Hepatitis B immune status of staff in smaller acute healthcare facilities
Alex Hoskins A , Leon James Worth A B , Michael James Malloy A , Katherine Walker A , Ann Bull A and Noleen Bennett A C *A Victorian Healthcare Associated Infection Surveillance System (VICNISS) Coordinating Centre, Doherty Institute for Infection and Immunity, Melbourne, Vic. 3000, Australia.
B Department of Medicine, University of Melbourne, Melbourne, Vic. 3065, Australia.
C Department of Nursing, Melbourne School of Health Sciences, University of Melbourne, Melbourne, Vic. 3065, Australia.
Australian Health Review 47(2) 254-257 https://doi.org/10.1071/AH22219
Submitted: 24 August 2022 Accepted: 9 February 2023 Published: 7 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of AHHA. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Objective To determine the proportion of staff employed in smaller Victorian public acute healthcare facilities with evidence of immunity to hepatitis B.
Methods For optimal long-term immunity, a completed hepatitis B vaccination course and post vaccination hepatitis B surface antibody (anti-HBs) level ≥10 mIU/mL is desirable for all high-risk staff employed in healthcare facilities. For the financial years 2016/17–2019/20, a standardised surveillance module developed by the Victorian Healthcare Associated Infection Surveillance System (VICNISS) Coordinating Centre was completed by the smaller Victorian public acute healthcare facilities (individual hospitals with <100 acute care beds or their multi-site health service). Staff were assessed as having evidence or no evidence of optimal immunity to hepatitis B. Those without optimal evidence were sub-classified as ‘incomplete vaccination course’, ‘no serology’, ‘contraindicated’, ‘non-responder’, ‘declined’ or ‘unknown’. Data were analysed to determine trends over time for healthcare facilities that participated more than once.
Results A total of 88 healthcare facilities reported hepatitis B immunity status of high-risk (Category A) staff (n = 29 920) at least once over 5 years; 55 healthcare facilities reported more than once. The aggregate proportion with evidence of optimal immunity was 66.3%. Healthcare facilities with 100–199 Category A staff employed reported the lowest evidence of optimal immunity (59.6%). Of all Category A staff with no evidence of optimal immunity, the majority had ‘unknown’ status (19.8%), with only 0.6% overall who declined vaccination.
Conclusions Our study found evidence of optimal staff hepatitis B immunity in only two-thirds of Category A staff working in surveyed healthcare facilities.
Keywords: healthcare facilities, healthcare workers, hepatitis B, immunisation, immunity, surveillance, survey, vaccination.
Introduction
Staff employed in healthcare facilities are at risk of exposure to a range of communicable and vaccine preventable diseases (VPD), including hepatitis B.1,2 To ensure the safety of staff and patients, healthcare facilities are required to have risk-based VPD policies and programs in accordance with national recommendations2 and jurisdictional requirements.3 In the state of Victoria, non-mandatory approaches to staff immunisation programs have generally been employed, and the impact of these has not been fully evaluated.
Since 2008, smaller Victorian public acute healthcare facilities (individual hospitals with <100 acute care beds or their multi-site health service) have periodically collected and submitted staff hepatitis B immunity survey data to the Victorian Healthcare Associated Infection Surveillance (VICNISS) Coordinating Centre. Immunisation programs within these healthcare facilities are generally coordinated by local Infection Prevention and Control (IPC) teams.
The objectives of this study were to evaluate the hepatitis B immunity status of staff employed in smaller Victorian public acute healthcare facilities, to determine if immunisation varied according to the number of staff employed, and to explore trends over time for facilities that completed serial surveys during a 5-year period (2016/17–2020/21).
Methods
For the financial years 2016/17 and 2019/20, smaller Victorian public acute healthcare facilities were required to participate in the VICNISS staff hepatitis B immunity module. Exemptions were permitted if in 2016/17 these healthcare facilities were participating in another specified VICNISS project4 or in 2019/20, IPC resources were limited because of the focus on coronavirus disease 2019 (COVID-19) responses. For financial years 2017/18, 2018/19 and 2020/21 participation was optional. Education regarding standardised methods and definitions were provided by the VICNISS Coordinating Centre and all data were submitted via a secure online portal.
Eligible healthcare facilities and staff
The participating healthcare facilities were permitted to submit staff hepatitis B immunity survey data as an individual hospital or if easier to collect data multi-site health service. A health service included all hospitals (acute and sub-acute) and aged care homes within the health serviceas a whole. All permanent, temporary or casual staff employed by the healthcare facility were included. Unpaid staff (e.g. university students, volunteers) and those employed by outside agencies (e.g. agency nurses) were excluded.
Classification of staff
Risk categories were applied based upon the level of contact staff had with patients and their risk of exposure to blood and body fluids:5
Category A: staff who had physical contact with patients or potential exposure to blood or body fluids (e.g. clinical nursing staff);
Category B: staff who had contact with patients but rarely had contact with blood or body substances (e.g. catering staff);
Category C: staff who had minimal patient contact and therefore had no greater risk of exposure to infectious diseases than the general public (e.g. gardening staff).
For optimal long-term immunity to hepatitis B, completion of a nationally recommended vaccination course and a post vaccination serological test showing hepatitis B surface antibody (anti-HBs) level ≥10 mIU/mL is desirable.2 For all Category A staff (required), and Category B and C staff (optional), their hepatitis B immunity status was reported as either:
Evidence of optimal hepatitis B immunity, or
No evidence of optimal hepatitis B immunity:
Incomplete: vaccination course not completed.
No serological testing: vaccination course completed however post vaccination serological testing not performed.
Contraindicated: vaccination contraindicated because documented evidence of anaphylaxis to previous hepatitis B vaccine or a component of the vaccine, or staff member hepatitis B surface antigen positive.3
Non-responder: serological testing anti-HBs level ≥10 mIU/mL not achieved.
Declined: vaccination course and serological testing offered but declined.
Unknown: none of the above criteria documented.
Data analysis
For the current study, survey data submitted between 1 July 2016 and 30 June 2021 were evaluated. All statistical analyses were performed in Stata/SE for Windows (Statacorp LP, College Station, TX, USA).
The overall proportion of staff with or without evidence of optimal hepatitis B immunity reported by participating healthcare facilities was determined. For those facilities that submitted more than one survey during a financial year, only the first survey was evaluated. To enable benchmarking between similarly-sized facilities, facility-level data were peer-grouped using an accepted reporting framework:6 <50, 50–99, 100–199, 200–499 or ≥500 Category A staff.
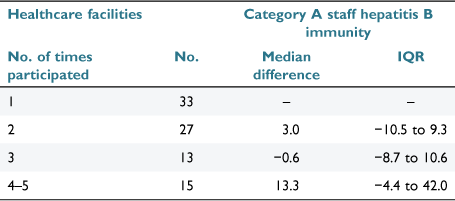
Healthcare facilities which participated in serial surveys were also grouped according to the number of times (1, 2, 3 or 4‒5) hepatitis B immunity data was submitted. With only a small number of facilities submitting data in all 5 years (n = 3), this group was combined with facilities that had participated for 4 years (n = 12). For each group except ‘1’ the median and interquartile range of difference from the first to last submission in Category A staff immunity proportions was calculated.
Ethics
Consistent with NHMRC-defined Quality Assurance activities, no staff-identifying data were collected, and pooled data were captured for purposes of quality improvement within participating healthcare facilities. Ethical approval was therefore not required.7
Results
Over the 5-year surveillance period, 88 healthcare facilities (73 hospitals and 15 healthcare services) submitted survey data regarding staff hepatitis B immunity at least once (Table 1); 55 facilities participated more than once. Of the 191 unique surveys submitted, 115 (60.2%) and 95 (49.7%) included optional Category B and C staff data, respectively. A total of 37 322 staff were represented, 80.2% (n = 29 920) of whom were designated as Category A staff.

|
Nearly two-thirds (62.7%, n = 23 389) of all surveyed staff had evidence of optimal hepatitis B immunity; 66.3% (n = 19 841) Category A staff had evidence of optimal hepatitis B immunity. Of Category A staff, about one-fifth (19.8%; n = 5936) had unknown hepatitis B immune status and 0.6% (n = 185) had declined vaccination or serological testing. In 2020–21 specifically, 14.9 and 0.3% Category A staff had unknown and declined status respectively.
Optimal hepatitis B immunity was highest in healthcare facilities with larger numbers of employed staff. Healthcare facilities with 100–199 Category A staff employed reported the lowest evidence of optimal immunity (59.6%). Incremental increases in Category A staff with evidence of optimal hepatitis B immunity over the 5 years were most evident in the 200–499 staff group, with 88.6% reported during the 2020–21 survey (an increase of 23.3% from 2016/17) (Table 1).
Healthcare facilities which submitted multiple surveys demonstrated modest improvements from the first year to the last year of participation. Those participating at least four times reported a median increase of 13.3% when initial and final surveys were compared (Table 2).
|
Discussion
Victoria is the only Australian jurisdiction with a standardised surveillance framework for monitoring the hepatitis B immunity status of staff employed in healthcare facilities. Our study analysed data reported over a 5-year period and found a relatively low proportion of high-risk (Category A) staff employed in smaller Victorian public acute healthcare facilities to have evidence of optimal hepatitis immunity (67.2%). Previous Australian studies have reported evidence of hepatitis B immunity in 83% of staff8 and 82% hepatitis B immunity for Category A staff.9 Internationally, reported hepatitis B immunity rates among healthcare workers have been variably reported (46.6–91.7%).10,11
Our aggregate findings demonstrated notable variation between some years in the proportion of Category A staff with evidence of optimal hepatitis B immunity. This variation may have been in part because the cohort of participating healthcare facilities was different each year. At the same time however, there was an increase in the aggregate proportion for those healthcare facilities which serially surveyed staff. This is consistent with ongoing use of data to support local quality improvement activities. Many strategies have been introduced by hospitals globally in an attempt to increase staff vaccination uptake. These include ensuring easy and free access to immune assessment and vaccines when required, and education regarding the benefits of vaccination.12
In the state of Victoria, legislation mandating the vaccination of healthcare staff has recently been passed, enabling the Department of Health to direct public acute care hospitals and other health service services to require the persons they employ or engage to be vaccinated against specified diseases.13 Implementation pathways are yet to be defined but it is likely documented follow up of those healthcare facility staff whose immune status is unknown will be required. We identified that approximately one-fifth (19.5%) of Category A staff had an unknown Hepatitis B immunity status. We propose that many of these unreported staff have evidence of optimal hepatitis B immunity, and hence the (true) overall proportion is higher. The low proportion of refusals is consistent with this assumption.
Our study has a number of limitations. First, monitoring was performed only in smaller Victorian public acute healthcare facilities. Looking ahead, the VICNISS staff hepatitis B immunity module is to be made available to all healthcare facilities, including larger public and private acute care hospitals. This will provide data representative of staff more broadly. Second, there is the potential for selection bias in those data submitted by participating healthcare facilities. IPC teams lacking resources to complete surveys may represent a specific cohort of facilities with unique needs for staff vaccination.
In conclusion, we report a modest proportion of staff with evidence of optimal hepatitis B immunity employed by smaller Victorian public acute healthcare facilities. To mitigate the risk of acquiring hepatitis B in the event of an occupational exposure to blood-borne pathogens incident, enhanced immunisation policies and programs are needed to support staff hepatitis B vaccination and serological testing; this includes documented follow up of those staff whose immune status is currently reported as unknown.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding
References
[1] Schillie S, Murphy TV, Sawyer M, Ly K, Hughes E, Jiles R, de Perio MA, Reilly M, Byrd K, Ward JW; Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep 2013; 62 1–19.[2] Australian Technical Advisory Group on Immunisation (ATAGI). Australian Immunisation Handbook. Canberra: Australian Government Department of Health; 2018.
[3] Australian Commission on Safety and Quality in Health Care. National Safety and Quality Health Service Standards. Sydney: ACSQHC; 2021.
[4] Hoskins AJ, Worth LJ, Imam N, Johnson SA, Bull AL, Richards MJ, Bennett NJ. Validation of healthcare-associated infection surveillance in smaller Australian hospitals. J Hosp Infect 2018; 99 85–8.
| Validation of healthcare-associated infection surveillance in smaller Australian hospitals.Crossref | GoogleScholarGoogle Scholar |
[5] Victorian Department of Health and Human Services. Vaccination for healthcare workers. 2014. Available at https://www.health.vic.gov.au/immunisation/vaccination-for-healthcare-workers [cited October 2021].
[6] Johnson SA, Wang D, Bennett N, Bull AL, Richards MJ, Worth LJ. Influenza vaccination of Australian healthcare workers: strategies to achieve high uptake. Aust N Z J Public Health 2017; 41 545–6.
| Influenza vaccination of Australian healthcare workers: strategies to achieve high uptake.Crossref | GoogleScholarGoogle Scholar |
[7] National Health and Medical Research Council. Ethical considerations in quality assurance and evaluation activities. 2014. Available at https://www.nhmrc.gov.au/about-us/resources/ethical-considerations-quality-assurance-and-evaluation-activities [cited 19 April 2018].
[8] Andrew EC, Gibney KB, Denholm J, Leder K. Seroprotection to vaccine-preventable diseases among workers at a Victorian tertiary hospital. Aust N Z J Public Health 2016; 40 284–9.
| Seroprotection to vaccine-preventable diseases among workers at a Victorian tertiary hospital.Crossref | GoogleScholarGoogle Scholar |
[9] Leung V, Harper S, Slavin M, Thursky K, Worth L. Are they protected? Immunity to vaccine-preventable diseases in healthcare workers at an Australian hospital. Aust N Z J Public Health 2014; 38 83–6.
| Are they protected? Immunity to vaccine-preventable diseases in healthcare workers at an Australian hospital.Crossref | GoogleScholarGoogle Scholar |
[10] Batra V, Goswami A, Dadhich S, Kothari D, Bhargava N. Hepatitis B immunization in healthcare workers. Ann Gastroenterol 2015; 28 276–80.
[11] Maltezou HC, Poland GA. Vaccination policies for healthcare workers in Europe. Vaccine 2014; 32 4876–80.
| Vaccination policies for healthcare workers in Europe.Crossref | GoogleScholarGoogle Scholar |
[12] FitzSimons D, Hendrickx G, Lernout T, Badur S, Vorsters A, Van Damme P. Incentives and barriers regarding immunization against influenza and hepatitis of health care workers. Vaccine 2014; 32 4849–54.
| Incentives and barriers regarding immunization against influenza and hepatitis of health care workers.Crossref | GoogleScholarGoogle Scholar |
[13] Health Services Amendment (Mandatory Vaccination of Healthcare Workers) Act 2020. 2020. Available at https://www.legislation.vic.gov.au/as-made/acts/health-services-amendment-mandatory-vaccination-healthcare-workers-act-2020


