Positive and negative effects of phoretic mites on the reproductive output of an invasive bark beetle
Lisa K. Hodgkin A B , Mark A. Elgar A and Matthew R. E. Symonds AA Department of Zoology, University of Melbourne, Parkville, Vic. 3010, Australia.
B Corresponding author. Email: l.hodgkin@pgrad.unimelb.edu.au
Australian Journal of Zoology 58(3) 198-204 https://doi.org/10.1071/ZO10034
Submitted: 13 May 2010 Accepted: 12 August 2010 Published: 23 September 2010
Abstract
When multiple species coexist upon a single host, their combined effect on the host can be unpredictable. We explored the effect of phoretic mites on the reproductive output of the five-spined bark beetle, Ips grandicollis. Using correlative approaches and experimental manipulation of mite numbers we examined how mite load affected the number, size and condition of bark beetle offspring produced. We found that mites have both negative and positive consequences on different aspects of bark beetle reproduction. Females from which mites were removed were more fecund and produced larger offspring than females with mites, implying a cost of mite loads. However, when mites were present on females, those bearing the highest mite loads produced offspring that were larger and in better condition, indicating a beneficial effect of mites. These data suggest that phoretic interactions between mites and bark beetles differ over the course of the host’s lifespan, with either the mites interacting in different ways with different life stages of the host (parasitic on adult, mutualistic with larvae), and/or the beetles being host to different mite assemblages over their lifetime.
Additional keywords: Acari, commensalism, ectoparasite, host reproduction, mutualism, Scolytinae, symbiosis.
Introduction
Phoresy, a form of ectosymbiosis, occurs when an organism gains the benefit of dispersal by superficially attaching to its host. Phoresy is often described as a form of commensalism, with the assumption that the host is unaffected by the interaction. However, the host is often strongly influenced by its phoretic companions due to the close association of the ectosymbiont (Houck and Cohen 1995). Phoretic ectosymbionts often synchronise with their host’s life cycle and must stay close to potential hosts in order to disperse (Okabe and Makino 2008). In some cases, this close association becomes mutualistic, but in most studies the interaction is costly to the host (Houck and Cohen 1995), and thus is more appropriately deemed parasitic. Costs can include reduction in host mating success (Forbes 1993), amplification of required reproductive and parental effort (Møller 1990), and increased offspring mortality (Lindquist 1969; Blackman and Evans 1994). In cases where the presence of ectosymbionts is beneficial to their hosts, typically a third species is involved (Proctor and Owens 2000; McLachlan 2006). For example, large numbers of phoretic mites on bumblebees are associated with low levels of parasitic nematodes (Walter and Proctor 1999), and the presence of feather mites increases the daily survival of cliff swallows, Petrochelidon pyrrhonota Vieillot (Passeriformes: Hirundinidae), possibly because mites are feeding on harmful bacteria (Brown et al. 2006).
Bark beetles (Coleoptera: Curculionidae: Scolytinae) are of particular ecological and economic interest because of their destructive potential for forestry (Kurz et al. 2008). These small (<5 mm) insects typically aggregate in large numbers, boring into the bark of coniferous trees and feeding on the phloem layer just beneath the bark (Wood 1982). They can coexist with many other organisms and microbes, including multiple species of mites, fungi and nematodes, which they often carry with them as they travel between trees (Moser et al. 2005; Cardoza et al. 2008). Although phoretic for at least a component of their life cycles, both mites and fungi can be either parasitic or mutualistic to their bark beetle hosts at other stages (Lombardero et al. 2003; Hofstetter et al. 2006). Some fungi are a food resource for developing beetles (Bentz and Six 2006) or help the beetle to overcome tree defences (Paine et al. 1997), while others may cause serious fitness costs to the beetle or its offspring (Cardoza et al. 2008). Similarly, some mites parasitise beetle eggs (Lindquist 1969; Blackman and Evans 1994) but others may help the beetle by feeding upon fungi (Moser et al. 2005) or nematodes (Karagoz et al. 2007) that are potentially harmful. Adding to this complexity, numerous species of mite can coexist on the body of a single bark beetle. The combined impact of these multiple mite species upon their host has seldom previously been explored.
Ips bark beetles are polygynous with a single male attracting between one and seven females in discrete harems (Kirkendall 1983). Females join a male, mate and construct elaborate gallery systems where they lay their eggs, and where their offspring grow to maturity. Ips grandicollis Eichhoff is native to North America, but was accidentally introduced into Australia in 1943 (Morgan 1967). In this study, four different species of mites, from four separate families, were identified as attaching phoretically to adult I. grandicollis (Stone and Simpson 1991; B. Halliday, pers. comm.). One of these mites, Macrocheles boudreauxi Krantz (Acarina: Macrochelidae), is an associate of I. grandicollis in North America, and is a predator, feeding primarily upon nematodes (Kinn and Witcosky 1977). Mite species present from three other families (Tarsonemidae, Acaridae and Histiostomatidae) could be identified only to family level, so their feeding behaviour cannot be defined with certainty. Typically, though, tarsonemid mites are fungivorous, while acarid mites are scavengers and histiostomatids are generally microbial filter feeders (Lindquist 1969; Stone and Simpson 1991; Moser et al. 2005; B. Halliday, pers. comm.). This community of mites therefore has the potential of both positively affecting bark beetles by removing parasites or pathogens such as nematodes and fungi, but also negatively affecting their host by preying on larvae or imposing energetic costs.
The aim of this study was to determine the overall costs or benefits of ectosymbiotic mite communities on I. grandicollis by examining their effect on host reproductive output. We examined how variation in mite load affected the reproductive output of beetles in a laboratory environment. By experimentally removing and manipulating mite loads, we were able to separate these mite effects from natural variation in beetle reproductive output. Although individual ectosymbiont effects have been studied in other systems (Lehmann 1993; Tompkins et al. 1996), there are surprisingly few studies of the effect of a collective ectosymbiotic community on its host.
Materials and methods
Beetle culture and measurement
All beetles came from laboratory cultures originally collected from a Pinus radiata plantation near Woodend, Victoria, 70 km north-west of Melbourne (37°35′S, 144°55′E) in February 2008. To initiate cultures, freshly cut P. radiata logs (average circumference = 55 cm, length = 40–50 cm) were laid out on the ground of the plantation as an attractant for beetle infestation.
Once logs had become suitably infested, as evidenced by the appearance of boreholes and orange frass, they were transported to the Department of Zoology, University of Melbourne. Cultures of beetles were maintained in logs that were stored in four plastic 50-L bins, at 30°C. Each bin was lined with paper towel, which was regularly replaced. The bark of each log was checked daily for emerging beetles. Newly eclosed adult beetles emerged from the logs and were refrigerated for storage in individually labelled 5-mL vials for up to 21 days before use (average = 13 days).
The length of all beetles was measured from the tip of the head to the end of the elytra, using digital callipers. Beetles were also weighed to 0.1 mg. The sex of beetles was determined by examination of the comparative sizes of the second and third declivital spine along the posterior margin of the beetle’s elytra, following Lanier and Cameron (1969). In cases of uncertainty, beetles were not included in sex-based analyses.
Experimental arrangement
Logs used in these experiments were collected during 2008 from the Woodend plantation. In all, 86 logs were cut from 16 different, recently fallen trees (determined as having fallen within 3 weeks, with pine needles still fresh). All logs were sawed to 30 cm in length and 30–40 cm in circumference and stored at 22°C. The time from when each log was stored until the experiment commenced indicates log freshness (i.e. age).
A single harem was established in each experimental log to ensure that offspring could be traced to their parents. Each harem consisted of one male and three females, reflecting the average harem size for natural populations of I. grandicollis in Australia (Latty et al. 2009). Colonisation was established by creating a small, 5-mm-deep hole in the bark in the middle of each log using a bent dissecting probe. A male was placed on the bark adjacent to the hole, and kept under an inverted 5-mL vial to prevent his escape. Prior to introduction, all males were cleared of mites using a mounted dissecting needle.
Logs were examined 3–5 days after the introduction of the male beetle. A male was deemed to have successfully established in the log if orange frass could be seen inside the attached vial. The introduction was deemed to have failed if there was no frass, or the beetle was visible on the surface of the log, in which case the male was removed (dug out if necessary), and the introduction process repeated with a new male on the reverse side of the log. If establishment failed a second time, the log was discarded and replaced with a new log and the establishment procedure repeated.
After successful introduction of a male, three females were introduced to the log, each on consecutive days. Females were introduced to the hole using the above process, after removing any surface frass and thus ensuring that the holes were visible and easily accessible. If a female beetle failed to enter the established entrance hole or a beetle inside actively blocked entry, she was removed and a different female was introduced 24 h later. After a successful harem was established, the log was placed within a plastic bag, measuring 56 cm long and 30 cm wide and containing two small air holes (5 mm diameter) covered with fine gauze. A layer of paper towel was placed underneath the log to absorb moisture. The bag was sealed and stored at a constant 24°C and 45% (±5%) humidity.
The effect of mite load on the reproductive output of beetles was investigated experimentally by comparing the reproductive output of females with natural mite loads (control) with females subjected to two treatments, in which mites were either removed from or added to the beetles. Control female beetles were initially separated, according to their natural mite loads, into harems bearing low (≤15) or high (≥25) mite loads.
For each of the two manipulation treatments, females were divided into either low (≤15) or high (≥25) mite loads according to their natural, or their added, load. For the removal treatment, all naturally occurring mites were removed from each female, using a mounted dissecting needle, before introduction of females into the log. In the addition treatment, females were randomly allocated either low or high mite quantities, irrespective of their initial natural mite load. All naturally attached mites were counted and then removed with a dissecting mounted needle. These cleared females were placed in individual Eppendorf tubes. New mites were obtained from beetles not used in any experimentation and transferred to the Eppendorf tube containing the clean female subject. The needle was rubbed against the beetle to dislodge mites and encourage attachment to the new host. Note that, because the beetles were placed in tubes containing mites derived directly from other beetles, the relative proportions of each mite species added to each individual was kept approximately constant. Beetles assigned to the low-mite group received a maximum of 15 mites into the Eppendorf tube. The females assigned to the high-mite group received a minimum of 70 mites to facilitate the attachment of over 25 mites. The low-mite group also received several extra manipulations with a clean needle to ensure that handling was consistent across groups. After 24 h, females were taken out of the Eppendorf tube, the number of attached mites counted, and the beetles introduced into an experimental log.
Each log was left for 88 days to allow beetles to breed and their offspring to reach maturity. During this time, the plastic bag was opened at least twice to collect any beetles that had emerged and to replace the paper towel with a fresh layer. After this period, each log was stripped of bark using a hobby knife and all beetles and larvae were collected in individual Eppendorf tubes. Adult beetles collected from underneath the bark were weighed immediately and their length measured within two days of collection. All offspring, regardless of their developmental stage, were frozen for preservation. Subsequently, the sex and mite load of each adult offspring was recorded. As before, offspring whose sexes could not be ascertained accurately were excluded from analysis (n = 111 of the 1423 examined; 8%). All collections of offspring, as well as measurements and weights, were performed blind, without knowledge of the experimental treatment of the harem.
Reproductive output of each harem was assessed through the number of offspring produced, body length and body condition. The number of offspring was calculated as the number collected per log, divided by the number of introduced female beetles. This procedure was necessary because 12 of the 86 logs contained only two female beetles that had established successfully (rather than the requisite three). This was due either to continual blocking of female entry by the male, the hole being blocked tight with frass, or multiple females refusing entry.
Length was used as a measure of offspring size because this is fixed at maturity (Grimaldi and Engel 2005). The body condition of offspring was calculated as the residual derived from the linear regression of ln(offspring weight) on ln(offspring length) (see Jakob et al. 1996). Residuals were obtained from separate regressions within each treatment group to control for across-treatment variability.
Data analysis
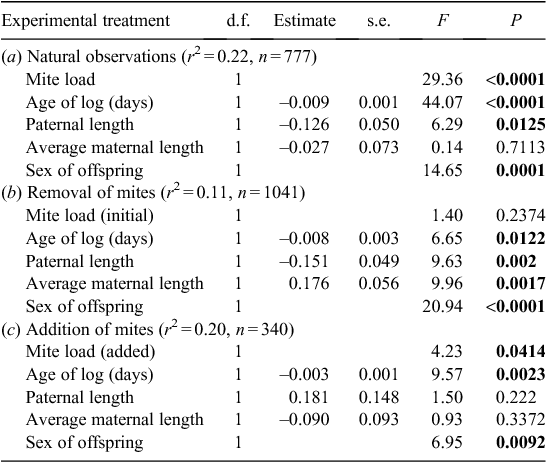
Data were analysed using JMP 7.0.1 2007 (SAS Institute, Cary, NC, USA). The distribution of the number of offspring produced by females was strongly skewed, and so models predicting number of offspring were run using a Poisson regression. Four models were run for each response variable: one to test across the three treatment groups (i.e. the unmanipulated beetles and the two manipulation treatments), and one each to test for the effect of maternal mite load within each of the three groups. Models explaining variation in the number of offspring produced included the factors of paternal length, average maternal length, age of log used, as well as treatment and mite load where appropriate.
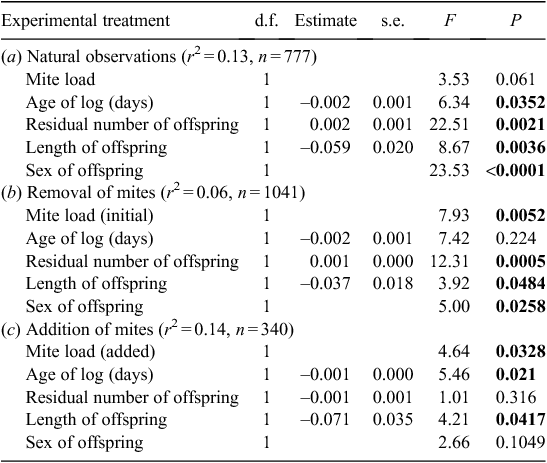
All individual measurements of offspring size and condition were used for the analysis of offspring size, rather than an average per log. The identity of the log was included as a random factor in all general linear models run at this level because offspring were effectively nested within logs. Models initially included age of each log, mite load, paternal length, average maternal length, residual number of offspring, sex of offspring and length of offspring, but factors not significant (P > 0.05) within any of the treatment groups were excluded from the final model. Logs that contained no larvae and had ≤4 adult beetles (the number of parent beetles introduced), present were not included in the analyses.
Models predicting offspring length included the number of offspring produced as a factor because of the possibility of trade-off between number and size of offspring (Smith and Fretwell 1974). However, number of offspring produced was not independent from the age of each log. Therefore, we used a residual measure for number of offspring, derived from the regression slope between age of log and number of offspring.
Results
The presence of mites, the age of each log and parental size significantly explained the variation in the number of offspring produced across the three treatments (Table 1). Notably, females that had their initial mites removed were significantly more fecund than females with natural or assigned mite loads (Fig. 1). The number of offspring produced was strongly negatively correlated with the age of the log. The variation in number of offspring produced within each treatment group was not explained by differences in maternal mite load (natural: χ2 = 0.09, P = 0.76; removal: χ2 = 0.35, P = 0.55; addition: χ2 = 0.35, P = 0.55).
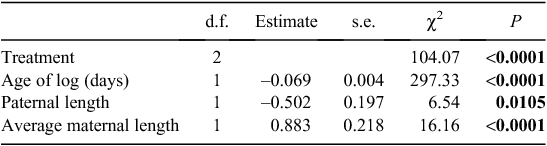
|
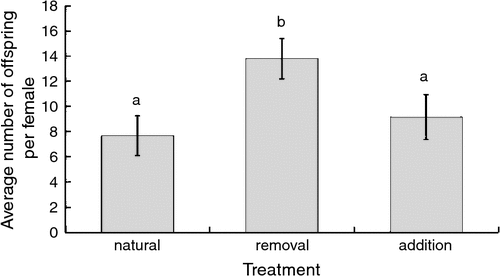
|
Average offspring size was affected by treatment, parental and log factors (Table 2). Females with mites removed produced larger offspring than those with natural mite loads, while females in the addition treatment produced smaller offspring than those in the other two treatments (Fig. 2). Moreover, the length of offspring was negatively correlated with both paternal length and age of log. In all treatments, male offspring were significantly larger than female offspring.
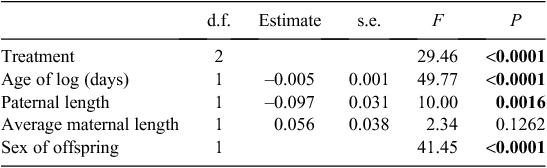
|
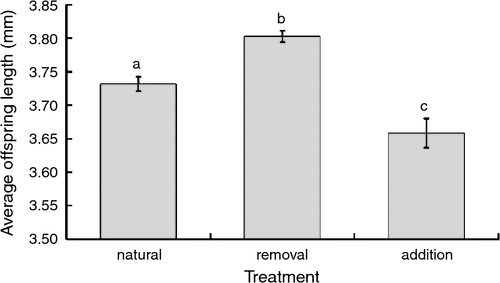
|
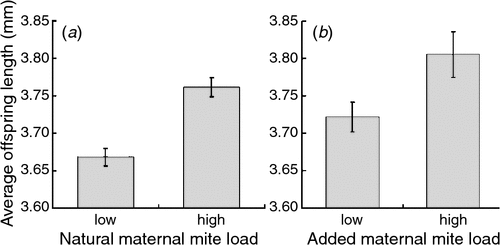
Within both the control and addition experimental groups, high mite loads had a positive effect on offspring size (Table 3); females with higher mite loads produced larger offspring (Fig. 3). This pattern was not related to initial mite load, as shown by the results of the removal treatment group (Table 3b). The age of the log negatively affected offspring size and condition (Tables 3, 4).
|
|
|
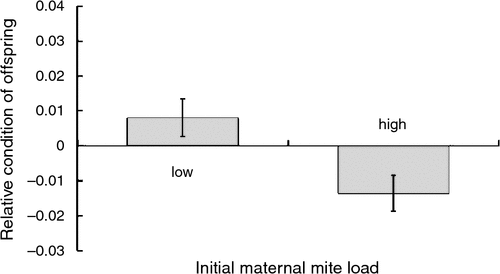
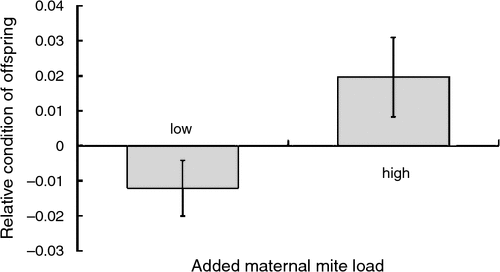
Maternal mite load also influenced offspring condition within both the removal and addition experimental groups (Table 4). In the former group, mothers who initially bore low mite loads produced offspring in better body condition than mothers with initially high mite loads (Fig. 4). In contrast, females given high mite loads tended to produce offspring in better condition than those given lower mite loads (Fig. 5).
|
|
The sex ratio of offspring did not differ significantly between any of the three experimental groups (F2,64 = 0.30, P = 0.74). Neither was there any effect of maternal mite load on offspring sex ratio across experimental groups (untreated: F1,23 = 0.01, P = 0.93; removal: F1,27 = 1.40, P = 0.25; addition: F1,14 = 1.29, P = 0.29).
There was a strong positive correlation between adult offspring length and the numbers of mites attached to them (F1,1115 = 17.94, P < 0.0001).
Discussion
The presence of phoretic organisms can have strong consequences for their host. These consequences can become complicated when considering the multitude of ectosymbionts that hosts may encounter in their natural environment. For this study we investigated the effect of a community of four different mite species on the reproduction of a bark beetle. Our study suggests that mite load can have both a negative and positive effect on aspects of host reproduction. While this finding presents an intriguing insight into the complexity of the interaction between the host beetle and its complement of phoretic mites, it is worth initially highlighting several difficulties with the interpretation of these results. The first of these lies in the inability to tease apart the effects that each individual mite species is having on bark beetles specifically. While our experimental manipulations involving the addition of mites endeavoured to fairly reproduce the relative numbers of mites and the ratio of mite species so that they adhered closely to the observed natural levels, there will obviously be inaccuracies that introduce noise into the analysis. Additionally, because mite communities are likely to vary in composition between populations of beetles, similar removal and addition experiments could yield different results in other populations. Second, the controlled nature of our study necessitated a laboratory-based investigation. Although mites, and heavily infested beetles, are readily seen in the field, particularly in dense beetle aggregations (M. Symonds, pers. obs.), it is possible that the number and proportion of mites cultured in the laboratory environment differs from that found in the natural environment. It is possible, then, that the effects we have observed here are mitigated or augmented in the natural environment.
We found that the presence of mites had a strong negative effect on the reproductive success of Ips grandicollis. All female beetles that entered the log with mites present showed a significant reduction in both number and size of offspring compared with beetles from which the mites were removed. We also found that the condition of beetle offspring was negatively correlated with the initial number of mites present on a female. These results indicate that the host beetle is being parasitised to some extent by their ectosymbiotic burden. This parasitism may be occurring in a variety of ways. Mites often travel upon bark beetles as a hypopus (a nymphal stage specialised with sucker plates or grasping organs for dispersal). A hypopus has no mouthparts, but studies suggest that some mite species may nevertheless extract nutrients from their host in this form (Houck and Cohen 1995). Using tritium radiolabelling, Houck and Cohen (1995) showed that material passed from Chilocorus cacti Linnaeus (Coleoptera: Coccinellidae) beetles into their attached astigmatid hypopodes through a small wound made by the ventral sucker plate of the mite. Thus, although they appear morphologically passive, these mites are functionally capable of parasitising their host. The majority of attached mites observed in this study were in a hypopus life stage, so a similar occurrence may exist in Ips grandicollis.
We also found that the condition of beetle offspring was negatively correlated with the initial number of mites present on a female. In the removal treatment, beetles that had previously experienced a low mite load produced offspring in better condition than those that had previously experienced a high mite load. These data suggest that either some of the mites are ectoparasites, or the distribution of mite load is not random. If the mites parasitise adult I. grandicollis, then the interesting conclusion is that mites are lowering the quality of their host before entry into a breeding log. Alternatively, this pattern may arise if mites preferentially attach to lower-quality beetles. Mites can actively choose their host (Lyon 1993; Krasnov et al. 2002), but it is typically in the opposite direction, preferring larger hosts, or those likely to be more fecund (Huck et al. 1998; Valera et al. 2004).
In contrast, the number of mites present during the experiment had a positive effect on bark beetle reproduction. Within experimental groups, females with a higher mite load produced larger and better-conditioned offspring than females carrying fewer mites. This pattern may be caused by the maternal investment strategies of I. grandicollis or by a direct effect of the mites themselves. In birds, mothers increase their current reproductive investment, at the expense of their lifetime reproductive success, in response to increased parasitism (Møller 1990; Fitze et al. 2004). Female I. grandicollis may similarly alter their reproductive effort to minimise the impact of ectoparasites (Forbes 1993). Alternatively, I. grandicollis and its associated mites may have a mutualistic relationship. Mites associated with Dendroctonus pine beetles are beneficial to their host’s reproductive success by feeding upon fungal strains that are antagonistic to the growth of beetle larvae (Cardoza et al. 2008). Several unidentified fungal species were present on logs used in these experiments, and their prevalence appeared to increase with the age of each log, which may partly explain the reduced reproductive success of beetles in these logs (previous field studies also suggest that Ips beetles prefer fresh wood: e.g. Hayes et al. 2008). Further, a more direct mutualistic interaction may occur, with mites actively stimulating growth or discouraging predation of larvae, analogous to the ants that protect lycaenid butterfly larvae from parasites and predators in exchange for liquid secretions produced by the larvae (Pierce et al. 2002). Larvae of the ant-tended butterfly Jalmenus evagoras Donovan (Lepidoptera: Lycaenidae) grow faster in the presence of ants (Pierce et al. 1987). Mites would benefit if the beetle larvae become larger and developed more quickly. Phoretic ectosymbionts prefer larger hosts that are in better condition (Valera et al. 2004; Grossman and Smith 2008). Indeed, there was a strong positive correlation between the size of Ips grandicollis offspring and their mite loads. Manipulating the numbers of individual mite species in Ips larval galleries would reveal whether the presence of mites influences larval growth.
A likely explanation of the seemingly disparate results obtained in this study is that the different mites present are affecting the host in different ways or at different times throughout their life cycle. For example, tarsonemid mites found upon species of Ips are often phoretic for one phase of their lives but detach upon arrival in a gallery to prey upon beetle eggs (Lindquist 1969). Indeed, females with a high reproductive value often bear the bulk of the phoretic burden within populations (Binns 1982). Asymmetrical ectosymbiotic interactions with different host life stages have been reported in astigmatid mites that are phoretic upon bees (Eickwort 1994). These mites are parasitic to adults, but do not harm juvenile hosts. Alternatively, the changing nature of the interaction between mites and adult and larval beetles may be a result of diverse interspecific interactions in the ectosymbiont community, with different mite species influencing the host in different ways at different times (Lindquist 1969). The four coexisting phoretic mite species in this study may influence the reproduction of their host bark beetle in contrasting ways, ranging from beneficial to detrimental. Finally, mite community composition may change in the balance of beneficial and parasitic species according to the level of mite infestation (e.g. highly infested females may have a greater proportion of mutualistic mites, resulting in better-conditioned offspring). However, our experimental manipulations should have balanced out relative proportions of mite species across heavily and lightly infested beetles, and reduced any such effect on offspring and condition in the addition treatment, but this was not the case.
The interaction between I. grandicollis and its associated phoretic mites appears to involve both parasitism and mutualism. Specifically, mites could be antagonistic to, and impose costs on, adult beetles while being beneficial to their larvae. This could stem from the behaviour of a single mite species or multiple species having disparate interactions with their host beetle. The exact mechanism underlying the interactions investigated here remains unknown: the interspecific relationships between the multiple mite species in the ectosymbiont community need to be disentangled to determine whether individual species are having independent impacts. Nevertheless, the intriguing and unexpected dual aspects obtained from this experiment highlight the importance and compelling nature of the ectosymbiont–host relationship in this invertebrate system.
Acknowledgements
We thank Dr Bruce Halliday (CSIRO Entomology, Canberra) for assistance in the identification of mite families and advice on mite biology. Hancocks Victorian Plantations allowed us access to the Woodend pine plantation. Michael Magrath provided statistical guidance and Therèsa Jones provided feedback on experimental design and interpretation. We also thank the three referees for their input, and the Animal Behaviour and Evolution Group at the University of Melbourne for general discussion. Financial support was provided by grants to MAE and MRES from the Australian Research Council. This research complies with the current laws in Australia.
Bentz, B. J. , and Six, D. L. (2006). Ergosterol content of fungi associated with Dendroctonus ponderosae and Dendroctonus rufipennis (Coleoptera: Curculionidae, Scolytinae). Annals of the Entomological Society of America 99, 189–194.
| Crossref | GoogleScholarGoogle Scholar | CAS |
Fitze, P. S. , Tschirren, B. , and Richner, H. (2004). Life history and fitness consequences of ectoparasites. Journal of Animal Ecology 73, 216–226.
| Crossref | GoogleScholarGoogle Scholar |
Grossman, J. D. , and Smith, R. J. (2008). Phoretic mite discrimination among male burying beetle (Nicrophorus investigator) hosts. Annals of the Entomological Society of America 101, 266–271.
| Crossref | GoogleScholarGoogle Scholar |
Wood, S. L. (1982). The bark and ambrosia beetles of north and central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Naturalist Memoirs 6, 1–1359.



