High levels of diversity for seed and forage production exist in Cullen australasicum, a potential new perennial forage legume for dry environments in southern Australia
Alan W. Humphries A D E , Stephen J. Hughes A D , Ramakrishnan M. Nair A B D , Eric Kobelt A and Graeme Sandral C DA SARDI, Waite Campus, PO Box 397, Adelaide, SA 5000, Australia.
B Present address: AVRDC – The World Vegetable Centre, South Asia, ICRISAT Campus, Patancheru, Hyderabad, AP 502 324, India.
C NSW Department of Primary Industries, Pine Gully Road, Wagga Wagga, NSW 2650, Australia.
D CRC for Future Farm Industries, University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
E Corresponding author. Email: alan.humphries@sa.gov.au
The Rangeland Journal 36(1) 41-51 https://doi.org/10.1071/RJ13055
Submitted: 23 May 2013 Accepted: 11 October 2013 Published: 19 November 2013
Abstract
The seed and forage production of a diverse group of the perennial forage legume Cullen spp., collected in southern Australia, was assessed with the aim of discovering diversity for exploitation in future breeding programs. Eighty ecotypes were assessed at the Waite Institute in South Australia, using replicated, spaced-plant field trials, between 2008 and 2012. Seed production in collected ecotypes of Cullen (Expt 1) ranged from 0 to 485 kg ha–1 for windrowed seed yield and from 0 to 790 kg ha–1 for total seed yield, which included vacuum-harvested seed from pods that had fallen to the ground. Individual plants were selected for seed production from their original populations, and the seed and fodder production of their progeny was evaluated in a further field experiment (Expt 2). Moderate to high heritability estimates were recorded for seed production traits. Seed production in progeny families ranged from 0 to1 423 kg ha–1 and was highly correlated with the number of seeds per inflorescence (r = 0.85) and forage yield (r = 0.59). Edible biomass, measured using the Adelaide visual appraisal method, ranged from 50 to 906 g dry weight (DW) plant–1 in parent ecotypes and from 404 to 1248 g DW plant–1 in the selected family progenies. Disease infection with anthracnose (Colletotrichum trifolii) caused considerable damage to plants in Expt 1, resulting in the death of all plants of 10 ecotypes, and infection with Alfalfa mosaic virus in Expt 2 was linked to the death of 67 individuals. The results are discussed in relation to breeding C. australasicum for increased seed yield and disease resistance to overcome these deficiencies as barriers to commercial adoption.
Additional keywords: Bullamon lucerne, domestication, scurf pea, seed production, tall verbine.
Introduction
New, drought-tolerant perennial forage legumes are being sought to prepare graziers for the onset of climate change, which is predicted to bring about an increased frequency of low rainfall and drought across large parts of southern Australia (Anon. 2007, cited in Hayes et al. 2009). Some Australian native legumes are well adapted to the climatic and edaphic conditions of the semi-arid agricultural regions of Australia, and are therefore being considered in the search to discover new, drought-hardy alternatives to the existing commercial legume species (Cocks 2001; Dear et al. 2007; Bennett et al. 2011). Cullen spp., typically semi-herbaceous perennial legumes, are one example of a genus native to Australia that is currently under consideration for use in extensive grazing systems (Cocks 2001; Dear et al. 2007; Hayes et al. 2009; Suriyagoda et al. 2013). Members of the Cullen genus are found in Africa, Spain, Portugal, Italy, Asia Minor, and southern Asia, with 25 of the 32 species occurring naturally in Australia (Grimes 1997). Early evaluation of the former taxonomic group, Psoralea eriantha-patens, by Skerman (1957), Kerridge and Skerman (1968), and Britten and De Lacy (1979) highlighted the high forage yield potential of this group relative to lucerne (Medicago sativa L.) and its value as a fodder for cattle during periods of drought. Recent studies have focussed on the species C. australasicum (Schltdl.) J.W. Grimes and confirmed that its forage production (Dear et al. 2007; Bennett et al. 2012) and persistence (Li et al. 2008; Suriyagoda et al. 2013) in low-rainfall environments can be similar to exotic perennial pastures such as lucerne. Comparable field performance to that of industry-leading lucerne varieties over 2–3-year periods has also been documented in Australia, in New South Wales (Dear et al. 2007; Hayes et al. 2009; Boschma et al. 2011) and Western Australia (Bennett et al. 2012), leading researchers to conclude that C. australasicum is a plant of considerable promise. In terms of potential for commercial development, C. australasicum has many agronomic qualities of a valuable forage plant. Its green leaf tissue has a similar digestibility (Dear et al. 2007; Bennett et al. 2012) and crude protein concentration (Bennett et al. 2012) to lucerne; it has the ability to preserve green leafy tissue during the onset of drought (Kerridge and Skerman 1968; Boschma et al. 2011); and field observations suggest that it is tolerant to damage from most insect pests (Dear et al. 2007; Hayes et al. 2009; Bennett et al. 2012).
Studies on the breeding system of C. australasicum led researchers to conclude that C. australasicum is mostly a self-pollinating species, with varying degrees of out-crossing potential among ecotypes (Kroiss et al. 2009; Wang et al. 2010). This suggests that a variety of breeding strategies could be implemented for plant improvement, from simple ecotype selection to a range of inter- and intra-population breeding methods modified from those used by inbred or out-crossing species. Aspects of C. australasicum genetics (or agronomy) that require better understanding before strategic plant breeding and commercialisation of this species can be considered include preference and utilisation by sheep under grazing (Dear et al. 2007; Hayes et al. 2009) and strategies for improving seed production (Kobelt et al. 2011).
The price of seed is a major barrier to the adoption of any new pasture species, and consequently, improved seed production technologies and harvestability traits must be developed to ensure that a viable commercial variety can exist. A major constraint to the seed production of Cullen spp. is that large seed losses can occur from low pod retention and uneven ripening of the seed. Cullen australasicum flowers prolifically throughout the year following sufficient rainfall (Grimes 1997), making harvest-timing decisions difficult. Nevertheless, seed production from a single accession of C. australasicum has been successful, with up to 700 kg ha–1 harvested using small-scale commercial machinery (Kobelt et al. 2011). However, seed production was not reliable, due to indeterminate flowering and loss of mature seed from the vine resulting from low pod retention. Kobelt et al. (2011) found that dry conditions improved the synchrony of flowering and, conversely, that irrigation during flowering promoted an extended flowering period, resulting in lower seeds yields and making it difficult to determine the best timing for harvest. The genetic diversity for seed production potential in C. australasicum was unknown before this study.
Here, we predict that variability for seed and forage production traits exists within a collection of Cullen spp. sourced from a diverse range of environments in southern Australia. In combination with improved agronomic practices, superior genotypes with desirable ‘domestication’ characteristics could contribute towards increased seed production and support the commercial success of the species.
Methods
Eighty populations of Cullen spp. (see Supplementary file), assembled by the South Australian Genetic Resource Centre and representing the distribution of this genus throughout Australia, were assessed for a range of morphological traits that relate to seed and forage production. A replicated experiment (Expt 1) was sown in July 2008, and assessment of germplasm was completed on established plants between April 2009 and January 2010. Progeny from individual plants, selected from approximately 2% of the populations, were then advanced in a second experiment (Expt 2) to confirm the heritability of seed production traits. Expt 2 was sown in September 2010, and characterisation of seed production traits and forage yield occurred between December 2011 and January 2012.
Location and soil type
The field site was in the SARDI Genetic Resources field nursery at the Waite Campus, Urrbrae, Adelaide, South Australia (34.97°S, 138.63°E; elevation 110 m). The fine sandy loam at this site is a red-brown earth (Stace et al. 1968) of the non-sodic Urrbrae series (Litchfield 1951). The upper 0.10 m contains 18% clay, increasing to 32% in the A2 horizon (Prescott 1931). Soil pH (in CaCl2) was 6.2 and there was negligible calcium carbonate (Grace et al. 1995). The site had subsurface drip irrigation, with two lines running 0.5 m apart, 0.2 m beneath each plot, and with drip intervals of 0.5 m. For Expt 1, irrigation was with weekly applications equivalent to 25 mm of rainfall between November and May. No irrigation was used in Expt 2.
Climate
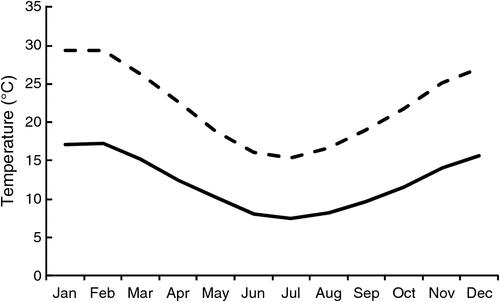
The climate in Adelaide is Mediterranean, characterised by hot, dry summers and a frost-free winter. The Waite Campus has a winter-dominant rainfall, with a long-term (130 years; Glen Osmond weather station) mean of 627 mm. Observations for mean daily minimum and maximum temperatures for Adelaide are shown in Fig. 1. Daylength peaks at 14.3 h in December and falls to 9.7 h in June. Rainfall for both experiments was close to long-term averages, with the exception of July 2009, when monthly rainfall of 131 mm was in the 85th percentile for Adelaide, 47.4 mm above the median rainfall of 83.6 mm. This event caused minor flooding on the experimental site, and waterlogged conditions remained for ~10 days. In November of the same year, the mean maximum temperature of 30.8°C and the mean minimum temperature of 18°C were the highest on record in Adelaide.
|
Experiment 1: Seed and forage production in a diverse range of Cullen spp. germplasm
Germplasm
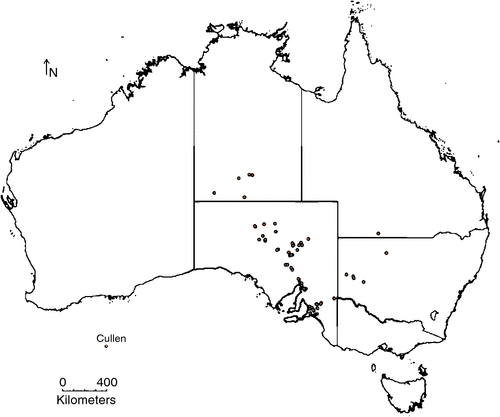
A map of the native origin of Cullen spp. germplasm in Australia used in Expt 1 is shown in Fig. 2. The germplasm represents collections of C. australasicum, and ecotypes of closely related species that required their taxonomy to be confirmed in this study, collected at latitudes 23.7–35.3°S, from a diverse range of soils and climates (Supplementary file). Accession SA4966 was used as a standard entry due to its previous evaluation in a range of studies (Dear et al. 2007; Li et al. 2008; Hayes et al. 2009; Bennett et al. 2012), including the research by Kobelt et al. (2011) to develop harvest technologies for C. australasicum.
|
Experimental design and culture
Eighty ecotypes were evaluated in a randomised grid with 15 columns and 16 rows, using three replications (each with five columns and 16 rows). The ecotypes were germinated in July 2008 in Petri dishes and seedlings were transferred to small, biodegradable pots. The seedlings were grown in a greenhouse until they produced two trifoliate leaves. Ten plants from each accession were transplanted into each plot containing one row, with 30 cm between plants. For calculations of unit area of production, a dimension 1 m by 3 m for each plot was used. The distance between plots was 1.2 m across the length and width directions. No observations were made in the first seedling year, and a herbage cut was used in April 2009 to reduce potential differences that resulted from transplant, recovery, and seedling-year growth.
Experiment 2: Confirmation of seed production traits in selected individual plants
Germplasm
Seed from 42 individual plants displaying a range of seed and forage production traits was harvested from 38 of the populations evaluated in Expt 1 (Tables 1 and 2). A further 15 ‘families’ were included from selections of field evaluation trials, plus seven ecotypes repeated from Expt 1, identified by their good performance in regional row nurseries (unpublished data), and from glasshouse aphid screening (Tables 1 and 2).

|
|
Experimental design and culture
The 64 entries were sown in a completely randomised and blocked grid with 64 rows and four columns, with four replications (one replication per column). Seedlings were raised as in Expt 1, and two seedlings from each family were space-planted 0.7 m apart on weed matting in September 2010. Individual plants were considered an experimental unit, and 512 plants (eight plants per line) were successfully established. Seed was harvested from all entries on 7 December 2011.
Evaluation protocols
The measurement protocols for forage yield and seed production components used in Expts 1 and 2 are presented in Table 3. Many of the variables were measured using visual assessment methods from rigorous, well-developed protocols. The use of 1 January as a reference date for flowering date was an arbitrary choice.

|
Statistical analyses
Means of fixed entry effects for each response variate (edible biomass, canopy density, flowering intensity, anthracnose resistance, days from flowering to harvest, days to harvest, and seed yield in Expt 1; and forage yield, seeds per inflorescence, and seed yield in Expt 2) were calculated using spatial linear mixed models performed by Genstat 15 (Lawes Agricultural Trust 2012). Diagnostic plots of sample variograms and residuals were used in conjunction with REML log-likelihood ratios and Wald tests to fit new models that compartmentalised and removed random and fixed effects of variation (Smith et al. 2005). Estimates of genetic variance were made using entry as a component of the random model. Heritability was calculated using the equation h2 = Yg/(Yg + Ye), where Yg is genotypic variance and Ye is residual variance (Yg + Ye = phenotypic variance; Mather and Jinks 1982). Correlation analysis was used to determine linear relationships between forage and seed yield components.
Results
Experiment 1
Edible biomass for Cullen ecotypes ranged from 70 to 420 g dry weight (DW) plant–1 with a mean of 251 g DW plant–1. Accession SA42965 had the highest autumn edible biomass (420 g DW plant–1) and canopy density (5.0), whereas accession SA4966 had the highest spring edible biomass (906 g DW plant–1). Accession SA42965 was also the last entry to flower (269 days) but had less than the median of days to harvest (351 days v. the median value of 368 days). Heritability for edible biomass was high, with estimates of 0.68 for autumn and 0.43 for spring. Anthracnose (caused by Colletotrichum trifolii) had a severe impact on plant survival, resulting in the death of 14 of the ecotypes before any measurements on seed production. The remaining entries expressed low to moderate symptoms for this disease and received damage scores of 0.8−2.9.
Cullen pallidum entry SA44108 had moderately high autumn (281 g DW plant–1) and spring (260 g DW plant–1) edible biomass but a low seed yield (31 g DW plant–1). The cross between C. pallidum and C. australasicum, SA42596, also had moderately high edible biomass (303 g DW plant–1 for autumn and 283 g DW plant–1 for spring) and a low seed yield (16 kg ha–1). The C. discolor, C. discolor × australasicum, and C. graveolens entries displayed relatively low forage yield (35−280 g DW plant–1) and seed yield (86–151 kg ha–1).
Seed production for Cullen ecotypes was highly variable, with 0−790 kg ha–1 harvested from the windrow and ground (Table 4). Within C. australasicum, the highest total seed yield of 790 kg ha–1 was produced by SA42564 and the highest seed yield, harvested directly from the windrow of 485 kg ha–1, was produced by SA42766. There was significant variation for pod retention, with the percentage of total seed harvested from the vine ranging from 0 to 99% (Table 4). Accession SA42772 had the highest ranking for number of inflorescences (23.4 out of a possible visual score of 30), and produced a high total seed yield of 594 kg ha–1. Genetic variance for seed production was large relative to residual variance, representing a high percentage of the phenotypic variance, or heritability (h2 = 0.53 for windrow-harvested seed yield and 0.77 for total seed yield).
The relationships between seed and forage production traits are presented in a correlation matrix (Table 5). Windrow-harvested seed yield was positively correlated with total seed yield (r = 0.85), autumn edible biomass (r = 0.40), and spring edible biomass (r = 0.55). Autumn edible biomass was highly correlated with spring edible biomass (r = 0.62). Anthracnose damage had a high negative correlation with autumn edible biomass (r = –0.45) and spring edible biomass (r = –0.46), and a high positive correlation with the number of days to harvest (r = 0.52). Anthracnose damage score was negatively correlated with windrow-harvested seed yield (r = –0.32) but not total seed yield. Windrow-harvested seed yield was negatively correlated with the number of days to harvest (r = –0.32) but not with the number of days to flowering or the number of days between first flower and harvest.
Experiment 2
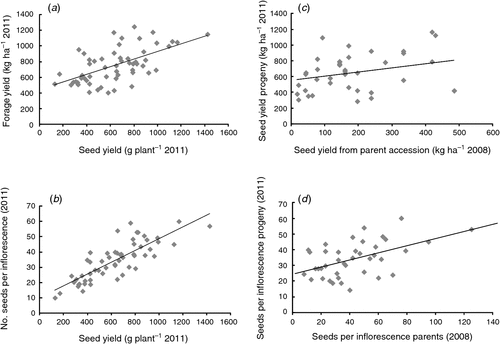
Seed yield of progeny families ranged from 128 to 1423 kg ha–1, and was highly correlated (r = 0.59) with forage yield, which ranged from 404 to 1248 g DW plant–1 (Fig. 3a, Table 2). Individual plants grew to up to 2.5 m high and 1.2 m2 in area, such that the plants with the highest forage yield produced up to 10.4 t DW ha–1 (based on the distribution of spaced plants). Seed yield per plant was also highly correlated with the number of seeds per inflorescence (r = 0.82; Fig. 3b), and heritability for this trait was moderate (h2 = 0.35). The seed yield of progenies harvested in Expt 2 was positively correlated with the seed yield of their parent accession in Expt 1 (r = 0.30, Fig. 3c), and the number of seeds per inflorescence in these progeny rows was highly correlated with that of their parents in Expt 1 (r = 0.51, Fig. 3d).
|
Alfalfa mosaic virus (AMV, genus Alfamovirus, family Bromoviridae) significantly reduced the forage and seed production of individual plants in Expt 2. Of the 564 individuals, 133 displayed severe yellow mosaic and leaf distortion symptoms, similar to those observed by Nair et al. (2009), and in 67 of these individuals the distortion was followed by stunting, necrosis, and death (all 167 diseased plants were treated as missing values in the statistical analysis). There was no significant effect of variety on plant damage, which appeared to be randomly distributed through the experiment (data not shown).
Discussion
Selecting/breeding Cullen australasicum with high seed yield
Seed production in C. australasicum is very diverse, with mechanically harvestable seed yield from windrows of 0−485 kg ha–1 in Expt 1, and 128−1 423 kg ha–1 in Expt 2. The ratio of genetic to environmental variance for seed production was moderate to high (h2 = 0.50−0.77), indicating that this trait would respond to improvement from selection and breeding. Levels of seed production in this experiment were similar to those achieved by Kobelt et al. (2011), where up to 700 kg ha–1 of windrow-harvested seed was produced. The results show that high levels of seed production, ~1 t ha–1, can be produced under rain-fed conditions.
Seed production in Expt 2, under rain-fed conditions, was higher than the seed yields achieved in Expt 1, where rainfall was supplemented with irrigation. The change in management practice followed a recommendation by Kobelt et al. (2011) that reducing soil moisture during flowering improved the synchrony of flowering, pod set, and maturation, resulting in improved seed yields. In this study, the improved management in Expt 2 is confirmed by the y-intercept of 550 kg ha–1 on the trend line in Fig. 3c, which compares with the seed production of related plants in each experiment. In faba beans (Vicia faba L.), a water shortage during flowering can increase seed yield by 20−60%, and water supply patterns can account for >90% of the variation in seed yield (Grashoff 1990). Forage yield, as a surrogate measure of water use, explained 75−85% of seed yield (Grashoff 1990). In this study, windrow-harvestable seed yield was also highly defined by spring forage yield, with 55% of the variation attributable to this measurement (Table 5).
Cullen spp. has the potential to be a high-yielding forage plant for medium-rainfall environments in southern Australia. In this study, mature plant edible biomass in second-year stands of up to 1248 g DW plant–1 (equal to ~10.4 t DW ha–1) was measured on plants growing 2.5 m high. The heritability of edible biomass was moderate to high (0.68 in Expt 1 and 0.32 in Expt 2), indicating that forage production should also respond to improvement from selection and breeding, but sources of environmental error (possibly associated with AMV infection in Expt 2) need to be minimised. Accession SA4966 had the highest edible spring biomass in this study, and was previously ranked in the top three ecotypes of Cullen spp. for high spring leaf (Bennett et al. 2012) and total forage (Hayes et al. (2009) production in experiments evaluating diverse collections of Cullen spp. in low–medium-rainfall environments. This accession also performed well relative to lucerne in national evaluation trials at five locations (Li et al. 2008), and in two additional sites in the low–medium-rainfall wheatbelt of southern New South Wales (Dear et al. 2007). A selection from SA4966, denoted pa29/5b, was also the highest yielding line in Expt 2, indicating that this line should feature prominently in future breeding activities.
Cullen pallidum, C. discolor, and C. graveolens ecotypes produced less seed and forage than C. australasicum ecotypes assessed in this study, and had higher levels of damage from anthracnose. The superior productivity of C. australasicum is supported by the findings of Hayes et al. (2009) and Bennett et al. (2012), which compared the yield and persistence of Cullen spp. ecotypes in low-rainfall environments. Despite their relatively low field production, C. pallidum and C. discolor can form inter-specific hybrids with C. australasicum and may, therefore, add value to future breeding programs by contributing genes for specific traits.
For the future development of Cullen as a forage plant, our results indicate that selection for forage yield will often result in increased seed production, due to the positive relationship found between these traits in both experiments (Table 5, Fig. 3a). Seed-production traits recorded moderate to high heritability, and selection for the number of mature seeds per inflorescence at harvest provides a quick and easy proxy for seed yield (Fig. 3b). The number of mature seeds per inflorescence is influenced strongly by the capacity of the plant to retain mature pods on the vine. The timing of the harvest in Expt 2 was successful in capturing variation for the number of mature seeds per inflorescence, and similar techniques have been used by breeders to exploit variation in pod retention and harvestable seed yield of lupin (Lupinus angustifolius L.; French and Buirchell 2005) and lentil (Lens culinaris Medik; Erskine 1985). The identification of germplasm with high seed yield in this study suggests that the only major hurdle in the development of Cullen as a commercial species is now likely to be associated with selection for disease resistance or its management through improved agronomy.
Potential influence of diseases on commercial development
Cullen was severely damaged by anthracnose in Expt 1, as previously reported by Nair et al. (2010). The variation in damage scores for anthracnose, and the high estimate of heritability for this score (h2 = 0.75), suggest that disease infection was uniform across the site, and that damage can be reduced through plant selection. These observations for plant resistance or tolerance should be confirmed in a further study using a controlled environment. The Waite Campus received rainfall well above average and minor flooding in July 2009, and this is likely to have increased the severity of anthracnose infection. No visual symptoms of anthracnose damage were observed in the following experiment, which also had a recent history of lucerne production and therefore a likely source of inoculum. It is therefore likely that this impediment to seed production can be overcome with either the use of resistant lines and/or the use of seed production ground with low levels of anthracnose inoculum.
The severe yellow mosaic and leaf distortion symptoms identified in Expt 2 are most likely associated with AMV, as reported by Nair et al. (2009), but further work is required to ascertain whether AMV was the sole causal agent of the mosaic disease, and whether the stunting, necrosis, and death in many of the plants displaying these symptoms was caused by a secondary agent. Intolerance to AMV is a threat to the use of this species in Australia, given the severe damage caused in Expt 2 and the widespread use of annual Medicago and Trifolium pastures, lucerne, and crop legumes that can provide sources of inoculum for transmission of the virus (Latham and Jones 2001a, 2001b). A lucerne nursery was directly adjacent to Expt 2, and infection from this area with sap-sucking insects (Hiruki and Hampton 1990) may have increased the rate of infection above what is likely to occur in commercial production. Other factors, such as sowing infected seed stock (Latham and Jones 2001b) and infection with machinery (Hiruki and Hampton 1990), may also account for the source and rapid spread of AMV infection in Expt 2, but transmission of AMV through mechanical inoculation or seed has not been observed in preliminary experiments (Nair et al. 2009). Although previous reports have identified a high incidence of AMV infection (~80%; Nair et al. 2009), this is the first report of infected plants becoming stunted, necrotic, and dead. Plant death from AMV infection is uncommon in other pasture legumes, but does occur in biserrula (Biserrula pelecinus L.), and other plants, such as French serradella (Ornithopus sativus Brot.), are also considered susceptible and highly sensitive (Latham and Jones 2001b). Selection for resistance or tolerance to AMV has been found in many other legumes species (Latham and Jones 2001b) and would clearly need to be considered in any future genetic improvement strategy for Cullen. Aphid resistance is likely to contribute in reducing AMV infection (Garran and Gibbs 1982), but aphid-resistant Cullen pallidum accession SA42722 (Hayes et al. 2009) had all eight of its plants killed by AMV in Expt 2. Another strategy that may contribute to a solution is to develop a Cullen variety that has the capacity to regenerate through seedling recruitment, providing that seed transmission is practically insignificant.
The concept of developing a Cullen variety with the capacity to recruit seedlings under favourable conditions is supported by observations of natural recruitment in arid environments. Although the low level of pod retention observed within most Cullen ecotypes is a negative trait for commercial seed production of Cullen, it has undoubtedly contributed to the success of the species in arid environments. Seed dispersal, combined with high levels of hard seededness, are necessary adaptations of plants to environments with highly variable rainfall patterns (Crawford et al. 1989; Real et al. 2012), and there is some potential to mimic the ley farming system success of annual medics (Medicago spp.), whereby these traits are critical factors to the success of the farming system (Crawford et al. 1989). The breeder’s line ‘Recruiter’, evaluated in Expt 2, is a field selection identified for its ability to recruit seedlings at Barmedman, New South Wales. The Recruiter line had high forage production (906 g DW plant–1), but the compromise in seed yield (375 kg ha–1) and low level of pod retention (19 seeds per inflorescence) may not represent an acceptable balance for commercial production. Further studies are required to show whether this or other Cullen germplasm may be successful in a system based on self-regeneration via seedling recruitment.
Conclusions
High commercial seed yields of >1 t ha–1, directly harvested from a windrow, are achievable for C. australasicum using a combination of the best genetics and seed production management practices. This should ensure that the production of low-cost seed is not a limiting factor to the adoption of this species as a forage plant in low-input systems. Further research is required to investigate possible cultural or genetic strategies to reduce AMV infection or damage before this species can be considered a viable forage option. Overall, the high fodder and seed production potential demonstrated in this study indicates that C. australasicum may emerge as a viable, drought-tolerant alternative for graziers in semi-arid to medium-rainfall environments of southern Australia.
Acknowledgements
This project was supported by the Rural Industry Research and Development Corporation, as part of the research program of the Cooperative Research Centre for Future Farm Industries. The authors thank Adrian Williams and Trevor Rowe from SARDI and Yan-Jing Wang from the Institute of Grassland Science Jilin, China, for their assistance with data collection and seed harvest.
References
Andrew, M., Noble, I., and Lange, R. (1979). A non-destructive method for estimating the weight of forage on shrubs. Australian Rangeland Journal 1, 225–231.| A non-destructive method for estimating the weight of forage on shrubs.Crossref | GoogleScholarGoogle Scholar |
Anon. (2007). Climate change in Australia: Regional impacts and adaptation, managing the risk for Australia. Report from PMSEIC Independent Working Group for Prime Minister’s Science, Engineering and Innovation Council, Canberra, ACT. Available at: www.innovation.gov.au/Science/PMSEIC/Documents/ClimateChangeinAustralia.pdf
Bennett, R., Ryan, M., Colmer, T., and Real, D. (2011). Prioritisation of novel pasture species for use in water-limited agriculture: a case study of Cullen in the Western Australian wheatbelt. Genetic Resources and Crop Evolution 58, 83–100.
| Prioritisation of novel pasture species for use in water-limited agriculture: a case study of Cullen in the Western Australian wheatbelt.Crossref | GoogleScholarGoogle Scholar |
Bennett, R. G., Colmer, T. D., Real, D., Renton, M., and Ryan, M. H. (2012). Phenotypic variation for productivity and drought tolerance is widespread in germplasm collections of Australian Cullen species. Crop & Pasture Science 63, 656–671.
| Phenotypic variation for productivity and drought tolerance is widespread in germplasm collections of Australian Cullen species.Crossref | GoogleScholarGoogle Scholar |
Boschma, S. P., Lodge, G. M., and Harden, S. (2011). Seasonal production of lucerne and other perennial legumes and herbs in a summer-dominant rainfall zone. New Zealand Journal of Agricultural Research 54, 105–114.
| Seasonal production of lucerne and other perennial legumes and herbs in a summer-dominant rainfall zone.Crossref | GoogleScholarGoogle Scholar |
Britten, E. J., and De Lacy, I. (1979). Assessment of the genetic potential for pasture purposes of the Psoralea eriantha-patens complex, a native legume of the semi-arid zone. Australian Journal of Experimental Agriculture and Animal Husbandry 19, 53–58.
| Assessment of the genetic potential for pasture purposes of the Psoralea eriantha-patens complex, a native legume of the semi-arid zone.Crossref | GoogleScholarGoogle Scholar |
Cocks, P. S. (2001). Ecology of herbaceous perennial legumes: a review of characteristics that may provide management options for the control of salinity and waterlogging in dryland cropping systems. Australian Journal of Agricultural Research 52, 137–151.
| Ecology of herbaceous perennial legumes: a review of characteristics that may provide management options for the control of salinity and waterlogging in dryland cropping systems.Crossref | GoogleScholarGoogle Scholar |
Crawford, E. J., Lake, A. W. H., and Boyce, K. G. (1989). Breeding annual Medicago for semiarid conditions in southern Australia. Advances in Agronomy 42, 399–437.
| Breeding annual Medicago for semiarid conditions in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Dear, B. S., Li, G. D., Hayes, R. C., Hughes, S. J., Charman, N., and Ballard, R. A. (2007). Cullen australasicum (syn. Psoralea australasica): a review and some preliminary studies related to its potential as a low rainfall perennial pasture legume. The Rangeland Journal 29, 121–132.
| Cullen australasicum (syn. Psoralea australasica): a review and some preliminary studies related to its potential as a low rainfall perennial pasture legume.Crossref | GoogleScholarGoogle Scholar |
Erskine, W. (1985). Selection for pod retention and pod indehiscence in lentils. Euphytica 34, 105–112.
| Selection for pod retention and pod indehiscence in lentils.Crossref | GoogleScholarGoogle Scholar |
French, R. J., and Buirchell, B. J. (2005). Lupin: the largest grain legume crop in Western Australia, its adaptation and improvement through plant breeding. Australian Journal of Agricultural Research 56, 1169–1180.
| Lupin: the largest grain legume crop in Western Australia, its adaptation and improvement through plant breeding.Crossref | GoogleScholarGoogle Scholar |
Garran, J., and Gibbs, A. J. (1982). Studies on alfalfa mosaic virus and alfalfa aphids. Australian Journal of Agricultural Research 33, 657–664.
| Studies on alfalfa mosaic virus and alfalfa aphids.Crossref | GoogleScholarGoogle Scholar |
Gholinejad, H., PourBabaei, H., Farajollahi, A., and Parvane, E. (2012). Assessment and comparison of different methods for estimating forage production (Case Study: Rangeland of Kurdistan Province). Journal of Rangeland Science 2, 483–489.
Grace, P. R., Oades, J. M., Keith, H., and Hancock, T. W. (1995). Trends in wheat yields and soil organic carbon in the permanent Rotation Trial at the Waite Agricultural Research Institute, South Australia. Australian Journal of Experimental Agriculture 35, 857–864.
| Trends in wheat yields and soil organic carbon in the permanent Rotation Trial at the Waite Agricultural Research Institute, South Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xhs1yiu7k%3D&md5=c1ab9881afc9cd47f0e149ac2ed067f1CAS |
Grashoff, C. (1990). Effect of pattern of water supply on Vicia faba L. 2. Pod retention and filling, and dry matter partitioning, production and water use. Netherlands Journal of Agricultural Science 38, 131–143.
Grimes, J. W. (1997). A revision of Cullen (Leguminosae: Papilionoideae). Australian Systematic Botany 10, 565–648.
| A revision of Cullen (Leguminosae: Papilionoideae).Crossref | GoogleScholarGoogle Scholar |
Hayes, R. C., Li, G. D., Dear, B. S., Humphries, A. W., and Tidd, J. R. (2009). Persistence, productivity, nutrient composition, and aphid tolerance of Cullen spp. Crop & Pasture Science 60, 1184–1192.
| Persistence, productivity, nutrient composition, and aphid tolerance of Cullen spp.Crossref | GoogleScholarGoogle Scholar |
Hiruki, C., and Hampton, R. O. (1990). Alfalfa mosaic. In: ‘Compendium of alfalfa diseases’. 2nd edn. (Eds D. L. Stuteville and D. C. Erwin.) p. 54. (APS Press: St. Paul, MN.)
Kerridge, P. C., and Skerman, P. J. (1968). The distribution and growth characteristics of the native legume Psoralea eriantha in Western Queensland. Tropical Grasslands 2, 41–50.
Kobelt, E., Hughes, S. J., and Humphries, A. W. (2011). Developing harvest technologies for Cullen australasicum. Publication No. 11/027. RIRDC, Australian Government, ACT. Available at: https://rirdc.infoservices.com.au/downloads/11-027.
Kroiss, L., Moody, M., Barker, S., Byrne, M., and Ryan, M. (2009). Development, characterization and transferability of microsatellite markers for Cullen australasicum (Leguminosae). Conservation Genetics 10, 1803–1805.
| Development, characterization and transferability of microsatellite markers for Cullen australasicum (Leguminosae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFSrtLfN&md5=d413f890111ac11ae19159ceb47fc9b5CAS |
Latham, L. J., and Jones, R. A. C. (2001a). Incidence of infection in experimental plots, commercial crops, and seed stocks of cool season crop legumes. Australian Journal of Agricultural Research 52, 397–413.
| Incidence of infection in experimental plots, commercial crops, and seed stocks of cool season crop legumes.Crossref | GoogleScholarGoogle Scholar |
Latham, L. J., and Jones, R. A. C. (2001b). Alfalfa mosaic and pea seed-borne mosaic viruses in cool season crop, annual pasture, and forage legumes: susceptibility, sensitivity, and seed transmission. Australian Journal of Agricultural Research 52, 771–790.
| Alfalfa mosaic and pea seed-borne mosaic viruses in cool season crop, annual pasture, and forage legumes: susceptibility, sensitivity, and seed transmission.Crossref | GoogleScholarGoogle Scholar |
Lawes Agricultural Trust (2012). ‘Genstat Release 15.’ (VSN International: Hemel Hempstead, UK)
Li, G. D., Lodge, G. M., Moore, G. A., Craig, A. D., Dear, B. S., Boschma, S. P., Albertsen, T. O., Miller, S. M., Harden, S., Hayes, R. C., Hughes, S. J., Snowball, R., Smith, A. B., and Cullis, B. C. (2008). Evaluation of perennial pasture legumes and herbs to identify species with high herbage production and persistence in mixed farming zones in southern Australia. Australian Journal of Experimental Agriculture 48, 449–466.
| Evaluation of perennial pasture legumes and herbs to identify species with high herbage production and persistence in mixed farming zones in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Litchfield, W. H. (1951). Soil survey of the Waite Agricultural Research Institute, Glen Osmond, South Australia. Divisional Report No. 2/51. CSIRO Division of Soils, Adelaide, S. Aust.
Mather, K., and Jinks, J. L. (1982). ‘Biometrical genetics.’ 3rd edn. (Chapman and Hall: London.)
Nair, R. M., Habili, N., and Randles, J. W. (2009). Infection of Cullen australasicum (syn. Psoralea australasica) with Alfalfa mosaic virus. Australasian Plant Disease Notes 4, 46–48.
| 1:CAS:528:DC%2BD1MXns1WgtLc%3D&md5=b7bb184891a23cd358a8fbfe18535be6CAS |
Nair, R. M., Wilmshurst, C., Russ, M. H., Williams, A., and Priest, M. (2010). First report of anthracnose (Colletotrichum trifolii) on Cullen australasicum (syn. Psoralea australasica). Australasian Plant Disease Notes 5, 34–36.
| First report of anthracnose (Colletotrichum trifolii) on Cullen australasicum (syn. Psoralea australasica).Crossref | GoogleScholarGoogle Scholar |
Prescott, J. A. (1931). The soils of Australia in relation to vegetation and climate. Council for Science and Industrial Research, Bulletin No. 52. CS and IR, Canberra, ACT.
Real, D., Sandral, G. A., Rebuffo, M., Hughes, S. J., Kelman, W. M., Mieres, J. M., Dods, K., and Crossa, J. (2012). Breeding of an early-flowering and drought-tolerant Lotus corniculatus L. variety for the high-rainfall zone of southern Australia. Crop & Pasture Science 63, 848–857.
| Breeding of an early-flowering and drought-tolerant Lotus corniculatus L. variety for the high-rainfall zone of southern Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVSkt7vF&md5=576357a206cac3f6ffb78983f48e0712CAS |
Skerman, P. J. (1957). Bullamon lucerne (Psoralea eriantha Benth). A plant worth watching. Journal of the Australian Institute of Agricultural Science 23, 337–339.
Smith, A. B., Cullis, B. R., and Thompson, R. (2005). The analysis of crop cultivar breeding and evaluation trials: an overview of current mixed model approaches. Journal of Agricultural Science, Cambridge 143, 449–462.
| The analysis of crop cultivar breeding and evaluation trials: an overview of current mixed model approaches.Crossref | GoogleScholarGoogle Scholar |
Stace, H. C. T., Hubble, G. D., Brewer, R., Northcote, K. H., Sleeman, J. R., Mulcahy, M. J., and Hallsworth, E. G. (1968). ‘A handbook of Australian soils.’ (Rellim Technical Publications: Adelaide, S. Aust.)
Suriyagoda, L. D. B., Real, D., Renton, M., Lambers, H., and Ryan, M. H. (2013). Establishment, survival, and herbage production of novel, summer-active perennial pasture legumes in the low-rainfall cropping zone of Western Australia as affected by plant density and cutting frequency. Crop & Pasture Science 64, 71–85.
| Establishment, survival, and herbage production of novel, summer-active perennial pasture legumes in the low-rainfall cropping zone of Western Australia as affected by plant density and cutting frequency.Crossref | GoogleScholarGoogle Scholar |
Wang, Y. J., Nair, R. M., Mu, C. S., and Dundas, I. S. (2010). Floral morphology and pollination system in the native Australian perennial pasture legume Cullen australasicum (syn. Psoralea australasica). Crop & Pasture Science 61, 1001–1008.
| Floral morphology and pollination system in the native Australian perennial pasture legume Cullen australasicum (syn. Psoralea australasica).Crossref | GoogleScholarGoogle Scholar |




