Leech breach: a first record of the invasive freshwater leech Helobdella europaea (Hirudinea: Glossiphoniidae) in Fiji
Bindiya Rashni A , Kelly T. Brown
A , Kelly T. Brown  B , Patricia A. McLenachan C , Peter J. Lockhart
B , Patricia A. McLenachan C , Peter J. Lockhart  C , Paul C. Southgate D and Monal M. Lal
C , Paul C. Southgate D and Monal M. Lal  B D *
B D *
A Institute of Applied Science, School of Agriculture, Geography, Environment, Ocean and Natural Sciences, University of the South Pacific, Lower Laucala Campus, Laucala Bay Road, Suva, Fiji.
B Discipline of Marine Studies, School of Agriculture, Geography, Environment, Ocean and Natural Sciences, University of the South Pacific, Lower Laucala Campus, Laucala Bay Road, Suva, Fiji.
C School of Natural Sciences, College of Sciences, Massey University, Palmerston North 4442, New Zealand.
D Australian Centre for Pacific Islands Research and School of Science, Technology and Engineering, University of the Sunshine Coast, Maroochydore, Qld 4558, Australia.
Abstract
The freshwater flat leech Helobdella europaea Kutschera, 1987 is a small annelid indigenous to South America. This invasive species feeds on the haemolymph of host aquatic invertebrates, with occurrences reported from Europe, USA, Taiwan, North Africa, Hawai‘i, Australia and New Zealand. A large number of individuals were discovered in the Ba River catchment, Fiji, during a 2015–2020 freshwater biodiversity survey, raising concerns of potential impacts on endemic Fijian aquatic invertebrate fauna and ecosystem integrity.
To facilitate assessments of its spread and ethology, this study employed morphological and phylogenetic analyses for verification of taxonomic identity.
Phylogenetic trees were constructed using a 658 bp fragment of the mitochondrial DNA cox1 (COI) gene. The first complete mitochondrial genome sequence of H. europaea was also determined using selective multiple displacement amplification and Oxford Nanopore Technology to provide a reference for future comparative analyses and source tracking of spread to other regions.
Morphological and COI analyses identified all Fijian leech specimens collected (n = 16) as H. europaea, reporting the first occurrence of this species on a south-west Pacific Island. The complete mitochondrial genome was sequenced.
Confirmation of its presence in Fiji is a national biosecurity concern and will guide the Biosecurity Authority of Fiji and national agencies in further ecosystem assessment and response strategies.
With the complete mitochondrial genome of H. europaea now available, transmission pathway traceability is possible in other regions where this species may be detected.
Keywords: biosecurity, DNA barcoding, freshwater, invasive, leech, mitochondrial DNA, taxonomic identification, traceability.
Introduction
Globally over 50% of the 917 described species of leeches (Mãgalhaes et al. 2021) inhabit freshwater and are divided among 91 genera (Sket and Trontelj 2008). Some freshwater leeches are highly specialised and play important roles as invertebrate predators. The family Glossiphoniidae is the sole lineage within the order Rhynchobdellida (proboscis-bearing leeches) that is predominantly confined to freshwater, although some taxa display temporary salinity tolerance (Sawyer 1974). Of all freshwater leeches, the genus Helobdella (Blanchard, 1896) (Annelida: Clitellata: Rhynchobdellida: Glossiphoniidae) is the most speciose, with 35 described species (Sket and Trontelj 2008). This genus has the highest diversity in South America with 20 species described (Sawyer 1986) and has received the most attention in the fields of annelid developmental biology, ecology, ethology and population genetic studies (Weisblat and Huang 2001; Seaver 2003).
Over the last 40 years the invasive freshwater flat leech, Helobdella europaea Kutschera 1987 has recorded occurrences in locations extending from the Palaearctic to the Australasian and Oceanian realms. Helobdella europaea belongs to the Helobdella triserialis species complex, members of which are indigenous to South America (Sawyer 1986). This species is relatively small (1–8 cm long), two eyed, flat, with a generally grey coloured body and light pigment spots. It is hermaphroditic, feeds on the haemolymph of host aquatic invertebrates (oligochaetes, insects and gastropods), and conducts brood care of its young. While its ecological niche varies from still waters to slow flowing hydrosystems, it is often epiphytic to algae and macrophytes, and has the capability of colonising artificial drainages and canals, slow streams, irrigation and drainage ditches and polluted open sewers (Lai et al. 2009; Málnás et al. 2016; Morhun et al. 2021).
H. europaea was first described as Helobdella striata from Germany (Kutschera 1985), but the latter name was preoccupied and this species was renamed (Kutschera 1987). Since then it has been reported in other European countries including the Netherlands (van Haaren et al. 2004), Spain (Jueg 2008; Reyes-Prieto et al. 2014), Hungary (Málnás et al. 2016) and more recently Ukraine (Morhun et al. 2021). Besides Europe, it is also present in California (Kutschera et al. 2013), Taiwan (Lai et al. 2009), North Africa (Mabrouki et al. 2019), South Africa, Hawai‘i, New Zealand (Siddall and Budinoff 2005), and Australia (Govedich and Davies 1998 – referred to as H. papillornata; Pfeiffer et al. 2004; Siddall and Budinoff 2005).
Freshwater leech studies in Fiji are still in their infancy, with previous work being limited to the identification of native specimens in the family Salifidae (Rashni 2013; Rashni 2014a, 2014b; Rashni 2015). The presence of Helobdella. cf. europaea in Fijian river systems on the island of Viti Levu is the first record of this species in the country. Individuals have been observed on three occasions in Fijian waterways: in 2015 during an environmental impact assessment study at Qalinabulu Creek, Bavu village, Nadroga Province; in 2015 and 2019 at Balevuto village, Ba Province; and in 2019 in the upper- and mid-Wainamau sub-catchment waterways (Rashni 2020). This last field visit in 2019 by authors to Koroboya village, Ba Province led to in situ population observations and preliminary live specimen morphological verification using established descriptions (Kutschera 2004; Lai et al. 2009; Kutschera et al. 2013). Anecdotal reports suggest the presence of H. cf. europaea has been known to local communities for at least a decade (Semisi Qamese, pers. comm., 2015).
Considering that (1) H. europaea is a recognised invasive species also recorded from neighbouring Australia and New Zealand (GBIF 2020), and (2) it parasitises aquatic invertebrates (Kutschera 2004; Málnás et al. 2016); it is important to confirm the taxonomic identity of Fijian Helobdella leech specimens. Therefore, the current research reports on the taxonomic verification of Fijian H. cf. europaea, and presents the complete mitochondrial genome of this species, which can be used as a source tracking tool should it spread to new localities. These data will inform interventions relating to possible ecosystem health impacts associated with this leech, both in Fiji and where occurrences are reported elsewhere.
Methodology
Study area and sample collection
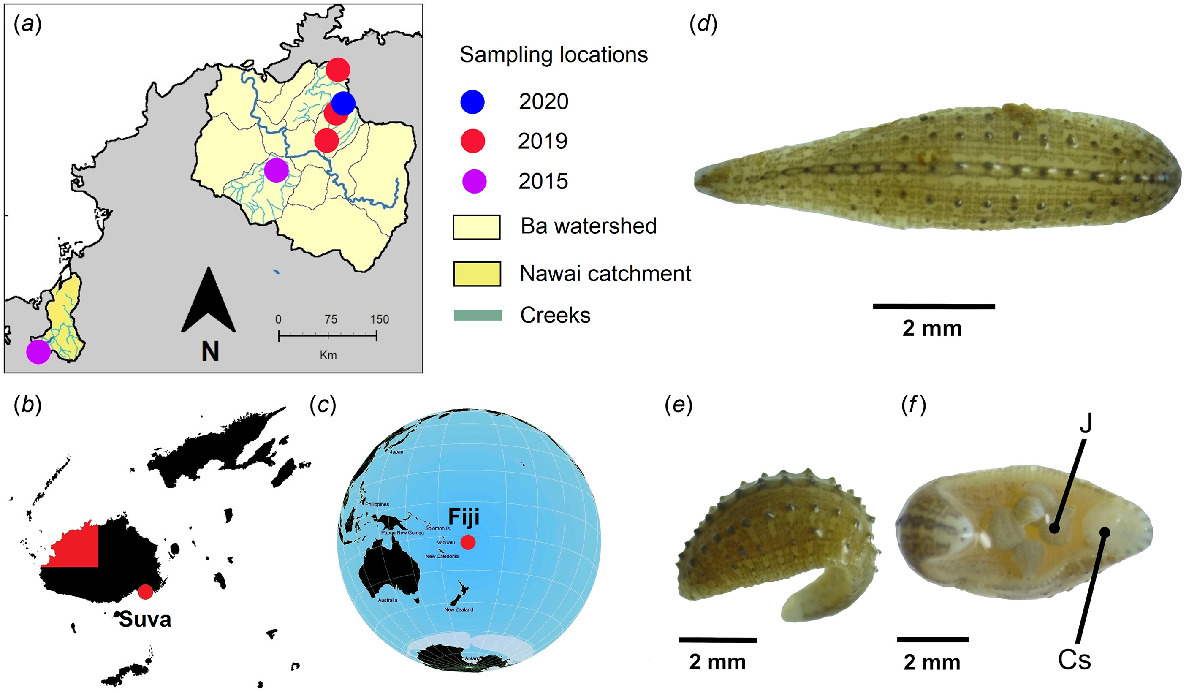
A kick-netting technique (Stark et al. 2001) was used to collect leech specimens (n = 50) for genetic analyses from the Koroboya village stream system, Ba Province, Viti Levu, Fiji in 2020 (Fig. 1). The specimens were preserved whole in 80% ethanol.
Collection locations and representative specimens of H. cf. europaea from Fiji. (a) Koroboya village stream system and wider Ba Province watershed annotated with coloured sampling locations and dates, (b) the location of Ba Province (red) in Fiji and (c) Fiji relative to the South Pacific region. Fijian specimen features shown include a (d) dorsal view with five rows of distinct black-tipped papillae; (e) cone-shaped dorsal papillae, dark longitudinal stripes and white chromatophores; and (f) ventral view with fully developed young attached – (J), a pair of eyes with (Cs) caudal sucker. Insets (a–c) were generated using QGIS v 3.18.3-Zürich and open source data obtained from the Humanitarian Data Exchange (https://data.humdata.org/dataset/cod-ab-fji) and Natural Earth (https://www.naturalearthdata.com/).
Morphological analyses
Additional specimens obtained from Bavu village in the Nadroga-Navosa province (year 2015) and Ba province (years 2015, 2019, and 2020, n = 52) were morphologically identified as H. europaea following Kutschera (2004), Lai et al. (2009) and Kutschera et al. (2013). Examinations involved measuring specimen sizes (body length at rest), observing form (body shape, presence of a convex dorsum, anterior sucker, posterior (caudal) sucker and pair of eyes with annulation along the axis of the body), colour and pattern.
Phylogenetic analyses
Total DNA was extracted from individual whole leech specimens (n = 32/50 specimens collected) using a CTAB (cetyltrimethylammonium bromide) chloroform/isoamyl alcohol protocol (Adamkewicz and Harasewych 1996), modified as per Lal et al. (2016) at the University of the South Pacific, Suva, Fiji. Purified DNA was quantified with a QUBIT flourometer (Life Technologies, MA, USA) using the Broad Range DNA assay. Subsequently, 16 samples were further processed for Polymerase Chain Reaction (PCR) and Sanger sequencing at the School of Natural Sciences at Massey University in New Zealand.
A 658 bp mitochondrial cytochrome c oxidase subunit I (cox1) DNA fragment was targeted for amplification using the universal primers (Folmer et al. 1994): LCO1490 (5’-GGT CAA CAA ATC ATA AAG ATA TTG G-3’) and HCO2198 (5’-TAA ACT TCA GGG TGA CCA AAA AAT CA-3’). Amplification was carried out on a Whatman T1 (Whatman Biometra, Göttingen, Germany) thermal cycler, with each reaction using EmeraldAmp GT Master Mix (Takara, Kusatsu, Shiga, Japan), 10 pmol of each primer and 2 ng template DNA. Cycling conditions were as follows: 94°C for 3 min; 35 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 40 s, and a final extension step at 72°C for 5 min with a hold at 10°C. PCR products were assayed using 1.5% agarose gel electrophoresis before clean-up with a SAP-Exo kit (Jena Bioscience, Jena, Germany). Bi-directional sequencing was performed on an ABI 3730 DNA Analyzer (Applied Biosystems, Waltham, MA, USA) following standard instructions.
Chromatograms were edited using CodonCode Aligner ver. 3.7.1–6.0.2 (CodonCode Co., Centerville, MA, USA) in the Geneious v.9.0.4 package (Kearse et al. 2012). Gaps in the sequences were treated as missing data, and final alignment for sequences was by MUltiple Sequence Comparison by Log-Expectation (MUSCLE, (Edgar 2004). The final alignment was subjected to substitution model testing using jModelTest (Posada 2008), with Bayesian Information Criterion (BIC) scores including the negative log likelihood (−lnL) and BIC difference (delta parameter) used to select the optimal model. Edited and aligned forward and reverse sequences were then subjected to a megaBLAST search to retrieve matching cox1 Helobdella sequences from the NCBI GenBank (Table 1). Sequences belonging to Placobdella phalera and Glossiphonia elegans were used as outgroups as per Lai et al. (2009).
| Species | GenBank accession number | Collection location | Reference | |
|---|---|---|---|---|
| H. europaea | AF329052 | Magill Creek, Brisbane, Australia | Reyes-Prieto et al. (2014) | |
| H. europaea | AY856047 | Aura Vale Lake, Australia | Reyes-Prieto et al. (2014) | |
| H. europaea | AY856048 | South Africa | Reyes-Prieto et al. (2014) | |
| H. europaea | AY856049 | New Zealand | Reyes-Prieto et al. (2014) | |
| H. europaea | DQ995297 | Galt, California, USA | Reyes-Prieto et al. (2014) | |
| H. europaea | DQ995298 | Galt, California, USA | Reyes-Prieto et al. (2014) | |
| H. europaea | DQ995304 | Berkeley, California, USA | Bely and Weisblat (2006) | |
| H. europaea | FJ000349 | Taipei, Taiwan | Reyes-Prieto et al. (2014) | |
| H. europaea | FJ000350 | Taipei, Taiwan | Reyes-Prieto et al. (2014) | |
| H. europaea | FJ000351 | Taipei, Taiwan | Reyes-Prieto et al. (2014) | |
| H. europaea | FJ000352 | Taipei, Taiwan | Reyes-Prieto et al. (2014) | |
| H. europaea | KC904241 | Castellon, Valencia, Spain | Reyes-Prieto et al. (2014) | |
| H. europaea | KC904242 | Castellon, Valencia, Spain | Reyes-Prieto et al. (2014) | |
| H. europaea | KC904243 | Castellon, Valencia, Spain | Reyes-Prieto et al. (2014) | |
| H. europaea | KU738724 | Debrecen, Hungary | Langguth and Kutschera (2016) in Morhun et al. (2021) | |
| H. europaea | MF804537 | Mississippi, USA | Richardson et al. (2017) | |
| H. europaea | MG976140 | Victoria, Australia | Carew et al. (2018) | |
| H. europaea | MN335875 | Spain | Perera et al. (2019) | |
| H. europaea | MT258557 | Kharkiv, Ukraine | Morhun et al. (2021) | |
| H. modesta | AF329040 | Ohio, USA | Reyes-Prieto et al. (2014) | |
| H. modesta | HQ179853 | Washington, USA | Reyes-Prieto et al. (2014) | |
| H. modesta | HQ179854 | Washington, USA | Reyes-Prieto et al. (2014) | |
| H. modesta | JF319988 | Connecticut, USA | Reyes-Prieto et al. (2014) | |
| H. modesta | JF319989 | Connecticut, USA | Reyes-Prieto et al. (2014) | |
| H. modesta | JF319990 | Connecticut, USA | Reyes-Prieto et al. (2014) | |
| H. modesta | JF319991 | Connecticut, USA | Reyes-Prieto et al. (2014) | |
| H. modesta | JF319992 | Connecticut, USA | Reyes-Prieto et al. (2014) | |
| H. modesta | JF319993 | Connecticut, USA | Reyes-Prieto et al. (2014) | |
| H. modesta | JF319994 | Connecticut, USA | Reyes-Prieto et al. (2014) | |
| H. modesta | JF319995 | Connecticut, USA | Reyes-Prieto et al. (2014) | |
| H. modesta | JF319996 | Connecticut, USA | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | FJ000342 | Taipei, Taiwan | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | FJ000343 | Taipei, Taiwan | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | FJ000344 | Taipei, Taiwan | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | FJ000345 | Taipei, Taiwan | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | FJ000346 | Taipei, Taiwan | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | FJ000347 | Taipei, Taiwan | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | FJ000348 | Taipei, Taiwan | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | HQ179855 | Querétaro, Mexico | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | HQ179856 | Ameca, Jalisco, Mexico | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | HQ179857 | Hidalgo, Mexico | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | HQ179858 | Guanajuato, Mexico | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | HQ179859 | Tabasco, Mexico | Reyes-Prieto et al. (2014) | |
| H. octatestisaca | HQ179860 | South Africa | Reyes-Prieto et al. (2014) | |
| H. papillata | AF329042 | Michigan, USA | Reyes-Prieto et al. (2014) | |
| H. papillata | AF329043 | Michigan, USA | Reyes-Prieto et al. (2014) | |
| H. papillata | AF329046 | Virginia, USA | Reyes-Prieto et al. (2014) | |
| H. papillata | KP176607 | USA | Richardson et al. (2015) | |
| H. papillata | MK416023 | USA | Beresic-Perrins et al. (2019) in Morhun et al. (2021) | |
| H. papillata | MK416024 | USA | Beresic-Perrins et al. (2019) in Morhun et al. (2021) | |
| H. papillata | MK416025 | USA | Beresic-Perrins et al. (2019) in Morhun et al. (2021) | |
| H. robusta | DQ995299 | California, USA | Reyes-Prieto et al. (2014) | |
| H. robusta | DQ995300 | California, USA | Reyes-Prieto et al. (2014) | |
| H. robusta | DQ995301 | California, USA | Reyes-Prieto et al. (2014) | |
| H. robusta | DQ995302 | California, USA | Reyes-Prieto et al. (2014) | |
| H. robusta | DQ995306 | Austin, Texas, USA | Reyes-Prieto et al. (2014) | |
| H. robusta | DQ995307 | Austin, Texas, USA | Reyes-Prieto et al. (2014) | |
| H. robusta | DQ995308 | Austin, Texas, USA | Reyes-Prieto et al. (2014) | |
| H. robusta | DQ995309 | Austin, Texas, USA | Reyes-Prieto et al. (2014) | |
| H. robusta | DQ995310 | Austin, Texas, USA | Reyes-Prieto et al. (2014) | |
| H. socimulcensis | DQ995311 | Mexico | Reyes-Prieto et al. (2014) | |
| H. socimulcensis | HQ179866 | Mexico | Reyes-Prieto et al. (2014) | |
| H. socimulcensis | HQ179867 | Mexico | Reyes-Prieto et al. (2014) | |
| H. socimulcensis | HQ179868 | Mexico | Reyes-Prieto et al. (2014) | |
| H. socimulcensis | HQ179869 | Mexico | Reyes-Prieto et al. (2014) | |
| H. socimulcensis | HQ179870 | Mexico | Reyes-Prieto et al. (2014) | |
| H. socimulcensis | HQ179871 | Mexico | Reyes-Prieto et al. (2014) | |
| H. socimulcensis | HQ179872 | Mexico | Reyes-Prieto et al. (2014) | |
| H. socimulcensis | MG821615 | Mexico | Bely and Weisblat (2006) | |
| H. socimulcensis | MG821616 | Mexico | Tessler et al. (2018) | |
| H. socimulcensis | MK208676 | Mexico | Jiménez-Armenta and Oceguera-Figueroa (2019) | |
| H. socimulcensis | MK208677 | Mexico | Jiménez-Armenta and Oceguera-Figueroa (2019) | |
| H. socimulcensis | MK208678 | Mexico | Jiménez-Armenta and Oceguera-Figueroa (2019) | |
| H. socimulcensis | MK208679 | Mexico | Jiménez-Armenta and Oceguera-Figueroa (2019) | |
| H. socimulcensis | MK208680 | Mexico | Jiménez-Armenta and Oceguera-Figueroa (2019) | |
| H. triserialis | AF329054 | Bolivia | Siddall and Borda (2003) | |
| H. triserialis | DQ995303 | USA | Bely and Weisblat (2006) | |
| H. triserialis | KC771417 | USA | Schmerer et al. (2013) | |
| Outgroups | ||||
| Placobdella phalera | AF003278 | Siddall and Burreson (1998) | ||
| Glossiphonia elegans | AF003258 | Siddall and Burreson (1998) | ||
Species, collection location, sequence accession data and citing references are reported.
Bayesian inference was used for phylogenetic reconstruction in the MrBayes v3.2 package (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003; Ronquist et al. 2012). Parameter settings for the data block were set as follows: lset applyto = (all), nst = 2, rates = gamma; prset applyto = (all), lset applyto = (all). Each analysis incorporated two runs of 100 000 000 generations, with four independent chains for each run, a temperature of 0.10 for the heated chains, sampling frequency of 1000 and burn-in fraction of 20%. The burn-in threshold was selected on the basis that both independent runs had achieved stationarity (i.e. stable log likelihood values reached for all sampled trees, indicated by the average standard deviation of split frequencies <0.01). Convergence was also assessed using Tracer v.1.6 independently (Rambaut et al. 2003). Final trees were retained by selecting only those post-burn in trees producing the highest individual (p) and cumulative posterior (P) probabilities during Markov Chain Monte Carlo (MCMC) computations, of p = 0.000 and P ≥ 0.995 respectively. A consensus tree was then constructed from the final credible sets of trees using the strict consensus method in Dendroscope 3.5.7 (Huson et al. 2007). All phylograms were visualised, inspected and edited in FigTree (https://tree.bio.ed.ac.uk/) v.1.4.2 (Rambaut 2014). Net evolutionary divergence estimates between the different Helobdella sp. taxa, as well as among H. europaea sampled from different collection sites were computed in MEGA6 (Tamura et al. 2013). Standard error estimates were obtained following a bootstrap procedure with replication.
The mitochondrial genome of H. europaea
Ten nanograms of H. europaea total genomic DNA from a Fijian specimen and 62.5 μM of the selective multiple displacement amplification (MDA) primers TATT*A*A and TAAT*T*A (* indicates exonuclease resistant phosphorothioate bonds) were denatured in 5 μL of 1× EquiPhi Reaction Buffer at 95°C for 3 min. The mix was cooled on ice for 3–5 min then added to 20 μL of 1× reaction mix containing EquiPhi29 DNA polymerase Reaction Buffer, DTT, dNTPs and EquiPhi29 DNA Polymerase as per the protocol: https://www.thermofisher.com/order/catalog/product/A39390. The reaction was incubated at 37°C for 3 h followed by an inactivation step at 65°C for 10 min.
After whole genome amplification, impurities were removed from the amplified DNA using a DNA Genomic Clean and Concentrator Column (Zymo Research). The amplified DNA was eluted from the column in 15 μL of water and the concentration of the eluate (290 ng/μL) determined by QUBIT (ThermoFisher). A MinION PCR-free library was then prepared using the Oxford Nanopore Technologies (ONT) rapid barcoding kit (SQK-RBK004) and sequenced on a MinION flongle (R9.4.1) using an ONT Mk1C MinION device running MinKNOW v21.10.8. H. europaea total genomic DNA was also sequenced without MDA on a flongle on the ONT Mk1C MinION device.
Bases were called using Guppy v5.0.17. Adaptors were trimmed using Porechop v0.2.4 (Wick 2018). Assembly of the mitochondrial genome was made using Trycycler (Wick et al. 2021). Short and low-quality reads were removed with NanoFilt https://github.com/wdecoster/nanofilt to produce two filtered read sets: (1) reads of length ≥1000 bases and (2) reads of length ≥5000 bases. BLASTn searches https://anaconda.org/bioconda/blast of read sets against the mitochondrial genome of Placobdella lamothei NC_030269.1 and against the assembled H. europaea mitochondrial genome were used to identify and count mitochondrial reads in MDA enriched and non-enriched DNA. Flye v2.9 was used to assemble contigs from read sets https://anaconda.org/bioconda/flye, Minimap2 v2.24 was used to map reads to linear contigs https://anaconda.org/bioconda/minimap2 and Geneious Prime 2022.2.1 www.geneious.com to map reads to a circular reference. Mapping to a circular reference was necessary as reads that spanned the 5′ and 3′ ends of the assembled mitochondrial genome were not well mapped using a linear mapper.
Comparing the counts of mitochondrial DNA (mtDNA) reads in amplified and unamplified DNA allowed us to calculate the amount of enrichment of mtDNA produced by selective MDA. Annotations to the mitochondrial genome were made using Geneious 9.1.8 https://www.geneious.com and these were compared to mitochondrial genome sequences for P. lamothei NC_030269.1 and Helobdella robusta AF178680.1. The mitochondrial genome sequence of H. europaea is available from the NCBI GenBank under accession number: NC_072606.1.
Results
Morphological analyses
Preserved adult specimens are depicted in Fig. 1d–f. Microscopic observations of morphological features revealed visible segments (Fig. 1d), a prominent ‘bumpy’ dorsal surface with distinguishing features (Fig. 1e) and a suction disc at the tail end (Fig. 1f). Other features observed included a moderately flattened ovate-lanceolate body, one pair of punctiform to triangular eyes in segment III (3rd annulus), five rows of distinct, black-tipped papillae of the dorsum, dorsal pigmentation arranged in numerous longitudinal dark grey stripes and the absence of a nuchal scute. All specimens collected from Koroboya village stream had a size range of 3–8 mm (Rashni and Brown 2021) and features were concordant with H. europaea.
Ecological characteristics
The Fijian specimens collected from Ba and Nadroga provinces inhabit slow flowing freshwater streams with heavily modified riparian vegetation. The Koroboya stream is 3–5 m wide and the channel depth range is 0.10–1.0 m with a silted streambed. The streambed was mostly bare during sampling and some areas were covered with Chara sp. (a green macroalga) and leaf litter. Large numbers of leeches were found on silt covered Chara sp. leaves and instream leaf litter dominated by Mangifera indica (mango) leaves. Previously, Helobdella leeches were found on silted Potamogeton crispus (a freshwater aquatic plant) beds in the Qalinabulu creek, Balevuto village, Nadroga.
Phylogenetic analyses
Sixteen samples of leech total DNA from Fiji amplified and were sequenced successfully, returning identical sequences. A megaBLAST search produced 100% identity matches to 12 catalogued H. europaea sequences: AY856049.1, DQ995298.1, AF329052.1, DQ995297.1, MF804537.1, AY856048.1, MG976140.1, FJ000349.1, KC904241.1, KC904242.1, MN335875.1 and DQ995304.1. Given that the 16 Fijian samples contained identical sequences, a consensus sequence (Supplementary Material 3) was generated for further analyses.
The consensus Fiji sequence was pooled with 80 Helobdella and outgroup sequences (Table 1) for alignment and substitution model testing. The Hasegawa–Kishino–Yano model with gamma-shaped rate variation and invariable sites (HKY + G + I) was determined to have the best fit from jModelTest results (−ln L = 2076.5, BIC = 5835.7 and AICc = 4491.6 [corrected Akaike information criterion]), and was subsequently used for Bayesian reconstruction of phylogenetic relationships. Two parallel runs of MrBayes were used to generate the final tree, where the final average standard deviation of split frequencies achieved was 0.0028 with average potential scale reduction factor (PSRF) for parameter values remaining at 1.000. In total, 16 002/20 002 trees were sampled from the parallel MrBayes runs and a burn-in set (20%) discarded to generate an initial subset of 12 802 trees. Subsequently, 89 highly credible trees were retained (posterior probability p ≤ 0.000 and cumulative posterior probability P ≤ 0.995) and a strict consensus final tree generated (Fig. 2).
Bayesian phylogenetic reconstruction based on 81 cytochrome c oxidase (cox1) nucleotide sequences. A consensus sequence was generated for the Fijian samples from 16 individual sequences. The tree presented is a strict consensus of 89 highly credible trees (cumulative posterior P ≤ 0.995). Node support values for major clades indicate posterior probabilities while the scale bar represents the number of substitutions per site. The major clade resolved for Helobdella europaea is highlighted in red, while the larger clade for the H. triserialis series sensu stricto as per Morhun et al. (2021) is presented in grey. Taxon images depict H. modesta (a), H. stagnalis (b), H. octatestisaca (c), H. papillata (d), H. robusta (e), H. triserialis (f) and H. europaea (g). Outgroup taxa used were Glossiphonia elegans (h) and Desserobdella phalera syn. Placobdella phalera (i). Images (b, c, f–h) are used with permissions from iNaturalist.org users mentioned in the acknowledgements section. The remaining images are adapted from www.boldsystems.org, with the following attributions: (d) and (i), no rights reserved/public domain; (a) and (e) CC BY CBG Photography Group.
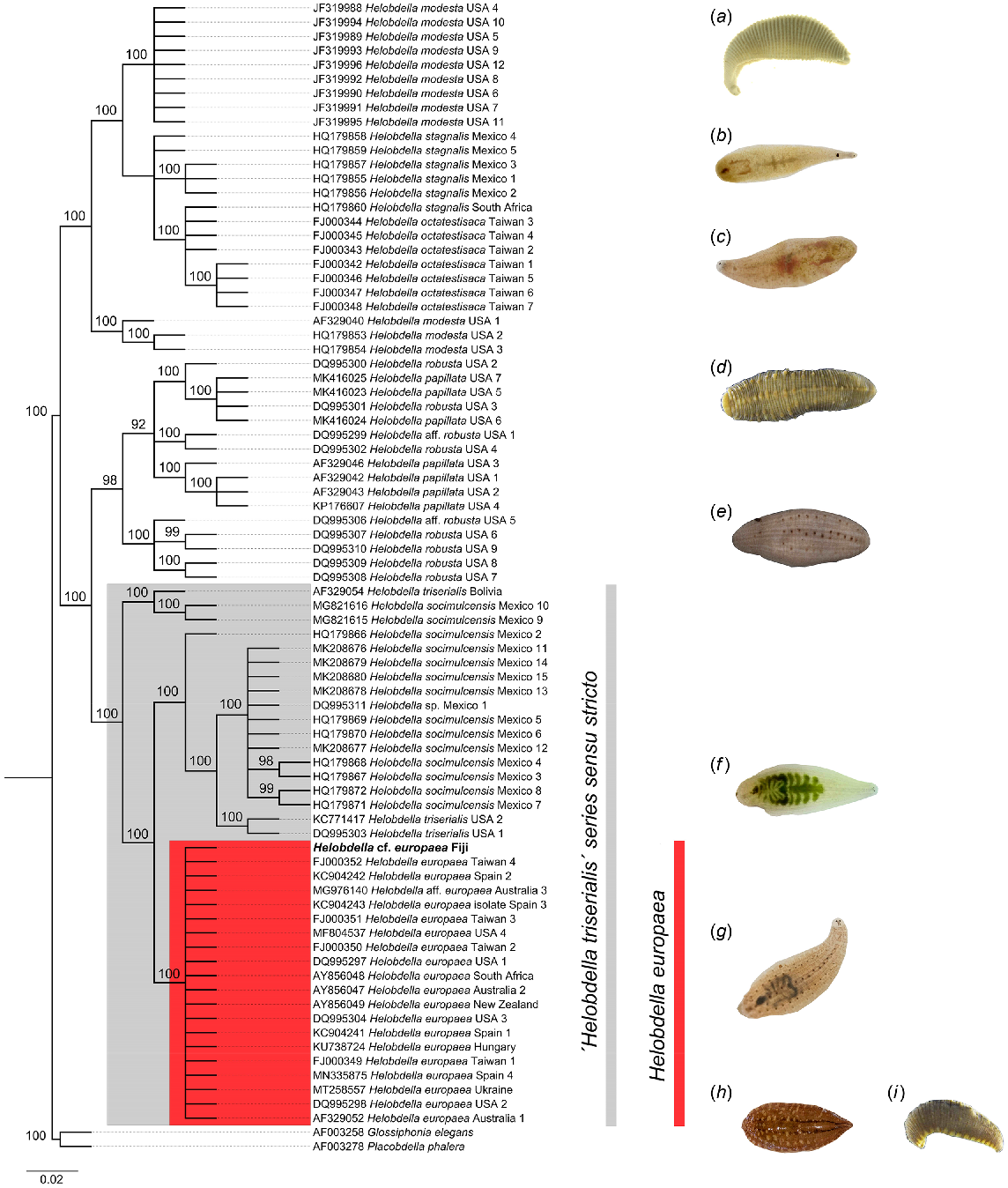
Our reconstruction confirms the assignment of the Fijian leech samples to Helobdella europaea, as the consensus sequence resolved a strong monophyletic clade with H. europaea specimens from Australia, New Zealand, Spain, Taiwan, South Africa, Hungary, Ukraine and the USA. This clade has short internal branch lengths and high posterior probability node support (100). The H. europaea clade is paraphyletic within a larger grouping comprising the sister taxa H. triserialis and H. socimulcensis. Two other large paraphyletic groups were identified, highlighting relationships between H. robusta and H. papillata; and H. modesta with H. stagnalis and H. octatestisaca, respectively. All clades were resolved with high posterior probabilities.
Evolutionary divergence estimates
Relationships identified in the Bayesian tree topology were supported in the pairwise net evolutionary divergence estimates computed between taxa and collection locations (Tables 2 and 3). Pairwise divergence estimates between the Fijian H. europaea sample sequence and all other H. europaea sequences were effectively zero (Table 3), supporting conspecificity of these taxa. A minor degree of divergence was detected between the Fijian and Australian sequences (0.0015 ± 0.0011), however the Australian samples also reflected an identical degree of separation from other conspecific sequences from Spain, Taiwan, the USA, Hungary and South Africa. The largest but still small degree of separation was evident between the Ukrainian sequence (MT258557) and all others (0.0022–0.0037).
| H. robusta | H. papillata | H. stagnalis | G. elegans | H. socimulcensis | P. phalera | H. triserialis | H. europaea | H. octatestisaca | H. modesta | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H. robusta | – | 0.0054 | 0.0310 | 0.0366 | 0.0240 | 0.0353 | 0.0232 | 0.0280 | 0.0317 | 0.0274 | |
| H. papillata | 0.0277 | – | 0.0293 | 0.0391 | 0.0247 | 0.0353 | 0.0237 | 0.0281 | 0.0298 | 0.0272 | |
| H. stagnalis | 0.2039 | 0.1967 | – | 0.0408 | 0.0340 | 0.0372 | 0.0315 | 0.0329 | 0.0035 | 0.0318 | |
| G. elegansA | 0.2579 | 0.2693 | 0.2757 | – | 0.0319 | 0.0294 | 0.0334 | 0.0354 | 0.0424 | 0.0339 | |
| H. socimulcensis | 0.1480 | 0.1501 | 0.2230 | 0.2238 | – | 0.0303 | 0.0046 | 0.0079 | 0.0338 | 0.0232 | |
| P. phaleraA | 0.2443 | 0.2453 | 0.2566 | 0.2090 | 0.2061 | – | 0.0277 | 0.0322 | 0.0364 | 0.0316 | |
| H. triserialis | 0.1316 | 0.1323 | 0.2049 | 0.2234 | 0.0041 | 0.1818 | – | 0.0087 | 0.0323 | 0.0221 | |
| H. europaea | 0.1733 | 0.1698 | 0.2198 | 0.2391 | 0.0297 | 0.2174 | 0.0304 | – | 0.0349 | 0.0252 | |
| H. octatestisaca | 0.2150 | 0.2027 | 0.0084 | 0.2901 | 0.2284 | 0.2584 | 0.2159 | 0.2382 | – | 0.0323 | |
| H. modesta | 0.1816 | 0.1765 | 0.2062 | 0.2244 | 0.1485 | 0.2210 | 0.1381 | 0.1667 | 0.2094 | – |
The number of base substitutions per site from estimation of net average between groups of sequences are shown. Standard error estimates are shown above the diagonal in blue and were obtained by a bootstrap procedure (1000 replicates). Analyses were conducted using the Tamura–Nei model (Tamura and Nei 1993) with the rate variation among sites modelled with a gamma distribution (shape parameter = 1). The analysis involved 81 nucleotide sequences, all positions containing gaps and missing data were eliminated with a total of 414 positions in the final dataset. Analyses were conducted in MEGA6 (Tamura et al. 2013).
AOutgroup taxa.
| Spain | Taiwan | Australia | USA | Hungary | South Africa | Fiji | New Zealand | Ukraine | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Spain | – | 0 | 0.0011 | 0 | 0 | 0 | 0 | 0 | 0.0022 | |
| Taiwan | 0 | – | 0.0011 | 0 | 0 | 0 | 0 | 0 | 0.0022 | |
| Australia | 0.0015 | 0.0015 | – | 0.0011 | 0.0011 | 0.0011 | 0.0011 | 0.0011 | 0.0024 | |
| USA | 0 | 0 | 0.0015 | – | 0 | 0 | 0 | 0 | 0.0022 | |
| Hungary | 0 | 0 | 0.0015 | 0 | – | 0 | 0 | 0 | 0.0022 | |
| South Africa | 0 | 0 | 0.0015 | 0 | 0 | – | 0 | 0 | 0.0022 | |
| Fiji | 0 | 0 | 0.0015 | 0 | 0 | 0 | – | 0 | 0.0022 | |
| New Zealand | 0 | 0 | 0.0015 | 0 | 0 | 0 | 0 | – | 0.0022 | |
| Ukraine | 0.0022 | 0.0022 | 0.0037 | 0.0022 | 0.0022 | 0.0022 | 0.0022 | 0.0022 | – |
The number of base substitutions per site from estimation of net average between groups of sequences are shown. Standard error estimates are shown above the diagonal in blue and were obtained by a bootstrap procedure (5000 replicates). Analyses were conducted using the Tamura–Nei model (Tamura and Nei 1993) with the rate variation among sites modelled using a gamma distribution (shape parameter = 1). The analysis involved 20 nucleotide sequences, all positions containing gaps and missing data were eliminated with a total of 414 positions in the final dataset. Analyses were conducted in MEGA6 (Tamura et al. 2013).
The close affinity of H. europaea with H. socimulcensis (0.0297 ± 0.0079) and H. triserialis (0.0304 ± 0.0086) provides support that these three taxa may comprise a species complex (Table 2, values in bold), which is further discussed by Morhun et al. (2021). Similarly, pairwise divergence estimates were low between H. robusta with H. papillata (0.0277 ± 0.0054), and H. stagnalis and H. octatestisaca (0.0084 ± 0.0035), respectively. Estimates were highest between H. octatestisaca and all other taxa with the exception of H. stagnalis, ranging from 0.2027 ± 0.0298 (H. papillata) to 0.2382 ± 0.0349 (H. europaea).
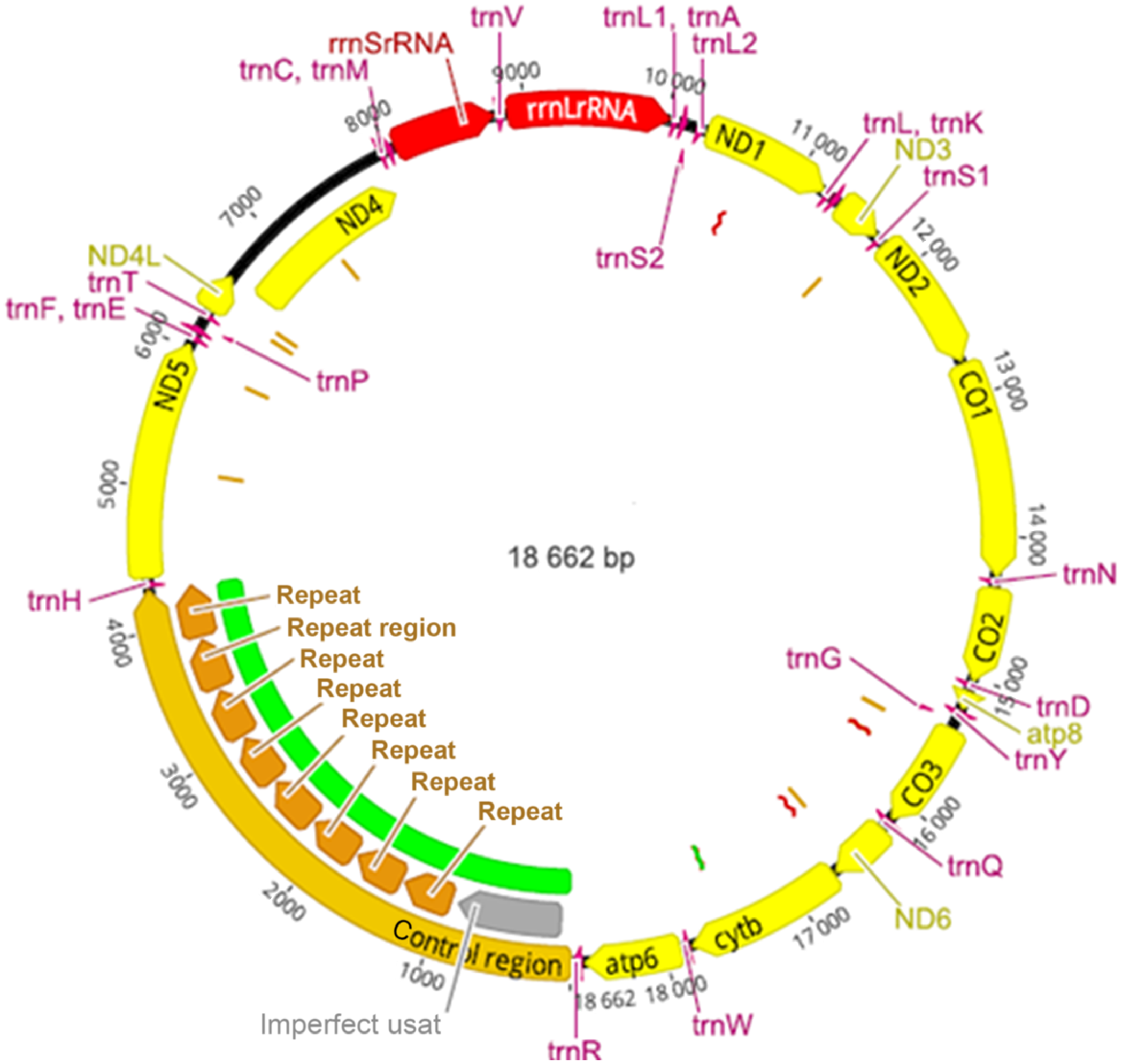
Mitochondrial genome of H. europaea
The genome sequence determined for H. europaea was assembled from high coverage of ONT MinION sequenced MDA mitochondrial DNA reads using Flye v. 2.9 based on the tutorial developed for Trycyler (Wick et al. 2021). Selective MDA using the protocol and primers described enriched mtDNA from 1% to more than 28% reads of the reads ≥1 kb in length. Details on enrichment and genome coverage have been provided in Supplementary Material 1 and 2.
The assembled annotated genome shown in Fig. 3 was found to be very similar in organisation to that of P. lamothei NC_030269.1 and H. robusta AF178680.1. The complete mitochondrial genome sequence is available online under accession NC_072606.1 (https://www.ncbi.nlm.nih.gov/nuccore/NC_072606.1) and as Supplementary Material 4. Unique to H. europaea was the presence of repeats in the control region. These repeats were identified in both amplified and unamplified H. europaea DNA that were sequenced on the MK1C minion device. It was not determined whether the number of repeats were variable within and between individuals of H. europaea.
Discussion
The independent morphological and molecular approaches employed confirm assignment of the Fijian leech samples to H. europaea, establishing the first record of this invasive annelid in the Pacific Islands region. The complete mitochondrial genome sequence reported here will also be a valuable resource for traceability studies involving spread of this species, and for future comparative analyses.
Morphological and ecological insights
The external morphology of samples collected in Fiji is consistent with descriptors for H. europaea reported in other studies (Kutschera 2004; Kutschera et al. 2013) and further studies examining specimen gut contents may reveal potential impacts on native aquatic invertebrate fauna. Our observations suggest that H. europaea in Fiji prefers soft-bottomed sections of streams inhabited by macrophytes (P. crispus and Chara sp.) and leaf litter.
Molecular barcoding
Resolution of the Fijian leech samples in a monophyletic clade, strong sequence identity matches and evolutionary divergence computations with catalogued H. europaea specimens confirm this species is now present in Fiji. Results reported here underscore the utility of molecular barcoding tools for identifying invasive or potentially invasive taxa. The cox1 locus has successfully been used to identify H. europaea in various other parts of the world where concerns have been raised on its presence, including Ukraine (Morhun et al. 2021), Spain (Reyes-Prieto et al. 2014) and New Zealand (Siddall and Budinoff 2005).
Molecular barcoding approaches have further characterised evolutionary relationships in the genus Helobdella, which has provided much needed taxonomic clarity. Siddall and Budinoff (2005) validated the current species name of H. europaea, after reporting this leech was originally named H. striata based on specimens collected in Germany and subsequently independently described as Helobdella papillornata from Australia – see Govedich and Davies (1998). Given that H. europaea is endemic to South America and affinities are apparent with other South American Helobdella spp., and also that unfortunately the appropriate name of Helobdella (triserialis) lineata is already assigned to a separate North American species, this name is valid (Siddall and Budinoff 2005).
Several studies have suggested that H. europaea is part of a species complex, which includes H. triserialis and H. socimulcensis (Siddall and Borda 2003; Siddall and Budinoff 2005; Morhun et al. 2021). Reconstructions generated during the current study show support for this H. triserialis complex, given the paraphyly of all H. europaea sequences included with H. triserialis and H. socimulcensis sequences for comparison.
The mitochondrial genome of H. europaea
The genome sequence determined for H. europaea is the first mitochondrial genome reported for the species and genus. Our MDA protocol and primers have also been used to achieve similar levels of mitochondrial (mt) genome enrichment with Papilio (butterfly) DNA, and we anticipate the protocol is applicable for mt genome enrichment in other invertebrates. Assembly and length determination of the control region in H. europaea was challenging, as this region comprises repeat sequences that were a feature of both MDA amplified and unamplified DNA. Dinucleotide repeats also occurred that are also a feature of the control region in Placobdella lamothei (NC_030269). Variation in the length of control region repeats has previously been observed in other animal species (Munwes et al. 2011), and it is possible that variation in the length of the control region also exists within individuals and populations of H. europaea.
The selective multiple displacement amplification and rapid barcoding Oxford Nanopore sequencing protocols used to sequence the mitochondrial genome of H. europaea provide a cost-effective means for sequencing the mitochondrial genomes of invertebrates. These protocols require a heating block and a microcentrifuge, but are PCR-free (i.e. do not require a PCR machine). Thus the protocol has potential applications for biodiversity and biosecurity assessments in a low infrastructure setting.
Implications for watershed management
The presence of H. europaea in the Ba River catchment is concerning given the potential negative impacts (Kutschera 1989; Kutschera 2004; Pfeiffer et al. 2004) this organism might have on the native invertebrate fauna of Fiji. Several area-endemic taxa are present in the Ba River catchment, with aquatic insects, molluscs and worms (Kutschera 2004; Málnás et al. 2016) potentially being vulnerable. A total of 73 freshwater macroinvertebrate taxa have been recorded from this catchment area, of which 14% are confirmed as endemic to Fiji with a further 47% being possibly endemic pending confirmation (Rashni 2020). These endemic taxa include five caddisflies (Abacaria fijiana, Abacaria ruficeps, Anisocentropus fijianus, Goera fijiana and Oxyethira fijiensis), a damselfly, Nesobasis spp., a shrimp (Caridina fijiana), a micro-water strider Fijivelia sp., a water cricket (Hydropedecticus vitiensis) and spring snails Fluviopupa spp. The presence of H. europaea may pose the greatest potential risk to area-endemic spring snails belonging to the genus Fluviopupa (Gastropoda: Tateidae), which are of high conservation significance. Currently a total of 28 Fluviopupa species are recorded from Fiji, all of which are area- and national- endemics (Zielske and Haase 2014), and are included in the Fiji Endangered and Protected Species (EPS) Act 2017.
The likely mode of introduction of H. europaea to Fiji remains unknown at the present time, and a thorough bioassessment of this species across the connected riverine network of both the Ba and Nadroga provinces is strongly recommended to understand the extent of spread and potential impacts on resident aquatic biodiversity. In this regard for Fiji and elsewhere this species may occur, the newly determined mitochondrial genome sequence for H. europaea provides a genome reference for higher resolution comparative analyses and source tracking. Such data will further assist the Fiji Invasive Species Taskforce (FIST) in their decision making for evaluating the status of H. europaea in Fiji.
Although H. europaea has been recognised as an invasive species globally (Kutschera 2004; Málnás et al. 2016; Morhun et al. 2021) and is alien to South Pacific Island countries, at this stage it can only be given the status of an introduced species in Fiji according to the corroborating definitions of Invasive Alien Species (IAS) via the Fiji National Biodiversity Strategies and Action Plans (NBSAPs), Convention on Biological Diversity (CBD) and the IUCN (International Union for Conservation of Nature) (Government of Fiji 2014; IUCN 2018; Department of Environment and Government of Fiji 2020). According to the Fiji NBSAP and CBD definitions, IAS are organisms found outside of their native geographical ranges that have spread and become invasive in their new habitats, and cause harm to biodiversity and other things that humans value (Government of Fiji 2014; Department of Environment and Government of Fiji 2020).
Results presented here offer a foundation on which further research assessing the extent of spread, ecology and ethology of H. europaea in Fiji and elsewhere may be based. In Fiji, outcomes of these future studies will further support the Biosecurity Authority of Fiji, the Ba and Nadroga-Navosa Provincial Administration and the Ba and Nadroga Yaubula Management Support Team to implement containment response protocols and formulate eradication strategies when/if required. Regionally in the Pacific and also globally, through the availability of the complete mitochondrial genome sequence for this species via this study, diagnosing its spread to other regions through traceability investigations is now possible.
Data availability
The datasets generated and/or analysed during the current study are available at the NCBI GenBank repository (https://www.ncbi.nlm.nih.gov/nuccore/NC_072606.1), included in this published article as supplementary files and also available from the corresponding author on reasonable request.
Declaration of funding
This research was financially supported by a grant from the New Zealand Royal Society Catalyst Fund to Peter Lockhart.
Author contributions
Bindiya Rashni: conceptualisation; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; validation; visualisation; writing – original draft; writing – review and editing. Kelly T. Brown: conceptualisation; data curation; formal analysis; investigation; methodology; project administration; resources; software; validation; visualisation; writing – original draft; writing – review and editing. Patricia A. McLenachan: data curation; formal analysis; investigation; methodology; resources; software; validation; visualisation; writing – original draft; writing – review and editing. Peter J. Lockhart: conceptualisation; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualisation; writing – original draft; writing – review and editing. Paul C. Southgate: methodology; resources; validation; visualisation; writing – original draft; writing – review and editing. Monal M. Lal: conceptualisation; data curation; formal analysis; investigation; methodology; project administration; resources; software; supervision; validation; visualisation; writing – original draft; writing – review and editing.
Acknowledgements
We gratefully acknowledge the field team (Apaitia Liga, Jone Raituva, Suliano Kanavakatini and Ambika Nandan) and the Turaga ni Koro (village headman) of Koroboya village together with the Fiji Ministry of iTaukei Affairs (MTA) for granting a research support permit (MTA-4/99/8-2). We also thank Nunia Thomas-Moko (Director, NatureFiji-MareqetiViti and FIST member) for her comments on the draft manuscript. Our gratitude also extends to Kalioni Taukena Bogiva for generation of the site map. We acknowledge the following iNaturalist (www.inaturalist.org) users for granting permission for use of their images in Fig. 2: Edgardo Soriano-Vargas (username: e-soriano-vargas), for Helobdella stagnalis (2b), all rights reserved; Chen-Yao Lin (username: chenyao2), for Helobdella octatestisaca (2c) all rights reserved; Zulema Trejo (username: Zule T), for Helobdella triserialis (2f), all rights reserved; Darrell Crisp (username: crispychipp) for H. europaea (2g), CC-BY-NC; Owen Ridgen (username: oridgen10) for G. elegans (2h), all rights reserved.
References
Adamkewicz SL, Harasewych MG (1996) Systematics and biogeography of the genus Donax (Bivalvia: Donacidae) in eastern North America. American Malacological Bulletin 13, 97-103.
| Google Scholar |
Bely AE, Weisblat DA (2006) Lessons from leeches: a call for DNA barcoding in the lab. Evolution & Development 8, 491-501.
| Crossref | Google Scholar |
Carew ME, Coleman RA, Hoffmann AA (2018) Can non-destructive DNA extraction of bulk invertebrate samples be used for metabarcoding? PeerJ 6, e4980.
| Crossref | Google Scholar |
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792-1797.
| Crossref | Google Scholar |
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit i from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294-299.
| Google Scholar |
GBIF (2020) GBIF occurrence download. Global Biodiversity Information Facility. Available at https://doi.Org/10.15468/dl.Anfxjg[Accessed 17 August 2020]
Govedich FR, Davies RW (1998) The first record of the genus Helobdella (Hirudinoidea: Glossiphoniidae) from Australia, with a description of a new species, Helobdella papillornata. Hydrobiologia 389, 45-49.
| Crossref | Google Scholar |
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754-755.
| Crossref | Google Scholar |
Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, Rupp R (2007) Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinformatics 8, 460.
| Crossref | Google Scholar |
Jiménez-Armenta J, Oceguera-Figueroa A (2019) Leeches from Mexico City, remnants of the ancient lake. Mitochondrial DNA Part A 30, 632-642.
| Crossref | Google Scholar |
Jueg U (2008) Alboglossiphonia iberica nov. sp. – eine neue Egelart von der Iberischen halbinsel (Hirudinea: Glossiphoniidae). Lauterbornia 65, 43-61.
| Google Scholar |
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647-1649.
| Crossref | Google Scholar |
Kutschera U (1985) Beschreibung einer neuen egelart, Helobdella striata nov. sp. (Hirudinea: Glossiphoniidae). Zoologische Jahrbücher. Abteilung für Systematik, Ökologie und Geographie der Tiere 112, 469-476.
| Google Scholar |
Kutschera U (1987) Notes on the taxonomy and biology of leeches of the genus Helobdella Blanchard 1896 (Hirudinea: Glossiphoniidae). Zoologischer Anzeiger 219, 321-323.
| Google Scholar |
Kutschera U (1989) Reproductive behaviour and parental care of the leech Helobdella californica (Hirudinea: Glossiphoniidae). Zoologischer Anzeiger 222, 122-128.
| Google Scholar |
Kutschera U (2004) The freshwater leech Helobdella europaea (Hirudinea: Glossiphoniidae): an invasive species from South America? Lauterbornia 52, 153-162.
| Google Scholar |
Kutschera U, Langguth H, Kuo D-H, Weisblat DA, Shankland M (2013) Description of a new leech species from North America, Helobdella austinensis n. sp. (hirudinea: Glossiphoniidae), with observations on its feeding behaviour. Zoosystematics and Evolution 89, 239-246.
| Crossref | Google Scholar |
Lai Y-T, Chang C-H, Chen J-H (2009) Two new species of Helobdella Blanchard 1896 (Hirudinida: Rhynchobdellida: Glossiphoniidae) from Taiwan, with a checklist of hirudinea fauna of the island. Zootaxa 2068, 27-46.
| Crossref | Google Scholar |
Lal MM, Southgate PC, Jerry DR, Zenger KR (2016) Fishing for divergence in a sea of connectivity: the utility of ddRADseq genotyping in a marine invertebrate, the black-lip pearl oyster Pinctada margaritifera. Marine Genomics 25, 57-68.
| Crossref | Google Scholar |
Mabrouki Y, Ahmed RB, Taybi AF, Rueda J (2019) An annotated checklist of the leech (Annelida: Hirudinida) species of the Moulouya River basin, Morocco, with several new distribution records and a historical overview. African Zoology 54, 199-214.
| Crossref | Google Scholar |
Mãgalhaes WF, Hutchings P, Oceguera-Figueroa A, Martin P, Schmelz RM, Wetzel MJ, Wiklund H, Maciolek NJ, Kawauchi GY, Williams JD (2021) Segmented worms (Phylum Annelida): a celebration of twenty years of progress through Zootaxa and call for action on the taxonomic work that remains. Zootaxa 4979, 190-211.
| Crossref | Google Scholar |
Málnás K, Kovács K, Ficsór M, Juhász P, Müller Z (2016) Appearances of the non-indigenous Helobdella europaea Kutschera, 1987 (Hirudinea, Glossiphoniidae) in Hungarian watercourses. Folia Historico-Naturalia Musei Matraensis 40, 17-20.
| Google Scholar |
Morhun H, Sidorovskyi S, Khomenko A, Mazepa G, Utevsky S (2021) First Ukrainian record of the invasive leech Helobdella europaea (Hirudinea: Glossiphoniidae) from an aquarium in Kharkiv: morphological variability and phylogenetic relationships. Biologia 76, 193-202.
| Crossref | Google Scholar |
Munwes I, Geffen E, Friedmann A, Tikochinski Y, Gafny S (2011) Variation in repeat length and heteroplasmy of the mitochondrial DNA control region along a core–edge gradient in the eastern spadefoot toad (Pelobates syriacus). Molecular Ecology 20, 2878-2887.
| Crossref | Google Scholar |
Perera A, Hernandez-Sastre P, Ayres C (2019) Hitch me a ride: first report of the alien leech Helobdella octatestisaca in Europe associated with freshwater turtles. Biological Invasions 21, 3467-3471.
| Crossref | Google Scholar |
Pfeiffer I, Brenig B, Kutschera U (2004) The occurrence of an Australian leech species (genus Helobdella) in German freshwater habitats as revealed by mitochondrial DNA sequences. Molecular Phylogenetics and Evolution 33, 214-219.
| Crossref | Google Scholar |
Posada D (2008) jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25, 1253-1256.
| Crossref | Google Scholar |
Rambaut A (2014) Figtree v.1.4.2: tree drawing tool. Available at https://github.com/rambaut/figtree/releases
Rambaut A, Suchard M, Drummond AJ (2003) Tracer (v.1.6.). Available at https://github.com/beast-dev/tracer/releases
Rashni B (2014b) Chapter 6: Freshwater macroinvertebrates. In: Pene S, Tuiwawa M, FPAM (2014) Biological and Socio-economical baseline report for the establishment of the greater Delaikoro protected area, Vanua Levu, Fiji Islands. A rapid biodiversity assessment, socioeconomic study and archaeological survey of the greater Delaikoro area, June 2014, Suva, Fiji, FPAM-2014-Biodiversity-01. Institute of Applied Science. pp. 53–72.
Rashni B (2020) Freshwater invertebrate assemblages and ecological status of the Ba River, Fiji. In ‘First series technical consultation of the regional scientific and technical committee for the GEF Pacific Ridge to Reef programme. Nadi, Fiji’, p. 27. GEF Pacific Ridge to Reef Programme. Available at https://www.pacific-r2r.org/sites/default/files/2020-05/First_Series_Technical_Consultation_of_the_RSTC.pdf
Rashni B, Brown KT (2021) Hellobdella europaea occurrence in Fiji. Occurrence dataset. The University of the South Pacific: GBIF. Available at https://doi.org/10.15468/c2yhjc
Reyes-Prieto M, Oceguera-Figueroa A, Snell S, Negredo A, Barba E, Fernández L, Moya A, Latorre A (2014) DNA barcodes reveal the presence of the introduced freshwater leech Helobdella europaea in Spain. Mitochondrial DNA 25, 387-393.
| Crossref | Google Scholar |
Richardson DJ, Moser WE, Hammond CI, Lazo-Wasem EA (2015) New host and geographic distribution records for Glossiphoniid leeches associated with turtles in southern New England. Comparative Parasitology 82, 240-243.
| Crossref | Google Scholar |
Richardson DJ, Moser WE, Hammond CI, Lazo-Wasem EA, McAllister CT, Pulis EE (2017) A new species of leech of the genus Placobdella (Hirudinida, Glossiphoniidae) from the American alligator (Alligator mississippiensis) in Mississippi, USA. ZooKeys 667, 39-49.
| Crossref | Google Scholar |
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572-1574.
| Crossref | Google Scholar |
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61, 539-542.
| Crossref | Google Scholar |
Schmerer MW, Null RW, Shankland M (2013) Developmental transition to bilaterally symmetric cell divisions is regulated by Pax-mediated transcription in embryos of the leech Helobdella austinensis. Developmental Biology 382, 149-159.
| Crossref | Google Scholar |
Seaver EC (2003) Segmentation: mono- or polyphyletic? International Journal of Developmental Biology 47, 583-595.
| Google Scholar |
Siddall ME, Borda E (2003) Phylogeny and revision of the leech genus Helobdella (Glossiphoniidae) based on mitochondrial gene sequences and morphological data and a special consideration of the triserialis complex. Zoologica Scripta 32, 23-33.
| Crossref | Google Scholar |
Siddall ME, Budinoff RB (2005) DNA-barcoding evidence for widespread introductions of a leech from the South American Helobdella triserialis complex. Conservation Genetics 6, 467-472.
| Crossref | Google Scholar |
Siddall ME, Burreson EM (1998) Phylogeny of leeches (Hirudinea) based on mitochondrial cytochrome c oxidase subunit I. Molecular Phylogenetics and Evolution 9, 156-162.
| Crossref | Google Scholar |
Sket B, Trontelj P (2008) Global diversity of leeches (Hirudinea) in freshwater. Hydrobiologia 595, 129-137.
| Crossref | Google Scholar |
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10, 512-526.
| Crossref | Google Scholar |
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30, 2725-2729.
| Crossref | Google Scholar |
Tessler M, Siddall ME, Oceguera-Figueroa A (2018) Leeches from Chiapas, Mexico, with a new species of Erpobdella (Hirudinida: Erpobdellidae). American Museum Novitates 3895, 1-15.
| Crossref | Google Scholar |
van Haaren T, Hop P, Soes M, Tempelman D (2004) The freshwater leeches (Hirudinea) of the Netherlands. Lauterbornia 52, 113-131.
| Google Scholar |
Weisblat DA, Huang FZ (2001) An overview of glossiphoniid leech development. Canadian Journal of Zoology 79, 218-232.
| Crossref | Google Scholar |
Wick RR (2018) Porechop. Available at https://github.com/rrwick/Porechop
Wick RR, Judd LM, Cerdeira LT, Hawkey J, Méric G, Vezina B, Wyres KL, Holt KE (2021) Trycycler: consensus long-read assemblies for bacterial genomes. Genome Biology 22, 266.
| Crossref | Google Scholar |
Zielske S, Haase M (2014) New insights into tateid gastropods and their radiation on Fiji based on anatomical and molecular methods (Caenogastropoda: Truncatelloidea). Zoological Journal of the Linnean Society 172, 71-102.
| Crossref | Google Scholar |