Intracolony relatedness and polydomy in the Australian meat ant, Iridomyrmex purpureus
Ellen van Wilgenburg A B , Raoul A. Mulder A and Mark A. Elgar AA Department of Zoology, The University of Melbourne, Parkville, Vic. 3010, Australia.
B Corresponding author. Email: ellenv@unimelb.edu.au
Australian Journal of Zoology 54(2) 117-122 https://doi.org/10.1071/ZO05075
Submitted: 15 December 2005 Accepted: 8 March 2006 Published: 11 May 2006
Abstract
In polydomous ants, individuals belonging to a single colony occupy a variable number of neighbouring nests. Polydomy is frequently associated with polygyny and species are often both facultatively polydomous and facultatively polygynous. In this study we test the generality of this association by investigating the genetic and spatial structure of polydomous colonies of Iridomyrmex purpureus in New South Wales, Australia. Genetic analysis of 15 colonies revealed high relatedness within all but one of the colonies, indicating that the workers are mostly produced by one, singly inseminated queen. Polydomy in this population therefore is not associated with polygyny. Intriguingly, our behavioural data suggests that the colony with low within-colony relatedness had been recently formed by colony fusion. While genotypes were not distributed homogenously throughout this newly formed colony, there was an obvious exchange of genotypes between the nests of the two former colonies. During 2 years of field observations in which we observed 140 colonies comprising over 1000 nests, we observed colony fusion only twice. We discuss these findings in relation to the current theories on the relationship between polydomy and polygyny.
Introduction
Colonies of social Hymenoptera (ants, bees and wasps) comprise individuals that cooperate in brood-rearing, foraging and nest defence. While most social insect colonies consist of one egg-laying queen and her progeny occupying a single nest, there are many variations on this simple colony structure. Colonies may harbour multiple egg-laying queens (polygyny) and can occupy multiple nests (polydomy). In polydomous colonies several nests are connected by the interchange of workers, food and brood (Rosengren and Pamilo 1983; Pedersen and Boomsma 1999). Several ecological factors may favour the spatial subdivision of nests, including improved foraging efficiency (McIver 1991; Herbers and Banschbach 1999; Holway and Case 2000), reduced predation costs (Droual 1984) and improved regulation of nest temperature (Banschbach et al. 1997). Alternatively, polydomy may be a consequence of polygyny. Polygynous colonies often reproduce by budding, thereby forming, at least temporarily, polydomous colonies (Hölldobler and Wilson 1977; Rosengren and Pamilo 1983; Bourke and Franks 1995). In budding species, workers accompany newly mated queens to establish daughter nests in the vicinity of the parent colony and these newly formed nests often remain in social contact with the parent colony (Rosengren and Pamilo 1983; Bourke and Franks 1995). Polydomy is frequently associated with polygyny (Rosengren and Pamilo 1983; Ross and Fletcher 1985; Keller 1991; Herbers 1993) and several species occur in both a monogynous and monodomous form and a polygynous and polydomous form (Alloway et al. 1982; Rosengren and Pamilo 1983; Greenberg et al. 1985). The interaction of polydomy and polygyny adds new intricacy to the organisation of social insects and thus it is important to determine the generality of this association.
To quantify the frequency of polydomy and polygyny, detailed study of relatedness and colony boundaries is essential. However, colony boundaries are often difficult to detect because (1) species can be small and cryptic (Herbers 1986, 1991), and (2) the frequency of interaction between the nests of a polydomous colony can be variable (Rosengren and Pamilo 1983; Herbers 1993; Pedersen and Boomsma 1999). The polygynous and facultatively polydomous meat ant Iridomyrmex purpureus is an ideal model species for the study of polydomy because colony boundaries are unambiguous. Colonies are characterised by mounds connected by trails of workers and the territorial borders of colonies are often demarcated by workers engaging in ritual display (Ettershank and Ettershank 1982; van Wilgenburg et al. 2005). There is considerable variation in the number of nests within colonies of I. purpureus. In a well documented population of meat ants in Belair, South Australia, most colonies occupied one nest each (Greenslade 1975; Greenslade and Halliday 1983; Halliday 1983) while a colony in Morgan, South Australia, occupied more than 85 nests (Halliday 1983). Several studies investigating the level of polygyny within colonies of I. purpureus, using either allozymes (Halliday 1975, 1983) or mitochondrial DNA (Carew et al. 1997), or by excavating nests (Greaves and Hughes 1974; Hölldobler and Carlin 1985), show that a substantial proportion of I. purpureus colonies are polygynous. However, no accurate estimates of relatedness within I. purpureus colonies have been obtained.
In this study, we re-examine the genetic and spatial structure of I. purpureus in a population in New South Wales, Australia, using both microsatellite markers and a long-term observational study of colony boundaries. Microsatellites are especially useful for the study of relatedness because of their high levels of polymorphism in ants (Evans 1993). We investigated the relationship between polydomy and polygyny in I. purpureus by (1) determining the level of relatedness within colonies of I. purpureus with known levels of polydomy, and (2) investigating colony fluidity, measured through colony budding and fusion.
Methods
The study site is located on a sheep station near Hillston, New South Wales. From January 2002 until October 2004 we mapped all the nest mounds within a 1 × 1 km area using a detailed aerial photograph and a geographic positioning system. The gravel-covered nest mounds contain between a few and 100 entrance holes and, within a colony, they are connected by trails that are typically clear of vegetation. Using these interconnecting trails and the location of territorial borders, we identified 140 colonies. We monitored all the colonies during a 2-year period, from April 2002 until April 2004. Surveys were conducted every 3 months in the first year and every 6 months in the second year. We determined the size of a colony by counting the number of entrance holes of each nest, which is a good measure of the number of individuals occupying a nest (Greaves and Hughes 1974). Colonies in this population have, on average, seven nests per colony (E. van Wilgenburg, unpublished data).
For the isolation of microsatellite loci, DNA was extracted using a DNeasy kit (Qiagen, Melbourne, Vic., Australia) following the manufacturer’s instructions. Genomic DNA was enriched for GA and GT repeat-containing fragments in polymerase chain reactions (PCRs), using the method described by Gardner et al. (1999). Additional modifications were made as detailed in Adcock and Mulder (2002). Positive clones were amplified in 50-µL reactions and the DNA extracted using Qiaquick PCR purification kit (Qiagen) following the manufacturer’s instructions. PCR products were sequenced commercially (Supamac, Sydney, NSW, Australia). From 335 colonies screened in the GT-enriched genomic library, 24 positive clones were sequenced. Primers were designed (Life Technologies, Gaithersburg, MD, USA) for seven of these that contained five repeats or more and had a suitable flanking sequence. From 141 colonies screened in the GA-enriched genomic library, 15 positive clones were sequenced. Primers were developed for four loci. Primer pairs that gave consistent, specific products were tested for polymorphism. One primer in each pair was manufactured with a 5-M13 (5CACGACGTTGTAAAACGAC) tail for use in the universal dye-labelling method described by Boutin-Ganache et al. (2001).
Collections for our genetic analysis took place during October 2004. In a 200 × 200 m area we selected 15 colonies that varied in the number of nests (between 1 and 16). For each colony we collected ~30 workers per nest and stored them in 75% ethanol. We maximised the chance of detecting genetic differentiation within colonies by collecting ants early in the morning, since meat ant workers of different nests within polydomous colonies mingle during the day, but always return to the same nest at night (Greaves and Hughes 1974). Alcohol-preserved workers were soaked in water for 1 h. Heads were crushed and kept in 10% Chelex (Bio-Rad, Hercules, CA, USA) solution at 55°C for 1 h, after which we boiled them for 15 min. The supernatant was kept in –20°C until needed.
Reactions were run in 0.2-mL microtitre plate wells layered with a drop of mineral oil (Sigma, St Louis, MO, USA) on a Corbett Research PC-960C thermocycler. Reactions (10 µL) contained an M13 primer (200 nm) 5-labelled with a dye (D2, D3 or D4; Beckman Coulter, Gladesville, NSW, Australia), and the locus-specific tailed (15 nm) and untailed (200 nm) primer, 1 µL Chelex-extracted DNA and the optimal concentration of MgCl2. All PCR used Taq polymerase (0.25 units per 10 µL), MgCl2 (see Table 1 for concentrations), and a reaction buffer (10 mm Tris-HCl, 50 mm KCl, 0.1% Triton X-100) and dNTPs (200 µm) supplied by Promega (Annandale, NSW, Australia). In total, 37 cycles of amplification were run: one cycle of 120 s at 92°C followed by 35 cycles of 92°C for 50 s, 57°C for 20 s and 72°C for 60 s followed by 1 cycle of 72°C for 5 min. PCR products (0.25 µL) were electrophoresed on a Beckman Coulter 8000XL automated sequencer using the CEqn 2000XL fragment analysis kit (Beckman Coulter) according to the manufacturer’s instructions. Fragment sizes were estimated using the Beckman Coulter 8000XL fragment analysis software. Two loci were polymorphic. The primer sequences and optimum annealing temperatures of the polymorphic loci are listed in Table 1. The primer sequences of the other loci are available on request from the first author. In addition to the two primer pairs developed especially for I. purpureus, we tested primer pairs developed for the argentine ant (Linepithema humile) (Lhum-11, -13, -19, -28, -35, -39 and -52 by Krieger and Keller (1999) and Lihu-H, -L, -N, -O and -P by Ingram and Palumbi (2002)). All the L. humile loci we tested cross-amplified in I. purpureus, and some appeared polymorphic, but we could reliably score only Lhum 19 (GenBank accession number AF093522). In total, 325 workers were genotyped.
Relatedness among colony mates was calculated using the program Relatedness (Queller and Goodnight 1989; http://es.rice.edu/projects/Bios321/relatedness.html, accessed October 2005). Colonies were weighted equally and standard errors were obtained by jack-knifing over colonies. We used a t-test to examine whether relatedness significantly deviated from 0.75, the expected relatedness value in monogynous, monandrous colonies. The effective number of queens (f) was estimated from the relatedness among workers (r) with the formula f = 3/(4r).
Results
The three tested loci revealed 15, 16 and 19 alleles per locus and allele frequencies ranged from 0.002 to 0.270 (see Table 1). The mean average genetic relatedness among colony-mate workers was 0.74 (s.e. = 0.03, 95% CI = 0.67–0.80; see Table 2), which is not significantly different from the value of 0.75 expected in monogynous and monandrous colonies (two-tailed t-test, d.f. = 14; relatedness: t = 0.48, P > 0.2). This relatedness value corresponds to an effective queen number of 1.02. Overall relatedness among all genotyped workers was 0.004 (s.e. = 0.008). We found no significant correlation between the number of nests per colony and the level of intracolony relatedness (Pearson correlation = –0.243, n = 15, P = 0.383).
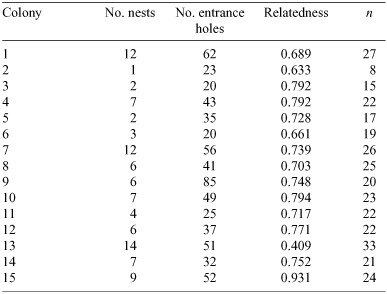
|
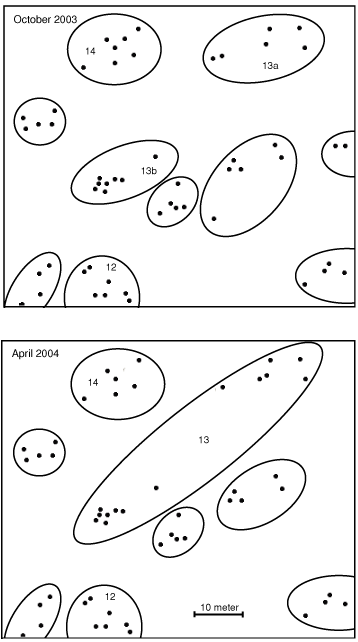
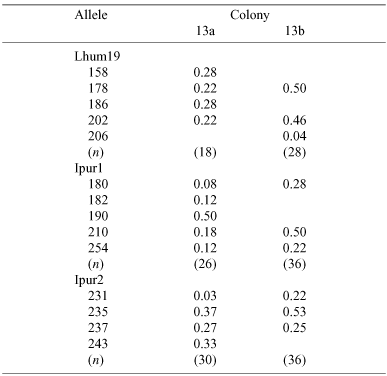
One colony (Colony 13) had a maximum of five alleles for each locus. Intriguingly, the nests of this colony used to be separated into two colonies (see Fig. 1). Fighting was observed between ants from the two groups of nests on several occasions between April 2002 and October 2003. However, the territorial border was absent in April and October 2004, and we observed exchange of workers between nests of the former colonies. Our genetic analysis reveals that the genotypes of the workers of this colony were not distributed evenly throughout the colony (Table 3). The mean average relatedness of workers (r) in the nests that formerly constituted Colony 13a was lower than in the nests that formerly constituted Colony 13b (r ± s.e. = 0.71 ± 0.07 and 0.37 ± 0.05, respectively). Some of the workers in the nests that had formerly constituted Colony 13a had the same genotypes as the workers in the nests that formerly constituted Colony 13b, while none of the workers of former Colony 13b had the same genotype as the majority of the workers in former Colony 13a.
|
|
During two years of observation we recorded no instances of colonies budding into multiple fractions. We did record two instances of apparent colony fusion (including that described above). Eleven colonies were using nests that had previously been used by other colonies. In two of these cases, the nests were clearly abandoned before subsequently being used by the other colony, while it was unclear whether the nests of the remaining colonies were taken over forcefully or were taken over after they were abandoned.
Discussion
Our genetic analysis reveals that the relatedness of polydomous colonies of I. purpureus in a population in New South Wales does not deviate from that expected under monandry and monogyny. This result contrasts with other studies on relatedness in I. purpureus colonies. Using allozymes, Halliday (1983) showed that in Sourth Australia five of 113 nests contained different homozygous genotypes, which, taking into account the low polymorphism of the markers, indicates a lower 95% limit of 40% polygyny among the colonies. Furthermore, Carew et al. (1997) showed, using mitochondrial DNA markers, that a large proportion of colonies may harbour unrelated queens. The social structure of colonies of several other ant species varies in both the number of queens and nests (Alloway et al. 1982; Rosengren and Pamilo 1983; Elmes and Keller 1993; Banschbach and Herbers 1996). However, in most of these species, polydomy and polygyny are correlated because polygynous colonies reproduce by budding (Alloway et al. 1982; Rosengren and Pamilo 1983; Greenberg et al. 1985). While I. purpureus is both facultatively polygynous and polydomous, our data reveal no such correlation, contradicting the generality of this rule. Moreover, the absence of such a correlation indicates that the ecological factors promoting polygyny in I. purpureus differ from those promoting polydomy.
Newly mated queens may seek adoption in established colonies if the chances of successfully founding a colony are low, which may occur under conditions of nest-site limitation or habitat saturation (Rosengren and Pamilo 1983; Herbers 1986). Nest density and the degree of habitat saturation are expected to increase with increasing duration of occupation. Polygynous colonies may therefore be more common in populations that have been established for longer (Rosengren et al. 1993; Sundström 1993; Seppä and Pamilo 1995). Our study population is subject to occasional flooding, with the last flood occurring in 1990. Even though I. purpureus nests can survive complete immersion for several days, long periods of flood may result in the death of all colonies in a flooded area and repopulation may take several years (Greaves 1971). While we do not have information on the age of the colonies studied by Halliday (1983) and Carew et al. (1997), the colonies on our field site are likely to be less than 14 years old and the difference in intracolony relatedness may thus be an age effect.
Our results show that polydomy in I. purpureus is independent of polygyny, suggesting other ecological benefits to a spatial separation of nests. First, the decentralisation of nests within colonies of I. purpureus is associated with the distribution of trees containing honeydew-producing homoptera (E. van Wilgenburg, unpublished data) and I. purpureus colonies may thus build extra nests to harvest resources more efficiently. Second, the proportion of nests that falls prey to echidna (Tachyglossus aculeatus) is negatively correlated with the number of nests, and so the probability that one or more nests avoid predation increases with increasing nest number (E. van Wilgenburg, unpublished data).
Within-colony relatedness was substantially lower in Colony 13 than in the other colonies. Intriguingly, worker genotypes were not distributed homogenously throughout the colony. While some genotypes were present throughout the whole colony, part of the nests (former Colony 13a) had a larger variety of genotypes than the rest of the colony (former Colony 13b). There are several possible explanations for this finding. First, we may not have correctly identified Colony 13, and it actually constitutes two colonies that share genotypes, one monogynous and the other polygynous or polyandrous. We do not think this is the case because we observed a clear exchange of workers between the nests on more than one occasion. Second, one of the former colonies may have died and the other colony may have taken over its nests (but not its inhabitants). This possibility also seems unlikely because the size of Colony 13 (measured in the number of entrance holes) is almost twice that of the two former colonies. In addition, the adoption of unoccupied nests would not explain the heterogenous distribution of genotypes throughout the colony. We therefore argue that it is most likely that Colony 13 is the result of colony fusion. Meat ant workers show nest fidelity: workers of different nests mingle during the day but return to the same nest every night (Halliday 1983). We suggest that I. purpureus workers show fidelity to the nest at which they eclosed. Colonies may therefore only homogenise by brood transfer, which would take time and may explain why we found a non-homogenous distribution of genotypes. We collected the workers for our genetic analysis early in the morning, but it would be interesting to investigate the extent of worker exchange of the two former colonies during the day.
Colony fusion, the merger of two independent social units into one, is poorly understood. While colony fusion has been observed in laboratory populations (Alloway et al. 1982; Foitzik and Heinze 1998), to our knowledge this is the first observation of colony fusion in ants in the field. We suggest that colony fusion may be an underreported phenomenon, probably because it is hard to distinguish from the reuniting of fragments of a polydomous colony. Several species show seasonal polydomy, in which colonies fragment in summer, but reunite in winter (Higashi 1976; Rosengren and Pamilo 1983; Foitzik and Heinze 2001). Additionally, colony fusion may be easily confused with acquisition of the nests of one colony by another, after either abandonment or removal of the occupants. To observe colony fusion, it is thus necessary to have long-term knowledge of the colony boundaries, both before and after the fusion event.
Colony fusion may be adaptive. A colony whose queen has died will benefit from a merger with another colony if it increases the chances of the remaining sexual brood surviving to adulthood and dispersing. The other colony may benefit from adopting the territory and resources of the queenless colony. Meat ant colonies in our study population often had access to one or two trees containing honeydew-secreting insects, which form the main food source of I. purpureus. These trees often changed owner, and the loss of one of these trees may have significant consequences for colony growth and survival (E. van Wilgenburg, unpublished data). Alternatively, colony fusion may be a consequence of an imperfect system for recognising colony mates. Workers of polydomous colonies not only have to recognise colony mates of their own nest, but also those of other nests. As a consequence, they may have a higher recognition threshold than ants occupying a single nest, which may result in a greater occurrence of recognition mistakes.
Acknowledgments
We thank Emile van Lieshout for help in the field and Ian and Linda Mclean for their generosity in permitting fieldwork on their property. We are grateful to Dr K. Ingram for providing us with microsatellite primers. This research was supported by an International Postgraduate Research Scholarship awarded by the Australian Government, a Melbourne International Research Scholarship awarded by the University of Melbourne, the Holsworth Wildlife Research Fund and the Joyce W. Vickery Scientific Research Fund of the Linnean Society of New South Wales.
Adcock, G. J. , and Mulder, R. A. (2002). Polymorphic microsatellite loci for paternity analysis in the Madagascar paradise flycatcher (Terpsiphone mutata: Aves). Molecular Ecology Notes 2, 287–289.
| Crossref | GoogleScholarGoogle Scholar |
Boutin-Ganache, I. , Raposo, M. , Raymond, M. , and Descepper, C. F. (2001). M13-tailed primers improve the readability and usability of microsatellite analyses performed with two different allele-sizing methods. BioTechniques 31, 1–3.
Ettershank, G. , and Ettershank, J. A. (1982). Ritualised fighting in the meat ant Iridomyrmex purpureus (Smith) (Hymenoptera: Formicidae). Journal of the Australian Entomological Society 21, 97–102.
Herbers, J. M. , and Banschbach, V. S. (1999). Plasticity of social organization in a forest ant species. Behavioral Ecology and Sociobiology 45, 451–465.
| Crossref | GoogleScholarGoogle Scholar |
Ross, K. G. , and Fletcher, D. J. C. (1985). Comparative study of genetic and social structure in two forms of the fire ant Solenopsis invicta (Hymenoptera: Formicidae). Behavioral Ecology and Sociobiology 17, 349–356.
| Crossref | GoogleScholarGoogle Scholar |

Seppä, P. , and Pamilo, P. (1995). Gene flow and population viscosity in Myrmica ants. Heredity 74, 200–209.

Sundström, L. (1993). Genetic population structure and sociogenetic organisation in Formica truncorum (Hymenoptera; Formicidae). Behavioral Ecology and Sociobiology 33, 345–354.
| Crossref | GoogleScholarGoogle Scholar |

van Wilgenburg, E. , van Lieshout, L. , and Elgar, M. A. (2005). Conflict resolution strategies in meat ants Iridomyrmex purpureus: ritualised displays versus lethal fighting. Behaviour 142, 701–716.
| Crossref | GoogleScholarGoogle Scholar |




