Two new species of burrowing scorpions (Urodacidae: Urodacus) from the Pilbara region of Western Australia with identical external morphology†
Bruno A. Buzatto A B C D * , Huon L. Clark B C , Mark S. Harvey
A B C D * , Huon L. Clark B C , Mark S. Harvey  C D and Erich S. Volschenk
C D and Erich S. Volschenk  C E F
C E F
A
B
C
D
E
F
Abstract
Two new species of urodacid scorpion are described from the Pilbara region in Western Australia, where they are both patchily distributed along creek lines in the north-east of the region. Urodacus uncinus sp. nov. and Urodacus lunatus sp. nov. are indistinguishable based on external morphology: adults are medium-sized, yellow burrowing scorpions with remarkable sexual dimorphism in the telson, in which males have a uniquely swollen vesicle and an aculeus that is more strongly curved than other known species of Urodacus. The species are superficially similar to Urodacus similis L.E. Koch, 1977 and Urodacus yaschenkoi Birula, 1903 in the morphology of the first four metasomal segments, which are extremely short and not much longer than high. The two new species can only be discerned from each other based on the morphology of their hemispermatophores, which highlights the extremely conserved morphology of species in the genus and suggests that many new species await description with careful examination of their genitalia.
Keywords: Arachnology, arid uplands, Iurida, Scorpiones, Scorpionoidea, sexual sting, short-range endemism, taxonomy.
Introduction
The genus Urodacus Peters, 1861 is endemic to mainland Australia, where it represents the largest radiation of scorpions on the continent (Koch 1977). Most species in the group live in deep burrows, which has enabled them to successfully persist in arid ecosystems, where scorpion diversity and abundance result in a large biomass and an ecologically significant assemblage (Polis 1990). The importance of Urodacus scorpions in Australia, especially in arid areas, was previously recognised by Volschenk et al. (2010), and a few studies are available on their behaviour (Quinlan et al. 1995; Roldan and Gaffin 2018) and ecophysiology (Zwicky 1968; Withers and Smith 1993; White 2001). Urodacus does not represent a medically important group of scorpions (Isbister et al. 2004), but the venom of some species has nevertheless been relatively well studied (Luna-Ramírez et al. 2013, 2015; Luna-Ramirez et al. 2017a). In contrast, the taxonomic diversity of the genus remains understudied despite numerous new species being collected across the continent in the past three or four decades (ESV, unpubl. data).
The phylogenetic position of Urodacus has been controversial (Fet and Soleglad 2005; Prendini and Wheeler 2005; Soleglad et al. 2005), but a close relationship with the genus Heteroscorpion Birula, 1903 (Heteroscorpionidae), from Madagascar, has been inferred using morphological data (Volschenk and Prendini 2008). The Western Australian endemic species U. planimanusPocock, 1893, has also been used in recent higher-level phylogenetic studies of the order Scorpiones (Sharma et al. 2015, 2018). Three species of Urodacus (family Urodacidae) were used in a phylogenomic study of the order Scorpiones, which placed Urodacidae as sister to the clade comprising Diplocentridae + Scorpionidae + some Hormuridae (Santibáñez-López et al. 2022). Despite the attention that the phylogenetic position of the genus has received, the species-level taxonomic framework for Urodacus is still very weak. The most significant contribution to the taxonomy of the genus came from the revision included in Koch (1977), which synonymised 13 of the previously described species and described seven new species, leading to 19 recognised species. In the following 45 years, only two additional species have been described, namely U. mckenziei Volschenk, Smith & Harvey (Volschenk et al. 2000) and U. butleri Volschenk, Harvey & Prendini (Volschenk et al. 2000, 2012), leading to 21 currently recognised species. The Western Australian Museum collection contains numerous undescribed species (unpubl. data), with nine undescribed species being revealed in a single survey in the Pilbara region (Volschenk et al. 2010). Moreover, there is strong evidence that some of the described species represent complexes of multiple somatically similar or even cryptic species, including a recent molecular study on Urodacus yaschenkoi (Birula, 1903) (Luna-Ramirez et al. 2017b).
The limits of most species included in Koch’s (1977) revision are broadly and vaguely defined, so a complete reassessment of the validity and diagnosis of each nominate taxon is urgently needed. Until this is completed, descriptions of new species are hampered by the fact that newly collected species fit the concept of one of the broadly defined species, which is particularly problematic for the current concepts applied to U. yaschenkoi and U. armatus Pocock, 1888. In fact, the last few decades saw only the most clearly distinctive new species described, and here we describe two unmistakably new species of Urodacus. Males of these species, here named Urodacus uncinus sp. nov. and Urodacus lunatus sp. nov., have remarkable telsons, with a swollen vesicle and an aculeus that is much more strongly curved than in other species in the genus. The combined morphology of the vesicle and the aculeus render the telson a unique shape that is unambiguously distinct from that of any other urodacid scorpion. U. uncinus sp. nov. and U. lunatus sp. nov. are, however, indistinguishable from each other externally, and can only be separated based on the morphology of the hemispermatophore. The most evident difference is that the hemispermatophore of U. lunatus sp. nov. bears a laminar hook that is distally sickle shaped and extends past the tip of the conical process. The two new species occur along creek lines (Fig. 1) in the north-east portion of the Pilbara (Fig. 2), and seem to both be relatively rare species, underrepresented in the WAM collection when compared to other morphospecies more commonly found in the numerous biodiversity surveys linked to mining proposals and developments in the area. Documenting the biodiversity of scorpions, a group that is renowned for containing short-range endemic species (sensuHarvey 2002), is especially important in a landscape under intense mining pressure. Moreover, the two new species emphasise the conserved external morphology in the genus Urodacus, further increasing the importance of developing their taxonomic framework for conservation.
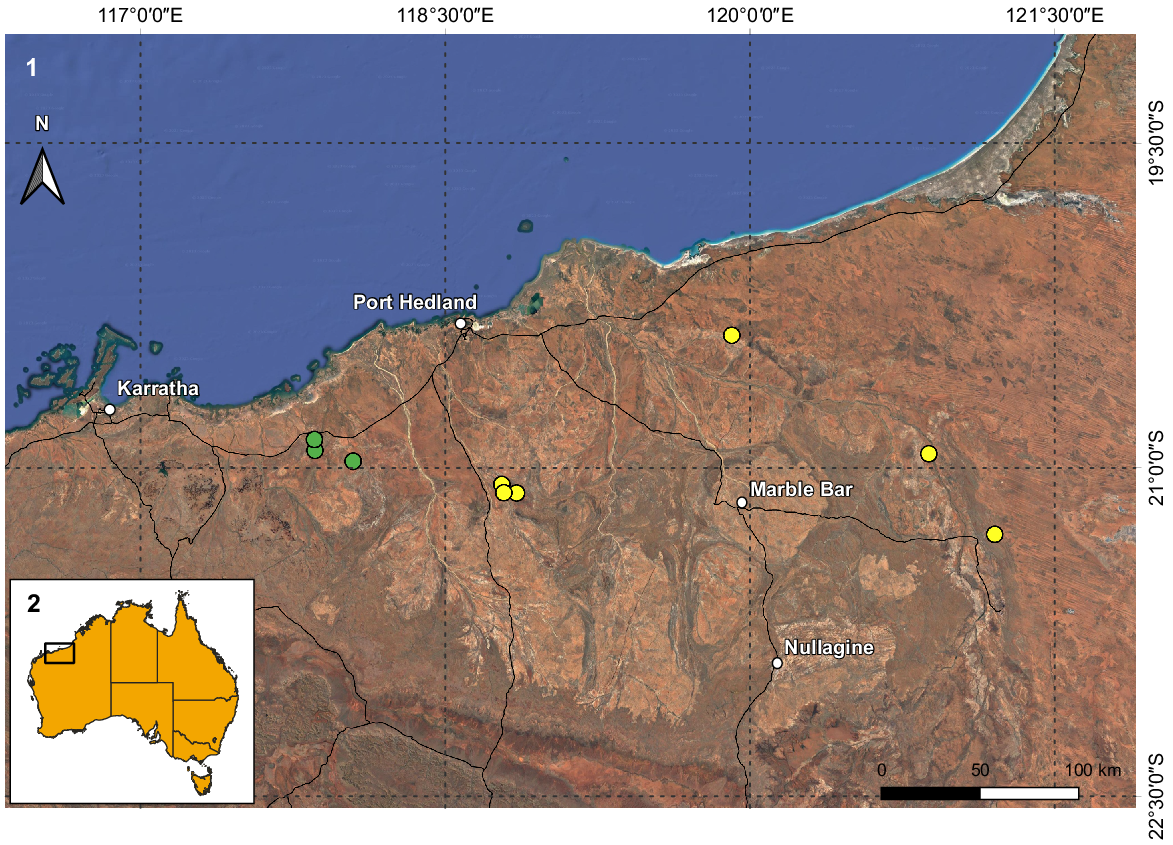
(1) Map of the northern coast of the Pilbara region in Western Australia with the known records of Urodacus uncinus sp. nov. (yellow circles) and U. lunatus sp. nov. (green circles). (2) Inset illustrates Australia and the extent of Fig. 1.
Materials and methods
The material we examined is deposited in the collection of the Western Australian Museum, Perth (WAM). We conducted microscopic examinations using a Leica MZ6 (Leica Microsystems, Wetzlar, Germany) and a Nikon SMZ1270 (Nikon Corporation, Tokyo, Japan) stereo dissecting microscopes and recorded measurements for two males of U. lunatus sp. nov. (WAM T79449 and T79450; females of this species are unknown), as well as three males (WAM T79442, T79444, T79446) and four females (WAM T107077, T113700, T113701 and T113703) of U. uncinus sp. nov., with an ocular micrometer attached to the Leica microscope and calibrated with a Leica 0.01 mm calibration slide stage. We drew sketches of trichobothrial maps for one male of U. lunatus sp. nov. (WAM T79450), as well as two males (WAM T79442 and T79444) and two females (WAM T107077 and T113703) of U. uncinus sp. nov., using a camera lucida attached to the microscope. We dissected and prepared the hemispermatophores of the holotypes of U. uncinus sp. nov. (WAM T79444) and U. lunatus sp. nov. (WAM T79450), as well as an additional five males of U. uncinus sp. nov. (WAM T79442, T153275, T158904, T158905 and T158906) and two males of U. lunatus sp. nov. (WAM T79449 and T158910), fixing and preserving them in 75% ethanol, and using a method modified from Monod and Volschenk (2004).
We use the general terminology found in Hjelle (1990) and Prendini (2000). For specific terminology, we followed Vachon (1974) for trichobothria, Loria and Prendini (2014) for ocelli and Prendini (2000) for carination. For hemispermatophores, we adapted the terminology from Monod and Volschenk (2004) and Monod et al. (2017). Our mensuration followed Stahnke (1970), and included a sample size of nine specimens (n = 3♂, 4♀ for U. uncinus sp. nov. and 2♂ for U. lunatus sp. nov.). For each species, we present the ranges of measurements, followed by the mean, in square brackets.
We obtained molecular sequence data from two specimens of U. uncinus, using techniques outlined in Huey et al. (2019). We also generated distribution maps for both species in QGIS (ver. 3.26.0).
Systematics
Genus Urodacus Peters
Urodacus Peters, 1861: 511.
Ioctonus Thorell, 1876: 14. Type species: Ioctonus manicatus Thorell, 1876, by monotypy. Synonymised by Pocock (1898 : p. 60).
Iodacus Pocock, 1891: 245. Type species: Iodacus darwinii Pocock, 1891 (junior synonym of Urodacus excellens Pocock, 1888, by monotypy). Synonymised by Pocock (1893 : p. 320).
Hemihoplopus Birula, 1903: xxxiii. Type species: Hemihoplopus yaschenkoi Birula, 1903, by original designation. Synonymised by Kraepelin (1908 ; p. 95).
Diagnosis
(Figs 3–6, 7–39, 41, 43; Tables 1–3)
https://zoobank.org/NomenclaturalActs/956CB7F3-AA00-498E-986C-8BACAF3280EB
Urodacus sp. 2: Volschenk et al. (2010 : 276–280) (in part).
Urodacus uncinus sp. nov., habitus. (3, 4) Holotype ♂ (WAM T79444), dorsal and ventral aspects. (5, 6) Paratype ♀ (WAM T107077), dorsal and ventral aspects. Scale bar = 10 mm.

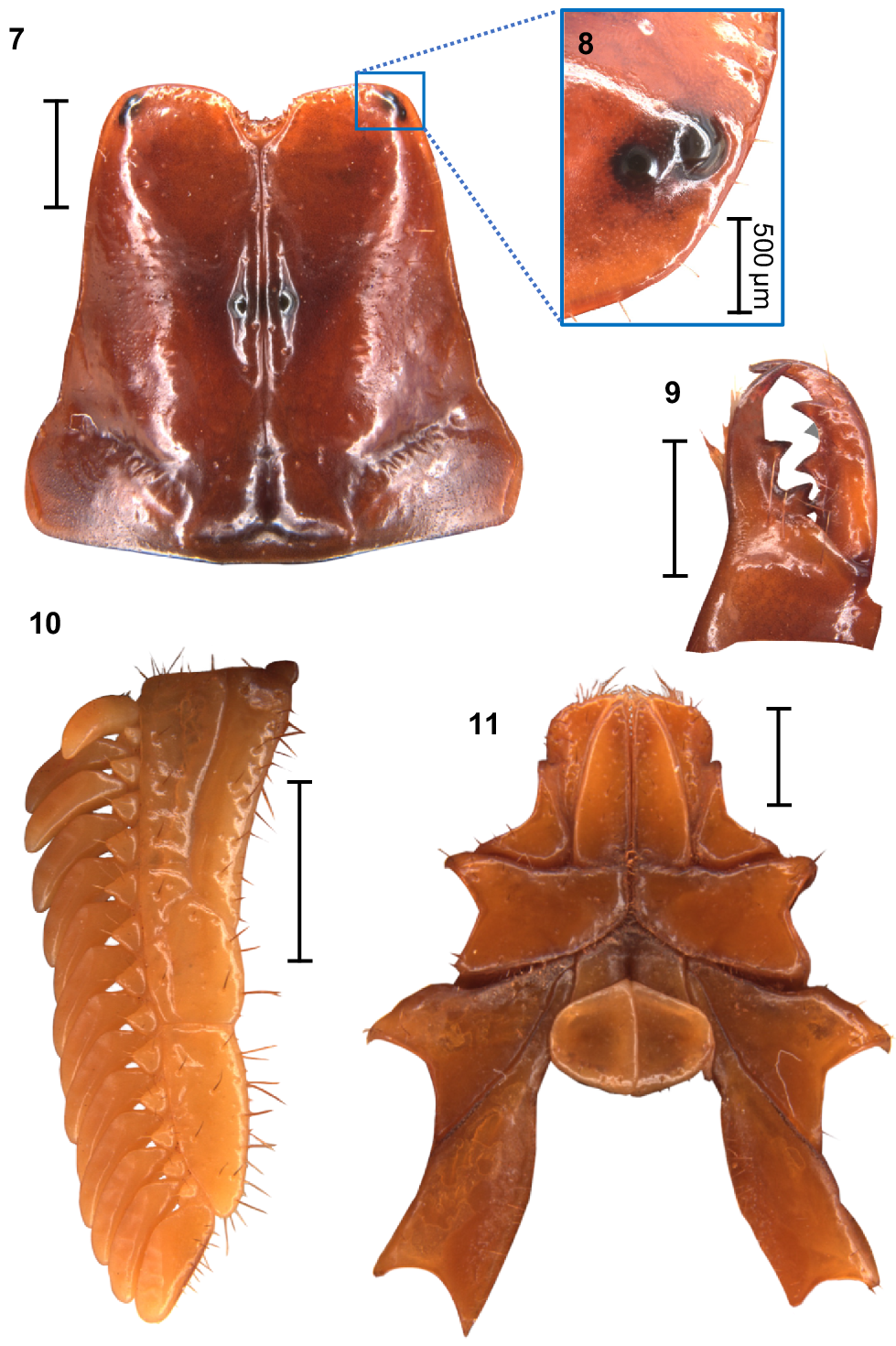
Urodacus uncinus sp. nov., holotype ♂ (WAM T79444). (7) Carapace, dorsal aspect. (8) Right pair of lateral ocelli, lateral aspect. (9) Dextral chelicerae, dorsal aspect, note that one of the subdistal teeth is broken, with original size drawn in grey. (10) Left pecten, ventral aspect. (11) Prosoma, ventral aspect, showing the coxae of pedipalps and all legs, the sternum, and the genital operculum. Scale bars always represent 2 mm, unless otherwise specified (Fig. 8).
| U. uncinus sp. nov. males | U. uncinus sp. nov. females | U. lunatus sp. nov. males | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | n | Range | Mean | n | Range | n | ||
| Carapace | |||||||||
| Total length | 6.80–9.10 | 8.27 | 3 | 8.90–9.90 | 9.43 | 4 | 7.60–8.10 | 2 | |
| Anterior eye length | 2.34–3.60 | 3.13 | 3 | 3.36–3.88 | 3.63 | 4 | 2.94–3.20 | 2 | |
| Posterior eye length | 3.72–5.06 | 4.53 | 3 | 4.80–5.38 | 5.17 | 4 | 4.15–4.30 | 2 | |
| Anterior width | 5.00–6.06 | 5.63 | 3 | 6.00–6.56 | 6.28 | 4 | 5.13–5.31 | 2 | |
| Posterior width | 7.44–9.60 | 8.65 | 3 | 9.10–10.64 | 10.01 | 4 | 8.50–8.60 | 2 | |
| Eye diameter | 0.38–0.51 | 0.44 | 3 | 0.37–0.51 | 0.45 | 4 | 0.39–0.39 | 2 | |
| Interocular distance | 0.46–0.60 | 0.55 | 3 | 0.57–0.70 | 0.64 | 4 | 0.38–0.52 | 2 | |
| Metasoma | |||||||||
| Metasoma I length | 4.15–5.63 | 4.88 | 3 | 4.05–4.60 | 4.35 | 4 | 4.44–4.75 | 2 | |
| Metasoma I width | 3.40–4.60 | 4.10 | 2 | 4.00–4.40 | 4.23 | 4 | 3.52–3.60 | 2 | |
| Metasoma I depth | 2.72–3.95 | 3.37 | 3 | 3.20–3.48 | 3.39 | 4 | 2.72–2.78 | 2 | |
| Metasoma II length | 4.45–5.75 | 5.21 | 3 | 4.15–4.85 | 4.65 | 4 | 4.60–5.19 | 2 | |
| Metasoma II width | 3.40–4.40 | 4.03 | 3 | 3.95–4.45 | 4.24 | 4 | 3.52–3.56 | 2 | |
| Metasoma II depth | 2.66–4.05 | 3.37 | 3 | 3.08–3.48 | 3.35 | 4 | 2.78–3.12 | 2 | |
| Metasoma III length | 5.00–6.40 | 5.76 | 3 | 4.65–5.38 | 5.04 | 4 | 4.80–5.38 | 2 | |
| Metasoma III width | 3.20–4.30 | 3.88 | 3 | 3.85–4.38 | 4.16 | 4 | 3.44–3.60 | 2 | |
| Metasoma III depth | 2.91–3.92 | 3.50 | 3 | 3.24–3.64 | 3.49 | 4 | 2.81–2.84 | 2 | |
| Metasoma IV length | 4.94–6.48 | 5.89 | 3 | 4.75–5.44 | 5.11 | 4 | 5.06–5.69 | 2 | |
| Metasoma IV width | 2.96–3.88 | 3.55 | 3 | 3.52–3.95 | 3.78 | 4 | 3.12–3.32 | 2 | |
| Metasoma IV depth | 2.81–3.72 | 3.36 | 3 | 3.00–3.56 | 3.29 | 4 | 2.72–2.91 | 2 | |
| Metasoma V length | 6.88–9.10 | 8.09 | 3 | 6.56–7.60 | 6.92 | 4 | 8.1–8.5 | 2 | |
| Metasoma V width | 2.75–3.48 | 3.22 | 3 | 2.78–3.20 | 3.01 | 4 | 2.94–2.97 | 2 | |
| Metasoma V depth | 2.03–2.91 | 2.49 | 3 | 2.10–2.50 | 2.35 | 4 | 2.05–2.33 | 2 | |
| Telson | |||||||||
| Total length | 7.20–9.00 | 8.20 | 3 | 6.19–7.44 | 6.97 | 4 | 7.44 | 1 | |
| Vesicle width | 3.60–4.60 | 4.25 | 3 | 2.75–3.12 | 2.96 | 4 | 3.95 | 1 | |
| Vesicle depth | 2.75–3.90 | 3.28 | 3 | 2.03–2.50 | 2.28 | 4 | 2.84–2.88 | 2 | |
| Aculeus length | 1.19–1.44 | 1.33 | 3 | 1.72–1.82 | 1.79 | 4 | 1.38 | 1 | |
| Pedipalp | |||||||||
| Femur length | 4.10–5.31 | 4.80 | 3 | 4.75–5.13 | 4.96 | 4 | 4.10–4.30 | 2 | |
| Femur width | 2.28–2.80 | 2.63 | 3 | 2.22–2.81 | 2.51 | 4 | 1.88–2.45 | 2 | |
| Patella length | 5.56–7.60 | 6.73 | 3 | 6.80–7.52 | 7.28 | 4 | 6.06–6.19 | 2 | |
| Patella width | 2.56–3.60 | 3.13 | 3 | 3.40–3.76 | 3.54 | 4 | 2.80–3.03 | 2 | |
| Patella depth | 2.96–4.05 | 3.62 | 3 | 3.60–4.25 | 4.04 | 4 | 3.28–3.28 | 2 | |
| Chela dorsal length | 9.90–12.95 | 11.93 | 3 | 12.44–13.72 | 13.30 | 4 | 10.77–10.77 | 2 | |
| Ventral manus length | 5.44–7.12 | 6.53 | 3 | 6.72–7.36 | 7.18 | 4 | 5.60–5.92 | 2 | |
| Chela width | 4.60–5.63 | 5.28 | 3 | 5.19–6.06 | 5.70 | 4 | 4.25–4.69 | 2 | |
| Chela depth | 4.94–6.72 | 5.75 | 3 | 5.84–6.56 | 6.26 | 4 | 5.38–5.44 | 2 | |
| Fixed finger length | 4.60–5.94 | 5.39 | 3 | 5.63–6.31 | 6.09 | 4 | 4.75–4.90 | 2 | |
| Movable finger length | 6.40–8.16 | 7.52 | 3 | 8.10–8.70 | 8.48 | 4 | 6.80–6.96 | 2 | |
For U. uncinus sp. nov., three males and four females were measured (although sample sizes can be smaller if a structure was damaged and could not be measured in a specimen), and the range and mean of the measurements are presented. For U. lunatus sp. nov., two males were measured (females are unknown), and therefore only the range is presented.
| Dorsal and prolateral surfaces | Retrolateral surface | Ventral surface | Totals | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| i | d | eb | esb | ea | Est | et | V | |||||
| Patella | ||||||||||||
| U. uncinus males | 1 | 2 | 13 | 1 | 24–27 | 0 | 0 | 11–14 | 52–58 | |||
| U. uncinus females | 1 | 2 | 10–12 | 1 | 21–26 | 0 | 0 | 11 | 46–53 | |||
| U. lunatus male | 1 | 2 | 11 | 1 | 22 | 0 | 0 | 12 | 49 | |||
| I | D | Eb | Esb | Em | Est | Et | v | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Manus | ||||||||||||
| U. uncinus males | 2 | 2 | 5 | 1 | 12 | 1 | 5 | 17 | 45 | |||
| U. uncinus females | 2 | 2 | 6 | 3 | 10 | 1 | 5 | 16–17 | 45–46 | |||
| U. lunatus male | 2 | 2 | 5 | 1 | 12 | 1 | 5 | 15 | 43 | |||
| db | dsb | dst | dt | eb | esb | est | et | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed finger | ||||||||||||
| U. uncinus males | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |||
| U. uncinus females | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |||
| U. lunatus male | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |||
Ranges are given where appropriate, and a single value is given when we found no variation in U. uncinus sp. nov. and for all counts in U. lunatus sp. nov., given that only one male was examined (females of this species are unknown).
| U. uncinus sp. nov. males | U. uncinus sp. nov. females | U. lunatus sp. nov. males | |||||
|---|---|---|---|---|---|---|---|
| Range; mode | n | Range; mode | n | Range | n | ||
| Pectines | |||||||
| Left | 14–15; 14 | 3 | 10–12; 12 | 4 | 13–14 | 2 | |
| Right | 12–16 | 3 | 11–12; 12 | 4 | 13–13 | 2 | |
| Leg I (right) macrosetae | |||||||
| Basitarsus retrolateral | 5–5; 5 | 3 | 5–5; 5 | 4 | 4–5 | 2 | |
| Telotarsus retrolateral | 9–10; 10 | 3 | 9–10; 10 | 4 | 9–9 | 2 | |
| Telotarsus prolateral | 6–6; 6 | 3 | 6–6; 6 | 4 | 5–5 | 2 | |
| Tibia retrolateral | 2–3; 3 | 3 | 2–3; 3 | 4 | 3–3 | 2 | |
| Leg II (right) macrosetae | |||||||
| Basitarsus retrolateral | 5–5; 5 | 3 | 5–5; 5 | 4 | 4–5 | 2 | |
| Telotarsus retrolateral | 9–10; 10 | 3 | 9–10; 9 | 4 | 8–9 | 2 | |
| Telotarsus prolateral | 6–7; 6 | 3 | 6–7; 6 | 4 | 6–7 | 2 | |
| Tibia retrolateral | 2–3; 2 | 3 | 2–3; 2 | 4 | 3–3 | 2 | |
| Leg III (right) macrosetae | |||||||
| Basitarsus retrolateral | 4–5; 4 | 3 | 3–4; 4 | 4 | 4–4 | 2 | |
| Telotarsus retrolateral | 9–10; 10 | 3 | 9–9; 9 | 4 | 9–9 | 2 | |
| Telotarsus prolateral | 6–8 | 3 | 6–7; 7 | 4 | 6–6 | 2 | |
| Tibia retrolateral | 1–1; 1 | 3 | 1–1; 1 | 4 | 1–1 | 2 | |
| Leg IV (right) macrosetae | |||||||
| Basitarsus retrolateral | 2–3; 2 | 3 | 2–3; 2 | 4 | 2–3 | 2 | |
| Telotarsus retrolateral | 9–11 | 3 | 10–11; 10 | 3 | 9–10 | 2 | |
| Telotarsus prolateral | 6–7; 7 | 3 | 6–7; 6 | 3 | 6–6 | 2 | |
| Tibia retrolateral | 1–1; 1 | 3 | 0–1; 1 | 4 | 1–1 | 2 | |
For U. uncinus sp. nov., three males and four females were inspected (although sample sizes can be smaller if a structure was damaged and could not be inspected in a specimen), and the range and mode of the counts are presented. Modes are omitted if there were no repeated numbers in the counts for a given set of individuals. For U. lunatus sp. nov., two males were inspected (females are unknown), and therefore only the range of counts is presented.
Material examined
Australia: Western Australia: male, 20 km ENE of Wodgina, Pilbara Biological Survey site MBW06, 21°06′53″S 118°51′06″E, ethylene glycol pitfall trap, 23 September 2005–15 May 2006, CALM staff (Pilbara Biological Survey) (WAM T79444).
Australia: Western Australia: one male, same data as holotype (WAM T153275); three females, ~15 km NE of Wodgina mine site, 21°06′48.3″S 118°47′19.1″E, 14 April 2010, hand collected in drainage line (first female on site H2–SRE–D, second on site H2–SRE–E and third on site H2–SRE–F), P. Bolton (Outback Ecology), first two females sequenced (NCB 2015) (WAM T107077–T107079); one subadult male, ~15 km NE of Wodgina Mine site, 21°04′31.6″S 118°46′44″E, 3 April 2011, hand collected in drainage line (site *10–41), A. Rakimov (Outback Ecology) (WAM T113699); three females, same data as last subadult male (site *10–41 for first female, *10–62 and *10–38 for the other females) (WAM T113700, T113701 and T113703); one juvenile, same data as last four individuals (site *10–40) (WAM T113702).
Australia: Western Australia: four males, 41 km ESE of Goldsworthy, Pilbara Biological Survey site PHYC02, 20°23′27″S 119°54′31″E, ethylene glycol pitfall trap, 28 July 2005–15 May 2006, CALM staff (Pilbara Biological Survey) (WAM T79441, T158904–T158906); one male, 78 km E of Meentheena Outcamp, Pilbara Biological Survey site NE11, 21°18′15.9″S 121°12′01.3″E, ethylene glycol pitfall trap, 15 November 2003–17 May 2004, CALM staff (Pilbara Biological Survey) (WAM T79442); one male, 20 km ESE of Warrawagine HS, Pilbara Biological Survey site PHYE07, 20°56′08″S 120°52′35″E, ethylene glycol pitfall trap, 3 July 2005–19 May 2006, CALM staff (Pilbara Biological Survey) (WAM T79446).
Diagnosis
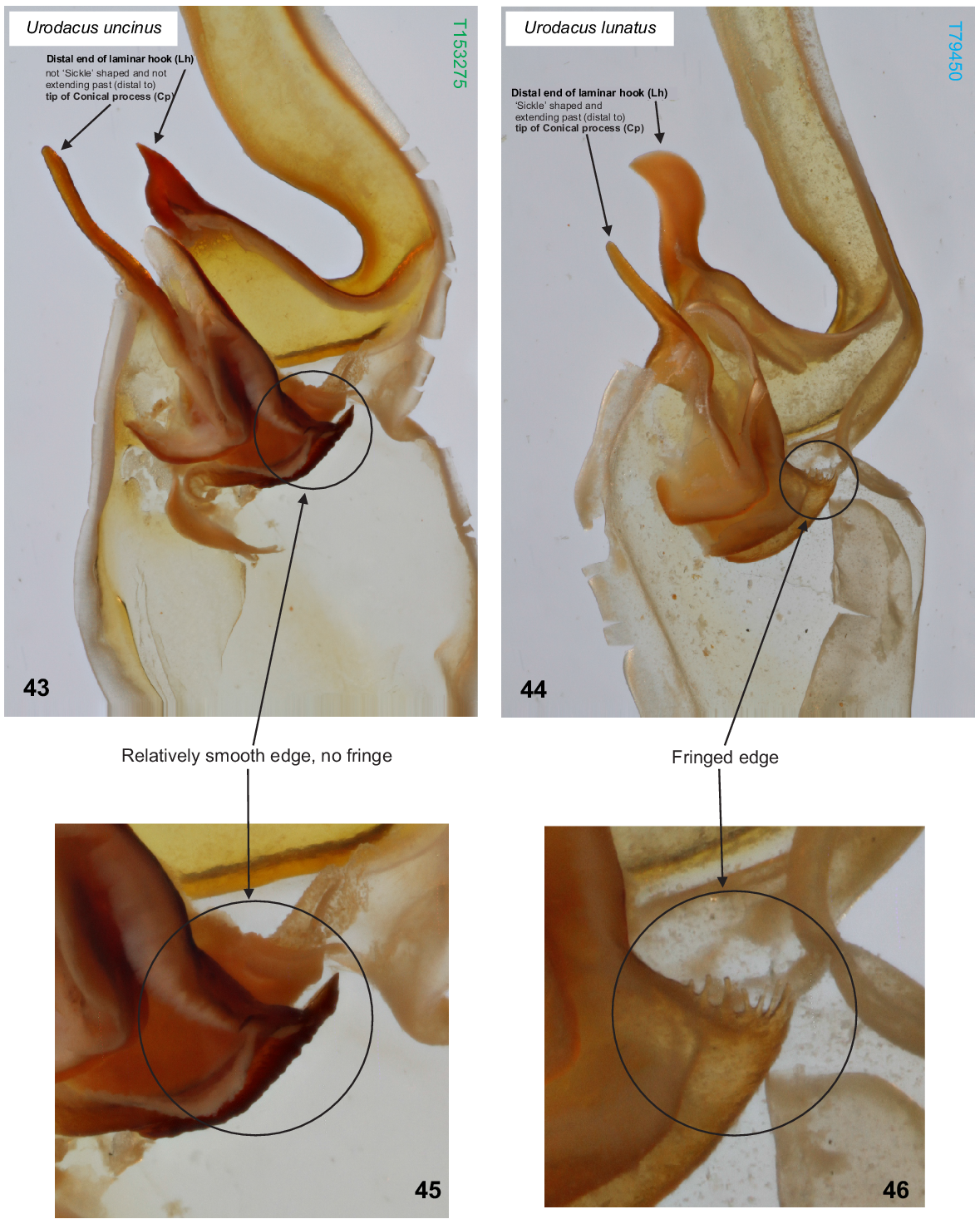
U. uncinus sp. nov. differs from all other described Urodacus species, except U. lunatus sp. nov., Urodacus similis L.E. Koch, 1977 and U. yaschenkoi Birula, 1903 by the very short first four metasomal segments, which are not much longer than high (Figs 3–6, 12–15). It can be separated from U. similis and U. yaschenkoi by numerous features, including the unusual shape of the telson, which in both sexes has an elliptic (rather than globose) vesicle and an aculeus that is more curved than in other species (Figs 19–22). These telson modifications are also present in the female but are much more pronounced in the male. The species can be further distinguished from U. similis and U. yaschenkoi by the near absence of secondary serrations on the chelicerae (Fig. 9); smaller dorsal carinae (almost absent in the female, rendering segments a round shape) on metasomal segments I–IV (Figs 3–6, 12–15); and a very different hemispermatophore (Figs 35–37), which has a longer and thinner distal lamina that is terminally more curved, no laminar hook process/inner lobe tooth, and no flap structure mentioned in Koch (1977: 286, fig. 117). Males of U. uncinus sp. nov. are indistinguishable from males of U. lunatus sp. nov. based on external morphology. These two species can be distinguished based on the morphology of the hemispermatophore, which in U. uncinus sp. nov. bears a laminar hook that is conically shaped (rather than shaped as a sickle) and does not extend past the tip of the conical process (’clasper’ sensuMonod et al. (2017)) (Fig. 43). Moreover, in U. uncinus sp. nov., the hemispermatophore has a relatively smooth (rather than fringed) subex margin (Figs 43, 45).
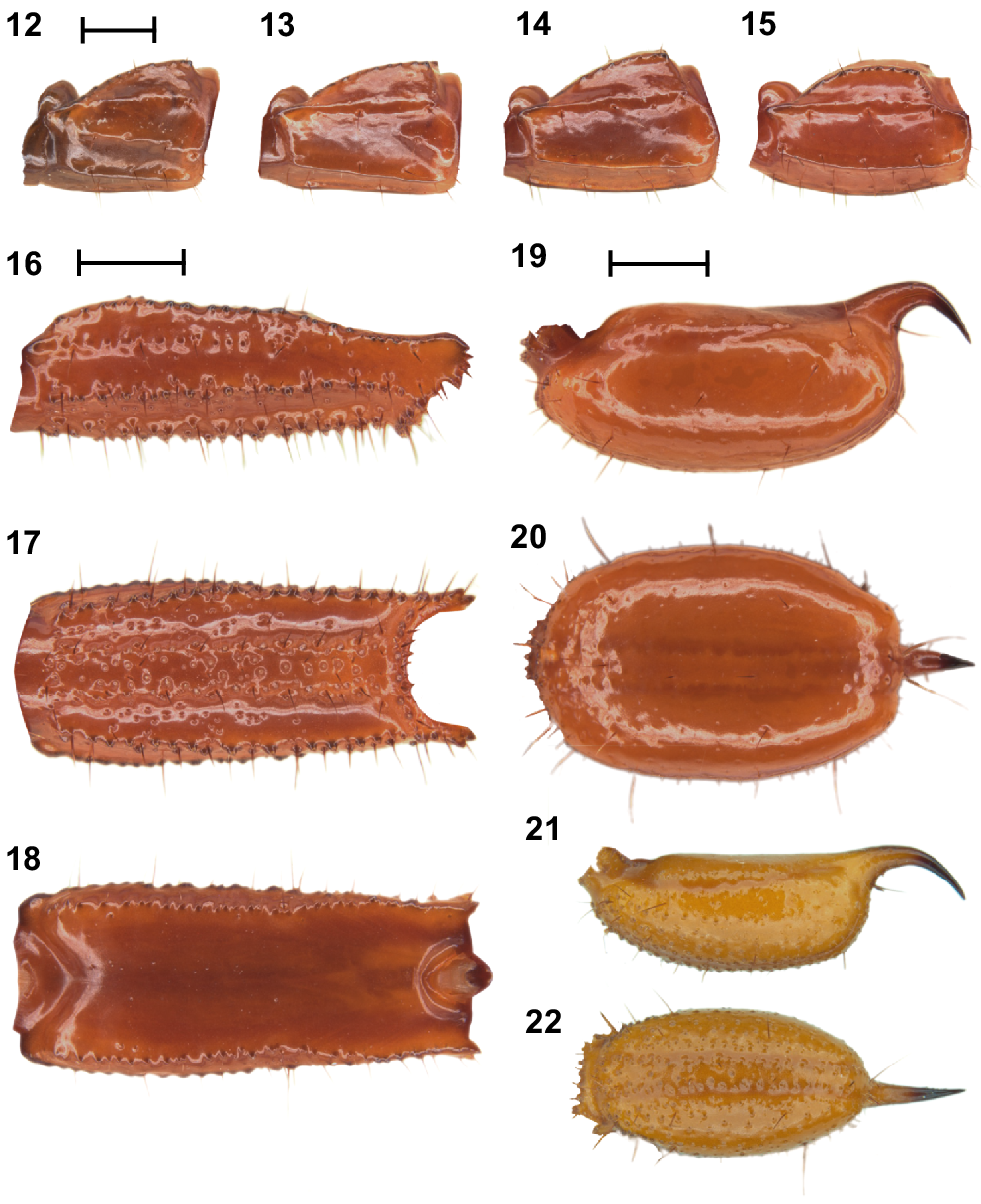
Urodacus uncinus sp. nov., holotype ♂ (WAM T79444). (12–15) Metasomal segments I – IV, lateral aspect (same scale). (16–18) Metasomal segment V, lateral, ventral, and dorsal aspects (same scale). (19, 20) Telson, lateral and ventral aspects. (21, 22) Paratype ♀ (WAM T107077), telson, lateral and ventral aspects. Scale bars represent 2 mm.
Description
Based on holotype and paratypes.
Colouration: Carapace darker orange to brown in older preserved specimens (Figs 3, 5, 7), becoming paler in anterior tenth. Mesosoma of similar colour to carapace. Metasoma and telson tending dark orange in older preserved specimens (Figs 3–6, 12–18). Aculeus black. Pedipalps dark orange to dark brown (Figs 23–29, 47). Legs light orange, femur and patella with darker spot at the terminal tip in the prolateral face (Figs 3, 5).
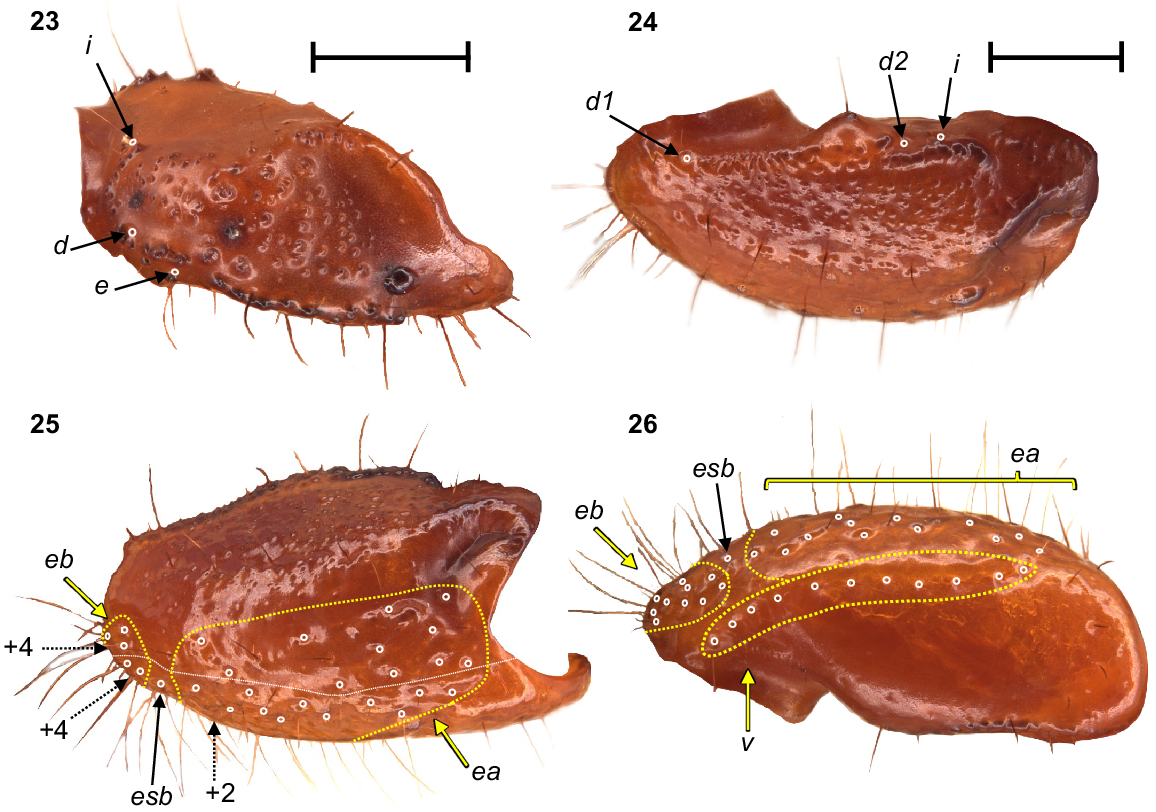
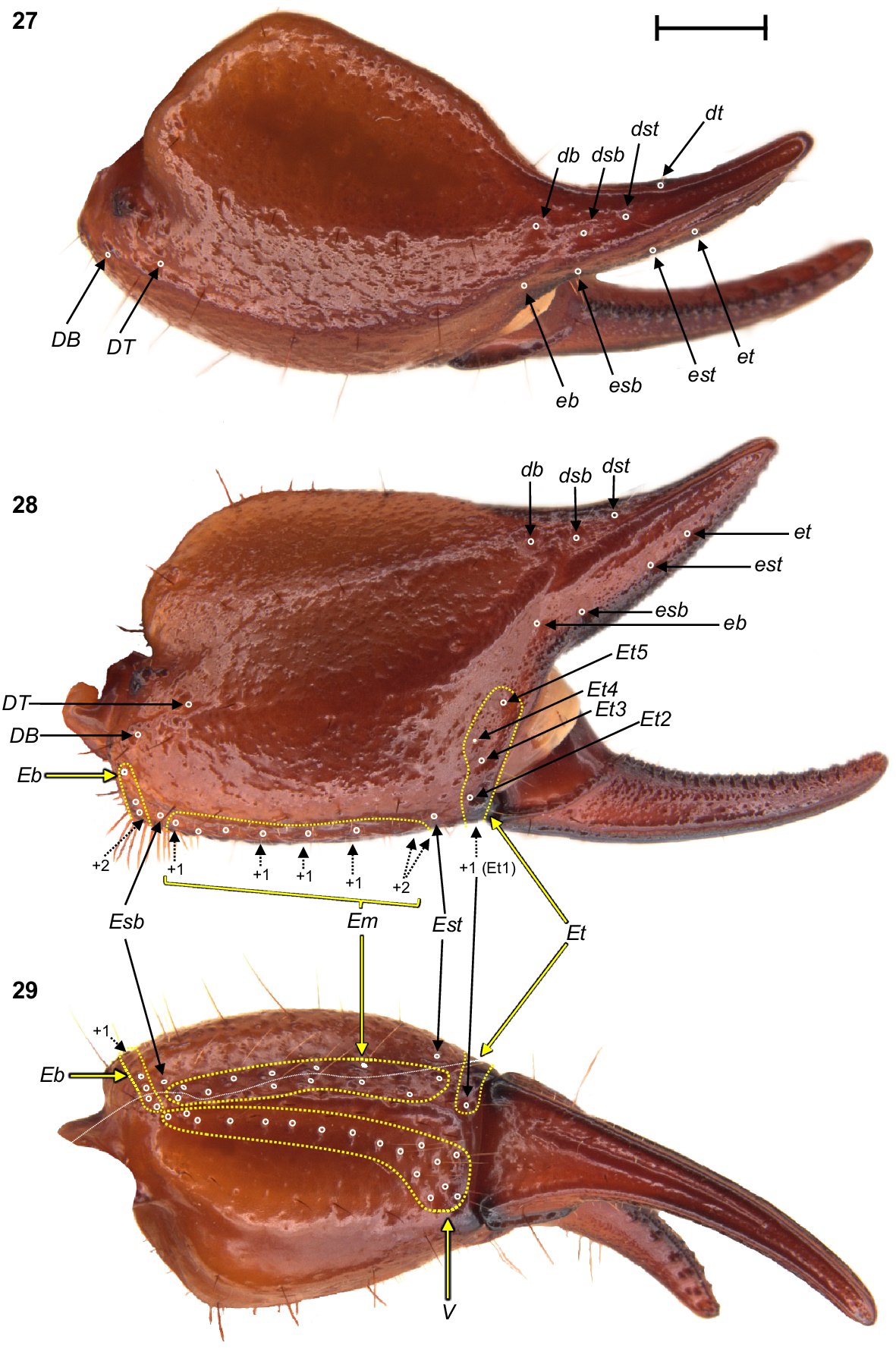
Urodacus uncinus sp. nov., holotype ♂ (WAM T79444). (23) Dextral pedipalp femur, dorsal aspect. (24–26) Dextral pedipalp patella, dorsal, retrolateral, and ventral aspects (same scale). Trichobothria (TB) are highlighted in white open circles, trichobothria groups are contained in dashed yellow lines and indicated in yellow arrows (single TB in black arrow), and additional TB that are not visible in each aspect are indicated with dashed arrows. A thin dashed white line in the retrolateral aspect of the patella separates TB that are visible only in the retrolateral aspect (above line) from TB visible in the retrolateral and ventral aspects (below line). Scale bars represent 2 mm.
Urodacus uncinus sp. nov., holotype ♂ (WAM T79444), dextral pedipalp chela, dorsal, retrolateral, and ventral aspects. Trichobothria (TB) are highlighted in white open circles, trichobothria groups are contained in dashed yellow lines and indicated in yellow arrows (single TB in black arrow), and additional TB that are not visible in each aspect are indicated with dashed arrows. A thin dashed white line in the ventral aspect separates TB that are visible only in the ventral aspect (below line) from TB visible in the retrolateral and ventral aspects (above line). Scale bar represents 2 mm.
Carapace: Relatively square with subparallel margins in the anterior first three quarters and becoming wider with rounded lateral margins posteriorly; anterior margin with well developed median notch that is wider than deep; frontal lobes with anterior margins slightly rounded (Fig. 7). Two pairs of lateral ocelli situated on the anterolateral margins, with the anterior ocelli almost twice as large as posterior ocelli (Fig. 8), consistent with type 2B in Loria and Prendini (2014); median ocular tubercle raised, situated anteromedially (Fig. 7). Anterior transverse, anterolateral and median lateral sulci absent; median longitudinal sulcus thin and deep, bifurcated anteriorly; posterolateral transverse sulcus distinct, wide, and deep; posteromarginal depression deep, extending anteriorly halfway to the median ocular tubercle (Fig. 7). Anterolateral margins, interocular, median, and posteromedian surfaces smooth and glossy; median lateral and posterolateral surfaces matt, sparsely and finely granular in places (Fig. 7). Measurements in Table 1.
Chelicerae: Distal tooth on moveable finger more curved than distal tooth on fixed finger; teeth with almost no secondary serrations, just small undulations on medial teeth. Movable finger with two subdistal teeth (note that the right chelicera of the holotype has one of these broken; original size drawn in grey in Fig. 9). Fixed finger without subdistal tooth; proximal edge of median tooth slightly incurved; basal tooth distinctively bilobed (Fig. 9).
Pedipalps: Coxa, ventroprolateral surface smooth, with several large and pointy granules medially. Femur, dorsoprolateral carinae vestigial, reduced to a row of granules in the distal third of the segment; dorsoretrolateral and ventroprolateral carinae well developed, costate–granular, comprising granules of irregular size, more pronounced in the middle half for the dorsoretrolateral carinae and in the proximal half for the ventroprolateral carinae; dorsal intercarinal surface sparsely covered with granules of equal size; prolateral intercarinal surface smooth and without any granules (Fig. 23). Patella, dorsoprolateral carina well developed, costate–granular; ventroretrolateral carina reduced to a smooth ridge more evident anteriorly; ventroprolateral carina reduced to a row of granules in the distal third of the segment; dorsoretrolateral and ventromedian carinae absent; dorsal intercarinal surface densely covered with small granules; dorsoprolateral process prominent and round, with a terminal large granule; ventroprolateral process wider but lower, less pronounced, and smooth (Figs 24–26). Chela manus, digital carina costate; dorsomarginal carinae very poorly developed; ventroretrolateral well developed and costate; dorsal secondary carina obsolete, comprising a few granules proximally; retrolateral secondary carina absent; dorsoprolateral carina absent (Fig. 27). Dorsal and retrolateral intercarinal surfaces granular–reticulate, with moderate to dense, irregular depressions formed by reticulate arrangement of small granules (Figs 27, 28); prolateral intercarinal surfaces sparsely covered with small granules. Fixed finger without distal diastema and hooklike terminal denticle; dentate margin with 2–3 denticle rows proximally and single row distally; seven retrolateral accessory denticles and 10–11 prolateral accessory denticles (Figs 27–29). Movable finger without distal diastema and hooklike terminal denticle; dentate margin entire, not scalloped, without basal lobe or concavity; 2–3 denticle rows proximally and single row distally; 4–5 retrolateral accessory denticles and 10–11 prolateral accessory denticles (Figs 27–29). Measurements in Table 1.
Trichobothria: Variation in trichobothria number and arrangement can be found in Table 2, whereas the following description is based on the holotype only. Pedipalp neobothriotaxic, Type C. Femur always with 3 trichobothria: i situated dorsobasally on prolateral surface; d situated basally and retrolaterally on dorsal surface, close to dorsoretrolateral carina; e situated dorsobasally on retrolateral surface, close to dorsoretrolateral carina (Fig. 23). Patella with 52 trichobothria: 1 i, 2 d, 38 e, and 11 v (Figs 24–26); d1 situated basally on dorsal surface, at the basal end of dorsoprolateral carina; d2 situated in the distal third of dorsal surface, between trichobothrium i and dorsoprolateral process (Fig. 24); retrolateral trichobothria divided into 13 eb, 1 esb and 24 ea (Figs 25, 26); ventral trichobothria in one group (Fig. 26). Chela with 53 trichobothria: eight situated on fixed finger, 4 d and 4 e; db situated at base of finger on dorsal surface; dsb situated in proximal fifth of finger on dorsal surface; dst situated medially on finger on dorsal surface; dt situated in distal half of finger on prolateral surface; eb situated at base of finger, opposite db; esb situated in proximal fifth of finger, opposite dsb; est situated medially on finger, opposite dst; et situated in distal half of finger, opposite dt (Figs 27, 28). 45 situated on manus: DB, DT, 5 Eb, Esb, 12 Em, Est, 5 Et (Et4 clearly petite) and 17 V (Figs 27–29); DB and DT situated basally and retrolaterally on dorsal surface of manus (Figs 27, 28); Eb group with 3 trichobothria visible retrolaterally (Fig. 28) and 2 visible ventrally (Fig. 29); Em group with 6 trichobothria visible retrolaterally (Fig. 28) and 6 visible ventrally (Fig. 29); Est situated terminoventrally on retrolateral surface of manus (Figs 28, 29); Et group with 4 trichobothria visible retrolaterally (Fig. 28) and 1 ventrally (Fig. 29); V group with 10 trichobothria almost regularly spaced along the basal–terminal axis, with 7 additional trichobothria clustered in the terminal end of the ventral surface (Fig. 29); It and Ib situated distally on manus, close to base of fixed finger; trichobothrial sulci absent.
Mesosoma: Tergites I–VII, dorsomedian carina vestigial, reduced to low, smooth, and slightly glossy mounds; dorsosubmedian and dorsolateral carinae absent; lateral surfaces matt, finely and evenly granular (Fig. 3). Sternites III–VII, ventral surfaces entirely smooth; VII with ventrosubmedian carinae absent and ventrolateral carinae costate. Book lung spiracles slit shaped (Fig. 4).
Pectines: Three marginal lamellae and three median lamellae; 12–16 pectinal teeth (Fig. 10). Counts of pectinal teeth and variation in males and females in Table 3.
Coxosternal sclerites: Coxapophysis I expanded anteriorly, subtriangular, and fused to coxa of leg I (Fig. 11). Coxapophysis II extending to anterior end of coxapophysis I, twice as long as coxa of leg II, and fused to it; triangular with slightly rounded margins (Fig. 11).
Sternum: Subpentagonal, nearly twice wider than long with a furrow running medially through the posterior half (Fig. 11). Genital operculum about 30% wider than long, divided in males (Fig. 11) and fused in females. Male genital papillae mostly hidden beneath the operculum, visible only from a posterior angle (Fig. 11).
Metasoma: Metasomal segments I–IV not much longer than high (measurements in Table 1), dorsal surfaces smooth; dorsosubmedian carinae slightly costate and granular, continuous along length of segments, without prominent spiniform posterior granule; dorsolateral carinae vestigial, reduced to a low smooth and continuous mound along length of segments; median lateral carinae distinctly costate and smooth along length of segments (Figs 12–15). Metasomal segment V, dorsal surface smooth, shiny; dorsosubmedian carinae absent; dorsolateral carinae with serration that looks sharp dorsally (Fig. 18) and originating from large granules laterally (Fig. 16), continuous along length of segment; median lateral carinae granular and restricted to anterior half of segment (Fig. 16); ventrolateral carinae with large granules, continuous along length of segment (Figs 16, 17); ventrosubmedian carinae absent; ventromedian carina raised, wide and granular, continuous along length of segment; ventral intercarinal surfaces with few scattered granules (Fig. 17).
Telson: Vesicle enlarged and elliptical in both dorsal (Figs 20, 22) and lateral aspects (Figs 19, 21), especially in males (Figs 19, 20); ventral surface smooth in the male (Fig. 20) and densely granular in the female (Fig. 22), without ventromedian carina; lateral surfaces similarly smooth in the male (Fig. 19) and densely granular in the female (Fig. 22); few macrosetae sparsely and evenly distributed. Aculeus stout, strongly curved in the female (Fig. 21), and extremely curved (90°) in the male (Fig. 19). Measurements in Table 1.
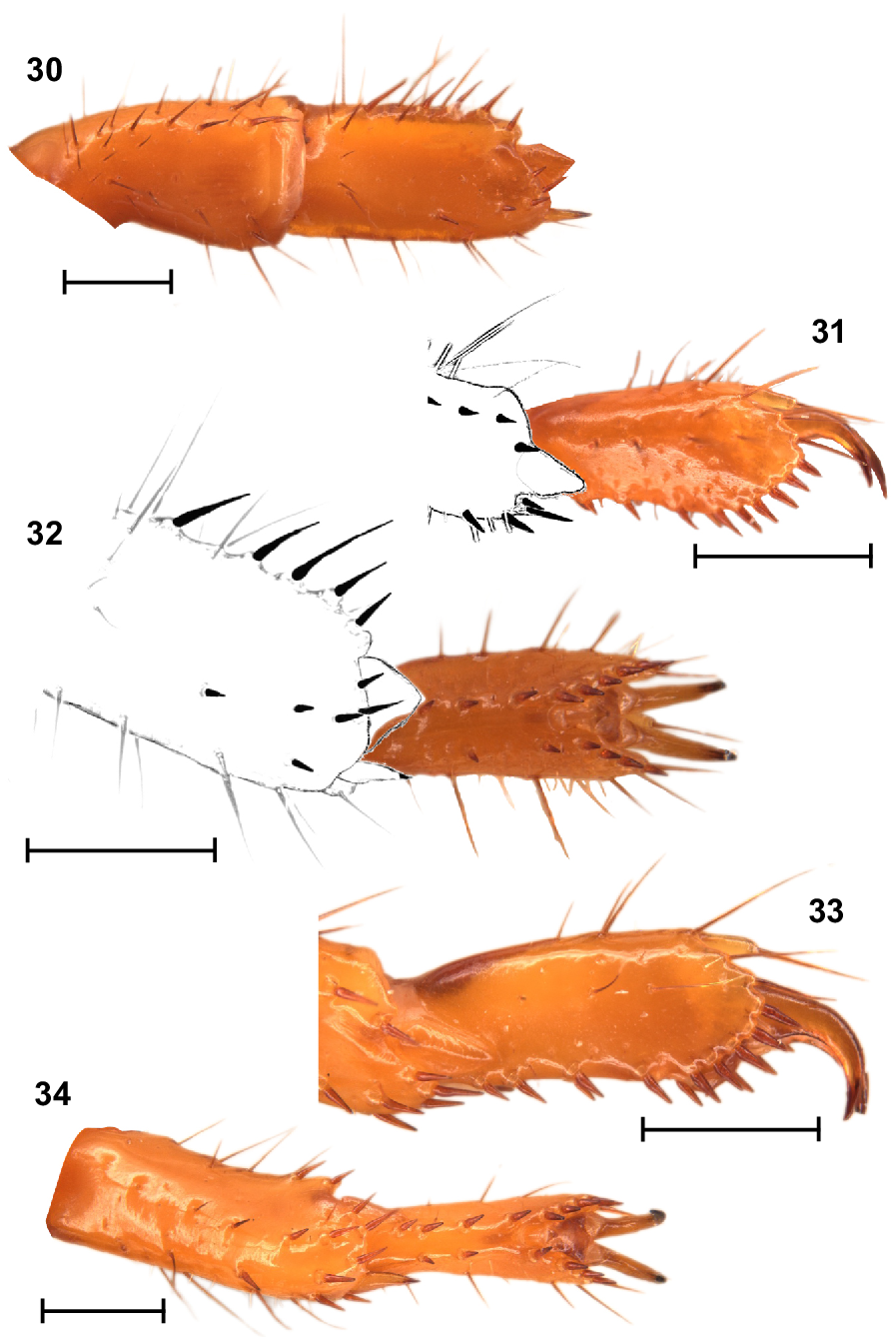
Legs: Legs I, tibia and basitarsus, each with comb-like retrolateral row of macrosetae (Fig. 30); basitarsus slightly broadened and dorsoventrally compressed (Fig. 30); telotarsus, retroventral row of spiniform macrosetae with more setae than proventral row (Figs 31, 32). Legs IV, basitarsus without a comb-like retrolateral row of macrosetae, but with two distal macrosetae (Figs 33, 34); telotarsus, retroventral row of spiniform macrosetae with more setae than proventral row (Fig. 34). Counts of macrosetae and spiniform macrosetae in Table 3.
Urodacus uncinus sp. nov., holotype ♂ (WAM T79444), spiniform macrosetae on dextral legs I and IV. (30) Leg I tibia and basitarsus, ventral aspect. (31, 32) Leg I telotarsus, lateral and ventral aspects, with terminal end of basitarsus sketched. (33, 34) Leg IV telotarsus, lateral and ventral aspects, showing the terminal end of basitarsus. Scale bars represent 1 mm.
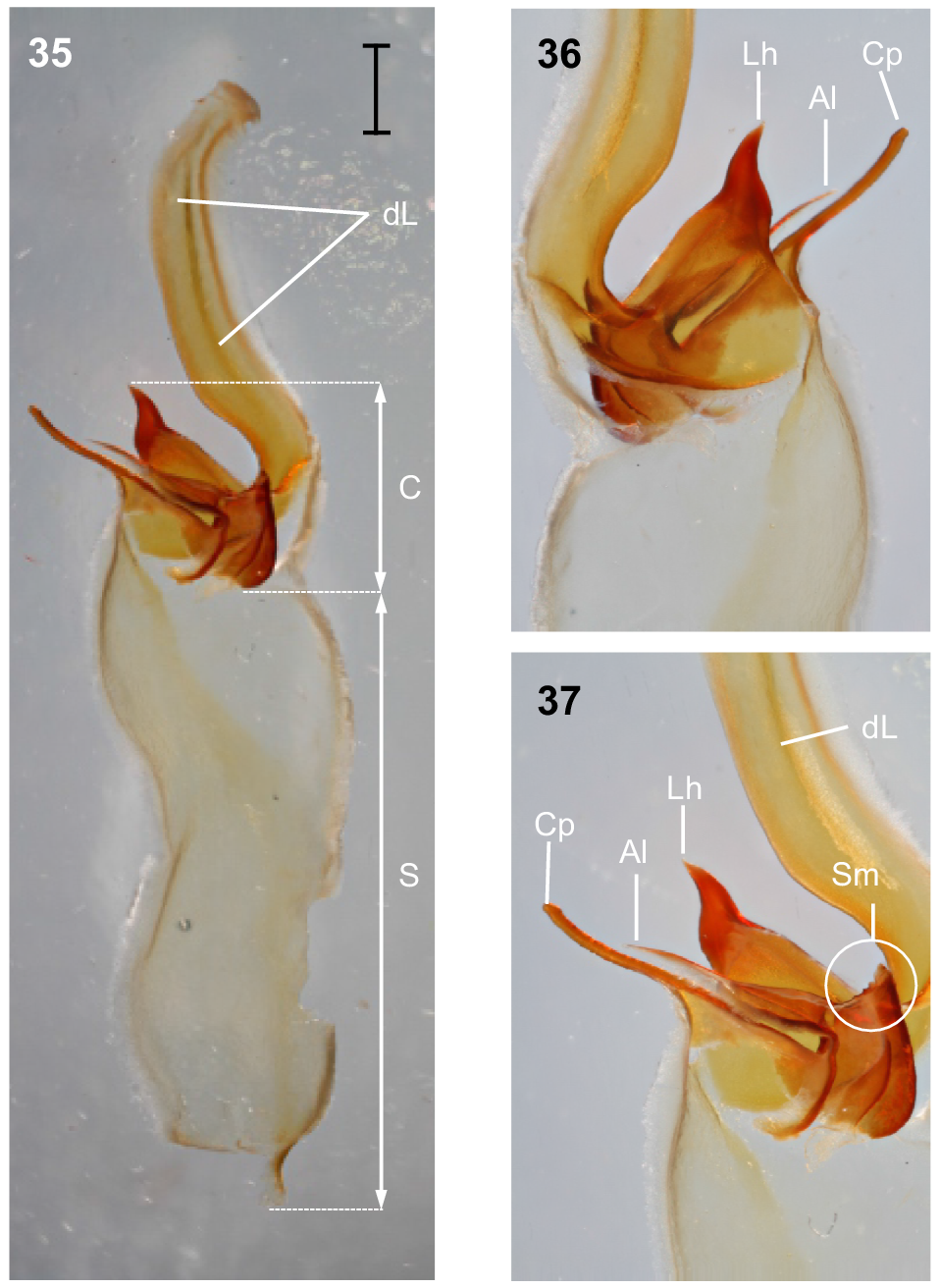
Hemispermatophore: Distal lamina just over twice the length of capsule, rounded distally and directed away from capsule (Fig. 35); distal crest absent; laminar hook broad at base and no more than twice longer than wide, situated proximally, conically shaped; distal transverse ridge costate, without laminar hook process (Figs 35–37); capsule orientation (lateral aspect) in same plane as distal lamina; anterior lobe (‘tectum’ sensuMonod et al. (2017)) thin and terminally rounded, without process; conical process (‘clasper’ sensuMonod et al. (2017)) thin and pointy, as long or longer than laminar hook (Figs 35–37, 43); subex margin relatively smooth terminally (Fig. 45).
Urodacus uncinus sp. nov., holotype ♂ (WAM T79444). (35) Dextral hemispermatophore, dorsal aspect; scale bar = 1 mm. (36) Detail of capsule, ventral aspect. (37) Detail of capsule, dorsal aspect. Abbreviations: Al, anterior lobe (‘tectum’ sensuMonod et al. (2017)); C, capsule; Cp, conical process (‘clasper’ sensuMonod et al. (2017)); dL, distal lamina (‘stalk’ sensuMonod et al. (2017)); Lh, laminar hook; Sm, subex margin; S, stem.
Sexual dimorphism
The most striking form of sexual dimorphism in U. uncinus sp. nov. is in the telson of adult males, which is more enlarged and dorsally elliptical than that of females and bears an aculeus that is more curved in males (Figs 19–22). Adult females possess slightly shorter and broader metasomal segments, but the granulation is similar between sexes (Figs 3–6), and neither sex has prominent spiniform granules at the posterior edge of the dorsosubmedian carinae on segments II–IV (as common in males of other Urodacus species). The pedipalps of adult females are less granular and their carinae less developed than those of the adult males, especially on the femur and patella segments. Finally, as is typical in Urodacus, the female pectines are significantly shorter than the male pectines (Figs 4, 6).
Distribution and remarks
U. uncinus sp. nov. is known only from 16 specimens collected in six locations in the north-eastern portion of the Pilbara (Fig. 2). The distance between the locations furthest to the west (15 km NE of Wodgina Mine site) and to the east (site NE11, 78 km E of Meentheena Outcamp) is 252 km, resulting in a known extent of occurrence of approximately 12 000 km2. Nevertheless, the species is rare and underrepresented in the WAM collection, especially when compared to most other Urodacus morphospecies known from the Pilbara region, which are very numerous. This could result from U. uncinus sp. nov. having a unique habitat requirement (see fig. 6 in Volschenk et al. 2010), which currently seems to restrict the species to creeks and drainage lines, generating a very patchy distribution across the species’ range. Therefore, it is still possible that the real species distribution represents less than 10 000 km2, rendering it short-range endemic status (Harvey 2002).
This species has been known as Urodacus ‘SCO035’ and Urodacus ‘Pilbara 2’ in the Western Australian Museum database.
Ecology
All males of U. uncinus sp. nov. collected so far were caught in pitfall traps, whereas females and juveniles were hand collected in drainage lines (at night using ultraviolet light detection). This probably indicates that males disperse over longer distances when looking for mating opportunities, as is usual in Urodacus. A few specimens were caught from burrows (using the cup trap method: Locket 1993) in the Woodie Woodie mine site (~80 km south of the furthest south-eastern confirmed occurrence location in Fig. 2), but their conspecificity with U. uncinus sp. nov. could not be confirmed due to lack of males. Nevertheless, the retrolateral rows of stout macrosetae on the basitarsi of legs I (Figs 30, 32) and robust habitus (Figs 3–6) corroborate that this species is fossorial.
Etymology
The specific name is taken from the Latin uncinus (meaning ‘hook’), in reference to the male aculeus, which is much more strongly hooked than in any other previously described Urodacus species.
(Figs 38–42, 44, 46; Tables 1–3)
https://zoobank.org/NomenclaturalActs/1766B0C7-2370-486D-BD6A-55D5AE6B0E20
Urodacus sp. 2: Volschenk et al. (2010 : 276–280) (in part).
Urodacus lunatus sp. nov., habitus. Holotype ♂ (WAM T79450), dorsal and ventral aspects. Scale bar = 10 mm.

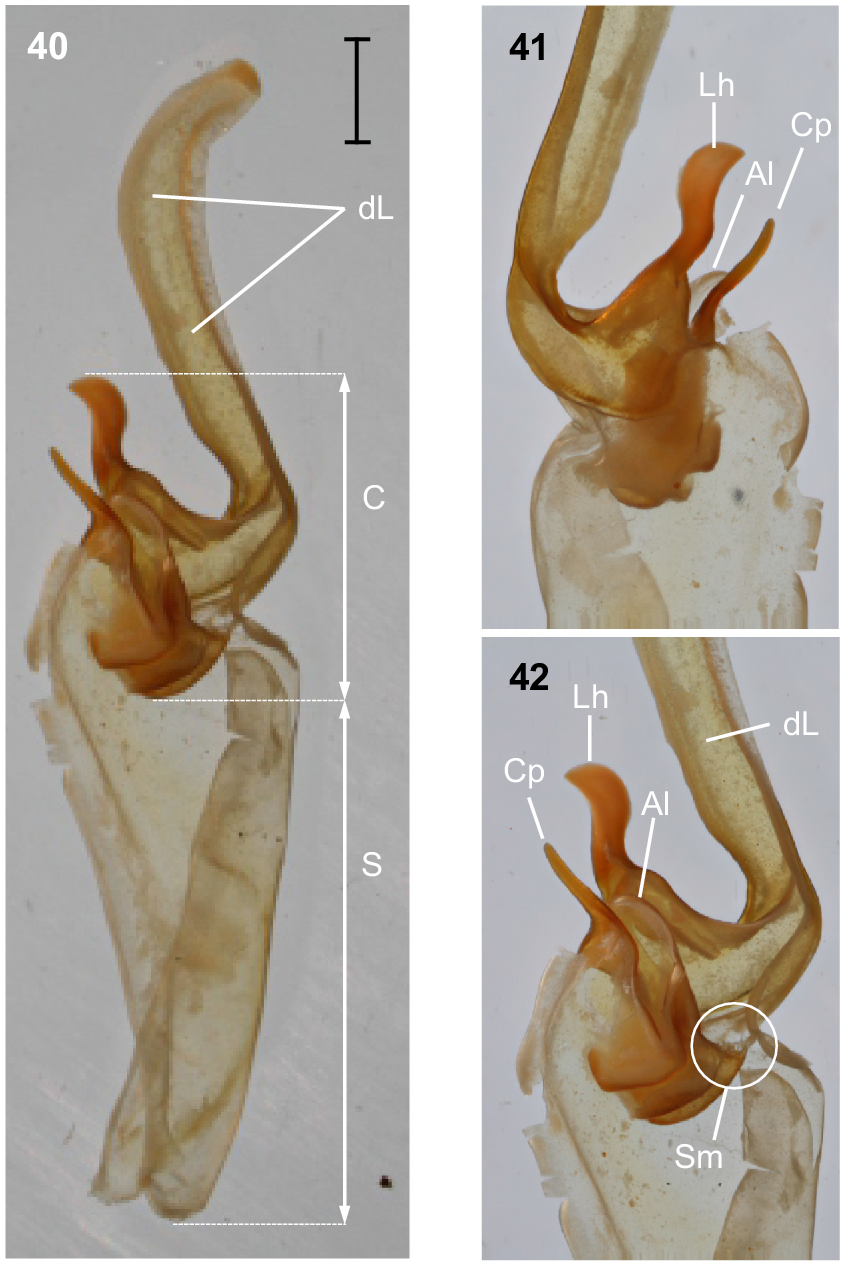
Urodacus lunatus sp. nov., holotype ♂ (WAM T79450). (40) Dextral hemispermatophore, dorsal aspect. (41) Detail of capsule, ventral aspect. (42) Detail of capsule, dorsal aspect. Abbreviations: Al, anterior lobe (‘tectum’ sensuMonod et al. (2017)); C, capsule; Cp, conical process (‘clasper’ sensuMonod et al. (2017)); dL, distal lamina (‘stalk’ sensuMonod et al. (2017)); Lh, laminar hook; Sm, subex margin; S, stem.
(43, 45) Urodacus uncinus sp. nov., paratype ♂ (WAM T153275), dextral hemispermatophore, detail of capsule, dorsal aspect; scale bar = 1 mm. Note that laminar hook does not extend past the tip of the conical process, and its distal end is not sickle/moon shaped. (45) Note that the subex margin is relatively smooth. (44, 46) U. lunatus sp. nov., holotype ♂ (WAM T79450), dextral hemispermatophore, detail of capsule, dorsal aspect. Note that laminar hook extends well past the tip of the conical process, and its distal end is sickle/moon shaped. (46) Note that the subex margin is conspicuously fringed.
Recently collected male from Toweranna (close to Whim Creek Hotel), showing the natural yellow colouration of Urodacus uncinus sp. nov. and U. lunatus sp. nov. that darkens into orange and reddish brown in the preserved specimens. The collection site is more consistent with the distribution of U. lunatus sp. nov., but the hemispermatophore of this male was not fully formed, and we hence refrain from assigning it to one of the species described here.

Material examined
Australia: Western Australia: male, 6 km SE of Whim Creek Hotel, Pilbara Biological Survey site DRE09, 20°52′11.7″S 117°51′31.6″E, ethylene glycol pitfall trap, 11 July 2003–3 October 2004, CALM staff (Pilbara Biological Survey) (WAM T79450).
Australia: Western Australia: two males, 10 km S of Mallina Homestead, Pilbara Biological Survey site DRE13, 20°58′10.4″S 118°02′54″E, ethylene glycol pitfall trap, 14 November 2003–13 May 2004, CALM staff (Pilbara Biological Survey) (WAM T79447); five males, 11 km SSE of Whim Creek Hotel, Pilbara Biological Survey site DRE10, 20°55′11.4″S 117°51′40.6″E, ethylene glycol pitfall trap, 11 July 2003–3 October 2004, CALM staff (Pilbara Biological Survey) (WAM T158907–T158910).
Diagnosis
U. lunatus sp. nov. differs from all other described Urodacus species, except U. similis L.E. Koch, 1977, U. uncinus sp. nov. and U. yaschenkoi Birula, 1903 by the very short first four metasomal segments, which are not much longer than high (Figs 38, 39). It can be separated from U. similis and U. yaschenkoi by numerous features, including the unusual shape of the telson, which has an elliptical (rather than globose) vesicle and an aculeus that is more curved than in other species (Figs 38, 39). These telson modifications are probably also present in the female but in a less pronounced manner than in the male. The species can be further distinguished from U. similis and U. yaschenkoi by the near absence of secondary serrations on the chelicerae; smaller dorsal carinae on metasomal segments I–IV (Figs 38, 39); and a very different hemispermatophore (Figs 40–42), which has a longer and thinner distal lamina that is terminally more curved, an inner lobe/laminar hook that is broader and shaped as a waxing crescent half-moon (rather than narrow and pointy), no laminar hook process/inner lobe tooth, and no flap structure mentioned in Koch (1977: 286, fig. 117). Males of U. lunatus sp. nov. are indistinguishable from males of U. uncinus sp. nov. based on external morphology. These two species can be distinguished based on the morphology of the hemispermatophore, which in U. lunatus sp. nov. bears a laminar hook that is shaped as a waxing crescent half-moon (rather than conically shaped) and extends well past the tip of the conical process (‘clasper’ sensuMonod et al. (2017)) (Fig. 44). Moreover, in U. lunatus sp. nov., the hemispermatophore has an obviously fringed (rather than relatively smooth) subex margin (Figs 44, 46).
Description
Based on holotype and paratypes.
Colouration: Carapace dark orange to brown in older preserved specimens, becoming paler in anterior tenth (Figs 38, 39). Mesosoma of similar colour to carapace. Metasoma and telson tending dark orange in older preserved specimens (Figs 38, 39). Aculeus black. Pedipalps dark orange to dark brown. Legs light yellow, femur and patella with darker spot at the terminal tip in the prolateral face (Fig. 38).
Carapace: Relatively square with subparallel margins in the anterior first three quarters and becoming wider with rounded lateral margins posteriorly; anterior margin with well developed median notch that is wider than deep; frontal lobes with anterior margins slightly rounded (Fig. 38). Two pairs of lateral ocelli situated on the anterolateral margins, with the anterior ocelli almost twice as large as posterior ocelli, consistent with type 2B in Loria and Prendini (2014); median ocular tubercle raised, situated anteromedially. Anterior transverse, anterolateral and median lateral sulci absent; median longitudinal sulcus thin and deep, bifurcated anteriorly; posterolateral transverse sulcus distinct, wide, and deep; posteromarginal depression deep, extending anteriorly halfway to the median ocular tubercle. Anterolateral margins, interocular, median, and posteromedian surfaces smooth and glossy; median lateral and posterolateral surfaces matt, sparsely and finely granular in places. Measurements in Table 1.
Chelicerae: Distal tooth on moveable finger more curved than distal tooth on fixed finger; teeth with almost no secondary serrations, just small undulations on medial teeth. Movable finger with two subdistal teeth. Fixed finger without subdistal tooth; proximal edge of median tooth slightly incurved; basal tooth distinctively bilobed.
Pedipalps: Coxa, ventroprolateral surface smooth, with several large and pointy granules medially. Femur, dorsoprolateral carinae vestigial, reduced to a row of granules in the distal third of the segment; dorsoretrolateral and ventroprolateral carinae well developed, costate–granular, comprising granules of irregular size, more pronounced in the middle half for the dorsoretrolateral carinae and in the proximal half for the ventroprolateral carinae; dorsal intercarinal surface sparsely covered with granules of equal size; prolateral intercarinal surface smooth and without any granules. Patella, dorsoprolateral carina well developed, costate–granular; ventroretrolateral carina reduced to a smooth ridge more evident anteriorly; ventroprolateral carina reduced to a row of granules in the distal third of the segment; dorsoretrolateral and ventromedian carinae absent; dorsal intercarinal surface densely covered with small granules; dorsoprolateral process prominent and round, with a terminal large granule; ventroprolateral process wider but lower, less pronounced, and smooth. Chela manus, digital carina costate; dorsomarginal carinae very poorly developed; ventroretrolateral well developed and costate; dorsal secondary carina obsolete, comprising a few granules proximally; retrolateral secondary carina absent; dorsoprolateral carina absent. Dorsal and retrolateral intercarinal surfaces granular–reticulate, with moderate to dense, irregular depressions formed by reticulate arrangement of small granules; prolateral intercarinal surfaces sparsely covered with small granules. Fixed finger without distal diastema and hooklike terminal denticle. Movable finger without distal diastema and hooklike terminal denticle; dentate margin entire, not scalloped, without basal lobe or concavity. Measurements in Table 1.
Trichobothria: The following description is based on the holotype only. Pedipalp neobothriotaxic, Type C. Femur always with 3 trichobothria: i situated dorsobasally on prolateral surface; d situated basally and retrolaterally on dorsal surface, close to dorsoretrolateral carina; e situated dorsobasally on retrolateral surface, close to dorsoretrolateral carina. Patella with 49 trichobothria: 1 i, 2 d, 34 e, and 12 v; d1 situated basally on dorsal surface, at the basal end of dorsoprolateral carina; d2 situated in the distal third of dorsal surface, between trichobothrium i and dorsoprolateral process; retrolateral trichobothria divided into 11 eb, 1 esb and 22 ea; ventral trichobothria in one group. Chela with 51 trichobothria: eight situated on fixed finger, 4 d and 4 e; db situated at base of finger on dorsal surface; dsb situated in proximal fifth of finger on dorsal surface; dst situated medially on finger on dorsal surface; dt situated in distal half of finger on prolateral surface; eb situated at base of finger, opposite db; esb situated in proximal fifth of finger, opposite dsb; est situated medially on finger, opposite dst; et situated in distal half of finger, opposite dt. 43 situated on manus: DB, DT, 5 Eb, Esb, 12 Em, Est, 5 Et (Et4 clearly petite) and 15 V; DB and DT situated basally and retrolaterally on dorsal surface of manus; Eb group with 3 trichobothria visible retrolaterally and 2 visible ventrally; Em group with 6 trichobothria visible retrolaterally and 6 visible ventrally; Est situated terminoventrally on retrolateral surface of manus; Et group with 4 trichobothria visible retrolaterally and 1 ventrally; V group with 10 trichobothria almost regularly spaced along the basal–terminal axis, with 7 additional trichobothria clustered in the terminal end of the ventral surface; It and Ib situated distally on manus, close to base of fixed finger; trichobothrial sulci absent.
Mesosoma: Tergites I–VII, dorsomedian carina vestigial, reduced to low, smooth, and slightly glossy mounds; dorsosubmedian and dorsolateral carinae absent; lateral surfaces matt, finely and evenly granular. Sternites III–VII, ventral surfaces entirely smooth; VII with ventrosubmedian carinae absent and ventrolateral carinae costate. Book lung spiracles slit shaped.
Pectines: Three marginal lamellae and three median lamellae; 14 pectinal teeth on left side (13 on right) in the holotype. Counts of pectinal teeth and variation within males in Table 3.
Coxosternal sclerites: Coxapophysis I expanded anteriorly, subtriangular, and fused to coxa of leg I. Coxapophysis II extending to anterior end of coxapophysis I, twice as long as coxa of leg II, and fused to it; triangular with slightly rounded margins.
Sternum: Subpentagonal, nearly twice wider than long with a furrow running medially through the posterior half. Genital operculum about 30% wider than long, divided in males. Male genital papillae mostly hidden beneath the operculum, visible only from a posterior angle.
Metasoma: Metasomal segments I–IV not much longer than high (measurements in Table 1), dorsal surfaces smooth; dorsosubmedian carinae slightly costate and granular, continuous along length of segments, without prominent spiniform posterior granule; dorsolateral carinae vestigial, reduced to a low smooth and continuous mound along length of segments; median lateral carinae distinctly costate and smooth along length of segments. Metasomal segment V, dorsal surface smooth, shiny; dorsosubmedian carinae absent; dorsolateral carinae with serration that looks sharp dorsally and originating from large granules laterally, continuous along length of segment; median lateral carinae granular and restricted to anterior half of segment; ventrolateral carinae with large granules, continuous along length of segment; ventrosubmedian carinae absent; ventromedian carina raised, wide and granular, continuous along length of segment; ventral intercarinal surfaces with few scattered granules.
Telson: Vesicle enlarged and elliptical in both dorsal and lateral aspects; ventral surface smooth, without ventromedian carina; lateral surfaces similarly smooth; few macrosetae sparsely and evenly distributed. Aculeus stout, extremely curved (90°) in the male. Measurements in Table 1.
Legs: Legs I, tibia and basitarsus, each with comb-like retrolateral row of macrosetae; basitarsus slightly broadened and dorsoventrally compressed; telotarsus, retroventral row of spiniform macrosetae with more setae than proventral row. Legs IV, basitarsus without a comb-like retrolateral row of macrosetae, but with two distal macrosetae; telotarsus, retroventral row of spiniform macrosetae with more setae than proventral row. Counts of macrosetae and spiniform macrosetae in Table 3.
Hemispermatophore: Distal lamina just over twice the length of capsule, rounded distally and directed away from capsule (Figs 40–42); distal crest absent; laminar hook broad (only three times longer than wide), situated proximally, and modified into a waxing crescent half-moon, posterior margin with low eminence (Figs 40–42); distal transverse ridge costate, without laminar hook process; capsule orientation (lateral aspect) in same plane as distal lamina; anterior lobe (‘tectum’ sensuMonod et al. (2017)) thin and terminally rounded, without process (Figs 40–42); conical process (‘clasper’ sensuMonod et al. (2017)) thin and pointy, shorter than laminar hook (Figs 40–42, 44); subex margin fringed terminally (Figs 42, 44, 46).
Distribution and remarks
U. lunatus sp. nov. is known only from nine males (females are unknown) collected in three locations in the north-eastern portion of the Pilbara (Fig. 2). The distance between the locations furthest apart (site DRE09, 6 km SE of Whim Creek Hotel and Site DRE13, 10 km S of Mallina Homestead) is just under 23 km, resulting in a known extent of occurrence of only 50 km2. Similarly to U. uncinus sp. nov., U. lunatus sp. nov. is very rare and underrepresented in the WAM collection, which could result from a unique habitat requirement (see fig. 6 in Volschenk et al. 2010), which might restrict the species to creeks and drainage lines. It is very likely that the species distribution is well under 10 000 km2, making it a short-range endemic (Harvey 2002).
This species has also been known as Urodacus ‘SCO035’ and Urodacus ‘Pilbara 2’ in the Western Australian Museum database.
Ecology
All specimens of U. lunatus sp. nov. collected so far are males caught in pitfall traps, suggesting that males disperse over longer distances when looking for mating opportunities, as is usual in Urodacus. No specimens so far were caught from burrows, but the retrolateral rows of stout macrosetae on the basitarsi of legs I and robust habitus (identical to U. uncinus sp. nov.) corroborates that this species is fossorial.
Etymology
The specific name is taken from the Latin lunatus (meaning ‘lunate’ or ‘crescent shaped’), in reference to the shape of the distal end of the laminar hook in the hemispermatophore, which is crescent shaped and represents the most obvious morphological difference between this species and U. uncinus sp. nov.
Discussion
Burrowing scorpions in the genus Urodacus are widespread in Australia, and might represent a large biomass in some arid areas of the continent (Volschenk et al. 2010). The genus is probably an order of magnitude more diverse than the 21 currently recognised species suggest, as multiple new species have been collected in the last 40 years, the vast majority of which are still awaiting formal description. The last comprehensive revision of Urodacus proposed five species-groups on the basis of morphological patterns in the chelicerae, telson vesicle, denticle rows on the pedipalps’ movable finger, relative lengths of terminal claws of legs IV, and the paraxial organ (Koch 1977). These species groups have never been tested phylogenetically, and later species descriptions refrained from assigning new species to them (Volschenk et al. 2000, 2012), mostly because specimens presented combinations of traits from more than one group. We also leave U. uncinus sp. nov. and U. lunatus sp. nov. ungrouped, given their mixture of traits from Koch’s species-groups, including no secondary serrations on the chelicerae, tarsal claws of almost equal lengths, very large vesicles, and the moveable finger on pedipalps with 2–3 denticle rows proximally and a single row distally. The two new species possess a uniquely shaped telson, which is strongly sexually dimorphic. It is possible that the venom produced is also sexually dimorphic (Sentenská et al. 2017; Olguín-Pérez et al. 2021; Krämer et al. 2022), in which case the enlargement of the male telson could be related to the production of sex-specific peptides involved in mating. However, nothing is currently known about the mating behaviour of U. uncinus sp. nov. or U. lunatus sp. nov., so whether sexual stinging occurs in these species remains to be determined.
Despite the fossorial ecology of most Urodacus species, the group is well known to naturalists and very popular in the pet trade, where three species in the genus featured in the top 10 most popular invertebrate species in online pet stores (Lassaline et al. 2023). The potential conservation impacts of the pet trade via the harvest of live animals is concerning, especially when one of the most traded species, U. yaschenkoi, has recently been found to represent a species complex (Luna-Ramirez et al. 2017b). The description of two new species that are identical in external morphology further supports the notion that Urodacus is much more diverse than currently recognised. Furthermore, these new species seem restricted to creeks and drainage lines, probably rendering them short-range endemic status (Harvey 2002). As a result, the conservation of burrowing scorpions in Australia will require urgent taxonomic revision, especially considering the occurrence of multiple undescribed species in landscapes under intense mining pressure, as well as the conserved external morphology that results in species complexes, some of which are currently targeted by the pet trade.
Data availability
All data used in this publication are available in the main manuscript, in tables, figures or in the lists of material examined and therefore no extra data are available elsewhere.
Acknowledgements
We are grateful to the CALM (currently DBCA) staff involved in the Pilbara Biological Survey and to the Outback Ecology staff for collecting most of the specimens examined here. We are also in debt to Julianne Waldock for help and support while accessing the collection of the Western Australian Museum. The molecular sequence data were obtained via a Net Conservation Benefits grant administered by the Western Australian Department of Biodiversity, Conservation and Attractions. We are very grateful to Joel Huey and Mia Hillyer for supplying the sequences.
References
Fet V, Soleglad ME (2005) Contributions to scorpion systematics. I. On recent changes in high-level taxonomy. Euscorpius 31, 1-13.
| Google Scholar |
Harvey MS (2002) Short-range endemism among the Australian fauna: some examples from non-marine environments. Invertebrate Systematics 16, 555-570.
| Crossref | Google Scholar |
Huey JA, Hillyer MJ, Harvey MS (2019) Phylogenetic relationships and biogeographic history of the Australian trapdoor spider genus Conothele (Araneae: Mygalomorphae: Halonoproctidae): diversification into arid habitats in an otherwise tropical radiation. Invertebrate Systematics 33, 628-643.
| Crossref | Google Scholar |
Isbister GK, Volschenk ES, Seymour JE (2004) Scorpion stings in Australia: five definite stings and a review. Internal Medicine Journal 34, 427-430.
| Crossref | Google Scholar | PubMed |
Koch LE (1977) The taxonomy, geographic distribution and evolutionary radiation of Austalo-Papauan scorpions. Records of the Western Australian Museum 5, 83-367.
| Google Scholar |
Krämer J, Pommerening R, Predel R (2022) Equipped for sexual stings? Male-specific venom peptides in Euscorpius italicus. International Journal of Molecular Sciences 23, 11020.
| Google Scholar | PubMed |
Lassaline CR, Stringham OC, Moncayo S, Toomes A, Cassey P (2023) Untangling the web: dynamics of Australia’s online terrestrial invertebrate trade. Austral Entomology 62(3), 372-387.
| Crossref | Google Scholar |
Locket NA (1993) Scorpion distribution in a dune and swale mallee environment. Memoirs of the Queensland Museum 33(2), 593-598.
| Google Scholar |
Loria SF, Prendini L (2014) Homology of the lateral eyes of Scorpiones: a six-ocellus model. PLoS ONE 9(12), e112913.
| Crossref | Google Scholar | PubMed |
Luna-Ramírez K, Quintero-Hernández V, Vargas-Jaimes L, Batista CVF, Winkel KD, Possani LD (2013) Characterization of the venom from the Australian scorpion Urodacus yaschenkoi: molecular mass analysis of components, cDNA sequences and peptides with antimicrobial activity. Toxicon 63, 44-54.
| Google Scholar | PubMed |
Luna-Ramírez K, Quintero-Hernández V, Juárez-González VR, Possani LD (2015) Whole transcriptome of the venom gland from Urodacus yaschenkoi scorpion. PLoS ONE 10(5), e0127883.
| Crossref | Google Scholar | PubMed |
Luna-Ramirez K, Tonk M, Rahnamaeian M, Vilcinskas A (2017a) Bioactivity of natural and engineered antimicrobial peptides from venom of the scorpions Urodacus yaschenkoi and U. manicatus. Toxins 9(1), 22.
| Crossref | Google Scholar |
Luna-Ramirez K, Miller AD, Rašić G (2017b) Genetic and morphological analyses indicate that the Australian endemic scorpion Urodacus yaschenkoi (Scorpiones: Urodacidae) is a species complex. PeerJ 5, e2759.
| Crossref | Google Scholar | PubMed |
Monod L, Volschenk ES (2004) Liocheles litodactylus (Scorpiones: Liochelidae): an unusual new Liocheles species from the Australian Wet Tropics (Queensland). Memoirs of the Queensland Museum 49(2), 675-690.
| Google Scholar |
Monod L, Cauwet L, González-Santillán E, Huber S (2017) The male sexual apparatus in the order Scorpiones (Arachnida): a comparative study of functional morphology as a tool to define hypotheses of homology. Frontiers in Zoology 14, 51.
| Crossref | Google Scholar | PubMed |
Olguín-Pérez L, Francke OF, Carbajal-Saucedo A (2021) Evidence of piercing and sexual differences in venom composition in a sexual stinging scorpion (Scorpiones: Euscorpiidae). The Journal of Arachnology 49, 98-107.
| Crossref | Google Scholar |
Pocock RI (1893) LIII.—Notes on the classification of scorpions, followed by some observations upon synonymy, with descriptions of new genera and species. Annals and Magazine of Natural History 12(70), 303-330.
| Crossref | Google Scholar |
Pocock RI (1898) VIII.—The Australian scorpions of the genus Urodacus, Pet. Annals and Magazine of Natural History 2(7), 59-67.
| Crossref | Google Scholar |
Prendini L (2000) Phylogeny and classification of the superfamily Scorpionoidea Latreille 1802 (Chelicerata, Scorpiones): an exemplar approach. Cladistics 16(1), 1-78.
| Crossref | Google Scholar | PubMed |
Prendini L, Wheeler WC (2005) Scorpion higher phylogeny and classification, taxonomic anarchy, and standards for peer review in online publishing. Cladistics 21, 446-494.
| Crossref | Google Scholar | PubMed |
Quinlan TG, Smith GT, Calver MC (1995) Relationships between morphology and feeding behaviour in the syntopic scorpions Urodacus armatus Pocock and Urodacus novaehollandiae Peters (Scorpiones: Scorpionidae). Australian Journal of Entomology 34, 277-279.
| Crossref | Google Scholar |
Roldan NMR, Gaffin DD (2018) New, sensitive behavioral assay shows scorpions are attracted to multiple wavelengths of light. Journal of Arachnology 46(3), 432-437.
| Crossref | Google Scholar |
Santibáñez-López CE, Aharon S, Ballesteros JA, Gainett G, Baker CM, González-Santillán E, Harvey MS, Hassan MK, Abu Almaaty AH, Aldeyarbi SM, Monod L, Ojanguren-Affilastro A, Pinto-da-Rocha R, Zvik Y, Gavish-Regev E, Sharma PP (2022) Phylogenomics of scorpions reveal contemporaneous diversification of scorpion mammalian predators and mammal-active sodium channel toxins. Systematic Biology 71, 1281-1289.
| Crossref | Google Scholar | PubMed |
Sentenská L, Graber F, Richard M, Kropf C (2017) Sexual dimorphism in venom gland morphology in a sexually stinging scorpion. Biological Journal of the Linnean Society 122, 429-443.
| Crossref | Google Scholar |
Sharma PP, Fernández R, Esposito LA, González-Santillán E, Monod L (2015) Phylogenomic resolution of scorpions reveals multilevel discordance with morphological phylogenetic signal. Proceedings of the Royal Society B: Biological Sciences 282(1804), 20142953.
| Crossref | Google Scholar |
Sharma PP, Baker CM, Cosgrove JG, Johnson JE, Oberski JT, Raven RJ, Harvey MS, Boyer SL, Giribet G (2018) A revised dated phylogeny of scorpions: phylogenomic support for ancient divergence of the temperate Gondwanan family Bothriuridae. Molecular Phylogenetics and Evolution 122, 37-45.
| Crossref | Google Scholar | PubMed |
Soleglad ME, Fet V, Kovařík F (2005) The systematic position of the scorpion genera Heteroscorpion Birula, 1903 and Urodacus Peters, 1861 (Scorpiones: Scorpionoidea). Euscorpius 20, 1-38.
| Google Scholar |
Stahnke HL (1970) Scorpion nomenclature and mensuration. Entomological News 81, 297-316.
| Google Scholar |
Vachon M (1974) Étude des caractères utilisés pour classer les familles et les genres de Scorpions (Arachnides). 1. La trichobothriotaxie en Arachnologie. Sigles trichobothriaux et types de trichobothriotaxie chez les Scorpions. Bulletin du Muséum National d’Historie Naturelle, Paris 3(140), 857-958.
| Google Scholar |
Volschenk ES, Prendini L (2008) Aops oncodactylus, gen. et sp. nov., the first troglobitic urodacid (Urodacidae:Scorpiones), with a re-assessment of cavernicolous, troglobitic and troglomorphic scorpions. Invertebrate Systematics 22, 235-257.
| Crossref | Google Scholar |
Volschenk ES, Smith GT, Harvey MS (2000) A new species of Urodacus from Western Australia, with additional descriptive notes for Urodacus megamastigus (Scorpiones). Records of the Western Australian Museum 20, 57-67.
| Google Scholar |
Volschenk ES, Burbidge AH, Durrant BJ, Harvey MS (2010) Spatial distribution patterns of scorpions (Scorpiones) in the arid Pilbara region of Western Australia. Records of the Western Australian Museum, Supplement 78(1), 271-284.
| Crossref | Google Scholar |
Volschenk ES, Harvey MS, Prendini L (2012) A new species of Urodacus (Scorpiones: Urodacidae) from Western Australia. American Museum Novitates 3748(3748), 1-18.
| Crossref | Google Scholar |
White CR (2001) The energetics of burrow excavation by the inland robust scorpion, Urodacus yaschenkoi (Birula, 1903). Australian Journal of Zoology 49, 663-674.
| Crossref | Google Scholar |
Withers PC, Smith GT (1993) Effect of temperature on the metabolic rate and evaporative water loss of the scorpion Urodacus armatus. Journal of Thermal Biology 18(1), 13-18.
| Crossref | Google Scholar |
Zwicky KT (1968) A light response in the tail of urodacus, a scorpion. Life Sciences 7, 257-262.
| Crossref | Google Scholar | PubMed |
Footnotes
1 ZooBank Registration: https://zoobank.org/References/67C9F761-2425-4B41-AE7D-21419D16271F.


