Maternal care and juvenile development of captive-bred short-beaked echidnas (Tachyglossus aculeatus acanthion) at Perth Zoo, Western Australia
Arthur Ferguson A * and Belinda Laming A
A * and Belinda Laming A
A Perth Zoo, Zoological Parks Authority, 20 Labouchere Road, South Perth, WA 6151, Australia.
Australian Journal of Zoology 70(2) 43-55 https://doi.org/10.1071/ZO22013
Submitted: 24 March 2022 Accepted: 10 October 2022 Published: 1 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
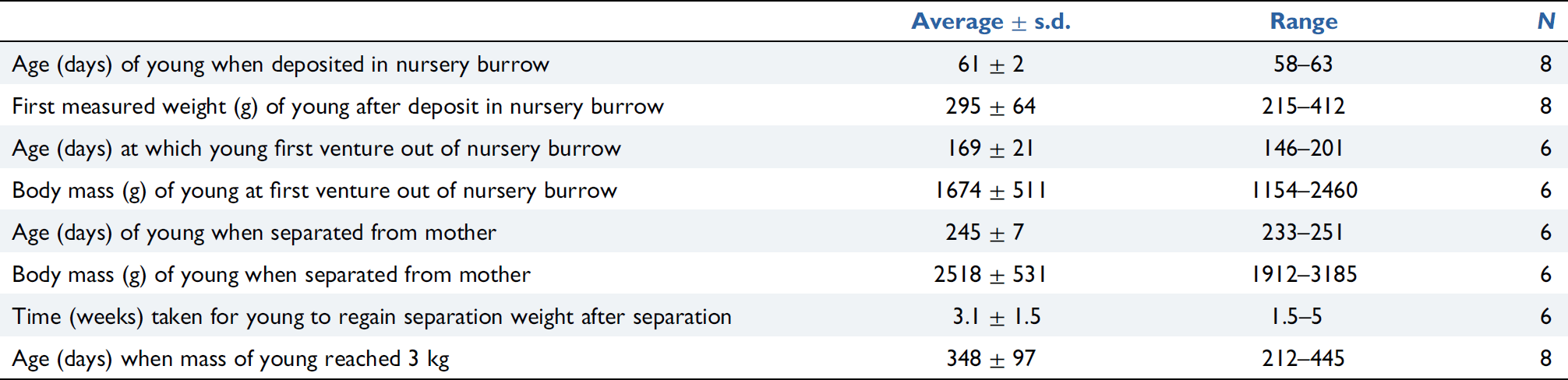
Successful breeding of short-beaked echidnas (Tachyglossus aculeatus acanthion) occurred at Perth Zoo on eight occasions between 2007 and 2012. Here we report the methods used for monitoring and managing breeding females and their young from hatching through to weaning. Growth and development of the young during burrow-life was quantified through regular weighing and maternal care was monitored using video camera surveillance. All young hatched between early August and mid-September and were deposited in nursery burrows in October–November at 58–63 days of age at an average mass of 295 ± 64.3 g. Mothers suckled their young, on average, every 3.3 ± 1.1 days. Young first emerged from their nursery burrow from mid-January into February at an average 169 ± 21 days of age and weighing 1674 ± 511 g, and reached 3 kg in mass at 348 ± 97 days of age. Sexual maturity of two female offspring was attained at 4 years of age. Our observations of maternal care and development of the captive-bred young are consistent with published observations made on wild echidnas. We suggest that important factors for the successful rearing of captive-bred echidnas are enclosure set-up, daily monitoring, combined with a suitably designed and managed nursery burrow that provides a suitable substrate and microenvironment.
Keywords: captive management, echidna, growth, maternel behaviour, monotreme, reproductive biology, surveillance, Tachyglossus aculeatus.
Introduction
The unique reproductive biology of the monotremes has long been of interest to scientists. As one of the few extant monotreme species, the short-beaked echidna (Tachyglossus aculeatus) has been the subject of many of these studies. However, the cryptic, semifossorial nature of the species has limited data collection and our understanding of their reproduction and development. Due to the difficulty of accessing echidna young when they are deposited in a nursery burrow, information on the growth and development of wild echidnas is limited (Griffiths 1978; Abensperg-Traun 1989; Beard and Grigg 2000; Rismiller and McKelvey 2003, 2009; Morrow and Nicol 2013). Captive breeding of echidnas provides an opportunity to better understand aspects of maternal care and development that are otherwise difficult to obtain from wild echidnas. Information documented on nursery burrow environments, weight gain and nursing frequency of young may be of assistance for wildlife carers, providing a guide to support the hand rearing for rehabilitation of orphaned young.
Little information is available on the reproduction of echidnas (Tachyglossus aculeatus lawesii and Zyglossus sp.) in New Guinea, and studies of echidnas in Tasmania (Morrow et al. 2009; Morrow and Nicol 2013) have identified some significant variations, such as males mating with females in deep hibernation, and a shorter lactation period than that of other subspecies. In the wild, breeding is seasonal, occurring from June to mid-September, with females producing a single egg 16–24 days after mating (Beard and Grigg 2000; Rismiller and McKelvey 2000; Nicol and Andersen 2007; Dutton-Regester et al. 2021). The egg is incubated in the pouch of the female for 10–11 days before hatching and the young is carried and suckled in the pouch for a further 23–63 days, before being deposited in a nursery burrow (Griffiths 1989; Abensperg-Traun 1989; Beard and Grigg 2000; Rismiller and McKelvey 2000; Morrow and Nicol 2013). The young remains in the burrow with the mother returning every 3–6 days to nurse it (Beard and Grigg 2000; Rismiller and McKelvey 2000; Nicol and Andersen 2007). Young vacate the nursery burrows and are weaned at 130–214 days of age (Abensperg-Traun 1989; Beard and Grigg 2000; Nicol and Andersen 2007; Rismiller and McKelvey 2009; Morrow and Nicol 2013).
Breeding success of captive echidnas has been poor until recent times (Ferguson and Turner 2013; Wallage et al. 2015), with the majority of births prior to this being the result of chance rather than deliberate breeding attempts (Olney and Fisken 1997). Few captive-bred young have survived to independence and captive growth has not been documented in detail for those that have. At Perth Zoo, successful breeding and rearing to independence of eight short-beaked echidnas (Tachyglossus aculeatus acanthion) was achieved over a 6-year period from 2007 to 2012. Captive management protocols to achieve breeding were described in Ferguson and Turner (2013). The aim of this study was to quantify growth rate and requirements of the captive-bred echidna young during burrow life through to 2 years of age. We also describe observations of maternal behaviour in captivity and compare these with published field observations. Two of the described breeding events involved second- generation 4-year-old Perth Zoo-bred females, providing evidence of the age of female sexually maturity in this species.
Materials and methods
Our study was conducted at Perth Zoo between 2007 and 2014 and involved a total of five females (three wild-born and two captive-born), and eight young (two of which subsequently bred during this study). The four enclosures housing echidnas ranged in size from 36 m2 to ∼100 m2 and included one public display enclosure and three off-display enclosures (Ferguson and Turner 2013).
Hatching and pouch life of young
Date of birth (hatching) of the young was assumed to be the day before the females’ resumption of activity and emergence from their burrows after an extended period of inactivity (Ferguson and Turner 2013). From 2009 we placed two sticks at the entrance of the general hide/shelter burrows (Fig. 1) once the female had entered to commence incubation which was evident with lack of food consumption. A fallen stick then prompted review of recorded CCTV footage to verify the timing of the resumption of activity.
|
|
Pouch checks were typically carried out when the young were expected to be at least three weeks of age and were completed quickly to minimise disturbance. Females were gently removed from their burrow by hand and carefully manipulated to view the pouch area or, with the support of veterinarians, they were lightly anaesthetised with isoflurane (mask down on 5% isoflurane and 2 L/min oxygen and then as soon as they show signs of sedation, recumbency and lack of withdrawal response undertake pouch check; if needed, reduce to 1% isoflurane and keep the oxygen flow rate at 2 L/min for maintenance). The light sedation relaxed the female, providing quick access to the pouch. Young were observed during these inspections but were not removed from the pouch for weighing during the entirety of pouch life (with two exceptions, once each in 2009 and 2012).
The nursery burrow
Maternal females were provided with at least one general/hide shelter burrow (see Ferguson and Turner 2013) as well as a purpose-built nursery burrow box (Fig. 2). The nursery boxes were insulated marine plywood sandwich construction (1200 mm × 600 mm × 400 mm) with a 250 mm × 2250 mm arched entry hole at one end for providing access to a 280 mm wide internal chamber, as described in Ferguson and Turner (2013). These nursery boxes were installed directly on top of the below-ground mesh barrier so the echidnas could not dig underneath, and they were prepared by prefilling the boxes (∼250 mm deep, slightly damp sandy loam). Nursery burrows were shaded from direct sunlight with shade cloth suspended overhead when temperatures increased towards the end of spring through summer. From 2008 onwards insulation material (Kingspan Insulation Air-cell Retroshield®) was added over the top of the nursery burrow boxes to help further support a stable internal temperature. From 2009, females were given access to the nursery burrow box approximately halfway through pouch-life, to provide the opportunity to discover a new unused box, and adequate time to construct/prepare the nursery burrow prior to depositing their young.
From 2007 to 2009, temperature loggers (DS1922L Thermochron iButton®) were used to record ambient temperature (Ta, °C) every 15 min within nursery burrows (and within enclosures in 2007). Equipment failure resulted in loss of some enclosure data in 2008 and 2009. Temperature loggers within enclosures were in exposed locations receiving full sun at times. Data from the loggers was downloaded in the enclosures at intervals of approximately 30 days using a laptop computer (DS1402D-DR8 Blue Dot Receptor 1-Wire® Cable attached to a DS9490R USB Port Adapter). A digital thermometer with stainless steel sensor probe (with 0.1°C resolution and accuracy of ±1°C) was used for real-time monitoring of ambient conditions within nursery burrows. This allowed keepers to monitor temperatures within nursery burrows on hot days (>30°C) and prompted activation of the enclosure cooling misters/sprinkler system when burrow temperatures approached, and were expected to exceed, 25°C.
Weighing of the deposited young in 2007 was conducted weekly or when opportunity presented. In 2008–2012 young were weighed daily except when the females were inside the nursery burrow with burrow young to prevent disturbance to the nursing process. Deposited young were weighed using KH Classik Chef 5000 g × 1 g and Nuweigh JAD 525 6 kg × 1 g balances.
Maternal behaviour and activity of females was filmed continuously, until young were weaned and separated from their mothers. Six infrared cameras (four Ness 100-372 Mini IR Tube® and two Ness 100-639 Ultimate IR LED Varifocal®) connected to two video storage units (NESS EDR410H CCTV®) were used to record and store camera imagery. For each breeding enclosure, one camera was positioned inside each nursery burrow, one camera was positioned outside the entrance of nursery burrows, and two were positioned at opposite ends of each enclosure, maximising coverage of enclosures. Recordings were analysed to provide information on the frequency and duration of nursery burrow visits (accuracy ±1 min). General observations on maternal behaviour and development of the young were also recorded. It was hoped that exact measurements could be made on the length of suckling bouts, but this proved too difficult to determine due to animals’ positioning within the nursery burrow and frequent obstruction of the camera lens with soil. Instead, the length of time from the female entering the nursery burrow to exiting the burrow was measured for young raised in 2007–2009. The recorded time included the time taken to unplug the burrow entrance, suckle the young and plug the burrow entrance closed again.
Analysis
Summary statistics are presented as mean ± standard deviation.
Results
Hatching
Behaviours of a maternal female that indicated that an egg had hatched and a pouch young was present were similar to those behaviours seen during gestation (Ferguson and Turner 2013). Following 11–13 days of inactivity associated with incubation, females (n = 3) were observed within the general hide/shelter rolling on their side, curling their heads towards the pouch with limbs outstretched and tending to the pouch area by probing gently with their beaks.
One of the five females that bred produced young in three successive years and one other female produced more than one young during the study.
Pouch life
Following completion of egg incubation and emergence from the general hide/shelter burrow, females with pouch young (n = 5) appeared to exhibit less activity in the first week compared to subsequent weeks. One female was active for less than 3 h and was observed to feed on only two occasions during the first week after hatching, as opposed to subsequent weeks, when general activity and feeding occurred daily.
Towards the end of pouch life, the growing young could no longer be fully contained in the pouch and the young echidna’s hindquarters were visible hanging out from underneath the female on the nursery burrow camera or the camera focused on the nursery burrow entrance (Fig. 3). All young had outgrown the pouch and were deposited in one of the provided nursery burrow boxes at 58–63 days of age (Table 1).
|
|
|
Nursery burrow life – maternal behaviour and young development
Approximately halfway through pouch life of the young, females usually began digging extensively in the enclosure to the maximum substrate depth (80–150 mm), signalling the desire to construct a nursery burrow. The diggings were distinctive, with freshly turned soil evident and presented in the form of a shallow trench as an attempt to construct a burrow. Observation of these behaviours informed the decisions for keepers to provide access to the purpose-built nursery burrow box from 2009 onwards.
Young were weighed at the first opportunity at 60–66 days of age, after the females had vacated the nursery burrow following caching of their young. Young weighed 295.3 ± 64.3 g (Table 1). At this age small black spines (<3 mm) were visible emerging across the dorsal surface (Fig. 4).
|
|
The way in which females managed and backfilled their nursery burrows varied. Three females backfilled their nursery burrow entrance with substrate, creating a secure plug leaving an open void in the nest chamber where the young resided (Fig. 5). On several occasions, two other females completely buried their young in the substrate during the process of backfilling the nursery burrow, and one female made very little effort to backfill the nursery burrow and did not adequately plug it during the entirety of nursery burrow life of her two young.
The routine weighing of nursery burrow young provided an opportunity to check the young and nursery burrow conditions most days during the entirety of nursery burrow life. When young were found buried in substrate they were uncovered, and a small chamber was created for the young by staff. For the two young in burrows where their mothers failed to plug the nursery burrow, staff would create a soil plug inside the nursery burrow box to contain the young and to discourage it from attempting to wander out of the burrow.
When not active between nursery burrow visits, females would reside in one of their general hide/shelter burrows. Entry to the nursery burrow by three females for feeding five of their young occurred from mid-afternoon to mid-morning, with 81.5% of burrow visits beginning between 18:00 and 03:00 hours (Fig. 6).
When a female intended to enter the nursery burrow, entry occurred without hesitation. The females would walk up to the burrow entrances and proceed to dig through the burrow substrate plug. The time taken for the females to enter the nursery burrow prior to suckling was considerable quicker (less than 5 min) than the time taken to exit and plug the burrow after suckling.
Suckling of the young occurred during all burrow visits made by females, with one exception. The smallest of the eight young (F1Ba) at deposit failed to gain weight and was subsequently removed for hand raising at 75 days of age when it weighed 188 g.
Interactions between one nursery burrow young and her mother (Fig. 7) were observed in detail during a nursery burrow visit when the young was 67 days of age and weighed 400 g. For this nursery burrow visit the mother conducted six pre-entry visits (an approach to the nursery burrow with no attempt to enter) during a 47-min period from 19:11 hours. She then moved to the nursery burrow entrance at 19:58 hours and was seen inside the nursery burrow 3 min later at 20:01 hours. The mother was observed tending to the young and gently nuzzling it with her beak and was observed to help guide the young with her beak under her body towards her pouch. The mother would lift up one of her forelimbs to give the young access to her pouch area. The young would then manoeuvre itself onto its back to access the pouch for suckling. Forty-two minutes after nursery burrow entry the female was observed gently probing/tending to the young’s cloaca area with her beak for 17 s in what could have been toileting stimulation behaviour (Fig. 7). The mother exited the nursery burrow at 23:18 hours and was observed to leave the nursery burrow entrance area at 01:20 hours the following day, indicating that she spent 3 h and 17 min inside the nursery burrow with her young and 2 h and 2 min backfilling and plugging the nursery burrow entrance area. In the morning, the young weighed 513 g, having gained 113 g or 28.3% in body mass during the overnight feeding bout.
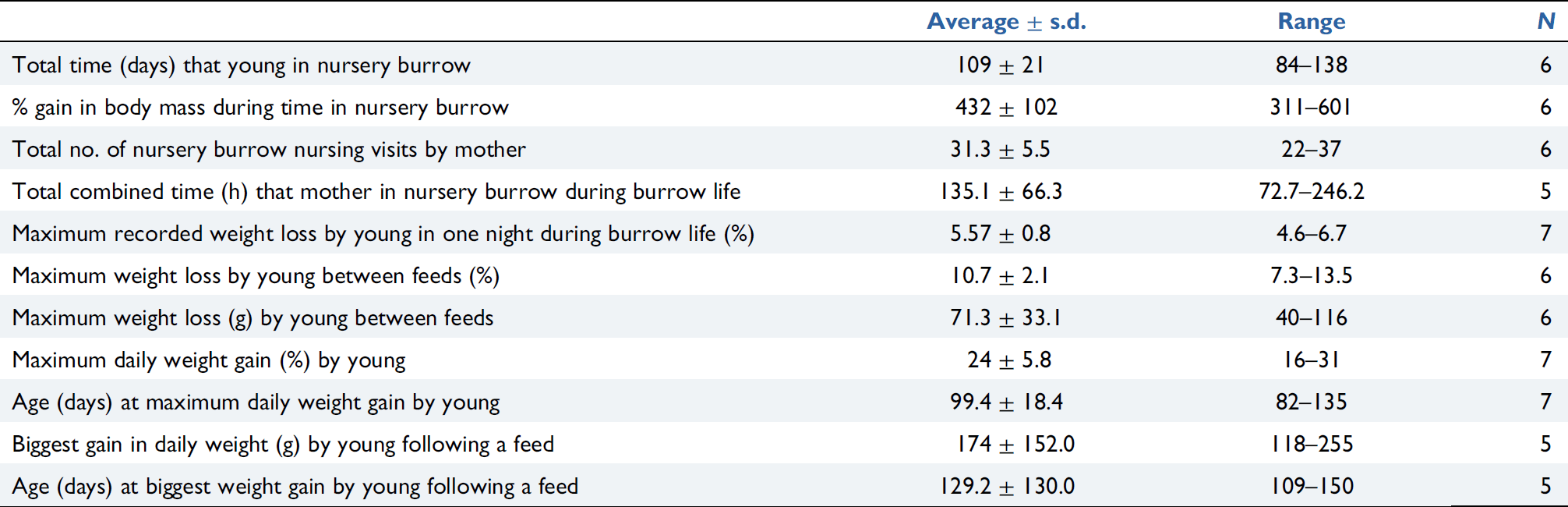
Young resided in the nursery burrows for an average 109.2 ± 20.8 days with attending females visiting the nursery burrow on an average 31.3 ± 5.5 occasions to feed their young (Table 2).
|
The average interval between nursery burrow visits/suckling for six young was 3.3 ± 1.1 days (n = 179, range 1–6 days) (Fig. 8). The length of nursery burrow visits for five young ranged from 32 min to 23.6 h and averaged 5.1 ± 1.7 h (n = 133). Visits were less frequent in the early stages of burrow life and became more frequent in the later stages of burrow life, especially from early January. The average total combined time that the females spent inside the nursery burrow with their young during nursery burrow life was 135.1 ± 66.3 h (Table 2).
Growth in the nursery burrow
There was marked variation in the growth of the eight young during burrow life. The smallest of the eight young when deposited failed to gain weight over the subsequent 2-week period and the decision was made to remove it for hand-raising at 75 days of age. Weight gain for the other seven young followed a linear trajectory during burrow life (Fig. 9).
The maximum daily weight gain as a percentage of body mass was an average 24.3 ± 5.8% (range 16–30.8%, n = 6) which occurred at between 82 and 109 days of age in the seven young studied (Table 2). The average maximum weight gain of young after a single nursery burrow visit feeding bout was 174.4 ± 152 g (range 118–255 g, n = 5) and occurred at between 109 and 150 days of age (Table 2), giving an indication of the volume of milk females provided for their young at this age. The average maximum daily weight loss in 1 day was 5.6 ± 0.8% (range 4.6–6.7%, n = 7) and the average maximum weight loss between nursery burrow visit feeding bouts (i.e. for feeding intervals >1 day) was 10.2 ± 2.4% (range 7.3–13.5%, n = 6) (Table 2).
Nursery burrow temperature
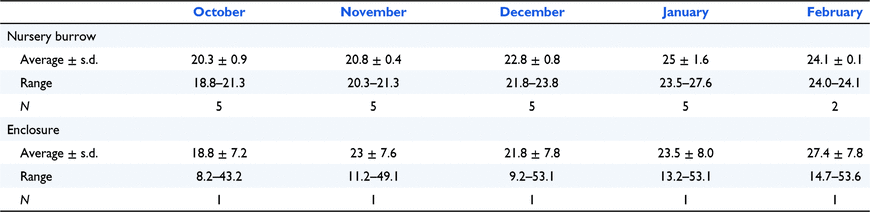
The average temperatures within nursery burrows increased each month from October to February during the three breeding seasons (2007–2009) but showed little variation across any 24-h period (Table 3).
|
Emergence from the nursery burrow
The six parent-reared young emerged from their nursery burrows from mid-January into February at an average 169 ± 21 days of age (range 146–201 days) and with body mass of 1673.5 ± 511.1 g (range 1154–2460 g) (Table 1: noting that the data from the two mother-young groups that were subject to keeper intervention during burrow life, were excluded from this analysis).
During the later stages of burrow life, burrow young became increasingly mobile and capable of digging. Young were observed digging through nursery burrow substrate plugs in the lead up to burrow emergence and this behaviour was considered part of the developmental process signalling readiness to emerge. Despite self-emergence of burrow young, two females continued to try and plug young back in the nursery burrow, with one female persisting with this behaviour for 9 and 11 days respectively for her two young until finally accepting her youngs’ independence. Following final emergence from the nursery burrow the young would often reside in the general hide/shelter burrow with their mother when she was not active.
One young was found out of the nursery burrow at only 92 days of age and 558 g and required staff to create the nursery burrow soil plugs to help contain the young. This continued until the young was 142 d of age and weighed 1171 g. The female’s nursery burrow visits became much more frequent and she was later removed from the young due to concerns with her health. The weight loss of this young (Fig. 10) from 149 days of age was likely attributed to the health status of the female. Despite the weight loss associated with the early removal of the mother at 157 days of age, the young echidna was weaned early and successfully reared. The weight loss observed in this young was more pronounced, but similar to that seen after the separation of other young from their mothers.
|
|
Weaning and separation of young
Two females were observed to continue to suckle their young on multiple occasions up to 34 and 39 days after nursery burrow emergence.
Young were offered diet containing crumbled termite mound containing live termites, (Coptotermes spp. and Nasutitermes spp.) in association with a small portion of the adults’ diet (canned cat-food, crushed primate leafeater and omnivore pellets, water, liquid multivitamin and chopped mealworms: Ferguson and Turner 2013) in addition to that provided for mothers. The diet was offered after young had emerged from the nursery burrow. Young were observed showing interest in the diet and gradually took to the diet over a period of an average 39.3 ± 26 days (range 12–82 days after burrow emergence, n = 6) which equated to 180–248 days of age.
Young were separated from their mothers at an average 245 ± 7.1 days of age (range 233–251 days of age, n = 6; Table 1) in the period April–May. Average body mass at the time of separation was 2518 ± 531 g (range 1912–3185 g, n = 6; Table 1). All weaned young lost weight following separation from their mothers and the average time taken to regain that lost weight was 3.1 ± 1.5 weeks (range 1.5–5 weeks, n = 6; Table 1).
The time taken for young to reach body mass of 3 kg varied, with an average of 11.1 ± 3.2 months (range 7–15 months, n = 6) of age (Table 1). By two years of age, six of the seven young had exceeded 4 kg in body mass (Fig. 10), with the seventh young exceeding 4 kg in mass three months after that.
Discussion
Echidnas are an uncommon species in zoological institutions around the world. At the commencement of this study, fewer than 90 echidnas were recorded in Zoo and Aquarium Association, Australasia (ZAA; formally known as ARAZPA)-member zoos (Turner 2008). Perth Zoo was one of only nine zoos holding both sexes, and one of only four zoos to hold seven or more individuals of the species (Turner 2008). The sample size used in this study was small by most standards; however, it was based on one of the largest captive colonies of echidnas in the world at that time.
The captive breeding of eight echidna young at Perth Zoo has provided an opportunity to document successful methods used for monitoring mothers and their young, from hatching of the egg through to weaning and independence. We have gained detailed information on development and nursing frequency of young, and nursery burrow environments suitable for successful rearing. Behaviours of mothers have also been described. This information provides a foundation of knowledge to support further successful captive breeding attempts in this species, and potentially other members of the Tachyglossidae.
Pouch life
The captive-bred echidna young hatched in August and September, conforming to the strong seasonal pattern of reproduction observed in wild echidnas, with breeding occurring between late-June and mid-September (Griffiths 1978; Beard et al. 1992; Rismiller and McKelvey 1996; Beard and Grigg 2000).
The use of strategically positioned cameras enabled identification of behaviour of maternal females through incubation, at hatching and glimpses of developing pouch young. With this knowledge, handling females for the purpose of confirming the presence of advanced pouch young may not be necessary.
Approximately halfway through their young’s post-hatch pouch life, females began digging extensively in the enclosure substrate, signalling they were looking for a suitable site to construct a nursery burrow. To facilitate ready access to the females and their young in their nursery burrows, all enclosures were fitted with wire mesh buried below the substrate at a maximum depth of between 80 and 150 mm, to encourage the females to deposit their young in the purpose-built nursery burrow box. Based on the timing when the females started to dig a nursery burrow, we recommend giving the female access to the nursery burrow box when the young are expected to be one month of age or as soon as the first nursery burrow diggings are identified. This practice was also informed by reports that in the wild females rarely reused the same nursery burrow site in successive years (Rismiller and McKelvey 2009; Morrow and Nicol 2013).
Young were deposited in the nursery burrow at 58–63 days of age. Field observations record echidnas being carried in the pouch for 45–50 days in south-east Queensland (Beard and Grigg 2000), 50–55 days in Western Australia (Abensperg-Traun 1989), 45–55 days on Kangaroo Island (Rismiller and McKelvey 2000), 48–63 days in New South Wales (Griffiths 1978) and 23–43 days in Tasmania (Morrow and Nicol 2013). Our slightly longer pouch-life is within the parameters reported by Griffiths (1978) and is comparable to a captive bred echidna at St Louis Zoo (USA), which was deposited in the nursery burrow at 60 days of age (Fieseler and Junge 1997). It has been suggested that the young is evicted from the pouch when it can no longer be safely carried by the female (Beard and Grigg 2000) due to the development of the young’s spines. In all cases for the captive-bred young at Perth Zoo, the hindquarters of the young were observed to be hanging out of the pouch prior to deposition, indicating that the young had outgrown the pouch and could no longer be securely transported by females.
Nursery burrow life
Soon after the young were deposited in the nursery burrow, they weighed 215–412 g. This is comparable with recorded mass of wild burrow young measured on Kangaroo Island, which ranged from 180 to 270 g (Rismiller and McKelvey 2003) and is similar with that of a wild mainland echidna observed by Griffiths (1965) with a 62-day-old young weighing 400 g. The range in weights seen in our young may be partly attributed to their age and timing of when the young were weighed after deposit in the nursery burrow, as not all of our young were weighed on the day they were deposited (range 0–4 days after deposit) at 60–66 days of age.
The observed behaviour of mothers approaching the nursery-burrow without entering has not previously been reported and could be a strategy used to disguise the burrow entrance from would-be predators or to ensure there are no threats close by prior to committing to entering the nursery burrow to feed the young. On each occasion when the mother entered the nursery burrow, suckling is believed to have occurred, except in the case of the young that required hand-raising due to lack of weight gain.
The majority of maternal nursery burrow entries occurred nocturnally and were often preceded by several hours of activity and even basking behaviour on occasions during the cooler weeks of early burrow-life. It has been reported that burrow young may become torpid at times between feeds (Griffiths et al. 1988) and that the mother may need to warm the infant prior to feeding. Beard and Grigg (2000) observed that females had a higher active body temperature during visits to feed burrow young. The basking behaviour during the cooler months of early burrow-life and the observed increase in maternal activity prior to feeding could therefore lead to a higher body temperature of the mother, which may facilitate warming the young for feeding. We did not record body temperature of young during this study and therefore could not evaluate torpor.
The time between nursery burrow visits ranged from 1 to 6 days and averaged 3.3 days, with visits occurring more frequently later in burrow life. This is comparable to field observations by Beard and Grigg (2000), who reported nursery burrow visit intervals of 3–6 days, increasing in frequency towards the end of burrow life, and Rismiller and McKelvey’s (2009) record of intervals of 3–6 days, and Morrow and Nicol’s (2013) records of intervals of 2–6 days. The slightly shorter interval reported in our study could reflect the availability of food in relative proximity to the nursery burrow. The time taken for the females’ mammary glands to refill and become engorged with milk may also be a trigger that prompts the females to return to the nursery burrow to suckle their young.
Due to the females shifting the nursery burrow substrate upon entry and exit from the nursery burrow, the internal nursery burrow camera was often obscured by substrate during maternal visits, and it was not always possible to view maternal interactions during this time. Similar observations of captive platypus (Ornithorhynchus anatinus) have been reported from burrow camera observations (Thomas et al. 2018). However, at times when the camera was not obscured, this provided excellent views of the behaviour within the nursery burrow and helped confirm ongoing maternal care of the young. Cameras provided information of nursing frequency and duration in our study animals. We observed an interaction between a female and her young that may have been toileting behaviour. No direct evidence was observed of faecal matter within the nursery burrow environments; however, due to the regular shifting of substrate this could have easily become buried or mixed into the soil substrate. The hand-reared young was observed to defaecate both with and without cloacal stimulation.
When the females were not inside the nursery burrow tending to their young or they were not active, they would seek refuge in the general hide/shelter burrows. We believe the provision of the general hide/shelter burrows provides an important retreat for the females during times of rest and this is an important provision for the females with young.
Growth in nursery burrow
Growth rates differed between the eight young. Some young grew rapidly and thrived while one young failed to thrive and required supportive hand-rearing by staff. The reason for this variation is not clear but may be influenced by the volume of milk produced by the female or the body temperature and metabolism of the young. The mother of the young that failed to thrive and which was subsequently hand-raised was a first-time inexperienced 4-year-old female and this may be attributed to the rearing complications early in nursery burrow life.
Although comparative data are scant, growth rates during burrow life for some of the captive-bred young appear higher than those seen in the wild. The daily weighing of the young in 2008–2012 provided an opportunity to closely monitor and observe weight gain following each feed and weight loss between nursing feeds (Table 2, Figs 9 and 10). Daily weights helped confirm the duration in days between nursery burrow visits/feeds. Further to this we now know that weight loss over consecutive days between nursing feeds is typical and should be followed by weight gain after the subsequent nursing feeds. The biggest gain in daily weight (118–255 g) of nursery burrow young provides a guide for the volume of milk females are capable of delivering to their young. Females delivered this large volume of milk for three of the young between 3 and 20 January whereas the other two young received their largest volume of milk on 22 November and 11 December.
Emergence from the nursery burrow
Towards the end of burrow-life we observed young to become increasingly inquisitive and bold, with young pushing/digging through nursery burrow substrate plugs. The young were typically determining when they were ready to leave the nursery burrow. Rismiller and McKelvey (2009) report that females discontinued backfilling nursery burrows at weaning and Morrow and Nicol (2013) reported that weaning appears not to be an abrupt event. We found that females continued to attempt to plug the young in the nursery burrow after their young’s first emergence. This strategy could further ensure that the young is strong enough and ready to permanently vacate the nursery burrow. The strength of the echidna is one of its key evolutionary attributes that likely helps determine its access to food resources. Ensuring the young has matured sufficiently could aid its survival after weaning. One young lost weight following early nursery burrow emergence and then separation from the mother due to a change in her health status. This female had previously raised a young successfully to independence, so we know she was an experienced mother. It is likely the mother’s health combined with the early separation of the mother from the young contributed to the young’s weight loss at this time.
Weaning and separation of young
As reported by Morrow and Nicol (2013), we found that weaning is not always abrupt and it is not tied directly to when the young first emerge from the nursery burrow. We observed that young would continue to suckle from their mothers after they had vacated the nursery burrow. The time taken for young to accept the diet offered varied. It is unclear why there was such variation in the time taken to accept the diet. Young were separated after they were confirmed to be reliably eating the diet and all young lost weight following separation. Once the young had regained their separation weight, they were considered fully weaned and independent. Based on estimates of wild juveniles dispersing at 12 months of age (Abensperg-Traun 1991) there may be benefits to delaying separation from their mothers beyond that implemented in our study.
Rearing of young to independence
The timing of captive breeding conformed to the strong seasonal pattern of reproduction observed in wild echidna, therefore captive breeding under natural lighting should be concentrated around June to mid-September in the southern hemisphere. The design of the nursery burrow box provided excellent access to the young for observation and monitoring. Having identified that females occasionally completely bury their young in substrate, the easy access to the young provided an opportunity to closely monitor the impact of this behaviour on the young. This system provided us with the opportunity for timely intervention to remove the young that was not thriving and required hand-raising. Further to this we were able to manipulate the burrow substrate to maintain mouldability by adding a small amount of water as needed. For females that failed to adequately plug their young in the nursery burrow, we could mould a plug out of the substrate to support containment of the young as necessary.
Burrow cameras provide an opportunity to learn more about the behaviour of young during nursery burrow life and associated interactions and behaviours of maternal echidnas. Temperature within the nursery burrow box should be monitored and managed to achieve a buffered temperature range to guard against external temperature extremes. It is not yet clear if temperature directly influences the growth and development of the young; however, the temperature ranges we have recorded can be used as a guide.
Two females that were born in 2008 produced their own young in 2012 respectively. This provides the first record of 4-year-old females producing young, adding further data on age at sexual maturity for female echidnas. Of the eight young born at Perth Zoo during this study, seven were confirmed as females and one as male (Perry et al. 2019). The ratio of females to males is of interest and further study may be required to investigate potential influences of sex determination.
For species that have traditionally not thrived or bred well in captivity like short-beaked echidnas, it is worth exploring opportunities to refine and enhance enclosure set-ups and provisions that complement the evolutionary needs of the species, and husbandry practices that complement the social needs and behavioural sensitivities of the animals in care. Use of modern technology such as CCTV cameras combined with practical management strategies, provides a great opportunity to implement a highly effective system for monitoring animal behaviour. This type of monitoring can provide significant insight into animal behaviour and care needs that may otherwise be difficult to obtain.
The western long-beaked echidna (Zaglossus bruijnii) and Sir David’s long-beaked echidna (Zaglossus attenboroughi) of New Guinea are close relatives of the short-beaked echidna and are classified as critically endangered species (IUCN 2016). Knowledge of behaviour and reproduction of these species is limited, and it is hoped that by unravelling the secrets of the short-beaked echidna, learned methods could be applied to support conservation efforts for these species in their native homeland in future.
Ethical approval
This research was conducted with Perth Zoo Animal Ethics Committee approvals 2007-8 and 2010-2.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
Thanks to all the staff involved with this project at Perth Zoo, in particular Aaron Gove, Peter Mawson and Matyas Liptovszky for technical write up support, all echidna keepers, Petra Hancock, Silke Tyler, John Lemon, Graham Moore, Pat Cooke and Helen Robertson. Thanks also to Todd Sinander, Peggy Rismiller, Stewart Nicol, Steve Johnston, Alice Seyfried, James McLaughlin, Jane Cook and Karen Payne for their support and encouragement. Sophie Walsh kindly spent many hours reviewing recorded video footage.
References
Abensperg-Traun, M (1989). Some observations on the duration of lactation and movements of a Tachyglossus aculeatus acanthion (Monotremata: Tachyglossidae) from Western Australia. Australian Mammalogy 12, 33–34.| Some observations on the duration of lactation and movements of a Tachyglossus aculeatus acanthion (Monotremata: Tachyglossidae) from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Abensperg-Traun, M (1991). A study of home range, movements and shelter use in adult and juvenile echidnas, Tachyglossus aculeatus (Monotremata: Tachyglossidae), in Western Australian wheatbelt reserves. Australian Mammalogy 14, 13–22.
| A study of home range, movements and shelter use in adult and juvenile echidnas, Tachyglossus aculeatus (Monotremata: Tachyglossidae), in Western Australian wheatbelt reserves.Crossref | GoogleScholarGoogle Scholar |
Beard, LA, and Grigg, GC (2000). Reproduction in the short-beaked echidna, Tachyglossus aculeatus: field observations at an elevated site in south-east Queensland. Proceedings of the Linnaean Society of New South Wales 122, 89–99.
Beard LA, Grigg GC, Augee ML (1992) Reproduction by echidnas in a cold climate. In ‘Platypus and Echidnas’. (Ed. ML Augee) pp. 93–100. (Royal Zoological Society of New South Wales: Sydney, NSW, Australia)
Dutton-Regester, K, Keeley, T, Fenelon, JC, Roser, A, Meer, H, Hill, A, Pyne, M, Renfree, MB, and Johnston, S (2021). Plasma progesterone secretion during gestation of the captive short-beaked echidna. Reproduction 162, 267–275.
| Plasma progesterone secretion during gestation of the captive short-beaked echidna.Crossref | GoogleScholarGoogle Scholar |
Ferguson, A, and Turner, B (2013). Reproductive parameters and behaviour of captive short-beaked echidna (Tachyglossus aculeatus acanthion) at Perth Zoo. Australian Mammalogy 35, 84–92.
| Reproductive parameters and behaviour of captive short-beaked echidna (Tachyglossus aculeatus acanthion) at Perth Zoo.Crossref | GoogleScholarGoogle Scholar |
Fieseler CA, Junge RE (1997) The management and husbandry of the short-beaked echidna (Tachyglossus aculeatus) at the St. Louis Zoo. (St Louis Zoo: St. Louis, MO, USA)
Griffiths, M (1965). Rate of growth and intake of milk in a suckling echidna. Comparative Biochemistry and Physiology 16, 383–392.
| Rate of growth and intake of milk in a suckling echidna.Crossref | GoogleScholarGoogle Scholar |
Griffiths M (1978) ‘The Biology of the Monotremes.’ (Academic Press: New York, USA)
Griffiths M (1989) Tachyglossidae. In ‘Fauna of Australia. Vol. 1B. Mammalia’. (Eds DW Walton, BJ Richardson) pp. 407–435. (Australian Government Publishing Service: Canberra)
Griffiths, M, Kristo, F, Green, B, Fogerty, AC, and Newgrain, K (1988). Observations on free-living, lactating echidnas, Tachyglossus aculeatus (Monotremata: Tachyglossidae), and sucklings. Australian Mammalogy 11, 135–144.
IUCN (2016) The IUCN Red List of Threatened Species. Version 2016-2. Available at www.iucnredlist.org [Accessed 21 March 2022]
Morrow, GE, and Nicol, SC (2013). Maternal care in the Tasmanian echidna (Tachyglossus aculeatus setosus). Australian Journal of Zoology 60, 289–298.
| Maternal care in the Tasmanian echidna (Tachyglossus aculeatus setosus).Crossref | GoogleScholarGoogle Scholar |
Morrow, G, Andersen, NA, and Nicol, SC (2009). Reproductive strategies of the short-beaked echidna – a review with new data from a long-term study on the Tasmanian subspecies (Tachyglossus aculeatus setosus). Australian Journal of Zoology 57, 275–282.
| Reproductive strategies of the short-beaked echidna – a review with new data from a long-term study on the Tasmanian subspecies (Tachyglossus aculeatus setosus).Crossref | GoogleScholarGoogle Scholar |
Nicol, S, and Andersen, NA (2007). The life history of an egg-laying mammal, the echidna (Tachyglossus aculeatus). Ecoscience 14, 275–285.
| The life history of an egg-laying mammal, the echidna (Tachyglossus aculeatus).Crossref | GoogleScholarGoogle Scholar |
Olney, PJS, and Fisken, A (1997). Census of rare animals in captivity. International Zoo Yearbook 35, 463–464.
| Census of rare animals in captivity.Crossref | GoogleScholarGoogle Scholar |
Perry, T, Toledo-Flores, D, Kang, WX, Ferguson, A, Laming, B, Tsend-Ayush, E, Lim, SL, and Grützner, F (2019). Non-invasive genetic sexing technique for analysis of short-beaked echidna (Tachyglossus aculeatus) populations. Reproduction, Fertility and Development 31, 1289–1295.
| Non-invasive genetic sexing technique for analysis of short-beaked echidna (Tachyglossus aculeatus) populations.Crossref | GoogleScholarGoogle Scholar |
Rismiller PD, McKelvey MW (1996) Sex, torpor and activity in temperate climate echidnas. In ‘Adaptations to the cold’. (Eds F Geiser, AJ Hulbert, SC Nicol) pp. 23–30. (University of New England Press: Armidale, NSW, Australia)
Rismiller, PD, and McKelvey, MW (2000). Frequency of breeding and recruitment in the short-beaked echidna, Tachyglossus aculeatus. Journal of Mammalogy 81, 1–17.
| Frequency of breeding and recruitment in the short-beaked echidna, Tachyglossus aculeatus.Crossref | GoogleScholarGoogle Scholar |
Rismiller, PD, and McKelvey, MW (2003). Body mass, age and sexual maturity in short-beaked echidna, Tachyglossus aculeatus. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology 136, 851–865.
| Body mass, age and sexual maturity in short-beaked echidna, Tachyglossus aculeatus.Crossref | GoogleScholarGoogle Scholar |
Rismiller, PD, and McKelvey, MW (2009). Activity and behaviour of lactating echidnas (Tachyglossus aculeatus multiaculeatus) from hatching of egg to weaning of young. Australian Journal of Zoology 57, 265–273.
| Activity and behaviour of lactating echidnas (Tachyglossus aculeatus multiaculeatus) from hatching of egg to weaning of young.Crossref | GoogleScholarGoogle Scholar |
Thomas, J, Handasyde, K, Parrott, ML, and Temple-Smith, P (2018). The platypus nest: burrow structure and nesting behaviour in captivity. Australian Journal of Zoology 65, 347–356.
| The platypus nest: burrow structure and nesting behaviour in captivity.Crossref | GoogleScholarGoogle Scholar |
Turner B (2008) Program outline for short-beaked echidna, Tachyglossus aculeatus. (Australasian Regional Association of Zoological Parks and Aquaria: Sydney)
Wallage, A, Clarke, L, Thomas, L, Pyne, M, Beard, L, Ferguson, A, Lisle, A, and Johnston, S (2015). Advances in the captive breeding and reproductive biology of the short-beaked echidna (Tachyglossus aculeatus). Australian Journal of Zoology 63, 181–191.
| Advances in the captive breeding and reproductive biology of the short-beaked echidna (Tachyglossus aculeatus).Crossref | GoogleScholarGoogle Scholar |


