Obstacles in establishing a national disease registry in Australia: lessons from the development of the CHAANZ Congenital Heart Disease Registry
Larissa K. Lloyd A B C , Reeja Nasir B C , Calum Nicholson A B C , Geoff Strange A B C and David S. Celermajer A B C *A Clinical Research Group, Heart Research Institute, Sydney, NSW, Australia.
B Cardiology Department, Royal Prince Alfred Hospital, Camperdown, Sydney, NSW 2050, Australia.
C Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia.
Australian Health Review 47(4) 410-417 https://doi.org/10.1071/AH23063
Submitted: 14 March 2023 Accepted: 26 April 2023 Published: 16 May 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of AHHA. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Objectives To provide insights into the obstacles which pose challenges to the set-up of any National Registry in Australia.
Methods An analysis of our experience in executing a Multi-Institutional Agreement (MIA) and obtaining ethics and governance approvals, post-award of a large Medical Research Futures Fund grant in June 2020.
Results From July 2020, our timeline to an executed MIA was 283 days, despite full-time staff working towards this goal. Subsequently, after lead site ethics approval, time to site governance approvals ranged from 9 to 291 days. A total of 214 emails were sent during the MIA development and signing. There were 11–71 emails sent to individual governance offices and the number of requested points of additional information ranged from 0 to 31 queries.
Conclusions There were considerable time delays in executing the initial (pre-research) stages of a National Federal Government funded Registry project which required substantial time and resources. We report a wide variation in requirements between different states and institutions. We propose several strategies which could be implemented to facilitate a more streamlined approach to research ethics and governance. This centralised approach would allow for better use of funding and facilitate better progress in medical research.
Keywords: ethics, governance, multi-institutional agreement, registry, research.
Introduction
Ethics approval is a fundamental first step in undertaking human medical research. The necessity of this review process is proven; however, the ensuing administrative process (‘governance’) can be time consuming and is often inconsistent between sites.
In 2007, the National Health and Medical Research Council (NHMRC) of Australia established the National Approach to Single Ethical Review of Multi-Centre Research.1 This was developed to expedite ethics approval processes in multi-site research projects, allowing one Human Research Ethics Committee (HREC) to provide ethical approval recognised by all participating sites, in Australia. As of late 2020, all Australian State and Territory jurisdictions now participate in National Mutual Acceptance (NMA), having signed a Memorandum of Understanding for the mutual acceptance of ethical and scientific review of multi-centre human research projects undertaken in public health organisations.2 The NMA scheme facilitates acceptance of a single ethics review of multi-centre human research projects, conducted by a NHMRC certified HREC.2
A written research agreement is a requirement of the Australian Code for the Responsible Conduct of Research (2018).3 NHMRC grant-funded research that involves collaboration between two or more sites must have a Multi-Institutional Agreement (MIA) in place, outlining how NHMRC funds will be distributed between the parties, and detailing intellectual property ownership, publication rights, insurance and indemnity obligations.4 Unfortunately, under current rules, projects cannot commence until every party to an MIA has signed and executed the Agreement.
A review of congenital heart disease (CHD) management across Australia outlined the necessity of a National CHD Registry to improve service delivery and to optimise patient outcomes.5 The Congenital Heart Alliance of Australia and New Zealand (CHAANZ) CHD Registry will improve care for CHD patients, capturing data on all patients diagnosed with a congenital heart defect.6 The Registry aims to quantify the lifelong burden of disease for patients.6 In the past 18 months, CHAANZ has developed the framework to govern the 11 major adult and paediatric CHD centres in Australia, from which we wish to gather data for the Registry.
In developing this framework, we have encountered inconsistencies between required documentation, submission processes and application review time. Lengthy administrative hold-ups threatened the timely delivery of this federal government funded research. In this report, we provide insights into the system’s inefficiencies. In particular, systemic problems challenge establishing the necessary MIA and obtaining the mandated approvals from the various nationwide research governance offices, who operate independently of any centralised process. We found that research outcomes are dependent on governance offices with inefficient processes and no clear accountabilities for performance metrics or timelines.
Methods
The ethics approval process commenced with submission of a Human Research Ethics Application (HREA) to a NHMRC certified HREC. Our research proposal had been approved at the time we were notified of our successful Medical Research Future Fund (MRFF) grant application. After obtaining ethics approval, research projects are submitted to research governance offices for site-specific assessment (SSA).
We analysed our experience in executing an MIA involving 12 institutions (11 participating institutions, and one administering institution; the University of Sydney). Once MRFF funding had been awarded in June 2020, the administering institution determined that a MIA was necessary, instead of multiple bilateral agreements between each participating site and the University of Sydney.
1. The MIA process. This began with internal discussions with the administering institution to develop the agreement. Once the agreement had been drafted, the research team then contacted the participating institutions for review. Once all parties had reviewed the first version of the MIA and provided comments, a second version was circulated with the necessary changes. A second review process followed, then a third version was circulated that all sites agreed upon, resulting in the fully executed MIA. Funding cannot be released for external sites to participate in data collection for the project, until every party to the MIA has executed the agreement.
We reviewed all correspondence during MIA development between the funding body and the administering institution, the participating institutions and the administering institution, the research team and the lead HREC, and the research team and governance offices at participating sites. Review time was calculated from the date the MIA was circulated to the date of the institution’s response.
2. The process of obtaining ethics approval from the lead HREC and governance approval from participating sites was also examined. We recorded the requirements of submission and subsequent requests from governance offices. Time to approval was calculated from the date of complete submission to notification of approval. Any party, excluding the coordinating research team, required to review a SSA or agreement and provide a signature of support was recorded. All submitted documents were reviewed, and the communications required for each submission recorded. Any requests for further information were recorded, including the nature of these requests. The 11th site (H11) is not a part of the MIA, and therefore is not included in all analysis.
Results
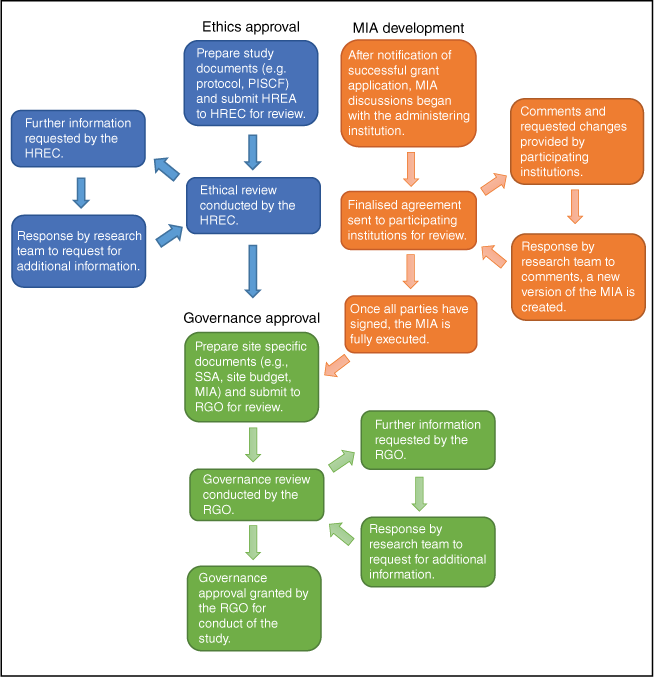
Eleven sites across Australia participated in the CHAANZ CHD Registry, with 10 of these sites party to the MIA outlining how MRFF funding is to be distributed throughout the study. Fig. 1 summarises the general processes involved in obtaining ethics approval, executing the MIA and obtaining governance approval. Most delays occurred in the review cycles, with no limit to how many times these additional requests for information could occur.
An overview of the post-award process of executing a Multi-Institutional Agreement, and obtaining ethical, scientific and governance approval in the CHAANZ Registry project. HREA, Human Research Ethics Application; HREC, Human Research Ethics Committee; MIA, Multi-Institutional Agreement; PISCF: participant information and consent form; RGO, research governance office; SSA, site-specific assessment.
A total of 11 governance applications were completed. At the time of notification of successful MRFF application, three sites had already received governance approval. Three of the seven remaining governance approvals were approved within 2 weeks of the MIA being executed, and a fourth approved within 2 months (Fig. 2).
Timeline in executing a Multi-Institutional Agreement and obtaining ethics and site governance approval for the CHAANZ Congenital Heart Disease Registry. AIR, additional information request; H, hospital; HREC, Human Research Ethics Committee; MIA, Multi-Institutional Agreement; MRFF, Medical Research Future Fund.
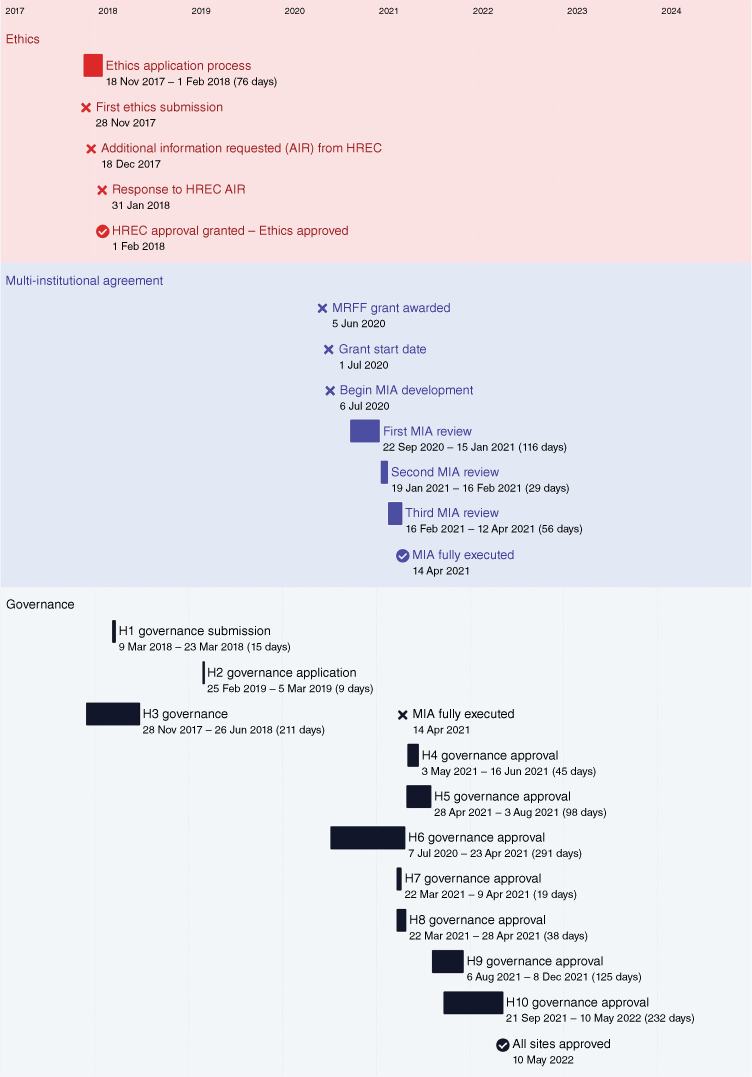
Table 1 outlines the various documentation required with submission, as well as the number of authorisations required, and communications with the relevant parties.
| Process | Type of application | Number of sites | Documents | Authorisation required | Communication |
|---|---|---|---|---|---|
| MIA | – | 10 | 1 | 12 signatures (authorised signatory from each party) | 214 emails, 2 resubmissions |
| Ethics approval | HREA | 1 | 10 documents | 52 emails, 1 resubmission | |
| Governance approval | SSA – overall | 11 | 226 documents | 3–8 for each SSA | 411 emails |
| SSA (NSW) | 2 | 13 documents | 3–5 for each SSA (site PI, site AI, heads of supporting departments, information manager, RGO) | 23 emails | |
| SSA (VIC) | 2 | 40 documents | 3–5 for each SSA (site PI, site AIs, heads of supporting departments, RGO) | 69 emails | |
| SSA (QLD) | 2 | 47 documents | 4–8 for each site (site PI, site AIs, head of supporting departments, site contact, divisional director, director of health and innovation, site research finance, RGO) | 74 emails, 1 resubmission | |
| SSA (SA) | 2 | 57 documents | 5–7 for each site (site PI, site AIs, head of supporting departments, site research finance, chief operating officer, RGO) | 102 emails | |
| SSA (WA) | 2 | 69 documents | 5 for each site (coordinating PI, site PI, CEO of CHAANZ, head of supporting departments, data custodian, RGO) | 142 emails, 2 meetings, 6 resubmissions |
AI, associate investigator; CEO, chief executive officer; CHAANZ, Congenital Heart Alliance of Australia and New Zealand; HREA, Human Research Ethics Application; HREC, Human Research Ethics Committee; NSW, New South Wales; PI, principal investigator; QLD, Queensland; RGO, research governance office; SA, South Australia; SSA, site-specific assessment; VIC, Victoria; WA, Western Australia. Where documents were required to be submitted with clean and tracked versions, this has only been counted as one document. Where edits were made to documents and resubmitted, new versions of the document were not counted as an additional document.
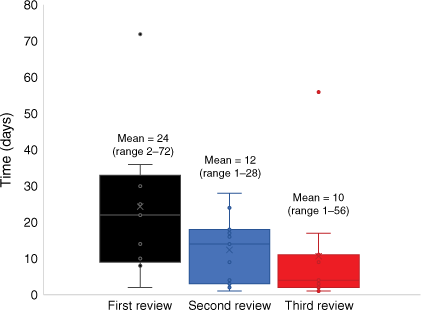
The execution of the MIA was an iterative process. From July 2020, our timeline to an executed MIA was 283 days, with lead ethics already approved. A total of 214 emails were sent during the MIA development and signing (Table 1). Review times varied between institutions, with the first review of the MIA ranging from 2 to 72 days, with the mean review time of 24 days. The second and third review times were generally shorter, with ranges of 1–28 days (mean = 12 days), and 1–56 days (mean = 10 days), respectively (Fig. 3).
Variation in review time of the Multi-Institutional Agreement (MIA) across the 11 participating institutions. Duration of MIA review time (in days).
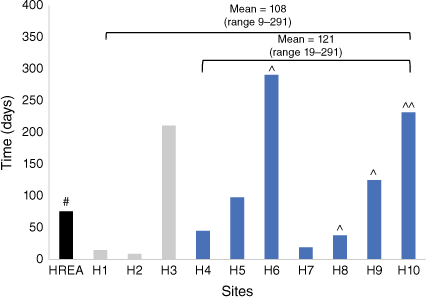
Governance approval times varied substantially between sites, with overall time from submission to approval ranging from 9 to 291 days. The median approval time was 108 days. Governance approval times did not notably improve after execution of the MIA, ranging from 19 to 291 days, with a median of 98 days (Fig. 4).
Duration of review of HREA and SSAs across the 10 sites participating in the Multi-Institutional Agreement. Duration of application review (in days). Grey bars represent sites with governance approval pre-award. Blue bars represent sites with governance approval post-award. #Denotes additional information request from the lead HREC. ^Sites where an additional information request was made by the governance office. ^^Sites where multiple additional information requests were made by the governance office.
Emails sent to governance offices ranged from 11 to 76, with the number of requested points of additional information ranging from 0 to 31 per site (Table 2). Authorisation required, not including the research team, ranged from 3 to 8 signatures, per site. Documents uploaded with submissions varied substantially between jurisdictions, with both New South Wales sites requiring 13 documents in total, while other states ranged from 40 to 69 (Table 1). No governance office at any site indicated an expected/mandated timeline for responses.
Discussion
For Australia to be competitive in this important research area of multi-institutional projects, and to optimise use of research expenditure, a centralised and streamlined approach to multi-institutional projects, ethics and governance is required.
The overall timelines for obtaining ethical and governance review for the CHAANZ CHD Registry project, as outlined in Fig. 1 and Table 1, were lengthy. Resources are therefore diverted from data collection to initial administrative tasks.
Execution of an MIA requires all participating parties to provide review and sign the required legal documentation. This means that this process can only occur as fast as the slowest respondent. Furthermore, even after the MIA was fully executed, there were many additional requests for information, as outlined in Table 2. For example, the requirement to sign subsequent data agreements led to quite extensive delays. Sites that required subsequent data agreements had an average of 187 days to approval compared to 34 days to approval at sites without these requirements.
Although the lead HREC had provided approval for a waiver of consent for retrospective data collection, one governance office required submission of further documentation regarding this, requesting statements from the lead HREC specifying it was granted under a specific section of the National Statement. Site specific requests such as this, questioning the decisions of the lead HREC, caused numerous time delays and increased administrative burden on the lead HREC.
For sites using the same ethics management system as the lead HREC – Research Ethics Governance Information System (REGIS), less documentation was required for submission as governance officers could access associated forms already approved by the lead HREC in REGIS (Table 1). Sites using other ethics management sites (such as Ethics Review Manager in Victoria and Queensland, Research Governance Service in Western Australia, Governance and Ethics Management System in South Australia), created a far greater administrative burden on research staff; resulting in substantial duplication in the documentation submitted (Table 1). Learning new management systems was often time and labour intensive, at times requiring further correspondence with governance offices. Considerable time was also spent interpreting the specific document requirements of each institution and developing the documents to meet them. Another major concern was the evident lack of performance goals or metrics.
Our documented experience demonstrates that the processes involved in achieving multi-site ethics and governance approval for nationally projects is in urgent need of reform. Many of our experiences are consistent with those reported by other research groups.7–11 We echo the suggestions of these groups, including a recommendation of clarification from the NHMRC on its national statement guidelines on ethical conduct to minimise subjective interpretation by HRECs,8 and development of governance guidelines similar to the National Statement.12
Streamlining the number of documents required and reducing variation in the requirements of these forms between jurisdictions could be achieved by having a standardised set of documents required with all SSAs.13 Clay-Williams et al. suggested a framework involving a single ethics and governance review process accepted by all Australian states.14 A centralised ethics and governance review framework such as this has been implemented in the United Kingdom since 2016, requiring the submission of only one application.15
We propose the implementation of a national body to expedite MIA and governance approvals. The effect that a few outlier institutions had on the timeline of our project was substantial and the only available course of action was to send further follow-up communications that had negligible influence on expediting review times. Our suggestions for improving ethics and governance for multi-centre human research projects, and their rationale are outlined in Table 3.
| Suggestion | Rationale |
|---|---|
| A national body to expedite MIAs and governance approvals. Taking that responsibility from the administering institution and allocating it to the Department of Health or NHMRC, for example. | To accelerate this process. Ensure customer accountability from research governance offices, which is currently lacking. |
| MIA system rule change to allow projects to commence at any site once that site had agreed to the MIA. | Reduce delays as study team is not required to wait for every site to execute the agreement. |
| Include clauses surrounding data transfer and other issues that are often found in template bilateral agreements provided by governance offices in MIAs. | Negate delays experienced when subsequent data agreements are requested during governance applications. |
| Clarification from the NHMRC on its national statement guidelines on ethical conduct. | To minimise subjective interpretation by ethics and governance offices that result in additional requests causing time delays and increased administrative burden on the lead HREC and research team. |
| Development of governance guidelines similar to the National Statement. | Limit the scope of local input from governance offices to truly local matters. Introduce reasonable timelines for tasks and responses, and accountabilities by staff at the participating centres, at all stages of the approvals process. |
| Establishing a standardised set of documents required with all SSAs. | Streamline the number of documents required and reduce variation in the requirements of these forms between jurisdictions. |
| Introduction of a centralised ethical review framework with local research governance approval. | Reduce time and labour costs in obtaining ethical approval for research. |
| Policy initiatives to harmonise interstate differences in legislation. | Increased synchronicity between jurisdictions. |
| The use of one ethics management system across Australia. A central portal for all applications and fulfilling monitoring requirements. | Reduced duplication of documentation required between ethics and governance applications. Reduced risk of inadvertent errors in meeting, monitoring and reporting requirements. |
In summary, this paper demonstrates an inefficient system which lacks key accountabilities. The result of this is a high administrative burden and delays to project commencement. Repeated publications highlighting this have not resulted in systems change. The inefficient practices documented here and by others highlight the need for more centralised systems in place to manage research. By streamlining the processes involved, researchers can redirect their focus and funding to achieving their research aims.
Data availability
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Declaration of funding
The development of a comprehensive ANZ CHD Registry, and the diagnosis coding solutions described, was initially funded by philanthropic donations from HeartKids Australia and the Kinghorn Foundation. Additional funding has been provided by an Australian Department of Health grant through the Medical Research Future Fund, (grant code ARGCHDG0000028).
References
1 National Health and Medical Research Council. National Statement on Ethical Conduct in Human Research (2007) Updated 2018. Canberra: NHMRC; 2007. Available at https://www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2007-updated-2018#toc__2102 [accessed May 2022].
2 NSW Government Department of Health. National Mutual Acceptance. Sydney: NSW Health; 2021. Available at https://www.medicalresearch.nsw.gov.au/national-mutual-acceptance/ [accessed May 2022].
3 National Health and Medical Research Council. Australian Code for the Responsible Conduct of Research. Canberra: NHMRC; 2018. Available at https://www.nhmrc.gov.au/research-policy/ethics/national-certification-scheme-ethics-review-multi-centre-research [accessed May 2022].
4 Victorian Comprehensive Cancer Centre. Types of Agreements: Clinical Trial Research Agreements. Melbourne: VCCC Alliance; 2022. Available at https://vcccalliance.org.au/our-work/research-and-translation/clinical-trial-innovations/investigator-initiated-trials/clinical-trial-agreements/types-of-agreements/# [accessed May 2022].
6 Celermajer D, Strange G, Cordina R, et al. Congenital Heart Disease Requires a Lifetime Continuum of Care: A Call for a Regional Registry. Heart Lung Circ 2016; 25(8): 750-4.
| Crossref | Google Scholar |
7 Foot H, Scott IA, Russell GM, et al. Ethics and site-specific governance approvals for multi-centre, inter-sector health care research. Med J Aust 2018; 209(4): 175-6.
| Crossref | Google Scholar |
8 De Smit E, Kearns LS, Clarke L, et al. Heterogeneity of Human Research Ethics Committees and Research Governance Offices across Australia: An observational study. Australas Med J 2016; 9(2): 33-9.
| Crossref | Google Scholar |
9 Duplancic C, Crough T, Bell SC, Australian Non-tuberculous Mycobacteria in Cystic Fibrosis Study Group . Multi-centre ethics and research governance review can impede non-interventional clinical research. Intern Med J 2019; 49(6): 722-8.
| Crossref | Google Scholar |
10 Buck K, Nolte L, Kelly H, et al. Challenges in obtaining research ethics and governance approvals for an Australian national intersector, multisite audit study. Aust Health Rev 2020; 44(5): 799-805.
| Crossref | Google Scholar |
11 Barnett AG, Campbell MJ, Shield C, et al. The high costs of getting ethical and site-specific approvals for multi-centre research. Res Integr Peer Rev 2016; 1: 16.
| Crossref | Google Scholar |
12 Vajdic CM, Meagher NS, Hicks SC, et al. Governance approval for multisite, non-interventional research: what can Harmonisation of Multi-Centre Ethical Review learn from the New South Wales experience? Intern Med J 2012; 42(2): 127-31.
| Crossref | Google Scholar |
13 White VM, Bibby H, Green M, et al. Inconsistencies and time delays in site-specific research approvals hinder collaborative clinical research in Australia. Intern Med J 2016; 46(9): 1023-9.
| Crossref | Google Scholar |
14 Clay-Williams R, Taylor N, Braithwaite J. Potential solutions to improve the governance of multicentre health services research. Med J Aust 2018; 208(4): 152-4.
| Crossref | Google Scholar |
15 Health Research Authority. HRA Approval. London: NHS; 2021. https://www.hra.nhs.uk/approvals-amendments/what-approvals-do-i-need/hra-approval/ [accessed July 2022].


