Going global: the adoption of the World Health Organization’s enabling recommendation on oral pre-exposure prophylaxis for HIV
Ioannis Hodges-Mameletzis A C , Shona Dalal A , Busisiwe Msimanga-Radebe B , Michelle Rodolph A and Rachel Baggaley AA World Health Organization, Headquarters, Avenue Appia 20, 1202 Geneva, Switzerland.
B World Health Organization, South Africa Country Office, 7th Floor Metro Park Building, 351 Francis Baard Street, Pretoria, 0002, South Africa.
C Corresponding author. Email: yanni.hodges@gmail.com
Sexual Health 15(6) 489-500 https://doi.org/10.1071/SH18125
Submitted: 29 June 2018 Accepted: 8 October 2018 Published: 29 November 2018
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
In September 2015, the World Health Organization (WHO) launched evidence-based guidelines by recommending that any person at substantial HIV risk should be offered oral pre-exposure prophylaxis (PrEP) containing tenofovir disoproxil fumarate (TDF) as an additional prevention choice. Since 2017, PrEP medicines have also been listed in the WHO’s Essential Medicines List, including TDF/emtricitabine (FTC) and TDF in combination with lamivudine (3TC). A descriptive policy review and analysis of countries adopting WHO’s 2015 recommendation on oral PrEP was conducted. As of June 2018, we identified 35 countries that had some type of policy on oral PrEP, and an additional five countries where a specific policy on PrEP is currently pending. A total of 19 high-income countries (HICs) and 21 low- and middle-income countries (LMICs) have adopted or have a pending policy. Most countries that have adopted or pending PrEP are in the European (42.9%) or African (30.0%) region. TDF/FTC is the most commonly recommended PrEP drug in the guidelines reviewed, although seven countries, namely in sub-Saharan Africa (6/7), are also recommending the use of TDF/3TC for PrEP. In sum, by the end of 2018, at least 40 countries (20.6%) are anticipated to have adopted WHO’s oral PrEP recommendation. Nonetheless, policy uptake does not reflect broader programmatic coverage of PrEP services, which remain limited across all settings, irrespective of income status. Enhancing global partnerships is needed to support and track ongoing policy adoption and to ensure that policy is translated into meaningful implementation of PrEP services.
Additional keywords: antiretroviral, policy, PrEP, prevention.
Introduction: HIV prevention still matters
From a public health perspective, the global response to HIV/AIDS has been marked by impressive gains in antiretroviral treatment (ART) scale-up, attributed to the interplay of advocacy, funding commitments, political will, drug optimisation, evidence-based normative guidance and innovative service delivery models. Nonetheless, HIV transmission persists, particularly in key populations, including men who have sex with men (MSM) across all regions.1 Adolescent girls and young women in high-burden countries in eastern and southern Africa also have been recognised as a population experiencing high levels of HIV acquisition. Many people with HIV also live in serodiscordant relationships and providing PrEP to the negative partner until the person with HIV is fully suppressed on ART has been shown to be acceptable and effective.2 This strategy of using PrEP as a bridge to ART is potentially highly effective and cost-effective, as supported by mathematical modelling that examined the impact and cost-effectiveness of different strategies for HIV-1 prevention for serodiscordant couples.3
Prioritising HIV prevention for these populations has varied across countries. There has been growing recognition that reinvigorating old interventions while introducing novel approaches remains vital in order to achieve a global elimination of new HIV infections, in not just children, but also in adults.4 The World Health Organization’s (WHO) Global Sector Strategy on HIV outlines the need to accelerate and focus on HIV combination prevention, including the introduction of pre-exposure prophylaxis (PrEP).5 PrEP is currently synonymous with oral-based PrEP (namely, oral tablets containing tenofovir disoproxil fumarate (TDF) as the key HIV drug). However, several other products are being investigated in the clinical research pipeline, including the dapivirine vaginal ring and a long-acting form of cabotegravir, which belongs to a class of HIV drugs called integrase inhibitors.
The latest Joint United Nations Programme on HIV/AIDS (UNAIDS) estimates highlight that, despite some encouraging declines in HIV incidence in high-burden countries in eastern and southern Africa, an overall ‘plateau’ is observed in the trajectory of HIV transmission.6 Epidemiologically, it is evident that HIV incidence is increasing in certain settings and populations, particularly among certain key populations.7–16 For instance, the latest report from the WHO and the European Centre for Disease Prevention and Control indicates worrisome trends in parts of eastern and central Europe over the last decade.7 The WHO European Region remains the only region worldwide where the number of new HIV infections is rising, which is attributed to late diagnoses of HIV infection and the unmet need for HIV testing, and expanded access to evidence-based interventions such as harm reduction, condom programming, PrEP and HIV self-testing (HIVST).
From evidence to recommendations: the evolution of the WHO’s clinical and implementation guidance
Developing evidence-based recommendations by the WHO requires a careful assessment of all available clinical evidence across several domains. The data on efficacy and the potential benefits and harms of an intervention, such as a drug for treatment of HIV infection or for PrEP, is appraised through systematic literature reviews and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework, which is then assessed by an independent group of experts convened by the WHO to develop recommendations.17 The WHO recommendations also consider the values and preferences of end users (e.g. people at risk for HIV that could benefit from PrEP) and healthcare providers, as well as the feasibility, acceptability, resources required and equity associated with a given intervention.
The WHO first produced PrEP guidance for public health stakeholders in 2012, specifically recommending daily oral PrEP in the context of demonstration projects for men and transgender women who have sex with men, and serodiscordant heterosexual couples.2,18 The WHO proceeded to publish additional guidance in 2014 by recommending daily oral PrEP for MSM beyond demonstration projects and research.19 Modelling estimates at that time suggested that, globally, 20–25% reductions in HIV incidence among MSM could be achieved through PrEP, and thereby averting up to 1 million new infections among this group over 10 years. The basis for the 2014 recommendation by the WHO was the iPrEx trial, a Phase III trial,20 evaluating the safety and efficacy of once-daily oral TDF/FTC, as compared with placebo for the prevention of HIV acquisition among MSM. The trial was conducted among 2499 participants across six countries: Peru, Ecuador, South Africa, Brazil, Thailand and the United States (USA).
In 2015, additional evidence from two critical studies, PROUD and Ipergay,21,22 both reporting 86% reduction in HIV acquisition risk in those receiving TDF/FTC, was analysed alongside all other clinical trials and open-label extension studies as part of a WHO meta-analysis.23 The systematic review by Fonner et al.23 was the basis for the WHO’s 2015 recommendation,24 which opened the door for PrEP to be considered for any person at risk for HIV, irrespective of gender. That recommendation was also included in the 2016 WHO Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection.25
In 2017, the WHO published an implementation tool that was structured in a series of modules (chapters), each focused on the key implementing stakeholders, including clinicians, regulators, pharmacists and strategic information personnel. For example, the clinical module provides evidenced-base suggestions for those who would be providing PrEP in clinical settings.26 It describes important considerations when starting PrEP in an individual and monitoring PrEP use, including the required laboratory tests that need to be undertaken.
Methods
This paper is a descriptive policy review and analysis of the countries that have adopted WHO’s 2015 oral PrEP recommendation. We searched for published documents considered to reflect the adoption of that recommendation for the period 1 January 2010 to 1 May 2018. We included documents from government websites; the WHO regional and country websites; and online searches in Google (including Google Scholar, with use of the combination of search terms preexposure prophylaxis, pre-exposure prophylaxis, PrEP, HIV, Truvada, tenofovir, antiretroviral, guidelines, protocol and guidance). In addition, we communicated with HIV program managers and HIV focal points at the WHO regional offices to request any unpublished PrEP policies. We sought to identify documents, published and unpublished, including national antiretroviral (ARV) guidelines by government agencies where PrEP was recommended (e.g. Handbook of the Botswana 2016 Integrated HIV Clinical Care Guidelines), stand-alone PrEP guidelines (e.g. USA Centers for Disease Control and Prevention), clinical protocols and guidelines produced by clinical associations (e.g. European AIDS Clinical Society). Across all WHO regions, WHO staff and other implementing partners in HIV prevention were contacted to provide and confirm the status of national policies on PrEP. A data extraction sheet was developed to capture key indicators of interest in policy documents that were reviewed, including: (1) populations eligible for PrEP; (2) PrEP drugs recommended; (3) use of PrEP drugs in pregnancy and breastfeeding; (4) HIV testing; (5) testing for sexually transmissible infections (STIs); (6) renal function testing; and (7) an age restriction for PrEP.
Results
Which countries have adopted the PrEP WHO recommendation?
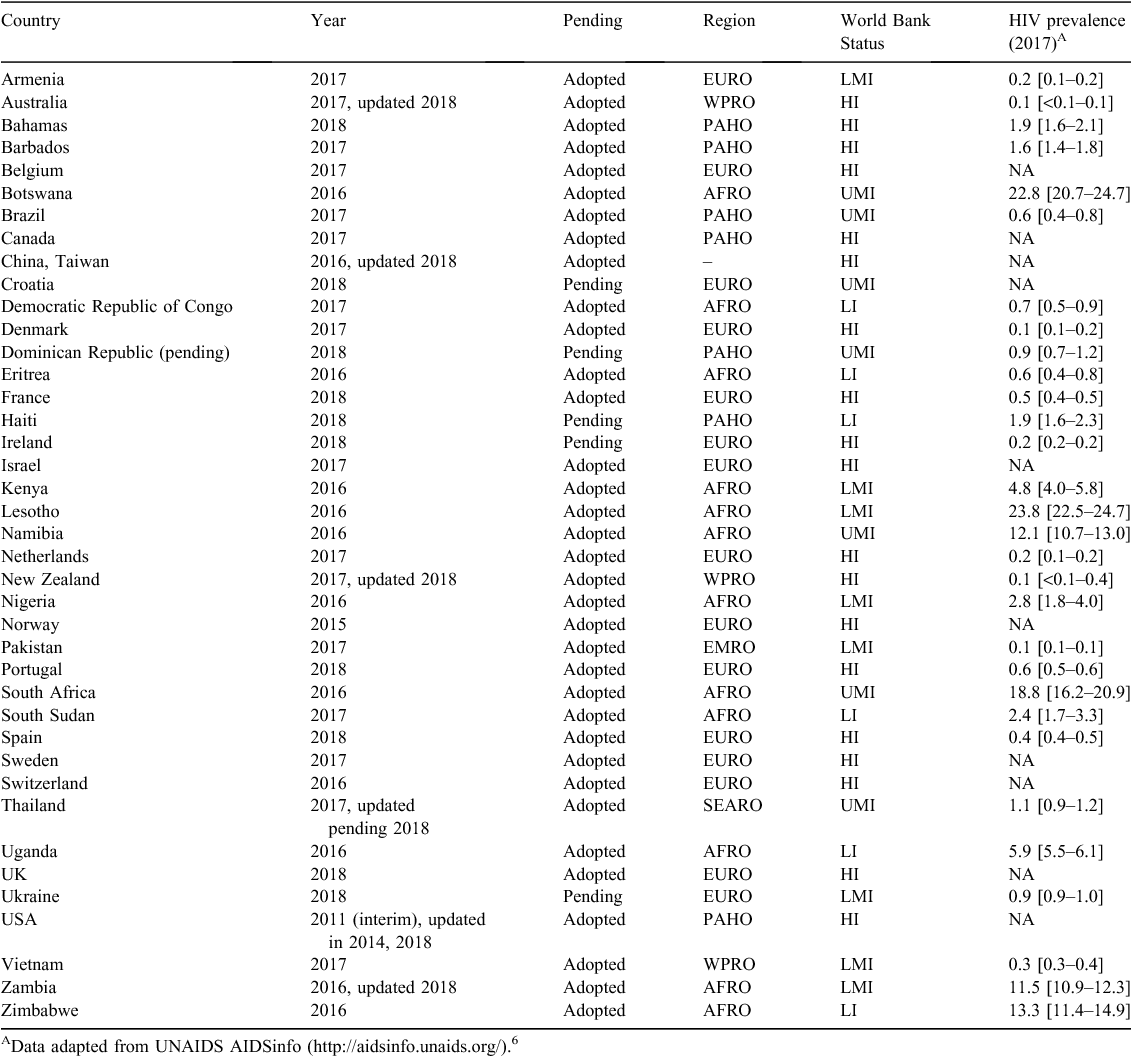
As of 1 May 2018, we identified 34 WHO Member States (out of a total of 194) that had some type of policy on oral PrEP containing TDF, and an additional five countries where a specific policy on PrEP is pending (Figure 1). An additional non-Member State, China, Taiwan, has had a policy in place since 2016, and has updated its recommendation in 2018 to include event-driven PrEP for MSM, coupled with daily dosing for MSM, transgender women, the negative partner in a serodiscordant relationship, high-risk heterosexual couples and people who inject drugs. Based on World Bank income status (2018), a total of 19 high-income countries (HICs) and 21 low- and middle-income countries (LMICs) have adopted or have a pending PrEP policy. From a regional perspective, the majority of countries that have adopted PrEP are in the European (n = 15) and African (n = 12) regions. The USA was the first country to issue interim PrEP guidance in 2011, with additional updates.27–29

|
We also identified three clinical society guidelines. First, the European AIDS Clinical Society (EACS) produces the European Guidelines for treatment of HIV-positive adults in Europe, which have recommended PrEP in two updates (2016, 2017).30,31 The latest EACS guidelines provide links to online video lectures on PrEP, as part of the EACS online course Clinical Management of HIV.32 In 2012, Southern African HIV Clinicians Society (SAHCS) published PrEP guidelines for MSM,33 and then expanded its guidelines to recommend PrEP more broadly in 2016 for other populations, including heterosexual men and women, sex workers, transgender persons and adolescents.34 Finally, the Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM) PrEP guidelines were first issued in 2015, and updated twice (2017 and 2018).35,36 Australia and New Zealand follow these guidelines, and for the purposes of our policy analysis, we have designated the two countries as individual units.
Table 1 lists the WHO Member States that have adopted the WHO recommendation on oral PrEP, by the WHO region and by income status based on the World Bank’s classification.
Populations prioritised and eligible for PrEP
In reviewing the policy documents, most countries indicated that key populations, and in particular MSM and transgender women, were groups that could benefit from the provision of PrEP, while African guidelines highlighted eligibility for adolescent girls and young women. Another relevant population that was noted across most guidelines were HIV-negative partners within serodiscordant relationships, where four country documents (Kenya, Botswana, USA, Brazil) specifically noted that PrEP could be prescribed for safer conception. Kenya’s criteria were clear on how PrEP can benefit a HIV-negative partner by stating ‘when HIV-positive partner is not on ART, or on ART <6 months, or suspected poor adherence to ART, or most recent viral load is detectable’. South Africa’s guidelines made a nuanced point on how HIV risk is cross-cutting when thinking about populations by stating ‘young MSMs are even more vulnerable to HIV as they may engage in overlapping risk behaviours, such as injecting drugs and selling sex’. South Sudan’s policy recommends PrEP for any individual at substantial risk of HIV, as per the WHO’s definition of substantial risk (HIV incidence >3%), while making reference of subpopulations such as sex workers and their clients, fishermen, long-distance truck drivers, MSM, uninformed forces and adolescents and young women engaged in transactional sex. In the Nigerian guidelines, although MSM are not explicitly stated as a PrEP eligible population, they are covered under ‘individuals who engage in anal sex on a prolonged and regular basis’. In South Sudan’s guidelines, the ‘eligibility criteria for PrEP’ do not include MSM, but do state ‘individuals who engage in anal sex’. The French guidelines have clear language on serodiscordant couples; ‘when the HIV-positive partner takes ARV therapy and has a viral load undetectable for more than 6 months, treatment is the first-line prevention intervention. In other situations, the prescription of PrEP may be considered’. The latest update to the UK guidelines states that PrEP is ‘not recommended for people who inject drugs (PWID) where needle exchange and opiate substitution programs are available’. Other guideline documents are more permissive to the use of PrEP in PWID, including the US, Thailand and Taiwanese guidelines, for instance. The ASHM PrEP guidelines point out that the International Network of People who Use Drugs has issued cautions against prioritising PrEP at the expense of other evidence-based interventions, namely harm-reduction services.
French guidelines also prioritise PrEP for adolescents at high risk for HIV sexual acquisition, especially within sexual health centers.37 The French recommendation is aligned with the extension of the indication for PrEP, as of 14 December 2017, by the European Medicines Agency. We also note that the French guidelines state that PrEP can be offered on a case-by-case basis to PWID, sex workers and any vulnerable person having ‘unprotected’ sex with a high risk of HIV transmission. The Swiss guidelines, issued early in January 2016 by the Swiss Federal Commission for Sexual Health, were one of the first guidelines to recognise that PrEP can be offered during a ‘season or moment of risk’,38 a term initially described in the literature by Grant and Glidden39 [‘may also be appropriate to prescribe PrEP for a limited period if the risk is temporarily increased (e.g. sex tourism/sex parties in countries/cities with high HIV prevalence)’].
Pre-exposure prophylaxis drugs recommended
The WHO recommendation from 2015 is permissive to TDF-containing PrEP, and allows flexibility in country considerations on which drug to recommend within national guidelines and to procure within national HIV programs. In 2017, the WHO Essential Medicines List (EML) was updated to include PrEP drugs, specifically noting TDF/FTC, TDF/3TC and TDF alone.40 The EML is a key reference and public health vehicle used by many countries to increase access to medicines and guide decisions about which products should be made available for their populations.
In our analysis of both published and unpublished documents, only one (Eritrea) out of 32 policies reviewed did not provide guidance on choice of PrEP drug. The majority of countries (29/32) recommended TDF/FTC as the preferred formulation; one policy (Democratic Republic of Congo (DRC)) recommended TDF/3TC/EFV as the preferred drug for PrEP (300 mg/150 mg/600 mg), which has not been considered by the WHO in its discussion and no PrEP study to our knowledge has evaluated a three-drug, fixed-dose combination (FDC) for PrEP. WHO post-exposure prophylaxis (PEP) guidelines do currently recommend, however, a three-drug FDC.41
Six countries recommend TDF/3TC for PrEP in addition to TDF/FTC (Pakistan, South Sudan, Namibia, Kenya, Zambia and Zimbabwe), while Lesotho’s guidelines recommend exclusively TDF/3TC. Spain and Portugal, although clearly recommending TDF/FTC as the key PrEP regimen, state that TDF alone (daily administration) can be prescribed in cases of intolerance or toxicity to FTC. The latest USA guidelines recommend TDF alone as an alternative for PWID and heterosexually active adults, but not for MSM,29 while the UK guidelines state that TDF alone ‘may be considered’ for heterosexual men and women only.42 Eleven countries and the EACS guidelines included recommendations and information on event-based dosing. Event-driven dosing is being offered alongside daily dosing in Europe, influenced by the EACS guidelines, which state both dosing approaches. Although the WHO currently recommends daily dosing for PrEP based on its latest guidelines, the agency is currently reviewing data emerging from event-driven PrEP for MSM in the Netherlands, France, Belgium, Canada, Norway and the UK.
PrEP use in pregnancy and breastfeeding
The WHO released a technical brief in 2017, based on a review of the data for safety of PrEP during pregnancy and breastfeeding.43 Within that brief, the WHO advises that PrEP should not be stopped during pregnancy and breastfeeding if women wish to continue their PrEP use. The brief also describes the possibility of offering PrEP to complement established HIV prevention strategies for pregnant and breastfeeding women as part of a comprehensive package to reduce HIV infections among women and transmission from mothers to infants in settings with very high HIV incidence. Our policy analysis revealed that in 18 out of the 32 national guidelines reviewed, it was stated that pregnancy was not considered a contraindication, and thereby PrEP was permissive. In the remaining documents, PrEP in pregnancy was either not mentioned or not clear, although three country documents implied use of PrEP in pregnancy, as it was recommended for serodiscordant couples wishing to conceive. Regarding use in breastfeeding, eight country documents clearly did not contraindicate PrEP during this heightened period of HIV acquisition in women. The Kenyan guidelines are particularly clear on this, and recommend the provision of PrEP within antenatal clinics, maternal and child health, and reproductive health services.44
The Spanish guidelines recognise that their setting may not be as relevant to the use of oral PrEP in pregnancy and breastfeeding, but they clearly reference the increased risk of HIV during pregnancy.45 According to these guidelines, PrEP is not recommended for women wanting to conceive where the HIV-positive male partner is on ART and virally suppressed for at least 6 months, while there is no mention of any information on breastfeeding. Portugal’s guidelines are permissive on the use of PrEP during pregnancy and breastfeeding, but they flag that a clinical evaluation is required on the increased risk of toxicity for PrEP in situations such as pregnancy and breastfeeding (e.g. along with individuals with risk factors for chronic kidney disease and those with bone disease).46
HIV testing
Pre-exposure prophylaxis service delivery requires HIV testing services to be in place before initiating PrEP and during the course of PrEP use, so as to confirm people using PrEP are HIV negative. In our review, all national PrEP guidelines included HIV testing as a requirement in confirming HIV-negative status before starting PrEP. A total of 31 of 32 policies recommended follow-up HIV testing at specified intervals, generally every 3 months. In one document, this was not clear. We observed that there was some variability as to the whether an HIV test at 1 month after initiating PrEP was recommended. The 1-month follow-up visit can serve as an adherence check for new PrEP users, but also as an opportunity to determine acute infection that may have not been identified at the onset of PrEP.
Fourth-generation HIV rapid tests are not readily available in most resource-limited settings. Of note, China, Taiwan recommends fourth or third generation ELISA tests, and its guidelines state that providers can consider nucleic acid testing (NAT) for PrEP clients who may have symptoms of acute retroviral syndrome.47 Portugal also follows a similar approach, where it recommends fourth generation testing on the same day or 7 days before the start of PrEP, and with NAT in the case of suspecting acute infection.
Sexually transmissible infection testing
Offering PrEP can be an opportunity to increase access to STI screening and testing for syphilis, gonorrhoea and chlamydia. The WHO recognises the added value of PrEP in the context of a broader sexual health package,48 and its 2017 PrEP implementation tool suggests that the frequency of STI screening and testing may be every 3 or 6 months depending on population and national policy. Our review revealed that in most policy documents, STI screening is recommended before starting PrEP, with follow-up STI screening and testing also recommended as part of routine clinical visits, or at specified intervals. Availability of molecular testing of STIs remains limited and this is reflected in our PrEP guidance review. For instance, the UK guidelines recommend that every 3 months, nucleic acid testing is carried out to detect gonococcal and chlamydial infection at sites of exposure and syphilis serology. The USA Centers for Disease Control and Prevention (CDC) guidelines recommend testing for bacterial STIs every 3–6 months. The French guidelines, in addition to screening and treatment for STIs, have clear instructions on STI prevention through vaccination; for example, human papillomavirus (HPV), hepatitis B virus (HBV), hepatitis A virus (HAV) and vaccination against invasive meningococcal infections C.
Syndromic management for STIs has particular limitations, and from both a public health perspective and an individual-medical approach, appropriate STI testing, especially in key populations, has not been achieved at coverage levels in most LMICs.
Renal function monitoring
TDF has been used extensively worldwide and is the most prescribed ARV drug for treatment of HIV infection. The extensive data available demonstrate this drug is well tolerated and has a favourable safety profile. It is estimated that one in every 200 PrEP users will have an elevation of serum creatinine during PrEP use.49 Therefore, renal function assessment has been recognised as an important element in prescribing PrEP. The WHO stated in its 2016 ARV guidelines that serum creatinine testing is preferred before starting PrEP and at quarterly visits during PrEP use for the first 12 months, then annually thereafter. The WHO’s PrEP implementation tool describes that serum creatinine testing on follow up be conducted every 6 months, but also makes note that it can be considered more frequently if there is a history of conditions affecting the kidney, including diabetes or hypertension.
In 27 of the 32 policies, creatinine testing before initiation of PrEP was recommended. Some guidelines, such as the French guidelines, call for ‘enhanced monitoring’ of renal function in people with risk factors for impaired renal function. The Kenyan guidelines recommend a baseline creatinine test, and one annually thereafter, which reflects an attempt to simplify the laboratory testing required when delivering PrEP.
Age restriction
The WHO’s recommendation on oral PrEP does not have an age-specific restriction. The USA Food and Drug Administration (FDA), along with the European Medicines Agency, recently approved PrEP for use in at-risk adolescents.50,51 There is growing recognition that certain adolescents may be at higher risk for HIV, and therefore could benefit from the provision of PrEP. There are, however, outstanding research gaps, particularly regarding models of implementation and support for adherence, in this population. The current literature suggests adolescents may require additional support for ensuring adequate levels of adherence while on PrEP.52,53 In our review, there was a trend for not including an age restriction in guidelines from most African countries. Of note, Kenya had a contraindication for PrEP in adolescents under the age of 15 years, or weighing less than 35 kg. The USA CDC guidelines recommend PrEP for individuals aged 18 years and over (implied as ‘adults’ and specified in the recommendation). In addition, the American guidelines state that the risks and benefits of PrEP for adolescents should be weighed carefully in the ‘context of local laws and regulations about autonomy in healthcare decision-making by minors’. Some guidelines also specify weight as a contraindication, as is the case in Namibia’s guidelines where adolescents weighing <35 kg or aged <15 years who are not Tanner stage 3 or greater should not be on TDF-containing PrEP. The French national guidelines also make the public health argument for PrEP in adolescents.
Alternative mechanisms for PrEP access
Despite the WHO’s strong emphasis for oral PrEP provision to people at substantial HIV risk, and the high quality of evidence that supports the crafting of that recommendation, availability of PrEP in many countries was initially limited to pilot research and demonstration projects. Once evidence was clear of the efficacy of PrEP, interest in PrEP grew, particularly from MSM communities. PrEP is increasingly available in the private sector, but high drug costs in most settings make this unaffordable to the majority of those who could benefit. As a consequence, alternative mechanisms to access PrEP drugs for individuals not enrolled in projects have flourished across many countries. Most notably, the online sales of PrEP generic medicines (e.g. TDF/FTC, which is a fraction of the cost of the originator product) has been a vehicle by which individuals, especially in Europe, have accessed PrEP where formal PrEP services were lacking or limited.54 In the UK, for instance, it is legal for individuals to purchase and import PrEP as long as it is for personal use.42 The latest UK guidelines have a section on the purchase of generic medicines, referencing the Medicines and Healthcare products Regulatory Agency (MHRA), which advises on the legality of buying up to 3 months of medicines from outside the European Union for personal use.
Advocacy organisations such as Prepster (UK), i-base (UK) and AIDES (France) and related websites (e.g. https://www.iwantprepnow.co.uk/) have been critical in sharing information about online access to generic PrEP drugs, with specific guidance for potential and active PrEP users on how to safely purchase drugs online.55–57 In England, oral PrEP is currently available for free through the IMPACT clinical trial, and for those who are not able to join the trial, online access remains an alternative option.58,59
Outstanding questions and concerns remain by public health authorities and other stakeholders around quality assurance of medicines when purchased online. Appropriate services should be accessible for individuals who purchase PrEP online, so as to ensure clinical monitoring and support during PrEP use. As Coleman and Prins have argued, ‘online purchasing is neither a viable or safe long-term substitute for national PrEP programs, nor legal in all countries’.54
Which regulatory authorities have approved TDF-based products for PrEP?
In the history of the HIV response, drugs for prevention of mother-to-child transmission (PMTCT) and post-exposure prophylaxis have traditionally not required market authorisation for use by stringent regulatory authorities (SRAs) and national medical regulatory authorities (NMRAs). For ARV medicines in the context of PEP, the lack of controlled trials of PEP efficacy have made the submission of regulatory submissions difficult and, consequently, PEP is widely used, but based on recommendations from public health authorities and medical societies. For PrEP, there is an evolving regulatory approval landscape for both the originator product (Truvada, by Gilead Sciences) and generic products. Truvada was the first PrEP drug to receive a PrEP indication by a SRA back in 2012 by the USA Food and Drug Administration.60 Since then, Truvada has been approved by NMRAs in both high-income and resource-limited countries worldwide (European Medicines Agency, France, Canada, Australia, New Zealand, China-Taiwan, Israel, South Korea, Brazil, Chile, Kenya, Malawi, Peru, South Africa, Thailand, Tanzania, Zambia, Zimbabwe).61 Generic manufacturers of PrEP regimens have also started becoming important players in access to PrEP services. The European Medicines Agency has approved generic versions by Mylan, Zentiva (Sanofi) and Krka for PrEP.62 As of June 2018, Cipla, Mylan, Hexal, Dr Reddy’s Laboratories, Lupin, Sandoz, Thailand’s Government Pharmaceutical Organisation (GPO), Biogaran, Teva, Zentiva and Ratiopharm have market authorisation to supply PrEP in several countries.61
When the initial PrEP efficacy was reported from the iPrex trial in 2010, the USA CDC issued interim guidance in 2011 for healthcare providers, and recommended PrEP for MSM, even in the absence of a PrEP indication on the originator product. In Switzerland, despite no PrEP indication on a PrEP product thus far by Swissmedic (the Swiss Agency for Therapeutic Products), the prescription of PrEP is undertaken by physicians as off-label, as per the Swiss 2016 PrEP guidance.
As noted earlier, the US FDA extended a PrEP indication for Truvada to adolescents. Data from ATN 113, a US-based study in young MSM, were included in the FDA submission by Gilead Sciences, which supported the applicant’s dossier. Published in 2018, the French guidelines also prioritise PrEP for adolescents at high HIV risk, and thus are aligned with the extension of the indication for PrEP as of December 2017 by the European Medicines Agency.
In South Africa, PrEP is currently contraindicated for both pregnancy and breastfeeding despite concerning levels of HIV incidence in pregnancy and breastfeeding.63 The current guidelines issued by the Southern African HIV Clinicians Society state that there is limited safety data for TDF-containing PrEP in these periods, and it is noted that ‘onus is on the clinician to discuss potential risks and benefits of PrEP initiation or maintenance during pregnancy’ with a woman.
Discussion
The WHO’s strong recommendation on the provision of oral PrEP containing TDF has been a catalyst for the development of national PrEP guidance. Our policy review concludes that, as of June 2018, 3 years after the release of WHO’s guidelines, 35 countries had some form of policy on oral PrEP, with an appreciable representation of sub-Saharan African countries (n = 12), with nine countries being in eastern and southern Africa. Although encouraging, most countries (79.3%) still lack national guidance and formal access to PrEP services. Since this review was conducted, additional countries were identified with policies which were not included in our analysis, including Tanzania, Swaziland and Mexico.
In reviewing specific indicators of interest across the policy documents, most guidelines remain generally clear on the additional procedures required before PrEP initiation, and their frequency during PrEP use; for example, HIV testing, renal function monitoring and STI testing. The WHO’s 2015 recommendation on oral PrEP was accompanied with a ‘Treat-All’ recommendation, where WHO recommended ART be offered to all adults living with HIV, regardless of WHO clinical stage and the CD4 cell count. An analysis by WHO indicated that as of the end of 2017, 70% of LMICs had adopted the ‘Treat-All’ policy when at the same time less than 11% had considered PrEP.64
Our review underscores that most countries with a PrEP policy in place are recommending daily TDF/FTC, and therefore are aligned with the WHO guidelines. We identified seven countries that are also recommending TDF/3TC for PrEP. Kenya’s guidelines are particularly clear that TDF/3TC is an alternative to TDF/FTC,65 while Lesotho remains the only country recommending exclusively TDF/3TC. Launched in 2017, the national programmatic rollout of PrEP in Kenya, which includes the Bridge-to-Scale initiative, is procuring TDF/FTC.61 Notably, both Haiti and Lesotho are currently developing their own policies on PrEP, with TDF/3TC being considered as a PrEP option (personal communication).61 An additional phase II study in MSM is evaluating TDF/3TC in Brazil and will provide further evidence on effectiveness.66 The crafting of the WHO recommendation on PrEP containing TDF and the 2017 update to the EML suggest that countries are enabled to consider not only TDF/FTC for PrEP procurement, but also TDF alone, and the fixed-dose combination of TDF/3TC. The WHO hosted a technical consultation in Geneva, Switzerland in 2016 that provided further evidence and discussion on the indirect evidence for TDF/3TC effectiveness on PrEP. As most countries within their ART programs procure TDF/3TC, and not TDF/FTC, there is a potential supply chain advantage with the introduction of TDF/3TC for PrEP within countries.
The current WHO recommendation, which calls for PrEP being offered to any person at risk for HIV, rests on evidence for TDF-containing regimens of daily dosing, both in MSM and also heterosexual populations. The WHO ARV guidelines from 2016 do recognise the efficacy data from the Ipergay placebo-controlled trial conducted in France and Canada, although there is as yet no GRADE-based recommendation by WHO calling for the provision of event-driven PrEP for MSM. Furthermore, the WHO’s PrEP implementation tool cautions that the effectiveness of event-driven dosing among women and heterosexual men has not yet been evaluated. A range of national PrEP guidance documents we reviewed included an additional recommendation on the use of TDF/FTC as an ‘on-demand’ dosing for MSM only. Ongoing studies of event-driven PrEP are underway, including Prevenir, an open-label study of TDF/FTC currently enrolling 3000 participants in the Paris region, where the primary outcome of interest is reduction in new HIV diagnoses.67 An interim analysis from Prevenir reported no incident HIV infections when PrEP was taken in either daily or on-demand dosing (mean follow up of 7 months in 1594 high-risk individuals), with approximately half of participants choosing the on-demand dosing.68 We recognise that the current literature is not consistent in the use of the terminology around on-demand or event-driven PrEP (e.g. ‘non-daily dosing’, ‘intermittent PrEP’, ‘periodic PrEP’), and future guidance should be clear on such terms.
One of the recurrent concerns by governments and other stakeholders within countries is the underlying cost of PrEP services, which can explain the limited countries that have PrEP guidance in place. Cost considerations include commodities, from the drug itself to the additional testing required to ensure PrEP is offered effectively and safely. Nonetheless, costs of TDF-containing regimens in LMICs have been on the decline, largely the result of competition by generic manufacturers, larger volumes of TDF-containing regimens for ART and the catalytic effects of access initiatives such as Medicines Patent Pool (MPP). According to the WHO’s Global Price Reporting Mechanism, TDF/FTC had a median cost of ~48.83 USD per person per year in 2017, while TDF/3TC was at a median cost of 36.76 USD per person per year.69
Of note, the WHO maintains that PrEP service delivery can be led not only by clinicians, but also by nurses and clinical officers, across diverse clinical settings. In South Africa, for instance, the National Department of Health (NDoH) is offering PrEP as part of a national rollout using nurses that have been trained on Nurse Initiated Management of Antiretroviral Treatment (NIMART) and have prescribing privileges of ART, including TDF/FTC.70
As there is an observed shift towards PrEP policy adoption and ultimately services that can be available and accessible within health systems, documenting the public health effect of PrEP rollout will become essential for monitoring and evaluation purposes. Public health effect could be defined and characterised by a range of outcomes, including identifying HIV-infected persons with expanded HIV testing and swift linkage to ART, improving coverage of STI management where PrEP is being offered, and ultimately seeing a reduction in HIV incidence. Some documented ‘success stories’ of PrEP having a role in the reduction of new HIV diagnoses have been described for Sydney, San Francisco, New York City and London.71–74 Cities, as health jurisdictions, are well placed to respond to HIV, and several so-called Fast-Track cities are now including PrEP in their planning and HIV responses.
Capturing programmatic data on provision of other services can also be informative, including provision for family planning, HBV testing and vaccination and other STI services. The benefits of PrEP should go beyond those of prescribing the drug. Many people who initially attend a PrEP service may not choose PrEP, but may benefit from other health services. Currently, in many settings, PrEP programs offer a range of other services. For example, the PrEP package for women should include contraception, other HIV-prevention options (e.g. male and female condoms, STI case management, support and linkages to intimate partner violence services). To realise and demonstrate the full impact of PrEP services, is it important that these consequences are captured, including those who test HIV positive during the initial PrEP screening and their linkage to ART.
At the country level, policy, as defined by having a clinical guidelines document, is not enough. Formal policy also does not coincidence with PrEP implementation beyond small scale efforts and sufficient levels of PrEP coverage for those individuals and groups that remain at higher risk for HIV. There are positive examples, however, where guidelines have been translated into rapid implementation of PrEP services, as has been observed in South Africa, Kenya and Australia. The transition from a discussion on policy shifted to a discussion on who pays, and how. The Pharmaceutical Benefits Advisory Committee (PBAC) in Australia announced in February 2018, that PrEP would be listed on its Pharmaceutical Benefits Scheme (PBS) as of April 2018.75 France is an interesting example, conversely, where the Ministry of Health, with support from civil society, chose to rollout PrEP within its national health system before having national PrEP guidelines. France became the first country in Europe to offer PrEP outside a clinical trial or demonstration project in January 2016.76 In 2013, the group, AIDES, submitted a request for a temporary recommendation for use (RTU) to the Agence nationale de sécurité du médicament (ANSM; the French National Agency for Drug and Health Product Safety). This request led eventually to an RTU, which was published in December 2015. This preceded the European Medicines Agency approval of Truvada in 2016, the first PrEP indication in Europe.
In recent years, the WHO has recognised that a mechanism of partnership and effective coordination at the global level could facilitate and track adoption of its WHO recommendation of PrEP, while sharing lessons on policy and programmatic development. Established in 2017, WHO’s Global PrEP Coalition (GPC) has been providing the global platform to ensure a more harmonised approach in policy adoption and implementation.77 The GPC, as a WHO-led forum, was designed to facilitate global dialogue and foster collaboration between PrEP stakeholders. The concept of such a coalition was first articulated in 2010 by Kim et al.,78 making the case that global coordination and collaboration would require the ‘ongoing collection of data, assessments, and monitoring and evaluation to develop and share lessons learned’. The GPC is focusing on influencing three key players: providers, policymakers and PrEP users through a series of regional consultations, webinars and targeted dissemination of existing WHO clinical and implementation guidance on PrEP.
Sound policy is contingent on and further strengthened by sound evidence. The WHO’s guidelines on oral PrEP outline the key clinical research gaps that persist and which should be addressed. These include the need for further evidence on event-based dosing, long-term consequences on renal function, risk of development of HIV drug resistance and how adherence to PrEP could be enhanced. From an implementation research perspective, the ‘early adopter’ countries will be providing further evidence on how to optimise and simplify service delivery for PrEP, all while safety and effectiveness are ensured for individuals. For PrEP to be feasible, affordable and acceptable in many LMIC settings, it will be essential to explore models of service delivery that are community-driven. Ways to minimise the need to monitoring elements such a renal function are being explored; for example, in Kenya, and results from these programs will be critical to inform how implementation can be simplified safely.
Conclusion
Learning from ongoing implementation will further enable PrEP programs to support people in identifying their own HIV risks and understand the benefits of PrEP, cope with initial start-up side-effects, support adherence and help decide when to stop and potentially re-start PrEP as their personal circumstances change. The WHO remains committed to assessing the learning from ongoing implementation projects to support countries to adapt their PrEP guidance for these and other areas where there is little current evidence. Meaningful HIV combination prevention efforts will require linking PrEP policy development to concrete implementation plans by public health authorities if we are to realise PrEP’s true potential.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We acknowledge the important contribution to this policy review by Mary Henderson, who conducted the initial review of each guidelines document and extraction of data. Ms. Henderson also provided insightful feedback to the draft manuscript. The Bill and Melinda Gates Foundation and Unitaid have also awarded the WHO with grant support to enable the development of this manuscript.
References
[1] Baggaley R, Dalal S, Johnson C, Macdonald V, Mameletzis I, Rodolph M, Figueroa C, Samuelson J, Verster A, Doherty M, Hirnschall G. Beyond the 90-90-90: refocusing HIV prevention as part of the global HIV response. J Int AIDS Soc 2016; 19 21348| Beyond the 90-90-90: refocusing HIV prevention as part of the global HIV response.Crossref | GoogleScholarGoogle Scholar |
[2] Heffron R, Ngure K, Odoyo J, Bulya N, Tindimwebwa E, Hong T, Kidoguchi L, Donnell D, Mugo NR, Bukusi EA, Katabira E, Asiimwe S, Morton J, Morrison S, Haugen H, Mujugira A, Haberer JE, Ware NC, Wyatt MA, Marzinke MA, Frenkel LM, Celum C, Baeten JM, Partners Demonstration Project Team. Pre-exposure prophylaxis for HIV-negative persons with partners living with HIV: uptake, use, and effectiveness in an open-label demonstration project in East Africa. Gates Open Res. 2018; 1 3
| Pre-exposure prophylaxis for HIV-negative persons with partners living with HIV: uptake, use, and effectiveness in an open-label demonstration project in East Africa.Crossref | GoogleScholarGoogle Scholar |
[3] Hallett TB, Baeten JM, Heffron R, Barnabas R, De Bruyn G, Cremin Í, Delany S, Garnett GP, Gray G, Johnson L, McIntyre J, Rees H, Celum C. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLoS Med 2011; 8 e1001123
| Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study.Crossref | GoogleScholarGoogle Scholar |
[4] Dehne KL, Dallabetta G, Wilson D, Garnett GP, Laga M, Benomar E, Fakoya A, Baggaley RC, Nelson LJ, Kasedde S, Bermejo A, Warren M, Benedikt C, Global Prevention Focal Point Group. HIV Prevention 2020: a framework for delivery and a call for action. Lancet HIV 2016; 3 e323–32.
| HIV Prevention 2020: a framework for delivery and a call for action.Crossref | GoogleScholarGoogle Scholar |
[5] World Health Organization. Global Health Sector Strategy on HIV 2016–2021. Geneva: World Health Organization; 2016.
[6] Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS Data 2018. Geneva: UNAIDS; 2018. Available online at: http://www.unaids.org/en/resources/documents/2018/unaids-data-2018 [verified 1 November 2018].
[7] European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2017–2016 Data. Stockholm: ECDC; 2017.
[8] Lam CR, Holtz TH, Leelawiwat W, Mock PA, Chonwattana W, Wimonsate W, Varangrat A, Thienkrua W, Rose C, Chitwarakorn A, Curlin ME. Subtypes and risk behaviors among incident HIV cases in the Bangkok Men Who Have Sex With Men Cohort Study, Thailand, 2006-2014. AIDS Res Hum Retroviruses 2017; 33 1004–12.
| Subtypes and risk behaviors among incident HIV cases in the Bangkok Men Who Have Sex With Men Cohort Study, Thailand, 2006-2014.Crossref | GoogleScholarGoogle Scholar |
[9] Ross AG, Ditangco RA, Belimac JG, Olveda RM, Mercado ES, Chau TN, Crowe SM. The dire sexual health crisis among MSM in the Philippines: an exploding HIV epidemic in the absence of essential health services. Int J Infect Dis 2015; 37 6–8.
| The dire sexual health crisis among MSM in the Philippines: an exploding HIV epidemic in the absence of essential health services.Crossref | GoogleScholarGoogle Scholar |
[10] Thienkrua W, van Griensven F, Mock PA, Dunne EF, Raengsakulrach B, Wimonsate W, Howteerakul N, Ungsedhapand C, Chiwarakorn A, Holtz TH. Young men who have sex with men at high risk for HIV, Bangkok MSM Cohort Study, Thailand 2006–2014. AIDS Behav 2018; 22 2137–46.
| Young men who have sex with men at high risk for HIV, Bangkok MSM Cohort Study, Thailand 2006–2014.Crossref | GoogleScholarGoogle Scholar |
[11] van Griensven F, Guadamuz TE, de Lind van Wijngaarden JW, Phanuphak N, Solomon SS, Lo Y-R. Challenges and emerging opportunities for the HIV prevention, treatment and care cascade in men who have sex with men in Asia Pacific. Sex Transm Infect 2017; 93 356–62.
| Challenges and emerging opportunities for the HIV prevention, treatment and care cascade in men who have sex with men in Asia Pacific.Crossref | GoogleScholarGoogle Scholar |
[12] De Boni R, Veloso VG, Grinsztejn B. Epidemiology of HIV in Latin America and the Caribbean. Curr Opin HIV AIDS 2014; 9 192–8.
| Epidemiology of HIV in Latin America and the Caribbean.Crossref | GoogleScholarGoogle Scholar |
[13] Rasmussen D, Wilkinson E, Vandormael A, Tanser F, Pillay D, Stadler T, de Oliveira T. External introductions helped drive and sustain the high incidence of HIV-1 in rural KwaZulu-Natal, South Africa. bioRxiv 2017; 119826
| External introductions helped drive and sustain the high incidence of HIV-1 in rural KwaZulu-Natal, South Africa.Crossref | GoogleScholarGoogle Scholar |
[14] Justman J, Reed JB, Bicego G, Donnell D, Li K, Bock N, Koler A, Philip NM, Mlambo CK, Parekh BS, Duong YT, Ellenberger DL, El-Sadr WM, Nkambule R. Swaziland HIV Incidence Measurement Survey (SHIMS): a prospective national cohort study. Lancet HIV 2017; 4 e83–92.
| Swaziland HIV Incidence Measurement Survey (SHIMS): a prospective national cohort study.Crossref | GoogleScholarGoogle Scholar |
[15] Thomson KA, Hughes J, Baeten JM, John-Stewart G, Celum C, Cohen CR, Ngure K, Kiarie J, Mugo N, Heffron R. Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis 2018; 218 16–25.
| Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners.Crossref | GoogleScholarGoogle Scholar |
[16] Kamarulzaman A, Reid SE, Schwitters A, Wiessing L, El-Bassel N, Dolan K, Moazen B, Wirtz AL, Verster A, Altice FL. Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners. Lancet 2016; 388 1115–26.
| Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners.Crossref | GoogleScholarGoogle Scholar |
[17] World Health Organization. WHO Handbook for Guideline Development, 2nd edn. Geneva: World Health Organization; 2014.
[18] World Health Organization. Guidance on pre-exposure prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk for HIV: recommendations for use in the context of demonstration projects. Geneva: World Health Organization; 2012.
[19] World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva: World Health Organization; 2014.
[20] Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallás EG, Amico KR, et al Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363 2587–99.
| Preexposure chemoprophylaxis for HIV prevention in men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[21] McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, Sullivan AK, Clarke A, Reeves I, Schembri G, Mackie N, Bowman C, Lacey CJ, Apea V, Brady M, Fox J, Taylor S, Antonucci S, Khoo SH, Rooney J, Nardone A, Fisher M, McOwan A, Phillips AN, Johnson AM, Gazzard B, Gill ON. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387 53–60.
| Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial.Crossref | GoogleScholarGoogle Scholar |
[22] Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, Tremblay C, Le Gall JM, Cua E, Pasquet A, Raffi F, Pintado C, Chidiac C, Chas J, Charbonneau P, Delaugerre C, Suzan-Monti M, Loze B, Fonsart J, Peytavin G, et al On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373 2237–46.
| On-demand preexposure prophylaxis in men at high risk for HIV-1 infection.Crossref | GoogleScholarGoogle Scholar |
[23] Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, Koechlin FM, Rodolph M, Hodges-Mameletzis I, Grant RM. Effectiveness and safety of oral HIV pre-exposure prophylaxis (PrEP) for all populations: a systematic review and meta-analysis. AIDS 2016; 30 1973–83.
| Effectiveness and safety of oral HIV pre-exposure prophylaxis (PrEP) for all populations: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[24] World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: World Health Organization; 2015.
[25] World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization; 2016.
[26] World Health Organization. WHO implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection. Geneva: World Health Organization; 2017.
[27] Centers for Disease Control and Prevention. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep 2011; 60 65–8.
[28] Centers for Disease Control and Prevention: US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2014: a clinical practice guideline. Atlanta: CDC; 2014.
[29] Centers for Disease Control and Prevention: US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2017 update: a clinical practice guideline. Atlanta: CDC; 2018. Available online at: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf [verified 1 November 2018].
[30] European AIDS Clinical Society. European AIDS Clinical Society Treatment Guidelines, 2016. Brussels: EACS; 2016. Available online at: http://www.eacsociety.org/files/guidelines_8.1-english.pdf [verified 1 November 2018].
[31] European AIDS Clinical Society. European AIDS Clinical Society Treatment Guidelines, 2017. Brussels: EACS; 2017. Available online at: http://www.eacsociety.org/files/guidelines_9.0-english.pdf [verified 1 November 2018].
[32] European AIDS Clinical Society. Clinical Management of HIV PrEP Online Course. Brussels: EACS; 2018. Available online at: http://www.eacsociety.org/education/clinical-management-of-hiv-online-course/clinical-management-of-hiv-online-course.html [verified 23 October 2018].
[33] Southern African HIV Clinicians Society Consensus Committee. Southern African guidelines for the safe use of pre-exposure prophylaxis in men who have sex with men who are at risk for HIV infection. South Afr J HIV Med 2012; 13 40–55.
[34] Bekker LG, Rebe K, Venter F, Maartens G, Moorhouse M, Conradie F, Wallis C, Black V, Harley B, Eakles R. Southern African guidelines on the safe use of pre-exposure prophylaxis in persons at risk of acquiring HIV-1 infection. South Afr J HIV Med 2016; 17 455
[35] Wright E, Grulich A, Roy K, Boyd M, Cornelisse V, Russell D, O’Donnell D, Whittaker B, Crooks L, Zablotska I. Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine HIV pre-exposure prophylaxis: clinical guidelines. J Virus Erad 2017; 3 168–84.
[36] Wright E, Grulich A, Roy K, Boyd M, Cornelisse V, Russell D, O’Donnell D, Whittaker B, Crooks L, Zablotska I. Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine HIV pre-exposure prophylaxis: clinical guidelines. Update April 2018. J Virus Erad 2018; 4 143–59.
[37] National Agency for Research on AIDS and Viral Hepatitis (ANRS). Medical care of people living with HIV - prevention and screening (April 2018). Paris: The National Council on AIDS and viral hepatitis (CNS); 2018. Available online at: https://cns.sante.fr/wp-content/uploads/2018/04/experts-vih_prevention-depistage.pdf [verified 1 November 2018]. [In French]
[38] Swiss Federal Commission for Sexual Health (FCSH). Recommendations of the FCSH on pre-exposure prophylaxis (PrEP) for HIV prevention. Bern: Federal Office of Public Health; 2016. Available online at: https://www.bag.admin.ch/dam/bag/en/dokumente/ mt/p-und-p/richtlinien-empfehlungen/prep-empfehlungen-der-eksg-januar-2016.pdf.download.pdf/fcsh-recommendations-prep-hiv.pdf [verified 1 November 2018].
[39] Grant RM, Glidden DV. HIV moments and pre-exposure prophylaxis. Lancet 2016; 387 1507–8.
| HIV moments and pre-exposure prophylaxis.Crossref | GoogleScholarGoogle Scholar |
[40] World Health Organization. WHO Model List of Essential Medicines: 20th List (March 2017). Geneva: World Health Organization; 2017. Available online at: http://apps.who.int/iris/bitstream/handle/10665/273826/EML-20-eng.pdf?ua=1 [verified 23 October 2018].
[41] Ford N, Mayer KH, World Health Organization Postexposure Prophylaxis Guideline Development Group World Health Organization guidelines on postexposure prophylaxis for HIV: recommendations for a public health approach. Clin Infect Dis 2015; 60 S161–4.
| World Health Organization guidelines on postexposure prophylaxis for HIV: recommendations for a public health approach.Crossref | GoogleScholarGoogle Scholar |
[42] British HIV Association. Update of BHIVA/BASHH guidelines on the use of HIV pre-exposure prophylaxis (PrEP), 2018. London: British HIV Association; 2018. Available online at: https://www.bhiva.org/file/5b729cd592060/2018-PrEP-Guidelines.pdf [verified 23 October 2018].
[43] World Health Organization. Preventing HIV during pregnancy and breastfeeding in the context of PrEP – technical brief. Geneva: World Health Organization; 2017. Available online at: http://apps.who.int/iris/bitstream/handle/10665/255866/WHO-HIV-2017.09-eng.pdf;jsessionid=3F00F80ECAA456FA41F786522011E0E3?sequence=1 [verified 1 November 2018].
[44] Ministry of Health, National AIDS & STI Control Programme. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya, 2016 edn. Nairobi, Kenya: NASCOP; 2016. Available online at: https://aidsfree.usaid.gov/sites/default/files/kenya_art_2016.pdf [verified 23 October 2018].
[45] AIDS Study Group (GeSIDA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Consensus document on control and monitoring of HIV infection, 2018. Madrid: GeSIDA; 2018. [In Spanish]
[46] Department of Quality in Health, Portuguese National Program for HIV, AIDS and Tuberculosis. Pre-exposure prophylaxis of HIV infection in adults. Lisbon: Department of Quality in Health, Portuguese National Program for HIV, AIDS and Tuberculosis; 2017. [In Portuguese]
[47] Taiwan AIDS Society. Guidelines for use of PrEP in Taiwan. Taipei: Taiwan AIDS Society; 2018.
[48] World Health Organization. Global Health Sector Strategy on Sexually Transmitted Infections, 2016–2021. Geneva: World Health Organization; 2016. Available online at: http://apps.who.int/iris/bitstream/handle/10665/246296/WHO-RHR-16.09-eng.pdf;jsessionid= 83B1BDB16B0097FA7E9E10C35C775C44?sequence=1 [verified 23 October 2018].
[49] World Health Organization. WHO implementation tool for pre-exposure prophylaxis (PrEP) of HIV infection – clinical module. Geneva: World Health Organization; 2017. Available online at: http://apps.who.int/iris/bitstream/handle/10665/255889/WHO-HIV-2017.17-eng.pdf?sequence=1 [verified 23 October 2018].
[50] United States Food and Drug Administration. Approval letter for adolescent indication. Rockville: United States Food and Drug Administration; 2018. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/021752Orig1s055ltr.pdf [verified 23 October 2018].
[51] European Medicines Agency. Summary of opinion on Truvada. London: European Medicines Agency; 2017. Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_ of_opinion/human/000594/WC500240366.pdf [verified 23 October 2018].
[52] Hosek SG, Landovitz RJ, Kapogiannis B, Siberry GK, Rudy B, Rutledge B, Liu N, Harris DR, Mulligan K, Zimet G, Mayer KH, Anderson P, Kiser JJ, Lally M, Brothers J, Bojan K, Rooney J, Wilson CM. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr 2017; 171 1063–71.
| Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States.Crossref | GoogleScholarGoogle Scholar |
[53] Machado DM, de Sant’Anna Carvalho AM, Riera R. Adolescent pre-exposure prophylaxis for HIV prevention: current perspectives. Adolesc Health Med Ther 2017; 8 137–48.
| Adolescent pre-exposure prophylaxis for HIV prevention: current perspectives.Crossref | GoogleScholarGoogle Scholar |
[54] Coleman R, Prins M. Options for affordable pre-exposure prophylaxis (PrEP) in national HIV prevention programmes in Europe. Euro Surveill 2017; 22 17-00698
| Options for affordable pre-exposure prophylaxis (PrEP) in national HIV prevention programmes in Europe.Crossref | GoogleScholarGoogle Scholar |
[55] Prepster. Prepster. London: Prepster; 2018. Available online at: http://prepster.info/pdf [verified 23 October 2018].
[56] HIV i-Base. UK guide to PrEP. London: HIV i-Base; 2018. Available online at: http://i-base.info/guides/prep [verified 23 October 2018].
[57] AIDES. La PrEP. Paris: AIDES; 2018. Available online at: https://www.aides.org/prep [verified 23 October 2018].
[58] Public Health England. PrEP impact trial protocol. London: Public Health England; 2017. Available online at: https://docs.wixstatic.com/ugd/f75c00_f1c229feb1f1471189bc99c9d697b0e5.pdf [verified 23 October 2018].
[59] PrEP in Europe. PrEP in Europe summit. London: PrEP in Europe; 2018. Available online at: http://www.prepineurope.org/en/summit [verified 23 October 2018].
[60] United States Food and Drug Administration. Truvada PrEP indication approval. Rockville: United States Food and Drug Administration; 2012. Available online at: https://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM312290.pdf [verified 23 October 2018].
[61] World Health Organization, HIV Department. Internal records. Geneva: World Health Organization; 2018.
[62] European Medicines Agency. Truvada PrEP indication approval. London: European Medicines Agency; 2016. Available online at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2016/07/news_detail_002578.jsp&mid=WC0b01ac058004d5c1 [verified 23 October 2018].
[63] National Department of Health, Republic of South Africa. National Policy on HIV Pre-exposure Prophylaxis (PrEP) and Test and Treat (T&T). Pretoria: National Department of Health, Republic of South Africa; 2016. Available online at: http://www.sahivsoc.org/Files/PREP%20and%20TT%20Policy%20-%20Final%20Draft%20-%205%20May%202016%20(HIV%20news).pdf [verified 23 October 2018].
[64] Ford N, Vitoria M, Doherty M. Providing antiretroviral therapy to all who are HIV positive: the clinical, public health and programmatic benefits of Treat All. J Int AIDS Soc 2018; 21 e25078
| Providing antiretroviral therapy to all who are HIV positive: the clinical, public health and programmatic benefits of Treat All.Crossref | GoogleScholarGoogle Scholar |
[65] National AIDS & STI Control Programme (NASCOP), Ministry of Health. Framework for the Implementation of Pre-exposure Prophylaxis of HIV in Kenya. Nairobi, Kenya: NASCOP; 2017.
[66] Federal University of Minas Gerais Medical School. Pesquisa vai comparar efeitos da PrEP importada com a nacional. Belo Horizonte: Federal University of Minas Gerais Medical School; 2018. Available online at: https://site.medicina.ufmg.br/inicial/pesquisa-vai-comparar-efeitos-da-prep-importada-com-a-nacional/ [verified 23 October 2018]. [In Portuguese]
[67] US National Library of Medicine. Prevenir protocol, identifier NCT03113123. Bethesda: US National Library of Medicine; 2017. Available online at: https://clinicaltrials.gov/ct2/show/NCT03113123 [verified 23 October 2018].
[68] Molina JM, Ghosn J, Béniguel L, Rojas-Castro D, Algarte-Genin M, Pialoux G, Delaugerre C, Yazdanpanah Y, Katlama C, Ségouin C, Morel S, Pintado C, Loze B, Le Mestre S, Gibowski S, Dore V, Assoumou L, Spire B, Costagliola D, Prévenir ANRS study group. Incidence of HIV-infection in the ANRS Prévenir study in Paris region with daily or on-demand PrEP with TDF/FTC. Proceedings of the 22nd International AIDS Conference (AIDS 2018); 23–27 July 2018; Amsterdam, Netherlands. Geneva: AIDS 2018; 2018. Abstract WEAE0406LB. Available online at: http://programme.aids2018.org/Abstract/Abstract/13278 [verified 23 October 2018].
[69] World Health Organization. WHO Global Price Reporting Mechanism; 2018. Geneva: World Health Organization; 2018.
[70] Pillay Y. South Africa’s experience in bringing PrEP to scale for a range of populations. Proceedings of the 9th International AIDS Society (IAS) Conference; 23–26 July 2017; Paris, France. Geneva: IAS; 2017. Abstract SUSA0703.
[71] San Francisco City Health Department. Annual HIV epidemiological report. San Francisco: San Francisco City Health Department; 2017.
[72] New York City Department of Health and Mental Hygiene, HIV Epidemiology and Field Services Program. HIV surveillance annual report, 2016. New York, NY: New York City Department of Health and Mental Hygiene; 2017. Available online at: https://www1.nyc.gov/assets/doh/downloads/pdf/dires/hiv-surveillance-annualreport-2016.pdf [verified 9 November 2018].
[73] Grulich AE, Guy R, Amin J, Jin F, Selvey C, Holden J, Schmidt HA, Zablotska I, Price K, Whittaker B, Chant K, Cooper C, McGill S, Telfer B, Yeung B, Levitt G, Ogilvie EE, Dharan NJ, Hammoud MA, Vaccher S, et al Population-level effectiveness of rapid, targeted, high-coverage roll-out of HIV pre-exposure prophylaxis in men who have sex with men: the EPIC-NSW prospective cohort study. Lancet HIV 2018;
| Population-level effectiveness of rapid, targeted, high-coverage roll-out of HIV pre-exposure prophylaxis in men who have sex with men: the EPIC-NSW prospective cohort study.Crossref | GoogleScholarGoogle Scholar |
[74] Nwokolo N, Hill A, McOwan A, Pozniak A. Rapidly declining HIV infection in MSM in central London. Lancet HIV 2017; 4 e482–3.
| Rapidly declining HIV infection in MSM in central London.Crossref | GoogleScholarGoogle Scholar |
[75] Pharmaceutical Benefits Scheme (PBS), Australian Government Department of Health. December 2017 decision of PrEP. Canberra: Commonwealth of Australia; 2017. Available online at: https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2017-12/files/tenofovir-emtricitabine-prep-psd-december-2017.docx [verified 23 October 2018].
[76] Delanoe C, Coulomb D. French announcement for PrEP coverage, November, 23, 2015. Malakoff: Le Quotidien du Médecin; 2015. Available online at: https://www.lequotidiendumedecin.fr/actualites/article/2015/11/23/marisol-touraine-dit-oui-la-prep-et-annonce-son-remboursement-en-france-en-janvier-2016_782261 [verified 23 October 2018].
[77] World Health Organization. Global PrEP Coalition. Geneva: World Health Organization; 2018. Available online at: http://www.who.int/hiv/prep/global-prep-coalition/ [verified 23 October 2018].
[78] Kim SC, Becker S, Dieffenbach C, Hanewall BS, Hankins C, Lo Y-R, Mellors JW, O’Reilly K, Paxton L, Roffenbender JS, Warren M, Piot P, Dybul MR. Planning for pre-exposure prophylaxis to prevent HIV transmission: challenges and opportunities. J Int AIDS Soc 2010; 13 24
| Planning for pre-exposure prophylaxis to prevent HIV transmission: challenges and opportunities.Crossref | GoogleScholarGoogle Scholar |