Financial costs associated with monopolies on biologic medicines in Australia
Deborah Gleeson A F , Belinda Townsend B , Ruth Lopert C , Joel Lexchin D and Hazel Moir EA School of Psychology and Public Health, La Trobe University, Vic. 3068, Australia.
B School of Regulation and Global Governance, College of Asia and the Pacific, Australian National University, ACT 0200, Australia. Email: belinda.townsend@anu.edu.au
C Department of Health Policy and Management, Milken Institute School of Public Health, George Washington University, Washington, DC, USA. Email: rlopert@gwu.edu
D School of Health Policy and Management, York University, 4700 Keele Street, Toronto, Ontario M3J 1P3, Canada. Email: jlexchin@yorku.ca
E Centre for European Studies, College of Arts and Social Sciences, Australian National University, Canberra, ACT 0200, Australia. Email: hazel.moir@anu.edu.au
F Corresponding author. Email: d.gleeson@latrobe.edu.au
Australian Health Review 43(1) 36-42 https://doi.org/10.1071/AH17031
Submitted: 9 February 2017 Accepted: 28 September 2017 Published: 9 November 2017
Journal Compilation © AHHA 2019 Open Access CC BY-NC-ND
Abstract
Objectives The aim of the study was to estimate the potential savings to the Pharmaceutical Benefits Scheme (PBS) and the Repatriation Pharmaceutical Benefits Scheme (RPBS) in 2015–16 if biosimilar versions of selected biologic medicines (biologics) had been available and listed on the PBS.
Methods The research involved retrospective analysis of Australian Medicare expenditure data and PBS price data from 2015–16 for biologics, for which biosimilar competition may be available in future, listed on the PBS.
Results Australian Government expenditure on biologics on the PBS and RPBS was estimated at A$2.29 billion dollars in 2015–16. If biosimilar versions of these medicines had been listed on the PBS in 2015–16, at least A$367 million dollars would have been saved in PBS and RPBS subsidies. Modelling based on price decreases following listing of biosimilars on the PBS suggests that annual PBS outlays on biologics could be reduced by as much as 24% through the timely introduction of biosimilars.
Conclusions Biologic medicines represent a large proportion of government expenditure on pharmaceuticals. Reducing the length of monopoly protections on these medicines could generate savings of hundreds of millions of dollars per year.
What is known about the topic? Biologics take up an increasing share of pharmaceutical expenditure, but no previous published studies have examined Australian Government expenditure on biologics or the potential savings from reducing the duration of monopoly protection.
What does this paper add? This paper provides new evidence about Australian Government expenditure on biologics and potential savings for selected medicines that are still subject to monopoly protection and thus are not yet subject to biosimilar competition. In 2015–16 Australian Government expenditure on biologics through the PBS and RPBS was estimated at A$2.29 billion dollars. If biosimilar versions of these medicines had been listed on the PBS at that time, at least A$367 million dollars would have been saved.
What are the implications for practitioners? Reducing the duration of monopoly protection on biologic medicines could save hundreds of millions of dollars annually that could be redirected to other areas of the healthcare system.
Additional keywords: biologics, biosimilar medicines, biosimilars, data exclusivity, pharmaceutical expenditure, Pharmaceutical Benefits Scheme, Trans-Pacific Partnership Agreement, TPP.
Introduction
The aim of this paper is to estimate the potential savings that could be made by Australia’s Pharmaceutical Benefits Scheme (PBS) and Repatriation Pharmaceutical Benefits Scheme (RPBS) through the timely introduction of biosimilar medicines (biosimilars), i.e. ‘follow-on products that are able to demonstrate a high and constant degree of similarity’ to the reference (original) product.1
Biologic medicines (biologics) are produced from living organisms through biotechnological processes.1,2 They include many new medicines for cancer and other serious illnesses, such as rheumatoid arthritis and multiple sclerosis.2 These complex products tend to be very expensive, particularly while they are under patent.1 For example, pembrolizumab (Keytruda, Merck Sharp & Dohme), a drug for metastatic melanoma, cost patients approximately A$150 000 for a year’s treatment before it was subsidised by Australia’s PBS.3
Biologics make up an increasing share of the pharmaceutical market and were projected to comprise 19–20% of the global market by 2017.4 The high costs associated with these medicines will place increasing strain on health systems unless there are sound processes to facilitate the introduction of biosimilars.5 In Australia, the government has committed A$20 million over 3 years for biosimilar awareness-raising and educational activities intended to increase their uptake and improve the sustainability of the PBS.6
Once the monopoly period is over, biosimilar medicines can be produced and made available at a lower cost. The pharmaceutical industry, however, has been lobbying for longer monopolies on biologics through trade deals such as the proposed Trans-Pacific Partnership (TPP).2,7,8 The final text of the TPP, signed in 2016, included a novel set of provisions specifically targeting biologics.7 Although it seems probable that the TPP will not proceed in its original form following the withdrawal of the United States, the remaining 11 TPP countries are seeking to revive the deal, albeit possibly with the biologics provisions suspended pending re-entry of the US9. It is however unlikely that the pharmaceutical industry will relinquish its efforts to have countries, including Australia, adopt longer monopoly protections for this class of drugs. Lengthening market exclusivity for biologics has also been a long-term goal of Medicines Australia, the organisation representing the research-based pharmaceutical industry in Australia.10
The primary mechanism through which the pharmaceutical industry has sought to extend monopolies on biologics is known as ‘data protection’ or ‘data exclusivity’. When an originator manufacturer applies to the Therapeutic Goods Administration for approval to market a drug in Australia, it is required to submit clinical trial data to demonstrate the drug’s safety and efficacy. Subsequently manufacturers of generic or biosimilar medicines may rely on these data to register their own products for sale. Under Section 25A of Australia’s Therapeutic Goods Act (1989), the Therapeutic Goods Administration is not permitted to rely on the clinical trial data submitted by an originator to support the approval of a generic or biosimilar version for a period of 5 years from the date of the originator’s marketing approval.11 The TPP biologics provisions set out two options: 8 years of data exclusivity, or 5 years of data exclusivity together with other (unspecified) measures to deliver ‘comparable outcomes’.7
Importantly, data protection applies irrespective of the patent status of the reference product, and unlike a patent, cannot be revoked or challenged in the courts.2,12 It thus provides a market exclusivity period that is distinct from that conferred by a patent. In most cases, pharmaceutical companies can expect 14–15 years of market exclusivity based on patent protection.12 Usually patent and data protection terms are concurrent, but if the commercial development phase has been particularly long the data protection term may continue to block competition after relevant patents have expired.
The pharmaceutical industry argues that the guaranteed market exclusivity period provided by data protection is necessary to stimulate innovation.13 Although there is some empirical evidence that fixed patent terms provide a disincentive to private company investment in long-term research,14 the bulk of this type of research and development is actually undertaken in the public sector.15,16 There is no evidence that incremental increases to monopoly protections stimulate additional innovation.17 Furthermore, an international empirical comparative study found no relationship between the existence of data exclusivity and the amount of pharmaceutical industry investment in a country.18
Two recent reviews commissioned by the Australian Government found no evidence that market exclusivity for biologic products needs to be extended.8,12 Furthermore, a 2015 study by IP Australia found that 43% of Patent Cooperation Treaty applications by Australian applicants were for biologic medicines, a figure considerably higher than the global average of 29%.19 The number of applications for new biologics remained steady from 2004 to 2012,19 suggesting that research and development (R&D) in this area was not adversely affected by Australia’s current duration of data protection.20
Data exclusivity has been shown to delay the entry of biosimilar drugs elsewhere. In September 2013, a biosimilar of infliximab (Remicade, Janssen) was approved by the European Medicines Agency, but could not be marketed in most of Europe until February 2015 because of an extension to the market exclusivity period granted to the originator product.21 However, in Norway, the introduction of biosimilar infliximab in late 2013 led to an initial price reduction of 39% of the originator product that later fell to 69%, with the market share for the biosimilar medicine rising from 20–30% in 2014 to more than 50% by March 2015.22
A 2014 Quintiles IMS Institute for Healthcare Informatics study of price reductions of biosimilars in Europe found that between 2006 and 2013, the reduction in treatment price for biosimilar erythropoietin was as high as 81% in Croatia, with a median reduction of 35% (on a volume-weighted price-per-defined-daily-dose basis, indexed relative to 2006).23
A study modelling potential savings from biosimilars in eight European countries estimated cumulative savings for three classes of biosimilars (erythropoietins, granulocyte-colony stimulating factors and monoclonal antibodies) of €11.8–33.4 billion over 2007–2020, 5.2–14.6% of the estimated expenditure in each country.5 Savings would be much higher if biosimilars were available immediately after patent expiry, in comparison with a scenario in which market entry was delayed. A 2013 USA study predicted that biosimilars would lead to a US$44.2 billion reduction in direct spending on biologic drugs from 2014–2024, ~4% of the total USA expenditure on biologics in this period.24
To date, there have been few studies that have estimated public expenditure on biologics in Australia or the potential savings that could be generated from biosimilars. Gleeson et al. found that adalimumab (Humira, AbbVie) cost the PBS A$272.2 million in the 2013–14 financial year, and calculated that listing of a follow-on product would have resulted in savings of A$43.6 million in that year alone.2 In a submission to the Department of Foreign Affairs and Trade, Gleeson et al. estimated that 10 biologic medicines had cost over A$1.29 billion in PBS and RPBS expenditure in 2013–14.25 Biosimilars for these 10 biologics could have saved more than A$205.9 million in the 2013–14 financial year alone.
The present study addresses three research questions:
-
What was the Australian Government’s expenditure on biologic medicines (for which it is conceivable that a follow-on product may be listed in future) through the PBS and the RPBS in the 2015–16 financial year?
-
How much would have been saved in PBS and RPBS expenditure in the 2015–16 financial year if biosimilar versions of these medicines had been listed on the PBS and RPBS?
-
For those biologics with biosimilars already listed on the PBS, what have been the price reductions to date resulting from statutory price reductions and price disclosure? If similar price falls are seen when biosimilar versions of high-cost biologics are listed on the PBS, what is the scale of the potential savings?
Methods
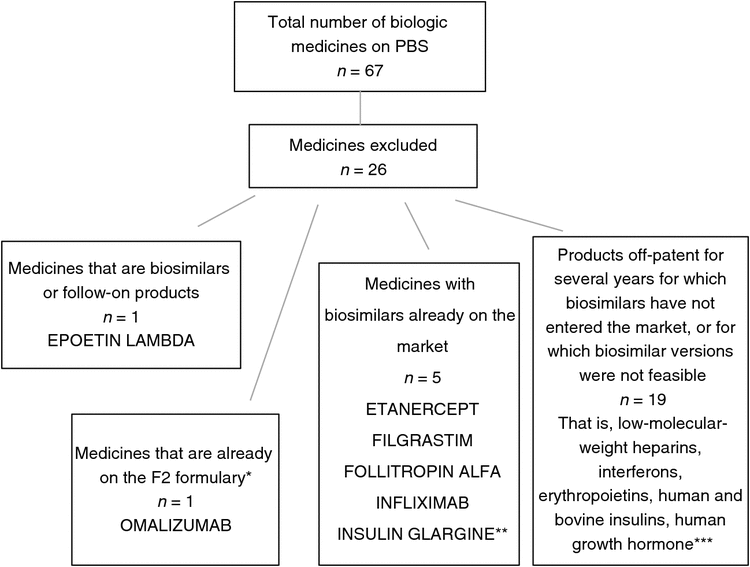
Using the PBS A–Z medicine listing,26 we identified 67 biologic medicines currently listed on the PBS.
We excluded 26 medicines from our analysis because they were: (i) medicines for which biosimilars were already available; (ii) medicines that were themselves biosimilars or follow-on versions of existing products; or (iii) medicines off-patent for several years and for which biosimilars have not entered the market, or for which biosimilars are unlikely or infeasible. The application of exclusion criteria is shown in Fig. 1.
We then collated PBS and RPBS Medicare claims data27 for each of the remaining 41 drugs using the relevant item numbers obtained from the PBS website for the financial year 2015–16. This data source provides a gross estimate of the expenditure on these medicines and does not take into account any special pricing arrangements, such as price-volume agreements or confidential rebates.
In Australia, a 16% price reduction is applied to all brands of a medicine as soon as the first generic or biosimilar version is listed on the PBS. Applying this price reduction enabled us to calculate the minimum savings that would have been generated in PBS and RPBS subsidies if follow-on versions of these drugs had been listed on the PBS in the year 2015–16.
Next, we examined those drugs that have biosimilar versions listed on the PBS (filgrastim (Neupogen, Amgen), follitropin alfa (Gonal-f, Merck Serono) and infliximab (Remicade, Janssen-Cilag)) and had been subject to statutory price reductions or been subject to price disclosure,A to calculate how much prices had decreased. We used these price changes to model how much might be saved over time for four biologic medicines: adalimumab (Humira, AbbVie), rituximab (Mabthera, Roche), ranibizumab (Lucentis, Novartis) and aflibercept (Eylea, Bayer). These medicines had the highest PBS expenditure in 2015–16 and are used to treat diseases such as macular degeneration and diabetic macular oedema (aflibercept, ranibizumab), rheumatoid and psoriatic arthritis (adalimumab) and autoimmune diseases and cancer (rituximab).
Results
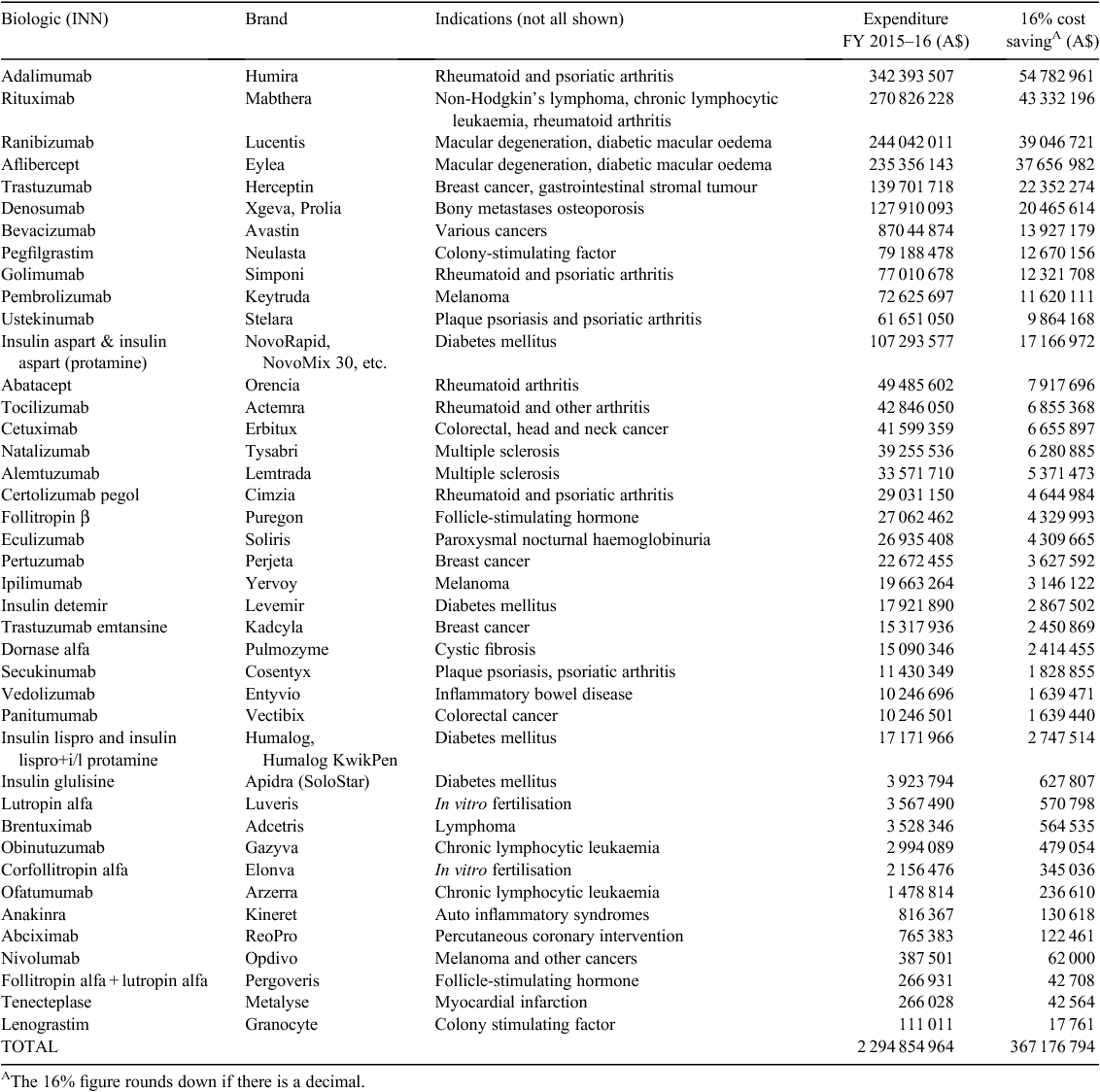
Table 1 shows the gross expenditure on the 41 biologics included in the analysis and the savings that would have been generated had a biosimilar been listed on the PBS and the 16% statutory price reduction applied. Australian Government expenditure on biologic medicines through the PBS and RPBS in 2015–16 was estimated at A$2.29 billion dollars.B This represents ~21.1% of the A$10.84 billion total PBS expenditure.28 If biosimilar versions of these medicines had been listed on the PBS in 2015–2016, at least A$367 million dollars would have been saved in PBS and RPBS subsidies.
The price for 20 syringes each containing 300 micrograms of filgrastim (approved in November 1995) was A$3054 in December 2010; this fell by 16% to A$2562 in August 2011 when the 16% statutory price reduction was applied following the introduction of two biosimilar versions. In November 2014, price disclosure was applied and the price fell by a further 15% and again by 47% over the 2 years to November 2016. The current price of A$1158 is ~38% of the initial price before the first biosimilar was listed on the PBS.
The originator brand of follitropin alfa (Gonal-F, Merck Serono) was first registered in December 2003. A biosimilar, Bemfola (Gedeon Richter), obtained marketing approval in November 2015 and PBS listing in August 2016. The price in December 2015, before PBS listing of the biosimilar, was A$731 for three 450IU doses. In August 2016 it fell by the statutory 16%. By December 2016 it had fallen a further 6% due to price disclosure, giving a total price decrease of A$144.15, or ~20% of the original cost since biosimilar entry. Infliximab (Remicade, Janssen-Cilag) was first registered on the Australian Register of Therapeutic Goods in August 2000. The biosimilar Inflectra (Pfizer) was approved in August 2015 and listed on the PBS in December 2015. From November to December 2015 the price fell 27%.
For follitropin alfa and infliximab the entry of biosimilars led to price falls of between 20 and 27%. This compares with the 62% price fall for filgrastim, for which three competing biosimilars had entered the market. A conservative general estimate for the price decrease consequent to biosimilar entry would be for a further fall of 10% from the already 16%-reduced price, leading to an overall reduction of 24% from the original price. A conservative estimate is appropriate given that the price reductions resulting from price disclosure are largely dependent on the degree of market competition, which may be less for other biologics than for filgrastim, for which a significant number of brands have entered the market.
In 2015–16, PBS and RPBS outlays on adalimumab were more than A$342 million. An initial price fall of 16% would provide savings of A$54 million, assuming the volume of prescriptions remained constant. A further 10% price fall would save an additional A$28 million. Similarly, for rituximab expected savings are A$43 million in the first year of biosimilar availability and a further A$23 million in the second year. Expected savings for ranibizumab would be A$19 million in the first year and a further A$20 million in the second year. For aflibercept the corresponding savings would be A$37 million and then a further A$19 million. Overall, the cross-section of all current medicines where biosimilar entry can be anticipated indicates savings could be between A$367 and A$560 million a year (see Table 2), depending on the level of market penetration of biosimilars.
Discussion
Our findings show that biologics make up more than 20% of government spending on pharmaceuticals in Australia, and price savings could be between A$367 and A$560 million a year by bringing biosimilars onto the market in a timely fashion. Extending market exclusivity, as the industry has sought, would have major cost implications for Australia’s PBS, as it would create unnecessary delays in the availability of less expensive follow-on products.
The manufacturing costs associated with biosimilars, their complexity and the likelihood of only small numbers of follow-on products for most biologic drugs mean the savings are likely to be more modest than those from generic small-molecule (non-biologic) drugs.1,29 However, given the high prices of many biologic medicines, even small reductions could generate significant savings1 particularly in a country such as Australia with a single-payer system and a single national drug formulary.
Limiting or reducing the period of data exclusivity is unlikely to have unanticipated negative effects on social welfare, particularly in the Australian context. Prices are independent of the duration of market monopoly for several reasons. First, the money spent to bring a product to market is a sunk cost, and the revenue generated from products is used to finance the R&D for future products, not to recover what has already been spent. Second, the PBS is an effective monopsony, and the upper bound of the price is limited by the requirement to demonstrate adequate cost effectiveness at the price proposed (to the satisfaction of the Pharmaceutical Benefits Advisory Committee).30 There is thus little scope for firms to compensate for a shorter duration of market protection by setting higher prices. Threats by pharmaceutical companies not to bring their products to the Australian market are unlikely to be realised as Australia is a market with a very high penetration of patented products and is thus attractive.31 Reduced investment in research for innovative products is also unlikely given that little R&D is conducted in Australia; expenditure by the Australian pharmaceutical industry on pharmaceutical R&D amounted to only A$404 million dollars in 2011–12.32 Furthermore, Australia accounts for only 1% of the global market;33 thus any change in Australian prices would have a negligible effect on the return on investment that companies earn.
Of course, there is no guarantee that biosimilars will automatically be available when the originator’s monopoly is exhausted. Biosimilars face a range of barriers to market entry.29 A 2016 European Union study identified four barriers to biosimilar entry over and above intellectual property issues: the complexity of the manufacturing process; regulatory barriers; difficulties with interchangeability and substitution; and close relationships between originator companies, physicians and health services.21
Slow uptake of biosimilars in Europe has been ascribed to national differences in generic use, low physician knowledge and slow acceptance of the concept of biosimilarity.21 Market penetration of biosimilars in Europe varies widely; for human growth hormone in Norway it is 2% whereas in Poland it is 99%. Biosimilar erythropoietin penetration ranges from 1% in Croatia to 62% in Bulgaria, and granulocyte colony-stimulating factor uptake varies from 2% in Belgium to nearly 100% in Croatia, Czech Republic, Hungary and Romania.4 Market penetration levels in Australia remain to be seen. However, Australia has effective processes for encouraging the use of generic medicines and the government’s biosimilars awareness program6 suggests similar procedures might be put in place for biosimilars. For filgrastim, the only medicine with substantial biosimilar competition, 52% of total PBS expenditure in 2015–16 was for biosimilar versions. This provides a useful indicator of what could be expected in cases where multiple biosimilars are listed on the PBS for several years.
Limitations to the methods used in this study arise from the nature of the data (the effects of rebates and confidential special pricing arrangements are not able to be gauged) and the unknown future market penetration of biosimilars, which means that we can only estimate the potential savings due to biosimilar entry. Other limitations of the paper include that it neither allows for the timing of biosimilar entry nor examines how much data protection contributes to the monopoly period in comparison with patent protection. The data protection issue in particular, is an area that needs further study.
Conclusion
This paper provides the first comprehensive estimate of Australian Government expenditure on biologic medicines and the potential savings from timely entry of biosimilars. Despite the hurdles for biosimilar market entry, ensuring timely entry of biosimilars is one important strategy to ensure that savings from biosimilars are realised.21,29 Unnecessarily long monopoly periods for medicines are expensive. There is no evidence to support claims that long monopoly periods create incentives for increased local R&D investment. On the other hand, the cost of delayed biosimilar entry is substantial. Savings in the order of A$367 to A$560 million a year could be anticipated if biosimilars were listed on the PBS for each of the biologic products in Table 1. Annual combined PBS and RPBS outlays of A$A2.2 billion on biologic medicines could be reduced by as much as 24%.
Competing interests
DG and BT often represent the Public Health Association of Australia in relation to trade agreements and public health. In 2015–16 JL received payment for being a consultant on two projects – one looking at indication-based prescribing and a second examining what drugs should be provided free of charge by general practitioners. He also was paid for being on a panel that discussed expanding drug insurance in Canada. He is on the Foundation Board of Health Action International. The views expressed in this paper are not the views of any organisation with which the authors are affiliated. The authors have no other competing interests to declare.
Acknowledgements
This research was supported by a grant from the School of Psychology and Public Health, La Trobe University.
References
[1] Megerlin F, Lopert R, Taymor K, Trouvin J-H. Biosimilars and the European experience: implications for the United States. Health Aff 2013; 32 1803–10.| Biosimilars and the European experience: implications for the United States.Crossref | GoogleScholarGoogle Scholar |
[2] Gleeson DH, Moir HVJ, Lopert R. Costs to Australian taxpayers of pharmaceutical monopolies and proposals to extend them in the Trans-Pacific Partnership Agreement. Med J Aust 2015; 202 306–8.
| Costs to Australian taxpayers of pharmaceutical monopolies and proposals to extend them in the Trans-Pacific Partnership Agreement.Crossref | GoogleScholarGoogle Scholar |
[3] ABC News. ‘Revolutionary’ melanoma drug worth $150,000 a year listed on PBS, saving Australian patients thousands. ABC News [Internet]. 2015. Available at: http://www.abc.net.au/news/2015-06-28/melanoma-drug-listed-on-pbs-saving-patients-thousands/6578554 [verified 9 December 2016].
[4] IMS Institute for Healthcare Informatics. The global use of medicines: outlook through 2017.Parsippany NJ: IMS Health; 2013.
[5] Haustein R, de Millas C, Hoer A, Haussler B. Saving money in the European healthcare systems with biosimilars. GaBI Journal 2012; 1 120–6.
| Saving money in the European healthcare systems with biosimilars.Crossref | GoogleScholarGoogle Scholar |
[6] Australian Government Department of Health. Biosimilar Awareness Initiative: Project Management Plan. 2016. Available at: https://health.gov.au/internet/main/publishing.nsf/Content/0CE13CDB4B59ACB0CA2580810077E68F/$File/biosimilar-project-management-plan.pdf [verified 9 December 2016].
[7] Lexchin J, Gleeson D. The Trans Pacific Partnership Agreement and pharmaceutical regulation in Canada and Australia. Int J Health Serv 2016; 46 597–613.
| The Trans Pacific Partnership Agreement and pharmaceutical regulation in Canada and Australia.Crossref | GoogleScholarGoogle Scholar |
[8] Productivity Commission. Intellectual property arrangements, draft report. Canberra: Productivity Commission; 2016. Available at: http://www.pc.gov.au/inquiries/completed/intellectual-property/draft/intellectual-property-draft.pdf [verified 9 October 2017].
[9] Yamazaki J. ‘TPP 11’ to freeze drug data protection demanded by US: Remaining members thrashing out details to revive trade pact. Nikkei Asian Review, 31 August 2017. https://asia.nikkei.com/Politics-Economy/International-Relations/TPP-11-to-freeze-drug-data-protection-demanded-by-US [verified 22 October 2017].
[10] Medicines Australia. Submission to the consultation on the issues paper by the Productivity Commission on Intellectual Property (IP) Arrangements in Australia. 2015. Available at: http://www.pc.gov.au/__data/assets/pdf_file/0011/194537/sub044-intellectual-property.pdf [verified 9 December 2016].
[11] Therapeutic Goods Act. (1989). Available at: https://www.legislation.gov.au/Details/C2016C01117 [verified 9 December 2016].
[12] Harris T, Nicol D, Gruen N. Pharmaceutical patents review report. Canberra: Commonwealth of Australia; 2013. Available at: http://www.ipaustralia.gov.au/pdfs/2013-05-27_PPR_Final_Report.pdf [verified January 2017].
[13] Lybecker KM. Essay: when patents aren’t enough: why biologics necessitate date exclusivity protection. William Mitchell Law Rev 2014; 40 Article 7
[14] Budish E, Roin BN, Williams H. Do firms underinvest in long-term research? Evidence from cancer clinical trials. Am Econ Rev 2015; 105 2044–85.
| Do firms underinvest in long-term research? Evidence from cancer clinical trials.Crossref | GoogleScholarGoogle Scholar |
[15] Stevens AJ, Jensen JJ, Wyler K, Kilgore PC, Chatterjee S, Rohrbaugh ML. The role of public-sector research in the discovery of drugs and vaccines. N Engl J Med 2011; 364 535–41.
| The role of public-sector research in the discovery of drugs and vaccines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhvVygur0%3D&md5=ba4701021908d0e12298ffdb8b43b65fCAS |
[16] Kesselheim AS, Tan YT, Avorn J. The roles of academia, rare diseases, and repurposing in the development of the most transformative drugs. Health Aff (Millwood) 2015; 34 286–93.
| The roles of academia, rare diseases, and repurposing in the development of the most transformative drugs.Crossref | GoogleScholarGoogle Scholar |
[17] Marchini KA. Patents and innovation in the pharmaceutical industry. Grove City College Journal of Law & Public Policy 2013; 4 47–59.
[18] Palmedo M. Do pharmaceutical firms invest more heavily in countries with data exclusivity? Currents International Trade Law Journal 2013; 21 38–47.
[19] IP Australia. A patent analytics study on the Australian pharmaceutical industry. Canberra: IP Australia; 2015.
[20] Wong J. Data protection for biologics – should the data exclusivity period be increased to 12 years? UNSWLJ Student Series 2016; No 16–07. Available at: https://ssrn.com/abstract=2831262 [verified 16 December 2016].
[21] Moorkens E, Jonker-Exler C, Huys I, Declerck P, Simoens S, Vulto AG. Overcoming barriers to the market access of biosimilars in the European Union: the case of biosimilar monoclonal antibodies. Front Pharmacol 2016; 7
| Overcoming barriers to the market access of biosimilars in the European Union: the case of biosimilar monoclonal antibodies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhsV2gtb7O&md5=ba7aacc415cdbf8a0ff3643b491cc693CAS |
[22] Mack A. Norway, biosimilars in different funding systems. What works? GaBI Journal 2015; 4 90–2.
| Norway, biosimilars in different funding systems. What works?Crossref | GoogleScholarGoogle Scholar |
[23] IMS Institute for Healthcare Informatics. Assessing biosimilar uptake and competition in European markets. Parsippany, NJ: IMS Health; 2014. Available at: https://www.imshealth.com/files/web/IMSH%20Institute/Healthcare%20Briefs/Assessing_biosimilar_uptake_and_competition_in_European_markets.pdf [verified 9 December 2016].
[24] Mulcahy AW, Predmore Z, Mattke S. The cost savings potential of biosimilar drugs in the United States. Santa Monica CA: Rand Corporation; 2014. Available at: https://www.rand.org/content/dam/rand/pubs/perspectives/PE100/PE127/RAND_PE127.pdf [verified 16 December 2016].
[25] Gleeson D, Lopert R, Moir HVJ. Proposals for extending data protection for biologics in the TPPA: Potential consequences for Australia. Submission to the Department of Foreign Affairs and Trade. 2014. Available at: http://dfat.gov.au/trade/agreements/tpp/negotiations/Documents/tpp_sub_gleeson_lopert_moir.pdf [verified 11 December 2016].
[26] Australian Government Department of Health. The Pharmaceutical Benefits Scheme: A-Z medicine listing. 2016. Available at: https://www.pbs.gov.au/browse/medicine-listing [verified November 2016].
[27] Australian Government Department of Human Services. Medicare Australia statistics: Pharmaceutical Benefits Schedule item reports 2016. Available at: http://medicarestatistics.humanservices.gov.au/statistics/pbs_item.jsp [verified November 2016].
[28] Department of Health. Expenditure and prescriptions: twelve months to 30 June 2016. Canberra: Department of Health; 2016.
[29] Sarpatwari A, Avorn J, Kesselheim AS. Progress and hurdles for follow-on biologics. N Engl J Med 2015; 372 2380–2.
| Progress and hurdles for follow-on biologics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtlert7rE&md5=431343647dcb14a94f98d03a870e9e38CAS |
[30] Australian Government Department of Health. The Pharmaceutical Benefits Advisory Committee guidelines. Available at: https://pbac.pbs.gov.au/ [verified July 2017].
[31] Economist Intelligence Unit. Benchmarking study of the characteristics of the Australian and international pharmaceuticals industries. Final report. London: The Economist; 2005. Available at: http://apo.org.au/system/files/5785/apo-nid5785-63926.pdf [verified 20 July 2017].
[32] Australian Government Department of Industry. Innovation and science. Australian pharmaceuticals industry data card. Canberra; 2014. Available at: https://industry.gov.au/industry/IndustrySectors/PharmaceuticalsandHealthTechnologies/Pharmaceuticals/Pages/PharmaceuticalsIndustryDataCard.aspx [verified 20 July 2017].
[33] Medicines Australia. Medicines Australia FactsBook 4th ed. Medicines Australia, Deakin ACT: 2015. Available at: https://medicinesaustralia.com.au/wp-content/uploads/sites/52/2010/11/MAFactsBook4_update2015.pdf [verified 20 July 2017].
A APrice disclosure requires suppliers of multi-source Pharmaceutical Benefits Scheme medicines to report to government the actual prices at which medicines are supplied to pharmacies (taking into account actual and in-kind discounts). The reimbursement to pharmacy for all brands of multi-source medicines is then adjusted to reflect more closely the price at which the products are supplied. (http://www.pbs.gov.au/info/industry/pricing/eapd/price-disclosure-faq).
B BThis estimate does not take into account any confidential risk sharing arrangements or special pricing agreements that apply, which cannot be estimated. Adalimumab, rituximab, ranibizumab, and aflibercept are all subject to special pricing arrangements for at least some indications.