Avian community changes following drought-induced canopy collapse in a Mediterranean-type forest
Sean Smithies A , Patricia A. Fleming A B , Philip W. Bateman
A B , Philip W. Bateman  C , Giles E. St. J. Hardy A and Shannon J. Dundas
C , Giles E. St. J. Hardy A and Shannon J. Dundas  A B *
A B *
A Terrestrial Ecosystem Science and Sustainability, Harry Butler Institute, Murdoch University, Murdoch, WA 6150, Australia.
B NSW Department of Primary Industries, Orange, NSW, Australia.
C Behavioural Ecology Laboratory, School of Molecular and Life Sciences, Curtin University, Bentley, WA, Australia.
Pacific Conservation Biology 29(4) 312-324 https://doi.org/10.1071/PC22005
Submitted: 9 February 2022 Accepted: 21 August 2022 Published: 19 September 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Extreme drought can result in the widespread die-off of forests and dramatically altered ecosystem structure. Such changes are likly to influence fauna using resouces within these forests.
Aims: Following a record hot and dry year/summer in 2010/11, large-scale canopy collapse occurred within a Mediterranean-type mixed jarrah (Eucalyptus marginata)–marri (Corymbia calophylla) forest in south-west Western Australia. We investigated the effects of this collapse on bird assemblages in 2016, 5 years after the initial collapse.
Methods: We carried out bird surveys using a standardised search method for five paired drought-affected and adjacent healthy forest plots.
Key results: A total of 3042 records of 51 bird species were observed across all surveys. Overall, the pooled (mean ± s.d.) reporting rates for drought-affected plots (13.84 ± 0.60 individuals/survey) were significantly less than the reporting rates for healthy plots (34.44 ± 1.03 individuals/survey) (PERMANOVA: F1 = 54.94, R2 = 0.31, P = 0.001). Species diversity was also higher in healthy plots (t26 = 11.21, P < 0.001). Foliage-searching birds were the most abundant guild across all plots and were reported less often in drought-affected plots (t6 = 2.70, P < 0.04).
Conclusions: Drought-affected jarrah forest plots exhibited significant differences in bird assemblages compared to healthy plots. Overall, the drought-affected forest provides a less favourable habitat for birds compared to healthy forest.
Implications: With marked variability and extreme climate events predicted for the future, understanding the impacts of such changes will contribute to how we manage forest ecosystems.
Keywords: avian, climate change, dieback, drought, jarrah forest, nectarivore, tree decline, woodland birds.
Introduction
Ongoing changes in climate have resulted in increased frequency, duration and severity of drought and heat stress incidents across a range of forest habitats globally, with climate-related forest mortality increasingly recorded (reviewed by Allen et al. 2010). In the southern hemisphere, climate-induced tree declines are evident across all terrestrial habitat types, including alpine, tropical, Mediterranean and sub-Antarctic landscapes (Hoffmann et al. 2019). The scale of these events varies according to the surrounding landscape, life-history traits and tolerances of individual tree species within forests, with differential mortality rates reported (e.g. Ruthrof et al. 2015). Drought events result in significant changes to forest structure such as large, old trees being replaced with small stems (Matusick et al. 2016; Restaino et al. 2019), mass loss of overstorey trees (Breshears et al. 2005; Floyd et al. 2015) and changes to leaf litter and coarse woody debris fuel loads (Floyd et al. 2015; Ruthrof et al. 2016). The incidence of tree loss due to climate change is predicted to increase in the future (Allen et al. 2015), suggesting that such changes could be permanent features of affected landscapes.
Such significant habitat changes resulting from climate-induced forest mortality are likely to have secondary effects on fauna (Fleming et al. 2021). Leaf litter, for example, is an important resource within forest ecosystems, with litter depth and complexity benefiting ground-living invertebrates (Bultman and Uetz 1984; Bengtsson et al. 1997; Langellotto and Denno 2004; Nittérus and Gunnarsson 2006) and vertebrates (Dundas et al. 2021) by providing shelter and habitat (Uetz 1979; Oliver et al. 2006). The loss of canopy cover due to drought-induced stress is likely to influence those species foraging in the mid to top canopy in forests. In Victoria, Australia, severe defoliation of manna gums (Eucalyptus viminalis) resulted in lower bird species richness and altered bird assemblages compared to trees with intact canopies (Whisson et al. 2018). Nectar and pollen food resources used by fauna can also be compromised, with drought-stressed trees less likely to flower (Mac Nally et al. 2009) and nectarivores becoming significantly more scarce in the presence of tree declines (Fleming et al. 2021).
Drought events and heatwaves can have immediate and direct effects on avifauna pushed beyond a survival threshold (McKechnie and Wolf 2010; McKechnie et al. 2012), but birds also respond to longer-term environmental changes as a consequence of climate. Their mobility means that bird presence is a strong reflection of the availability of food and shelter resources, and therefore the composition and abundance of bird communities are linked with habitat factors through their diet, foraging and nesting guilds (Fleming et al. 2021). Consequently birds are excellent indicators of environmental perturbances (Fumy and Fartmann 2021), including changes to forest structure related to climate-induced forest decline, with drought resulting in significant shifts in avian community composition across large landscape scales. In southern Europe, increased defoliation and mortality of oak woodlands in response to drier conditions resulted in a 50–60% decline in abundance and species richness of woodland birds, particularly tree foraging species, as a result of reduced food resources and lower tree cover (Correia et al. 2015). Similarly, in Sierra Nevada, USA, warmer and drier conditions coupled with widespread mortality of trees is predicted to have a negative effect on bird species with 36% of 45 species likely to decline in response to higher tree mortality and drier conditions (Roberts et al. 2019). Changing climatic conditions can also cause species range shifts. In conifer forests in California, USA, neotropical migrant birds showed a shift in range to higher elevations over a 14-year period where May temperatures increased annually by 0.037°C (Furnas 2020).
Short-term impacts of drought are likely to reflect a deficit of water, which compromises the ability of birds to deal with heat. Changing climate is predicted to result in increasingly more frequent heatwaves, with sustained high temperatures having detrimental effects on birds, especially smaller species living in Australian arid regions (McKechnie et al. 2012; Conradie et al. 2020). Longer term impacts of drought on bird communities are likely to be due to vegetation changes. Several studies report decreased bird abundance in response to sustained drought conditions, which likely reflects movement to more favourable sites for mobile species, or a lack of resources for survival or successful reproduction for residents. For example, abundance of common bird species in the Warrumbungle Mountains in New South Wales (NSW), Australia, declined over two decades, with reduced summer–autumn rainfall identified as the likely cause (Stevens and Watson 2013). In north-central Victoria, ongoing drought conditions resulted in widespread reductions in abundance of woodland birds across survey sites (Haslem et al. 2015). On the floodplains of the Murray Darling Basin, Australia, longer periods of drought compared to flood periods over the past two decades has resulted in reduced occurrence of woodland birds (Reid et al. 2022).
Not surprisingly, therefore, changes in bird species richness and community composition have been reported in response to sustained drought conditions. Long term analysis of birds in south-eastern Australia (Mac Nally et al. 2009) revealed that the reporting rate of 74% of 82 monitored bird species declined over a 12-year period (1995/7–2004/5), over which time a marked reduction in rainfall was also recorded; the decline was particularly marked for insectivorous and nectarivore species. A 17-year study of sites spanning 15 states in the USA reported the greatest negative impact of drought on neotropical migrants, while resident species appeared to increase in abundance (Albright et al. 2010). Similarly, bird communities in remnant woodlands in the central wheatbelt of NSW were increasingly dominated by larger, generalist species, including Australian Magpies and Galahs, during declared drought years (Ellis and Taylor 2013). Different responses recorded between avian guilds suggests varying mechanisms by which drought affects birds.
In the northern jarrah forest, an important Mediterranean-type forest, the ongoing declines in annual precipitation and groundwater levels have resulted in water stress for jarrah (Eucalyptus marginata) and marri (Corymbia calophylla) trees, the two dominant canopy species (Petrone et al. 2010; Hughes et al. 2012). The warming and drying trend peaked in 2010/11, producing the driest and second hottest year on record at that time (BOM 2011). The stress on the northern jarrah forest resulted in a sudden and unprecedented collapse in patches of forest (Matusick et al. 2013). It was estimated that 16 515 ha of forest experienced canopy collapse, equivalent to ~1.5% of the northern jarrah forest, leaving large patches of drought-affected forest surrounded by healthy forest regions (Matusick et al. 2013). The rapid and dramatic changes in canopy tree species across the jarrah forest, resulting in a markedly altered habitat, are hypothesised to influence the activity and persistence of avifauna at these sites. Our research aims were therefore to identify whether there were differences between drought-affected and healthy plots for: (1) overall bird reporting rates; (2) avian species diversity; and (3) reporting rates by feeding guild.
Materials and methods
Study sites
Surveys were conducted at five sites ~30 km south-east of Perth, Western Australia, within the northern jarrah forest (Fig. 1). The bioregion experiences a Mediterranean-type climate, with cool, wet winters and hot and dry summers (Gentilli 1989). The forest is dominated by mixed jarrah and marri canopy trees with a mid-storey comprised of bull banksia (Banksia grandis) and grasstrees (Xanthorrhoea preissii) (Dell and Havel 1989). The bioregion has experienced historical native timber harvesting, clearing for plantations of pine (Pinus radiata) (Stoneman et al. 1989), and bauxite mining followed by forest rehabilitation (Nichols et al. 1985).
Drought-affected and healthy forest plots
The study sites formed part of long-term monitoring project to assess recovery and ongoing changes to drought-affected forest (Matusick et al. 2013). Severe drought-affected sites were characterised by significant loss of canopy cover (Matusick et al. 2013) and increased mortality of both canopy and understorey species (Steel et al. 2019). Drought-affected sites had more fine coarse woody debris and standing dead trees (fig. 2a, Ruthrof et al. 2016), less leaf litter accumulation, and experienced more extreme temperature differences throughout the day compared to healthy sites (Dundas et al. 2021). The drought-affected sites were not randomly distributed in the jarrah forest and affected canopy trees tended to be on rockier soils with lower water holding capacity (Brouwers et al. 2013). Despite this, we ensured that vegetation assemblages at the paired drought-affected and healthy sites were comparable, with the same canopy and understorey species composition. Healthy forest plots had relatively full canopies of jarrah and marri (Fig. 1b), with a thick leaf litter layer that was constantly being added to with natural leaf fall (Dundas et al. 2021).
Bird surveys
At each of five sites, we marked out one drought-affected plot (~2 ha in size) and paired it with an adjacent healthy plot located 0.1–1 km away. Drought-affected and healthy plots (10 plots in total) were carefully selected to ensure they consisted of the same habitat type (same understorey and canopy species). Some marri trees and bull banksia were flowering during the surveys.
A total of 126 bird surveys were conducted between January and April 2016 across the 10 plots. All surveys commenced within 1 h of sunrise and were finished by no later than 1100 hours. All 10 plots were surveyed approximately every 7 days, avoiding days when the weather was not optimal (e.g. raining, strong winds). Each pair of drought-affected and healthy plots were surveyed the same morning on each survey day. Survey methods were based on a standardised search method with a results-based stopping rule, as suggested by Watson (2003). Briefly, one observer slowly walked through each plot for 20 min and recorded all bird species seen or heard within a 250 m radius of their location. If two or more previously unencountered species were identified during a survey, both the drought and healthy paired plots for that site were searched for an additional 20 min, which allowed us to account for rarer species. To account for potential changes in birds’ activity at different times during the morning, the order in which plots were surveyed was randomised across the survey period. Birds flying over the plots were excluded from the count. Using this method, each site was surveyed an average of 13 ± 4 times.
Assignment of feeding guild
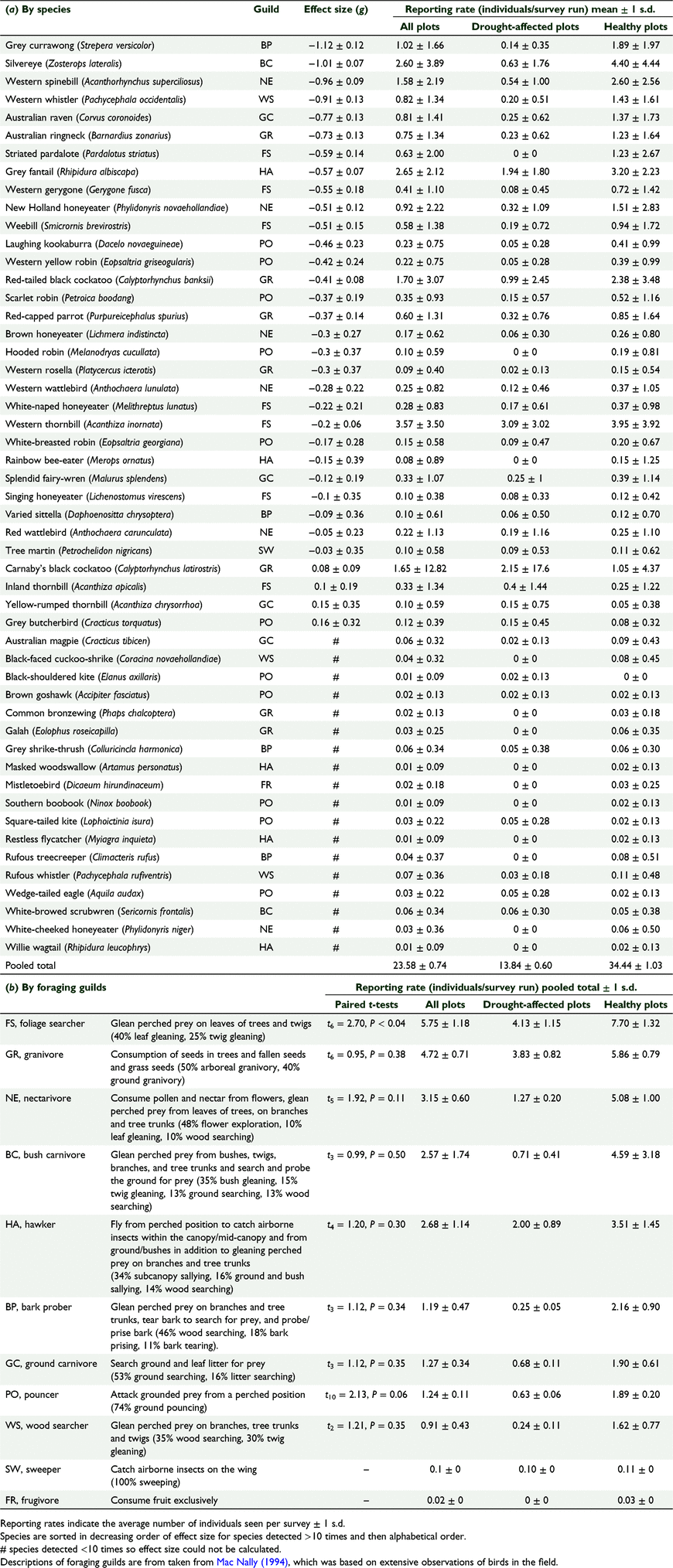
Observed species were grouped into foraging guilds as described by Mac Nally (1994). For species not listed in Mac Nally (1994), we assigned birds to guilds based on similar species with additional reference to Morcombe (2003). Foraging guilds as described by Mac Nally (1994) are in Table 1b.
|
Statistical analysis
Total numbers were converted to reporting rates (number of birds recorded per survey) to standardise values across all plots; reporting rates were used in all analyses. Visual assessment of avian community assemblages between sites and treatments (drought-affected/healthy plots) was carried out using multidimensional scaling (MDS) using PAST 3.11 (Bray–Curtis similarity index) (Hammer et al. 2001). To assess species diversity, we calculated Shannon’s Diversity Index for each individual survey and tested for significant differences between drought-affected and healthy plots using a paired t-test. A permutational ANOVA (PERMANOVA, Bray–Curtis similarity index) using the adonis function in vegan package (Oksanen et al. 2020) in R (R Core Team 2022) was used to investigate differences in reporting rates between treatments (drought-affected/healthy plots). Paired t-tests were carried out to determine if reporting rates of birds in each of the 11 foraging guilds were significantly different between drought-affected and healthy sites. Effect sizes were calculated using published formulae (Hedge’s g; bias-corrected standardised mean difference corrected for small sample size) (Borenstein et al. 2011) and the escalc function in the R package ‘metafor’ (Viechtbauer 2020; Harrer et al. 2021) to transform data into Hedges’ g with 95% confidence intervals. Values are presented as means ± 1 s.d.
Results
There were 3042 records of 51 bird species for the total 126 surveys carried out across all plots. Reporting rates were significantly higher in healthy plots compared to drought-affected plots (PERMANOVA: F1 = 54.94, R2 = 0.31, P = 0.001). Species diversity was also higher for healthy plots (t62 = 11.21, P < 0.001). The bird communities in healthy plots were similar (Fig. 2), indicating consistency and similarity in community structure across these plots, while there were marked differences in community composition at the drought-affected plots.
Across the 11 foraging guilds, only the foliage-searching foraging guild had significantly more birds reported in healthy plots compared to drought-affected plots (t6 = 2.70, P = <0.04). Of the 51 bird species observed across all surveys, 43 bird species were reported more frequently in healthy plots, with 20 of the 33 species most commonly seen species (>10 observations) having a significant negative effect size, capturing their lower reporting rate on drought-affected plots (Fig. 3). These included three large omnivores, the Grey currawong (g = −1.12), Australian Raven (g = −0.77), and Laughing kookaburra (g = −0.46). Significant negative effects were also recorded for seven leaf, twig and bush gleaning insectivores (Silvereye g = −1.01, Western whistler g = −0.91, Striated pardalote g = −0.59, Western gerygone g = −0.55, Weebill g = −0.51, White-naped honeyeater g = −0.22, Western thornbill g = −0.20), a hawking insectivore (Grey fantail g = −0.57) and pouncing insectivores (Western yellow robin g = −0.42, Scarlet robin g = −0.37). Significant negative effects were also evident for four nectarivores (Western spinebill g = −0.96, New Holland honeyeater g = −0.51, Brown honeyeater g = −0.30, Western wattlebird g = −0.28) and three granivore species (Australian ringneck g = −0.73, Red-tailed black cockatoo g = −0.41, Red-capped parrot g = −0.37).
|
|
In contrast, Grey Butcherbirds (g = 0.16), Yellow-rumped thornbills (g = 0.15), Inland thornbills (g = 0.10) and Carnaby’s black cockatoos (g = 0.08) were recorded more often in drought-affected plots, although the effect sizes for all these species were not significantly different from zero.
Discussion
Overall, reporting rates of birds was significantly higher in healthy plots compared to drought-affected plots. Similarly, species diversity was higher in healthy plots with all but one (Black-shouldered kite) of the total 51 bird species recorded overall seen in healthy plots compared to 38 species seen in drought-affected plots. Bird communities in healthy plots were more similar to each other, while bird communities in drought-affected plots showed greater dissimilarity between plots. Birds are likely to be influenced by climate changes through direct impacts of temperature extreme or reduced rainfall, as well as indirect changes in available habitat due to decline in vegetation growth and resilience. A global meta-analysis of the abundance responses of 186 bird species showed an overall increase in bird abundance in response to tree declines (Fleming et al. 2021). The marked detrimental impact recorded by multiple studies in response to drought suggests that the impact of drought is likely to have more marked effects on avian communities than tree declines due to other causes.
Drought-induced changes to vegetation
Healthy jarrah forest sites offer greater canopy coverage and substantial leaf litter cover compared to drought-affected sites (Dundas et al. 2021). In comparison, drought-affected plots exhibited ongoing structural changes after the initial drought-induced collapse, including replacement of mature trees with small stems and resprouting of trees (Matusick et al. 2016). Similar reduced bird activity has been reported in response to loss of canopy cover and susceptible understorey species in forests and woodland affected by the plant pathogen Phytophthora cinnamomi (Armstrong and Nichols 2000; Davis et al. 2014). Additionally, manna gums (E. viminalis) defoliated by koalas (Phascolarctos cinereus) show decreased bird species richness in proportion to the severity of defoliated canopy (Whisson et al. 2018). Direct anthropogenic changes to forest and woodland have some similar impacts as seen for drought and plant pathogens. In Mexico, compared to uncut forest, removal of shade trees for coffee farming has resulted in significant reductions in the abundance and species richness of insectivorous birds, attributed to the loss of canopy cover and canopy depth (Philpott and Bichier 2012). For small, insectivorous birds, healthy foliage offers suitable habitat for foraging as well as protection from predation.
Change due to tree declines is not unconditionally detrimental, as drought-stressed trees can provide some short-term benefits to birds. For example, growth of eucalypt leaves from epicormic shoots are generally of superior dietary quality compared to those of healthy trees (Landsberg and Wylie 1983; Landsberg 1990a, 1990b), which may initially benefit folivorous insects (Landsberg 1988; Recher et al. 1996) and, potentially, leaf-gleaning insectivorous birds. Dead tree branches offer good vantage points for predatory birds pouncing on prey such as robins, butcherbirds and larger birds of prey. Dead trees were also infested with native Eucalyptus longhorned borer beetles, (Phoracantha semipunctata) (Seaton et al. 2015) the larvae of which are likely attractive food for birds. Unfortunately, beneficial resources available in drought-affected sites are likely to be short-lived as standing dead trees fall, and affected areas, which are more fire-prone (Ruthrof et al. 2016), are more readily burnt.
Landscape position can influence bird assemblages, with forests varying in productivity as a consequence of elevation and soil type. Trees affected by drought in our study were more likely to be in areas of rockier soil, and were more likely to be warmer and receive more rainfall than were trees in unaffected areas of jarrah forest (Brouwers et al. 2013). It is possible the underlying differences in vegetation productivity as a result of these subtle landscape differences may have some influenced bird communities in these affected areas, even before the drought. The productivity-based hypothesis for declines of woodland birds proposed by Watson (2011) suggests that poorer soils support a reduced biomass of decomposers, which in turn reduces the number of ground-dwelling insects that are food resources for woodland birds. This hypothesis was evident in smaller patches of intact vegetation across agricultural land in northeast NSW offering reduced food resources for birds, which had flow on effects for breeding success (Zanette et al. 2000). Topography can also influence bird presence; for example, higher elevations are associated with declining occurrence of Mallee emu wrens (Stipiturus mallee), possibly as a consequence of decreased soil nutrients and water availability at higher elevations (Verdon et al. 2019).
Reduction in food resources
The impact of vegetation changes due to drought are likely to manifest through reduction in canopy cover, flowering and fruiting activities (Fig. 4). Reduced rainfall negatively impacts food and habitat resources, especially when periods of drought continue for years. Meta-analysis of bird responses to tree declines show substantial variability, with a strong influence of diet (as well as nesting guild) on bird responses (Fleming et al. 2021). Drought is likely to increase the foraging costs for birds dependent on forest habitats, increasing movement distances required in order to meet their energy requirements. Bird populations may therefore decrease overall, or animals may avoid the affected area, resulting in reduced activity. For example, in northern Victoria, declines in bird populations have been evident in both remnant vegetation and cleared sites surveyed over a 9-year period of reduced rainfall (Mac Nally et al. 2009). In some cases, birds can adapt to changed conditions and may thrive; for example, during drought years, bird communities at survey sites in NSW were dominated by larger, generalist species including Australian Magpies and Galahs (Ellis and Taylor 2013). The specific resources used by bird species are likely to reflect their responses to drought changes.
|
|
Foliage-searching insectivore species were the most common guild of birds observed in the current study. Many of the small bird species that search for prey on leaves and wood (e.g. Silvereyes, Western whistlers, Striated pardalotes, Western gerygones, Weebills, White-naped honeyeaters and Western thornbills) were recorded significantly less often in drought-affected sites. The most marked difference in activity was for Silvereyes. Silvereyes have similarly been observed less frequently in P. cinnamomi-affected banksia woodland (Davis et al. 2014). Projected foliage cover of wandoo (Eucalyptus wandoo) woodlands, which have been in decline in response to reduced rainfall, was found to be a good predictor for the number of canopy insect gleaning Yellow-plumed honeyeaters (Lichenostomus ornatus) (Angel and Bradley 2021).
The lower numbers of nectarivore reported in drought-affected jarrah forest sites is likely related to reduced availability of food resources due to reduced flowering effort as well as death of the plants. Mortality of flowering plants, including marri (7%), jarrah (19%) and bull banksia (59%) trees, was greater in severely affected jarrah forest drought sites (Steel et al. 2019), resulting in fewer food resources available at these sites for nectarivores. In the northern jarrah forest, Western wattlebirds and Western spinebills forage almost exclusively on bull banksia (Abbott and Van Heurck 1985), a plant species that was present principally in healthy plots in the present study. Lower nectarivore abundance has also been observed in sites where tuart (Eucalyptus gomphocephala) trees exhibited canopy decline as a result of Phytophthora multivora infestation (Wentzel 2010). Similarly, Eastern spinebills (Acanthorhynchus tenuirostris) and Red wattlebirds (Anthochaera carunculata) are less common in patches of yellow box (Eucalyptus melliodora) and Blakely’s red gum (Eucalyptus blakelyi) affected by dieback as a result of defoliating insects near Canberra, ACT (Er 1997).
Reduction in fruit availablility was also likely to influence bird activity at drought-affected sites. Australian ringnecks and Red-capped parrots were observed feeding in healthy jarrah forest plots. Healthy forest plots offer green eucalypt fruits, which are readily fed upon by native parrot species (Long 1984; Cooper et al. 2003). Red-capped parrots also selectively feeding on bull banksia in the jarrah forest (Abbott and Van Heurck 1985). Red-capped parrots were observed visiting perches and hollows in dead jarrah trees, including in drought sites (we note that the availability of tree hollows would have preceded the present canopy collapse, although these sites may be more vulnerable to frequent cycles of canopy collapse; Matusick et al. 2013; Ruthrof et al. 2016). Similarly, Armstrong and Nichols (2000) observed Red-tailed black cockatoos exclusively using large, dead Eucalyptus trees in Phytophthora-affected jarrah forest.
Healthy jarrah forest is more likely to support small vertebrates (Dundas et al. 2021) and invertebrate prey species (Nichols and Burrows 1985; Postle et al. 1986) that would be likely to attract birds. Similarly, in banksia woodland sites affected by P. cinnamomi, Australian ravens (Davis et al. 2014), Grey fantails, Laughing kookaburras and Western yellow robins were observed less frequently in affected sites compared to heathy sites (Armstrong and Nichols 2000). In wandoo woodland sites, Western yellow robins show preference for sites with high canopy density and high leaf density (Cousin 2004).
Longer term effects of drought on woodland birds and associated habitat
Ongoing changing climate results in two key environmental factors that are likely to affect bird communities: reduced rainfall and extreme temperatures (both hot and cold). Intact vegetation could provide vital thermal refuges for birds, especially as changing climate results in increased temperatures. Measurements of air temperatures at microhabitats at these same jarrah forest sites showed that more extreme temperature fluctuations were experienced in drought-affected sites across the day compared to healthy sites (Dundas et al. 2021). In Western Oklahoma, USA, Northern bobwhite (Colinus virginianus) retreated to tall, woody vegetation on the hottest days, where temperatures were on average up to 16.5°C cooler (Carroll et al. 2017). Similarly in the southern Kalahari desert, birds showed a preference for trees with the greatest canopy density when temperatures were >35°C (Martin et al. 2015). Loss of canopy from jarrah forest is therefore likely to reduce the benefit of this vegetation as a thermal refuge for birds.
By contrast with the long-term changes due to drought, extreme events such as heat waves or unseasonal cold snaps are more sudden and can quickly push birds beyond their survival threshold, often resulting in mass mortalities (McKechnie and Wolf 2010; McKechnie et al. 2012). For example, mass deaths of migrating birds is often attributed to unseasonal cold weather at breeding areas as migrant birds are not habituated to the cold (Newton 2007). An extreme heatwave along the Western Australian south coast resulted in mass mortalities of Carnaby’s black cockatoo (Calyptorhynchus latirostris) and other bird species (Saunders et al. 2011). The effects of these events are likely to be exacerbated when birds are already under stress due to drought conditions and low food supply.
Future drought periods and heatwave events are inevitable (Fischer et al. 2013; Perkins-Kirkpatrick et al. 2016) and can kill younger regenerating trees and new seedlings (Gazol et al. 2018; Qie et al. 2019), potentially resulting in these drought-affected areas remaining open and exposed. Additionally, legacy effects are evident from chronically reduced long-term precipitation at these drought sites, thereby increasing the probability of tree mortality in the future (Matusick et al. 2018). In north-east Alberta, Canada, predictive modelling of boreal bird communities has demonstrated a transition from boreal forest to grass or shrub-dominated vegetation over the next 100 years (Cadieux et al. 2020). This would result in a subsequent shift towards bird communities associated with open and deciduous vegetation and a decline in bird species associated with coniferous and mixed wood forest (Cadieux et al. 2020).
Persistent drought periods are likely to have negative, ongoing, and broader scale effects on bird assemblages. Longer drought periods over the past two decades has resulted in declines of woodland birds associated with the river red gum (Eucalyptus camaldulensis) floodplains in the Murray Darling Basin (Reid et al. 2022). In terms of effects of drought on breeding, low breeding success for woodland birds was attributed to reduced eucalypt flowering events during 12 years of drought (Mac Nally et al. 2009). Similarly, offspring survival for Lesser kestrels (Falco naumanni) in southern Portugal decreased by 12% during drought events and predictive modelling indicate that ongoing and more frequent extreme drought events are likely to negate recent population recoveries (Marcelino et al. 2020). Similarly, analysis of 59 bird species in England over 45 years showed a negative impact of hot, dry summers on population growth (Pearce-Higgins et al. 2015). For Eastern bluebills (Sialia sialis) in North America, hatching and fledgling rates decreased as drought severity increased (Carleton et al. 2019). These longer-term effects are likely to influence bird populations beyond the initial drought event.
Limitations of this study
Detectability of birds can vary during surveys, and it is possible that bird sightings in healthy vegetation sites may be hindered by increased vegetative cover and refuge for individuals. The more exposed drought-sites made it easier to observe birds. If visibility did compromise our activity records, then the statistically significant results recorded are a conservative measure of the changes that were evident at these sites. It is also possible that the observer was also more visible at drought-affected sites, which potentially scared off birds that were present. Concealed sit-and-wait survey methods to account for shyer species, or following target individuals to identify their habitat choices (Moore et al. 2013), would be a worthwhile addition to more active surveys in the future.
Conclusion
We found drought-affected forest plots supported lower reporting rates and less diverse bird assemblages than healthy forest plots. It is likely the resources provided within healthy jarrah forest influence utilisation of these areas by woodland birds and would also provide suitable refugia during extreme weather events such as heatwaves. As availability of resources changes over time, ongoing extreme weather events are likely to have long-term effects on forest structure (Paz-Kagan et al. 2017; Restaino et al. 2019) and therefore bird communities. Forest management to protect intact forest should be prioritised, and any management action also needs to take into account vulnerability of forested areas to potential effects of future drought.
Data availability
Data are available on request from the authors.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was financially supported by Murdoch University.
Acknowledgements
This study was carried out with the approval of the Murdoch University Animal Ethics Committee RW2769/15. Thanks to Katinka Ruthrof and Emma Steel for specific advice about the field sites, Josh Collard, Hannah Ashbil, Michael Childs, Nicki Miglori, Jack Banister, Lynne Smithies, and Hugh Smithies for field assistance.
References
Abbott, I, and Heurck, P (1985). Tree species preferences of foraging birds in jarrah forest in Western Australia. Wildlife Research 12, 461–466.| Tree species preferences of foraging birds in jarrah forest in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Albright, TP, Pidgeon, AM, Rittenhouse, CD, Clayton, MK, Flather, CH, Culbert, PD, Wardlow, BD, and Radeloff, VC (2010). Effects of drought on avian community structure. Global Change Biology 16, 2158–2170.
| Effects of drought on avian community structure.Crossref | GoogleScholarGoogle Scholar |
Allen, CD, Macalady, AK, Chenchouni, H, Bachelet, D, McDowell, N, Vennetier, M, Kitzberger, T, Rigling, A, Breshears, DD, Hogg, EH, Gonzalez, P, Fensham, R, Zhang, Z, Castro, J, Demidova, N, Lim, J-H, Allard, G, Running, SW, Semerci, A, and Cobb, N (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259, 660–684.
| A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests.Crossref | GoogleScholarGoogle Scholar |
Allen, CD, Breshears, DD, and McDowell, NG (2015). On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6, 1–55.
| On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene.Crossref | GoogleScholarGoogle Scholar |
Angel, AS, and Bradley, JS (2021). Impact of a prolonged decline in rainfall on eucalypt woodlands in southwestern Australia and its consequences for avifauna. Pacific Conservation Biology , .
| Impact of a prolonged decline in rainfall on eucalypt woodlands in southwestern Australia and its consequences for avifauna.Crossref | GoogleScholarGoogle Scholar |
Armstrong, KN, and Nichols, OG (2000). Long-term trends in avifaunal recolonisation of rehabilitated bauxite mines in the jarrah forest of south-western Australia. Forest Ecology and Management 126, 213–225.
| Long-term trends in avifaunal recolonisation of rehabilitated bauxite mines in the jarrah forest of south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Bengtsson, J, Persson, T, and Lundkvist, H (1997). Long-term effects of logging residue addition and removal on macroarthropods and enchytraeids. Journal of Applied Ecology 34, 1014–1022.
| Long-term effects of logging residue addition and removal on macroarthropods and enchytraeids.Crossref | GoogleScholarGoogle Scholar |
BOM (2011) ‘Western Australia in 2010: a very dry year in southwest western Australia.’ (Commonwealth of Australia, Bureau of Meteorology)
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2011) ‘Introduction to meta-analysis.’ (John Wiley & Sons: Chichester)
Breshears, DD, Cobb, NS, Rich, PM, Price, KP, Allen, CD, Balice, RG, Romme, WH, Kastens, JH, Floyd, ML, Belnap, J, Anderson, JJ, Myers, OB, and Meyer, CW (2005). Regional vegetation die-off in response to global-change-type drought. Proceedings of the National Academy of Sciences 102, 15144–15148.
| Regional vegetation die-off in response to global-change-type drought.Crossref | GoogleScholarGoogle Scholar |
Brouwers, N, Matusick, G, Ruthrof, K, Lyons, T, and Hardy, G (2013). Landscape-scale assessment of tree crown dieback following extreme drought and heat in a Mediterranean eucalypt forest ecosystem. Landscape Ecology 28, 69–80.
| Landscape-scale assessment of tree crown dieback following extreme drought and heat in a Mediterranean eucalypt forest ecosystem.Crossref | GoogleScholarGoogle Scholar |
Bultman, TL, and Uetz, GW (1984). Effect of structure and nutritional quality of litter on abundances of litter-dwelling arthropods. American Midland Naturalist 111, 165–172.
| Effect of structure and nutritional quality of litter on abundances of litter-dwelling arthropods.Crossref | GoogleScholarGoogle Scholar |
Cadieux, P, Boulanger, Y, Cyr, D, Taylor, AR, Price, DT, Sólymos, P, Stralberg, D, Chen, HYH, Brecka, A, and Tremblay, JA (2020). Projected effects of climate change on boreal bird community accentuated by anthropogenic disturbances in western boreal forest, Canada. Diversity and Distributions 26, 668–682.
| Projected effects of climate change on boreal bird community accentuated by anthropogenic disturbances in western boreal forest, Canada.Crossref | GoogleScholarGoogle Scholar |
Carleton, RE, Graham, JH, Lee, A, Taylor, ZP, and Carleton, JF (2019). Reproductive success of Eastern Bluebirds (Sialia sialis) varies with the timing and severity of drought. PLoS ONE 14, e0214266.
| Reproductive success of Eastern Bluebirds (Sialia sialis) varies with the timing and severity of drought.Crossref | GoogleScholarGoogle Scholar |
Carroll, JM, Davis, CA, Elmore, RD, and Fuhlendorf, SD (2017). Using a historic drought and high-heat event to validate thermal exposure predictions for ground-dwelling birds. Ecology and Evolution 7, 6413–6422.
| Using a historic drought and high-heat event to validate thermal exposure predictions for ground-dwelling birds.Crossref | GoogleScholarGoogle Scholar |
Conradie, SR, Woodborne, SM, Wolf, BO, Pessato, A, Mariette, MM, and McKechnie, AE (2020). Avian mortality risk during heat waves will increase greatly in arid Australia during the 21st century. Conservation Physiology 8, coaa048.
| Avian mortality risk during heat waves will increase greatly in arid Australia during the 21st century.Crossref | GoogleScholarGoogle Scholar |
Cooper, C, Withers, P, Mawson, PR, Johnstone, R, Kirkby, T, Prince, J, Bradshaw, SD, and Robertson, H (2003). Characteristics of marri (corymbia calophylla) fruits in relation to the foraging behaviour of the forest red-tailed black cockatoo (calyptorhynchus banksii naso). Journal of the Royal Society of Western Australia 86, 139–142.
Correia, RA, Haskell, WC, Gill, JA, Palmeirim, JM, and Franco, AMA (2015). Topography and aridity influence oak woodland bird assemblages in southern Europe. Forest Ecology and Management 354, 97–103.
| Topography and aridity influence oak woodland bird assemblages in southern Europe.Crossref | GoogleScholarGoogle Scholar |
Cousin, JA (2004). Habitat selection of the Western Yellow Robin (Eopsaltria griseogularis) in a Wandoo woodland, Western Australia. Emu - Austral Ornithology 104, 229–234.
| Habitat selection of the Western Yellow Robin (Eopsaltria griseogularis) in a Wandoo woodland, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Davis, RA, Valentine, LE, Craig, MD, Wilson, B, Bancroft, WJ, and Mallie, M (2014). Impact of Phytophthora-dieback on birds in Banksia woodlands in south west Western Australia. Biological Conservation 171, 136–144.
| Impact of Phytophthora-dieback on birds in Banksia woodlands in south west Western Australia.Crossref | GoogleScholarGoogle Scholar |
Dell B, Havel JJ (1989) The jarrah forest, an introduction. In ‘The jarrah forest: a complex mediterranean ecosystem’. (Eds B Dell, JJ Havel, N Malajczuk). (Kluwer Academic Publishers: Dordrecht, The Netherlands)
Dundas, SJ, Ruthrof, KX, Hardy, GESJ, and Fleming, PA (2021). Some like it hot: drought-induced forest die-off influences reptile assemblages. Acta Oecologica 111, 103714.
| Some like it hot: drought-induced forest die-off influences reptile assemblages.Crossref | GoogleScholarGoogle Scholar |
Ellis, M, and Taylor, J (2013). Birds in remnant woodland vegetation in the central wheatbelt of New South Wales during the drought declared years 2005 to 2009. Australian Zoologist 36, 332–348.
| Birds in remnant woodland vegetation in the central wheatbelt of New South Wales during the drought declared years 2005 to 2009.Crossref | GoogleScholarGoogle Scholar |
Er, KBH (1997). Effects of eucalypt dieback on bird species diversity in remnants of native woodland. Corella 21, 101–111.
Fischer, EM, Beyerle, U, and Knutti, R (2013). Robust spatially aggregated projections of climate extremes. Nature Climate Change 3, 1033–1038.
| Robust spatially aggregated projections of climate extremes.Crossref | GoogleScholarGoogle Scholar |
Fleming, PA, Wentzel, JJ, Dundas, SJ, Kreplins, TL, Craig, MD, and Hardy, GESJ (2021). Global meta-analysis of tree decline impacts on fauna. Biological Reviews 96, 1744–1768.
| Global meta-analysis of tree decline impacts on fauna.Crossref | GoogleScholarGoogle Scholar |
Floyd, ML, Romme, WH, Rocca, ME, Hanna, DP, and Hanna, DD (2015). Structural and regenerative changes in old-growth piñon–juniper woodlands following drought-induced mortality. Forest Ecology and Management 341, 18–29.
| Structural and regenerative changes in old-growth piñon–juniper woodlands following drought-induced mortality.Crossref | GoogleScholarGoogle Scholar |
Fumy, F, and Fartmann, T (2021). Climate and land-use change drive habitat loss in a mountain bird species. Ibis 163, 1189–1206.
| Climate and land-use change drive habitat loss in a mountain bird species.Crossref | GoogleScholarGoogle Scholar |
Furnas, BJ (2020). Rapid and varied responses of songbirds to climate change in California coniferous forests. Biological Conservation 241, 108347.
| Rapid and varied responses of songbirds to climate change in California coniferous forests.Crossref | GoogleScholarGoogle Scholar |
Gazol, A, Camarero, JJ, Sangüesa-Barreda, G, and Vicente-Serrano, SM (2018). Post-drought resilience after forest die-off: shifts in regeneration, composition, growth and productivity. Frontiers in Plant Science 9, 1546.
| Post-drought resilience after forest die-off: shifts in regeneration, composition, growth and productivity.Crossref | GoogleScholarGoogle Scholar |
Gentilli J (1989) Climate of the jarrah forest. In ‘The jarrah forest.’ pp. 23–40. (Springer)
Hammer, Ø, Harper, DAT, and Ryan, PD (2001). Past: paleontological statistics software package for education and data analysis. Palaeontolia Electronica 4, 9.
Harrer M, Cuijpers P, Furukawa TA, Ebert DD (2021) ‘Doing meta-analysis with r: a hands-on guide.’ 1st edn. (Chapman & Hall/CRC Press: Boca Raton, FL and London)
Haslem, A, Nimmo, DG, Radford, JQ, and Bennett, AF (2015). Landscape properties mediate the homogenization of bird assemblages during climatic extremes. Ecology 96, 3165–3174.
| Landscape properties mediate the homogenization of bird assemblages during climatic extremes.Crossref | GoogleScholarGoogle Scholar |
Hoffmann, AA, Rymer, PD, Byrne, M, Ruthrof, KX, Whinam, J, McGeoch, M, Bergstrom, DM, Guerin, GR, Sparrow, B, Joseph, L, Hill, SJ, Andrew, NR, Camac, J, Bell, N, Riegler, M, Gardner, JL, and Williams, SE (2019). Impacts of recent climate change on terrestrial flora and fauna: some emerging Australian examples. Austral Ecology 44, 3–27.
| Impacts of recent climate change on terrestrial flora and fauna: some emerging Australian examples.Crossref | GoogleScholarGoogle Scholar |
Hughes, JD, Petrone, KC, and Silberstein, RP (2012). Drought, groundwater storage and stream flow decline in southwestern Australia. Geophysical Research Letters 39, L03408.
| Drought, groundwater storage and stream flow decline in southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
Landsberg, J (1988). Dieback of rural eucalypts: Tree phenology and damage caused by leaf-feeding insects. Australian Journal of Ecology 13, 251––267.
Landsberg, J (1990a). Dieback of rural eucalypts: Does insect herbivory relate to dietary quality of tree foliage? Australian Journal of Ecology 15, 73––87.
Landsberg, J (1990b). Dieback of rural eucalypts: Response of foliar dietary quality and herbivory to defoliation. Australian Journal of Ecology 15, 89––96.
Landsberg, J, and Wylie, FR (1983). Water stress, leaf nutrients and defoliation: a model of dieback of rural eucalypts. Australian Journal of Ecology 8, 27––41.
Langellotto, GA, and Denno, RF (2004). Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. Oecologia 139, 1–10.
| Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis.Crossref | GoogleScholarGoogle Scholar |
Long, JL (1984). The diets of three species of parrots in the south of western Australia. Wildlife Research 11, 357–371.
| The diets of three species of parrots in the south of western Australia.Crossref | GoogleScholarGoogle Scholar |
Mac Nally, R (1994). Habitat-specific guild structure of forest birds in south-eastern Australia: a regional scale perspective. Journal of Animal Ecology 63, 988–1001.
| Habitat-specific guild structure of forest birds in south-eastern Australia: a regional scale perspective.Crossref | GoogleScholarGoogle Scholar |
Mac Nally, R, Bennett, AF, Thomson, JR, Radford, JQ, Unmack, G, Horrocks, G, and Vesk, PA (2009). Collapse of an avifauna: climate change appears to exacerbate habitat loss and degradation. Diversity and Distributions 15, 720–730.
| Collapse of an avifauna: climate change appears to exacerbate habitat loss and degradation.Crossref | GoogleScholarGoogle Scholar |
Marcelino, J, Silva, JP, Gameiro, J, Silva, A, Rego, FC, Moreira, F, and Catry, I (2020). Extreme events are more likely to affect the breeding success of lesser kestrels than average climate change. Scientific Reports 10, 7207.
| Extreme events are more likely to affect the breeding success of lesser kestrels than average climate change.Crossref | GoogleScholarGoogle Scholar |
Martin, RO, Cunningham, SJ, and Hockey, PAR (2015). Elevated temperatures drive fine-scale patterns of habitat use in a savanna bird community. Ostrich 86, 127–135.
| Elevated temperatures drive fine-scale patterns of habitat use in a savanna bird community.Crossref | GoogleScholarGoogle Scholar |
Matusick, G, Ruthrof, KX, Brouwers, NC, Dell, B, and Hardy, GSJ (2013). Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in southwestern Australia. European Journal of Forest Research 132, 497–510.
| Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
Matusick, G, Ruthrof, KX, Fontaine, JB, and Hardy, GESJ (2016). Eucalyptus forest shows low structural resistance and resilience to climate change-type drought. Journal of Vegetation Science 27, 493–503.
| Eucalyptus forest shows low structural resistance and resilience to climate change-type drought.Crossref | GoogleScholarGoogle Scholar |
Matusick, G, Ruthrof, KX, Kala, J, Brouwers, NC, Breshears, DD, and Hardy, GESJ (2018). Chronic historical drought legacy exacerbates tree mortality and crown dieback during acute heatwave-compounded drought. Environmental Research Letters 13, 095002.
| Chronic historical drought legacy exacerbates tree mortality and crown dieback during acute heatwave-compounded drought.Crossref | GoogleScholarGoogle Scholar |
McKechnie, AE, and Wolf, BO (2010). Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biology Letters 6, 253–256.
| Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves.Crossref | GoogleScholarGoogle Scholar |
McKechnie, AE, Hockey, PAR, and Wolf, BO (2012). Feeling the heat: Australian landbirds and climate change. Emu - Austral Ornithology 112, i–vii.
| Feeling the heat: Australian landbirds and climate change.Crossref | GoogleScholarGoogle Scholar |
Moore, TL, Valentine, LE, Craig, MD, Hardy, GESJ, and Fleming, PA (2013). Do woodland birds prefer to forage in healthy Eucalyptus wandoo trees? Australian Journal of Zoology 61, 187–195.
| Do woodland birds prefer to forage in healthy Eucalyptus wandoo trees?Crossref | GoogleScholarGoogle Scholar |
Morcombe MK (2003) ‘Field guide to Australian birds.’ (Steve Parish Publishing)
Newton, I (2007). Weather-related mass-mortality events in migrants. Ibis 149, 453–467.
| Weather-related mass-mortality events in migrants.Crossref | GoogleScholarGoogle Scholar |
Nichols, OG, and Burrows, R (1985). Recolonisation of revegetated bauxite mine sites by predatory invertebrates. Forest Ecology and Management 10, 49–64.
| Recolonisation of revegetated bauxite mine sites by predatory invertebrates.Crossref | GoogleScholarGoogle Scholar |
Nichols, OG, Carbon, BA, Colquhoun, IJ, Croton, JT, and Murray, NJ (1985). Rehabilitation after bauxite mining in south-western Australia. Landscape Planning 12, 75–92.
| Rehabilitation after bauxite mining in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Nittérus, K, and Gunnarsson, B (2006). Effect of microhabitat complexity on the local distribution of arthropods in clear-cuts. Environmental Entomology 35, 1324–1333.
| Effect of microhabitat complexity on the local distribution of arthropods in clear-cuts.Crossref | GoogleScholarGoogle Scholar |
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) ‘Vegan: community ecology package, R package version 2.5-7.’ Available at https://cran.r-project.org/web/packages/vegan/vegan.pdf
Oliver, IAN, Pearce, S, Greenslade, PJM, and Britton, DR (2006). Contribution of paddock trees to the conservation of terrestrial invertebrate biodiversity within grazed native pastures. Austral Ecology 31, 1–12.
| Contribution of paddock trees to the conservation of terrestrial invertebrate biodiversity within grazed native pastures.Crossref | GoogleScholarGoogle Scholar |
Paz-Kagan, T, Brodrick, PG, Vaughn, NR, Das, AJ, Stephenson, NL, Nydick, KR, and Asner, GP (2017). What mediates tree mortality during drought in the southern Sierra Nevada? Ecological Applications 27, 2443–2457.
| What mediates tree mortality during drought in the southern Sierra Nevada?Crossref | GoogleScholarGoogle Scholar |
Pearce-Higgins, JW, Eglington, SM, Martay, B, and Chamberlain, DE (2015). Drivers of climate change impacts on bird communities. Journal of Animal Ecology 84, 943–954.
| Drivers of climate change impacts on bird communities.Crossref | GoogleScholarGoogle Scholar |
Perkins-Kirkpatrick, SE, White, CJ, Alexander, LV, Argüeso, D, Boschat, G, Cowan, T, Evans, JP, Ekström, M, Oliver, ECJ, Phatak, A, and Purich, A (2016). Natural hazards in Australia: heatwaves. Climatic Change 139, 101–114.
| Natural hazards in Australia: heatwaves.Crossref | GoogleScholarGoogle Scholar |
Petrone, KC, Hughes, JD, Van Niel, TG, and Silberstein, RP (2010). Streamflow decline in southwestern Australia, 1950–2008. Geophysical Research Letters 37, L11401.
| Streamflow decline in southwestern Australia, 1950–2008.Crossref | GoogleScholarGoogle Scholar |
Philpott, SM, and Bichier, P (2012). Effects of shade tree removal on birds in coffee agroecosystems in Chiapas, Mexico. Agriculture, Ecosystems & Environment 149, 171–180.
| Effects of shade tree removal on birds in coffee agroecosystems in Chiapas, Mexico.Crossref | GoogleScholarGoogle Scholar |
Postle, AC, Majer, JD, and Bell, DT (1986). Soil and litter invertebrates and litter decomposition in jarrah (Eucalyptus marginata) forest affected by jarrah dieback fungus (Phytophthora cinnamomi). Pedobiologia 29, 47–69.
Qie, L, Telford, EM, Massam, MR, Tangki, H, Nilus, R, Hector, A, and Ewers, RM (2019). Drought cuts back regeneration in logged tropical forests. Environmental Research Letters 14, 045012.
| Drought cuts back regeneration in logged tropical forests.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2022) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria)
Recher, HF, Majer, JD, and Ganesh, S (1996). Eucalypts, arthropods and birds: on the relation between foliar nutrients and species richness. Forest Ecology and Management 85, 177––195.
Reid, T, Lada, H, Selwood, KE, Horrocks, GFB, Thomson, JR, and Mac Nally, R (2022). Responses of floodplain birds to high-amplitude precipitation fluctuations over two decades. Austral Ecology 47, 828–840.
| Responses of floodplain birds to high-amplitude precipitation fluctuations over two decades.Crossref | GoogleScholarGoogle Scholar |
Restaino, C, Young, DJN, Estes, B, Gross, S, Wuenschel, A, Meyer, M, and Safford, H (2019). Forest structure and climate mediate drought-induced tree mortality in forests of the Sierra Nevada, USA. Ecological Applications 29, e01902.
| Forest structure and climate mediate drought-induced tree mortality in forests of the Sierra Nevada, USA.Crossref | GoogleScholarGoogle Scholar |
Roberts, LJ, Burnett, R, Tietz, J, and Veloz, S (2019). Recent drought and tree mortality effects on the avian community in southern Sierra Nevada: a glimpse of the future? Ecological Applications 29, e01848.
| Recent drought and tree mortality effects on the avian community in southern Sierra Nevada: a glimpse of the future?Crossref | GoogleScholarGoogle Scholar |
Ruthrof, KX, Matusick, G, and Hardy, GESJ (2015). Early differential responses of co-dominant canopy species to sudden and severe drought in a mediterranean-climate type forest. Forests 6, 2082–2091.
| Early differential responses of co-dominant canopy species to sudden and severe drought in a mediterranean-climate type forest.Crossref | GoogleScholarGoogle Scholar |
Ruthrof, KX, Fontaine, JB, Matusick, G, Breshears, DD, Law, DJ, Powell, S, and Hardy, G (2016). How drought-induced forest die-off alters microclimate and increases fuel loadings and fire potentials. International Journal of Wildland Fire 25, 819–830.
| How drought-induced forest die-off alters microclimate and increases fuel loadings and fire potentials.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA, Mawson, P, and Dawson, R (2011). The impact of two extreme weather events and other causes of death on Carnaby’s Black Cockatoo: a promise of things to come for a threatened species? Pacific Conservation Biology 17, 141–148.
| The impact of two extreme weather events and other causes of death on Carnaby’s Black Cockatoo: a promise of things to come for a threatened species?Crossref | GoogleScholarGoogle Scholar |
Seaton, S, Matusick, G, Ruthrof, K, and Hardy, G (2015). Outbreak of Phoracantha semipunctata in response to severe drought in a mediterranean Eucalyptus forest. Forests 6, 3868–3881.
| Outbreak of Phoracantha semipunctata in response to severe drought in a mediterranean Eucalyptus forest.Crossref | GoogleScholarGoogle Scholar |
Steel, EJ, Fontaine, JB, Ruthrof, KX, Burgess, TI, and Hardy, GESJ (2019). Changes in structure of over- and midstory tree species in a Mediterranean-type forest after an extreme drought-associated heatwave. Austral Ecology 44, 1438–1450.
| Changes in structure of over- and midstory tree species in a Mediterranean-type forest after an extreme drought-associated heatwave.Crossref | GoogleScholarGoogle Scholar |
Stevens, HC, and Watson, DM (2013). Reduced rainfall explains avian declines in an unfragmented landscape: incremental steps toward an empty forest? Emu - Austral Ornithology 113, 112–121.
| Reduced rainfall explains avian declines in an unfragmented landscape: incremental steps toward an empty forest?Crossref | GoogleScholarGoogle Scholar |
Stoneman GL, Bradshaw FJ, Christensen PES (1989) Silviculture. In ‘The jarrah forest: a complex mediterranean ecosystem’. (Eds B Dell, JJ Havel, N Malajczuk). (Kluwer Academic Publishers: Dordrecht, The Netherlands)
Uetz, GW (1979). The influence of variation in litter habitats on spider communities. Oecologia 40, 29–42.
| The influence of variation in litter habitats on spider communities.Crossref | GoogleScholarGoogle Scholar |
Verdon, SJ, Watson, SJ, and Clarke, MF (2019). Modeling variability in the fire response of an endangered bird to improve fire-management. Ecological Applications 29, e01980.
| Modeling variability in the fire response of an endangered bird to improve fire-management.Crossref | GoogleScholarGoogle Scholar |
Viechtbauer W (2020) ‘Metafor v2.4-0 package for R.’ Available at https://www.quantargo.com/help/r/latest/packages/metafor/2.4-0/forest
Watson, DM (2003). The ‘standardized search’: An improved way to conduct bird surveys. Austral Ecology 28, 515––525.
Watson, DM (2011). A productivity-based explanation for woodland bird declines: poorer soils yield less food. Emu - Austral Ornithology 111, 10–18.
| A productivity-based explanation for woodland bird declines: poorer soils yield less food.Crossref | GoogleScholarGoogle Scholar |
Wentzel JJ (2010) Is tuart (Eucalyptus gomphocephala) decline detrimental for fauna? PhD Thesis, Murdoch University, Perth, WA, Australia.
Whisson, DA, Orlowski, A, and Weston, MA (2018). Tree canopy defoliation impacts avifauna. Forest Ecology and Management 428, 81–86.
| Tree canopy defoliation impacts avifauna.Crossref | GoogleScholarGoogle Scholar |
Zanette, L, Doyle, P, and Trémont, SM (2000). Food shortage in small fragments: evidence from an area-sensitive passerine. Ecology 81, 1654–1666.
| Food shortage in small fragments: evidence from an area-sensitive passerine.Crossref | GoogleScholarGoogle Scholar |


