Introduced red foxes (Vulpes vulpes) driving Australian freshwater turtles to extinction? A critical evaluation of the evidence
Bruce C. Chessman A *
A *
A Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, UNSW Sydney, Sydney, NSW 2052, Australia.
Pacific Conservation Biology 28(6) 462-471 https://doi.org/10.1071/PC21058
Submitted: 1 September 2021 Accepted: 21 November 2021 Published: 14 December 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
It has been asserted that introduced red foxes (Vulpes vulpes) destroy ∼95% of nests of freshwater turtles in south-eastern Australia, are more efficient predators of freshwater turtle nests than Australian native predators, and are driving Australian freshwater turtle species to extinction. Available information was reviewed and analysed to test these assertions. Nest predation rates for all predators including foxes averaged 70% across Australia and 76% for south-eastern Australia compared to 72% for North America where freshwater turtles co-exist with many native predators, including foxes. Predation rates on Australian freshwater turtle nests did not differ significantly where foxes were included in the identified nest predators and where they were not, but sample sizes were very small. Evidence was lacking of foxes being the primary driver of population declines of Australian freshwater turtles, and some turtle populations are stable or increasing despite exposure to fox predation. Australian native species can be effective nest predators, and their role has probably been usurped by foxes to some degree. Where research shows that increased recruitment is necessary to conserve Australian freshwater turtle populations, strategies such as electric fencing of nesting beaches, nest protection cages and ex situ incubation of turtle eggs will probably be more cost-effective than efforts to reduce fox numbers. Further research is also needed to better understand the biological and environmental factors that regulate nest predation rates.
Keywords: Australia, extinction, freshwater, invasive species, nest, predation, turtle, Vulpes vulpes.
Introduction
More than half of the world’s species of turtles and tortoises are judged to be threatened with extinction (Rhodin et al. 2018). Australian freshwater turtles are no exception, with 44% of recognised species formally listed as Vulnerable, Endangered or Critically Endangered by Australian national, state and territory governments or by the International Union for Conservation of Nature (van Dyke et al. 2018). Knowledge of the conservation status of Australian freshwater turtles is also incomplete, with some species listed as Data Deficient in one or more jurisdictions.
Globally, freshwater turtles face a plethora of threats, including the loss, degradation and fragmentation of their habitats, overharvesting for food, traditional medicines or the pet trade, climate change, road traffic, predation and disease (Stanford et al. 2020). Australian species are vulnerable to all of these threats, but predation by introduced red foxes (Vulpes vulpes) has drawn particular attention since Thompson’s (1983) estimate that foxes destroyed 93% of nests of eastern long-necked turtles (Chelodina longicollis) and Macquarie turtles (Emydura macquarii). This figure was based on data from just three locations in South Australia obtained during one or two nesting seasons, but has been extrapolated to assert that ‘nest predation rates are extremely high in south-eastern Australia, with >90% of nests destroyed each year’ (Spencer 2018) and that ‘invasive foxes destroy 95% or more of all turtle nests present in the Murray catchment’ (Petrov et al. 2018). It has also been used to parameterise population models projecting that foxes will drive some Australian freshwater turtle species to extinction (Spencer et al. 2016, 2017).
Vulpes vulpes was introduced to Australia in 1871 and has had a devastating predatory impact on many species of Australian wildlife (Dickman 1996; Saunders et al. 2010). Fox predation on eggs of Australian freshwater turtles was reported more than a century ago (Adelaide Advertiser, 3 January 1910, p. 8), and foxes also kill turtles emerging on land to nest or migrate between water bodies (Spencer 2002; Dawson et al. 2016). Fox control, principally by poison baiting, is widely practised across Australia but its effectiveness in reducing destruction of freshwater turtle nests is questionable (Robley et al. 2016). It is important that assertions that foxes are driving Australian freshwater turtle species to extinction are critically evaluated, because over-statement of the risk posed by foxes could result in resources for turtle conservation being devoted to efforts to reduce fox numbers when they would be more effectively directed elsewhere.
This paper reviews and analyses available information in order to critically evaluate hypotheses that foxes routinely destroy ∼95% of nests of Australian freshwater turtles, are more efficient predators of freshwater turtle nests than Australian native predators, and are driving Australian freshwater turtles toward extinction. It then discusses options for increasing recruitment of Australian freshwater turtles where necessary.
Materials and methods
Books, journal papers, reports and theses were consulted for evidence of population trends of Australian freshwater turtles and their causes. Rates of predation on nests of Australian freshwater turtle species, either provided by authors or able to be calculated from data presented, were compiled. For comparative purposes, predation rates were also tabulated for freshwater turtle nests in North America, where foxes and nearly all other nest predators are indigenous. Relevant information was located by combing through the author’s collection of documents on Australian and other freshwater turtles, a Google Scholar search using the words ‘turtle’, ‘nest’ and ‘predation’, and screening the reference lists of documents selected by those processes. For North America, the estuarine diamondback terrapin (Malaclemys terrapin) and semi-aquatic species such as wood turtles (Glyptemys insculpta) were included along with strictly freshwater species. North American studies occasionally reported nest predation rates as proportions of eggs rather than nests, but the two statistics were considered broadly comparable.
Analysis of variance (ANOVA) and the non-parametric Mann–Whitney test were used to compare rates of predation on nests of Australian freshwater turtles between instances where the identified predators included foxes and instances where they did not. Data were not sufficient to include factors such as geographic region or turtle species. ANOVA and the Mann–Whitney test were also used to compare nest predation rates between Australia and North America. The deviation of ANOVA residuals from a normal distribution was assessed with the Shapiro–Wilk test.
Experimental studies monitoring artificial nests constructed by investigators were excluded from these analyses because studies of bird nests cast doubt on the equivalence of predation rates on artificial and natural nests (Berry and Lill 2003). In addition, predation rates on artificial nests can vary considerably depending on nest contents and construction (Spencer 2002; Buzuleciu et al. 2016; Edmunds et al. 2018). The analyses also excluded rates that were likely to be influenced by artificial reduction, inhibition or exclusion of predators, as well as instances in which losses from predation could not be separated from losses due to other factors such as floods or trampling by cattle. In order to ensure independence of observations, rates for the same turtle species and study area (e.g. for different years or sites) were averaged. Statistical analyses were performed with XLSTAT (Addinsoft 2021).
Results
Reported introduced predators of Australian freshwater turtle nests include cats (Felis catus), dogs (Canis familiaris) and pigs (Sus scrofa), as well as red foxes (V. vulpes) (Table 1; see also Parmenter 1985). Native nest predators include Australian magpies (Gymnorhina tibicen), crows and ravens (Corvus spp.), lapwings (Vanellus sp.), monitor lizards (Varanus spp.), rakali (Hydromys chrysogaster) and southern brown bandicoots (Isoodon obesulus) (Table 1; see also Burbidge 1981; Micheli-Campbell et al. 2013). Harrington (1933) reported that rats raided nests of C. longicollis, but did not specify the rat species or whether it was introduced or native.

|
Nest predation rates for Australian freshwater turtles (Table 1; n = 10) ranged from 20 to 97% and averaged 70 ± 7% overall (mean ± s.e.) or 76 ± 5% for south-eastern Australia only (New South Wales, South Australia and Victoria). Rates were higher where the identified predators included foxes (78 ± 5%) than where they did not (59 ± 15%), but the difference was not statistically significant (ANOVA: F1,8 = 1.91; P = 0.21; Mann–Whitney test: P = 0.26). ANOVA residuals deviated only slightly from a normal distribution (Shapiro–Wilk test: W = 0.97).
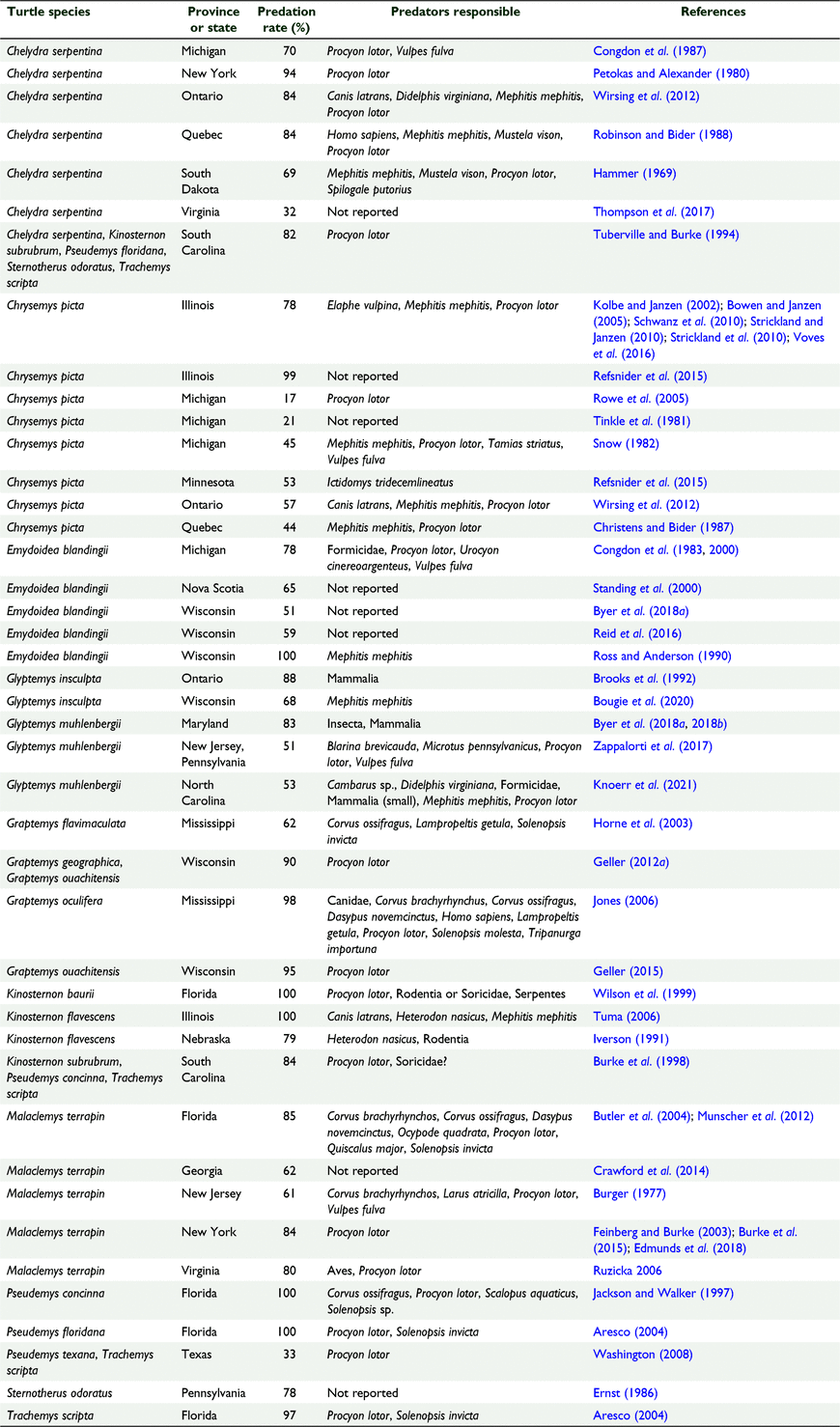
Nest predation rates for North American freshwater turtles (Table 2; n = 43) ranged from 17 to 100% and averaged 72 ± 3%. Nest predation rates in Australia and North America did not differ significantly (ANOVA: F1,51 = 0.09; P = 0.77; Mann–Whitney test: P = 0.71). ANOVA residuals deviated somewhat more from a normal distribution for this analysis (Shapiro–Wilk test: W = 0.93).
|
No evidence was found of foxes being the primary driver of population declines of Australian freshwater turtles. For example, decreases in numbers of western swamp turtles (Pseudemydura umbrina) in Western Australia since the 1960s have been attributed mainly to habitat loss and contraction of the turtle’s annual activity period due to a sharp decrease in winter rainfall, with fox predation noted as a contributing factor (Burbidge 1981; Kuchling 2000; Bouma et al. 2020). Excessive collection of eggs for the pet trade in the 1960s and 1970s has been primarily blamed for the low current abundance of the Mary River turtle (Elusor macrurus) in Queensland, although anecdotal evidence suggests an increase in nest predation since the 1960s (Flakus 2002). A long-term decline in catch per unit effort (CPUE) of C. longicollis in southern New South Wales was attributed primarily to extreme drought in association with anthropogenic flow alteration, whereas the cause of a co-incident decline in CPUE of E. macquarii could not be established (Chessman 2011). In eastern New South Wales, a sudden decline of ∼90% in the population of Bellinger River snapping turtles (Myuchelys georgesi) was caused by an epidemic (Chessman et al. 2020).
Moreover, some Australian freshwater turtle populations appear to be stable or increasing, despite exposure to fox predation. For example, no long-term change in CPUE of broad-shelled turtles (Chelodina expansa) was observed by Chessman (2011). In the Bellinger River, the population of translocated E. macquarii has increased from a few individuals to ∼500 in about 30 years (Chessman et al. 2020), despite the presence of foxes (Blamires et al. 2005). Emydura macquarii has also proliferated in other Australian river systems where it has been introduced (Chessman 2021).
Discussion
This review and analysis did not support the hypothesis that foxes routinely destroy ∼95% of nests of Australian freshwater turtles. The average nest predation rate across studies was 70%, or 76% for south-eastern Australia only, and these figures include predation by all species, not just foxes. Moreover, nest predation rates may have been overestimated in some studies because depredated nests with excavated soil and scattered eggshells are highly conspicuous (Chessman 2018; Petrov et al. 2018), whereas intact nests can be cryptic, particularly if some time has elapsed since nesting (Turner 2004; Spencer 2018). In addition, markers used to facilitate nest monitoring may attract predators (Rollinson and Brooks 2007). Alternatively, however, predation rates may be underestimated if nests are not monitored until the end of the period of hatchling emergence.
Studies of artificial nests were not included in the statistical analysis, but also failed to support a figure of ∼95% nest predation by foxes or even by all nest predators combined. For example, Spencer (2002) reported ∼55–80% predation on artificial nests containing turtle or quail eggs at Lake Mulwala, New South Wales. Blamires et al. (2005) recorded a 73% loss of artificial nests containing chicken eggs on the Bellinger River, New South Wales, from a combination of predation by foxes and monitor lizards, trampling by cattle and flooding. Dawson et al. (2014) reported 48–53% predation on artificial nests containing chicken eggs at Chittering Lakes, Western Australia. Robley et al. (2016) recorded 84% loss of artificial nests containing quail eggs at Hattah Lakes, Victoria, in the absence of fox control measures. Data graphed by Spencer et al. (2017) suggest an average depredation of ∼80% for artificial nests containing chicken or quail eggs in New South Wales and Victoria. As for natural nests, the average over all of these artificial-nest studies is ∼70% for all predators combined.
Supporting evidence was also lacking for the proposition that foxes are more efficient nest predators than Australian native birds, marsupials, monitor lizards and rodents. The lack of a statistically significant difference in Australian nest predation rates between instances where foxes were among the identified nest predators and instances where they were not might reflect the very small sample size, the role of other introduced predators, or incomplete detection of predator species, as well as the efficiency of native nest predators. However, it is clear that native species can destroy a large proportion of turtle nests. For example, monitor lizards had raided 11 of 12 nests of the pig-nosed turtle (Carettochelys insculpta) in Kakadu National Park, Northern Territory (Georges and Kennett 1989), and 18 of 24 nests of C. longicollis at Lake Cowal, New South Wales (Vestjens 1977). Lyndon (1966) and Beck (1991) both reported ravens (Corvus spp.) as voracious predators of C. longicollis nests. It is also likely that foxes have partly substituted for Australian native nest predators, because foxes have reduced or even eliminated populations of native species that are known or likely to prey on turtle nests, such as bandicoots, monitor lizards and quolls (Dasyurus spp.) (Dickman 1996; Saunders et al. 2010). Thus, fox suppression can increase populations of these native nest predators (Bowler 2016; Hu et al. 2019; Madden Hof et al. 2020). Large native marsupial predators (Sarcophilus harrisii and Thylacinus cynocephalus), which became extinct on mainland Australia soon after the introduction of dogs (dingoes) about 3500 years ago, might also have preyed on turtle nests (Burbidge 1981; Thompson 1983).
Nest predation rates also did not differ significantly between Australia and North America, where freshwater turtle nests are preyed on by a plethora of native arthropod, avian, mammalian and reptilian nest predators, including coyotes (Canis latrans), crows (Corvus spp.), mink (Mustela vison), North American red foxes (Vulpes fulva, sometimes assigned to V. vulpes), raccoons (Procyon lotor), and striped skunks (Mephitis mephitis) (Table 2). Some of these predators also take nesting turtles (e.g. Karson et al. 2018; Mullin et al. 2020).
If average nest predation rates are equivalent in Australia and North America, and North American turtles have been able to persist for millennia in sympatry with foxes and a panoply of other predators, why would foxes be able to drive Australian freshwater turtles to extinction? Spencer et al. (2016) maintain that Australian turtles such as E. macquarii are especially vulnerable to fox predation because they employ an ‘arribada’ strategy of synchronous and spatially concentrated mass nesting, like that of some sea turtles (Eckrich and Owens 1995). They suggest that this strategy evolved to protect some nests by saturating native nest predators, but fails to do so in the case of foxes, thereby constituting an ‘ethological trap’ that will eventually drive E. macquarii to extinction. However, an empirical study in Québec, Canada, concluded that synchronous nesting increased survival of nests of snapping turtles (Chelydra serpentina) that were preyed on by a suite of efficient predators including M. mephitis, M. vison and P. lotor (Robinson and Bider 1988). Moreover, nesting of E. macquarii is spread over about 2 months in the south of its distribution and 4 months further north, with females producing up to three clutches of eggs annually (Chessman 1978; Georges 1983; Booth 2010). Its nesting is triggered by rainfall (McCosker 2002; Bowen et al. 2005; Booth 2010), as in many other freshwater turtle species (Czaja et al. 2018), and is therefore concentrated during rain events. However, there is no evidence that E. macquarii migrates to nest communally in the manner of some sea turtles. Nesting during rain is probably adaptive regardless of the species of nest predator, because heavy rain softens soil to facilitate nest excavation, and can disguise nests from predators (Legler 1954; Bowen and Janzen 2005; Czaja et al. 2018).
Although the impact of foxes on nests of Australian freshwater turtles appears to have been exaggerated, it is clear that foxes have a substantial impact on turtle recruitment and survival, except in the far north of the continent where foxes are absent and feral pigs are the chief introduced predator (Fordham et al. 2006). In addition, some fox populations may be enhanced by mesopredator release due to suppression of Australia’s apex terrestrial predators, dingoes and other wild dogs (Wallach et al. 2010; Letnic et al. 2011; though see Allen et al. 2013). Furthermore, foxes may benefit from anthropogenic food subsidies (Oro et al. 2013), which they widely exploit (Fleming et al. 2021). Consequently, the pressure of fox predation on turtle nests is likely to be spatially variable and intense in some regions.
How should nest predation be curtailed where research shows that increased recruitment is necessary for the persistence or recovery of Australian freshwater turtle populations? Many methods are used to control foxes across Australia for both wildlife conservation and livestock protection, including exclusion fencing, den fumigation, shooting, trapping and stationing of guard animals. However, the deployment of meat baits poisoned with sodium fluoroacetate (1080) is most popular because of its cost effectiveness (Saunders et al. 2010). Controlling fox numbers with poison baits has been found to lower predation on nests of both freshwater and marine turtles in Australia (Spencer 2002; Madden Hof et al. 2020), but a major fox-baiting programme at the Hattah Lakes in Victoria did not significantly reduce predation on artificial turtle nests (Robley et al. 2016). Spencer et al. (2017) suggested that poison baiting may be ineffective unless it results in the near elimination of the fox population because predation on artificial nests was independent of fox abundance unless the latter was extremely low. In addition, baits are often consumed by non-target species (Dundas et al. 2014; Millar et al. 2015), and foxes can rapidly immigrate to re-populate areas where baiting has been undertaken (Gentle et al. 2007), or develop bait avoidance or toxin tolerance (Allsop et al. 2017). Thus, effective reduction of turtle nest predation by fox baiting suffers from many practical constraints.
Excluding foxes from nesting areas through fencing can be effective (Palmer-Allen et al. 1991), but can also produce perverse outcomes. For example, a predator-exclusion fence surrounding a reserve in the Australian Capital Territory intercepted and killed migrating C. longicollis, apparently by making them overheat (Ferronato et al. 2014). In Western Australia, the installation of fox-exclusion fences around reserves to protect P. umbrina led to population increases of other predators inside the reserves: native bandicoots (I. obesulus) and ravens (Corvus coronoides), and introduced rats (Rattus spp.) (Kuchling 2000; Bowler 2016). Electric fencing of nesting areas (Geller 2012b) can provide a targeted way to reduce nest predation, and such fences can be removed if necessary once hatchlings have emerged.
Locating intact turtle nests and covering them with predator-exclusion screens or cages can also be effective for inhibiting both foxes and other predators (Graham 1997; Riley and Litzgus 2013). This technique proved more effective than lethal fox control via trapping and den fumigation for protecting eggs of Australian sea turtles (O’Connor et al. 2017). However, predation on hatchlings after their emergence from protected nests may limit the benefits in some circumstances (Campbell et al. 2020), and care may be needed to avoid attracting illegal egg or hatchling collectors (Flakus 2002).
Another option for increasing turtle recruitment is to bypass reproduction-related predation entirely by capturing gravid females in the wild and inducing their oviposition with hormone injections (Ewert and Legler 1978). The eggs so obtained can be artificially incubated, and the resulting hatchlings can be released in habitats where they have the best chance of survival, or else head-started; i.e. kept in captivity until they grow big enough to resist most predators (Buhlmann et al. 2015; Carstairs et al. 2019). In extreme cases, captive breeding may be necessary (Kuchling 2000; Chessman et al. 2020).
Research is needed to better understand the biological and environmental factors that regulate recruitment and survival of Australian freshwater turtles, and why some turtle populations are able to increase despite exposure to fox predation, while others seem unable to do so. Spencer et al. (2016) suggested that C. expansa is less vulnerable than E. macquarii to nest predation by foxes because nests of C. expansa are more widely scattered. However, available evidence shows population stability for C. expansa (Chessman 2011) and increases for E. macquarii in some instances (Chessman et al. 2020; Chessman 2021). It is important that assessments of nest predation rates extend for several years, because inter-annual variation in rates can be enormous (e.g. Congdon et al. 2000; Schwanz et al. 2010).
Conclusions and recommendations
Assertions that red foxes consistently cause ∼95% depredation of the nests of freshwater turtles in south-eastern Australia and are thereby driving turtle species to extinction, are not supported by the available evidence. Documented declines of Australian freshwater turtle populations have mainly been attributed to factors other than fox predation, and population models forecasting fox-induced extinction are based on what appear to be inflated nest predation rates. Where research shows that increased recruitment is necessary for the persistence or recovery of populations of Australian freshwater turtles, strategies such as electric fencing of nesting beaches, caging of nests, artificial incubation of eggs, and head-starting of juvenile turtles will probably be more cost-effective than efforts to reduce fox numbers.
Data availability
The data analysed are available in the cited works.
Conflicts of interest
The author declares no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The anonymous reviewers are thanked for their helpful comments on the initial submission of this paper.
References
Addinsoft (2021) XLSTAT. Available at https://www.xlstat.com/en/. [Accessed 24 June 2021]Allen, BL, Allen, LR, Engeman, RM, and Leung, LK-P (2013). Intraguild relationships between sympatric predators exposed to lethal control: predator manipulation experiments. Frontiers in Zoology 10, e39.
| Intraguild relationships between sympatric predators exposed to lethal control: predator manipulation experiments.Crossref | GoogleScholarGoogle Scholar |
Allsop, SE, Dundas, SJ, Adams, PJ, Kreplins, TL, Bateman, PW, and Fleming, PA (2017). Reduced efficacy of baiting programs for invasive species: some mechanisms and management implications. Pacific Conservation Biology 23, 240–257.
| Reduced efficacy of baiting programs for invasive species: some mechanisms and management implications.Crossref | GoogleScholarGoogle Scholar |
Aresco, MJ (2004). Reproductive ecology of Pseudemys floridana and Trachemys scripta (Testudines: Emydidae) in northwestern Florida. Journal of Herpetology 38, 249–256.
| Reproductive ecology of Pseudemys floridana and Trachemys scripta (Testudines: Emydidae) in northwestern Florida.Crossref | GoogleScholarGoogle Scholar |
Beck, RG (1991). The common longnecked tortoise Chelodina longicollis (Shaw 1802) (Testudinata: Chelidae): a comparative study of the morphology and field behaviour of disjunct populations. South Australian Naturalist 66, 4–22.
Berry, L, and Lill, A (2003). Do predation rates on artificial nests accurately predict predation rates on natural nests? The effects of nest type, egg type and nest-site characteristics. Emu – Austral Ornithology 103, 207–214.
| Do predation rates on artificial nests accurately predict predation rates on natural nests? The effects of nest type, egg type and nest-site characteristics.Crossref | GoogleScholarGoogle Scholar |
Blamires, SJ, Spencer, R-J, King, P, and Thompson, MB (2005). Population parameters and life-table analysis of two coexisting freshwater turtles: are the Bellinger River turtle populations threatened? Wildlife Research 32, 339–347.
| Population parameters and life-table analysis of two coexisting freshwater turtles: are the Bellinger River turtle populations threatened?Crossref | GoogleScholarGoogle Scholar |
Booth, D (2010). The natural history of nesting in two Australian freshwater turtles. Australian Zoologist 35, 198–203.
| The natural history of nesting in two Australian freshwater turtles.Crossref | GoogleScholarGoogle Scholar |
Bougie, TA, Byer, NW, Lapin, CN, Peery, MZ, Woodford, JE, and Pauli, JN (2020). Wood turtle (Glyptemys insculpta) nest protection reduces depredation and increases success, but annual variation influences its effectiveness. Canadian Journal of Zoology 98, 715–724.
| Wood turtle (Glyptemys insculpta) nest protection reduces depredation and increases success, but annual variation influences its effectiveness.Crossref | GoogleScholarGoogle Scholar |
Bouma, A, Kuchling, G, Zhai, SY, and Mitchell, N (2020). Assisted colonisation trials for the western swamp turtle show that juveniles can grow in cooler and wetter climates. Endangered Species Research 43, 75–88.
| Assisted colonisation trials for the western swamp turtle show that juveniles can grow in cooler and wetter climates.Crossref | GoogleScholarGoogle Scholar |
Bowen, KD, and Janzen, FJ (2005). Rainfall and depredation of nests of the painted turtle, Chrysemys picta. Journal of Herpetology 39, 649–652.
| Rainfall and depredation of nests of the painted turtle, Chrysemys picta.Crossref | GoogleScholarGoogle Scholar |
Bowen, KD, Spencer, R-J, and Janzen, FJ (2005). A comparative study of environmental factors that affect nesting in Australian and North American freshwater turtles. Journal of Zoology 267, 397–404.
| A comparative study of environmental factors that affect nesting in Australian and North American freshwater turtles.Crossref | GoogleScholarGoogle Scholar |
Bowler H (2016) Conservation catch 22: are threatened southern brown bandicoots (Isoodon obesulus) predating critically endangered western swamp turtle (Pseudemydura umbrina) nests?. BSc(Hons) thesis, University of Western Australia, Perth.
Brooks, RJ, Shilton, CM, Brown, GP, and Quinn, NWS (1992). Body size, age distribution, and reproduction in a northern population of wood turtles (Clemmys insculpta). Canadian Journal of Zoology 70, 462–469.
| Body size, age distribution, and reproduction in a northern population of wood turtles (Clemmys insculpta).Crossref | GoogleScholarGoogle Scholar |
Buhlmann, KA, Koch, SL, Butler, BO, Tuberville, TD, Palermo, VJ, Bastarache, BA, and Cava, ZA (2015). Reintroduction and head-starting: tools for Blanding’s turtle (Emydoidea blandingii) conservation. Herpetological Conservation and Biology 10, 436–454.
Burbidge, AA (1981). The ecology of the western swamp tortoise Pseudemydura umbrina (Testudines: Chelidae). Australian Wildlife Research 8, 203–223.
| The ecology of the western swamp tortoise Pseudemydura umbrina (Testudines: Chelidae).Crossref | GoogleScholarGoogle Scholar |
Burger, J (1977). Determinants of hatching success in diamond-back terrapin, Malaclemys terrapin American Midland Naturalist 97, 444–464.
| Determinants of hatching success in diamond-back terrapin, Malaclemys terrapinCrossref | GoogleScholarGoogle Scholar |
Burke, VJ, Rathbun, SL, Bodie, JR, and Gibbons, JW (1998). Effect of density on predation rate for turtle nests in a complex landscape. Oikos 83, 3–11.
| Effect of density on predation rate for turtle nests in a complex landscape.Crossref | GoogleScholarGoogle Scholar |
Burke, RL, Vargas, M, and Kanonik, A (2015). Pursuing pepper protection: habanero pepper powder does not reduce raccoon predation of terrapin nests. Chelonian Conservation and Biology 14, 201–203.
| Pursuing pepper protection: habanero pepper powder does not reduce raccoon predation of terrapin nests.Crossref | GoogleScholarGoogle Scholar |
Butler, JA, Broadhurst, C, Green, M, and Mullin, Z (2004). Nesting, nest predation and hatchling emergence of the Carolina diamondback terrapin, Malaclemys terrapin centrata, in northeastern Florida. American Midland Naturalist 152, 145–155.
| Nesting, nest predation and hatchling emergence of the Carolina diamondback terrapin, Malaclemys terrapin centrata, in northeastern Florida.Crossref | GoogleScholarGoogle Scholar |
Buzuleciu, SA, Crane, DP, and Parker, SL (2016). Scent of disinterred soil as an olfactory cue used by raccoons to locate nests of diamondbacked terrapins (Malaclemys terrapin). Herpetological Conservation and Biology 11, 539–551.
Byer, NW, Reid, BN, Seigel, RA, and Peery, MZ (2018a). Applying lessons from avian ecology to herpetological research: techniques for analyzing nest survival due to predation. Herpetological Conservation and Biology 13, 517–532.
Byer, NW, Smith, SA, and Seigel, RA (2018b). Microgeographic variation in bog turtle nesting ecology. Journal of Herpetology 52, 228–233.
| Microgeographic variation in bog turtle nesting ecology.Crossref | GoogleScholarGoogle Scholar |
Campbell, MA, Connell, MJ, Collett, SJ, Udyawer, V, Crewe, TL, McDougall, A, and Campbell, HA (2020). The efficacy of protecting turtle nests as a conservation strategy to reverse population decline. Biological Conservation 251, e108769.
| The efficacy of protecting turtle nests as a conservation strategy to reverse population decline.Crossref | GoogleScholarGoogle Scholar |
Carstairs, S, Paterson, JE, Jager, KL, Gasbarrini, D, Mui, AB, and Davy, CM (2019). Population reinforcement accelerates subadult recruitment rates in an endangered freshwater turtle. Animal Conservation 22, 589–599.
| Population reinforcement accelerates subadult recruitment rates in an endangered freshwater turtle.Crossref | GoogleScholarGoogle Scholar |
Chessman BC (1978) Ecological studies of freshwater turtles in south-eastern Australia. PhD thesis, Monash University, Melbourne.
Chessman, BC (2011). Declines of freshwater turtles associated with climatic drying in Australia’s Murray–Darling Basin. Wildlife Research 38, 664–671.
| Declines of freshwater turtles associated with climatic drying in Australia’s Murray–Darling Basin.Crossref | GoogleScholarGoogle Scholar |
Chessman, BC (2018). Freshwater turtle hatchlings that stay in the nest: strategists or prisoners? Australian Journal of Zoology 66, 34–40.
| Freshwater turtle hatchlings that stay in the nest: strategists or prisoners?Crossref | GoogleScholarGoogle Scholar |
Chessman BC (2021) A creeping threat? Introduced Macquarie turtles and the future of endangered helmeted turtles in southern Australia. Aquatic Conservation: Marine and Freshwater Ecosystems 10.1002/aqc.3723
Chessman, BC, McGilvray, G, Ruming, S, Jones, HA, Petrov, K, Fielder, DP, Spencer, R-J, and Georges, A (2020). On a razor’s edge: status and prospects of the critically endangered Bellinger River snapping turtle, Myuchelys georgesi. Aquatic Conservation: Marine and Freshwater Ecosystems 30, 586–600.
| On a razor’s edge: status and prospects of the critically endangered Bellinger River snapping turtle, Myuchelys georgesi.Crossref | GoogleScholarGoogle Scholar |
Christens, E, and Bider, JR (1987). Nesting activity and hatching success of the painted turtle (Chrysemys picta marginata) in southwestern Quebec. Herpetologica 43, 55–65.
Congdon, JD, Tinkle, DW, Breitenbach, GL, and van Loben Sels, RC (1983). Nesting ecology and hatching success in the turtle Emydoidea blandingi. Herpetologica 39, 417–429.
Congdon, JD, Breitenbach, GL, van Loben Sels, RC, and Tinkle, DW (1987). Reproductive and nesting ecology of snapping turtles (Chelydra serpentina) in southeastern Michigan. Herpetologica 43, 39–54.
Congdon, JD, Nagle, RD, Kinney, OM, Osentoski, M, Avery, HW, van Loben Sels, RC, and Tinkle, DW (2000). Nesting ecology and embryo mortality: implications for hatching success and demography of Blanding’s turtles (Emydoidea blandingii). Chelonian Conservation and Biology 3, 569–579.
Crawford, BA, Maerz, JC, Nibbelink, NP, Buhlmann, KA, and Norton, TM (2014). Estimating the consequences of multiple threats and management strategies for semi-aquatic turtles. Journal of Applied Ecology 51, 359–366.
| Estimating the consequences of multiple threats and management strategies for semi-aquatic turtles.Crossref | GoogleScholarGoogle Scholar |
Czaja, RA, Kanonik, A, and Burke, RL (2018). The effect of rainfall on predation of diamond-backed terrapin (Malaclemys terrapin) nests. Journal of Herpetology 52, 402–405.
| The effect of rainfall on predation of diamond-backed terrapin (Malaclemys terrapin) nests.Crossref | GoogleScholarGoogle Scholar |
Dawson, SJ, Adams, PJ, Huston, RM, and Fleming, PA (2014). Environmental factors influence nest excavation by foxes. Journal of Zoology 294, 104–113.
| Environmental factors influence nest excavation by foxes.Crossref | GoogleScholarGoogle Scholar |
Dawson, SJ, Crawford, HM, Huston, RM, Adams, PJ, and Fleming, PA (2016). How to catch red foxes red handed: identifying predation of freshwater turtles and nests. Wildlife Research 43, 615–622.
| How to catch red foxes red handed: identifying predation of freshwater turtles and nests.Crossref | GoogleScholarGoogle Scholar |
Dickman, CR (1996). Impact of exotic generalist predators on the native fauna of Australia. Wildlife Biology 2, 185–195.
| Impact of exotic generalist predators on the native fauna of Australia.Crossref | GoogleScholarGoogle Scholar |
Doody, JS, Georges, A, and Young, JE (2004). Determinants of reproductive success and offspring sex in a turtle with environmental sex determination. Biological Journal of the Linnean Society 81, 1–16.
| Determinants of reproductive success and offspring sex in a turtle with environmental sex determination.Crossref | GoogleScholarGoogle Scholar |
Doody, JS, Green, B, Sims, R, Rhind, D, West, P, and Steer, D (2006). Indirect impacts of invasive cane toads (Bufo marinus) on nest predation in pig-nosed turtles (Carettochelys insculpta). Wildlife Research 33, 349–354.
| Indirect impacts of invasive cane toads (Bufo marinus) on nest predation in pig-nosed turtles (Carettochelys insculpta).Crossref | GoogleScholarGoogle Scholar |
Dundas, SJ, Adams, PJ, and Fleming, PA (2014). First in, first served: uptake of 1080 poison fox baits in south-west Western Australia. Wildlife Research 41, 117–126.
| First in, first served: uptake of 1080 poison fox baits in south-west Western Australia.Crossref | GoogleScholarGoogle Scholar |
Eckrich, CE, and Owens, DW (1995). Solitary versus arribada nesting in the olive ridley sea turtles (Lepidochelys olivacea): a test of the predator-satiation hypothesis. Herpetologica 51, 349–354.
Edmunds, SE, Kasparov, CN, Yoon, JB, Kanonik, AK, and Burke, RL (2018). Twelve years later: reassessing visual and olfactory cues raccoons use to find diamondback terrapin nests. Journal of Herpetology 52, 307–312.
| Twelve years later: reassessing visual and olfactory cues raccoons use to find diamondback terrapin nests.Crossref | GoogleScholarGoogle Scholar |
Ercolano E (2008) Aquatic and terrestrial habitat use of the Australian freshwater turtle, Chelodina expansa. Report for Australia: Natural and Cultural Ecology, SIT Study Abroad. University of Canberra, Canberra.
Ernst, CH (1986). Ecology of the turtle, Sternotherus odoratus, in southeastern Pennsylvania. Journal of Herpetology 20, 341–352.
| Ecology of the turtle, Sternotherus odoratus, in southeastern Pennsylvania.Crossref | GoogleScholarGoogle Scholar |
Ewert, MA, and Legler, JM (1978). Hormonal induction of oviposition in turtles. Herpetologica 34, 314–318.
Feinberg, JA, and Burke, RL (2003). Nesting ecology and predation of diamondback terrapins, Malaclemys terrapin, at Gateway National Recreation Area, New York. Journal of Herpetology 37, 517–526.
| Nesting ecology and predation of diamondback terrapins, Malaclemys terrapin, at Gateway National Recreation Area, New York.Crossref | GoogleScholarGoogle Scholar |
Ferronato, BO, Roe, JH, and Georges, A (2014). Reptile bycatch in a pest-exclusion fence established for wildlife reintroductions. Journal for Nature Conservation 22, 577–585.
| Reptile bycatch in a pest-exclusion fence established for wildlife reintroductions.Crossref | GoogleScholarGoogle Scholar |
Flakus SP (2002) Ecology of the Mary River turtle, Elusor macrurus. PhD thesis, University of Queensland, Brisbane.
Fleming, PA, Crawford, HM, Stobo-Wilson, AM, Dawson, SJ, Dickman, CR, Dundas, SJ, Gentle, MN, Newsome, TN, O’Connor, J, Palmer, R, Riley, J, Ritchie, EG, Speed, J, Saunders, G, Stuart, J-MD, Thompson, E, Turpin, JM, and Woinarski, JCZ (2021). Diet of the introduced red fox Vulpes vulpes in Australia: analysis of temporal and spatial patterns. Mammal Review 51, 508–527.
| Diet of the introduced red fox Vulpes vulpes in Australia: analysis of temporal and spatial patterns.Crossref | GoogleScholarGoogle Scholar |
Fordham, D, Georges, A, Corey, B, and Brook, BW (2006). Feral pig predation threatens the indigenous harvest and local persistence of snake-necked turtles in northern Australia. Biological Conservation 133, 379–388.
| Feral pig predation threatens the indigenous harvest and local persistence of snake-necked turtles in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Freeman A (2010) ‘Saving a living fossil: identification and mitigation of threats to the conservation status of the freshwater turtle, Elseya lavarackorum.’ (Department of Environment and Resource Management: Atherton)
Geller, GA (2012a). Notes on the nest predation dynamics of Graptemys at two Wisconsin sites using trail camera monitoring. Chelonian Conservation and Biology 11, 197–205.
| Notes on the nest predation dynamics of Graptemys at two Wisconsin sites using trail camera monitoring.Crossref | GoogleScholarGoogle Scholar |
Geller, GA (2012b). Reducing predation of freshwater turtle nests with a simple electric fence. Herpetological Review 43, 398–403.
Geller, GA (2015). A test of substrate sweeping as a strategy to reduce raccoon predation of freshwater turtle nests, with insights from supplemental artificial nests. Chelonian Conservation and Biology 14, 64–72.
| A test of substrate sweeping as a strategy to reduce raccoon predation of freshwater turtle nests, with insights from supplemental artificial nests.Crossref | GoogleScholarGoogle Scholar |
Gentle, MN, Saunders, GR, and Dickman, CR (2007). Poisoning for production: how effective is fox baiting in south-eastern Australia? Mammal Review 37, 177–190.
| Poisoning for production: how effective is fox baiting in south-eastern Australia?Crossref | GoogleScholarGoogle Scholar |
Georges, A (1983). Reproduction of the Australian freshwater turtle Emydura krefftii (Chelonia: Chelidae). Journal of Zoology 201, 331–350.
| Reproduction of the Australian freshwater turtle Emydura krefftii (Chelonia: Chelidae).Crossref | GoogleScholarGoogle Scholar |
Georges, A, and Kennett, R (1989). Dry-season distribution and ecology of Carettochelys insculpta (Chelonia: Carettochelydidae) in Kakadu National Park, northern Australia. Australian Wildlife Research 16, 323–335.
| Dry-season distribution and ecology of Carettochelys insculpta (Chelonia: Carettochelydidae) in Kakadu National Park, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Graham, T (1997). Effective predator excluders for turtle nests. Herpetological Review 28, 76.
Hammer, DA (1969). Parameters of a marsh snapping turtle population Lacreek Refuge, South Dakota. The Journal of Wildlife Management 33, 995–1005.
| Parameters of a marsh snapping turtle population Lacreek Refuge, South Dakota.Crossref | GoogleScholarGoogle Scholar |
Harrington, KH (1933). Breeding habits of the Australian long-necked tortoise (Chelodina longicollis). South Australian Naturalist 15, 25–27.
Horne, BD, Brauman, RJ, Moore, MJC, and Seigel, RA (2003). Reproductive and nesting ecology of the yellow-blotched map turtles, Graptemys flavimaculata: implications for conservation and management. Copeia 2003, 729–738.
| Reproductive and nesting ecology of the yellow-blotched map turtles, Graptemys flavimaculata: implications for conservation and management.Crossref | GoogleScholarGoogle Scholar |
Hu, Y, Gillespie, G, and Jessop, TS (2019). Variable reptile responses to introduced predator control in southern Australia. Wildlife Research 46, 64–75.
| Variable reptile responses to introduced predator control in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Iverson, JB (1991). Life history and demography of the yellow mud turtle, Kinosternon flavescens. Herpetologica 47, 373–395.
| Life history and demography of the yellow mud turtle, Kinosternon flavescens.Crossref | GoogleScholarGoogle Scholar |
Jackson, DR, and Walker, RN (1997). Reproduction in the Suwannee cooter, Pseudemys concinna suwanniensis. Bulletin of the Florida Museum of Natural History 41, 69–167.
Jones, RL (2006). Reproduction and nesting of the endangered ringed map turtle, Graptemys oculifera, in Mississippi. Chelonian Conservation and Biology 5, 195–209.
| Reproduction and nesting of the endangered ringed map turtle, Graptemys oculifera, in Mississippi.Crossref | GoogleScholarGoogle Scholar |
Karson, A, Ango, SYJ, and Davy, CM (2018). Depredation of gravid freshwater turtles by raccoons (Procyon lotor). Canadian Field-Naturalist 132, 122–125.
| Depredation of gravid freshwater turtles by raccoons (Procyon lotor).Crossref | GoogleScholarGoogle Scholar |
Knoerr, MD, Graeter, GJ, and Barrett, K (2021). Hatch success and recruitment patterns of the bog turtle. The Journal of Wildlife Management 85, 293–302.
| Hatch success and recruitment patterns of the bog turtle.Crossref | GoogleScholarGoogle Scholar |
Kolbe, JJ, and Janzen, FJ (2002). Spatial and temporal dynamics of turtle nest predation: edge effects. Oikos 99, 538–544.
| Spatial and temporal dynamics of turtle nest predation: edge effects.Crossref | GoogleScholarGoogle Scholar |
Kuchling, G (2000). Conservation strategies for remnant turtle populations: the Western Australian swamp turtle Pseudemydura umbrina recovery programme. Stapfia 69, 179–188.
Legler, JM (1954). Nesting habits of the western painted turtle, Chrysemys picta bellii (Gray). Herpetologica 10, 137–144.
Letnic, M, Greenville, A, Denny, E, Dickman, CR, Tischler, M, Gordon, C, and Koch, F (2011). Does a top predator suppress the abundance of an invasive mesopredator at a continental scale? Global Ecology and Biogeography 20, 343–353.
| Does a top predator suppress the abundance of an invasive mesopredator at a continental scale?Crossref | GoogleScholarGoogle Scholar |
Lyndon, E (1966). Freshwater tortoises at sale. Victorian Naturalist 83, 170–171.
Madden Hof, CA, Shuster, G, McLachlan, N, McLachlan, B, Giudice, S, Limpus, C, and Egichi, T (2020). Protecting nests of the critically endangered South Pacific loggerhead turtle Caretta caretta from goanna Varanus spp. predation. Oryx 54, 323–331.
| Protecting nests of the critically endangered South Pacific loggerhead turtle Caretta caretta from goanna Varanus spp. predation.Crossref | GoogleScholarGoogle Scholar |
McCosker, J (2002). Chelodina expansa (broad shell river turtle) and Emydura signata (Brisbane shortneck turtle) reproduction. Herpetological Review 33, 198–199.
Micheli-Campbell, MA, Baumgartl, T, Booth, DT, Campbell, HA, Connell, M, and Franklin, CE (2013). Selectivity and repeated use of nesting sites in a freshwater turtle. Herpetologica 69, 383–396.
| Selectivity and repeated use of nesting sites in a freshwater turtle.Crossref | GoogleScholarGoogle Scholar |
Millar, A, Gentle, M, and Leung, LK-P (2015). Non-target species interaction with sodium fluoroacetate (1080) meat bait for controlling feral pigs (Sus scrofa). Pacific Conservation Biology 21, 158–162.
| Non-target species interaction with sodium fluoroacetate (1080) meat bait for controlling feral pigs (Sus scrofa).Crossref | GoogleScholarGoogle Scholar |
Mullin, DI, White, RC, Lentini, AM, Brooks, RJ, Bériault, KR, and Litzgus, JD (2020). Predation and disease limit population recovery following 15 years of headstarting an endangered freshwater turtle. Biological Conservation 245, e108496.
| Predation and disease limit population recovery following 15 years of headstarting an endangered freshwater turtle.Crossref | GoogleScholarGoogle Scholar |
Munscher, EC, Kuhns, EH, Cox, CA, and Butler, JA (2012). Decreased nest mortality for the Carolina diamondback terrapin (Malaclemys terrapin centrata) following removal of raccoons (Procyon lotor) from a nesting beach in northeastern Florida. Herpetological Conservation and Biology 7, 176–184.
O’Connor, JM, Limpus, CJ, Hofmeister, KM, Allen, BL, and Burnett, SE (2017). Anti-predator meshing may provide greater protection for sea turtle nests than predator removal. PLOS ONE 12, e0171831.
| Anti-predator meshing may provide greater protection for sea turtle nests than predator removal.Crossref | GoogleScholarGoogle Scholar | 28187181PubMed |
Oro, D, Genovart, M, Tavecchia, G, Fowler, MS, and Martínez-Abraín, A (2013). Ecological and evolutionary implications of food subsidies from humans. Ecology Letters 16, 1501–1514.
| Ecological and evolutionary implications of food subsidies from humans.Crossref | GoogleScholarGoogle Scholar | 24134225PubMed |
Palmer-Allen, M, Beynon, F, and Georges, A (1991). Hatchling sex ratios are independent of temperature in field nests of the long-necked turtle, Chelodina longicollis (Testudinata: Chelidae). Wildlife Research 18, 225–231.
| Hatchling sex ratios are independent of temperature in field nests of the long-necked turtle, Chelodina longicollis (Testudinata: Chelidae).Crossref | GoogleScholarGoogle Scholar |
Parmenter CJ (1985) Reproduction and survivorship of Chelodina longicollis (Testudinata: Chelidae). In ‘Biology of Australasian frogs and reptiles’. (Eds G Grigg, R Shine, H Ehmann) pp. 53–61. (Royal Zoological Society of New South Wales: Sydney)
Petokas, PJ, and Alexander, MM (1980). The nesting of Chelydra serpentina in northern New York. Journal of Herpetology 14, 239–244.
| The nesting of Chelydra serpentina in northern New York.Crossref | GoogleScholarGoogle Scholar |
Petrov, K, Stricker, H, Van Dyke, JU, Stockfeld, G, West, P, and Spencer, R-J (2018). Nesting habitat of the broad-shelled turtle (Chelodina expansa). Australian Journal of Zoology 66, 4–14.
| Nesting habitat of the broad-shelled turtle (Chelodina expansa).Crossref | GoogleScholarGoogle Scholar |
Refsnider, JM, Reedy, AM, Warner, DA, and Janzen, FJ (2015). Do trade-offs between predation pressures on females versus nests drive nest-site choice in painted turtles? Biological Journal of the Linnean Society 116, 847–855.
| Do trade-offs between predation pressures on females versus nests drive nest-site choice in painted turtles?Crossref | GoogleScholarGoogle Scholar |
Reid, BN, Thiel, RP, and Peery, MZ (2016). Population dynamics of endangered Blanding’s turtles in a restored area. The Journal of Wildlife Management 80, 553–562.
| Population dynamics of endangered Blanding’s turtles in a restored area.Crossref | GoogleScholarGoogle Scholar |
Rhodin, AGJ, Stanford, CB, van Dijk, PP, et al. (2018). Global conservation status of turtles and tortoises (Order Testudines). Chelonian Conservation and Biology 17, 135–161.
| Global conservation status of turtles and tortoises (Order Testudines).Crossref | GoogleScholarGoogle Scholar |
Riley, JL, and Litzgus, JD (2013). Evaluation of predator-exclusion cages used in turtle conservation: cost analysis and effects on nest environment and proxies of hatchling fitness. Wildlife Research 40, 499–511.
| Evaluation of predator-exclusion cages used in turtle conservation: cost analysis and effects on nest environment and proxies of hatchling fitness.Crossref | GoogleScholarGoogle Scholar |
Robinson, C, and Bider, JR (1988). Nesting synchrony – a strategy to decrease predation of snapping turtle (Chelydra serpentina) nests. Journal of Herpetology 22, 470–473.
| Nesting synchrony – a strategy to decrease predation of snapping turtle (Chelydra serpentina) nests.Crossref | GoogleScholarGoogle Scholar |
Robley, A, Howard, K, Lindeman, M, Cameron, R, Jardine, A, and Hiscock, D (2016). The effectiveness of short-term fox control in protecting a seasonally vulnerable species, the eastern long-necked turtle (Chelodina longicollis). Ecological Management & Restoration 17, 63–69.
| The effectiveness of short-term fox control in protecting a seasonally vulnerable species, the eastern long-necked turtle (Chelodina longicollis).Crossref | GoogleScholarGoogle Scholar |
Rollinson, N, and Brooks, RJ (2007). Marking nests increases the frequency of nest depredation in a northern population of painted turtles (Chrysemys picta). Journal of Herpetology 41, 174–176.
| Marking nests increases the frequency of nest depredation in a northern population of painted turtles (Chrysemys picta).Crossref | GoogleScholarGoogle Scholar |
Ross, DA, and Anderson, RK (1990). Habitat use, movements, and nesting of Emydoidea blandingi in central Wisconsin. Journal of Herpetology 24, 6–12.
| Habitat use, movements, and nesting of Emydoidea blandingi in central Wisconsin.Crossref | GoogleScholarGoogle Scholar |
Rowe, JW, Coval, KA, and Dugan, MR (2005). Nest placement, nest-site fidelity and nesting movements in midland painted turtles (Chrysemys picta marginata) on Beaver Island, Michigan. American Midland Naturalist 154, 383–397.
| Nest placement, nest-site fidelity and nesting movements in midland painted turtles (Chrysemys picta marginata) on Beaver Island, Michigan.Crossref | GoogleScholarGoogle Scholar |
Ruzicka VA (2006) The influence of predation on the nesting ecology of diamondback terrapins (Malaclemys terrapin) in the Lower Chesapeake Bay. MSc thesis, College of William & Mary, Williamsburg.
Saunders, GR, Gentle, MN, and Dickman, CR (2010). The impacts and management of foxes Vulpes vulpes in Australia. Mammal Review 40, 181–211.
| The impacts and management of foxes Vulpes vulpes in Australia.Crossref | GoogleScholarGoogle Scholar |
Schwanz, LE, Spencer, R-J, Bowden, RM, and Janzen, FJ (2010). Climate and predation dominate juvenile and adult recruitment in a turtle with temperature-dependent sex determination. Ecology 91, 3016–3026.
| Climate and predation dominate juvenile and adult recruitment in a turtle with temperature-dependent sex determination.Crossref | GoogleScholarGoogle Scholar | 21058561PubMed |
Snow, JE (1982). Predation on painted turtle (Chrysemys picta) nests: nest survival as a function of nest age. Canadian Journal of Zoology 60, 3290–3292.
| Predation on painted turtle (Chrysemys picta) nests: nest survival as a function of nest age.Crossref | GoogleScholarGoogle Scholar |
Spencer, R-J (2002). Experimentally testing nest site selection: fitness trade-offs and predation risk in turtles. Ecology 83, 2136–2144.
| Experimentally testing nest site selection: fitness trade-offs and predation risk in turtles.Crossref | GoogleScholarGoogle Scholar |
Spencer, R-J (2018). How much long-term data are required to effectively manage a wide-spread freshwater turtle? Australian Zoologist 39, 568–575.
| How much long-term data are required to effectively manage a wide-spread freshwater turtle?Crossref | GoogleScholarGoogle Scholar |
Spencer, R-J, and Thompson, MB (2005). Experimental analysis of the impact of foxes on freshwater turtle populations. Conservation Biology 19, 845–854.
| Experimental analysis of the impact of foxes on freshwater turtle populations.Crossref | GoogleScholarGoogle Scholar |
Spencer, R-J, van Dyke, JU, and Thompson, MB (2016). The ethological trap: functional and numerical responses of highly efficient invasive predators driving prey extinctions. Ecological Applications 26, 1969–1983.
| The ethological trap: functional and numerical responses of highly efficient invasive predators driving prey extinctions.Crossref | GoogleScholarGoogle Scholar | 27755718PubMed |
Spencer, R-J, van Dyke, JU, and Thompson, MB (2017). Critically evaluating best management practices for preventing freshwater turtle extinctions. Conservation Biology 31, 1340–1349.
| Critically evaluating best management practices for preventing freshwater turtle extinctions.Crossref | GoogleScholarGoogle Scholar | 28319283PubMed |
Standing, KL, Herman, TB, Shallow, M, Power, T, and Morrison, IP (2000). Results of the nest protection program for Blanding’s turtles in Kejimkujik National Park, Canada: 1987–1997. Chelonian Conservation and Biology 3, 637–642.
Stanford, CB, Iverson, JB, Rhodin, AGJ, et al. (2020). Turtles and tortoises are in trouble. Current Biology 30, R721–R735.
| Turtles and tortoises are in trouble.Crossref | GoogleScholarGoogle Scholar | 32574638PubMed |
Strickland, JT, and Janzen, FJ (2010). Impacts of anthropogenic structures on predation of painted turtle (Chrysemys picta) nests. Chelonian Conservation and Biology 9, 131–135.
| Impacts of anthropogenic structures on predation of painted turtle (Chrysemys picta) nests.Crossref | GoogleScholarGoogle Scholar |
Strickland, J, Colbert, P, and Janzen, FJ (2010). Experimental analysis of effects of markers and habitat structure on predation of turtle nests. Journal of Herpetology 44, 467–470.
| Experimental analysis of effects of markers and habitat structure on predation of turtle nests.Crossref | GoogleScholarGoogle Scholar |
Thompson, MB (1983). Populations of the Murray River tortoise, Emydura (Chelodina): the effect of egg predation by the red fox, Vulpes vulpes. Australian Wildlife Research 10, 363–371.
| Populations of the Murray River tortoise, Emydura (Chelodina): the effect of egg predation by the red fox, Vulpes vulpes.Crossref | GoogleScholarGoogle Scholar |
Thompson, MM, Coe, BH, Congdon, JD, Stauffer, DF, and Hopkins, WA (2017). Nesting ecology and habitat use of Chelydra serpentina in an area modified by agricultural and industrial activity. Herpetological Conservation and Biology 12, 292–306.
Tinkle, DW, Congdon, JD, and Rosen, PC (1981). Nesting frequency and success: implications for the demography of painted turtles. Ecology 62, 1426–1432.
| Nesting frequency and success: implications for the demography of painted turtles.Crossref | GoogleScholarGoogle Scholar |
Tuberville, TD, and Burke, VJ (1994). Do flag markers attract turtle nest predators? Journal of Herpetology 28, 514–516.
| Do flag markers attract turtle nest predators?Crossref | GoogleScholarGoogle Scholar |
Tuma, MW (2006). Range, habitat use, and seasonal activity of the yellow mud turtle (Kinosternon flavescens) in northwestern Illinois: implications for site-specific conservation and management. Chelonian Conservation and Biology 5, 108–120.
| Range, habitat use, and seasonal activity of the yellow mud turtle (Kinosternon flavescens) in northwestern Illinois: implications for site-specific conservation and management.Crossref | GoogleScholarGoogle Scholar |
Turner, G (2004). Nesting behaviour in the white-throated snapping turtle Elseya sp. aff. dentata from the Johnstone River, north Queensland. Herpetofauna 34, 48–58.
Van Dyke, JU, Ferronato, BO, and Spencer, R-J (2018). Current conservation status of Australian freshwater turtles. Australian Journal of Zoology 66, 1–3.
| Current conservation status of Australian freshwater turtles.Crossref | GoogleScholarGoogle Scholar |
Vestjens WJM (1977) Status, habitats and food of vertebrates at Lake Cowal, N.S.W. CSIRO Division of Wildlife Research, Technical Memorandum No. 12, Canberra.
Voves, KC, Mitchell, TS, and Janzen, FJ (2016). Does natural visual camouflage reduce turtle nest predation? American Midland Naturalist 176, 166–172.
| Does natural visual camouflage reduce turtle nest predation?Crossref | GoogleScholarGoogle Scholar |
Wallach, AD, Johnson, CN, Ritchie, EG, and O’Neill, AJ (2010). Predator control promotes invasive dominated ecological states. Ecology Letters 13, 1008–1018.
| Predator control promotes invasive dominated ecological states.Crossref | GoogleScholarGoogle Scholar | 20545732PubMed |
Washington AC (2008) Site selection and survival of Pseudemys texana and Trachemys scripta elegans nests at Spring Lake in San Marcos, Texas. MSc thesis, Texas State University, San Marcos.
Wilson, DS, Mushinsky, HR, and McCoy, ED (1999). Nesting behavior of the striped mud turtle, Kinosternon baurii (Testudines: Kinosternidae). Copeia 1999, 958–968.
| Nesting behavior of the striped mud turtle, Kinosternon baurii (Testudines: Kinosternidae).Crossref | GoogleScholarGoogle Scholar |
Wirsing, AJ, Phillips, JR, Obbard, ME, and Murray, DL (2012). Incidental nest predation in freshwater turtles: inter- and intraspecific differences in vulnerability are explained by relative crypsis. Oecologia 168, 977–988.
| Incidental nest predation in freshwater turtles: inter- and intraspecific differences in vulnerability are explained by relative crypsis.Crossref | GoogleScholarGoogle Scholar | 22009341PubMed |
Zappalorti, RT, Tutterow, AM, Pittman, SE, and Lovich, JE (2017). Hatching success and predation of bog turtle (Glyptemys muhlenbergii) eggs in New Jersey and Pennsylvania. Chelonian Conservation and Biology 16, 194–202.
| Hatching success and predation of bog turtle (Glyptemys muhlenbergii) eggs in New Jersey and Pennsylvania.Crossref | GoogleScholarGoogle Scholar |


