Do people with multiple sclerosis receive appropriate support from the National Disability Insurance Scheme matching their level of disability? A description of disease ‘burden and societal cost in people with multiple sclerosis in Australia’ (BAC-MS)
Jeannette Lechner-Scott A B D , Penny Reeves B , Karen Ribbons B , Bente Saugbjerg B and Rodney Lea B C
A B D , Penny Reeves B , Karen Ribbons B , Bente Saugbjerg B and Rodney Lea B C
A John Hunter Hospital, Department of Neurology, New Lambton Heights, NSW 2305, Australia.
B Hunter Medical Research Institute, University of Newcastle, New Lambton Heights, NSW 2305, Australia. Email: penny.reeves@hmri.org.au; karen.ribbons@newcastle.edu.au; Bente.Saugbjerg@health.nsw.gov.au; Rodney.a.lea@gmail.com
C Queensland University of Technology, Brisbane, Qld 4000, Australia.
D Corresponding author. Email: jeannette.lechner-scott@health.nsw.gov.au
Australian Health Review 45(6) 745-752 https://doi.org/10.1071/AH21056
Submitted: 19 February 2021 Accepted: 24 May 2021 Published: 21 September 2021
Journal Compilation © AHHA 2021 Open Access CC BY-NC-ND
Abstract
Objective This study is the first to assess if the National Disability Insurance Scheme (NDIS) package allocated to people with multiple sclerosis (pwMS) is correlated with the disability level measured by standardised neurological assessment.
Methods We aimed to recruit 10 pwMS per expanded disability status score (EDSS) step, including EDSS 0 (no disability) up to 9 (bedridden), and requested information about their NDIS application. Value of their packages was compared with mobility, cognition and psychological impact.
Results Out of 186 pwMS, only 49% of all patients had an NDIS package approved. The mean values of the annual allowance were AU$30 318 for patients with mild disability, AU$38 361 for moderate disability and AU$115 113 for severe disability. There was a striking variability in packages approved, but restricted mobility seems to be the driving factor. Rejection rates were <20% in patients with mild and moderate disability and none in those with severe disability. The package value correlated with EDSS steps, cognitive impairment and physical impact, but not psychological impact.
Conclusions This is the first study to assess if NDIS packages correlate with internationally accepted disability scales. The NDIS support was correlated with disability measured by EDSS steps and cognition, but not psychological impact of the disease.
What is known about the topic? There are over 25 000 Australians living with multiple sclerosis, which is one of the most common neurological diseases leading to disability in early age. The National Disability Insurance Scheme has been introduced since 2013 to particularly assist young disabled Australians to participate in the community. Whether the approved package correlates with internationally accepted disability scores has not yet been assessed.
What does this paper add? This study is the first to correlate disability, as assessed by the Expanded Disability Severity Scale (EDSS), with the approved package value.
What are the implications for practitioners? Multiple sclerosis is a very variable disease affecting quality of life not only due to impairment of mobility, but also cognition and mental health. Although the NDIS package value was correlated with an EDSS and cognition, the psychological impact of the disease is often neglected.
Introduction
There are 4.3 million Australians living with permanent disability.1 The National Disability Insurance Scheme (NDIS) was created to financially support Australians aged <65 years with intellectual, physical, sensory, cognitive and psychosocial disability. The purpose of the NDIS is to support eligible participants to achieve their goals, including increased independence, greater social participation and employment opportunities.2 In 2013, a staged roll out commenced to replace the old support services with a national approach. We selected the region that was chosen as one of the first trial areas to study the success of the scheme. In 2015, a citizen jury assessed the viability of the scheme, but no objective evaluation has been performed to determine if the funds are being disbursed according to formally assessed disability.3 In 2018–19, the NDIS budget was AU$11.9 billion. During this period, 300 000 people with disabilities received support under this scheme.4 Only one-third were new recipients of support services, whereas two-thirds had previously received funding provided by the states and territories.
Neurological disorders are now the leading cause of disability globally, with MS affecting young adults in particular.5 The total NDIS expenditure for MS has accumulated to AU$378 million annually, or an average plan of ~AU$90 000 per patient.4 The incidence and prevalence of MS are rising in most developed countries, but this varies according to latitude.6 In the Hunter region, Australia, where we recruited for this study, the prevalence has more than doubled in the past 15 years and is now estimated to be 124/100 000.7 Despite advances in treatment, the excess mortality of patients with MS has not changed over the last 50 years.8 In highly disabled patients, mortality is eight-fold higher than in the general population.9 Life expectancy, in contrast, is only reduced by ~7 years,9,10 which often translates into decades of dependency. Although the societal impact of MS in Australia has been reported,11 these estimates omit the NDIS-specific costs. Furthermore, these reports generally split patients into mild, moderate or severe categories, but do not associate the cost with each EDSS steps.
NDIS support is divided into three pillars: core support; capital costs; and capacity building.1 Core support is meant to enable patients to participate in work and communities. Core support can fund activities of daily living (showering, dressing, grooming, meal time assistance, toileting) and independent activities of daily living (shopping, transport, etc.). The second pillar, capital costs, are investments in home modification and assistance technology. The third pillar, capacity building, supports coordination, assists with improved life choices (diet, exercise programs), as well as activities to find a job or social interactions.2 Many participants apply online and then they have a face-to-face meeting with a Local Area Coordinator to assess/review their plan. This takes place in an interview format, with the focus on the participant stating what their goals are and what supports they need to achieve their goals.
Neurologists monitor disease progress with standardised disability assessments for people with MS according to the Expanded Disability Status Scale (EDSS), which indicates disability steps ranging from 0 (no disability or signs) to 10 (death due to MS). The EDSS was introduced in 1955 by JF Kurtzke as a (1) 10-point scale;12 and (2) enlarged with half steps in 1983.13 The EDSS is the most commonly used outcome measure in therapeutic MS trials14 and, despite its limitations, most patient registries rely on monitoring via the use of this disability measure. EDSS steps are not equally distributed, and participants tend to spend more time around scores of 3 and 3.5 and then at around 6 and 6.5.15 An EDSS of 0 reflects normal neurological examination, but not necessarily no impairment as impaired mood does not change the EDSS. An EDSS of 1 represents findings of which the patient is not aware. A functional score of 2 represents mild disability, a functional score of 3 moderate disability in any given function. A reduced walking distance determines the EDSS from 4 (>500 m) to 6.5 (bilateral assistance required). Scores >6.5 reflect dependence on a wheelchair or other assistance. In order to improve consistency in these assessments, a worldwide training module has been developed.16 Despite the high prevalence of cognitive decline observed in MS patients,17 a formal cognitive assessment is not part of routine EDSS.16
Health technology assessment of new treatment options requires a deeper understanding of the socioeconomic impacts of disability prevention or even disability improvement as measured by the EDSS for each country. Worldwide, there is a clear correlation with cost and disability measured by EDSS,18 but support structures differ from country to country. No study has so far assessed the cost of disability for patients with MS since the introduction of the NDIS for disability. We therefore aimed, in this study, to assess the relationship between EDSS and NDIS package value in a cohort of patients from the Hunter region.
Methods
Patients for this study were recruited from a single centre in a regional MS clinic, where over 1100 patients are prospectively followed and biannually evaluated by the EDSS.
Only patients with a confirmed clinically definite diagnosis of MS as evidenced by multiple lesions on MRI and clinical symptoms and aged at least 18 years were included. If the participants were aged <65 years and had an EDSS level of at least 3.5, they must have previously been assessed for an NDIS package to be included. To ensure stable disease, we excluded participants with a relapse (new symptoms) within 6 months of the assessment date.
The Expanded Disability Status Score (EDSS)19 was performed by a Neurostatus-approved rater. Mild disability was defined as an EDSS of 0–3.5, moderate 4.0–6.0 and 6.5–9 was considered severe disability. The study aimed to recruit 10 patients per EDSS step to cover the breadth of disability with MS (n = 180). Following informed consent, data were collected for disease course, severity and treatment, as well as for quality of life, employment status and productivity, support needs, receipt of services and overall costs. Cognitive assessment was collected within 12 months of the EDSS assessment via an Audio Recorded Cognitive Score (ARCS), which assesses five domains including attention, memory, visual construction, fluency and language.20 When participants were unable to attend the clinic due to disability, EDSS was assessed by phone.21,22 In addition, data on the patient-reported physical and psychological impact of MS was assessed by the Multiple Sclerosis Impact Scale (MSIS-29),23 and patient and carer-reported quality of life and health state utility was rated by the five-level EuroQoL (EQ-5D-5 L) questionnaire24 as well as work productivity and activity impairment, assessed by the Work Productivity and Activity Impairment General Health (WPAI-GH) questionnaire25 and resource utilisation, captured in the modified Client Services Receipt Inventory (CSRI),26 which was collected as part of a larger study, but will be analysed separately and reported on in a separate publication. This study focused on the NDIS resource utilisation, recorded across each of the three package components (i.e. core, capacity building and capital). Copies of the NDIS plan were collected if the participant was eligible for support. NDIS package value was correlated with EDSS, cognitive function, quality of life and employment status.
Statistical analysis was performed using SPSS (SPSS Inc.).
Results
Cohort summary
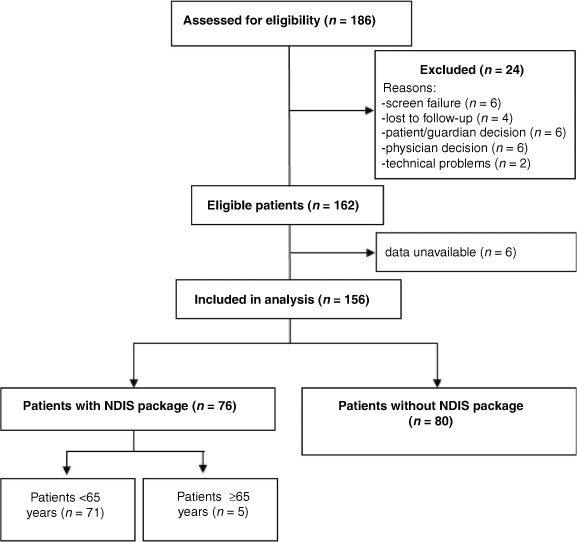
For this study, 186 patients were recruited in 2018–19, 162 patients were eligible for analysis and 156 participants were included in this analysis; two patients awaited outcome of their application and four did not provide details of their package (Fig. 1 and Table 1). The mean age of the cohort was 51.4 years (s.d. = 13.6), and 117 (75%) were female. The mean disease duration was 12.5 years (s.d. = 9.5) and 112 patients (72%) were on disease-modifying therapies (DMTs). The MS disease course distribution at the time of recruitment was: 94 (60%) with relapsing remitting, 45 (29%) with secondary progressive and 17 (11%) with primary progressive disease. Of the cohort, 68 (44%) patients had mild disability, 40 (26%) moderate and 48 (30%) severe disability according to EDSS. In total, 76 patients (49%) were receiving an NDIS package and 126 (81%) were aged <65 years (Table 1).
|

|
The disability profile of the selected cohort was comparable to the total patient registry of the clinic.23 Compared with the total international MS Base cohort, more advanced-stage MS patients are seen in this clinic.23 This was of benefit for selecting patients with higher EDSS steps, although we had difficulties recruiting participants in the EDSS categories 3.5, 5.0 and, in particular, with severe disability 9.0 (Fig. 2).
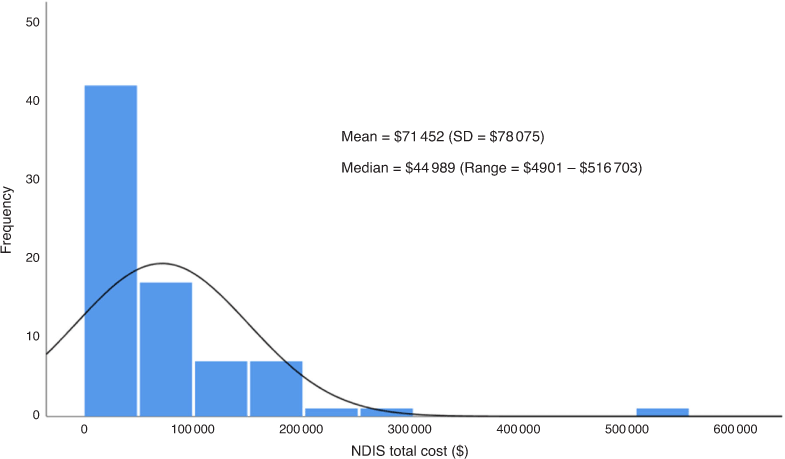
Of the 156 patients, only 49% were currently on a NDIS plan. Twenty-two patients were aged >65 years and might have received other support than from NDIS. Thirty-two patients had an EDSS of less than two, which is identified as less than mild disability, but some of this group still had a plan supporting exercise programs and household support. The range in package value of packages was highly skewed, with one package valued at AU$516 703 (an extreme outlier). This resulted in quite a substantial difference between mean (AU$71 452) and median (AU$44 989) package value (Fig. 3).
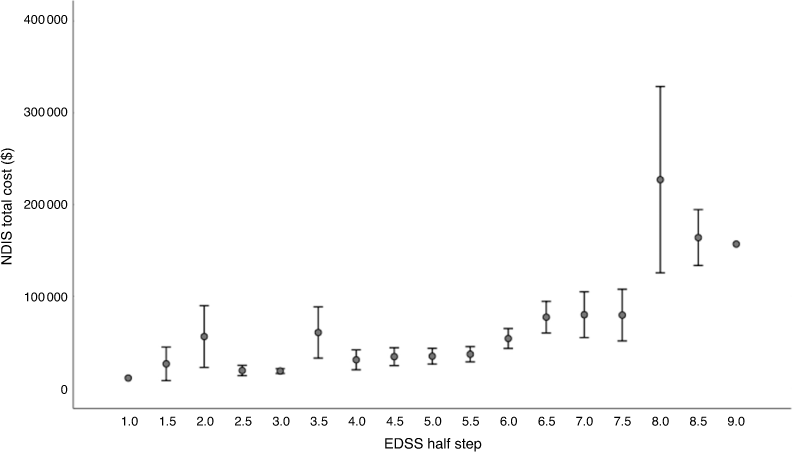
The mean values of the NDIS package were AU$34 224 for patients with mild disability, AU$40 342 for moderate disability and AU$114 585 for severe disability (Fig. 4). The total NDIS package value for the severe disability group was statistically significantly higher than the two less severe groups (P < 0.001). When plotting the NDIS package value per EDSS step, it is apparent that mobility influences the packages. For an EDSS of below four, there is great variability in the NDIS packages, but when a walking aid is required (≥ EDSS of 6.0) the plan value rises (P = 0.0001). When a wheelchair is required and assistance to transfer (≥ EDSS of 8) is needed, another significant increase can be observed (P < 0.0001) (Fig. 5).
Of the group that were aged <65 years, seven patients were rejected for a NDIS plan, including two patients with mild disability and five with moderate disability according to EDSS; none of the patients with severe disability had their application rejected (Table 1).
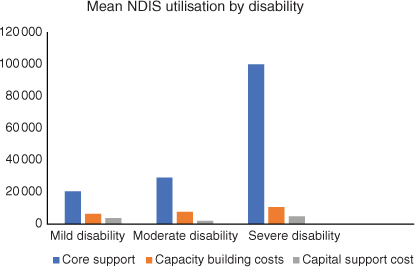
The largest part of the NDIS package was spent on core support. For milder disability, this support was used for house cleaning or gardening, or in the higher disability category, this support was used for self-care, transport, etc. Capacity-building costs were similar in all three categories; only capital costs, which are usually used for equipment like wheelchairs, are higher for the severely disabled patients, but not significantly so (Fig. 6).
|
Having received an NDIS package was associated with a higher EDSS, longer disease duration, higher physical and psychological impact of the disease, as well as lower cognitive function and quality of life (P < 0.05). The increased mean age of the group receiving a NDIS package was not statistically significant (P = 0.1) (Table 2).
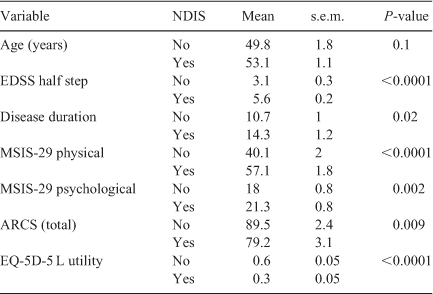
|
The total value of the NDIS package correlated moderately with EDSS (r = 0.51) and physical impact (r = 0.47) and quality of life (r = –0.58), but not with age, disease duration or psychological impact. Cognition as measured by the ARCS was also moderately correlated with the size of the package, in particular memory (r = –0.41), attention (r = –0.26) and speed of writing (r = –0.41). All correlations were statistically significant (P < 0.05).
The rate of PwMS not employed out of the total cohort (aged <65 years) was 56%. There was a significantly higher unemployment rate for the NDIS group compared with the non-NDIS group (73% vs 35%, P < 0.0001), but also, the mean NDIS package value was higher for the unemployed group: unemployed (n = 52) = AU$88 439 vs employed (n = 19) = AU$33 881 (P = 0.0003).
The NDIS sets out to increase work participation for people with disabilities. Therefore, we attempted to address this in the small subgroup of patients on NDIS packages who were employed. Employment was negatively correlated with age, EDSS and duration of disease (P < 0.05). When we adjusted for NDIS, the strong correlation with EDSS became statistically non-significant.
Discussion
This is the first study to report on the value of NDIS packages for people with MS in Australia. Here, we assess the correlation of NDIS package value with formally assessed disability (EDSS) as used in clinical trial outcomes. In our study, participants were selected by EDSS in order to have a wide range of disability rather than the bimodal distribution typical for this scale.27 Importantly, we were successful in including patients with higher EDSS, three of which were in residential age care facilities.
Not surprisingly, the NDIS package value was significantly larger in the severe compared with the mild disability group. The total value of the package was moderately correlated with the EDSS step as well as cognitive assessments, although the support varied significantly. Core support costs for mildly disabled patients varied from AU$5000 to AU$72 000. One patient with severe disability received a package of more than AU$500 000. This might be because of varying individual functional deficits or surrounding support structures, but it might also reflect that there are no disease-specific guidelines given to the NDIS assessor. The likelihood of being accepted for a package was surprisingly higher in the mild than the moderate disability group, which might reflect better tenacity in the mildly impaired group than a different range of deficits. None of the patients with high disability were rejected, but the value of the package varied widely.
Unfortunately, the majority of patients who had a NDIS package NDIS were unemployed. But the fact that the strong correlation of employment with EDSS becomes non-significant after adjusting for NDIS package value suggests that the support that NDIS offers does change the disparity caused by disability. This analysis is, of course, limited by the low numbers. Already at moderate EDSS (3.5), only 50% of patients are employed, which is similar to the level in other countries.28 Larger and prospective studies are required to determine conclusively whether NDIS improves work-related engagement.
The EDSS is not a perfect reflection of MS disability, especially in the range of EDSS 4.0 to 7.5 where it is very much dependent on mobility. In addition, cognitive dysfunction is not sufficiently accounted for by EDSS and does warrant formal testing. It is reassuring that cognition seems to have been taken into account as much as physical disability when evaluating patients for a support package. In contrast, the psychological impact, as measured by MSIS, does not seem to influence the value of support. This is despite up to 60% of people with MS suffering from anxiety and depression, which has been shown to also have an impact on their cognitive function.29 Interestingly, the majority of NDIS-supported patients are not employed, although one of the stated aims of NDIS is to increase the work participation of disabled citizens. Also, the support employed MS patients were receiving is significantly lower. Unfortunately, our study was not longitudinally designed to investigate whether receiving support actually does improve work participation and quality of life.
One major limitation of this study is that only patients from a single centre were included and so generalisation of findings to a national level is not possible. The location was chosen for the fact that this region has been a trial centre for NDIS. One would expect that the majority of patients would have at least applied for funding, but more than half of the cohort do not have a package and even with moderate disability, 17% of applications were rejected. This regional MS clinic has the advantage in that it includes ~85% of the region’s population with MS.7 If this study can be replicated in other regions, it would strengthen the evidence of the benefit of NDIS with regards to work productivity of MS patients in Australia.
Conclusion
The NDIS provides a vital infrastructure for people with multiple sclerosis, although this study has not prospectively assessed the benefit of this support. Support provided by NDIS correlated with disability as measured by EDSS, and it also correlated with measures of cognitive impairment (ARCS) used at our centre. As cognitive impairment is not captured with standard EDSS measures, it underscores the importance of clinicians assessing cognition with validated tools as this may be incorporated into NDIS eligibility considerations. However, psychological impact of the disease was not associated with a NDIS package. More can be done to improve a more individual disability-related value of packages.
Competing interests
The authors declare no competing interests.
Acknowledgements
This non-interventional study was sponsored and funded by Novartis Pharmaceuticals Australia, Pty Limited. Novartis employee, Mary-Ann Bonney, was involved in the study design and drafted the protocol and clinical study report; and Novartis employee, Patricia Berry, managed the study contracts and development of the electronic clinical report form and study database.
References
[1] Australian Institute of Health and Welfare. People with disability in Australia. 2019. Available at: https://www.aihw.gov.au/ [verified May 2020].[2] Nugent HM. Corporate Plan NDIS. 2018–2022. Available at: https://www.ndis.gov.au/about-us/publications/corporate-plan#past-corporate-plans. [verified April 2021].
[3] People with Disability Australia. Citizen Jury score card. 2018. Available at: https://pwd.org.au/ndis-citizens-jury-scorecard. [verified April 2021].
[4] The National Disability Insurance Agency. Budget NDIS. 2018/2019. Updated September 2019. Available at: https://www.ndis.gov.au/news/3731-national-disability-insurance-agency-statement-ndis-expenditure
[5] Feigin VL, Abajobir AA, Abate KH, et al Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017; 16 877–97.
| Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015.Crossref | GoogleScholarGoogle Scholar |
[6] Simpson S, Wang W, Otahal P, et al Latitude continues to be significantly associated with the prevalence of multiple sclerosis: an updated meta-analysis. J Neurol Neurosurg Psychiatry 2019; 90 1193–200.
| Latitude continues to be significantly associated with the prevalence of multiple sclerosis: an updated meta-analysis.Crossref | GoogleScholarGoogle Scholar | 31217172PubMed |
[7] Ribbons K, Lea R, Tiedeman C, et al Ongoing increase in incidence and prevalence of multiple sclerosis in Newcastle, Australia: A 50-year study. Mult Scler 2017; 23 1063–71.
| Ongoing increase in incidence and prevalence of multiple sclerosis in Newcastle, Australia: A 50-year study.Crossref | GoogleScholarGoogle Scholar | 27682228PubMed |
[8] Manouchehrinia A, Tanasescu R, Tench CR, et al Mortality in multiple sclerosis: meta-analysis of standardised mortality ratios. J Neurol Neurosurg Psychiatry 2016; 87 324–31.
| Mortality in multiple sclerosis: meta-analysis of standardised mortality ratios.Crossref | GoogleScholarGoogle Scholar | 25935887PubMed |
[9] Leray E, Moreau T, Fromont A, Edan G. Epidemiology of multiple sclerosis. Rev Neurol 2016; 172 3–13.
| Epidemiology of multiple sclerosis.Crossref | GoogleScholarGoogle Scholar | 26718593PubMed |
[10] Palmer AJ, van der Mei I, Taylor BV, et al Modelling the impact of multiple sclerosis on life expectancy, quality-adjusted life years and total lifetime costs: Evidence from Australia. Mult Scler 2020; 26 411–20.
| Modelling the impact of multiple sclerosis on life expectancy, quality-adjusted life years and total lifetime costs: Evidence from Australia.Crossref | GoogleScholarGoogle Scholar | 30806569PubMed |
[11] Palmer AJ, Colman S, O’Leary B, Taylor BV, Simmons RD. The economic impact of multiple sclerosis in Australia in 2010. Mult Scler 2013; 19 1640–6.
| The economic impact of multiple sclerosis in Australia in 2010.Crossref | GoogleScholarGoogle Scholar | 23652216PubMed |
[12] Kurtzke JF. A new scale for evaluating disability in multiple sclerosis. Neurology 1955; 5 580–3.
| A new scale for evaluating disability in multiple sclerosis.Crossref | GoogleScholarGoogle Scholar | 13244774PubMed |
[13] Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33 1444–52.
| Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS).Crossref | GoogleScholarGoogle Scholar | 6685237PubMed |
[14] Cohen JA, Reingold SC, Polman CH, Wolinsky JS. Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol 2012; 11 467–76.
| Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects.Crossref | GoogleScholarGoogle Scholar | 22516081PubMed |
[15] Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology 1995; 45 251–5.
| Disease steps in multiple sclerosis: a simple approach to evaluate disease progression.Crossref | GoogleScholarGoogle Scholar | 7854521PubMed |
[16] D’Souza M, Yaldizli O, John R, et al Neurostatus e-Scoring improves consistency of Expanded Disability Status Scale assessments: A proof of concept study. Mult Scler 2017; 23 597–603.
| Neurostatus e-Scoring improves consistency of Expanded Disability Status Scale assessments: A proof of concept study.Crossref | GoogleScholarGoogle Scholar | 27364325PubMed |
[17] Grzegorski T, Losy J. Cognitive impairment in multiple sclerosis – a review of current knowledge and recent research. Rev Neurosci 2017; 28 845–60.
| Cognitive impairment in multiple sclerosis – a review of current knowledge and recent research.Crossref | GoogleScholarGoogle Scholar | 28787275PubMed |
[18] Kobelt G, Eriksson J, Phillips G, Berg J. The burden of multiple sclerosis 2015: Methods of data collection, assessment and analysis of costs, quality of life and symptoms. Mult Scler 2017; 23 4–16.
| The burden of multiple sclerosis 2015: Methods of data collection, assessment and analysis of costs, quality of life and symptoms.Crossref | GoogleScholarGoogle Scholar | 28643592PubMed |
[19] Kappos L, D’Souza M, Lechner-Scott J, Lienert C. On the origin of Neurostatus. Mult Scler Relat Disord 2015; 4 182–5.
| On the origin of Neurostatus.Crossref | GoogleScholarGoogle Scholar | 26008933PubMed |
[20] Lechner-Scott J, Kerr T, Spencer B, et al The Audio Recorded Cognitive Screen (ARCS) in patients with multiple sclerosis: a practical tool for multiple sclerosis clinics. Mult Scler 2010; 16 1126–33.
| The Audio Recorded Cognitive Screen (ARCS) in patients with multiple sclerosis: a practical tool for multiple sclerosis clinics.Crossref | GoogleScholarGoogle Scholar | 20621944PubMed |
[21] Collins CD, Ivry B, Bowen JD, et al A comparative analysis of Patient-Reported Expanded Disability Status Scale tools. Mult Scler 2016; 22 1349–58.
| A comparative analysis of Patient-Reported Expanded Disability Status Scale tools.Crossref | GoogleScholarGoogle Scholar | 26564998PubMed |
[22] Lechner-Scott J, Kappos L, Hofman M, et al Can the Expanded Disability Status Scale be assessed by telephone? Mult Scler 2003; 9 154–9.
| Can the Expanded Disability Status Scale be assessed by telephone?Crossref | GoogleScholarGoogle Scholar | 12708811PubMed |
[23] Hobart J, Lamping D, Fitzpatrick R, et al The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain 2001; 124 962–73.
| The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure.Crossref | GoogleScholarGoogle Scholar | 11335698PubMed |
[24] Herdman M, Gudex C, Lloyd A, et al Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D–5L). Qual Life Res 2011; 20 1727–36.
| Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D–5L).Crossref | GoogleScholarGoogle Scholar | 21479777PubMed |
[25] Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4 353–65.
| The validity and reproducibility of a work productivity and activity impairment instrument.Crossref | GoogleScholarGoogle Scholar | 10146874PubMed |
[26] Feagan BG, Reilly MC, Gerlier L, et al Clinical trial: the effects of certolizumab pegol therapy on work productivity in patients with moderate-to-severe Crohn’s disease in the PRECiSE 2 study. Aliment Pharmacol Ther 2010; 31 1276–85.
| Clinical trial: the effects of certolizumab pegol therapy on work productivity in patients with moderate-to-severe Crohn’s disease in the PRECiSE 2 study.Crossref | GoogleScholarGoogle Scholar | 20298497PubMed |
[27] MSBase. 2020. Available at: https://registry.msbase.org/patient-demographics [verified May 2020].
[28] Flachenecker P, Kobelt G, Berg J, et al New insights into the burden and costs of multiple sclerosis in Europe: Results for Germany. Mult Scler 2017; 23 78–90.
| New insights into the burden and costs of multiple sclerosis in Europe: Results for Germany.Crossref | GoogleScholarGoogle Scholar | 28643593PubMed |
[29] Ribbons K, Lea R, Schofield PW, Lechner-Scott J. Anxiety Levels Are Independently Associated With Cognitive Performance in an Australian Multiple Sclerosis Patient Cohort. J Neuropsychiatry Clin Neurosci 2017; 29 128–34.
| Anxiety Levels Are Independently Associated With Cognitive Performance in an Australian Multiple Sclerosis Patient Cohort.Crossref | GoogleScholarGoogle Scholar | 27899051PubMed |