Epicuticular waxes and plant primary metabolites on the surfaces of juvenile Eucalyptus globulus and E. nitens (Myrtaceae) leaves
Martin J. Steinbauer A D , Noel W. Davies B , Cyril Gaertner C and Sylvie Derridj CA Co-operative Research Centre for Sustainable Production Forestry/CSIRO Entomology, Canberra and Department of Zoology, La Trobe University, Melbourne, Victoria 3086, Australia.
B Central Science Laboratory, University of Tasmania, Private Bag 74, Hobart, Tasmania 7001, Australia.
C Institut National de la Recherche Agronomique, Unité 1272, Physiologie de l’Insecte-Signalisation et Communication, F 78 000 Versailles, France.
D Corresponding author. Email: M.Steinbauer@latrobe.edu.au
Australian Journal of Botany 57(6) 474-485 https://doi.org/10.1071/BT09108
Submitted: 19 June 2009 Accepted: 22 September 2009 Published: 9 November 2009
Abstract
Our knowledge of the composition of the waxes on the surfaces of Eucalyptus leaves is growing but that of plant primary metabolites has been completely overlooked. The diffusion of primary metabolites above the cuticle exposes them to a variety of herbivorous taxa and has the potential to influence their responses to that plant. Juvenile leaves of two families of Eucalyptus globulus Labill. ssp. globulus and two families of E. nitens (Deane & Maiden) Maiden had 11 out of 16 of the epicuticular waxes that were detected in common. However, two phenylethyl esters (waxes) were only detected on leaves of one family of E. globulus and two benzyl esters (waxes) were not detected or were uncommon in samples from E. nitens. Wax compounds were generally found in samples from both leaf surfaces but a few were only detected in samples from particular sides. Species and families of eucalypt did not differ significantly in the concentrations of free sugars, polyols, malic acid or γ-aminobutyric acid (GABA) (all plant primary metabolites) collected from the surfaces of leaves. However, concentrations of all these metabolites were usually higher in collections from the upper surfaces of leaves. High wax abundance, especially on the lower surfaces of E. globulus leaves, is suspected to have hindered dissolution of all the primary metabolites quantified. Several free amino acids exhibited significant species-level differences in concentrations, namely the aromatic, amide and sulfur-containing amino acids as well as proline; family-level differences in amino acid concentrations were not significant. Australian and overseas evidence showing that differences in waxes and primary metabolites can be influential in plant susceptibility to herbivorous taxa is considered with respect to the threats posed by the autumn gum moth and Mycosphaerella leaf spot fungi.
Introduction
The products of plant biochemistry are not confined to the tissues beneath the cuticle. Whatever the mechanisms by which these metabolites diffuse from the plant’s tissues (e.g. Schreiber 2006), their exposure and subsequent interception by other taxa catalyse a suite of diverse trophic interactions. Epicuticular waxes are highly apparent and very well studied products of plant metabolism that overlay the cuticle (Jeffree 2006). In addition to their role in the adaptation of plants to harsh environmental conditions (e.g. in Eucalyptus see Lamberton 1964; Hallam and Chambers 1970; Carr et al. 1985; Wirthensohn and Sedgley 1996), they have been shown to mediate several interactions with insects and plant pathogens (Leveau 2006; Müller 2006). Less appreciated is the occurrence of plant primary metabolites on the surfaces of leaves. Quantification and documentation of the occurrence of primary metabolites on the surfaces of plant leaves has been led by researchers concerned with the interactions of plants with herbivorous insects (Derridj et al. 1989, 1996a, 1996b; Fiala et al. 1990, 1993; Lombarkia and Derridj 2002, 2008; Maher et al. 2006) and plant pathogens (Ruan et al. 1995; Mercier and Lindow 2000; Marcell and Beattie 2002). It is probably because entomologists and plant pathologists need to understand why some plants are more susceptible than others to particular taxa, but also because cuticular permeability is not yet well understood, that researchers in these disciplines lead advances in understanding the role of surface properties in species interactions more than do botanists and plant physiologists (Müller and Riederer 2005).
Although host specialist insects may obtain an adequate diet from all plants of a preferred species, there are likely to be significant advantages (in terms of survival and growth) to being able to detect those that are nutritionally superior to neighbouring conspecifics. Consequently, many insects have the ability to assess plants for the composition of their primary metabolites. Typically, sugars act as phagostimulants, while the influence of amino acids is more variable, but includes action as phagostimulants (Chapman 2003; Schoonhoven et al. 2005). Primary metabolites can also influence oviposition behaviour (Derridj et al. 1989; Lombarkia and Derridj 2008). Lombarkia and Derridj (2008) found that variations in the proportional representation of fructose, sorbitol and myo-inositol could explain differences in oviposition by Cydia pomonella (L.) (Lepidoptera: Tortricidae) on resistant and susceptible apple cultivars. Epicuticular waxes can also act as insect feeding and oviposition stimulants – as well as deterrents (Eigenbrode and Espelie 1995; Schoonhoven et al. 2005). For example, foliar waxes from wheat leaves act as attractants and oviposition stimulants of female hessian flies (Foster and Harris 1992; Morris et al. 2000). Steinbauer et al. (2004) suggested that eucalypt epicuticular waxes are oviposition stimulants of the economically important autumn gum moth, Mnesampela privata (Guenée) (Lepidoptera: Geometridae). For plant pathogens, the structure of the epicuticular waxes can influence the ability of bacteria to colonize different genotypes of host leaf (Marcell and Beattie 2002). Not surprisingly, the availability of sugars on host leaf surfaces can determine the size of bacterial populations (Mercier and Lindow 2000; Leveau and Lindow 2001). Interactions between primary and secondary metabolites (as exudates) can also influence the responses to some pathogenic microbes (Nelson and Hsu 1994).
Given the potential implications of the constituents of the surfaces of eucalypt leaves to pest and disease problems, and our specific interest in their potential to influence insect herbivores, we sought to conduct the first comprehensive assessment of the primary metabolites found on the surfaces of leaves of families of two commercially important species, namely Eucalyptus globulus and E. nitens (grown extensively throughout temperate Australia primarily for pulp production). We also sought to expand our knowledge of familial (genetic) variations in epicuticular waxes that occur within eucalypts. Recent research has attributed variations in epicuticular wax composition to differential oviposition on families of E. globulus by M. privata (Jones et al. 2002; Rapley et al. 2004; Steinbauer et al. 2004). In contrast, no plant metabolites have been implicated in differential attack of genotypes of Eucalyptus by Mycosphaerella leaf disease or other plant pathogens (Carnegie et al. 1994, 2004; Dungey et al. 1997). Our intention is to highlight the potential for variations in metabolites on leaf surfaces to contribute to ongoing insect and pathogen problems with a view to stimulating an emphasis on understanding the mechanistic bases of resistance in eucalypts to these taxa.
Materials and methods
Eucalypts
Two species of Eucalyptus (Eucalyptus globulus ssp. globulus, hereafter E. globulus, and E. nitens), each comprising two families, were studied. Details of the species and families studied are provided in Table 1. Trees belonging to the same family are individuals grown from seed harvested from one or more parent trees which were pollinated by neighbouring trees. Eucalypt seed was bought from the Australian Tree Seed Centre in Canberra and sent to France for sowing. Following germination, plants were grown individually in 230 mm diameter plastic pots in a potting mixture comprising equal parts sand, vermiculite and loam. A plant nutrient solution (made using 12.5 kg Duclos® fertilizer Lunel Viel 34403-France) [12% nitric acid, 6% ammonia, 6% P2O5, 26% K2O and 2% MgO], 1.38 kg calcium nitrate, 4.49 L nitric acid and 1 L Kanieltra 10 Fe® Yara Nanterre 92751 France trace element mix [0.202% boron, 0.027% copper, 0.910% chelated iron, 0.582% manganese, 0.024% molybdenum and 0.193% zinc], diluted at the rate of 5 L in 1000 L of water, was given to plants at weekly intervals. Once a month, the nutrient solution was supplemented with 500 mL of chelated iron. Plants were grown under 16 h of combined artificial (400 W Philips high pressure sodium lamps) and natural light in a controlled temperature glasshouse (day temperature 28 ± 5°C, night temperature 18 ± 0°C, ambient humidity 40 ± 8%) at the Institut National de la Recherche Agronomique (INRA), Versailles, France.
|
Juvenile leaves from four trees of each family were harvested when trees were 1.0–1.5 m tall, i.e. ~9–11 months after germination. Juvenile leaves of E. globulus and E. nitens are produced in opposing pairs. Leaves were harvested in pairs (i.e. two leaves per tree by four families per species giving eight leaves per family and species; half for analyses of adaxial metabolites and half for abaxial) and waxes sampled from the upper and lower surfaces of one and primary metabolites from the upper and lower surfaces of the other. It was hoped that by collecting metabolites from leaf surfaces in this way, variations due to leaf age would be minimized.
Collection and analysis: epicuticular waxes
Collections of epicuticular waxes from fresh leaves were taken at INRA and then sent to Australia for chemical analysis. Leaves were sprayed with n-hexane RS-grade at the rate of 10 mL per 100 cm2 of leaf surface area to remove waxes using the same equipment as used to remove primary metabolites (see next). Consequently, we sprayed smaller leaves for ~5 sec per side (with 3–6 mL of hexane depending upon leaf area) and larger leaves for ~8 sec per side (with 3–8 mL of hexane depending upon leaf area). The runoff was collected in a beaker before letting the hexane evaporate at an ambient temperature over the course of 2–3 h. When all the hexane had evaporated, the wax residue was dissolved from the walls of the beaker using two 1-mL aliquots of hexane and transferred to gas chromatograph (GC) vials. The hexane was allowed to evaporate overnight at ambient temperature. The GC vials were sealed and kept at −80°C before being sent to Australia for analysis.
At the Central Science Laboratory (University of Tasmania), 1 mL of hexane containing 50 μg of heptadecane (as internal standard) was added to each dried wax sample. The samples were analysed using a Varian 3800 GC coupled directly to a Varian 1200 triple-quad mass spectrometer (Walnut Creek, CA, USA). The column was a 30 m Varian Factor Four VF-5ms (0.25 mm inner diameter, 0.25 μm film). A high flow rate of 3.5 mL sec–1 in constant flow mode was used to assist the elution of the wax compounds from the column. A 1177 injector was used in splitless mode, at a temperature of 275°C. The column oven temperature at the start of an analysis was 60°C, held for 1 min, rising to 220°C at 30°C min–1 and then rising to 310°C at 10°C min–1 with an 8-min hold time at the final temperature. The temperature of the transfer line was 310°C and the ion source 240°C; 0.2 μL of sample was used in each analysis.
All the monitoring was by MS in full scan mode scanning from m/z (mass to charge ratio) 35 to 600 at three scans sec–1. Diagnostic ions were used to track data for specific compounds (this avoids the problems of co-eluting or partially co-eluting peaks). This enabled easy comparison of the relative amounts of individual components by using the ratio of the area of the diagnostic ion mass to that of the internal standard peak area. Diagnostic ions used were: m/z 100 (β-diketones), 104 (phenylethyl esters), 108 (benzyl esters), 71 (hydrocarbons), 82 (n-hexadecanal) and 58 (heptadecan-2-one). For comparison of absolute percentage of total ion current (TIC), these diagnostic ion peak areas were converted to TIC using the known ratio of diagnostic ion to TIC from a clean mass spectrum (i.e. a spectrum not contaminated by partial or complete co-elution with another compound) of each compound. These percentages were used to rank the compounds identified in order of their abundance relative to those of other compounds in comparable samples.
Compounds that were either not detected or just detected but below the quantitation limit in individual analyses are effectively treated as missing data. This assumption is also applied to the results for analyses of primary metabolites. That is, any compound not detected is treated as missing data rather than as a zero.
Collection and analysis: free sugars, polyols and malic acid
Analyses of primary metabolites were undertaken at INRA. Accidental collection of internal leaf fluids such as sap was prevented by sealing the petiole after harvesting with melted (40°C) paraffin. Metabolites were dissolved and removed from leaves by spraying with ultra-pure water as per Fiala et al. (1990) and concentrations of sugars, polyols and malic acid quantified as per the technique given in Lombarkia and Derridj (2008).
Collection and analysis: free γ-aminobutyric acid (GABA), amino acids and ornithine
The method for removal of metabolites from the surfaces of leaves was the same as used for the sugars and polyols. Samples were then purified using ion exchange chromatography. Dried samples were each dissolved using a 1-mL aliquot of a 50 : 50 water and alcohol mixture followed by addition of 4 mL of Milli-Q water. The ion exchange resin (AG 50W-X8 in an Econo-column – supplied by Bio-Rad, Marnes la Coquette 92 430 France) was preconditioned with 1 mL of 0.1 M HCl. Amino acids were eluted from the column using 4 mL of 6 M NH4OH. Eluents were freeze-dried and then dissolved in 1 mL of a mixture of 0.1 M HCl and 0.1 M norleucine (latter as internal standard). Solutions were passed through a 0.45 μm polyvinylidenefluoride (PVDF) Luer Lock filter before being freeze-dried. Residues were treated with 100 μL acetonitrile and 50 μL N-methyl-N-(t-butyldimethylsilyl)trifluoroacetamide (MTBSTFA). Prior to analysis, samples were heated at 75°C for 30 min.
Gas chromatography – flame ionisation detector (GC-FID) analyses were performed using the equipment described above. Samples were injected with the column at 70°C then after a 2-min delay it was increased to 220°C at 6°C min–1, then to 310°C at 8°C min–1 then held at the final temperature for 2 min; the total run time was just over 40 min. The injector and detector temperatures were 310°C and 300°C, respectively.
Identification of individual amino acids was by retention time compared to standards. Peak areas for amino acid derivatives relative to the norleucine internal standard were used to calculate quantities. Derivatisation does not permit estimation of actual quantities but does allow comparisons of relative amounts of different primary metabolites.
Statistical analyses
Data pertaining to the epicuticular waxes of each family of eucalypt relate to composition and are treated qualitatively. That is, the compounds detected, their occurrence across samples, the side of leaf from which they were obtained and their ranking based on their relative abundance in each sample are tabulated, but no statistical analyses were conducted.
Data pertaining to the primary metabolites found on leaf surfaces relate not only to composition but also to their quantity, hence, these data are treated quantitatively, that is, they were subjected to statistical analysis. To compare data, sugars, polyols and amino acids were combined; malic acid, GABA and ornithine were analysed individually because none belong to any of the aforementioned groupings. Within the amino acids, data were also combined according to the type of side chain each possesses; the following groupings were used: amino acids with aliphatic side chains (alanine, glycine, valine, leucine, isoleucine), with basic side chains (lysine, arginine, histidine), with aromatic side chains (tyrosine, phenylalanine, tryptophan), with acidic side chains (aspartic and glutamic acids), with amide side chains (asparagine, glutamine), with aliphatic hydroxyl side chains (serine, threonine), with sulfur-containing side chains (cysteine, methionine) (Stryer 1981). Proline, which has a secondary amino group, could not be grouped with any other amino acid and was also analysed individually.
Analysis of variance (ANOVA), using a General Linear Model (GLM) approach, was chosen to compare the quantities of primary metabolites collected from the surfaces of leaves because of the unbalanced nature of the data caused by missing values. The model used species, family and leaf surface as factors with family nested in species and leaf surface nested in family and species; all were treated as fixed factors because we wished to determine those characteristics that differentiated each plant from the others. Leaf (equivalent to tree) was not included in ANOVAs because we were not interested in making comparisons at this level. Statistically significant results obtained using GLM were examined further by means of post hoc one-way ANOVAs. The results of the one-way ANOVAs are presented in the text; the GLM results are tabulated.
Because primary metabolites were collected from either the upper or lower surface of the same leaf, post hoc comparisons of differences between leaf surfaces (within families) were by means of paired t-tests; probabilities presented are for two-tailed tests. The number of measurements able to be used for these tests (maximum of n = 8) was determined by the degree of correspondence in missing values.
Results
Epicuticular waxes
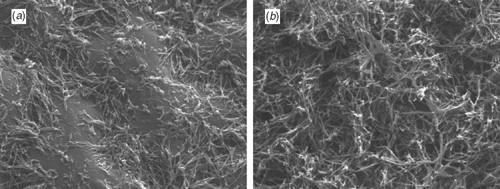
Wax production by each of the two species when grown in the INRA glasshouse in France was as observed under field conditions in Australia. That is, epicuticular waxes were more abundant on the lower than on the upper surfaces of the E. globulus leaves and more uniformly distributed on the two surfaces of the leaves of E. nitens (see Fig. 1). A visual ranking of the families relative to one another on the glaucousness (or waxiness, i.e. the extent and uniformity of development of the wax layer) of their leaves would order them thus: G99 more than G81 more than N52 more than N39. This is reflected in the diversity of wax compounds in samples from each family (Table 2). The distribution and abundance of the waxes are considered to have had a significant influence on our ability to collect water-soluble primary metabolites from the leaf surfaces. Specifically, the detection of sugars and polyols was inconsistent (especially in samples from lower leaf surfaces) and the concentrations of many primary metabolites (but especially of the polyols and amino acids) were lower in samples from E. globulus than in samples from E. nitens, that is, in opposition to the glaucousness of each species (see results in next section).
|
Of the 16 wax compounds quantified, 11 were common to the leaves of both species and all four families (Table 2). The most diagnostic wax compounds were phenylethyl n-hexacosanoate and phenylethyl n-octacosanoate, which were only detected in samples from leaves of G99. Two benzyl esters (benzyl n-hexacosanoate and benzyl n-octacosanoate) were not detected in samples from N39 and were also only detected in a quarter of the samples from N52. Indeed, these two ester groups showed the greatest differentiation of species and families because the three β-diketones (n-hentriacontan-14,16-dione, n-tritriacontan-16,18-dione, n-pentatriacontan-16,18-dione), n-hexadecanal, 2-heptadecanone and n-nonacosane were ubiquitous in samples and, except for n-hexadecanal, among the most abundant wax compounds (see rankings in Table 2).
Primary metabolites: free sugars, polyols, malic acid and GABA
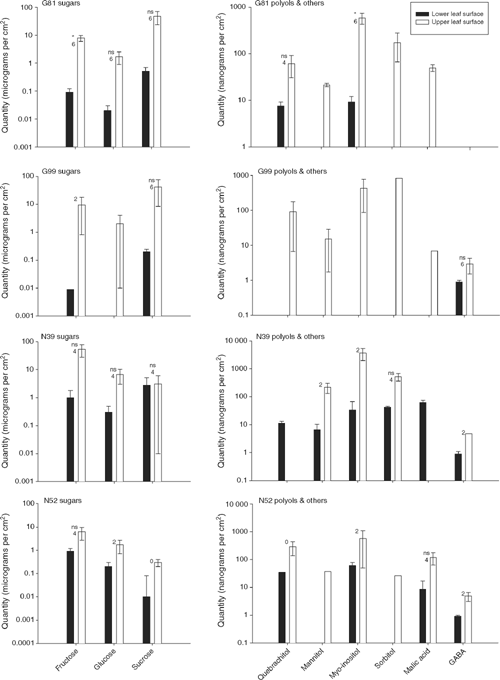
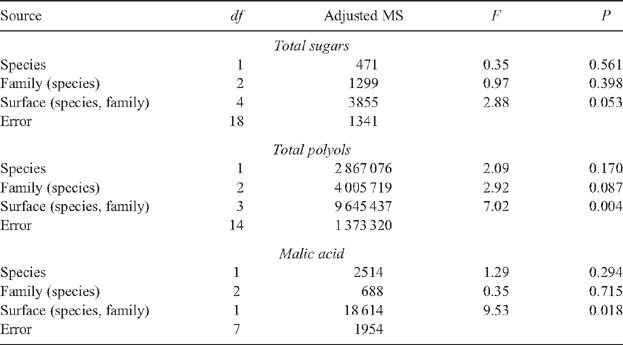
Comparisons of the concentrations of sugars, polyols, malic acid and GABA using the entire dataset, revealed no significant species, or family-level differences. Only in the case of total polyol concentrations did the differences between families within species approach statistical significance (P = 0.087). The only statistically significant results obtained were for differences in concentrations of polyols (P = 0.004) and malic acid (P = 0.018) associated with leaf surface (Appendix 1). Moreover, differences in concentrations of sugars and GABA associated with the different leaf surfaces were close to statistical significance (P = 0.053 and 0.064, respectively).
Post hoc one-way ANOVAs of statistically significant results in Appendix 1 permit the ranking of species and leaf surfaces relative to one another on the basis of differences in concentrations of polyols and malic acid. In the case of total polyols, the mean for E. nitens upper leaf surface was greater than E. globulus upper leaf surface which was greater than E. nitens lower leaf surface which was greater than E. globulus lower leaf surface; differences in concentrations of polyols using this analysis remained statistically significant (df 3 and 17, F = 4.66, P = 0.015). By contrast, differences in concentrations of malic acid, as compared using one-way ANOVA, were just outside statistical significance (df 2 and 9, F = 4.04, P = 0.056). The ranking of mean concentrations of malic acid were the same as in the case of total polyols; however, the mean for E. globulus lower leaf surface could not be placed because malic acid was treated as missing in these samples.
Mean concentrations of sugars, polyols, malic acid and GABA were always greater in collections from upper leaf surfaces than in collections from lower leaf surfaces (Fig. 2). Missing values significantly affected our opportunities to conduct post hoc paired t-tests using individual pairs of measurements of concentrations of sugars, polyols, malic acid and GABA from either leaf surface. For example, of a possible 36 tests, there were only sufficient paired measurements for 12 tests (Fig. 2). Of those 12 tests, only one was statistically significant, that is, fructose on G81 leaves. High variability between individual measurements, including pairs with measurements in opposition to others, resulted in most tests failing to achieve statistical significance.
|
Primary metabolites: free amino acids and ornithine
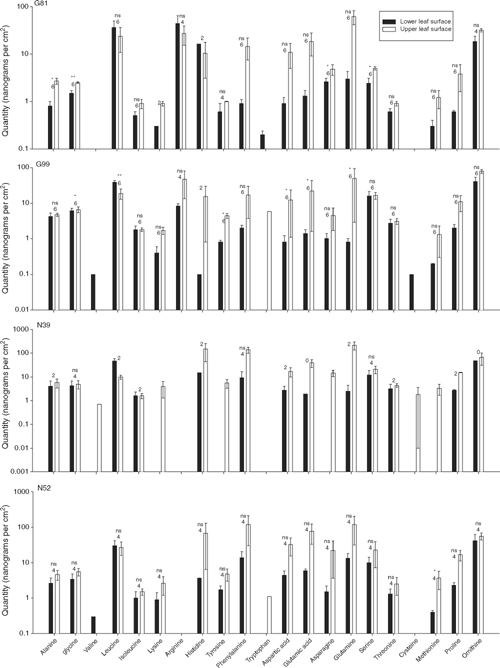
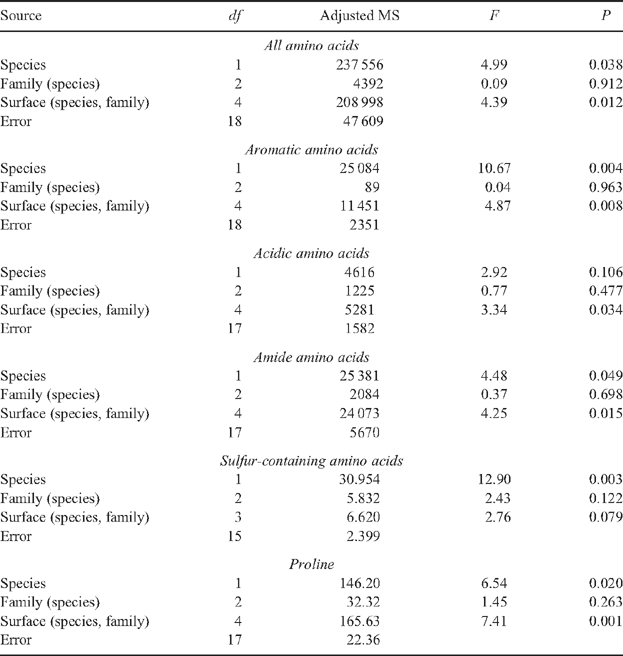
There was some consistency between the statistical results relating to the sugars, polyols, malic acid and GABA and those relating to the amino acids and ornithine in that no significant family-level differences were obtained. Only in the case of the aliphatic hydroxyl amino acids and ornithine did the differences between families within species approach statistical significance (P = 0.065 and 0.078, respectively). However, there was a significant species-level difference in the combined concentration of amino acids collected from leaves (P = 0.038; Appendix 2). This result reflects statistically significant differences in the concentrations of aromatic (P = 0.004), amide (P = 0.049) and sulfur-containing amino acids (P = 0.003) and proline (P = 0.020) collected from the leaves of the two species. Again, leaf surface was associated with several statistically significant differences in the combined concentration of amino acids (P = 0.012) as well as the concentrations of various groupings of amino acids, including the aromatic (P = 0.008), acidic (P = 0.034) and amide amino acids (P = 0.015) as well as proline (P = 0.001).
Post hoc one-way ANOVAs of statistically significant results in Appendix 2 permit the ranking of species and leaf surfaces relative to one another on the basis of differences in concentrations of their amino acid. For example, samples from E. nitens contained greater concentrations of amino acids than those from E. globulus, although this difference did not attain statistical significance using one-way ANOVA (df 1 and 24, F = 3.34, P = 0.080). The one-way ANOVA comparing amino acid concentrations on each leaf surface according to species revealed that the mean for E. nitens upper leaf surface was greater than the mean for E. globulus upper leaf surface which was greater than the mean for E. nitens lower leaf surface which was greater than the mean for E. globulus lower leaf surface (df 3 and 22, F = 8.82, P < 0.001). Both these patterns of ranking of species and leaf surfaces were repeated for each of the one-way ANOVAs conducted; however, some species comparisons failed to attain statistical significance. The statistical probabilities for the magnitude of the differences in the concentrations of aromatic, amide and sulfur-containing amino acids and proline associated with the two species were P = 0.015 (F = 6.83, df 1 and 24), 0.063 (F = 3.82, df 1 and 23), 0.016 (F = 6.87, df 1 and 20) and 0.073 (F = 3.52, df 1 and 23), respectively. The statistical probabilities for the magnitude of the differences in the concentrations of aromatic, acidic and amide amino acids and proline associated with the two leaf surfaces were P < 0.001 (F = 12.04, df 3 and 22), = 0.006 (F = 5.50, df 3 and 21), 0.001 (F = 8.22, df 3 and 21) and <0.001 (F = 12.34, df 3 and 21), respectively.
Mean concentrations of amino acids and ornithine were almost always greater in collections from upper leaf surfaces than in collections from lower leaf surfaces (Fig. 3). The exceptions were leucine (in aliphatic grouping), arginine and histidine (both in basic grouping); mean concentrations of leucine were greater on lower leaf surfaces than on upper leaf surfaces for all families, whereas concentrations of arginine and histidine were only greater on the lower surfaces of G81 leaves than on their upper surfaces. Missing values were less of a hindrance to post hoc paired t-tests of the amino acids and ornithine than for the sugars, polyols and so on. For example, of 84 possible tests, there were sufficient paired measurements for 53 tests. Nevertheless, the high variability of the measurements meant that only 11 tests attained statistical significance, that is, alanine, glycine, asparagine and serine on G81 leaves, glycine, leucine, tyrosine, aspartic and glutamic acids and glutamine on G99 leaves and methionine on N52 leaves. Of the four families, the lower surfaces of N39 leaves had the least diverse amino acid composition, that is, only 12 amino acids and ornithine were detected in collections compared to 17–19 amino acids as well as ornithine in collections from the lower surfaces of leaves of the other three families.
|
Discussion
While the composition of the epicuticular waxes of a few species of eucalypt has received modest attention, and their role in mediating some plant-insect interactions is beginning to be revealed, the composition of primary metabolites on the surfaces of eucalypt leaves has never before been investigated. As in the case of epicuticular waxes (e.g. Steinbauer et al. 2004), we have shown that there are significant between-species differences in the concentrations of certain amino acids on leaf surfaces. Our findings also suggest that there are significant differences in the concentrations of most primary metabolites according to the leaf surface considered; greater quantities of many of the primary metabolites considered were collected from the upper surfaces of leaves. Nevertheless, we suggest that this trend needs to be interpreted with caution because it seems highly likely that the denser wax layer on the undersides of leaves, most noticeable in the case of E. globulus, has hindered the complete removal of several metabolites, if not all. We suggest that it is conceivable that there is either no difference in the concentrations of primary metabolites according to leaf surface (e.g. amino acids) or that lower surfaces may have higher concentrations (e.g. sugars) as a consequence of their diffusion across the cuticle in association with the crystallisation of the epicuticular waxes. Hallam (1964) considered that wax precursors reached the surface of the leaves of E. cinerea via anastomosed channels formed between cuticular lamellae, not via stomata. The existence of cuticular channels providing egress of primary metabolites to the surfaces of leaves has been inferred for a Prunus species (see Stammitti et al. 1995). Consequently, if primary metabolites such as sugars diffuse onto the surfaces of eucalypt leaves in association with wax precursors, they would seem likely to concentrate where wax crystallisation is most active, not where stomata are most abundant. Indeed, if primary metabolites reached the leaf surface primarily via the stomata, they would not be found on the upper surfaces of juvenile E. globulus leaves because stomata are all but absent on this side of the leaf (Johnson 1926). Note that in the wild, the upper sides of leaves are likely to be ‘polluted’ with exogenous free sugars such as from honeydew (see Short and Steinbauer 2004) than are the lower sides of leaves (and probably to an extent greater than possible via endogenous sources). However, the INRA glasshouse was free of sucking insects.
Genotypic variation in plant secondary metabolite composition has been suggested to be one of the primary factors that determine insect specificity for plant species (see original hypothesis by Fraenkel 1959). Although around two-thirds of the epicuticular wax compounds detected in samples from the surfaces of the leaves of the four families of eucalypt were ubiquitous, some were unique to trees of particular families (phenylethyl n-hexacosanoate and phenylethyl n-octacosanoate to G99) while others were apparently absent or only infrequently represented in that family’s waxes (benzyl n-hexacosanoate and benzyl n-octacosanoate were absent from N39 and detected in only a quarter of samples from N52). Such differences provide part (acting in concert with terpenoid metabolites) of the means by which some insects are able to discriminate between the two species and the two families in situations where they co-occur. For example, laboratory choice experiments have shown that female M. privata prefer to lay their eggs on E. globulus rather than E. nitens – possibly due to differences in wax abundance (Östrand et al. 2008). Under field conditions, another family of E. globulus from Otway National Park (G25; i.e. same collection locality as G81) was less preferred by females for oviposition to a family of E. globulus from Jeeralang North (G19; i.e. from a comparable geographic region to G99) while, in other laboratory choice experiments, a family of E. nitens from southern New South Wales (N64; i.e. from the same Australian State as N39) was less preferred for oviposition to a family of E. nitens from Toorongo (N63; i.e. proximal collection locality to N52) (Östrand et al. 2008). Hence, the differences in wax composition reported herein are expected to reflect the preference rankings of female M. privata for these same families (i.e. G81 is expected to be less preferred than G99 and N39 is expected to be less preferred than N52), although the specific wax compounds mediating these preferences have not yet been elucidated.
It is widely held that, unlike plant secondary metabolites, the ubiquity and variability (due to developmental and environmental factors) of plant primary metabolites means they are unlikely to determine insect host specificity (Schoonhoven et al. 2005). This is not to say that the internal and even leaf surface composition of plant primary metabolites cannot be genotype-specific (see Yeoh et al. 1984; Soldaat et al. 1996; Özcan 2006). Plant secondary metabolites are thought to determine host specificity through their tendencies to be perceived at distance from the source (cf. contact chemoreception of primary metabolites) and their capacity to adversely affect insect survival and/or growth, thereby imposing a selective pressure on herbivorous insects to utilise suitable hosts. Nevertheless, because variations in amounts of primary metabolites can influence insect survival and growth, it is quite possible that an insect such as M. privata may alter the numbers of eggs laid on families of E. globulus and E. nitens depending upon the primary metabolites they encounter on leaf surfaces. Importantly, Calas et al. (2009) have shown that female M. privata are capable of contact chemoreception of leaf-surface primary metabolites by virtue of taste sensilla on their fifth tarsomeres. One pair of these sensilla exhibits notably larger responses to the amino acids alanine and serine than the other sensilla. Interestingly, female M. privata would encounter greater quantities of both these amino acids on leaves of G99 than on leaves of G81, that is, the family of E. globulus suggested to be preferred on the basis of its waxes. The role of leaf primary metabolites in host acceptance by M. privata, as well as their interactions with epicuticular waxes, clearly warrants further investigation.
Although the influence of plant metabolites on insect host specificity for species of Eucalyptus is beginning to be revealed, it is not possible to make the same statement in relation to eucalypt–pathogen interaction research, let alone the potential for plant primary metabolites to influence pathogenicity or plant susceptibility. In the case of Mycosphaerella, there does not even seem to be published information on differential susceptibility of leaf surfaces to the fungus or whether infection occurs via stomatal openings. In the absence of specific information, it must be assumed that phenomena known to occur in exotic host–pathogen associations may have some parallels in Australian systems. Certainly there already appear to be some similarities between the specificity of insects for certain host plants and the pathogenicity of at least one fungus. For example, Carnegie et al. (1994) showed that E. globulus subspecies bicostata (Maiden, Blakeley & J.Simm) Kirkpatr. and globulus were more susceptible to Mycosphaerella than subspecies maidenii (F.Muell.) Kirkpatr. and pseudoglobulus (Naudin ex Maiden) Kirkpatr. Furthermore, the severity of leaf necrosis due to Mycosphaerella varies within genotypes of the same subspecies of eucalypt (see Dungey et al. 1997; Carnegie et al. 2004). However, as yet the explanations posited for the mechanistic foundations of variations in susceptibility have been related to environment rather than composition. For example, Carnegie et al. (1994) noted that genotypes of E. globulus endemic to regions of higher summer rainfall were less susceptible than those endemic to lower rainfall regions. Applying this hypothesis to the families of E. globulus and E. nitens we studied, G99 and N52 should be expected to be less susceptible to Mycosphaerella than G81 and N39, that is, the opposite of the expected preference rankings for M. privata (see rainfall statistics given in Table 1).
Notwithstanding the hypotheses proposed by plant pathologists for explaining Mycosphaerella infection, the environmental characteristics of a genotype’s region of endemism are likely to have produced unique biochemical adaptations that benefit that particular genotype. For example, the leaves of Eucalyptus urnigera J.D.Hook. growing at high altitude are waxier and reflect more light than those growing at low altitude (Thomas and Barber 1974; Close et al. 2007). Moreover, leaves of trees at high altitude had lower tannin concentrations than those from trees at high altitude (Close et al. 2007). Interestingly, genotypes of Eucalyptus pilularis Smith from high-altitude locations suffered less Mycosphaerella damage than trees from low-altitude locations (Carnegie et al. 2004). Could this indicate that genotypes of E. pilularis with better developed epicuticular wax layers are less susceptible and/or that trees with higher tannin concentrations are more susceptible to this fungus? Carnegie et al. (1994, 2004) also reported that high tree growth rates were negatively related to the severity of Mycosphaerella infection. Does this mean that fast-growing trees are more efficient in the allocation of primary metabolites to their tissues, with less photosynthate making its way onto leaf surfaces for possible use by microbes, thereby making them less suitable hosts? The answers to such questions are readily obtained using techniques already well established in chemical ecology research. It is to be hoped that knowing the sheer diversity of food available to microbes on leaf surfaces, together with an appreciation that host-specificity mechanisms applicable to insects may be relevant to pathogens, might induce application of these techniques to a different consideration of the mechanisms of eucalypt susceptibility to diseases such as Mycosphaerella.
Acknowledgements
Financial support for MJS to work at INRA in August–September 2003 was provided by the Australian Academy of Science under the Academy’s Scientific Visits to Europe program. The GC-MS used for the wax analyses was purchased with funds from the Australian Research Council’s Linkage Infrastructure, Equipment and Facilities scheme (reference number LE0345743). We thank Prof. Dr Caroline Müller (University of Bielefeld, Germany) for her advice on terminology and an anonymous reviewer for their constructive comments on an earlier draft of the manuscript.
Calas D,
Marion-Poll F, Steinbauer MJ
(2009) Tarsal taste sensilla of the autumn gum moth, Mnesampela privata: Morphology and electrophysiological activity. Entomologia Experimentalis et Applicata 133, 186–192.
| Crossref | GoogleScholarGoogle Scholar |

Carnegie AJ,
Johnson IG, Henson M
(2004) Variation among provenances and families of blackbutt (Eucalyptus pilularis) in early growth and susceptibility to damage from leaf spot fungi. Canadian Journal of Forest Research 34, 2314–2326.
| Crossref | GoogleScholarGoogle Scholar |

Carnegie AJ,
Keane PJ,
Ades PK, Smith IW
(1994) Variation in susceptibility of Eucalyptus globulus provenances to Mycosphaerella leaf disease. Canadian Journal of Forest Research 24, 1751–1757.
| Crossref | GoogleScholarGoogle Scholar |

Carr DJ,
Carr SGM, Lenz JR
(1985) Oriented arrays of epicuticular wax plates in Eucalyptus species. Protoplasma 124, 205–212.
| Crossref | GoogleScholarGoogle Scholar |

Chapman RF
(2003) Contact chemoreception in feeding by phytophagous insects. Annual Review of Entomology 48, 455–484.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Close DC,
Davidson NJ,
Shields CB, Wiltshire R
(2007) Reflectance and phenolics of green and glaucous leaves of Eucalyptus urnigera. Australian Journal of Botany 55, 561–567.
| Crossref | GoogleScholarGoogle Scholar |

Derridj S,
Boutin JP,
Fiala V, Soldaat LL
(1996a) Composition en métabolites primaires de la surface foliaire du poireau: étude comparative, incidence sur la sélection de la plante hôte pour pondre par un insecte. Acta Botanica Gallica 143, 125–130.

Derridj S,
Wu BR,
Stammitti L,
Garrec JP, Derrien A
(1996b) Chemicals on the leaf surface, information about the plant available to insects. Entomologia Experimentalis et Applicata 80, 197–201.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Derridj S,
Gregoire V,
Boutin JP, Fiala V
(1989) Plant growth stages in the interspecific oviposition preference of the European corn borer and relations with chemicals present on the leaf surfaces. Entomologia Experimentalis et Applicata 53, 267–276.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Dungey HS,
Potts BM,
Carnegie AJ, Ades PK
(1997) Mycosphaerella leaf disease: Genetic variation in damage to Eucalyptus nitens, Eucalyptus globulus, and their F1 hybrid. Canadian Journal of Forest Research 27, 750–759.
| Crossref | GoogleScholarGoogle Scholar |

Eigenbrode SD, Espelie KE
(1995) Effects of plant epicuticular lipids on insect herbivores. Annual Review of Entomology 40, 171–194.
| Crossref | GoogleScholarGoogle Scholar |

Fiala V,
Boutin JP,
Barry P, Derridj S
(1993) Les métabolites de surface foliaire (phylloplan): présence et rôle dans les relations plante-insecte. Acta Botanica Gallica 140, 207–216.
|
CAS |

Fiala V,
Glad C,
Martin M,
Jolivet E, Derridj S
(1990) Occurrence of soluble carbohydrates on the phylloplane of maize (Zea mays L.): variations in relation to leaf heterogeneity and position on the plant. The New Phytologist 115, 609–615.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Foster SP, Harris MO
(1992) Foliar chemicals of wheat and related grasses influencing oviposition by Hessian fly, Mayetiola destructor (Say) (Diptera, Cecidomyiidae). Journal of Chemical Ecology 18, 1965–1980.
| Crossref | GoogleScholarGoogle Scholar |

Fraenkel GS
(1959) The raison d’être of secondary plant substances. Science 129, 1466–1470.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Hallam ND
(1964) Sectioning and electron microscopy of eucalypt leaf waxes. Australian Journal of Biological Sciences 17, 587–590.

Hallam ND, Chambers TC
(1970) The leaf waxes of the genus Eucalyptus L’Héritier. Australian Journal of Botany 18, 335–386.
| Crossref | GoogleScholarGoogle Scholar |

Johnson ED
(1926) A comparison of the juvenile and adult leaves of Eucalyptus globulus. The New Phytologist 25, 202–212.
| Crossref | GoogleScholarGoogle Scholar |

Jones TH,
Potts BM,
Vaillancourt RE, Davies NW
(2002) Genetic resistance of Eucalyptus globulus to autumn gum moth defoliation and the role of cuticular waxes. Canadian Journal of Forest Research 32, 1961–1969.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Lamberton JA
(1964) The occurrence of 5-hydroxy-7,4′-dimethoxy-6-methylflavone in Eucalyptus waxes. Australian Journal of Chemistry 17, 692–696.
|
CAS |

Leveau JHJ, Lindow SE
(2001) Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proceedings of the National Academy of Sciences of the United States of America 98, 3446–3453.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Lombarkia N, Derridj S
(2008) Resistance of apple trees to Cydia pomonella egg-laying due to leaf surface metabolites. Entomologia Experimentalis et Applicata 128, 57–65.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Lombarkia N, Derridj S
(2002) Incidence of apple fruit and leaf surface metabolites on Cydia pomonella oviposition. Entomologia Experimentalis et Applicata 104, 79–87.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Maher N,
Thiery D, Städler E
(2006) Oviposition by Lobesia botrana is stimulated by sugars detected by contact chemoreceptors. Physiological Entomology 31, 14–22.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Marcell LM, Beattie GA
(2002) Effect of leaf surface waxes on leaf colonization by Pantoea agglomerans and Clavibacter michiganensis. Molecular Plant-Microbe Interactions 15, 1236–1244.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Mercier J, Lindow SE
(2000) Role of leaf surface sugars in colonisation of plants by bacterial epiphytes. Applied and Environmental Microbiology 66, 369–374.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Morris BD,
Foster SP, Harris MO
(2000) Identification of 1-octacosanal and 6-methoxy-2-benzoxazolinone from wheat as ovipositional stimulants for Hessian fly, Mayetiola destructor. Journal of Chemical Ecology 26, 859–873.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Müller C, Riederer M
(2005) Plant surface properties in chemical ecology. Journal of Chemical Ecology 31, 2621–2651.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Nelson EB, Hsu JST
(1994) Nutritional factors affecting responses of sporangia of Phythium ultimum to germination stimulants. Phytopathology 84, 677–683.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Östrand F,
Wallis IR,
Davies NW,
Matsuki M, Steinbauer MJ
(2008) Causes and consequences of host expansion by Mnesampela privata. Journal of Chemical Ecology 34, 153–167.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Özcan T
(2006) Total protein and amino acid compositions in the acorns of Turkish Quercus L. taxa. Genetic Resources and Crop Evolution 53, 419–429.
| Crossref | GoogleScholarGoogle Scholar |

Rapley LP,
Allen GR, Potts BM
(2004) Susceptibility of Eucalyptus globulus to Mnesampela privata defoliation in relation to a specific foliar wax compound. Chemoecology 14, 157–163.
|
CAS |

Ruan YJ,
Kotraiah V, Straney DC
(1995) Flavonoids stimulate spore germination in Fusarium solani pathogenic on legumes in a manner sensitive to inhibitors of cAMP-dependent protein kinase. Molecular Plant-Microbe Interactions 8, 929–938.
|
CAS |

Short MW, Steinbauer MJ
(2004) Floral nectar versus honeydew as food for wasp parasitoids: implications for pest management in eucalypt plantations. Australian Forestry 67, 199–203.

Soldaat LL,
Boutin JP, Derridj S
(1996) Species-specific composition of free amino acids on the leaf surface of four Senecio species. Journal of Chemical Ecology 22, 1–12.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Stammitti L,
Garrec JP, Derridj S
(1995) Permeability of isolated cuticles of Prunus laurocerasus to soluble carbohydrates. Plant Physiology and Biochemistry 33, 319–326.
|
CAS |

Steinbauer MJ,
Schiestl FP, Davies NW
(2004) Monoterpenes and epicuticular waxes help female autumn gum moth differentiate between waxy and glossy Eucalyptus and leaves of different ages. Journal of Chemical Ecology 30, 1117–1142.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Thomas DA, Barber HN
(1974) Studies on leaf characteristics of a cline of Eucalyptus urnigera from Mount Wellington, Tasmania. II. Reflection, transmission and absorption of radiation. Australian Journal of Botany 22, 701–707.
| Crossref | GoogleScholarGoogle Scholar |

Wirthensohn MG, Sedgley M
(1996) Epicuticular wax structure and regeneration on developing juvenile Eucalyptus leaves. Australian Journal of Botany 44, 691–704.
| Crossref | GoogleScholarGoogle Scholar |

Yeoh HH,
Wee YC, Watson L
(1984) Systematic variation in leaf amino-acid compositions of leguminous plants. Phytochemistry 23, 2227–2229.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

|
|



