Self-reported anal symptoms and their association with anal pathology among gay and bisexual men: a cross-sectional observational analysis
Sian L. Goddard A B M , I. Mary Poynten A , Kathy Petoumenos C , Fengyi Jin A , Richard J. Hillman D , Carmella Law D , Jennifer M. Roberts E , Christopher K. Fairley F G , Suzanne M. Garland H I J , Andrew E. Grulich A , David J. Templeton A K L and on behalf of the Study of the Prevention of Anal Cancer (SPANC) Research Team
A B M , I. Mary Poynten A , Kathy Petoumenos C , Fengyi Jin A , Richard J. Hillman D , Carmella Law D , Jennifer M. Roberts E , Christopher K. Fairley F G , Suzanne M. Garland H I J , Andrew E. Grulich A , David J. Templeton A K L and on behalf of the Study of the Prevention of Anal Cancer (SPANC) Research Team
A HIV Epidemiology and Prevention Program, The Kirby Institute, UNSW Sydney, Level 6,Wallace Wurth Building, High Street, Sydney, NSW 2052, Australia.
B Infection and Immunity, Ambrose King Centre, Barts Health NHS Trust, London, E1 2BB, UK.
C Biostatistics and Databases Program, The Kirby Institute, UNSW Sydney, Sydney, NSW 2052, Australia.
D Dysplasia and Anal Cancer Services, HIV Immunology and Infectious Disease Department, St Vincent’s Hospital, Sydney, NSW 2010, Australia.
E Cytology Department, Douglass Hanly Moir Pathology, Sydney, NSW 2113, Australia.
F Central Clinical School, Monash University, Melbourne, Vic. 3044, Australia.
G Melbourne Sexual Health Centre, Melbourne, Vic. 3053, Australia.
H Centre for Women’s infectious Diseases, Royal Women’s Hospital, Melbourne, Vic. 3052, Australia.
I Infection Immunity, Murdoch Children’s Research Institute, Parkville, Vic. 3052, Australia.
J Department of Obstetrics and Gynaecology, The University of Melbourne, Parkville, Vic. 3052, Australia.
K Department of Sexual Health Medicine and Sexual Assault Medicine, Sydney Local Health District, Camperdown, NSW 2050, Australia.
L Sydney Medical School, The University of Sydney, NSW 2006, Australia.
M Corresponding author. Email: sgoddard@kirby.unsw.edu.au
Sexual Health 18(2) 123-129 https://doi.org/10.1071/SH20104
Submitted: 13 June 2020 Accepted: 9 September 2020 Published: 12 March 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
Background: Anal symptoms may indicate serious pathology. Receptive anal intercourse (RAI) and sexually transmissible infections (STIs) may contribute to a higher prevalence of symptoms among gay and bisexual men (GBM). This study investigated associations with anal symptoms among GBM. Methods: The Study of the Prevention of Anal Cancer was a longitudinal study of anal human papillomavirus and related lesions in Sydney, Australia. GBM aged ≥35 years were recruited from community settings between September 2010 and August 2015. Information about anal symptoms (discharge, itch, pain defecating, lump, bleeding, ‘sores’, tearing, tenesmus), STIs and sexual behaviours was collected. High-resolution anoscopy (HRA) and STI testing were performed. Logistic regression analyses on baseline data were performed to assess associations with each symptom. Results: Among 616 participants (median age 49 years, 35.9% HIV positive), 35.3% reported at least one anal symptom within the past week and 65.3% were diagnosed with fistula, fissure, ulcer, warts, haemorrhoids and/or perianal dermatoses at HRA. Anal symptoms were not associated with anal chlamydia, gonorrhoea, warts or syphilis. Self-reported ‘sores’ were associated with previous anal herpes simplex virus (HSV; P < 0.001). ‘Sores’ (P < 0.001), itch (P = 0.019), discharge (P = 0.032) and lump (P = 0.028) were independently associated with ulceration. Among participants diagnosed with fissure, fistulae, haemorrhoids and perianal dermatoses, 61.9%, 100%, 62.0% and 63.9% respectively were asymptomatic. Only self-reported anal tear was independently associated with recent RAI. Conclusions: Previous anal HSV was the only STI associated with any symptom. Anal pathology was highly prevalent, but often asymptomatic. Anal symptoms do not appear to be useful markers of most anal pathology in GBM.
Keywords: anal sex, gay men, prevalence, sexually transmissible infections.
Introduction
Anal symptoms are extremely common1 and are sometimes associated with serious pathology.2 Although a frequent reason for presentation to healthcare services,3,4 anal symptoms remain underreported to clinicians.1,5–7 One in two gay and bisexual men (GBM) sampled from two community-recruited studies in Sydney, Australia, reported anal symptoms within the past year when questioned directly.8
The Study of the Prevention of Anal Cancer (SPANC) was a prospective cohort study of anal human papillomavirus (HPV) and related lesions among older GBM in Sydney, Australia. There was a high prevalence of anal symptoms among SPANC participants,9 and we hypothesised that receptive anal sexual practices10,11 and a high prevalence of anal sexually transmissible infections (STIs)12 may contribute to anal symptoms among GBM. To the best of our knowledge, no published analysis has considered a broad range of potential causes and associations with anal symptoms among GBM or shown how self-reported symptoms relate to abnormal anal findings. We aimed to investigate the predictors of anal symptoms and their association with anal pathology among SPANC participants.
Methods
Study population and procedures
The SPANC study design and methods have been published previously.13 Briefly, GBM aged ≥35 years who reported previous sex with another man were recruited (mainly via gay community organisations, social events and gay media) between September 2010 and August 2015. Because of the known higher prevalence of anal HPV and anal cancer among HIV-positive men who have sex with men (MSM), the aim was to recruit three HIV-negative participants for every two HIV-positive participants. Men who had undergone previous high-resolution anoscopy (HRA) or had been diagnosed with anal cancer, were excluded. Self-reported symptoms did not affect study eligibility. At baseline, participants completed a computer-assisted self-interview (Questionnaire Development System (QDS); NOVA Research Company, Bethesda, MD, USA). An anal swab was collected for cytology and participants were tested for anal chlamydia and gonorrhoea, as well as syphilis.12 HRA was performed on all participants, with biopsy of any suspected HPV-associated lesion. A standardised data collection tool was used to record the location of any abnormality. For this analysis, a diagnosis of warts was based on visual inspection. Haemorrhoids were recorded as present or absent, but were not graded by severity.
Statistical analysis
The prevalence of each of the anal symptoms and a composite outcome of ‘at least one symptom’ within the past week, past month and past 6 months was calculated. Pearson’s Chi-squared test was used to compare the prevalence of anal symptoms among HIV-negative and -positive participants. The prevalence of each anal pathology diagnosed during HRA was calculated and Pearson’s Chi-squared tests were used to compare the prevalence of anal pathology among HIV-negative and -positive participants.
Logistic regression analyses were performed to evaluate the univariable association of each predictor variable with the presence of each anal symptom within the past week only. Covariates with P < 0.2 in univariable analysis were included in the multivariable model and removed in a backward stepwise fashion.
Data analyses were performed using STATA version 14.2 (StataCorp, College Station, TX, USA).
Ethics approval
Ethics approval for the SPANC study was obtained from the St Vincent’s Hospital Ethics Committee (HREC/09/SVH/168). All participants provided informed consent.
Results
Of 617 SPANC participants seen at baseline, one person did not tolerate HRA and was excluded from the present analysis. Among the remaining 616 participants, the median age was 49 years (range 35–79 years) and almost all (n = 602; 97.7%) self-identified as ‘gay’, ‘bisexual’ or ‘homosexual’. One-third (n = 221; 35.9%) were HIV-positive and 93.6% (n = 206) were taking antiretroviral therapy. Among the 208 HIV-positive participants with available data, 135 (64.9%) reported their most recent CD4 count to be >500 cells mm–3, 63 (30.3%) reported a CD4 count of 201–500 cells mm–3 and 10 (4.8%) reported a CD4 count of <200 cells mm–3.
Receptive anal intercourse (RAI) within the past week was reported by 192 (31.4%) participants. Other anoreceptive sexual behaviours reported within the past week included rimming (n = 132; 21.5%), fingering (n = 130; 21.1%), fisting (n = 13; 2.1%) and sex toy use (n = 48; 7.8%). When comparing HIV-positive and -negative participants, there was no significant difference between the reported frequencies of these sexual behaviours (data not shown).
Approximately one-fifth (n = 115; 19.2%) of participants reported they had previously been diagnosed with anal herpes simplex virus (HSV), of whom 51 (44.4%) reported an episode within the past year. A previous diagnosis of anal HSV was more frequent among HIV-positive than HIV-negative participants (29.8% vs 13.3%; P < 0.001). As reported previously, anal chlamydia, anal gonorrhoea and syphilis were diagnosed at the baseline SPANC visit in 14 (2.3%), 3 (0.5%) and 5 (0.9%) participants respectively.12
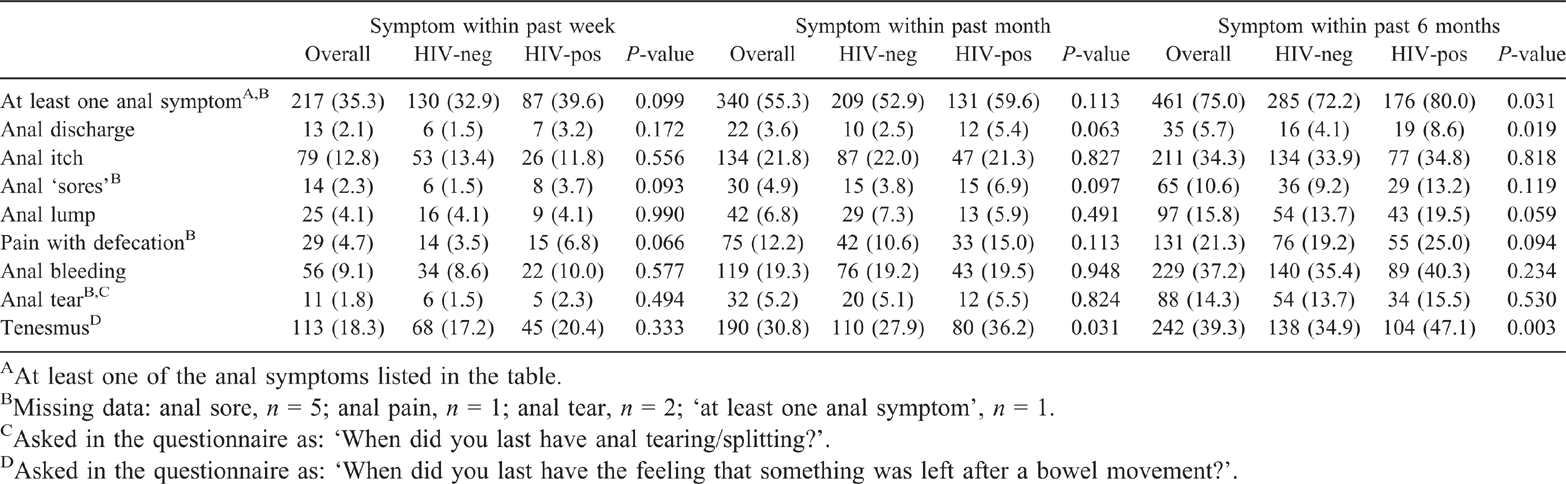
The self-reported history of anal symptoms within the past week, past month and past 6 months is presented in Table 1. One-third of participants reported at least one anal symptom within the past week. The most frequently reported anal symptoms within the past week were tenesmus (18.3%), itch (12.8%) and bleeding (9.1%).
Compared with HIV-negative participants, a slightly higher proportion of HIV-positive participants reported at least one anal symptom within the past week, past month and past 6 months, but this was only statistically significant for symptoms reported within the past 6 months (80.0% vs 72.2%; P = 0.031; Table 1). Similarly, the proportion of HIV-positive participants who reported anal discharge in the past week, past month and past 6 months, was twofold higher than that of HIV-negative participants. This was only significant for discharge reported within the past 6 months (8.6% vs 4.1%; P = 0.019; Table 1). The proportion of HIV-positive participants reporting anal ‘sores’ and pain with defecation within the past week, past month and past 6 months was higher among HIV-positive than HIV-negative participants, but the differences did not reach statistical significance (Table 1). Tenesmus was more common among HIV-positive than HIV-negative participants, but this was only statistically significant for tenesmus reported within the past month (36.2% vs 27.9%; P = 0.031) and past 6 months (47.1% vs 34.9%; P = 0.003).
Predictors of self-reported anal symptoms within the past week
STIs and anal symptoms
In univariable analysis, anal chlamydia, anal gonorrhoea and syphilis were not significantly associated with any anal symptom investigated. Of 14 participants diagnosed with anal chlamydia, only two (14%) reported any anal symptom (one each with bleeding and tenesmus). Of three participants with anal gonorrhoea, one reported tenesmus. None of the five participants diagnosed with syphilis reported any anal symptom.
In univariable analysis, there was no significant association between any anal symptom investigated and either peri- or intra-anal warts. The prevalence of self-reported anal discharge was over twofold higher among participants with than without intra-anal warts, but this difference did not reach statistical significance (3.6% v 1.6%; odds ratio (OR) 2.40, 95% confidence interval (CI) 0.79–7.23; P = 0.122). The prevalence of self-reported itch and lump was higher among participants with than without perianal warts, but neither difference was statistically significant (itch: 19.6% vs 12.2%, OR 1.75, 95% CI 0.84–3.66, P = 0.135; lump: 7.8% vs 3.7%, OR 2.20, 95% CI 0.73–6.69, P = 0.163).
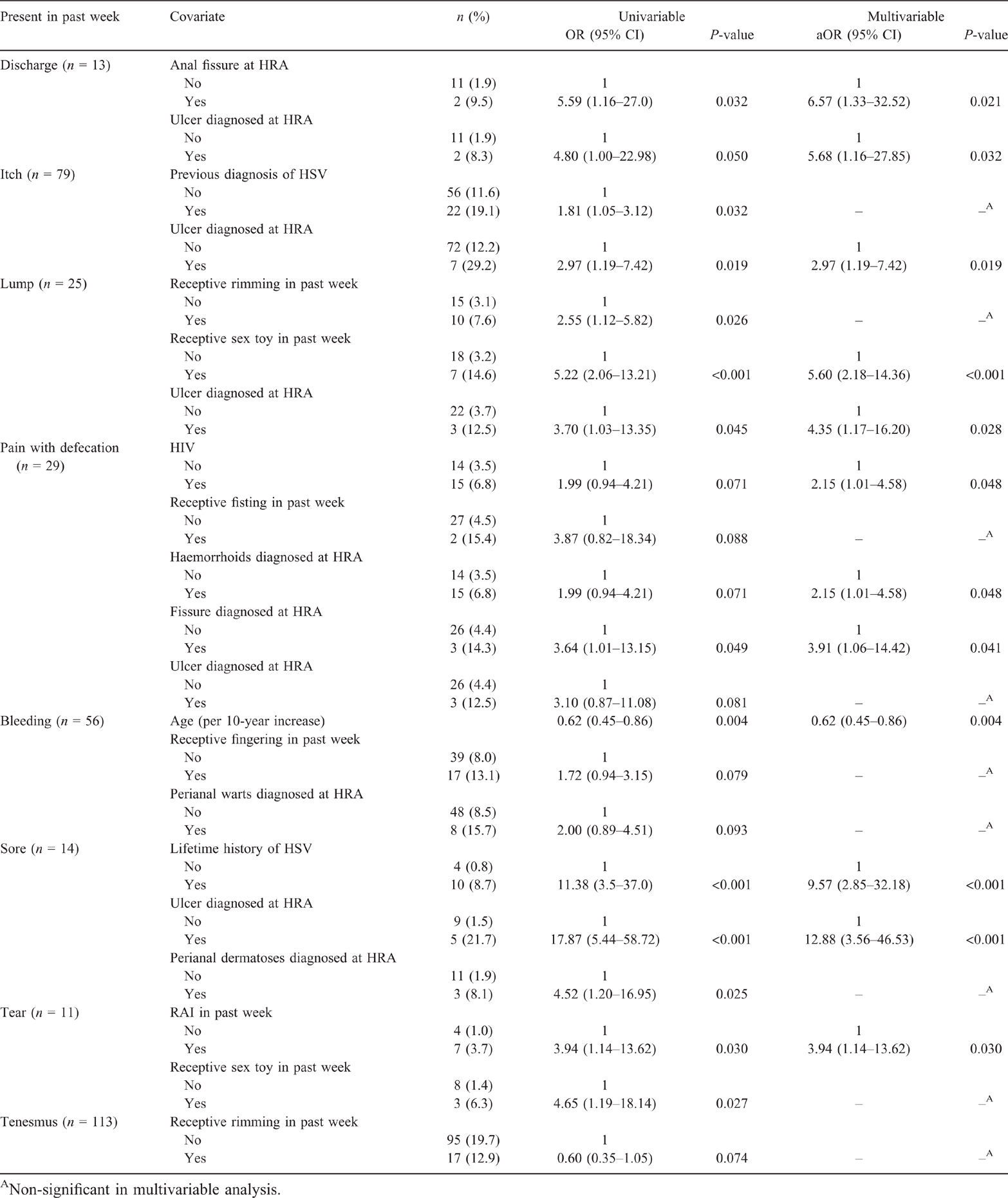
Self-reported anal ‘sores’ were independently associated with a previous diagnosis of HSV (Table 2). The significant univariable association between anal itch and a previous diagnosis of anal HSV did not persist in multivariable analysis (Table 2). When excluding 24 participants with anal ulceration detected at HRA, there was a univariable borderline significant association between anal itch and a previous diagnosis of HSV (OR 1.75, 95% CI 0.99–3.11; P = 0.056).
Sexual behaviours and anal symptoms
Among participants who did and did not report RAI in the past week, there was no significant difference in the proportion of men who reported anal bleeding (10.4% vs 8.6%; OR 1.24, 95% CI 0.70–2.20; P = 0.469) or anal pain (5.7% vs 4.3%; OR 1.35, 95% CI 0.63–2.92; P = 0.445). Self-reported anal tear was independently associated with RAI in the past week (Table 2). Self-reported anal lump was independently associated with receptive sex toy use in the past week (Table 2).
Prevalent anal pathology and anal symptoms
The prevalence of anal pathology is presented in Table 3. Approximately two-thirds (n = 402; 65.3%) of participants had at least one type of anal pathology detected at HRA. Haemorrhoids (n = 221; 35.9%) and anal warts (n = 193; 31.3%) were the most frequently diagnosed anal pathologies. Among 115 participants with a previous diagnosis of HSV, nine (7.8%) had anal ulceration detected at HRA, compared with 15 (3.1%) participants with no previous diagnosis of HSV and anal ulceration (P = 0.020). Among 24 participants with anal ulceration, nine (37.5%) had a history of anal HSV.
Among 401 participants with at least one type of anal pathology detected and with data available, there was no significant difference in the proportion who reported at least one anal symptom compared with the 214 participants without anal pathology (36.7% vs 32.7%; OR 1.19, 95% CI 0.84–1.69; P = 0.329).
Nearly two-thirds (62.0%) of participants with haemorrhoids detected at HRA did not report any anal symptom within the past week. Notably, there was also no significant association between the detection of haemorrhoids at HRA and anal bleeding in the past week (11.3% vs 7.9%; OR 1.50, 95% CI 0.86–2.61; P = 0.154). Haemorrhoids were independently associated with pain with defecation (Table 2).
In univariable and multivariable analysis, itch, lump, ‘sores’ and discharge were significantly associated with the detection of intra- and/or perianal ulceration at HRA (Table 2).
Anal discharge and pain with defecation were both independently associated with anal fissure (Table 2). However, of 21 participants with anal fissure detected at HRA, only three (14.3%) reported pain with defecation in the past week and only one (4.8%) self-reported anal tear. Most (n = 13; 61.9%) participants with anal fissure did not report any anal symptom in the past week. None of the three patients with anal fistula detected at HRA reported any anal symptom in the past week.
None of the anal symptoms investigated was independently associated with perianal dermatoses (Table 2). Most (n = 23; 63.9%) participants with perianal dermatoses diagnosed at HRA did not report any anal symptom.
Discussion
There was a high prevalence of self-reported anal symptoms at study entry among this cohort of GBM aged ≥35 years, with one-third reporting at least one anal symptom within the past week. Anal pathology was also common, with almost two-thirds of participants having an abnormality diagnosed at HRA. However, most participants diagnosed with prevalent anal pathology and/or bacterial STIs were asymptomatic. Self-reported symptoms were independently associated with both a previous diagnosis of anal HSV and anal ulceration identified at HRA. In addition, self-reported anal tear was significantly associated with recent RAI.
Compared with the one-third of SPANC participants who reported at least one anal symptom within the past week, the prevalence of anal symptoms was much lower (4.5%) in two large retrospective studies of GBM attending STI testing services in the Netherlands.14,15 This may be because retrospective studies are limited by reliance on recorded data, or because anal symptoms are often not enquired about systematically and/or disclosed during a clinical consultation.6 Three-quarters of GBM who underwent STI testing at a sexual health clinic in Australia reported anal symptoms via questionnaire, but only 16% had reported symptoms to the clinician.5 The use of a computer-assisted self-questionnaire in SPANC may also explain the high prevalence of tenesmus among SPANC participants (18% within the past week), which was substantially higher than the 4% of 165 GBM who presented to a STI testing clinic in Italy.16 However, SPANC participants were asked ‘Have you ever a feeling that something was left after a bowel movement?’, which may have contributed to an overestimation of the prevalence of tenesmus, depending on how the question was interpreted by participants who were experiencing difficulty with bowel evacuation for other reasons (e.g. as a consequence of constipation). A similar overall prevalence of anal symptoms to that reported in SPANC was found in a prospective study of anal HPV prevalence in Thailand (two-thirds within the past 6 months),17 and half of 300 community-recruited GBM from two prospective cohort studies in Australia also reported anal symptoms within the past year.8 These studies support our finding of anal symptoms being very common in this group.
Among 338 men and women (mean age 46 years) who were randomly selected from the electoral roll of a suburb in Sydney, Australia, and completed a postal survey, one in five reported anal bleeding within the past year.18 However more than one-third of SPANC participants reported bleeding in the past 6 months. Receptive anal practices could be expected to contribute to a high prevalence of anal bleeding. Approximately 40% of GBM attending a Mexican HIV testing centre reported bleeding ‘sometimes’ during RAI.11 However, in SPANC, RAI in the past week was associated with self-reported anal tear, but was not associated with anal bleeding. This is perhaps surprising because anal fissure would be expected to result in bleeding, but the number of participants reporting anal tear (n = 11) was much lower than the number reporting anal bleeding (n = 56). This could be because there are multiple possible causes of anal bleeding, including common pathology such as haemorrhoids. Compared with older participants, younger participants were more likely to report anal bleeding. A higher prevalence of anal bleeding among younger people was also reported in the postal survey of men and women in a suburb of Sydney,18 suggesting that the association between age and anal bleeding may be a true finding. Possible explanations for this could include changing physiology or a reduced likelihood of reporting symptoms with older age.19 Nevertheless, anal bleeding is a symptom that may indicate serious underlying pathology that is more common in older age groups and should prompt further investigation, yet most people with anal bleeding do not seek medical review.18,20
Although anal chlamydia, anal gonorrhoea and syphilis may cause proctitis,21 our finding that these infections were not predictive of any anal symptom is not surprising because most anal chlamydia and gonorrhoea diagnoses are asymptomatic22 and anal ulceration due to primary syphilis is often unnoticed.23 However, our ability to detect any association would also have been limited by the small number of chlamydia, gonorrhoea and syphilis study visit diagnoses in SPANC.12 As reported previously,12 the incidence of anal chlamydia and gonorrhoea was high among SPANC participants, which highlights the importance of regular STI testing among older GBM, regardless of symptoms. Although anal warts can cause pain, bleeding and itch,24 they are considered to be mostly asymptomatic.25 This was supported by our finding that no anal symptom investigated was independently associated with either intra- or peri-anal warts. In contrast, it is likely that recurrent anal HSV may cause symptoms, even in the absence of visible ulceration.26 Our findings suggest that anal itch is likely to be associated with recurrent anal HSV and consideration should be given to testing for HSV when investigating this symptom.
Nearly two-thirds of SPANC participants had at least one form of anal pathology detected. This is higher than half the HIV-positive GBM who underwent standard anoscopy while attending routine HIV care in France,10 which may be because HRA can more easily detect anal pathology under magnification. To the best of our knowledge, this is the first published study to report the prevalence of a range of anal pathology in a cohort of GBM that includes both HIV-positive and -negative participants. In SPANC, HIV-positive participants were more likely to report at least one symptom in the past 6 months. They were also more likely than HIV-negative participants to be diagnosed with anal warts. Anal warts were detected in more than one-third of HIV-positive participants, which is consistent with the findings in a cohort of HIV-positive MSM who underwent routine anal examination in France (36.5%).10 Although some anal pathology was associated with anal symptoms, anal pathology was mostly asymptomatic. For example, anal pain was independently associated with the detection of anal fissure at HRA, but nearly two-thirds of participants with fissure were asymptomatic. We hypothesise that anal health was of high importance to SPANC participants, among whom anoreceptive sexual practices were common. If true, this could contribute to the high frequency of anal symptoms reported by participants, because they may have been more likely to notice changes in the anal canal. Thus, the high proportion of asymptomatic anal pathology is even more notable in this context.
The major strength of this study was the use of a questionnaire to systematically collect data about a range of anal symptoms and their potential predictors. The detailed anal examination with HRA allowed for the detection and recording of a range of anal pathology. Recruitment of GBM from community settings allowed for an estimation of the prevalence of symptoms within the community. Although men may have been more likely to enrol in SPANC due to concerns about anal symptoms, only 11 participants (1.8%) specifically reported past or current anal symptoms as their primary motivation for participation in the study, with an additional 14 participants (2.3%) reporting past or current anal warts as the reason for participation. Our analyses may be limited by recall bias and the small numbers recorded for some variables may have limited our ability to detect associations between symptoms and pathology. Anoreceptive sexual practices (which were frequent among SPANC participants) may have resulted in anal pathology that had already been treated before the baseline SPANC visit, but the 1-week time frame used in this analysis should minimise this possibility. We were unable to investigate anal douching as a potential predictor of anal symptoms because of collinearity with receptive anal sex. In addition, we were unable to delineate the origin of anal bleeding, which may also occur from the colon. Finally, clinicians did not grade the severity of haemorrhoids20 detected at HRA, nor did they consistently document the location as intra- or peri-anal, which limits our ability to interpret any association with symptoms.
In summary, anal symptoms and pathology were highly prevalent among this cohort of older GBM. Anal bleeding in particular may warrant further investigation, yet nearly one in 10 participants reported anal bleeding in the past week and more than one-third reported bleeding within the past 6 months. No anal symptom was significantly associated with anal chlamydia, gonorrhoea, syphilis or anal warts. Self-reported anal itch should be considered a symptom of recurrent anal HSV. Receptive anal sexual practices should be considered when evaluating the cause of anal symptoms. Anal pathology was mostly asymptomatic, which suggests that anal symptoms are not reliable as a marker of most anal pathology in GBM.
Conflicts of interest
Andrew E. Grulich has received honoraria and research funding from CSL Biotherapies, honoraria and travel funding from Merck and research funding from Gilead. Christopher K. Fairley has received research funding from CSL and Merck and owns shares in CSL Biotherapies; he is also an Editor for Sexual Health. Suzanne M. Garland has received advisory board fees, grant support through her institution from Merck, lecture fees from Merck for work performed in her own time and is an active member of the Merck Global Advisory Board as well as the Merck Scientific Advisory Board. Kathy Petoumenos has received consultancy fees from ViiV Healthcare. Richard J. Hillman has received research funding from CSL, Merck and Hologic. I. Mary Poynten has received travel funding from Sequiris. The remaining authors declare no conflicts of interest.
Acknowledgements
The authors thank all the participants. In addition to the coauthors of this manuscript, the SPANC study team includes Annabelle Farnsworth, Clare Biro, Alyssa Cornall, Adele Richards, Julia Thurloe, Deborah Ekman, Ross McDonald, Marjorie Adams, Sepehr Tabrizi, Samuel Phillips, Monica Molano Luque, Simon Comben, Amber Ellis, Kirsten McCaffery, Kirsten Howard, Patrick Kelly, Daniel Seeds, Andrew Carr, Lance Feeney, Russ Gluyas, Garrett Prestage, Matthew Law, Brian Acraman, Patrick McGrath, Robert Mellor, Piero Pezzopane, Rick Varma, Julian Langton-Lockton and Winnie Tong. The SPANC study was supported by the National Health and Medical Research Council (Grant number 568971) and Cancer Council New South Wales (Grant number 13-11). Cytological testing materials were provided by Hologic Pty Ltd, Marlborough, MA, USA. The Kirby Institute is affiliated with the Faculty of Medicine, UNSW, and funded by the Australian Government of Health and Ageing.
References
[1] Tournu G, Abramowitz L, Couffignal C, Juguet F, Senejoux A, Berger S, et al Prevalence of anal symptoms in general practice: a prospective study. BMC Fam Pract 2017; 18 78| Prevalence of anal symptoms in general practice: a prospective study.Crossref | GoogleScholarGoogle Scholar | 28774265PubMed |
[2] Read TR, Huson KL, Millar JL, Haydon A, Porter IW, Grulich AE, et al Size of anal squamous cell carcinomas at diagnosis: a retrospective case series. Int J STD AIDS 2013; 24 879–82.
| Size of anal squamous cell carcinomas at diagnosis: a retrospective case series.Crossref | GoogleScholarGoogle Scholar | 23970608PubMed |
[3] Manavi K, McMillan A, Young H. The prevalence of rectal chlamydial infection amongst men who have sex with men attending the genitourinary medicine clinic in Edinburgh. Int J STD AIDS 2004; 15 162–4.
| The prevalence of rectal chlamydial infection amongst men who have sex with men attending the genitourinary medicine clinic in Edinburgh.Crossref | GoogleScholarGoogle Scholar | 15038860PubMed |
[4] Charles J, Miller G, Fahridin S. Perianal problems. Aust Fam Physician 2010; 39 365
| 20628672PubMed |
[5] Lister NA, Chaves NJ, Phang CW, Smith A, Fairley CK. Clinical significance of questionnaire-elicited or clinically reported anorectal symptoms for rectal Neisseria gonorrhoeae and Chlamydia trachomatis amongst men who have sex with men. Sex Health 2008; 5 77–82.
| Clinical significance of questionnaire-elicited or clinically reported anorectal symptoms for rectal Neisseria gonorrhoeae and Chlamydia trachomatis amongst men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 18361859PubMed |
[6] Rosa-Cunha I, Cardenas GA, Dickinson G, Metsch LR. Addressing anal health in the HIV primary care setting: a disappointing reality. AIDS Patient Care STDS 2010; 24 533–8.
| Addressing anal health in the HIV primary care setting: a disappointing reality.Crossref | GoogleScholarGoogle Scholar | 20731611PubMed |
[7] Chiu S, Joseph K, Ghosh S, Cornand RM, Schiller D. Reasons for delays in diagnosis of anal cancer and the effect on patient satisfaction. Can Fam Physician 2015; 61 e509–16.
| 26889506PubMed |
[8] Vajdic CM, van Leeuwen MT, Jin F, Prestage G, Medley G, Hillman RJ, et al Anal human papillomavirus genotype diversity and co-infection in a community-based sample of homosexual men. Sex Transm Infect 2009; 85 330–5.
| Anal human papillomavirus genotype diversity and co-infection in a community-based sample of homosexual men.Crossref | GoogleScholarGoogle Scholar | 19342375PubMed |
[9] Goddard SL, Templeton DJ, Petoumenos K, Jin F, Hillman RJ, Law C, et al Association of anal symptoms with anal high grade squamous intraepithelial lesions (HSIL) among men who have sex with men: baseline data from the study of the prevention of anal cancer (SPANC). Cancer Epidemiol 2019; 58 12–16.
| Association of anal symptoms with anal high grade squamous intraepithelial lesions (HSIL) among men who have sex with men: baseline data from the study of the prevention of anal cancer (SPANC).Crossref | GoogleScholarGoogle Scholar | 30439602PubMed |
[10] Abramowitz L, Benabderrahmane D, Baron G, Walker F, Yeni P, Duval X. Systematic evaluation and description of anal pathology in HIV-infected patients during the HAART era. Dis Colon Rectum 2009; 52 1130–6.
| Systematic evaluation and description of anal pathology in HIV-infected patients during the HAART era.Crossref | GoogleScholarGoogle Scholar | 19581857PubMed |
[11] Coplan PM, Gortmaker S, Hernandez-Avila M, Spiegelman D, Uribe-Zuniga P, Mueller NE. Human immunodeficiency virus infection in Mexico City: rectal bleeding and anal warts as risk factors among men reporting sex with men. Am J Epidemiol 1996; 144 817–27.
| Human immunodeficiency virus infection in Mexico City: rectal bleeding and anal warts as risk factors among men reporting sex with men.Crossref | GoogleScholarGoogle Scholar | 8890660PubMed |
[12] Goddard SL, Poynten IM, Petoumenous K, Jin F, Hillman RJ, Law C, et al Prevalence, incidence and predictors of anal Chlamydia trachomatis, anal Neisseria gonorrhoeae and syphilis among older gay and bisexual men in the longitudinal Study for the Prevention of Anal Cancer (SPANC). Sex Transm Infect 2019; 95 477–83.
| Prevalence, incidence and predictors of anal Chlamydia trachomatis, anal Neisseria gonorrhoeae and syphilis among older gay and bisexual men in the longitudinal Study for the Prevention of Anal Cancer (SPANC).Crossref | GoogleScholarGoogle Scholar | 31018992PubMed |
[13] Machalek DA, Grulich AE, Hillman RJ, Jin F, Templeton DJ, Tabrizi SN, et al The Study of the Prevention of Anal Cancer (SPANC): design and methods of a three-year prospective cohort study. BMC Public Health 2013; 13 946
| The Study of the Prevention of Anal Cancer (SPANC): design and methods of a three-year prospective cohort study.Crossref | GoogleScholarGoogle Scholar | 24107134PubMed |
[14] Peters RP, Verweij SP, Nijsten N, Ouburg S, Mutsaers J, Jansen CL, et al Evaluation of sexual history-based screening of anatomic sites for Chlamydia trachomatis and Neisseria gonorrhoeae infection in men having sex with men in routine practice. BMC Infect Dis 2011; 11 203
| Evaluation of sexual history-based screening of anatomic sites for Chlamydia trachomatis and Neisseria gonorrhoeae infection in men having sex with men in routine practice.Crossref | GoogleScholarGoogle Scholar | 21791061PubMed |
[15] van Liere GA, van Rooijen MS, Hoebe CJ, Heijman T, de Vries HJ, Dukers-Muijrers NH. Prevalence of and factors associated with rectal-only chlamydia and gonorrhoea in women and in men who have sex with men. PLoS One 2015; 10 e0140297
| Prevalence of and factors associated with rectal-only chlamydia and gonorrhoea in women and in men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 26713628PubMed |
[16] Foschi C, Gaspari V, Sgubbi P, Salvo M, D’Antuono A, Marangoni A. Sexually transmitted rectal infections in a cohort of ‘men having sex with men’. J Med Microbiol 2018; 67 1050–7.
| Sexually transmitted rectal infections in a cohort of ‘men having sex with men’.Crossref | GoogleScholarGoogle Scholar | 29927376PubMed |
[17] Ruanpeng D, Chariyalertsak S, Kaewpoowat Q, Supindham T, Settakorn J, Sukpan K, et al Cytological anal squamous intraepithelial lesions associated with anal high-risk human papillomavirus infections among men who have sex with men in northern Thailand. PLoS One 2016; 11 e0156280
| Cytological anal squamous intraepithelial lesions associated with anal high-risk human papillomavirus infections among men who have sex with men in northern Thailand.Crossref | GoogleScholarGoogle Scholar | 27227684PubMed |
[18] Eslick GD, Kalantar JS, Talley NJ. Rectal bleeding: epidemiology, associated risk factors, and health care seeking behaviour: a population-based study. Colorectal Dis 2009; 11 921–6.
| Rectal bleeding: epidemiology, associated risk factors, and health care seeking behaviour: a population-based study.Crossref | GoogleScholarGoogle Scholar | 19175652PubMed |
[19] Gott CM, Rogstad KE, Riley V, Ahmed-Jushuf I. Delay in symptom presentation among a sample of older GUM clinic attenders. Int J STD AIDS 1999; 10 43–6.
| Delay in symptom presentation among a sample of older GUM clinic attenders.Crossref | GoogleScholarGoogle Scholar | 10215129PubMed |
[20] Royal College of Surgeons of England. Commissioning guide: rectal bleeding. 2013. Available online at: https://www.rcseng.ac.uk/library-and-publications/rcs-publications/docs/rectal-bleeding-guide/ [verified 21 October 2020].
[21] Klausner JD, Kohn R, Kent C. Etiology of clinical proctitis among men who have sex with men. Clin Infect Dis 2004; 38 300–2.
| Etiology of clinical proctitis among men who have sex with men.Crossref | GoogleScholarGoogle Scholar | 14699467PubMed |
[22] Kent CK, Chaw JK, Wong W, Liska S, Gibson S, Hubbard G, et al Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis 2005; 41 67–74.
| Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003.Crossref | GoogleScholarGoogle Scholar | 15937765PubMed |
[23] Read PJ, Donovan B. Clinical aspects of adult syphilis. Intern Med J 2012; 42 614–20.
| Clinical aspects of adult syphilis.Crossref | GoogleScholarGoogle Scholar | 22697151PubMed |
[24] Metcalf A. Anorectal disorders: five common causes of pain, itching, and bleeding. Postgrad Med 1995; 98 81–94.
| Anorectal disorders: five common causes of pain, itching, and bleeding.Crossref | GoogleScholarGoogle Scholar | 29224554PubMed |
[25] Gilson R, Mayura N, Sonnex C, Lazaro N, Keirs T. UK national guidelines on the management of anogenital warts 2015. 2015. Available online at: https://www.bashhguidelines.org/media/1075/uk-national-guideline-on-warts-2015-final.pdf [verified 16 March 2020].
[26] Hoentjen F, Rubin DT. Infectious proctitis: when to suspect it is not inflammatory bowel disease. Dig Dis Sci 2012; 57 269–73.
| Infectious proctitis: when to suspect it is not inflammatory bowel disease.Crossref | GoogleScholarGoogle Scholar | 21994137PubMed |