Facultative sex and reproductive strategies in response to male availability in the spiny stick insect, Extatosoma tiaratum
Angela Schneider A and Mark A. Elgar A BA Department of Zoology, University of Melbourne, Vic. 3010, Australia.
B Corresponding author. Email: m.elgar@unimelb.edu.au
Australian Journal of Zoology 58(4) 228-233 https://doi.org/10.1071/ZO10012
Submitted: 17 February 2010 Accepted: 14 September 2010 Published: 13 October 2010
Abstract
Facultative thelytoky, in which females can reproduce both sexually and asexually, offers a promising model system to understand the evolutionary significance of sex, by providing insights into whether the different reproductive modes reflect an adaptive life-history response to varying environmental conditions. Females of the spiny stick insect, Extatosoma tiaratum, can reproduce both sexually or asexually. We show that virgin females signal their reproductive state: males respond to signals produced by virgin females that have not commenced ovipositing, but fail to respond to ovipositing virgin females. Virgin females reared under different social environments varied their reproductive output: virgin females reared in the absence of males laid more eggs over a seven-day period than virgin females reared in the presence of males. The reproductive output of mated females over a seven-day period was higher than that of virgin females. These data suggest that female E. tiaratum adjust several life-history strategies in conjunction with facultative thelytoky.
Additional keywords: parthenogenetic, asexual, sex, oviposition, sexual signals, phasmatid.
Introduction
The taxonomically widespread distribution of sexual reproduction is a paradox for evolutionary theory: while asexually reproducing individuals produce all female offspring that contribute directly to the following generation, sexually reproducing individuals expend part of their reproductive effort on males (Maynard Smith 1978). Empirical studies that attempt to account for the dominance of sexual reproduction (Bell 1982; Kondrashov 1993; West et al. 1999; Otto and Nuismer 2004) typically compare the performance of asexually and sexually reproducing populations (e.g. Lively 1987; Loyning 2000). However, the observed differences between asexual and sexual reproduction may reflect differences between hybrids and non-hybrids rather than necessarily reproductive mode, because asexuality is often confounded with hybridisation and polyploidy (Kearney and Shine 2004).
Facultative thelytoky, in which females can reproduce both sexually and asexually (Bell 1982), occurs in a broad range of insects (e.g. Goldschmidt 1917; Roth and Willis 1956; Goto 1960; Nur 1971; Kurup and Prabhoo 1977; Corley and Moore 1999; Kramer and Templeton 2001; Matsuura and Nishida 2001; Ball 2002) and represents a promising model system to understand the evolutionary significance of genetic systems (Normark 2003). In particular, these species can provide insights into whether the different reproductive modes reflect an adaptive response to varying environmental conditions and/or life-history strategies (see Hadany and Otto 2007; Cáceres et al. 2009).
Parthenogenesis is thought to be favoured in environments that are stable (e.g. Hoffmann et al. 2008) and/or in which sex is difficult or impossible (e.g. Stalker 1956; Kramer and Templeton 2001; Schwander and Crespi 2009). The latter predicts facultative thelytoky in species in which female access to males is both temporally and spatially highly variable – including an absence of males. Accordingly, female reproductive strategies may vary according to their mode of reproduction. For example, virgin females (and females with nearly exhausted sperm supplies) might initially signal their sexual receptivity to males, but then cease signalling and proceed to produce eggs by parthenogenesis. Depending upon the cost of egg production, females may also adjust their output of asexual offspring according to the likelihood of mating, which may be informed by their detection of males during growth. Females that mature in an environment in which males are present may produce fewer asexual offspring in order to retain resources that can be used subsequently to produce sexual offspring. In contrast, females have nothing to gain by this strategy if there is little or no prospect of mating during their lifetime.
The Australian spiny stick insect, Extatosoma tiaratum, is facultatively thelytokic. This highly sexually dimorphic phasmatid is found in the rainforests of tropical and subtropical Queensland and northern New South Wales (Gurney 1947). The large (~12 cm long) female is heavy-bodied and brachypterous, and typically hangs inverted among the foliage with her procryptic abdomen curled over her back (Key 1991). The smaller (~8 cm long) male has large mottled wings and relatively longer antennae (Key 1991). The colouration, shape and behaviour of 1st-instar nymphs bear a close resemblance to ants of the genus Leptomyrmex (Key 1991), which are also distributed along the eastern seaboard of Australia (Shattuck 1999). Females may live up to 18 months after maturation, while males live 3–6 months after maturation (Peter Miller, pers. comm.). The duration of copulation is lengthy, as with most phasmatids, and can last up to 15 h (Carlberg 1983). Females oviposit continuously and may initiate asexual reproduction if left unmated (Carlberg 1983, 1984). It is not known whether these parthenogenetic offspring are produced by apomixis or automixis, but the former may be more likely, given this is the mechanism for other stick insects (Mantovani et al. 1996). Although E. tiaratum is a globally popular species for maintaining in captivity by the general public, and often features in live exhibits, remarkably little is known about its reproductive biology.
Here, we investigate whether the reproductive strategies of female E. tiaratum, including sexual signalling and oviposition behaviour, covaries with reproductive mode. Specifically, we examine whether female sexual signalling persists following the onset of asexual reproduction. Further, we examine whether the early reproductive output of asexually reproducing females is influenced by exposure to males during juvenile development. Finally, we compare the short-term reproductive performance of sexually and asexually reproducing females.
Methods
Animal maintenance
A stock population of stick insects was derived from eggs and juveniles obtained from existing cultures maintained by the Melbourne Zoological gardens and the Melbourne Museum. The precise, original source of these cultures is not known. Eggs were incubated in peat moss and misted with water every second day, and the emerging hatchlings were housed in cylindrical containers (height: 23 cm; diameter: 25 cm), enclosed by a fine mesh.
The sex of juvenile stick insects can be determined following their first moult by inspecting the posterior of the abdomen for spines, which are present in females but absent in males. Males and females were housed separately in 40-L tubs and fed on fresh leaves of various eucalypt species, which were replaced as required. Animals were watered at the time of feeding.
Sex pheromones, courtship and copulation
Sexual signalling by females was assessed using a Y-maze olfactometer. The Y-maze was constructed using 100-mm-diameter PVC tubing, with a 15-mm plastic rod enclosed in mesh running along the centre of the tube, which allowed males to travel easily within the maze. Each of the three arms of the maze were 50 cm long, with the Y forming a 60° angle. Tubular containers (14 cm diameter by 20 cm deep) were attached to each arm of the maze, and these containers were also lined with mesh, allowing the females to hang in their natural position and minimise the production of chemical secretions associated with stress (Carlberg 1981). The adjoining end of the container was covered with a fine black cotton cloth, which prevented males from making physical contact with the female. An air compressor was used to blow a gentle air current through the source containers and into the Y-maze towards the male, who was placed in a container at the basal arm of the maze. The male could walk from the container to the intersection and thus choose an arm leading to a container that was either empty or housed a female.
Trials were conducted within 4 h after dark. The apparatus was cleaned before each trial using commercial detergent, and food and water were placed in the containers. Participating individuals were placed in their containers and allowed to acclimate for 20 min before their container was attached to the Y-maze apparatus. The source containers (one empty and the other with a test female) were randomly attached to the arms of the Y-maze apparatus. A gentle stream of air was blown through the olfactometer for 20 min before the container with the male was attached to the base of the Y-maze. Trials ran for 30 min, and a positive response was recorded when males travelled at least 10 cm from the base of the maze. For those males that responded, we deemed a choice to have occurred if he travelled 10 cm up one arm of the maze.
All of the test females were virgin, but some females had commenced ovipositing. Males were randomly allocated to one of two treatments, in which the source container of one arm of the Y-maze held a female that either had or had not commenced ovipositing, and the container at the end of the other arm was empty. Note that these experiments do not reveal male choices between females of differing reproductive activity, but rather test whether females of different reproductive activity emit signals that elicit a male response.
Mating trials took place in clear plastic cylindrical cages (height: 40 cm; diameter: 25 cm) that also contained a few small branches of eucalypt leaves. All males had mated previously, and all of the females were virgins; of these, nine had initiated ovipositing. Males were randomly assigned to females; some males in the ‘non-ovipositing female’ treatment may have been used more than once. Pairs were introduced 2 h before dark and the trial was terminated if copulation had not commenced after 6 h. Observations of courtship were made before the initiation of copulation, and the duration of copulation, if it occurred, was recorded. Copulation was observed using a Sony digital video camera (model-TRVI5E) that had an inbuilt night vision setting, which allowed an accurate determination of the initiation and termination of copulation.
Fecundity of females reared under different sex ratios
Females were raised in one of three treatments that differed in their exposure to males. For each replicate, six animals were placed in a clear plastic cylindrical cage (see above) that offered similar access to space and food. The treatments comprised (1) six females; (2) five females and one male; and (3) three females and three males. Three cages were used for treatment (1) and two each for the other treatments. The ends of the cylinder were enclosed with plastic food wrap and the cages were then placed under a positive air pressure (5-mm inflow tubing connected to an outdoor air compressor, and 3-mm outflow tubing connected to the building ventilation system) to prevent the transfer of male and female pheromones between cages. Each cage had to be disconnected from the airflow system during feeding, so animals from treatments with lower numbers of males were fed first.
High levels of mortality among very young instars meant that most individuals commenced the experiment as second and third instars, and individuals were randomly allocated to each treatment. Males and females were separated before their allocation to the experimental treatments, so females had no contact with males before the experiment commenced. A black nylon mesh (0.5 mm) placed inside the container separated males and females: males were placed on the inflow side of the container, maximising exposure of any odour they may have produced.
The date of sexual maturation, determined when the wings are fully developed, was noted for both males and females. Sexual maturation was determined when wings were fully developed. The initiation of ovipositing could not be determined accurately: eggs are clearly visible in the ovipositor of females before ovipositing, but the precise timing of ovipositing was difficult to predict. While ovipositors were checked every 1–2 days, ovipositing may have been overlooked for some females if they had just oviposited and/or had low ovipositing rates.
After all females within a replicate group had initiated ovipositing, they were placed in separate clear plastic cylindrical cages (see above) and the remaining males placed in 40-L tubs. Egg production and weight of eggs was measured for each female over a seven-day period.
Ovipositing by mated females
Five females and 10 males were used to obtain measurements of ovipositing rates and egg weight of mated females. Sexually mature females were maintained with two males in separate clear plastic cylindrical cages (see above). Males were randomly reassigned to females every seven days over a period of two months, which ensured that females mated multiple times with multiple males. The number and weight of eggs were then measured for each female over a seven-day period.
Data analysis
Patterns of variation in the data were analysed by least-squares models in JMP (ver. 7.0.2: 2007 SAS Institute Inc., Cary, USA). Female weight and tibia length were initially included in the models, but were subsequently dropped if they did not explain any significant variation in the parameter tested. Values given are means ± s.e., unless specified otherwise.
Results
Female signals, courtship and copulation
The Y-maze experiment demonstrated that females signal their reproductive state. Males were less likely to respond if the female had commenced ovipositing (three of 13 males responded) than if she had not (seven of 10 males responded: Fisher’s exact probability = 0.027). Of the trials in which males moved beyond the junction of the Y-maze, the males typically chose the arm leading to the female (female not ovipositing: males proceeded to the female in six of the seven trials in which males responded; female not ovipositing: males proceeded to the female in one of the three trials in which males responded).
We conducted 18 mating trials and observed courtship behaviour that involved, in all trials, the male pulling at the female’s abdomen. Copulation followed courtship in 10 trials, involving females that had and had not commenced ovipositing. The mean duration of copulation was 7.6 ± 0.9 h, and did not differ between females that had or had not commenced ovipositing (F1,8 = 0.77, P = 0.41).
Fecundity of females reared under different sex ratios
Females commenced ovipositing between 19 and 38 days after moulting to sexual maturity. The mean time to commence ovipositing was not influenced by the number of males in the cage (no males: 27.6 ± 1.1 days (n = 12); one male: 24.3 ± 1.2 days (n = 10); three males: 27.8 ± 2.8 days (n = 5); F2,19 = 0.99, P = 0.39), and there was no significant nested (cage) effect (F4,19 = 1.51, P = 0.24) or female body-size effect (F1,19 = 0.03, P = 0.86).
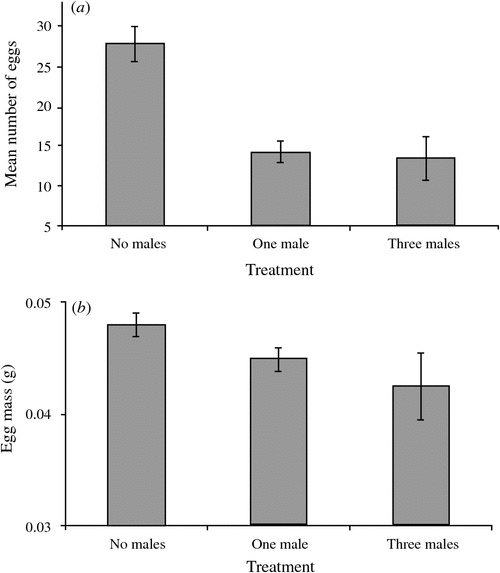
The social environment experienced during development influenced the reproductive output of females. The number of males in the cage influenced the number of eggs produced during a seven-day period (main effect: F2,20 = 17.68, P < 0.001; nested (cage) effect: F4,20 = 1.35, P = 0.28; female weight: F1,20 = 2.88, P = 0.11) (Fig. 1a). Analysis of the variation in mean egg weight revealed a significant nested (cage) effect (F4,20 = 7.51, P = 0.001) and female size effect (F1,20 = 4.66, P = 0.049). The treatment effect was therefore tested against the variance between cages, and was not significant (F2,4 = 1.27, P = 0.37) (Fig. 1b).
|
Fecundity of mated and unmated females
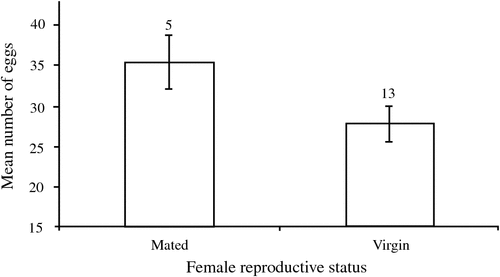
We compared the reproductive potential of females that were reproducing sexually with those reproducing asexually in two ways. First, we compared the reproductive output of females given unlimited mating opportunities with that of virgin females that had been exposed to males during their development. Mated females produce significantly more eggs in the seven-day period than virgin females (mating status: F1,32 = 11.50, P = 0.002), but there was no significant difference in the weight of these eggs (mating status: F1,32 = 1.62, P = 0.21). In the second analysis, we excluded those females that had been exposed to males during their immature stages. It is unclear whether these virgin females differed from mated females in either the size (F1,17 = 3.44, P = 0.08) or number (F1,17 = 3.50, P = 0.08) of eggs (Fig. 2). Female body size was not a significant factor in any of the above analyses (P > 0.25) and was removed from the models.
|
Discussion
Several reproductive strategies, including the production of mate-attracting signals and ovipositing behaviour, apparently covary with the mode of reproduction in the facultatively thelytokic spiny stick insect E. tiaratum. Virgin females that have not commenced ovipositing attract males, but cease to do so after they commence ovipositing. Females exposed to males during their juvenile stages have a lower ovipositing rate over a seven-day period than females that were not exposed to males, suggesting that females may temporarily retard the production of eggs in the expectation of future mating opportunities. More generally, the reproductive output of virgin females over a seven-day period is lower than that of mated females.
The signal produced by virgin females of E. tiaratum is most likely chemical in nature. Our experimental design excluded the possibility of visual cues, and auditory signals (or the capacity to detect such signals) have not been reported for phasmatids (see Hoy and Robert 1996; Stumpner and von Helversen 2001). While sex pheromones have not been identified for phasmatids, the production of alarm pheromones to deter predators is well known in this group (see Carlberg 1981; Dossey et al. 2008). Significantly, virgin females suspend signalling after they initiate oviposition, perhaps to minimise or eliminate the costs of mate attraction while reproducing asexually. While direct evidence of the metabolic cost of chemical production has proved elusive (Wyatt 2003; Johansson and Jones 2007), there are numerous examples of natural enemies locating victims by using their chemical signals as cues (see Zuk and Kolluru 1998; Wyatt 2003). While virgin females may cease signalling, they do not become sexually unreceptive: females copulate after they have commenced oviposition, and the duration of copulation is unaffected by whether the female has commenced oviposition.
Virgin females of E. tiaratum adjust their asexual ovipositing rate in response to their exposure to males during their growth and development: females that had been exposed to males laid, over a seven-day period, almost 50% fewer eggs than those reared without males. This difference is unlikely to arise through differences in nutrient acquisition, as each container had the same number of individuals, and the observed patterns were significant after controlling for female size, which is likely to reflect nutrient acquisition. Rather, the presence of males may provide some indication of the likelihood of mating, and virgin females initially lay fewer eggs in anticipation of producing sexual eggs at some later stage. In contrast, females that fail to encounter males during their immature stages may be less likely to encounter a male during their adult lifetime and thus produce offspring at a higher rate. Indeed, the mean number of eggs laid over a seven-day period by virgin females that had not been exposed to males (27.7 ± 2.21) is comparable to that of mated females (35.4 ± 3.33) (Fig. 2). This explanation assumes that the prospects of sexually produced eggs are greater than that of asexually produced eggs, for which there is some evidence in phasmatids (see Bedford 1978 for review) and other insects (e.g. Corley and Moore 1999; Kramer and Templeton 2001; but see Matsuura and Kobayashi 2007; Morgan-Richards et al. 2010). Indeed, females of many insects adjust their ovipositing rates in response to environmental conditions that may affect the survival of offspring (e.g. Mitrovski and Hoffmann 2001), and perhaps females of E. tiaratum similarly adjust their reproductive output. It is surprising that virgin females reared in the absence of males did not commence ovipositing earlier than females that had been reared with males, as might be expected in theory.
The mechanism by which virgin females perceived, while immature, the presence of males is unclear, but they could utilise a combination of tactile and chemical cues. It seems unlikely that females can determine the number of males; the difference in reproductive output was strongest between the presence and absence of males, rather than between one and three males. The difference in reproductive output of mated and virgin females may be a simple response to the presence of male accessory products transferred during copulation. Numerous studies report increases in female reproductive output as a result of male accessory products (e.g. Gillott 2003), although these effects are unknown for E. tiaratum.
Our results are broadly consistent with the view that selection favours facultative thelytoky in environments in which sex is difficult or impossible (e.g. Stalker 1956; Kramer and Templeton 2001; Ball 2002). Ecological data from natural populations are required, but securing a mating partner may be challenging for female E. tiaratum because the relatively shorter lived males may be widely dispersed and uncommon. While females are potentially long-lived, thereby increasing their chances of securing a sexual partner, their cryptic manner and chemical defences suggest that the risk of predation is generally high and thus there would be strong selection to commence ovipositing shortly after attaining sexual maturity. Facultative thelytoky may provide some reproductive output while not excluding the possibility of producing higher-quality offspring following mating. Indeed, facultative thelytoky may allow females to avoid the cost of mate choice associated with remaining unmated. Finally, asexual reproduction in E. tiaratum has interesting implications for sex allocation, because an increased frequency of asexual reproduction will create a more female-biased operational sex ratio. Perhaps mated females compensate by producing relatively more sons.
Acknowledgements
We thank Alan Henderson (Melbourne Museum) and Patrick Honan (Melbourne Zoo) for providing insects; and Rob Day, Michael Kearney, Therésa Jones, Peter Miller and several anonymous referees for helpful comments and advice.
Ball, S. L. (2002). Population variation and ecological correlates of tychoparthenogenesis in the mayfly, Stenonema femoratum. Biological Journal of the Linnean Society. Linnean Society of London 75, 101–123.
| Crossref | GoogleScholarGoogle Scholar |
Cáceres, C. E. , Hartway, C. , Kimberly, A. , and Paczolt, K. A. (2009). Inbreeding depression varies with investment in sex in a facultative parthenogen. Evolution 63, 2474–2480.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Kondrashov, A. S. (1993). Classification of hypotheses on the advantage of amphimixis. The Journal of Heredity 84, 372–387.
| CAS | PubMed |
Mitrovski, P. , and Hoffmann, A. A. (2001). Postponed reproduction as an adaptation to winter conditions in Drosophila melanogaster: evidence for clinal variation under semi-natural conditions. Proceedings. Biological Sciences 268, 2163–2168.
| Crossref | GoogleScholarGoogle Scholar | CAS |
Stalker, H. D. (1956). On the evolution of parthenogenesis in Lonchoptera (Diptera). Evolution 10, 345–359.
| Crossref | GoogleScholarGoogle Scholar |
Zuk, M. , and Kolluru, G. R. (1998). Exploitation of sexual signals by predators and parasitoids. The Quarterly Review of Biology 73, 415–438.
| Crossref | GoogleScholarGoogle Scholar |



