Syndromic management of sexually transmissible infections in resource-poor settings: a systematic review with meta-analysis of the abnormal vaginal discharge flowchart for Neisseria gonorrhoea and Chlamydia trachomatis
Caroline van Gemert A B H , Margaret Hellard A B , Catriona S. Bradshaw C D , Freya J. I. Fowkes A B E F , Paul A. Agius A B , Mark Stoove A B and Catherine M. Bennett GA Burnet Institute, 85 Commercial Road, Melbourne, Vic. 3004, Australia.
B Department of Epidemiology and Preventative Medicine, Monash University, The Alfred Centre, 99 Commercial Road, Melbourne, Vic. 3004, Australia.
C Central Clinical School, The Alfred Centre, 99 Commercial Road, Melbourne, Vic. 3004, Australia.
D Melbourne Sexual Health Clinic, 580 Swanston Street, Carlton, Vic. 3053, Australia.
E Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Parkville, Vic. 3010, Australia.
F Department of Infectious Diseases, Central Clinical School, Monash University, Level 2, The Burnet Institute, 85 Commercial Road, Melbourne, Vic. 3004, Australia.
G Centre for Population Health Research, Deakin University, Geelong, Vic. 3220, Australia.
H Corresponding author. Email: caroline.vangemert@burnet.edu.au
Sexual Health 15(1) 1-12 https://doi.org/10.1071/SH17070
Submitted: 30 March 2017 Accepted: 19 June 2017 Published: 25 August 2017
Journal compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Background: Syndromic management of sexually transmissible infections is commonly used in resource-poor settings for the management of common STIs; abnormal vaginal discharge (AVD) flowcharts are used to identify and treat cervical infection including Neisseria gonorrhoea and Chlamydia trachomatis. A systematic review and meta-analysis was undertaken to measure the diagnostic test performance of AVD flowcharts, including both World Health Organization (WHO)- and locally-adapted AVD flowcharts. Methods: A systematic search of multiple electronic databases was conducted to locate eligible studies published between 1991 and 2014. Flowcharts were categorised into one of 14 types based on: 1) use of WHO guidelines or locally-adapted versions; 2) use of risk assessment, clinical examination or both; and 3) symptomatic entry. Summary diagnostic performance measures calculated included summary sensitivity, summary specificity and diagnostic odds ratio. Results: Thirty-six studies, including data on 99 flowcharts, were included in the review. Summary sensitivity estimates for WHO flowcharts ranged from 41.2 to 43.6%, and for locally adapted flowcharts from 39.5 to 74.8%. Locally adapted flowcharts performed slightly better than the WHO flowcharts. A difference in performance was not observed between use of risk assessment or clinical examination. The AVD flowchart performed slightly better when it was not restricted to symptomatic women only. Conclusions: There was considerable variation in the performance of the AVD flowchart but overall it was a poor diagnostic tool regardless of whether risk assessment or clinical examination was included, or whether the flowchart was WHO or locally developed. Many women were treated unnecessarily and many women with cervical infection were not detected. We caution against their continued use for management of cervical infection.
Introduction
Women in resource-poor settings are disproportionately affected by curable sexually transmissible infections (STIs), dominated by Neisseria gonorrhoea (NG) and Chlamydia trachomatis (CT).1,2 Untreated cervical infections can cause serious complications including infertility, cervical cancer, spontaneous abortion, premature delivery and low birthweight.3 Syndromic management of STIs is commonly used in settings where laboratory resources and capacity for etiological diagnoses are limited in an effort to expedite care and treatment.4–6 Syndromic management of STIs is the presumptive diagnosis and subsequent treatment of STIs based on the identification of consistent groups of symptoms and easily recognised signs, with point-of-care therapies used to treat the majority of organisms responsible for producing specific syndromes without a laboratory-confirmed result. The World Health Organization (WHO) developed its first guidelines for syndromic management of STIs in 1991;7 flowcharts were developed for the syndromic detection and treatment of abnormal vaginal discharge (AVD), urethral discharge, lower abdominal pain and genital ulcers, among other symptoms.
The AVD flowchart was developed to identify and treat both cervical (NG and CT) and vaginal infections (Trichomonas vaginalis, Candida albicans, bacterial vaginosis [BV]). The first WHO guidelines for self-reported AVD, confirmed by clinical examination and inspection of the cervix, prompted presumptive treatment for either trichomoniasis and BV or CT and NG, depending on characteristics of observed discharge.7 Where clinical examination was not possible, patients presenting with AVD were treated for trichomoniasis and BV as well as CT and NG if the local prevalence exceeded 10–20%.7 Due to the asymptomatic nature of cervical CT and NG infections, the majority of AVD identified through WHO guidelines were due to vaginal infection rather than cervical infection8–10 resulting in over-treatment of many women for vaginal infections they did not have, and under-treatment of women with asymptomatic cervical infection. In response, WHO added the use of risk scores to the flowchart in 1993 to improve its diagnostic performance and distinguish women with cervical infection from those with vaginal infection.8,10,11 Several sociodemographic and behavioural characteristics demonstrated to be associated with cervical infection were used in the risk score, such as age, marital status, condom use by the partner, and number of partners.10 WHO also recommended local modification of the AVD flowchart based on local epidemiology.11 In 2003, WHO released updated guidelines for the management of STIs including updated flowcharts for AVD incorporating risk assessment.10
Several validation studies have assessed the diagnostic performance in specific settings and key populations, however to our knowledge a systematic review of both WHO and locally-developed AVD flowcharts has not been conducted to compute an overall measure of their performance in detecting cervical infection. A previous systematic review with meta-analysis of WHO-based AVD flowcharts for cervical and vaginal infections concluded that the diagnostic performance for identifying cervical infections was low, and that the AVD flowchart should focus on management of vaginal infection only.12 Prior literature reviews have also reported a lower than acceptable performance of the AVD flowchart.6,13,14 A formal synthesis of data using both WHO and locally-developed AVD flowcharts is paramount for the review and possible revision of clinical guidelines. The objective of this review was to calculate summary estimates of the diagnostic test performance of AVD flowcharts, including both WHO and locally-developed flowcharts.
Methodology
Data sources and literature reviews
A systematic search was conducted of electronic databases for the period 1 January 1991–30 April 2014 to identify all relevant studies; the start date was chosen based on the release of the 1991 WHO guidelines. Searches were conducted in April 2009 and repeated in April 2012 and May 2014. Electronic databases searched included PubMed, Ovid MEDLINE, Scopus, African Medicus Index and LILACs. The search strategy used combinations of subject-specific terms relating to STI screening and testing; for example, the search strategy used for PubMed database was ‘sexually transmitted diseases/ classification’ (MeSH) OR ‘sexually transmitted diseases/diagnosis’ (MeSH) OR ‘sexually transmitted diseases/epidemiology’ (MeSH) AND ‘flowchart’ OR ‘syndromic*’ AND ‘chlamydia’ OR ‘gonorrhoea’ AND ‘sensitivity’. Non-peer-reviewed literature was also sourced by searching the bibliographies of eligible studies and broad Internet searches.
Study selection
One independent reviewer (CvG) performed all aspects of the search strategy, assessed retrieved abstracts against inclusion criteria, and reviewed the full text articles in detail. Studies were included if they: 1) reported measures of diagnostic test accuracy (including sensitivity and specificity) where the diagnostic test was an AVD flowchart and laboratory testing for CT by nucleic acid amplification tests or enzyme immune assay or NGby culture or enzyme immune assay constituted the gold standard reference; 2) reported sufficient data to calculate true positives, false positives, false negatives and true negatives; 3) did not include HIV-positive women (or data could be identified for HIV-negative women); 4) included women aged 16 years and older; 5) were conducted in resource-poor settings; 6) were published between 1991 and April 2014; and 7) were published in English language. Women with HIV were excluded as an association between BV and HIV acquisition has been observed in Africa and AVD is a symptom of BV.15 In studies where several clinical indicators were used to detect AVD, only data for clinical identification of AVD were used.
Methodological review
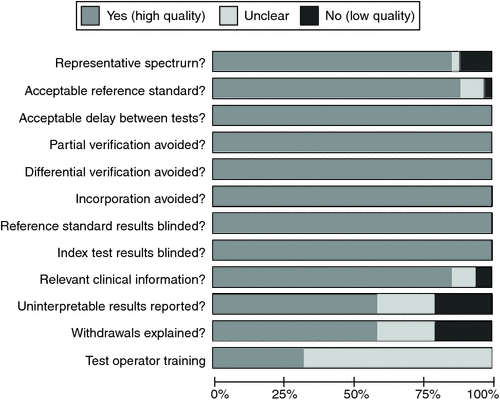
The Quality Assessment for Diagnostic Accuracy Studies tool (QUADAS)16,17 was used for quality assessment. Each study was assessed against 11 recommended QUADAS items: 1) representative spectrum; 2) acceptable reference standard; 3) acceptable delay between tests; 4) partial verification avoided; 5) differential verification avoided; 6) incorporation avoided 7) reference standard results blinded; 8) index test results blinded; 9) relevant clinical information; 10) uninterpretable results reported; 11) withdrawals explained. One additional QUADAS item was also included in the methodological assessment: 12) test operators training.17 Data items for methodological quality were also extracted into Review Manager 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark) for descriptive analysis.
Data collection and measurements
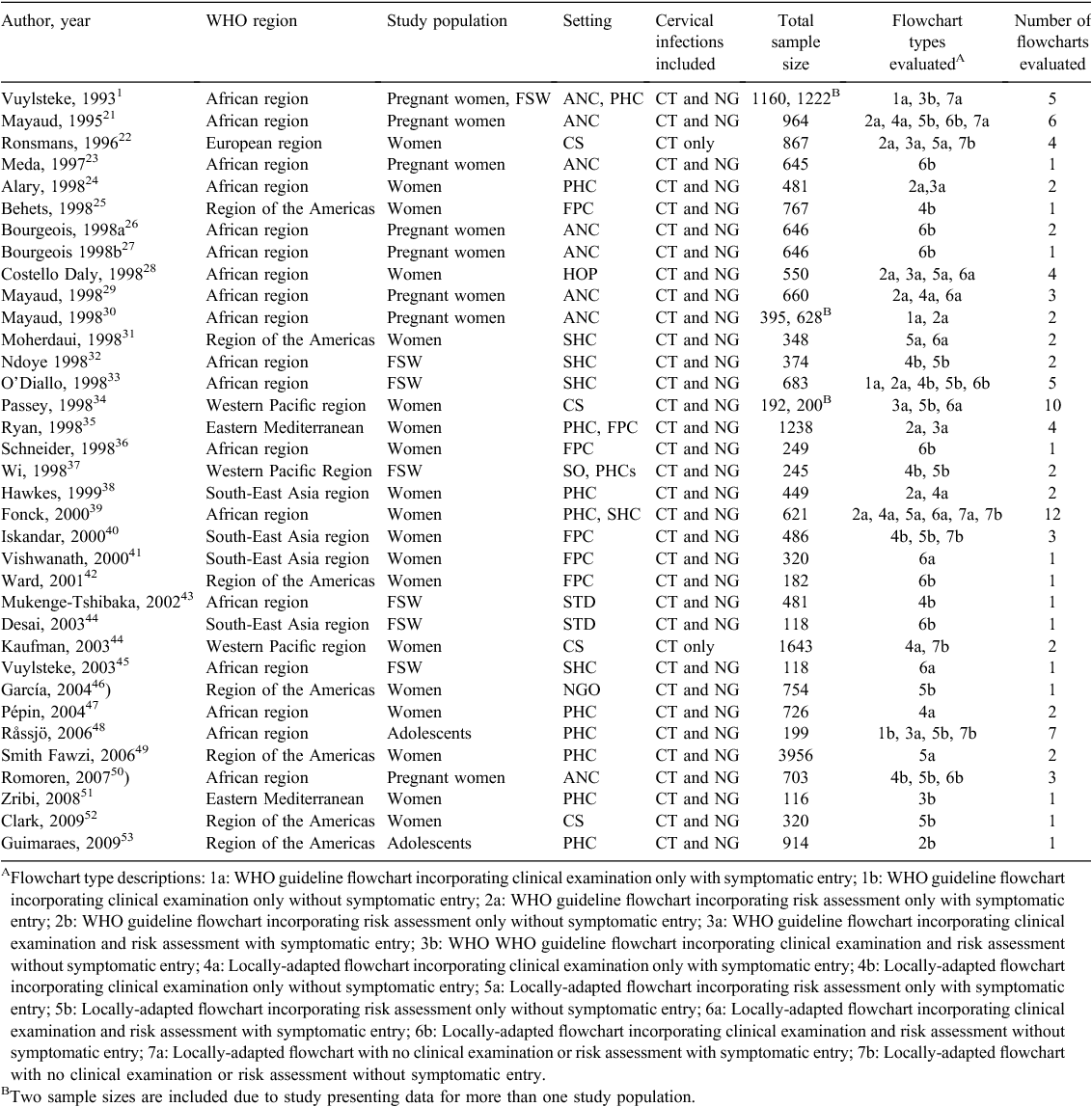
Due to the variation in flowcharts used in the studies, flowcharts were categorised into one of 14 flowchart types. Each study may have reported the diagnostic test performance for one or more flowchart types. The first level of classification was based on whether flowcharts were WHO-based or locally-adapted. The second level of classification was based on whether they incorporated clinical examination or risk assessment, or neither clinical examination or risk assessment. Clinical examination refers to genital examination by a medical practitioner or nurse, with or without use of speculum. Risk assessment refers to identifying risk factors most closely associated with CT and NG infection; these include specific demographic factors (i.e. age), sexual history, partner’s sexual history and physical signs and symptoms. A risk score is calculated by summing the number of affirmative responses to risk assessment questions, and a cut-off score is used to determine if treatment is indicated or not. Risk score cut-offs differed in each study. The third level of classification was based on whether patient eligibility to be assessed by the flowchart included self-reported symptoms (yes/no, referred to as ‘symptomatic entry’ hereafter. The flowchart categories were:
-
Flowchart 1a: WHO guideline flowchart incorporating clinical examination only with symptomatic entry
-
Flowchart 1b: WHO guideline flowchart incorporating clinical examination only without symptomatic entry
-
Flowchart 2a: WHO guideline flowchart incorporating risk assessment only with symptomatic entry
-
Flowchart 2b: WHO guideline flowchart incorporating risk assessment only without symptomatic entry
-
Flowchart 3a: WHO guideline flowchart incorporating clinical examination and risk assessment with symptomatic entry
-
Flowchart 3b: WHO guideline flowchart incorporating clinical examination and risk assessment without symptomatic entry
-
Flowchart 4a: Locally-adapted flowchart incorporating clinical examination only with symptomatic entry
-
Flowchart 4b: Locally-adapted flowchart incorporating clinical examination only without symptomatic entry
-
Flowchart 5a: Locally-adapted flowchart incorporating risk assessment only with symptomatic entry
-
Flowchart 5b: Locally-adapted flowchart incorporating risk assessment only without symptomatic entry
-
Flowchart 6a: Locally-adapted flowchart incorporating clinical examination and risk assessment with symptomatic entry
-
Flowchart 6b: Locally-adapted flowchart incorporating clinical examination and risk assessment without symptomatic entry
-
Flowchart 7a: Locally-adapted flowchart with no clinical examination or risk assessment with symptomatic entry
-
Flowchart 7b: Locally-adapted flowchart with no clinical examination or risk assessment without symptomatic entry
Data extraction and management
Data were extracted for each eligible study and entered into Review Manager 5.3. Where studies evaluated more than one flowchart, information was extracted and evaluated for each flowchart. The following data points were extracted for each flowchart: country of study, year of publication, study setting (primary health clinic, hospital outpatient department, sexual health clinic, antenatal clinic, family planning clinic), population of interest (female sex workers, young females, pregnant women, other women), self-reported AVD entry to the flowchart (yes or no), risk assessment score cut-off (number), risk population (sex workers, pregnant women or young people), use of speculum in clinical examination (binary), sample size, prevalence of CT or NG in the study population, flowchart sensitivity, flowchart specificity and flowchart positive predictive value. The number of true positives, false negatives, true negatives and false positives and associated 95% confidence intervals were manually calculated using reported data when not explicitly provided. For each flowchart, the sample prevalence of cervical infection was calculated as (true positives + false negatives/true negatives + false positives)×100.
Data analysis
Meta-analysis was conducted at the flowchart level; due to variation between flowcharts used it was not possible to pool data for different categories such as key population, study setting or region. Using METANDI and MIDAS, STATA-user-written programs, pooled meta-analysis of the AVD flowchart diagnostic accuracy was conducted through application of a bivariate multilevel random effects modelling approach.18 The bivariate random-effect model accounts for both the correlation between study sensitivity and the specificity estimates, and also unobserved between-study heterogeneity in test performance through specification of a multilevel bivariate normal regression approach. The bivariate model estimates pooled sensitivity and specificity simultaneously by modelling both diagnostic parameters as random effects (accounting for between-study heterogeneity in estimates) with a covariance term to account for the within-study dependence between these estimates. Summary diagnostic performance measures estimated from the meta-analysis models included summary sensitivity (SSe), summary specificity (SSp), positive and negative likelihood ratios and diagnostic odds ratio (DOR). Levels of study heterogeneity in pooled analyses of diagnostic performance (sensitivity and specificity) were quantified using intraclass correlation coefficient (ICC) estimates for sensitivity and specificity.19 In order to assess heterogeneity, subgroup analyses (based on symptomatic entry) were performed; meta-analyses were conducted for each flowchart (stratified by symptomatic entry) in turn. Diagnostic statistics (Cook’s distance statistics [using a five parameter model based cut-off]20 and standardised residual plots) from multi-level bivariate random-effect models were examined to assess pooled sensitivity and specificity estimates for outlier bias.20
STATA version 13 was used in all statistical analyses (STATA Corporation, College Station, TX, USA). Summary estimates were estimated when four or more flowcharts could be included in the analysis, Review Manager 5.3 was used to generate forest plots to display sensitivity and specificity estimates. Open source dot program Graphviz(http://www.graphviz.org) was used to present the study flow diagram.
Results
Database search results
A total of 393 studies were retrieved from the database searches, of which 78 were duplicates, resulting in the identification of 315 unique studies (Fig. 1). The titles and abstracts of these studies were then assessed against the inclusion criteria; a total of 250 studies were excluded on the basis of not being relevant (n = 172), not meeting the eligibility criteria (n = 75) or full text not able to be retrieved (n = 3). Sixty-five studies were then reviewed in full; additional excluded studies did not provide sufficient data (n = 5) or did not meet eligibility criteria (n = 24). A total of 36 met the eligibility criteria and were included.
Methodological review
The methodological quality of the 36 included studies was high for the majority of study characteristics with the exception of test operator training (Fig. 2); approximately one-quarter of studies had a sufficient level of test operator training. For the majority of studies the actual level of training achieved was unclear.
|
Description of included studies
Study characteristics are presented in Table 1. Half of the included studies (n = 18) were conducted in Africa. Nearly all (n = 30) of the studies were conducted in a healthcare setting; the most common healthcare settings were primary health clinics (n = 10), antenatal clinics (n = 7), and sexual health clinics (n = 7). Studies were published between 1993 and 2009 (median 1996). Study sample sizes ranged from 116 to 1643 (median 449). Study prevalence of cervical infection ranged between 0.7% and 35% (median 12%). We note that summary estimates could not be calculated using broad descriptors used here due to variation in flowcharts used.
Assessment of heterogeneity
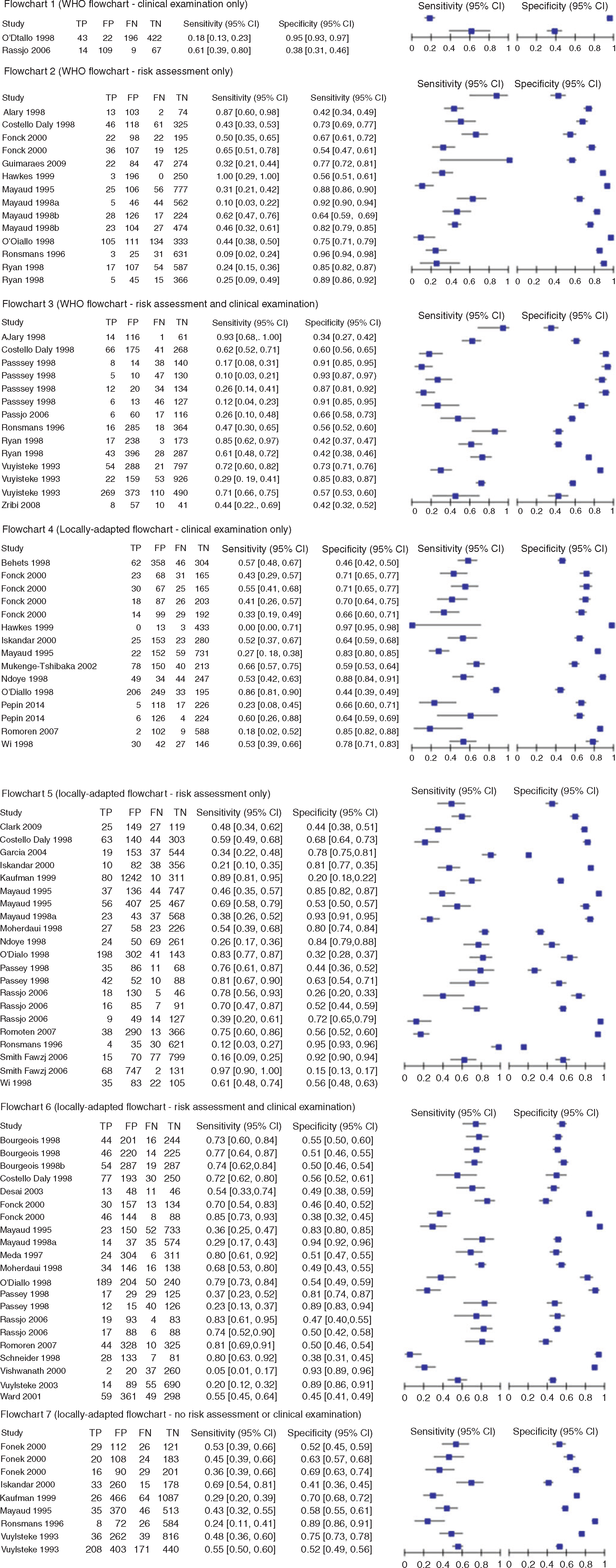
Of the 14 potential flowchart types there were four that had four or fewer studies describe that flowchart; hence Cook’s distance statistics could not be calculated. Cook’s distance statistics could be calculated for studies using the 10 other flowchart types; three flowchart types had Cook’s distance statistics greater than the established cut-off value indicating outliers within the flowchart grouping and these outlier studies were excluded from the calculation of summary statistics, one study was excluded from Flowchart 4a, two studies were excluded from Flowchart 5b and two studies were excluded from Flowchart 6b (Fig. 3). There was considerable heterogeneity between studies for all flowcharts included in meta-analyses, with an intraclass correlation coefficient range of 0.01–0.54 for sensitivity and 0.03–0.45 for specificity (Table 2).
Description of flowcharts and summary estimates
Key study parameters of individual flowchart types including flowchart sensitivity and specificity are presented in Fig. 4. Table 2 describes included flowcharts and summary estimates calculated for each flowchart type. Ninety-nine individual flowcharts were identified from the 36 studies; the majority (n = 59, 70%) of included flowcharts were locally-adapted. Among WHO flowcharts, the greatest number of included studies used risk assessment only (n = 14). Among locally-developed flowcharts, the greatest number of included studies also used risk assessment only (n = 20). SSe among WHO flowcharts ranged from 41.2% (Flowchart 3a) to 43.6% (Flowchart 2a). SSp among WHO-developed flowcharts ranged from 68.7% (Flowchart 3a) to 76.2% (Flowchart 2a). Summary estimates for DOR among WHO-developed flowcharts ranged from 1.5% (Flowchart 3a) to 2.5% (Flowchart 2a). SSe among locally-adapted flowcharts ranged from 39.5% (Flowchart 4a) to 74.8% (Flowchart 6b). SSp among locally-adapted flowcharts ranged from 53.6% (Flowchart 6b) to 75.6% (Flowchart 5a and 6a). Summary estimates for DOR among locally-adapted flowcharts ranged from 1.2% (Flowchart 7a) to 3.4 (Flowchart 5b). Overall, the flowchart with the greatest DOR (a ratio of the odds of a test being positive if the subject was a true case relative to the odds of a test being positive if the subject was not a true case) was 6b (locally-adapted flowchart incorporating clinical examination and risk assessment without symptomatic entry).
Comparison of WHO and locally-adapted flowcharts
Due to the limited number of similar WHO and locally-adapted flowcharts, comparisons for WHO-based and locally-adapted flowcharts could only be made between Flowchart 2a and Flowchart 5a (both using risk assessment only with symptomatic entry), and between Flowchart 3a and Flowchart 6a (both using clinical examination and risk assessment with symptomatic entry). For both comparisons, the locally-adapted flowcharts performed slightly better than the WHO equivalent. For example, Flowchart 5a (locally-adapted flowchart incorporating risk assessment only with symptomatic entry) had a higher DOR than Flowchart 2a (WHO guideline flowchart incorporating risk assessment only with symptomatic entry, DOR = 3.3 v. DOR = 2.5). Flowchart 6a (locally-adapted flowchart incorporating clinical examination and risk assessment with symptomatic entry) had similar pooled sensitivity and specificity to Flowchart 3a (WHO guideline flowchart incorporating clinical examination and risk assessment with symptomatic entry), however the DOR was higher for Flowchart 6a (DOR = 2.4) compared with Flowchart 3a (DOR = 1.5).
Comparison of flowcharts using risk assessment, clinical examination or both
A difference was not observed between the performance of flowcharts that included clinical assessment or risk assessment; however the flowchart that incorporated neither (Flowchart 7a) had the lowest DOR of all flowcharts included in this study. Flowchart 7a also had low SSe (47.8%) and moderate SSp (64.0%).
Comparison of symptomatic entry
Pooled diagnostic performance estimates suggest improved performance when the AVD flowchart was not restricted to participants with self-reported AVD symptoms (Table 2). Pooled estimates of flowcharts dependent on self-reported symptoms by study participants could be calculated for Flowcharts 4–6 (all locally-adapted); the pooled sensitivity estimate was higher when flowcharts were not restricted to those participants with self-reported AVD symptoms. For example, the SSe for Flowchart 4a and 4b were 39.5% and 63.6%), respectively. The specificity, however, was higher when flowcharts were validated by participants self-reporting AVD symptoms; for example, the SSp for Flowchart 5a and 5b were 75.6% and 61.9%, respectively.
Discussion
The present study provides a much-needed summary of the evidence on the performance of WHO and locally-adapted AVD flowcharts to detect cervical infection. Although some flowcharts performed better than others, summary diagnostic performance estimates were consistently low for all flowcharts, regardless of whether flowcharts incorporated risk assessment or clinical examination, if they were WHO-based or locally-adapted, or if they were only applied to women self-reporting AVD symptoms. These findings call for a revision of the inclusion of the AVD flowchart in WHO and local guidelines for STI management.
The results suggest the inclusion of risk assessment or clinical examination in locally-adapted flowcharts improves the ability of flowcharts to correctly identify women with cervical infection (sensitivity) and correctly identify women as not having cervical infection (specificity). The improvement in sensitivity was greatest if the flowchart was used at a population level rather than selectively among women self-reporting AVD symptoms. However, the improvement was marginal and the level of sensitivity and specificity remained low.
Overall, AVD flowcharts performed better in terms of SSe and DOR when they were not dependent on women self-reporting symptoms of AVD. Although DOR was higher among flowcharts which were not dependent on women self-reporting AVD symptoms, the corresponding SSe remained low, with only 60–75% of women with cervical infection being detected through the flowchart. As a result, up to 40% of women with likely cervical infection would remain untreated using these flowcharts. With any screening or diagnostic test, it is important to weigh up the consequences of leaving cases undetected and untreated against incorrect classification and treatment of false positive cases. False positive cases can lead to unnecessary expense for the healthcare system on drug expenditure, potential side effects for participants and unnecessary notification of sexual partners, which in some settings puts women at risk. A high specificity is important to reduce unnecessary treatment whereas a high sensitivity will reduce false negative cases and ensure people with infection receive appropriate treatment and follow-up, including partner notification and STI counselling. The serious public health consequences of undiagnosed and untreated cervical infection in the community suggest that trading specificity for higher sensitivity is arguably warranted. In situations where both sensitivity and specificity are unacceptably low a screening tool should not be used as they provide a false impression about the ability to accurately diagnose infection. With this in mind, the results of this review support the removal of the AVD flowchart for the syndromic management of STIs and in national STI control policies in resource-poor settings globally. In settings of high prevalence of chlamydia where laboratory diagnosis is not possible, it may be warranted to implement a mass treatment program rather than screening.54 It is important to note, however, that laboratory diagnosis of STIs is the gold standard and where possible should be used rather than presumptive treatment. For example, new molecular-based point-of-care tests for the simultaneous detection of CT and NG in community-health settings in remote Australia have been evaluated to have high sensitivity and specificity and operational benefits in terms of resources and ease of use55 and may offer a more suitable alternative to syndromic management in resource-poor settings globally.
Several limitations should be considered in the interpretation of the present study. First there was considerable heterogeneity across studies and within flowchart categories. Included studies represented populations that were diverse in geography, risk profile and setting. Although efforts were made to compare homogenous studies in terms of flowchart description and dependence on self-reported symptoms, it was not feasible to further stratify due to the limited number of flowcharts identified. Additional analysis explored differences in flowchart performance by cervical infection prevalence (low, medium and high) and risk profile (sex workers, pregnant women) but did not find a difference and these results are not reported here. Second, flowcharts were not consistently described and reported and it is possible that flowcharts were misclassified; the effect of misclassification cannot be estimated. Similarly, flowchart test performance measures were also not consistently reported leading to potential errors in the derived number of true positives and false positives. It was also not possible to estimate the effect of this potential bias. Only English language publications were included in this review, and one reviewer retrieved the studies and extracted data.
Conclusion
Various iterations of AVD flowcharts are currently used in STI guidelines in resource-poor settings globally, including WHO and locally- adapted versions, due to their low cost and lack of reliance on laboratory diagnostics. Our systematic review of the diagnostic performance of the AVD flowchart provides further evidence that the performance of the AVD is both inadequate and lacking in evidence. Many women were treated unnecessarily and many women with cervical infection were not detected and therefore not treated. We therefore caution against the continued use of these flowcharts as they create a false impression of being able to identify and improve the management of women infected with chlamydia and gonorrhoea. We recommend that in future the WHO Guidelines for the Management of Sexually Transmitted Infections10 exclude syndromic management of AVD to detect cervical infection.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
Part of this work was conducted while Caroline van Gemert was a Master of Public Health student at the University of Melbourne. Caroline van Gemert receives support through an Australian Government Research Training Program Scholarship. Freya Fowkes is supported by an Australian Research Council Future Fellowship. Margaret Hellard is supported by a National Health and Medical Research Council Principal Research Fellowship. The Burnet Institute gratefully acknowledge the contribution to this work of the Victorian Operational Infrastructure Support Program.
References
[1] World Health Organization. Global prevalence and incidence of selected curable sexually transmitted infections. Geneva: World Health Organization; 2001.[2] Mayaud P, Mabey D. Approaches to the control of sexually transmitted infections in developing countries: old problems and modern challenges. Sex Transm Infect 2004; 80 174–82.
| Approaches to the control of sexually transmitted infections in developing countries: old problems and modern challenges.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c3ntFWisA%3D%3D&md5=cf92ceb65608b8255cb2db0e219311eeCAS |
[3] UNAIDS, World Health Organization. Consultation on STD interventions for preventing HIV: What is the evidence? Geneva: World Health Organization; 2000.
[4] Low N, Broutet N, Adu-Sarkodie Y, Barton P, Hossain M, Hawkes S. Global control of sexually transmitted infections. Lancet 2006; 368 2001–16.
| Global control of sexually transmitted infections.Crossref | GoogleScholarGoogle Scholar |
[5] Dallabetta GA, Gerbase AC, Holmes KK. Problems, solutions, and challenges in syndromic management of sexually transmitted diseases. Sex Transm Infect 1998; 74 S1–S11.
[6] Pettifor A, Walsh J, Wilkins V, Raghunathan P. How effective is syndromic management of STDs? A review of current studies. Sex Transm Dis 2000; 27 371–85.
| How effective is syndromic management of STDs? A review of current studies.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2FmvFSrtg%3D%3D&md5=14e87b2de908fbdef79407fdf22a097aCAS |
[7] World Health Organization. Management of patients with sexually transmitted diseases. Geneva: World Health Organization; 1991.
[8] Pépin J, Deslandes S, Khonde N, Kintin DF, Diakite S, Sylla M, et al Low prevalence of cervical infections in women with vaginal discharge in west Africa: implications for syndromic management. Sex Transm Infect 2004; 80 230–5.
| Low prevalence of cervical infections in women with vaginal discharge in west Africa: implications for syndromic management.Crossref | GoogleScholarGoogle Scholar |
[9] Vuylsteke B, Laga M, Alary M, Gerniers MM, Lebughe JP, Nzila N, et al Clinical algorithms for the screening of women for gonococcal and chlamydial infection: evaluation of pregnant women and prostitutes in Zaire. Clin Infect Dis 1993; 17 82–8.
| Clinical algorithms for the screening of women for gonococcal and chlamydial infection: evaluation of pregnant women and prostitutes in Zaire.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3szlslChsQ%3D%3D&md5=325a88bb6279a05fb88b6ff2a81ca43fCAS |
[10] World Health Organisation. Guidelines for the management of sexually transmitted infections. Geneva: World Health Organization; 2003.
[11] World Health Organization. Report of the informal technical working group meeting on STD activities in GPA; Geneva: World Health Organization; 1993.
[12] Zemouri C, Wi TE, Kiarie J, Seuc A, Mogasale V, Latif A, et al The performance of the vaginal discharge syndromic management in treating vaginal and cervical infection: a systematic review and meta-analysis. PLoS One 2016; 11 e0163365
| The performance of the vaginal discharge syndromic management in treating vaginal and cervical infection: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[13] Gergen C, Wilkins V, Ragunathan P, Walsh J. When is syndromic management of sexually transmitted diseases useful? An analysis of the literature. 2000. Available online at: https://www.hsph.harvard.edu/ihsg/publications/pdf/No-83.PDF
[14] Cucuzza L. Vaginal discharge: syndromic management in family planning programs. A review of the literature. World Health and Population 2001; 4
[15] Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, et al HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 1997; 350 546–50.
| HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2svjtVKluw%3D%3D&md5=2b35e918a73a21977aab4f87bcd5e74bCAS |
[16] Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003; 3 25–38.
| The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews.Crossref | GoogleScholarGoogle Scholar |
[17] Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
[18] Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005; 58 982–90.
| Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews.Crossref | GoogleScholarGoogle Scholar |
[19] Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21 1539–58.
| Quantifying heterogeneity in a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[20] Rabe-Hesketh S, Skrondal A, Pickles A. GLLAMM Manual. Working Paper 160. UC Berkeley Division of Biostatistics Working Paper Series. October 2004.
[21] Mayaud P, Grosskurth H, Changalucha J, Todd J, West B, Gabone R, et al Risk assessment and other screening options for gonorrhoea and chlamydial infections in women attending rural Tanzanian antenatal clinics. Bull World Health Organ 1995; 73 621–30.
| 1:STN:280:DyaK287hsVentQ%3D%3D&md5=f063f5d0cbe5b83f16e642f6fbe2066dCAS |
[22] Ronsmans C, Bulut A, Yolsal N, Agacfidan A, Filippi V. Clinical algorithms for the screening of Chlamydia trachomatis in Turkish women. Genitourin Med 1996; 72 182–6.
| 1:STN:280:DyaK28zjsV2gtQ%3D%3D&md5=f665bf4666f0b4666ba9ca3b0e0ebca7CAS |
[23] Meda N, Sangare L, Lankoande S, Sanou PT, Compaore PI, Catraye J, et al Pattern of sexually transmitted diseases among pregnant women in Burkina Faso, west Africa: potential for a clinical management based on simple approaches. Genitourin Med 1997; 73 188–93.
| 1:STN:280:DyaK2svlt1Whug%3D%3D&md5=2d5f320e592e95c73fdd2581036418f2CAS |
[24] Alary M, Baganizi E, Guedeme A, Padonou F, Davo N, Adjovi C, et al Evaluation of clinical algorithms for the diagnosis of gonococcal and chlamydial infections among men with urethral discharge or dysuria and women with vaginal discharge in Benin. Sex Transm Infect 1998; 74 S44–9.
[25] Behets FM, Ward E, Fox L, Reed R, Spruyt A, Bennett L, et al Sexually transmitted diseases are common in women attending Jamaican family planning clinics and appropriate detection tools are lacking. Sex Transm Infect 1998; 74 S123-7
[26] Bourgeois A, Henzel D, Dibanga G, Malonga-Mouelet G, Peeters M, Coulaud JP, et al Prospective evaluation of a flow chart using a risk assessment for the diagnosis of STDs in primary healthcare centres in Libreville, Gabon. Sex Trans Infect 1998; 74 S128-32
[27] Bourgeois A, Henzel D, Malonga-Mouelet G, Dibanga G, Tsobou C, Peeters M, et al Clinical algorithms for the screening of pregnant women for STDs in Libreville, Gabon: which alternatives? Sex Transm Infect 1998; 74 35–9.
| Clinical algorithms for the screening of pregnant women for STDs in Libreville, Gabon: which alternatives?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3ns1GmsA%3D%3D&md5=8953897c0d41f61e0baa873c91ffe6ceCAS |
[28] Costello Daly C, Wangel AM, Hoffman IF, Canner JK, Lule GS, Lema VM, et al Validation of the WHO diagnostic algorithm and development of an alternative scoring system for the management of women presenting with vaginal discharge in Malawi. Sex Transm Infect 1998; 74 S50–8.
[29] Mayaud P, Uledi E, Cornelissen J, et al Risk scores to detect cervical infections in urban antenatal clinic attenders in Mwanza, Tanzania. Sex Transm Infect 1998a; 74 S139–46.
[30] Mayaud P, et al Validation of a WHO algorithm with risk assessment for the clinical management of vaginal discharge in Mwanza, Tanzania. Sex Transm Infect 1998b; 74 S77–84.
[31] Moherdaui F, Vuylsteke B, Siqueira LF, dos Santos MQ, Jardim ML, de Brito AM, et al Validation of national algorithms for the diagnosis of sexually transmitted diseases in Brazil: results from a multicentre study. Sex Transm Infect 1998; 74 S38–43.
[32] Ndoye I, Mboup S, De Schryver A, Van Dyck E, Moran J, Samb ND, et al Diagnosis of sexually transmitted infections in female prostitutes in Dakar, Senegal. Sex Transm Infect 1998; 74 S112–7.
[33] Diallo MO, Ghys PD, Vuylsteke B, Ettiegne-Traore V, Gnaore E, Soroh D, et al Evaluation of simple diagnostic algorithms for Neisseria gonorrhoeae and Chlamydia trachomatis cervical infections in female sex workers in Abidjan, Cote d’Ivoire. Sex Transm Infect 1998; 74 S106–11.
[34] Passey M, Mgone CS, Lupiwa S, Tiwara S, Lupiwa T, Alpers MP. Screening for sexually transmitted diseases in rural women in Papua New Guinea: are WHO therapeutic algorithms appropriate for case detection? Bull World Health Organ 1998; 76 401–11.
| 1:STN:280:DyaK1M%2FitVCgsA%3D%3D&md5=7634c96689f5a605298982fbb3ab817cCAS |
[35] Ryan CA, Zidouh A, Manhart LE, Selka R, Xia M, Moloney-Kitts M, et al Reproductive tract infections in primary healthcare, family planning, and dermatovenereology clinics: evaluation of syndromic management in Morocco. Sex Transm Infect 1998; 74 S95–105.
[36] Schneider H, Coetzee DJ, Fehler HG, Bellingan A, Dangor Y, Radebe F, et al Screening for sexually transmitted diseases in rural South African women. Sex Transm Infect 1998; 74 S147–52.
[37] Wi T, Mesola V, Manalastas R, Tuazon C, Mugrditchian DS, Perine P, et al Syndromic approach to detection of gonococcal and chlamydial infections among female sex workers in two Philippine cities. Sex Transm Infect 1998; 74 S118–22.
[38] Hawkes S, Morison L, Foster S, Gausia K, Chakraborty J, Peeling RW, et al Reproductive-tract infections in women in low-income, low-prevalence situations: assessment of syndromic management in Matlab, Bangladesh. Lancet 1999; 354 1776–81.
| Reproductive-tract infections in women in low-income, low-prevalence situations: assessment of syndromic management in Matlab, Bangladesh.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FktlGksw%3D%3D&md5=b1e2fb2c8c188ae17e42f296d7d3a8dbCAS |
[39] Fonck K, Kidula N, Jaoko W, Estambale B, Claeys P, Ndinya-Achola J, et al Validity of the vaginal discharge algorithm among pregnant and non-pregnant women in Nairobi, Kenya. Sex Transm Infect 2000; 76 33–8.
| Validity of the vaginal discharge algorithm among pregnant and non-pregnant women in Nairobi, Kenya.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c3nslWlsQ%3D%3D&md5=62c48fcba3593cd8001fe0f9598ed5dbCAS |
[40] Iskandar MB, Patten JH, Qomariyah SN, Vickers C, Molyneaux SI. Detecting cervical infection among family planning clients: difficulties at the primary health-care level in Indonesia. Int J STD AIDS 2000; 11 180–6.
| Detecting cervical infection among family planning clients: difficulties at the primary health-care level in Indonesia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7ovFCktQ%3D%3D&md5=b001871414f1bdee259efae7d679d10dCAS |
[41] Vishwanath S, Talwar V, Prasad R, Coyaji K, Elias CJ, de Zoysa I. Syndromic management of vaginal discharge among women in a reproductive health clinic in India. Sex Transm Infect 2000; 76 303–6.
| Syndromic management of vaginal discharge among women in a reproductive health clinic in India.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3cvns1yiuw%3D%3D&md5=451607c91b34d87d580b4728d833295aCAS |
[42] Ward E, Spruyt A, Fox L, Johnson L, Wong E, Behets F, et al Strategies for Detection of Sexually Transmitted Infection Among Family Planning Clients in Jamaica. International Family Planning Perspectives 2001; 27 201–7.
| Strategies for Detection of Sexually Transmitted Infection Among Family Planning Clients in Jamaica.Crossref | GoogleScholarGoogle Scholar |
[43] Mukenge-Tshibaka L, Alary M, Lowndes CM, Van Dyck E, Guedou A, Geraldo N, et al Syndromic versus laboratory-based diagnosis of cervical infections among female sex workers in Benin: implications of nonattendance for return visits. Sex Transm Dis 2002; 29 324–30.
| Syndromic versus laboratory-based diagnosis of cervical infections among female sex workers in Benin: implications of nonattendance for return visits.Crossref | GoogleScholarGoogle Scholar |
[44] Desai VK, Kosambiya JK, Thakor HG, Umrigar DD, Khandwala BR, Bhuyan KK. Prevalence of sexually transmitted infections and performance of STI syndromes against aetiological diagnosis, in female sex workers of red light area in Surat, India. Sex Transm Infect 2003; 79 111–5.
| Prevalence of sexually transmitted infections and performance of STI syndromes against aetiological diagnosis, in female sex workers of red light area in Surat, India.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3s7ntFylsQ%3D%3D&md5=a977472172ac21c96583aef6e0c3a8a6CAS |
[45] Vuylsteke BL, Ettiegne-Traore V, Anoma CK, Bandama C, Ghys PD, Maurice CE, et al Assessment of the validity of and adherence to sexually transmitted infection algorithms at a female sex worker clinic in Abidjan, Cote d’Ivoire. Sex Transm Dis 2003; 30 284–91.
| Assessment of the validity of and adherence to sexually transmitted infection algorithms at a female sex worker clinic in Abidjan, Cote d’Ivoire.Crossref | GoogleScholarGoogle Scholar |
[46] García PJ, Chavez S, Feringa B, Chiappe M, Li W, Jansen KU, et al Reproductive tract infections in rural women from the highlands, jungle, and coastal regions of Peru. Bull World Health Organ 2004; 82 483–92.
[47] Pépin J, Deslandes S, Khonde N, Kintin DF, Diakite S, Sylla M, et al Low prevalence of cervical infections in women with vaginal discharge in west Africa: implications for syndromic management. Sex Transm Infect 2004; 80 230–5.
| Low prevalence of cervical infections in women with vaginal discharge in west Africa: implications for syndromic management.Crossref | GoogleScholarGoogle Scholar |
[48] Råssjö EB, Kambugu F, Tumwesigye MN, Tenywa T, Darj E. Prevalence of sexually transmitted infections among adolescents in Kampala, Uganda, and theoretical models for improving syndromic management. J Adolesc Health 2006; 38 213–21.
| Prevalence of sexually transmitted infections among adolescents in Kampala, Uganda, and theoretical models for improving syndromic management.Crossref | GoogleScholarGoogle Scholar |
[49] Smith Fawzi MC, Lambert W, Singler J, Leandre F, Nevil P, Bertrand D, et al Identification of chlamydia and gonorrhoea among women in rural Haiti: maximising access to treatment in a resource poor setting. Sex Transm Infect 2006; 82( 175–81.
| Identification of chlamydia and gonorrhoea among women in rural Haiti: maximising access to treatment in a resource poor setting.Crossref | GoogleScholarGoogle Scholar |
[50] Romoren M, Sundby J, Velauthapillai M, Rahman M, Klouman E, Hjortdahl P. Chlamydia and gonorrhoea in pregnant Batswana women: time to discard the syndromic approach? BMC Infect Dis 2007; 7 27
| Chlamydia and gonorrhoea in pregnant Batswana women: time to discard the syndromic approach?Crossref | GoogleScholarGoogle Scholar |
[51] Zribi M, Mansour KB, Abid F, Masmoudi A, Fendri C. Syndromic approach to sexually transmitted infections in Tunisian women: bacteriological validation. Int J STD AIDS 2008; 19 112–4.
| Syndromic approach to sexually transmitted infections in Tunisian women: bacteriological validation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c7ns1WjsQ%3D%3D&md5=0881b2fcc246f47746f511e44b4654deCAS |
[52] Clark JL, Lescano AG, Konda KA, Leon SR, Jones FR, Klausner JD, et al 2009; 4 e7201
[53] Guimarães EM, Guimaraes MD, Vieira MA, Bontempo NM, Seixas MS, Garcia MS, et al Lack of utility of risk score and gynecological examination for screening for sexually transmitted infections in sexually active adolescents. BMC Med 2009; 7 8
| Lack of utility of risk score and gynecological examination for screening for sexually transmitted infections in sexually active adolescents.Crossref | GoogleScholarGoogle Scholar |
[54] Ndoye I, Mboup S, De Schryver A, Van Dyck E, Moran J, Samb ND, et al Diagnosis of sexually transmitted infections in female prostitutes in Dakar, Senegal. Sex Transm Infect 1998; 74 S112–7.
[55] Causer LM, Hengel B, Natoli L, Tangey A, Badman SG, Tabrizi SN, et al A field evaluation of a new molecular-based point-of-care test for chlamydia and gonorrhoea in remote Aboriginal health services in Australia. Sex Health 2015; 12 27–33.
| A field evaluation of a new molecular-based point-of-care test for chlamydia and gonorrhoea in remote Aboriginal health services in Australia.Crossref | GoogleScholarGoogle Scholar |