Do disasters predict international pharmacy legislation?
Kaitlyn E. Watson A B C , Judith A. Singleton A B , Vivienne Tippett A B and Lisa M. Nissen A B
A B C , Judith A. Singleton A B , Vivienne Tippett A B and Lisa M. Nissen A B
A School of Clinical Sciences, Queensland University of Technology, Brisbane, Qld 4000, Australia. Email: judith.singleton@qut.edu.au; vivienne.tippett@qut.edu.au; l.nissen@qut.edu.au
B Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, Qld 4059, Australia.
C Corresponding author. Email: kewatson@ualberta.ca
Australian Health Review 44(3) 392-398 https://doi.org/10.1071/AH19093
Submitted: 18 April 2019 Accepted: 4 September 2019 Published: 5 December 2019
Journal Compilation © AHHA 2020 Open Access CC BY-NC-ND
Abstract
Objective The aim of this study was to explore whether a relationship exists between the number of disasters a jurisdiction has experienced and the presence of disaster-specific pharmacy legislation.
Methods Pharmacy legislation specific to disasters was reviewed for five countries: Australia, Canada, UK, US and New Zealand. A binary logistic regression test using a generalised estimating equation was used to examine the association between the number of disasters experienced by a state, province, territory or country and whether they had disaster-specific pharmacy legislation.
Results Three of six models were statistically significant, suggesting that the odds of a jurisdiction having disaster-specific pharmacy legislation increased as the number of disasters increased for the period 2007–17 and 2013–17. There was an association between the everyday emergency supply legislation and the presence of the extended disaster-specific emergency supply legislation 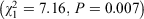 .
.
Conclusions It is evident from this review that there are inconsistencies as to the level of assistance pharmacists can provide during times of crisis depending on their jurisdiction and location of practice. It is not a question of whether pharmacists have the skills and capabilities to assist, but rather what legislative barriers are preventing them from being able to contribute further to the disaster healthcare team.
What is known about the topic? The contributing factors to disaster-specific pharmacy legislation has not previously been explored in Australia. It can be postulated that the number of disasters experienced by a jurisdiction increases the likelihood of governments introducing disaster-specific pharmacy legislation based on other countries.
What does this paper add? This study compared five countries and their pharmacy legislation specific to disasters. It identified that as the number of disasters increases, the odds of a jurisdiction having disaster-specific emergency supply or disaster relocation or mobile pharmacy legislation increases. However, this is likely to be only one of many factors affecting the political decisions of when and what legislation is passed in relation to pharmacists’ roles in disasters.
What are the implications for practitioners? Pharmacists are well situated in the community to be of assistance during disasters. However, their ability to help patients with chronic disease management or providing necessary vaccinations in disasters is limited by the legislation in their jurisdiction. Releasing pharmacists’ full potential in disasters could alleviate the burden of low-acuity patients on other healthcare services. This could subsequently free up other healthcare professionals to treat high-acuity patients and emergencies.
Introduction
Health care during disasters is often provided by hospitals and emergency services. However, access to these healthcare services can become overwhelmed or compromised, and hospitals can be overcrowded. The most common adverse health outcome as a result of a disaster is shifting from acute injuries to chronic disease exacerbations. In a disaster, disaster-affected individuals can often become displaced from their medications, prescriptions or their regular pharmacy.1 Other healthcare services may already be overburdened, affecting their capacity to handle the increase in medical needs from disaster-affected individuals. Pharmacists are regarded as the most easily accessible healthcare professional and are the third largest healthcare provider after doctors and nurses.2–7 Pharmacists working in primary care settings have the potential to reduce pressure on the healthcare system during disasters. Pharmacies are well situated in a community, being more accessible than supermarkets, banks or medical centres.8 Pharmacists provide essential access to medications and medical advice on a daily basis, as well as in times of crisis.
There are two possible scenarios in which pharmacy services or medication supply can be affected during a disaster. The pharmacy infrastructure may be affected directly, requiring a pharmacy to temporarily close and relocate to provide pharmacy services. The other situation is where a pharmacy itself may be operational, but the community is significantly affected by the disaster and requires additional pharmaceutical needs and services. These additional needs and services could include treatment for minor ailments, first aid, referral to other healthcare professionals and emergency supplies of chronic disease medications due to patients’ inability to access prescriptions, medications or funds to cover new prescriptions. If a pharmacy premise becomes temporarily non-operational during a disaster, this does not prevent the pharmacists employed at that pharmacy from assisting their community. Pharmacists can continue to provide services by relocating to temporary premises, supplying services in an evacuation centre or by operating mobile pharmacies. Pharmacies have been identified as one of the fastest community healthcare services to re-establish operations after a disaster.9 Following Hurricane Sandy in the US, 80% of the pharmacies in five severely affected counties were operational with pharmacists providing healthcare services within 1 week of the disaster.9
To ensure pharmacists are able to continue to provide these healthcare services during times of disasters and declared states of emergency, support is essential from pharmacy legislation. There are currently three areas of legislation that affect pharmacists’ ability to assist their community during a declared state of emergency or disaster: (1) disaster-specific emergency supply or refill; (2) disaster-specific vaccination; and (3) temporary relocation or mobile pharmacies. These three areas often overlap during a disaster and a pharmacist can undertake these additional roles once the legislation has been temporarily enacted. Only the legislation covering the temporary relocation of pharmacy premises refers to the ‘bricks and mortar’ of the pharmacy building; the other pieces of legislation covering disaster-specific emergency supply, disaster-specific vaccinations and operating mobile pharmacies refer to a pharmacist’s skill set and can be enacted in other contexts, such as evacuation centres.
Emergency supply legislation
Some countries allow pharmacists to assist in short-term emergencies by using a 3-day emergency supply rule to ensure the continuity of medication supply.10,11 This rule enables pharmacists to supply medications at their discretion to individuals who do not have a valid prescription and when the pharmacist is unable to contact the prescriber for authorisation in circumstances where not supplying a medication could lead to patient harm.10 Under this rule, in everyday individual emergencies, pharmacists are able to provide a patient with a 3-day supply of their regular ongoing medication or a single dosing unit of devices or products such as insulin pens, inhalers or creams.10,11 A 3-day supply was initially introduced because this number of days covered a patient over weekends and public holidays and allowed them time to arrange a physician’s appointment for a new prescription.12,13 A clinical audit in England found patients most often accessing community pharmacies for an emergency supply were elderly patients requiring refills of their long-term chronic disease medications.12 Allowing pharmacists to provide emergency supplies was found to reduce the burden on other areas of the healthcare system, including after-hours general practitioners and hospitals.12
To address population-wide disruptions, a state of emergency or disaster can be declared by a government. Due to the significant community service disruptions that occur during a disaster, the 3-day emergency supply rule is not generally adequate because it can take community services longer than 3 days to return to operational.11 Some states in the US have recognised this and have adopted emergency supply legislation specific to state-declared disasters.13 This legislation gives pharmacists the authority to provide a longer emergency supply to patients, with some states allowing up to 30 days supply.13,14 This could alleviate some of the health care burden during disasters from lower-acuity patients crowding tertiary hospitals and emergency departments requiring refills of their chronic disease medications, freeing up doctors’ and nurses’ time to treat the disaster emergencies. Following Hurricane Katrina in Alabama in the US, when the hurricane was labelled a state-declared disaster, pharmacists had the authority to provide evacuees increased quantities (30 days) of emergency medication supplies to help alleviate the burden on the healthcare system.15,16 In 2014, a review found more than 50% of US states did not have this disaster-specific emergency supply legislation in place.13
Vaccination legislation
The second area of pharmacy legislation reviewed regarding disasters was vaccinations. In many countries, pharmacists are able to administer vaccinations as part of their daily practice, thereby increasing the community’s access to the service. It has been reported that pharmacy-led vaccination services increase vaccination rates, especially in those who have previously not been vaccinated.17–19 In a state-declared emergency or disaster, vaccinations may be required for affected patients (e.g. tetanus, measles, pertussis and influenza vaccinations). In a pandemic or bioterrorism event, prophylactic or treatment vaccinations may be required for mass populations (e.g. pandemic influenza vaccine or antidote). In a simulated hospital bioterrorism event using annual flu vaccinations, the number of people able to be vaccinated within 48 h increased significantly once pharmacists were added to the point-of-dispensing team as vaccinators.20 The expected number of vaccinations to sufficiently cover the target population within 48 h of the simulated bioterrorism threat could not be reached until pharmacists were added to these point-of-dispensing teams as vaccinators, increasing the available healthcare resources.20
The ability of a pharmacist to contribute in disasters by providing vaccinations to the public varies substantially depending on the jurisdiction in which the pharmacist is registered to practise. There is a large difference between the limitations applying to vaccinations performed by Australian pharmacists and their counterparts in Canada or the US, who are able to administer intramuscular vaccines, subcutaneous vaccines and vaccinations to children.21–23 In most Australian states, pharmacists are allowed to vaccinate adults (depending on the state or territory) and only a limited number of intramuscular vaccines depending on the specific state legislation (e.g. pertussis, influenza and the measles, mumps and rubella vaccines).21,22 At the time of writing, Western Australia had just allowed pharmacists to vaccinate children as young as 10 years of age.24 In Canada, depending on the province, pharmacists can administer intramuscular and subcutaneous vaccinations.23 In the US, some states allow for any vaccine to be administered by pharmacists, and some states allow pharmacists to vaccinate children as young as 3 years of age.
Currently the legislation is ambiguous on the translation of a pharmacist’s ability to vaccinate in a disaster setting. This raises the question as to whether the legislation that allows a pharmacist to administer a seasonal influenza vaccine extends to allow pharmacists to administer a specific influenza vaccine during a pandemic.25
Temporary relocation legislation
The third area of pharmacy legislation reviewed was the ability for pharmacies to temporarily relocate or operate mobile pharmacies during a declared state of emergency or disaster. In a disaster, a pharmacy’s premises may be damaged and not be safe for operations. However, this does not mean the pharmacist and pharmacy staff cannot assist their communities. There are two legislation options that can be enacted during a declared state of emergency or disaster for pharmacists to continue operating their pharmacy: (1) take mobile pharmacies into a disaster zone operating under the licence of an existing premises; or (2) suspend their licence and temporarily relocate their premises to a new facility (usually for no longer than 6 months). It depends on the country as to which legislative option is preferred. In Australia, pharmacies are approved by both state and federal government legislation. They are able to apply for temporary relocation to continue providing services until their original premises are operational again under Federal government National Health Act 1953. In the US, pharmacies typically operate mobile pharmacies from their existing premises into disaster zones.26
Recent studies have found that the disaster health community is accepting of pharmacists undertaking more clinical roles in disasters, but a significant barrier of legislation was identified.27 Most of the published research to date exploring disaster-specific pharmacy legislation has been conducted in the US. There is currently no research in Australia reviewing pharmacy legislation relevant to disasters or literature that compares the disaster-specific pharmacy legislation across multiple countries. This review was conducted to determine where advances in utilising pharmacists’ full scope of practice in a disaster have occurred and which countries lag in preparing their pharmacy workforce for disasters in terms of legislative power. In addition, no literature has been published on the potential relationship between the number of disasters a jurisdiction has previously experienced and their level of preparedness in terms of pharmacy legislation (i.e. if a state in a country experiences more disasters than another state, are they more likely to have disaster-specific pharmacy legislation?).
The aim of this study was to update and expand previous research on current disaster-specific pharmacy legislation. The first research objective was to compare pharmacy legislation in five countries, namely Australia, Canada, UK, New Zealand (NZ) and the US. The second research objective was to investigate whether there was a relationship between the number of disasters a state, territory, province or country has experienced in the past 5 and 10 years and the presence of disaster-specific pharmacy legislation.
Methods
Context
Pharmacy legislation is regulated by governments within every country. However, the level of government at which the legislation is regulated differs depending on the country. Only Western countries were included in the present disaster pharmacy legislation review because their legislation was obtainable online (see Table S1, available as Supplementary Material to this paper) and written in English. Australia, UK, NZ and Canada all have similar healthcare systems for easy comparisons of pharmacy services. The US was included because it is one of the leading countries in disaster-specific pharmacy legislation. Table 1 outlines the countries involved in the present disaster pharmacy legislation review and the level of government at which the pharmacy legislation is regulated.

|
Data collection
The legal documents pertaining to pharmacy were reviewed in May 2018 for 51 states of the US (including the District of Columbia), 13 provinces and territories of Canada, eight states and territories of Australia, the UK and NZ. Four specific pieces of pharmacy legislation were reviewed within each of the legislative documents: (1) everyday emergency supply rule (commonly known as the ‘3-day supply’ rule); (2) disaster-specific emergency supply rule (quantity >3 days); (3) disaster-specific vaccination rule; and (4) temporary relocation or mobile pharmacy rule.
The number of disasters for each state, territory, province and country was collected for the periods 2007–17 (10 years) and 2013–17 (5 years). The 10-year period was originally chosen as the reference to account for the fluctuations in partisan attention, spanning multiple political terms in each country and the length of time it takes to get legislation passed in the different parliamentary systems within the countries of interest. As a comparison, data for the more recent 5-year period (2013–17) was also collected to see whether there was a difference in a single political cycle (or two depending on the country) and the increased uptake of pharmacists into public health roles including disasters in the past few years.
The disaster data for the UK and NZ were obtained from the Centre for Research on the Epidemiology of Disasters (CRED) Emergency Events Database (EM-DAT; http://www.emdat.be/disaster_trends/index.html, accessed 28 May 2018). However, the CRED EM-DAT database could not be used for comparisons across all the countries because it does not provide data for disasters at the state level. Therefore, those countries with state-based legislation required a different disaster database source. The Canadian government’s Canadian Disaster Database (http://cdd.publicsafety.gc.ca/srchpg-eng.aspx?dynamic=false, accessed 28 May 2018) was used to obtain the number of disasters experienced by each province and territory for the two time periods of interest. Information on US state-declared disasters was obtained from a US database provided by the Federal Emergency Management Agency (FEMA) website (https://www.fema.gov/disasters, accessed 28 May 2018). The Australian Institute for Disaster Resilience Knowledge Hub DisasterMapper (https://knowledge.aidr.org.au/disasters/, accessed 28 May 2018) was used to determine the number of disasters experienced by each state and territory in Australia for the time periods of interest. Because there is no universal definition of a disaster, each database uses a slightly different definition.28–31 The major difference in the disaster definitions used was that the Australian and Canadian databases provided a disaster definition similar to that of the CRED database used for the UK and NZ. Whereas, the FEMA database only recorded major declared states of emergencies or disasters and did not provide a specific disaster definition. This involved a government representative or governor declaring the local area a state of emergency or disaster. Each jurisdiction included an all-hazard approach to its disaster definition. Disaster-specific pharmacy legislation often uses the terminology ‘declared state of emergency’ to describe emergencies and disasters that have significantly disrupted a community or region.
Data analysis
The data obtained from the pharmacy legislation documents and disaster databases were entered into the IBM SPSS Statistics software version 25 (IBM Corp., Armonk, NY, USA). Pearson Chi-squared tests of independence were performed to determine whether there was a relationship between the four individual pharmacy legislations reviewed, specifically the relationship between the disaster-specific emergency supply rule and the other pieces of disaster pharmacy legislation. Where the expected cell count was below five, Fisher’s exact test was used.
A binary logistic regression test using a generalised estimating equation (GEE) was used to test the association between the number of disasters experienced by a jurisdiction and whether they had disaster-specific emergency supply legislation, vaccination legislation or temporary relocation and mobile pharmacy legislation. To account for possible within-variable correlation due to the different levels of government that regulate pharmacy legislation in a country, a GEE model was used to cluster data for the states, provinces and territories within countries. These GEE models were simulated in IBM SPSS Statistics software version 25 for the disaster variables ‘10 years’ and ‘5 years’, producing six different models.
Results
International legislation comparison
There were 74 data points. Table 2 depicts the frequency of disaster-specific pharmacy legislation within each of the country profiles.
The everyday ‘3-day emergency supply’ rule was found in 74.3% (55/74) of pharmacy legislations from all countries. However, this was extended to an increased quantity to cover state-declared disasters only in 41.9% (31/74) of cases. Disaster-specific vaccination rules were found in 15.1% (11/73) of legislations. The disaster-specific vaccination legislation had to clearly state it was applicable for disasters or be generic and overarching so as to not restrict pharmacists practising in disasters. Disaster pharmacy temporary relocation or mobile pharmacy legislation was found in 23% (17/74) of legislations.
There was a relationship between the everyday emergency supply legislation and the presence of the extended disaster-specific emergency supply legislation 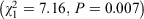 . This is not surprising because most jurisdictions with disaster-specific legislation have worded their legislation to be an extension of the existing 3-day emergency supply rule. There was also a relationship between the presence of disaster-specific emergency supply legislation and disaster-specific vaccination legislation (Fisher’s exact test, P = 0.04). There was no relationship between disaster-specific emergency supply legislation and the relocation or mobile pharmacies legislation
. This is not surprising because most jurisdictions with disaster-specific legislation have worded their legislation to be an extension of the existing 3-day emergency supply rule. There was also a relationship between the presence of disaster-specific emergency supply legislation and disaster-specific vaccination legislation (Fisher’s exact test, P = 0.04). There was no relationship between disaster-specific emergency supply legislation and the relocation or mobile pharmacies legislation 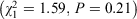 .
.
Effects of disasters on pharmacy legislation
Each disaster-specific pharmacy legislation was tested for an association with the number of disasters experienced by the jurisdictions over a 5- (2013–17) and 10-year (2007–17) period using a GEE binary logistics regression (Table 3). There were six models in total.
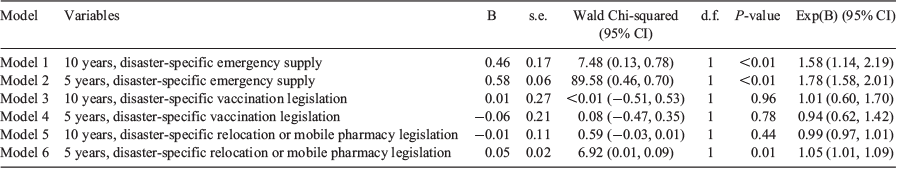
|
Models 1 and 2 propose there was a significant association between disaster-specific emergency supply (quantity >3 days) in both the 5- and 10-year periods. Model 1 suggests the odds of a country with a higher number of disasters in the 10-year period is 1.58-fold more likely to have disaster specific emergency supply legislation (odds ratio (OR) 1.58; 95% confidence interval (CI) 1.14–2.19; P < 0.01). Model 2 proposes that, based on the more recent 5-year period, these odds increase to 1.78-fold more likely (OR 1.78; 95% CI 1.58–2.01; P < 0.01). Model 6 predicts there is a significant association between the number of disasters a jurisdiction has experienced in the more recent 5-year period (2013–17) and the presence of disaster-specific pharmacy relocation or mobile pharmacy legislation. This advocates that as the number of disasters increases, the odds of having disaster-specific pharmacy relocation or mobile pharmacy legislation increases 1.05-fold (95% CI 1.01–1.09; P = 0.01).
Discussion
Before this study, disaster pharmacy disaster legislation had only been reviewed in the US. In 2014, <50% of US states had developed disaster-specific emergency supply legislation.13 In the present study, 4 years later, this is still the case, with 66.67% of US states still not having disaster-specific legislation. Ford et al.14 performed a content analysis on the uptake by US State Board of Pharmacies of pharmacy emergency preparedness and response guidelines into legislation as was suggested by the US National Association of Boards of Pharmacy.32 The two most common guidelines adopted by states were: (1) allowing out-of-state pharmacists and pharmacy personnel to practise in the affected state during the disaster; and (2) if affected by a disaster, the pharmacy should have a reporting procedure to the board.14 A survey of the uptake of these US National Association of Boards of Pharmacy guidelines by US State Board of Pharmacies was conducted in 2014.33 Of the 18 boards surveyed, 16 allowed for the temporary establishment of mobile pharmacies and nine allowed for emergency refill supplies to be dispensed more than once by a pharmacist.33
The present study compared five countries and their pharmacy legislation specific to disasters. It identified that as the number of disasters increases, the odds of a state, province, territory or country having disaster-specific emergency supply or disaster relocation or mobile pharmacy legislation increase. This provides a possible explanation for the variation in disaster-specific pharmacy legislation across the different jurisdictions. However, this is likely to be only one of many factors affecting the political decisions of when and what legislation is passed in relation to pharmacists’ roles in disasters.
Pharmacists need to be aware of the different legislation supporting their roles in disasters, which can differ significantly depending on the location of their practice. Supportive legislation (e.g. emergency supply, vaccination and relocation or mobile pharmacies) has the ability to empower pharmacists in disasters to better serve disaster-affected communities and increases the overall healthcare resources available. Hurricane Katrina highlighted the impact pharmacists could have in reducing the burden on the healthcare system, emergency departments and evacuation centres by providing continuity of medication care through increased quantity of emergency supplies.15,16,34,35 The Anthrax crisis in 2001 in the US illustrates how dissemination of prophylactic medications to the general public required pharmacists at the different stages of the triage process.36,37
Limitations
This research project was limited to the five countries included in the analysis because their legislation was available publicly online and was written in the English language. Although only Western countries were included in this study, this allows for easy comparison because these countries have similar pharmacy services and pharmacist roles in disasters. Another limitation of the present study was that a single disaster database could not be used due to the varying levels of government that regulate pharmacy legislation. Having a single disaster database (like CRED EM-DAT; http://www.emdat.be/disaster_trends/index.html, accessed 28 May 2018) would have reduced the variability in disaster definitions used by each database. However, due to the need for state-level disaster information, this was not feasible. There were only three areas of legislation that were explored within this study; further research needs to explore the barriers and enablers of other legislation regarding pharmacists working in disasters (i.e. compulsory licensing of essential medicines, emergency supplies of controlled drugs).
Conclusion
It is evident from this review of international disaster pharmacy legislation that there are inconsistencies as to the level of assistance pharmacists can provide during times of crisis depending on their jurisdiction and location of practice. It is not a question of whether pharmacists have the skills and capabilities to assist, but rather what legislative barriers are preventing them from contributing further to the disaster healthcare team.
Competing interests
The authors declare no competing interests.
Acknowledgements
The authors acknowledge the assistance and support of the Research Methods Group at Queensland University of Technology. This research did not receive any specific funding.
References
[1] Ochi S, Hodgson S, Landeg O, Mayner L, Murray V. Disaster-driven evacuation and medication loss: a systematic literature review. PLoS Curr 2014; 1| Disaster-driven evacuation and medication loss: a systematic literature review.Crossref | GoogleScholarGoogle Scholar |
[2] Patwardhan A, Duncan I, Murphy P, Pegus C. The value of pharmacists in health care. Popul Health Manag 2012; 15 157–62.
| The value of pharmacists in health care.Crossref | GoogleScholarGoogle Scholar | 22313438PubMed |
[3] Paolini N, Rouse MJ. Scope of contemporary pharmacy practice: roles, responsibilities, and functions of pharmacists and pharmacy technicians – executive summary. Am J Health Syst Pharm 2010; 67 1030–1.
| Scope of contemporary pharmacy practice: roles, responsibilities, and functions of pharmacists and pharmacy technicians – executive summary.Crossref | GoogleScholarGoogle Scholar | 20516475PubMed |
[4] Lai E, Trac L, Lovett A. Expanding the pharmacist’s role in public health. Univers J Public Health 2013; 1 79–85.
[5] Mossialos E, Naci H, Courtin E. Expanding the role of community pharmacists: policymaking in the absence of policy-relevant evidence? Health Policy 2013; 111 135–48.
| Expanding the role of community pharmacists: policymaking in the absence of policy-relevant evidence?Crossref | GoogleScholarGoogle Scholar | 23706523PubMed |
[6] Cooksey JA, Knapp KK, Walton SM, Cultice JM. Challenges to the pharmacist profession from escalating pharmaceutical demand. Health Aff (Millwood) 2002; 21 182–8.
| Challenges to the pharmacist profession from escalating pharmaceutical demand.Crossref | GoogleScholarGoogle Scholar | 12224881PubMed |
[7] International Pharmaceutical Federation (FIP). FIP global pharmacy workforce report. The Hague: International Pharmaceutical Federation; 2009.
[8] The Pharmacy Guild of Australia. Submission in response to the competition policy review draft report. Barton, ACT: National Secretariat; 2014.
[9] Mullen E. The essential role of pharmacists in disasters. eInsight Magazine 11 August 2014. Available at: http://www.webcitation.org/76KqdD62T [verified 9 May 2018].
[10] Department of Health, Government of Western Australia. Emergency supply of medicines. Available at: https://ww2.health.wa.gov.au/Articles/A_E/Emergency-supply-of-medicines [verified 2 April 2019].
[11] Pharmaceutical Guild of Australia. Medicine access in emergencies. 2019. Available at: https://www.guild.org.au/news-events/news/forefront/v09n03/medicine-access-in-emergencies [verified 5 April 2019].
[12] Morecroft CW, Mackridge AJ, Stokes EC, Gray NJ, Wilson SE, Ashcroft DM, Mensah N, Pickup GB. Emergency supply of prescription-only medicines to patients by community pharmacists: a mixed methods evaluation incorporating patient, pharmacist and GP perspectives. BMJ Open 2015; 5 e006934
| Emergency supply of prescription-only medicines to patients by community pharmacists: a mixed methods evaluation incorporating patient, pharmacist and GP perspectives.Crossref | GoogleScholarGoogle Scholar | 26163029PubMed |
[13] Kim J. A review of state emergency prescription refill protocols. Healthcare Ready Blog 5 November 2014. Available at: https://www.healthcareready.org/blog/state-emergency-refills [verified 22 June 2017].
[14] Ford H, Trent S, Wickizer S. An assessment of state board of pharmacy legal documents for public health emergency preparedness. Am J Pharm Educ 2016; 80 20
| An assessment of state board of pharmacy legal documents for public health emergency preparedness.Crossref | GoogleScholarGoogle Scholar | 27073273PubMed |
[15] Traynor K. Pharmacy, public health intersect in Alabama disaster plans. Am J Health Syst Pharm 2007; 64 1998–9.
| Pharmacy, public health intersect in Alabama disaster plans.Crossref | GoogleScholarGoogle Scholar | 17893407PubMed |
[16] Ford JH. Pharmacists in disasters. PhD thesis, University of Georgia, Athens; 2013.
[17] Warner JG, Portlock J, Smith J, Rutter P. Increasing seasonal influenza vaccination uptake using community pharmacies: experience from the Isle of Wight, England. Int J Pharm Pract 2013; 21 362–7.
| Increasing seasonal influenza vaccination uptake using community pharmacies: experience from the Isle of Wight, England.Crossref | GoogleScholarGoogle Scholar | 23581450PubMed |
[18] Anderson C, Thornley T. Who uses pharmacy for flu vaccinations? Population profiling through a UK pharmacy chain. Int J Clin Pharm 2016; 38 218–22.
| Who uses pharmacy for flu vaccinations? Population profiling through a UK pharmacy chain.Crossref | GoogleScholarGoogle Scholar | 26821372PubMed |
[19] Nissen L, Glass B, Lau E, Rosenthal M. Queensland pharmacist immunisation pilot. Phase 1. Pharmacist vaccination – influenza. Final report. 2015. Available at: https://core.ac.uk/download/pdf/33505826.pdf [verified 6 November 2019].
[20] Veltri KT, Yaghdjian V, Morgan-Joseph T, Prlesi L, Rudnick E. Hospital emergency preparedness: push-POD operation and pharmacists as immunizers. J Am Pharm Assoc (2003) 2012; 52 81–5.
| Hospital emergency preparedness: push-POD operation and pharmacists as immunizers.Crossref | GoogleScholarGoogle Scholar |
[21] Hope D, Yeates G, King M. Vaccinations: fragmented federation: inconsistent interstate requirements for pharmacist vaccine administration. Australas J Pharm 2016; 97 18–22.
[22] Hoey J. Making a point: pharmacist immunisation in 2018. 2018. Available at: https://www.australianpharmacist.com.au/making-a-point/ [verified 23 March 2018].
[23] Canadian Pharmacists Association. Pharmacists’ expanded scope of practice in Canada. 2016. Available at: https://www.pharmacists.ca/pharmacy-in-canada/scope-of-practice-canada/ [verified 7 August 2018].
[24] Haggan M. WA pharmacists to vaccinate kids. AJP News 19 June 2019. Available at: https://ajp.com.au/news/wa-pharmacists-to-vaccinate-kids/ [verified 23 August 2019].
[25] McCourt E. Improving pharmacist involvement in pandemic influenza planning and response in Australia. Issues Brief 25. Brisbane: Deeble Institute of Health Policy Research, Australian Healthcare and Hospitals Association (AHHA); 2018. Available at: https://apo.org.au/sites/default/files/resource-files/2018/03/apo-nid136141-1180126.pdf [verified 6 November 2019].
[26] California State Board of Pharmacy. 4062. Furnishing dangerous drugs during emergency; mobile pharmacy. In: 2019 Lawbook for Pharmacy (The Pharmacy Law Business and Professions Code 4000 et seq.). 2019. Available at: https://www.pharmacy.ca.gov/laws_regs/lawbook.pdf [verified 6 November 2019].
[27] Watson KE, Tippett V, Singleton JA, Nissen LM. Disaster health management: do pharmacists fit in the team? Prehosp Disaster Med 2019; 34 30–7.
| Disaster health management: do pharmacists fit in the team?Crossref | GoogleScholarGoogle Scholar |
[28] Centre for Research on the Epidemiology of Disasters. EM-DAT glossary. 2009. Available at: https://www.emdat.be/Glossary#letter_d [verified 29 May 2019].
[29] Australian Institute for Disaster Resilience. Australian disaster resilience glossary – disasters. 2019. Available at: https://knowledge.aidr.org.au/glossary/?wordOfTheDayId=&keywords=&alpha=D&page=3&results=50&order=AZ [verified 29 May 2019].
[30] Government of Canada. The Canadian disaster database. 2018. Available at: https://www.publicsafety.gc.ca/cnt/rsrcs/cndn-dsstr-dtbs/index-en.aspx [verified 29 May 2019].
[31] Federal Emergency Management Agency. The disaster declaration process. 2018. Available at: https://www.fema.gov/disaster-declaration-process [verified 29 May 2019].
[32] National Association of Boards of Pharmacy (NABP). Emergency and disaster preparedness and response planning: a guide for boards of pharmacy. Mount Prospect, IL: NABP; 2006.
[33] Lowe R. Roles and regulations for pharmacists in state-level disaster relief efforts [Thesis]. Oxford: The University of Mississippi; 2014.
[34] Currier M, King DS, Wofford MR, Daniel BJ. A Katrina experience: lessons learned. Am J Med 2006; 119 986–92.
| A Katrina experience: lessons learned.Crossref | GoogleScholarGoogle Scholar | 17071168PubMed |
[35] Hogue MD, Hogue HB, Lander RD, Avent K, Fleenor M. The nontraditional role of pharmacists after Hurricane Katrina: process description and lessons learned. Public Health Rep 2009; 124 217–23.
| The nontraditional role of pharmacists after Hurricane Katrina: process description and lessons learned.Crossref | GoogleScholarGoogle Scholar | 19320363PubMed |
[36] Montello MJ, Ostroff C, Frank EC, Haffer AST, Rogers JR. 2001 anthrax crisis in Washington, DC: pharmacists’ role in screening patients and selecting prophylaxis. Am J Health Syst Pharm 2002; 59 1193–9.
| 2001 anthrax crisis in Washington, DC: pharmacists’ role in screening patients and selecting prophylaxis.Crossref | GoogleScholarGoogle Scholar | 12073861PubMed |
[37] Partridge R, Alexander J, Lawrence T, Suner S. Medical counterbioterrorism: the response to provide anthrax prophylaxis to New York city US postal service employees. Ann Emerg Med 2003; 41 441–6.
| Medical counterbioterrorism: the response to provide anthrax prophylaxis to New York city US postal service employees.Crossref | GoogleScholarGoogle Scholar | 12658240PubMed |



