Impact of a prolonged decline in rainfall on eucalypt woodlands in southwestern Australia and its consequences for avifauna
A. Sara Angel A * and J. Stuart Bradley A
A * and J. Stuart Bradley A
A School of Veterinary and Life Science, Murdoch University, South Street Campus, Murdoch, WA, Australia.
Pacific Conservation Biology 28(6) 491-504 https://doi.org/10.1071/PC20078
Submitted: 30 March 2021 Accepted: 3 September 2021 Published: 18 October 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Aims: Our objective was to establish a relationship between long-term variation in the climatic environment, tree canopy decline and observed effects on the population dynamics of avifauna in the Dryandra Woodlands in southwestern Australia. These geographically isolated remnant woodlands are rich in endemic species and sustain a diverse range of ecological communities, but are threatened by habitat degradation and a decline in rainfall.
Methods: We used annual rainfall data, averaged from a series of weather stations within 100 km of the Dryandra Woodlands and a time series analysis to investigate long-term changes in annual rainfall. Satellite spectral observations of eight study sites at Dryandra was used to measure changes in Projected Foliage Cover (PFC) of old growth Eucalyptus wandoo at all sites. Our mist-net trapping study across three years and all eight sites, targeted two focal species; the rufous treecreeper (Climacteris rufa) and yellow-plumed honeyeater (Ptilotula ornata). We investigated the relationship between the captures of each species and variation in PFC, between sites and across years. Also in a separate demographic study, capture-mark-recapture data was used to estimate the apparent survival rate of each species, following the robust design for open and closed populations.
Key results: We demonstrate a long-term and continuing decline in average annual rainfall that is accelerating. We found the rainfall trend is concomitant with a long-term decline in PFC of E. wandoo and that the previous year’s annual rainfall is a predictor of average PFC across all sites. Additionally, we discovered that the PFC at each site, in each year, is a predictor of the number of yellow-plumed honeyeaters which prefer feeding on canopy insects and not a predictor of the predominantly ground-foraging rufous treecreeper. We also found a substantial difference in the apparent survival rates between the two species, with the apparent survival of yellow-plumed honeyeaters being approximately half that of rufous treecreepers. This difference was partially attributed to the likely movement outside of the study area due to decreasing habitat quality.
Conclusions and implications: Overall, our results do suggest that some impacts of long-term rainfall trends can be traced to particular species through PFC variation, but the response between species to habitat change will differ and depend on species-specific habitat requirements. As increasing greenhouse emissions are associated with declining rainfall in southwestern Australia, this study shows if rainfall decline and habitat degradation continue, it will have catastrophic consequences for woodland ecosystems.
Keywords: bird ecology, canopy, climate change, climate modelling, ecosystem change, extreme climate events, Eucalyptus spp., Eucalyptus wandoo, rufus treecreeper, yellow-plumed honeyeater.
Introduction
Global climate change is generating a major impact on natural ecosystems. Although most species can recover from short term climate variability; longer term shifts in mean climate, or an increased frequency in extreme events are altering the quantity, quality and distribution of suitable habitats for many species (Thomas and Hanski 2004; IPCC 2007, 2014). In southwestern Australia, rapidly declining rainfall (CSIRO 2005; BOM 2016 [2015]) is having a severe impact on eucalypt woodlands (Close and Davidson 2004; Veneklaas and Manning 2007; Matusick et al. 2013) and many woodland avifauna through changes in phenology (Saunders et al. 2013) and an overall reduction in survival (Chambers et al. 2005). If southwestern Australia continues to experience declining rainfall patterns with an increasing frequency and intensity of drought and heatwaves as expected (CSIRO 2005, 2015; Matusick et al. 2018), it is important to recognise a critical timeframe beyond which the increasing mortality of trees in old growth eucalypt woodlands will catastrophically change their unique ecological communities. As Bradshaw (2012) states, ‘Without clear policies to regenerate degraded forests and protect existing tracts at a massive scale, Australia stands to lose a large proportion of its remaining endemic biodiversity’.
For over 50 years, southwestern Australia has undergone extreme climatic shifts with a gradual increase in temperature and substantial decrease in winter rainfall (IOCI 2002; CSIRO 2005; BOM 2016 [2015]). Data from 1960 to 1990 shows there was a 16% decrease in mean annual rainfall (CSIRO 2005), and projections show a further 20% decrease in rainfall by 2030 (SRES 2000) and by 2070, up to a 60% decrease in winter rainfall (CSIRO 2005). This reduction in rainfall is associated with changes in large scale atmospheric circulation termed El Niño Southern Oscillation events (ENSO) that are driven by the greenhouse effect and human activity on a global scale (Risbey et al. 2009; IPCC 2014). Climate modelling indicates that there will be a doubling in the occurrences of ENSO-related extreme weather events in response to global warming (Cai et al. 2014). The warmer and dryer weather in southwestern Australia is reducing the total carrying capacity of eucalypt woodlands and other remnant native vegetation that provides habitat for a diverse range of endemic species and ecological communities (Saunders 1989; Bradshaw 2012; Mitchell et al. 2014).
Southwestern Australia is one of 34 global biodiversity hotspots because it is rich in endemic species while particularly threatened by vast stretches of agricultural land known as the wheatbelt (Myers et al. 2000; Bradshaw 2012). Since European settlement in the 1890s, over 93% of the native vegetation in the central wheatbelt, including 97% of the salmon gum (Eucalyptus salmonophloia), York gum (E. loxophleba) and wandoo (E. wandoo) woodlands, has been cleared for agriculture (Saunders 1989). Also, agricultural practices have led to fundamental changes in nutrient inputs and hydrology of remaining native vegetation which has also caused physical, chemical and biological changes to woodland soils, driving reductions in the abundance of soil and litter dwelling invertebrates, which are a major food source for many woodland birds (Watson 2011). Consequently, most of the remnant native vegetation in the wheatbelt exists as small, isolated patches and fragmented habitat that are connected by narrow strips of vegetation (Hobbs 2002). Despite such little native vegetation remaining, extensive bird banding studies have shown an apparent ease of dispersal between remnants using these narrow strips of vegetation, which can also be used as habitat (Ford et al. 2001). However, it is still unclear which structural features of connectivity facilitate movement and to what extent birds disperse between habitat patches (Ford et al. 2001; Doerr et al. 2011). Whether a species will use a particular type of functional connectivity or not, depends on a species dispersal capacity (Brooker and Brooker 2002), interspecific competition (Ford 2011), species movement behaviour and how and at what scales individuals search their landscapes for breeding or foraging opportunities (Doerr et al. 2011).
Structurally, woodlands in southwestern Australia contain trees with a projected foliage cover (PFC) of 10–30%, where crowns do not touch and tree density is about 5–20 trees per hectare (Harvey and Keighery 2012). However, for over four decades, E. wandoo has shown an increase in crown decline, which is characterised by a thinning of the foliage that begins at the branch ends, progresses towards the trunk (Close and Davidson 2004) and can eventually kill the tree (DBCA 2021). It is caused by various aerial canker fungi, which usually enter the tree at the site of mechanical damage or insect attack (DBCA 2021), but had not been noticed on a large scale until the mid-1980s, when it was discovered to coincide with a decrease in annual rainfall (Veneklaas and Manning 2007). Although there are 24 provenances of E. wandoo that grow within a range of rainfall between 333 and 1016 mm (Dalmaris 2012), the E. wandoo within the Dryandra woodlands have a climatic range in between the isohyets of 400 mm and 600 mm, but closer to the 600 mm isoline (Zdunic et al. 2012). Mercer (2003) observed that this zone displayed the most severe crown decline, with better health found in areas with higher rainfall. Additionally, Brouwers et al. (2013) found isolated and fragmented remnants of E. wandoo with a relatively large perimeter/area ratio were declining because of a negative fragmentation effect, or edge effects (Hobbs 2002). This is where the edges of isolated fragments are exposed to different microclimatic, hydrological, nutrient, and chemical exposures resulting in biotic changes when compared to the interior of the fragment (Hobbs 2002). Eucalyptus wandoo have developed specific survival mechanisms to cope with drought and attack by insects or pathogens (Batini 2004; Veneklaas and Manning 2007). The usual recovery from drought and crown decline takes the form of vegetative epicorms that sprout along the main branches after good rains and eventually replenishes the tree (Veneklaas and Manning 2007; Mercer 2008; Dalmaris 2012). However, when a combination of negative impacts are sustained over long periods of time, these adaptive defence mechanisms are compromised and may fail (Batini 2004).
In order to assess the risks of catastrophic degradation and species loss imposed on ecological communities, a knowledge of environmental processes and of how species respond to their environment is important (Austin 2002). As native vegetation is dynamic and changes in response to seasons and disturbances, a single time of observation may not reflect its true condition (Wallace et al. 2006), which includes the changes it is undergoing. To accurately capture changes within a landscape, observations at multiple points in time are required and a time series of Landsat satellite imagery can provide accurate information on changes in vegetation at different times and spatial scales (Wallace et al. 2006). The Landsat Thematic Mapper (TM) satellite imagery allows the detection of vegetation trends over time, utilising a vegetation index produced from spectral maps with on-ground reference points and an interpolation modelling procedure that extrapolates the point based spectral data across the landscape (Garkaklis and Behn 2009). This technique has been successfully used to map forest cover in Western Australia, to examine the dynamics of crown decline of E. wandoo in southwestern Australia (Zdunic et al. 2012) and within the Dryandra woodlands (Garkaklis and Behn 2009). To assess the response of species to landscape and ecosystem change, avifaunal focal species (Lambeck 1997) are useful ecological indicators (Brooker et al. 2001; Doerr et al. 2011) that can inform of the presence of other species (Leibold and Miller 2004) and, since they are sensitive to vegetation structure (Saunders 1989), including crown decline and changes in temperature and rainfall (Saunders et al. 2013), they are appropriate surrogates for assessing the impacts of climate change (Chambers et al. 2005).
Our study began with the aim of exploring the landscape genetics and population viability of declining avifauna in the remnant old growth E. wandoo woodlands of Dryandra (Angel 2015). A mist-net trapping pilot study assessed different field sites for a range of bird species and the most consistent recapture rates across the range of E. wandoo within Dryandra, was for the rufous treecreeper, RTC (Climacteris rufa) and the yellow-plumed honeyeater, YPH (Ptilotula ornata). Therefore, the selection of sampling sites and these two focal species was based on the ability to gather enough field data to generate robust statistical analyses. Also, since previous ecological studies presented evidence for differences in the foraging strategies of these two species (Luck et al. 2001; Wilson and Recher 2001), it provided an opportunity to find a comparative relationship with E. wandoo tree canopy cover.
The RTC is a resident, co-operatively breeding territorial passerine (Luck 2002) that is generally sedentary after natal dispersal (Ford et al. 2001). According to banding data, the RTC has an estimated life expectancy of 11.5 years, with a movement ability of 12 km (ABBBS 2021). This species displays seasonal differences in foraging behaviour, but is a predominately ground dwelling, insectivorous species that forages dead wood, tree bark (Luck et al. 2001) and rarely tree foliage (Recher and Davis 1998). Within Dryandra, treecreepers are found near old growth E. wandoo trees that contain adequately sized tree hollows required for nesting (Rose 1993; Luck 2002), which can take between 150 and 180 years to form (DBCA 2021).
The YPH has been described as loosely colonial within the Dryandra woodlands, (Wilson and Recher 2001), a rare vagrant in extensively cleared districts (Saunders and Ingram 1995) and displays edge avoiding behaviour in remnant vegetation (Luck 1996). Banding data for the yellow-plumed honeyeater indicates it has a movement ability of 94 km and an estimated life expectancy of just over 9 years (ABBBS 2021). Within Dryandra, the distribution of the YPH depends on relatively undisturbed habitat for foraging and has preference for mature E. wandoo trees that provide a wide variety of substrates (Wilson and Recher 2001). They predominately forage by gleaning foliage arthropods in the canopy of trees and to lesser extent bark and flowers, when nectar is available (Wilson 1997). Because these birds do not occupy all available habitat and tend to follow the temporal and spatial variation in local food resources, it is likely that they respond to and utilise foods in different proportions in different parts of their range (Wilson and Recher 2001).
The effects of climate change in southwestern Australia has been seen in seasonal shifts in rainfall and an overall reduction in rainfall, which, in combination with habitat loss and environmental degradation, has reduced the amount and quality of food available for many birds (Ford et al. 2001; Watson 2011; Saunders et al. 2013). Consequently, where environmental conditions have become unpredictable, some birds have been forced to undertake movements outside their home range to survive (Mettke-Hofmann 2017), wheres other more habitat-specific species have diminishing population sizes with shrinking distributional ranges (Saunders 1989; Watson 2011). The YPH and RTC have disappeared from fragmented districts but remain abundant in districts with extensive areas of remaining habitat (Ford 2011).
In this investigation of the Dryandra woodlands, we aim to establish a relationship between the variation in the climatic environment, the observed effects on tree canopy decline and the population dynamics of focal woodland avifauna. Long term rainfall data, a time series of satellite imagery to monitor tree foliage changes and variation in species apparent survival rates inferred from mist netting captures and recaptures, provides the opportunity to explore these relationships.
We thus take the opportunity to examine some of the effects of climate change, as exemplified by the declining rainfall in southwestern Australia, when applied to the avifauna of a temperate and geographically isolated woodland, as demonstrated by the RTC and YPH.
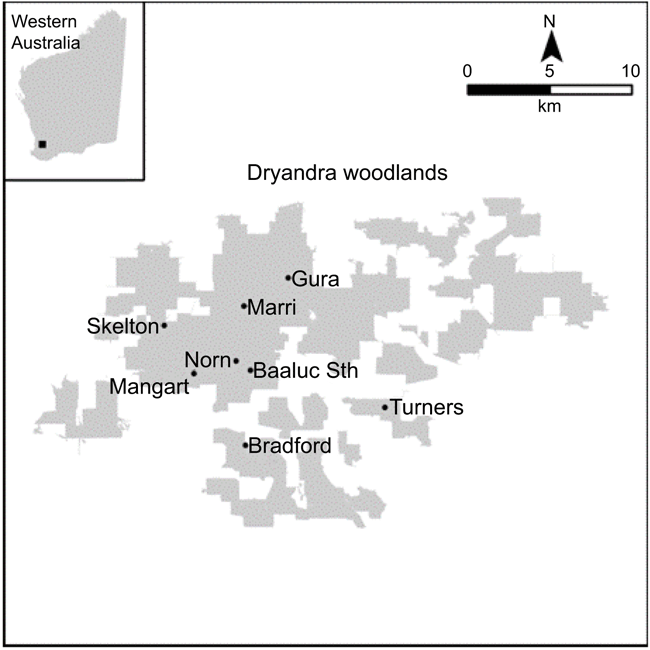
Methods
Study area
The Dryandra woodlands comprises 10 individual blocks with a combined area of 28 066 ha, which are the largest native vegetation remnants in the central western wheatbelt (CALM 1995). These remnants contain 12 different types of vegetation communities including 800 native plants that inhabit 24 mammal, 41 reptile, 8 frog and of the 98 bird species (CALM 1995); not all are residents and as such, some are found within a wider range (Saunders 1989; Saunders and Ingram 1995). As E. wandoo trees are found in gravelly or sandy loam (Harvey and Keighery 2012), their distribution within the Dryandra woodlands varies (Fig. 1). Our eight sampling sites are located within this distribution (Fig. 2), since the RTC and the YPH prefer E. wandoo habitat (Wilson 1997; Luck et al. 2001). Initially 12 sites were selected for a pilot study, but four were discarded on the basis of not being able to capture enough individuals for a robust statistical capture–mark–recapture analysis.

|
Rainfall data and analysis
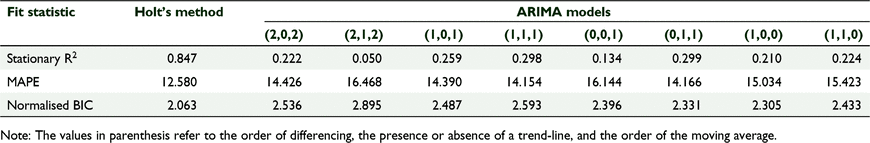
Predictions of future annual rainfall was generated using the IBM-SPSS® Time Series Modelling module (SPSS 2016). A time-series is essentially a data set in which an observation or measure is identified at the time at which it was taken. Although such data can be treated as a series of independent observations, the assumption of mutual independence is seldom sustainable (Diggle 1990). Thus, time-series analysis assumes a degree of dependence in the data, either from cyclic phenomena or from correlations between adjacent observations, and seeks to model trends and generate predictions whilst accounting for any inherent cyclic or seasonal effects. The Holt’s linear trend time series model was used to identify any linear trends in the data. This model uses exponential smoothing (Diggle 1990) and is similar to an autoregressive integrated moving average (ARIMA) process with no autoregression, but with two orders of differencing and two orders of moving average. Holt’s linear trend model is the most general of the available exponential smoothing models and makes the fewest assumptions concerning conditioning of the data. It does not assume seasonality in the time-series, which, since the observations consisted of annual total rainfall averaged over a number of sites, was not to be expected. However, large-scale cyclic, climatic events such as ENSO, which is known to have substantial effects on Australian rainfall patterns (Cai et al. 2014), could be expected to produce serial correlations. Thus a very general but non-seasonal model was considered the optimum approach for modelling the data. We compared the fit of Holt’s model to a series of ARIMA models varying in the orders of difference and moving average, and with and without trend lines to establish if it did indeed represent the best descriptive model of the time-series.
In addition, since the time series analysis did establish the existence of a decreasing trend in the data, we undertook further analysis of the time-series to establish if the trend showed any increasing or slowing tendencies. A quadratic regression model was fitted to the time-series to establish any significant non-linearity in identified trends, i.e. whether a quadratic function produced a significant improvement in fit over a linear regression.
Tree foliage cover and analysis
The TM satellite information obtained from the Leeuwin Centre for Earth Sensing Technologies (Supplementary material, Excel sheet 2), provided an accurate and measurable description of the E. wandoo canopy foliage in each of the 1 ha sampling sites within the Dryandra woodlands (Fig. 2) between 1988 and 2010. This method applies a vegetation index (TM Band 3 + TM Band 5)/2, to spectral maps where, TM Band 3 is the visible red waveband and TM Band 5 is in the short-wavelength infrared (SWIR) (Garkaklis and Behn 2009). The pixel size is 25 m, with a resolution of at least several trees and shrubs per pixel and several pixels per homogeneous area (Garkaklis and Behn 2009). The index image from each date is compiled into a sequence file and the imagery is calibrated using a number of ground-truthed reference points, to establish the percentage of actual crown cover (Garkaklis and Behn 2009). The relationship between the averaged spectral index values and observed crown cover estimate is defined by a regression equation, where the values are then applied to the index image to relate the image reflectance to the predicted PFC.
For the purposes of this study the spectral index image data between 1988 and 2010 at each sampling site within the Dryandra woodlands was used to estimate a PFC value for each site in each year.
Bird mist netting data and analysis
A demographic study involving capture–recapture data was used to firstly investigate the effects of canopy cover on the capture rates of RTC and YPH populations at the sites and secondly to estimate the annual population apparent survival rates, using a capture–mark–recapture model. It should be noted that capture–recapture models of themselves cannot distinguish between loss due to mortality and loss due to emigration without additional movement data (White et al. 2002), and hence survival really measures the tendency of individuals to be found within the same site and is generally termed apparent survival.
Mist netting was conducted on five sampling occasions, one during October in 2003, three during September, October and November in 2004 and one in October 2007 in eight (1 ha) replicate sampling sites within Dryandra (Fig. 2) The sites were selected: (1) for their proximity to E. wandoo trees; (2) their accessibility via available dirt tracks within the woodlands; (3) the presence of RTC bird calls; (4) their distribution across wandoo forest blocks at Dryandra to ensure the captures broadly sampled the entire Dryandra population; (5) a sufficiently high recapture rate of the RTC at each site established in a pilot study. In a capture–recapture analysis, the accuracy of the population estimates (in our case the apparent survival rate) depends critically on the recapture rate (Pollock and Raveling 1982) and it is important to ensure that this is sufficiently high to produce robust estimates. The eight sites selected from the pilot study were considered to provide sufficient replication for robust estimates. Each site was visited twice a day from 6 to 11 am and 4 to 7 pm where species were captured using nine 12-m and three 9-m mist nets. Each captured individual had an identification band (metal ring) attached to its leg before release. Thus, the trapping effort was the same for each site on each sampling occasion within each year.
A linear model was used to model the response of avifauna to percentage E. wandoo foliage cover (SPSS 2016). The model examined if canopy cover at each site within each year was a significant predictor of the number of RTC and YPH captures from the mist-netting program. The annual number of captures for each species was log-transformed using log(x + 1) to stabilise the count data variance. The specific model applied was a repeat measure analysis of covariance since the captures at each site represented a repeated, and hence an inherently potentially correlated, variable across the years and the canopy cover at each site within each year was the covariate of interest. The SPSS ‘MIXED’ command was used to set up the repeat measures with year as the repeated factor. The counts from the three sampling occasions in 2004 were averaged for each site to ensure the same sampling intensity applied in each year within sites.
The MARK® software program (White and Burnham 1999) was used to estimate an apparent survival rate and was based on the capture history of individuals between sampling occasions. The data was analysed using the Cormack–Jolly–Seber (CJS) model, which builds a multinomial distribution of captures and recaptures for single aged, open populations. One of the assumptions of this model is that the time between sampling occasions is kept short to minimise deaths, recruitment and movement out of the study area (Williams et al. 2002). Another is that probability of capture and probability of loss should be the same for all individuals in the population, and it is a pre-requisite to sample each population at least three times (Southwood and Henderson 2000). The analysis uses a parsimony procedure, which selects the smallest possible number of parameters to model a situation that maximises the probability of the observed data (Southwood and Henderson 2000; Williams et al. 2002). The goodness of fit procedure uses sample data to investigate the mathematical structure of a distribution rather than using specific values for its parameters (Williams et al. 2002). If there is prior knowledge of the degree of emigration and hence the value of a parameter describing the emigration rate can be assumed, then true survival rather than apparent survival can be estimated by the model (Williams et al. 2002). However, in our case these data were not available and hence, the apparent survival rates only were estimated. Because of the unequal intervals between sampling periods, we followed the robust design estimation procedure outlined by Pollock and Raveling (1982), which uses a closed population model for captures collected close together in time (i.e. months apart in our case) and an open population model to captures collected over greater time intervals (i.e. several years apart in our case). This approach combines Jolly–Seber estimators with closed population estimators to reduce bias caused by unequal catchability and provide estimators for parameters unidentifiable by the unmodified CJS model alone.
Results
Rainfall forecast
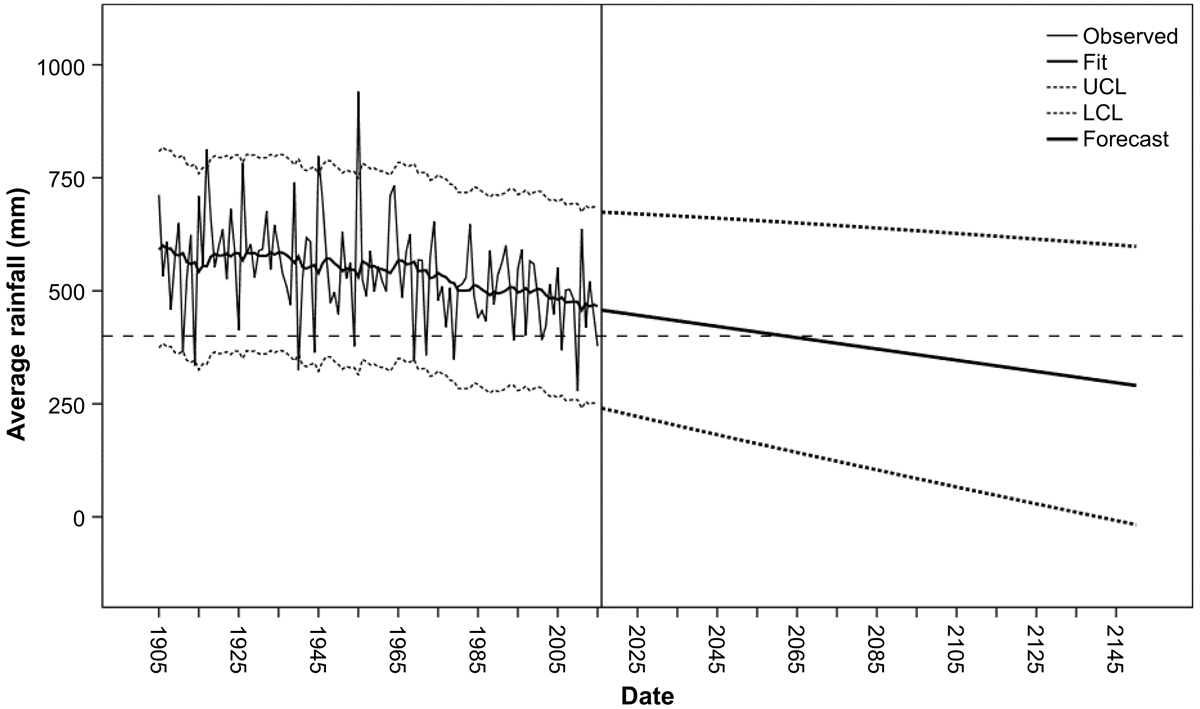
Average annual rainfall measurements between 1905 and 2015 were collated from a total of 13 Bureau of Meterology weather stations (Supplementary material, Excel sheets 3 and 4) across a range of 100 km, encompassing and including the Dryandra woodlands (BOM 2016 [2015]). A time-series analysis using Holt’s method produced a trajectory of average rainfall from 2015 to 2145 (Fig. 3). The goodness-of-fit statistics for Holt’s method in comparison with a suite of other applicable ARIMA models are presented in Table 1. The lowest normalised Bayesian information criterion (BIC) value, highest stationary R2 (coefficient of determination) value and lowest mean absolute percentage error (MAPE) value indicate that Holt’s method is a much better fit to the data than the alternative models. Although the trend identified by Holt’s method has wide confidence limits, it predicts average annual rainfall will fall below 400 mm by 2063 (Fig. 3), which is the minimum average rainfall in the climatic range of E. wandoo in the Dryandra woodlands (Zdunic et al. 2012).
|
|
When linear and quadratic regressions were applied to decreasing average rainfall (Y) and time (X) sequence data (Fig. 4), the quadratic curve was a significantly better fit to the observed data. Although both regression models produced significant fits (Table 2), the quadratic model produced a highly significant decrease in the residual variance over the linear model (F1,109 = 7.23; P = 0.008), indicating an element of non-linearity in the trend and an accelerating decline in rainfall. In addition, the ΔAIC value for the linear regression was substantial, producing a very low relative likelihood for this model as opposed to the quadratic model which had a relative likelihood close to one.

|
Tree foliage cover trends and relationship to rainfall
The PFC of E. wandoo from eight (1 ha) sampling sites within the Dryandra woodlands between 1988 and 2010 appeared to have a similar fluctuating pattern, with an overall declining trend (Fig. 5). At site 3 (Skelton), the foliage cover did not appear to recover after falling below 10% on several occasions. The PFC from each sampling site was then averaged for every year and a linear regression fitted, which shows a significant decreasing trend in foliage cover with time between 1988 and 2010 (Fig. 5).
Since, similar to the average rainfall for the region, the mean PFC was also observed to decline with time, we tested for the occurrence of a significant relationship between the mean PFC and rainfall measurements. We found a significant linear relationship for average PFC and the previous year’s total average rainfall (Fig. 6), but not the rainfall in the current year (F1,14 = 0.164, P = 0.692) or the rainfall in the winter months only of the previous year (F1,14 = 0.001, P = 0.976).
Bird captures in relation to tree foliage cover
Using repeat-measures analysis of covariance, we found that the foliage cover at each site was a significant predictor of YPH captures but not of RTC captures (Fig. 7). However, the significance of the trend-line slope was not high (P = 0.03), indicating that the power of this analysis to detect effects was not particularly strong. Hence the lack of a significant effect in the case of the RTC does not imply that foliage cover is not a determining factor in this species’ abundance.
Bird capture–mark–recapture data and apparent survival rates
Mist net trapping of the RTC over five sampling occasions between 2003 and 2007 at eight sampling sites (Fig. 2), resulted in 162 RTC individual captures and a recapture rate of 16.66%. Mist net trapping of the YPH over the same time and sampling sites, resulted in 216 individual captures and a recapture rate of 12.03%. A test of association of the log-transformed capture data of RTC and YPH was conducted with a Pearson correlation (two-tailed test) and found a correlation coefficient (r) of 0.539 (P = 0.007). Both species had a positive (non-random) relationship to each other across sampling sites and since the trapping effort at each site was consistent, the relationship between species captures at each site was significant. Apparent survival rates for the RTC and YPH were estimated to be 0.653 (±0.131) and 0.303 (±0.086) with a 95% confidence interval respectively.
Discussion
The results of this study demonstrate that, within the Dryandra woodlands, there is a long term and continuing decline in rainfall and that, in recent years, the rate of decline has accelerated. Concomitant with the rainfall decline, the PFC of E. wandoo across eight sampling sites has also declined. We also found that the previous year’s annual rainfall is a predictor of average foliage cover. Results from our mist net trapping program show that the PFC at each site in each year was a predictor of the number of YPH captured, but not a predictor of the number of RTC. Finally, based on the feeding preference for tree canopy insects by the YPH, opposed to the predominately ground foraging behaviour of the RTC, the impact of PFC decline on woodland avifauna was assessed using data from a capture–mark–recapture study. We found a substantial difference in the apparent survival rate between the two species, with the apparent survival of the YPH being approximately half that of the RTC. Overall, our results suggest that some impacts of long term rainfall trends can be traced to particular species in some instances through foliage cover variation, but the responses between species to these changes will differ and depend on species-specific habitat requirements.
The time-series analysis of rainfall data (Fig. 3) forecasts that average rainfall will continue to decline and by 2063, permanently fall below an average 400 mm rainfall. The decline in rainfall (Fig. 4) and forecast declining trend (Fig. 3) is consistent with projected changes in rainfall for southwestern Australia, using atmospheric CO2 stabilisation at 550 ppm, according to the Wigley, Richels and Edmons stabilisation curve (CSIRO 2005; Wigley et al. 1996) and the more recent CMIP5 multi-model mean simulations of precipitation over the globe in 2081–2100 relative to 1986–2005, under the Representative Concentration Pathway 8.5-high greenhouse gas emissions scenario (CSIRO 2015).
The spatial distribution modelling of E. wandoo conducted by Dalmaris (2012), inferred that recent declines in drier areas may represent contraction from areas of previous expansion during more mesic conditions since the last glacial maximum (Hoffmann et al. 2019) approximately 21 000 years ago (Nolan et al. 2018). Detailed management strategies and restoration programs, such as climate adjusted provenancing (Prober et al. 2015) and assisted gene migration (Aitken and Whitlock 2013), have been proposed to mitigate the impact of climatic range shifts (Hoffmann et al. 2019). However it is unlikely that these strategies will be adequate, if the forecast rainfall in Dryandra falls below an average of 400 mm by 2063 (Fig. 3), which is the minimum average rainfall in the climatic range for E. wandoo (Blakely) (Dalmaris 2012). Also, considering E. wandoo are slow growing, can live to 400 years and take 150–180 years to form tree hollows for nesting birds (DBCA 2021), it is unlikely that regeneration of E. wandoo from dryer rainfall areas will be able to mature and provide suitable habitat within a short period of time. Studies modelling future projections of climate impacts on the native flora of southwestern Australia, even under relatively moderate climate change scenarios and optimistic assumptions of species migrations, is likely to be large with wide scale range contractions (Fitzpatrick et al. 2008). Yates et al. (2010) also concludes that at some point in a hotter and dryer climate, the climatic tolerances of many species will be exceeded. Based on simulations from the Intergovernmental Panel on Climate Change (IPCC) Fourth Assessment Report, using climate sensitivities from 2 to 4°C, a complete biome shift is expected in southwestern Australia, where woodlands may be transformed into shrublands by the end of the 21st century (Bergengren et al. 2011).
PFC estimates from eight sites within the Dryandra woodlands between 1988 and 2010 found a significant decline in PFC (Fig. 4). In a similar study, Zdunic et al. (2012) also found a declining trend for E. wandoo for the same time period. Interpolated rainfall data and area calculations showed a gaining and declining trend but could not establish the timing of these events (Zdunic et al. 2012). We found a significant relationship between annual PFC and the previous year’s rainfall (Fig. 6) but not the current year’s rainfall. Although Mercer (2008), also found no relationship between the annual tree foliage cover of E. wandoo and annual average rainfall, this study indicates the loss of foliage cover has progressed over a longer term than that covered by the satellite data and that it tends to be more impacted by the longer term effects of declining rainfall patterns.
In another study of eucalypt species growing in higher rainfall areas in southwestern Australia (Smettem et al. 2013), found the average annual leaf area index (LAI) of E. globulus, E. calophylla and E. diversicolor was linearly related to mean annual rainfall, but interannual changes to the LAI in response to rainfall was less than expected from the long term trend. This buffered response was investigated and attributed to the presence of deep soil moisture and groundwater storage systems which are also impacted by a reduction in seasonal rainfall (Smettem et al. 2013) and have been dropping consistently by 0.05–0.48 m year−1 for the last four decades (Hughes et al. 2012). Other experiments conducted by Barbeta et al. (2015) and Liu et al. (2018) on groundwater dependent Mediterranean-type forests, have shown that isohydric species experience increased crown defoliation and synergistic mortality effects during acute drought if they have already experienced chronic drought. The Dryandra woodlands are subject to: (1) a long term and increasing decline in rainfall (Figs 3 and 4); (2) the loss of foliage cover of E. wandoo associated with the longer term effects of declining rainfall (Fig. 6); (3) reduced groundwater availability (Hughes et al. 2012) combined with an expected increase in drought and heatwave events (Mitchell et al. 2014; Matusick et al. 2018). All these factors will have accumulative impacts, that in other studies have shown to result in the amplification of crown dieback and tree mortality (Close and Davidson 2004; Matusick et al. 2013, 2018).
Woodlands in southwestern Australia are defined by trees with a PFC of 10–30% (Harvey and Keighery 2012) and since site 3 (Skelton) was not able to recover to 10% PFC from 2007 (Fig. 5), a closer examination of its geographic location within Dryandra (Fig. 2), shows the site is situated on the apex of an agricultural field. This likely results in this site being exposed to edge effects which are known to profoundly impact the microclimatic, hydrology and biota within it (Hobbs 2002), including the crown decline of E. wandoo (Brouwers et al. 2013). These edge effects, in combination with an overall loss of foliage cover impacted by the longer term effects of declining rainfall (Figs 5 and 6), are the most likely reason for the 5% PFC at site 3 (Skelton).
The impact of variation in PFC on the numbers of RTC and YPH captured is shown in Fig. 7. The PFC was not found to be a significant predictor of RTC captures but was a significant and positive predictor of YPH captures. In the case of RTC there is no evidence that a positive relationship exists between number of captures and PFC, rather the fitted line, though not significant and therefore not illustrated, had a negative slope, indicating that the role of canopy cover as a possible indicator of habitat quality is quite different between the two species.
This result may reflect major differences between the foraging strategies and movement patterns of the species. Though both feed largely on insects, RTC forages mainly on the ground, with a lesser gleaning effort on tree boles and very little, only seasonal, foraging occurs in the canopy (Luck et al. 2001). In contrast YPH forage mainly by gleaning and probing foliage and bark within the canopy and also hawking and hovering. (Wilson and Recher 2001). Despite being a honeyeater, the YPH exhibited very little nectar feeding although feeding on lerp and manna were recorded. The RTC is a cooperative-breeding, territorial resident species (Luck 2002). At Dryandra, fidelity to territory is high and dispersal rates are low. In contrast, less is known about the movements and territoriality of YPH at Dryandra, but Wilson and Recher (2001) point out that they exhibit clumped distributions and are gregarious, suggesting that they be described as loosely colonial.
These patterns suggest that YPH abundance would more strongly reflect variation in PFC, both between years and between sites, on the basis that the quality of the habitat in which it feeds (i.e. within foliage) varies on a more rapid time-scale, with annual variation in cover reflecting directly annual variation in the preceding year’s rainfall, and on the basis that YPH exhibits greater movement to congregate in sites retaining a higher habitat quality. Thus, numbers decrease in sites exhibiting habitat quality loss when rainfall is poor and there is a general lessening of habitat quality and hence available resources. Lindenmayer et al. (2019) in a study of 13 species of birds in temperate woodlands in south-eastern Australia identify variation in rainfall as a key driver of increase of occurrence, especially in small-bodied species, pointing out that higher rainfall can result in resource pulses such as nectar, seeds and insect prey.
An indicator of the movement of the two populations was the estimated apparent survival rates from capture–recapture data using the CJS model for open populations. A preliminary analysis of mist net trapping data found a greater number of YPH to RTC captures. Since Log-transformed capture totals of both species were significantly correlated to each other across sampling sites, this confirmed that both species were, to some extent, reflecting variations in density due to common features of habitat quality rather than random chance influencing the captures at any site. Between 2003 and 2007 the apparent survival rate estimate for the RTC was 0.653 (±0.131) and 0.303 (±0.086) for the YPH. In a previous study conducted in Dryandra between 1997 and 1999 by Luck (2000), the RTC survival rate was 0.77 (±0.06) for primary males and 0.75 (±0.05) for primary females. There are no previous survival estimates for YPH at Dryandra.
The survival values for RTC produced by this study from capture–recapture, and by Luck (2000), using observation of identifiable individuals at nests are sufficiently close to ‘ground-truth’ the capture–recapture technique, and thus to add credence to the low apparent survival rate of YPH. Adult survival rates for temperate woodland passerines in the Southern Hemisphere have generally been found to be in the order of 0.75 (Rowley and Russell 1991), and we consider it is highly unlikely that mortality alone is forcing the YPH apparent survival estimate down to this extent, especially since three separate years of data were involved.
Therefore, the most likely interpretation is that YPH exhibited significantly more movement than RTC. This does not preclude some increased mortality in YPH as a consequence of declining habitat quality. However, if such low apparent survival truly reflected only mortality and not movement, we would not see the relatively high number of captures at some sites in 2007 (see Fig. 7b), since such low survival would have led to a population collapse over the 4 years from 2003. It is also likely that decreasing habitat quality, reflecting declining PFC, would increase movement, since, even in sedentary species, territories and foraging distances would expand to accommodate a declining resource density. Catastrophic collapse of PFC, such as that exhibited by site 3 (Skelton), may well lead to a total dispersal since resources densities for viable territories would likely not be available.
The argument that movement produced the positive association between YPH and cover, rather than increased reproductive success in sites with higher habitat quality, needs to be treated with care. Movement can certainly produce an association between habitat quality and density as individuals move from lower quality habitat to higher, but it effectively buffers individuals in the short term from the longer-term effects of widespread habitat decline. Sedentary species with rapid breeding can, just as quickly, produce a strong association on the basis of annual variation in habitat quality. A classic example was discovered in the honey possum (Tarsipes rostratus) population of the Fitzgerald National Park in southwestern Australia (Wooller et al. 1998), where the number of captures per 1000 trap nights, over a 13 year period, was very strongly correlated with rainfall in the preceding year. Wooller et al. (1998), point out that honey possums are extremely sedentary and cannot escape the nectar shortage brought on by low rainfall by changing their diet from nectar or moving elsewhere. The honey possum is short-lived, but produces young throughout the year, and hence can increase rapidly as nectar flow increases. The question of why we do not see a similar response in the more sedentary RTC is likely to be a consequence of its feeding below the canopy, where the insect resources available to it are less dependent on immediate canopy growth and lerp and nectar production, and more dependent on decaying detritus and litter. Consequently, the impact on productivity of annual variations in rainfall would be spread over several years. In addition, the reproductive rate of both species, even under ideal conditions, is substantially less than that of honey possums, who can achieve breeding status very quickly because of their small body size and rapid growth. For this reason also, movement would be predicted to be a stronger determinant of the positive response seen in YPH than is reproductive success.
But the above argument also depends upon the variability in the magnitude of the differences between site foliage cover from year to year. If the correlation with previous years PFC (autocorrelation) is high within sites and therefore the rankings of the sites with regard to cover tend not to change from year to year even if the actual PFC values change markedly, then the relationship will tend to be more strongly driven by breeding success than by movement. In this scenario, habitat quality fluctuates from year to year, but a consistently better site will sustain more successful breeding of YPH than other sites, and there is no advantage in dispersing in a poor year because the quality of other sites will tend to fall pro-rata. Sites with a consistent higher ranking in foliage cover will therefore consistently sustain a higher number of individuals, and this will provide the basis for the relationship. We therefore have two mechanisms that might explain the relation found in YPH: (1) dispersal of individuals to available sites usually within the home range with a higher PFC; (2) consistently higher quality sites that sustain more YPH despite the vagaries of rainfall. These mechanisms are not mutually exclusive.
The rankings of the sites in fact do exhibit a degree of consistency (Fig. 5). For example, site 6 (Baaluc) has the highest (or joint highest) PFC in all 15 years, and site 4 (Bradford) is usually second. Site 3 (Skelton) has almost always the lowest PFC and from 2007 onwards becomes an outlier with a value in the region of 5%, most likely due to edge effects. In sites with PFCs closer to the yearly average, however, the rankings do shift, indicating that the degree of consistency is intermediate rather than absolute. A substantial degree of within site autocorrelation is to be expected since the population of trees bearing significant foliage will not change much from year-to-year but will vary over a longer time scale. Shifts in the rankings between sites may be driven by local variation in rainfall at the scale of between site distances, and also by ongoing shifts in the vegetation structure within sites. Until more data are available on movement and dispersal in the case of YPH we are not in a position to determine the relative roles of movement vs year-to-year consistency in PFC values in explaining why PFC values predict capture rates of YPH.
However, it should be borne in mind that recent radiotracking data has revealed that breeding adults of passerine species normally considered as residents with clearly defined territories and home ranges are capable of forays substantially beyond their normal home range. Doerr et al. (2011), in a study of functional connectivity in a fragmented Australian woodland, attached radio transmitters to five passerine species; three resident: brown treecreepers (Climacteris picumnus), white-throated treecreepers (Cormobates leucophaeus), eastern yellow robins (Eopsaltria australis), and two considered semi-nomadic: white-plumed honeyeaters (Ptilotula penicillata) and fuscous honeyeaters (P. fusca). They found that, surprisingly, the foray distances were largest in the brown treecreepers (mean 1099 m, longest 2.6 km) than in the other species (mean 545 m). A white-plumed honeyeater exhibited the longest distance in the remaining species of 2 km, and all five species tracked exhibited substantial foray distances. On the basis of this, Doerr et al. (2011) suggest that decision rules for movement in passerines have been shaped by variability in the landscape and therefore movement behaviour may be less species-specific than previously assumed. It should also be noted that a molecular taxonomy study of honeyeaters (Nyári and Joseph 2011) has established that white-plumed honeyeaters and YPH are much more closely related to each other than to other members of genus Ptilotula and are thus likely to share some movement characteristics.
Lindenmayer et al. (2019) point out that the occurrence of woodland birds is influenced by habitat attributes, climate and short-term weather, and that these drivers are likely to interact, but are often considered separately and quantified independently. Our study considers all of these factors in terms of a long-term decline in rainfall, the short term relationship between PFC and the previous year’s rainfall, and the different habitats strata inhabited by being a ground feeder as opposed to a canopy feeder. Our results suggest that interactions between these factors do take place, and that studies designed to examine the detailed impact of climate change on the population dynamics and viability of species need to have both long term local weather data sets and long term local abundance data for individual species over a number of sites to deal with habitat variability and its interaction with long and short term climate variability. This is an expensive ask in terms of research effort, but if general models of interactions underpinning climate change effects are to emerge, then this effort is required. The payoff would be predictive models useful for a range of species and habitat types in managing the conservation of species under a changing climate. The 13 year multi-site and multi-species study described by Lindenmayer et al. (2019) is an exemplar of this approach.
Supplementary material
Supplementary material is available online.
Data availability
Any data not included in supplementary materials can be found in Thesis of Angel (2015).
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was funded by the CSIRO Centre for Environment and Life Science, Perth Western Australia and Birdlife’s Stuart Leslie Bird Research Award.
Acknowledgements
Appreciation expressed to Division of Research and Development Research Ethics Office at Murdoch University, Perth Western Australia for the provision of permit W997/03 to conduct mist net trapping, John Ingram for technical assistance in field work and the Leeuwin Centre for Earth Sensing Technologies for satellite imagery data of the Dryandra woodlands. The authors also wish to thank Dr. Kenneth J. Pollock for his supervision of the survival rate estimation presented in the text.
References
ABBBS (2021) ‘Australian Bird and Bat Banding Scheme’. (Australian Government Department of Environment: Canberra, ACT, Australia). Available at https://www.environment.gov.au/science/bird-and-bat-banding/banding-data/search-abbbs-database [Accessed 7 August 2021].Aitken, SN, and Whitlock, MC (2013). Assisted gene flow to facilitate local adaptation to climate change. Annual Review of Ecology, Evolution, and Systematics 44, 367–388.
| Assisted gene flow to facilitate local adaptation to climate change.Crossref | GoogleScholarGoogle Scholar |
Angel AS (2015) Landscape genetics and effects of climate change on the population viability of declining avifauna in the fragmented eucalypt woodlands of the West Australian wheatbelt. PhD thesis, Murdoch University, Perth, WA, Australia.
Austin MP (2002) Case studies of the use of environmental gradients in vegetation and fauna modelling: theory and practice in Australia and New Zealand. In ‘Predicting species occurrences issues of accuracy and scale’. (Eds JM Scott, PJ Heglund, ML Morrison) pp. 73–82. (Island Press: London, UK)
Barbeta, A, Mejía-Chang, M, Ogaya, R, Voltas, J, Dawson, TE, and Peñuelas, J (2015). The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Global Change Biology 21, 1213–1225.
| The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest.Crossref | GoogleScholarGoogle Scholar | 25359123PubMed |
Batini, F (2004). Comparison of changes to water levels in Deep bores 1975–2004 – Helena Catchment, Western Australia. Western Wildlife 8, 4.
Bergengren, JC, Waliser, DE, and Yung, YL (2011). Ecological sensitivity: a biospheric view of climate change. Climatic Change 107, 3–4.
| Ecological sensitivity: a biospheric view of climate change.Crossref | GoogleScholarGoogle Scholar |
BOM (2016 [2015]) Australian Government Bureau of Meteorology. Available at http://www.bom.gov.au/climate/data/index.shtml [Accessed 2015 and 2016].
Bradshaw, CJA (2012). Little left to lose: deforestation and forest degradation in Australia since European colonization. Journal of Plant Ecology 5, 109–120.
| Little left to lose: deforestation and forest degradation in Australia since European colonization.Crossref | GoogleScholarGoogle Scholar |
Brooker L, Atkins L, Ingram J (2001) Enhancing biodiversity values in agricultural lands morbinning sub-catchment and surrounds August 2001. A CSIRO report commissioned by Greening Australia, Western Australia.
Brooker, LC, and Brooker, MG (2002). Dispersal and population dynamics of the blue-breasted fairy-wren, Malurus pulcherrimus, in fragmented habitat in the Western Australian wheatbelt. Wildlife Research 29, 225–233.
| Dispersal and population dynamics of the blue-breasted fairy-wren, Malurus pulcherrimus, in fragmented habitat in the Western Australian wheatbelt.Crossref | GoogleScholarGoogle Scholar |
Brouwers, NC, Mercer, J, Lyons, T, Poot, P, Veneklaas, E, and Hardy, G (2013). Climate and landscape drivers of tee decline in a Mediterranean ecoregion. Ecology and Evolution 3, 67–79.
| Climate and landscape drivers of tee decline in a Mediterranean ecoregion.Crossref | GoogleScholarGoogle Scholar |
Cai, W, Borlace, S, Lengaigne, M, van Rensch, P, Collins, M, Vecchi, G, Timmermann, A, Santoso, A, McPhaden, M, Wu, L, England, MH, Wang, G, Guilyardi, E, and Jin, F-F (2014). Increasing frequency of extreme El Niño events due to greenhouse warming. Nature Climate Change 4, 111–116.
| Increasing frequency of extreme El Niño events due to greenhouse warming.Crossref | GoogleScholarGoogle Scholar |
CALM (1995) Dryandra Woodland Management Plan 1995–2005 – management plan no. 30. Department of Conservation and Land Management, National Parks and Nature Conservation and Lands and Forests Commission, Perth, WA, Australia.
Chambers, LE, Hughes, L, and Weston, MA (2005). Climate change and its impact on Australia’s avifauna. Emu – Austral Ornithology 105, 1–20.
| Climate change and its impact on Australia’s avifauna.Crossref | GoogleScholarGoogle Scholar |
Close, DC, and Davidson, NJ (2004). Review of rural tree decline in a changing Australian climate. Tasforests , 1–18.
Coates A (1993) Vegetation survey of Dryandra forest. Prepared for the Department of Conservation and Land Management, Perth Western Australia.
CSIRO (2005) Ryan, B., Smith, I. and Bates, B., Indian Ocean Climate Initiative (IOCI): how will the future climate of south-western Australia be affected by the greenhouse signature? A presentation given in Melbourne, 17th November 2005. CSIRO Marine and Atmospheric Research, Clayton, Vic., Australia.
CSIRO (2015) Climate change in Australia-projection for Australia’s NRM regions. Climate change in Australia technical report. (CSIRO and Bureau of Meteorology: Clayton, Vic., Australia) Available at https://www.climatechangeinaustralia.gov.au/media/ccia/2.2/cms_page_media/168/CCIA_2015_NRM_TR_Chapter%203.pdf
Dalmaris E (2012) Eucalyptus wandoo: tolerance to drought and salinity in relation to provenance and evolutionary history in southwestern Australia. PhD thesis, University of Western Australia, Perth, WA, Australia.
DBCA (2021) ‘Wandoo woodlands.’ (The Department of Biodiversity Conservation and Attractions) Available at https://library.dbca.wa.gov.au/static/FullTextFiles/019398 [Accessed 12 January 2021].
Diggle PJ (1990) ‘Time series: a biostatistical introduction.’ (Oxford University Press: Oxford, UK)
Doerr, VAJ, Doerr, ED, and Davies, MJ (2011). Dispersal behaviour of Brown Treecreeper predicts functional connectivity for several other woodland birds. Emu – Austral Ornithology 111, 71–83.
| Dispersal behaviour of Brown Treecreeper predicts functional connectivity for several other woodland birds.Crossref | GoogleScholarGoogle Scholar |
Fitzpatrick, MC, Gove, AD, Saunders, NJ, and Dunn, RR (2008). Climate change, plant migration, and range collapse in a global biodiversity hotspot: the Banksia (Proteaceae) of Western Australia. Global Change Biology 14, 1337–1352.
| Climate change, plant migration, and range collapse in a global biodiversity hotspot: the Banksia (Proteaceae) of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Ford, HA (2011). The causes of decline of birds of eucalypt woodlands: advances in our knowledge over the last 10 years. Emu – Austral Ornithology 111, 1–9.
| The causes of decline of birds of eucalypt woodlands: advances in our knowledge over the last 10 years.Crossref | GoogleScholarGoogle Scholar |
Ford, HA, Barrett, GW, Saunders, DA, and Recher, HF (2001). Why have birds in the woodlands of Southern Australia declined? Biological Conservation 97, 71–88.
| Why have birds in the woodlands of Southern Australia declined?Crossref | GoogleScholarGoogle Scholar |
Garkaklis M, Behn G (2009) Assessment of Eucalyptus wandoo (Wandoo) and other tree canopy decline using Landsat Trend Analysis. (Report to Department of Environment and Conservation: WA, Australia)
Harvey J, Keighery G (2012) Wheatbelt Baselining Project. Benchmarking wheatbelt vegetation. Classification and description of eucalypt woodlands. (Report by the Department of Environment and Conservation: Perth, WA, Australia) Available at https://www.dpaw.wa.gov.au/images/about/science/wheatbelt_woodland_communities_report.pdf [Accessed 15 March 2021].
Hobbs RJ (2002) Chapter 3: The ecological context: a landscape perspective. In ‘Handbook of ecological restoration volume one-principles of restoration’. (Eds MR Perrow, AJ Davy) pp. 24–45. (Cambridge University Press: Cambridge, UK)
Hoffmann, AA, Rymer, PD, Byrne, M, Ruthrof, KX, Whinam, J, McGeoch, M, Bergstrom, DM, Guerin, GR, Sparrow, B, Joseph, L, Hill, SJ, Andrew, NR, Camac, J, Bell, N, Riegler, M, Gardner, JL, and Williams, SE (2019). Impacts of recent climate change on terrestrial flora and fauna: some emerging Australian examples. Austral Ecology 44, 3–27.
| Impacts of recent climate change on terrestrial flora and fauna: some emerging Australian examples.Crossref | GoogleScholarGoogle Scholar |
Hughes, JD, Petrone, KC, and Silberstein, RP (2012). Drought, groundwater storage and stream flow decline in southwestern Australia. Geophysical Research Letters 39, L03408.
| Drought, groundwater storage and stream flow decline in southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
IOCI (2002) ‘Living with climate change – an overview of potential climate change impacts on Australia.’ (Australian Department of the Environment & Heritage, Australian Greenhouse Office) Available at http://www.greenhouse.gov.au [Accessed 4 September 2009].
IPCC (2007) Inter-Governmental Panel on Climate Change Fourth Assessment Report, Working Group II Report ‘Impacts, Adaptation and Vulnerability’. Available at www.ipcc.ch/pccreports/ar4-wg2.htm [Accessed 28 November 2007].
IPCC (2014) Inter-Governmental Panel on Climate Change Fifth Assessment Report, Working Group I ‘The Physical Science Basis’. Available at http://www.climatechange.gov.au/climate-change/climate-science/intergovernmental-panel-climate-change [Accessed 7 August 2014].
Lambeck, RJ (1997). Focal species: a multi-species umbrella for nature conservation. Conservation Biology 11, 849–856.
| Focal species: a multi-species umbrella for nature conservation.Crossref | GoogleScholarGoogle Scholar |
Leibold MA, Miller TE (2004) From metapopulations to metacommunities. In ‘Ecology, genetics, and evolution of metapopulations’. (Eds I Hanski, OE Gaggiotti) pp. 133–150. (Elsevier Academic Press: London, UK)
Lindenmayer, DB, Lane, P, Crane, M, Florance, D, Foster, CN, Ikin, K, Michael, D, Sato, CF, Scheele, BC, and Westgate, M (2019). Weather effects on birds of different size are mediated by long-term climate and vegetation type in endangered temperate woodlands. Global Change Biology 25, 675–685.
| Weather effects on birds of different size are mediated by long-term climate and vegetation type in endangered temperate woodlands.Crossref | GoogleScholarGoogle Scholar | 30431211PubMed |
Liu, D, Ogaya, R, Barbeta, A, Yang, X, and Peñuelas, J (2018). Long-term experimental drought combined with natural extremes accelerate vegetation shift in a Mediterranean holm oak forest. Environmental and Experimental Botany 151, 1–11.
| Long-term experimental drought combined with natural extremes accelerate vegetation shift in a Mediterranean holm oak forest.Crossref | GoogleScholarGoogle Scholar |
Luck G (1996) Bird population responses and artificial nest predation at inherent and induced edges in the Murray Mallee, South Australia. Honours thesis, University of Adelaide, Adelaide, SA, Australia.
Luck, G, Charmantier, A, and Ezanno, P (2001). Seasonal and landscape differences in the foraging behaviour of the Rufous Treecreeper Climacteris rufa. Pacific Conservation Biology 7, 9–20.
| Seasonal and landscape differences in the foraging behaviour of the Rufous Treecreeper Climacteris rufa.Crossref | GoogleScholarGoogle Scholar |
Luck GW (2000) Landscape differences in the ecology of the Rufous Treecreeper Climacteris rufa. PhD thesis, Edith Cowan University, Perth, WA, Australia.
Luck, GW (2002). The habitat requirements of the Rufous Treecreeper (Climacteris rufa). 1. Preferential habitat use demonstrated at multiple spatial scales. Biological Conservation 105, 383–394.
| The habitat requirements of the Rufous Treecreeper (Climacteris rufa). 1. Preferential habitat use demonstrated at multiple spatial scales.Crossref | GoogleScholarGoogle Scholar |
Matusick, G, Ruthoff, KX, Kala, J, Brouwers, NC, Breshears, DD, and Hardy, GJ (2018). Chronic historical drought legacy exacerbates tree mortality and crown dieback during acute heatwave-compounded drought. Environmental Research Letters 13, 095002.
| Chronic historical drought legacy exacerbates tree mortality and crown dieback during acute heatwave-compounded drought.Crossref | GoogleScholarGoogle Scholar |
Matusick, G, Ruthroff, KX, Brouwers, NC, Dell, B, and Hardy, GJ (2013). Sudden forest canopy collapse corresponding with extreme drought and heat in a Mediterranean-type eucalypt forest in southwestern Australia. European Journal of Forest Research 132, 497–510.
| Sudden forest canopy collapse corresponding with extreme drought and heat in a Mediterranean-type eucalypt forest in southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
Mercer JW (2003) Survey of Eucalyptus wandoo decline. Report prepared for CALM Science Division, Como, Western Australia, Australia.
Mercer JW (2008) Second survey of Eucalyptus wandoo decline. Final report on wandoo decline on behalf of the Wandoo Recovery Group. (Department of the Environment and Conservation and WWF-Australia)
Mettke-Hofmann, C (2017). Avian movements in a modern world: cognitive challenges. Animal Cognition 20, 77–86.
| Avian movements in a modern world: cognitive challenges.Crossref | GoogleScholarGoogle Scholar | 27287625PubMed |
Mitchell, PJ, O’Grady, AP, Hayes, KR, and Pinkard, EA (2014). Exposure of trees to drought-induced die-off is defined by a common climatic threshold across different vegetation types. Ecology and Evolution 4, 1088–1101.
| Exposure of trees to drought-induced die-off is defined by a common climatic threshold across different vegetation types.Crossref | GoogleScholarGoogle Scholar | 24772285PubMed |
Myers, N, Mittermeier, RA, Mittermeier, CG, da Fonseca, GAB, and Kent, J (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
| Biodiversity hotspots for conservation priorities.Crossref | GoogleScholarGoogle Scholar | 10706275PubMed |
Nolan, C, Overpeck, JT, Allen, JRM, Anderson, PM, Betancourt, JL, Binney, HL, Brewer, S, Bush, MB, Chase, BM, Cheddadi, R, Djamali, M, Dodson, J, Edwards, ME, Gosling, WD, Haberle, S, Hotchkiss, SC, Huntley, B, Ivory, SJ, Kershaw, AP, Kim, SH, Latorre, C, Leydet, M, Lézine, A, Liu, KB, Liu, Y, Lozhkin, AV, McGlone, MS, Marchant, RA, Momohara, A, Moreno, PI, Müller, S, Otto-Bliesner, BL, Shen, C, Stevenson, J, Takahara, H, Tarasov, PE, Tipton, J, Vincens, A, Weng, C, Xu, Q, Zheng, Z, and Jackson, ST (2018). Past and future global transformation of terrestrial ecosystems under climate change. Science 361, 920–923.
| Past and future global transformation of terrestrial ecosystems under climate change.Crossref | GoogleScholarGoogle Scholar | 30166491PubMed |
Nyári, ÁS, and Joseph, L (2011). Systematic dismantlement of Lichenostomus improves the basis for understanding relationships within the honeyeaters (Meliphagidae) and the historical development of Australo-Papuan bird communities. Emu – Austral Ornithology 111, 202–211.
| Systematic dismantlement of Lichenostomus improves the basis for understanding relationships within the honeyeaters (Meliphagidae) and the historical development of Australo-Papuan bird communities.Crossref | GoogleScholarGoogle Scholar |
Pollock, KH, and Raveling, DG (1982). Assumptions of modern band-recovery models, with emphasis on heterogeneous survival rates. The Journal of Wildlife Management 46, 88–98.
| Assumptions of modern band-recovery models, with emphasis on heterogeneous survival rates.Crossref | GoogleScholarGoogle Scholar |
Prober, SM, Byrne, M, McLean, EH, Steane, DA, Potts, BM, Vaillancourt, RE, and Stock, WD (2015). Climate adjusted provenancing: a strategy forclimate-resilient ecological restoration. Frontiers in Ecology and Evolution 3, 65.
| Climate adjusted provenancing: a strategy forclimate-resilient ecological restoration.Crossref | GoogleScholarGoogle Scholar |
Recher, HF, and Davis, WE (1998). The foraging profile of a wandoo woodland avifauna in early spring. Australian Journal of Ecology 23, 514–527.
| The foraging profile of a wandoo woodland avifauna in early spring.Crossref | GoogleScholarGoogle Scholar |
Risbey, JS, Pook, MJ, McIntosh, PC, Wheeler, MC, and Hendon, HH (2009). On the remote drivers of rainfall variability in Australia. Monthly Weather Review 137, 3233–3253.
| On the remote drivers of rainfall variability in Australia.Crossref | GoogleScholarGoogle Scholar |
Rose PW (1993) Production of habitat hollows by wheatbelt Eucalyptus. Final report – project R053. (Department of Conservation and Land Management (CALM): Perth, WA, Australia)
Rowley I, Russell E (1991) Demography of passerines in the temperate Southern Hemisphere. In ‘Bird population studies: Relevance to conservation management’. (Eds C Perrins, JD Lebreton, GJM Hirons) pp. 22–44. (Oxford University Press: Oxford, UK)
Saunders DA, Ingram JA (1995) ‘Birds of southwestern Australia.’ (Surrey Beatty & Sons: Chipping Norton, UK)
Saunders, D, Wintle, BA, Mawson, PR, and Dawson, R (2013). Egg-laying and rainfall synchrony in an endangered bird species: implications for conservation in a changing climate. Biological Conservation 161, 1–9.
| Egg-laying and rainfall synchrony in an endangered bird species: implications for conservation in a changing climate.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA (1989). Changes in the avifauna of a region, district and remnant as a result of fragmentation of native vegetation: the wheatbelt of Western Australia. A case study. Biological Conservation 50, 99–135.
| Changes in the avifauna of a region, district and remnant as a result of fragmentation of native vegetation: the wheatbelt of Western Australia. A case study.Crossref | GoogleScholarGoogle Scholar |
Smettem, KRJ, Waring, RH, Callow, JN, Wilson, M, and Mu, Q (2013). Satellite-derived estimates of forest leaf area index in southwest Western Australia are not tightly coupled to interannual variations in rainfall: implications for groundwater decline in a drying climate. Global Change Biology 19, 2401–2412.
| Satellite-derived estimates of forest leaf area index in southwest Western Australia are not tightly coupled to interannual variations in rainfall: implications for groundwater decline in a drying climate.Crossref | GoogleScholarGoogle Scholar |
Southwood TRE, Henderson PA (2000) ‘Ecological methods,’ 3rd edn. (Blackwell Science Ltd, London, UK)
SPSS (2016) IBM Corp. Released 2013. IBM SPSS statistics for Windows (version 22.0). (IBM Corp: Armonk, NY, USA)
SRES (2000) Special report on emissions scenarios, a special report of working group III of the intergovernmental panel on climate change (Cambridge University Press: Cambridge, UK) Available at https://www.ipcc.ch/site/assets/uploads/2018/03/emissions_scenarios-1.pdf
Thomas CD, Hanski I (2004) Metapopulation dynamics in changing environments: butterfly responses to habitat and climate change. In ‘Ecology, genetics and evolution of metapopulations’. (Eds I Hannski, OE Gaggiotti) pp. 489–514. (Elsevier Academic Press: London, UK)
Veneklaas, E, and Manning, L (2007). Wandoo crown decline linked to a changing environment? Landscope 22, 17–22.
Wallace, J, Behn, G, and Furby, S (2006). Vegetation condition assessment and monitoring from sequences of satellite imagery. Ecological Management & Restoration 7, S31–S36.
| Vegetation condition assessment and monitoring from sequences of satellite imagery.Crossref | GoogleScholarGoogle Scholar |
Watson, DM (2011). A productivity-based explanation for woodland bird declines: poorer soils yield less food. Emu – Austral Ornithology 111, 10–18.
| A productivity-based explanation for woodland bird declines: poorer soils yield less food.Crossref | GoogleScholarGoogle Scholar |
White, GC, and Burnham, KP (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139.
| Program MARK: survival estimation from populations of marked animals.Crossref | GoogleScholarGoogle Scholar |
White GC, Franklin AB, Shenk TM (2002) Estimating parameters of PVA models from data on marked animals. In ‘Population viability analysis’. (Eds SR Beissinger, DR McCullough) pp. 169–190. (The University of Chicago Press: Chicago, IL, USA)
Wigley, TML, Richels, R, and Edmonds, JA (1996). Economic and environmental choices in the stabilization of atmospheric CO2 concentrations. Nature 379, 240–243.
| Economic and environmental choices in the stabilization of atmospheric CO2 concentrations.Crossref | GoogleScholarGoogle Scholar |
Williams BK, Nichols JD, Conroy MJ (2002) Combining closed and open mark-recapture models: the robust design. In ‘Analysis and management of animal populations’. pp. 523–554. (Academic Press)
Wilson K (1997) The foraging ecology and habitat selection of the Yellow-plumed Honeyeater (Lichenostomus omatus) at Dryandra Woodland, Western Australia. PhD thesis, Edith Cowan University, Perth, WA, Australia.
Wilson, K, and Recher, HF (2001). Foraging ecology and habitat selection of the Yellow-plumed Honeyeater, Lichenostomus ornatus, in a Western Australian woodland: implications for conservation. Emu – Austral Ornithology 101, 89–94.
| Foraging ecology and habitat selection of the Yellow-plumed Honeyeater, Lichenostomus ornatus, in a Western Australian woodland: implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Wooller, RD, Richardson, KC, Garavanta, CAM, Saffer, VM, Anthony, C, and Wooller, SJ (1998). The influence of annual rainfall upon capture rates of a nectar-dependent marsupial. Wildlife Research 25, 165–169.
| The influence of annual rainfall upon capture rates of a nectar-dependent marsupial.Crossref | GoogleScholarGoogle Scholar |
Yates, CJ, McNeill, A, Elith, J, and Midgley, GF (2010). Assessing the impacts of climate change and land transformation on Banksia in the South West Australian Floristic Region. Diversity and Distributions 16, 187–201.
| Assessing the impacts of climate change and land transformation on Banksia in the South West Australian Floristic Region.Crossref | GoogleScholarGoogle Scholar |
Zdunic K, Behn G, van Dongen R (2012) Investigating the dynamics of wandoo crown decline with time series Landsat imagery. In ‘International archives of the photogrammetry, remote sensing and spatial information sciences, XXXIX-B3, XXII ISPRS congress, 25 August–1 September 2012, Melbourne, Australia’.