Maximising the value of clinical registry information through integration with a health service clinical governance framework: a case study
Susannah Ahern A C , Robert Feiler B and Susan Sdrinis BA Department of Epidemiology and Preventive Medicine, Monash University, Level 2, 553 St Kilda Road, Melbourne, Vic. 3004, Australia.
B Alfred Health, 55 Commercial Road, Melbourne, Vic. 3004, Australia. Email: Robert.Feiler@alfred.org.au; S.Sdrinis@alfred.org.au
C Corresponding author. Email: Susannah.ahern@monash.edu
Australian Health Review 44(3) 421-426 https://doi.org/10.1071/AH19004
Submitted: 9 January 2019 Accepted: 29 August 2019 Published: 11 February 2020
Journal Compilation © AHHA 2020 Open Access CC BY-NC-ND
Abstract
This initiative sought to identify unit participation in clinical registries within a large metropolitan health service and to develop approaches to integration of registry reporting within the organisational clinical governance framework to maximise potential quality improvement benefits. The initiative, led by the Medical Services Department at Alfred Health, initially involved identifying health service participation in clinical registries via a range of mechanisms, including one-on-one meetings with clinical registry investigators. In conjunction with the Clinical Governance Unit, tools to summarise and track clinical registry information at Alfred Health over time were developed and piloted. Alfred Health identified 69 clinical registries in which its units participated. These were heterogeneous in terms of clinical area and purpose, as well as the nature and frequency of reporting. Engagement with clinicians led to the establishment of a registry interest group, a calendar of clinical quality registry reports, and a guideline and reporting template and dashboard. Clinician engagement and medical leadership were critical to the development of this initiative. The reporting tool and dashboard have had initial success, with long-term success ultimately being measured by the routine incorporation of registry outcomes into clinical governance reporting over time.
What is known about the topic? Health service clinical governance systems require the collection, analysis and ongoing monitoring of clinical performance and quality improvement information. These data may be from internally derived clinical indicators or from external datasets, such as clinical registries. However, although clinical registries have traditionally provided information at the unit level, mechanisms to systematically incorporate these clinical measures into health service clinical governance systems have been lacking.
What does this paper add? This paper provides a case study of the steps taken by one large health service to identify, engage clinicians and incorporate disease-specific clinical registry indicators into its organisational clinical governance framework. It highlights the complexity of the task through the time taken to identify, translate and summarise key clinical information into a format suitable for organisational committee reporting.
What are the implications for practitioners? This paper highlights to health service managers the importance of initial and ongoing engagement of clinicians in the development of a shared approach to organisational use of clinical registry data. It outlines potential steps that can be taken within a health service to engage clinicians in sharing registry information, and processes that can assist in systematically incorporating registry information into actionable organisational-level reporting as part of clinical governance.
Introduction
Health service clinical governance ‘involves a complex set of leadership behaviours, policies, procedures, and monitoring and improvement mechanisms that are directed towards ensuring good clinical outcomes’.1 In practice, this typically occurs through the setting of a strategic clinical governance framework by the organisation’s governing board and executive, and the review of performance of high-level organisational clinical indicators aligned with the National Model Clinical Governance Framework of the Australian Commission on Safety and Quality in Health Care (ACSQHC) (https://www.safetyandquality.gov.au/publications-and-resources/resource-library/national-model-clinical-governance-framework, accessed 21 November 2019). In addition, the National Safety and Quality Health Service Standards (NSQHS)2 require that health services have systems to monitor variation in practice against expected standards, and identify unwarranted clinical variation to inform clinical practice improvements. Many of the data used to inform health system improvements are internally derived performance and clinical data, but external datasets, such as from clinical audits and registries, are increasingly being considered.
Clinical registries are multisite initiatives that collect disease- or procedure-specific information for epidemiological, quality, research and other purposes. Clinical quality registries (CQRs) are internationally recognised as a significant quality improvement tool because they provide benchmarked key quality indicator information to participating sites.3,4 In particular, CQRs can monitor variation in practice and outcomes between participating sites and over time, which may not necessarily be easily identified from routine health service data.5,6 When mature, CQRs can monitor variation in mortality rates, a critical safety measure particularly in elective cardiac or bariatric surgery,7,8 and can identify persistent statistical outlying clinical units that may warrant internal review or further investigation. CQR outputs have been associated with improvements in both process measures9 and outcome measures, particularly when associated with public disclosure of outcomes.10
The ACSQHC has provided guidance on CQR development in Australia over the past decade through the publication of key documents ,including the Operating Principles and Technical Standards for Clinical Quality Registries (2008),11 the Framework for Australian Clinical Quality Registries (2014)12 and the Prioritised list of Clinical Domains for Clinical Quality Registry development (2016).13 Within Victoria, a recent Department of Health and Human Services review of state-wide safety and quality systems similarly recognised the importance of CQRs by recommending improved access to and use of clinical registry data by health services.14
In line with these national and state-based clinical governance expectations, health service managers are increasingly seeking clinical registry information. In particular, health service clinicians and managers value the benchmarked reports of key clinical process measures (e.g. compliance with best practice guidelines) and outcome measures (e.g. postoperative complications) with other participating health services that many registries produce. Significantly, it has been found that the engagement of both managers and clinicians in clinical registries is correlated with clinical staff utilisation of registry information for quality improvement.15,16 However, as tools established primarily for local clinical quality improvement purposes, registries have not necessarily highlighted key messages or recommendations at the organisational level.
There is currently no national ‘register of clinical registries’ in Australia to assist health services in identifying and accessing their clinical registry reports, although a recent study of the characteristics of clinical registries in Australia included 40 clinical registries, which is likely an underrepresentation of the true number.17 Further, there is little evidence that clinical registry information is regularly made available to health service boards or executives, or incorporated within health service clinical governance frameworks. A review at a Melbourne tertiary health service that participated in over 40 clinical registries notes that although there was ‘very high level of medical staff participation in Clinical Registries, there is a lack of systematic reporting of Registries data into quality committees beyond unit level’.18
With limited information regarding how health services may systematically seek to maximise the quality improvement benefits that arise from effective organisational use of clinical registry data, this case study provides an example of a health service’s development of a more structured approach.
Objectives
The objectives of this initiative were to: (1) identify clinician and unit participation in clinical registries at an Australian quaternary health service; (2) investigate opportunities to engage health service clinicians to share clinical registry information within the organisation; and (3) integrate CQR reporting within the health service clinical governance framework in order to maximise the value of CQR information for health service quality improvement.
Methods
Setting
This initiative was undertaken within a public quaternary health service in Melbourne, namely Alfred Health (AH). AH provides for a local population of 700 000 people and, in 2017–18, provided over 115 000 episodes of in-patient care.19 The AH Clinical Governance and Quality Framework (Alfred Health, Melbourne, Vic., Australia, pers. comm.) is consistent with the National Model Clinical Governance Framework,1 the National Safety and Quality Health Service Standards2 and jurisdictional and community services frameworks. The initiative took place over an 18-month period.
Participants
This initiative was sponsored by the Medical Services Department (comprising a medical administrator and trainee), with support from the Clinical Governance Unit (CGU) of AH (comprising clinical risk and quality managers). The medical heads of departments and principal investigators of clinical registries were key stakeholders in the project. A project officer from the CGU was assigned to manage the project.
Methodology and sequence of events
Prior to this project, AH clinical units participating in CQRs variably reported CQR activity and outcomes to senior management, and via an annual clinical audit process. The Medical Services Department within AH identified that there was an opportunity to strengthen and systematically identify and track all registry activity undertaken at AH. Because this was a local quality improvement initiative, ethics and governance approval were not required.
The CQR initiative comprised three main components: (1) ascertaining AH participation in clinical registries; (2) engagement of clinicians in ongoing sharing of CQR outcomes; and (3) piloting facilitation of CQR reports into AH clinical governance reporting.
Ascertaining AH participation in clinical registries
In order to ascertain AH’s participation in clinical registries, the CGU project officer reviewed several sources, including AH Ethics Committee documentation of approved clinical registry projects and individual CQR site reports, a survey of all clinical units and via verbal feedback from units. From this process an initial list of clinical registries at AH was developed. Over 12 months, one-on-one meetings were held between the CGU project officer and AH registry principal investigators (clinicians) to better understand the duration of participation, the nature of the information collected and the reporting and analysis undertaken by each clinical registry. This information was regularly updated by the project officer and reviewed by Medical Services managers as the project sponsors.
Engagement of clinicians in ongoing sharing of CQR outcomes
Engaging clinicians in the registry project was considered critical to the broader success of the initiative’s aims of sharing registry outcomes for maximum benefit. Thus, following the one-on-one investigative meetings, the project officer and sponsors met with registry-participating clinicians in a variety of forums to highlight the organisational benefits of sharing of clinical registry information and to discuss potential processes, including policy and engagement activities, that may support this. Identified solutions were developed and rolled out over a 6-month period.
Piloting facilitation of CQR reports into AH clinical governance reporting
Following identification of potential options to support integration of CQR information into AH clinical governance reporting from the clinician forums, technical solutions for information sharing and a standardised approach to reporting were developed with the assistance of the CGU, and piloted for several registries.
Results
AH participation in clinical registries
The initiative identified the scope and breadth of clinical registry participation occurring at AH, namely participation in 69 clinical registries or external audits. Just over 50% (35 registries) were from the Cancer and Medical Program, 19 registries were from the Surgical Program and 14 registries were from the Emergency and Acute Medicine Program (Table 1).

|
Clinical registries at AH were classified based on their primary function (with many registries undertaking multiple functions). Over 50% (35 registries) reported quality of care and patient outcomes, whereas over one-third (27 registries) reported patterns of disease and treatment over time. Fewer registries monitored device safety or service delivery performance. Fig. 1 highlights the overlapping but variable nature of the purposes and reporting features of the clinical registries.
There was significant variability in relation to reports from the participating registries. Forty-two per cent (29) of the registries produced a public report, and 26% (18) produced a site report with clinical information benchmarked against other sites. The most common frequency of site reports was annual, with some registries providing quarterly, biannual or biennial reports. Several registries had not yet reached sufficient maturity to produce reports, and others provided site reports only on request. CQR reports varied from seven-page quarterly benchmarked reports to 300-page deidentified aggregate annual reports. Outside narrative-style executive summaries, few reports provided ‘at-a-glance’ summary overviews of key findings to facilitate organisational committee reporting.
Engagement of clinicians in ongoing sharing of CQR outcomes
Strategies developed following the internal forums to engage AH clinicians in ongoing sharing of CQR outcomes included:
establishment of a regular senior clinician Clinical Registry Interest Group, chaired by the Director Medical Services (Governance) as a forum for regular collaboration and discussion
development of a calendar schedule of CQR reports for monitoring by the CGU
incorporation of clinical registry site data into existing unit-based safety and quality presentations, such as the annual unit audits
development of a guideline requiring units participating in CQRs to provide an electronic copy of their CQR reports to the CGU, together with a summary of key report findings and action plan, if applicable, on a designated template.
Facilitation of CQR reports into AH clinical governance reporting
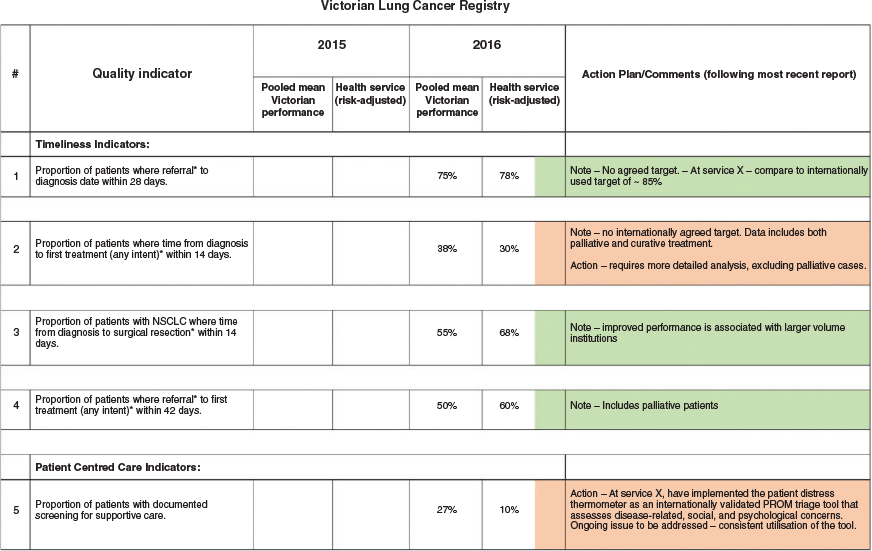
Following the development of the CQR summary document template, AH CGU staff developed a traffic light-style dashboard for each registry that allowed benchmarked report data to be tracked and visualised over time (for an example with ‘dummy’ data, see Fig. 2). This data was used to provide a regular report to the executive and Board Quality Committee on significant outliers and actions taken to address these. During the pilot, dashboards for 12 distinct registries were developed relating to a total of 25 benchmarked clinical reports.
|
Problems, conflicts and constraints
This initiative started with a relatively simple premise. However, it took longer to complete than first anticipated because we underestimated the difficulty of identifying all possible registries at AH, determining which could be classified as CQRs, and the time it would take both clinicians and the CGU to develop a reporting framework. Further, as with all change management projects, there were variable levels of clinician engagement, and building and sustaining interest in the collaborative approach took time.
The scope and scale of clinical registry participation was more significant than anticipated, as was the variability of clinical registry reports in terms of content, frequency and measures. This required AH clinical governance staff to develop a summary template to meaningfully translate voluminous CQR clinical information into succinct and useable outcome measures of performance and quality. For example, Fig. 2 (dummy data from the Victorian Lung Cancer Registry) is presented in the site registry report as a series of funnel plots. This has been translated into a dashboard template with AH data provided against the mean for each indicator and colour coded (green, red) in relation to benchmarked performance, Further, reporting that included complex clinical content and/or data that was not risk adjusted or had other statistical limitations required clinician translation to enable it to be meaningful to health service managers.
Discussion
This project was developed to support the core business of AH, namely to enhance the quality of clinical outcome reporting within the health service, and thus responsibility for this project was formalised within a CGU staff role. Providing a project officer to engage with clinicians in one-on-one meetings allowed a deeper understanding of the history, purpose and significance of each registry. Acknowledging the barriers, time and effort required for clinicians to input data into registries was a key focus of individual meetings, as was creating a culture of engagement. Although this is not necessarily a quick and efficient process, it is more likely to lead to sustainable engagement and alignment of purpose between the clinicians and the organisational executive. Local CQR clinician champions were critical in highlighting the benefits of sharing CQR results within the organisation. Such champions led by example, incorporating registry reports and feedback into regular local and organisation-wide audits and presentations.
Clinical registries can complement administrative and other datasets in providing meaningful, high-quality information that is useful for both clinicians delivering clinical care and health service managers to comply with governance standards and drive quality improvement. Implementing organisational reporting of CQR outcomes is in its early stages; however, it has the potential to significantly add value to an organisation’s understanding of its safety and quality performance across several key discipline areas. In particular, participation in clinical registries supports the National Accreditation Standard’s requirement for health services to monitor variation, provide feedback to clinicians and use this information to improve safety and quality.2
Although management support for clinician participation in clinical registries is a factor that leads to greater unit engagement, there is currently no systematic framework or model for how health service managers can appropriately access, interpret and use clinical registry information within their organisation. In addition, participation in CQRs is largely an unfunded activity at the health service level, with neither funder nor health service funding being allocated to collection or review of these data. Yet investment in this vital data infrastructure is necessary, not only for health service quality and safety purposes, but also increasingly for patients, governments and other funders, researchers and policy makers. Clinical registry custodians also have lessons to learn from this initiative: they should recognise that the health service executive is an important stakeholder in the registry reporting process and ensure that they report relevant data and key messages to meet clinical governance requirements and to inform health service quality improvement.
Conclusions
Clinical registries provide additional meaningful information to clinicians and health services about the quality and safety of their clinical services. This case study has described one large public health service’s efforts to identify its organisational contribution to external clinical registry activities and to maximise this value by developing an environment that fosters sharing of registry information throughout the organisation. This project identified health service participation in 69 clinical registries and external audits. Outcomes from the project comprised the establishment of a CQR interest group, development of a CQR reporting guideline, a calendar of CQR site reports and a CQR dashboard to monitor site CQR outcomes over time. Long-term success will be measured by routine incorporation of registry outcomes into AH clinical governance processes over time.
It is anticipated that this case study will inform the work of others addressing similar issues. Many health services across Australia are currently grappling with the introduction and use of a range of datasets including electronic medical records, as well as administrative data and reports from governments and other agencies. The management of clinical and quality improvement data at the health service level is a major challenge. Investment in both local and system-wide initiatives is required to ensure that such valuable clinical information is recognised and maximally used.
Competing interests
The authors declare no competing interests.
Acknowledgements
The authors have no specific acknowledgements. This research did not receive any specific funding.
References
[1] Australian Commission on Safety and Quality in Health Care (ACSQHC). National model clinical governance framework. Sydney: ACSQHC; 2017.[2] Australian Commission on Safety and Quality in Health Care (ACSQHC). National safety and quality health service standards. 2nd edn. Sydney: ACSQHC; 2017.
[3] Wilcox N, McNeil JJ. Clinical quality registries have the potential to drive improvements in the appropriateness of care. Med J Aust 2016; 205 S21–6.
| Clinical quality registries have the potential to drive improvements in the appropriateness of care.Crossref | GoogleScholarGoogle Scholar |
[4] Hoque DME, Kumari V, Hoque M, Ruseckaite R, Romero L, Evans S. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS One 2017; 12 e0183667
| Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review.Crossref | GoogleScholarGoogle Scholar | 29228050PubMed |
[5] Evans SM, Scott IA, Johnson NP, Cameron PA, McNeil JJ. Development of clinical-quality registries in Australia: the way forward. Med J Aust 2011; 194 360–3.
| Development of clinical-quality registries in Australia: the way forward.Crossref | GoogleScholarGoogle Scholar | 21470087PubMed |
[6] Ovretveit J, Nelwon E, James B. Building a learning health system using clinical registers: a non-technical introduction. J Health Organ Manag 2016; 30 1105–18.
| Building a learning health system using clinical registers: a non-technical introduction.Crossref | GoogleScholarGoogle Scholar | 27700477PubMed |
[7] Lefkovits J, Brennan A, Dinh D, Driscoll A, Stub D, Carruthers H, Doyle J, Tytler K, Reid C, on behalf of the VCOR. Annual public report 2017. Victorian Cardiac Outcomes Registry. 2018. Available at: http://www.vcor.org.au/sites/default/files/VCOR%20Annual%20Report%202017.pdf [verified 10 May 2019].
[8] Bariatric Surgery Registry. Bariatric surgery registry 2017/18 report. 2018. Available at: https://www.monash.edu/__data/assets/pdf_file/0003/1481943/Bariatric-Surgery-Registry-2018_FINAL.pdf [verified 10 May 2019].
[9] Asrar Ul Haq M, Tsay IM, Dinh DT, Brennan A, Clark D, Cox N, Harper R, Nadurata V, Andrianopoulos N, Reid C, Duffy SJ, Lefkovits J, van Gaal WJ. Prevalence and outcomes of trans-radial access for percutaneous coronary intervention in contemporary practise. Int J Cardiol 2016; 221 264–8.
| Prevalence and outcomes of trans-radial access for percutaneous coronary intervention in contemporary practise.Crossref | GoogleScholarGoogle Scholar | 27404687PubMed |
[10] Bridgewater B, Hickey G L, Cooper G, Deanfield J. Publishing cardiac surgery mortality rates: lessons for other specialties. BMJ 2013; 346 f1139
| Publishing cardiac surgery mortality rates: lessons for other specialties.Crossref | GoogleScholarGoogle Scholar | 23449673PubMed |
[11] Australian Commission on Safety and Quality in Health Care Care (ACSQHC). Operating principles and technical standards for clinical quality registries. Sydney: ACSQHC; 2008.
[12] Australian Commission on Safety and Quality in Health Care Care (ACSQHC). Framework for Australian clinical quality registries. Sydney: ACSQHC; 2014.
[13] Australian Commission on Safety and Quality in Health Care Care (ACSQHC). Prioritised list of clinical domains for clinical quality registry development: final report. Sydney: ACSQHC; 2016.
[14] Victorian Government. Targeting zero – supporting the Victorian hospital system to eliminate avoidable harm and strengthen quality of care. Report of the Review of Hospital Safety and Quality Assurance in Victoria. Melbourne: Victorian Government; 2016.
[15] Eldh AC, Wallin L, Fredriksson M, Vengberg S, Winblad U, Halford C, Dahlstrom T. Factors facilitating a national quality registry to aid clinical quality improvement: findings of a national survey. BMJ Open 2016; 6 e011562
| Factors facilitating a national quality registry to aid clinical quality improvement: findings of a national survey.Crossref | GoogleScholarGoogle Scholar | 28128099PubMed |
[16] Eldh AC, Fredriksson M, Vengberg S, Halford C, Wallin L, Dahlstrom T, Winblad U. Depicting the interplay between organisational tiers in the use of a national quality registry to develop quality of care in Sweden. BMC Health Serv Res 2015; 15 519
| Depicting the interplay between organisational tiers in the use of a national quality registry to develop quality of care in Sweden.Crossref | GoogleScholarGoogle Scholar | 26607344PubMed |
[17] Hoque DME, Ruseckaite R, Lorgelly P, McNeil JJ, Evans SM. Cross-sectional study of characteristics of clinical registries in Australia: a resource for clinicians and policy makers. Int J Qual Health Care 2018; 30 192–9.
| Cross-sectional study of characteristics of clinical registries in Australia: a resource for clinicians and policy makers.Crossref | GoogleScholarGoogle Scholar |
[18] Dwyer A, McNeil J. Are clinical registries actually used? The level of medical staff participation in clinical registries and reporting within a major tertiary teaching hospital. Asia Pacific Journal of Health Management 2016; 11 56–64.
[19] Alfred Health. Annual report 2017–18. Melbourne: Alfred Health; 2018. Available at: https://www.alfredhealth.org.au/contents/resources/corporate-publications/Annual-Report/Alfred-Health-Annual-Report-2017-2018.pdf [verified 15 May 2019].



