Distress responses during handling in urban and exurban bandiny, the New Holland honeyeater (Phylidonyris novaehollandiae), in southwestern Western Australia
M. Pearmain-Fenton A B * , L. N. Gilson
A B * , L. N. Gilson  B , B. J. Saunders A and P. W. Bateman
B , B. J. Saunders A and P. W. Bateman  B
B
A School of Molecular and Life Sciences, Curtin University, Perth, WA, Australia.
B Behavioural Ecology Laboratory, School of Molecular and Life Sciences, Curtin University, Perth, WA, Australia.
Pacific Conservation Biology 29(5) 419-428 https://doi.org/10.1071/PC22014
Submitted: 22 March 2022 Accepted: 12 September 2022 Published: 7 October 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Birds appear to be better suited than mammals or reptiles to adapt to fast-changing landscapes because of their greater mobility; however, the behavioural effects of urbanisation on birds in Australia remain broadly unexplored.
Aims: This study aimed to investigate the effects of urbanisation on behavioural responses exhibited by a common and widespread meliphagid, the bandiny or New Holland honeyeater (Phylidonyris novaehollandiae) while undergoing standard bird banding processes.
Methods: Five non-invasive techniques (alarm calling, wriggling, biting, breathing rate, and tonic immobility) were explored for efficacy in identifying underlying differences in distress arising from mist-netting at urban and exurban localities in southwestern Western Australia.
Key results: Breathing rate was the most important variable for identifying differences in post-capture distress response. The breathing rate of urban bandiny following capture was lower than those of exurban areas. All other parameters proved suboptimal for detecting differential behavioural responses to handling between urban and exurban populations, despite having been successfully used in other international studies.
Conclusions: We suggest that urban honeyeaters exhibit lower mean breathing rates due to chronic overstimulation in response to urban lifestyles and are not able to further elevate this behaviour in response to stressful stimuli. The failure of other approaches explored highlights the need to apply ecosystem-appropriate methods for investigating urbanisation within an Australian context.
Implications: Our results suggest that behavioural approaches to quantifying avian stress developed internationally require additional consideration when applied to the ecosystems of Australia, whose birds are evolved to accommodate a different regime of seasonality that has shaped them behaviourally and morphologically.
Keywords: adaptive, behaviour, bird banding, distress, handling stress, Meliphagidae, passerine, urbanisation.
Introduction
Urbanisation is a form of habitat degradation that is particularly relevant to contemporary environmental research because of its rapid expansion across many previously unaltered landscapes (Tsurim et al. 2008). Birds may be better suited for adaptation to these fast-changing landscapes than mammals or reptiles due to the greater mobility afforded by flight (Møller 2009; Luck and Smallbone 2010). Birds are among the most successful urban adapters (Møller 2009) and as a result, provide a range of essential ecosystem services in urban areas. While bird community structure can serve as a reliable indicator of the health and functionality of urban areas as habitats for other organisms (Sandström et al. 2006), the effects of urbanisation on birds, especially the perching songbirds or passerines, remain broadly unexplored in Australia.
Due to the direct link between stress and survival, there remains a need to investigate causal relationships between the processes of human-induced landscape fragmentation and avian stress responses (Brearley et al. 2013). Stress in birds can be directly measured via tissue (blood and feather) sampling. Susceptibility to disease and parasites, such as avian malaria, also may indicate ambient stress level in an organism (Aharon-Rotman et al. 2016). By contrast, stress in birds can be inferred through the non-invasive observation of indirect behavioural indicators, such as flight initiation distance, handling aggression, and exploration/neophobia of novel environments. (Evans et al. 2009; Charmantier et al. 2017). For birds in the hand, stress can also be gauged by physiological phenomena such as breathing rate (Carere and van Oers 2004; Torné-Noguera et al. 2014). Differences in the intensity of distress behaviour exhibited by an organism can serve as a proxy for underlying environmentally-mediated stress.
The majority of studies investigating the effects of urbanisation on passerines have concentrated on Europe and North America, and tend to support ‘urban effect’ hypotheses (Møller and Ibáñez-Álamo 2012; Torné-Noguera et al. 2014; Abolins-Abols et al. 2016); that is, urban birds differ behaviourally and physiologically as a result of their urban residency. Research related to stress physiology in Australia, by contrast, has not always supported the hypothesis that urbanised landscapes produce physiologically unique populations (Amos et al. 2013; McGiffin et al. 2013). Amos et al. (2013) found little evidence that stress indicators, such as body condition (residual body mass), haematocrit, or whole blood haemoglobin concentration were related to landscape variation among eastern Australian woodland passerines and argued that such methods might not be suitable on this continent. For example, fleeing behaviours of Common Mynas (Acridotheres tristis) in and around Melbourne were not correlated with estimated human population density across an urban/exurban gradient, and vigilance effort also did not vary between zones within this same gradient (McGiffin et al. 2013).
The Australian Meliphagidae (honeyeaters) are primarily nectarivorous (Astheimer and Buttemer 2002) and have evolved structural adaptations of the tongue, which enable them to specialise on nectar. All the Meliphagidae rely largely on nectar-producing plants to survive, although many also forage on non-nectar resources, including lerp, honeydew, and small insects (Recher et al. 2016). Populations may be either sedentary or nomadic depending on the availability of nectar-producing trees in the surrounding environment.
In the Northern Hemisphere, urban-adapter passerines – those taxa thriving in highly urbanised landscapes – are almost always sedentary, omnivorous, and widely distributed, with large wingspans, and high nesting sites (Møller 2009; Torné-Noguera et al. 2014; Serrano-Davies et al. 2017). Australian native bird species that successfully exploit urbanised environments are, by contrast, almost exclusively opportunistic foragers (Sol et al. 2012), taking advantage of the patchy, year-round flowering of Australian shrubs and trees. Quantifying stress responses of meliphagids in the hand during routine bird banding at both urban and rural sites therefore serves as a simple means of investigating behavioural differences between urban and exurban honeyeater populations.
We used non-invasive behavioural observations to assess differences in handling stress responses in the bandiny – the southwestern Kongal-Marawar Noongar name for the New Holland honeyeater (Phylidonyris novaehollandiae) (Klesch and Spehn-Jackson 2014), on whose land we carried out this research. As Carere and van Oers (2004) found that increased breathing rate in Great Tits (Parus major) was a good proxy of increased stress, Torné-Noguera et al. (2014) found that urban Great Tits had a higher breathing rate in the hand than did exurban conspecifics. Møller and Ibáñez-Álamo (2012) also found that urban birds across several species demonstrated longer tonic immobility (a temporary state of motor inhibition/paralysis; Wang et al. 2013) and decreased biting and screaming behaviour in the hand than did exurban conspecifics. We subsequently made the following hypothesis: urban and exurban bandiny populations will exhibit significantly different reactions to handling stress as measured by breathing rate, wriggling, biting, alarm/distress calling and tonic immobility (proxies for this stress) when in the hand. Specifically, sensu Torné-Noguera et al. (2014), urban bandiny will have a higher breathing rate in the hand than exurban bandiny, and, sensu Møller and Ibáñez-Álamo (2012), urban bandiny will demonstrate longer tonic immobility in the hand, and decreased screaming, wriggling and biting behaviour than will exurban bandiny.
Materials and methods
Site description
This research was conducted in collaboration with five existing bird banding projects in southwestern Western Australia (Fig. 1). The three urban bird banding stations in the Perth Metropolitan Region were situated within the Swan Coastal Plain Key Biodiversity Area (KBA; Birdlife Australia 2021); the two exurban sites were situated in the Avon Wheatbelt Region of Western Australia (Fig. 1). A brief description of each site, including the dominant vegetation communities, principal land uses, and the Indigenous Country on which each is located, is provided as justification for assigning ‘urban’ or ‘exurban’ status to sites across the landscape (Table 1).
|
|

|
Data collection
Birds were captured using mist nets, following the guidelines provided by the Australian Bird Bander’s Manual (Lowe 1989) and all actions involving the capture, handling, and processing of birds were performed under the direct supervision of ABBBS (Australian Bird and Bat Banding Scheme) licenced A-class supervisors at each site (Animal Ethics Committee (AEC) #: ARE2021-1). Nets were placed in full or partial shade if temperatures exceeded 20°C. Banding operations ceased when temperatures fell outside the acceptable range of 5–25°C, following Australian and international best-practice guidelines (Lowe 1989; Smith et al. 1997). Banding activities began at dawn and continued until dusk on private properties and until midday on public properties (Table 1). Birds were removed from nets and immediately placed into cotton bags. Individuals waiting to be processed were left in bags for no longer than 5 min and were held out of direct sunlight and wind. Individuals were processed in the order that they were removed from nets, following routine banding protocols of attaching ABBBS-issued bird bands to the tarsus then collecting morphometric measures of head-bill length, wing length, tail length, and body mass (Lowe 1989). The age and sex of each bird were determined when possible following the Handbook of Australian, New Zealand and Antarctic Birds (Higgins et al. 2001). Data were collected over 8 months in 2021, from Birak to Djilba – the Noongar seasonal equivalent of January to August (South-west Aboriginal Land and Sea Council 2021). Data gathering ceased at the beginning of Djilba (the ‘season of conception’) to avoid the influence of breeding condition (McFarland 1996) on behavioural responses to standard banding procedures.
Distress responses were evaluated throughout the banding process, beginning with the recording of initial fear/alarm expressed at removal from the holding bag and ending with the assessment of tonic immobility immediately prior to release. Alarm calling during processing or upon release was recorded as either 0 = no call, or 1 = call produced. Rates of wriggling/resistance to being held were scored as 0 = no movement, 1 = moves rarely, 2 = moves regularly, but not always, and 3 = moves continuously, following Crino et al. (2017). Biting was scored as either 0 = no biting attempts, or 1 = biting attempt. After banding and morphometric measurements had been completed, the bird was then repositioned in the hand so that its abdomen could be seen clearly, with legs held loosely to stabilise the body while filming breathing rate. The bird’s inhalations were video recorded using a mobile phone camera for 30–60 s, allowing for the capture of at least 30 s of observable breathing rate (Fig. 2). Breathing rate was quantified as the number of breaths taken within the clearest 30-s period of video, following Torné-Noguera et al. (2014).
|
|
Tonic immobility was quantified as time elapsed between release of the bander’s grip and a bird’s departure from the open hand (Møller and Ibáñez-Álamo 2012). After breathing rate was recorded, the bander’s grip was released [fingers withdrawn from around the bird’s neck and shoulders so that only the bird’s back remained in contact with the bander’s hand (Fig. 3)]. While the observer continued to record the video, the legs were released so that the bird was unrestrained. If the bird did not immediately fly freely from the palm, the open hand holding the bird was gently raised and lowered 10–15 cm to rouse the bird from its tonic state. If the individual still did not fly after 30 s, it was turned upright, placed on a branch and monitored until it flew.
Data analysis
Statistical analyses were performed using both R (ver. 4.0.5, R Core Team 2021) run through RStudio (ver. 1.4.1 2021) and StatistiXL (ver. 2.0 2021). Mann–Whitney tests were used to evaluate differences in the categorical alarm, wriggle, and bite scores and the continuous breathing rate and tonic immobility measures across the urban/exurban divide. As the five banding locations produced unequal sample sizes, assumptions of normality and homogeneity of variance were violated, and a non-parametric approach was necessary.
To further assess the variation in breathing rate for bandiny at urban versus exurban sites, modelling of explanatory variables was performed using a Generalised Linear Model (GLM) with urban/exurban (two levels) as a fixed factor and Site (nested within urban/exurban) as a random factor. Additional explanatory variables which were assessed during the modelling process were air temperature (Ta), sex (fixed factor, male or female), wing length (WL), head-bill length (HB), and tail length (TL). A preliminary investigation of the data indicated that the morphometric variables WL, HB, and TL were highly correlated (Pearson correlation >0.87). As the most repeatable morphometric measurement, HB was retained in the model. The GLM was initially constructed using a Poisson rather than Gaussian distribution because the dataset violated parametric assumptions of homogeneity of variance (Levene test, F(d.f.=1,48) = 5.256, P = 0.027) (Zuur et al. 2007). However, the residuals of this model were over-dispersed, so models were fitted using a negative binomial distribution with a log link function (Zuur et al. 2007). The model with the lowest Akaike information criterion (AIC) was then selected from all possible combinations of the explanatory variables. As there were no alternative models within three AICs of the final model, only the final model is reported.
Ethical statement
The research and results presented in this paper were conducted in compliance with The Australian Code for the Care and Use of Animals for Scientific Purposes (8th edition). Permission to conduct research involving the use of live animals was granted by the Curtin Animal Ethics Committee (ARE2021-1). The C-class Bird Banding Authority (Authority number 3515) was granted to MPF by the Australian Bird and Bat Banding Scheme. Permission to band birds in Western Australia is regulated via the Department of Biodiversity, Conservation and Attractions, which operates using the Biodiversity Conservation Act 2016 as well as the Biodiversity Conservation Regulations 2018.
Results
No instances of alarm calling or biting were recorded at any of the banding locations. No difference was observed with regard to median wriggle score (U(d.f.=29,29) = 483.000, P = 0.338) or median duration of tonic immobility (U(d.f.=29,29) = 490.500, P = 0.279). However, the median breathing rate was significantly higher for bandiny in exurban than urban sites (exurban 142 breaths/min ± 7.80 s.e. and urban 114 breaths/min ± 3.73 s.e., U(d.f.=29,29) = 585.500, P = 0.010).
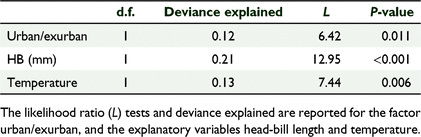
The most parsimonious negative binomial model included the morphometric variable HB (mm), the environmental variable air temperature (°C), and the locality factor urban/exurban (explained deviance = 0.374, AIC = 408.9). Each variable explained a statistically significant proportion of the overall variance in breathing rate, with HB (as a proxy for body size variation) carrying the highest explained deviance overall. HB and temperature were negatively correlated with breathing rate, and breathing rate was lower in urban environments than in exurban (Tables 2, 3).

|
|
Discussion
Breathing rate as an indicator of distress
Breathing rate has long been recognised as an accurate and reliable indicator of acute stress in passerines (Carere and van Oers 2004). As Torné-Noguera et al. (2014) found that urban Great Tit (Parus major) exhibited significantly higher mean breathing rates than did exurban ones while in the hand, we predicted a similar pattern in breathing rate between urban and exurban honeyeaters but did not obtain a similar direction in our results. Urban bandiny demonstrated lower breathing rates than did exurban ones, which suggests that lower breathing rates are indicative of habituation to humans. Exurban bandiny were much less likely to have encountered humans, such that their elevated breathing rate upon being caught and handled suggests a stronger perception of threat. Other Australian studies have shown that honeyeaters living in highly urbanised landscapes demonstrate greater resilience and acclimatisation to human cohabitation (Myers et al. 2010; Davis and Wilcox 2013; Cleary et al. 2016; Recher et al. 2016) and may be less sensitised to predators (Atwell et al. 2012); this may explain why urban birds should present as less stressed when captured and handled by bird-banders.
Bite score and alarm calling as means of quantifying distress
Our prediction of reduced biting and screaming demonstrated by urban over exurban bandiny was not supported. Although biting and alarm calling are commonly observed during the banding of passerine birds, both while in the hand during processing and also upon release, neither of these behaviours were displayed by any of the bandiny handled in our study. The short, strong bills of Great Tits (which do bite in the hand) are suitable for capturing insects via foliage gleaning but are also used in conjunction with the feet for consuming larger items, such as hazelnuts, through a process called ‘hold hammering’ (Serrano-Davies et al. 2017). This hammering action is deployed as an antipredator tactic when in the hand (Preiszner et al. 2017). Honeyeaters have evolved entirely different bill morphologies to accommodate their nectarivorous diet (McFarland 1996): their long, slender bills, curved for accessing nectar from tubular-flowered plants, are not effective at delivering a powerful blow against either a tough food item or a potential (perceived) threat. Therefore, we suggest that honeyeaters may only rarely, if ever, bite as a defensive reaction or a distress response.
Alarm calling was also not exhibited by any of the meliphagids handled in this research. In contrast, Møller and Ibáñez-Álamo (2012) demonstrate that urban Great Tits showed much higher rates of alarm-calling during bird banding than did exurban birds. The intensity and duration of alarm calls towards human ‘threats’ are known to be positively associated with exploratory behaviours in novel environments, such as newly established urban habitats (Hollander et al. 2008). However, despite bandiny often producing alarm calls, this did not occur when handled and therefore does not appear to be a defence tactic of bandiny intended to startle a predator into releasing the caller (Stefanski and Falls 1972; Starkey and Starkey 1973; Conover 1994).
Wriggle and escape behaviour
Wriggling while in the hand acts as an attempt to escape from a perceived threat and is an indicator of distress that requires little training to recognise. Wriggling behaviour was exhibited by bandiny at both urban and exurban areas, though no significant difference in median wriggle score was identified. Møller and Ibáñez-Álamo (2012) found that urban Great Tits wriggled less than did exurban conspecifics. This agrees with similar studies on other passerines in the Northern Hemisphere, which mostly identify urban birds as having higher tolerances to disturbance and other stressors (Davies and Sewall 2016; Charmantier et al. 2017; Ducatez et al. 2017).
Tonic immobility and departure from the hand
The longer a bird remains in tonic immobility (TI) and attempts to ‘play dead’, the more fearful or distressed it is assumed to be (Wang et al. 2013). Often described as an ‘unlearnt catatonic state’ (Flores et al. 2019, p. 3), TI can be understood as the ultimate stage within a chain of anti-predator behaviour patterns (Mills and Faure 1991), and therefore TI duration is recognised as a reliable indicator of underlying fearfulness (Boissy 1995; Hazard et al. 2008). However, our prediction that urban bandiny would show longer periods of TI than exurban ones was not supported: capture locality did not affect the duration of TI. Individuals in this study either flew away immediately (TI of 0 s) or not at all (TI beyond 30 s, requiring repositioning and standard release from the hand). This tendency toward a bimodal pattern suggests two distinct escape strategies, though we are unable to identify the exact biological drivers of this decision.
Diet, behaviour, and landscape interact to shape bandiny/P. novaehollandiae movement
Honeyeaters are described as irruptive migrants, responding to seasonal fluctuations in food resources (McFarland 2002), a pattern more prevalent in Australian birds rather than the seasonally synchronised, long-distance migrations observed in other hemispheres and climate types. Individual honeyeaters may travel long distances to find food in dry season months when the flowering of nectar-producing plants is low, but will do so only if they do not already occupy territories with plentiful resources (Keast 1968; Yates et al. 2007). The bandiny is one of only a few meliphagid species where territoriality has been adequately researched, with demonstrated flexibility in both breeding and non-breeding territory acquisition and defence (McFarland 1994, 1996, 2002). Owners of feeding territories held during the breeding cycle may remain site-faithful as the territories are often close to valuable nesting sites, even though they do not always provide sufficient energy supplies post-fledgling (McFarland 1994). Within a population of bandiny, some individuals may remain within the same territory their whole lives, while others may migrate in times of need (Recher et al. 2016). This behavioural plasticity contrasts sharply with that of the Great Tit, which is strictly non-migratory (Demeyrier et al. 2016; Charmantier et al. 2017; Serrano-Davies et al. 2017). While urban and exurban Great Tit populations are sedentary and discrete, urban and exurban bandiny ‘populations’ are not reliably sedentary and therefore less discrete. As a result, birds captured by mist-netting at any time of year could be either residents of or migrants to the locality. This factor potentially confounds the assignment of birds in this study as ‘urban’ and ‘exurban’ based on capture locations, as such descriptors are not truly relevant to irruptive migrants that exploit both localities when they are potential sources of nectar.
Recommendations for future study
For the scope of this field-based study, a simple urban versus exurban classification was created based on the proximity of sites to the metropolitan centre of Perth (Fig. 1). However, research and policy focusing on habitat degradation through changing land use are often limited in their ability to accurately describe the full range of possible landscape configurations created by a variety of disturbances across the landscape (Davis et al. 2013), and it may be unwise to resort to a simple binary classification of habitat versus non-habitat/urbanised landscape within a management context. Collecting data over multiple years will likely also reduce the effect of seasonally fluctuating food resources on behaviour. It would also be advantageous to incorporate more ecologically buffered or undamaged exurban sites into the study design, as opposed to the highly fragmented Wheatbelt sites included in this study (sites in this study were restricted by the availability of established bird banding projects). Additionally, including a larger sample size across multiple bird families may reveal large-scale impacts of urbanisation that have not been previously acknowledged. Future investigation into the mechanisms influencing avian adaptation in urban environments may reveal other species-specific changes that are unique to Australian ecosystems. It is necessary for researchers and policymakers to understand that the methods constructed for use on birds situated in the Northern Hemisphere are not guaranteed to produce the same results worldwide. This is particularly the case for meliphagid species whose ecologies (both behavioural and physiological) are coadapted to the ecologies of the food plants they rely on, as the wide variety of nectar-producing flora promotes irruptive migratory feeding behaviours as the standard foraging tactic for honeyeaters. The identification of ecologically appropriate variables that are tailored to Australian passerine physiologies and behaviours should be used in combination with the methods explored in this study to benefit future researchers. The low resource demand and simple training requirements of these methods in comparison to more invasive techniques, such as corticosteroid analysis through blood or feather sampling, would facilitate a broader field study of Australian birds’ ecologically constrained stress-response behaviours. It is also important to recognise that a large proportion of Northern Hemisphere based studies investigating stress physiology on captive-bred birds are, in fact modelled on a Southern Hemisphere species: the Zebra Finch (Taeniopygia guttata). This species is now the most widely researched bird outside those used in agricultural production (Crino et al. 2017; Griffith et al. 2021), thanks to their low maintenance requirements and fast maturation. Model species are an essential part of biological studies across many disciplines, and those that can be researched in controlled conditions remain a crucial part of contemporary biology. However, it is critical to continue to study a variety of additional non-model species in ecologically appropriate contexts. This combination of learning from laboratory studies as well as wild species will together produce a broader and more thorough understanding of avian biology.
Conclusions
With the exception of breathing rate, the results of this study suggest that several behavioural approaches used in Northern Hemisphere bird taxa to indicate underlying stress as a consequence of urbanisation are not applicable to meliphagid species in southwestern Western Australia. Although urbanisation is recognised as negatively impactful to wildlife, its role as an evolutionary driver of adaptation has not been fully explored until recently, and the need for species and ecosystem-specific research, particularly in already vulnerable areas of the Southern Hemisphere, remains critical. Being able to accurately record and analyse the responses of multiple species to their environments is crucial to better understand the long-term impacts of urbanisation. Birds serve as ideal candidates for this research because of their already highly adapted urban lifestyles and as providers of essential ecosystem services, but all birds are not equivalents of each other. The continuing global encroachment of urbanisation necessitates investigation into how animals adapt locally. It is our responsibility as scientists to also ensure these urbanised areas remain functional and serve as suitable habitats for native species.
Data availability
The data that supports the findings of this study are available from the corresponding author, MPF, upon reasonable request.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
We acknowledge the Traditional Owners of the Boodja (Country) upon which this research was carried out and where this document was written: the Whadjuk and Wiilman people of the Noongar Nation. We pay our respects to Elders past, present, and emerging and recognise the importance of First Nations’ knowledge in connection to Country. We choose to use Noongar names for our study species occurring in Noongar Boodja. The authors extend their appreciation to the banding project collaborators who supported this work: the Herdsman Lake Bird Banding Group and Whiteman Park Management, for allowing us to conduct research on their grounds; Marion Massam, for providing additional training to MPF in ‘all things bird banding’; and the Bamford and Metcalfe families for access to their home properties as research sites.
References
Abolins-Abols, M, Hope, SF, and Ketterson, ED (2016). Effect of acute stressor on reproductive behavior differs between urban and rural birds. Ecology and Evolution 6, 6546–6555.| Effect of acute stressor on reproductive behavior differs between urban and rural birds.Crossref | GoogleScholarGoogle Scholar |
Aharon-Rotman, Y, Buchanan, KL, Clark, NJ, Klaassen, M, and Buttemer, WA (2016). Why fly the extra mile? Using stress biomarkers to assess wintering habitat quality in migratory shorebirds. Oecologia 182, 385–395.
| Why fly the extra mile? Using stress biomarkers to assess wintering habitat quality in migratory shorebirds.Crossref | GoogleScholarGoogle Scholar |
Amos, JN, Balasubramaniam, S, Grootendorst, L, Harrisson, KA, Lill, A, Mac Nally, R, Pavlova, A, Radford, JQ, Takeuchi, N, Thomson, JR, and Sunnucks, P (2013). Little evidence that condition, stress indicators, sex ratio, or homozygosity are related to landscape or habitat attributes in declining woodland birds. Journal of Avian Biology 44, 45–54.
| Little evidence that condition, stress indicators, sex ratio, or homozygosity are related to landscape or habitat attributes in declining woodland birds.Crossref | GoogleScholarGoogle Scholar |
Arnold, GW, and Weeldenburg, JR (1990). Factors determining the number and species of birds in road verges in the wheatbelt of Western Australia. Biological Conservation 53, 295–315.
| Factors determining the number and species of birds in road verges in the wheatbelt of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Astheimer, LB, and Buttemer, WA (2002). Changes in latitude, changes in attitude: a perspective on ecophysiological studies of Australian birds. Emu - Austral Ornithology 102, 19–27.
| Changes in latitude, changes in attitude: a perspective on ecophysiological studies of Australian birds.Crossref | GoogleScholarGoogle Scholar |
Atwell, JW, Cardoso, GC, Whittaker, DJ, Campbell-Nelson, S, Robertson, KW, and Ketterson, ED (2012). Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behavioral Ecology 23, 960–969.
| Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation.Crossref | GoogleScholarGoogle Scholar |
Australian Institute of Aboriginal and Torres Strait Islander Studies (1996) Map of Indigenous Australia. Available at https://aiatsis.gov.au/explore/map-indigenous-australia
Birdlife Australia (2021) Key biodiversity areas: nature’s hotspots. Available at https://www.keybiodiversityareas.org.au
Boissy, A (1995). Fear and fearfulness in animals. The Quarterly Review of Biology 70, 165–191.
| Fear and fearfulness in animals.Crossref | GoogleScholarGoogle Scholar |
Brearley, G, Rhodes, J, Bradley, A, Baxter, G, Seabrook, L, Lunney, D, Liu, Y, and McAlpine, C (2013). Wildlife disease prevalence in human-modified landscapes. Biological Reviews 88, 427–442.
| Wildlife disease prevalence in human-modified landscapes.Crossref | GoogleScholarGoogle Scholar |
Brunton C (2001) CALM Regional Parks usage survey. Department of Conservation and Land Management, Perth.
Carere, C, and van Oers, K (2004). Shy and bold great tits (Parus major): body temperature and breath rate in response to handling stress. Physiology & Behavior 82, 905–912.
| Shy and bold great tits (Parus major): body temperature and breath rate in response to handling stress.Crossref | GoogleScholarGoogle Scholar |
Charmantier, A, Demeyrier, V, Lambrechts, M, Perret, S, and Grégoire, A (2017). Urbanization is associated with divergence in pace-of-life in great tits. Frontiers in Ecology and Evolution 5, 53.
| Urbanization is associated with divergence in pace-of-life in great tits.Crossref | GoogleScholarGoogle Scholar |
Cleary, GP, Parsons, H, Davis, A, Coleman, BR, Jones, DN, Miller, KK, and Weston, MA (2016). Avian assemblages at bird baths: a comparison of urban and rural bird baths in Australia. PLoS ONE 11, e0150899.
| Avian assemblages at bird baths: a comparison of urban and rural bird baths in Australia.Crossref | GoogleScholarGoogle Scholar |
Conover MR (1994) How birds interpret distress calls: implications for applied uses of distress call playbacks. Proceedings of the Vertebrate Pest Conference 16, 232–234.
Crino, OL, Buchanan, KL, Trompf, L, Mainwaring, MC, and Griffith, SC (2017). Stress reactivity, condition, and foraging behavior in zebra finches: effects on boldness, exploration, and sociality. General and Comparative Endocrinology 244, 101–107.
| Stress reactivity, condition, and foraging behavior in zebra finches: effects on boldness, exploration, and sociality.Crossref | GoogleScholarGoogle Scholar |
Davies, S, and Sewall, KB (2016). Correction to ‘Agonistic urban birds: elevated territorial aggression of urban song sparrows is individually consistent within a breeding period’. Biology Letters 12, 20160900.
| Correction to ‘Agonistic urban birds: elevated territorial aggression of urban song sparrows is individually consistent within a breeding period’.Crossref | GoogleScholarGoogle Scholar |
Davis, RA, and Wilcox, JA (2013). Adapting to suburbia: bird ecology on an urban-bushland interface in Perth, Western Australia. Pacific Conservation Biology 19, 110–120.
| Adapting to suburbia: bird ecology on an urban-bushland interface in Perth, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Davis, RA, Gole, C, and Roberts, JD (2013). Impacts of urbanisation on the native avifauna of Perth, Western Australia. Urban Ecosystems 16, 427–452.
| Impacts of urbanisation on the native avifauna of Perth, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Demeyrier, V, Lambrechts, MM, Perret, P, and Grégoire, A (2016). Experimental demonstration of an ecological trap for a wild bird in a human-transformed environment. Animal Behaviour 118, 181–190.
| Experimental demonstration of an ecological trap for a wild bird in a human-transformed environment.Crossref | GoogleScholarGoogle Scholar |
Department of Planning and Urban Development, Western Australia (1993) The natural resources of the Darling Ranges. Darling Range Regional Park supplementary report, no. 2. Department of Planning and Urban Development, Western Australia, Perth, WA.
Ducatez, S, Audet, J-N, Rodriguez, JR, Kayello, L, and Lefebvre, L (2017). Innovativeness and the effects of urbanization on risk-taking behaviors in wild Barbados birds. Animal Cognition 20, 33–42.
| Innovativeness and the effects of urbanization on risk-taking behaviors in wild Barbados birds.Crossref | GoogleScholarGoogle Scholar |
Evans, KL, Gaston, KJ, Frantz, AC, and Simeoni, M (2009). Independent colonization of multiple urban centres by a formerly forest specialist bird species. Proceedings of the Royal Society B: Biological Sciences 276, 2403–2410.
| Independent colonization of multiple urban centres by a formerly forest specialist bird species.Crossref | GoogleScholarGoogle Scholar |
Flores, R, Penna, M, Wingfield, JC, Cuevas, E, Vásquez, RA, and Quirici, V (2019). Effects of traffic noise exposure on corticosterone, glutathione and tonic immobility in chicks of a precocial bird. Conservation Physiology 7, coz061.
| Effects of traffic noise exposure on corticosterone, glutathione and tonic immobility in chicks of a precocial bird.Crossref | GoogleScholarGoogle Scholar |
Gentilli J, Bekle H (1993) ‘History of the Perth Lakes. Vol. 10.’ (Royal Western Australian Historical Society)
Griffith, SC, Ton, R, Hurley, LL, McDiarmid, CS, and Pacheco-Fuentes, H (2021). The ecology of the Zebra Finch makes it a great laboratory model but an outlier amongst passerine birds. Birds 2, 60–76.
| The ecology of the Zebra Finch makes it a great laboratory model but an outlier amongst passerine birds.Crossref | GoogleScholarGoogle Scholar |
Hazard, D, Couty, M, Richard, S, and Guémené, D (2008). Intensity and duration of corticosterone response to stressful situations in Japanese quail divergently selected for tonic immobility. General and Comparative Endocrinology 155, 288–297.
| Intensity and duration of corticosterone response to stressful situations in Japanese quail divergently selected for tonic immobility.Crossref | GoogleScholarGoogle Scholar |
Higgins P, Peter JM, Steele WK (2001) ‘Handbook of Australia, New Zealand and Antarctic birds: tyrant-flycatchers to chats. Vol. 5.’ 2nd edn. (Oxford University Press Australia & New Zealand: Melbourne)
Hollander, FA, Van Overveld, T, Tokka, I, and Matthysen, E (2008). Personality and nest defence in the great tit (Parus major). Ethology 114, 405–412.
| Personality and nest defence in the great tit (Parus major).Crossref | GoogleScholarGoogle Scholar |
Keast, A (1968). Seasonal movements in the Australian honeyeaters (Meliphagidae) and their ecological significance. Emu - Austral Ornithology 67, 159–209.
| Seasonal movements in the Australian honeyeaters (Meliphagidae) and their ecological significance.Crossref | GoogleScholarGoogle Scholar |
Kinnear, A, and Garnett, P (1999). Water chemistry of the wetlands of the Yellagonga Regional Park, Western Australia. Journal of the Royal Society of Western Australia 82, 79–87.
Klesch M, Spehn-Jackson L (2014) ‘Djerap: Noongar birds.’ (Batchelor Institute Press: Perth, WA)
Lowe KW (1989) ‘The Australian bird bander’s manual’. (CPP Communications Ltd).
Luck GW, Smallbone LT (2010) Chapter 5: Species diversity and urbanisation: patterns, drivers and implications. In ‘Urban ecology’. (Ed. KJ Gaston) pp. 88–119. (Cambridge University Press: Cambridge)
McFarland, DC (1994). Responses of territorial New Holland honeyeaters Phylidonyris novaehollandiae to short-term fluctuations in nectar productivity. Emu - Austral Ornithology 94, 193–200.
| Responses of territorial New Holland honeyeaters Phylidonyris novaehollandiae to short-term fluctuations in nectar productivity.Crossref | GoogleScholarGoogle Scholar |
McFarland, DC (1996). Aggression and nectar use in territorial non-breeding New Holland honeyeaters Phylidonyris novaehollandiae in eastern Australia. Emu - Austral Ornithology 96, 181–188.
| Aggression and nectar use in territorial non-breeding New Holland honeyeaters Phylidonyris novaehollandiae in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
McFarland, DC (2002). Non-breeding territoriality in the New Holland honeyeater, Phylidonyris novaehollandiae, in an unpredictable environment—short-term energy costs for possible long-term reproductive benefits. Emu - Austral Ornithology 102, 315–321.
| Non-breeding territoriality in the New Holland honeyeater, Phylidonyris novaehollandiae, in an unpredictable environment—short-term energy costs for possible long-term reproductive benefits.Crossref | GoogleScholarGoogle Scholar |
McGiffin, A, Lill, A, Beckman, J, and Johnstone, CP (2013). Tolerance of human approaches by Common Mynas along an urban-rural gradient. Emu - Austral Ornithology 113, 154–160.
| Tolerance of human approaches by Common Mynas along an urban-rural gradient.Crossref | GoogleScholarGoogle Scholar |
Mills, AD, and Faure, J-M (1991). Divergent selection for duration of tonic immobility and social reinstatement behavior in Japanese quail (Coturnix coturnix japonica) chicks. Journal of Comparative Psychology 105, 25–38.
| Divergent selection for duration of tonic immobility and social reinstatement behavior in Japanese quail (Coturnix coturnix japonica) chicks.Crossref | GoogleScholarGoogle Scholar |
Møller, AP (2009). Successful city dwellers: a comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 159, 849–858.
| Successful city dwellers: a comparative study of the ecological characteristics of urban birds in the Western Palearctic.Crossref | GoogleScholarGoogle Scholar |
Møller, AP, and Ibáñez-Álamo, JD (2012). Escape behaviour of birds provides evidence of predation being involved in urbanization. Animal Behaviour 84, 341–348.
| Escape behaviour of birds provides evidence of predation being involved in urbanization.Crossref | GoogleScholarGoogle Scholar |
Myers, S, Brown, G, and Kleindorfer, S (2010). Divergence in New Holland honeyeaters (Phylidonyris novaehollandiae): evidence from morphology and feeding behavior. Journal of Ornithology 151, 287–296.
| Divergence in New Holland honeyeaters (Phylidonyris novaehollandiae): evidence from morphology and feeding behavior.Crossref | GoogleScholarGoogle Scholar |
Preiszner, B, Papp, S, Pipoly, I, Seress, G, Vincze, E, Liker, A, and Bókony, V (2017). Problem-solving performance and reproductive success of great tits in urban and forest habitats. Animal Cognition 20, 53–63.
| Problem-solving performance and reproductive success of great tits in urban and forest habitats.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2021) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna) Available at https://www.rstudio.com/products/rstudio/
Recher, HF, Calver, MC, and Davis, WE (2016). Ecology of honeyeaters (Meliphagidae) in Western Australian Eucalypt Woodlands I: resource allocation among species in the Great Western Woodland during spring. Australian Zoologist 38, 130–146.
| Ecology of honeyeaters (Meliphagidae) in Western Australian Eucalypt Woodlands I: resource allocation among species in the Great Western Woodland during spring.Crossref | GoogleScholarGoogle Scholar |
Sandström, UG, Angelstam, P, and Mikusiński, G (2006). Ecological diversity of birds in relation to the structure of urban green space. Landscape and Urban Planning 77, 39–53.
| Ecological diversity of birds in relation to the structure of urban green space.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA (1989). Changes in the Avifauna of a region, district and remnant as a result of fragmentation of native vegetation: the wheatbelt of western Australia. A case study. Biological Conservation 50, 99––135.
Serrano-Davies, E, O’Shea, W, and Quinn, JL (2017). Individual foraging preferences are linked to innovativeness and personality in the great tit. Behavioral Ecology and Sociobiology 71, 161.
| Individual foraging preferences are linked to innovativeness and personality in the great tit.Crossref | GoogleScholarGoogle Scholar |
Smith H, McCracken J, Shepherd D, Velez P (1997) ‘The mist netter’s bird safety handbook.’ (The Institute for Bird Populations)
Sol, D, Bartomeus, I, and Griffin, AS (2012). The paradox of invasion in birds: competitive superiority or ecological opportunism? Oecologia 169, 553––564.
South-west Aboriginal Land and Sea Council (2021) Six seasons - Nyungar life on the Coastal Plain. Available at https://www.derbalnara.org.au/boodjar-six-seasons
Starkey, EE, and Starkey, JF (1973). Description of an aerial predator alarm call for mallard (Anas platyrhynchos) ducklings. The Condor 75, 364–366.
| Description of an aerial predator alarm call for mallard (Anas platyrhynchos) ducklings.Crossref | GoogleScholarGoogle Scholar |
Stefanski, RA, and Falls, JB (1972). A study of distress calls of song, swamp, and white-throated sparrows (Aves: Fringillidae). I. Intraspecific responses and functions. Canadian Journal of Zoology 50, 1501–1512.
| A study of distress calls of song, swamp, and white-throated sparrows (Aves: Fringillidae). I. Intraspecific responses and functions.Crossref | GoogleScholarGoogle Scholar |
Torné-Noguera, A, Pagani-Núñez, E, and Senar, JC (2014). Great tit (Parus major) breath rate in response to handling stress: urban and forest birds differ. Journal of Ornithology 155, 315–318.
| Great tit (Parus major) breath rate in response to handling stress: urban and forest birds differ.Crossref | GoogleScholarGoogle Scholar |
Tsurim, I, Abramsky, Z, and Kotler, BP (2008). Foraging behavior of urban birds: are human commensals less sensitive to predation risk than their nonurban counterparts? The Condor 110, 772–776.
| Foraging behavior of urban birds: are human commensals less sensitive to predation risk than their nonurban counterparts?Crossref | GoogleScholarGoogle Scholar |
Wang, S, Ni, Y, Guo, F, Fu, W, Grossmann, R, and Zhao, R (2013). Effect of corticosterone on growth and welfare of broiler chickens showing long or short tonic immobility. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 164, 537–543.
| Effect of corticosterone on growth and welfare of broiler chickens showing long or short tonic immobility.Crossref | GoogleScholarGoogle Scholar |
Western Australian Planning Commission (2017) Whiteman Park strategic plan 2017–2021. Western Australian Planning Commission.
Yates, CJ, Coates, DJ, Elliott, C, and Byrne, M (2007). Composition of the pollinator community, pollination and the mating system for a shrub in fragments of species rich kwongan in south-west Western Australia. Biodiversity and Conservation 16, 1379–1395.
| Composition of the pollinator community, pollination and the mating system for a shrub in fragments of species rich kwongan in south-west Western Australia.Crossref | GoogleScholarGoogle Scholar |
Zuur AF, Ieno EN, Smith GM (2007) Chapter 7: Additive and generalised additive modelling. In ‘Analysing ecological data. Statistics for biology and health’. (Eds AF Zuur, EN Ieno, GM Smith) pp. 97–124. (Springer, New York, NY)


