Phylogeography of the Australian sugar glider (Petaurus breviceps): evidence for a new divergent lineage in eastern Australia
Mansoureh Malekian A D E , Steven J. B. Cooper B C D and Susan M. Carthew DA Department of Natural Resources, Isfahan University of Technology, Isfahan 84156-83111, Iran.
B Australian Centre for Evolutionary Biology and Biodiversity, The University of Adelaide, SA 5005, Australia.
C Evolutionary Biology Unit, South Australian Museum, North Terrace, Adelaide, SA 5000, Australia.
D School of Earth & Environmental Sciences, The University of Adelaide, Adelaide, SA 5005, Australia.
E Corresponding author. Email: mmalekian@cc.iut.ac.ir
Australian Journal of Zoology 58(3) 165-181 https://doi.org/10.1071/ZO10016
Submitted: 27 February 2010 Accepted: 15 July 2010 Published: 23 September 2010
Abstract
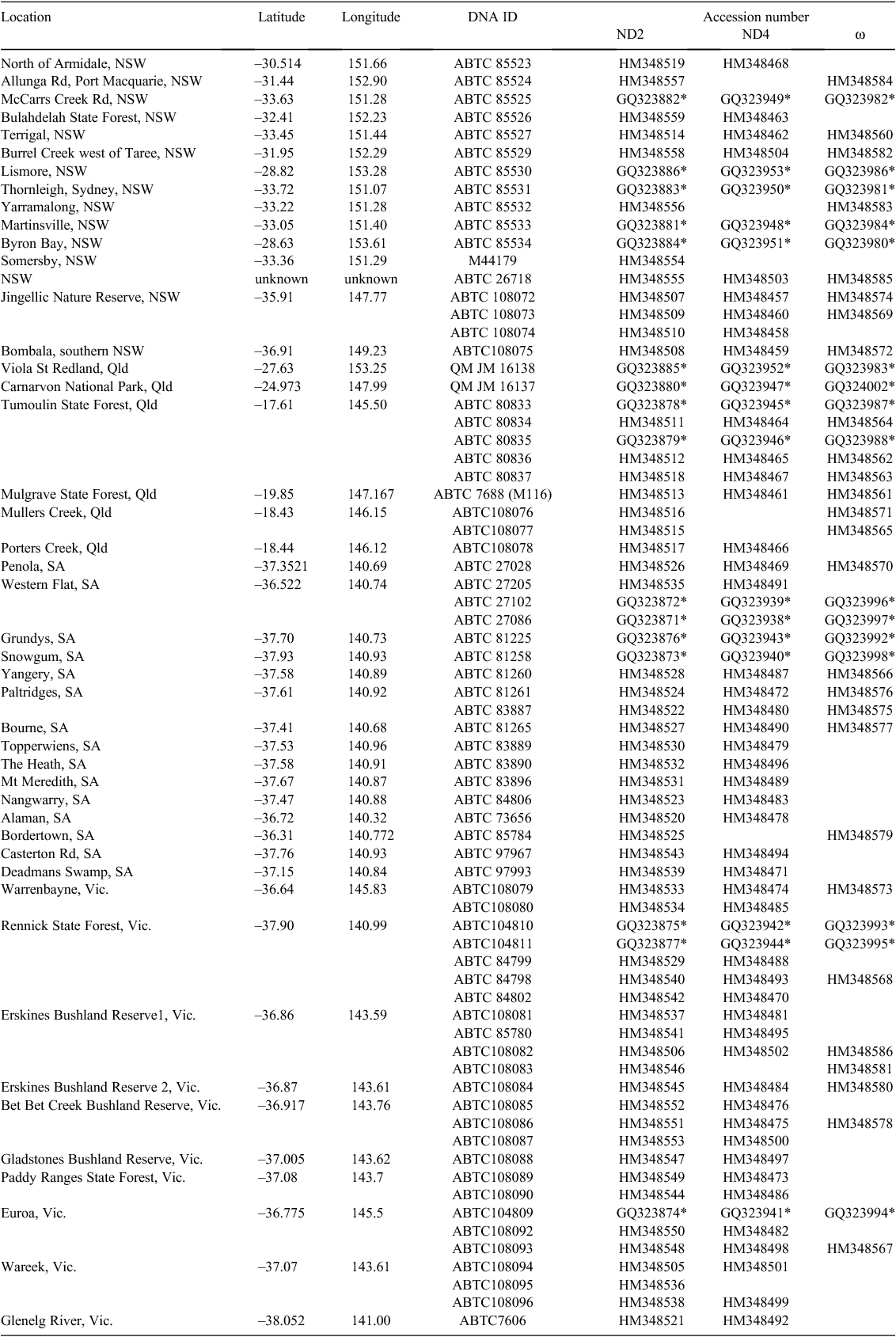
The sugar glider (Petaurus breviceps) shows considerable variation in external morphology and mitochondrial DNA (mtDNA) diversity across its distribution in New Guinea and Australia. Here we investigate the phylogeography of P. breviceps in Australia using data from two mitochondrial genes (ND2 and ND4) and a nuclear gene (ω-globin). Phylogenetic analyses revealed the existence of two divergent mtDNA clades that are distributed over distinct geographical regions, one from coastal New South Wales and south-eastern Queensland and a second over the remaining distributional range of the species in Australia. The two groups generally had distinct ω-globin haplotypes that differed by one or two mutational steps. Analyses of Molecular Variation further supported the presence of at least two populations, accounting for 84.8% of the total mtDNA variation and 44% of the ω-globin variation. The general concordance of phylogeographic and population analyses suggests that population subdivision, possibly resulting from the combined influences of aridification after the Pliocene and uplift of the Great Dividing Range has impacted the evolution of P. breviceps. Our results also show that the geographical distribution of the two evolutionary lineages does not correspond with the distribution of the current morphological subspecies and we further propose that they be considered as separate Evolutionarily Significant Units for the purposes of conservation management.
Additional keywords: Evolutionarily Significant Unit, population structure.
Acknowledgements
We acknowledge the following people for providing the samples and specimens used in this study: Dennis O’Meally (Australian Museum), David Stemmer and Cath Kemper (South Australian Museum), Dan Harley, Trish Kendal, Andrea Taylor, Rodney van der Ree and Meredeth Brown. We also thank Terry Bertozzi and Kathy Saint for laboratory assistance. Finally, we acknowledge two anonymous reviewers for detailed comments that have considerably improved the manuscript. This research was supported by funding from The Department of Environment and Heritage (Wildlife Conservation Fund) in South Australia, ANZ Holsworth Wildlife Research Fund, Mark Mitchell Foundation, Hancock Victorian Timber Plantations, The University of Adelaide and South Australian Museum.
Arbogast, B. S. , and Kenagy, G. J. (2001). Comparative phylogeography as an integrative approach to historical biogeography. Journal of Biogeography 28, 819–825.
| Crossref | GoogleScholarGoogle Scholar |
Arevalo, E. , Davis, S. K. , and Sites, J. W. (1994). Mitochondrial-DNA sequence divergence and phylogenetic relationships among 8 chromosome races of the Sceloporus–Grammicus complex (Phrynosomatidae) in Central Mexico. Systematic Biology 43, 387–418.
Brown, M. , Cooksley, H. , Carthew, S. M. , and Cooper, S. J. B. (2006). Conservation units and phylogeographic structure of an arboreal marsupial, the yellow-bellied glider (Petaurus australis). Australian Journal of Zoology 54, 305–317.
| Crossref | GoogleScholarGoogle Scholar |
Donnellan, S. C. , McGuigan, K. , Knowles, R. , Mahony, M. , and Moritz, C. (1999). Genetic evidence for species boundaries in frogs of the Litoria citropa species-group (Anura: Hylidae). Australian Journal of Zoology 47, 275–293.
| Crossref | GoogleScholarGoogle Scholar |
Fraser, D. J. , and Bernatchez, L. (2001). Adaptive evolutionary conservation: towards a unified concept for defining conservation units. Molecular Ecology 10, 2741–2752.
Hudson, R. R. (1987). Estimating the recombination parameter of a finite population model without selection. Genetical Research 50, 245–250.
| Crossref | GoogleScholarGoogle Scholar |
Hugall, A. , Moritz, C. , Moussalli, A. , and Stanisic, J. (2002). Reconciling paleodistribution models and comparative phylogeography in the Wet Tropics rainforest land snail Gnarosophia bellendenkerensis (Brazier 1875). Proceedings of the National Academy of Sciences of the United States of America 99, 6112–6117.
| Crossref | GoogleScholarGoogle Scholar |
Malekian, M. , Cooper, S. J. B. , Norman, J. A. , Christidis, L. , and Carthew, S. M. (2010). Molecular systematics and evolutionary origin of genus Petaurus, in Australia and New Guinea. Molecular Phylogenetics and Evolution 54, 122–135.
| Crossref | GoogleScholarGoogle Scholar |
Meikle, R. D. (1957). What is the subspecies? Taxon 6, 102–105.
| Crossref | GoogleScholarGoogle Scholar |
Sunnucks, P. , Blacket, M. J. , Taylor, J. M. , Sands, C. J. , Ciavaglia, S. A. , Garrick, R. C. , Tait, N. N. , Rowell, D. M. , and Pavlova, A. (2006). A tale of two flatties: different responses of two terrestrial flatworms to past environmental climatic fluctuations at Tallaganda in montane southeastern Australia. Molecular Ecology 15, 4513–4531.
| Crossref | GoogleScholarGoogle Scholar |
Symula, R. , Keogh, J. S. , and Cannatella, D. C. (2008). Ancient phylogeographic divergence in southeastern Australia among populations of the widespread common froglet, Crinia signifera. Molecular Phylogenetics and Evolution 47, 569–580.
| Crossref | GoogleScholarGoogle Scholar |

Taberlet, P. , Fumagalli, L. , Wust-Saucy, A. G. , and Cosson, J. F. (1998). Comparative phylogeography and postglacial colonization routes in Europe. Molecular Ecology 7, 453–464.
| Crossref | GoogleScholarGoogle Scholar |

Tajima, H. (1989). Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123, 585–595.

Templeton, A. R. , Crandall, K. A. , and Sing, C. F. (1992). A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. 3. Cladogram estimation. Genetics 132, 619–633.

Thompson, J. D. , Gibson, T. J. , Plewniak, F. , Jeanmougin, F. , and Higgins, D. G. (1997). The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25, 4876–4882.
| Crossref | GoogleScholarGoogle Scholar |

Wheeler, D. , Hope, R. , Cooper, S. J. B. , Dolman, G. , Webb, G. C. , Bottema, C. D. K. , Gooley, A. A. , Goodman, M. , and Holland, R. A. B. (2001). An orphaned mammalian [beta]-globin gene of ancient evolutionary origin. Proceedings of the National Academy of Sciences of the United States of America 98, 1101–1106.
| Crossref | GoogleScholarGoogle Scholar |

Yang, Z. H. (1996). Among-site rate variation and its impact on phylogenetic analyses. Trends in Ecology & Evolution 11, 367–372.
| Crossref | GoogleScholarGoogle Scholar |

Zink, R. M. (2004). The role of subspecies in obscuring avian biological diversity and misleading conservation policy. Proceedings of the Royal Society of London. Series B. Biological Sciences 271, 561–564.
| Crossref | GoogleScholarGoogle Scholar |