Socioeconomic and psychosocial factors are associated with poor treatment outcomes in Australian adults living with HIV: a case-control study
Krista J. Siefried A B G , Stephen Kerr A , Robyn Richardson A , Limin Mao C , John Rule D E , John McAllister A , John de Wit C F and Andrew Carr A
A B G , Stephen Kerr A , Robyn Richardson A , Limin Mao C , John Rule D E , John McAllister A , John de Wit C F and Andrew Carr A
A St Vincent’s Centre for Applied Medical Research, St Vincent’s Hospital, 390 Victoria Street, Sydney, NSW 2010, Australia.
B National Centre for Clinical Research on Emerging Drugs, University of New South Wales, Sydney, NSW 2052, Australia.
C Centre for Social Research in Health, University of New South Wales, Sydney, NSW 2052, Australia.
D National Association of People with HIV Australia, 1 Erskineville Road, Newtown, NSW 2042, Australia.
E School of Public Health and Community Medicine, University of New South Wales, Sydney, NSW 2052, Australia.
F Department of Interdisciplinary Social Science, Utrecht University, PO Box 80125, 3508 TC Utrecht, The Netherlands.
G Corresponding author. Email: krista.siefried@svha.org.au
Sexual Health 16(6) 548-553 https://doi.org/10.1071/SH18138
Submitted: 30 July 2018 Accepted: 16 April 2019 Published: 13 September 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Background: A substantial minority of patients living with HIV refuse or cease antiretroviral therapy (ART), have virological failure (VF) or develop an AIDS-defining condition (ADC) or serious non-AIDS event (SNAE). It is not understood which socioeconomic and psychosocial factors may be associated with these poor outcomes. Methods: Thirty-nine patients with poor HIV treatment outcomes, defined as those who refused or ceased ART, had VF or were hospitalised with an ADC or SNAE (cases), were compared with 120 controls on suppressive ART. A self-report survey recorded demographics, physical health, life stressors, social supports, HIV disclosure, stigma or discrimination, health care access, treatment adherence, side effects, health and treatment perceptions and financial and employment status. Socioeconomic and psychosocial covariates significant in bivariate analyses were assessed with conditional multivariable logistic regression, adjusted for year of HIV diagnosis. Results: Cases and controls did not differ significantly with regard to sex (96.2% (n = 153) male) or age (mean (± s.d.) 51 ± 11 years). Twenty cases (51%) had refused or ceased ART, 35 (90%) had an HIV viral load >50 copies mL–1, 12 (31%) were hospitalised with an ADC and five (13%) were hospitalised with a new SNAE. Three covariates were independently associated with poor outcomes: foregoing necessities for financial reasons (adjusted odds ratio (aOR) 3.1, 95% confidence interval (95% CI) 1.3–7.6, P = 0.014), cost barriers to accessing HIV care (aOR 3.1, 95% CI 1.0–9.6, P = 0.049) and lower quality of life (aOR 3.8, 95% CI 1.5–9.7, P = 0.004). Conclusions: Despite universal health care, socioeconomic and psychosocial factors are associated with poor HIV outcomes in adults in Australia. These factors should be addressed through targeted interventions to improve long-term successful treatment.
Additional keywords: antiretroviral therapy, ART failure, finance, HIV cascade.
Introduction
The HIV treatment cascade is used to model the stages of the HIV care continuum, namely HIV diagnosis, engagement in HIV healthcare, receiving antiretroviral therapy (ART) and achieving and maintaining a suppressed HIV viral load.1 Based on surveillance data to December 2017, it is estimated that 27 545 people are living with HIV in Australia. Of these, an estimated 89% are diagnosed, 85% are retained in care, 78% are receiving ART and 74% have achieved viral suppression.2 The Joint United Nations Programme on HIV/AIDS (UNAIDS) ‘90–90–90’ targets are that 90% of patients with HIV are diagnosed, 90% of those diagnosed are treated with ART and 90% of those treated are virologically suppressed.3 Public health policies and community organisation campaigns are implemented to improve outcomes at each step of the HIV treatment cascade.
If the goal of successful ART is virological suppression, regression in the treatment cascade at the individual (patient) level (e.g. from virological suppression to stopping ART) could be considered a poor treatment outcome. For patients who have initiated ART after a confirmed HIV diagnosis, poor outcomes could comprise not being on ART or being on ART but experiencing virological failure (i.e. a detectable HIV viral load). Improving the HIV cascade in Australia requires increasing the number of patients retained in care with sustained viral suppression. Furthermore, attention should be directed towards improving health and quality of life outcomes, a proposed ‘fourth 90’.4 This refers to the importance of maintaining high quality of life of people with HIV, which could be achieved by preventing progression to AIDS5 or the development of a serious non-AIDS event (SNAE).6
Although socioeconomic disadvantage has been shown to be associated with suboptimal HIV outcomes, this has primarily been studied in low- and middle-income countries.7 In high-income settings, many studies examining the relationship between socioeconomic indicators and virological outcomes were conducted before recommendations of lifelong ART,8,9 or before single-tablet ART,10–14 and may be less relevant in the current ART era. More recent longitudinal studies in high-income settings have found lower household income to be associated with virologic failure.15 In addition, some, but not all, studies showed that lower education was related to worse virologic outcomes.16,17 Studies examining covariates of adherence or treatment outcomes often limit eligibility to specific groups or populations, for example enrolling only people who inject drugs11,12 or women,15 or excluding men who have sex with men.17 This may limit the generalisability of findings.
In the contemporary ART era, most studies assessing associations between socioeconomic factors and HIV treatment outcomes have been conducted in the US. The effects of socioeconomic status may be greater in the US than in high-income countries with universal health care.18 Nevertheless, an Australian single-site cross-sectional survey reported that 30% of patients who had difficulties meeting pharmacy dispensing costs had ceased ART.19 However, that self-report survey was completed anonymously and could not be linked to clinical endpoints. It remains unknown whether socioeconomic covariates are associated with HIV treatment outcomes in a high-income country with publicly subsidised health care. Therefore, the aim of the present study was to assess associations between socioeconomic and psychosocial variables and poor HIV treatment outcomes in Australia, a high-income setting with subsidised health care.
Methods
We implemented a case-control study of adult participants living with HIV through St Vincent’s Public Hospital, Sydney, Australia, from 2014 to 2016. All eligible patients that study staff were aware of (e.g. through hospital admission systems and out-patient clinics) were approached and offered participation in the study. Participants were defined as eligible cases if, at the time of entry, they had one or more of the following: not taking ART, a detectable HIV viral load at ≥50 copies mL–1 or hospitalised with an AIDS-defining illness or a new SNAE.6 Participants were matched to controls enrolled at the same site in a nationwide cohort study of HIV-infected adults on ART with an undetectable viral load (<20 copies mL–1) at study entry and on stable ART for at least 3 months.20 Participants were matched for site of enrolment (the controls from the larger cohort were selected from the same site as the cases) and sex.
All participants completed the same study assessments, with participants who were enrolled as cases completing a single study visit that mirrored the baseline visit completed by the controls. The visit consisted of completing a study questionnaire and a cognitive screening test. The participant self-completed questionnaire incorporated a series of measures assessing sociodemographics, financial and employment status, health care and treatment access, physical health, mental health, quality of life, drug and alcohol use, life stressors, social supports, HIV disclosure, HIV stigma, ART regimen (use, side effects and adherence), concomitant medication use and ART-related necessity beliefs and concerns.19,21–30 Participants completed a brief neurocognitive screening (CogState).31 Data collected by study coordinators included medical and HIV history, SNAEs,6 comorbidities, sexually transmissible infections and laboratory data. The study assessments are described in detail elsewhere.20
Human research ethics approval was obtained from the St Vincent’s Hospital Human Research and Ethics Committee. All participants provided written informed consent before enrolment. Participants were offered an A$20 meal voucher in return for participation.
Sample size calculation and statistical analysis
We initially planned for inclusion of 37 cases and 111 controls to enable detection, with 90% power, of an odds ratio (OR) of ≥3.5, with a target ratio of 1 : 3 cases to controls. This was based on the assumption that financial strain (as a marker of socioeconomic disadvantage) would lead to poorer HIV treatment outcomes (as defined) in approximately 29% of cases, based on data derived from a separate sample enrolled at the same site.19
We purposefully assessed a range of socioeconomic and psychosocial variables identified in the literature, testing these for associations in bivariate analyses comparing cases and controls using a significance level of P < 0.05 (two-tailed). The significance of differences in continuous variables normally distributed in each group was analysed using independent samples t-tests. The significance of differences in non-normally distributed continuous variables was assessed using the Mann–Whitney U-test. The significance of differences in categorical variables was analysed using Chi-squared tests, and ORs were generated with a 95% confidence intervals (CIs). Life stressors and quality of life scores were dichotomised according to their mean and median value respectively.
Socioeconomic and psychosocial variables significantly associated with poor outcomes in bivariate analyses were entered in an initial conditional multivariable logistic regression model. A forced-entry stepwise hierarchical model reduction approach was used to identify independent covariates. Length of time living with HIV (recoded as a binary variable using median split: diagnosed before 1999 vs diagnosed since 1999) was included and retained throughout model reduction. Socioeconomic and psychosocial variables significantly associated with poor outcomes in the final model were considered independently associated with poor HIV treatment outcomes as defined. All statistical analyses were conducted using IBM SPSS Statistics Version 23.0 (IBM Corp., Armonk, NY, USA).
Unless indicated otherwise, data are presented as the mean ± s.d.
Results
Participants
Thirty-nine cases were enrolled and compared with 120 controls. The characteristics of the cases are presented in Table 1. There were no significant differences between the cases and controls in terms of age (50.3 ± 9.7 vs 51.4 ± 11.6 years respectively) or sex (37 (94.9%) vs 116 (6.7%) male respectively). Significant differences between cases and controls in bivariate analyses were found for financial assistance required for cost-of-living expenses (56.4% (n = 22) vs 25.8% (n = 31) respectively), receiving income from social benefits (61.5% (n = 24) vs 35% (n = 42) respectively) and cost being a barrier to accessing HIV care (28.2% (n = 11) vs 9.2% (n = 11) respectively).

|
Thirty-five cases (90%) had a viral load >50 copies mL–1 (either not taking ART or on ART with virologic failure). Nineteen of 39 cases (48.7%) had previously recorded viral suppression since ART initiation. No case met all four inclusion criteria; 10 cases (25.6%) met three criteria, 19 (48.7%) met two criteria and 10 (25.6%) only met one criterion. Ten cases (25.6%) were recruited while they were hospital in-patients. No control had an active AIDS-defining illness, although 39 (32.5%) had a history of an AIDS-defining illness. No controls were hospitalised. Seventy controls (58.3%) had a known comorbidity, mostly cardiovascular disease (n = 33 (27.5%)).
Multivariable analysis
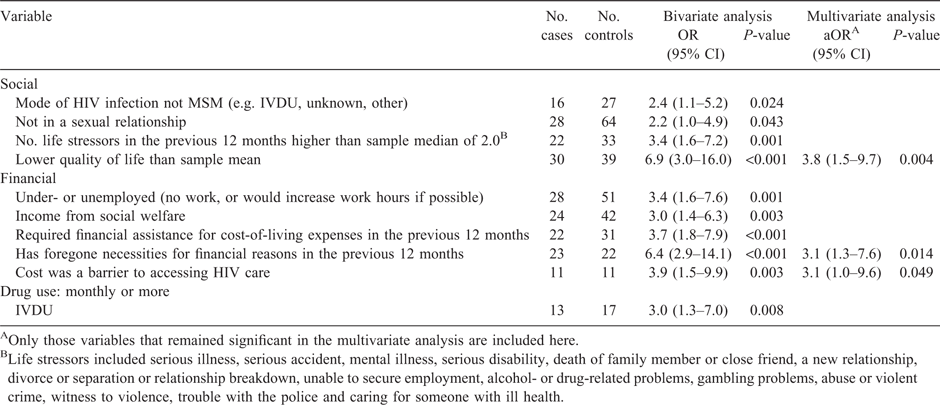
Ten socioeconomic and psychosocial variables examined were significantly associated with poor outcomes as defined (i.e. with being a case) in bivariate analysis: mode of HIV transmission, not being in a sexual relationship, two or more life stressors in the previous 12 months, quality of life lower than the sample mean, being under- or unemployed, income from social benefits, foregoing necessities for financial reasons, requiring financial assistance for cost-of-living expenses in the previous 12 months, cost as a barrier to accessing HIV care and injection drug use (see Table 2).
Variables significant in bivariate analyses were entered into a multivariable model that was statistically significant (χ102 = 46.175, P < 0.001), correctly classified 79.9% of cases and explained 37.5% of the variance (Nagelkerke R2) in poor treatment outcomes. Three covariates were independently associated with poor treatment outcomes: foregoing necessities for financial reasons (adjusted OR (aOR) 3.1, 95% CI 1.3–7.6, P = 0.014), cost barriers to accessing HIV care (aOR 3.1, 95% CI 1.0–9.6, P = 0.049) and lower quality of life (aOR 3.8, 95% CI 1.5–9.7, P = 0.004).
Discussion
In this study, three socioeconomic and psychosocial covariates were independently associated with poor treatment outcomes: foregoing necessities for financial reasons, cost barriers to accessing HIV care and low quality of life. Importantly, poor treatment outcomes encompassed HIV viraemia, as well as not taking ART or hospitalisation with an AIDS-defining illness or new SNAE.
The association of socioeconomic variables, notably financial strain and cost barriers to accessing HIV care (e.g. pharmaceutical copayments and medical practitioner gap payments), with poor treatment outcomes is consistent with previous Australian findings that patients in a hospital out-patient setting who have difficulty paying for ART-related out-of-pocket expenses are more likely to cease ART and that a smaller subset of these patients also had difficulty meeting travel costs to attend the clinic.19 Despite the importance of financial constraints, less than 5% of patients in the previous study were asked by healthcare workers whether they had difficulty meeting these expenses.19 Further attesting to the importance of socioeconomic factors in HIV treatment outcomes, a large cross-sectional study in the UK, a country with a national health system similar to that in Australia, found that virological failure was most likely in participants experiencing the highest rate of financial hardship.16 In addition, a systematic review found an association between unemployment and virological non-suppression or changes in viral load in four of five included studies.18
In the present study, 100% of the control participants and 77% of the case participants were enrolled before a state-wide abolition of the pharmaceutical copayment for ART for HIV. Prior to its removal, this was A$37.70 for full paying patients per medication, capped at A$1494.90 annually. This policy change may have resulted in a reduction in the cost barriers to accessing HIV care, and further studies since the policy change are required.
Some social disadvantages may contribute to vulnerability to HIV, including social disparities in health (e.g. environmental resources or constraints, socioeconomic position, access to care).32 From our data, it is not possible to tell whether a patient’s HIV serostatus contributed to their socioeconomic disadvantage or whether the disadvantage became more pronounced following their HIV diagnosis.
Lower self-reported quality of life was also associated with poor treatment outcomes. As with all associations, we are unable to infer the direction of this association. It may be that as patients become more unwell, with detectable virus and opportunistic infections, there is a decline in quality of life. However, it may also be that those with a lower quality of life are less likely to adhere to treatments or engage in their health care. This further supports the incorporation of the ‘fourth 90’ (representing good health-related quality of life).4 It has been proposed that the ‘fourth 90’ encompass comorbidities and self-perceived quality of life, a model of person-centred chronic care acknowledging that virological suppression is not the only measure of successful treatment.4
The present study has several limitations. Recruitment was at a single hospital site, and predominantly male patients were enrolled; hence, the findings are not necessarily generalisable to other settings and populations (e.g. women or youth) in Australia or elsewhere in high-income countries. Recruitment was challenging in this patient population, because many potential participants were disengaged from care, unwell or had limited ability to consent to participate because of substance use. This may have compounded the potential for selection bias.
The sample of cases in this study was relatively small (n = 39), and this is reflected in wide CIs. Statistical power for the study was set to detect an OR of 3.5, which may have limited the possibility of detecting smaller effects. Future research would benefit from a larger sample size, which would have allowed analysis of the separate components of the composite endpoint encompassing not being on ART, virological failure while on ART or hospitalisation due to an ADC or a new SNAE. In addition, other statistical techniques could be used with a larger sample, notably principle component analysis to examine interitem correlations and establish multi-item scales that contribute a lower risk of multicollinearity.33–36 Further, larger studies in other settings are required.
Conclusions
This study found that, in a high-income setting with a universal healthcare system providing highly subsidised health care and pharmaceuticals, socioeconomic and psychosocial variables are associated with poorer treatment outcomes in adults with HIV. Targeted policy and interventions that ensure the provision of financial and social support to those who need it likely contributes to mitigating critical socioeconomic and psychosocial barriers to successful HIV treatment. This, in turn, may contribute to increasing the number of people with HIV who are on ART, promote sustained viral suppression, and reduce hospitalisations due to ADC or new SNAEs.
Conflicts of interest
Krista J. Siefried has received a fellowship grant from Gilead Sciences, travel and conference sponsorships from Gilead Sciences and an Australian Government Research Training Program (RTP) Scholarship. John McAllister has received lecture fees from ViiV Healthcare and conference and travel sponsorships from ViiV Healthcare and MSD. John de Wit has received lecture sponsorship from BMS Australia. Andrew Carr has received research funding from BMS, Gilead Sciences and ViiV Healthcare; consultancy fees from Gilead Sciences and ViiV Healthcare; lecture and travel sponsorships from BMS, Gilead Sciences and ViiV Healthcare; and has served on advisory boards for Gilead Sciences, MSD, and ViiV Healthcare. The remaining authors have no potential conflicts of interest to declare.
Acknowledgements
The authors thank all the study participants for their time. This study was funded by a Gilead Fellowship Grant and The Balnaves Foundation.
References
[1] Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52 793–800.| The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection.Crossref | GoogleScholarGoogle Scholar | 21367734PubMed |
[2] The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2018. Sydney: University of New South Wales; 2018.
[3] The Joint United Nations Programme on HIV/AIDS (UNAIDS). 90–90–90: an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014.
[4] Lazarus JV, Safreed-Harmon K, Barton SE, Costagliola D, Dedes N, Del Amo Valero J, et al. Beyond viral suppression of HIV – the new quality of life frontier. BMC Med 2016; 14 94
| Beyond viral suppression of HIV – the new quality of life frontier.Crossref | GoogleScholarGoogle Scholar | 27334606PubMed |
[5] Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2018. Available online at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf [verified 19 June 2019].
[6] Lifson AR, Belloso WH, Davey RT, Duprez D, Gatell JM, Hoy JF, et al. Development of diagnostic criteria for serious non-AIDS events in HIV clinical trials. HIV Clin Trials 2010; 11 205–19.
| Development of diagnostic criteria for serious non-AIDS events in HIV clinical trials.Crossref | GoogleScholarGoogle Scholar | 20974576PubMed |
[7] McNairy ML, Abrams EJ, Rabkin M, El-Sadr WM. Clinical decision tools are needed to identify HIV-positive patients at high risk for poor outcomes after initiation of antiretroviral therapy. PLoS Med 2017; 14 e1002278
| Clinical decision tools are needed to identify HIV-positive patients at high risk for poor outcomes after initiation of antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 29112963PubMed |
[8] Pence BW, Ostermann J, Kumar V, Whetten K, Thielman N, Mugavero MJ. The influence of psychosocial characteristics and race/ethnicity on the use, duration, and success of antiretroviral therapy. J Acquir Immune Defic Syndr 2008; 47 194–201.
| The influence of psychosocial characteristics and race/ethnicity on the use, duration, and success of antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 17971712PubMed |
[9] Zaragoza-Macias E, Cosco D, Nguyen ML, Del Rio C, Lennox J. Predictors of success with highly active antiretroviral therapy in an antiretroviral-naive urban population. AIDS Res Hum Retroviruses 2010; 26 133–8.
| Predictors of success with highly active antiretroviral therapy in an antiretroviral-naive urban population.Crossref | GoogleScholarGoogle Scholar | 20156096PubMed |
[10] Legarth R, Omland LH, Kronborg G, Larsen CS, Pedersen C, Gerstoft J, et al. Educational attainment and risk of HIV infection, response to antiretroviral treatment, and mortality in HIV-infected patients. AIDS 2014; 28 387–96.
| Educational attainment and risk of HIV infection, response to antiretroviral treatment, and mortality in HIV-infected patients.Crossref | GoogleScholarGoogle Scholar | 24670524PubMed |
[11] Milloy MJ, Kerr T, Bangsberg DR, Buxton J, Parashar S, Guillemi S, et al. Homelessness as a structural barrier to effective antiretroviral therapy among HIV-seropositive illicit drug users in a Canadian setting. AIDS Patient Care STDS 2012; 26 60–7.
| Homelessness as a structural barrier to effective antiretroviral therapy among HIV-seropositive illicit drug users in a Canadian setting.Crossref | GoogleScholarGoogle Scholar | 22107040PubMed |
[12] Milloy MJ, Kerr T, Buxton J, Rhodes T, Krusi A, Guillemi S, et al. Social and environmental predictors of plasma HIV RNA rebound among injection drug users treated with antiretroviral therapy. J Acquir Immune Defic Syndr 2012; 59 393–9.
| Social and environmental predictors of plasma HIV RNA rebound among injection drug users treated with antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 22134149PubMed |
[13] Shacham E, Nurutdinova D, Onen N, Stamm K, Overton ET. The interplay of sociodemographic factors on virologic suppression among a U.S. outpatient HIV clinic population. AIDS Patient Care STDS 2010; 24 229–35.
| The interplay of sociodemographic factors on virologic suppression among a U.S. outpatient HIV clinic population.Crossref | GoogleScholarGoogle Scholar | 20397898PubMed |
[14] Simoni JM, Yard SS, Huh D. Prospective prediction of viral suppression and immune response nine months after ART initiation in Seattle, WA. AIDS Care 2013; 25 181–5.
| Prospective prediction of viral suppression and immune response nine months after ART initiation in Seattle, WA.Crossref | GoogleScholarGoogle Scholar | 22639986PubMed |
[15] McFall AM, Dowdy DW, Zelaya CE, Murphy K, Wilson TE, Young MA, et al. Understanding the disparity: predictors of virologic failure in women using highly active antiretroviral therapy vary by race and/or ethnicity. J Acquir Immune Defic Syndr 2013; 64 289–98.
| Understanding the disparity: predictors of virologic failure in women using highly active antiretroviral therapy vary by race and/or ethnicity.Crossref | GoogleScholarGoogle Scholar | 23797695PubMed |
[16] Burch L, Smith C, Anderson J, Sherr L, Rodger A, O’Connell R, et al. Socio-economic factors and virological suppression among people diagnosed with HIV in the United Kingdom: results from the ASTRA study. J Int AIDS Soc 2014; 17 19533
| Socio-economic factors and virological suppression among people diagnosed with HIV in the United Kingdom: results from the ASTRA study.Crossref | GoogleScholarGoogle Scholar | 25394042PubMed |
[17] Rosin C, Elzi L, Thurnheer C, Fehr J, Cavassini M, Calmy A, et al. Gender inequalities in the response to combination antiretroviral therapy over time: the Swiss HIV Cohort Study. HIV Med 2015; 16 319–25.
| Gender inequalities in the response to combination antiretroviral therapy over time: the Swiss HIV Cohort Study.Crossref | GoogleScholarGoogle Scholar | 25329751PubMed |
[18] Burch LS, Smith CJ, Phillips AN, Johnson MA, Lampe FC. Socioeconomic status and response to antiretroviral therapy in high-income countries: a literature review. AIDS 2016; 30 1147–61.
| Socioeconomic status and response to antiretroviral therapy in high-income countries: a literature review.Crossref | GoogleScholarGoogle Scholar | 26919732PubMed |
[19] McAllister J, Beardsworth G, Lavie E, Macrae K, Carr A. Financial stress is associated with reduced treatment adherence in HIV-infected adults in a resource-rich setting. HIV Med 2013; 14 120–4.
| Financial stress is associated with reduced treatment adherence in HIV-infected adults in a resource-rich setting.Crossref | GoogleScholarGoogle Scholar | 22780330PubMed |
[20] Siefried KJ, Mao L, Kerr S, Cysique LA, Gates TM, McAllister J, et al. Socioeconomic factors explain suboptimal adherence to antiretroviral therapy among HIV-infected Australian adults with viral suppression. PLoS One 2017; 12 e0174613
| Socioeconomic factors explain suboptimal adherence to antiretroviral therapy among HIV-infected Australian adults with viral suppression.Crossref | GoogleScholarGoogle Scholar | 28369066PubMed |
[21] Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16 606–13.
| The PHQ-9: validity of a brief depression severity measure.Crossref | GoogleScholarGoogle Scholar | 11556941PubMed |
[22] Mao L, Kippax SC, Newman CE, Andrews G, Rogers G, Saltman DC, et al. Rates of depression among men attending high-HIV-caseload general practices in Australia. Ment Health Fam Med 2008; 5 79–83.
| 22477852PubMed |
[23] Duracinsky M, Herrmann S, Berzins B, Armstrong AR, Kohli R, Le Coeur S, et al. The development of PROQOL-HIV: an international instrument to assess the health-related quality of life of persons living with HIV/AIDS. J Acquir Immune Defic Syndr 2012; 59 498–505.
| The development of PROQOL-HIV: an international instrument to assess the health-related quality of life of persons living with HIV/AIDS.Crossref | GoogleScholarGoogle Scholar | 22205438PubMed |
[24] Knobel H, Alonso J, Casado JL, Collazos J, Gonzalez J, Ruiz I, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study. AIDS 2002; 16 605–13.
| Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study.Crossref | GoogleScholarGoogle Scholar | 11873004PubMed |
[25] Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA 1984; 252 1905–7.
| Detecting alcoholism: the CAGE questionnaire.Crossref | GoogleScholarGoogle Scholar | 6471323PubMed |
[26] Guck TP, Goodman MD, Dobleman CJ, Fasanya HO, Tadros MB. Relationship between acceptance of HIV/AIDS and functional outcomes assessed in a primary care setting. AIDS Care 2010; 22 89–95.
| 20390485PubMed |
[27] Horne R, Buick D, Fisher M. Doubts about necessity and concerns about adverse effects: identifying the types of beliefs that are associated with non-adherence to HAART. Int J STD AIDS 2004; 15 38–44.
| Doubts about necessity and concerns about adverse effects: identifying the types of beliefs that are associated with non-adherence to HAART.Crossref | GoogleScholarGoogle Scholar | 14769170PubMed |
[28] Prestage G, Mao L, Kippax S, Jin F, Hurley M, Grulich A, et al. Use of viral load to negotiate condom use among gay men in Sydney, Australia. AIDS Behav 2009; 13 645–51.
| Use of viral load to negotiate condom use among gay men in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 19199021PubMed |
[29] Prestage G, McCann P, Hurley M, Bradley J, Down I, Brown G. Pleasure and sexual health: the PASH study. Sydney: The National Centre in HIV Epidemiology and Clinical Research; 2009.
[30] Stutterheim SE, Pryor JB, Bos A, Hoogendjik R, Muris P, Schaalma H. HIV-related stigma and psychological stress: the harmful effects of specific stigma manifestations in various social settings. AIDS 2009; 23 2353–7.
| HIV-related stigma and psychological stress: the harmful effects of specific stigma manifestations in various social settings.Crossref | GoogleScholarGoogle Scholar | 19741478PubMed |
[31] Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol 2009; 24 165–78.
| Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex.Crossref | GoogleScholarGoogle Scholar | 19395350PubMed |
[32] Pellowski JA, Kalichman SC, Matthews KA, Adler N. A pandemic of the poor: social disadvantage and the U.S. HIV epidemic. Am Psychol 2013; 68 197–209.
| A pandemic of the poor: social disadvantage and the U.S. HIV epidemic.Crossref | GoogleScholarGoogle Scholar | 23688088PubMed |
[33] Tabachnick BG, Fidell LS. Using multivariate statistics. 6th edn. Boston: Allyn & Bacon; 2012.
[34] Pearson RH, Mundform DJ. Recommended sample size for conducting exploratory factor analysis on dichotomous data. J Mod Appl Stat Methods 2010; 9 359–68.
| Recommended sample size for conducting exploratory factor analysis on dichotomous data.Crossref | GoogleScholarGoogle Scholar |
[35] Comrey AL, Lee HB. A first course in factor analysis. Hillsdale: L. Erlbaum Associates; 1992.
[36] Kass RA, Tinsley HEA. Factor analysis. J Leis Res 1979; 11 120–38.
| Factor analysis.Crossref | GoogleScholarGoogle Scholar |