.
| Effect of egg location and respiratory gas concentrations on development success in nests of leatherback turtles, Dermochelys coriacea.Crossref | GoogleScholarGoogle Scholar |
Reina, RD, and Cooper, PD (2000). Control of salt gland activity in the hatchling green sea turtle, Chelonia mydas. Journal of Comparative Physiology B 170, 27–35.
| Control of salt gland activity in the hatchling green sea turtle, Chelonia mydas.Crossref | GoogleScholarGoogle Scholar |
Salehi, MH, Beni, OH, Harchegani, HB, Borujeni, IE, and Motaghian, HR (2011). Refining soil organic matter determination by loss-on-ignition. Pedosphere 21, 473–482.
| Refining soil organic matter determination by loss-on-ignition.Crossref | GoogleScholarGoogle Scholar |
Sato, JH, de Figeiredo, CC, Marchão, RL, Madari, BE, Benedito, LEC, Busato, JG, and de Souza, DM (2014). Methods of soil organic carbon determination in Brazilian savannah soils. Scientia Agricola 71, 302–308.
| Methods of soil organic carbon determination in Brazilian savannah soils.Crossref | GoogleScholarGoogle Scholar |
Schmidt-Nielsen K (1997) ‘Animal Physiology Adaptation and Environment,’ 5th edn. (Cambridge University Press: Cambridge, UK)
Smith, CE, Booth, DT, Crosby, A, Miller, JD, Staines, MN, Versace, H, and Madden-Hof, CA (2021). Trialling seawater irrigation to combat the high nest temperature feminisation of green turtle (Chelonia mydas) hatchlings. Marine Ecology Progress Series 667, 177–190.
| Trialling seawater irrigation to combat the high nest temperature feminisation of green turtle (Chelonia mydas) hatchlings.Crossref | GoogleScholarGoogle Scholar |
Stewart, TA, Booth, DT, and Rusli, MU (2019). Influence of sand grain size and nest microenvironment on incubation success, hatchling morphology and locomotion performance of green turtles (Chelonia mydas) at the Chagar Hutang Turtle Sanctuary, Redang Island, Malaysia. Australian Journal of Zoology 66, 356–368.
| Influence of sand grain size and nest microenvironment on incubation success, hatchling morphology and locomotion performance of green turtles (Chelonia mydas) at the Chagar Hutang Turtle Sanctuary, Redang Island, Malaysia.Crossref | GoogleScholarGoogle Scholar |
Suss, JS, Patel, S, Neeman, N, Panagopoulou, A, Margaritoulis, D, O’Connor, MP, and Spotila, JR (2012). Beach characteristics affect the gas exchange environment for sea turtle nests. Integrative and Comparative Biology 52, E335.
Valverde, RA, Cornelius, SE, and Mo, CL (1998). Decline of the olive ridley sea turtle Lepidochelys olivacea) nesting assemblage at Nancite Beach, Santa Rosa National Park, Costa Rica. Chelonian Conservation and Biology 3, 58–63.
Wallace, BP, Sotherland, PR, Spotila, JR, Reina, R, Franks, BF, and Paladino, F (2004). Biotic and abiotic factors affect the nest environment of embryonic leatherback turtles, Dermochelys coriacea. Physiological and Biochemical Zoology 77, 423–432.
| Biotic and abiotic factors affect the nest environment of embryonic leatherback turtles, Dermochelys coriacea.Crossref | GoogleScholarGoogle Scholar | 15286916PubMed |
Wood, DW, and Bjorndal, KA (2000). Relation of temperature, moisture, salinity, and slope to nest site selection in loggerhead sea turtles. Copeia 2000, 119.
| Relation of temperature, moisture, salinity, and slope to nest site selection in loggerhead sea turtles.Crossref | GoogleScholarGoogle Scholar |
Yntema, CL, and Mrosovsky, N (1980). Sexual differentiation in hatchling loggerheads (Caretta caretta) incubated at different controlled temperatures. Herpetologica 36, 33–36.


