Could late-latent syphilis be treated with a single subcutaneous infusion of long-acting penicillin?
Thel K. Hla A B C * , Sam Salman A B D , Joseph Kado A B , Brioni R. Moore A B E and Laurens Manning A B C
A B C * , Sam Salman A B D , Joseph Kado A B , Brioni R. Moore A B E and Laurens Manning A B C
A
B
C
D
E
Abstract
Syphilis is an important global health threat and little has changed in its treatment since the mid-20th century. For late-latent or syphilis infection of unknown duration, the standard treatment of multiple intramuscular injections of benzathine penicillin G (BPG) are associated with significant pain and distress to clients and caregivers, negatively impacting on treatment completion. Based on pharmacokinetic modelling from a Phase I study of subcutaneous infusion of high dose BPG (SCIP), we present its feasibility, safety and tolerability for treatment of syphilis in a single infusion. SCIP leads to more sustained penicillin concentrations above the desired target with less reported pain and reduced clinic visits.
Keywords: antibiotics, benzathine penicillin G, late latent syphilis, population pharmacokinetics, subcutaneous penicillin, syphilis, syphilis of unknown duration, treatment of syphilis.
An estimated 19.9 million people are living with syphilis globally.1 The World Health Organization has set a key strategic target for elimination of congenital syphilis via mother-to-child transmission (MTCT) in 50 countries by 2025. Currently only 15 countries have achieved this, highlighting the need for improved treatments.2 The Australian syphilis epidemic continues and a national target to eliminate congenital syphilis by 2022 was not achieved.3
For those with late-latent or syphilis infection of unknown duration, three intramuscular (i.m.) injections of benzathine penicillin G (BPG; 2.4 million units [MU]) given on consecutive weeks are recommended, a regimen which has changed little since the 1950s.4 Data are scarce on multi-dose completion rates, but appear low (43–69%), likely owing to the associated pain and the need for multiple clinic visits, which impede the overall efforts to reduce global burden and MTCT of syphilis.5,6 Pharmacokinetic modelling suggests that current three-dose regimen given a week apart (using Bicillin®L-A formulation, Pfizer) result in penicillin plasma concentrations above 18 ng/mL (a historical therapeutic target) for approximately 6 weeks (Fig. 1; orange).7–9
Simulation of 7.2 MU subcutaneous infusion of benzathine penicillin (as Bicillin®L-A) compared to three separate weekly 2.4 MU intramuscular injections modelled from Kado et al.9 MU, million units; SCIP, subcutaneous infusion of penicillin.
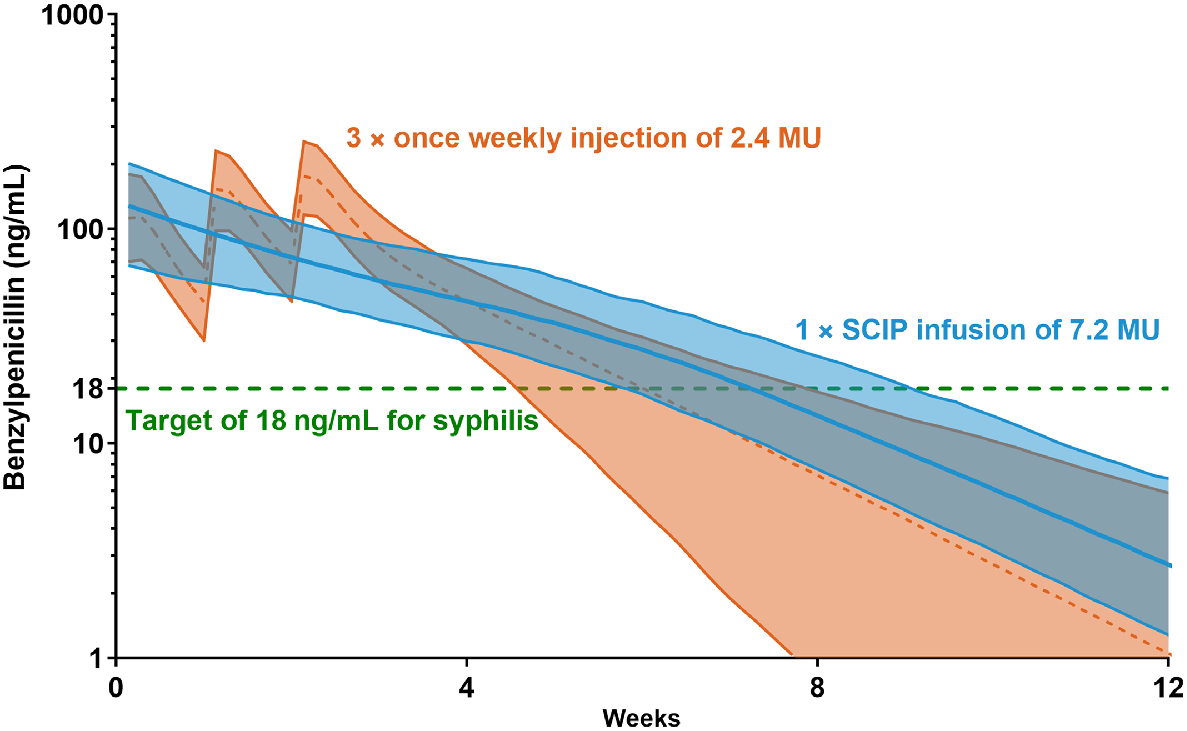
Subcutaneous (SC) delivery of BPG, delivered to the lower abdominal area using a SC catheter at a rate of 0.5–1 mL/min, delays systemic absorption of penicillin which could be exploited to deliver the entire treatment course at a single visit.9 Here we show the pharmacological profiles following SC infusion of 7.2 MU BPG (SCIP), delivered in 10 healthy adult volunteers who participated in a Phase I study9 (Fig. 1; blue), which focused on secondary prophylaxis for acute rheumatic fever, a condition for which BPG is also indicated. Compared with standard treatment, SCIP leads to increased time (7 extra days) above 18 ng/mL. There were no serious adverse events throughout the 16 weeks follow-up and majority reported minimal pain, substantiated via quantitative pain scores and qualitative interviews.9,10
Therefore, SCIP appears safe and tolerable for syphilis, potentially providing a more sustained therapeutic penicillin exposure and less pain compared to standard i.m. therapy. In late-latent or syphilis of unknown duration, it confers added benefit of treatment completion at a single visit, emphasising patient-centred delivery to improve treatment completion rates. Equivalent drug costs can be assumed as the total dose delivered is the same (7.2 MU), while the additional low cost of a SC catheter is offset by reduced demand on material and personnel resources from reduced number of visits. Further evaluation is currently underway in a Phase IIa trial to explore safety, tolerability, and treatment efficacy of SCIP in non-central nervous system syphilis (ACTRN12622000349741), the results of which have the potential to improve syphilis treatment and adherence.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Declaration of funding
Phase I study (ACTRN12621000135819) funded by Cure Kids NZ (Grant number 7012). The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication. TKH is supported by a Research Program Training Scholarship and a Post Graduate Research Scholarship at the University of Western Australia, partly funded by the Athelstan Saw Bequest Fund. JK is supported by a PhD Scholarship and a Scholarship for International Research Fees at the University of Western Australia (UWA). LM is supported by an Investigator Award from the NHMRC (GNT1197177).
References
1 Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 2019; 97(8): 548-562P.
| Crossref | Google Scholar |
4 Magnuson HJ, Thomas EW, Olansky S, Kaplan BI, De Mello L, Cutler JC. Inoculation syphilis in human volunteers. Medicine 1956; 35(1): 33-82.
| Crossref | Google Scholar |
5 Mangone E, Bell J, Khurana R, Taylor MM. Treatment completion with three-dose series of benzathine penicillin among people diagnosed with late latent and unknown duration syphilis, Maricopa County, Arizona. Sex Transm Dis 2023; 50(5): 298-303.
| Crossref | Google Scholar | PubMed |
6 Wu M, Seel M, Britton S, Dean JA, Lazarou M, Safa H, et al. Addressing the crisis of congenital syphilis: key findings from an evaluation of the management of syphilis in pregnancy and the newborn in South-East Queensland. Aust N Z J Obstet Gynaecol 2022; 62: 91-97 10.1111/ajo.13424.
| Google Scholar |
7 Rein MF. Biopharmacology of syphilotherapy. J Am Vener Dis Assoc 1976; 3(2 Pt 2): 109-27.
| Google Scholar | PubMed |
8 Kampmeier RH. The introduction of penicillin for the treatment of syphilis. Sex Transm Dis 1981; 8(4): 260-265.
| Crossref | Google Scholar | PubMed |
9 Kado J, Salman S, Hla TK, Enkel S, Henderson R, Hand RM, et al. Subcutaneous infusion of high-dose benzathine penicillin G is safe, tolerable, and suitable for less-frequent dosing for rheumatic heart disease secondary prophylaxis: a phase 1 open-label population pharmacokinetic study. Antimicrob Agents Chemother 2023; 67: e0096223.
| Crossref | Google Scholar |
10 Enkel SL, Kado J, Hla TK, Salman S, Bennett J, Anderson A, et al. Qualitative assessment of healthy volunteer experience receiving subcutaneous infusions of high-dose benzathine penicillin G (SCIP) provides insights into design of late phase clinical studies. PLoS ONE 2023; 18(4): e0285037.
| Crossref | Google Scholar | PubMed |


