Management of acute sexual assault presenting to a large Australian sexual health clinic in 2012–2021: a retrospective clinical audit
Aleah Kink A B * , Janet M. Towns
A B * , Janet M. Towns  A C , Christopher K. Fairley
A C , Christopher K. Fairley  A C , Tiffany R. Phillips
A C , Tiffany R. Phillips  A C , Catriona S. Bradshaw
A C , Catriona S. Bradshaw  A C D and Eric P. F. Chow
A C D and Eric P. F. Chow  A C D
A C D
A
B
C
D
Abstract
The incidence of sexual assault continues to rise in Australia. This study aimed to describe the nature of assault, HIV/STI positivity, and its management at a sexual health clinic.
We performed a chart review of 516 sexual assault cases presenting to Melbourne Sexual Health Centre between 2012 and 2021, collecting data on victim demographics, details of assault, HIV/STI testing and positivity, police involvement, and offer of counselling.
We included 516 cases: 124 males (24.0%); 384 females (74.4%); and eight transgender (1.6%) victims. The proportion of assault cases presenting to Melbourne Sexual Health Centre increased from 0.1% (37/37,070) in 2012 to 0.2% (56/36,514) in 2021 (Ptrend = 0.006). HIV post-exposure prophylaxis was prescribed for 64.5% (80/124) of males and 12.5% (48/384) of females. Among victims, 69.4% (358/516) were tested for HIV and no one tested positive, while 71.9% (371/516) were tested for syphilis, with 1.6% (6/371) positive. Gonorrhoea and chlamydia were tested at the oropharynx (44.8% [231/516] vs 28.7% [148/516]), genitals (83.7% [432/516] vs 92.4% [477/516]) and anorectum (35.3% [182/516] vs 35.3% [182/516]). Positivity for gonorrhoea and chlamydia were: 2.6% (6/231) vs 2.0% (3/148) at oropharynx, 1.4% (6/432) vs 2.9% (14/477) at genitals, and 5.5% (10/182) vs 7.1% (13/182) at anorectum. According to clinical records, 25.2% (130/516) of victims sought police involvement, and 71.7% (370/516) were offered counselling.
Sexual assault was an uncommon presentation at Melbourne Sexual Health Centre, with diverse circumstances surrounding assault; however, clinical documentation varied, indicating a need for a standard primary care protocol for clients presenting with acute sexual assault.
Keywords: human immunodeficiency virus, primary care, rape, sexual assault, sexual harassment, sexual violence, sexually transmitted infection, violence exposure.
Introduction
The incidence of sexual assault continues to rise in Australia, with the number of police-reported cases increasing by 13.0% from 2020 to 2021, the tenth annual rise in a row.1 An estimated one in six females, and one in 25 males, have experienced at least one incidence of sexual assault since the age of 15 years.2 The definition of sexual assault is broad, but generally it describes a form of sexual violence, that may or may not involve physical contact, in which consent is not obtained. It can have a profound impact on the physical and mental health of the victim, affecting functional capacity.3,4 Understanding the context and nature of an assault is important for clinicians to provide appropriate management, support, crisis care, and counselling for victims.
Previous literature has focused on sexually transmitted infection (STI) and human immunodeficiency virus (HIV) positivity in the context of assault, rather than examining the circumstances of assault, with minimal evidence to suggest that assault places a victim at higher risk of STI exposure.5–7 A study undertaken from 2005 to 2016 at the Amsterdam STI Clinic determined that female (n = 1066) victims of sexual assault did not have an increased odds ratio of STI prevalence compared to non-sexual assault clients, and male (n = 135) victims had a decreased odds ratio for STI prevalence.8
It is difficult to estimate the true prevalence of sexual assault due to low reporting rates and a focus on the female experience of assault.9 The state of Victoria currently lacks a centralised service offering both forensic and medical services for victims of assault, potentially impacting presentation to services, continuity of care, and accurate reporting of assault rates.
This study aimed to examine HIV and STI positivity in victims of sexual assault and assess the nature of assault. Additionally, we aimed to assess temporal trends in incidence of sexual assault presentations over a decade, and potential influence of the coronavirus disease 2019 (COVID-19) pandemic on case presentations. Results may inform the need for a standard primary care protocol for clients presenting with acute sexual assault.
Materials and methods
Study setting and population
This study was conducted at Melbourne Sexual Health Centre (MSHC), the only public sexual health clinic in Victoria to provide free community sexual health services, including diagnosis and treatment of STIs, and provision of sexual and reproductive health care after assault.10 MSHC is not a specialist sexual assault service.
This was a retrospective clinical audit of clients attending MSHC between 2012 and 2021, who reported being sexually assaulted. We defined sexual assault as sexual contact or behaviour occurring without the explicit consent of a victim, in which there is a potential STI transmission risk. People who experience sexual assault may be both victims and survivors, but we will refer to the study population as ‘victims’ throughout this paper.
We included individuals aged 16 years or above, who had a diagnosis code in the electronic medical record (EMR) of ‘sexual assault’ between 1 January 2012 and 31 December 2021. All genders were included. We excluded individuals assaulted greater than 3 months prior to presentation, or the assault did not carry an STI risk, such as indecent exposure. We refer to ‘assault’ or ‘assailant(s)’ but acknowledge that the assault may be ‘alleged’ with ‘alleged assailant(s)’; we do not collect detail regarding potential legal proceedings.
This study was approved by the Alfred Hospital Ethics Committee, Melbourne, Australia (199/22).
Data collection
Clinicians were required to select from a pre-determined list of clinical diagnostic codes in the EMR in order to complete a consultation. For clients with a diagnostic code of ‘sexual assault’ during the study period, we extracted the following: consultation date; age; gender; marital status; sex worker status; country of birth; Aboriginal and Torres Strait Islander status; history of injecting drug use; HIV status; laboratory testing and positivity for syphilis, gonorrhoea, chlamydia, trichomoniasis, Mycoplasma genitalium, beta-human chorionic gonadotropin (β-hCG); and other related diagnoses.
Manual chart review was performed by AK, with the following extracted from clinical notes: location of initial assault-related care, anatomical site penetrated, body part of assailant used in the assault, use of barrier protection, characteristics of the assailant, reported drug and/or alcohol intoxication, reported memory loss, police involvement, physical examination, reported symptoms, treatment administered, including prescription of HIV post-exposure prophylaxis (PEP), use of emergency contraception, and counselling referral. There is no standard template for clinician reviews, therefore, several data points were missing, or victims were unable to recall details of the alleged assault. Prior presentation and treatment at other medical services such as general practices or hospitals were included; however, presentation to the Centre Against Sexual Assault or Resourcing Health and Education was excluded as we had variable data collection regarding the extent of service involvement.
We changed the diagnostic method for gonorrhoea and trichomoniasis from culture to nucleic acid amplification test in March 2015 and August 2018, respectively. We also changed the diagnostic assay for chlamydia from Strand Displacement Amplification to Transcription-Mediated Amplification.11,12
Statistical analyses
Descriptive statistics were calculated. Median and interquartile range (IQR) were reported for continuous variables. Frequency and proportion were reported for categorical variables. The date of the assault was stratified into three groups: (1) within 7 days; (2) 1–2 weeks; and (3) between 2 weeks and 3 months prior to presentation to MSHC. We determined these stratification parameters based on STI testing window periods. Characteristics of assault presentation were reported and stratified by gender. Fisher’s exact test was used to compare the characteristics between female and male victims. The annual proportion of sexual assault cases was calculated as the number of annual sexual assault cases divided by the number of annual consultations at MSHC. The χ2 trend test was used to examine the changes in the annual proportion of sexual assault cases over the study period. All statistical analyses were conducted in Stata (ver. 17, College Station, TX, USA). Figures were generated in R (ver. 4.2.3, R Foundation for Statistical Computing, Vienna, Austria).
Results
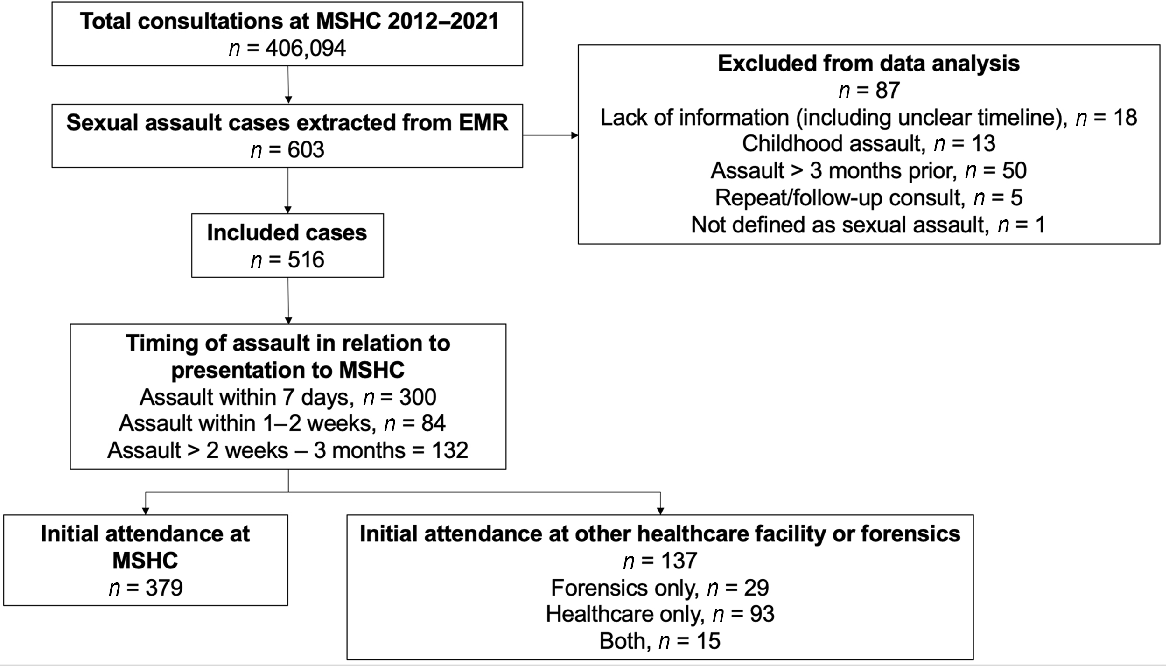
There were 406,094 consultations at MSHC between 2012 and 2021, of which 603 were sexual assault cases. After applying our inclusion criteria, 87 cases were excluded (Fig. 1). Of the remaining 516 cases, 58.1% (n = 300) of victims presented to MSHC within 7 days of the assault, 16.3% (n = 84) within 1–2 weeks, and 25.6% (n = 132) in the 2 weeks to 3 months following the assault.
Study inclusion criteria flow chart. MSHC, Melbourne Sexual Health Centre; EMR, electronic medical record.
The median age of the 516 victims was 26 years (IQR 22–32) (Table 1), with 74.4% of victims identifying as female (n = 384), 24.0% (n = 124) as male, and 1.6% (n = 8) as transgender.
| Characteristics | n/N (%) | |
|---|---|---|
| Median age in years (interquartile range) | 26 (22–32) | |
| Gender | ||
| Male | 124 (24.0) | |
| Female | 384 (74.4) | |
| Transgender | 8 (1.6) | |
| Marital status | ||
| Single or never married | 380 (73.6) | |
| Married | 20 (3.9) | |
| De facto | 18 (3.5) | |
| Divorced or separated | 32 (6.2) | |
| Widowed | 1 (0.2) | |
| Missing | 65 (12.6) | |
| Current sex worker | ||
| Yes | 30 (5.8) | |
| No | 366 (70.9) | |
| Missing | 120 (23.3) | |
| Region of birth13 | ||
| Oceania and AntarcticaA | 267 (51.7) | |
| North-West Europe | 70 (13.6) | |
| Southern and Eastern Europe | 9 (1.7) | |
| North Africa and the Middle East | 7 (1.4) | |
| South-East Asia | 28 (5.4) | |
| North-East Asia | 27 (5.2) | |
| Southern and Central Asia | 12 (2.3) | |
| Americas | 43 (8.3) | |
| Sub-Saharan Africa | 13 (2.5) | |
| Missing | 40 (7.8) | |
| Aboriginal and/or Torres Strait Islander status | ||
| Aboriginal and/or Torres Strait Islander peoples | 8 (1.6) | |
| Neither Aboriginal nor Torres Strait Islander peoples | 429 (83.1) | |
| Missing | 79 (15.3) | |
| Injecting drugs | ||
| Never injected | 371 (71.9) | |
| Current or previously injected | 17 (3.3) | |
| Missing | 128 (24.8) | |
| People living with HIV | ||
| Yes | 4 (0.8) | |
| No | 512 (99.2) | |
Among victims, 73.6% (n = 380) were single at the time of the assault. A total of 30 victims (5.8%) identified as sex workers, and 17 (3.3%) were current or previous people who inject drugs. There were four victims who were people living with HIV at the time of presentation.
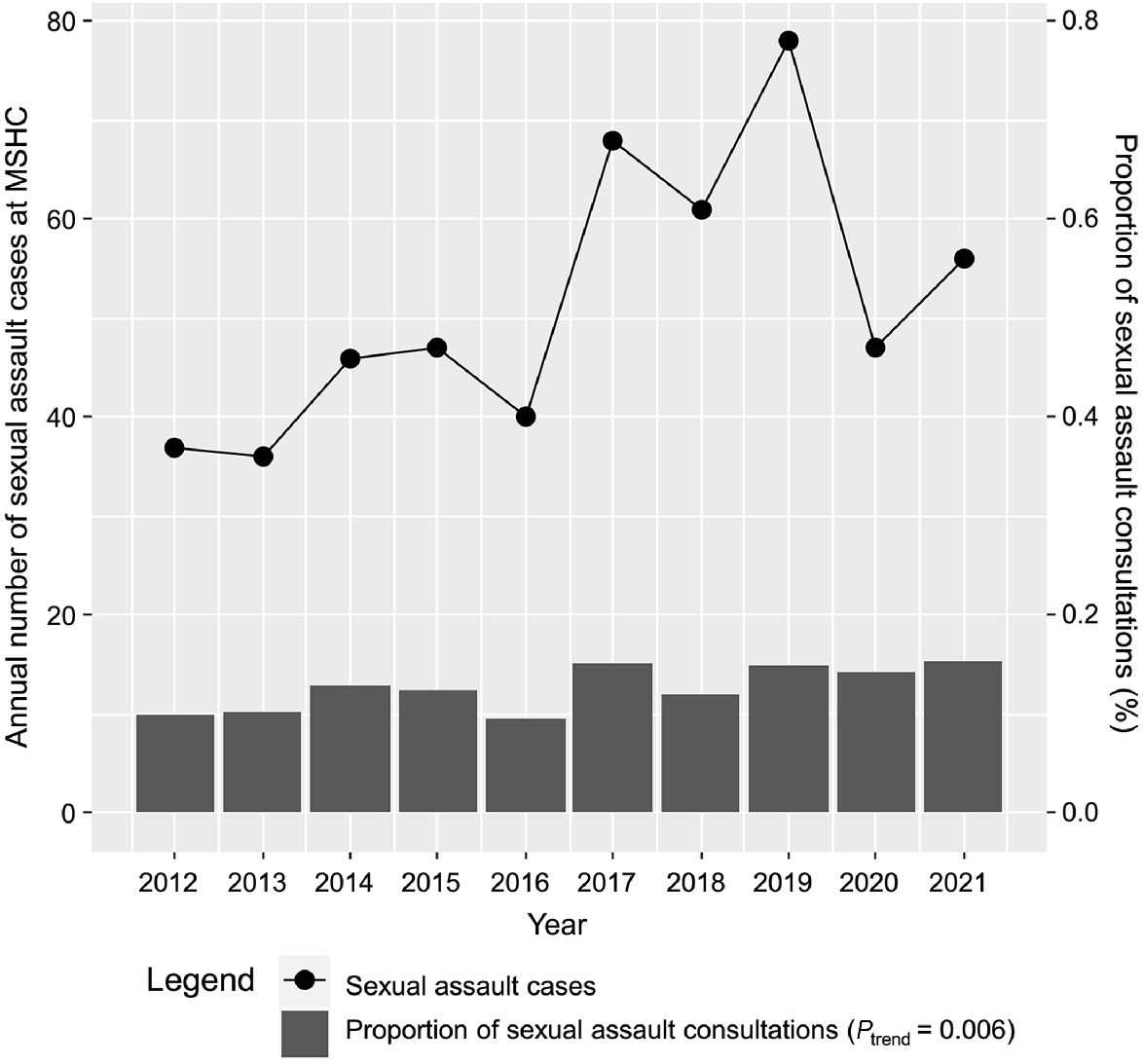
Fig. 2 shows a significant increase in the proportion of sexual assault cases presenting to MSHC from 0.1% (37/37,070) in 2012 to 0.2% (56/36,514) in 2021 (Ptrend = 0.006).
The number of acute sexual assault cases, and proportion of sexual assault cases presenting to the Melbourne Sexual Heath Centre, 2012–2021. MSHC, Melbourne Sexual Health Centre.
There were some significant differences in the relationships with the assailant(s) and how the victims met the assailant(s) between female and male victims (P < 0.001, Table 2). For example, most female victims (29.7% [114/384]) knew the alleged assailant(s) compared to males, but most male victims (28.2% [35/124]) were sexually assaulted by a casual social acquaintance (Table 2). Females most often met the assailant(s) in a social setting (24.5% [94/384]), home setting (i.e. partner, roommate) (13.3% [51/384]), and while performing sex work (9.6% [37/384]). Among sex workers, 73.3% (22/30) of assailant(s) were reported clients. Males most often met the assailant(s) in a social setting (27.4% [34/124]), online or via a smartphone app (16.1% [20/124]), and while travelling overseas (9.7% [12/124]).
| Sexual assault characteristics | Male, n/N (%), N = 124 | Female, n/N (%), N = 384 | P-valueA | Transgender, n/N (%), N = 8 | |
|---|---|---|---|---|---|
| Anatomical site penetratedB | |||||
| Vaginal | Not applicable | 219 (57.0) | – | 2 (25.0) | |
| Oral | 25 (20.2) | 61 (15.9) | 0.273 | 2 (25.0) | |
| Anal | 83 (66.9) | 55 (14.3) | <0.001 | 6 (75.0) | |
| Unable to recallC | 23 (18.5) | 71 (18.5) | 1.000 | 1 (12.5) | |
| OtherD | 4 (3.2) | 6 (1.6) | 0.268 | 0 (0.0) | |
| Missing | 11 (8.9) | 75 (19.5) | 0.006 | 0 (0.0) | |
| Body part of assailant(s) used in the assaultB | |||||
| Penis | 76 (61.3) | 242 (63.0) | 0.749 | 5 (62.5) | |
| Anus or vagina | 5 (4.0) | 0 (0.0) | 0.001 | 1 (12.5) | |
| Mouth | 13 (10.5) | 9 (2.3) | <0.001 | 0 (0.0) | |
| Digital | 8 (6.5) | 16 (4.2) | 0.330 | 1 (12.5) | |
| Foreign object | 3 (2.4) | 3 (0.8) | 0.159 | 0 (0.0) | |
| Unable to recallC | 22 (17.7) | 71 (18.5) | 0.895 | 1 (12.5) | |
| Missing | 11 (8.9) | 63 (16.4) | 0.041 | 0 (0.0) | |
| Reported use of condom barrier protection | 0.271 | ||||
| Yes | 1 (0.8) | 7 (1.8) | – | 0 (0.0) | |
| NoE | 48 (38.7) | 135 (35.2) | – | 4 (50.0) | |
| Removed | 4 (3.2) | 25 (6.5) | – | 0 (0.0) | |
| Not applicableF | 4 (3.2) | 4 (1.0) | – | 1 (12.5) | |
| Unable to recallC | 35 (28.2) | 96 (25.0) | – | 1 (12.5) | |
| Missing | 32 (25.8) | 117 (30.5) | – | 2 (25.0) | |
| Relationship between victim and assailant(s) | <0.001 | ||||
| Known | 19 (15.3) | 114 (29.7) | – | 2 (25.0) | |
| Acquaintance | 35 (28.2) | 72 (18.8) | – | 1 (12.5) | |
| Sex work client | 1 (0.8) | 35 (9.1) | – | 1 (12.5) | |
| Unable to recallC | 47 (37.9) | 97 (25.3) | – | 2 (25.0) | |
| Missing | 22 (17.7) | 66 (17.2) | – | 2 (25.0) | |
| Source of meeting assailant(s) | <0.001 | ||||
| Apps or online | 20 (16.1) | 15 (3.9) | – | 1 (12.5) | |
| Home | 8 (6.5) | 51 (13.3) | – | 1 (12.5) | |
| In hospital | 1 (0.8) | 0 (0.0) | – | 0 (0.0) | |
| In public or outdoors | 10 (8.1) | 21 (5.5) | – | 0 (0.0) | |
| Overseas | 12 (9.7) | 27 (7.0) | – | 0 (0.0) | |
| Sex work/brothel | 3 (2.4) | 37 (9.6) | – | 1 (12.5) | |
| Social setting | 34 (27.4) | 94 (24.5) | – | 0 (0.0) | |
| Workplace | 2 (1.6) | 15 (3.9) | – | 0 (0.0) | |
| Unable to recallC | 4 (3.2) | 14 (3.6) | – | 0 (0.0) | |
| Missing | 30 (24.2) | 110 (28.6) | – | 5 (62.5) | |
| Gender of assailant(s) | <0.001 | ||||
| Male | 84 (67.7) | 332 (86.5) | – | 7 (87.5) | |
| Female | 4 (3.2) | 1 (0.3) | – | 1 (12.5) | |
| Transgender | 5 (4.0) | 0 (0) | – | 0 (0.0) | |
| Multiple alleged assailants | 22 (17.7) | 21 (5.5) | – | 0 (0.0) | |
| Unable to recallC | 6 (4.8) | 13 (3.4) | – | 0 (0.0) | |
| Missing | 3 (2.4) | 17 (4.4) | – | 0 (0.0) | |
| Drug and/or alcohol use reported | <0.0001 | ||||
| Yes | 82 (66.1) | 178 (46.4) | – | 1 (12.5) | |
| No | 42 (33.9) | 206 (53.6) | – | 7 (87.5) | |
| Memory loss reported | 0.299 | ||||
| Yes | 58 (46.8) | 159 (41.4) | – | 1 (12.5) | |
| No | 66 (53.2) | 225 (58.6) | – | 7 (87.5) | |
| Police contactG | 0.253 | ||||
| Yes | 28 (22.6) | 100 (26.0) | – | 2 (25.0) | |
| No | 87 (70.2) | 269 (70.1) | – | 6 (75.0) | |
| Intent to report | 9 (7.3) | 15 (3.9) | – | 0 (0.0) | |
Over half of the assaults involved a male assailant, with assaults on female victims less likely to involve multiple assailants (5.5% [21/384]) compared to males (17.7% [22/124], P < 0.001). Most female victims were vaginally penetrated (57.0% [219/384]), while males were most often anally penetrated (66.9% [83/124]) during an assault.
About two-thirds (62.6% [323/516]) of victims were penetrated with the assailant(s)’ penis. Seven (1.8% [7/384]) female victims and one (0.8% [1/124]) male victim could recall definitive use of condoms during the assault, with 29 (5.6%) recorded cases of stealthing (deliberate removal of a condom during sex without the partner’s knowledge or consent).14 Nine (31.0% [9/29]) of these victims identified as sex workers.
Fewer females (46.4% [178/384]) reported intoxication with drugs and/or alcohol at the time of the assault compared to males (66.1% [82/124], P < 0.0001). The proportion of females (41.4% [159/384]) who reported memory loss was similar to males (46.8% [58/124], P = 0.299).
Only one-quarter (25.2% [130/516]) of victims had involved police prior to presentation to MSHC, in the form of forensic examination, filing of a police report, or an informal discussion regarding the assault.
Most victims (69.4% [358/516]) underwent HIV testing; there was no difference in HIV testing between female and male victims (67.2% [258/384] vs 74.2% [92/124], P = 0.149), and none tested positive for HIV (Table 3). Almost 50.0% (58/124) of male victims were prescribed HIV PEP at MSHC (Table 4). Similarly, most victims (71.9% [371/516]) were tested for syphilis. There was a significantly higher proportion of male victims who were tested for syphilis compared to female victims (81.5% [101/124] vs 68.2% [262/384], P < 0.001); however, there was no significant difference in syphilis positivity between male and female victims (4.0% [3/101] vs 0.8% [2/262], P = 0.134).
| Diagnosis | Male n/N (%) | Female n/N (%) | P-valueA | Transgender n/N (%) | |
|---|---|---|---|---|---|
| Laboratory testingB | |||||
| SyphilisC | 3/101 (3.0) | 2/262 (0.8) | 0.134 | 1/8 (12.5) | |
| New HIVD | 0/92 (0.0) | 0/258 (0.0) | 1.000 | 0/8 (0.0) | |
| Chlamydia | |||||
| GenitalE | 1/108 (0.9) | 13/361 (3.6) | 0.206 | 0/8 (0.0) | |
| Oropharyngeal | 1/60 (1.7) | 2/82 (2.4) | 1.000 | 0/6 (0.0) | |
| Anorectal | 10/97 (10.3) | 3/78 (3.8) | 0.148 | 0/7 (0.0) | |
| Gonorrhoea | |||||
| GenitalE | 4/74 (5.4) | 2/350 (0.6) | 0.010 | 0/8 (0.0) | |
| Oropharyngeal | 4/102 (3.9) | 1/122 (0.8) | 0.180 | 1/7 (14.3) | |
| Anorectal | 7/97 (7.2) | 3/78 (3.8) | 0.515 | 0/7 (0.0) | |
| Trichomoniasis | |||||
| GenitalE | 0/0 (0.0) | 2/194 (1.0) | 1.000 | 0/2 (0.0) | |
| Mycoplasma genitalium | |||||
| GenitalE | 0/16 (0.0) | 0/83 (0.0) | 1.000 | 0/1 (0.0) | |
| Anorectal | 1/16 (6.3) | 1/10 (10.0) | 1.000 | 0/1 (0.0) | |
| Pregnancy testB,F | NA | 0/148 (0.0) | – | 0/1 (0.0) | |
| Clinical diagnosesG | |||||
| Acute non-gonococcal urethritis | 7/124 (5.6) | 0/384 (0.0) | <0.001 | 0 (0.0) | |
| Bacterial vaginosis | NA | 30/384 (7.8) | – | 0 (0.0) | |
| Balanitis | 1/124 (0.8) | NA | – | 0 (0.0) | |
| Candidiasis | NA | 23/384 (6.0) | – | 0 (0.0) | |
| Cervicitis | NA | 3/384 (0.8) | – | 0 (0.0) | |
| Dermatosis | 1/124 (0.8) | 10/384 (2.6) | 0.309 | 1 (12.5) | |
| Genital warts | 0/124 (0.0) | 1/384 (0.3) | 1.00 | 0 (0.0) | |
| Pelvic inflammatory disease | NA | 8/384 (2.1) | – | 0 (0.0) | |
| Proctitis | 4/124 (3.2) | 1/384 (0.3) | 0.014 | 0 (0.0) | |
| Urinary tract infection/cystitis | 0/124 (0.0) | 9/384 (2.3) | 0.122 | 0 (0.0) | |
| Vaginitis | NA | 1/384 (0.3) | – | 0 (0.0) | |
| Physical examination performedG | 88/124 (71.0) | 283/384 (73.7) | 0.562 | 5 (62.5) | |
HIV, human immunodeficiency virus; β-hCG, beta-human chorionic gonadotropin; NA, not applicable.
| Male, n (%), N = 124 | Female, n (%), N = 384 | P-valueA | Transgender, n (%), N = 8 | ||
|---|---|---|---|---|---|
| Symptoms reported | |||||
| Genital discomfort, pain, or itch | 8 (6.5) | 119 (31.0) | <0.001 | 1 (12.5) | |
| Urinary symptoms | 11 (8.9) | 49 (12.8) | 0.267 | 0 (0.0) | |
| Vaginal discharge | NA | 92 (24.0) | – | 0 (0.0) | |
| Vaginal bleeding | NA | 42 (10.9) | – | 1 (12.5) | |
| Anal pain or discomfort | 29 (23.4) | 28 (7.3) | <0.001 | 2 (25.0) | |
| Anal bleeding or discharge | 19 (15.3) | 14 (3.6) | <0.001 | 0 (0.0) | |
| Rash or lesion | 7 (5.6) | 22 (5.7) | 1.000 | 1 (12.5) | |
| Physical injury | 12 (9.7) | 50 (13.0) | 0.430 | 1 (12.5) | |
| OtherB | 27 (21.8) | 86 (22.4) | 1.000 | 0 (0.0) | |
| Antibiotics prescribed | |||||
| Oral | 27 (21.8) | 128 (33.3) | 0.018 | 0 (0.0) | |
| Parenteral | 0 (0.0) | 2 (0.5) | 1.000 | 0 (0.0) | |
| Antivirals prescribed | |||||
| Oral | 5 (4.0) | 12 (3.1) | 0.576 | 1 (12.5) | |
| Antifungal prescribed | |||||
| Oral | 0 (0.0) | 38 (9.9) | <0.001 | 0 (0.0) | |
| Topical | 2 (1.6) | 16 (4.2) | 0.265 | 0 (0.0) | |
| Oral and topical | 0 (0.0) | 11 (2.9) | 0.074 | 0 (0.0) | |
| OtherC | |||||
| Oral | 2 (1.6) | 8 (2.1) | 1.000 | 0 (0.0) | |
| Topical | 2 (1.6) | 15 (3.9) | 0.266 | 1 (12.5) | |
| STI or supportive treatment reportedly given elsewhereD | 0.168 | ||||
| Yes | 8 (6.5) | 43 (11.2) | – | 1 (12.5) | |
| No | 116 (93.5) | 341 (88.8) | – | 7 (87.5) | |
| Emergency contraceptionE | – | ||||
| Yes | NA | 128 (33.3) | – | 1 (12.5) | |
| No | NA | 175 (45.6) | – | 1 (12.5) | |
| Missing | NA | 81 (21.1) | – | 1 (12.5) | |
| Offer of counselling recorded | 0.320 | ||||
| Yes | 69 (55.6) | 219 (57.0) | – | 5 (62.5) | |
| No | 32 (25.8) | 114 (29.7) | – | 0 (0.0) | |
| Declined | 23 (18.5) | 51 (13.3) | – | 3 (37.5) | |
| HIV PEP prescribed | <0.001 | ||||
| MSHC | 58 (46.8) | 31 (8.1) | – | 4 (50.0) | |
| Other facility | 13 (10.5) | 10 (2.6) | – | 0 (0.0) | |
| Initiated at other facility then continued at MSHC | 9 (7.3) | 7 (1.8) | – | 0 (0.0) | |
| Already on HIV PEP/PrEP | 2 (1.6) | 1 (0.3) | – | 0 (0.0) |
MSHC, Melbourne Sexual Health Centre; NA, not applicable; STI, sexually transmitted infection; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis.
More female victims (94.0% [361/384]) were tested for genital chlamydia compared to male victims (87.1% [108/124], P = 0.019); however, more male victims were tested for extragenital chlamydia compared to female victims (all P < 0.001, Table 3). The overall victim positivity for genital, oropharyngeal, and anorectal chlamydia was 2.9% (14/477), 2.0% (3/148), and 7.1% (13/182), respectively. There were no significant differences in chlamydia positivity between female and male victims.
Similar to chlamydia, more female victims (91.1% [350/384]) were tested for genital gonorrhoea compared to males (59.7% [74/124], P < 0.001); however, more male victims were tested for extragenital gonorrhoea compared to female victims (all P < 0.001, Table 3). The overall victim positivity for genital, oropharyngeal, and anorectal gonorrhoea was 1.4% (6/432), 2.6% (6/231), and 5.5% (10/182), respectively. There were no significant differences in extragenital gonorrhoea positivity between female and male victims (all P > 0.05); however, male victims had a higher genital gonorrhoea positivity (5.4% [4/74]) compared to female victims (0.6% [2/350], P = 0.010).
One-third of females (33.3% [128/384]) sought or were prescribed emergency contraception, while 148 (38.5%) underwent β-hCG pregnancy testing, of which none returned a positive test.
Reported symptoms and treatment administered are listed in Table 4. Close to 60.0% (306/516) of victims reported symptoms at the time of presentation to MSHC, with genital itch, pain, or discomfort common in females (31.0% [119/384]), and anal pain or discomfort common in males (23.4% [29/124]).
A majority (56.8% [293/516]) of victims presenting to MSHC had a clear offer of counselling services either at MSHC or with other providers, cited in the clinical record.
Discussion
To our knowledge, this clinical audit is the largest study examining the nature of sexual assaults, and consequent clinical management of victims of all genders in the state of Victoria. There was an increase in the proportion of sexual assault consultations at MSHC between 2012 and 2021, with a diversity in assault characteristics. Our data shows that female victims were usually assaulted by someone they knew, but male victims were assaulted by casual social acquaintance assailant(s). HIV/STI testing was performed in a majority of victims but there were some disparities in STI testing patterns between female and male victims. We found that more male victims were tested for extragenital chlamydia or gonorrhoea compared to female victims. This may be due to most male victims being assaulted by male assailants, and that extragenital chlamydia or gonorrhoea screening is recommended among men who have sex with men but not among females.15 Furthermore, there were no differences in HIV/STI positivity between female and male victims except male victims had a higher genital gonorrhoea positivity compared to female victims. Varied clinical documentation of assault supports the implementation of a standardised primary care approach to clinical assessment and management of sexual assault victims.
There was a sharp spike in the number of cases presenting in 2017, potentially correlated with the introduction of the #MeToo campaign and increased awareness of assault incidence and empowerment to report.16 There were fewer presentations after 2019 and the onset of the COVID-19 pandemic; victims may have been reluctant to seek medical and police attention due to breach of lockdown curfew laws at the time of assault, or may have perceived an inability to access health services.17,18 Past studies have found that there was a reduction in casual sexual activity during the COVID-19 pandemic, which resulted in changes in the nature of clients’ presentations to MSHC, with a drop in asymptomatic STI screening and diagnoses.19,20 However, there was no change in the time to seek healthcare for symptomatic clients as a result of the pandemic.21 Despite an overall reduction in consultations at MSHC during the COVID-19 pandemic, our findings suggest that the porportion of sexual assault presentations continued to increase.
Our findings align with research at both Danish and Australian specialised sexual health centres, in that male victims were more likely to be assaulted by an acquaintance rather than known perpetrator and have multiple assailants.22,23 However, a study conducted at a Sexual Assault Centre in the Netherlands found that there was no significant difference in victim or assault characteristics between males and females, although, unlike our study, this paper contained a small sample size of males in comparison to females.24 Future research could consider vulnerability factors to improve our understanding of client populations most at risk of assault.
There were 29 victims that reported ‘stealthing’, an act which was only formally criminalised in Victoria in 2022.25 Nine of these victims identified as sex workers. A previous study conducted at MSHC found that females who experienced stealthing were more likely to be a current sex worker, highlighting the need for improved laws and workplace standards to protect this population.26
Early presentation following sexual assault is important for crisis support and management. However, there may be a reduced likelihood of detecting an assault related STI in the acute period due to incubation periods exceeding 14 days.5,27 Baseline STI testing dependent on sexual practice can be useful to diagnose pre-existing infections; however, it cannot conclude that a positive STI result following a sexual assault is directly correlated. Therefore, we did not analyse the relationship between anatomical site penetrated during the assault, and detection of STIs, as the presence of an STI could not be definitively linked to the assault event.
Although the majority of victims attended for acute care within 7 days of the assault, one-quarter attended MSHC at least 2 weeks following the assault. This could have been due to a reluctance to seek care, attending for non-assault related care, or a lack of access to care. For example, in the case of 39 victims, the assault occurred overseas, in which the victims may have accessed local treatment initially, or travel plans may have limited their ability to attend to a medical or sexual health service in a timely manner. As a result, a delayed time to presentation at MSHC may not necessarily be indicative of a reluctance to seek care.
The Australian STI guidelines offer no recommendation for STI prophylaxis in the context of sexual assault.28,29 A higher proportion of victims of all genders were prescribed antibiotics (n = 157) at MSHC compared to those who tested positive (n = 53) for genital, oropharyngeal or anorectal chlamydia, gonorrhoea, or Mycoplasma genitalium, suggesting that prophylaxis was often administered opportunistically, potentially due to the known high rate of loss to follow up.5
A 2005 study on uptake of HIV PEP following sexual assault, conducted at the Eastern and Central Sexual Assault Service in Sydney, found that 7.7% (9/117) of victims were prescribed PEP.30 There were comparatively higher rates of prescription at MSHC, with almost 50.0% (58/124) of males prescribed HIV PEP. No victims tested positive for HIV in the wake of the assault, however, as follow-up testing was not included in this analysis, there could have been cases of HIV positivity at a later testing interval.
Limitations of our study include missing data of up to 29.3% for some key variables (e.g. sex worker status, use of barrier protection) due to the nature of retrospective data collection, and potential impact of recall bias, secondary to trauma or memory loss. During the study period, there was no standardised template at MSHC for clinicians to fill out when conducting a consult related to sexual assault. It is possible that clinicians did not ask questions regarding the nature of assault, or that these questions were discussed, but only relevant positives were recorded in the clinical notes. We cannot conclude that presentations were for the purpose of acute assault management rather than for symptomatic treatment, and therefore it is possible that the incidence of STI positivity may have been inflated if most consultations were for symptomatic treatment, rather than assault-specific care.
Additionally, potential of selection bias might have occurred. We observed that a majority of victims were female, which may support existing evidence that females are disproportionately affected by sex crimes.28 However, we were unable to capture all sexual assaults occurring in the state of Victoria, only those who presented to MSHC and disclosed the assault, and therefore, the sample proportion of females and males in our study may not reflect the true population. Nevertheless, similar publications, and Australian Bureau of Statistics data, do not report on male victims of assault due to a small number of reported cases.2 A strength of our study is the large number of male victims (n = 124), addressing an important gap in the literature. Only eight victims identified as transgender and thus, we cannot draw conclusions regarding assault management for this population.
Furthermore, 56.8% of victims were offered a counselling service; however, we did not assess the uptake of such services. Lastly, more cases of gonorrhoea, chlamydia, and trichomoniasis might have been diagnosed in later years due to the change in the diagnostic method during the study period.11,12 However, due to the small number of STI cases in this study, we were unable to examine the temporal trends.
Conclusion
Understanding the circumstances surrounding sexual assault is important for clinicians to provide appropriate clinical management, support, crisis care, and counselling for victims. Sexual assault was an uncommon reason for clients to attend MSHC, however, several key details of assault were missing from the clinical record, highlighting the need for a standard primary care protocol for clients presenting with acute sexual assault.
Data availability
Data for this clinical audit is presented in the figures and tables. Access to the original dataset can be made available upon a formal and reasonable request to the corresponding author. Further inquiries can be directed to the corresponding author, and the availability of the data will be subjected to the permission of the Alfred Hospital Ethics Committee.
Conflicts of interest
EPFC and TRP are Associate Editors of Sexual Health. To mitigate this potential conflict of interest, they were blinded from the review process. The authors declare no other conflicts of interest.
Declaration of funding
EPFC is supported by an Australian National Health and Medical Research Council (NHMRC) Emerging Leadership Investigator Grant (GNT1172873). CKF and CSB are supported by an Australian NHMRC Leadership Investigator Grant (GNT1172900 and GNT1173361, respectively). JMT is supported by an Australian NHMRC Partnership Grant (GNT2003399). The funders of the study had no role in the design or conduct of the study, including data collection, management, analysis, or interpretation of the results; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author contributions
EPFC and JMT conceived and designed the study and assisted with data interpretation and writing of the manuscript. EPFC also assisted with data analysis and preparation of the ethics application. AK prepared the ethics application, performed data collection and data analysis, and wrote the original manuscript. CKF, CSB, and TRP reviewed and edited the manuscript for important intellectual content, and approved the final version.
Acknowledgements
The authors thank Afrizal Afrizal at the Melbourne Sexual Health Centre for assistance with data extraction.
References
1 Australian Bureau of Statistics. Sexual assaults increase for tenth year in a row. Australian Bureau of Statistics; 2022. Available at https://www.abs.gov.au/media-centre/media-releases/sexual-assaults-increase-tenth-year-row [accessed 10 September 2023]
2 Australian Institute of Health and Welfare. Sexual assault in Australia. Australian Institute of Health and Welfare; 2020. Available at https://www.aihw.gov.au/reports/domestic-violence/sexual-assault-in-australia [accessed 10 September 2023]
3 Boyd C. The impacts of sexual assault on women. Australian Institute of Family Studies; 2011. Available at https://apo.org.au/node/24540 [accessed 10 September 2023]
4 Dworkin ER, Menon SV, Bystrynski J, Allen NE. Sexual assault victimization and psychopathology: a review and meta-analysis. Clin Psychol Rev 2017; 56: 65-81.
| Crossref | Google Scholar | PubMed |
5 Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(Rr-03): 1-137 Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6403a1.htm.
| Google Scholar | PubMed |
6 Gibb AM, McManus T, Forster GE. Should we offer antibiotic prophylaxis post sexual assault? Int J STD AIDS 2003; 14(2): 99-102.
| Crossref | Google Scholar | PubMed |
7 Cybulska B, Forster G, Welch J, Lacey H, Rogstad K, Lazaro N. UK national guidelines on the management of adult and adolescent complainants of sexual assault. British Association for Sexual Health and HIV; 2012. Available at https://www.bashhguidelines.org/media/1079/4450.pdf [accessed 10 September 2023]
8 van Rooijen MS, Schim van der Loeff MF, van Kempen L, de Vries HJC. Sexually transmitted infection positivity rate and treatment uptake among female and male sexual assault victims attending the Amsterdam STI clinic between 2005 and 2016. Sex Transm Dis 2018; 45(8): 534-541.
| Crossref | Google Scholar |
9 Krug EG, Dahlberg LL, Mercy JA, Zwi AB, Lozano R. World report on violence and health. World Health Organization; 2002. Available at https://apps.who.int/iris/bitstream/handle/10665/42495/9241545615_eng.pdf [accessed 10 September 2023]
10 Melbourne Sexual Health Centre. Our services. Melbourne Sexual Health Centre; 2023. Available at https://www.mshc.org.au/clinics-services/our-services [accessed 10 September 2023]
11 Cornelisse VJ, Chow EPF, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44(2): 114-117.
| Crossref | Google Scholar | PubMed |
12 Abraham E, Fairley CK, Denham I, et al. Positivity and risk factors for Trichomonas vaginalis among women attending a sexual health clinic in Melbourne, 2006 to 2019. Sex Transm Dis 2022; 49(11): 762-768.
| Crossref | Google Scholar | PubMed |
13 Australian Bureau of Statistics. Standard Australian Classification of Countries (SACC). Australian Bureau of Statistics; 2023. Available at https://www.abs.gov.au/statistics/classifications/standard-australian-classification-countries-sacc/latest-release [accessed 21 September 2023]
14 Maullin S. Stealthing isn’t a ‘sex trend’. It’s sexual assault – and it happened to me. The Guardian; 2017. Available at https://www.theguardian.com/commentisfree/2017/may/22/stealthing-sex-trend-sexual-assault-crime [accessed 10 September 2023]
15 Chow EPF, Fairley CK, Kong FYS. STI pathogens in the oropharynx: update on screening and treatment. Curr Opin Infect Dis 2024; 37(1): 35-45.
| Crossref | Google Scholar |
16 Ro’ee L, Mattsson M. The effects of social movements: evidence from #MeToo. Social Science Research Network; 2019. Available at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3496903 [accessed 10 September 2023]
17 Muldoon KA, Denize KM, Talarico R, et al. COVID-19 pandemic and violence: rising risks and decreasing urgent care-seeking for sexual assault and domestic violence survivors. BMC Med 2021; 19(1): 20.
| Crossref | Google Scholar | PubMed |
18 Kaswa R. The impact of the COVID-19 pandemic on healthcare service access for the victims of sexual assault. S Afr Fam Pract 2021; 63(1): e1-e4.
| Crossref | Google Scholar | PubMed |
19 Coombe J, Kong FYS, Bittleston H, Williams H, Tomnay J, Vaisey A, et al. Love during lockdown: findings from an online survey examining the impact of COVID-19 on the sexual health of people living in Australia. Sex Transm Infect 2021; 97(5): 357-362.
| Crossref | Google Scholar | PubMed |
20 Chow EPF, Hocking JS, Ong JJ, Phillips TR, Fairley CK. Sexually transmitted infection diagnoses and access to a sexual health service before and after the national lockdown for COVID-19 in Melbourne, Australia. Open Forum Infect Dis 2021; 8(1): ofaa536.
| Crossref | Google Scholar | PubMed |
21 Farquharson RM, Fairley CK, Ong JJ, Phillips TR, Chow EPF. Time to healthcare-seeking following the onset of STI-associated symptoms during two waves of the COVID-19 pandemic in Melbourne, Australia. Sex Transm Infect 2022; 98(5): 388-389.
| Crossref | Google Scholar | PubMed |
22 Larsen M-L, Hilden M. Male victims of sexual assault; 10 years’ experience from a Danish Assault Center. J Forensic Leg Med 2016; 43: 8-11.
| Crossref | Google Scholar | PubMed |
23 Zilkens RR, Smith DA, Mukhtar SA, Semmens JB, Phillips MA, Kelly MC. Male sexual assault: Physical injury and vulnerability in 103 presentations. J Forensic Leg Med 2018; 58: 145-151.
| Crossref | Google Scholar | PubMed |
24 Covers MLV, Teeuwen J, Bicanic IAE. Male victims at a Dutch sexual assault center: a comparison to female victims in characteristics and service use. J Interpers Violence 2022; 37(15–16): NP14772-NP14786.
| Crossref | Google Scholar |
25 Australian Associated Press. Victoria passes laws banning stealthing and requiring affirmative consent. The Guardian; 2022. Available at https://www.theguardian.com/australia-news/2022/aug/31/victoria-passes-laws-banning-stealthing-and-requiring-affirmative-consent [accessed 10 September 2023]
26 Latimer RL, Vodstrcil LA, Fairley CK, et al. Non-consensual condom removal, reported by patients at a sexual health clinic in Melbourne, Australia. PLoS ONE 2018; 13(12): e0209779.
| Crossref | Google Scholar | PubMed |
27 The Department of Health. Gonorrhoea. The Victorian State Government; 2022. Available at https://www.health.vic.gov.au/infectious-diseases/gonorrhoea#incubation-period-of-neisseria-gonorrhoeae [accessed 1 September 2023]
28 Freedman E. Clinical management of patients presenting following a sexual assault. Aust J Gen Pract 2020; 49(7): 406-411.
| Crossref | Google Scholar |
29 Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM). Australian STI management guidelines: For use in primary care. ASHM; 2021. Available at https://sti.guidelines.org.au/ [accessed 21 July 2023]
30 Templeton DJ, Davies SC, Garvin AL, Garsia RJ. The uptake of HIV post-exposure prophylaxis within a sexual assault setting in Sydney, Australia. Int J STD AIDS 2005; 16(2): 108-111.
| Crossref | Google Scholar | PubMed |


