Molecular-genetic analyses of dispersal and breeding behaviour in the Australian termite Coptotermes lacteus: evidence for non-random mating in a swarm-dispersal mating system
Graham J. Thompson A D E , Michael Lenz B , Ross H. Crozier C and Bernard J. Crespi AA Department of Biological Science, Simon Fraser University, Burnaby, BC V5S 1S6, Canada.
B CSIRO Entomology, GPO Box 1700, Canberra, ACT 2601, Australia.
C School of Tropical Biology, James Cook University, Townsville, Qld 4811, Australia.
D Present address: School of Biological Science, University of Sydney, Macleay Building A12, Sydney, NSW 2006, Australia.
E Corresponding author. Email: gthompson@usyd.edu.au
Australian Journal of Zoology 55(4) 219-227 https://doi.org/10.1071/ZO07023
Submitted: 30 April 2007 Accepted: 30 July 2007 Published: 24 September 2007
Abstract
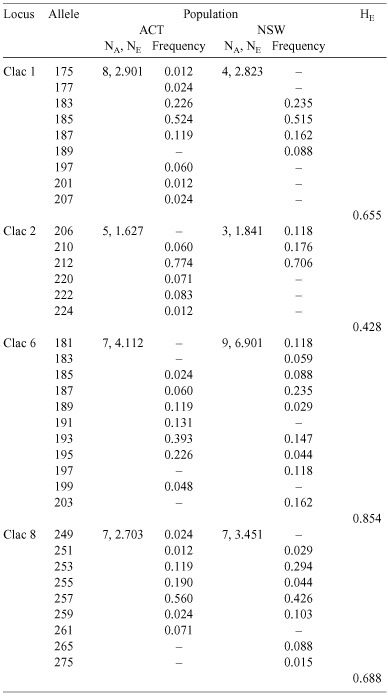
We used microsatellite DNA markers to infer the dispersal and breeding behaviour of Coptotermes lacteus, a termite whose large mounds are a conspicuous feature of Australia’s central east coast. We genotyped a subsample of neuter offspring for each of 38 colonies sampled over two spatially separated populations, one in a natural forest and the other in an exotic radiata pine plantation. All colonies showed offspring genotype frequencies consistent with a single reproductive pair. This result confirms that stable monogamy is the normal breeding arrangement for this species and that multi-reproductive colonies are rare. The two study populations were significantly differentiated and the distance separating them (~150 km) is therefore an effective constraint on gene flow. The populations themselves, however, were not noticeably subdivided above the level of colony. This lack of within-population viscosity is unexpected for weakly dispersing species and suggests that local gamete dispersal is in fact quite effective in C. lacteus. Nonetheless, dispersing sexuals do not appear to mate randomly. Instead, all four microsatellite loci are deficient in heterozygotes, indicating that populations are substantially inbred, irrespective of habitat. Evidence from hierarchical F-statistics, spatial genetic autocorrelation and relatedness calculations suggests that deviations from Hardy–Weinberg equilibrium may result from either a preference for non-sibling relatives over totally unrelated mates, or from random mating with viscosity – though evidence for the latter hypothesis was not detected. These findings suggest that swarm-dispersal mating systems, usually considered to produce outbreeding and panmixia, can instead involve a notable degree of non-random mating.
Additional keywords: Isoptera, microsatellites, population genetics, social insects.
Acknowledgements
We thank Patrick V. Gleeson and Theo Evans for help and advice collecting termites and Michael A. D. Goodisman, Nathan Lo, Silke Nebel, Edward L Vargo and two anonymous reviewers for constructive comments. This work was funded by an NSERC Discovery Grant to BJC and an NSERC Postdoctoral Research Fellowship to GJT.
Brandl, R. , Hacker, M. , Epplen, J. T. , and Kaib, M. (2005). High gene flow between populations of Macrotermes michaelseni (Isoptera, Termitidae). Insectes Sociaux 52, 344–349.
| Crossref | GoogleScholarGoogle Scholar |
Goudet, J. (2005). HIERFSTAT, a package for R to compute and test hierarchical F-statistics. Molecular Ecology Notes 5, 184–186.
| Crossref | GoogleScholarGoogle Scholar |
Husseneder, C. , Messenger, M. T. , Su, N. Y. , Grace, J. K. , and Vargo, E. L. (2005). Colony social organization and population genetic structure of an introduced population of Formosan subterranean termite from New Orleans, Louisiana. Journal of Economic Entomology 98, 1421–1434.
| PubMed |
Peakall, R. , and Smouse, P. E. (2006). GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes 6, 288–295.
| Crossref | GoogleScholarGoogle Scholar |
Roisin, Y. , and Lenz, M. (2002). Origin of male-biased sex allocation in orphaned colonies of the termite, Coptotermes lacteus. Behavioral Ecology and Sociobiology 51, 472–479.
| Crossref | GoogleScholarGoogle Scholar |
Shellman-Reeve, J. S. (2001). Genetic relatedness and partner preference in a monogamous, wood-dwelling termite. Animal Behaviour 61, 869–876.
| Crossref | GoogleScholarGoogle Scholar |
Yang, R. C. (1998). Estimating hierarchical F-statistics. Evolution 52, 950–956.
| Crossref | GoogleScholarGoogle Scholar |

|
|


