Group structure in a social huntsman spider (Delena cancerides) reveals seasonal variation in group complexity
Vanessa Penna-Gonçalves A *
A *
A
Abstract
Sociality in spiders has evolved independently multiple times with diverse expressions. Delena cancerides, an Australian huntsman spider, shows some sociality but has been classified variably as social, subsocial, or non-social. Previous classifications were based on evidence like outbreeding, balanced sex ratios, and colonies primarily consisting of one mother and her offspring. However, studies, including this one, have found colonies with multiple adult females, males, and juveniles at certain times of the year. The data show that D. cancerides colonies were more diverse in summer, with multiple adult females, males, and juveniles, compared with spring, when colonies mainly consisted of one adult female and juveniles. Although all huntsman spiderlings cohabit briefly before dispersing, D. cancerides spiderlings shared prey beyond this period, especially larger prey. This suggests that the species’ social structure is more complex than previously thought, varying with time and possibly related to colony composition, warranting further study.
Keywords: Delena cancerides, feeding behaviour, huntsman spiders, parasitism, predatory behaviour, seasonal variation, sociality, social behaviour, spiders.
Introduction
Sociality is an extremely rare phenomenon in spiders, occurring in only 0.1% of species (Whitehouse and Lubin 2005; Yip and Rayor 2013). It is believed that sociality in spiders evolved from the extension of maternal care and delayed dispersal of juveniles (‘subsocial route’: Wickler and Seibt 1993; Avilés 1997; Lubin and Bilde 2007), in association with a lack of aggression and increased tolerance between individuals (Schneider 1995). Sociality in spiders has evolved independently multiple times, including in web builders relying on prey-capture webs and cursorial hunters not utilising capture webs to capture prey (Avilés 1997). The species-specific social systems vary broadly from periodic to permanent group living (see Avilés and Guevara 2017).
Although ‘sociality’ is a complex phenomenon with many different forms of living together, the social systems classification in spiders is based on two main criteria: (1) territoriality (whether individuals are in proximity but maintain individual territories or not) and (2) the duration of living together (periodic or permanent) (see Avilés and Guevara 2017 for a review). Here I (and others) consider the word ‘social’ in its broader context, describing spiders living in groups that tolerate others, often siblings, beyond the early instars (Beavis et al. 2007). It is important to note that freshly hatched spiderlings of most species (solitary and social) cohabit for a period before dispersal (Foelix 2011). During this time, they may forage together but, equally, sibling cannibalism is also common (e.g. Modanu et al. 2014). Perhaps not surprisingly, the period of cohabitation in most spider species is brief (e.g. pholcid spiders cohabit for about two weeks after emergence (Bristowe 1971).
Avilés and Guevara (2017) characterise four primary expressions of spider sociality: (1) non-territorial permanent social spiders, in which the colony has multiple females with multiple offspring generations [for example, some species of Anelosimus (Theridiidae) live in a communal web where offspring do not disperse but remain in the natal web to mate and reproduce (Avilés 1997). They cooperate in prey capture and share offspring care (see Caponera et al. 2021 for a summary). The gene flow among the colonies is low, resulting in a highly inbred system and a female sex bias (Avilés 1993). It is important to note that Anelosimus also includes species that are entirely solitary, subsocial or transitional between subsocial and social (e.g. A. jabaquara: Avilés and Harwood 2012)]; (2) territorial permanent social spiders, in which multiple individuals aggregate in communal webs that last for generations, where individuals connect their discrete webs by frame lines (Uetz and Hieber 1997) and all colony members build and repair the overall web (e.g. Metepeira spinipes (Araneidae): Uetz and Burgess 1979); (3) territorial periodic social spiders, in which offspring have their own territory but live within a communal web and disperse before reproduction [for example, in the web-building spiders Eriophora bistriata (Araneidae) each spider has its orbicular web, which is communally connected (Fernández Campón 2007). The mating system is outbred with a balanced sex ratio (Avilés and Guevara 2017)]; (4) non-territorial periodic social spiders, in which the colony consists of only one adult female and her offspring, which disperse before reproduction, such as in Xysticus bimaculatus (Thomisidae: Ruch et al. 2014a, 2014b) and Delena cancerides (Sparassidae).
In addition to this very basic definition of ‘social’, other authors have attempted to describe more subtle forms of social behaviour in spiders, namely ‘subsocial’ or ‘prolonged subsocial’ behaviour, where juveniles remain with their mother for a prolonged period prior to their own reproduction (Yip and Rayor 2014).
In this paper, I will refer to spiders as being either solitary (living alone and not tolerating other individuals beyond the early instars) or social (living in groups and tolerating other group members beyond the early instars).
In Australia, huntsman spiders are represented by almost 100 described species (Agnarsson and Rayor 2013). They are mostly nocturnal and hunt small insects. Delena cancerides Walckenaer, 1837 (Sparassidae) is a cursorial spider that lives under the bark of trees in groups of up to 300 individuals (Rowell and Avilés 1995; Beavis et al. 2007). They are considered panmictic (i.e. they display random mating within the breeding population) due to outbreeding and a balanced sex ratio (Rowell and Avilés 1995).
Delena cancerides was first described as social in 1985 (Rowell 1985) and then refined as subsocial by Agnarsson et al. (2006) due to outbreeding and dispersal prior to maturity. Further arguments favouring the reclassification were based on the observation that multiple adults in the same colony were rare (Lubin and Bilde 2007). Rayor and Taylor (2006) suggested the term ‘prolonged subsocial’ for arachnids, which was later applied to D. cancerides (Yip and Rayor 2014), and other five species of huntsman spiders (Gorneau et al. 2022), due to the long-term social bonds between mothers and their offspring, or among siblings, that can extend beyond the sexual maturity of the offspring. However, these social associations remain centred on mothers and their offspring and do not develop into more complex group structures with multiple breeding adults (Rayor and Taylor 2006). However, groups containing up to 12 adults of both sexes mixed with multiple generations of juveniles have also been reported (Rowell and Avilés 1995; Yip and Rayor 2011, 2014).
Despite the definition as subsocial in 2006 (Agnarsson et al. 2006), many subsequent studies still use the broader term of ‘social’ to describe D. cancerides (Beavis et al. 2007; Yip and Rayor 2011, 2013; Foelix 2011; Hurst and Rayor 2021). Furthermore, in a recent phylogenetic study on the evolution of sociality in spiders, D. cancerides was classified as a non-social species due to its phylogenetic position among other non-social spiders (Tong et al. 2022). Despite their phylogenetic position, the behaviour of D. cancerides is social and involves group living and communal prey capture and prey sharing (e.g. Avilés 1997; Yip and Rayor 2013). It is possible that social feeding is a common expression of early-stage spiderlings in huntsman spiders generally, which is retained by social huntsman spiders but lost by solitary huntsman spiders after dispersal from the nest.
The broader aim of this study is to contribute further information on the social structure and behaviour of D. cancerides, with two specific objectives: (1) to validate if groups of D. cancerides contain multiple adults or mainly consist of mother–offspring association; and (2) if social feeding is exclusive to D. cancerides from an early developmental stage or if it is also expressed in spiderlings of solitary huntsman species.
Methods
Study species
To date, four species of Delena have been described as prolonged subsocial: Delena cancerides, Delena lapidicola (Hirst, 1991), Delena melanochelis (Strand, 1913) and Delena spenceri (Hogg, 1903) (Gorneau et al. 2022). Here, I focused on D. cancerides only. Collections and experiments on D. cancerides (social species) (Fig. 1a), Isopoda villosa and Holconia immanis (solitary species) (Fig. 1b, c, respectively) were conducted between December 2022 and September 2024. Delena cancerides has also been the focus of recent neurobiological investigations, linking brain volume and structure to social status (Penna-Gonçalves et al. 2025a, Penna-Gonçalves et al. 2025b).
Colony size and composition
To quantify the size and composition of the social spider colonies I collected eight colonies of D. cancerides during the Australian summer (December, minimum 13°C to maximum 30°C) and five colonies in the spring (September, minimum 10°C to maximum 14°C) in Victoria, Australia (Braeside Park, −37.79361, 145.08639) in the same area (90 m2) for about 6 h for three days in both seasons. The spiders were collected from behind the bark of eucalyptus trees (mainly Eucalyptus viminalis subsp. pryoriana) and dead Acacia sp. trees, and all individuals within a nest were collected, transported to the laboratory at Macquarie University, Sydney, and classified into adult female (externally visible epigynum) or male (palpal bulbs fully developed), subadult males (palpal bulbs visible but not yet developed) and juveniles of unknown sex. I also noted if the females had an egg sack with them. Colonies were collected during the day to ensure all colony members were in the retreat and not hunting outside. I defined a colony to be more than one individual found in the same bark tree retreat, even though the female may have been absent.
Additionally, I collected 25 solitary Isopoda villosa huntsman females from under the bark of Eucalyptus trees on the campus of Macquarie University (−33.770338, 151.113395), and nine solitary Holconia immanis huntsman from under the bark of Eucalyptus trees in Brisbane, Australia (Corra-Mulling Park, −27.47278, 153.02778). Solitary spiders were collected during the night (with some exceptions), and I never found more than one individual at any time. Egg sacs were either collected in the field or bred in the lab. All spiders were collected in public areas, which did not require permits.
Feeding experiments
I conducted feeding experiments on 165 individuals from 25 egg sacs of D. cancerides, 150 individuals from 25 egg sacs of Isopoda and 55 individuals from 9 egg sacs of Holconia. All spiderlings were in the 2nd instar (after their second moult outside the egg sac). Five spiderlings per family were randomly selected, and each group of five was placed into an arena (petri dish, radius = 85 mm) (Fig. 2a). Arenas were placed on top of grid paper (5 mm), and the groups were randomly allocated to receive either one large prey (cricket, 1 cm) or five small prey items (Drosophila). Spiderling groups were allowed to settle in the Petri dishes for 5 min before the prey was introduced, and the spiderling’s behaviour was recorded using an Olympus Tough TG-5. For small prey groups, once one individual captured the prey, I continued recording for an additional 30 min. For large prey, the total recording was 2 h, regardless of whether the prey was captured or not. Videos were scored blinded to species, noting if the group captured prey or not and, if they did, whether prey was shared (i.e. more than one individual was feeding on it; see Fig. 2b). Each spiderling was used only once in the feeding trials.
Data analysis
To test whether the two solitary species (H. immanis and I. villosa) differed in their capture and sharing behaviour I used 2 × 2 contingency tables with Yates’ correction in R-studio, which confirmed that the two species did not differ in their prey capture (larger prey capture P = 0.4719; small prey capture P = 0.3894). For prey sharing, none of the solitary species shared large prey and I observed only one case of sharing small prey in I. villosa between two individuals. Based on the similarities between the two solitary species, I pooled them into one ‘solitary’ category for our comparison with the social D. cancerides. All remaining comparisons were conducted using Pearson’s Chi-square test with Yates’ continuity correction using R-studio (R Core Team 2020).
Results
Colony size and composition
Summer: D. cancerides colonies (n = 8) ranged in size from two to 32 individuals. Seven of the eight collected colonies contained multiple adult females, three colonies also contained adult males, and five colonies contained up to four unhatched egg sacs (Table 1).
| Adult female with egg sac | Adult female without egg sac | Adult male | Subadult male | Juveniles (unknown sex) | Total | ||
|---|---|---|---|---|---|---|---|
| Summer (2022) | |||||||
| Colony 1 | 1 | 2 | 0 | 5 | 17 | 25 | |
| Colony 2 | 1 | 1 | 0 | 12 | 18 | 32 | |
| Colony 3 | 1 | 4 | 0 | 6 | 12 | 23 | |
| Colony 4 | 0 | 2 | 1 | 0 | 0 | 3 | |
| Colony 5 | 2 | 0 | 2 | 1 | 2 | 7 | |
| Colony 6 | 1 | 1 | 1 | 0 | 2 | 5 | |
| Colony 7 | 1 | 0 | 0 | 0 | 1 | 2 | |
| Colony 8 | 2 | 0 | 0 | 0 | 1 | 3 | |
| Spring (2024) | |||||||
| Colony 1 | 0 | 1 | 1 | 0 | 17 | 19 | |
| Colony 2 | 0 | 0 | 0 | 0 | 25 | 25 | |
| Colony 3 | 0 | 1 | 0 | 0 | 26 | 27 | |
| Colony 4 | 0 | 1 | 0 | 0 | 18 | 19 | |
| Colony 5 | 0 | 2 | 0 | 0 | 28 | 30 |
Spring: D. cancerides colonies (n = 5) ranged in size from 19 to 30 individuals. Colonies were composed mainly of one female with juveniles, except for one colony that contained two adult females and another colony with a mature male and female (Table 1).
The main observed difference in colony composition between the two seasons was the presence of multiple adults (male and female), and egg sacs, which only occurred during summer.
In 2022 only, I found single individuals under the bark of Eucalyptus trees, including one female with an egg sac, three females without egg sacs, two adult males, two subadult males and five juveniles, all of which were heavily parasitised by mites (Fig. 3). It is unknown whether these individuals had just dispersed or had become separated from a colony during a foraging trip.
Feeding experiments
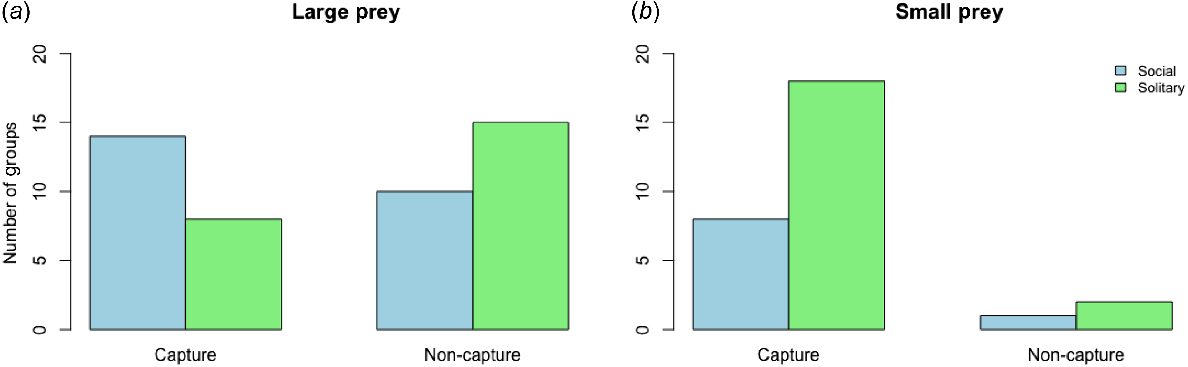
I found no significant difference in the number of groups that captured large prey between social and solitary huntsman spiderlings (Chi-square = 1.755, d.f. = 1, P = 0.1851) (Fig. 4a), although, on a few occasions, social huntsman spiderlings captured large prey cooperatively. Similarly, for small prey capture, there was no significant difference in the number of social or solitary spiderling groups that successfully captured prey (Chi-square = 3.9045 × 1031, d.f. = 1, P = 1) (Fig. 4b).
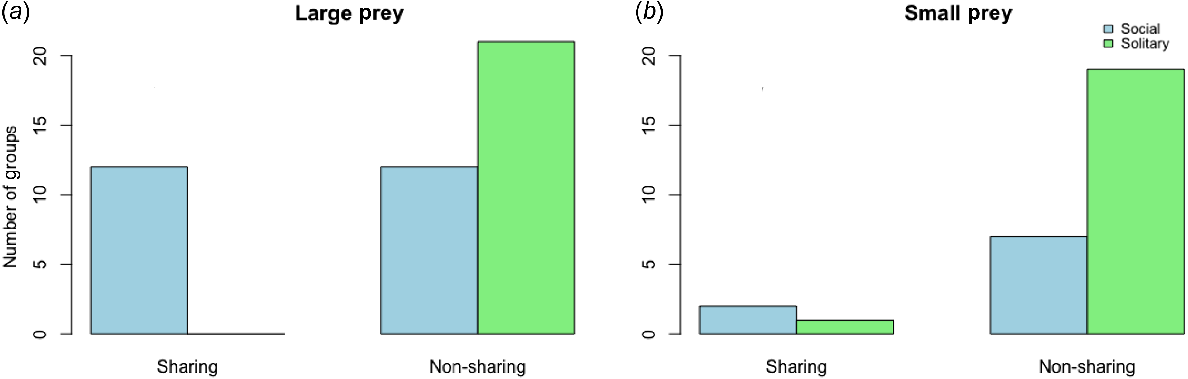
Bar plot depicting the number of groups that captured or did not capture large prey (cricket) (a) and small prey (Drosophila) (b) for social (Delena cancerides) and solitary (Holconia immanis and Isopoda villosa) huntsman spiderlings.
When it came to sharing prey, half of the social spiderling groups shared large prey, but none of the solitary species shared any large prey (Chi-square = 11.876, d.f. = 1 and P = 0.0005) (Fig. 5a). Prey sharing was considered to occur if more than two individuals shared an item; it occurred in most of the cases involving three to five individuals (85%). However, small prey was generally not shared by either social or solitary spiderlings, with no significant difference between them (Chi-square = 0.562, d.f. = 1, P = 0.4533) (Fig. 5b).
Discussion
Observations of D. cancerides colonies indicate varying levels of social complexity ranging from small mother–offspring-only groups through to large groups composed of multiple adult males, reproductive females and offspring. This variation could possibly be a result of seasonal differences, where in spring, colonies are mostly mother–offspring groups whereas later in summer more complex groupings emerge. Similarly, Beavis et al. (2007) collected D. cancerides colonies from February (late summer) to September (early spring) and reported mostly mother–offspring groups. Other collections of D. cancerides colonies also appear to have contained multiple adults (e.g. Rowell 1985; Rowell and Avilés 1995) but lack detailed descriptions of colony composition.
I was not able to determine whether the additional male and female adults I observed in summer were the matured offspring of original mother–offspring groups or if unrelated adults had joined the colony. It is unlikely that I sampled multiple colonies in proximity on the same tree, as I have never observed more than one colony per tree, and the trees were narrow with limited space for multiple colonies. Although the inclusion of unrelated juveniles in D. cancerides colonies is quite common (Rowell and Avilés 1995), adults are so far not known to enter the colonies of unrelated groups (Yip et al. 2012). As kin recognition is highly developed in this species and alien intruders are aggressively attacked (Beavis et al. 2007; Yip et al. 2009), the multiple adults observed were more likely to be mature offspring that did not disperse. The sheer presence of multiple related adults, however, could increase the likelihood of sibling matings and, thus, inbreeding. Although inbreeding has not been observed in D. cancerides (Rowell and Avilés 1995), under laboratory conditions, when D. cancerides individuals are kept together in groups beyond maturation, sibling matings do occur and produce viable offspring (V Penna-Gonçalves pers. obs.). It is interesting to note that the limited number of D. cancerides individuals that were found in solitary conditions were all heavily parasitised by mites, raising two equally intriguing possibilities: (1) parasitised individuals are evicted from colonies, or (2) when individuals accidentally become isolated from colonies, they are at higher risk of parasitism due to lack of grooming by other nestmates (see Wilson et al. 2020). The risk of parasitism could also have contributed to the evolution of sociality in spiders as proposed for other social species (e.g. Wilson et al. 2020).
The feeding trials confirm that prey sharing is not a common behaviour in most species of huntsman spiderlings and thus far has only been observed in D. cancerides, from the youngest instars (my experiments) through to subadults (V Penna-Gonçalves pers. obs.) (Yip and Rayor 2013). Although sharing prey is a common behaviour in many species of social spiders (Avilés 1997; Schneider and Bilde 2008; Dumke et al. 2018), it seems to be rare in solitary species, having been described only in solitary Zodarion spiders when they capture large ants (Pekár et al. 2005). However, unlike solitary huntsman spiders, solitary Zodarion spiders are found in high densities with more than a hundred individuals hunting in the same ant nest, where cooperation could increase capture efficiency (Pekár et al. 2005). There are clear fitness benefits from prey sharing in social spiders (Dumke et al. 2018), and specifically in D. cancerides, in which the younger siblings in particular benefit from sharing prey with their older siblings (Yip and Rayor 2013).
How do my results contribute to the current understanding of D. cancerides’ social structure? Sampling colonies in late summer has revealed greater colony complexity than has been described in many previous studies that collected colonies earlier in the season. These observations warrant more detailed investigations into how the adults interact with each other (e.g. inbreeding) and with juveniles in the group. Ultimately, such knowledge might help refine the level of sociality in D. cancerides, which has already seen many changes and iterations. Further detailed molecular analyses of the more complex groupings would contribute to our overall understanding of the social structure of D. cancerides.
Data availability
The data that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.14792217.
Declaration of funding
The Linnean Society of New South Wales, European Society for Evolutionary Biology, Macquarie University.
Acknowledgements
I acknowledge the traditional custodians of the Macquarie University land, the Wattamattagal clan of the Darug nation where the fieldwork and studies were conducted. I also acknowledge the traditional custodians of the Melbourne city land, the Wurundjeri Woi-wurrung and Bunurong/Boon Wurrung peoples of the Kulin nation where the fieldwork for this study was conducted. Thanks to Macquarie University for providing scholarships for the author of this paper. Thanks to Mariella Herberstein for being an incredible supervisor and for guiding me through this manuscript. Thanks to Marilia Erickson and Jess Herbert for assisting me with fieldwork. Thanks to Jess Ewes for assisting me with maintaining the spiders in the lab. Thanks to Lizzy Lowe, Jim Maclean and Michael Kelly for reviewing this manuscript.
References
Agnarsson I, Rayor LS (2013) A molecular phylogeny of the Australian huntsman spiders (Sparassidae, Deleninae): implications for taxonomy and social behaviour. Molecular Phylogenetics and Evolution 69, 895-905.
| Crossref | Google Scholar | PubMed |
Agnarsson I, Avilés L, Coddington JA, Maddison WP (2006) Sociality in theridiid spiders: repeated origins of an evolutionary dead end. Evolution 60, 2342-2351.
| Crossref | Google Scholar | PubMed |
Avilés L (1993) Interdemic selection and the sex ratio: a social spider perspective. The American Naturalist 142, 320-345.
| Crossref | Google Scholar |
Avilés L, Harwood G (2012) A quantitative index of sociality and its application to group-living spiders and other social organisms. Ethology 118, 1219-1229.
| Crossref | Google Scholar | PubMed |
Beavis AS, Rowell DM, Evans T (2007) Cannibalism and kin recognition in Delena cancerides (Araneae: Sparassidae), a social huntsman spider. Journal of Zoology 271, 233-237.
| Crossref | Google Scholar |
Caponera V, Avilés L, Barrett M, O’Donnell S (2021) Behavioral attributes of social groups determine the strength and direction of selection on neural investment. Frontiers in Ecology and Evolution 9, 733228.
| Crossref | Google Scholar |
Dumke M, Herberstein ME, Schneider JM (2018) Advantages of social foraging in crab spiders: groups capture more and larger prey despite the absence of a web. Ethology 124, 695-705.
| Crossref | Google Scholar |
Fernández Campón F (2007) Group foraging in the colonial spider Parawixia bistriata (Araneidae): effect of resource levels and prey size. Animal Behaviour 74, 1551-1562.
| Crossref | Google Scholar |
Gorneau JA, Rheims CA, Moreau CS, Rayor LS (2022) Huntsman spider phylogeny informs evolution of life history, egg sacs, and morphology. Molecular Phylogenetics and Evolution 174, 107530.
| Crossref | Google Scholar |
Hurst JA, Rayor LS (2021) Effects on running speed of changes in sexual size dimorphism at maturity in the cursorial huntsman spider, Delena cancerides (Sparassidae). Journal of Comparative Physiology A 207, 269-277.
| Crossref | Google Scholar |
Lubin Y, Bilde T (2007) The evolution of sociality in spiders. Advances in the Study of Behavior 37, 83-145.
| Crossref | Google Scholar |
Modanu M, Li LDX, Said H, Rathitharan N, Andrade MC (2014) Sibling cannibalism in a web-building spider: effects of density and shared environment. Behavioural Processes 106, 12-16.
| Crossref | Google Scholar | PubMed |
Pekár S, Hrušková M, Lubin Y (2005) Can solitary spiders (Araneae) cooperate in prey capture? Journal of Animal Ecology 74, 63-70.
| Crossref | Google Scholar |
Penna-Gonçalves V, Willmott NJ, Kelly MBJ, Black JR, Lowe EC, Herberstein ME (2025a) Comparing microCT staining and scanning methodology for brain studies in various sizes of spiders. Journal of Comparative Neurology 533, 1-13.
| Crossref | Google Scholar |
Penna-Gonçalves V, McLean DJ, Willmott NJ, Kelly MB, Lowe E, Herberstein ME (2025b) Volumetric comparison of overall brain and neuropil size between social and non-social spiders: exploring the social brain hypothesis. Integrative Zoology inz213033.
| Crossref | Google Scholar |
Rayor LS, Taylor LA (2006) Social behavior in amblypygids, and a reassessment of arachnid social patterns. The Journal of Arachnology 34, 399-421.
| Crossref | Google Scholar |
Rowell DM (1985) Complex sex-linked fusion heterozygosity in the Australian huntsman spider Delena cancerides (Araneae: Sparassidae). Chromosoma 93, 169-176.
| Crossref | Google Scholar |
Rowell DM, Avilés L (1995) Sociality in a bark-dwelling huntsman spider from Australia, Delena cancerides Walckenaer (Araneae: Sparassidae). Insectes Sociaux 42, 287-302.
| Crossref | Google Scholar |
Ruch J, Herberstein ME, Schneider JM (2014a) Offspring dynamics affect food provisioning, growth and mortality in a brood-caring spider. Proceedings of the Royal Society B: Biological Sciences 281, 20132180.
| Crossref | Google Scholar |
Ruch J, Riehl T, Michalik P (2014b) Re-description of Xysticus bimaculatus L. Koch, 1867 (Araneae, Thomisidae) and characterization of its subsocial lifestyle. ZooKeys [427] 1-19.
| Crossref | Google Scholar |
Schneider JM (1995) Survival and growth in groups of a subsocial spider (Stegodyphus lineatus). Insectes Sociaux 42, 237-248.
| Crossref | Google Scholar |
Schneider JM, Bilde T (2008) Benefits of cooperation with genetic kin in a subsocial spider. Proceedings of the National Academy of Sciences of the United States of America 105, 10843-10846.
| Crossref | Google Scholar |
Tong C, Avilés L, Rayor LS, Mikheyev AS, Linksvayer TA (2022) Genomic signatures of recent convergent transitions to social life in spiders. Nature Communications 13, 6967.
| Crossref | Google Scholar | PubMed |
Uetz GW, Burgess W (1979) Habitat structure and colonial behavior in Metepeira spinipes (Araneae: Araneidae), an orb weaving spider from Mexico. Psyche: A Journal of Entomology 86, 79-90.
| Google Scholar |
Whitehouse MEA, Lubin Y (2005) The functions of societies and the evolution of group living: spider societies as a test case. Biological Reviews 80, 347-361.
| Crossref | Google Scholar |
Wickler W, Seibt U (1993) Pedogenetic sociogenesis via the “sibling-route” and some consequences for Stegodyphus spiders. Ethology 95, 1-18.
| Crossref | Google Scholar |
Wilson SN, Sindi SS, Brooks HZ, Hohn ME, Price CR, Radunskaya AE, Williams ND, Fefferman NH (2020) How emergent social patterns in allogrooming combat parasitic infections. Frontiers in Ecology and Evolution 8, 54.
| Crossref | Google Scholar |
Yip EC, Rayor LS (2011) Do social spiders cooperate in predator defense and foraging without a web? Behavioral Ecology and Sociobiology 65, 1935-1947.
| Crossref | Google Scholar |
Yip EC, Rayor LS (2013) The influence of siblings on body condition in a social spider: is prey sharing cooperation or competition? Animal Behaviour 85, 1161-1168.
| Crossref | Google Scholar |
Yip EC, Rayor LS (2014) Maternal care and subsocial behaviour in spiders. Biological Reviews 89, 427-449.
| Crossref | Google Scholar | PubMed |
Yip EC, Clarke S, Rayor LS (2009) Aliens among us: nestmate recognition in the social huntsman spider, Delena cancerides. Insectes Sociaux 56, 223-231.
| Crossref | Google Scholar |
Yip EC, Rowell DM, Rayor LS (2012) Behavioural and molecular evidence for selective immigration and group regulation in the social huntsman spider, Delena cancerides. Biological Journal of the Linnean Society 106, 749-762.
| Crossref | Google Scholar |