Hospital admissions caused by adverse drug events: an Australian prospective study
Alexandra L. Phillips A B , Olimpia Nigro C , Karen A. Macolino C , Kirsty C. Scarborough C , Christopher J. Doecke C D F , Manya T. Angley E and Sepehr Shakib CA Noarlunga Health Service, Alexander Kelly Drive, Noarlunga Centre, SA 5168, Australia.
B School of Pharmacy and Medical Sciences, University of South Australia, Adelaide, SA 5000, Australia. Email: alexandra.phillips@health.sa.gov.au
C Royal Adelaide Hospital, Adelaide, SA 5000, Australia. Email: olimpia.nigro@health.sa.gov.au; karen.macolino@health.sa.gov.au; kirsty.scarborough@health.sa.gov.au; sepehr.shakib@health.sa.gov.au
D School of Pharmacy and Medical Sciences, University of South Australia, Adelaide, SA 5000, Australia.
E Sansom Institute for Health Research, School of Pharmacy and Medical Sciences, University of South Australia, Adelaide, SA 5000, Australia. Email: manya.angley@unisa.edu.au
F Corresponding author. Email: chris.doecke@health.sa.gov.au
Australian Health Review 38(1) 51-57 https://doi.org/10.1071/AH12027
Submitted: 3 December 2012 Accepted: 8 October 2013 Published: 19 December 2013
Abstract
Objective To assess the frequency of adverse drug event (ADE)-related admissions (ADE-RAs) during a prospective medical record review of patients admitted to a metropolitan tertiary referral hospital.
Methods Potential ADE-RA cases were identified by examination of case records of randomly selected patients. Cases were assessed by an expert panel to measure study outcomes, which were the frequency (ADEs and ADE-RAs) as well as type, likelihood of causality, severity, avoidability and detection of ADEs.
Results Of the 370 subjects, 59 (16.0%) had a confirmed ADE-RA, with 15 (4.1%) of these serious and preventable. The 59 ADE-RAs were a result of 72 discreet ADEs. Adverse drug reactions were the most common type of ADE, followed by non-compliance. Of the 72 discreet ADEs, 31.9% were classified as ‘probable’ or ‘highly probable’. Most ADEs (54.2%) were classified as ‘definitely avoidable’, 34.7% were classified as ‘severe’ and 21.8% were classified as both ‘definitely avoidable’ and ‘severe’. Half the ADEs were detected after the patient had been admitted and most were detected by medical practitioners. Antineoplastics followed by antidiabetic agents were most frequently implicated.
Conclusions Implementing a systems approach that involves multiple strategies, such as improving tertiary-to-primary care information transfer and promoting medication adherence through education programs, is necessary to tackle the problem of avoidable ADE-RAs and the associated cost burden.
What is known about the topic? It is estimated that 2–3% of Australian hospital admissions are due to adverse drug events (ADEs), but recent data are lacking. According to the Australian Statistics on Medicines, over 250 million prescriptions were dispensed in 2007, compared with just under 180 million in 1997. This 40% increase in drug utilisation over the 10 years surpasses the Australian population growth of 14% in the same period. An increase in drug use per person indicates that the rate of ADEs and possible ADE-related admissions (ADE-RAs) is likely to have increased.
What does this paper add? This prospective study was conducted at a large Australian metropolitan teaching hospital and we report that 59 of 370 participants (16.0%) presenting to the Emergency Department had a confirmed ADE-RA, with 15 (4.1%) presenting with a serious and preventable ADE-RA.
What are the implications for practitioners? The findings of this study support implementing a systems approach involving multiple strategies to tackle the problem of avoidable ADE-RAs and the associated cost burden. This study reveals that half the ADEs were not detected until after the admission process, which reinforces the importance of focusing efforts towards preventing ADE-RAs and detecting ADE-RAs through measures such as those recommended in the Australian Pharmaceutical Advisory Council guiding principles.
Introduction
Adverse drug events (ADEs) incorporate many types of drug-related problems, including adverse drug reactions (ADRs). Although an ADE relates to any unintentional harm to a patient caused by drug use, misuse or non-use, an ADR is specifically a response to a drug that is unintended, unexpected, undesired or excessive.1
Miller et al. found that of 8215 general practitioner (GP) encounters, 10.4% of patients reported experiencing an ADE over a 6-month period in 2003–04.2 Roughead and Lexchin estimated that 2 million Australians experience an ADE annually, of which half are moderate or severe with 138 000 requiring hospitalisation.3 Further, a recent review by Roughead and Semple4 concluded that 2–3% of Australian hospital admissions are medication related, representing an estimated 190 000 medication-related hospital admissions per year, with associated costs of A$660 million. Studies by Galbraith5 and Dartnell et al.6 indicate that unplanned admissions via the Emergency Department (ED) are even more likely to be medication related, with the authors reporting that ADEs account for 6.4% and 5.7% of admissions, respectively. In addition, a 2009 report by the National Prescribing Service concluded that approximately 5.6% of general hospital admissions and 30.4% of hospital admissions in patients 75 years and older in Australia are related to ADEs.7 Of the Australian studies that have assessed preventability, Roughead and Semple4 found that approximately 50% of ADE-related admissions (ADE-RAs) were potentially preventable.
ADE-RAs can be determined by various prospective and retrospective methods. A review of the international literature shows the frequency of ADE-RAs being 1.4–30.4% when determined prospectively.6,8–15 When Capuano et al.,8 Chan et al.,9 Dartnell et al.6 and Malhotra et al.12 examined causality between drug and ADE, they found that events were either ‘definitely’ or ‘probably’ due to drugs in 8.9%, 61.4%, 62.0% and 27.7% of cases, respectively. Each study used a different method to determine causality: Capuano et al.6 used the Naranjo Algorithm,16 which is commonly used to assess causality of ADRs, Dartnell et al.6 used criteria modified from Karch and Lasagna,17 whereas Chan et al.7 and Malhotra et al.10 defined their own criteria for causality.
Studies examining age and ADE-RAs have demonstrated that older patients are more likely to be hospitalised due to ADEs.7,14,15 Conversely, no clear link has been found between the likelihood of ADEs and gender.9,13,15
The few studies that have investigated the severity of ADE-RAs have shown that 15.7–38.8% of ADEs that lead to admission are severe.9,13,15 Several studies have also evaluated avoidability, reporting that 5.5–55.5% of these ADEs are avoidable.6,9,13 Substantial variation in the reported frequency of ADE-RAs and the lack of published studies investigating the severity and avoidability of ADEs necessitates further research.
According to the Australian Statistics on Medicines, over 250 million prescriptions were dispensed in 2007, compared with almost 180 million in 1997.18,19 This 40% increase in drug utilisation over the 10 years surpasses the Australian population growth of 14% in the same period,20,21 indicating that the rate of ADEs and possible ADE-RAs may also have increased. Data from hospital ADR reports and the Pharmaceutical Benefits Scheme support this theory, demonstrating that as drug utilisation increases, so do ADR rates.22
Finally, investigation into the detection of ADEs is lacking. In particular, only one known study6 has examined which health professionals detect ADEs, and none has determined how long after presentation to the ED the detection of ADEs occurs.
The present prospective study investigated the frequency of ADEs and ADE-Ras, as well as the likelihood of causality, severity, avoidability, timing and details of personnel involved in the detection of ADEs.
Methods
Patients admitted via the ED of the Royal Adelaide Hospital, a 640-bed metropolitan public tertiary acute care adult teaching hospital, were randomly selected to undergo a prospective review to detect possible ADE-RAs over an 8-week period between May and July 2009.
Ethics approval was obtained for the study from the Royal Adelaide Hospital and the University of South Australia Human Research Ethics Committees.
Participants were identified via a hospital-generated list of all ED admissions each working day through the careconnect.sa clinical reporting system. All patients were assigned a consecutive number and 10 subjects were selected each day using a computerised random number generator.
All patients admitted via the ED during the study period were eligible for inclusion, except for those whose admission was associated with intentional overdose or poisoning, accidental poisoning with non-therapeutic substances or alcohol or illicit drug intoxication. This distinction was made to distinguish patients with accidental medication-related overdoses from those who were intentionally trying to cause self-harm, or where therapeutic medications were not involved. In addition, patients admitted during the weekend or on public holidays and discharged before the next working day were excluded from the study.
Medical records, including drug charts, were reviewed immediately after admission and demographic and clinical data were recorded in a purposefully developed form. Information from careconnect.sa clinical information (using Open Architecture Clinical Information System) and separation summaries were also used when applicable. Cases of potential ADE-RAs were identified by the presence of one or more of the following key indicators: (1) diagnosis of a drug-related condition; (2) pre-hospital use of a medication known to cause the admitting diagnosis; (3) omission of a medication, where omission is known to cause the admitting diagnosis; and (4) presence of drug interactions known to cause the admitting diagnosis. The time of detection of the possible ADE and the type of health professional involved in documentation were also recorded. The progress of all subjects was followed to Day 3, with the patients’ clinical pharmacists notifying the project team if an ADE was detected after Day 3. All participants with a suspected ADE-RA were followed to discharge. Possible ADE-RA cases that were initially identified were reviewed by at least two other project clinical pharmacists. Following consensus agreement among the investigators, possible ADE-RA cases were forwarded to an independent panel for review. In the absence of adequate supporting information, the potential ADE-RA was not forwarded.
The study outcomes were the frequency (ADEs and ADE-RAs) as well as type, likelihood of causality, severity, avoidability and detection (time and health professional involved) of ADEs.
Data were analysed according to subject demographics and drug class involved in the ADE-RAs. Drug classes were grouped according to the World Health Organization’s anatomical therapeutic chemical (ATC) code for drug utilisation.23
The panel included a clinical pharmacologist, a cardiologist and two clinical pharmacists. The panel received specific training before independently assessing each suspected ADE according to the study outcomes. Where there were two or more ADEs for one subject that were equally judged as the primary cause of admission, they were assessed individually. Where there were two or more drugs that contributed to an ADE, they were assessed individually, unless they caused the ADE via the same pharmacodynamic mechanism or there was evidence or a likelihood that the ADE could be caused by the combination of drugs. Discrepancies between panel members’ independent assessments were discussed in a group consisting of each panel member, the principal investigator and one other project investigator. The majority of differences resulted from various interpretations of the case summaries and, once the details were clarified, the assessment was made unanimously. Aside from these, discrepancies were resolved through debate of the current guidelines and clinical opinion until consensus was reached.
Any ADE that was confirmed by the panel as being a major contributor to the primary cause of admission was included as an ADE-RA and contributed to overall frequency.
ADEs were classified according to Hepler and Strand,24 based on previous work by Strand et al.,25 who categorised drug-related problems as ‘Untreated indications’, ‘Improper drug selection’, ‘Subtherapeutic dosage’, ‘Failure to receive drugs’, ‘Overdosage’, ‘Adverse drug reactions’, ‘Drug interactions’ and ‘Drug use without indication’. ADEs were allowed to be assigned to more than one category if necessary (e.g. if a patient with known peptic ulcer disease was prescribed a non-steroidal anti-inflammatory drug and developed a bleeding ulcer, the ADE would be categorised as both ‘Improper drug selection’ and ‘Adverse drug reaction’).
The probability that the ADE was caused by a particular drug or group of drugs was identified in two ways. Where the ADE was classified as an ADR, Jones’ algorithm26 was used, which classifies the likelihood of causality as ‘highly probable’, ‘probable’, ‘possible’ or ‘remote’. Where the ADE was classified as any other type of ADE, Jones’ algorithm was found to be inappropriate and causality was instead assessed using clinical judgement. Where more than one drug was ceased or recommenced simultaneously, causality for all drugs was either ‘remote’ or ‘possible’, unless that particular combination of drugs was a plausible cause for the ADE in question.
The severity of suspected ADEs was assessed using a modified Pearson classification,9 with ADEs classified as either ‘moderate’ (the ADE was the major contributor to the reason for hospital admission, with or without requiring a change in therapy or specific treatment) or ‘severe’ (the ADE was life threatening, caused permanent damage or required intensive care). The ‘mild’ classification was not used because, by definition, these ADEs were not a major contributor or did not lead to admission.
Suspected ADEs were assessed for avoidability using the criteria of Chan et al.9 The ADEs were classified as ‘not avoidable’, ‘possibly avoidable’ or ‘definitely avoidable’.
Where the panel confirmed an ADE, the details surrounding ADE detection were included for analysis. The time of detection was classified as ‘before presentation’ (e.g. if detection was made by the patient’s GP), ‘ED admission’, ‘medical admission’ or by the number of days since admission. The health professionals who detected ADEs were grouped by profession or job title.
Results were analysed using descriptive statistics and are expressed as the median with the interquartile range (IQR) in parentheses, where applicable, and/or as the mean ± 95% confidence interval (CI). Differences in age, sex, length of stay and number of medications taken before admission between the ADE-RA and non-ADE-RA groups were analysed using unpaired two-tailed Student’s t-tests in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA); P < 0.05 was considered significant.
Results
During the study period, 2815 patients were admitted to the Royal Adelaide Hospital via the ED. Of the 400 patients randomly selected to participate in the study, 370 were eligible for inclusion.
There were 199 (54%) men and 171 (46%) women. The overall median (IQR) age of the subjects was 64 (46–80) years (mean ± 95% CI 61.19 ± 2.15 years); the median (IQR) number of medications taken before admission was 5.5 (2–10), with a mean (± 95% CI) of 6.44 ± 0.55 medications. The median (IQR) length of stay was 5 (3–11) days (mean ± 95% CI 9.51 ± 1.36 days). The mortality rate was 4.8% (n = 18); however, only one of the deceased was assessed as having an ADE-RA.
There were 84 suspected discrete ADEs detected in 65 of the 370 participants (17.6%). Of these, 72 ADEs were confirmed in 59 patients (16% of all patients) following expert panel review. There were 47 patients who had one confirmed ADE, 11 patients who had two ADEs and one patient who had three ADEs.
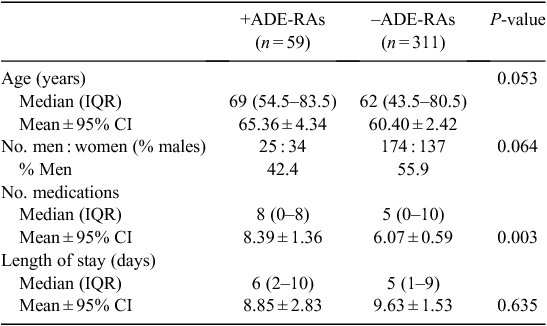
Relationships between participant characteristics and the presence of an ADE-RA are given in Table 1. Patients with an ADE-RA tended to be older (mean age 65.36 vs 60.40 years), but this trend was not supported statistically (P = 0.053). Females tended to have a higher chance of an ADE-RA than males (n = 34 vs 25); however, this difference did not reach statistical significance (P = 0.064). A greater number of medications taken before admission was significantly correlated with the risk of an ADE-RA (P = 0.003). Admissions that were ADE related were not found to have a greater length of stay than non-ADE-RAs (P = 0.634).
|
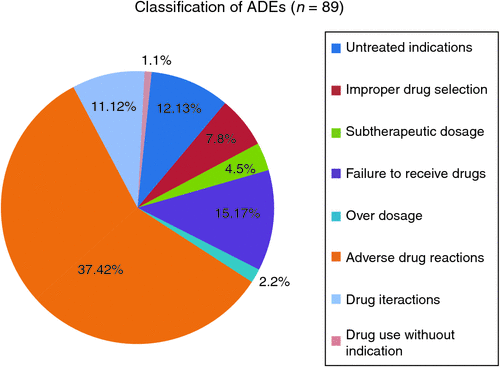
The 72 confirmed ADEs were classified by category, using the eight categories of drug-related problem identified by Hepler and Strand.24 Because 11 of the ADEs fit into two different categories of ADE and three were applicable to three different categories, there was a total of 89 discrete classifications. Figure 1 shows the frequency of ADE according to category.
|
With respect to the likelihood of causality, most (66.7%) of the confirmed ADEs were classified by the panel as ‘possible’. A further 22.2% were assessed as ‘probable’ and 9.7% were classified as ‘highly probable’. Only one ADE (1.4%) was classified as ‘remote’.
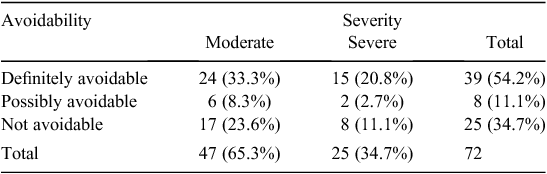
Most of the confirmed ADEs (65.3%) were classified as ‘moderate’, with the remainder (34.7%) classified as ‘severe’.
Most of the confirmed ADEs (54.2%) were classified by the panel as ‘definitely avoidable’, with 11.1% classified as ‘possibly avoidable’ and the remainder (34.7%) classified as ‘not avoidable’.
Table 2 shows the relationship between severity and avoidability of confirmed ADEs (n = 72).
|
Examination of the individual ADE-RA cases revealed that 15 of 59 were associated with at least one ADE classified as causing moderate or severe harm, assessed as ‘definitely avoidable’ and classified with a likelihood of causality as probable or highly probable, which amounts to more than one in 25 of all admissions (15/370; 4.1%) being serious and preventable. Examples of cases demonstrating assessment of the outcomes are given in Table 3.

|
Half the ADEs (36/72) were detected during the ED process by ED staff or medical or surgical admitting doctors. The other half (36/72) were not detected until after the patient had been admitted, with most of these (24%) being detected within the first 2 days after admission. Nine (13%) ADEs were detected after discharge during the panel review, where the newly identified ADE was in addition to another ADE already put forward to the panel (e.g. where a panel expert identified a drug interaction in addition to an ADR that equally contributed to the ADE).
ADEs were most frequently detected by resident medical officers and registrars (50%), followed by consultants (18%) and interns (10%). Of the remaining ADEs, 6% were detected by pharmacists, 1% were detected by a nurse, 1% were detected by a project investigator, 13% were detected by the panel and 1% were unknown (not recorded).
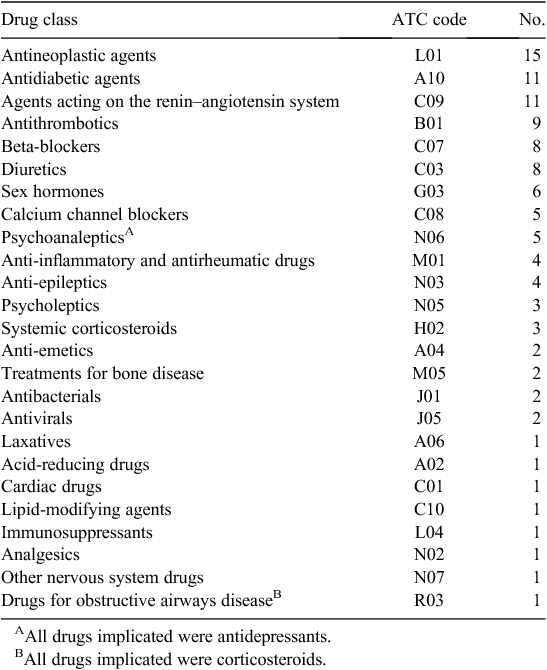
Of the 72 confirmed ADEs contributing to admissions, 109 individual drugs were implicated as causative agents. Table 4 shows the frequency of drug classes (ATC codes) implicated in ADE-RAs.
|
Discussion
In the present study we report that 59 of 370 patients (16.0%) from a random selection of ED admissions had a confirmed ADE-RA. Although there is considerable variability between the frequencies reported in previous prospective studies of unplanned or ED admissions (range 2.4–30.4%),6,8,9,12–15 the mean rate of ADE-RAs is 9.2%, which is comparable to the findings of the present study. If we were to remove an Australian study, which was restricted to ADE-RAs in elderly patients,9 the mean frequency is reduced to 5.7%, much lower than our reported figure.
Almost one-third (23/72) of all ADEs identified were classified as ‘probable’ or ‘highly probable’. Of these, more than half of those detected were classified as ‘definitely avoidable’ (15/23), and almost half were classified as ‘severe’ (11/23), with one-third (8/23) classified as both ‘definitely avoidable’ and ‘severe’. Importantly, almost one-tenth (35/370) of all admissions were associated with at least one ADE classified as causing moderate to severe harm that was assessed as ‘definitely avoidable’. According to the Australian Hospital Statistics, there were 1.65 million admissions to Australian hospitals via the ED in 2007–08;27 hence, an estimated 66 891 hospitalisations per year can be classified as ‘definitely avoidable’ ADE-RAs.
The prospective nature and the small sample size of the study (n = 370), as well as the extensive experience of the panel members, enabled a highly intensive review of each case, reducing the likelihood of overlooking any ADEs, thus potentially contributing to a higher ADE-RA frequency than reported previously. The classification of ADEs was broader than used in previous studies, which also may have contributed to the higher frequency. Furthermore, 31.9% of ADEs were classified as either ‘probable’ or ‘highly probable’. If the ADE-RAs are restricted to these cases, the frequency is reduced to 5.1% (i.e. 31.9% of 16%), which is comparable to most previous studies, in particular those Australian studies by Galbraith5 and Dartnell et al.,6 which also focused on all unplanned ED admissions (6.4% and 5.7%, respectively).
In contrast with some studies,7,14,15 we report no significant differences in the median age in the ADE-RA versus non-ADE-RA group. Although there was a trend for more women having an ADE-RA (34/171; 19.9%) than men (25/199; 12.6%), the difference did not reach statistical significance. These findings are consistent with other studies in which age and gender were identified as poor predictors of the occurrence of an ADE.9,13
Consistent with Malhotra et al.12 and Chan et al.,9 who studied elderly populations, we found the number of medications taken before admission was significantly higher in the ADE-RA compared with the non-ADE-RA group.
A distinctive aspect of the present study was to identify when and by whom ADEs were first detected. Although patients with ADE-RAs were not shown to have longer lengths of stay than non-ADE-RAs, it is plausible to argue that where there is an ADE-RA, early detection may result in a shorter hospital stay and reduced harm to the patient. Only one known study by Dartnell et al.6 has reported the rate of drug-related problems identified by the medical team (87%) or the pharmacist (13%); however, they did not detail the time to detection or elaborate on the pharmacist’s role in ADE identification. We found most ADEs were detected by medical staff during the admission process while compiling a comprehensive medical and medication history and determining the presenting complaint. However, half the ADEs were detected after admission and had been hindered by an incomplete medication history. It has been shown that a pharmacist compiling comprehensive medication histories and conducting medication reconciliation on admission reduces the likelihood of ADRs occurring,28 suggesting that earlier involvement by pharmacists may lead to earlier detection of more ADE-RAs. Implementation of the Australian Pharmaceutical Advisory Council (APAC) guiding principles is intended to ensure the early completion of medication histories and reconciliation,29 and enable earlier detection of ADE-RAs.
The likelihood of causality of ADEs contributing to admission was classified as ‘probable’ or ‘highly probable’ for 31.9% of cases. Capuano et al.,8 Chan et al.,9 Dartnell et al.6 and Malhotra et al.12 reported that 8.9%, 61.4%, 62.0% and 14.4%, respectively, of ADEs investigated had a likelihood of at least ‘probable’. These authors used different classifications, which could account for the wide variation in the reported likelihood of causality.
The proportion of ADEs classified as ‘severe’ in the present study was 34.7%, which is higher than that reported by Chan et al.9 (15.7%) but lower than that reported by Jha et al.10 (77.6%). Our findings are similar to those of Raschetti et al.13 and Trifiro et al.,15 who reported 37.7% and 38.8% of all ADEs as severe, respectively.
The proportion of ADEs in the present study deemed ‘definitely avoidable’ was 54.2%. Our results are similar to those of the prospective studies by Chan et al.9 and Raschetti et al.,13 who reported that 53.4% and 55.5% of all ADEs were ‘definitely avoidable’, respectively, but in contrast with those of Dartnell et al.6 and Jha et al.,10 who reported rates of 5.5% and 27.6%, respectively. Again, the variability in the results may be attributed to differences in methodology, in particular the quality of the criteria used to classify avoidability.
We used a similar approach to Chan et al.9 to classify severity and avoidability. Our results are in contrast with those reported by Chan et al.9 with respect to severity (34.7% vs 15.7%), but similar with respect to avoidability (54.2% vs 53.4%). Variation in severity between the studies may be attributed to the fact that the classifications from Chan et al.9 were adapted slightly in our study. Further, because Chan et al.9 restricted their study to the elderly, it is plausible that older patients are admitted with less severe ADEs more frequently for precautionary reasons.
Concordant with other reports,6,9,11,13,30,31 ADRs were the most prevalent type of ADE, accounting for 37.4% of ADEs overall. Despite many studies now using Hepler and Strand’s categorisation of ADEs,24 methodologies and definitions continue to vary from study to study, leading to difficulties in evaluating the cause of ADE-RAs.
Of concern is the proportion of ADEs attributed to patients not taking medications due to adherence issues or untreated indications. The combination of ‘failure to receive drugs’ and ‘untreated indications’ accounted for 37 of 89 of all ADEs (41.6%), warranting the development of prevention strategies and further investigation. A systems-based approach should be used to reduce admissions caused by ADEs (e.g. optimisation of information transfer between tertiary and primary care providers, campaigns to improve consumer medication literacy and adherence and computerised decision-making support software to enable optimised prescribing).
Antineoplastics were the agents most often involved in an ADE-RA, followed closely by antidiabetic agents, agents acting on the renin–angiotensin system and antithrombotic agents. Because previous studies did not follow a standardised code to group drugs, it is difficult to make comparisons as to which drugs cause ADE-RAs; however, cardiovascular drugs are consistently reported as being major contributors.6,8–13,30 In addition, anti-inflammatory, antithrombotic, anti-infective and antidiabetic agents are commonly implicated in other studies.6,8–13
A major limitation of the present study was the use of Jones’ algorithm,26 which proved to be unsuitable for assessing the causality of ADEs that were not ADRs. Thus, clinical judgement was used to classify all other ADEs, which could have led to underestimating the probability of causality. Further, without access to GP and/or private hospital records, it is possible that some ADE-RAs were overlooked or were unable to be forwarded to the panel due to insufficient supporting information, hence underestimating the ADE-RA frequency rate. The substantial time and effort required to conduct a prospective audit such as this may limit the ability of some institutions to replicate our work; however, the valuable data gained through such a study may balance these inconveniences. In fact, in the clinical setting, the cost of employing more pharmacists to detect and rectify ADEs earlier may reduce overall hospital costs by minimising patient morbidity and length of stay. Finally, the modest sample size of the study and the single study site limits the generalisability of the results, although it perhaps allowed a more intensive review of each case.
In conclusion, the present Australian study reports a higher frequency of ADE-RAs (16%) than reported previously in international prospective studies.6,8,12–15 Further, more than one-quarter of ADE-RA cases were associated with at least one ADE classified as causing moderate or severe harm, assessed as ‘definitely avoidable’ and classified with a likelihood of causality as probable or highly probable, which amounts to more than one in 25 of all admissions being serious and preventable. The findings of the present study support implementing a systems approach involving multiple strategies not only to detect ADE-RAs earlier in the admission, thus potentially reducing patient morbidity and length of stay, but also to tackle the problem of avoidable ADE-RAs and the associated cost burden.
Competing interests
The authors declare there are no competing interests.
Acknowledgements
The authors acknowledge the expertise and time of Ivanka Hendrix, Simon LaForgia and Dr Darryl Leong in participating in the review panel.
References
[1] American Society of Health-System Pharmacists Suggested definitions and relationships among medication misadventures, medication errors, adverse drug events, and adverse drug reactions. Am J Health Syst Pharm 1998; 55 165–6.| 9465983PubMed |
[2] Miller GC, Britt HC, Valenti L. Adverse drug events in general practice patients in Australia. Med J Aust 2006; 184 321–4.
| 16584364PubMed |
[3] Roughead EE, Lexchin J. Adverse drug events: counting is not enough, action is needed. Med J Aust 2006; 184 315–6.
| 16584361PubMed |
[4] Roughead EE, Semple SJ. Medication safety in acute care in Australia: where are we now? Part 1: a review of the extent and causes of medication problems 2002–2008. Aust New Zealand Health Policy 2009; 6 18
| Medication safety in acute care in Australia: where are we now? Part 1: a review of the extent and causes of medication problems 2002–2008.Crossref | GoogleScholarGoogle Scholar | 19671158PubMed |
[5] Galbraith KJ. Is there a role for a clinical pharmacist in the emergency department? Melbourne: Victorian College of Pharmacy; 1993.
[6] Dartnell JGA, Anderson RP, Chohan V, Galbraith KJ, Lyon MEH, Nestor PJ, et al Hospitalisation for adverse events related to drug therapy: incidence, avoidability and costs. Med J Aust 1996; 164 659–62.
| 1:STN:280:DyaK283lslejsA%3D%3D&md5=3a438b3a482148c3673cef17b2eb9838CAS |
[7] Easton K, Morgan T, Williamson M. Medication safety in the community: a review of the literature. Sydney: National Prescribing Service Limited; 2009.
[8] Capuano A, Motola G, Russo F, Avolio A, Filippelli A, Rossi F, Mazzeo F. Adverse drug events in two emergency departments in Naples, Italy: an observational study. Pharmacol Res 2004; 50 631–6.
| Adverse drug events in two emergency departments in Naples, Italy: an observational study.Crossref | GoogleScholarGoogle Scholar | 15501703PubMed |
[9] Chan M, Nicklason F, Vial J. Adverse drug events as a cause of hospital admission in the elderly. Int Med J 2001; 31 199–205.
| Adverse drug events as a cause of hospital admission in the elderly.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MvgsFGmtw%3D%3D&md5=855df5fca88f145ddfd106db733b21cbCAS |
[10] Jha AK, Kuperman GJ, Rittenberg E, Teich JM, Bates DW. Identifying hospital admissions due to adverse drug events using a computer-based monitor. Pharmacoepidemiol Drug Saf 2001; 10 113–19.
| Identifying hospital admissions due to adverse drug events using a computer-based monitor.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mvltlaltg%3D%3D&md5=8103ee9767c15e9dc5fb9c3e912e1260CAS | 11499849PubMed |
[11] Major S, Badr S, Bahlawan L, Hassan G, Khogaoghlanian T, Khalil R, Melhem A, Richani R, Younes F, Yeretzian J, Khogali M, Sabra R. Drug-related hospitalization at a tertiary teaching center in Lebanon: incidence, associations, and relation to self-medicating behavior. Clin Pharmacol Ther 1998; 64 450–61.
| Drug-related hospitalization at a tertiary teaching center in Lebanon: incidence, associations, and relation to self-medicating behavior.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M%2FhsVGgtA%3D%3D&md5=37e8d8acb9aee911d9f57aa1eae9ee5fCAS | 9797802PubMed |
[12] Malhotra S, Karan RS, Pandhi P, Jain S. Drug related medical emergencies in the elderly: role of adverse drug reactions and non-compliance. Postgrad Med J 2001; 77 703–7.
| Drug related medical emergencies in the elderly: role of adverse drug reactions and non-compliance.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MrntVWitg%3D%3D&md5=cd2b489672070b1481e4b6d8ab3efc56CAS | 11677279PubMed |
[13] Raschetti R, Morgutti M, Menniti-Ippolito F, Belisari A, Rossignoli A, Longhini P, et al Suspected adverse drug events requiring emergency department visits or hospital admissions. Eur J Clin Pharmacol 1999; 54 959–63.
| Suspected adverse drug events requiring emergency department visits or hospital admissions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M3gs12ktA%3D%3D&md5=0c368f3c5d9cbe15b06a3a6ed9c0d7d4CAS | 10192758PubMed |
[14] Schneeweiss S, Hasford J, Gottler M, Hoffmann A, Riethling AK, Avorn J. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. Eur J Clin Pharmacol 2002; 58 285–91.
| Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study.Crossref | GoogleScholarGoogle Scholar | 12136375PubMed |
[15] Trifirò G, Calogero G, Ippolito FM, Cosentino M, Giuliani R, Conforti A, et al Adverse drug events in emergency department population: a prospective Italian study. Pharmacoepidemiol Drug Saf 2005; 14 333–40.
| Adverse drug events in emergency department population: a prospective Italian study.Crossref | GoogleScholarGoogle Scholar | 15682429PubMed |
[16] Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30 239–45.
| A method for estimating the probability of adverse drug reactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXltVygsLk%3D&md5=ac251813c543aa41ce131d213214cf76CAS | 7249508PubMed |
[17] Karch FE, Lasagna L. Adverse drug reactions. A critical review. JAMA 1975; 234 1236–41.
| Adverse drug reactions. A critical review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE28%2Fnt1ehug%3D%3D&md5=f9d2172039ba666da09f6eb401a43ac2CAS | 1242749PubMed |
[18] Department of Health and Ageing. Australian statistics on medicines 1997. Canberra: Commonwealth of Australia; 1998.
[19] Department of Health and Ageing. Australian statistics on medicines 2007. Canberra: Commonwealth of Australia; 2009.
[20] Australian Bureau of Statistics. Population by age and sex, Australian states and territories: June 1997 to 2002. Catalogue no. 3201.0. Canberra: Australian Bureau of Statistics; 2003.
[21] Australian Bureau of Statistics. Population, Australian states and territories, December 2007. Catalogue no. 3239.0.55.001. Canberra: Australian Bureau of Statistics; 2008.
[22] Runciman WB, Roughead EE, Semple SJ, Adams RJ. Adverse drug events and medication errors in Australia. Int J Qual Health Care 2003; 15 i49–59.
| Adverse drug events and medication errors in Australia.Crossref | GoogleScholarGoogle Scholar | 14660523PubMed |
[23] WHO Collaborating Centre for Drug Statistics Methodology. Anatomical therapeutic chemical index. Available at http://www.whocc.no/atc_ddd_index/ [verified September 2012]
[24] Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm 1990; 47 533–43.
| 1:STN:280:DyaK3c7ps1Sgsw%3D%3D&md5=971e77a985104410cd820c5af818418bCAS | 2316538PubMed |
[25] Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: their structure and function. Ann Pharmacother 1990; 24 1093–7.
| 1:STN:280:DyaK3M7ht1Wluw%3D%3D&md5=fe23bce6f756639a443e197c671ae468CAS |
[26] Jones JK. Adverse drug reactions in the community health setting: approaches to recognizing, counseling, and reporting. Fam Community Health 1982; 5 58–67.
| Adverse drug reactions in the community health setting: approaches to recognizing, counseling, and reporting.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL383htFWitQ%3D%3D&md5=ee81f115695d424ec10af6cd895ce5e2CAS | 10278126PubMed |
[27] Australian Institute of Health and Welfare (AIHW). Australian hospital statistics 2007–08. Health services series no. 33. Catalogue no. HSE 71. Canberra: AIHW; 2009. Available at http://www.aihw.gov.au/publications/index.cfm/title/10776 [verified September 2012]
[28] Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals. Pharmacotherapy 2006; 26 735–47.
| Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD283ovVOhuw%3D%3D&md5=78023d9cc6733ec1712d0c7b5347d8d7CAS | 16716127PubMed |
[29] Australian Pharmaceutical Advisory Council. Guiding principles to achieve continuity in medication management. Canberra: Commonwealth of Australia; 2005.
[30] Einarson TR. Drug-related hospital admissions. Ann Pharmacother 1993; 27 832–40.
| 1:STN:280:DyaK3sznt1Cnsw%3D%3D&md5=880d01fc50a2ad5a1b1167089592311bCAS | 8364259PubMed |
[31] Malpass A, Helps SC, Runciman WB. An analysis of Australian adverse drug events. J Qual Clin Pract 1999; 19 27–30.
| An analysis of Australian adverse drug events.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M7psVertw%3D%3D&md5=c42c5b8b65aba4e9097a6bcecb1f5db6CAS | 10096721PubMed |


