Gastrointestinal helminth parasites of the grey kangaroos, Macropus fuliginosus and M. giganteus
Ian Beveridge A B *
A B *
A
B
Abstract
The helminth parasites of Macropus fuliginosus and Macropus giganteus are reported based on examination of a total of 285 animals extending, for the first time, across the entire geographical range of both species and including, where possible, data from previous regional studies. A total of 64 species of helminths was found including 42 species of strongyloid nematodes in the stomach, seven species of trichostrongyloid nematodes in the pylorus and small intestine and seven species of nematodes in the terminal ileum and large intestine, one species of spirurid nematode in the stomach and six species of cestodes and one species of trematode. Forty-three species were encountered in both M. fuliginosus and M. giganteus. The helminth communities of the two kangaroo species exhibited a similarity of 85.4% based on all helminth species encountered or 91.4% if only the species specific to grey kangaroos were considered. Interchange of helminths between the two species of kangaroos revealed several different patterns with instances both of transfer and lack of transfer in areas of host sympatry as well as transfers beyond the zone of sympatry. The findings are discussed in relationship to the phylogeography of the host species.
Keywords: grey kangaroos, helminth, host species, Macropus fuliginosus, Macropus giganteus, parasite.
Introduction
The helminth parasites of the grey kangaroos, Macropus fuliginosus and Macropus giganteus, have been relatively well studied, with the findings summarised by Spratt and Beveridge (2016). Since then several additions, including new species and generic changes, have been made to the known helminth fauna of these kangaroo species by Beveridge (2020a), Sukee et al. (2020a, 2020b, 2021a) and Beveridge et al. (2021).
The first survey of parasites of the grey kangaroos was that of Beveridge and Arundel (1979) providing prevalence and intensity data for the first time, but covering only eastern Australia, with no data for M. fuliginosus in Western Australia. For several genera of nematodes (e.g. Cloacina, Labiosimplex), the data for individual species were not provided. Since their publication, there have been substantial taxonomic changes in parasite taxonomy, complicating comparisons with current data.
Beveridge et al. (1998) provided prevalence but not intensity data for a sample of 28 M. giganteus from north and central Queensland in a comparative study of the helminth communities of macropodids in the same region, but in this study data were provided for individual helminth species for the first time. Subsequently, several localised studies on similarly small numbers of animals have provided both prevalence and intensity data at helminth species level: Webley et al. (2004) for 25 M. fuliginosus on Kangaroo Island, South Australia, Aussavy et al. (2011) for both M. fuliginosus and M. giganteus in the Grampian Ranges of Victoria (10 M. fuliginosus, 18 M. giganteus) and Vendl and Beveridge (2014) for gastric nematodes in 16 M. giganteus at Portland, Victoria. Spratt et al. (2017) reported prevalence and intensities of gastric helminths from 24 M. giganteus from several coastal and montane sites of south-eastern New South Wales.
The aim of the present study was to provide, for the first time, an overview of the helminths of both species of grey kangaroo across their entire geographical ranges, including previously published studies for which the data from individual animals were available as well as from a significant number of animals from which data have not previously been published.
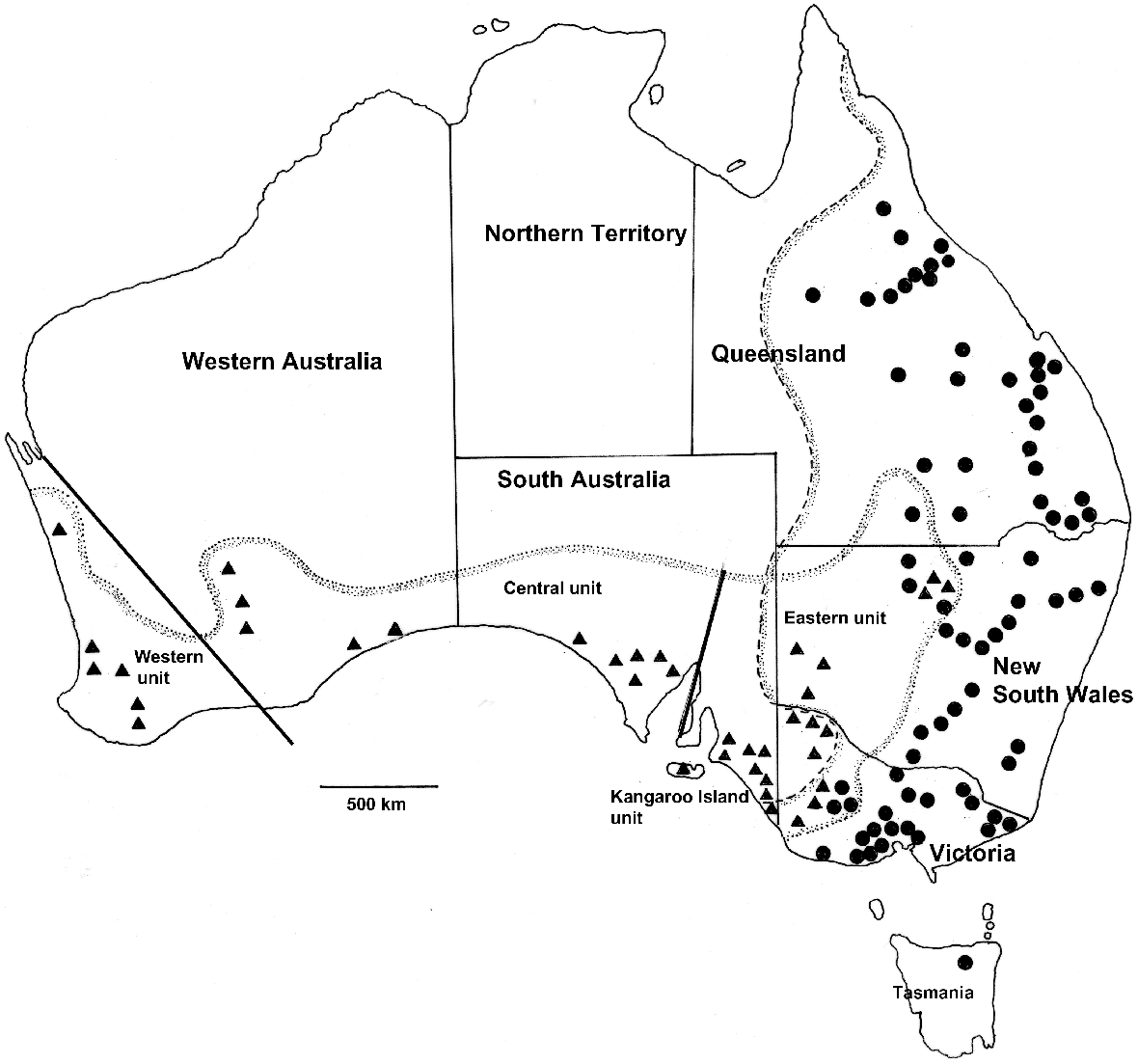
A particular interest in analysing the helminth communities of the two species of grey kangaroos was the potential to compare the helminth communities of two closely related kangaroo species with the phylogeography of their hosts and the extent to which they currently share helminth species. M. fuliginosus is thought to have evolved in the south-west of Western Australia, possibly 2 million years ago (Meredith et al. 2008), while separated from eastern populations during changes in sea levels in the Nullarbor region (Maynes 1989). Subsequent periods have allowed the eastward migration of M. fuliginosus so that it now also occurs in South Australia, western New South Wales and south-western Queensland, often in sympatry with M. giganteus. Neaves et al. (2009) recognised four genetic units within M. fuliginosus: a ‘western’ unit comprising kangaroos in the south-west of Western Australia, a ‘central’ unit extending from Kalgoorlie in the west, across the Nullarbor Plain to the Flinders Ranges in South Australia, a ‘Kangaroo Island unit’ and an ‘eastern unit’ comprising eastern South Australia, north-western Victoria, western New South Wales and south-western Queensland (Neaves et al. 2009) (Fig. 1) The ‘Kangaroo Island’ unit was recognised as a subspecies by Jackson and Groves (2015). By contrast, M. giganteus is thought to have evolved in south-eastern Australia and migrated north, based on evidence of decreased genetic diversity in northern populations (Zenger et al. 2003), but with no clear genetic subdivisions within the populations.
Sites from which Macropus fuliginosus (▴) and M. giganteus (•) were examined for helminth parasites with the distributions of the host species based on van Dyck and Strahan (2008). The dotted line represents the distribution of M. fuliginosus and the dashed line that of M. giganteus. Coordinates and numbers of animals examined at each site are provided in Tables S1 and S2. Genetic subdivisions of M. fuliginosus (‘western’, ‘central’, ‘eastern’ and ‘Kangaroo Island’) are based on Neaves et al. (2009).
The helminth parasite communities of the two species of kangaroos are examined here both in terms of their geographical distributions and the extent to which the helminth communities reflect the phylogeography of the hosts.
Materials and methods
The methods used were broadly similar to those used in previously published studies (Beveridge and Arundel 1979; Beveridge et al. 1998; Webley et al. 2004; Aussavy et al. 2011; Vendl and Beveridge 2014). Kangaroos were collected opportunistically either as fresh road-kills, from professional kangaroo shooters, from property owners with destruction permits or in association with other studies on grey kangaroos. Kangaroos were identified based on the morphological features described in van Dyck and Strahan (2008). If there was any doubt as to specific identity, photographs were taken and the opinion of highly experienced colleagues was sought. For most animals in the zone of sympatry, frozen tissues were collected in case they were required to confirm a specific identification, and have been deposited in the South Australian Museum, Adelaide. Some of the specimens were included in a study of hybridisation between the two species of kangaroo in the zone of sympatry (Neaves et al. 2010).
At autopsy, the liver was examined for cestodes in the bile ducts; the small intestine contents were collected, washed in water or saline, sedimented and any helminths fixed in 10% formalin or 70% ethanol. The large intestine was examined grossly. Large nematodes were fixed in formalin or ethanol; if oxyurids were observed, a sample of content was preserved and nematodes extracted subsequently in the laboratory. The stomach was opened and either a random sample of nematodes was collected (Beveridge et al. 1998), fixed and then subsequently sorted in the laboratory, or a 5% or 10% sample of the stomach contents was fixed and all nematodes in it were subsequently sorted and stored in ethanol in the laboratory (Aussavy et al. 2011; Vendl and Beveridge 2014). The pulmonary system was not routinely examined for helminths. Filarioid nematodes encountered in the body cavities, the subcutis and the intermuscular connective tissues were collected when observed but were not sought systematically. No attempt was made to locate parasites in the vascular system such as the nematode Durikainema or the subcutaneous tissues for filarioid nematodes, nor to identify infections with Strongyloides sp., which requires the use of specialised techniques (Speare 1986).
Wherever possible, voucher specimens were retained and have been deposited in the collections of the South Australian Museum, Adelaide (Supplementary Table S1).
Nematodes were cleared in lactophenol for identification. Cestodes were stained in Celestine blue, dehydrated in an ethanol series, cleared in methyl salicylate and mounted in Canada balsam. Helminths were identified using the following sources: Nematoda: Strongyloidea: Cloacininae: publications summarised in Beveridge and Smales (2022); Phascolostrongylinae: Beveridge and Mawson (1978); Sukee et al. (2020a, 2021a); Trichostrongyloidea: Cassone and Baccam (1985), Beveridge and Spratt (1988), Beveridge and Durette-Desset (2010), Durette-Desset and Beveridge (2012); Oxyuroidea: Mawson (1964); Filarioidea: Spratt and Varughese (1975), Spratt (2011); Spiruroidea: Spratt (2023); Cestoda: Beveridge (1976, 2009); Trematoda: Jones (2005).
In some kangaroos, females of the nematode genera Labiosimplex, Alocostoma, Filarinema and Austrostrongylus were encountered without accompanying males and could not be identified to species. The common nematode Rugopharynx australis has recently been shown to include a cryptic species, Rugopharynx moennigi (Beveridge et al. 2021). Where possible, voucher specimens have been used to separate these two species. However, in some instances, voucher specimens were lacking or only female nematodes were present and in both cases, the nematodes have been included in a category ‘R. australis or R. moennigi’. The cestode species Progamotaenia festiva, Progamotaenia macropodis, Triplotaenia undosa and Wallabicestus ewersi currently include cryptic species not separable using morphological characters (Hu et al. 2005; Beveridge 2009; Beveridge and Shamsi 2009; Hardman et al. 2012) but it has been presumed, for simplicity, that those present in the grey kangaroos represent a single genotype.
The use of the terms prevalence and intensity follow Bush et al. (1997). Not all organs were examined in every kangaroo collected, usually due to damage of some organs during collection or inadequate facilities for a full examination. Prevalences are based on the number of organs examined rather than the total number of animals collected. The overall similarity of the helminth communities was calculated using Sorenson’s Index (Magurran 1988) and the diversity of the communities in the two kangaroo host species was compared using the reciprocal of Simpson’ Index (Magurran 1988). The significance of differences in the sex ratio of the host samples and the prevalence of each helminth species between M. fuliginosus and M. giganteus was tested using the Chi-square test.
Each helminth species was allocated to one of seven groups based on its host range and prevalence:
Specific to both M. fuliginosus and M. giganteus either at low prevalences but not differing by more than 10% (a) or, in both species at a high prevalence >80% (b). The subdivision of this group was considered advisable in case different factors were involved in the role these individual helminth species played within the community, those occurring at low prevalences being accidental infections and those at a high prevalence, core species of the community.
Specific to M. fuliginosus.
Specific to M. giganteus.
Primarily parasitic in M. fuliginosus but present in M. giganteus in areas of host sympatry.
Primarily parasitic in M. giganteus but present in M. fuliginosus in areas of host sympatry.
Parasitic in several other sympatric host species.
Incidental infections from a sympatric host species (with host identity indicated).
Most helminth species in macropodoids are host specific, occurring primarily in a single species or in two closely related host species (Beveridge et al. 2010), but specificity is not always absolute. Occasional infections from sympatric hosts do occur at low prevalences and intensities (e.g. Beveridge 2016, 2020a, 2020b) and this has been taken into account in assessing specificity in instances in which hosts other than the two species of grey kangaroos are involved. If a parasite occurred at a high prevalence in the grey kangaroos but was found uncommonly in sympatric hosts, the parasite species was still considered to be host specific to the grey kangaroo host.
For both M. fuliginosus and M. giganteus, the number of helminth species in each 10% prevalence class was plotted. Due to a lack of abundance data, the correlation between prevalence and abundance, a prerequisite for the analysis of ‘core’ and ‘satellite’ species (Hanski 1982) could not be calculated. However, Webley et al. (2004) established such a correlation for a smaller sample of M. fuliginosus, as did Aussavy et al. (2011) for M. giganteus, and so it was assumed that the same relationship would also apply to the present larger sample sizes.
For M. fuliginosus, the number of grey kangaroo–specific species in each of the four genetic subdivisions of the species (‘western’, ‘central’, ‘Kangaroo Island’ and ‘eastern’) identified by Neaves et al. (2009) was calculated. In the case of M. giganteus, Zenger et al. (2003) identified a reduction in genetic diversity in northern populations, but with no discrete barriers identified as they compared only two populations in south-eastern New South Wales and central and northern Queensland. For this analysis, the distribution of each grey kangaroo–specific species was plotted on a map and a division made into predominantly ‘northern’ and predominantly ‘southern’ species. As outliers occurred in some species, the northern or southern extent of the species was noted as well as the presence of outliers. In cases where distribution maps for the helminth species have been published, references are provided.
Host nomenclature follows Jackson and Groves (2015) and the geographical ranges of the host species shown in figs 1, 3, and 4 are from van Dyck and Strahan (2008).
Results
In total, 285 grey kangaroos for which sufficiently detailed data were available were included in the study; they comprised 109 M. fuliginosus and 176 M. giganteus. Among the M. fuliginosus, 46 were males, 31 were females and there were 32 animals for which sex was not recorded. There was no significant bias towards males from specimens from which the sex was recorded (χ2 = 2.9; P < 0.1). In the case of M. giganteus, 80 were males, 65 females and the sex was not recorded for 31. There was no significant bias towards males (χ2 = 03.19; P, 0.1). Of the 109 M. fuliginosus, 21 were collected in New South Wales, 17 in Victoria, 43 in South Australia and 29 in Western Australia (Table S2). In the case of the 178 M. giganteus, 66 were collected in Queensland, 49 in New South Wales, 59 in Victoria and 2 in Tasmania (Table S3). All kangaroos examined were adults but no attempt was made to ascertain ages. The distribution of collection sites is shown in Fig. 1 and details of coordinates and numbers of animals collected at each site are presented in Tables S2 and S3.
Some of the animals included in this study have also been included in previously published studies. In the case of M. fuliginosus, original data are provided for 65 animals together with 44 animals from previous studies (Beveridge and Arundel 1979 (9); Webley et al. 2004 (25); Aussavy et al. 2011 (10)). For M. giganteus, new data from 102 animals were included in addition to 74 from previous studies (Beveridge and Arundel 1979 (28); Beveridge et al. 1998 (22); Aussavy et al. 2011 (18); Vendl and Beveridge 2014 (6)).
The stomach contents of every kangaroo were sampled. In the case of bile duct cestodes, livers were frequently absent in material obtained from shooters and totals of 90 from M. fuliginosus and 160 from M. giganteus were examined. For the small intestine, 68 were examined from M. fuliginosus and 93 from M. giganteus. In the case of the large intestines, 86 M. fuliginosus and 119 M. giganteus were examined for strongyloid nematodes (Macropostrongyloides, Torquenema) and 78 M. fuliginosus and 103 M. giganteus were examined for oxyuroids.
A total of 64 species of helminths was found (excluding unidentifiable females and specimens that were either Rugopharynx australis or R. moennigi) with two species of cestodes in the bile ducts, 42 species of strongyloid nematodes and one species of spirurid nematode in the stomach, four species of cestode and seven species of trichostrongyloid nematode in the pylorus and small intestine (primarily duodenum) and seven species of nematode and one species of trematode in the terminal ileum and large intestine (Table 1). Helminths were assigned to seven categories described above, based on their prevalence in each host species (Table 1).
| Parasite | Macropus fuliginosus | Macropus giganteus | Site in host | P-value (χ2) | Classification (see text) | |
|---|---|---|---|---|---|---|
| Trematoda | ||||||
| Macropotrema n. sp. | 1.3 | 0 | Large int. | <0.05 | 2 | |
| Cestoda | ||||||
| Anoplocephalidae | ||||||
| Progamotaenia effigia | 25.5 | 0 | Bile duct | <0.01 | 2 | |
| Progamotaenia festiva | 5.6 | 40.7 | Bile duct | <0.01 | 5 | |
| Progamotaenia macropodis | 5.5 | 9.1 | Small int. | <0.1 | 1aA | |
| Triplotaenia fimbriata | 5.5 | 3.0 | Small int. | <0.1 | 1a | |
| Triplotaenia undosa | 17.8 | 9.0 | Small int. | <0.05 | 1aA | |
| Wallabicestus ewersi | 9.6 | 4.0 | Small int. | <0.01 | 1aA | |
| Nematoda | ||||||
| Cloacinidae | ||||||
| Phascolostrongylinae | ||||||
| Hypodontus macropi | 9.3 | 1.7 | Ileum, large int. | <0.025 | 7 (O. rufus) | |
| Macropicola ocydromi | 12.2 | 0 | Ileum | <0.01 | 2 | |
| Macropostrongyloides mawsonae | 3.6 | 41.9 | Large int. | <0.01 | 5 | |
| Macropostrongyloides yamagutii | 36.1 | 5.0 | Large int. | <0.01 | 4 | |
| Paramacropostrongylus iugalis | 5.8 | 21.0 | Stomach | <0.01 | 4 | |
| Paramacropostrongylus typicus | 20.2 | 1.8 | Stomach | <0.01 | 5 | |
| Torquenema toraliforme | 0 | 21.0 | Large int. | <0.01 | 3 | |
| Cloacininae | ||||||
| Labiostrongylinea | ||||||
| Labiosimplex bipapillosus | 0.9 | 39.1 | Stomach | <0.01 | 5 | |
| Labiosimplex kungi | 24.3 | 14.4 | Stomach | <0.1 | 1a | |
| Labiosimplex laterilabellosus | 1.9 | 0.6 | Stomach | <0.1 | 1a | |
| Labiosimplex longispicularis | 0 | 1.7 | Stomach | <0.1 | 7 (O. rufus) | |
| Labiosimplex major | 8.4 | 7.5 | Stomach | <0.1 | 1a | |
| Labiosimplex occidentalis | 1.9 | 0 | Stomach | <0.01 | 2 | |
| Labiosimplex females or immatures | 5.6 | 5.2 | Stomach | na | – | |
| Pharyngostrongylinea | ||||||
| Pharyngostrongylus kappa | 2.8 | 46.7 | Stomach | <0.01 | 5 | |
| Pharyngostrongylus lambda | 1.9 | 14.4 | Stomach | <0.01 | 6 | |
| Rugopharynx australis | 11.2 | 16.1 | Stomach | <0.1 | 6 | |
| Rugopharynx moennigi | 19.7 | 1.7 | Stomach | <0.01 | 4 | |
| Either R. australis or R. moennigi | 9.3 | 4.6 | Stomach | na | – | |
| Rugopharynx macropodis | 44.9 | 44.8 | Stomach | <0.1 | 1a | |
| Rugopharynx disjunctus | 10.3 | 0 | Stomach | <0.01 | 2 | |
| Rugopharynx rosemariae | 4.7 | 28.7 | Stomach | <0.01 | 5 | |
| Cloacininea | ||||||
| Cloacina ares | 0 | 0.6 | Stomach | <0.1 | 7 (O. rufus) | |
| Cloacina artemis | 34.6 | 31.6 | Stomach | <0.1 | 1a | |
| Cloacina cf. artemis | 1.9 | 0 | Stomach | <0.1 | 2 | |
| Cloacina australis | 0 | 1.1 | Stomach | <0.1 | 7 (N. agilis) | |
| Cloacina expansa | 34.6 | 44.3 | Stomach | <0.1 | 1a | |
| Cloacina feronia | 0 | 0.6 | Stomach | <0.1 | 7 (O. robustus) | |
| Cloacina hera | 13.1 | 11.5 | Stomach | <0.1 | 1a | |
| Cloacina hermes | 52.3 | 19.5 | Stomach | <0.01 | 4 | |
| Cloacina herceus | 15.9 | 87.9 | Stomach | <0.01 | 5 | |
| Cloacina hestia | 26.2 | 9.2 | Stomach | <0.01 | 4 | |
| Cloacina hydriformis | 5.6 | 0.6 | Stomach | <0.025 | 7 (O. rufus) | |
| Cloacina kartana | 1.9 | 0 | Stomach | <0.1 | 7 (N. eugenii) | |
| Cloacina macropodis | 0.9 | 0 | Stomach | <0.1 | 7 (O. robustus) | |
| Cloacina leto | 0 | 5.2 | Stomach | <0.025 | 3 | |
| Cloacina magnipapillata | 63.6 | 34.5 | Stomach | <0.01 | 1b | |
| Cloacina obtusa | 78.3 | 52.3 | Stomach | <0.025 | 1b | |
| Cloacina pelops | 15.9 | 23.0 | Stomach | <0.01 | 1a | |
| Cloacina selene | 35.5 | 18.4 | Stomach | <0.025 | 1b | |
| Cloacina typhon | 0 | 20.7 | Stomach | <0.01 | 3 | |
| Macropostrongylinea | ||||||
| Alocostoma clelandi | 2.8 | 14.4 | Stomach | <0.01 | 6 | |
| Alocostoma propinquum | 2.8 | 8.0 | Stomach | <0.1 | 6 | |
| Alocostoma females | 0.9 | 2.3 | Stomach | na | – | |
| Macroponema arundeli | 0 | 14.9 | Stomach | <0.01 | 3 | |
| Macroponema comani | 1.9 | 25.3 | Stomach | <0.01 | 5 | |
| Coronostrongylinea | ||||||
| Papillostrongylus barbatus | 10.3 | 9.2 | Stomach | <0.1 | 6 | |
| Popovastrongylus macropodis | 1.9 | 14.9 | Stomach | <0.01 | 6 | |
| Popovastrongylus pearsoni | 25.2 | 2.3 | Stomach | <0.01 | 7 (N. eugenii) | |
| Zoniolaiminea | ||||||
| Wallabinema cobbi | 0.9 | 0 | Stomach | <0.1 | 7 (O. rufus) | |
| Trichostrongylidae | ||||||
| Filarinema dissimile | 4.0 | 0 | Stomach | <0.025 | 6 | |
| Filarinema females | 5.0 | 0 | Stomach | na | ||
| Herpetostrongylidae | ||||||
| Austrostrongylus chandleri | 1.4 | 1.9 | Small int. | <0.1 | 7 (N.rufogriseus) | |
| Austrostrongylus incurvispiculum | 4.1 | 0 | Small int. | <0.025 | 7 (N. irma) | |
| Austrostrongylus smalesae | 4.1 | 1.9 | Small int. | <0.1 | 7 (N. rufogriseus | |
| Austrostrongylus females | 2.7 | 3.7 | Small int. | na | – | |
| Globocephaloides affinis | 0 | 0.9 | Small int. | <0.1 | 7 (N. dorsalis) | |
| Globocephaloides macropodis | 0 | 0.6 | Small int. | <0.1 | 7 (N. agilis) | |
| Globocephaloides trifidospicularis | 23.3 | 22.2 | Small int. | <0.1 | 6 | |
| Oxyuridae | ||||||
| Macropoxyuris spp. | 60.3 | 33.9 | Large int. | na | – | |
| Macropoxyuris brevigularis | 53.8 | 37.1 | Large int. | <0.01 | 1b | |
| Macropoxyuris longigularis | 38.5 | 36.0 | Large int. | <0.1 | 1a | |
| Gongylonematidae | ||||||
| Gongylonema macropodum | 0 | 0.9 | Stomach | <0.1 | 7 (N. agilis) | |
Six species occurred exclusively in M. fuliginosus (group 2: Table 1) and four exclusively in M. giganteus (group 3: Table 1). Twenty-seven species occurred in both M. fuliginosus and M. giganteus (groups 1, 4, 5: Table 1), although with differing prevalences. Ten of these were helminth species, which occurred primarily in one species of grey kangaroo, but occurred in the reciprocal host species at low prevalences at sites where M. fuliginosus and M. giganteus were sympatric (groups 4, 5: Table 1). Seven species occurred in several sympatric hosts species (usually Osphranter robustus and Osphranter rufus) in inland areas at moderate prevalences (group 6: Table 1), and 16 species were considered to be accidental infections from sympatric hosts, based on a low prevalence in grey kangaroos and at a much higher prevalence in sympatric species (group 7: Table 1). The sympatric species involved, with number of helminth species in parentheses, were Notamacropus agilis (2), Notamacropus dorsalis (1), Notamacropus eugenii (2), Notamacropus irma (1), Notamacropus rufogriseus (2), O. robustus (2), O. rufus (5). One species, Popovastrongylus pearsoni, has a relatively wide host range, but has its highest prevalence in N. eugenii (Smales and Mawson 1978). Globocephaloides trifidospicularis was found predominantly in M. giganteus, with fewer infections in M. fuliginosus. However, it also occurs in the sympatric hosts N. eugenii, N. rufogriseus and Wallabia bicolor (Beveridge 1979) and the level of interchange between these host species is not currently understood.
Additional species encountered incidentally were the filarioid nematodes Pelecitus roemeri, from the intermuscular connective tissues surrounding the femoro-tibial joint in both M. fuliginosus and M. giganteus, Breinlia robertsi in the abdominal cavity of M. fuliginosus, and Breinlia mundayi, and Breinlia dentonensis both in the abdominal cavity of M. giganteus. Metacestodes of Echinococcus granulosus were found in the lungs of M. giganteus.
Similarity between the helminth communities of M. fuliginosus and M. giganteus, estimated using Sorenson’s Index was 85.4%. In the case of helminth species specific to the grey kangaroos, the similarity index was 91.4%. Diversity within the communities from each host species, estimated using Simpson’s Index, were: M. fuliginosus 34.0 and M. giganteus 31.8.
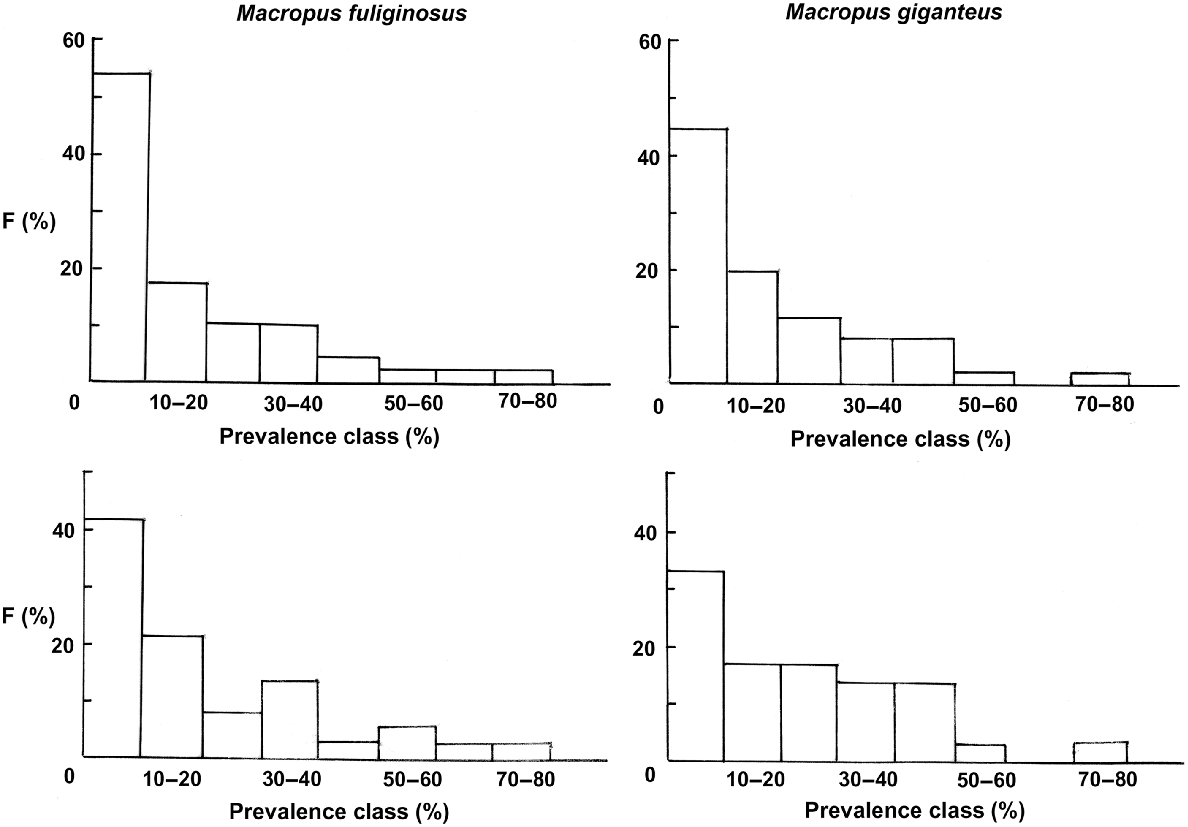
Plots of the frequency distribution of prevalences in 10% prevalence classes were similar between M. fuliginosus and M. giganteus, whether all helminth species or whether only those specific to the grey kangaroos were included. In each case, there was a decline in frequency from low to high prevalence classes (Fig. 2).
Relationships between helminth prevalence classes (%) (axis) and frequency of that prevalence class (F%) (abscissa) for the helminth parasites of Macropus fuliginosus (left) and M. giganteus (right). Upper figures include all helminth species, lower figures those found primarily in M. fuliginosus and M. giganteus.
The geographical distributions of grey kangaroo – specific helminth species in M. fuliginosus (total 18 species) revealed similar numbers of species (15–16) in each of the ‘western’, ‘central’ and ‘eastern’ genetic subdivisions of the host species, but only six species in the ‘Kangaroo Island’ subdivision (Table 2). In addition, R. moennigi and Cloacina hera were absent in the ‘western’ unit, Macropicola ocydromi from the ‘eastern’ unit and Rugopharynx disjunctus and Cloacina artemis from the ‘central, unit. Labiosimplex occidentalis was found only in the ‘western’ unit. In the case of M. giganteus, of the 23 species specific to this host, four were considered to have a predominantly northern distribution and 19 a predominantly southern distribution, with some species occurring in both regions: P. festiva, Labiosimplex bipapillosus, Pharyngostrongylus kappa, C. artemis, Cloacina expansa, Cloacina herceus, Cloacina leto, Macroponema comani and Macropoxyuris spp. (Table 3).
| Parasite species | ‘Western’ unit | ‘Central’ unit | ‘Eastern’ unit | ‘Kangaroo Island’ unit | |
|---|---|---|---|---|---|
| Progamotaenia effigia | + | + | + | − | |
| Macropicola ocydromi | + | + | − | − | |
| Macropostrongyloides yamagutii | + | + | + | − | |
| Paramacropostrongylus typicus | + | + | + | − | |
| Labiosimplex kungi | + | + | + | − | |
| Labiosimplex occidentalis | + | − | − | − | |
| Rugopharynx moennigi | − | + | + | − | |
| Rugopharynx disjunctus | + | − | + | − | |
| Rugopharynx macropodis | + | + | + | − | |
| Cloacina artemis | + | − | + | + | |
| Cloacina expansa | + | + | + | − | |
| Cloacina hera | − | + | + | − | |
| Cloacina hermes | + | + | + | + | |
| Cloacina hestia | + | + | + | − | |
| Cloacina magnipapillata | + | + | + | + | |
| Cloacina obtusa | + | + | + | + | |
| Cloacina selene | + | + | + | + | |
| Macropoxyuris spp. | + | + | + | + | |
| Total no. of taxa (18) | 16 | 15 | 16 | 6 |
| Parasite species | Northern | Southern | Comment | |
|---|---|---|---|---|
| Progamotaenia festiva | + | + | ||
| Macropostrongyloides mawsonae | − | + | Southern, north to Theodore, Qld, outlier in Townsville, Qld. Map: Sukee et al. (2020a). | |
| Paramacropostrongylus iugalis | + | − | Northern, southern limit Bourke, NSW. Fig. 4a. | |
| Torquenema toraliforme | − | + | Southern, northern limit Warwick, Qld. Map: Sukee et al. (2021a) | |
| Labiosimplex bipapillosus | + | + | ||
| Pharyngostrongylus kappa | + | + | ||
| Rugopharynx macropodis | − | + | Southern, northern limit Inglewood, Qld, outlier in Miles, Qld. Map: Beveridge (2020e). | |
| Rugopharynx rosemariae | − | + | Southern, northern limit Toowoomba, Qld | |
| Cloacina artemis | + | + | ||
| Cloacina expansa | + | + | ||
| Cloacina hera | − | + | Southern, northern limit Miles, Cunnamulla, Qld. | |
| Cloacina hermes | − | + | Southern, northern limit Armidale, NSW. | |
| Cloacina herceus | + | + | ||
| Cloacina hestia | − | + | Southern, northern limit Charleville, Qld. | |
| Cloacina leto | + | + | ||
| Cloacina magnipapillata | + | − | Northern, southern limit Jerilderie, NSW, outliers in Nagambie and Grampian Ranges, Vic. Fig. 4b. | |
| Cloacina obtusa | + | + | ||
| Cloacina pelops | − | + | Southern | |
| Cloacina selene | − | + | Southern, northern limit Jerilderie, NSW | |
| Cloacina typhon | + | − | Northern, outliers at Tidbinbilla, ACT and Dartmouth, Vic. | |
| Macroponema arundeli | + | − | Southern limit, Wollomombi, NSW. Map: Sukee et al. (2020b). | |
| Macroponema comani | + | + | Map: Sukee et al. (2020b). | |
| Macropoxyuris spp. | + | + | ||
| Total 23 | 4 | 19 |
Each parasite has been identified as ‘southern’ or ‘northern’ in its distribution, with limits and outliers indicated. Any published maps of parasite distribution have been cited.
Discussion
The present study represents the first attempt to describe the helminth communities of M. fuliginosus and M. giganteus across their entire geographical ranges based on hosts from which sufficiently detailed information was obtainable and to which contemporary parasite identifications could be applied given the major changes in the taxonomy of these helminths which have occurred since the original survey of eastern Australian representatives of these kangaroo species by Beveridge and Arundel (1979). The prevalence data presented here need to be treated with some degree of caution since the material was collected opportunistically, not all parts of the gastrointestinal tract could be investigated in every individual due to availability of suitable facilities at collecting sites and, in the case of the stomach-inhabiting cloacinine nematodes, which constituted 66% of the helminth species recovered, differing methods were used to determine the number of nematode species present. In some cases (e.g. Vendl and Beveridge 2014), all nematode species in a 10% sample of the stomach content were reported whereas in many other cases diversity was assessed based only on a random sample of the stomach nematodes. Vendl and Beveridge (2014) suggested that in the case of M. giganteus, 27–92 (mean 57) nematodes needed to be examined to recover all species in a 10% sample of contents. However, Beveridge (2020b) described a new species of Cloacina from an unrelated host, the black-striped wallaby, N. dorsalis, which constituted only 3% of the gastric nematode community, suggesting that similarly uncommon nematodes such as C. cf artemis from M. fuliginosus may also have been overlooked to date. For these reasons, either of the methods used may have underestimated the diversity of gastric nematode species in grey kangaroos, particularly small species.
Some species of Labiosimplex (Labiosimplex longispicularis in red kangaroos, O. rufus) are known to be highly seasonal in their occurrence (Mykytowycz and Dudzinski 1965), but equivalent data are lacking for the grey kangaroos and therefore the timing of collections may have affected the prevalence data. In addition, the prevalence of Globocephaloides infections is heavily influenced by age (Arundel et al. 1977), with higher prevalences in juvenile kangaroos, an age group that was effectively excluded from the present study. A further complication in the present data set is the recognition of cryptic species of some cestodes (P. festiva, P. macropodis; T. undosa; W. ewersi) (Hu et al. 2005; Beveridge 2009; Beveridge and Shamsi 2009; Hardman et al. 2012) which have not been fully resolved taxonomically, complicating comparisons across host species.
In addition, although every attempt was made to identify kangaroos correctly, Neaves et al. (2010) reported introgression between the two host species in the zone of sympatry in eastern Australia. In their study, a small number of kangaroos identified morphologically as M. fuliginosus proved to be M. giganteus backcrosses.
Given these caveats, the helminth communities in both species of grey kangaroos can now be defined relatively well in a very broad sense. Helminth species could be categorised as primarily parasites of the two species of grey kangaroos or from infections acquired from related host species occurring in sympatry, the latter indicated by very low prevalences in grey kangaroos (1–2%) compared with a high prevalence in a sympatric host species. Examples include Cloacina australis (prevalence in M. giganteus 1.1%, in N. agilis 87%: Speare et al. 1983), Cloacina macropodis (prevalence in M. fuliginosus 0.9%, in O. robustus 76%: Beveridge 2020c) and Wallabinema cobbi (prevalence in M. fuliginosus 0.9%, in O. rufus, 14%: Beveridge 2020d). In other instances, helminths such as Hypodontus macropi occurred at higher prevalences (9.3% in M. fuliginosus, 1.7% in M. giganteus), but these infections occurred only in areas where the grey kangaroos were sympatric with O. rufus, the main host of this parasite (Arundel et al. 1979, prevalence 93%) and in this study more M. fuliginosus than M. giganteus were collected in areas of sympatry with O. rufus. Although H. macropi is a species complex, molecular data indicate that nematodes from M. fuliginosus are identical to those in O. rufus (Chilton et al. 2012). A similar situation applies to R. australis, with a high prevalence in O. rufus (98% in Arundel et al. 1979) and lower prevalences in the grey kangaroos but exclusively in areas of sympatry with O. rufus (Beveridge et al. 2021).
In north-eastern Australia, several helminth species (Alocostoma clelandi, Alocostoma propinquum, Pharyngostrongylus lambda, Papillostrongylus barbatus, Popovastrongylus macropodis) occured in the grey kangaroos as well as in the sympatric host species O. rufus and O. robustus, sometimes at comparable prevalences (Beveridge 2020c, 2020d), providing difficulties in determining a possible primary host species using this criterion. For example, A. clelandi and P. lambda have comparable prevalences in O. robustus and M. giganteus (Beveridge 2020c) while P. macropodis occurs at similar prevalences in O. rufus and M. giganteus (Beveridge 2020d). As P. barbatus occurs at a prevalence of 43% in O. rufus (Beveridge 2020d), compared with only 9% in M. giganteus, and 4% in O. robustus (Beveridge 2020c), the former host is probably the principal host. P. pearsoni is particularly problematical, having been described originally from a rock wallaby, Petrogale lateralis pearsoni (Beveridge and Smales 2022), but having a high prevalence in Kangaroo Island wallabies, N. eugenii (Smales and Mawson 1978) and occurring in M. fuliginosus at a high prevalence on Kangaroo Island and to a lesser extent in both M. fuliginosus and M. giganteus on mainland Australia. Here, the primary host has been assumed to be N. eugenii, but based exclusively on prevalence data. Similarly, in south-eastern Australia, G. trifidospicularis is a common parasite of both species of grey kangaroos in south-eastern Australia, but also occurs in the sympatric species N. eugenii (prevalence 46%), N. rufogriseus (prevalence 27%) and W. bicolor (prevalence 35%) (Beveridge 1979). Cloacina typhon occurs in O. robustus at a prevalence of 23% (Beveridge 2020c) compared with 20.7% in M. giganteus. However, unpublished DNA sequence data suggest that two different species are currently included under the single name (S. Middleton, pers. comm.).
In five species of Cloacina (Cloacina hermes, Cloacina hestia, Cloacina magnipapillata, Cloacina pelops, Cloacina selene), the nematode species occurred at moderate prevalences in both species of kangaroos, but the differences between the two host species were statistically significant. Statistical analyses were undertaken for each helminth species to provide a more objective basis for assigning them to the different categories shown in Table 1. However, in these cases, the only apparent explanations for the significant differences in prevalence may relate simply to the geographical distribution of the kangaroo samples in relation to the geographical distributions of the helminth species (see below) or to the techniques by which gastric helminth diversity was assessed. Thus the comparative prevalences can be useful in assigning roles within the helminth community, but need to be treated with a degree of caution.
In spite of these qualifications, the communities of helminth parasites in both M. fuliginosus and M. giganteus conform with previous studies of these communities and those of several related host species (Beveridge et al. 1998; Beveridge 2016, 2020c, 2020d) with a unimodal, left-skewed distribution for the frequency of helminth prevalence classes (Fig. 2). Webley et al. (2004) found a bimodal pattern in M. fuliginosus on Kangaroo Island, but with only a small sample size (25). That this same pattern is as clearly illustrated in the helminth species specific to grey kangaroos as in the total helminth community suggests that as the lower prevalence classes are constituted both by host-specific species and infections from sympatric host species and that the frequency distributions cannot be used to effectively differentiate between ‘core’ and ‘satellite’ helminth species within the community (Hanski 1982). Clearly, the species occurring at a high prevalence can be considered ‘core’ species, but those occurring at lower prevalences may be less common but host-specific species or transfers from related host species.
The similarity between the two communities, using Sorenson’s index, was high (85.4%), similar to the 85% reported from kangaroos in western Victoria by Aussavy et al. (2011). The result is not surprising given the close phylogenetic relationships of the two host species (Meredith et al. 2008).
The diversity of the helminth communities as measured by Simpson’s Index was similar in both kangaroo species, 34.0 in M. fuliginosus and 31.8 in M. giganteus. These values are much higher than those reported earlier, 18.1 for M. fuliginosus (Aussavy et al. 2011) and 12.9–16.3 in M. giganteus (Beveridge et al. 1998; Aussavy et al. 2011; Vendl and Beveridge 2014), but the sample size in the present study was much larger (Beveridge et al. 1998: 28 M. giganteus; Aussavy et al. 2011: 10 M. fuliginosus, 18 M. giganteus; Vendl and Beveridge 2014: 16 M. giganteus) and the total number of helminth species, 64, was much greater than the numbers of species encountered in earlier studies (Beveridge et al. 1998, 18; Aussavy et al. 2011, 16; Vendl and Beveridge 2014, 15). The differences in scale between these studies most likely explains the obvious differences in Simpson’s Index.
Five helminth species (C. cf. artemis, L. occidentalis, M. ocydromi, Progamotaenia effigia, R. disjunctus) occured exclusively in M. fuliginosus and five (C. leto, C. typhon, Macroponema arundeli, P. festiva, Torquenema toraliforme) exclusively in M. giganteus. However, in the zones of host sympatry, some degree of interchange of parasites occured. In the Grampian Ranges of western Victoria, Aussavy et al. (2011) reported exchanges of P. festiva, M. comani, Macropostrongyloides mawsonae and C. pelops from M. giganteus to M. fuliginosus and of Macropostrongyloides yamagutii and Paramacropostrongylus typicus from M. fuliginosus to M. giganteus. In south-western Victoria, a single instance was found of L. bipapillosus in M. fuliginosus (Smales 1995), with all other records being from M. giganteus (Fig. 3a). In north-western New South Wales, Paramacropostrongylus iugalis, primarily parasitic in M. giganteus, was found in M. fuliginosus (Fig. 4a). However, in the latter case, Chilton et al. (1997) found evidence of hybridisation between P. iugalis and P. typicus in this region using allozyme electrophoresis, a result not supported by DNA sequence data (Sukee et al. 2021b). Pending resolution of this inconsistency, the data provided by the sequence data have been followed here. In the same region, R. moennigi, primarily a parasite of M. fuliginosus, also occurred in M. giganteus (Beveridge et al. 2021).
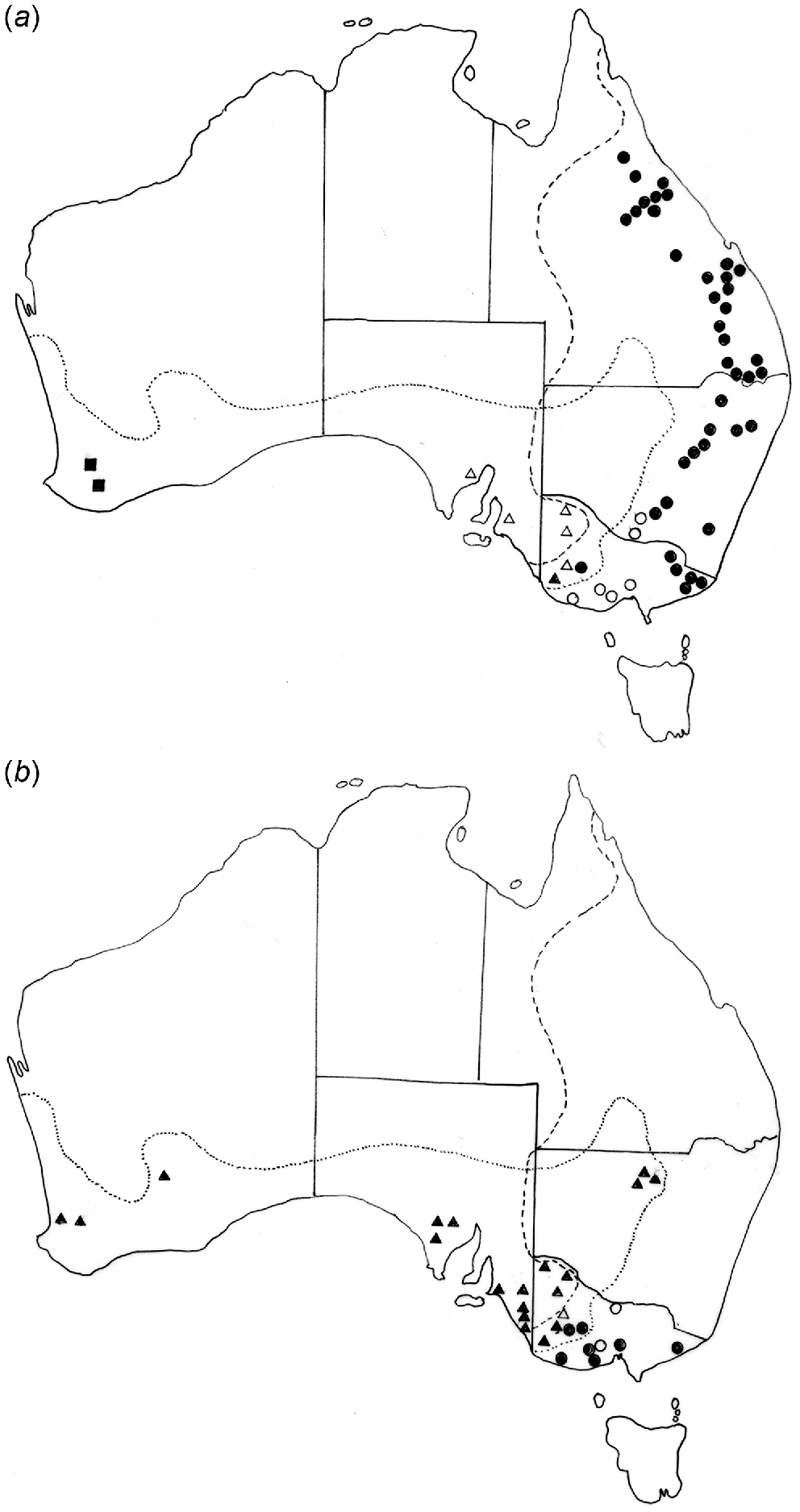
(a) Distribution of Labiosimplex bipapillosus in Macropus giganteus (•) and M. fuliginosus (▴), Labiosimplex major in Macropus giganteus (○) and M. fuliginosus (Δ) and Labiosimplex occidentalis in M. fuliginosus (▪). (b) Distribution of Labiosimplex kungi in Macropus giganteus (•) and M. fuliginosus (▴) and Labiosimplex laterilabellosus in Macropus giganteus (○) and M. fuliginosus (Δ).
(a) Distribution of Paramacropostrongylus iugalis in Macropus giganteus (○) and M. fuliginosus (Δ) and P. typicus in Macropus giganteus (•) and M. fuliginosus (▴). (b) Distribution of Cloacina magnipapillata in Macropus giganteus (•) and M. fuliginosus (▴) and of C. pelops in Macropus giganteus (○) and M. fuliginosus (Δ).
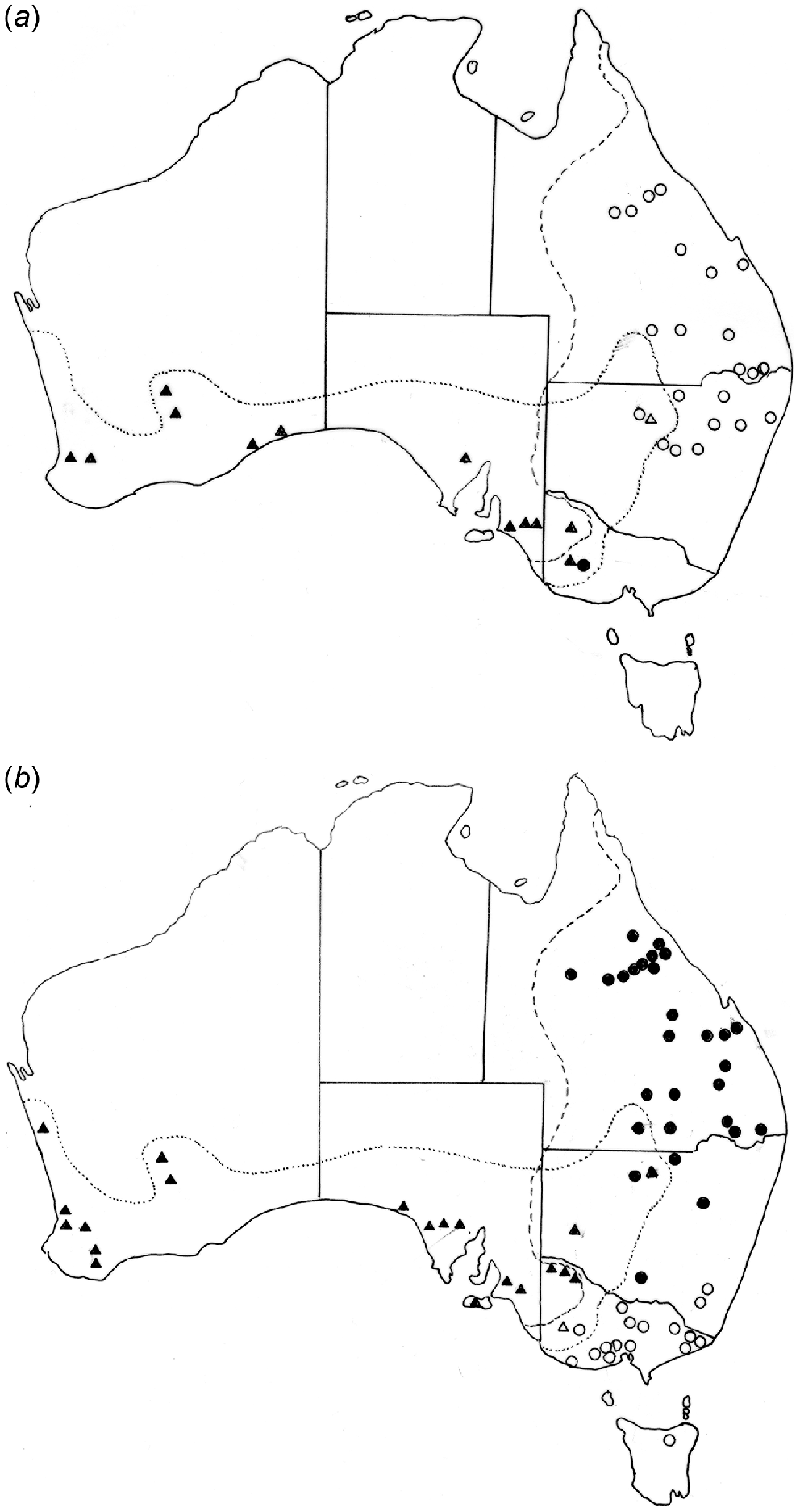
Additional helminth species appear to have been exchanged and subsequently spread beyond the immediate zone of sympatry. Rugopharynx rosemariae is primarily a parasite of M. giganteus in south-eastern Australia, but has extended its range in M. fuliginosus beyond the zone of sympatry to the Fleurieu Peninsula of South Australia. However, M. giganteus formerly occurred on Kangaroo Island (Seersholm et al. 2021) and therefore also presumably on the Fleurieu Peninsula. In addition, it was noted by Beveridge (1979) that this species appears to be limited by rainfall in south-eastern Australia, most collections falling within the 500 mm isohyet and this may be an additional factor in the limited distribution of this nematode species in M. fuliginosus, which tends to occupy areas of lower rainfall (Caughley et al. 1987). Rugopharynx macropodis, which also occurs in both grey kangaroo species, appears to be limited in its distribution by rainfall (Beveridge 2020e). Labiosimplex kungi is presumed to be a parasite primarily of M. fuliginosus based on its geographical distribution (Fig. 3b), but appears to have colonised M. giganteus in Victoria as far east as Gippsland. Although also present in north-western New South Wales, it has not been found in sympatric host species in that area to date, suggesting that factors additional to observed sympatry may be involved in the transfer of helminth species.
The interchange of parasites between M. fuliginosus and M. giganteus in areas of sympatry appears to be complex, particularly because of known genetic introgression between the two host species in the zone of sympatry (Neaves et al. 2010), but the current data are limited by inadequate sampling in areas of host sympatry and the topic warrants more detailed studies.
The influence of parasite species distributions on the collection of prevalence data has been relatively neglected in studies of the helminth communities of macropodids to date, in part because studies have been regional (e.g. Beveridge et al. 1989, 1992, 1998; Spratt et al. 2017) or because the geographical distribution of the parasite species has been poorly documented. This situation has been remedied to some extent in more recent studies (Beveridge 2016, 2020c, 2020d) and is considered here in the case of the grey kangaroos.
Neaves et al. (2009) divided M. fuliginosus into four genetic units (Fig. 1) and the principal conclusion derived from the present study is that the Kangaroo Island genetic unit stands out as singularly helminth species depauperate (Table 2), supporting the conclusions of Webley et al. (2004), who at the time lacked adequate comparable data from mainland populations. This may be due to a ‘founder’ effect with colonisation of an island by a small number of macropodid hosts although the climatic differences on the island and their possible effects on the development of larval nematode stages cannot be ruled out (Webley et al. 2004).
Although less definitive due to the difficulty of drawing boundaries between northern and southern populations, the data available for M. giganteus suggest a decrease in helminth species diversity in northern populations (Table 3), consistent with the decrease in genetic diversity in the host. The hypothesis that M. giganteus originated in south-eastern Australia and subsequently migrated northwards (Zenger et al. 2003) is consistent with a subsequent loss not only of genetic but also parasite diversity. The significance of geographical distributions of parasites on the potential determination of prevalences is best demonstrated using selected distribution maps of major helminth species, or those with an unusual distribution, some of which have already been indicated in Tables 2 and 3.
Labiosimplex bipapillosus is an example of an eastern Australian species extending from Queensland to Victoria (Fig. 3a). Species with similar distributions are P. kappa, C. herceus, and C. leto. Labiosimplex major occurs in the south-east of the continent but in both species of grey kangaroos (Fig. 3a), whereas Labiosimplex laterilabellosus has a similar distribution (Fig. 3b). L. occidentalis is an example of a species restricted to the ‘western’ genetic unit of M. fuliginosus (Fig. 3a). The two congeners, P. iugalis and P. typicus are distributed in the north-east and in the south-west, respectively (Fig. 4a), with one currently known area of sympatry, although this may be due to relatively limited collecting in New South Wales. Cloacina magnipapillata is an example of a species extending from the north-east to the south-west, in both grey kangaroo species, but is absent from the south-east where it is apparently replaced by the closely related species, C. pelops (Fig. 4b). Other species of Cloacina, C. expansa, C. hera, C. hermes, C. hestia, Cloacina obtusa, C. selene) have a broad distribution across the continent in both kangaroo species.
These examples indicate the need for some caution in interpreting the prevalence data provided in Table 1, as the overall prevalence for any given species is likely to be influenced by geographical distributions of the collection localities. In this study, collections were opportunistic and there are potential biases towards sites at which collection was easier, either due to host densities, in instances where climatic conditions prevented collection (the wet season in northern Australia), or where facilities used for processing host animals were more readily accessible.
A number of helminth species, for which reliable records of their occurrence in free-ranging hosts exist (Spratt and Beveridge 2016), were not encountered in the present study due to a number of reasons, primarily due to their rarity in these host species. Some species such as Globocephaloides macropodis and Globocephaloides affinis are common in sympatric host species, N. agilis and N. dorsalis, respectively, but have been found in M. giganteus on a single occasion in each instance (Beveridge et al. 1984; Fazenda et al. 2010). Records for the occurrence of the cloacinine nematode Zoniolaimus latebrosus in both M. fuliginosus and M. giganteus and the trichostrongylid nematode Filarinema beveridgei in M. fuliginosus are based on single findings (Spratt and Beveridge 2016) but with neither species being encountered in the present survey. Dunsmore and Howkins (1968) reported the presence of the larval cestode stage of Taenia serialis in the subcutaneous tissues of M. giganteus. The larval stage of this cestode occurs most commonly in rabbits, with canids as the definitive hosts, but has not been found since the original report.
In conclusion, the current study provides insights into the helminth communities of M. fuliginosus and M. giganteus across their entire geographical ranges for the first time. It also provides the basis for more detailed studies of the exchange of parasite species between two closely related host species which evolved in allopatry but now occur in sympatry in different parts of the continent. More generally, it provides insights into the way in which helminth parasites have been affected by the evolution of the two closely related host species.
Ethical statement
Where required, all specimens were collected under relevant state government permits.
Acknowledgements
Thanks are due to numerous colleagues who have helped in the collection of the data presented here, including Ross Andrews, (the late) Jack Arundel, Ian Barker, Neil Chilton, Graeme Coulson, Brian Coman, Jasmin Hufschmid, Robin Gasser, Abdul Jabbar, Peter Johnson, (the late) Barry Munday, John Nelson, Michael O’Callaghan, David Obendorf, Shane Middleton, (the late) Paul Presidente, Dave Spratt, Tanapan Sukee, Lesley Warner and Lee Webley. Lesley Warner is thanked particularly for identifications of the species of Labiosimplex and Dave Spratt for identification of filarioid nematodes. Graeme Coulson is thanked for comments on photographs of kangaroos in cases of uncertain specific identity and Dave Spratt for comments on a draft of the manuscript. The reviewers are thanked for making substantial improvements to the manuscript.
References
Arundel JH, Beveridge I, Presidente PJ (1979) Parasites and pathological findings in enclosed and free-ranging populations of Macropus rufus (Demarest) (Marsupialia) at Menindee, New South Wales. Australian Wildlife Research 6, 361-379.
| Crossref | Google Scholar |
Aussavy M, Bernardin E, Corrigan A, Hufschmid J, Beveridge I (2011) Helminth parasite communities in four species of sympatric macropodids in western Victoria. Australian Mammalogy 33, 13-20.
| Crossref | Google Scholar |
Beveridge I (1976) A taxonomic revision of the Anoplocephalidae, (Cestoda: Cyclophyllidea) of Australian marsupials. Australian Journal of Zoology Supplementary Series 24, 1-110.
| Crossref | Google Scholar |
Beveridge I (1979) A review of the Globocephaloidinae Inglis (Nematoda: Amidostomatidae) from macropodid marsupials. Australian Journal of Zoology 27, 151-175.
| Crossref | Google Scholar |
Beveridge I (2009) A re-description of Progamotaenia ewersi (Schmidt, 1975) (Cestoda: Anoplocephalidae) from wallabies and kangaroos (Macropodidae) with the description of a new species, Progamotaenia ualabati n. sp. Transactions of the Royal Society of South Australia 133, 1-17.
| Crossref | Google Scholar |
Beveridge I (2016) The gastro-intestinal helminth parasites of the swamp wallaby, Wallabia bicolor (Desmarest) (Marsupialia: Macropodidae), and their regional distribution. Transactions of the Royal Society of South Australia 140, 203-227.
| Crossref | Google Scholar |
Beveridge I (2020a) Four new species of Rugopharynx Mönnig, 1927 (Nematoda: Strongyloidea) parasitic in the stomachs of macropodid marsupials from Australia. Systematic Parasitology 97, 41-55.
| Crossref | Google Scholar | PubMed |
Beveridge I (2020b) Cloacina celata n. sp. and a new record of C. io Beveridge, 1998 (Nematoda: Strongyloidea) parasitic in the black-striped wallaby, Notamacropus dorsalis (Gray) from Queensland. Transactions of the Royal Society of South Australia 144, 215-223.
| Crossref | Google Scholar |
Beveridge I (2020c) Gastrointestinal helminth parasites of the common wallaroo or euro, Osphranter robustus (Gould) (Marsupialia: Macropodidae) from Australia. Journal of Helminthology 94, e114.
| Crossref | Google Scholar | PubMed |
Beveridge I (2020d) Gastrointestinal helminth parasites of the red kangaroo, Osphranter rufus (Desmarest) (Marsupialia: Macropodidae) and their regional distribution. Transactions of the Royal Society of South Australia 144, 200-214.
| Crossref | Google Scholar |
Beveridge I (2020e) Mammal parasites in arid Australia. International Journal for Parasitology: Parasites and Wildlife 12, 265-274.
| Crossref | Google Scholar | PubMed |
Beveridge I, Arundel JH (1979) Helminth parasites of grey kangaroos, Macropus giganteus Shaw and M. fuliginosus (Desmarest), in eastern Australia. Australian Wildlife Research 6, 69-77.
| Crossref | Google Scholar |
Beveridge I, Durette-Desset M-C (2010) Two new species of Filarinema Mönnig, 1929 (Nematoda: Trichostrongylina) parasitic in macropodid marsupials. Transactions of the Royal Society of South Australia 134, 164-171.
| Crossref | Google Scholar |
Beveridge I, Mawson PM (1978) A taxonomic revision of the genera Macropostrongyloides Yamaguti and Paramacropostrongylus Johnston & Mawson (Nematoda: Trichonematidae) from Australian marsupials. Australian Journal of Zoology 26, 763-787.
| Crossref | Google Scholar |
Beveridge I, Shamsi S (2009) Revision of the Progamotaenia festiva species complex (Cestoda: Anoplocephalidae) from Australasian marsupials, with the resurrection of P. fellicola (Nybelin, 1917) comb. nov. Zootaxa 1990, 1-29.
| Crossref | Google Scholar |
Beveridge I, Smales LR (2022) Review of the Cloacininae Stossich (Nemata: Strongyloidea) from Australasian marsupials (Marsupialia: Macropodoidea). MANTER: Journal of Parasite Biodiversity 19, 1-197.
| Crossref | Google Scholar |
Beveridge I, Spratt DM (1988) A redescription of Filarinema dissimile (Wood, 1931), with new records of other species of Filarinema Moennig, 1929 (Nematoda: Trichostrongyloidea) from macropodid marsupials. Transactions of the Royal Society of South Australia 112, 57-62.
| Google Scholar |
Beveridge I, Speare R, Johnson PM (1984) New records of Globocephaloidinae (Nematoda: Trichostrongyloidea) from Macropodidae in North Queensland. Transactions of the Royal Society of South Australia 108, 197-201.
| Google Scholar |
Beveridge I, Spratt DM, Close RL, Barker SC, Sharman GB (1989) Helminth parasites of rock-wallabies, Petrogale spp (Marsupialia), from Queensland. Australian Wildlife Research 16, 273-287.
| Crossref | Google Scholar |
Beveridge I, Speare R, Johnson PM, Spratt DM (1992) Helminth parasite communities of macropodoid marsupials of the genera Hypsiprymnodon, Aepyprymnus, Thylogale, Onychogale, Lagorchestes and Dendrolagus from Queensland. Wildlife Research 19, 359-376.
| Crossref | Google Scholar |
Beveridge I, Chilton NB, Johnson PM, Smales LR, Speare R, Spratt DM (1998) Helminth parasite communities of kangaroos and wallabies (Macropus spp. and Wallabia bicolor) from north and central Queensland. Australian Journal of Zoology 46, 473-495.
| Crossref | Google Scholar |
Beveridge I, Sukee T, Jabbar A (2021) Redescription of Rugopharynx australis (Mönnig, 1926) and the description of R. moennigi n. sp. (Nematoda: Strongyloidea) from kangaroos (Marsupialia: Macropodidae) in Australia. Systematic Parasitology 98, 679-695.
| Crossref | Google Scholar | PubMed |
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. The Journal of Parasitology 83, 575-583.
| Crossref | Google Scholar | PubMed |
Cassone J, Baccam D (1985) Le genre Filarinema Mönnig, 1929 (Nematoda, Trichostrongyloidea) parasites de marsupiaux Australiens. Bulletin du Muséum national d’Histoire naturelle, Paris, 4ème série 7, 349-382.
| Google Scholar |
Caughley G, Short J, Grigg GC, Nix H (1987) Kangaroos and climate: an analysis of distribution. Journal of Animal Ecology 56, 751-761.
| Crossref | Google Scholar |
Chilton NB, Beveridge I, Hoste H, Gasser RB (1997) Evidence for hybridisation between Paramacropostrongylus iugalis and P. typicus (Nematoda: Strongyloidea) in grey kangaroos, Macropus fuliginosus and M. giganteus, in a zone of sympatry in eastern Australia. International Journal for Parasitology 27, 475-482.
| Crossref | Google Scholar | PubMed |
Chilton NB, Jabbar A, Huby-Chilton F, Jex A, Gasser RB, Beveridge I (2012) Genetic variation within the Hypodontus macropi (Nematoda: Strongyloidea) complex from macropodid marsupial hosts in Australia. Electrophoresis 33, 3544-3554.
| Crossref | Google Scholar | PubMed |
Dunsmore JD, Howkins AB (1968) Coenurus serialis in a grey kangaroo. Australian Journal of Science 30, 465.
| Google Scholar |
Durette-Desset MC, Beveridge I (2012) Redescriptions and descriptions of new species of Austrostrongylus Chandler, 1924 (Nematoda: Trichostrongylina), from Australian marsupials with a comparative study of features of the synlophe. Zootaxa 3512, 1-41.
| Crossref | Google Scholar |
Fazenda I, Gasser R, Carvalho L, Beveridge I (2010) Resurrection and redescription of Globocephaloides wallabiae Johnston et Mawson, 1939 (Nematoda, Trichostrongyloidea) from macropodid marsupials in north-eastern Australia. Acta Parasitologica 55, 138-143.
| Crossref | Google Scholar |
Hanski I (1982) Dynamics of regional distribution: the core and satellite species hypothesis. Oikos 38, 210-221.
| Crossref | Google Scholar |
Hardman LM, Haukisalmi V, Beveridge I (2012) Phylogenetic relationships of the anoplocephaline cestodes of Australasian marsupials and resurrection of the genus Wallabicestus Schmidt, 1975. Systematic Parasitology 82, 49-63.
| Crossref | Google Scholar | PubMed |
Hu M, Gasser RB, Chilton NB, Beveridge I (2005) Genetic variation in the mitochondrial cytochrome c oxidase subunit 1 within three species of Progamotaenia (Cestoda: Anoplocephalidae) from macropodid marsupials. Parasitology 130, 117-129.
| Crossref | Google Scholar | PubMed |
Mawson PM (1964) Some Nematoda (Strongylina and Oxyurina) from kangaroos (Macropus spp.) from eastern Australia. Parasitology 54, 237-262.
| Crossref | Google Scholar | PubMed |
Meredith RW, Westerman M, Springer MS (2008) A phylogeny and timescale for the living genera of kangaroos and kin (Macropodiformes: Marsupialia) based on nuclear DNA sequences. Australian Journal of Zoology 56, 395-410.
| Crossref | Google Scholar |
Mykytowycz R, Dudzinski ML (1965) Sex ratio, weight, length and numbers of Labiostrongylus longispicularis (Wood), the large stomach worm of the red kangaroo (Megaleia rufa (Desmarest)), in relation to age of the host and season. Parasitology 55, 527-541.
| Crossref | Google Scholar |
Neaves LE, Zenger KR, Prince RIT, Eldridge MDB, Cooper DW (2009) Landscape discontinuities influence gene flow and genetic structure in a large, vagile Australian mammal, Macropus fuliginosus. Molecular Ecology 18, 3363-3378.
| Crossref | Google Scholar | PubMed |
Neaves LE, Zenger KR, Cooper DW, Eldridge MDB (2010) Molecular detection of hybridization between sympatric kangaroo species in south-eastern Australia. Heredity 104, 502-512.
| Crossref | Google Scholar | PubMed |
Seersholm FV, Grealy A, McDowell MC, Cole TL, Arnold LJ, Prideaux GJ, Bunce M (2021) Ancient DNA from bulk bone reveals past genetic diversity of vertebrate fauna on Kangaroo Island, Australia. Quaternary Science Reviews 262, 106962.
| Crossref | Google Scholar |
Smales LR (1995) A revision of the subgenus Labiostrongylus (Labiosimplex) (Nematoda: Cloacinidae) from macropodid marsupials, with descriptions of twelve new species and a key to the species of the subgenus. Invertebrate Taxonomy 9, 181-242.
| Crossref | Google Scholar |
Smales LR, Mawson PM (1978) Nematode parasites of the Kangaroo Island wallaby, Macropus eugenii (Desmarest). 1. Seasonal and geographical distribution. Transactions of the Royal Society of South Australia 102, 9-16.
| Google Scholar |
Speare R, Beveridge I, Johnson PM, Corner LA (1983) Parasites of the agile wallaby, Macropus agilis (Marsupialia). Australian Wildlife Research 10, 89-96.
| Crossref | Google Scholar |
Spratt DM (2011) New records of filarioid nematodes (Nematoda: Filarioidea) parasitic in Australasian monotremes, marsupials and murids, with descriptions of nine new species. Zootaxa 2860, 1-61.
| Crossref | Google Scholar |
Spratt DM (2023) Redescription of species of Gongylonema Molin, 1857 (Nematoda: Spiruroidea: Gongylonematidae) parasitic in some Australian vertebrate hosts and description of three new species. Zootaxa 5239, 204-220.
| Crossref | Google Scholar | PubMed |
Spratt DM, Beveridge I (2016) Helminth parasites of Australasian monotremes and marsupials. Zootaxa 4123, 1-198.
| Crossref | Google Scholar | PubMed |
Spratt DM, Varughese G (1975) A taxonomic revision of filarioid nematodes from Australian marsupials. Australian Journal of Zoology Supplementary Series 23, 1-99.
| Crossref | Google Scholar |
Spratt DM, Walter EL, Haycock P (2017) Oesophageal and stomach nematode communities in three sympatric macropodid species in coastal and montane environments in southeastern New South Wales. Transactions of the Royal Society of South Australia 141, 237-252.
| Crossref | Google Scholar |
Sukee T, Beveridge I, Jabbar A (2020a) New species of Macropostrongyloides Yamaguti, 1961 (Nematoda: Strongylida) and the redescription of Ma. baylisi (Wood, 1930) from Australian macropodid marsupials. Systematic Parasitology 97, 267-284.
| Crossref | Google Scholar | PubMed |
Sukee T, Jabbar A, Beveridge I (2020b) Revision of Macroponema Mawson, 1978 (Nematoda: Strongylida) from macropodid marsupials with the description of two new species. Parasites & Vectors 13, 298.
| Crossref | Google Scholar | PubMed |
Sukee T, Beveridge I, Jabbar A (2021a) Torquenema n. g., Wallabicola n. g., and Macropostrongyloides phascolomys n. sp.: new genera and a new species of nematode (Strongylida: Phascolostrongylinae) parasitic in Australian macropodid and vombatid marsupials. Animals 11, 175.
| Crossref | Google Scholar | PubMed |
Sukee T, Beveridge I, Sabir AJ, Jabbar A (2021b) Phylogenetic relationships within the nematode subfamily Phascolostrongylinae (Nematoda: Strongyloidea) from Australian macropodid and vombatid marsupials. Microorganisms 9, 9.
| Crossref | Google Scholar | PubMed |
Vendl C, Beveridge I (2014) Estimations of species richness in the complex communities of nematode parasites found in the stomachs of kangaroos and wallabies (Family Macropodidae). Transactions of the Royal Society of South Australia 138, 105-112.
| Crossref | Google Scholar |
Webley LS, Beveridge I, Coulson G (2004) Endoparasites of an insular subspecies of the western grey kangaroo, Macropus fuliginosus. Australian Journal of Zoology 52, 623-634.
| Crossref | Google Scholar |
Zenger KR, Eldridge MDB, Cooper DW (2003) Intraspecific variation, sex-biased dispersal and phylogeography of the eastern grey kangaroo (Macropus giganteus). Heredity 91, 153-162.
| Crossref | Google Scholar | PubMed |


